Nuclear Factor Erythroid 2-Related Factor 2 (NRF2) as a Biomarker for Radiation Dosimetry and Health Risk Assessment: A Review
Abstract
1. Introduction
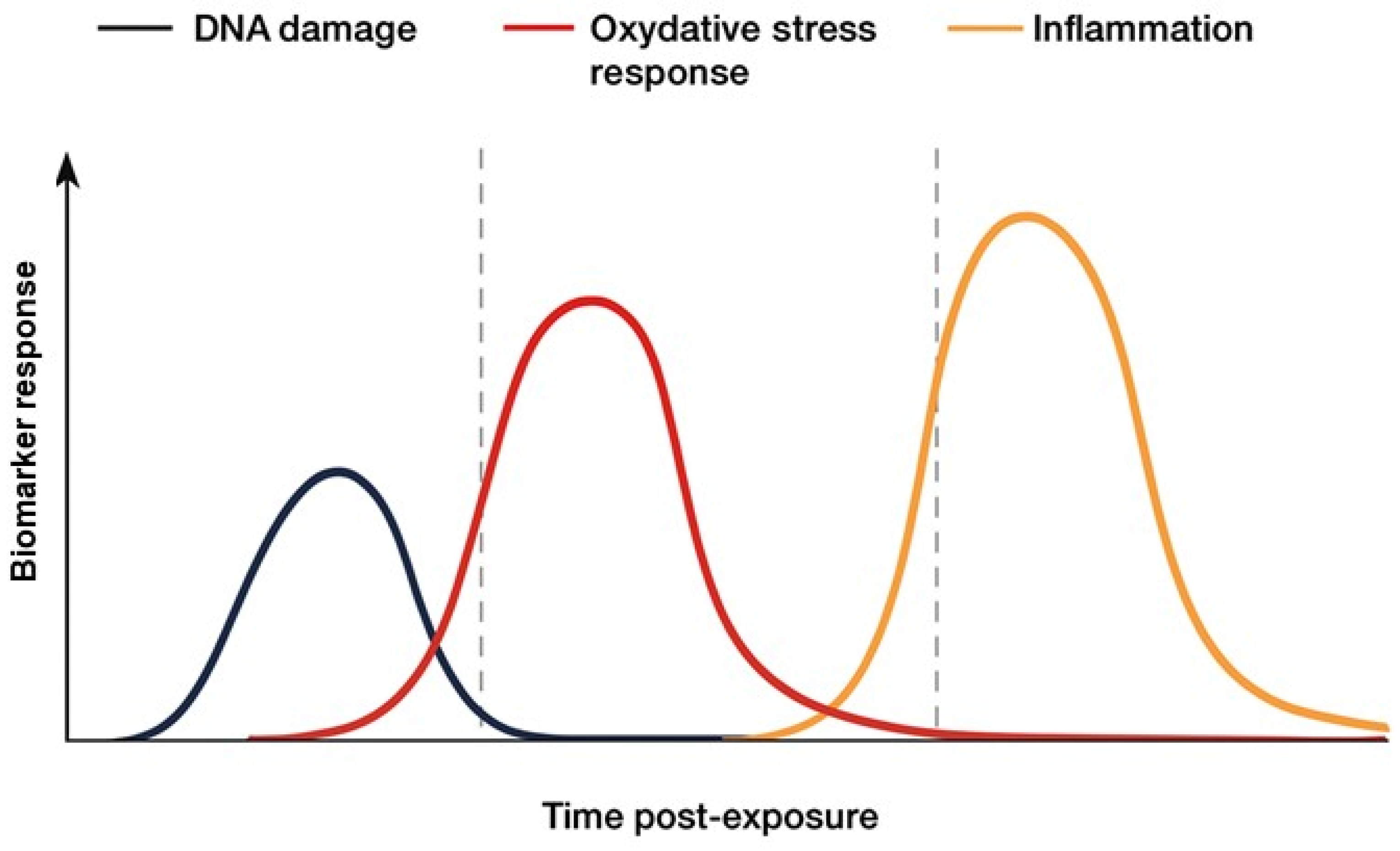
2. Biodosimetry (History, Advantages, and Disadvantages)
3. Mechanism of NRF2 Activation in Radiobiology
4. In Vitro, In Vivo, and Clinical Studies
5. Comparison of the NRF2 Marker with Other Biological Markers and Methods for Biological Dosimetry
| Method | Principal and Biomarker | Range of Doses | Best Time After Exposure (h) | References |
|---|---|---|---|---|
| Dicentric chromosome assay (DCA) | Unstable chromosomal aberrations | 0.1–5 Gy | 48–72 h | [72,73,74] |
| Premature chromosome condensation (PCC) assay | Unstable chromosomal aberrations | 0.2 to 20 Gy | For fusion PCC is 2–6 h/for chemical PCC is 40–72 h | [75] |
| Fluorescence in situ hybridization (FISH) translocation assay | Stable chromosomal aberrations | 0.25–4 Gy | 48–72 h (to obtain metaphases), but stable translocations can be detected months to years later | [76] |
| Cytokinesis-block micronucleus (CBMN) assay | Micronuclei in binucleated cells | 0.2–4 Gy | ~48–72 h | [77,78] |
| γ-H2AX foci | DNA double-strand break marker | <0.1–3 Gy | 0.5–6 h (ideal 0.5–1 h) (functional up to ~24 h) | [14,69] |
| ESR/EPR | Detects unpaired electrons in radicals or paramagnetic species | 0.1–9 Gy | Depends on material: radicals in soft tissues/fingernails—hours to days; tooth enamel or bone—any time (months–years) | [70,71] |
| NRF2 activation | Antioxidant gene activation (HO-1, NQO1) | 0.02–8 Gy | ~2–24 h (common peak 4–12 h) | [1,50] |
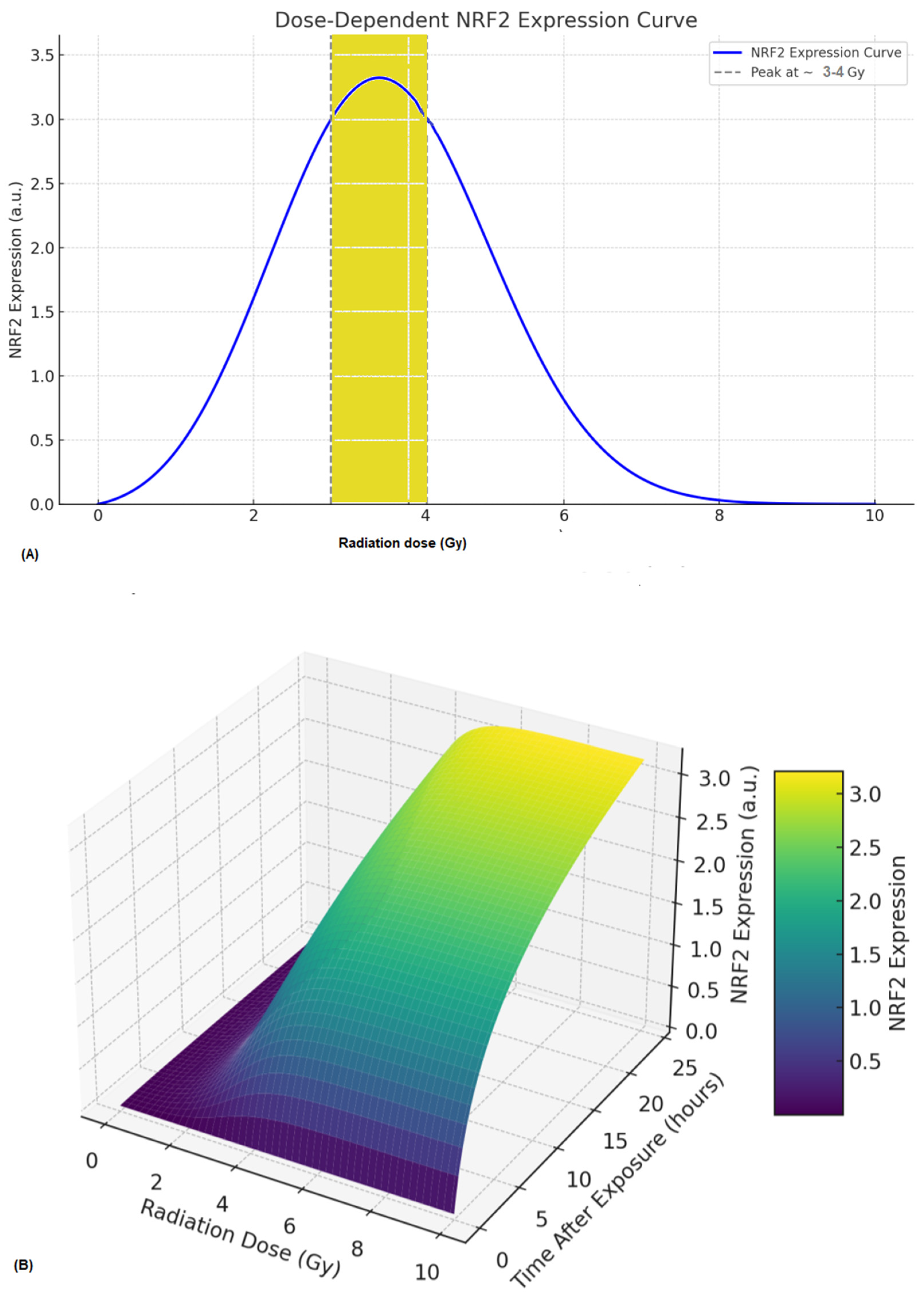
6. A Prediction Radiobiological Model of NRF2 Expression
- (a)
- dN(t)/dt = β × ROS(t) × (1 − N(t)/Nmax) − ˠ N(t)where N(t) = The quantity or level of NRF2 at time (t), β = rate of NRF2 activation by ROS, γ = degradation rate of NRF2 and Nmax = maximum NRF2 expression capacity.
- (b)
- ROS = αD × eλ1twhere D = Absorbed radiation dose (Gy), α = scaling factor, λ1 = ROS clearance rate. However, ROS generation increases with dose and decays over time [79,80]. Furthermore, the observed non-linear activation of NRF2 within the 0.5–4 Gy range likely reflects the complex interplay between direct DNA damage signaling and secondary oxidative stress responses. At lower doses (<1 Gy), transient ROS production induces modest NRF2 nuclear translocation, whereas higher doses (≥3–4 Gy) can suppress NRF2 activity through the oxidative degradation of KEAP1 or overwhelming cellular stress, resulting in a biphasic pattern. Several studies [19,23,50] support this non-linear dose dependence in human PBMCs and fibroblasts. Moreover, the NRF2 activation window (6–24 h) varies with cell type, redox status, and radiation quality, and high-LET radiation or metabolically active cells often exhibit earlier and more sustained activation.
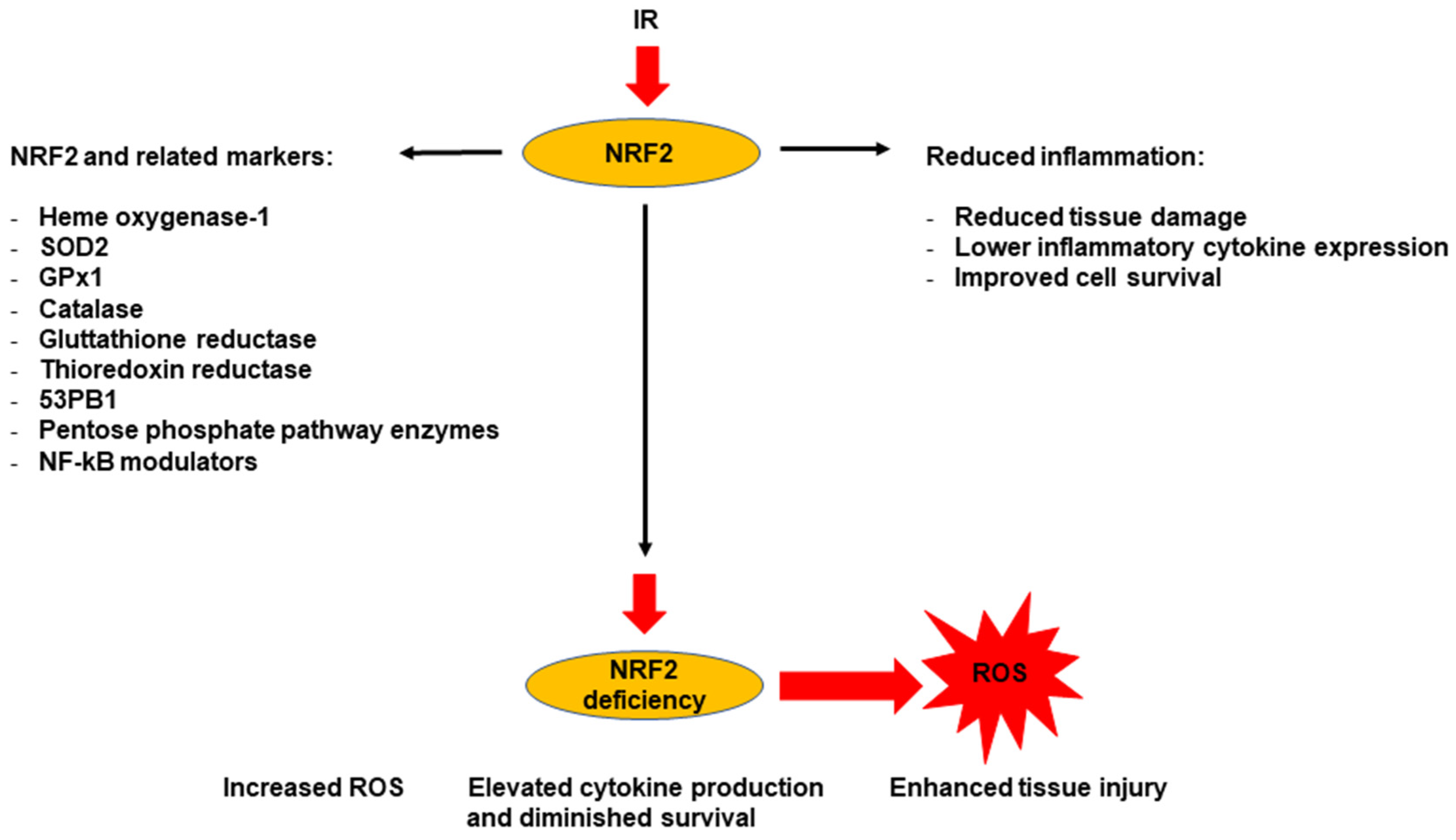
7. Use of NRF2 Signaling as a Marker for Radiation-Induced Chronic Oxidative Stress and Chronic Inflammation
7.1. NRF2 as a Marker for Radiation-Induced Oxidative Stress
7.2. NRF2 as a Marker for Radiation-Induced Inflammatory Response
| NRF2target | Tissue/Cell Line | Radiation Response | Inflammatory Role/Inflammation Time Response | References |
|---|---|---|---|---|
| HO-1 | Fibroblasts, breast cancer cells | Upregulated after radiation; absent in Nrf2-deficient cells | Antioxidant, cytoprotective/early response (hours to 1 day) | [50,59] |
| HO-1, p53-binding protein 1 (53BP1) | Colonic epithelium, crypts | Increased DNA repair, reduced apoptosis, improved survival | Anti-inflammatory, DNA repair/early (hours) | [85] |
| Glutathione reductase (GR), thioredoxin reductase 1 (TRXR1), pentose phosphate pathway (PPP) enzymes, nuclear factor kappa B (NF-κB) | Mouse embryonic fibroblasts, immune cells | Reduced transformation, lower NF-κB activation in wild-type | Antioxidant/early to intermediate (hours to days); NF-κB is often activated within hours | [68] |
| GPX1, SOD2, CAT, HO-1 | Lung | Reduced oxidative damage, lower pro-inflammatory cytokines, higher interleukin-10 (IL-10) | Antioxidant/early to intermediate (hours to a few days); antioxidant enzymes respond early to ROS | [91] |
| CDDO targets, delta Np63 (ΔNp63) | Crypts, lung | Attenuates crypt injury, modulates stem cell response | Modulates ROS, transforming growth factor beta (TGF-β)/Smad, collagen degradation/Intermediate (days) | [61] |
| SOD1, 53BP1, plasminogen activator inhibitor-1 (PAI-1) | Lung, bone, glioblastoma | Promotes DNA repair, detoxifies ROS, suppresses fibrosis | Modulates cytokines, suppresses TGF-β1 | [94,99] |
| NRF2 promotes radiation resistance by cooperating with TOPBP1 to regulate DNA repair | Human lung cancer cell lines (radioresistant derivatives, e.g., A549/A549R) and mouse xenografts | Evaluation of NRF2 protein, chromatin fractionation, functional assays (clonogenic survival), and γ-H2AX | Altered inflammatory gene expression in the tumor microenvironment in models, linking NRF2 to both radioresistance and radiation-associated inflammatory signaling. | [100] |
