Ferroptosis in Human Diseases: Fundamental Roles and Emerging Therapeutic Perspectives
Abstract
1. Introduction
2. Molecular Underpinnings of Ferroptosis: Core Mechanisms and Regulatory Pathways
2.1. Iron Metabolism Dysregulation
2.2. Lipid Peroxidation Mechanisms
| Regulator/Pathway | Role and Mechanism of Action | Impact on Ferroptosis Sensitivity |
|---|---|---|
| Iron Metabolism Dysregulation | ||
| Fenton Reaction [5] | Iron-catalyzed reaction (Fe2+ + H2O2 → Fe3+ + HO• + OH−) that generates highly reactive hydroxyl radicals (HO•) | Initiates LPO |
| TFRC [13,14,15] | Primary receptor responsible for the endocytic uptake of transferrin-bound iron. | Promotes |
| DMT1 [12,15] | Transports iron from the endosome to the cytosol and directly imports NTBI from outside the cell. | Promotes |
| FPN1 [17] | The sole known cellular iron exporter; actively transports iron out of the cytosol. | Inhibits |
| Hepcidin [18] | Binds to FPN1, inducing its internalization and degradation. This action traps iron intracellularly. | Promotes |
| Ferritin [19] | Safely blocks iron in a non-reactive form. | Inhibits |
| NCOA4 (Ferritinophagy) [19,20] | Selective autophagy cargo receptor that targets ferritin for lysosomal degradation, releasing large amounts of Fe2+ into the LIP. | Promotes |
| IRPs [6] | Iron-responsive element binding proteins. Post-transcriptionally regulate iron homeostasis. | Promotes |
| HO-1 [21] | Enzyme that catabolizes heme, releasing free iron and contributing to the LIP. | Promotes |
| PCBP1 [22,23] | Cytosolic chaperone that safely binds and traffics iron to its destinations, | Inhibits |
| LPO Mechanisms | ||
| ACSL4 [27,28] | Activates PUFAs (e.g., arachidonic acid) for membrane incorporation. | Promotes |
| LPCAT3 [29] | Inserts ACSL4-activated PUFAs into membrane phospholipids. | Promotes |
| LOXs [31] | Directly and enzymatically oxygenate membrane PUFAs to generate lipid hydroperoxides (LOOH). | Promotes |
| PEBP1 [31] | Scaffold protein that binds 15-LOX and guides it to membrane PUFAs | Promotes |
| Cell–Cell Propagation [33] | α-catenin-dependent cell contact for the transfer of LPO to adjacent cells. | Promotes (in adjacent cells) |
2.3. Antioxidant Defense Systems
| Regulator/Pathway | Role and Mechanism of Action | Impact on Ferroptosis Sensitivity |
|---|---|---|
| The GPX4-Dependent Axis | ||
| SLC7A11 [36] | Nrf2 target. Cystine/glutamate antiporter; imports cystine, the rate-limiting precursor for GSH synthesis. | Inhibits |
| GCL and GSS [37] | Nrf2 targets. Key enzymes for GSH synthesis. GCL (GCLC/GCLM) is the rate-limiting enzyme; GSS catalyzes the final step. | Inhibits |
| GPx4 [39,40,41] | A unique selenoprotein that utilizes GSH to directly detoxify lipid hydroperoxides (LOOH) into benign lipid alcohols (LOH). | Inhibits |
| HO-1 and FTH1/FTL [44,45] | Nrf2 targets. HO-1 catabolizes pro-oxidant heme; ferritin sequesters the released free iron, reducing the LIP. | Inhibits |
| GPX4-Independent Pathways | ||
| FSP1-CoQ10 Axis [46,47,48] | NAD(P)H-dependent reductase that regenerates coenzyme Q10 (Ubiquinone) to its active antioxidant form, Ubiquinol (CoQ10H2), which neutralizes the LOO• | Inhibits |
| GCH1-BH4 Pathway [50] | Rate-limiting enzyme for the synthesis of Tetrahydrobiopterin (BH4). BH4 acts as an endogenous RTA and promotes CoQ10 regeneration. | Inhibits |
| DHODH [51] | An inner mitochondrial membrane enzyme that reduces the mitochondrial CoQ10 pool, providing localized protection against LPO. | Inhibits |
| Substrate Remodeling | ||
| MBOAT1/MBOAT2 [52] | Incorporate MUFAs into membranes, competitively displacing the highly peroxidizable PUFA. | Inhibits |
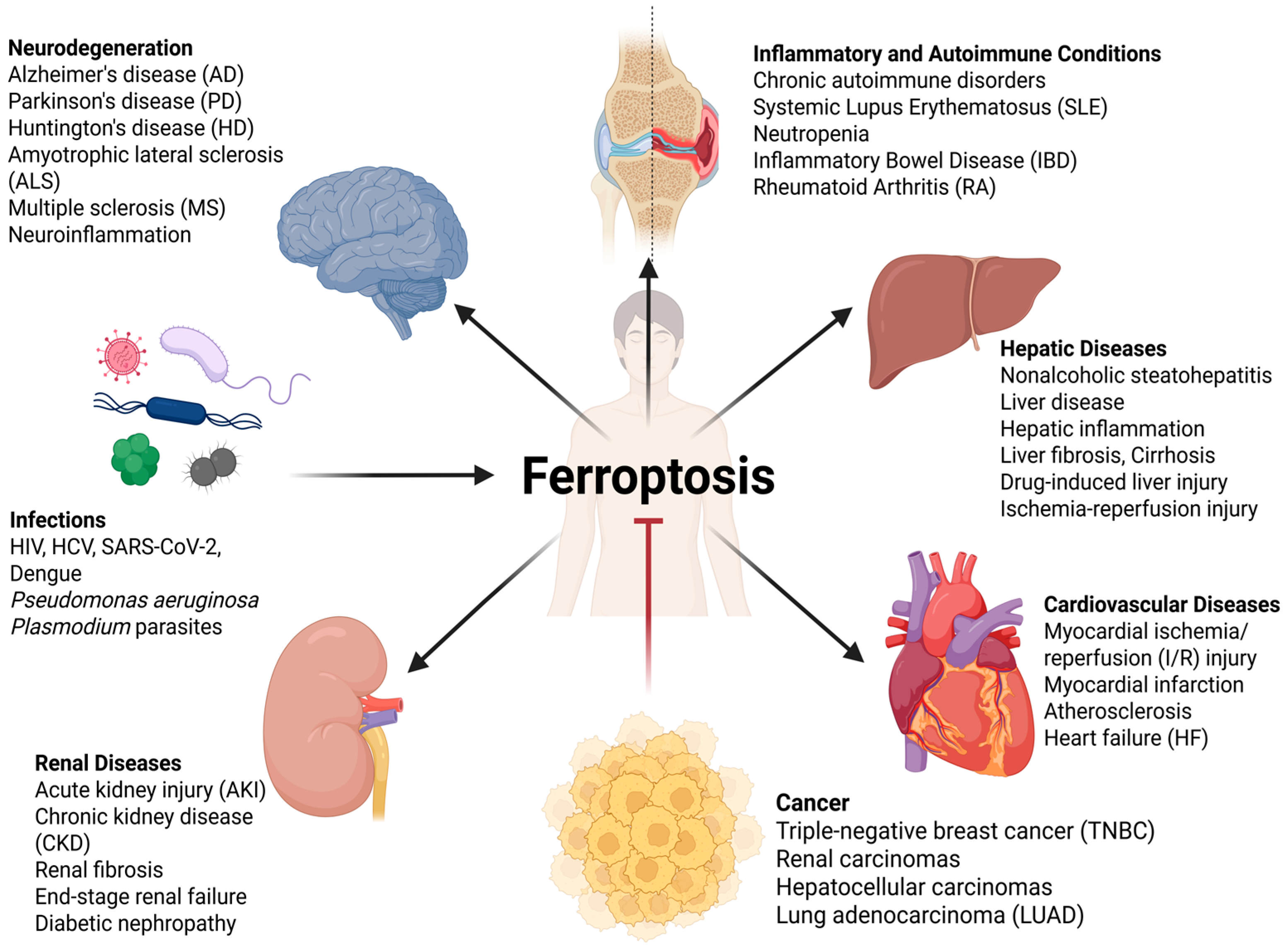
3. Ferroptosis in Disease Pathogenesis: A Fundamental Role Across Organ Systems
3.1. Cancer
3.2. Neurodegenerative Diseases
3.3. Cardiovascular Diseases
3.4. Renal Diseases
3.5. Hepatic Diseases
3.6. Inflammatory and Autoimmune Conditions
4. Emerging Therapeutic Strategies and Clinical Translation
4.1. Therapeutic Induction of Ferroptosis: A Weapon Against Malignancy
| Class | Compound | Mechanism of Action |
|---|---|---|
| GPx4 Inhibitors | RSL3 [154,155,156] | Direct GPx4 inhibitor. |
| ML162 [157] | Direct GPx4 inhibitor. | |
| FIN56 [158] | Direct GPx4 inhibitor (promotes degradation). | |
| ARP-246 [164] | P53 activator, induce ferroptosis by disrupting the GSH/GPx4 axis | |
| GSH Depletors | Erastin [159,160] | Blocks System Xc− antiporter, inhibiting cystine uptake. |
| Sorafenib [161,162,163] | Multi-kinase inhibitor; also blocks System Xc− antiporter. | |
| Sulfasalazine [168] | Induces ferroptosis (Nrf2 downregulation). | |
| GPx4-Independent Pathway Inhibitors | Brequinar [167] | DHODH inhibitor (targets mitochondrial CoQ10 metabolism). |
| Lysosome-Targeting Agents | Fentomycin-1 [72] | Phospholipid degrader specifically activated within the lysosome. |
| Immunotherapy (Combination) | Anti-PD-1 mAb [171] | Immune checkpoint inhibitor (synergizes with ferroptosis). |
| Anti-CTLA-4 mAb [171] | Immune checkpoint inhibitor (synergizes with ferroptosis). |
4.2. Therapeutic Inhibition of Ferroptosis: A Shield for Degenerative Disease and Injury
| Class | Compound | Primary Mechanism of Action |
|---|---|---|
| Iron Chelators | Deferoxamine (DFO) [174,175,176] | Iron chelator (systemic). |
| Deferiprone (DFP) [80,84,177,178] | Iron chelator (oral, brain-penetrant). | |
| Deferasirox (DFX) [179] | Iron chelator (oral). | |
| Dexrazoxane (DZR) [180,181,182] | Iron chelator; Topoisomerase II inhibitor. | |
| Natural Products (Polyphenols) | EGCG [184,185] | Natural polyphenol; iron chelator; antioxidant. |
| Curcumin [186,187,212] | Natural polyphenol; iron chelator; Nrf2 activator. | |
| Quercetin [188,190] | Natural flavonoid; iron chelator. | |
| Resveratrol [189,213] | Natural stilbenoid; iron chelator; Nrf2 activator. | |
| Radical-Trapping Antioxidants (RTAs) | Vitamin E (α-tocopherol) [197,198] | Physiological RTA (chain-breaking scavenger). |
| Ferrostatin-1 (Fer-1) [24,199] | Synthetic RTA (blocks LPO initiation and propagation). | |
| Liproxstatin-1 (Lip-1) [24,199] | Synthetic RTA (blocks LPO initiation and propagation). | |
| GSH/GPx4 Axis Support | N-acetylcysteine (NAC) [208] | GSH precursor. |
| Ebselen [209] | GPx4 mimetic (organoselenium compound). | |
| Nrf2 Activators | Sulforaphane [211] | Natural Nrf2 activator (isothiocyanate). |
| Dimethyl Fumarate (DMF) [214,215] | Synthetic Nrf2 activator (fumaric acid ester). | |
| Diroximel Fumarate (DRF) [214,215] | Synthetic Nrf2 activator (Prodrug of DMF). | |
| Bardoxolone Methyl [216] | Synthetic Nrf2 activator (triterpenoid). | |
| Omaveloxolone [216] | Synthetic Nrf2 activator (triterpenoid). |
5. Challenges and Future Perspectives in Ferroptosis Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 15-LOX | 15-lipoxygenase |
| 4-HNE | 4-hydroxynonenal |
| Aβ | amyloid-beta |
| ACSL4 | Acyl-CoA synthetase long-chain family member 4 |
| AD | Alzheimer’s disease |
| AKI | acute kidney injury |
| ALS | amyotrophic lateral sclerosis |
| AR | androgen receptor |
| ARE | antioxidant response element |
| BH4 | Tetrahydrobiopterin |
| CKD | chronic kidney disease |
| CMA | chaperone-mediated autophagy |
| CoQ10 | coenzyme Q10 |
| CoQH2/CoQ10H2 | Ubiquinol |
| CVDs | cardiovascular diseases |
| DAMPs | damage-associated molecular patterns |
| DFO | Deferoxamine |
| DFP | Deferiprone |
| DFX | Deferasirox |
| DHODH | dihydroorotate dehydrogenase |
| DMT1 | divalent metal transporter 1 |
| DMF | dimethyl fumarate |
| DRF | diroximel fumarate |
| DTP | drug-tolerant persister |
| DZR | Dexrazoxane |
| EGCG | epigallocatechin-3-gallate |
| EMT | epithelial-to-mesenchymal transition |
| ER | estrogen receptor |
| FADS2 | fatty acid desaturase 2 |
| Fer-1 | ferrostatin-1 |
| FPN1 | ferroportin |
| FSP1 | ferroptosis suppressor protein 1 |
| FTH1 | ferritin heavy chain |
| FTL | ferritin light chain |
| GCH1 | guanosine triphosphate cyclohydrolase 1 |
| GCL | glutamate–cysteine ligase |
| GPx4 | glutathione peroxidase 4 |
| GSH | glutathione |
| GSS | glutathione synthetase |
| GSSG | glutathione disulfide |
| HD | Huntington’s disease |
| HF | heart failure |
| HMGB1 | high-mobility group box 1 |
| HO-1 | heme oxygenase-1 |
| HSCs | hepatic stellate cells |
| IBD | inflammatory bowel disease |
| ICIs | immune checkpoint inhibitors |
