Hydralazine Attenuates Lipopolysaccharide-Induced Murine Myocardial Dysfunction by Inhibition of Semicarbazide-Sensitive Amine Oxidase
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animal
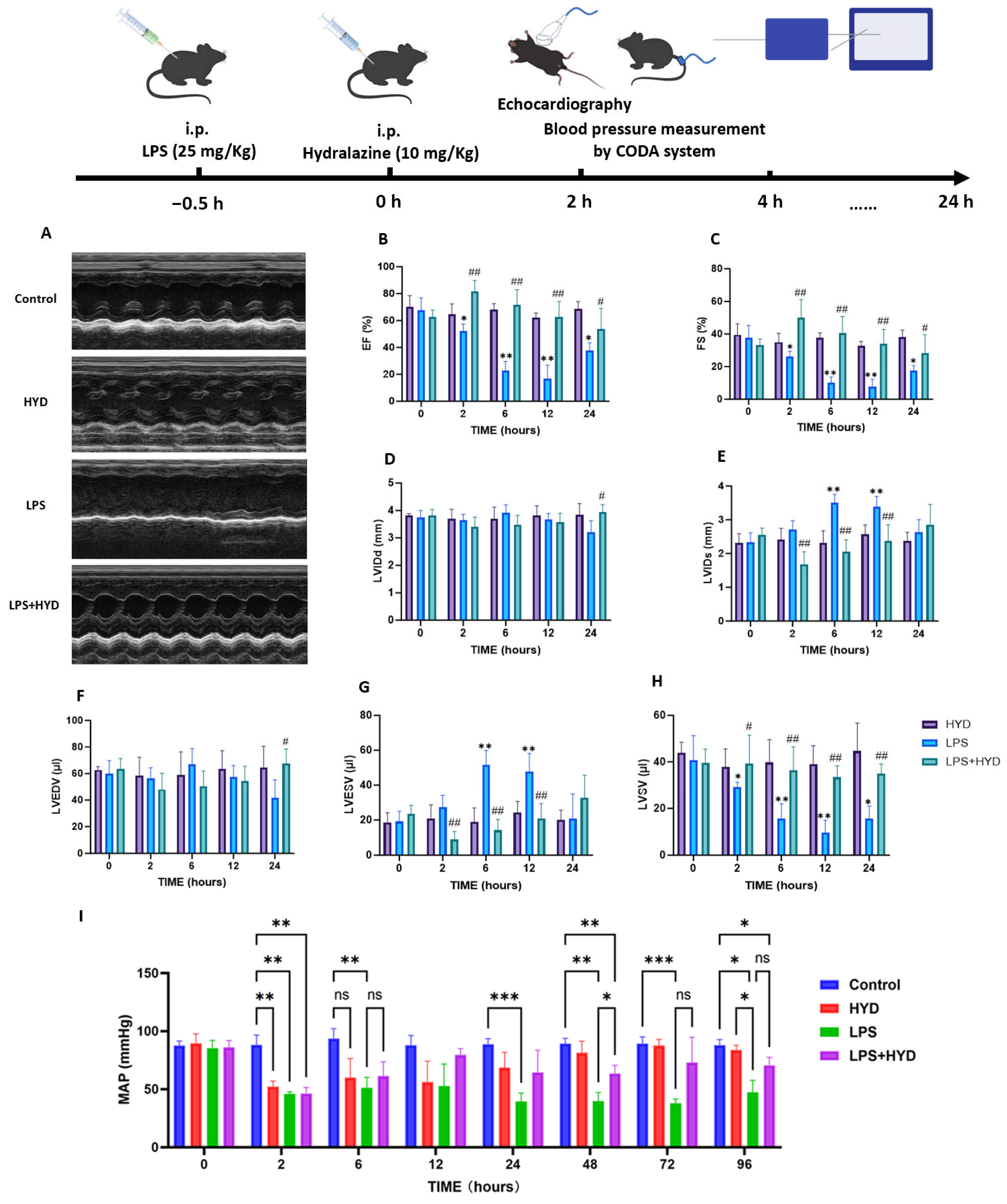
2.3. Experimental Protocols
2.4. Survival Study
2.5. Echocardiography
2.6. Blood Pressure Measurement
2.7. Blood Plasma Biochemical Analyses
2.8. Histological Evaluation
2.8.1. Hematoxylin-Eosin (H&E) Staining
2.8.2. Transmission Electron Microscopy (TEM)
2.9. Quantification of Blood Plasma Chemokines
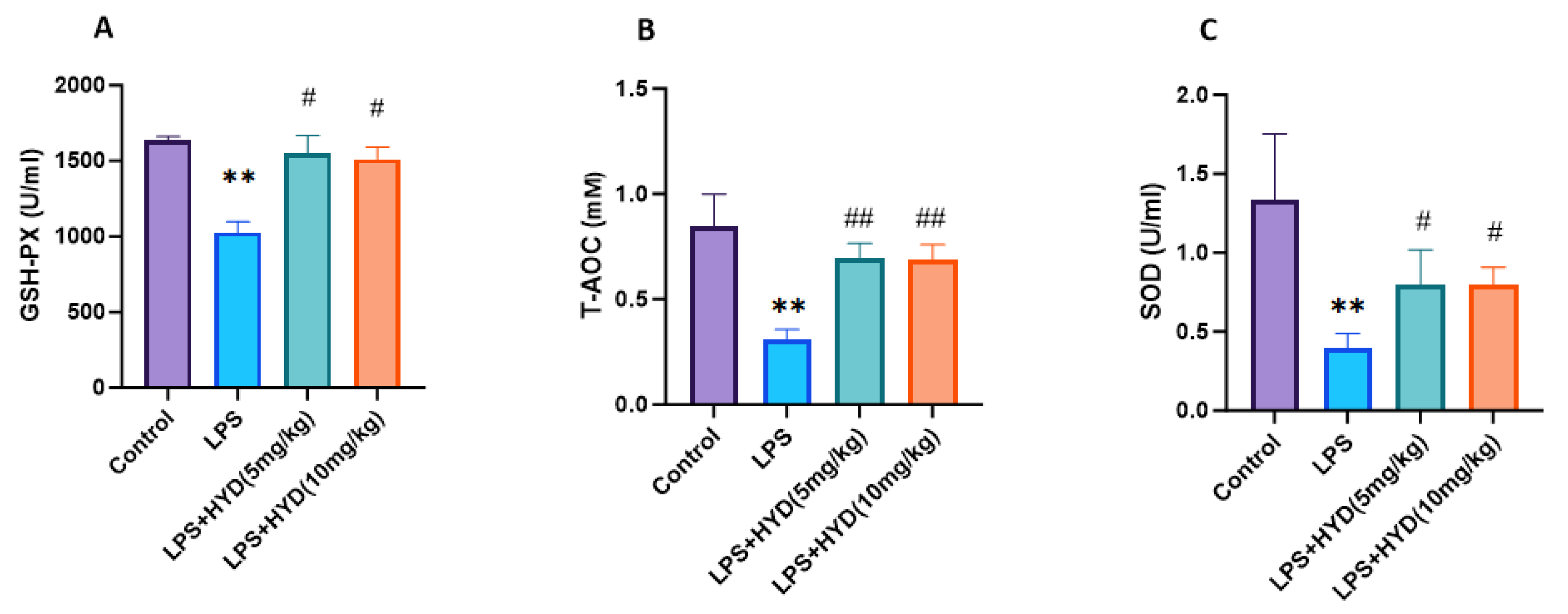
2.10. Plasma GSH-PX, T-AOC, SOD Activity Measurement
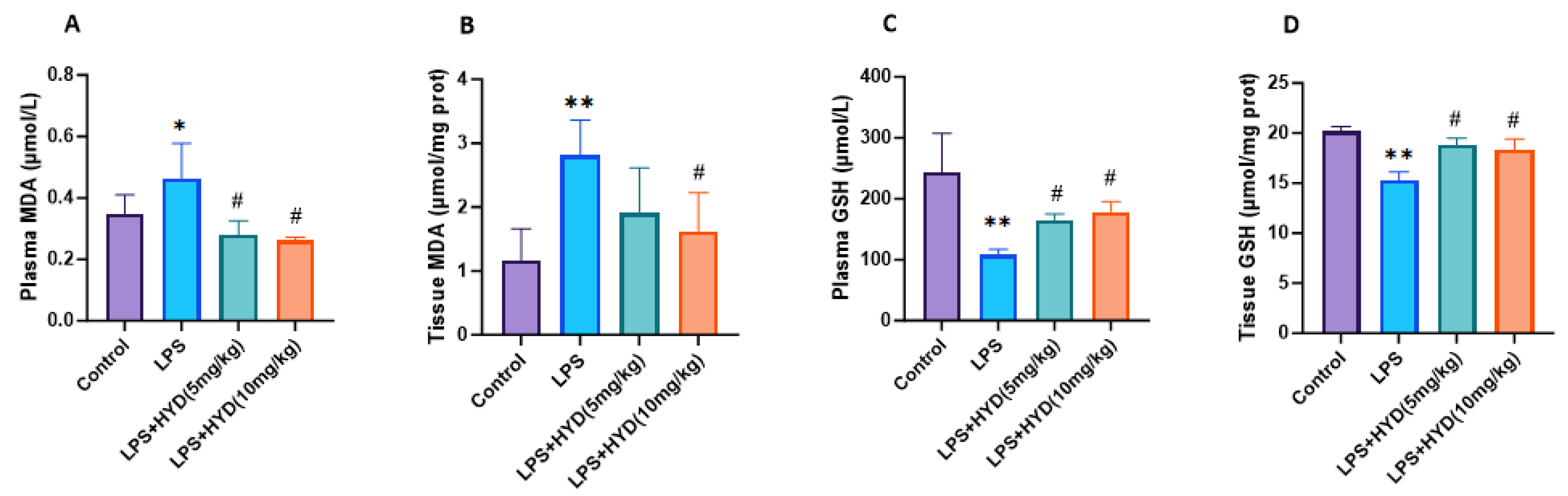
2.11. Determination of Malondialdehyde (MDA) and Glutathione (GSH) in Plasma and Cardiac Tissue
2.12. Measurement of Myocardial SSAO Activity
2.13. Statistical Analysis
3. Results
3.1. Hydralazine Improved the Survival Rates of Septic Mice Induced by LPS
3.2. Characterization of the LPS-Induced Sepsis Model and Its Impact on Cardiac Function and Hemodynamics
3.3. Hydralazine Improved Cardiac Function and Hemodynamics in Mice with SIMD
3.4. The Protection Effect of Hydralazine Against Tissue Damage Induced by Sepsis
3.5. Hydralazine Suppressed the Systemic Inflammatory Response in Sepsis
3.6. Hydralazine Enhanced Antioxidant Capacity and Attenuated Oxidative Stress
3.7. Hydralazine Inhibited Myocardial SSAO Activity
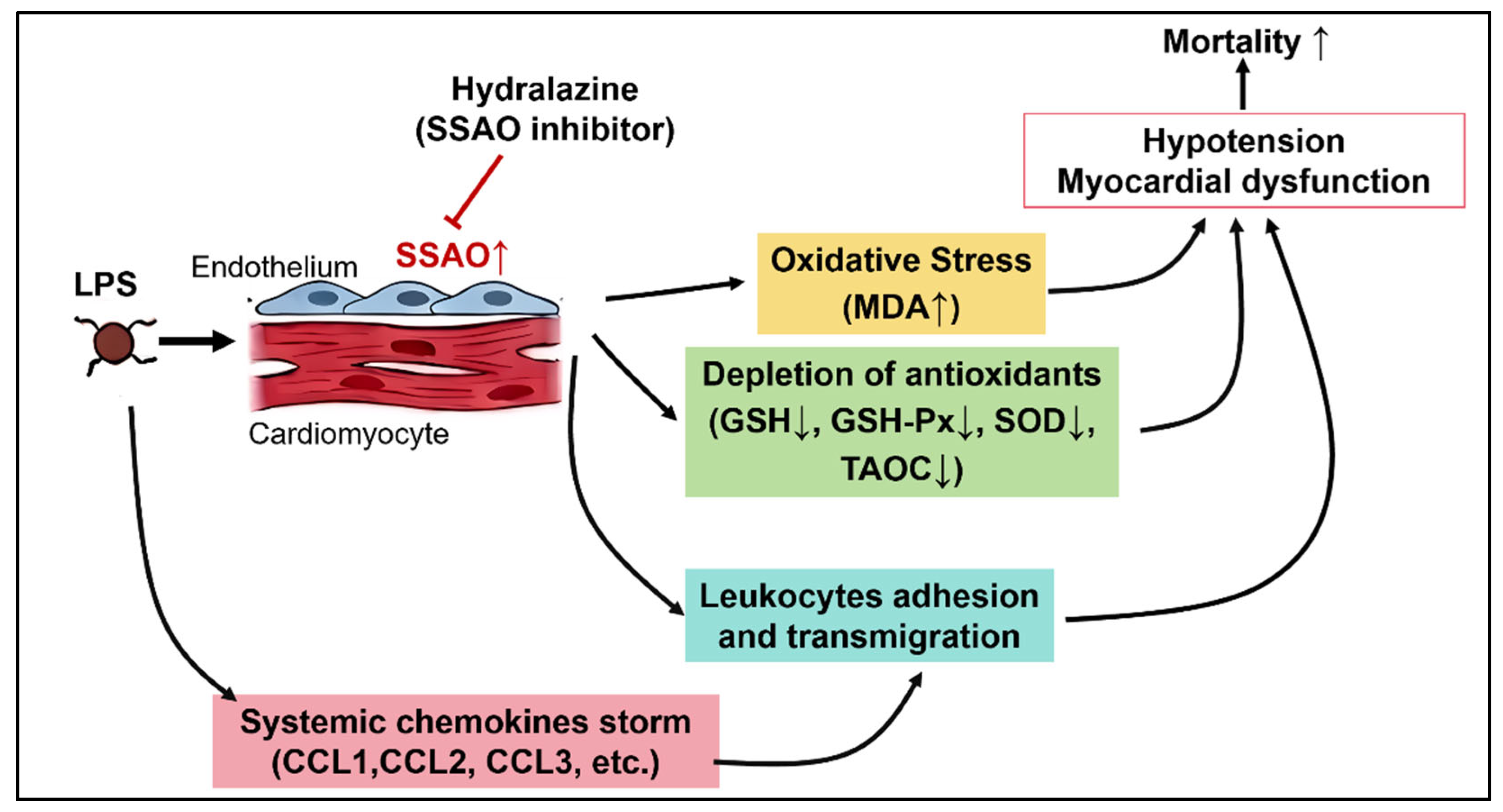
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SIMD | Sepsis-induced myocardial dysfunction |
| SSAO | Semicarbazide-sensitive amine oxidase |
| LPS | lipopolysaccharide |
| HYD | Hydralazine |
| VAP-1 | vascular adhesion protein-1 |
| T-AOC | total antioxidant capacity |
| SOD | Superoxide Dismutase |
| i.p. | intraperitoneal |
| LV | left ventricular |
| LVIDd | The left ventricular internal diameters at end-diastole |
| LVIDs | The left ventricular internal diameters at end-systole |
| LVEDV | left ventricular end-diastolic volume |
| LVESV | left ventricular end-systolic volume |
| LVSV | left ventricular stroke volume |
| EF | ejection fraction |
| FS | fractional shortening |
| CK | creatine kinase |
| CK-MB | creatine kinase-MB |
| LDH | lactate dehydrogenase |
| AST | aspartate aminotransferase |
| H&E | Hematoxylin and Eosin |
| TEM | Transmission electron microscopy |
| GSH-PX | glutathione peroxidase |
| MDA | Malondialdehyde |
| GSH | Glutathione |
| TBA | 2-thiobarbituric acid |
| CCL | C-C motif chemokine ligand |
| CXCL | C-X-C motif chemokine ligand |
