Effects of Emissions from Heated Tobacco Products and Reference Cigarettes on Gene Expression and Mitochondrial Function in Human Lung Epithelial BEAS-2B Cells † †
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of WCSCs
2.3. Cell Culture
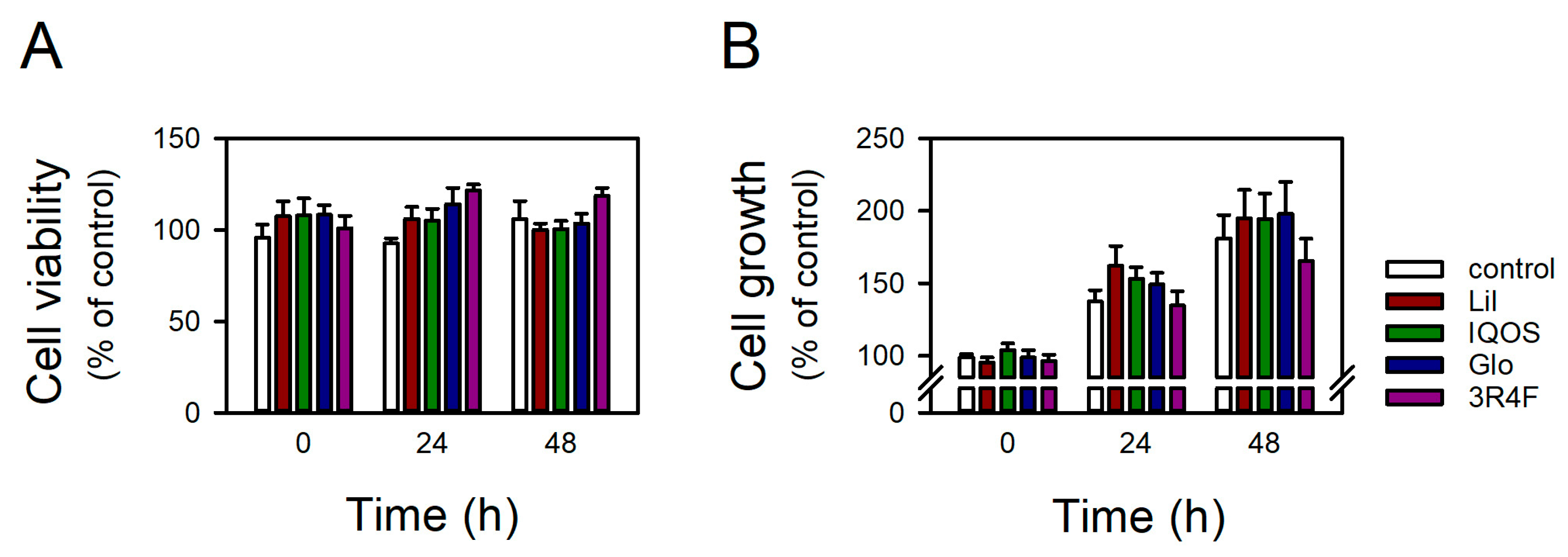
2.4. Assessment of Cell Viability and Growth
2.5. RNA Isolation and Library Preparation
2.6. RNA Sequencing Analysis
2.6.1. Preprocessing
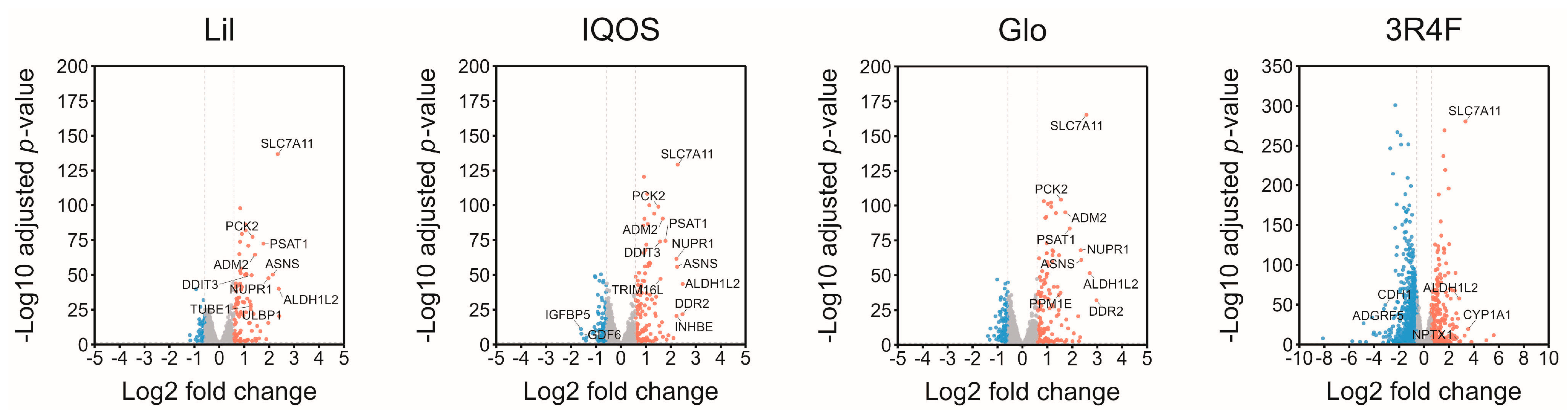
2.6.2. Differential Expression Analysis
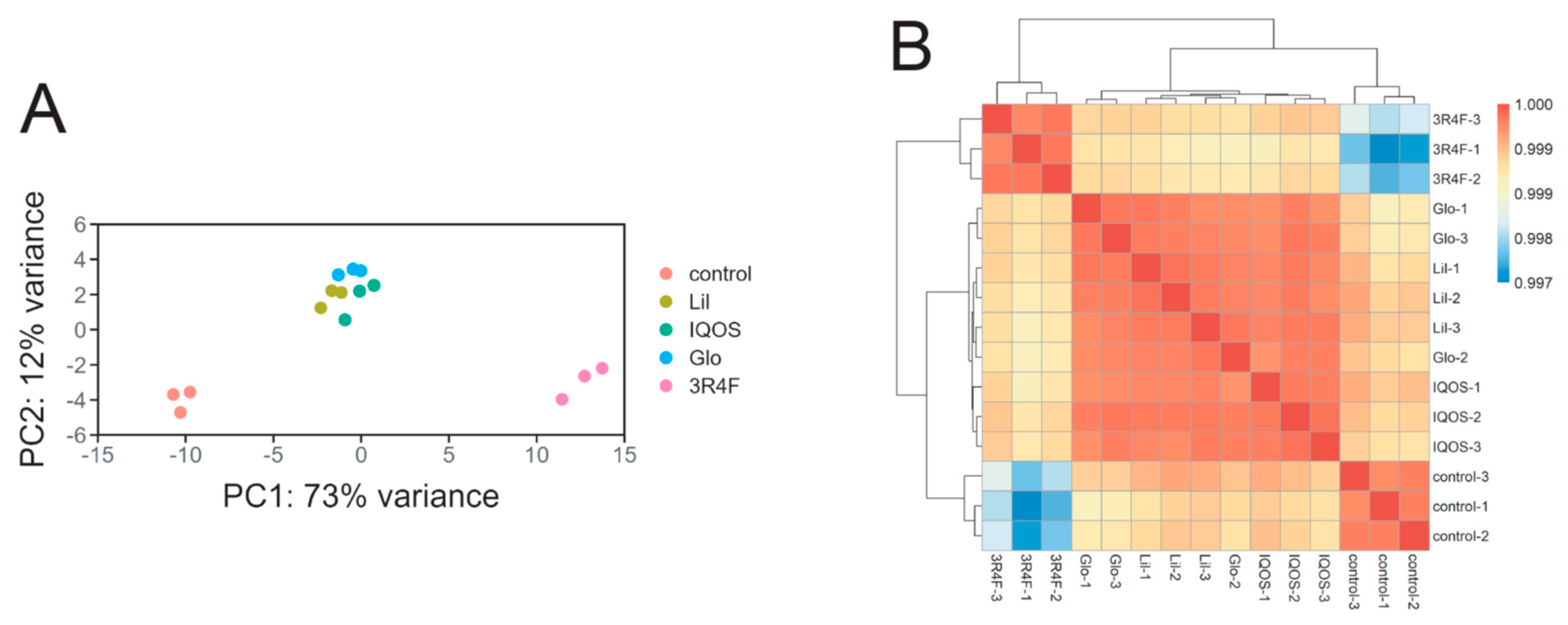
2.6.3. Principal Component Analysis (PCA) and Sample Correlation
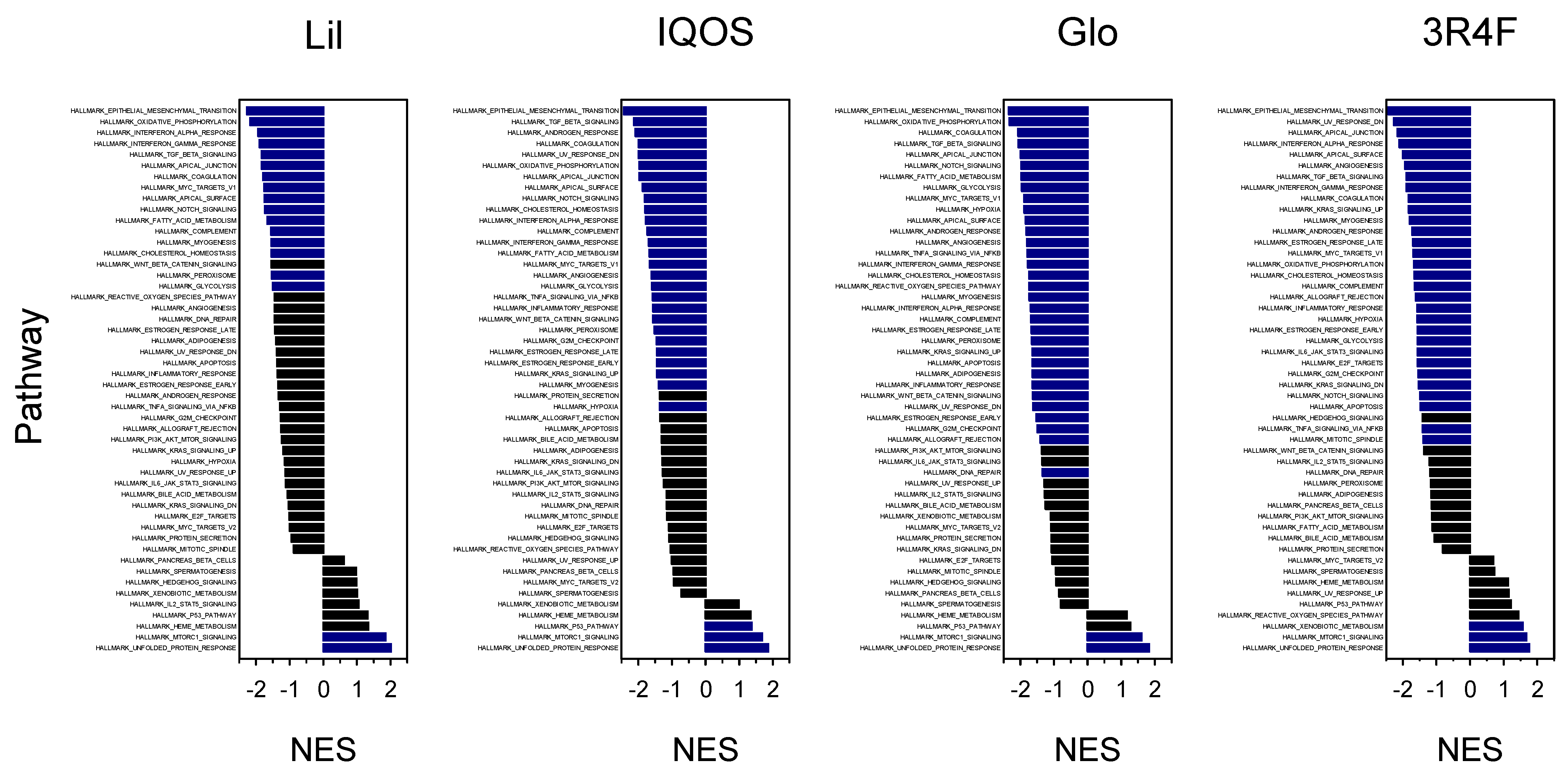
2.6.4. Gene Set Enrichment Analysis (GSEA)
2.7. Analysis of Mitochondrial Morphology
2.8. Immunoblot Analysis
2.9. Measurement of Mitochondrial ROS (mtROS), Intracellular ROS, and Mitochondrial Membrane Potential
2.10. Measurement of ATP Levels
2.11. Bioenergetic Profiling
2.12. Statistical Analysis
3. Results
3.1. Overall Effects of HTP-WCSCs on Gene Expression in BEAS-2B Cells
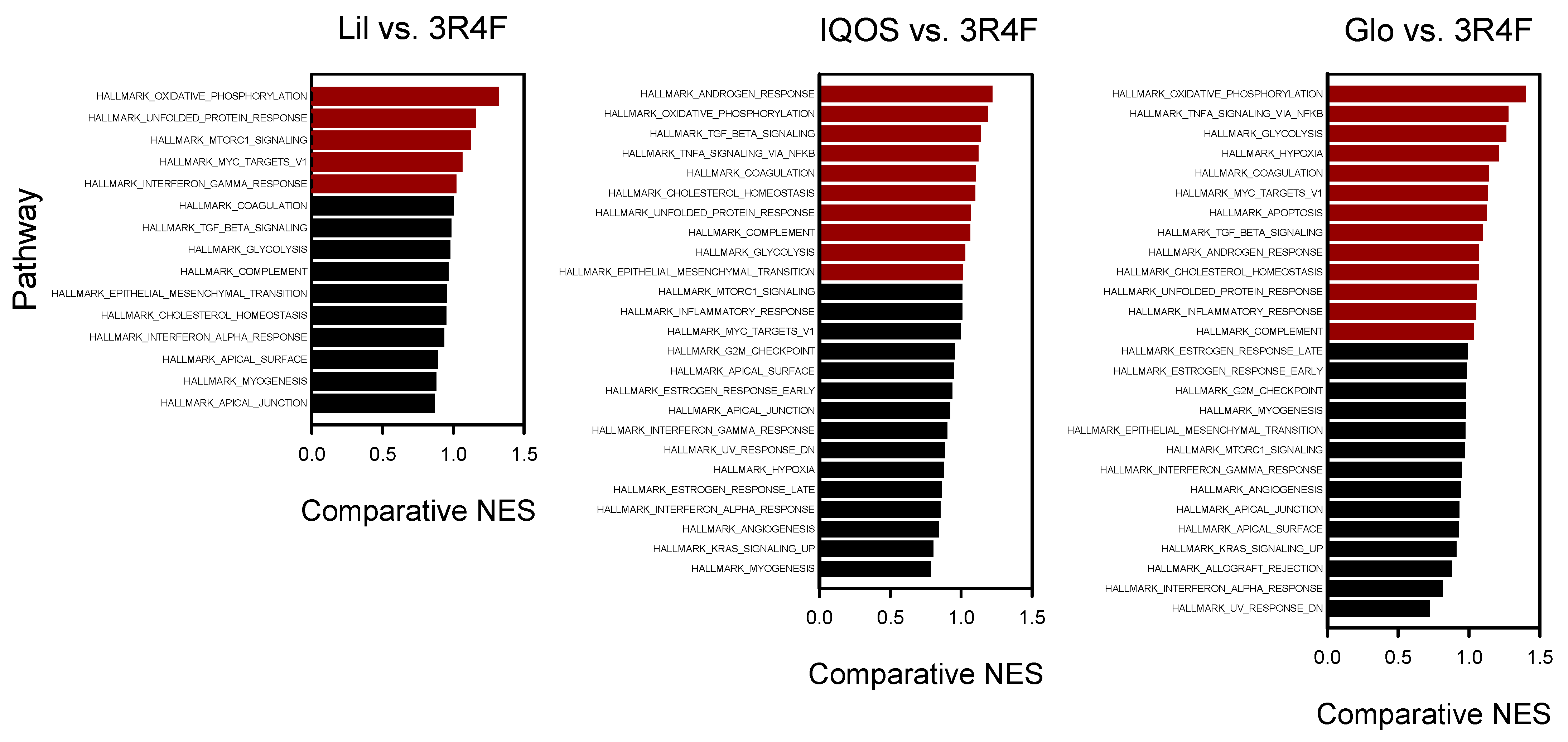
3.2. Enriched Biological Pathways Identified by GSEA
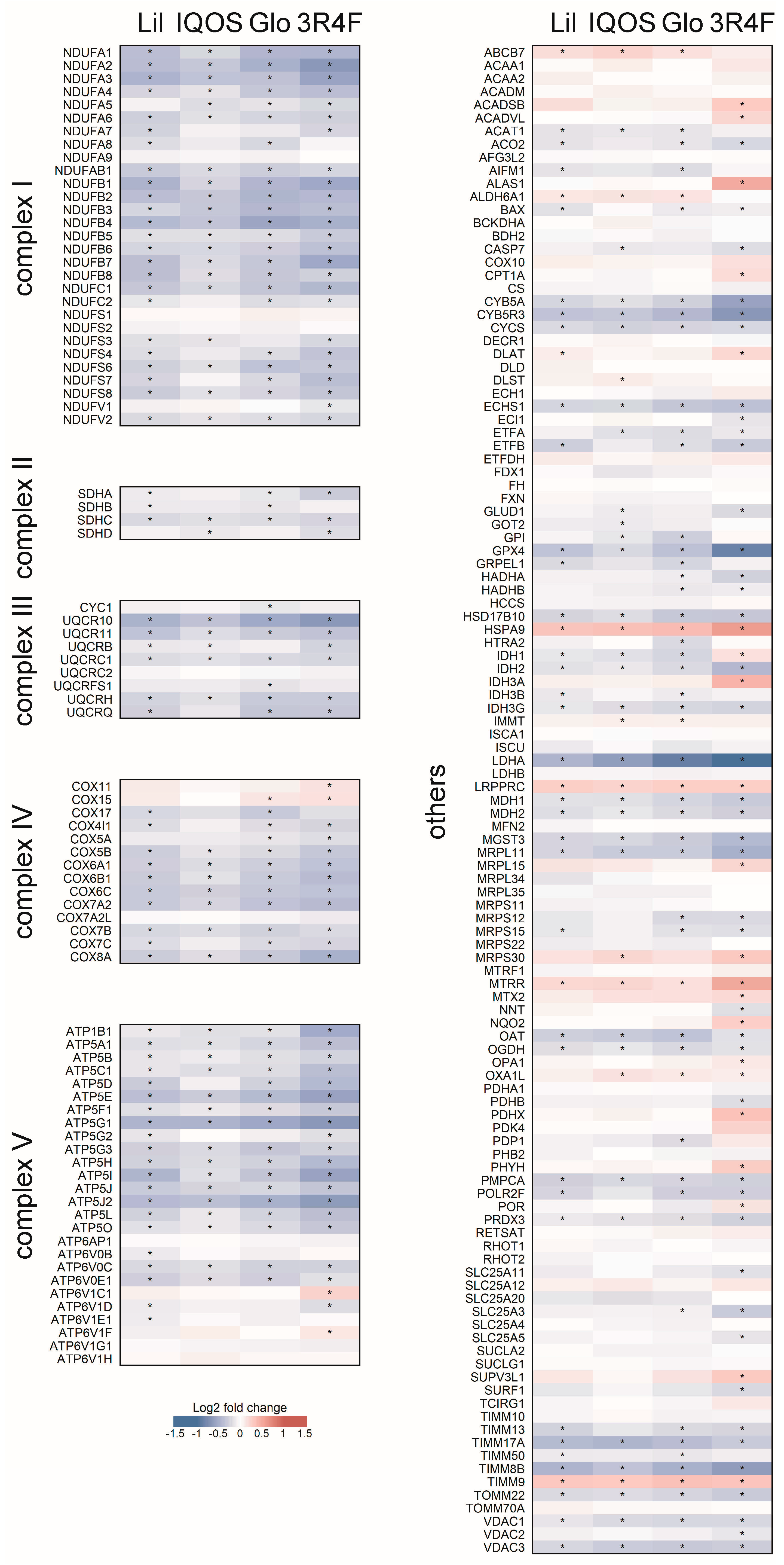
3.3. Changes in OXPHOS Pathway-Related Gene Expression
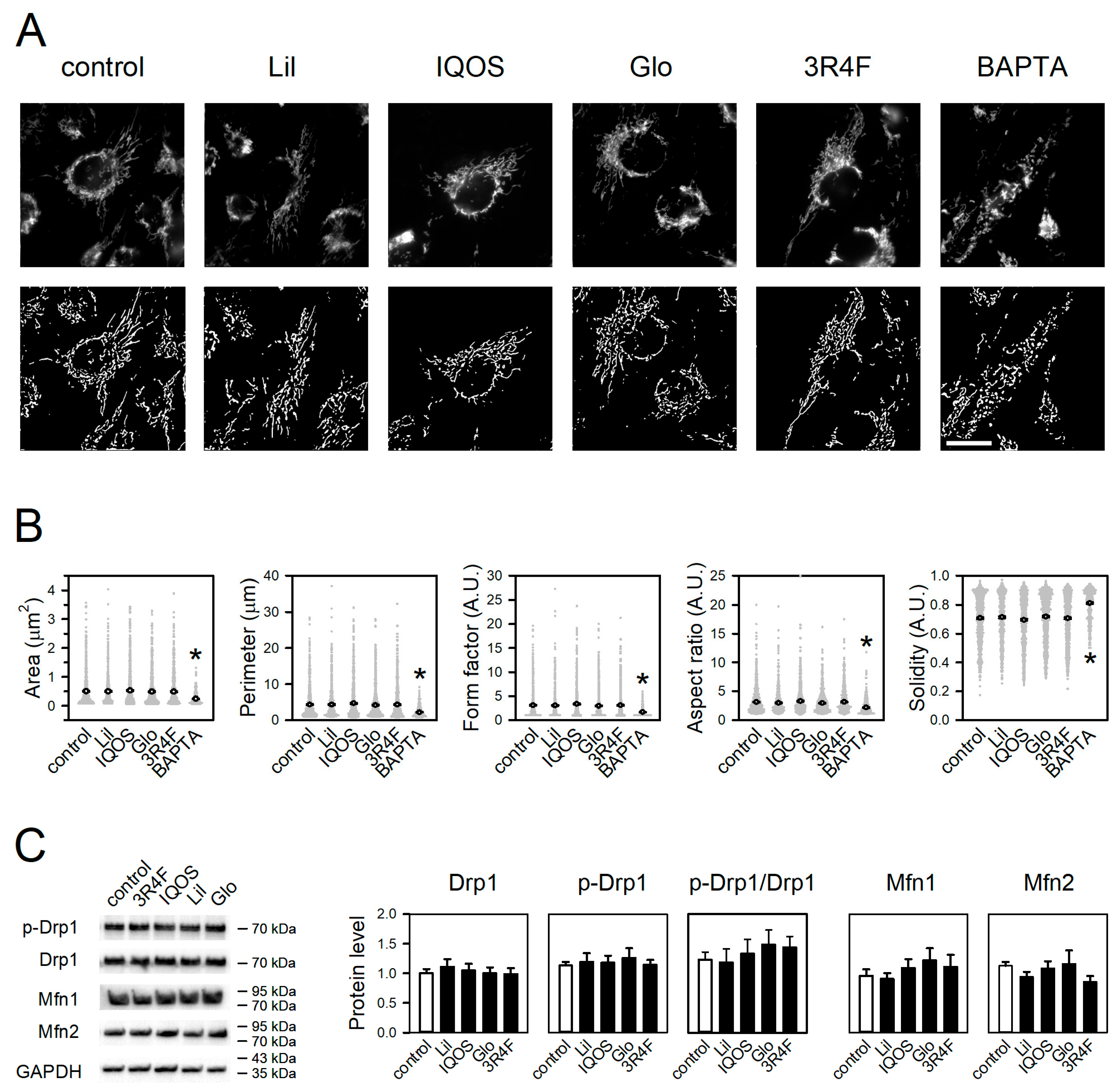
3.4. Effect of WCSCs on Mitochondrial Morphology and Dynamics
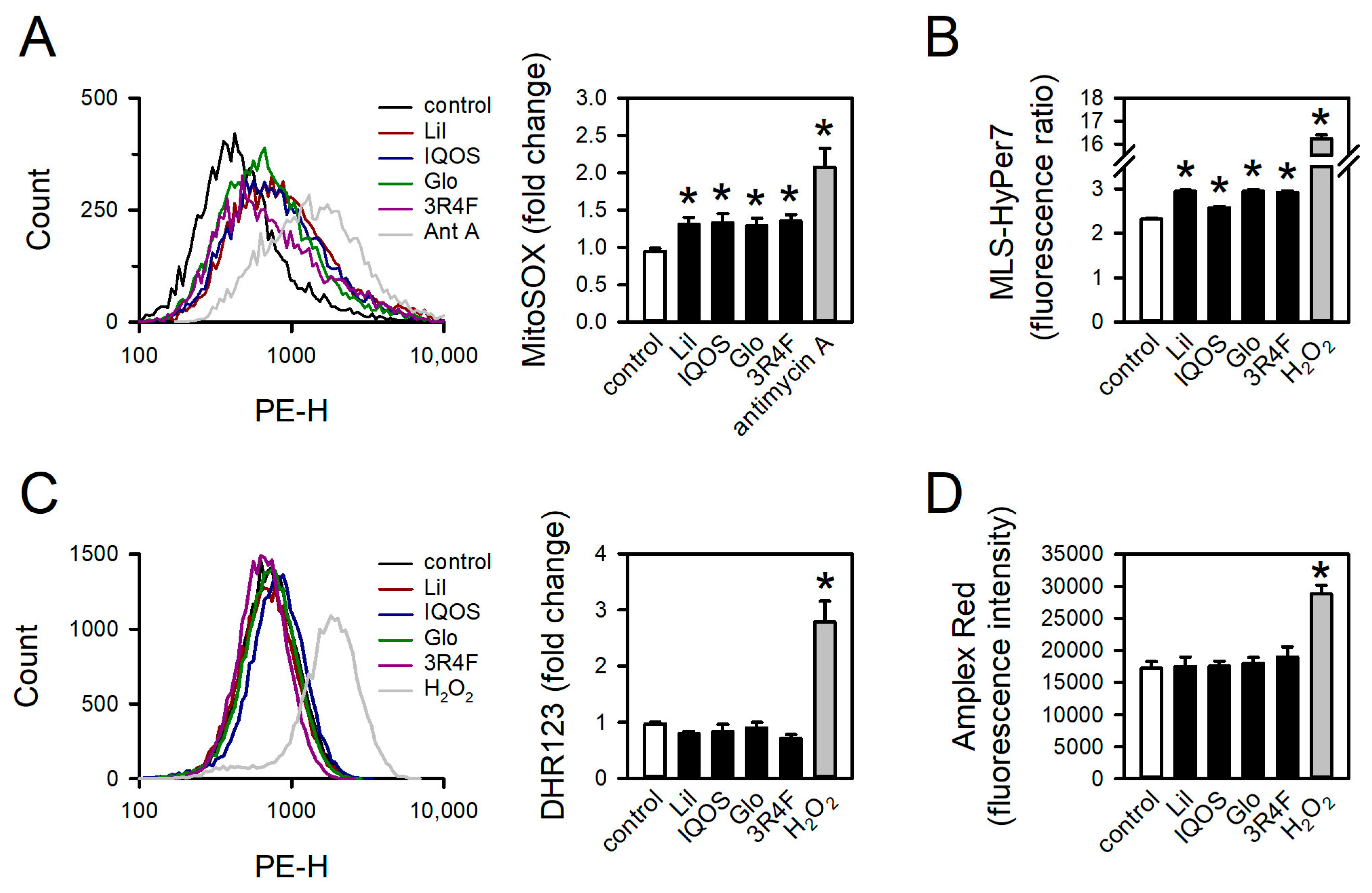
3.5. Effect of WCSCs on mtROS Formation
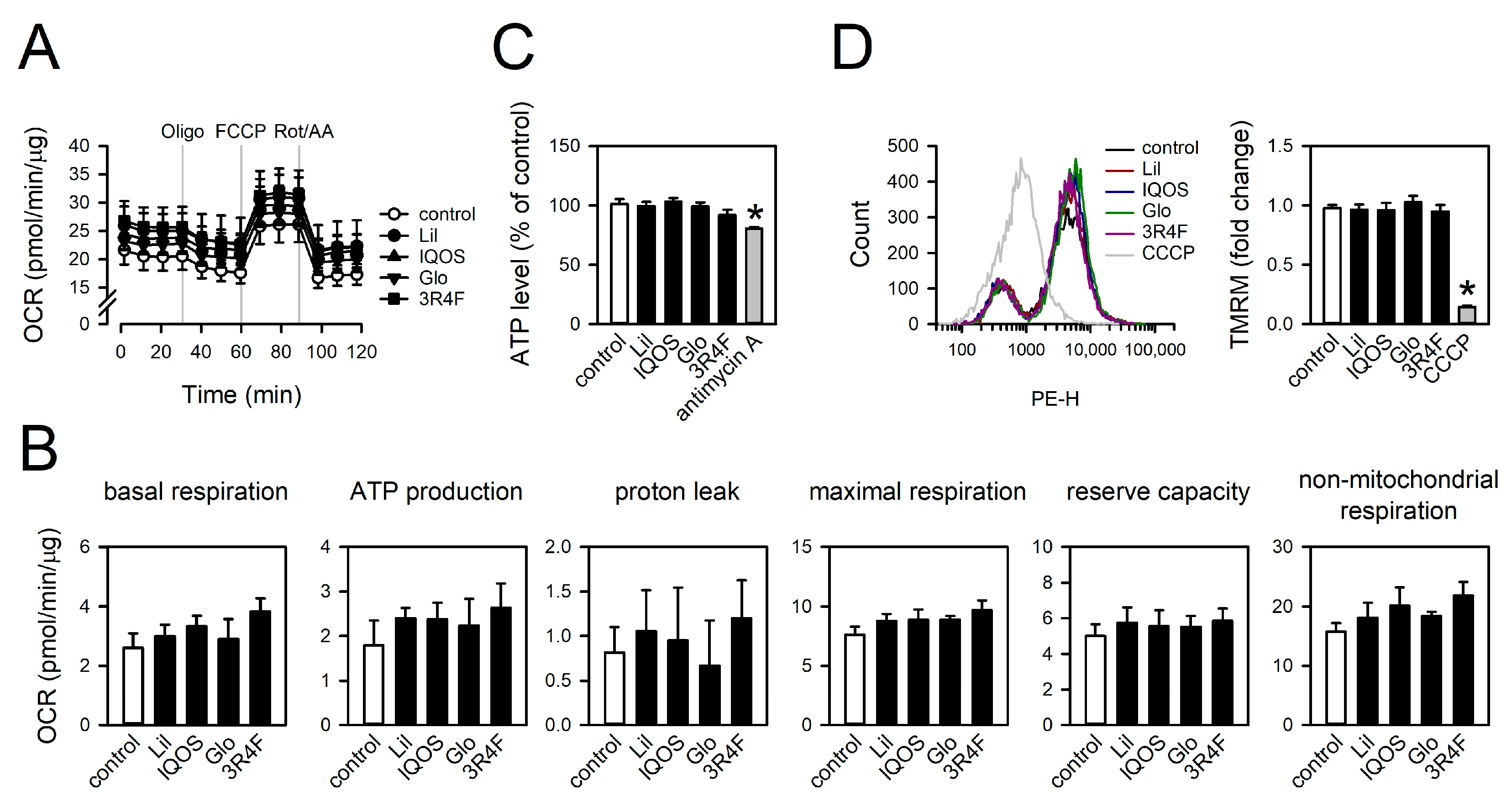
3.6. Effect of WCSCs on ATP Levels, Mitochondrial Respiration, and Membrane Potential
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HTPs | Heated tobacco products |
| WCSCs | Whole cigarette smoke condensates |
| OXPHOS | Oxidative phosphorylation |
| ROS | Reactive oxygen species |
| KT&G | Korea tomorrow & global |
| DHR123 | Dihydrorhodamine 123 |
| TMRM | Tetramethylrhodamine methyl ester |
| Amplex Red | 10-Acetyl-3,7-dihydroxyphenoxazine |
| BCA | Bicinchoninic acid |
| RPMI | Roswell Park Memorial Institute |
| FBS | Fetal bovine serum |
| HBSS | Hank’s balanced salt solution |
| Drp1 | Dynamin-related protein 1 |
| Mfn1 | Mitofusin-1 |
| Mfn2 | Mitofusin-2 |
| HRP | Horseradish peroxidase |
| CCCP | Carbonyl cyanide m-chlorophenyl hydrazone |
| RIPA | Radioimmuno-precipitation assay |
| CSE | Cigarette smoke extract |
| TPM | Total particulate matter |
| SDS | Sodium dodecyl sulfate |
| PCR | Polymerase chain reaction |
| GSEA | Gene set enrichment analysis |
| FDR | False discovery rate |
| NES | Normalized enrichment score |
| mtROS | Mitochondrial ROS |
| OCR | Oxygen consumption rate |
| FCCP | Carbonyl cyanide-4-(trifluoro-methoxy) phenylhydrazone |
| PCA | Principal component analysis |
| DEG | Differentially expressed gene |
| MsigDB | Molecular Signatures Database |
References
