Vaginal Administration of Progesterone in Twin Gestation: Influence on Bone Turnover and Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Blood Sampling
2.3. Bone Turnover
2.4. Alkaline Phosphatase
2.5. Total Antioxidant Capacity (TAC)
2.6. 8-Hydroxy-2’-deoxyguanosine (8-OHdG)
2.7. Thiobarbituric Acid–Reactive Substances (TBARS) Measurement
2.8. Statistical Analysis
3. Results
3.1. Maternal–Neonatal Characteristics and Clinical Outcomes
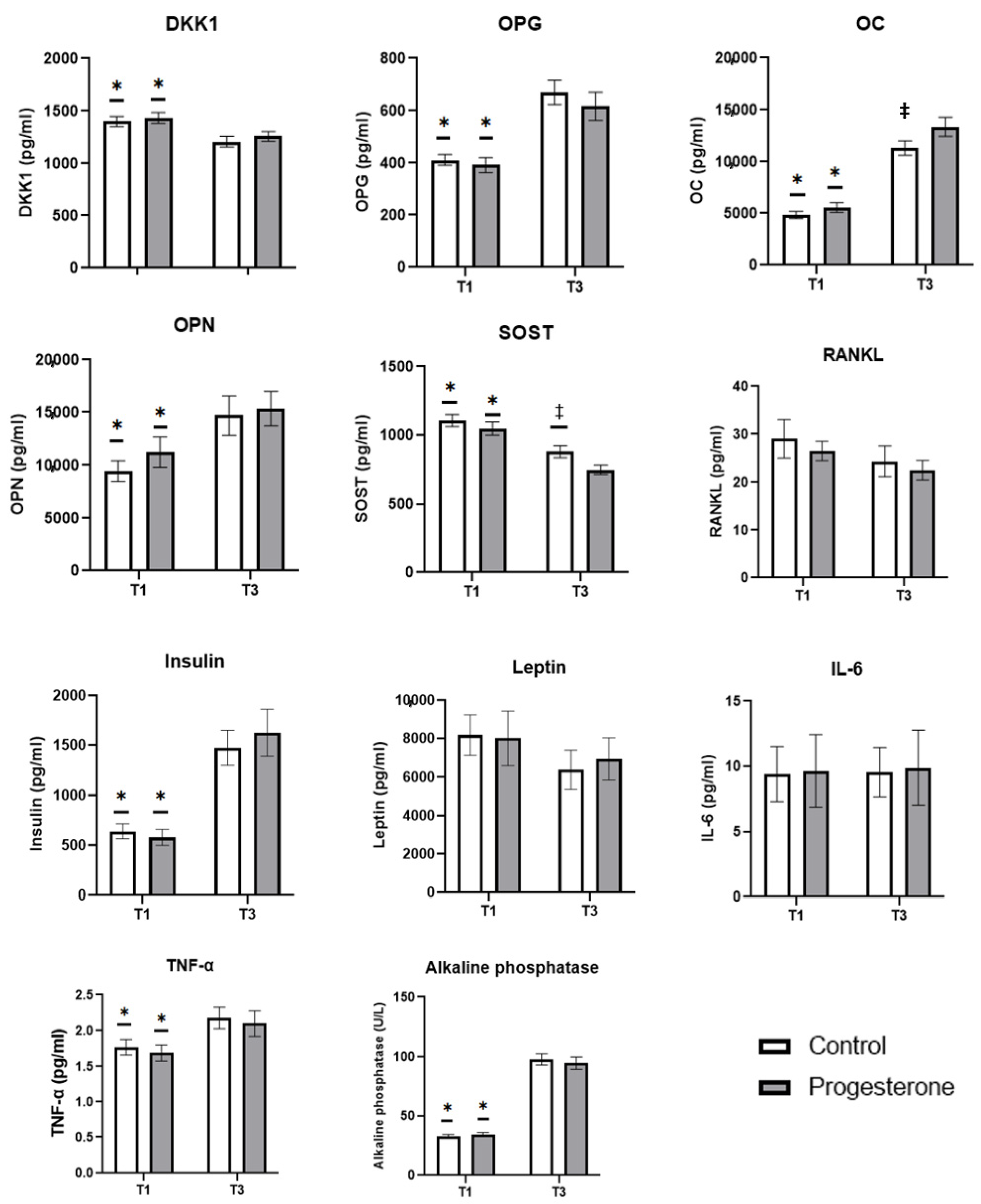
3.2. Biomarkers of Bone Turnover Studied
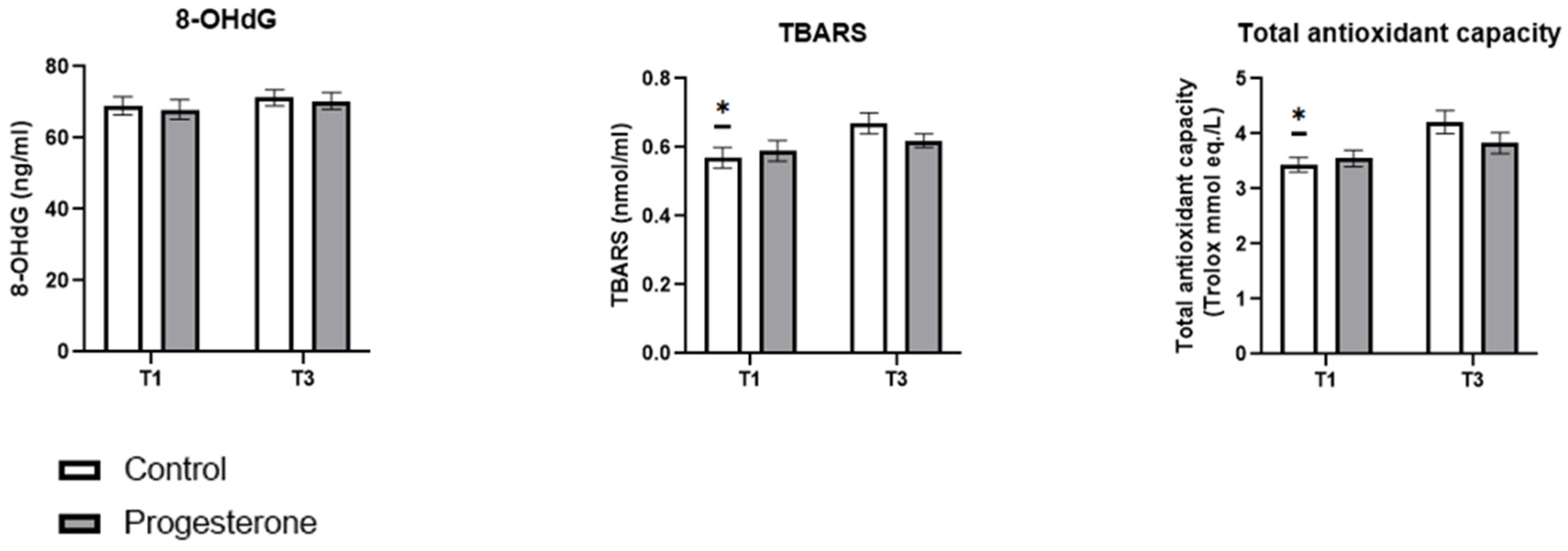
3.3. Biomarkers of Oxidative Stress Studied
3.4. Regressions Between Maternal and Neonatal Variables of Interest and the Biomarkers Studied
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blondel, B.; Kaminski, M. Trends in the occurrence, determinants, and consequences of multiple births. Semin. Perinatol. 2002, 26, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, M.; Greatholder, I.; Kilby, M.D.; Heazell, A.E.P. Risk factors for adverse outcomes in twin pregnancies: A narrative review. J. Matern. Fetal Neonatal Med. 2023, 36, 2240467. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.P.; Torloni, M.R.; Seuc, A.; Betrán, A.P.; Widmer, M.; Souza, J.P.; Merialdi, M. Maternal and perinatal outcomes of twin pregnancy in 23 low- and middle-income countries. PLoS ONE 2013, 8, e70549. [Google Scholar] [CrossRef]
- Lorenz, J.M. Neurodevelopmental outcomes of twins. Semin. Perinatol. 2012, 36, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Luke, B.; Brown, M.B. Contemporary risks of maternal morbidity and adverse outcomes with increasing maternal age and plurality. Fertil. Steril. 2007, 88, 283–293. [Google Scholar] [CrossRef]
- Chauhan, S.P.; Scardo, J.A.; Hayes, E.; Abuhamad, A.Z.; Berghella, V. Twins: Prevalence, problems, and preterm births. Am. J. Obstet. Gynecol. 2010, 203, 305–315. [Google Scholar] [CrossRef]
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.; Driscoll, A.K.; Mathews, T.J. Births: Final Data for 2015. Natl. Vital Stat. Rep. 2017, 66, 1. [Google Scholar]