| IFNγ | interferon–gamma |
| I/R | ischemia/reperfusion |
| IREs | iron-responsive elements |
| IRPs | iron-responsive element binding proteins |
| L• | lipid radical |
| Lip-1 | liproxstatin-1 |
| LIP | labile iron pool |
| LO• | alkoxyl radical |
| LOO• | lipid peroxyl radical |
| LOOH | lipid hydroperoxides |
| LOXs | lipoxygenases |
| LPCAT3 | lysophosphatidylcholine acyltransferase 3 |
| LPO | lipid peroxidation |
| LUAD | lung adenocarcinoma |
| MAO | monoamine oxidase |
| MBOAT | membrane-bound glycerophospholipid O-acyltransferase |
| MDA | malondialdehyde |
| mHTT | mutant huntingtin |
| MS | multiple sclerosis |
| MUFAs | monounsaturated fatty acids |
| NAC | N-acetylcysteine |
| NACs | natural active compounds |
| NASH | nonalcoholic steatohepatitis |
| NCOA4 | nuclear receptor coactivator 4 |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| NTBI | non-transferrin-bound ferrous iron |
| PCBP1 | poly(rC)-binding protein 1 |
| PD | Parkinson’s disease |
| PEBP1 | phosphatidylethanolamine-binding protein 1 |
| PUFA | polyunsaturated fatty acid |
| RA | rheumatoid arthritis |
| RA-FLS | RA-fibroblast-like synoviocytes |
| RCD | regulated cell death |
| ROS | reactive oxygen species |
| RTAs | radical-trapping antioxidants |
| SLE | systemic lupus erythematosus |
| TFRC | transferrin receptor 1 |
| TNBC | triple-negative breast cancer |
| TNF-α | tumor necrosis factor-alpha |
References
- Fuchs, Y.; Steller, H. Programmed Cell Death in Animal Development and Disease. Cell 2011, 147, 742–758. [Google Scholar] [CrossRef]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A Basic Biological Phenomenon with Wide-Ranging Implications in Tissue Kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Kepp, O.; Krautwald, S.; Kroemer, G.; Linkermann, A. Molecular Mechanisms of Regulated Necrosis. Semin. Cell Dev. Biol. 2014, 35, 24–32. [Google Scholar] [CrossRef]
- Man, S.M.; Karki, R.; Kanneganti, T.D. Molecular Mechanisms and Functions of Pyroptosis, Inflammatory Caspases and Inflammasomes in Infectious Diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, Biology and Role in Disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, J.; Chen, J.; Zhou, Z.; Lin, Q. Ferroptosis in Neurodegenerative Diseases: Potential Mechanisms of Exercise Intervention. Front. Cell Dev. Biol. 2025, 13, 1622544. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, X.Z.; Wang, Y.H.; Cheng, X.L.; Zhao, Y.; Zhou, L.Y.; Wang, K. Emerging Roles of Ferroptosis in Cardiovascular Diseases. Cell Death Discov. 2022, 8, 394. [Google Scholar] [CrossRef]
- Li, S.; Han, Q.; Liu, C.; Wang, Y.; Liu, F.; Pan, S.; Zuo, L.; Gao, D.; Chen, K.; Feng, Q.; et al. Role of Ferroptosis in Chronic Kidney Disease. Cell Commun. Signal. 2024, 22, 113. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Ge, C.; Min, J.; Wang, F. The Multifaceted Role of Ferroptosis in Liver Disease. Cell Death Differ. 2022, 29, 467–480. [Google Scholar] [CrossRef]
- Ru, Q.; Li, Y.; Chen, L.; Wu, Y.; Min, J.; Wang, F. Iron Homeostasis and Ferroptosis in Human Diseases: Mechanisms and Therapeutic Prospects. Signal Transduct. Target. Ther. 2024, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.L.; Gelbart, T.; West, C.; Halloran, C.; Beutler, E. The Human Nramp2 Gene: Characterization of the Gene Structure, Alternative Splicing, Promoter Region and Polymorphisms. Blood Cells Mol. Dis. 1998, 24, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Gruenheid, S.; Cellier, M.; Vidal, S.; Gros, P. Identification and Characterization of a Second Mouse Nramp Gene. Genomics 1995, 25, 514–525. [Google Scholar] [CrossRef]
- Liu, Q.; Barker, S.; Knutson, M.D. Iron and Manganese Transport in Mammalian Systems. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2021, 1868, 118890. [Google Scholar] [CrossRef]
- Yi, L.; Hu, Y.; Wu, Z.; Li, Y.; Kong, M.; Kang, Z.; Zuoyuan, B.; Yang, Z. TFRC Upregulation Promotes Ferroptosis in CVB3 Infection via Nucleus Recruitment of Sp1. Cell Death Dis. 2022, 13, 592. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Tang, D.; Wang, Y.; Li, X.; Bao, H.; Tang, C.; Dong, X.; Li, X.; Yang, Q.; Yan, Y.; et al. The Mechanism of Ferroptosis and Its Related Diseases. Mol. Biomed. 2023, 4, 33. [Google Scholar] [CrossRef]
- Camaschella, C.; Nai, A.; Silvestri, L. Iron Metabolism and Iron Disorders Revisited in the Hepcidin Era. Haematologica 2020, 105, 260–272. [Google Scholar] [CrossRef]
- Ohshima, T.; Yamamoto, H.; Sakamaki, Y.; Saito, C.; Mizushima, N. NCOA4 Drives Ferritin Phase Separation to Facilitate Macroferritinophagy and Microferritinophagy. J. Cell Biol. 2022, 221, e202203102. [Google Scholar] [CrossRef]
- Mancias, J.D.; Wang, X.; Gygi, S.P.; Harper, J.W.; Kimmelman, A.C. Quantitative Proteomics Identifies NCOA4 as the Cargo Receptor Mediating Ferritinophagy. Nature 2014, 509, 105–109. [Google Scholar] [CrossRef]
- Han, S.; Lin, F.; Qi, Y.; Liu, C.; Zhou, L.; Xia, Y.; Chen, K.; Xing, J.; Liu, Z.; Yu, W.; et al. HO-1 Contributes to Luteolin-Triggered Ferroptosis in Clear Cell Renal Cell Carcinoma via Increasing the Labile Iron Pool and Promoting Lipid Peroxidation. Oxidative Med. Cell Longev. 2022, 2022, 3846217. [Google Scholar] [CrossRef] [PubMed]
- Protchenko, O.; Baratz, E.; Jadhav, S.; Li, F.; Shakoury-Elizeh, M.; Gavrilova, O.; Ghosh, M.C.; Cox, J.E.; Maschek, J.A.; Tyurin, V.A.; et al. Iron Chaperone Poly RC Binding Protein 1 Protects Mouse Liver from Lipid Peroxidation and Steatosis. Hepatology 2021, 73, 1176–1193. [Google Scholar] [CrossRef]
- Wang, Y.; Protchenko, O.; Huber, K.D.; Shakoury-Elizeh, M.; Ghosh, M.C.; Philpott, C.C. The Iron Chaperone Poly(RC)-Binding Protein 1 Regulates Iron Efflux through Intestinal Ferroportin in Mice. Blood 2023, 142, 1658–1671. [Google Scholar] [CrossRef]
- Miotto, G.; Rossetto, M.; Di Paolo, M.L.; Orian, L.; Venerando, R.; Roveri, A.; Vučković, A.M.; Bosello Travain, V.; Zaccarin, M.; Zennaro, L.; et al. Insight into the Mechanism of Ferroptosis Inhibition by Ferrostatin-1. Redox Biol. 2020, 28, 101328. [Google Scholar] [CrossRef]
- Girotti, A.W. Lipid Hydroperoxide Generation, Turnover, and Effector Action in Biological Systems. J. Lipid Res. 1998, 39, 1529–1542. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free Radical Lipid Peroxidation: Mechanisms and Analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 Dictates Ferroptosis Sensitivity by Shaping Cellular Lipid Composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Kagan, V.E.; Mao, G.; Qu, F.; Angeli, J.P.F.; Doll, S.; Croix, C.S.; Dar, H.H.; Liu, B.; Tyurin, V.A.; Ritov, V.B.; et al. Oxidized Arachidonic and Adrenic PEs Navigate Cells to Ferroptosis. Nat. Chem. Biol. 2017, 13, 81–90. [Google Scholar] [CrossRef]
- Cui, J.; Wang, Y.; Tian, X.; Miao, Y.; Ma, L.; Zhang, C.; Xu, X.; Wang, J.; Fang, W.; Zhang, X. LPCAT3 Is Transcriptionally Regulated by YAP/ZEB/EP300 and Collaborates with ACSL4 and YAP to Determine Ferroptosis Sensitivity. Antioxid. Redox Signal. 2023, 39, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Wang, T.; Cao, C.; Li, X.; Li, H.; Gao, H.; Li, J.; Shen, H.; Chen, G. LPCAT3 Exacerbates Early Brain Injury and Ferroptosis after Subarachnoid Hemorrhage in Rats. Brain Res. 2024, 1832, 148864. [Google Scholar] [CrossRef]
- Manivarma, T.; Kapralov, A.A.; Samovich, S.N.; Tyurina, Y.Y.; Tyurin, V.A.; VanDemark, A.P.; Nowak, W.; Bayır, H.; Bahar, I.; Kagan, V.E.; et al. Membrane Regulation of 15LOX-1/PEBP1 Complex Prompts the Generation of Ferroptotic Signals, Oxygenated PEs. Free Radic. Biol. Med. 2023, 208, 458–467. [Google Scholar] [CrossRef]
- Hirata, Y.; Cai, R.; Volchuk, A.; Steinberg, B.E.; Saito, Y.; Matsuzawa, A.; Grinstein, S.; Freeman, S.A. Lipid Peroxidation Increases Membrane Tension, Piezo1 Gating, and Cation Permeability to Execute Ferroptosis. Curr. Biol. 2023, 33, 1282–1294.e5. [Google Scholar] [CrossRef] [PubMed]
- Roeck, B.F.; Lotfipour Nasudivar, S.; Vorndran, M.R.H.; Schueller, L.; Yapici, F.I.; Rübsam, M.; von Karstedt, S.; Niessen, C.M.; Garcia-Saez, A.J. Ferroptosis Spreads to Neighboring Cells via Plasma Membrane Contacts. Nat. Commun. 2025, 16, 2951. [Google Scholar] [CrossRef]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 Represses Nuclear Activation of Antioxidant Responsive Elements by Nrf2 through Binding to the Amino-Terminal Neh2 Domain. Genes. Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, S.; Jain, A.K.; Bloom, D.A.; Jaiswal, A.K. Bach1 Competes with Nrf2 Leading to Negative Regulation of the Antioxidant Response Element (ARE)-Mediated NAD(P)H:Quinone Oxidoreductase 1 Gene Expression and Induction in Response to Antioxidants. J. Biol. Chem. 2005, 280, 16891–16900. [Google Scholar] [CrossRef]
