SHP2: A Redox-Sensitive Regulator Linking Immune Checkpoint Inhibitor Therapy to Cancer Treatment and Vascular Risk
Abstract
1. Introduction
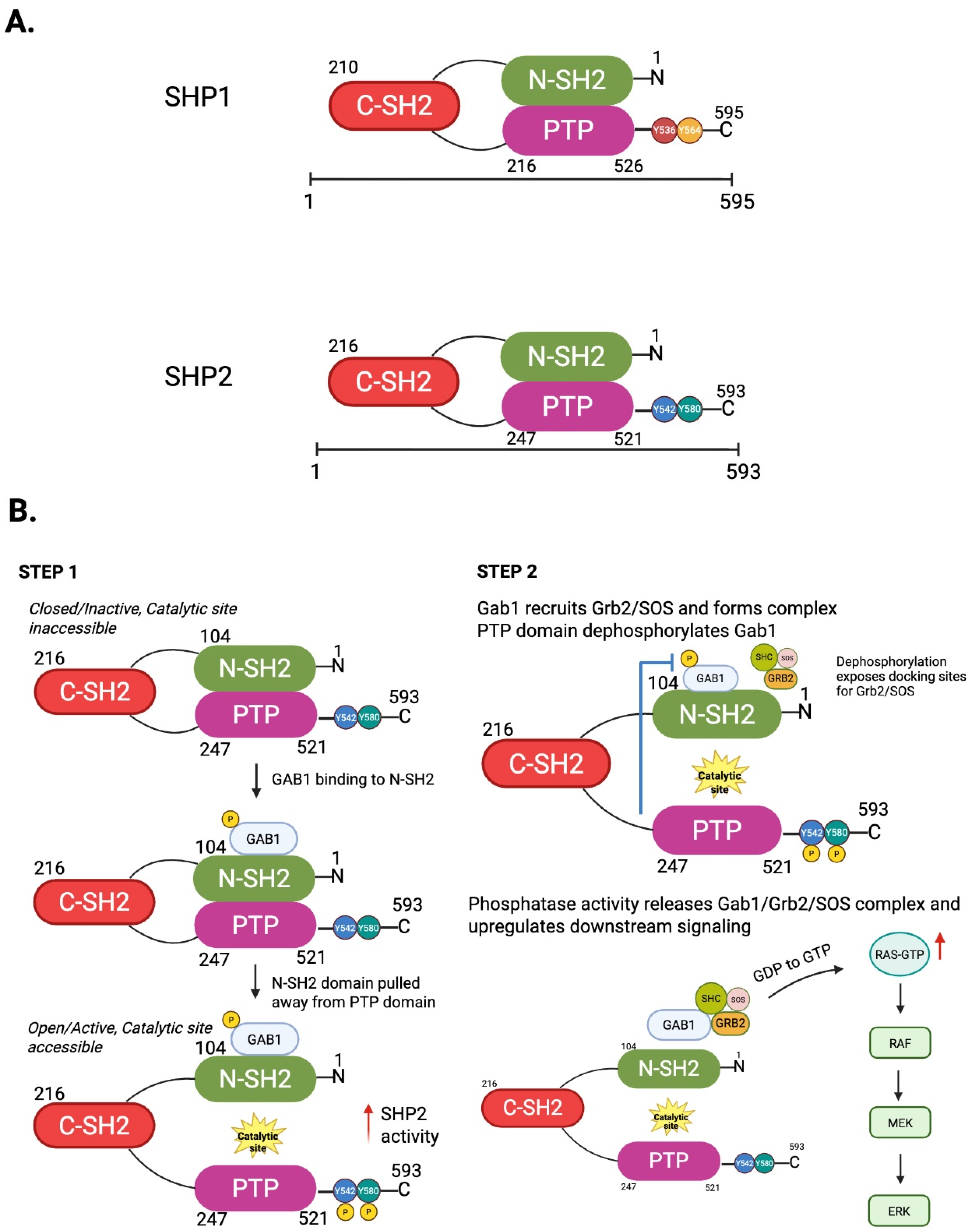
2. Structural and Functional Characteristics of SHP2: Mechanisms of Activation and Regulation
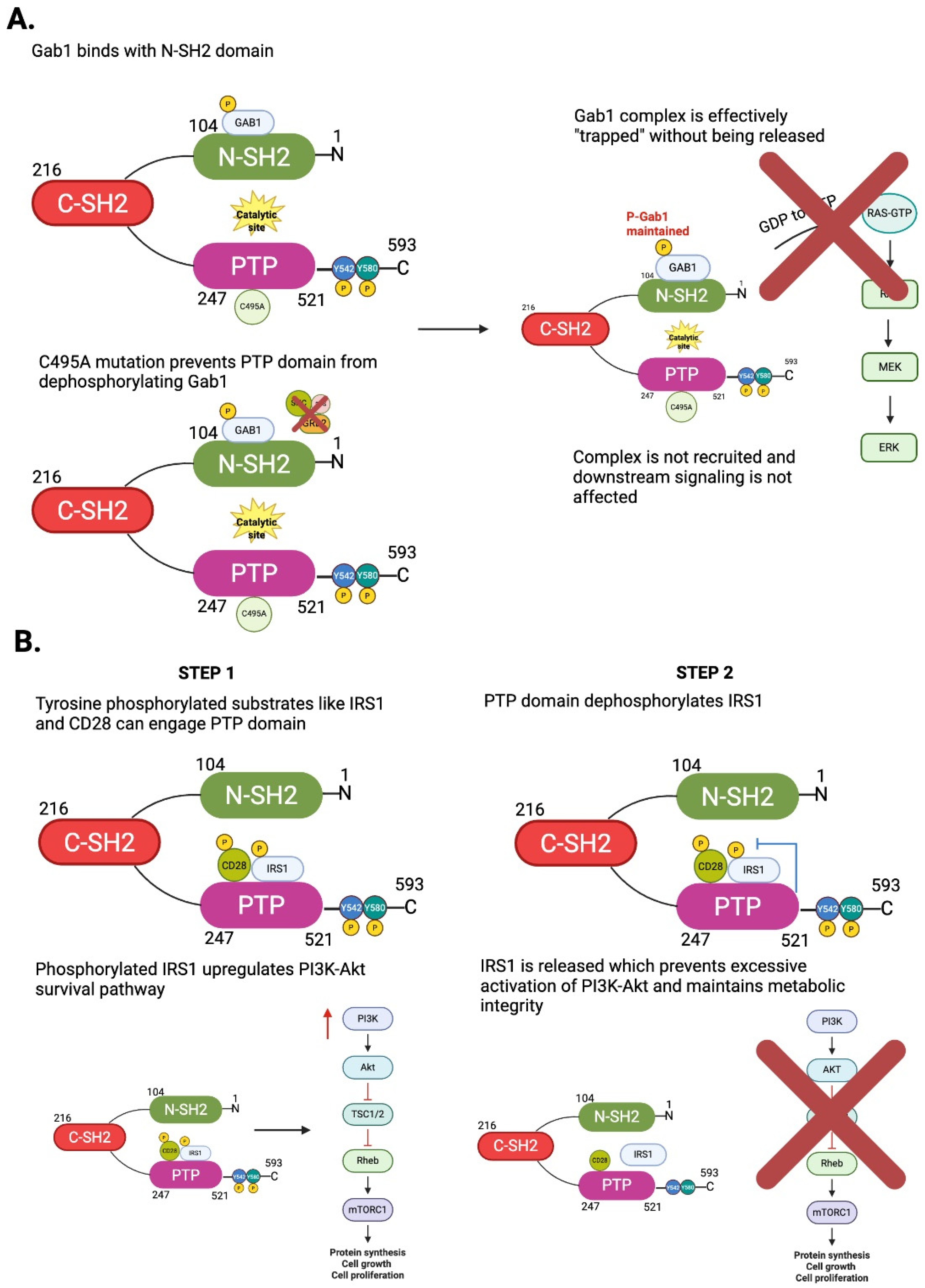
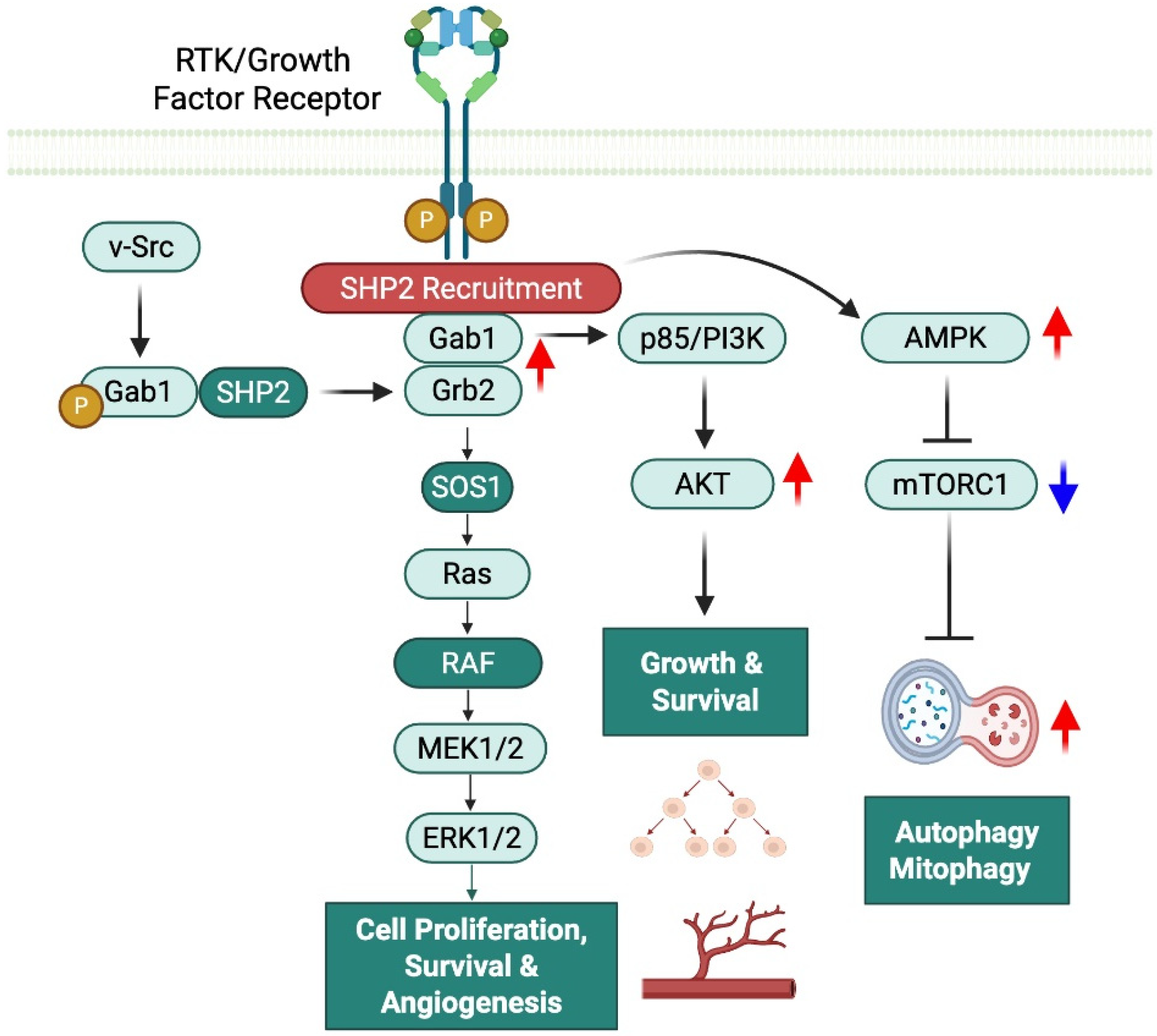
2.1. SHP2 as a Phosphatase and Scaffold Protein: Context-Dependent Regulatory Effects
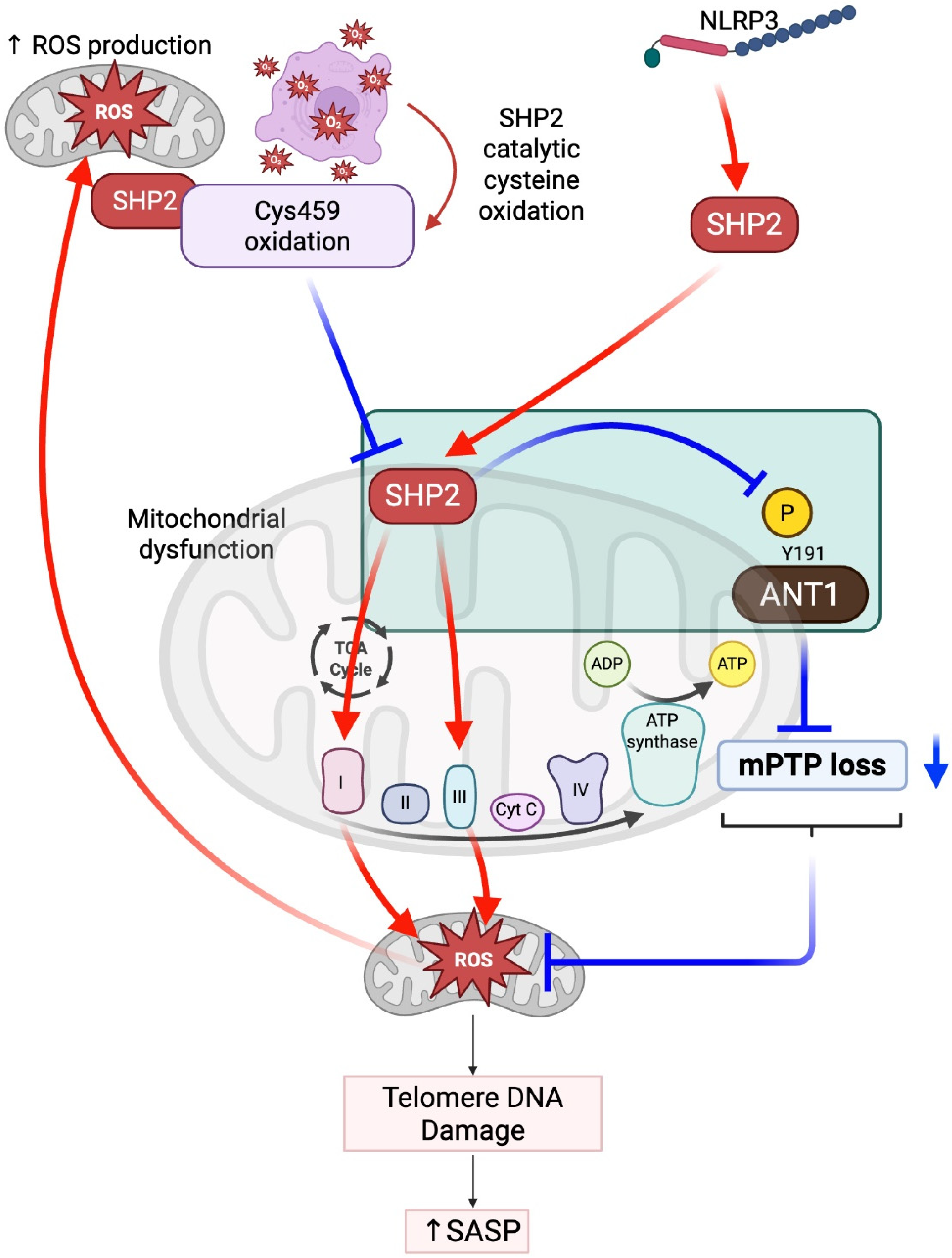
2.2. SHP2 in Mitochondria: Balancing ROS, Inflammation, and Senescence
3. SHP2 in Cancer and Cancer Treatments
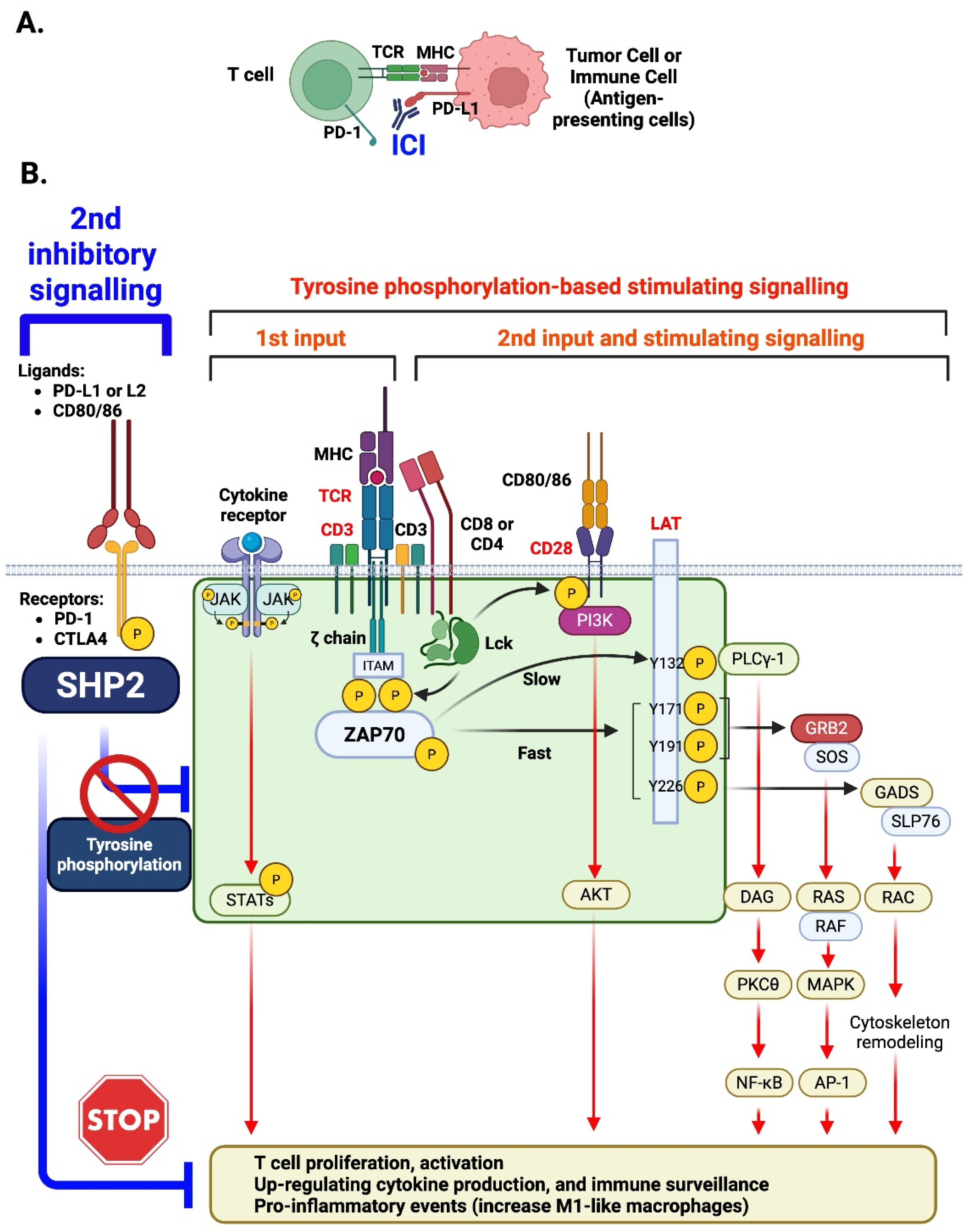
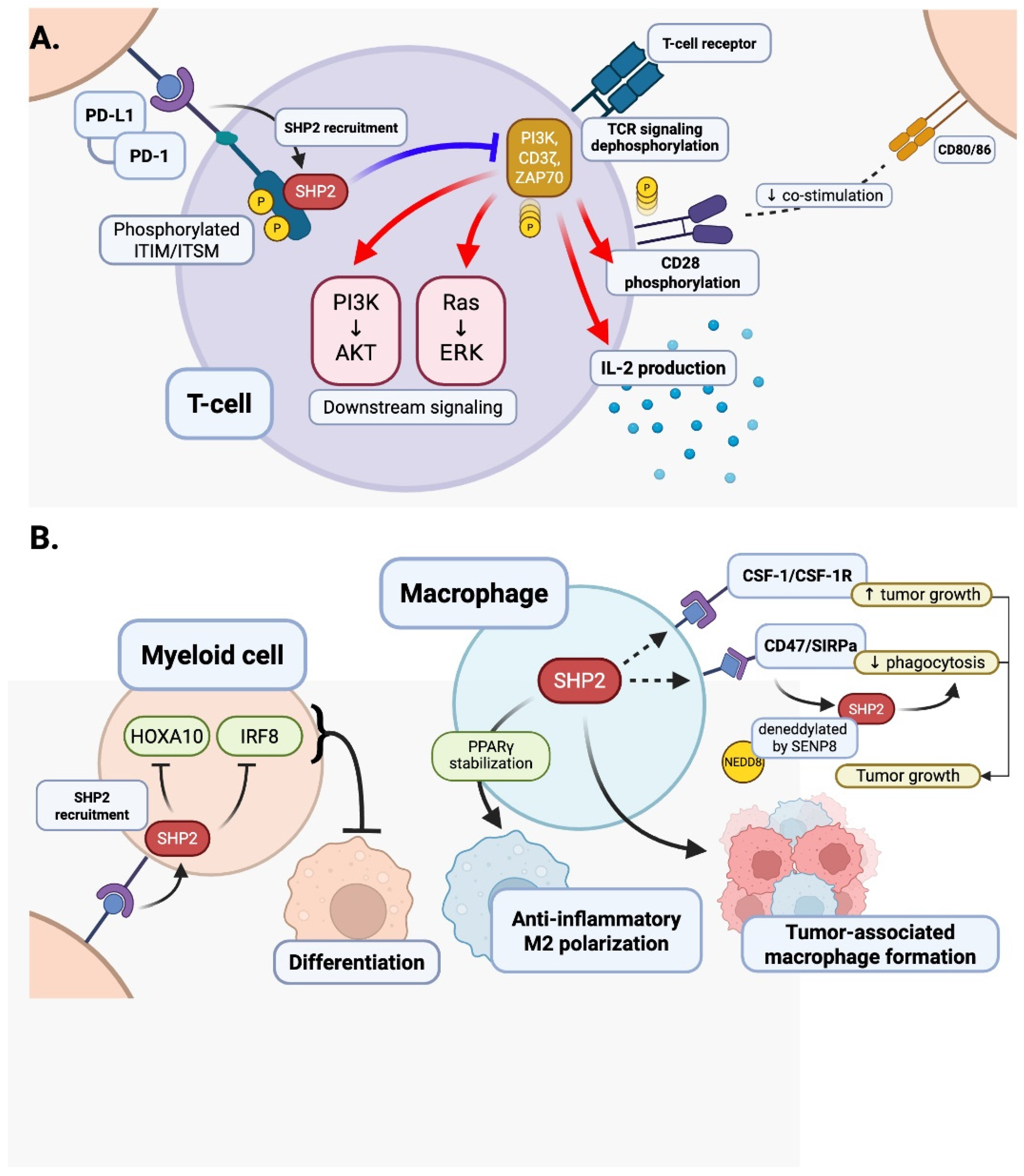
4. The Potential Roles of SHP2 in T Cells, Myeloid Cells, and Endothelial Cells, Leading to ICI-Induced CVD: Insights from Animal Models
4.1. T Cells
4.2. Myeloid Cells
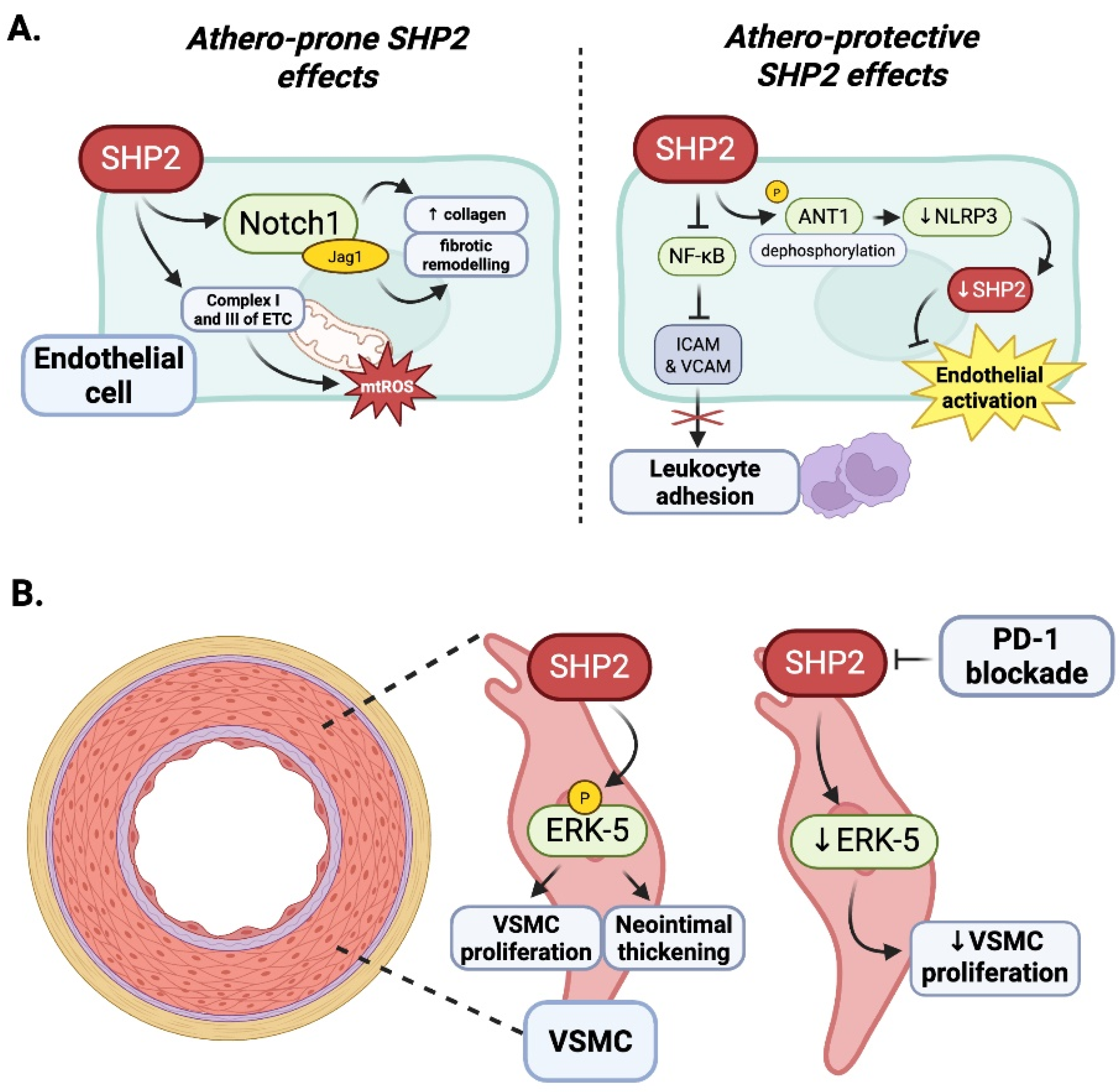
4.3. Contextual Duality of SHP2 in Endothelial Inflammation: The Influence of Oxidative Stress
4.4. Vascular Smooth Muscle Cells
4.5. Summary: Context-Dependent Roles of SHP2 in Atherosclerosis and ICI-Associated Vascular Diseases
5. Clinical Relevance and Therapeutic Implications
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, J.; Li, W.; Zhang, P.; Guo, F.; Liu, M. Current trends in sensitizing immune checkpoint inhibitors for cancer treatment. Mol. Cancer 2024, 23, 279. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Jo, W.; Won, T.; Daoud, A.; Cihakova, D. Immune checkpoint inhibitors associated cardiovascular immune-related adverse events. Front. Immunol. 2024, 15, 1340373. [Google Scholar] [CrossRef] [PubMed]
