Integrated Antioxidants, Nanoparticle, and Antifreeze Protein Strategies Synergistically Enhance Cryotop Vitrification Outcomes of Porcine Parthenogenetic Embryos
Abstract
1. Introduction
2. Materials and Methods
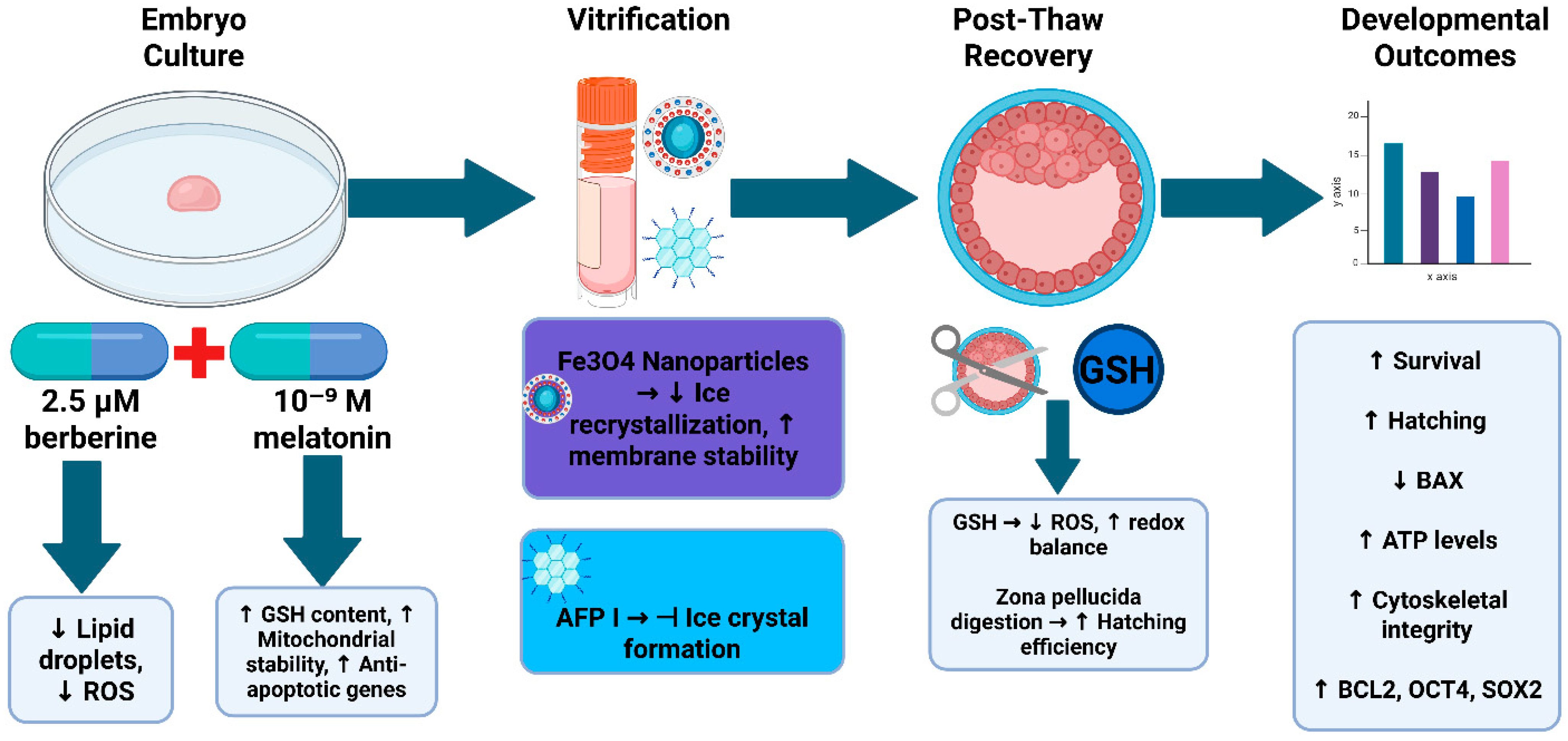
2.1. Experimental Design
2.2. Oocyte Collection and In Vitro Maturation (IVM)
2.3. Parthenogenetic Activation of Oocytes
2.4. Cryopreservation Procedure
2.5. Thawing and Post-Thaw Culture
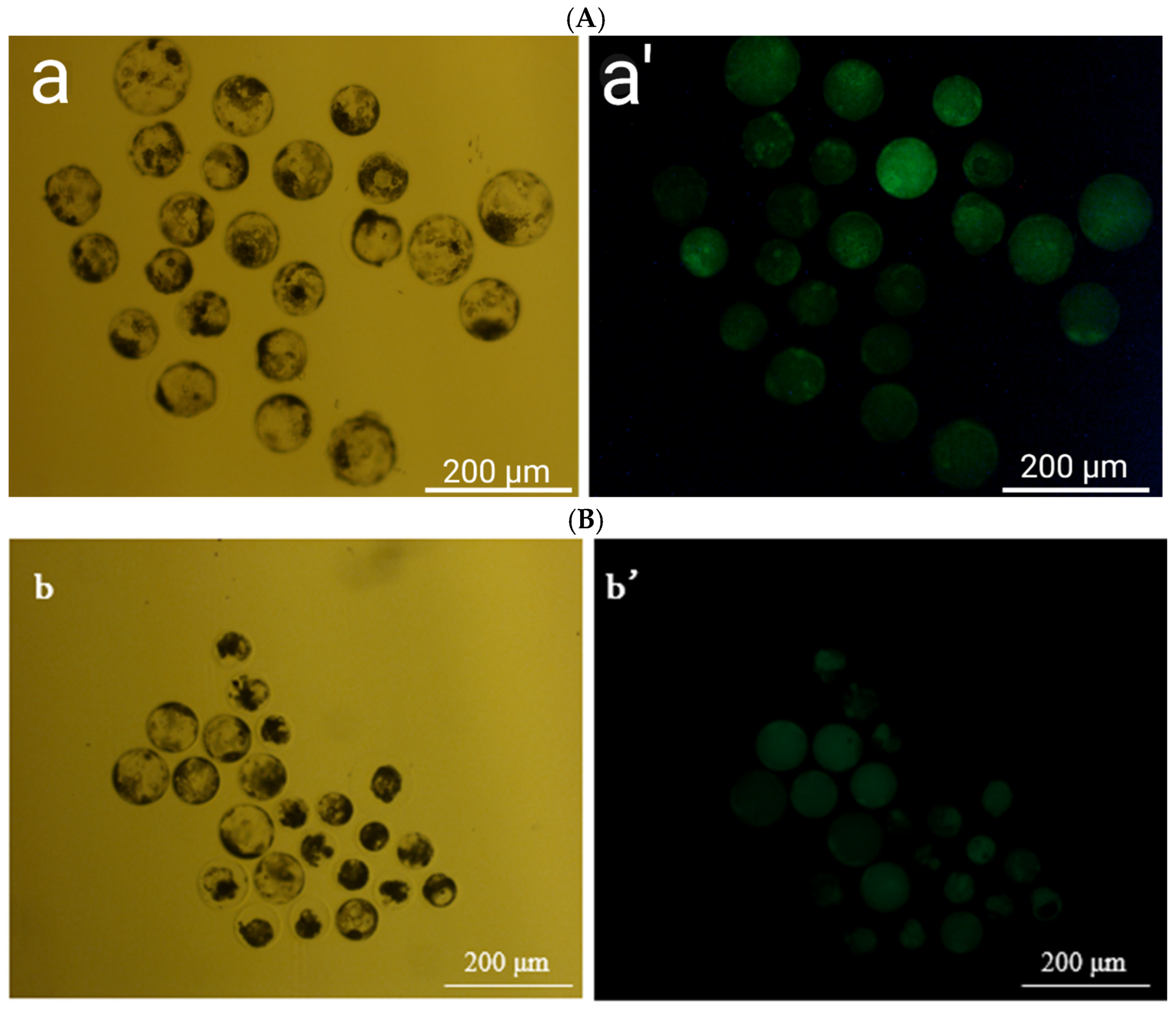
2.6. Survival and Hatching Rates
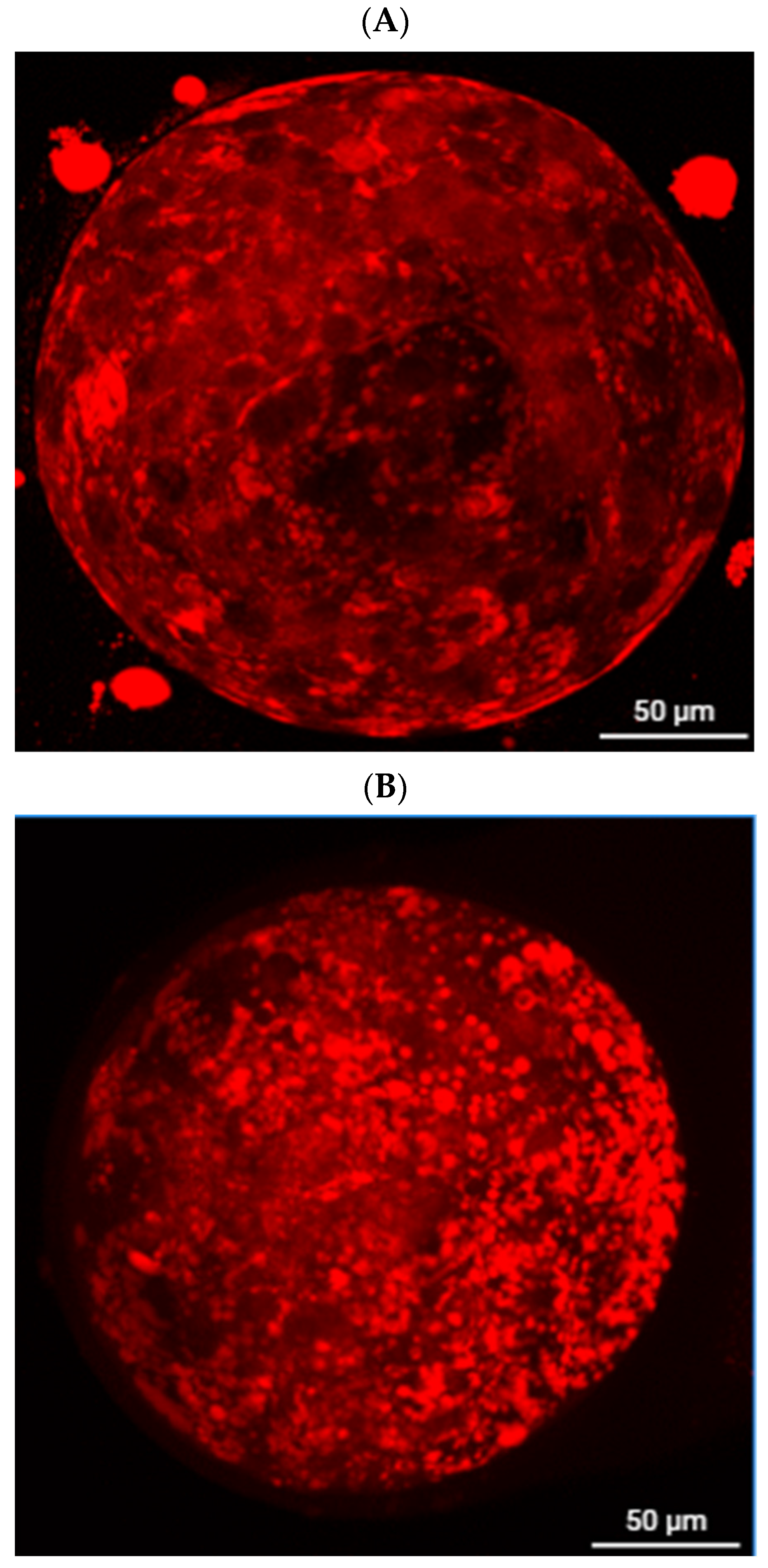
2.7. Embryo Lipid Droplet Staining
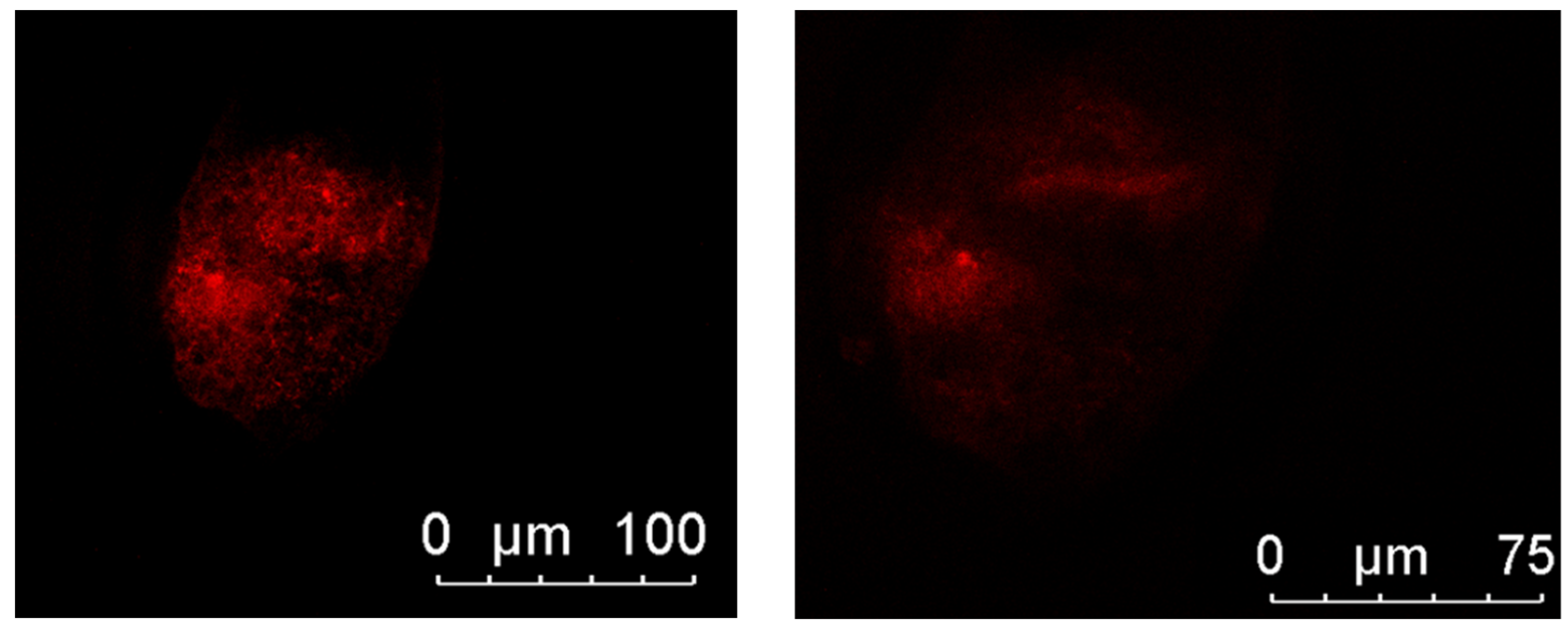
2.8. Embryo Cytoskeleton Integrity
2.9. Reactive Oxygen Species (ROS) Staining
2.10. ATP Measurement
2.11. Apoptosis Detection (TUNEL Assay)
2.12. Total RNA Extraction, Reverse Transcription (cDNA Synthesis), and RT-qPCR
2.13. Statistical Analysis
3. Results
3.1. Comparative Efficiency of Cryopreservation Methods on Post-Thaw Viability of Parthenogenetically Activated Pig Embryos
3.2. Effect of Berberine on Lipid Content of Porcine Parthenogenetically Activated Pig Embryos
3.3. Berberine and Melatonin Treatments on the Freezing Effect of Parthenogenetically Activated Porcine Embryos
3.4. Effects of Fe3O4 Nanoparticles and AFP I on the Frozen Survival Rate of Parthenogenetically Activated Porcine Embryos
3.5. Effect of Combined Treatment of Culture and Freezing Process on the Frozen Survival Rate of Parthenogenetically Activated Porcine Embryos
3.6. Effects of Optimization of Culture Medium After Thawing and Different Zona Pellucida Treatments on Hatching Rate of Parthenogenetically Activated Porcine Embryos
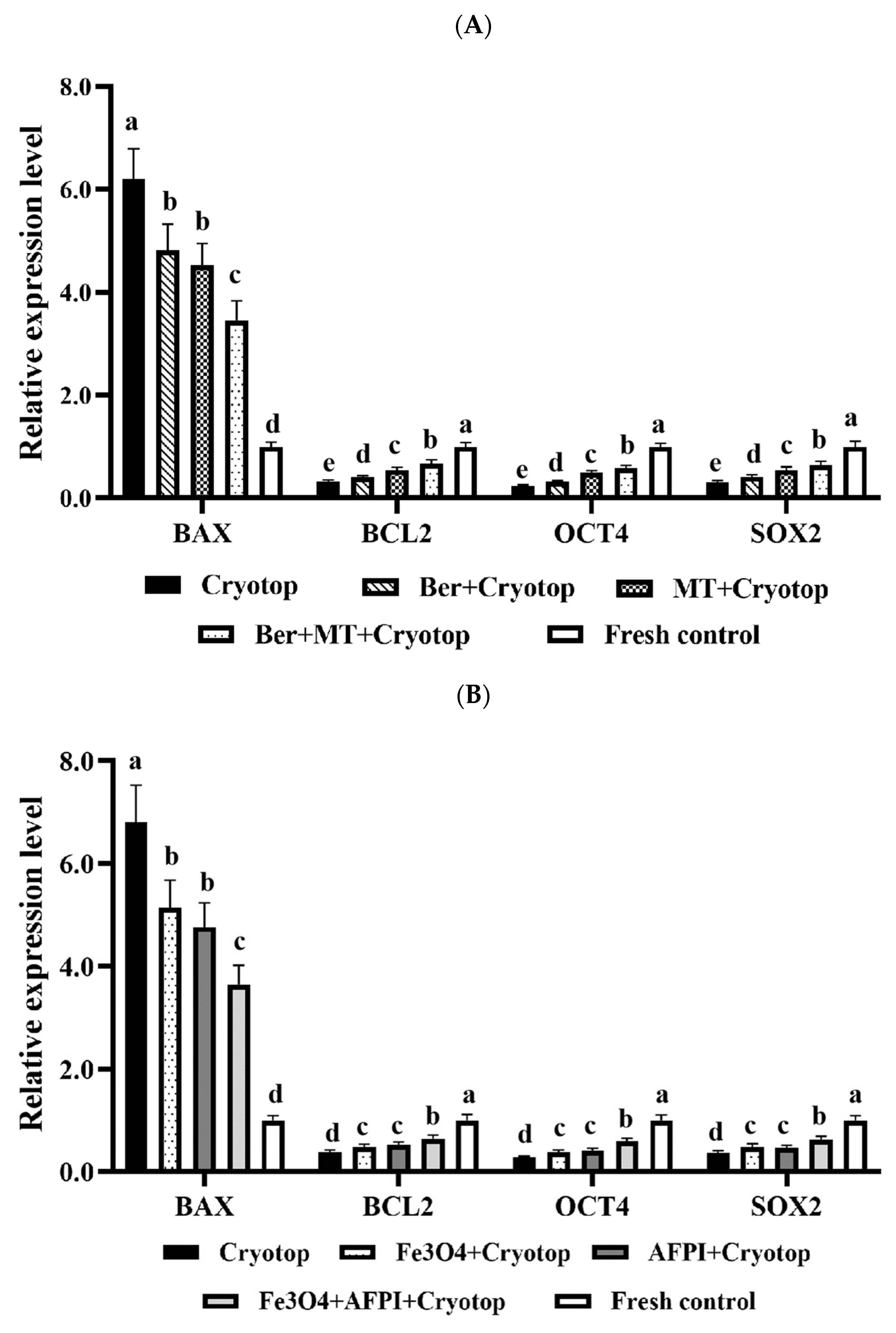
3.7. Relative Expression Analysis of Apoptosis and Development-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFP I | Antifreeze Protein I |
| ATP | Adenosine Triphosphate |
| ROS | Reactive Oxygen Species |
| BAX | Bcl-2–Associated X Protein |
| BCL2 | B-Cell Lymphoma 2 |
| OCT4 | Octamer-Binding Transcription Factor 4 |
| SOX2 | SRY-Box Transcription Factor 2 |
| BER | Berberine |
| GSH | Glutathione |
| ZP | Zona Pellucida |
| COCs | Cumulus-Oocyte Complexes |
| CHX | Cycloheximide |
| OPS | open-pulled straw |
| TL | Tyrode’s lactate (in TL-PVA solutions) |
| TL-PVA | TL-HEPES-polyvinyl alcohol solution |
| PVA | Polyvinyl Alcohol |
| DCFH-DA | 2′,7′-dichlorodihydrofluorescein diacetate (ROS probe) |
| RT-qPCR | Reverse-Transcriptase quantitative PCR |
| PBS | Phosphate-buffered saline (wash buffer) |
| DAPI | DNA-binding nuclear stain |
| cDNA | Complementary DNA |
| RNase/DNase I | Ribonuclease/Deoxyribonuclease I (used in RNA prep) |
| LH | Luteinizing hormone |
| FSH | Follicle-stimulating hormone |