- Sasaki, H.; Sato, H.; Kuriyama-Matsumura, K.; Sato, K.; Maebara, K.; Wang, H.; Tamba, M.; Itoh, K.; Yamamoto, M.; Bannai, S. Electrophile Response Element-Mediated Induction of the Cystine/Glutamate Exchange Transporter Gene Expression. J. Biol. Chem. 2002, 277, 44765–44771. [Google Scholar] [CrossRef]
- Lu, S.C. Glutathione Synthesis. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Maiorino, M.; Gregolin, C. The Selenoenzyme Phospholipid Hydroperoxide Glutathione Peroxidase. Biochim. Biophys. Acta 1985, 839, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Cozza, G.; Rossetto, M.; Bosello-Travain, V.; Maiorino, M.; Roveri, A.; Toppo, S.; Zaccarin, M.; Zennaro, L.; Ursini, F. Glutathione Peroxidase 4-Catalyzed Reduction of Lipid Hydroperoxides in Membranes: The Polar Head of Membrane Phospholipids Binds the Enzyme and Addresses the Fatty Acid Hydroperoxide Group toward the Redox Center. Free Radic. Biol. Med. 2017, 112, 1–11. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M. Lipid Peroxidation and Ferroptosis: The Role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef]
- Yang, W.S.; Sriramaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Roveri, A.; Di Giacinto, F.; Rossetto, M.; Cozza, G.; Cheng, Q.; Miotto, G.; Zennaro, L.; Di Paolo, M.L.; Arner, E.S.J.; De Spirito, M.; et al. Cardiolipin Drives the Catalytic Activity of GPX4 on Membranes: Insights from the R152H Mutant. Redox Biol. 2023, 64, 102806. [Google Scholar] [CrossRef]
- Wu, K.C.; Cui, J.Y.; Klaassen, C.D. Beneficial Role of Nrf2 in Regulating NADPH Generation and Consumption. Toxicol. Sci. 2011, 123, 590. [Google Scholar] [CrossRef]
- Liu, C.; Xu, X.; He, X.; Ren, J.; Chi, M.; Deng, G.; Li, G.; Nasser, M.I. Activation of the Nrf-2/HO-1 Signalling Axis Can Alleviate Metabolic Syndrome in Cardiovascular Disease. Ann. Med. 2023, 55, 2284890. [Google Scholar] [CrossRef]
- Anandhan, A.; Dodson, M.; Shakya, A.; Chen, J.; Liu, P.; Wei, Y.; Tan, H.; Wang, Q.; Jiang, Z.; Yang, K.; et al. NRF2 Controls Iron Homeostasis and Ferroptosis through HERC2 and VAMP8. Sci. Adv. 2023, 9, eade9585. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Grocin, A.G.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 Is a Glutathione-Independent Ferroptosis Suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Mishima, E.; Yamada, N.; Santos, A.; Mourão, D.; Trümbach, D.; Doll, S.; Wanninger, J.; Lytton, E.; Sennhenn, P.; et al. Integrated Chemical and Genetic Screens Unveil FSP1 Mechanisms of Ferroptosis Regulation. Nat. Struct. Mol. Biol. 2023, 30, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ Oxidoreductase FSP1 Acts Parallel to GPX4 to Inhibit Ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Espinosa-García, J. Theoretical Study of the Trapping of the OOH Radical by Coenzyme Q. J. Am. Chem. Soc. 2004, 126, 920–927. [Google Scholar] [CrossRef]
- Kraft, V.A.N.; Bezjian, C.T.; Pfeiffer, S.; Ringelstetter, L.; Müller, C.; Zandkarimi, F.; Merl-Pham, J.; Bao, X.; Anastasov, N.; Kössl, J.; et al. GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent. Sci. 2019, 6, 41. [Google Scholar] [CrossRef]
- Li, D.; Lu, X.; Xu, G.; Liu, S.; Gong, Z.; Lu, F.; Xia, X.; Jiang, J.; Wang, H.; Zou, F.; et al. Dihydroorotate Dehydrogenase Regulates Ferroptosis in Neurons after Spinal Cord Injury via the P53-ALOX15 Signaling Pathway. CNS Neurosci. Ther. 2023, 29, 1923. [Google Scholar] [CrossRef]
- Liang, D.; Feng, Y.; Zandkarimi, F.; Wang, H.; Zhang, Z.; Kim, J.; Cai, Y.; Gu, W.; Stockwell, B.R.; Jiang, X. Ferroptosis Surveillance Independent of GPX4 and Differentially Regulated by Sex Hormones. Cell 2023, 186, 2748–2764.e22. [Google Scholar] [CrossRef]
- Viswanathan, V.S.; Ryan, M.J.; Dhruv, H.D.; Gill, S.; Eichhoff, O.M.; Seashore-Ludlow, B.; Kaffenberger, S.D.; Eaton, J.K.; Shimada, K.; Aguirre, A.J.; et al. Dependency of a Therapy-Resistant State of Cancer Cells on a Lipid Peroxidase Pathway. Nature 2017, 547, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhu, L. The Role of Glutathione Peroxidase 4 in the Progression, Drug Resistance, and Targeted Therapy of Non-Small Cell Lung Cancer. Oncol. Res. 2025, 33, 863. [Google Scholar] [CrossRef]
- Jiang, H.; Muir, R.K.; Gonciarz, R.L.; Olshen, A.B.; Yeh, I.; Hann, B.C.; Zhao, N.; Wang, Y.H.; Behr, S.C.; Korkola, J.E.; et al. Ferrous Iron–Activatable Drug Conjugate Achieves Potent MAPK Blockade in KRAS-Driven Tumors. J. Exp. Med. 2022, 219, e20210739. [Google Scholar] [CrossRef]
- Lee, J.; Roh, J.L. Epithelial-Mesenchymal Plasticity: Implications for Ferroptosis Vulnerability and Cancer Therapy. Crit. Rev. Oncol. Hematol. 2023, 185, 103964. [Google Scholar] [CrossRef] [PubMed]
- Lorito, N.; Subbiani, A.; Smiriglia, A.; Bacci, M.; Bonechi, F.; Tronci, L.; Romano, E.; Corrado, A.; Longo, D.L.; Iozzo, M.; et al. FADS1/2 Control Lipid Metabolism and Ferroptosis Susceptibility in Triple-Negative Breast Cancer. EMBO Mol. Med. 2024, 16, 1533–1559. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xiao, Y.; Ding, J.H.; Jin, X.; Ma, D.; Li, D.Q.; Shi, J.X.; Huang, W.; Wang, Y.P.; Jiang, Y.Z.; et al. Ferroptosis Heterogeneity in Triple-Negative Breast Cancer Reveals an Innovative Immunotherapy Combination Strategy. Cell Metab. 2023, 35, 84–100.e8. [Google Scholar] [CrossRef]
- He, C.; Li, Q.; Wu, W.; Liu, K.; Li, X.; Zheng, H.; Lai, Y. Ferroptosis-Associated Genes and Compounds in Renal Cell Carcinoma. Front. Immunol. 2024, 15, 1473203. [Google Scholar] [CrossRef]
- Nie, J.; Lin, B.; Zhou, M.; Wu, L.; Zheng, T. Role of Ferroptosis in Hepatocellular Carcinoma. J. Cancer Res. Clin. Oncol. 2018, 144, 2329–2337. [Google Scholar] [CrossRef]
- Wu, Y.C.; Huang, C.S.; Hsieh, M.S.; Huang, C.M.; Setiawan, S.A.; Yeh, C.T.; Kuo, K.T.; Liu, S.C. Targeting of FSP1 Regulates Iron Homeostasis in Drug-Tolerant Persister Head and Neck Cancer Cells via Lipid-Metabolism-Driven Ferroptosis. Aging 2024, 16, 627. [Google Scholar] [CrossRef]
- Meng, X.; Peng, F.; Yu, S.; Chi, X.; Wang, W.; Shao, S. Knockdown of NADK Promotes LUAD Ferroptosis via NADPH/FSP1 Axis. J. Cancer Res. Clin. Oncol. 2024, 150, 228. [Google Scholar] [CrossRef]
- Rodriguez, R.; Schreiber, S.L.; Conrad, M. Persister Cancer Cells: Iron Addiction and Vulnerability to Ferroptosis. Mol. Cell 2022, 82, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Basuli, D.; Tesfay, L.; Deng, Z.; Paul, B.; Yamamoto, Y.; Ning, G.; Xian, W.; McKeon, F.; Lynch, M.; Crum, C.P.; et al. Iron Addiction: A Novel Therapeutic Target in Ovarian Cancer. Oncogene 2017, 36, 4089–4099. [Google Scholar] [CrossRef]
- Yang, Y.; Ning, Y.; Chen, Y.; Tian, T.; Gao, X.; Kong, Y.; Lei, K.; Cui, Z. Transferrin Receptor Promotes Endometrial Cancer Proliferation by Activating the Iron-Dependent PI3K/AKT/MTOR Signaling Pathway. Cancer Sci. 2025, 116, 1352–1365. [Google Scholar] [CrossRef]
- Feng, G.; Arima, Y.; Midorikawa, K.; Kobayashi, H.; Oikawa, S.; Zhao, W.; Zhang, Z.; Takeuchi, K.; Murata, M. Knockdown of TFRC Suppressed the Progression of Nasopharyngeal Carcinoma by Downregulating the PI3K/Akt/MTOR Pathway. Cancer Cell Int. 2023, 23, 185. [Google Scholar] [CrossRef] [PubMed]
- Si, Q.; Wang, Y.; Lu, W.; Liu, Z.; Song, Y.; Chen, S.; Xia, S.; Li, H.; Weng, P.; Jing, Y.; et al. Transferrin Receptor Uptakes Iron from Tumor-Associated Neutrophils to Regulate Invasion Patterns of OSCC. Cancer Immunol. Immunother. 2025, 74, 43. [Google Scholar] [CrossRef]
- Tan, Q.; Zhang, X.; Li, S.; Liu, W.; Yan, J.; Wang, S.; Cui, F.; Li, D.; Li, J. DMT1 Differentially Regulates Mitochondrial Complex Activities to Reduce Glutathione Loss and Mitigate Ferroptosis. Free Radic. Biol. Med. 2023, 207, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Barra, J.; Crosbourne, I.; Roberge, C.L.; Bossardi-Ramos, R.; Warren, J.S.A.; Matteson, K.; Wang, L.; Jourd’heuil, F.; Borisov, S.M.; Bresnahan, E.; et al. DMT1-Dependent Endosome-Mitochondria Interactions Regulate Mitochondrial Iron Translocation and Metastatic Outgrowth. Oncogene 2024, 43, 650–667. [Google Scholar] [CrossRef]