- Waheed, N.; Fradley, M.G.; DeRemer, D.L.; Mahmoud, A.; Shah, C.P.; Langaee, T.Y.; Lipori, G.P.; March, K.; Pepine, C.J.; Cooper-DeHoff, R.M.; et al. Newly diagnosed cardiovascular disease in patients treated with immune checkpoint inhibitors: A retrospective analysis of patients at an academic tertiary care center. Cardiooncology 2021, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Christofides, A.; Katopodi, X.L.; Cao, C.; Karagkouni, D.; Aliazis, K.; Yenyuwadee, S.; Aksoylar, H.I.; Pal, R.; Mahmoud, M.A.A.; Strauss, L.; et al. SHP-2 and PD-1-SHP-2 signaling regulate myeloid cell differentiation and antitumor responses. Nat. Immunol. 2023, 24, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Niogret, C.; Birchmeier, W.; Guarda, G. SHP-2 in Lymphocytes’ Cytokine and Inhibitory Receptor Signaling. Front. Immunol. 2019, 10, 2468. [Google Scholar] [CrossRef]
- Xu, F.; Xu, M.J.; Zhao, R.; Guerrah, A.; Zeng, F.; Zhao, Z.J. Tyrosine phosphatases SHP-1 and SHP-2 are associated with distinct tyrosine-phosphorylated proteins. Exp. Cell Res. 2002, 272, 75–83. [Google Scholar] [CrossRef]
- Tsui, H.W.; Hasselblatt, K.; Martin, A.; Mok, S.C.; Tsui, F.W. Molecular mechanisms underlying SHP-1 gene expression. Eur. J. Biochem. 2002, 269, 3057–3064. [Google Scholar] [CrossRef]
- Neel, B.G.; Gu, H.; Pao, L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 2003, 28, 284–293. [Google Scholar] [CrossRef]
- Sodir, N.M.; Pathria, G.; Adamkewicz, J.I.; Kelley, E.H.; Sudhamsu, J.; Merchant, M.; Chiarle, R.; Maddalo, D. SHP2: A Pleiotropic Target at the Interface of Cancer and Its Microenvironment. Cancer Discov. 2023, 13, 2339–2355. [Google Scholar] [CrossRef]
- Tojjari, A.; Saeed, A.; Sadeghipour, A.; Kurzrock, R.; Cavalcante, L. Overcoming Immune Checkpoint Therapy Resistance with SHP2 Inhibition in Cancer and Immune Cells: A Review of the Literature and Novel Combinatorial Approaches. Cancers 2023, 15, 5384. [Google Scholar] [CrossRef]
- Huang, Q.; Lerner-Marmarosh, N.; Che, W.; Ohta, S.; Osawa, M.; Yoshizumi, M.; Glassman, M.; Yan, C.; Berk, B.C.; Abe, J. The novel role of the C-terminal region of SHP-2. Involvement of Gab1 and SHP-2 phosphatase activity in Elk-1 activation. J. Biol. Chem. 2002, 277, 29330–29341. [Google Scholar] [CrossRef]