| EGF | Epidermal growth factor |
References
- Almiñana, C.; Dubuisson, F.; Bauersachs, S.; Royer, E.; Mermillod, P.; Blesbois, E.; Guignot, F. Unveiling how vitrification affects the porcine blastocyst: Clues from a transcriptomic study. J. Anim. Sci. Biotechnol. 2022, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Xingzhu, D.; Qingrui, Z.; Keren, C.; Yuxi, L.; Yunpeng, H.; Shien, Z.; Xiangwei, F. Cryopreservation of Porcine Embryos: Recent Updates and Progress. Biopreservation Biobanking 2021, 19, 210–218. [Google Scholar] [CrossRef]
- Gil, M.A.; Parrilla, I.; Cuello, C.; Martinez, E.A. Current status of nonsurgical embryo transfer in swine. Reprod. Fertil. Dev. 2024, 37, RD24137. [Google Scholar] [CrossRef]
- Blackburn, H.D.; Wilson, C.S.; Krehbiel, B. Conservation and utilization of livestock genetic diversity in the United States of America through gene banking. Diversity 2019, 11, 244. [Google Scholar] [CrossRef]
- Leroy, G.; Boettcher, P.; Besbes, B.; Danchin-Burge, C.; Baumung, R.; Hiemstra, S.J. Cryoconservation of animal genetic resources in Europe and two African countries: A gap analysis. Diversity 2019, 11, 240. [Google Scholar] [CrossRef]
- Hryhorowicz, M.; Lipiński, D.; Hryhorowicz, S.; Nowak-Terpiłowska, A.; Ryczek, N.; Zeyland, J. Application of genetically engineered pigs in biomedical research. Genes 2020, 11, 670. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, W.; Li, J.; Jin, Y.; Qiu, Z. Application of the transgenic pig model in biomedical research: A review. Front. Cell Dev. Biol. 2022, 10, 1031812. [Google Scholar] [CrossRef]
- Chen, P.R.; Redel, B.K.; Kerns, K.C.; Spate, L.D.; Prather, R.S. Challenges and considerations during in vitro production of porcine embryos. Cells 2021, 10, 2770. [Google Scholar] [CrossRef]
- Lima, P.F.; Oliveira, M.A.L.; Gonçalves, P.B.D.; Montagner, M.M.; Reichenbach, H.D.; Weppert, M.; Neto, C.C.C.; Pina, V.M.R.; Santos, M.H.B. Effects of retinol on the in vitro development of Bos Indicus embryos to blastocysts in two different culture systems. Reprod. Domest. Anim. 2004, 39, 356–360. [Google Scholar] [CrossRef]
- Amstislavsky, S.; Mokrousova, V.; Brusentsev, E.; Okotrub, K.; Comizzoli, P. Influence of Cellular Lipids on Cryopreservation of Mammalian Oocytes and Preimplantation Embryos: A Review. Biopreservation Biobanking 2019, 17, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Okotrub, K.A.; Omelchenko, A.N.; Chuyko, E.A.; Amstislavsky, S.Y.; Surovtsev, N.V. Irreversible lipid phase transition detected in a porcine oocyte at chilling. Cryobiology 2024, 114, 104850. [Google Scholar] [CrossRef]