- Bayanbold, K.; Singhania, M.; Fath, M.A.; Searby, C.C.; Stolwijk, J.M.; Henrich, J.B.; Pulliam, C.F.; Schoenfeld, J.D.; Mapuskar, K.A.; Sho, S.; et al. Depletion of Labile Iron Induces Replication Stress and Enhances Responses to Chemoradiation in Non-Small-Cell Lung Cancer. Antioxidants 2023, 12, 2005. [Google Scholar] [CrossRef]
- Loftus, L.V.; Rolle, L.T.A.; Wang, B.; Pienta, K.J.; Amend, S.R. Dysregulation of Labile Iron Predisposes Chemotherapy Resistant Cancer Cells to Ferroptosis. Int. J. Mol. Sci. 2025, 26, 4193. [Google Scholar] [CrossRef]
- Cañeque, T.; Baron, L.; Müller, S.; Carmona, A.; Colombeau, L.; Versini, A.; Solier, S.; Gaillet, C.; Sindikubwabo, F.; Sampaio, J.L.; et al. Activation of Lysosomal Iron Triggers Ferroptosis in Cancer. Nature 2025, 642, 492–500. [Google Scholar] [CrossRef]
- Qi, D.; Peng, M. Ferroptosis-Mediated Immune Responses in Cancer. Front. Immunol. 2023, 14, 1188365. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zou, J.; Liu, J.; Kang, R.; Tang, D. DAMPs in the Immunogenicity of Cell Death. Mol. Cell 2025, 85, 3874–3889. [Google Scholar] [CrossRef]
- Wang, W.; Green, M.; Choi, J.E.; Gijón, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.; Kryczek, I.; Sell, A.; et al. CD8+ T Cells Regulate Tumour Ferroptosis during Cancer Immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef]
- Gebicke-Haerter, P.J. The Computational Power of the Human Brain. Front. Cell Neurosci. 2023, 17, 1220030. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. Neuro-Vulnerability in Energy Metabolism Regulation: A Comprehensive Narrative Review. Nutrients 2023, 15, 3106. [Google Scholar] [CrossRef]
- Majerníková, N.; Marmolejo-Garza, A.; Salinas, C.S.; Luu, M.D.A.; Zhang, Y.; Trombetta-Lima, M.; Tomin, T.; Birner-Gruenberger, R.; Lehtonen, Š.; Koistinaho, J.; et al. The Link between Amyloid β and Ferroptosis Pathway in Alzheimer’s Disease Progression. Cell Death Dis. 2024, 15, 782. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.S.; Gao, L.; Han, Z.; Eleuteri, S.; Shi, W.; Shen, Y.; Song, Z.Y.; Su, M.; Yang, Q.; Qu, Y.; et al. Ferroptosis in Parkinson’s Disease: Molecular Mechanisms and Therapeutic Potential. Ageing Res. Rev. 2023, 91, 102077. [Google Scholar] [CrossRef]
- Jamal, M.H.; Dhupar, M.; Aran, K.R. Exploring the Role of Ferroptosis Pathways in Huntington’s Disease: Insight of Pathophysiology to Emerging Treatment. Brain Disord. 2025, 18, 100207. [Google Scholar] [CrossRef]
- Wang, T.; Tomas, D.; Perera, N.D.; Cuic, B.; Luikinga, S.; Viden, A.; Barton, S.K.; McLean, C.A.; Samson, A.L.; Southon, A.; et al. Ferroptosis Mediates Selective Motor Neuron Death in Amyotrophic Lateral Sclerosis. Cell Death Differ. 2022, 29, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.Y.; Liu, T.; Zhang, L.; Wang, M.J.; Yang, Y.; Gao, J. Role of Ferroptosis in Neurological Diseases. Neurosci. Lett. 2021, 747, 135614. [Google Scholar] [CrossRef] [PubMed]
- Van San, E.; Debruyne, A.C.; Veeckmans, G.; Tyurina, Y.Y.; Tyurin, V.A.; Zheng, H.; Choi, S.M.; Augustyns, K.; van Loo, G.; Michalke, B.; et al. Ferroptosis Contributes to Multiple Sclerosis and Its Pharmacological Targeting Suppresses Experimental Disease Progression. Cell Death Differ. 2023, 30, 2092–2103. [Google Scholar] [CrossRef]
- Levi, S.; Ripamonti, M.; Moro, A.S.; Cozzi, A. Iron Imbalance in Neurodegeneration. Mol. Psychiatry 2024, 29, 1139. [Google Scholar] [CrossRef]
- Ng, S.C.W.; Furman, R.; Axelsen, P.H.; Shchepinov, M.S. Free Radical Chain Reactions and Polyunsaturated Fatty Acids in Brain Lipids. ACS Omega 2022, 7, 25337–25345. [Google Scholar] [CrossRef]
- Bazinet, R.P.; Layé, S. Polyunsaturated Fatty Acids and Their Metabolites in Brain Function and Disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Benarroch, E. What Is the Role of Ferroptosis in Neurodegeneration? Neurology 2023, 101, 312. [Google Scholar] [CrossRef]
- Everett, J.; Brooks, J.; Lermyte, F.; O’Connor, P.B.; Sadler, P.J.; Dobson, J.; Collingwood, J.F.; Telling, N.D. Iron Stored in Ferritin Is Chemically Reduced in the Presence of Aggregating Aβ(1-42). Sci. Rep. 2020, 10, 10332. [Google Scholar] [CrossRef] [PubMed]
- Mathys, H.; Davila-Velderrain, J.; Peng, Z.; Gao, F.; Mohammadi, S.; Young, J.Z.; Menon, M.; He, L.; Abdurrob, F.; Jiang, X.; et al. Single-Cell Transcriptomic Analysis of Alzheimer’s Disease. Nature 2019, 570, 332–337, Erratum in Nature 2019, 571, E1. [Google Scholar] [CrossRef] [PubMed]
- Greenough, M.A.; Lane, D.J.R.; Balez, R.; Anastacio, H.T.D.; Zeng, Z.; Ganio, K.; McDevitt, C.A.; Acevedo, K.; Belaidi, A.A.; Koistinaho, J.; et al. Selective Ferroptosis Vulnerability Due to Familial Alzheimer’s Disease Presenilin Mutations. Cell Death Differ. 2022, 29, 2123–2136. [Google Scholar] [CrossRef]
- Rasool, A.; Manzoor, R.; Ullah, K.; Afzal, R.; Ul-Haq, A.; Imran, H.; Kaleem, I.; Akhtar, T.; Farrukh, A.; Hameed, S.; et al. Oxidative Stress and Dopaminergic Metabolism: A Major PD Pathogenic Mechanism and Basis of Potential Antioxidant Therapies. CNS Neurol. Disord. Drug Targets 2024, 23, 852–864. [Google Scholar] [CrossRef]
- Sofic, E.; Lange, K.W.; Jellinger, K.; Riederer, P. Reduced and Oxidized Glutathione in the Substantia Nigra of Patients with Parkinson’s Disease. Neurosci. Lett. 1992, 142, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Pearce, R.K.B.; Owen, A.; Daniel, S.; Jenner, P.; Marsden, C.D. Alterations in the Distribution of Glutathione in the Substantia Nigra in Parkinson’s Disease. J. Neural Transm. 1997, 104, 661–677. [Google Scholar] [CrossRef]
- Colamartino, M.; Duranti, G.; Ceci, R.; Sabatini, S.; Testa, A.; Cozzi, R. A Multi-Biomarker Analysis of the Antioxidant Efficacy of Parkinson’s Disease Therapy. Toxicol. Vitr. 2018, 47, 1–7. [Google Scholar] [CrossRef]
- Malik, M.Y.; Guo, F.; Asif-Malik, A.; Eftychidis, V.; Barkas, N.; Eliseeva, E.; Timm, K.N.; Wolska, A.; Bergin, D.; Zonta, B.; et al. Impaired Striatal Glutathione–Ascorbate Metabolism Induces Transient Dopamine Increase and Motor Dysfunction. Nat. Metab. 2024, 6, 2100–2117. [Google Scholar] [CrossRef]
- Niu, L.; Ye, C.; Sun, Y.; Peng, T.; Yang, S.; Wang, W.; Li, H. Mutant Huntingtin Induces Iron Overload via Up-Regulating IRP1 in Huntington’s Disease. Cell Biosci. 2018, 8, 41. [Google Scholar] [CrossRef]
- Wu, W.L.; Gong, X.X.; Qin, Z.H.; Wang, Y. Molecular Mechanisms of Excitotoxicity and Their Relevance to the Pathogenesis of Neurodegenerative Diseases—An Update. Acta Pharmacol. Sin. 2025, 46, 3129–3142. [Google Scholar] [CrossRef]
- Kwan, J.Y.; Jeong, S.Y.; van Gelderen, P.; Deng, H.X.; Quezado, M.M.; Danielian, L.E.; Butman, J.A.; Chen, L.; Bayat, E.; Russell, J.; et al. Iron Accumulation in Deep Cortical Layers Accounts for MRI Signal Abnormalities in ALS: Correlating 7 Tesla MRI and Pathology. PLoS ONE 2012, 7, e35241. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Ke, Y.; Luo, C.; Gozal, D.; Liu, R. Depletion of Reduced Glutathione Enhances Motor Neuron Degeneration In Vitro and In Vivo. Neuroscience 2006, 144, 991. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Wu, W.; Liu, J.; Lan, T.; Xiao, Z.; Gai, K.; Hu, L.; Luo, Z.; Wei, C.; Wang, X.; et al. Ferroptosis in Oligodendrocyte Progenitor Cells Mediates White Matter Injury after Hemorrhagic Stroke. Cell Death Dis. 2022, 13, 259. [Google Scholar] [CrossRef]
- Riedl, C.J.; Bormann, D.; Steinmaurer, A.; Novak, A.; Testa, G.; Poldlehner, E.; Haider, C.; Berger, T.; Mildner, M.; Höftberger, R.; et al. Inflammation Alters Myeloid Cell and Oligodendroglial Iron-Handling in Multiple Sclerosis. Acta Neuropathol. Commun. 2025, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.M.; Liu, N.; Qin, Z.H.; Wang, Y. Mitochondrial-Derived Damage-Associated Molecular Patterns Amplify Neuroinflammation in Neurodegenerative Diseases. Acta Pharmacol. Sin. 2022, 43, 2439–2447. [Google Scholar] [CrossRef]
- Fang, W.; Xie, S.; Deng, W. Ferroptosis Mechanisms and Regulations in Cardiovascular Diseases in the Past, Present, and Future. Cell Biol. Toxicol. 2024, 40, 17. [Google Scholar] [CrossRef]
- Luan, X.; Chen, P.; Miao, L.; Yuan, X.; Yu, C.; Di, G. Ferroptosis in Organ Ischemia–Reperfusion Injuries: Recent Advancements and Strategies. Mol. Cell. Biochem. 2025, 480, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, X.; Hu, B.; Rong, S. Expression Levels and Clinical Significance of Ferroptosis-Related Genes in Patients with Myocardial Infarction. Sci. Rep. 2024, 14, 1870. [Google Scholar] [CrossRef]