- Asmamaw, M.D.; Shi, X.J.; Zhang, L.R.; Liu, H.M. A comprehensive review of SHP2 and its role in cancer. Cell Oncol. 2022, 45, 729–753. [Google Scholar] [CrossRef] [PubMed]
- Dance, M.; Montagner, A.; Salles, J.P.; Yart, A.; Raynal, P. The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK1/2) pathway. Cell Signal. 2008, 20, 453–459. [Google Scholar] [CrossRef]
- Zeke, A.; Takacs, T.; Sok, P.; Nemeth, K.; Kirsch, K.; Egri, P.; Poti, A.L.; Bento, I.; Tusnady, G.E.; Remenyi, A. Structural insights into the pSer/pThr dependent regulation of the SHP2 tyrosine phosphatase in insulin and CD28 signaling. Nat. Commun. 2022, 13, 5439. [Google Scholar] [CrossRef]
- Myers, M.G., Jr.; Mendez, R.; Shi, P.; Pierce, J.H.; Rhoads, R.; White, M.F. The COOH-terminal tyrosine phosphorylation sites on IRS-1 bind SHP-2 and negatively regulate insulin signaling. J. Biol. Chem. 1998, 273, 26908–26914. [Google Scholar] [CrossRef]
- Liu, Q.; Qu, J.; Zhao, M.; Xu, Q.; Sun, Y. Targeting SHP2 as a promising strategy for cancer immunotherapy. Pharmacol. Res. 2020, 152, 104595. [Google Scholar] [CrossRef] [PubMed]
- Gavrieli, M.; Watanabe, N.; Loftin, S.K.; Murphy, T.L.; Murphy, K.M. Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of B and T lymphocyte attenuator required for association with protein tyrosine phosphatases SHP-1 and SHP-2. Biochem. Biophys. Res. Commun. 2003, 312, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Tajan, M.; de Rocca Serra, A.; Valet, P.; Edouard, T.; Yart, A. SHP2 sails from physiology to pathology. Eur. J. Med. Genet. 2015, 58, 509–525. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Niu, R. Functions of Shp2 in cancer. J. Cell. Mol. Med. 2015, 19, 2075–2083. [Google Scholar] [CrossRef]