8. A Summary on the Role of NRF2 as a Biomarker for Health Risk Assessment
9. Conclusions and Prospective View
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obrador, E.; Salvador-Palmer, R.; Villaescusa, J.I.; Gallego, E.; Pellicer, B.; Estrela, J.M.; Montoro, A. Nuclear and radiological emergencies: Biological effects, countermeasures and biodosimetry. Antioxidants 2022, 11, 1098. [Google Scholar] [CrossRef] [PubMed]
- Giussani, A.; Lopez, M.A.; Testa, A. The EURADOS work towards a review on retrospective dosimetry after incorporation of radionuclides. Radiat. Prot. Dosim. 2019, 186, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Giussani, A.; Lopez, M.A.; Romm, H.; Testa, A.; Ainsbury, E.A.; Degteva, M.; Della Monaca, S.; Etherington, G.; Fattibene, P.; Güclu, I. Eurados review of retrospective dosimetry techniques for internal exposures to ionising radiation and their applications. Radiat. Environ. Biophys. 2020, 59, 357–387. [Google Scholar] [CrossRef] [PubMed]
- Herate, C.; Sabatier, L. Retrospective biodosimetry techniques: Focus on cytogenetics assays for individuals exposed to ionizing radiation. Mutat. Res./Rev. Mutat. Res. 2020, 783, 108287. [Google Scholar] [CrossRef]
- Sotnik, N.; Rybkina, V.; Azizova, T. New approaches to biological dosimetry: Development of complex biodosimetric systems (review of foreign literature). Medico-Biol. Socio-Psychol. Probl. Saf. Emerg. Situat. 2019, 4, 90–96. [Google Scholar] [CrossRef]
- Piotrowski, I.; Dawid, A.; Kulcenty, K.; Suchorska, W.M. Use of biological dosimetry for monitoring medical workers occupationally exposed to ionizing radiation. Radiation 2021, 1, 95–115. [Google Scholar] [CrossRef]
- Fattibene, P.; Trompier, F.; Bassinet, C.; Ciesielski, B.; Discher, M.; Eakins, J.; Gonzales, C.A.B.; Huet, C.; Romanyukha, A.; Woda, C.; et al. Reflections on the future developments of research in retrospective physical dosimetry. Phys. Open 2023, 14, 100132. [Google Scholar] [CrossRef]
- Azariasl, S.; Yasuda, H. The effects of age and other individual factors on radiation induced ESR signals from fingernails. Front. Public Health 2025, 13, 1531253. [Google Scholar] [CrossRef]
- Yasuda, H.; Azariasl, S.; Trompier, F. Preliminary analysis of the integrated EPR signals of fingernails to validate the dosimetry method based on peak-to-peak amplitudes. Int. J. Radiat. Biol. 2025, 101, 1–8. [Google Scholar] [CrossRef]
- Garty, G.; Chen, Y.; Turner, H.C.; Zhang, J.; Lyulko, O.V.; Bertucci, A.; Xu, Y.; Wang, H.; Simaan, N.; Randers-Pehrson, G. The RABiT: A rapid automated biodosimetry tool for radiological triage. II. Technological developments. Int. J. Radiat. Biol. 2011, 87, 776–790. [Google Scholar] [CrossRef]
- Repin, M.; Garty, G.; Garippa, R.J.; Brenner, D.J. RABiT-III: An automated micronucleus assay at a non-specialized biodosimetry facility. Radiat. Res. 2024, 201, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.M.; Puzantian, B.; Mayenburg, J.M.; Li, M.; Rashid, M.; Wilkins, R.C.; Beaton-Green, L.A. Application of an imaging flow cytometry γ-H2AX assay for biodosimetry using supervised machine learning. Int. J. Radiat. Biol. 2025, 101, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sproull, M.; Camphausen, K. State-of-the-art advances in radiation biodosimetry for mass casualty events involving radiation exposure. Radiat. Res. 2016, 186, 423–435. [Google Scholar] [CrossRef]
- Raavi, V.; Perumal, V.; Paul, S.F. Potential application of γ-H2AX as a biodosimetry tool for radiation triage. Mutat. Res./Rev. Mutat. Res. 2021, 787, 108350. [Google Scholar] [CrossRef]
- Draeger, E.; Roberts, K.; Decker, R.D.; Bahar, N.; Wilson, L.D.; Contessa, J.; Husain, Z.; Williams, B.B.; Flood, A.B.; Swartz, H.M. In vivo verification of electron paramagnetic resonance biodosimetry using patients undergoing radiation therapy treatment. Int. J. Radiat. Oncol. Biol. Phys. 2024, 119, 292–301. [Google Scholar] [CrossRef]
- Wilkins, R.; Lloyd, D.; Maznyk, N.; Carr, Z. The international biodosimetry capacity, capabilities, needs and challenges: The 3rd WHO BioDoseNet survey results. Environ. Adv. 2022, 8, 100202. [Google Scholar] [CrossRef]
- Kulka, U.; Ainsbury, L.; Atkinson, M.; Barquinero, J.-F.; Barrios, L.; Beinke, C.; Bognar, G.; Cucu, A.; Darroudi, F.; Fattibene, P. Realising the European network of biodosimetry (RENEB). Radiat. Prot. Dosim. 2012, 151, 621–625. [Google Scholar] [CrossRef]
- Escalona, M.B.; Ryan, T.L.; Balajee, A.S. Current developments in biodosimetry tools for radiological/nuclear mass casualty incidents. Environ. Adv. 2022, 9, 100265. [Google Scholar] [CrossRef]
- Shimura, T.; Nakashiro, C.; Narao, M.; Ushiyama, A. Induction of oxidative stress biomarkers following whole-body irradiation in mice. PLoS ONE 2020, 15, e0240108. [Google Scholar] [CrossRef] [PubMed]
- Moloudi, K.; Haghdoost, S. An Update on Role of Ionizing Radiation to Enhance Proliferation and Differentiation of Normal Stem Cells via Activation of NRF2 Pathway. Antioxidants 2025, 14, 986. [Google Scholar] [CrossRef]