- Ouyang, S.; You, J.; Zhi, C.; Li, P.; Lin, X.; Tan, X.; Ma, W.; Li, L.; Xie, W. Ferroptosis: The Potential Value Target in Atherosclerosis. Cell Death Dis. 2021, 12, 782. [Google Scholar] [CrossRef]
- Yang, X.; Kawasaki, N.K.; Min, J.; Matsui, T.; Wang, F. Ferroptosis in Heart Failure. J. Mol. Cell Cardiol. 2022, 173, 141. [Google Scholar] [CrossRef]
- Li, T.; Wang, N.; Yi, D.; Xiao, Y.; Li, X.; Shao, B.; Wu, Z.; Bai, J.; Shi, X.; Wu, C.; et al. ROS-Mediated Ferroptosis and Pyroptosis in Cardiomyocytes: An Update. Life Sci. 2025, 370, 123565. [Google Scholar] [CrossRef]
- Bugger, H.; Pfeil, K. Mitochondrial ROS in Myocardial Ischemia Reperfusion and Remodeling. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165768. [Google Scholar] [CrossRef]
- Ma, X.H.; Liu, J.H.Z.; Liu, C.Y.; Sun, W.Y.; Duan, W.J.; Wang, G.; Kurihara, H.; He, R.R.; Li, Y.F.; Chen, Y.; et al. ALOX15-Launched PUFA-Phospholipids Peroxidation Increases the Susceptibility of Ferroptosis in Ischemia-Induced Myocardial Damage. Signal Transduct. Target. Ther. 2022, 7, 288. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.D.; Ikeda, M.; Ide, T.; Tadokoro, T.; Furusawa, S.; Abe, K.; Ishimaru, K.; Enzan, N.; Sada, M.; Yamamoto, T.; et al. Iron Overload via Heme Degradation in the Endoplasmic Reticulum Triggers Ferroptosis in Myocardial Ischemia-Reperfusion Injury. JACC Basic Transl. Sci. 2022, 7, 800–819, Erratum in JACC Basic Transl. Sci. 2022, 9, 162. [Google Scholar] [CrossRef]
- Buffon, A.; Santini, S.A.; Ramazzotti, V.; Rigattieri, S.; Liuzzo, G.; Biasucci, L.M.; Crea, F.; Giardina, B.; Maseri, A. Large, Sustained Cardiac Lipid Peroxidation and Reduced Antioxidant Capacity in the Coronary Circulation after Brief Episodes of Myocardial Ischemia. J. Am. Coll. Cardiol. 2000, 35, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Yuan, C.; Wu, X. Targeting Ferroptosis in Acute Kidney Injury. Cell Death Dis. 2022, 13, 182. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Huang, X.; Wang, S.; Wu, D.; Zhang, M.; Wei, W. The main molecular mechanisms of ferroptosis and its role in chronic kidney disease. Cell Signal. 2024, 121, 111256. [Google Scholar] [CrossRef]
- Zhang, Y.; Mou, Y.; Zhang, J.; Suo, C.; Zhou, H.; Gu, M.; Wang, Z.; Tan, R. Therapeutic Implications of Ferroptosis in Renal Fibrosis. Front. Mol. Biosci. 2022, 9, 890766. [Google Scholar] [CrossRef]
- Stadler, K.; Ilatovskaya, D.V. Renal Epithelial Mitochondria: Implications for Hypertensive Kidney Disease. Compr. Physiol. 2023, 14, 5225. [Google Scholar] [CrossRef]
- Ye, K.; Lan, R.; Chen, Z.; Lai, K.; Song, Y.; Li, G.; Ma, H.; Chen, H.; Xu, Y. Roles of ACSL4/GPX4 and FSP1 in Oxalate-Induced Acute Kidney Injury. Cell Death Discov. 2025, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Yu, X.; Shi, C.; Fang, Y.; Dai, C.; Zhou, Y. ACSL4 Predicts Rapid Kidney Function Decline in Patients with Diabetic Kidney Disease. Front. Endocrinol. 2025, 16, 1499555. [Google Scholar] [CrossRef]
- Mengstie, M.A.; Seid, M.A.; Gebeyehu, N.A.; Adella, G.A.; Kassie, G.A.; Bayih, W.A.; Gesese, M.M.; Anley, D.T.; Feleke, S.F.; Zemene, M.A.; et al. Ferroptosis in Diabetic Nephropathy: Mechanisms and Therapeutic Implications. Metab. Open 2023, 18, 100243. [Google Scholar] [CrossRef]
- Minami, Y.; Hoshino, A.; Higuchi, Y.; Hamaguchi, M.; Kaneko, Y.; Kirita, Y.; Taminishi, S.; Nishiji, T.; Taruno, A.; Fukui, M.; et al. Liver Lipophagy Ameliorates Nonalcoholic Steatohepatitis through Extracellular Lipid Secretion. Nat. Commun. 2023, 14, 4084. [Google Scholar] [CrossRef]
- Bellanti, F.; Villani, R.; Facciorusso, A.; Vendemiale, G.; Serviddio, G. Lipid Oxidation Products in the Pathogenesis of Non-Alcoholic Steatohepatitis. Free Radic. Biol. Med. 2017, 111, 173–185. [Google Scholar] [CrossRef]
- Borgne-Sanchez, A.; Fromenty, B. Mitochondrial Dysfunction in Drug-Induced Hepatic Steatosis: Recent Findings and Current Concept. Clin. Res. Hepatol. Gastroenterol. 2025, 49, 102529. [Google Scholar] [CrossRef] [PubMed]
- Prasun, P.; Ginevic, I.; Oishi, K. Mitochondrial Dysfunction in Nonalcoholic Fatty Liver Disease and Alcohol Related Liver Disease. Transl. Gastroenterol. Hepatol. 2021, 6, 4. [Google Scholar] [CrossRef]
- Boslem, E.; Reibe, S.; Carlessi, R.; Smeuninx, B.; Tegegne, S.; Egan, C.L.; McLennan, E.; Terry, L.V.; Nobis, M.; Mu, A.; et al. Therapeutic Blockade of ER Stress and Inflammation Prevents NASH and Progression to HCC. Sci. Adv. 2023, 9, eadh0831, Erratum in Sci. Adv. 2023, 9, eadl4279. [Google Scholar] [CrossRef]
- Sharma, R.S.; Harrison, D.J.; Kisielewski, D.; Cassidy, D.M.; McNeilly, A.D.; Gallagher, J.R.; Walsh, S.V.; Honda, T.; McCrimmon, R.J.; Dinkova-Kostova, A.T.; et al. Experimental Nonalcoholic Steatohepatitis and Liver Fibrosis Are Ameliorated by Pharmacologic Activation of Nrf2 (NF-E2 P45-Related Factor 2). Cell Mol. Gastroenterol. Hepatol. 2017, 5, 367. [Google Scholar] [CrossRef] [PubMed]
- Hino, K.; Nishina, S.; Yanatori, I. Ferroptosis: Biology and role in liver disease. J. Gastroenterol. 2025, 60, 1339–1361. [Google Scholar] [CrossRef]
- Yu, Y.; Jiang, L.; Wang, H.; Shen, Z.; Cheng, Q.; Zhang, P.; Wang, J.; Wu, Q.; Fang, X.; Duan, L.; et al. Hepatic Transferrin Plays a Role in Systemic Iron Homeostasis and Liver Ferroptosis. Blood 2020, 136, 726–739. [Google Scholar] [CrossRef] [PubMed]
- Peleman, C.; Francque, S.; Berghe, T. Vanden Emerging Role of Ferroptosis in Metabolic Dysfunction-Associated Steatotic Liver Disease: Revisiting Hepatic Lipid Peroxidation. EBioMedicine 2024, 102, 105088. [Google Scholar] [CrossRef]
- Yu, Q.; Song, L. Unveiling the Role of Ferroptosis in the Progression from NAFLD to NASH: Recent Advances in Mechanistic Understanding. Front. Endocrinol. 2024, 15, 1431652. [Google Scholar] [CrossRef]
- Okuda, M.; Lee, H.C.; Chance, B.; Kumar, C. Glutathione and Ischemia-Reperfusion Injury in the Perfused Rat Liver. Free Radic. Biol. Med. 1992, 12, 271–279. [Google Scholar] [CrossRef]
- Dimova, S.; Hoet, P.H.M.; Dinsdale, D.; Nemery, B. Acetaminophen Decreases Intracellular Glutathione Levels and Modulates Cytokine Production in Human Alveolar Macrophages and Type II Pneumocytes In Vitro. Int. J. Biochem. Cell Biol. 2005, 37, 1727–1737. [Google Scholar] [CrossRef]
- Roušar, T.; Pařík, P.; Kučera, O.; Bartoš, M.; Červinková, Z. Glutathione Reductase Is Inhibited by Acetaminophen-Glutathione Conjugate In Vitro. Physiol. Res. 2010, 59, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Luo, J.; Zhu, X.; Miao, P.; Tang, H.; Jian, Y.; Ruan, S.; Ling, F.; Tang, M. Recent Progress in the Effect of Ferroptosis of HSCs on the Development of Liver Fibrosis. Front. Mol. Biosci. 2023, 10, 1258870. [Google Scholar] [CrossRef]
- Wang, Y.; Kuang, S.; Li, K.; Chen, S.; Yang, M.; Deng, K.; Li, M.; Xie, S.; Chen, Q.; Wen, J.; et al. Astragalin Promotes HSCs Ferroptosis through NCOA4 Mediated Ferritinophagy to Alleviate Liver Fibrosis in Zebrafish and Mice. Commun. Biol. 2025, 8, 1081. [Google Scholar] [CrossRef]
- Zeng, L.; Yang, K.; Yu, G.; Hao, W.; Zhu, X.; Ge, A.; Chen, J.; Sun, L. Advances in Research on Immunocyte Iron Metabolism, Ferroptosis, and Their Regulatory Roles in Autoimmune and Autoinflammatory Diseases. Cell Death Dis. 2024, 15, 481, Erratum in Cell Death Dis. 2024, 15, 765. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, Z.M.; Yi, X.; Wei, X.; Jiang, D.S. The Interaction between Ferroptosis and Inflammatory Signaling Pathways. Cell Death Dis. 2023, 14, 205. [Google Scholar] [CrossRef]
- Eltyar, F.S.; El-Tanbouly, D.M.; Zaki, H.F.; El-Sayed, R.M. Crosstalk between Ferroptosis and NLRP3, a Possible Therapeutic Target in Experimentally-Induced Rheumatoid Arthritis: Role of P2Y12R Inhibition in Modulating P53/SLC7A11/ALOX15 Signaling. Inflammopharmacology 2025, 33, 3947. [Google Scholar] [CrossRef]
- Davaanyam, D.; Lee, H.; Seol, S.I.; Oh, S.A.; Kim, S.W.; Lee, J.K. HMGB1 Induces Hepcidin Upregulation in Astrocytes and Causes an Acute Iron Surge and Subsequent Ferroptosis in the Postischemic Brain. Exp. Mol. Med. 2023, 55, 2402–2416. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Chen, A.; Chen, W.; Cheng, S.; Lin, S.; Mei, R.; Mei, X. Knockout of TNF-α in Microglia Decreases Ferroptosis and Convert Microglia Phenotype after Spinal Cord Injury. Heliyon 2024, 10, e36488. [Google Scholar] [CrossRef]