- Zito, C.I.; Qin, H.; Blenis, J.; Bennett, A.M. SHP-2 regulates cell growth by controlling the mTOR/S6 kinase 1 pathway. J. Biol. Chem. 2007, 282, 6946–6953. [Google Scholar] [CrossRef] [PubMed]
- Kimura, N.; Tokunaga, C.; Dalal, S.; Richardson, C.; Yoshino, K.; Hara, K.; Kemp, B.E.; Witters, L.A.; Mimura, O.; Yonezawa, K. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells 2003, 8, 65–79. [Google Scholar] [CrossRef]
- Guo, W.; Xu, Q. Phosphatase-independent functions of SHP2 and its regulation by small molecule compounds. J. Pharmacol. Sci. 2020, 144, 139–146. [Google Scholar] [CrossRef]
- Noguchi, T.; Matozaki, T.; Horita, K.; Fujioka, Y.; Kasuga, M. Role of SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in insulin-stimulated Ras activation. Mol. Cell. Biol. 1994, 14, 6674–6682. [Google Scholar] [CrossRef]
- Kandadi, M.R.; Stratton, M.S.; Ren, J. The role of Src homology 2 containing protein tyrosine phosphatase 2 in vascular smooth muscle cell migration and proliferation. Acta Pharmacol. Sin. 2010, 31, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.K. The SHP-2 tyrosine phosphatase: Signaling mechanisms and biological functions. Cell Res. 2000, 10, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Tidyman, W.E.; Rauen, K.A. The RASopathies: Developmental syndromes of Ras/MAPK pathway dysregulation. Curr. Opin. Genet. Dev. 2009, 19, 230–236. [Google Scholar] [CrossRef]
- Yu, Z.H.; Zhang, R.Y.; Walls, C.D.; Chen, L.; Zhang, S.; Wu, L.; Liu, S.; Zhang, Z.Y. Molecular basis of gain-of-function LEOPARD syndrome-associated SHP2 mutations. Biochemistry 2014, 53, 4136–4151. [Google Scholar] [CrossRef]
- Yu, Z.H.; Xu, J.; Walls, C.D.; Chen, L.; Zhang, S.; Zhang, R.; Wu, L.; Wang, L.; Liu, S.; Zhang, Z.Y. Structural and mechanistic insights into LEOPARD syndrome-associated SHP2 mutations. J. Biol. Chem. 2013, 288, 10472–10482. [Google Scholar] [CrossRef]