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Wang, H. Pathophysiology of sepsis-induced myocardial dysfunction. Mil. Med. Res. 2016, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.; Tao, X.; Lin, Y.; Zheng, H.; Ning, L.; Lu, H.S.; Daugherty, A.; Shi, P.; Mullick, A.E.; Chen, S.; et al. Loss of Hepatic Angiotensinogen Attenuates Sepsis-Induced Myocardial Dysfunction. Circ. Res. 2021, 129, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.; Deem, S.; Bendjelid, K.; Treggiari, M.M. Characterization of cardiac dysfunction in sepsis: An ongoing challenge. Shock 2014, 41, 12–24. [Google Scholar] [CrossRef]
- Poveda-Jaramillo, R. Heart Dysfunction in Sepsis. J. Cardiothorac. Vasc. Anesth. 2021, 35, 298–309. [Google Scholar] [CrossRef]
- Merx, M.W.; Weber, C. Sepsis and the heart. Circulation 2007, 116, 793–802. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Gupta, R.G.; Hartigan, S.M.; Kashiouris, M.G.; Sessler, C.N.; Bearman, G.M. Early goal-directed resuscitation of patients with septic shock: Current evidence and future directions. Crit. Care 2015, 19, 286. [Google Scholar] [CrossRef]
- Shen, Y.L.; Shi, Y.Z.; Chen, G.G.; Wang, L.L.; Zheng, M.Z.; Jin, H.F.; Chen, Y.Y. TNF-alpha induces Drp1-mediated mitochondrial fragmentation during inflammatory cardiomyocyte injury. Int. J. Mol. Med. 2018, 41, 2317–2327. [Google Scholar] [CrossRef]
- Pan, P.; Wang, X.; Liu, D. The potential mechanism of mitochondrial dysfunction in septic cardiomyopathy. J. Int. Med. Res. 2018, 46, 2157–2169. [Google Scholar] [CrossRef]
- Becchi, S.; Buson, A.; Foot, J.; Jarolimek, W.; Balleine, B.W. Inhibition of semicarbazide-sensitive amine oxidase/vascular adhesion protein-1 reduces lipopolysaccharide-induced neuroinflammation. Br. J. Pharmacol. 2017, 174, 2302–2317. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Iyer, M.R. Therapeutic Potential of Vascular Adhesion Protein-1 (VAP-1)/Semicarbazide-Sensitive Amine Oxidase (SSAO) Inhibitors: Current Medicinal Chemistry and Emerging Opportunities. Med. Res. Rev. 2025, 45, 1564–1596. [Google Scholar] [CrossRef] [PubMed]
- Aalto, K.; Autio, A.; Kiss, E.A.; Elima, K.; Nymalm, Y.; Veres, T.Z.; Marttila-Ichihara, F.; Elovaara, H.; Saanijoki, T.; Crocker, P.R.; et al. Siglec-9 is a novel leukocyte ligand for vascular adhesion protein-1 and can be used in PET imaging of inflammation and cancer. Blood 2011, 118, 3725–3733. [Google Scholar] [CrossRef]
- Chen, C.; Zhong, W.; Zheng, H.; Zhao, W.; Wang, Y.; Dong, Q.; Shen, B. The role of VAP-1 in cardiovascular disease: A review. Front. Cardiovasc. Med. 2025, 12, 1549157. [Google Scholar] [CrossRef]