- Kong, R.; Wang, N.; Han, W.; Bao, W.; Lu, J. IFNγ-Mediated Repression of System Xc- Drives Vulnerability to Induced Ferroptosis in Hepatocellular Carcinoma Cells. J. Leukoc. Biol. 2021, 110, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Peng, M.; Ma, J.; Hu, R.; Xu, Q.; Hu, P.; Chen, L. Hepcidin Deficiency Disrupts Iron Homeostasis and Induces Ferroptosis in Zebrafish Liver. Fishes 2025, 10, 243. [Google Scholar] [CrossRef]
- Zhang, H.; Ostrowski, R.; Jiang, D.; Zhao, Q.; Liang, Y.; Che, X.; Zhao, J.; Xiang, X.; Qin, W.; He, Z. Hepcidin Promoted Ferroptosis through Iron Metabolism Which Is Associated with DMT1 Signaling Activation in Early Brain Injury Following Subarachnoid Hemorrhage. Oxidative Med. Cell. Longev. 2021, 2021, 9800794. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Kashanchi, F.; Foster, A.; Rotimi, J.; Turner, W.; Gordeuk, V.R.; Nekhai, S. Hepcidin Induces HIV-1 Transcription Inhibited by Ferroportin. Retrovirology 2010, 7, 104. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Gao, N.; Wang, Y.; Tian, Y.; Wu, J.; Zhang, J.; Zhu, J.; Fan, D.; An, J. Inhibitory Effect of Glutathione on Oxidative Liver Injury Induced by Dengue Virus Serotype 2 Infections in Mice. PLoS ONE 2013, 8, e55407. [Google Scholar] [CrossRef]
- Labarrere, C.A.; Kassab, G.S. Glutathione Deficiency in the Pathogenesis of SARS-CoV-2 Infection and Its Effects upon the Host Immune Response in Severe COVID-19 Disease. Front. Microbiol. 2022, 13, 979719. [Google Scholar] [CrossRef]
- Ding, L. Ferroptosis in Viral Infection: A Potential Therapeutic Target. Future Microbiol. 2024, 19, 519. [Google Scholar] [CrossRef]
- Ousingsawat, J.; Schreiber, R.; Gulbins, E.; Kamler, M.; Kunzelmann, K.P. Aeruginosa Induced Lipid Peroxidation Causes Ferroptotic Cell Death in Airways. Cell. Physiol. Biochem. 2021, 55, 590–604. [Google Scholar] [CrossRef] [PubMed]
- Dar, H.H.; Tyurina, Y.Y.; Mikulska-Ruminska, K.; Shrivastava, I.; Ting, H.C.; Tyurin, V.A.; Krieger, J.; Croix, C.M.S.; Watkins, S.; Bayir, E.; et al. Pseudomonas Aeruginosa Utilizes Host Polyunsaturated Phosphatidylethanolamines to Trigger Theft-Ferroptosis in Bronchial Epithelium. J. Clin. Investig. 2018, 128, 4639. [Google Scholar] [CrossRef] [PubMed]
- Kain, H.S.; Glennon, E.K.K.; Vijayan, K.; Arang, N.; Douglass, A.N.; Fortin, C.L.; Zuck, M.; Lewis, A.J.; Whiteside, S.L.; Dudgeon, D.R.; et al. Liver Stage Malaria Infection Is Controlled by Host Regulators of Lipid Peroxidation. Cell Death Differ. 2020, 27, 44–54. [Google Scholar] [CrossRef]
- Yamane, D.; Hayashi, Y.; Matsumoto, M.; Nakanishi, H.; Imagawa, H.; Kohara, M.; Lemon, S.M.; Ichi, I. FADS2-Dependent Fatty Acid Desaturation Dictates Cellular Sensitivity to Ferroptosis and Permissiveness for Hepatitis C Virus Replication. Cell Chem. Biol. 2022, 29, 799–810. [Google Scholar] [CrossRef]
- Li, P.; Jiang, M.; Li, K.; Li, H.; Zhou, Y.; Xiao, X.; Xu, Y.; Krishfield, S.; Lipsky, P.E.; Tsokos, G.C.; et al. Glutathione Peroxidase 4 Regulated Neutrophil Ferroptosis Induces Systemic Autoimmunity. Nat. Immunol. 2021, 22, 1107. [Google Scholar] [CrossRef]
- Niu, R.; Lan, J.; Liang, D.; Xiang, L.; Wu, J.; Zhang, X.; Li, Z.; Chen, H.; Geng, L.; Xu, W.; et al. GZMA Suppressed GPX4-Mediated Ferroptosis to Improve Intestinal Mucosal Barrier Function in Inflammatory Bowel Disease. Cell Commun. Signal. 2024, 22, 474. [Google Scholar] [CrossRef]
- Zhao, H.; Tang, C.; Wang, M.; Zhao, H.; Zhu, Y. Ferroptosis as an Emerging Target in Rheumatoid Arthritis. Front. Immunol. 2023, 14, 1260839. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Abreu, M.T.; Guan, J.; Khalid, U.; Ning, J.; Costa, M.R.; Chan, J.; Li, Q.; Fortin, J.P.; Wong, W.R.; Perampalam, P.; et al. Inhibition of GPX4 Enhances CDK4/6 Inhibitor and Endocrine Therapy Activity in Breast Cancer. Nat. Commun. 2024, 15, 9550. [Google Scholar] [CrossRef]
- Hangauer, M.J.; Viswanathan, V.S.; Ryan, M.J.; Bole, D.; Eaton, J.K.; Matov, A.; Galeas, J.; Dhruv, H.D.; Berens, M.E.; Schreiber, S.L.; et al. Drug-Tolerant Persister Cancer Cells Are Vulnerable to GPX4 Inhibition. Nature 2017, 551, 247. [Google Scholar] [CrossRef]
- Weïwer, M.; Bittker, J.A.; Lewis, T.A.; Shimada, K.; Yang, W.S.; MacPherson, L.; Dandapani, S.; Palmer, M.; Stockwell, B.R.; Schreiber, S.L.; et al. Development of Small-Molecule Probes That Selectively Kill Cells Induced to Express Mutant RAS. Bioorganic Med. Chem. Lett. 2012, 22, 1822–1826. [Google Scholar] [CrossRef]
- Wiernicki, B.; Maschalidi, S.; Pinney, J.; Adjemian, S.; Vanden Berghe, T.; Ravichandran, K.S.; Vandenabeele, P. Cancer Cells Dying from Ferroptosis Impede Dendritic Cell-Mediated Anti-Tumor Immunity. Nat. Commun. 2022, 13, 3676. [Google Scholar] [CrossRef]
- Sun, Y.; Berleth, N.; Wu, W.; Schlütermann, D.; Deitersen, J.; Stuhldreier, F.; Berning, L.; Friedrich, A.; Akgün, S.; Mendiburo, M.J.; et al. Fin56-Induced Ferroptosis Is Supported by Autophagy-Mediated GPX4 Degradation and Functions Synergistically with MTOR Inhibition to Kill Bladder Cancer Cells. Cell Death Dis. 2021, 12, 1028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, H.; Daniels, J.D.; Zandkarimi, F.; Liu, H.; Brown, L.M.; Uchida, K.; O’Connor, O.A.; Stockwell, B.R. Imidazole Ketone Erastin Induces Ferroptosis and Slows Tumor Growth in a Mouse Lymphoma Model. Cell Chem. Biol. 2019, 26, 623. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Stockwell, B.R. Synthetic Lethal Screening Identifies Compounds Activating Iron-Dependent, Nonapoptotic Cell Death in Oncogenic-RAS-Harboring Cancer Cells. Chem. Biol. 2008, 15, 234–245. [Google Scholar] [CrossRef]
- Dixon, S.J.; Patel, D.N.; Welsch, M.; Skouta, R.; Lee, E.D.; Hayano, M.; Thomas, A.G.; Gleason, C.E.; Tatonetti, N.P.; Slusher, B.S.; et al. Pharmacological Inhibition of Cystine-Glutamate Exchange Induces Endoplasmic Reticulum Stress and Ferroptosis. Elife 2014, 3, e02523. [Google Scholar] [CrossRef] [PubMed]
- Lachaier, E.; Louandre, C.; Godin, C.; Saidak, Z.; Baert, M.; Diouf, M.; Chauffert, B.; Galmiche, A. Sorafenib Induces Ferroptosis in Human Cancer Cell Originating from Different Solid Tumors. Anticancer Res. 2014, 34, 6417–6422. [Google Scholar]
- Zheng, J.; Sato, M.; Mishima, E.; Sato, H.; Proneth, B.; Conrad, M. Sorafenib Fails to Trigger Ferroptosis across a Wide Range of Cancer Cell Lines. Cell Death Dis. 2021, 12, 698. [Google Scholar] [CrossRef]
- Birsen, R.; Larrue, C.; Decroocq, J.; Johnson, N.; Guiraud, N.; Gotanegre, M.; Cantero-Aguilar, L.; Grignano, E.; Huynh, T.; Fontenay, M.; et al. APR-246 Induces Early Cell Death by Ferroptosis in Acute Myeloid Leukemia. Haematologica 2021, 107, 403. [Google Scholar] [CrossRef]
- Hendricks, J.M.; Doubravsky, C.E.; Wehri, E.; Li, Z.; Roberts, M.A.; Deol, K.K.; Lange, M.; Lasheras-Otero, I.; Momper, J.D.; Dixon, S.J.; et al. Identification of Structurally Diverse FSP1 Inhibitors That Sensitize Cancer Cells to Ferroptosis. Cell Chem. Biol. 2023, 30, 1090–1103.e7. [Google Scholar] [CrossRef]
- Xavier da Silva, T.N.; Friedmann Angeli, J.P. Sabotaging the Breaks: FSEN1 Expands the Toolbox of FSP1 Inhibitors. Cell Chem. Biol. 2023, 30, 1006–1008. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Liu, X.; Zhang, Y.; Lei, G.; Yan, Y.; Lee, H.; Koppula, P.; Wu, S.; Zhuang, L.; Fang, B.; et al. DHODH-Mediated Ferroptosis Defence Is a Targetable Vulnerability in Cancer. Nature 2021, 593, 586–590, Erratum in Nature 2021, 596, E13. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Zhang, H.; Lai, M.; Wei, J.; Qian, J.; Chen, X.; Wang, X.; Wang, Y. Sulfasalazine Induces Ferroptosis in Osteosarcomas by Regulating Nrf2/SLC7A11/GPX4 Signaling Axis. Sci. Rep. 2025, 15, 30197. [Google Scholar] [CrossRef]
- Belavgeni, A.; Tonnus, W.; Linkermann, A. Cancer Cells Evade Ferroptosis: Sex Hormone-Driven Membrane-Bound O-Acyltransferase Domain-Containing 1 and 2 (MBOAT1/2) Expression. Signal Transduct. Target. Ther. 2023, 8, 336. [Google Scholar] [CrossRef]
- Song, Q.; Peng, S.; Sun, Z.; Heng, X.; Zhu, X. Temozolomide Drives Ferroptosis via a DMT1-Dependent Pathway in Glioblastoma Cells. Yonsei Med. J. 2021, 62, 843–849. [Google Scholar] [CrossRef]
- Zamani, M.R.; Šácha, P. Immune Checkpoint Inhibitors in Cancer Therapy: What Lies beyond Monoclonal Antibodies? Med. Oncol. 2025, 42, 273. [Google Scholar] [CrossRef]