- Wu, C.J.; O’Rourke, D.M.; Feng, G.S.; Johnson, G.R.; Wang, Q.; Greene, M.I. The tyrosine phosphatase SHP-2 is required for mediating phosphatidylinositol 3-kinase/Akt activation by growth factors. Oncogene 2001, 20, 6018–6025. [Google Scholar] [CrossRef]
- Ivins Zito, C.; Kontaridis, M.I.; Fornaro, M.; Feng, G.S.; Bennett, A.M. SHP-2 regulates the phosphatidylinositide 3’-kinase/Akt pathway and suppresses caspase 3-mediated apoptosis. J. Cell Physiol. 2004, 199, 227–236. [Google Scholar] [CrossRef]
- Wei, X.; Zheng, L.; Tian, Y.; Wang, H.; Su, Y.; Feng, G.; Wang, C.; Lu, Z. Tyrosine phosphatase SHP2 in ovarian granulosa cells balances follicular development by inhibiting PI3K/AKT signaling. J. Mol. Cell Biol. 2022, 14, mjac048. [Google Scholar] [CrossRef]
- Kan, C.; Tan, Z.; Liu, L.; Liu, B.; Zhan, L.; Zhu, J.; Li, X.; Lin, K.; Liu, J.; Liu, Y.; et al. Phase separation of SHP2E76K promotes malignant transformation of mesenchymal stem cells by activating mitochondrial complexes. JCI Insight 2024, 9, e170340. [Google Scholar] [CrossRef] [PubMed]
- Salvi, M.; Stringaro, A.; Brunati, A.M.; Agostinelli, E.; Arancia, G.; Clari, G.; Toninello, A. Tyrosine phosphatase activity in mitochondria: Presence of Shp-2 phosphatase in mitochondria. Cell Mol. Life Sci. 2004, 61, 2393–2404. [Google Scholar] [CrossRef]
- Guo, W.; Liu, W.; Chen, Z.; Gu, Y.; Peng, S.; Shen, L.; Shen, Y.; Wang, X.; Feng, G.S.; Sun, Y.; et al. Tyrosine phosphatase SHP2 negatively regulates NLRP3 inflammasome activation via ANT1-dependent mitochondrial homeostasis. Nat. Commun. 2017, 8, 2168. [Google Scholar] [CrossRef]
- Kuilman, T.; Michaloglou, C.; Vredeveld, L.C.; Douma, S.; van Doorn, R.; Desmet, C.J.; Aarden, L.A.; Mooi, W.J.; Peeper, D.S. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008, 133, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Wiley, C.D.; Campisi, J. From Ancient Pathways to Aging Cells-Connecting Metabolism and Cellular Senescence. Cell Metab. 2016, 23, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Baker, D.J.; Wijshake, T.; Conover, C.A.; Campisi, J.; van Deursen, J.M. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 2016, 354, 472–477. [Google Scholar] [CrossRef]