- López, A.; Ducolomb, Y.; Casas, E.; Retana-Márquez, S.; Betancourt, M.; Casillas, F. Effects of Porcine Immature Oocyte Vitrification on Actin Microfilament Distribution and Chromatin Integrity During Early Embryo Development In Vitro. Front. Cell Dev. Biol. 2021, 9, 636765. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Otero, Y.; Yeste, M.; Damato, A.; Giaretta, E. Cryopreservation and oxidative stress in porcine oocytes. Res. Vet. Sci. 2021, 135, 20–26. [Google Scholar] [CrossRef]
- Wang, C.R.; Ji, H.W.; He, S.Y.; Liu, R.P.; Wang, X.Q.; Wang, J.; Huang, C.M.; Xu, Y.N.; Li, Y.H.; Kim, N.H. Chrysoeriol Improves In Vitro Porcine Embryo Development by Reducing Oxidative Stress and Autophagy. Vet. Sci. 2023, 10, 143. [Google Scholar] [CrossRef]
- Kang, H.G.; Lee, S.; Jeong, P.S.; Kim, M.J.; Park, S.H.; Joo, Y.E.; Park, S.H.; Song, B.S.; Kim, S.U.; Kim, M.K.; et al. Lycopene improves in vitro development of porcine embryos by reducing oxidative stress and apoptosis. Antioxidants 2021, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Cuello, C.; Martinez, C.A.; Cambra, J.M.; Parrilla, I.; Rodriguez-Martinez, H.; Gil, M.A.; Martinez, E.A. Effects of vitrification on the blastocyst gene expression profile in a porcine model. Int. J. Mol. Sci. 2021, 22, 1222. [Google Scholar] [CrossRef]
- Somfai, T.; Haraguchi, S.; Dang-Nguyen, T.Q.; Kaneko, H.; Kikuchi, K. Vitrification of Porcine Immature Oocytes and Zygotes Results in Different Levels of DNA Damage Which Reflects Developmental Competence to the Blastocyst Stage. PLoS ONE 2023, 18, e0282959. [Google Scholar] [CrossRef]
- Amini, M.; Benson, J.D. Technologies for Vitrification Based Cryopreservation. Bioengineering 2023, 10, 508. [Google Scholar] [CrossRef]
- Gonzalez-Plaza, A.; Cambra, J.M.; Parrilla, I.; Gil, M.A.; Martinez, E.A.; Martinez, C.A.; Cuello, C. The Open Cryotop System Is Effective for the Simultaneous Vitrification of a Large Number of Porcine Embryos at Different Developmental Stages. Front. Vet. Sci. 2022, 9, 936753. [Google Scholar] [CrossRef]
- Cuello, C.; Martinez, C.A.; Nohalez, A.; Parrilla, I.; Roca, J.; Gil, M.A.; Martinez, E.A. Effective vitrification and warming of porcine embryos using a pH-stable, chemically defined medium. Sci. Rep. 2016, 6, 33915. [Google Scholar] [CrossRef] [PubMed]
- González-Plaza, A.; Garcia-Canovas, M.; Parrilla, I.; Rodriguez-Martinez, H.; Gil, M.A.; Martinez, E.A.; Cuello, C. The Cryotop vitrification system is competent for the simultaneous cryopreservation of large numbers of pig in vitro-produced blastocysts. Reprod. Domest. Anim. 2024, 59, e14600. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.Q.; Quan, G.B.; Shao, Q.Y.; Lv, C.R.; Jiang, Y.T.; Zhao, Z.Y.; Hong, Q.H. Cryotop vitrification of porcine parthenogenetic embryos at the early developmental stages. Theriogenology 2016, 85, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, X.; Tao, R.; Bi, J.; He, X.; Zhu, F.; Liu, K.; Xu, Y.; Li, J. Optimal Stage for Cryotop Vitrification of Porcine Embryos. Cell. Reprogramming 2022, 24, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.A.; Martinez, C.A.; Nohalez, A.; Sanchez-Osorio, J.; Vazquez, J.M.; Roca, J.; Parrilla, I.; Gil, M.A.; Cuello, C. Nonsurgical deep uterine transfer of vitrified, in vivo-derived, porcine embryos is as effective as the default surgical approach. Sci. Rep. 2015, 5, 10587. [Google Scholar] [CrossRef]
- Martinez-Serrano, C.A.; Martinez, E.A. The swine revolution: Can embryo transfer transform the industry? Livest. Sci. 2025, 300, 105793. [Google Scholar] [CrossRef]
- Tajima, S.; Motoyama, S.; Wakiya, Y.; Uchikura, K.; Misawa, H.; Takishita, R.; Hirayama, Y.; Kikuchi, K. Piglet production by non-surgical transfer of vitrified embryos, transported to commercial swine farms and warmed on site. Anim. Sci. J. 2020, 91, e13476. [Google Scholar] [CrossRef]
- Huang, M.; Hu, M.; Cai, G.; Wei, H.; Huang, S.; Zheng, E.; Wu, Z. Overcoming ice: Cutting-edge materials and advanced strategies for effective cryopreservation of biosample. J. Nanobiotechnology 2025, 23, 187. [Google Scholar] [CrossRef]
- Choi, H.W.; Jang, H. Application of Nanoparticles and Melatonin for Cryopreservation of Gametes and Embryos. Curr. Issues Mol. Biol. 2022, 44, 4028–4044. [Google Scholar] [CrossRef]
- Xu, X.; Yang, B.; Zhang, H.; Feng, X.; Hao, H.; Du, W.; Zhu, H.; Khan, A.; Khan, M.Z.; Zhang, P.; et al. Effects of β-Nicotinamide Mononucleotide, Berberine, and Cordycepin on Lipid Droplet Content and Developmental Ability of Vitrified Bovine Oocytes. Antioxidants 2023, 12, 991. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Yu, C.; Tang, S.; Jiang, G.; Zhang, J.; Zhang, H.; Xu, J.; Xu, W. Berberine Regulation of Cellular Oxidative Stress, Apoptosis and Autophagy by Modulation of m6A mRNA Methylation through Targeting the Camk1db/ERK Pathway in Zebrafish-Hepatocytes. Antioxidants 2022, 11, 2370. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Q.; Cui, M.; Li, Q.; Mu, S.; Zhao, Z. Effect of melatonin on the in vitro maturation of porcine oocytes, development of parthenogenetically activated embryos, and expression of genes related to the oocyte developmental capability. Animals 2020, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Kim, G.A.; Taweechaipaisankul, A.; Lee, S.H.; Qasim, M.; Ahn, C.; Lee, B.C. Melatonin enhances porcine embryo development via the Nrf2/ARE signaling pathway. J. Mol. Endocrinol. 2019, 63, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, Y.; Hajiaghalou, S.; Baniasadi, F.; Mahabadi, V.P.; Ghalamboran, M.R.; Fathi, R. Fe3O4 magnetic nanoparticles improve the vitrification of mouse immature oocytes and modulate the pluripotent genes expression in derived pronuclear-stage embryos. Cryobiology 2021, 100, 81–89. [Google Scholar] [CrossRef]