- Chang, T.T.; Chiang, C.H.; Chen, C.; Lin, S.C.; Lee, H.J.; Chen, J.W. Antioxidation and Nrf2-mediated heme oxygenase-1 activation contribute to renal protective effects of hydralazine in diabetic nephropathy. Biomed. Pharmacother. 2022, 151, 113139. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sargisson, O.; Nguyen, D.T.; Parker, K.; Pyke, S.J.R.; Alramahi, A.; Thihlum, L.; Fang, Y.; Wallace, M.E.; Berzins, S.P.; et al. Effect of Hydralazine on Angiotensin II-Induced Abdominal Aortic Aneurysm in Apolipoprotein E-Deficient Mice. Int. J. Mol. Sci. 2023, 24, 15955. [Google Scholar] [CrossRef]
- Ruiz-Magana, M.J.; Martinez-Aguilar, R.; Lucendo, E.; Campillo-Davo, D.; Schulze-Osthoff, K.; Ruiz-Ruiz, C. The antihypertensive drug hydralazine activates the intrinsic pathway of apoptosis and causes DNA damage in leukemic T cells. Oncotarget 2016, 7, 21875–21886. [Google Scholar] [CrossRef] [PubMed]
- Nyolczas, N.; Dekany, M.; Muk, B.; Szabo, B. Combination of Hydralazine and Isosorbide-Dinitrate in the Treatment of Patients with Heart Failure with Reduced Ejection Fraction. Adv. Exp. Med. Biol. 2018, 1067, 31–45. [Google Scholar] [CrossRef]
- Taylor, A.L.; Ziesche, S.; Yancy, C.; Carson, P.; D’Agostino, R., Jr.; Ferdinand, K.; Taylor, M.; Adams, K.; Sabolinski, M.; Worcel, M.; et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N. Engl. J. Med. 2004, 351, 2049–2057. [Google Scholar] [CrossRef]
- Yang, W.; Li, H.; Luo, H.; Luo, W. Inhibition of semicarbazide-sensitive amine oxidase attenuates myocardial ischemia-reperfusion injury in an in vivo rat model. Life Sci. 2011, 88, 302–306. [Google Scholar] [CrossRef]
- Huang, H.C.; Hsiao, T.S.; Liao, M.H.; Tsao, C.M.; Shih, C.C.; Wu, C.C. Low-dose hydralazine improves endotoxin-induced coagulopathy and multiple organ dysfunction via its anti-inflammatory and anti-oxidative/nitrosative properties. Eur. J. Pharmacol. 2020, 882, 173279. [Google Scholar] [CrossRef]
- Santos, D.M.D.; Da Silva, E.A.P.; Oliveira, J.Y.S.; Marinho, Y.Y.M.; Santana, I.R.; Heimfarth, L.; Pereira, E.W.M.; Junior, L.J.Q.; Assreuy, J.; Menezes, I.A.C.; et al. The Therapeutic Value of Hydralazine in Reducing Inflammatory Response, Oxidative Stress, and Mortality in Animal Sepsis: Involvement of the PI3K/AKT Pathway. Shock 2021, 56, 782–792. [Google Scholar] [CrossRef] [PubMed]
- An, N.; Song, Y.; Zhang, X.; Ci, X.; Li, H.; Cao, Y.; Zhang, M.; Cui, J.; Deng, X. Pretreatment of mice with rifampicin prolongs survival of endotoxic shock by modulating the levels of inflammatory cytokines. Immunopharmacol. Immunotoxicol. 2008, 30, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Ozcelebi, E.; Iskit, A.B. Experimental Animal Models of Sepsis and Septic Shock. J. Crit. Intensive Care 2023, 14, 96–100. [Google Scholar] [CrossRef]