- Dempsey, R.; Rodrigo, G.; Vonmoos, F.; Gunduz, I.; Belushkin, M.; Esposito, M. Preliminary toxicological assessment of heated tobacco products: A review of the literature and proposed strategy. Toxicol. Rep. 2023, 10, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Rahman, M.; Johanson, G.; Palmberg, L.; Ganguly, K. Heated tobacco products: Insights into composition and toxicity. Toxics 2023, 11, 667. [Google Scholar] [CrossRef] [PubMed]
- Odani, S.; Tsuno, K.; Agaku, I.T.; Tabuchi, T. Heated tobacco products do not help smokers quit or prevent relapse: A longitudinal study in Japan. Tob. Control 2024, 33, 472. [Google Scholar] [CrossRef]
- Ratajczak, A.; Jankowski, P.; Strus, P.; Feleszko, W. Heat not burn tobacco product—A new global trend: Impact of heat-not-burn tobacco products on public health, a systematic review. Int. J. Environ. Res. Public Health 2020, 17, 409. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, J.; Zhang, J.; Chen, G.; Luo, C.; Huang, L. Research progress and prospect on the safety of heated tobacco products. Toxicol. 2024, 505, 153823. [Google Scholar] [CrossRef]
- Jankowski, M.; Brożek, G.M.; Lawson, J.; Skoczyński, S.; Majek, P.; Zejda, J.E. New ideas, old problems? Heated tobacco products—A systematic review. Int. J. Occup. Med. Environ. Health 2019, 32, 595–634. [Google Scholar] [CrossRef]
- Wang, H.T.; Wang, P.H.; Chen, C.Y.; Liu, T.Y.; Tsou, H.H. Comparison of carbonyls and tobacco-specific nitrosamines in aerosols of heated tobacco products and conventional cigarette smoke using both targeted and untargeted analytical methods. Regul. Toxicol. Pharmacol. 2025, 157, 105786. [Google Scholar] [CrossRef] [PubMed]
- Dusautoir, R.; Zarcone, G.; Verriele, M.; Garçon, G.; Fronval, I.; Beauval, N.; Allorge, D.; Riffault, V.; Locoge, N.; Lo-Guidice, J.M.; et al. Comparison of the chemical composition of aerosols from heated tobacco products, electronic cigarettes and tobacco cigarettes and their toxic impacts on the human bronchial epithelial BEAS-2B cells. J. Hazard. Mater. 2021, 401, 123417. [Google Scholar] [CrossRef]
- Auer, R.; Concha-Lozano, N.; Jacot-Sadowski, I.; Cornuz, J.; Berthet, A. Heat-not-burn tobacco cigarettes: Smoke by any other name. JAMA Intern. Med. 2017, 177, 1050–1052. [Google Scholar] [CrossRef]
- Eckford, R.; Severini, G.; Sebrié, E.M.; Muggli, M.E.; Beem, A.; Rosen, D.; Crosbie, E. United States Food and Drug Administration’s authorization of reduced exposure claims for IQOS®: Implications for regulation in Latin America. Rev. Panam. Salud Publica 2022, 46, e155. [Google Scholar] [CrossRef]
- Dean, J.L.; Zhao, Q.J.; Lambert, J.C.; Hawkins, B.S.; Thomas, R.S.; Wesselkamper, S.C. Editor’s highlight: Application of gene set enrichment analysis for identification of chemically induced, biologically relevant transcriptomic networks and potential utilization in human health risk assessment. Toxicol. Sci. 2017, 157, 85–99. [Google Scholar] [CrossRef]