- Xiao, C.; He, N.; Liu, Y.; Wang, Y.; Liu, Q. Research progress on biodosimeters of ionizing radiation damage. Radiat. Med. Prot. 2020, 1, 127–132. [Google Scholar] [CrossRef]
- Abend, M.; Blakely, W.; Ostheim, P.; Schuele, S.; Port, M. Early molecular markers for retrospective biodosimetry and prediction of acute health effects. J. Radiol. Prot. 2022, 42, 010503. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Yamaguchi, M.; Yoshino, H.; Nakai, Y.; Kashiwakura, I. Dose-dependent increase of Nrf2 target gene expression in mice exposed to ionizing radiation. Radiat. Res. 2019, 191, 176–188. [Google Scholar] [CrossRef]
- Amundson, S.A. Transcriptomics for radiation biodosimetry: Progress and challenges. Int. J. Radiat. Biol. 2023, 99, 925–933. [Google Scholar] [CrossRef]
- Liu, K.; Singer, E.; Cohn, W.; Micewicz, E.D.; McBride, W.H.; Whitelegge, J.P.; Loo, J.A. Time-Dependent Measurement of Nrf2-Regulated Antioxidant Response to Ionizing Radiation Toward Identifying Potential Protein Biomarkers for Acute Radiation Injury. Proteom.–Clin. Appl. 2019, 13, 1900035. [Google Scholar] [CrossRef]
- Sudprasert, W.; Belyakov, O.V.; Tashiro, S. Biological and internal dosimetry for radiation medicine: Current status and future perspectives. J. Radiat. Res. 2022, 63, 247–254. [Google Scholar] [CrossRef]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 627. [Google Scholar]
- Ozasa, K.; Grant, E.J.; Kodama, K. Japanese legacy cohorts: The life span study atomic bomb survivor cohort and survivors’ offspring. J. Epidemiol. 2018, 28, 162–169. [Google Scholar] [CrossRef]
- Bender, M.; Gooch, P. Somatic chromosome aberrations induced by human whole-body irradiation: The “Recuplex” criticality accident. Radiat. Res. 1966, 29, 568–582. [Google Scholar] [CrossRef]
- Ricoul, M.; Gnana-Sekaran, T.; Piqueret-Stephan, L.; Sabatier, L. Cytogenetics for biological dosimetry. In Cancer Cytogenetics: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2016; pp. 189–208. [Google Scholar]
- Johnson, R.; Rao, P. Mammalian cell fusion: Induction of premature chromosome condensation in interphase nuclei. Nature 1970, 226, 717. [Google Scholar] [CrossRef]
- Pantelias, G.E.; Maillie, H.D. A simple method for premature chromosome condensation induction in primary human and rodent cells using polyethylene glycol. Somat. Cell Genet. 1983, 9, 533–547. [Google Scholar] [CrossRef]
- Dyban, A.; De Sutter, P.; Verlinsky, Y. Okadaic acid induces premature chromosome condensation reflecting the cell cycle progression in one-cell stage mouse embryos. Mol. Reprod. Dev. 1993, 34, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, E.; Asakawa, Y.; Kosaka, H. Inhibition of protein serine/threonine phosphatases directly induces premature chromosome condensation in mammalian somatic cells. Biomed. Res. 1995, 16, 63–68. [Google Scholar] [CrossRef]
- Hayata, I.; Kanda, R.; Minamihisamatsu, M.; Furukawa, A.; Sasaki, M.S. Cytogenetical dose estimation for 3 severely exposed patients in the JCO criticality accident in Tokai-mura. J. Radiat. Res. 2001, 42, S149–S155. [Google Scholar] [CrossRef]
- Abend, M.; Ostheim, P.; Port, M. Radiation-induced gene expression changes used for biodosimetry and clinical outcome prediction: Challenges and promises. Cytogenet. Genome Res. 2023, 163, 223–230. [Google Scholar] [CrossRef]
- Hladik, D.; Bucher, M.; Endesfelder, D.; Oestreicher, U. The potential of omics in biological dosimetry. Radiation 2022, 2, 78–90. [Google Scholar] [CrossRef]
- Vuong, N.Q.; Khilji, S.; Williams, A.; Adam, N.; Flores, D.; Fulton, K.M.; Baay, I.; Twine, S.M.; Meier, M.J.; Kumarathasan, P. Integration of multi-omics and benchmark dose modeling to support adverse outcome pathways. Int. J. Radiat. Biol. 2025, 101, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Ting Goh, V.S.; Fujishima, Y.; Nakayama, R.; Takebayashi, K.; Yoshida, M.A.; Kasai, K.; Ariyoshi, K.; Miura, T. Manual scoring with shortened 48 h cytokinesis-block micronucleus assay feasible for triage in the event of a mass-casualty radiation accident. Radiat. Res. 2023, 199, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Vral, A.; Fenech, M.; Thierens, H. The micronucleus assay as a biological dosimeter of in vivo ionising radiation exposure. Mutagenesis 2011, 26, 11–17. [Google Scholar] [CrossRef]
- Selvan, G.T.; Venkatachalam, P. Potentials of cytokinesis blocked micronucleus assay in radiation triage and biological dosimetry. J. Genet. Eng. Biotechnol. 2024, 22, 100409. [Google Scholar] [CrossRef]
- International Organization for Standardization. Radiological Protection–Performance Criteria for Laboratories Using Fluorescence In Situ Hybridization (FISH) Translocation Assay for Assessment of Exposure to Ionizing Radiation; ISO Geneva: Geneva, Switzerland, 2019. [Google Scholar]
- Lindholm, C.; Edwards, A. Long-term persistence of translocations in stable lymphocytes from victims of a radiological accident. Int. J. Radiat. Biol. 2004, 80, 559–566. [Google Scholar] [CrossRef]
- Cho, M.S.; Lee, J.K.; Bae, K.S.; Han, E.-A.; Jang, S.J.; Ha, W.-H.; Lee, S.-S.; Barquinero, J.F.; Kim, W.T. Retrospective biodosimetry using translocation frequency in a stable cell of occupationally exposed to ionizing radiation. J. Radiat. Res. 2015, 56, 709–716. [Google Scholar] [CrossRef]