- Tian, C.; Shen, L.; Gong, C.; Cao, Y.; Shi, Q.; Zhao, G. Microencapsulation and nanowarming enables vitrification cryopreservation of mouse preantral follicles. Nat. Commun. 2022, 13, 7515. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, J.H.; Hur, Y.B.; Lee, C.W.; Park, S.H.; Koo, B.W. Marine antifreeze proteins: Structure, function, and application to cryopreservation as a potential cryoprotectant. Mar. Drugs 2017, 15, 27. [Google Scholar] [CrossRef]
- Chang, T.; Zhao, G. Ice Inhibition for Cryopreservation: Materials, Strategies, and Challenges. Adv. Sci. 2021, 8, 2002425. [Google Scholar] [CrossRef]
- Jo, J.W.; Jee, B.C.; Suh, C.S.; Kim, S.H. The beneficial effects of antifreeze proteins in the vitrification of immature mouse Oocytes. PLoS ONE 2012, 7, e37043. [Google Scholar] [CrossRef] [PubMed]
- Sirotinskaya, V.; Bar Dolev, M.; Yashunsky, V.; Bahari, L.; Braslavsky, I. Extended Temperature Range of the Ice-Binding Protein Activity. Langmuir 2024, 40, 7395–7404. [Google Scholar] [CrossRef]
- Robles, V.; Valcarce, D.G.; Riesco, M.F. The use of antifreeze proteins in the cryopreservation of gametes and embryos. Biomolecules 2019, 9, 181. [Google Scholar] [CrossRef]
- Correia, L.F.L.; Leal, G.R.; Brandão, F.Z.; Batista, R.I.T.P.; Souza-Fabjan, J.M.G. Effect of antifreeze protein I in the freezing solution on in vivo-derived sheep embryos. Res. Vet. Sci. 2024, 168, 105132. [Google Scholar] [CrossRef]
- Júnior, R.A.d.S.; Desenzi, R.; Ramires, M.M.d.S.; Souza, A.F.d.; Donato, M.A.M.; Peixoto, C.A.; Bartolomeu, C.C.; Batista, A.M. Use of Antifreeze Protein from Tenebrio molitor (TmAFP) in Vitrification of In Vitro-Produced Bovine Embryos: An Ultrastructural Study. Biopreservation Biobanking 2024, 22, 51–59. [Google Scholar] [CrossRef]
- Tas, R.P.; Hendrix, M.M.R.M.; Voets, I.K. Nanoscopy of single antifreeze proteins reveals that reversible ice binding is sufficient for ice recrystallization inhibition but not thermal hysteresis. Proc. Natl. Acad. Sci. USA 2023, 120, e2212456120. [Google Scholar] [CrossRef]
- García-Martínez, T.; Vendrell-Flotats, M.; Martínez-Rodero, I.; Ordóñez-León, E.A.; Álvarez-Rodríguez, M.; López-Béjar, M.; Yeste, M.; Mogas, T. Glutathione ethyl ester protects in vitro-maturing bovine oocytes against oxidative stress induced by subsequent vitrification/warming. Int. J. Mol. Sci. 2020, 21, 7547. [Google Scholar] [CrossRef]