- Prohens, L.; Rodríguez, N.; Segura, À.-G.; Martínez-Pinteño, A.; Olivares-Berjaga, D.; Martínez, I.; González, A.; Mezquida, G.; Parellada, M.; Cuesta, M.J.; et al. Gene expression imputation provides clinical and biological insights into treatment-resistant schizophrenia polygenic risk. Psychiatry Res. 2024, 332, 115722. [Google Scholar] [CrossRef]
- Rosati, D.; Palmieri, M.; Brunelli, G.; Morrione, A.; Iannelli, F.; Frullanti, E.; Giordano, A. Differential gene expression analysis pipelines and bioinformatic tools for the identification of specific biomarkers: A review. Comput. Struct. Biotechnol. J. 2024, 23, 1154–1168. [Google Scholar] [CrossRef]
- Yen, N.T.H.; Park, S.-M.; Thu, V.T.A.; Phat, N.K.; Cho, Y.-S.; Yoon, S.; Shin, J.-G.; Kim, D.H.; Oh, J.-H.; Long, N.P. Genome-wide gene expression analysis reveals molecular insights into the drug-induced toxicity of nephrotoxic agents. Life Sci. 2022, 306, 120801. [Google Scholar] [CrossRef] [PubMed]
- Haswell, L.E.; Corke, S.; Verrastro, I.; Baxter, A.; Banerjee, A.; Adamson, J.; Jaunky, T.; Proctor, C.; Gaça, M.; Minet, E. In vitro RNA-seq-based toxicogenomics assessment shows reduced biological effect of tobacco heating products when compared to cigarette smoke. Sci. Rep. 2018, 8, 1145. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.; Veljkovic, E.; Boué, S.; Schlage, W.K.; Vuillaume, G.; Martin, F.; Titz, B.; Leroy, P.; Buettner, A.; Elamin, A.; et al. An 8-month systems toxicology inhalation/cessation study in Apoe-/- mice to investigate cardiovascular and respiratory exposure effects of a candidate modified risk tobacco product, THS 2.2, compared with conventional cigarettes. Toxicol. Sci. 2016, 149, 411–432. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Hayashida, A.; Nozawa, A.; Ito, S. RNA sequencing analysis of early-stage atherosclerosis in vascular-on-a-chip and its application for comparing combustible cigarettes with heated tobacco products. Curr. Res. Toxicol. 2024, 6, 100163. [Google Scholar] [CrossRef]
- Sato, A.; Ishigami, A. Effects of heated tobacco product aerosol extracts on DNA methylation and gene transcription in lung epithelial cells. Toxicol. Appl. Pharmacol. 2023, 475, 116637. [Google Scholar] [CrossRef]
- Uehara, O.; Nakamoto, N.; Hiraki, D.; Paudel, D.; Sugiyama, N.; Morikawa, T.; Yoshida, K.; Kawano, Y.; Shimo, T.; Furuichi, Y.; et al. Effects of prolonged stimulation with heated tobacco products (Ploom TECH+) on gingival epithelial cells. J. Periodontal Res. 2023, 58, 553–563. [Google Scholar] [CrossRef]
- Thorley, A.J.; Tetley, T.D. Pulmonary epithelium, cigarette smoke, and chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2007, 2, 409–428. [Google Scholar]