- Oestreicher, U.; Endesfelder, D.; Gomolka, M.; Kesminiene, A.; Lang, P.; Lindholm, C.; Rößler, U.; Samaga, D.; Kulka, U. Automated scoring of dicentric chromosomes differentiates increased radiation sensitivity of young children after low dose CT exposure in vitro. Int. J. Radiat. Biol. 2018, 94, 1017–1026. [Google Scholar] [CrossRef]
- Gnanasekaran, T.S. Cytogenetic biological dosimetry assays: Recent developments and updates. Radiat. Oncol. J. 2021, 39, 159. [Google Scholar] [CrossRef]
- Ramakumar, A.; Subramanian, U.; Prasanna, P.G. High-throughput sample processing and sample management; the functional evolution of classical cytogenetic assay towards automation. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2015, 793, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.; Salma, R.; Balosso, J.; Rezvani, M.; Haghdoost, S. Role of oxidative stress signaling, Nrf2, on survival and stemness of human adipose-derived stem cells exposed to X-rays, protons and carbon ions. Antioxidants 2024, 13, 1035. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.T.; Kim, K.; Norris, A.J.; Vlashi, E.; Phillips, T.M.; Lagadec, C.; Della Donna, L.; Ratikan, J.; Szelag, H.; Hlatky, L. Ionizing radiation activates the Nrf2 antioxidant response. Cancer Res. 2010, 70, 8886–8895. [Google Scholar] [CrossRef]
- Hammad, M.; Raftari, M.; Cesário, R.; Salma, R.; Godoy, P.; Emami, S.N.; Haghdoost, S. Roles of oxidative stress and Nrf2 signaling in pathogenic and non-pathogenic cells: A possible general mechanism of resistance to therapy. Antioxidants 2023, 12, 1371. [Google Scholar] [CrossRef]
- Harris, I.S.; Treloar, A.E.; Inoue, S.; Sasaki, M.; Gorrini, C.; Lee, K.C.; Yung, K.Y.; Brenner, D.; Knobbe-Thomsen, C.B.; Cox, M.A. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer cell 2015, 27, 211–222. [Google Scholar] [CrossRef]
- Shrestha, J.; Limbu, K.R.; Chhetri, R.B.; Paudel, K.R.; Hansbro, P.M.; Oh, Y.S.; Baek, D.J.; Ki, S.-H.; Park, E.-Y. Antioxidant genes in cancer and metabolic diseases: Focusing on Nrf2, Sestrin, and heme oxygenase 1. Int. J. Biol. Sci. 2024, 20, 4888. [Google Scholar] [CrossRef]
- Morgenstern, C.; Lastres-Becker, I.; Demirdöğen, B.C.; Costa, V.M.; Daiber, A.; Foresti, R.; Motterlini, R.; Kalyoncu, S.; Arioz, B.I.; Genc, S. Biomarkers of NRF2 signalling: Current status and future challenges. Redox Biol. 2024, 72, 103134. [Google Scholar] [CrossRef] [PubMed]
- Binkley, M.S.; Jeon, Y.-J.; Nesselbush, M.; Moding, E.J.; Nabet, B.Y.; Almanza, D.; Kunder, C.; Stehr, H.; Yoo, C.H.; Rhee, S. KEAP1/NFE2L2 mutations predict lung cancer radiation resistance that can be targeted by glutaminase inhibition. Cancer Discov. 2020, 10, 1826–1841. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, H.E.; Barber, A.R.; Edwards, R.S.; Tyrrell, W.E.; George, M.E.; Dos Santos, S.N.; Baark, F.; Tanc, M.; Khalil, E.; Falzone, A. Imaging NRF2 activation in non-small cell lung cancer with positron emission tomography. Nat. Commun. 2024, 15, 10484. [Google Scholar] [CrossRef]
- Chen, M.; Carpenter, D.; Leng, J.; Qazi, J.; Wan, Z.; Niedzwiecki, D.; Alder, L.; Clarke, J.; Kirkpatrick, J.; Floyd, S. Impact of KEAP1/NFE2L2 Mutations on Local Recurrence in Patients Receiving Stereotactic Radiosurgery for Non-Small Cell Lung Cancer Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, e223. [Google Scholar] [CrossRef]
- Abdel-Aziz, N.; Haroun, R.A.-H.; Mohamed, H.E. Low-dose gamma radiation modulates liver and testis tissues response to acute whole body irradiation. Dose-Response 2022, 20, 15593258221092365. [Google Scholar] [CrossRef]
- Sangsuwan, T.; Khavari, A.P.; Blomberg, E.; Romell, T.; De, P.R.d.A.V.; Harms-Ringdahl, M.; Haghdoost, S. Oxidative stress levels and dna repair kinetics in senescent primary human fibroblasts exposed to chronic low dose rate of ionizing radiation. Front. Biosci.-Landmark 2023, 28, 296. [Google Scholar] [CrossRef]
- Bradfield, D.T.; Slaven, J.E.; Rittase, W.B.; Rusnak, M.; Symes, A.J.; Brehm, G.V.; Muir, J.M.; Lee, S.-H.; Anderson, J.A.; Day, R.M. Cell death and iron deposition in the liver in two murine models of acute radiation syndrome. PLoS ONE 2025, 20, e0324361. [Google Scholar] [CrossRef] [PubMed]
- Cameron, B.D.; Sekhar, K.R.; Ofori, M.; Freeman, M.L. The role of Nrf2 in the response to normal tissue radiation injury. Radiat. Res. 2018, 190, 99–106. [Google Scholar] [CrossRef]
- Tsukimoto, M.; Tamaishi, N.; Homma, T.; Kojima, S. Low-dose gamma-ray irradiation induces translocation of Nrf2 into nuclear in mouse macrophage RAW264. 7 cells. J. Radiat. Res. 2010, 51, 349–353. [Google Scholar] [CrossRef]
- Rodrigues-Moreira, S.; Moreno, S.G.; Ghinatti, G.; Lewandowski, D.; Hoffschir, F.; Ferri, F.; Gallouet, A.-S.; Gay, D.; Motohashi, H.; Yamamoto, M. Low-dose irradiation promotes persistent oxidative stress and decreases self-renewal in hematopoietic stem cells. Cell Rep. 2017, 20, 3199–3211. [Google Scholar] [CrossRef] [PubMed]