- Wang, J.; Uryga, A.K.; Reinhold, J.; Figg, N.; Baker, L.; Finigan, A.; Gray, K.; Kumar, S.; Clarke, M.; Bennett, M. Vascular Smooth Muscle Cell Senescence Promotes Atherosclerosis and Features of Plaque Vulnerability. Circulation 2015, 132, 1909–1919. [Google Scholar] [CrossRef]
- Salminen, A. Inhibitory immune checkpoints suppress the surveillance of senescent cells promoting their accumulation with aging and in age-related diseases. Biogerontology 2024, 25, 749–773. [Google Scholar] [CrossRef]
- Wang, Y.; Mohseni, M.; Grauel, A.; Diez, J.E.; Guan, W.; Liang, S.; Choi, J.E.; Pu, M.; Chen, D.; Laszewski, T.; et al. SHP2 blockade enhances anti-tumor immunity via tumor cell intrinsic and extrinsic mechanisms. Sci. Rep. 2021, 11, 1399. [Google Scholar] [CrossRef]
- Lan, L.; Holland, J.D.; Qi, J.; Grosskopf, S.; Rademann, J.; Vogel, R.; Gyorffy, B.; Wulf-Goldenberg, A.; Birchmeier, W. Shp2 signaling suppresses senescence in PyMT-induced mammary gland cancer in mice. EMBO J. 2015, 34, 1493–1508. [Google Scholar] [CrossRef]
- Lauriol, J.; Jaffre, F.; Kontaridis, M.I. The role of the protein tyrosine phosphatase SHP2 in cardiac development and disease. Semin. Cell Dev. Biol. 2015, 37, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Bentires-Alj, M.; Paez, J.G.; David, F.S.; Keilhack, H.; Halmos, B.; Naoki, K.; Maris, J.M.; Richardson, A.; Bardelli, A.; Sugarbaker, D.J.; et al. Activating mutations of the noonan syndrome-associated SHP2/PTPN11 gene in human solid tumors and adult acute myelogenous leukemia. Cancer Res. 2004, 64, 8816–8820. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zheng, H.; Yu, W.M.; Qu, C.K. Activating mutations in protein tyrosine phosphatase Ptpn11 (Shp2) enhance reactive oxygen species production that contributes to myeloproliferative disorder. PLoS ONE 2013, 8, e63152. [Google Scholar] [CrossRef] [PubMed]