- Tsou, H.-H.; Wang, P.-H.; Ting, T.-H.; Ping, Y.-H.; Liu, T.-Y.; Cheng, H.-W.; Wang, H.-T. Effect of heated tobacco products and traditional cigarettes on pulmonary toxicity and SARS-CoV-2-induced lung injury. Toxicol. 2022, 479, 153318. [Google Scholar] [CrossRef]
- Hashizume, T.; Ishikawa, S.; Matsumura, K.; Ito, S.; Fukushima, T. Chemical and in vitro toxicological comparison of emissions from a heated tobacco product and the 1R6F reference cigarette. Toxicol. Rep. 2023, 10, 281–292. [Google Scholar] [CrossRef]
- Kim, J.; Cho, Y.; Oh, G.-J.; Park, H.-B.; Yang, M.J.; Park, C.-M.; Kim, Y.-H.; Choi, K.-C.; Go, R.-E.; Kim, M.-S. Repeated intratracheal instillation of whole-cigarette smoke condensate to assess lung damage in a rat model. Environ. Toxicol. 2024, 39, 2304–2315. [Google Scholar] [CrossRef]
- ISO 3402:2023; Tobacco and Tobacco Products—Atmosphere for Conditioning and Testing. International Organization for Standardization: Geneva, Switzerland, 2023.
- Kim, Y.-H.; Kim, M.-S. Development and assessment of a novel standardized method for preparation of whole cigarette smoke condensate (WCSC) for toxicity testing of cigarette smoke. Microchem. J. 2023, 191, 108914. [Google Scholar] [CrossRef]
- Do, V.Q.; Park, K.H.; Park, J.M.; Lee, M.Y. Comparative in vitro toxicity study of docetaxel and nanoxel, a docetaxel-loaded micellar formulation using cultured and blood cells. Toxicol. Res. 2019, 35, 201–207. [Google Scholar] [CrossRef]
- Park, J.-M.; Park, S.; Seo, Y.-S.; Kim, J.-H.; Lee, M.-Y. Cytosolic zinc mediates the cytotoxicity of thiol-reactive electrophiles in rat vascular smooth muscle cells. Food Chem. Toxicol. 2024, 185, 114446. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-H.; Tseng, W.-W.; Hsu, C.-M.; Wei, A.-C. Image analysis of the mitochondrial network morphology with applications in cancer research. Front. Phys. 2022, 10, 855775. [Google Scholar] [CrossRef]
- Valente, A.J.; Maddalena, L.A.; Robb, E.L.; Moradi, F.; Stuart, J.A. A simple ImageJ macro tool for analyzing mitochondrial network morphology in mammalian cell culture. Acta Histochem. 2017, 119, 315–326. [Google Scholar] [CrossRef]
- Park, J.M.; Do, V.Q.; Seo, Y.S.; Kim, H.J.; Nam, J.H.; Yin, M.Z.; Kim, H.J.; Kim, S.J.; Griendling, K.K.; Lee, M.Y. NADPH oxidase 1 mediates acute blood pressure response to angiotensin II by contributing to calcium influx in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2022, 42, e117–e130. [Google Scholar] [CrossRef]
- Behrsing, H.; Aragon, M.; Adamson, J.; Sheehan, D.; Gaca, M.; Curren, R.; Hill, E. Characterization of a Vitrocell VC1 using nicotine dosimetry: An essential component toward standardized in vitro aerosol exposure of tobacco and next generation nicotine delivery products. Appl. In Vitro Toxicol. 2018, 4, 159–166. [Google Scholar] [CrossRef]