- Fréchard, T.; Bachelot, F.; Ménard, V.; Brizais, C.; Macé, L.; Elie, C.; Cailler Gruet, N.; Teulade, T.; Havet, C.; Voyer, F. Co-exposure to inhaled tungsten particles and low-dose gamma rays: Neurotoxicological outcome in rats. Sci. Rep. 2025, 15, 18307. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, J.-h.; Ryu, S.-H.; Yeom, J.; Ryu, J.-w.; Park, E.-Y.; Choi, K.-C.; Heo, S.-H.; Kim, K.H.; Ha, C.H. Radiation-induced phosphorylation of serine 360 of SMC1 in human peripheral blood mononuclear cells. Radiat. Res. 2019, 191, 262–270. [Google Scholar] [CrossRef]
- Li, S.; Lu, X.; Feng, J.-B.; Tian, M.; Wang, J.; Chen, H.; Chen, D.-Q.; Liu, Q.-J. Developing gender-specific gene expression biodosimetry using a panel of radiation-responsive genes for determining radiation dose in human peripheral blood. Radiat. Res. 2019, 192, 399–409. [Google Scholar] [CrossRef]
- Pantelias, G.E.; Maillie, H.D. The use of peripheral blood mononuclear cell prematurely condensed chromosomes for biological dosimetry. Radiat. Res. 1984, 99, 140–150. [Google Scholar] [CrossRef]
- Schaue, D.; Micewicz, E.D.; Ratikan, J.A.; Iwamoto, K.S.; Vlashi, E.; McDonald, J.T.; McBride, W.H. NRF2 mediates cellular resistance to transformation, radiation, and inflammation in mice. Antioxidants 2022, 11, 1649. [Google Scholar] [CrossRef]
- López, J.S.; Pujol-Canadell, M.; Puig, P.; Ribas, M.; Carrasco, P.; Armengol, G.; Barquinero, J.F. Establishment and validation of surface model for biodosimetry based on γ-H2AX foci detection. Int. J. Radiat. Biol. 2022, 98, 1–10. [Google Scholar] [CrossRef]
- Kubiak, T. Advances in EPR dosimetry in terms of retrospective determination of absorbed dose in radiation accidents. Curr. Top. Biophys. 2018, 41, 11–21. [Google Scholar] [CrossRef]
- Sevan’Kaev, A.; Khvostunov, I.; Lloyd, D.; Voisin, P.; Golub, E.; Nadejina, N.; Nugis, V.; Sidorov, O.; Skvortsov, V. The suitability of FISH chromosome painting and ESR-spectroscopy of tooth enamel assays for retrospective dose reconstruction. J. Radiat. Res. 2006, 47 (Suppl. A), A75–A80. [Google Scholar] [CrossRef]
- Ludovici, G.M.; Cascone, M.G.; Huber, T.; Chierici, A.; Gaudio, P.; de Souza, S.; d’Errico, F.; Malizia, A. Cytogenetic bio-dosimetry techniques in the detection of dicentric chromosomes induced by ionizing radiation: A review. Eur. Phys. J. Plus 2021, 136, 482. [Google Scholar] [CrossRef]
- Ryan, T.L.; Escalona, M.B.; Smith, T.L.; Albanese, J.; Iddins, C.J.; Balajee, A.S. Optimization and validation of automated dicentric chromosome analysis for radiological/nuclear triage applications. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2019, 847, 503087. [Google Scholar] [CrossRef]
- Anderson, D.; Abe, Y.; Ting, V.G.S.; Nakayama, R.; Takebayashi, K.; Thanh, M.T.; Fujishima, Y.; Nakata, A.; Ariyoshi, K.; Kasai, K. Cytogenetic biodosimetry in radiation emergency medicine: 5. The dicentric chromosome and its role in biodosimetry. Radiat. Environ. Med. 2023, 12, 121–139. [Google Scholar]
- World Health Organization. Cytogenetic Dosimetry: Applications in Preparedness for and Response to Radiation Emergencies; International Atomic Energy Agency, Department of Nuclear Safety and Security, Incident and Emergency Centre: Vienna, Austria, 2011. [Google Scholar]
- Goh, V.S.T.; Fujishima, Y.; Abe, Y.; Sakai, A.; Yoshida, M.A.; Ariyoshi, K.; Kasai, K.; Wilkins, R.C.; Blakely, W.F.; Miura, T. Construction of fluorescence in situ hybridization (FISH) translocation dose-response calibration curve with multiple donor data sets using R, based on ISO 20046: 2019 recommendations. Int. J. Radiat. Biol. 2019, 95, 1668–1684. [Google Scholar] [CrossRef] [PubMed]
- Beinke, C.; Port, M.; Riecke, A.; Ruf, C.G.; Abend, M. Adaption of the cytokinesis-block micronucleus cytome assay for improved triage biodosimetry. Radiat. Res. 2016, 185, 461–472. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Beaton-Green, L.A.; Wilkins, R.C. Validation of the cytokinesis-block micronucleus assay using imaging flow cytometry for high throughput radiation biodosimetry. Health Phys. 2016, 110, 29–36. [Google Scholar] [CrossRef]
- Feinendegen, L.E.; Pollycove, M.; Sondhaus, C.A. Responses to low doses of ionizing radiation in biological systems. Nonlinearity Biol. Toxicol. Med. 2004, 2, 15401420490507431. [Google Scholar] [CrossRef]
- Mollaheydar, E. A Multiscale Model of Tumor Growth, Pharmacokinetics and Radiobiology for Radiopharmaceutical Therapy Optimization. Doctoral Dissertation, University of British Columbia, Vancouver, BC, Canada, 2024. [Google Scholar]
- Marampon, F.; Codenotti, S.; Megiorni, F.; Del Fattore, A.; Camero, S.; Gravina, G.L.; Festuccia, C.; Musio, D.; De Felice, F.; Nardone, V. NRF2 orchestrates the redox regulation induced by radiation therapy, sustaining embryonal and alveolar rhabdomyosarcoma cells radioresistance. J. Cancer Res. Clin. Oncol. 2019, 145, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bodas, M.; Wakabayashi, N.; Bunz, F.; Biswal, S. Gain of Nrf2 function in non-small-cell lung cancer cells confers radioresistance. Antioxid. Redox Signal. 2010, 13, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Schmidlin, C.J.; de la Vega, M.R.; Perer, J.; Zhang, D.D.; Wondrak, G.T. Activation of NRF2 by topical apocarotenoid treatment mitigates radiation-induced dermatitis. Redox Biol. 2020, 37, 101714. [Google Scholar] [CrossRef]
