ROS-Driven STAT1 S-Glutathionylation Sustains IFNγ Signaling and Pro-Inflammatory Microglial Polarization
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Measurement of Intracellular Reactive Oxygen Species
2.4. Glutathione Content Quantification
2.5. Western Blot Analysis
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Immunoprecipitation and Identification of Glutathionylated Proteins
2.8. Immunofluorescence and Confocal Analysis
2.9. RT-qPCR Analysis
2.10. Statistical Analysis
3. Results
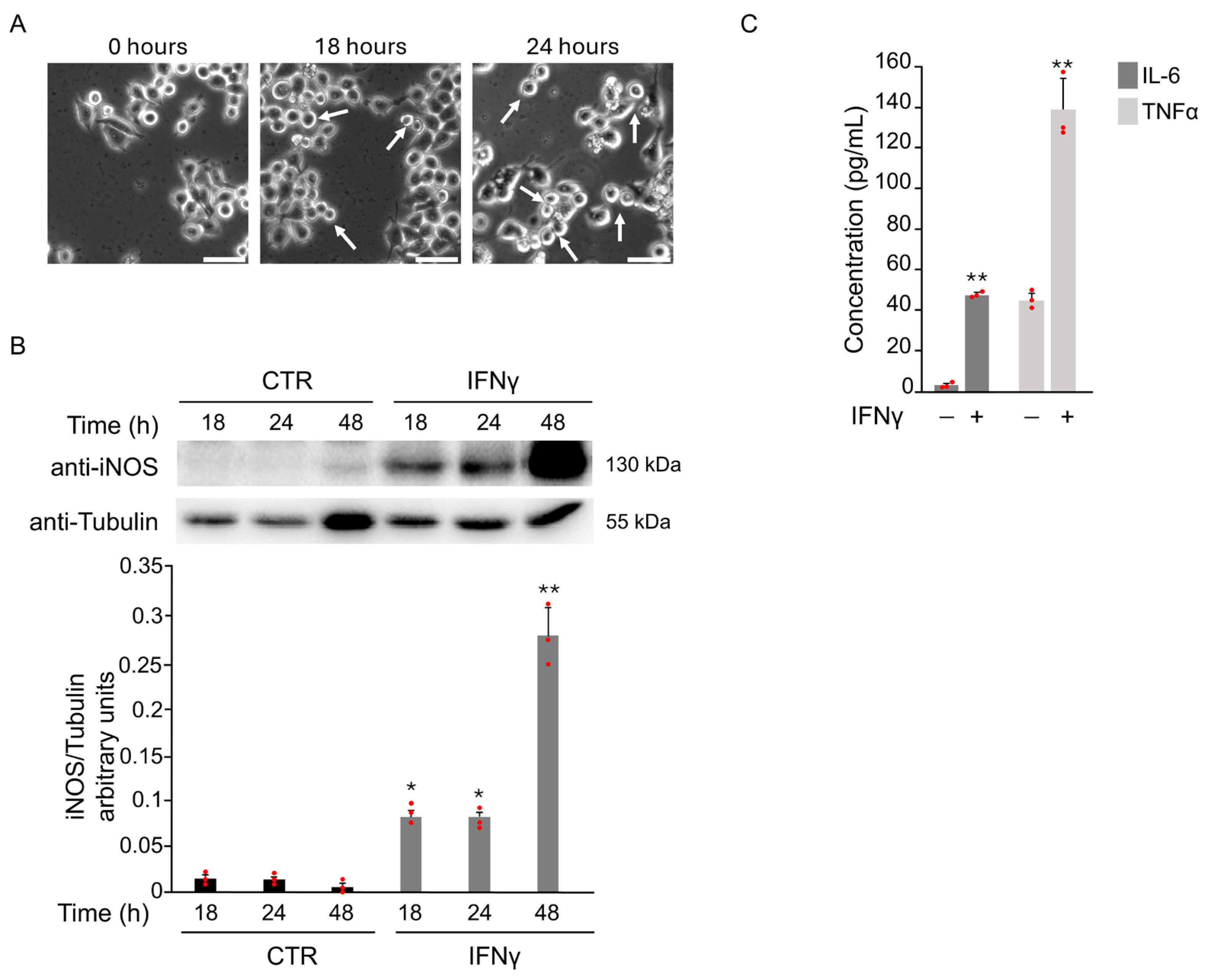
3.1. IFNγ-Induced M1 Phenotype Activation in BV2 Cells Through Oxidative Stress
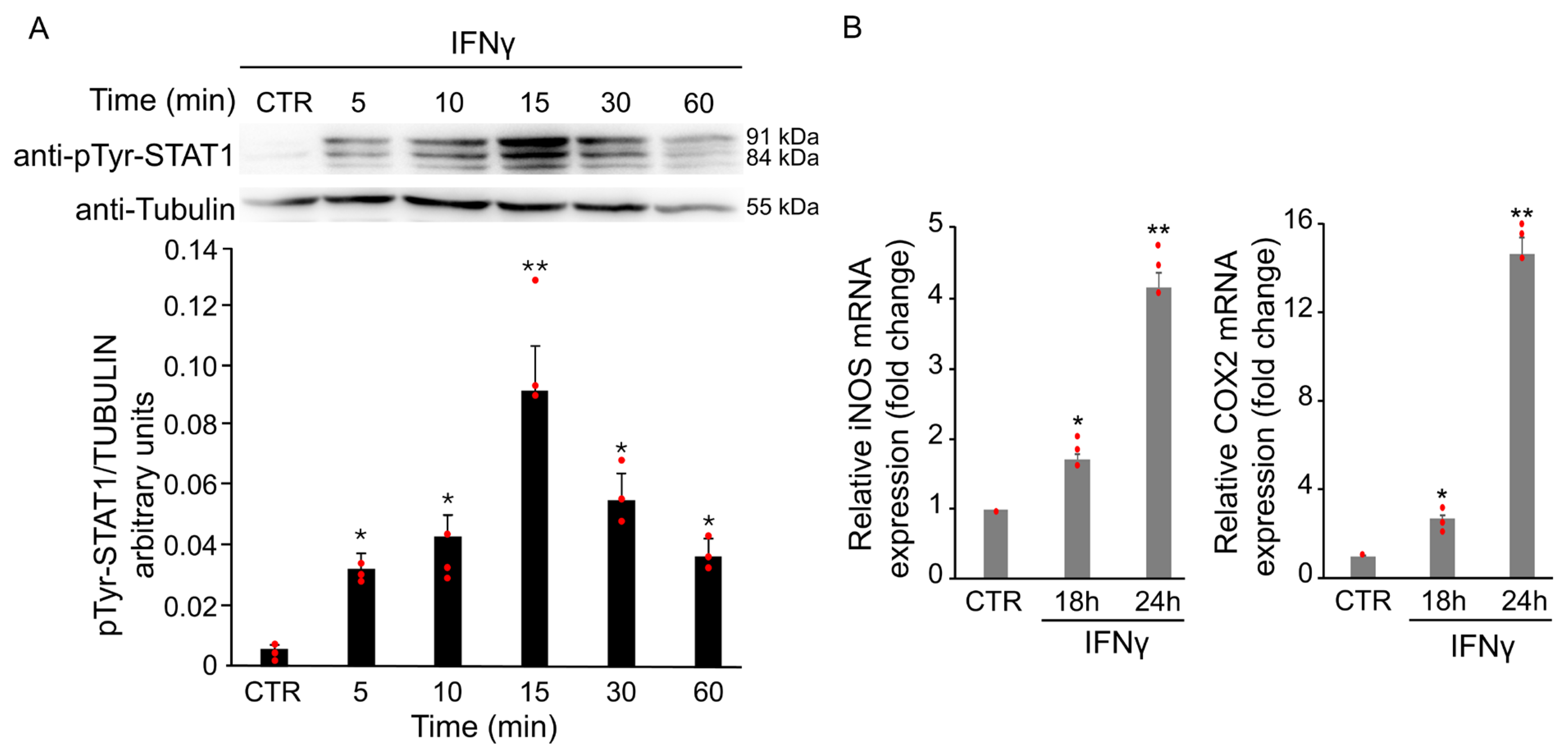
3.1.1. IFNγ Triggers Phosphorylation of STAT1 in BV2 Cells
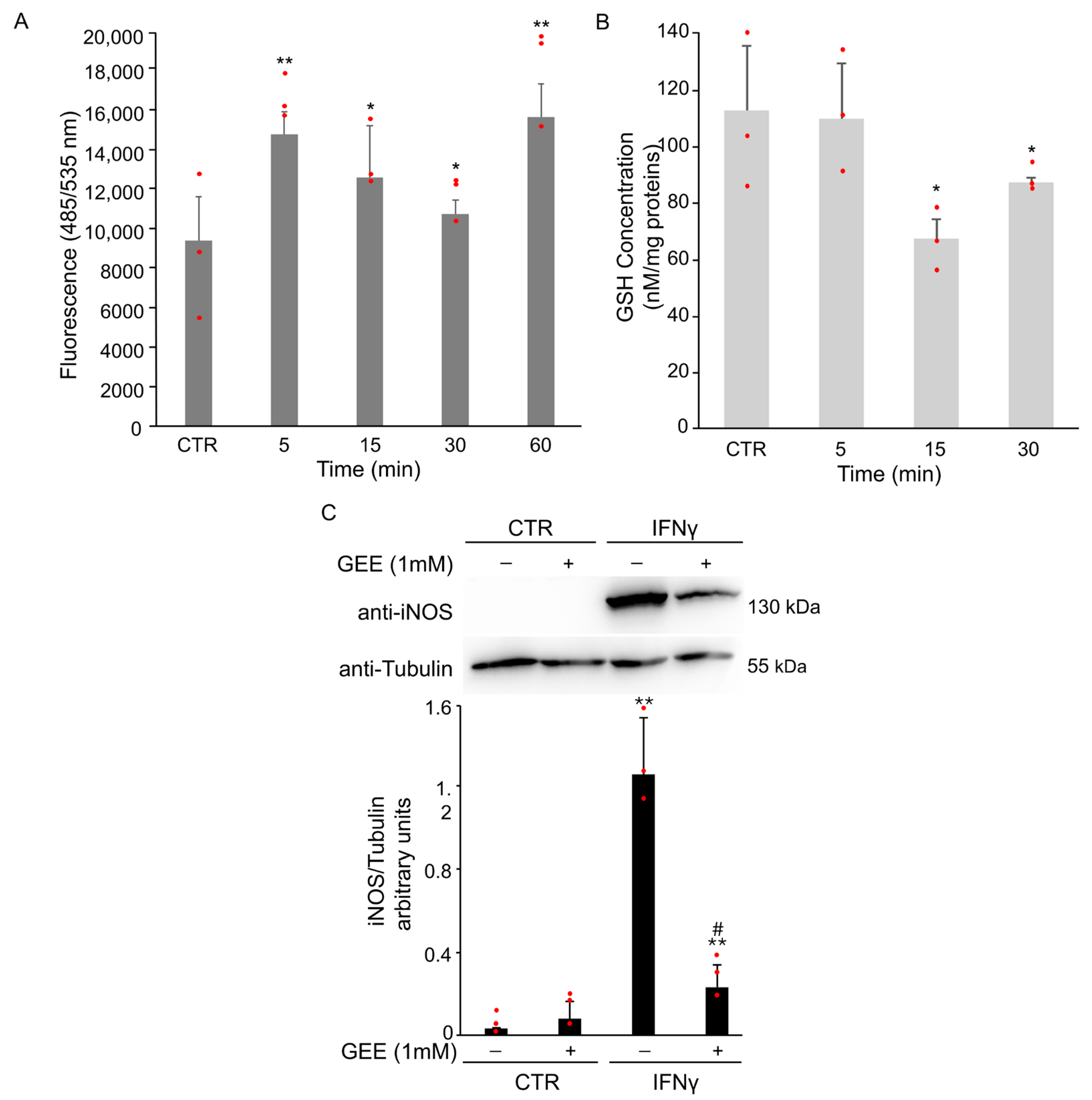
3.1.2. IFNγ Alters the Intracellular Redox State and Triggers STAT1 Phosphorylation in BV2 Cells
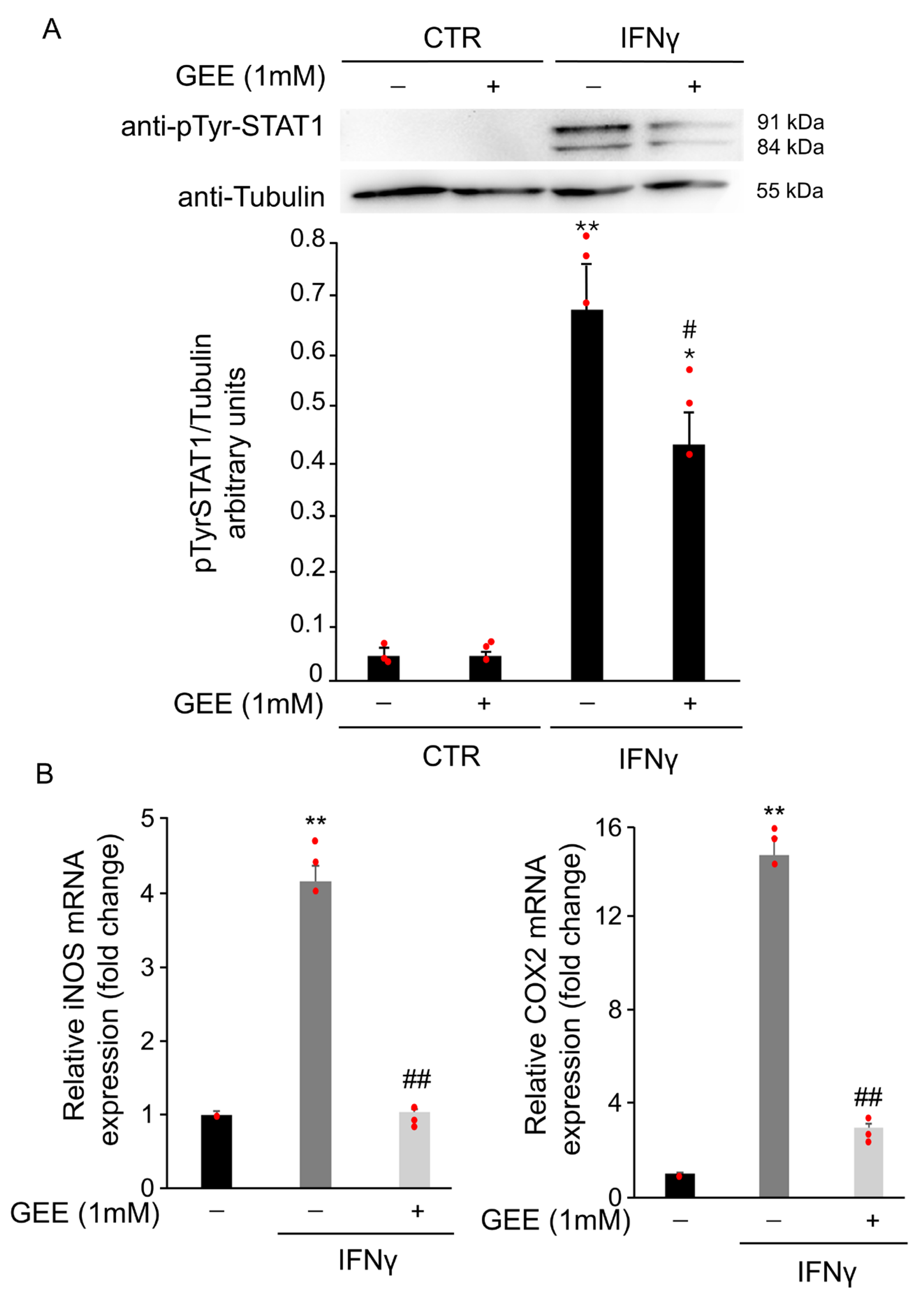
3.1.3. IFNγ Alters the Intracellular Redox State and Induces S-Glutathionylation of STAT1 in BV2 Cells
3.1.4. IFNγ Triggers M1 Phenotype in BV2 Cells Through the Activation of Oxidative Stress and STAT1 Signaling
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GEE | Glutathione Ethyl Ester |
| ROS | Reactive Oxygen Species |
| GSH | Glutathione |
| IFNγ | Interferon gamma |
| STAT1 | Signal transducer and activator of transcription 1 |
References
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef]