- Rudd, K.; Stevenson, M.; Wieczorek, R.; Pani, J.; Trelles-Sticken, E.; Dethloff, O.; Czekala, L.; Simms, L.; Buchanan, F.; O’Connell, G.; et al. Chemical composition and in vitro toxicity profile of a pod-based e-cigarette aerosol compared to cigarette smoke. Appl. In Vitro Toxicol. 2020, 6, 11–41. [Google Scholar] [CrossRef]
- Eldridge, A.; Betson, T.R.; Gama, M.V.; McAdam, K. Variation in tobacco and mainstream smoke toxicant yields from selected commercial cigarette products. Regul. Toxicol. Pharmacol. 2015, 71, 409–427. [Google Scholar] [CrossRef]
- Westrate, L.M.; Drocco, J.A.; Martin, K.R.; Hlavacek, W.S.; MacKeigan, J.P. Mitochondrial morphological features are associated with fission and fusion events. PLoS ONE 2014, 9, e95265. [Google Scholar] [CrossRef]
- Lai, Y.-S.; Chang, C.-C.; Chen, Y.-Y.; Nguyen, T.M.H.; Xu, J.; Chen, Y.-C.; Chang, Y.-F.; Wang, C.-Y.; Chen, P.-S.; Lin, S.-C.; et al. Optogenetically engineered Ca2+ oscillation-mediated DRP1 activation promotes mitochondrial fission and cell death. J. Cell Sci. 2023, 136, jcs260819. [Google Scholar] [CrossRef]
- Saoudi, Y.; Rousseau, B.; Doussière, J.; Charrasse, S.; Gauthier-Rouvière, C.; Morin, N.; Sautet-Laugier, C.; Denarier, E.; Scaïfe, R.; Mioskowski, C.; et al. Calcium-independent cytoskeleton disassembly induced by BAPTA. Eur. J. Biochem. 2004, 271, 3255–3264. [Google Scholar] [CrossRef]
- Chang, C.-R.; Blackstone, C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann. N. Y. Acad. Sci. 2010, 1201, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Schrepfer, E.; Scorrano, L. Mitofusins, from mitochondria to metabolism. Mol. Cell 2016, 61, 683–694. [Google Scholar] [CrossRef]
- Pak, V.V.; Ezeriņa, D.; Lyublinskaya, O.G.; Pedre, B.; Tyurin-Kuzmin, P.A.; Mishina, N.M.; Thauvin, M.; Young, D.; Wahni, K.; Martínez Gache, S.A.; et al. Ultrasensitive genetically encoded indicator for hydrogen peroxide identifies roles for the oxidant in cell migration and mitochondrial function. Cell Metab. 2020, 31, 642–653.e646. [Google Scholar] [CrossRef]
- Agriesti, F.; Tataranni, T.; Pacelli, C.; Scrima, R.; Laurenzana, I.; Ruggieri, V.; Cela, O.; Mazzoccoli, C.; Salerno, M.; Sessa, F.; et al. Nandrolone induces a stem cell-like phenotype in human hepatocarcinoma-derived cell line inhibiting mitochondrial respiratory activity. Sci. Rep. 2020, 10, 2287. [Google Scholar] [CrossRef] [PubMed]