- Rana, T.; Schultz, M.A.; Freeman, M.L.; Biswas, S. Loss of Nrf2 accelerates ionizing radiation-induced bone loss by upregulating RANKL. Free. Radic. Biol. Med. 2012, 53, 2298–2307. [Google Scholar] [CrossRef]
- Kim, S.B.; Pandita, R.K.; Eskiocak, U.; Ly, P.; Kaisani, A.; Kumar, R.; Cornelius, C.; Wright, W.E.; Pandita, T.K.; Shay, J.W. Targeting of Nrf2 induces DNA damage signaling and protects colonic epithelial cells from ionizing radiation. Proc. Natl. Acad. Sci. USA 2012, 109, E2949–E2955. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Ji, K.; Liu, Y.; Kong, Y.; Nie, S.; Li, N.; Hao, J.; Xie, Y.; Xu, C. NRF2 preserves genomic integrity by facilitating ATR activation and G2 cell cycle arrest. Nucleic Acids Res. 2020, 48, 9109–9123. [Google Scholar] [CrossRef]
- Rey, N.; Ebrahimian, T.; Gloaguen, C.; Kereselidze, D.; Magneron, V.; Bontemps, C.; Demarquay, C.; Olsson, G.; Haghdoost, S.; Lehoux, S. Exposure to low to moderate doses of ionizing radiation induces a reduction of pro-inflammatory Ly6chigh monocytes and a U-curved response of T cells in ApoE-/-mice. Dose-Response 2021, 19, 15593258211016237. [Google Scholar] [CrossRef]
- Akbari, A.; Jelodar, G.; Nazifi, S.; Afsar, T.; Nasiri, K. Oxidative stress as the underlying biomechanism of detrimental outcomes of ionizing and non-ionizing radiation on human health: Antioxidant protective strategies. Zahedan J. Res. Med. Sci. 2019, 21, e85655. [Google Scholar] [CrossRef]
- Mohammadgholi, M.; Hosseinimehr, S.J. Crosstalk between oxidative stress and inflammation induced by ionizing radiation in healthy and cancerous cells. Curr. Med. Chem. 2024, 31, 2751–2769. [Google Scholar] [CrossRef] [PubMed]
- Akhmedov, A.T.; Marín-García, J. Mitochondrial DNA maintenance: An appraisal. Mol. Cell. Biochem. 2015, 409, 283–305. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, F.; Luo, Y.; Ma, S.; Zhang, N.; Sun, Y.; You, C.; Tang, G.; Li, S.; Gong, Y. Protective role of nuclear factor-erythroid 2-related factor 2 against radiation-induced lung injury and inflammation. Front. Oncol. 2018, 8, 542. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Wang, M.; Zuo, C.-Y.; Mao, M.-X.; Peng, X.-C.; Cai, J. Nrf-2 as a novel target in radiation induced lung injury. Heliyon 2024, 10, e29492. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Guo, L.; Yang, Y.; Wang, Y.; Xia, S.; Gong, H.; Zhang, B.-K.; Yan, M. Dissecting the crosstalk between Nrf2 and NF-κB response pathways in drug-induced toxicity. Front. Cell Dev. Biol. 2022, 9, 809952. [Google Scholar] [CrossRef]
- Godoy, P.R.; Pour Khavari, A.; Rizzo, M.; Sakamoto-Hojo, E.T.; Haghdoost, S. Targeting NRF2, regulator of antioxidant system, to sensitize glioblastoma neurosphere cells to radiation-induced oxidative stress. Oxidative Med. Cell. Longev. 2020, 2020, 2534643. [Google Scholar] [CrossRef]
- Aebisher, D.; Przygórzewska, A.; Bartusik-Aebisher, D. Natural Photosensitizers in Clinical Trials. Appl. Sci. 2024, 14, 8436. [Google Scholar] [CrossRef]
- Luo, J.; Zhi, Q.; Li, D.; Xu, Y.; Zhu, H.; Zhao, L.; Ren, G.; Wang, J.; Liu, N. Low dose radiotherapy combined with immune checkpoint inhibitors induces ferroptosis in lung cancer via the Nrf2/HO-1/GPX4 axis. Front. Immunol. 2025, 16, 1558814. [Google Scholar] [CrossRef] [PubMed]
- Ramisetti, S.V.; Patra, T.; Munirathnam, V.; Sainath, J.V.; Veeraiyan, D.; Namani, A. NRF2 signaling pathway in chemo/radio/immuno-therapy resistance of lung cancer: Looking beyond the tip of the iceberg. Arch. Bronconeumol. 2024, 60, S59–S66. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.; Duennwald, M.L. Nrf2 and oxidative stress: A general overview of mechanisms and implications in human disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, K.R.; Freeman, M.L. Nrf2 promotes survival following exposure to ionizing radiation. Free. Radic. Biol. Med. 2015, 88, 268–274. [Google Scholar] [CrossRef]
- Sun, X.; Dong, M.; Li, J.; Sun, Y.; Gao, Y.; Wang, Y.; Du, L.; Liu, Y.; Ji, K.; He, N. NRF2 promotes radiation resistance by cooperating with TOPBP1 to activate the ATR-CHK1 signaling pathway. Theranostics 2024, 14, 681. [Google Scholar] [CrossRef]
- Haghdoost, S.; Hammad, M.; Balosso, J. The Role of Nrf2 Signaling in Surival and Differentiation of Primary Stem Cells Exposed to Particle Radiation. Int. J. Part. Ther. 2024, 12, 100215. [Google Scholar] [CrossRef]
- Yagishita, Y.; Gatbonton-Schwager, T.N.; McCallum, M.L.; Kensler, T.W. Current landscape of NRF2 biomarkers in clinical trials. Antioxidants 2020, 9, 716. [Google Scholar] [CrossRef]
- Rooney, J.P.; Chorley, B.; Hiemstra, S.; Wink, S.; Wang, X.; Bell, D.A.; van de Water, B.; Corton, J.C. Mining a human transcriptome database for chemical modulators of NRF2. PLoS ONE 2020, 15, e0239367. [Google Scholar] [CrossRef]
- Helou, D.G.; Martin, S.F.; Pallardy, M.; Chollet-Martin, S.; Kerdine-Römer, S. Nrf2 involvement in chemical-induced skin innate immunity. Front. Immunol. 2019, 10, 1004. [Google Scholar] [CrossRef]