- De Rocca Serra-Nedelec, A.; Edouard, T.; Treguer, K.; Tajan, M.; Araki, T.; Dance, M.; Mus, M.; Montagner, A.; Tauber, M.; Salles, J.P.; et al. Noonan syndrome-causing SHP2 mutants inhibit insulin-like growth factor 1 release via growth hormone-induced ERK hyperactivation, which contributes to short stature. Proc. Natl. Acad. Sci. USA 2012, 109, 4257–4262. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, V.E.; Luetteke, N.; Ren, Y.; Berns, H.; Chen, L.; Foroutan, P.; Martinez, G.V.; Haura, E.B.; Chen, J.; Coppola, D.; et al. SHP2E76K mutant promotes lung tumorigenesis in transgenic mice. Carcinogenesis 2014, 35, 1717–1725. [Google Scholar] [CrossRef]
- Fedele, C.; Ran, H.; Diskin, B.; Wei, W.; Jen, J.; Geer, M.J.; Araki, K.; Ozerdem, U.; Simeone, D.M.; Miller, G.; et al. SHP2 Inhibition Prevents Adaptive Resistance to MEK Inhibitors in Multiple Cancer Models. Cancer Discov. 2018, 8, 1237–1249. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Wherry, E.J.; Ahmed, R.; Freeman, G.J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007, 8, 239–245. [Google Scholar] [CrossRef]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef]
- Marasco, M.; Berteotti, A.; Weyershaeuser, J.; Thorausch, N.; Sikorska, J.; Krausze, J.; Brandt, H.J.; Kirkpatrick, J.; Rios, P.; Schamel, W.W.; et al. Molecular mechanism of SHP2 activation by PD-1 stimulation. Sci. Adv. 2020, 6, eaay4458. [Google Scholar] [CrossRef]