- Biswas, K. Microglia mediated neuroinflammation in neurodegenerative diseases: A review on the cell signaling pathways involved in microglial activation. J. Neuroimmunol. 2023, 383, 578180. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Gomes, C.; Vaz, A.R.; Brites, D. Exploring New Inflammatory Biomarkers and Pathways during LPS-Induced M1 Polarization. Mediat. Inflamm. 2016, 2016, 6986175. [Google Scholar] [CrossRef] [PubMed]
- Durafourt, B.A.; Moore, C.S.; Zammit, D.A.; Johnson, T.A.; Zaguia, F.; Guiot, M.C.; Bar-Or, A.; Antel, J.P. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia 2012, 60, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.L.; Ahmad Puzi, N.N.; Ooi, Y.Y.; Ramasamy, R.; Vidyadaran, S. Response Profiles of BV2 Microglia to IFN-γ and LPS Co-Stimulation and Priming. Biomedicines 2023, 11, 2648. [Google Scholar] [CrossRef]
- Ceccarelli, M.C.; Lai, L.; Carmignani, A.; Battaglini, M.; Ciofani, G. Polydopamine nanoparticles as immunomodulators: Inhibition of M1 microglial polarization. Front. Bioeng. Biotechnol. 2025, 13, 1672520. [Google Scholar] [CrossRef]
- Liu, J.; Li, T.; Sun, W.; Xu, J.; Miao, C.; Wang, Q.; Hou, L. CXCR2 mediates rotenone-induced neuroinflammation and neurodegeneration through neurotrophil infiltration and extracellular traps formation in mice. Int. J. Biol. Macromol. 2025, 330 Pt 4, 148305. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, T.; Zhang, L.; Yang, J.; Zhao, F.; Wang, Y.; Ouyang, Y.; Liu, J. TRAF6 promotes spinal microglial M1 polarization to aggravate neuropathic pain by activating the c-JUN/NF-kB signaling pathway. Cell Biol. Toxicol. 