- Plitzko, B.; Loesgen, S. Measurement of oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) in culture cells for assessment of the energy metabolism. Bio Protoc. 2018, 8, e2850. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, S.; He, B.; Li, L.; Li, L.; Wang, J.; Cai, T.; Chen, S.; Jiang, H. OXPHOS deficiency activates global adaptation pathways to maintain mitochondrial membrane potential. EMBO Rep. 2021, 22, e51606. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, A.O.; van den Heuvel, L.P.; Dijkman, H.B.P.M.; De Abreu, R.A.; Birkenkamp, K.U.; de Witte, T.; van der Reijden, B.A.; Smeitink, J.A.M.; Jansen, J.H. Bcl-2 prevents loss of mitochondria in CCCP-induced apoptosis. Exp. Cell. Res. 2004, 299, 533–540. [Google Scholar] [CrossRef]
- Ghazi, S.; Song, M.-A.; El-Hellani, A. A scoping review of the toxicity and health impact of IQOS. Tob. Induc. Dis. 2024, 22, 97. [Google Scholar] [CrossRef]
- Abroms, L.; Levine, H.; Romm, K.; Wysota, C.; Broniatowski, D.; Bar-Zeev, Y.; Berg, C. Anticipating IQOS market expansion in the United States. Tob. Prev. Cessat. 2022, 8, 4. [Google Scholar] [CrossRef]
- Goodall, S.; Gale, N.; Thorne, D.; Hadley, S.; Prasad, K.; Gilmour, I.; Miazzi, F.; Proctor, C. Evaluation of behavioural, chemical, toxicological and clinical studies of a tobacco heated product glo™ and the potential for bridging from a foundational dataset to new product iterations. Toxicol. Rep. 2022, 9, 1426–1442. [Google Scholar] [CrossRef]
- Keyser, B.M.; Leverette, R.; McRae, R.; Wertman, J.; Shutsky, T.; Jordan, K.; Szeliga, K.; Makena, P. In vitro toxicological evaluation of glo menthol and non-menthol heated tobacco products. Toxicol. 2024, 504, 153801. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S. Korean-made heated tobacco product, ‘lil’. Tob. Control 2019, 28, e156–e157. [Google Scholar] [CrossRef]
- Sansone, L.; Milani, F.; Fabrizi, R.; Belli, M.; Cristina, M.; Zagà, V.; de Iure, A.; Cicconi, L.; Bonassi, S.; Russo, P. Nicotine: From discovery to biological effects. Int. J. Mol. Sci 2023, 24, 14570. [Google Scholar] [CrossRef]
- Harris, C.C. Tobacco smoking, E-cigarettes, and nicotine harm. Proc. Nati. Acad. Sci. 2018, 115, 1406–1407. [Google Scholar] [CrossRef] [PubMed]
- Jaunky, T.; Adamson, J.; Santopietro, S.; Terry, A.; Thorne, D.; Breheny, D.; Proctor, C.; Gaça, M. Assessment of tobacco heating product THP1.0. Part 5: In vitro dosimetric and cytotoxic assessment. Regul. Toxicol. Pharmacol. 2018, 93, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Haswell, L.E.; Baxter, A.; Banerjee, A.; Verrastro, I.; Mushonganono, J.; Adamson, J.; Thorne, D.; Gaça, M.; Minet, E. Reduced biological effect of e-cigarette aerosol compared to cigarette smoke evaluated in vitro using normalized nicotine dose and RNA-seq-based toxicogenomics. Sci. Rep. 2017, 7, 888. [Google Scholar] [CrossRef]