- Terrando, N.; Rei Fidalgo, A.; Vizcaychipi, M.; Cibelli, M.; Ma, D.; Monaco, C.; Feldmann, M.; Maze, M. The impact of IL-1 modulation on the development of lipopolysaccharide-induced cognitive dysfunction. Crit. Care 2010, 14, R88. [Google Scholar] [CrossRef]
- Chensue, S.W.; Terebuh, P.D.; Remick, D.G.; Scales, W.E.; Kunkel, S.L. In vivo biologic and immunohistochemical analysis of interleukin-1 alpha, beta and tumor necrosis factor during experimental endotoxemia. Kinetics, Kupffer cell expression, and glucocorticoid effects. Am. J. Pathol. 1991, 138, 395–402. [Google Scholar]
- Pan, B.; Alam, H.B.; Chong, W.; Mobley, J.; Liu, B.; Deng, Q.; Liang, Y.; Wang, Y.; Chen, E.; Wang, T.; et al. CitH3: A reliable blood biomarker for diagnosis and treatment of endotoxic shock. Sci. Rep. 2017, 7, 8972. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Q.; Zhao, Y.; Xu, J.; Cui, Y. Sevoflurane, as opposed to pentobarbital anesthesia, attenuates LPS-induced myocardial injury by up-regulating TAF1D. Sci. Rep. 2025, 15, 36894. [Google Scholar] [CrossRef]
- Coldewey, S.M.; Rogazzo, M.; Collino, M.; Patel, N.S.; Thiemermann, C. Inhibition of IkappaB kinase reduces the multiple organ dysfunction caused by sepsis in the mouse. Dis. Models Mech. 2013, 6, 1031–1042. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, X.; Wang, Y.; Yan, Y.; Wang, Y.; Su, M.; Lv, H.; Li, K.; Hao, X.; Xing, X.; et al. Application of lipopolysaccharide in establishing inflammatory models. Int. J. Biol. Macromol. 2024, 279, 135371. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.; Rateri, D.; Hong, L.; Balakrishnan, A. Measuring blood pressure in mice using volume pressure recording, a tail-cuff method. J. Vis. Exp. 2009, 27, 1291. [Google Scholar] [CrossRef]
- Hadi, S.M.H.; Majeed, S.; Ghafil, F.A.; Altoraihi, K.; Hadi, N.R. Xanthohumol ameliorates cardiac injury induced by sepsis in a mice model: Role of toll-like receptor 4. J. Med. Life 2023, 16, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; George, K.; Duan, F.; Tong, T.K.; Tian, Y. Histological evidence for reversible cardiomyocyte changes and serum cardiac troponin T elevation after exercise in rats. Physiol. Rep. 2016, 4, e13083. [Google Scholar] [CrossRef]
- Wu, Q.; Fan, J.; Sheng, Q.; He, X. Rapamycin improves endometriosis-related infertility involving ovarian senescence via the PPARalpha/IGFBP2 pathway. Mol. Med. Rep. 2025, 33, 12. [Google Scholar] [CrossRef]
- Chu, M.; Jiang, S.; Xue, J.; Li, W.; Jing, G.; Li, H.; Zhang, J.; Xu, W. Premature renal epithelial cell senescence promoted by LXN/Rps3/p53 signaling pathway activation increases calcium oxalate crystal deposition by altering macrophage polarization. Front. Immunol. 2025, 16, 1658989. [Google Scholar] [CrossRef]