- Romero, R.; Conde-Agudelo, A.; El-Refaie, W.; Rode, L.; Brizot, M.L.; Cetingoz, E.; Serra, V.; Da Fonseca, E.; Abdelhafez, M.S.; Tabor, A.; et al. Vaginal progesterone decreases preterm birth and neonatal morbidity and mortality in women with a twin gestation and a short cervix: An updated meta-analysis of individual patient data. Ultrasound Obstet. Gynecol. 2017, 49, 303–314. [Google Scholar] [CrossRef]
- Azeez, J.M.; Susmi, T.R.; Remadevi, V.; Ravindran, V.; Sasikumar Sujatha, A.; Ayswarya, R.N.S.; Sreeja, S. New insights into the functions of progesterone receptor (PR) isoforms and progesterone signaling. Am. J. Cancer Res. 2021, 11, 5214–5232. [Google Scholar]
- Keelan, J.A. Intrauterine inflammatory activation, functional progesterone withdrawal, and the timing of term and preterm birth. J. Reprod. Immunol. 2018, 125, 89–99. [Google Scholar] [CrossRef]
- Kolatorova, L.; Vitku, J.; Suchopar, J.; Hill, M.; Parizek, A. Progesterone: A Steroid with Wide Range of Effects in Physiology as Well as Human Medicine. Int. J. Mol. Sci. 2022, 23, 7989. [Google Scholar] [CrossRef] [PubMed]
- Glover, M.M.; McKenna, D.S.; Downing, C.M.; Smith, D.B.; Croom, C.S.; Sonek, J.D. A randomized trial of micronized progesterone for the prevention of recurrent preterm birth. Am. J. Perinatol. 2011, 28, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Nicolaides, K.; Conde-Agudelo, A.; Tabor, A.; O’Brien, J.M.; Cetingoz, E.; Da Fonseca, E.; Creasy, G.W.; Klein, K.; Rode, L.; et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: A systematic review and metaanalysis of individual patient data. Am. J. Obstet. Gynecol. 2012, 206, 124.e1–124.e19. [Google Scholar] [CrossRef]
- Stewart, L.A.; Simmonds, M.; Duley, L.; Dietz, K.C.; Harden, M.; Hodkinson, A.; Llewellyn, A.; Sharif, S.; Walker, R.; Wright, K. Evaluating progestogens for prevention of preterm birth international collaborative (EPPPIC) individual participant data (IPD) meta-analysis: Protocol. Syst. Rev. 2017, 6, 235. [Google Scholar] [CrossRef]
- Rehal, A.; Benkő, Z.; De Paco Matallana, C.; Syngelaki, A.; Janga, D.; Cicero, S.; Akolekar, R.; Singh, M.; Chaveeva, P.; Burgos, J.; et al. Early vaginal progesterone versus placebo in twin pregnancies for the prevention of spontaneous preterm birth: A randomized, double-blind trial. Am. J. Obstet. Gynecol. 2021, 224, 86.e1–86.e19. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.G.; Yang, L.; Nielsen, M.F.; Kassem, M.; Dhillo, W.S.; Comninos, A.N. The Relationship Between Bone and Reproductive Hormones Beyond Estrogens and Androgens. Endocr. Rev. 2021, 42, 691–719. [Google Scholar] [CrossRef]