- Iskandar, A.R.; Mathis, C.; Schlage, W.K.; Frentzel, S.; Leroy, P.; Xiang, Y.; Sewer, A.; Majeed, S.; Ortega-Torres, L.; Johne, S.; et al. A systems toxicology approach for comparative assessment: Biological impact of an aerosol from a candidate modified-risk tobacco product and cigarette smoke on human organotypic bronchial epithelial cultures. Toxicol. In Vitro 2017, 39, 29–51. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Thorne, D.; Carr, T.; Breheny, D.; Walker, P.; Proctor, C.; Gaça, M. Assessment of novel tobacco heating product THP1.0. Part 6: A comparative in vitro study using contemporary screening approaches. Regul. Toxicol. Pharmacol. 2018, 93, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-M.; Jeong, H.; Seo, Y.-S.; Do, V.Q.; Choi, S.-J.; Lee, K.; Choi, K.-C.; Choi, W.J.; Lee, M.-Y. Cigarette smoke extract produces superoxide in aqueous media by reacting with bicarbonate. Toxics 2021, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Han, C.-H.; Lee, M.-Y. NADPH oxidase and the cardiovascular toxicity associated with smoking. Toxicol. Res. 2014, 30, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.-S.; Park, J.-M.; Kim, J.-H.; Lee, M.-Y. Cigarette smoke-induced reactive oxygen species formation: A concise review. Antioxidants 2023, 12, 1732. [Google Scholar] [CrossRef]
| Lil | IQOS | Glo | 3R4F | |
|---|---|---|---|---|
| TPM | 24.4 | 27.5 | 29.0 | 20.6 |
| Nicotine | 1.81 | 2.45 | 2.26 | 1.10 |
| FC > 1.5 | FC > 2 | |||
|---|---|---|---|---|
| Up | Down | Up | Down | |
| Lil | 90 | 37 | 24 | 0 |
| IQOS | 104 | 67 | 32 | 1 |
| Glo | 113 | 112 | 36 | 4 |
| 3R4F | 307 | 712 | 91 | 158 |
| Pathway | Rank (NES) | |||
|---|---|---|---|---|
| Lil | IQOS | Glo | 3R4F | |
| Epithelial–mesenchymal transition | 1 (−2.27) | 1 (−2.43) | 1 (−2.34) | 1 (−2.43) |
| Oxidative phosphorylation | 2 (−2.18) | 6 (−1.98) | 2 (−2.32) | 17 (−1.67) |
| Unfolded protein response | 3 (2.01) | 9 (1.86) | 12 (1.84) | 12 (1.76) |
| Interferon-α response | 4 (−1.94) | 12 (−1.78) | 20 (−1.70) | 4 (−2.11) |
| Interferon-γ response | 5 (−1.90) | 14 (−1.69) | 16 (−1.77) | 8 (−1.89) |
| MTORC1 signaling | 6 (1.85) | 15 (1.68) | 30 (1.61) | 16 (1.68) |
| TGF-β signaling | 7 (−1.84) | 2 (−2.14) | 4 (−2.06) | 7 (−1.90) |
| Apical junction | 8 (−1.83) | 7 (−1.97) | 5 (−1.99) | 3 (−2.16) |
| Coagulation | 9 (−1.80) | 4 (−2.00) | 3 (−2.06) | 9 (−1.83) |
| MYC targets_v1 | 10 (−1.77) | 17 (−1.66) | 9 (−1.89) | 15 (−1.69) |
| Apical surface | 11 (−1.76) | 8 (−1.88) | 11 (−1.84) | 5 (−2.01) |
| Notch signaling | 12 (−1.74) | 10 (−1.82) | 6 (−1.97) | false |
| Fatty acid metabolism | 13 (−1.67) | 16 (−1.67) | 7 (−1.97) | false |
| Complement | 14 (−1.56) | 13 (−1.74) | 21 (−1.69) | 19 (−1.65) |
| Myogenesis | 15 (−1.55) | 28 (−1.40) | 19 (−1.73) | 11 (−1.80) |
| Cholesterol homeostasis | 16 (−1.54) | 11 (−1.80) | 17 (−1.74) | 18 (−1.65) |
| Peroxisome | 17 (−1.53) | 23 (−1.52) | 23 (−1.66) | false |
| Glycolysis | 18 (−1.51) | 19 (−1.59) | 8 (−1.96) | 25 (−1.57) |
| Androgen response | false | 3 (−2.09) | 13 (−1.82) | 13 (−1.73) |
| UV response_down | false | 5 (−1.99) | 29 (−1.62) | 2 (−2.27) |