- Liu, X.; Sun, B.; Jiang, Y.; Guan, Z.; Li, H. Proteomics Insights Into Gingival Aging: The Role of Oxidative Stress and Key Signaling Pathways. Int. Dent. J. 2025, 75, 100903. [Google Scholar] [CrossRef]
- Jentzsch, A.M.; Bachmann, H.; Furst, P.; Biesalski, H.K. Improved analysis of malondialdehyde in human body fluids. Free Radic. Biol. Med. 1996, 20, 251–256. [Google Scholar] [CrossRef]
- Glowacki, R.; Stachniuk, J.; Borowczyk, K. A Simple HPLC-UV Method for Simultaneous Determination of Cysteine and Cysteinylglycine in Biological Fluids. Acta Chromatogr. 2016, 28, 333–346. [Google Scholar] [CrossRef]
- Chwatko, G.; Kuzniak, E.; Kubalczyk, P.; Borowczyk, K.; Wyszczelska-Rokiel, M.; Glowacki, R. Determination of cysteine and glutathione in cucumber leaves by HPLC with UV detection. Anal. Methods-UK 2014, 6, 8039–8044. [Google Scholar] [CrossRef]
- Li, H.; Luo, W.; Lin, J.; Lin, Z.; Zhang, Y. Assay of plasma semicarbazide-sensitive amine oxidase and determination of its endogenous substrate methylamine by liquid chromatography. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2004, 810, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhang, J.; Zhang, Z.; Turdi, S.; Han, X.; Liu, Q.; Hu, H.; Ye, H.; Dong, M.; Duan, Y.; et al. Cardiac-specific overexpression of catalase attenuates lipopolysaccharide-induced cardiac anomalies through reconciliation of autophagy and ferroptosis. Life Sci. 2023, 328, 121821. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O. The chemokine superfamily revisited. Immunity 2012, 36, 705–716. [Google Scholar] [CrossRef]
- Slim, H.B.; Black, H.R.; Thompson, P.D. Older blood pressure medications-do they still have a place? Am. J. Cardiol. 2011, 108, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Herr, S.A.; Gardeen, S.S.; Low, P.S.; Shi, R. Targeted delivery of acrolein scavenger hydralazine in spinal cord injury using folate-linker-drug conjugation. Free Radic. Biol. Med. 2022, 184, 66–73. [Google Scholar] [CrossRef]
- Duenas-Gonzalez, A.; Coronel, J.; Cetina, L.; Gonzalez-Fierro, A.; Chavez-Blanco, A.; Taja-Chayeb, L. Hydralazine-valproate: A repositioned drug combination for the epigenetic therapy of cancer. Expert. Opin. Drug Metab. Toxicol. 2014, 10, 1433–1444. [Google Scholar] [CrossRef]
- Tampe, B.; Steinle, U.; Tampe, D.; Carstens, J.L.; Korsten, P.; Zeisberg, E.M.; Muller, G.A.; Kalluri, R.; Zeisberg, M. Low-dose hydralazine prevents fibrosis in a murine model of acute kidney injury-to-chronic kidney disease progression. Kidney Int. 2017, 91, 157–176. [Google Scholar] [CrossRef]