- Chainy, G.B.N.; Sahoo, D.K. Hormones and oxidative stress: An overview. Free Radic. Res. 2020, 54, 1–26. [Google Scholar] [CrossRef]
- Nakayama, S.; Yasui, T.; Suto, M.; Sato, M.; Kaji, T.; Uemura, H.; Maeda, K.; Irahara, M. Differences in bone metabolism between singleton pregnancy and twin pregnancy. Bone 2011, 49, 513–519. [Google Scholar] [CrossRef]
- Sanz-Salvador, L.; García-Pérez, M.; Tarín, J.J.; Cano, A. Bone metabolic changes during pregnancy: A period of vulnerability to osteoporosis and fracture. Eur. J. Endocrinol. 2015, 172, R53–R65. [Google Scholar] [CrossRef]
- Fernandes, T.A.P.; Gonçalves, L.M.L.; Brito, J.A.A. Relationships between Bone Turnover and Energy Metabolism. J. Diabetes Res. 2017, 2017, 9021314. [Google Scholar] [CrossRef]
- Diaz-Castro, J.; Mira-Rufino, P.J.; Moreno-Fernandez, J.; Chirosa, I.; Chirosa, J.L.; Guisado, R.; Ochoa, J.J. Ubiquinol supplementation modulates energy metabolism and bone turnover during high intensity exercise. Food Funct. 2020, 11, 7523–7531. [Google Scholar] [CrossRef]
- Marcucci, G.; Domazetovic, V.; Nediani, C.; Ruzzolini, J.; Favre, C.; Brandi, M.L. Oxidative Stress and Natural Antioxidants in Osteoporosis: Novel Preventive and Therapeutic Approaches. Antioxidants 2023, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Toboła-Wróbel, K.; Pietryga, M.; Dydowicz, P.; Napierała, M.; Brązert, J.; Florek, E. Association of Oxidative Stress on Pregnancy. Oxidative Med. Cell. Longev. 2020, 2020, 6398520. [Google Scholar] [CrossRef] [PubMed]
- Seifert-Klauss, V.; Schmidmayr, M.; Hobmaier, E.; Wimmer, T. Progesterone and bone: A closer link than previously realized. Climacteric 2012, 15 (Suppl. S1), 26–31. [Google Scholar] [CrossRef] [PubMed]
- Prior, J.C. Progesterone for the prevention and treatment of osteoporosis in women. Climacteric 2018, 21, 366–374. [Google Scholar] [CrossRef]
- Trotter, A.; Maier, L.; Pohlandt, F. Management of the extremely preterm infant: Is the replacement of estradiol and progesterone beneficial? Paediatr. Drugs 2001, 3, 629–637. [Google Scholar] [CrossRef]