- Ma, M.; Zhang, L.; Liu, Z.; Teng, Y.; Li, M.; Peng, X.; An, L. Effect of blastocyst development on hatching and embryo implantation. Theriogenology 2024, 214, 66–72. [Google Scholar] [CrossRef]
- Buschiazzo, J.; Ríos, G.L.; Canizo, J.R.; Antollini, S.S.; Alberio, R.H. Free cholesterol and cholesterol esters in bovine oocytes: Implications in survival and membrane raft organization after cryopreservation. PLoS ONE 2017, 12, e0180451. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, H.; Shahzad, M.; Kolachi, H.A.; Li, Y.; Sheng, H.; Zhang, X.; Wan, P.; Zhao, X. Supplementation of Forskolin and Linoleic Acid During IVC Improved the Developmental and Vitrification Efficiency of Bovine Embryos. Int. J. Mol. Sci. 2025, 26, 4151. [Google Scholar] [CrossRef]
- Hara, S.; Shirasuna, K.; Iwata, H. A polysaccharide gel made of gellan gum improves oocyte maturation and embryonic development in pigs. J. Reprod. Dev. 2024, 70, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Plaza, A.; Cambra, J.M.; Garcia-Canovas, M.; Parrilla, I.; Gil, M.A.; Martinez, E.A.; Rodriguez-Martinez, H.; Martinez, C.A.; Cuello, C. Cryotop vitrification of large batches of pig embryos simultaneously provides excellent postwarming survival rates and minimal interference with gene expression. Theriogenology 2023, 206, 1–10. [Google Scholar] [CrossRef]
- Somfai, T. Vitrification of immature oocytes in pigs. Anim. Sci. J. 2024, 95, e13943. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, H.; Guo, F.; Wang, L.; Zhang, W.; Liu, R.; Zhang, X.; Dai, X. Vitrification affects the post-implantation development of mouse embryos by inducing DNA damage and epigenetic modifications. Clin. Epigenetics 2025, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Betancourt, M.; Ducolomb, Y.; Rodríguez, J.J.; Casas, E.; Bonilla, E.; Bahena, I.; Retana-Márquez, S.; Juárez-Rojas, L.; Casillas, F. DNA damage in cumulus cells generated after the vitrification of in vitro matured porcine oocytes and its impact on fertilization and embryo development. Porc. Health Manag. 2021, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Wang, F.T.; Chan, W.H. Dose-dependent beneficial and harmful effects of berberine on mouse oocyte maturation and fertilization and fetal development. Toxicol. Res. 2020, 9, 431–443. [Google Scholar] [CrossRef] [PubMed]
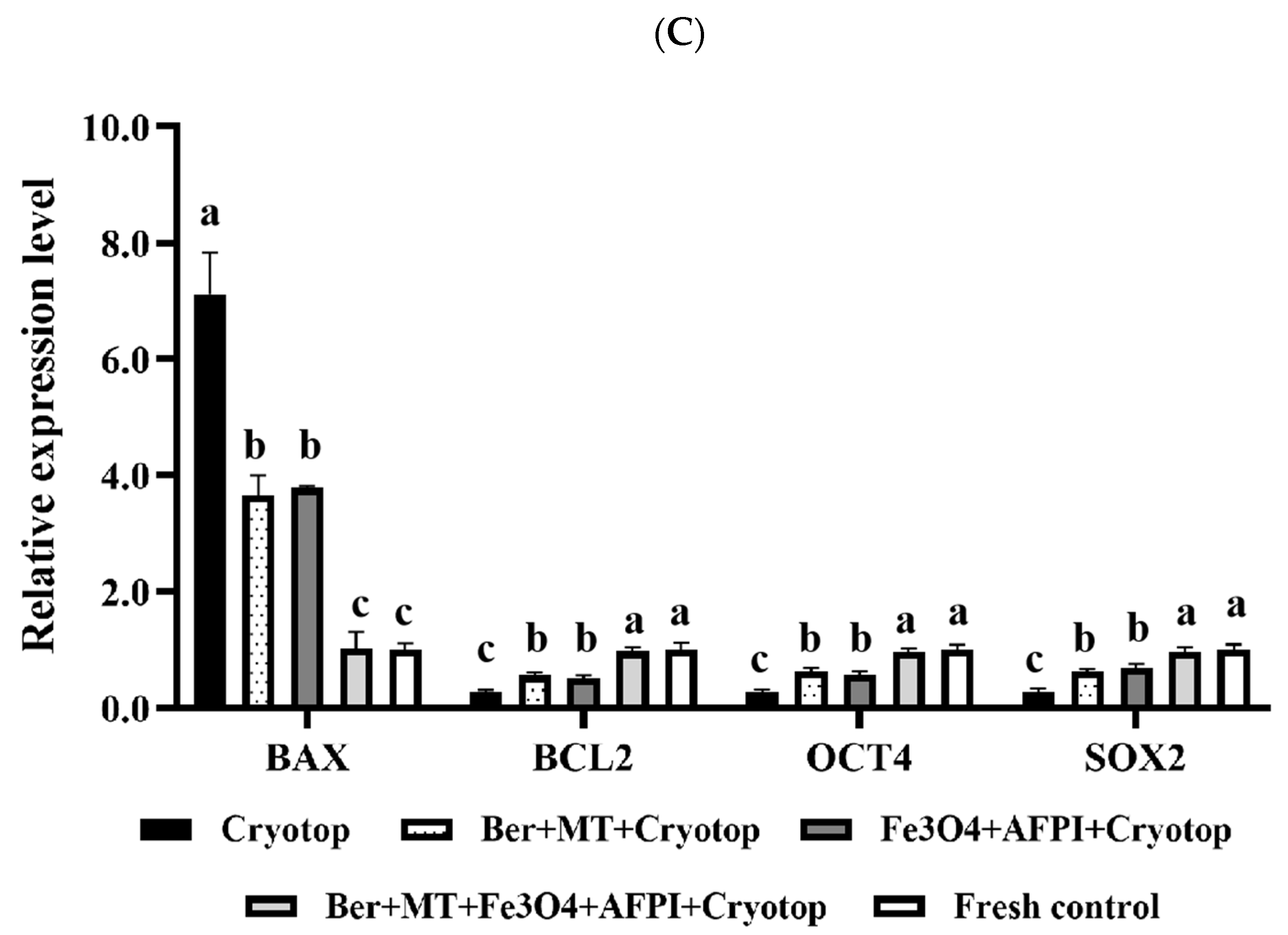
| Gene | Primer | Accession Number |
|---|---|---|
| BAX | F: TCTGAGCAGATCATGAAGACAGG R: GCAGCTCCATGTTACTGTCC | XM_003127290.5 |
| BCL2 | F: AGTTCGGTGGGGTCATGTG R: ATACAGCTCCACAAAGGCATC | XM_021099593.1 |
| OCT4 | F: CTATGACTTCTGCGGAGGGAT R: TTTGATGTCCTGGGACTCCTCG | NM_001113060.1 |
| SOX2 | F: ATGGGCTCAGTGGTCAAGTC R: AGAGAGGCAGTGTACCGTTG | NM_001123197.1 |