- Chiang, C.H.; Chen, C.; Fang, S.Y.; Lin, S.C.; Chen, J.W.; Chang, T.T. Xanthine oxidase/NADPH oxidase inhibition by hydralazine attenuates acute kidney injury and prevents the transition of acute kidney injury to chronic kidney disease. Life Sci. 2023, 327, 121863. [Google Scholar] [CrossRef]
- Romao, I.C.; Siqueira, S.M.C.; Silva Abreu, F.; Santos, H.S.D. Hydralazine and Hydrazine Derivatives: Properties, Applications, and Repositioning Potential. Chem. Biodivers. 2025, 22, e202401561. [Google Scholar] [CrossRef]
- Quan, X.; Yu, C.; Fan, Z.; Wu, T.; Qi, C.; Zhang, H.; Wu, S.; Wang, X. Hydralazine plays an immunomodulation role of pro-regeneration in a mouse model of spinal cord injury. Exp. Neurol. 2023, 363, 114367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lin, Z.; Yin, X.; Tang, L.; Luo, H.; Li, H.; Zhang, Y.; Luo, W. In vitro and in vivo study of hydralazine, a potential anti-angiogenic agent. Eur. J. Pharmacol. 2016, 779, 138–146. [Google Scholar] [CrossRef]
- Vidrio, H.; Medina, M.; Gonzalez-Romo, P.; Lorenzana-Jimenez, M.; Diaz-Arista, P.; Baeza, A. Semicarbazide-sensitive amine oxidase substrates potentiate hydralazine hypotension: Possible role of hydrogen peroxide. J. Pharmacol. Exp. Ther. 2003, 307, 497–504. [Google Scholar] [CrossRef]
- Lyles, G.A.; Callingham, B.A. Hydralazine is an irreversible inhibitor of the semicarbazide-sensitive, clorgyline-resistant amine oxidase in rat aorta homogenates. J. Pharm. Pharmacol. 1982, 34, 139–140. [Google Scholar] [CrossRef]
- Li, R.; Li, H.; Luo, H.J.; Lin, Z.X.; Jiang, Z.W.; Luo, W.H. SSAO inhibitors suppress hepatocellular tumor growth in mice. Cell Immunol. 2013, 283, 61–69. [Google Scholar] [CrossRef]
- de Padua Lucio, K.; Rabelo, A.C.S.; Araujo, C.M.; Brandao, G.C.; de Souza, G.H.B.; da Silva, R.G.; de Souza, D.M.S.; Talvani, A.; Bezerra, F.S.; Cruz Calsavara, A.J.; et al. Anti-Inflammatory and Antioxidant Properties of Black Mulberry (Morus nigra L.) in a Model of LPS-Induced Sepsis. Oxidative Med. Cell. Longev. 2018, 2018, 5048031. [Google Scholar] [CrossRef]
- Huang, S.H.; Xu, M.; Wu, H.M.; Wan, C.X.; Wang, H.B.; Wu, Q.Q.; Liao, H.H.; Deng, W.; Tang, Q.Z. Isoquercitrin Attenuated Cardiac Dysfunction Via AMPKalpha-Dependent Pathways in LPS-Treated Mice. Mol. Nutr. Food Res. 2018, 62, e1800955. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Wang, G.; Huang, R.; Liu, C.; Yushanjiang, F.; Mao, T.; Li, J. Astilbin protects from sepsis-induced cardiac injury through the NRF2/HO-1 and TLR4/NF-kappaB pathway. Phytother. Res. 2024, 38, 1044–1058. [Google Scholar] [CrossRef]