2024, 40, 54. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Chen, Y.; Liu, X.; Li, J.; Liu, F.; Wang, J.; Zhao, Y.; Zhang, K.; Sang, L.; et al. PD-1/PD-L1 inhibits M1 polarization of microglia and macrophages through STAT1/NF-κB and Akt/mTOR signaling pathways. Brain Res. Bull. 2025, 232, 111610. [Google Scholar] [CrossRef]
- Shuai, K.; Liu, B. Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 2003, 3, 900–911. [Google Scholar] [CrossRef]
- Chiba, T.; Yamada, M.; Sasabe, J.; Terashita, K.; Shimoda, M.; Matsuoka, M.; Aiso, S. Amyloid-beta causes memory impairment by disturbing the JAK2/STAT3 axis in hippocampal neurons. Mol. Psychiatry 2009, 14, 206–222. [Google Scholar] [CrossRef]
- Hsu, W.L.; Ma, Y.L.; Hsieh, D.Y.; Liu, Y.C.; Lee, E.H. STAT1 negatively regulates spatial memory formation and mediates the memory-impairing effect of Aβ. Neuropsychopharmacology 2014, 39, 746–758. [Google Scholar] [CrossRef]
- Boriero, D.; Carcereri de Prati, A.; Antonini, L.; Ragno, R.; Sohji, K.; Mariotto, S.; Butturini, E. The anti-STAT1 polyphenol myricetin inhibits M1 microglia activation and counteracts neuronal death. FEBS J 2021, 288, 2347–2359. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, Y.; Ohshima, M.; Suzuki, H.; Misawa, H. LAG-3 expression in microglia regulated by IFN-γ/STAT1 pathway and metalloproteases. Front. Cell Neurosci. 2023, 17, 1308972. [Google Scholar] [CrossRef] [PubMed]