| GAPDH | F: CGATGGTGAAGGTCGGAGTG R: TACGACCACCCCATCCAAGT | XM_021091114.1 |
| Group | Post-Thaw Survival Rate (%) |
|---|---|
| Straw Method | 52.94 ± 6.35 c (18/34) |
| OPS Method | 65.85 ± 7.52 b (27/41) |
| Cryotop | 70.27 ± 7.16 a (26/37) |
| Group | Embryo Lipid Content (Fluorescence Intensity/Embryo) |
|---|---|
| 2.5 μM Berberine group | 20.18 ± 2.51 b (n = 30) |
| Fresh control group | 62.63 ± 4.32 a (n = 30) |
| Group | Survival Rate (%) | Microtubule Normal (%) | ROS (Fluorescence/Embryo) | ATP (pmol/Blastocyst) | Hatching Rate (%) |
|---|---|---|---|---|---|
| Cryotop only | 70.00 ± 7.47 c (245/350) | 66.67 ± 7.45 e (22/33) | 86.32 ± 6.78 a (n = 30) | 0.21 ± 0.03 d (n = 30) | 70.73 ± 6.89 d (29/41) |
| 2.5 μM Berberine + Cryotop | 78.05 ± 8.46 b (224/287) | 72.73 ± 6.19 d (24/33) | 74.68 ± 7.15 b (n = 32) | 0.26 ± 0.03 c (n = 30) | 76.19 ± 8.16 c (32/42) |
| 10−9 M Melatonin + Cryotop | 81.09 ± 7.44 b (210/259) | 77.14 ± 7.26 c (27/35) | 66.32 ± 5.42 c (n = 34) | 0.27 ± 0.03 c (n = 30) | 77.55 ± 7.68 c (38/49) |
| 2.5 μM Berberine + 10−9 M MT + Cryotop | 87.80 ± 6.77 a (252/287) | 82.69 ± 8.78 b (43/52) | 52.85 ± 5.14 d (n = 33) | 0.32 ± 0.04 b (n = 30) | 83.90 ± 8.53 b (73/85) |
| Fresh control (no freeze) | — | 91.89 ± 8.32 a (34/37) | 25.67 ± 3.57 e (n = 31) | 0.38 ± 0.05 a (n = 30) | 92.59 ± 8.16 a (50/54) |
| Group | Survival Rate (%) | Hatching Rate (%) | Cytoskeleton Integrity (%) | ROS (Fluorescence/Embryo) | ATP (pmol/Blastocyst) |
|---|---|---|---|---|---|
| Cryotop only | 72.97 ± 5.65 c (197/270) | 71.15 ± 7.36 d (35/52) | 66.03 ± 7.36 d (35/53) | 94.72 ± 10.36 a (n = 30) | 0.20 ± 0.03 d (n = 30) |
| 1.0% Fe3O4 + Cryotop | 78.05 ± 6.48 b (192/246) | 76.92 ± 8.52 c (40/52) | 73.68 ± 6.28 c (28/38) | 76.32 ± 7.41 b (n = 30) | 0.28 ± 0.01 c (n = 30) |
| 500 ng/mL AFP I + Cryotop | 79.07 ± 8.42 b (204/258) | 75.00 ± 6.28 c (42/56) | 73.52 ± 7.16 c (25/34) | 74.28 ± 6.18 b (n = 30) | 0.27 ± 0.02 c (n = 30) |
| 1.0% Fe3O4 + 500 ng/mL AFP I + Cryotop | 84.44 ± 9.64 a (228/270) | 82.36 ± 6.72 b (42/51) | 78.94 ± 8.34 b (30/38) | 40.15 ± 8.32 c (n = 30) | 0.33 ± 0.04 b (n = 30) |
| Fresh control (no freeze) | — | 88.89 ± 9.16 a (32/36) | 92.30 ± 8.68 a (36/39) | 28.61 ± 3.05 d (n = 30) | 0.43 ± 0.02 a (n = 30) |
| Group | Survival Rate (%) | Hatching Rate (%) | Cytoskeleton Integrity (%) | ROS (Fluorescence/Embryo) | ATP (pmol/Blastocyst) |
|---|---|---|---|---|---|
| Cryotop only | 71.11 ± 4.44 c (160/225) | 68.42 ± 8.37 c (26/38) | 69.23 ± 6.48 c (27/39) | 87.65 ± 8.34 a (n = 30) | 0.22 ± 0.03 c (n = 30) |
| Berberine + MT + Cryotop | 83.33 ± 8.42 b (140/168) | 74.36 ± 6.48 b (29/39) | 73.68 ± 8.42 b (28/38) | 64.25 ± 6.46 b (n = 30) | 0.28 ± 0.02 b (n = 30) |