- Lukic, I.; Mihic, D.; Varzic, S.C.; Relatic, K.S.; Zibar, L.; Loinjak, D.; Curic, Z.B.; Klobucar, L.; Maricic, L. Septic Cardiomyopathy. Rev. Cardiovasc. Med. 2024, 25, 23. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, Y.; Zhang, Z. Sepsis-Induced Myocardial Dysfunction (SIMD): The Pathophysiological Mechanisms and Therapeutic Strategies Targeting Mitochondria. Inflammation 2020, 43, 1184–1200. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Su, Z.; Ge, L.; Chen, Y.; Chen, X.; Li, Y. Cardioprotection of hydralazine against myocardial ischemia/reperfusion injury in rats. Eur. J. Pharmacol. 2020, 869, 172850. [Google Scholar] [CrossRef] [PubMed]
- Danielli, M.; Thomas, R.C.; Quinn, L.M.; Tan, B.K. Vascular adhesion protein-1 (VAP-1) in vascular inflammatory diseases. Vasa 2022, 51, 341–350. [Google Scholar] [CrossRef]
- Li, H.; Du, S.; Niu, P.; Gu, X.; Wang, J.; Zhao, Y. Vascular Adhesion Protein-1 (VAP-1)/Semicarbazide-Sensitive Amine Oxidase (SSAO): A Potential Therapeutic Target for Atherosclerotic Cardiovascular Diseases. Front. Pharmacol. 2021, 12, 679707. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, Y.; Zhao, H.; Zhang, F.; Wang, J.; Liu, Y.; Lin, J.; Huang, Y.; Pan, W.; Qi, J.; et al. Midbrain FA initiates neuroinflammation and depression onset in both acute and chronic LPS-induced depressive model mice. Brain Behav. Immun. 2024, 117, 356–375. [Google Scholar] [CrossRef]
- Mihaljevic, O.; Zivancevic-Simonovic, S.; Jovanovic, D.; Drakulic, S.M.; Vukajlovic, J.T.; Markovic, A.; Pirkovic, M.S.; Srejovic, I.; Jakovljevic, V.; Milosevic-Djordjevic, O. Oxidative stress and DNA damage in critically ill patients with sepsis. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2023, 889, 503655. [Google Scholar] [CrossRef]
- Mantzarlis, K.; Tsolaki, V.; Zakynthinos, E. Role of Oxidative Stress and Mitochondrial Dysfunction in Sepsis and Potential Therapies. Oxid. Med. Cell Longev. 2017, 2017, 5985209. [Google Scholar] [CrossRef] [PubMed]
- Tsolaki, V.; Makris, D.; Mantzarlis, K.; Zakynthinos, E. Sepsis-Induced Cardiomyopathy: Oxidative Implications in the Initiation and Resolution of the Damage. Oxidative Med. Cell. Longev. 2017, 2017, 7393525. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Liu, L. Vitamin C as a treatment for organ failure in sepsis. Eur. J. Med. Res. 2023, 28, 222. [Google Scholar] [CrossRef]
- Xie, Y.; Gu, Y.; Li, Z.; Zhang, L.; Hei, Y. Effects of exercise on different antioxidant enzymes and related indicators: A systematic review and meta-analysis of randomized controlled trials. Sci. Rep. 2025, 15, 12518. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, Q.; Ren, K.; Wu, P.; Wang, Y.; Lv, C. ALDH2 mitigates LPS-induced cardiac dysfunction, inflammation, and apoptosis through the cGAS/STING pathway. Mol. Med. 2023, 29, 171. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.J.; Hanna-Jumma, S.; Carraretto, M.; Forni, L. The pathophysiological basis and consequences of fever. Crit. Care 2016, 20, 200. [Google Scholar] [CrossRef]