- Butturini, E.; Cozzolino, F.; Boriero, D.; Carcereri de Prati, A.; Monti, M.; Rossin, M.; Canetti, D.; Cellini, B.; Pucci, P.; Mariotto, S. S-glutathionylation exerts opposing roles in the regulation of STAT1 and STAT3 signaling in reactive microglia. Free Radic. Biol. Med. 2018, 117, 191–201. [Google Scholar] [CrossRef]
- Blasi, E.; Barluzzi, R.; Bocchini, V.; Mazzolla, R.; Bistoni, F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J. Neuroimmunol. 1990, 27, 229–237. [Google Scholar] [CrossRef]
- Eder, C. Ion channels in monocytes and microglia/brain macrophages: Promising therapeutic targets for neurological diseases. J. Neuroimmunol. 2010, 224, 51–55. [Google Scholar] [CrossRef]
- Butturini, E.; Carcereri de Prati, A.; Chiavegato, G.; Rigo, A.; Cavalieri, E.; Darra, E.; Mariotto, S. Mild oxidative stress induces S-glutathionylation of STAT3 and enhances chemosensitivity of tumoural cells to chemotherapeutic drugs. Free Radic. Biol. Med. 2013, 65, 1322–1330. [Google Scholar] [CrossRef]
- Thalappil, M.A.; Butturini, E.; Carcereri de Prati, A.; Bettin, I.; Antonini, L.; Sapienza, F.U.; Garzoli, S.; Ragno, R.; Mariotto, S. Pinus mugo Essential Oil Impairs STAT3 Activation through Oxidative Stress and Induces Apoptosis in Prostate Cancer Cells. Molecules 2022, 27, 4834. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wang, Y.; Manokaran, C.; Chen, J.; Gu, H.; Liu, D.; Bousso, P.; Zhao, J.J.; Roberts, T.M. Loss of the tumor suppressor PTEN activates cell-intrinsic interferon signaling to drive immune resistance. bioRxiv 2025, Preprint. [Google Scholar] [CrossRef]