| Angiogenesis | false | 18 (−1.61) | −14 (1.81) | 6 (−1.94) |
| TNFα signaling via NF-κB | false | 20 (−1.57) | 15 (−1.79) | 31 (−1.42) |
| Inflammatory response | false | 21 (−1.57) | 27 (−1.63) | 21 (−1.58) |
| WNTβ catenin signaling | false | 22 (−1.57) | 28 (−1.63) | false |
| G2M checkpoint | false | 24 (−1.46) | 32 (−1.50) | 28 (−1.55) |
| Estrogen response_late | false | 25 (−1.45) | 22 (−1.67) | 14 (−1.70) |
| Estrogen response_early | false | 26 (−1.45) | 31 (−1.53) | 24 (−1.57) |
| KRAS signaling_up | false | 27 (−1.45) | 24 (−1.65) | 10 (−1.83) |
| p53 pathway | false | 29 (1.37) | false | false |
| Hypoxia | false | 30 (−1.36) | 10 (−1.88) | 23 (−1.57) |
| Reactive oxygen species pathway | false | false | 18 (−1.74) | false |
| Apoptosis | false | false | 25 (−1.64) | 30 (−1.47) |
| Adipogenesis | false | false | 26 (−1.64) | false |
| Allograft rejection | false | false | 33 (−1.40) | 20 (−1.62) |
| DNA repair | false | false | 34 (−1.34) | false |
| Xenobiotic metabolism | false | false | false | 22 (1.57) |
| IL6-JAK-STAT3 signaling | false | false | false | 26 (−1.56) |
| E2F targets | false | false | false | 27 (−1.56) |
| KRAS signaling_down | false | false | false | 29 (−1.53) |
| Mitotic spindle | false | false | false | 32 (−1.40) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Kim, M.; Jin, W.; Yeo, J.Y.; Kim, J.-H.; Seo, Y.-S.; Park, J.-M.; Kim, J.; Kim, M.-S.; Kim, D.; et al. Effects of Emissions from Heated Tobacco Products and Reference Cigarettes on Gene Expression and Mitochondrial Function in Human Lung Epithelial BEAS-2B Cells †. Antioxidants 2025, 14, 1404. https://doi.org/10.3390/antiox14121404
Park S, Kim M, Jin W, Yeo JY, Kim J-H, Seo Y-S, Park J-M, Kim J, Kim M-S, Kim D, et al. Effects of Emissions from Heated Tobacco Products and Reference Cigarettes on Gene Expression and Mitochondrial Function in Human Lung Epithelial BEAS-2B Cells †. Antioxidants. 2025; 14(12):1404. https://doi.org/10.3390/antiox14121404
Chicago/Turabian StylePark, Suin, Miil Kim, Wei Jin, Ji Yun Yeo, Jae-Hyeong Kim, Yoon-Seok Seo, Jung-Min Park, Jinhee Kim, Min-Seok Kim, Donghyun Kim, and et al. 2025. "Effects of Emissions from Heated Tobacco Products and Reference Cigarettes on Gene Expression and Mitochondrial Function in Human Lung Epithelial BEAS-2B Cells †" Antioxidants 14, no. 12: 1404. https://doi.org/10.3390/antiox14121404
APA StylePark, S., Kim, M., Jin, W., Yeo, J. Y., Kim, J.-H., Seo, Y.-S., Park, J.-M., Kim, J., Kim, M.-S., Kim, D., Bae, O.-N., Lee, C., & Lee, M.-Y. (2025). Effects of Emissions from Heated Tobacco Products and Reference Cigarettes on Gene Expression and Mitochondrial Function in Human Lung Epithelial BEAS-2B Cells †. Antioxidants, 14(12), 1404. https://doi.org/10.3390/antiox14121404