- Pekovic-Vaughan, V.; Gibbs, J.; Yoshitane, H.; Yang, N.; Pathiranage, D.; Guo, B.; Sagami, A.; Taguchi, K.; Bechtold, D.; Loudon, A. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 2014, 28, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zeng, C.; Du, L.; Dong, C. Mechanism of circadian regulation of the NRF2/ARE pathway in renal ischemia-reperfusion. Exp. Ther. Med. 2021, 21, 190. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.R.; Donepudi, A.C.; Xu, J.; Wei, W.; Cheng, Q.C.; Driscoll, M.V.; Johnson, D.A.; Johnson, J.A.; Li, X.; Slitt, A.L. Fasting induces nuclear factor E2-related factor 2 and ATP-binding Cassette transporters via protein kinase A and Sirtuin-1 in mouse and human. Antioxid. Redox Signal. 2014, 20, 15–30. [Google Scholar] [CrossRef]
- Lettieri-Barbato, D.; Minopoli, G.; Caggiano, R.; Izzo, R.; Santillo, M.; Aquilano, K.; Faraonio, R. Fasting drives Nrf2-related antioxidant response in skeletal muscle. Int. J. Mol. Sci. 2020, 21, 7780. [Google Scholar] [CrossRef]
- Clifford, T.; Acton, J.P.; Cocksedge, S.P.; Davies, K.A.B.; Bailey, S.J. The effect of dietary phytochemicals on nuclear factor erythroid 2-related factor 2 (Nrf2) activation: A systematic review of human intervention trials. Mol. Biol. Rep. 2021, 48, 1745–1761. [Google Scholar] [CrossRef]
- Wu, X.; Wei, J.; Yi, Y.; Gong, Q.; Gao, J. Activation of Nrf2 signaling: A key molecular mechanism of protection against cardiovascular diseases by natural products. Front. Pharmacol. 2022, 13, 1057918. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef]
- Rothkamm, K.; Löbrich, M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc. Natl. Acad. Sci. USA 2003, 100, 5057–5062. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Pernot, E.; Hall, J.; Baatout, S.; Benotmane, M.A.; Blanchardon, E.; Bouffler, S.; El Saghire, H.; Gomolka, M.; Guertler, A.; Harms-Ringdahl, M. Ionizing radiation biomarkers for potential use in epidemiological studies. Mutat. Res./Rev. Mutat. Res. 2012, 751, 258–286. [Google Scholar] [CrossRef]
- Hall, J.; Jeggo, P.A.; West, C.; Gomolka, M.; Quintens, R.; Badie, C.; Laurent, O.; Aerts, A.; Anastasov, N.; Azimzadeh, O. Ionizing radiation biomarkers in epidemiological studies–An update. Mutat. Res./Rev. Mutat. Res. 2017, 771, 59–84. [Google Scholar] [CrossRef]
- Liu, T.; Lv, Y.-F.; Zhao, J.-L.; You, Q.-D.; Jiang, Z.-Y. Regulation of Nrf2 by phosphorylation: Consequences for biological function and therapeutic implications. Free. Radic. Biol. Med. 2021, 168, 129–141. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, Z.; Zhang, D.D. Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PLoS ONE 2009, 4, e6588. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Cao, J.; Wu, C.; Tang, L.; Bian, W.; Chen, Y.; Yu, L.; Wu, Y.; Li, S. Targeting epigenetic and post-translational modifications of NRF2: Key regulatory factors in disease treatment. Cell Death Discov. 2025, 11, 189. [Google Scholar] [CrossRef]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2: INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef] [PubMed]

| Advantages | Disadvantages |
|---|---|
| Reflects biological impact (Measures actual damage or cellular response, not just exposure) | Time-consuming (Traditional assays (like DCA) may take days to a week) |
| Useful when physical dosimeters are absent (Critical for accidents, triage, and emergencies) | Requires lab infrastructure (Needs specialized equipment and trained personnel) |
| Retrospective assessment (Some methods, e.g., ESR on teeth, allow dose estimation long after exposure) | Variability (Results may vary based on age, sex, health, and genetic background) |
| Applicable across radiation types (Works for gamma, X-rays, neutrons, and mixed fields) | Limited sensitivity window and unscheduled exposure (Some biomarkers are transient and require fast sampling post-exposure) |
| Multiple biomarkers available (Allows customization (cytogenetic, molecular, biochemical) depending on timeframe and context) | Low throughput (Many conventional methods are not scalable for mass casualty events) |
| Useful for internal and organ dosimetry | Not always dose-specific (Some markers may be non-specific to radiation) (e.g., NRF2 also responds to oxidative stress from non-radiation sources) |
| Genes | Function | Antioxidant Role |
|---|---|---|
| GCLC | GSH synthesis (catalytic) | Maintains redox balance |
| GCLM | GSH synthesis (modifier) | Enhances GCLC activity |
| HMOX1 | Heme degradation | Anti-inflammatory, cytoprotective |
| NQO1 | Detoxifies quinones | Prevents ROS generation |
| SRXN1 | Restores peroxiredoxins | Supports ROS clearance |
| TXNRD1 | Reduces thioredoxin | Supports DNA synthesis, detox |
| Radiation Source and Dose | Type of Study | Tissue or Organ | Findings | References |
|---|---|---|---|---|
| Gamma ray (40 mGy–4 Gy) | In vivo | Liver and testis | Exposure to 40 mGy before 4 Gy induced a significant increase in the levels of NRF2, NRF2 mRNA | [58] |
| 60Co (7.9 Gy and 6.85 Gy) | In vivo | Liver | Increased ferritin, HO-1, and inflammatory cytokine | [60] |