- Kandhaya-Pillai, R.; Yang, X.; Tchkonia, T.; Martin, G.M.; Kirkland, J.L.; Oshima, J. TNF-α/IFN-γ synergy amplifies senescence-associated inflammation and SARS-CoV-2 receptor expression via hyper-activated JAK/STAT1. Aging Cell 2022, 21, e13646. [Google Scholar] [CrossRef]
- Qin, H.; Roberts, K.L.; Niyongere, S.A.; Cong, Y.; Elson, C.O.; Benveniste, E.N. Molecular mechanism of lipopolysaccharide-induced SOCS-3 gene expression in macrophages and microglia. J. Immunol. 2007, 179, 5966–5976. [Google Scholar] [CrossRef]
- Kovarik, P.; Stoiber, D.; Novy, M.; Decker, T. Stat1 combines signals derived from IFN-gamma and LPS receptors during macrophage activation. EMBO J. 1998, 17, 3660–3668. [Google Scholar] [CrossRef]
- Cheng, F.; Yang, A.; Yan, F.; Liu, J.; Jiang, L. MiR-182 ameliorates neuropathic pain by enhancing the transition from M1 to M2 phenotype polarization via PI3K/AKT signaling. Folia Neuropathol. 2025, 63, 269–281. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Q.; Li, N.; Lin, W.; Cui, X.; Yang, F.; Ma, L.; Zhang, H.; Ma, H. HMGB1 promotes LPS-induced M1 polarization and apoptosis in microglia by mediating the expression of immune and inflammation-related genes. Brain Res. Bull. 2025, 233, 111615. [Google Scholar] [CrossRef] [PubMed]