- Stekovic, S.; Ruckenstuhl, C.; Royer, P.; Winkler-Hermaden, C.; Carmona-Gutierrez, D.; Fröhlich, K.U.; Kroemer, G.; Madeo, F. The neuroprotective steroid progesterone promotes mitochondrial uncoupling, reduces cytosolic calcium and augments stress resistance in yeast cells. Microb. Cell 2017, 4, 191–199. [Google Scholar] [CrossRef]
- Cagnacci, A.; Gazzo, I.; Stigliani, S.; Paoletti, A.M.; Anserini, P.; Londero, A.P.; Xholli, A. Oxidative Stress: The Role of Estrogen and Progesterone. J. Clin. Med. 2023, 12, 7304. [Google Scholar] [CrossRef]
- Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar]
- Naylor, K.E.; Rogers, A.; Fraser, R.B.; Hall, V.; Eastell, R.; Blumsohn, A. Serum osteoprotegerin as a determinant of bone metabolism in a longitudinal study of human pregnancy and lactation. J. Clin. Endocrinol. Metab. 2003, 88, 5361–5365. [Google Scholar] [CrossRef][Green Version]
- Kovacs, C.S. The role of vitamin D in pregnancy and lactation: Insights from animal models and clinical studies. Annu. Rev. Nutr. 2012, 32, 97–123. [Google Scholar] [CrossRef] [PubMed]
- Black, A.J.; Topping, J.; Durham, B.; Farquharson, R.G.; Fraser, W.D. A detailed assessment of alterations in bone turnover, calcium homeostasis, and bone density in normal pregnancy. J. Bone Miner. Res. 2000, 15, 557–563. [Google Scholar] [CrossRef]
- Ulrich, U.; Miller, P.B.; Eyre, D.R.; Chesnut, C.H., 3rd; Schlebusch, H.; Soules, M.R. Bone remodeling and bone mineral density during pregnancy. Arch. Gynecol. Obstet. 2003, 268, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Møller, U.K.; Streym, S.; Mosekilde, L.; Heickendorff, L.; Flyvbjerg, A.; Frystyk, J.; Jensen, L.T.; Rejnmark, L. Changes in calcitropic hormones, bone markers and insulin-like growth factor I (IGF-I) during pregnancy and postpartum: A controlled cohort study. Osteoporos. Int. 2013, 24, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Shibata, O.; Mizuno, A.; Kobayashi, F.; Higashio, K.; Morinaga, T.; Tsuda, E. Immunological study on circulating murine osteoprotegerin/osteoclastogenesis inhibitory factor (OPG/OCIF): Possible role of OPG/OCIF in the prevention of osteoporosis in pregnancy. Biochem. Biophys. Res. Commun. 2001, 288, 217–224. [Google Scholar] [CrossRef]
- Wei, J.; Karsenty, G. An overview of the metabolic functions of osteocalcin. Rev. Endocr. Metab. Disord. 2015, 16, 93–98. [Google Scholar] [CrossRef]