- Jiang, Z.; Lim, S.O.; Yan, M.; Hsu, J.L.; Yao, J.; Wei, Y.; Chang, S.S.; Yamaguchi, H.; Lee, H.H.; Ke, B.; et al. TYRO3 Induces Anti-PD-1/PD-L1 Therapy Resistance by Limiting Innate Immunity and Tumoral Ferroptosis. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, Y.; Chen, R.; Tang, E.; Tang, S. From Mechanisms to Therapy: Exploring the Role of Ferroptosis in Cervical Cancer Transformation and Treatment. Traffic 2025, 26, 7–9. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, Y.; Sun, C.; Xiang, Y.; Deng, Y. Deferoxamine Reduces Endothelial Ferroptosis and Protects Cerebrovascular Function after Experimental Traumatic Brain Injury. Brain Res. Bull. 2024, 207, 110878. [Google Scholar] [CrossRef]
- Huanga, H.; He, Z.; Roberts, L.J.; Salahudeen, A.K. Deferoxamine Reduces Cold-Ischemic Renal Injury in a Syngeneic Kidney Transplant Model. Am. J. Transplant. 2003, 3, 1531–1537. [Google Scholar] [CrossRef]
- Zager, R.A. Combined Mannitol and Deferoxamine Therapy for Myohemoglobinuric Renal Injury and Oxidant Tubular Stress. Mechanistic and Therapeutic Implications. J. Clin. Investig. 1992, 90, 711–719. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lau, Y.M.; Ng, K.M.; Lai, W.H.; Ho, S.L.; Tse, H.F.; Siu, C.W.; Ho, P.W.L. Efficient Attenuation of Friedreich’s Ataxia (FRDA) Cardiomyopathy by Modulation of Iron Homeostasis-Human Induced Pluripotent Stem Cell (HiPSC) as a Drug Screening Platform for FRDA. Int. J. Cardiol. 2016, 203, 964–971, Erratum in Int. J. Cardiol. 2016, 207, 393. [Google Scholar] [CrossRef]
- Soriano, S.; Llorens, J.V.; Blanco-Sobero, L.; Gutiérrez, L.; Calap-Quintana, P.; Morales, M.P.; Moltó, M.D.; Martínez-Sebastián, M.J. Deferiprone and Idebenone Rescue Frataxin Depletion Phenotypes in a Drosophila Model of Friedreich’s Ataxia. Gene 2013, 521, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Adel, N.; Mantawy, E.M.; El-Sherbiny, D.A.; El-Demerdash, E. Iron Chelation by Deferasirox Confers Protection against Concanavalin A-Induced Liver Fibrosis: A Mechanistic Approach. Toxicol. Appl. Pharmacol. 2019, 382, 114748. [Google Scholar] [CrossRef] [PubMed]
- Hasinoff, B.B.; Patel, D.; Wu, X. The Role of Topoisomerase IIβ in the Mechanisms of Action of the Doxorubicin Cardioprotective Agent Dexrazoxane. Cardiovasc. Toxicol. 2020, 20, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Upshaw, J.N.; Parson, S.K.; Buchsbaum, R.J.; Schlam, I.; Ruddy, K.J.; Durani, U.; Epperla, N.; Leong, D.P. Dexrazoxane to Prevent Cardiotoxicity in Adults Treated with Anthracyclines: JACC: CardioOncology Controversies in Cardio-Oncology. Cardio Oncol. 2024, 6, 322–324. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Liu, Z.; Du, K.; Lu, X. Protective Effects of Dexazoxane on Rat Ferroptosis in Doxorubicin-Induced Cardiomyopathy Through Regulating HMGB1. Front. Cardiovasc. Med. 2021, 8, 685434. [Google Scholar] [CrossRef]
- Scarano, A.; Laddomada, B.; Blando, F.; De Santis, S.; Verna, G.; Chieppa, M.; Santino, A. The Chelating Ability of Plant Polyphenols Can Affect Iron Homeostasis and Gut Microbiota. Antioxidants 2023, 12, 630. [Google Scholar] [CrossRef] [PubMed]
- Al Amin, M.; Zehravi, M.; Sweilam, S.H.; Shatu, M.M.; Durgawale, T.P.; Qureshi, M.S.; Durgapal, S.; Haque, M.A.; Vodeti, R.; Panigrahy, U.P.; et al. Neuroprotective Potential of Epigallocatechin Gallate in Neurodegenerative Diseases: Insights into Molecular Mechanisms and Clinical Relevance. Brain Res. 2025, 1860, 149693. [Google Scholar] [CrossRef] [PubMed]
- Settakorn, K.; Kongkarnka, S.; Chompupoung, A.; Svasti, S.; Fucharoen, S.; Porter, J.B.; Srichairatanakool, S.; Koonyosying, P. Effects of Green Tea Extract Treatment on Erythropoiesis and Iron Parameters in Iron-Overloaded β-Thalassemic Mice. Front. Physiol. 2022, 13, 1053060. [Google Scholar] [CrossRef]
- Jalali, M.; Mahmoodi, M.; Mosallanezhad, Z.; Jalali, R.; Imanieh, M.H.; Moosavian, S.P. The Effects of Curcumin Supplementation on Liver Function, Metabolic Profile and Body Composition in Patients with Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Med. 2020, 48, 102283. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Neuroprotective Effects of Curcumin in Neurodegenerative Diseases. Foods 2024, 13, 1774. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Chen, H.Y.; Tang, Q.Q.; Li, Y.F.; Liu, X.S.; Lu, F.H.; Gu, Y.Y. Protective Effect of Quercetin on Kidney Diseases: From Chemistry to Herbal Medicines. Front. Pharmacol. 2022, 13, 968226. [Google Scholar] [CrossRef]
- Gal, R.; Deres, L.; Toth, K.; Halmosi, R.; Habon, T. The Effect of Resveratrol on the Cardiovascular System from Molecular Mechanisms to Clinical Results. Int. J. Mol. Sci. 2021, 22, 10152. [Google Scholar] [CrossRef]
- Kato, K.; Takahashi, M.; Oh-hashi, K.; Ando, K.; Hirata, Y. Quercetin and Resveratrol Inhibit Ferroptosis Independently of Nrf2–ARE Activation in Mouse Hippocampal HT22 Cells. Food Chem. Toxicol. 2023, 172, 113586. [Google Scholar] [CrossRef]
- Wang, N.; Xue, X.; Zhang, Z.; Gao, M.; Yin, L.; Xu, L.; Zhao, X.; Peng, J. Curcumin-Loaded Nanoparticles for Renal Ischemia-Reperfusion Injuries: Triple-Play of Redox Homeostasis Accommodation, Lipid Metabolism Regulation, and Nuclear Magnetic Tracing. Mater. Today Bio 2025, 33, 101986. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Han, M.; Li, R.; Zhou, L.; Zhang, Y.; Duan, L.; Su, S.; Li, M.; Wang, Q.; Chen, T.; et al. Curcumin Nanoparticles Inhibiting Ferroptosis for the Enhanced Treatment of Intracerebral Hemorrhage. Int. J. Nanomed. 2021, 16, 8049. [Google Scholar] [CrossRef]
- Wei, H.; Qin, J.; Huang, Q.; Jin, Z.; Zheng, L.; Zhao, J.; Qin, Z. Epigallocatechin-3-Gallate (EGCG) Based Metal-Polyphenol Nanoformulations Alleviates Chondrocytes Inflammation by Modulating Synovial Macrophages Polarization. Biomed. Pharmacother. 2023, 161, 114366. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fan, Y.; Luo, Y.; Guo, C.; Hu, Y.; Guo, X.; Zhou, D.; Zhu, B. Mechanisms of Epigallocatechin-3-Gallate-Loaded Metal−organic Framework in Preventing Oxidative Degradation of Shrimp (Litopenaeus vannamei) Surimi Gel. Food Chem. 2025, 473, 143036. [Google Scholar] [CrossRef]
- Zhang, L.; Zhan, M.; Sun, H.; Zou, Y.; Laurent, R.; Mignani, S.; Majoral, J.P.; Cao, X.; Shen, M.; Shi, X. Mesenchymal Stem-Cell-Derived Exosomes Loaded with Phosphorus Dendrimers and Quercetin Treat Parkinson’s Disease by Modulating Inflammatory Immune Microenvironment. ACS Appl. Mater. Interfaces 2025, 17, 32013–32027. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, X.; Guo, B.; Song, J.; Bi, H. Quercetin-Loaded Exosomes Delivery System Prevents Myopia Progression by Targeting Endoplasmic Reticulum Stress and Ferroptosis in Scleral Fibroblasts. Mater. Today Bio 2025, 32, 101896. [Google Scholar] [CrossRef]
- Niki, E. Lipid Oxidation That Is, and Is Not, Inhibited by Vitamin E: Consideration about Physiological Functions of Vitamin E. Free Radic. Biol. Med. 2021, 176, 1–15. [Google Scholar] [CrossRef]
- Chiabrando, C.; Avanzini, F.; Rivalta, C.; Colombo, F.; Fanelli, R.; Palumbo, G.; Roncaglioni, M.C.; Pioltelli, M.B.; Capra, A.; Cristofari, M.; et al. Long-Term Vitamin E Supplementation Fails to Reduce Lipid Peroxidation in People at Cardiovascular Risk: Analysis of Underlying Factors. Curr. Control. Trials Cardiovasc. Med. 2002, 3, 5. [Google Scholar] [CrossRef]
- Zilka, O.; Shah, R.; Li, B.; Friedmann Angeli, J.P.; Griesser, M.; Conrad, M.; Pratt, D.A. On the Mechanism of Cytoprotection by Ferrostatin-1 and Liproxstatin-1 and the Role of Lipid Peroxidation in Ferroptotic Cell Death. ACS Cent. Sci. 2017, 3, 232–243. [Google Scholar] [CrossRef]
- Feng, Y.; Madungwe, N.B.; Imam Aliagan, A.D.; Tombo, N.; Bopassa, J.C. Ferroptosis Inhibitor, Liproxstatin-1, Protects the Myocardium against Ischemia/Reperfusion Injury by Decreasing VDAC1 Levels and Rescuing GPX4 Levels. Biochem. Biophys. Res. Commun. 2019, 520, 606. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Du, Y.; Zheng, J.; Tang, W.; Liang, Q.; Zheng, Z.; Liu, B.; Sun, H.; Wang, K.; Shao, C. Liproxstatin-1 Alleviated Ischemia/Reperfusion-Induced Acute Kidney Injury via Inhibiting Ferroptosis. Antioxidants 2024, 13, 182. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a Target for Protection against Cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef]