- Varinou, L.; Ramsauer, K.; Karaghiosoff, M.; Kolbe, T.; Pfeffer, K.; Müller, M.; Decker, T. Phosphorylation of the Stat1 transactivation domain is required for full-fledged IFN-gamma-dependent innate immunity. Immunity 2003, 19, 793–802. [Google Scholar] [CrossRef]
- Lim, C.M.; Kim, N.Y.; Kim, J.; Park, H.M.; Lee, H.P.; Hong, J.T.; Kwon, D.O.; Choi, K.; Yoon, D.Y. MMPP Attenuates the Inflammatory Response by Suppressing ROS and Proinflammatory Cytokine and Chemokine Production in TNF-α/IFN-γ-Stimulated Human Keratinocytes. J. Microbiol. Biotechnol. 2025, 35, e2508045. [Google Scholar] [CrossRef] [PubMed]
- Kiran, B.K.; Siddesh, B.M.; Sherapura, A.; Thirusangu, P.; Vijay Avin, B.R.; Vigneshwaran, V.; Kumaraswamy, H.M.; Suchetha Kumari, N.; Pramod, S.N.; Prabhakar, B.T. Allium sativum lectin as an immunotherapeutic agent: Eliciting IFN-γ production via “M1” macrophage polarization to trigger JAK1/STAT1 and DDR mediated lung cancer cell death. Int. J. Biol. Macromol. 2025, 330, 148076. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Suzumura, A.; Sawada, M.; Yamamoto, H.; Marunouchi, T. Transforming growth factor-beta suppresses activation and proliferation of microglia in vitro. J. Immunol. 1993, 151, 2150–2158. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, D.C.; Kim, H.M.; Kwon, H.J.; Kwon, S.J.; Kang, S.J.; Kim, S.C.; Choi, G.E. STAT1 deficiency redirects IFN signalling toward suppression of TLR response through a feedback activation of STAT3. Sci. Rep. 2015, 5, 13414. [Google Scholar] [CrossRef]