- Hu, K.; Jiang, P.; Hu, J.; Song, B.; Hou, Y.; Zhao, J.; Chen, H.; Xie, J. Dapagliflozin attenuates LPS-induced myocardial injury by reducing ferroptosis. J. Bioenerg. Biomembr. 2024, 56, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Ye, B.; Zhong, L.; Chen, Y.; Hong, G.; Zhao, G.; Su, L.; Lu, Z. GSDMD Mediates LPS-Induced Septic Myocardial Dysfunction by Regulating ROS-dependent NLRP3 Inflammasome Activation. Front. Cell Dev. Biol. 2021, 9, 779432. [Google Scholar] [CrossRef] [PubMed]





| Scores | Features |
|---|---|
| Myocardial injury severity scoring for H&E | |
| 0 | Normal myocardium |
| 1 | Mild interstitial edema with focal necrosis |
| 2 | Moderate myocardial cell swelling and diffuse necrosis |
| 3 | Severe ischemia with prominent neutrophil infiltration |
| 4 | Extensive damage, characterized by contraction band necrosis, leukocyte infiltration, ischemia, and hemorrhage |
| Mitochondria injury severity for TEM 1 | |
| 0 | Intact double membrane, compact orderly cristae, and a homogeneous dense matrix |
| 1 | Mitochondrial swelling |
| 2 | Lysis and breakage of mitochondrial cristae (cristolysis) |
| 3 | Mitochondrial matrix proteins disintegrate |
| 4 | Large dense granules in the mitochondrial matrix |
| 5 | Ruptured and fragmented mitochondria |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuang, Z.; Luo, H.; Li, H.; Zhou, Y.; Lin, Z.; Luo, W. Hydralazine Attenuates Lipopolysaccharide-Induced Murine Myocardial Dysfunction by Inhibition of Semicarbazide-Sensitive Amine Oxidase. Antioxidants 2025, 14, 1502. https://doi.org/10.3390/antiox14121502
Kuang Z, Luo H, Li H, Zhou Y, Lin Z, Luo W. Hydralazine Attenuates Lipopolysaccharide-Induced Murine Myocardial Dysfunction by Inhibition of Semicarbazide-Sensitive Amine Oxidase. Antioxidants. 2025; 14(12):1502. https://doi.org/10.3390/antiox14121502
Chicago/Turabian StyleKuang, Zejian, Hongjun Luo, Hui Li, Yongying Zhou, Zhexuan Lin, and Wenhong Luo. 2025. "Hydralazine Attenuates Lipopolysaccharide-Induced Murine Myocardial Dysfunction by Inhibition of Semicarbazide-Sensitive Amine Oxidase" Antioxidants 14, no. 12: 1502. https://doi.org/10.3390/antiox14121502
APA StyleKuang, Z., Luo, H., Li, H., Zhou, Y., Lin, Z., & Luo, W. (2025). Hydralazine Attenuates Lipopolysaccharide-Induced Murine Myocardial Dysfunction by Inhibition of Semicarbazide-Sensitive Amine Oxidase. Antioxidants, 14(12), 1502. https://doi.org/10.3390/antiox14121502