- Jing, S.; Liu, Y.; Ye, Z.; Ghaleb Al-bashari, A.A.; Zhou, H.; He, Y. Ferrostatin-1 Loaded Gelatin Methacrylate Scaffold Promotes Recovery from Spinal Cord Injury via Inhibiting Apoptosis and Ferroptosis. Nano TransMed 2023, 2, 100005. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Y.; He, C.; Yan, F.; Li, J.R.; Xu, H.Z.; Zhuang, J.F.; Zhou, H.; Peng, Y.C.; Fu, X.J.; et al. Selective Ferroptosis Inhibitor Liproxstatin-1 Attenuates Neurological Deficits and Neuroinflammation After Subarachnoid Hemorrhage. Neurosci. Bull. 2021, 37, 535. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.Y.; Pang, Y.L.; Li, W.X.; Zhao, C.X.; Zhang, Y.; Wang, X.; Ning, G.Z.; Kong, X.H.; Liu, C.; Yao, X.; et al. Liproxstatin-1 Is an Effective Inhibitor of Oligodendrocyte Ferroptosis Induced by Inhibition of Glutathione Peroxidase 4. Neural Regen. Res. 2020, 16, 561. [Google Scholar] [CrossRef]
- Li, Y.; Sun, M.; Cao, F.; Chen, Y.; Zhang, L.; Li, H.; Cao, J.; Song, J.; Ma, Y.; Mi, W.; et al. The Ferroptosis Inhibitor Liproxstatin-1 Ameliorates LPS-Induced Cognitive Impairment in Mice. Nutrients 2022, 14, 4599. [Google Scholar] [CrossRef] [PubMed]
- Naderi, S.; Motamedi, F.; Pourbadie, H.G.; Rafiei, S.; Khodagholi, F.; Naderi, N.; Janahmadi, M. Neuroprotective Effects of Ferrostatin and Necrostatin Against Entorhinal Amyloidopathy-Induced Electrophysiological Alterations Mediated by Voltage-Gated Ca2+ Channels in the Dentate Gyrus Granular Cells. Neurochem. Res. 2024, 49, 99–116. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, W.; Ito, J.; Henkelmann, B.; Xu, C.; Mishima, E.; Conrad, M. N-Acetyl-L-Cysteine Averts Ferroptosis by Fostering Glutathione Peroxidase 4. Cell Chem. Biol. 2025, 32, 767–775. [Google Scholar] [CrossRef]
- Conrad, M.; Proneth, B. Selenium: Tracing Another Essential Element of Ferroptotic Cell Death. Cell Chem. Biol. 2020, 27, 409–419. [Google Scholar] [CrossRef]
- Moretti, D.; Tambone, S.; Cerretani, M.; Fezzardi, P.; Missineo, A.; Sherman, L.T.; Munoz-Sajuan, I.; Harper, S.; Dominquez, C.; Pacifici, R.; et al. NRF2 Activation by Reversible KEAP1 Binding Induces the Antioxidant Response in Primary Neurons and Astrocytes of a Huntington’s Disease Mouse Model. Free Radic. Biol. Med. 2021, 162, 243–254. [Google Scholar] [CrossRef]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician’s Expectation Be Matched by the Reality? Oxid. Med. Cell. Longev. 2016, 2016, 7857186. [Google Scholar] [CrossRef]
- Badria, F.A.; Ibrahim, A.S.; Badria, A.F.; Elmarakby, A.A. Curcumin Attenuates Iron Accumulation and Oxidative Stress in the Liver and Spleen of Chronic Iron-Overloaded Rats. PLoS ONE 2015, 10, e0134156, Erratum in PLoS ONE 2020, 15, e0243398; PLoS ONE 2025, 20, e0321563. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Ye, Q.; Mi, X.; Jiao, D.; Zhang, S.; Cheng, R.; Fang, Z.; Fang, M.; Ye, X. Resveratrol Inhibits Ferroptosis via Activating NRF2/GPX4 Pathway in Mice with Spinal Cord Injury. Microsc. Res. Tech. 2023, 86, 1378–1390. [Google Scholar] [CrossRef]
- Jonasson, E.; Sejbaek, T. Diroximel Fumarate in the Treatment of Multiple Sclerosis. Neurodegener. Dis. Manag. 2020, 10, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Gales, S.M.; Obeidat, A.Z.; Neall, A.; Lloyd, K.A.; Belviso, N.; Levin, S.; Bozin, I.; Mendoza, J.P.; Lewin, J.B.; Kornberg, M.D. Lymphocyte Dynamics in Patients with Multiple Sclerosis Who Were Treated with Dimethyl Fumarate and Subsequently Switched to Diroximel Fumarate. Brain Disord. 2025, 18, 100229. [Google Scholar] [CrossRef]
- Pilotto, F.; Chellapandi, D.M.; Puccio, H. Omaveloxolone: A Groundbreaking Milestone as the First FDA-Approved Drug for Friedreich Ataxia. Trends Mol. Med. 2024, 30, 117–125. [Google Scholar] [CrossRef]
- Yang, J.S.; Morris, A.J.; Kamizaki, K.; Chen, J.; Stark, J.; Oldham, W.M.; Nakamura, T.; Mishima, E.; Loscalzo, J.; Minami, Y.; et al. ALDH7A1 Protects against Ferroptosis by Generating Membrane NADH and Regulating FSP1. Cell 2025, 188, 2569–2585. [Google Scholar] [CrossRef]
- Ladds, M.J.G.W.; Popova, G.; Pastor-Fernández, A.; Kannan, S.; van Leeuwen, I.M.M.; Håkansson, M.; Walse, B.; Tholander, F.; Bhatia, R.; Verma, C.S.; et al. Exploitation of Dihydroorotate Dehydrogenase (DHODH) and P53 Activation as Therapeutic Targets: A Case Study in Polypharmacology. J. Biol. Chem. 2021, 295, 17935–17949. [Google Scholar] [CrossRef]
- Ye, J.; Xie, Y.; Liang, Y.; Gao, S.; Hua, C. The Multifaceted Role of Exosomes in Ferroptosis-Associated Diseases and Its Potential Applications. Cell. Signal. 2025, 135, 111983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, Y.; Xie, J.; Lu, D.; Wang, L.; Zhao, S.; Zhou, J.; Cheng, Y.; Kou, T.; Wang, J.; et al. Ferroptosis in Neurodegenerative Diseases: Mechanisms and Therapeutic Potential of Stem Cell Derivatives. Front. Cell Dev. Biol. 2025, 13, 1577382. [Google Scholar] [CrossRef]
- Brooks, B.R.; Berry, J.D.; Ciepielewska, M.; Liu, Y.; Zambrano, G.S.; Zhang, J.; Hagan, M. Intravenous Edaravone Treatment in ALS and Survival: An Exploratory, Retrospective, Administrative Claims Analysis. EClinicalMedicine 2022, 52, 101590. [Google Scholar] [CrossRef]
- Fujisawa, A.; Yamamoto, Y. Edaravone, a Potent Free Radical Scavenger, Reacts with Peroxynitrite to Produce Predominantly 4-NO-Edaravone. Redox Rep. 2016, 21, 98–103. [Google Scholar] [CrossRef]
- Michaličková, D.; Öztürk, H.K.; Hroudová, J.; Ľupták, M.; Kučera, T.; Hrnčíř, T.; Canová, N.K.; Šíma, M.; Slanař, O. Edaravone Attenuates Disease Severity of Experimental Auto-Immune Encephalomyelitis and Increases Gene Expression of Nrf2 and HO-1. Physiol. Res. 2022, 71, 147–157. [Google Scholar] [CrossRef]
- Veroni, C.; Olla, S.; Brignone, M.S.; Siguri, C.; Formato, A.; Marra, M.; Manzoli, R.; Macario, M.C.; Ambrosini, E.; Moro, E.; et al. The Antioxidant Drug Edaravone Binds to the Aryl Hydrocarbon Receptor (AHR) and Promotes the Downstream Signaling Pathway Activation. Biomolecules 2024, 14, 443. [Google Scholar] [CrossRef]
- Sun, P.; Zhou, J.; Zhao, T.; Qi, H.; Qian, G. Zileuton Ameliorates Neuronal Ferroptosis and Functional Recovery After Spinal Cord Injury. Altern. Ther. Health Med. 2023, 29, 314–319. [Google Scholar] [PubMed]
- Tschuck, J.; Skafar, V.; Friedmann Angeli, J.P.; Hadian, K. The Metabolic Code of Ferroptosis: Nutritional Regulators of Cell Death. Trends Biochem. Sci. 2025, 50, 663–676. [Google Scholar] [CrossRef]
- Teng, L.S.; Ying, Z.D.; Sun, X.H.; Hou, H.C.; Qiu, S.D.; Liu, P.; Li, K.J.; Zhang, L.; Sheng, X.H. Formoterol, a Clinically Approved Drug, Inhibits Ferroptosis by Suppressing Lipid Peroxidation and Attenuates APAP-Induced Acute Liver Injury. Chem. Biol. Interact. 2025, 421, 111724. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Chen, X.; Zhong, H.; Wang, Y. Ferroptosis in Life: To Be or Not to Be. Biomed. Pharmacother. 2023, 159, 114241. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Q.; Zhou, L.; Xia, X. Ferroptosis: A Double-Edged Sword. Cell Death Discov. 2024, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Mishima, E.; Nakamura, T.; Doll, S.; Proneth, B.; Fedorova, M.; Pratt, D.A.; Pedro, J.; Angeli, F.; Dixon, S.J.; Wahida, A.; et al. Nature Reviews Molecular Cell Biology Expert Recommendation Check for Updates Recommendations for Robust and Reproducible Research on Ferroptosis. Nat. Rev. Mol. Cell Biol. 2025, 26, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Rubin, M.; Artusi, I.; Cozza, G.; Mattoscio, D.; Laselva, O.; Yang, F. Mapping the Oxidative Landscape in Cystic Fibrosis: Methodological Frontiers and Application. Front. Pharmacol. 2025, 16, 1632924. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artusi, I.; Rubin, M.; Cravin, G.; Cozza, G. Ferroptosis in Human Diseases: Fundamental Roles and Emerging Therapeutic Perspectives. Antioxidants 2025, 14, 1411. https://doi.org/10.3390/antiox14121411
Artusi I, Rubin M, Cravin G, Cozza G. Ferroptosis in Human Diseases: Fundamental Roles and Emerging Therapeutic Perspectives. Antioxidants. 2025; 14(12):1411. https://doi.org/10.3390/antiox14121411
Chicago/Turabian StyleArtusi, Ilaria, Michela Rubin, Giovanni Cravin, and Giorgio Cozza. 2025. "Ferroptosis in Human Diseases: Fundamental Roles and Emerging Therapeutic Perspectives" Antioxidants 14, no. 12: 1411. https://doi.org/10.3390/antiox14121411
APA StyleArtusi, I., Rubin, M., Cravin, G., & Cozza, G. (2025). Ferroptosis in Human Diseases: Fundamental Roles and Emerging Therapeutic Perspectives. Antioxidants, 14(12), 1411. https://doi.org/10.3390/antiox14121411