- Li, H.; Shen, Y.; Xiao, H.; Sun, W. Resveratrol attenuates rotenone-induced inflammation and oxidative stress via STAT1 and Nrf2/Keap1/SLC7A11 pathway in a microglia cell line. Pathol. Res. Pract. 2021, 225, 153576. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brattini, M.; Carcereri de Prati, A.; Passarini, C.; Menegazzi, M.; Fiore, A.; Mosaico, M.; D’Urso, M.; Mariotto, S.; Butturini, E. ROS-Driven STAT1 S-Glutathionylation Sustains IFNγ Signaling and Pro-Inflammatory Microglial Polarization. Antioxidants 2025, 14, 1395. https://doi.org/10.3390/antiox14121395
Brattini M, Carcereri de Prati A, Passarini C, Menegazzi M, Fiore A, Mosaico M, D’Urso M, Mariotto S, Butturini E. ROS-Driven STAT1 S-Glutathionylation Sustains IFNγ Signaling and Pro-Inflammatory Microglial Polarization. Antioxidants. 2025; 14(12):1395. https://doi.org/10.3390/antiox14121395
Chicago/Turabian StyleBrattini, Martina, Alessandra Carcereri de Prati, Carlotta Passarini, Marta Menegazzi, Alessandra Fiore, Maria Mosaico, Michelle D’Urso, Sofia Mariotto, and Elena Butturini. 2025. "ROS-Driven STAT1 S-Glutathionylation Sustains IFNγ Signaling and Pro-Inflammatory Microglial Polarization" Antioxidants 14, no. 12: 1395. https://doi.org/10.3390/antiox14121395
APA StyleBrattini, M., Carcereri de Prati, A., Passarini, C., Menegazzi, M., Fiore, A., Mosaico, M., D’Urso, M., Mariotto, S., & Butturini, E. (2025). ROS-Driven STAT1 S-Glutathionylation Sustains IFNγ Signaling and Pro-Inflammatory Microglial Polarization. Antioxidants, 14(12), 1395. https://doi.org/10.3390/antiox14121395