- Liang, M.; Liao, E.Y.; Xu, X.; Luo, X.H.; Xiao, X.H. Effects of progesterone and 18-methyl levonorgestrel on osteoblastic cells. Endocr. Res. 2003, 29, 483–501. [Google Scholar] [CrossRef]
- Jürimäe, J.; Vaiksaar, S.; Mäestu, J.; Purge, P.; Jürimäe, T. Adiponectin and bone metabolism markers in female rowers: Eumenorrheic and oral contraceptive users. J. Endocrinol. Invest. 2011, 34, 835–839. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Sato, A.Y.; Bellido, T. Role and mechanism of action of sclerostin in bone. Bone 2017, 96, 29–37. [Google Scholar] [CrossRef]
- Dimas, A.; Politi, A.; Bargiota, A.; Panoskaltsis, T.; Vlahos, N.F.; Valsamakis, G. The Gestational Effects of Maternal Bone Marker Molecules on Fetal Growth, Metabolism and Long-Term Metabolic Health: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 8328. [Google Scholar] [CrossRef]
- El Amrousy, D.; El-Afify, D.; Shabana, A. Relationship between bone turnover markers and oxidative stress in children with type 1 diabetes mellitus. Pediatr. Res. 2021, 89, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.H.; Lo, L.M.; Chiu, T.H.; Li, M.J.; Yeh, Y.L.; Chen, S.F.; Hsieh, T.T. A longitudinal study of oxidative stress and antioxidant status in women with uncomplicated pregnancies throughout gestation. Reprod. Sci. 2010, 17, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Buey, B.; Grasa, L.; Mesonero, J.E.; Latorre, E. Protective role of short-chain fatty acids on intestinal oxidative stress induced by TNF-α. Cell Stress Chaperones 2024, 29, 769–776. [Google Scholar] [CrossRef] [PubMed]

| Control | Progesterone | ||
|---|---|---|---|
| Maternal age (years) | 34.6 ± 0.7 | 33.9 ± 0.8 | |
| Height (cm) | 165.0 ± 1.0 | 164.0 ± 0.9 | |
| Weight (kg) | 69.8 ± 2.0 | 69.4 ± 2.2 | |
| BMI (kg/m2) | 25.5 ± 0.7 | 25.7 ± 0.7 | |
| Conception | In vitro fertilization | 16 (31.4%) | 13 (28.3%) |
| Ovulation drugs | 1 (2.0%) | 1 (2.2%) | |
| Natural | 34 (66.7%) | 32 (69.6%) | |
| Parity | Nulliparous | 25 (49.0%) | 24 (52.1%) |
| Multiparous | 26 (51.0%) | 23 (47.9%) | |
| Chorion | Dichorionic | 42 (82.4%) | 40 (87.0%) |
| Monochorionic | 9 (17.6%) | 6 (13.0%) | |
| Cervical Length (mm) | 36.7 ± 0.9 | 36.2 ± 0.8 | |