| Fe3O4 + AFP + Cryotop | 85.11 ± 8.64 b (160/188) | 75.56 ± 7.46 b (34/45) | 76.32 ± 7.46 b (29/38) | 62.34 ± 5.19 b (n = 30) | 0.31 ± 0.03 b (n = 30) |
| Berberine + MT + Fe3O4 + AFP + Cryotop | 93.75 ± 8.64 a (180/192) | 90.48 ± 9.45 a (38/42) | 90.70 ± 8.16 a (39/43) | 31.35 ± 6.31 c (n = 30) | 0.39 ± 0.04 a (n = 30) |
| Fresh control (no freeze) | 91.30 ± 8.68 a (42/46) | 92.68 ± 8.46 a (38/41) | 29.32 ± 3.42 c (n = 30) | — | 0.41 ± 0.05 a (n = 30) |
| Group | Hatching Rate (%) |
|---|---|
| 1 μM Resveratrol | 76.79 ± 7.64 b (43/56) |
| 5 mM Glutathione (GSH) | 82.54 ± 8.42 b (52/63) |
| Cryotop frozen control | 72.41 ± 7.48 c (42/58) |
| Fresh control (no freeze) | 88.52 ± 8.18 a (54/61) |
| Treatment | Hatching Rate (%) |
|---|---|
| Trypsin 45 s (partial digestion) | 85.00 ± 8.46 b (34/40) |
| Acid Tyrode’s solution 45 s | 84.31 ± 7.42 bc (43/51) |
| Zona piercing | 82.22 ± 8.54 c (37/45) |
| Cryotop frozen control | 80.77 ± 7.15 c (42/52) |
| Fresh control (no freeze) | 90.20 ± 8.18 a (46/51) |
| Treatment | Hatching Rate (%) |
|---|---|
| Trypsin 45 s + 5 mM GSH (post-thaw) | 90.70 ± 9.52% a (39/43) |
| Cryotop frozen control | 80.77 ± 8.64% b (42/52) |
| Fresh control (no freeze) | 89.58 ± 8.18% a (43/48) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayantoye, J.O.; Yang, B.; Dong, J.; Feng, X.; Shahzad, M.; Kolachi, H.A.; Wan, P.; Pan, H.; Zhao, X. Integrated Antioxidants, Nanoparticle, and Antifreeze Protein Strategies Synergistically Enhance Cryotop Vitrification Outcomes of Porcine Parthenogenetic Embryos. Antioxidants 2025, 14, 1412. https://doi.org/10.3390/antiox14121412
Ayantoye JO, Yang B, Dong J, Feng X, Shahzad M, Kolachi HA, Wan P, Pan H, Zhao X. Integrated Antioxidants, Nanoparticle, and Antifreeze Protein Strategies Synergistically Enhance Cryotop Vitrification Outcomes of Porcine Parthenogenetic Embryos. Antioxidants. 2025; 14(12):1412. https://doi.org/10.3390/antiox14121412
Chicago/Turabian StyleAyantoye, Jesse Oluwaseun, Baigao Yang, Jianhua Dong, Xiaoyi Feng, Muhammad Shahzad, Hubdar Ali Kolachi, Pengcheng Wan, Hongmei Pan, and Xueming Zhao. 2025. "Integrated Antioxidants, Nanoparticle, and Antifreeze Protein Strategies Synergistically Enhance Cryotop Vitrification Outcomes of Porcine Parthenogenetic Embryos" Antioxidants 14, no. 12: 1412. https://doi.org/10.3390/antiox14121412
APA StyleAyantoye, J. O., Yang, B., Dong, J., Feng, X., Shahzad, M., Kolachi, H. A., Wan, P., Pan, H., & Zhao, X. (2025). Integrated Antioxidants, Nanoparticle, and Antifreeze Protein Strategies Synergistically Enhance Cryotop Vitrification Outcomes of Porcine Parthenogenetic Embryos. Antioxidants, 14(12), 1412. https://doi.org/10.3390/antiox14121412