| Tungsten aerosol (80 mg/m3) plus low-dose radiation of gamma ray (50 mGy) | In vivo | Brain | NRF2 and pro-inflammatory cytokines (IL-1β and TNF-α) | [64] |
| Gamma ray (0.1–0.3 Gy) | In vivo | Blood (Mouse macrophage RAW264.7 cells) | NRF2, HMOX1, Ferritin heavy chain (Fth1), Nqo1, GCLC/M, Gsr, and Txnrd1 | [62] |
| X-ray (0.1–5 Gy) | In vivo | Peripheral lymphocytes | Parkin, NRF2, and DNA damage | [19] |
| Gamma ray (6 Gy) | In vivo | Bone marrow | Upregulation in antioxidant enzymes: NRF2, CAT (catalase), SOD1, and HO-1 | [25] |
| Gamma ray (0 to 2 Gy) | In vivo | Hematopoietic stem cells (HSCs) | NRF2 | [63] |
| X-rays and γ-rays (variable laboratory doses; typically 0.5–5 Gy) | In vitro | Peripheral blood mononuclear cells (PBMCs) | Demonstrated radiation-induced phosphorylation of Serine 360 of SMC1, establishing it as a sensitive molecular marker for radiation exposure. | [65] |
| X-rays (0.5–6 Gy range) | In vitro (ex vivo human PBMCs) | PBMCs | Specific Genes (*) (CDKN1A, BAX, MDM2, XPC, PCNA, FDXR, GDF-15, DDB2, TNFRSF10B, PHPT1, ASTN2, RPS27L, BBC3, TNFSF4, POLH, CCNG1, PPM1D and GADD45A) | [66] |
| γ-rays (0.5–8 Gy) | In vitro/translational | PBMCs | Introduced the prematurely condensed chromosome (PCC) assay in PBMCs | [67] |
| Type of Study | Cell Line/Animal | Radiation Dose | NRF2 Assessment Method | Outcomes | References |
|---|---|---|---|---|---|
| In vitro, in vivo | Human keratinocytes, SKH1 mice | 4 and 30 Gy | NRF2knockdown/activation (bixin), glutathione levels, DNA damage/oxidative stress markers | Radiation-induced dermatitis, DNA damage, oxidative stress, cell viability | [83] |
| In vitro, in vivo | Primary osteoblasts, C57BL/6J mice | 20 Gy | NRF2 knockout, ROS, glutathione (GSH), receptor activator of nuclear factor kappa-Β ligand (RANKL) | Bone loss, osteoblast mineralization, oxidative stress | [84] |
| In vitro, in vivo | MCF7, C57BL/6 mice | 2–8 Gyvia single/fractionated, whole-body | Antioxidant response element (ARE)-dependent transcription, NRF2-deficient vs. wild-type, HO-1 | Implicates NRF2 in modulating radiation-induced oxidative stress (with downstream implications for inflammatory responses). | [50] |
| In vitro | NSCLC, mouse embryonic fibroblasts | 0–20 Gy | NRF2 knockdown/overexpression, ROS, antioxidant gene expression | Radioresistance, ROS, cell survival, protein carbonyls | [82] |
| In vitro | Human rhabdomyosarcoma cell lines | >2 Gy | NRF2 gene expression, silencing, γ-H2AX | Clonogenic survival, ROS, DNA damage, antioxidant response | [81] |
| In vivo | C57BL/6NCrSlc mice | 0.1–5 Gywhole-body | NRF2 immunostaining, parkin, γ-H2AX | Oxidative stress biomarkers, DNA damage, dosimetry | [19] |
| In vivo | C57BL/6 mice | 0.5–3 Gy, whole-body | NRF2 target gene (ferritin heavy chain 1 (Fth1), Gsr mRNA expression | Dose–response of NRF2 target genes, biological damage | [23] |
| In vitro, in vivo | Mouse embryonic fibroblasts, C57BL/6 mice | 7–8.2 Gy whole-body (mice), 2–8 Gy targeted (cells) | NRF2 knockout, gene expression, ROS, γ-H2AX, immune markers | Transformation, inflammation, radioresistance, immune response | [68] |
| In vitro, in vivo | Human colonic epithelial cells, wild-type 129/Sv mice | 7.5–10 Gy whole-body | NRF2 activation (bardoxolone methyl (BARD)), ARE binding, HO-1, p53-binding protein 1 (53BP1), DNA repair foci | DNA damage signaling, cell survival, radioprotection | [85] |
| In vitro | A549 cell line | 8 Gy | NRF2 knockout/inhibition, protein localization, ataxia telangiectasia and Rad3-related/checkpoint kinase 1/cell division cycle 2 (ATR/CHK1/CDC2) pathway | ATR activation, G2 arrest, DNA repair, radiosensitivity | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moloudi, K.; Sangsuwan, T.; Monzen, S.; Fujishima, Y.; Anderson, D.; Frey, B.; Miura, T.; Azariasl, S.; Yasuda, H.; Haghdoost, S. Nuclear Factor Erythroid 2-Related Factor 2 (NRF2) as a Biomarker for Radiation Dosimetry and Health Risk Assessment: A Review. Antioxidants 2025, 14, 1393. https://doi.org/10.3390/antiox14121393
Moloudi K, Sangsuwan T, Monzen S, Fujishima Y, Anderson D, Frey B, Miura T, Azariasl S, Yasuda H, Haghdoost S. Nuclear Factor Erythroid 2-Related Factor 2 (NRF2) as a Biomarker for Radiation Dosimetry and Health Risk Assessment: A Review. Antioxidants. 2025; 14(12):1393. https://doi.org/10.3390/antiox14121393
Chicago/Turabian StyleMoloudi, Kave, Traimate Sangsuwan, Satoru Monzen, Yohei Fujishima, Donovan Anderson, Benjamin Frey, Tomisato Miura, Samayeh Azariasl, Hiroshi Yasuda, and Siamak Haghdoost. 2025. "Nuclear Factor Erythroid 2-Related Factor 2 (NRF2) as a Biomarker for Radiation Dosimetry and Health Risk Assessment: A Review" Antioxidants 14, no. 12: 1393. https://doi.org/10.3390/antiox14121393
APA StyleMoloudi, K., Sangsuwan, T., Monzen, S., Fujishima, Y., Anderson, D., Frey, B., Miura, T., Azariasl, S., Yasuda, H., & Haghdoost, S. (2025). Nuclear Factor Erythroid 2-Related Factor 2 (NRF2) as a Biomarker for Radiation Dosimetry and Health Risk Assessment: A Review. Antioxidants, 14(12), 1393. https://doi.org/10.3390/antiox14121393