| Preeclampsia | No | 46 (90.2%) | 42 (91.3%) |
| Yes | 5 (9.8%) | 4 (8.7%) | |
| Preterm Delivery (<37 weeks) | No | 30 (58.8%) | 25 (54.3%) |
| Yes | 21 (41.2%) | 21 (45.6%) |
| Control | Progesterone | ||||
|---|---|---|---|---|---|
| Twin 1 | Twin 2 | Twin 1 | Twin 2 | ||
| Length of largest fetus (week 12) | 13.2 ± 0.7 | 13.1 ± 0.7 | |||
| Cranial–caudal length | 68.8 ± 1.4 | 68.8 ± 1.4 | 67.8 ± 1.5 | 68.0 ± 1.4 | |
| Gestational age (week) | 36.9 ± 0.2 | 36.5 ± 0.3 | |||
| Birth weight (g) | 2537.6 ± 58.1 | 2537.6 ± 58.1 | 2426.9 ± 65.1 | 2398.4 ± 67.9 | |
| Neonatal Therapy | Admission to NICU | 3 (6.0%) | 2 (4.0%) | 4 (8.0%) | 3 (6.0%) |
| Need for ventilation | 5 (10.0%) | 4 (8.0%) | 5 (10.0%) | 5 (10.0%) | |
| Neonatal Morbidity | 1 (2.0%) | 1 (2.0%) | 1 (2.0%) | 1 (2.0%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puche-Juarez, M.; Toledano, J.M.; Moreno-Fernandez, J.; Diaz-Castro, J.; Sánchez-Romero, J.; Mar Gil, M.; Rolle, V.; Nieto-Díaz, A.; Ochoa, J.J.; De Paco Matallana, C. Vaginal Administration of Progesterone in Twin Gestation: Influence on Bone Turnover and Oxidative Stress. Antioxidants 2025, 14, 324. https://doi.org/10.3390/antiox14030324
Puche-Juarez M, Toledano JM, Moreno-Fernandez J, Diaz-Castro J, Sánchez-Romero J, Mar Gil M, Rolle V, Nieto-Díaz A, Ochoa JJ, De Paco Matallana C. Vaginal Administration of Progesterone in Twin Gestation: Influence on Bone Turnover and Oxidative Stress. Antioxidants. 2025; 14(3):324. https://doi.org/10.3390/antiox14030324
Chicago/Turabian StylePuche-Juarez, María, Juan M. Toledano, Jorge Moreno-Fernandez, Javier Diaz-Castro, Javier Sánchez-Romero, María Mar Gil, Valeria Rolle, Aníbal Nieto-Díaz, Julio J. Ochoa, and Catalina De Paco Matallana. 2025. "Vaginal Administration of Progesterone in Twin Gestation: Influence on Bone Turnover and Oxidative Stress" Antioxidants 14, no. 3: 324. https://doi.org/10.3390/antiox14030324
APA StylePuche-Juarez, M., Toledano, J. M., Moreno-Fernandez, J., Diaz-Castro, J., Sánchez-Romero, J., Mar Gil, M., Rolle, V., Nieto-Díaz, A., Ochoa, J. J., & De Paco Matallana, C. (2025). Vaginal Administration of Progesterone in Twin Gestation: Influence on Bone Turnover and Oxidative Stress. Antioxidants, 14(3), 324. https://doi.org/10.3390/antiox14030324