- Shimizu, K.; Sugiura, D.; Okazaki, I.M.; Maruhashi, T.; Takegami, Y.; Cheng, C.; Ozaki, S.; Okazaki, T. PD-1 Imposes Qualitative Control of Cellular Transcriptomes in Response to T Cell Activation. Mol. Cell 2020, 77, 937–950.e6. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Guo, C.; Ye, Q.; Shi, Y.; Sun, Y.; Zhang, J.; Huang, J.; Huang, Y.; Zeng, C.; Zhang, X.; et al. Endothelial deletion of SHP2 suppresses tumor angiogenesis and promotes vascular normalization. Nat. Commun. 2021, 12, 6310. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Goodwin, C.B.; Nabinger, S.C.; Richine, B.M.; Yang, Z.; Hanenberg, H.; Ohnishi, H.; Matozaki, T.; Feng, G.S.; Chan, R.J. Protein-tyrosine phosphatase Shp2 positively regulates macrophage oxidative burst. J. Biol. Chem. 2015, 290, 3894–3909. [Google Scholar] [CrossRef]
- Panchal, N.; Houghton, B.C.; Vassalou, E.; Thrasher, A.J.; Booth, C. Allosteric inhibition of SHP2 rescues functional T-cell abnormalities in SAP deficiency. J. Allergy Clin. Immunol. 2022, 150, 1507–1516.e7. [Google Scholar] [CrossRef]
- Yokosuka, T.; Takamatsu, M.; Kobayashi-Imanishi, W.; Hashimoto-Tane, A.; Azuma, M.; Saito, T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J. Exp. Med. 2012, 209, 1201–1217. [Google Scholar] [CrossRef]
- Rota, G.; Niogret, C.; Dang, A.T.; Barros, C.R.; Fonta, N.P.; Alfei, F.; Morgado, L.; Zehn, D.; Birchmeier, W.; Vivier, E.; et al. Shp-2 Is Dispensable for Establishing T Cell Exhaustion and for PD-1 Signaling In Vivo. Cell Rep. 2018, 23, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Cammann, C.; Israel, N.; Frentzel, S.; Jeron, A.; Topfstedt, E.; Schuler, T.; Simeoni, L.; Zenker, M.; Fehling, H.J.; Schraven, B.; et al. T cell-specific constitutive active SHP2 enhances T cell memory formation and reduces T cell activation. Front. Immunol. 2022, 13, 958616. [Google Scholar] [CrossRef]
- Foster, C.J.R.; Du, J.; Pundel, O.; Geer, M.J.; Ripert, R.C.; Liu, J.; Heim, T.A.; Araki, K.Y.; Lund, A.W.; Wang, J.; et al. T lymphocyte-specific deletion of SHP1 and SHP2 promotes activation-induced cell death of CD4(+) T cells and impairs antitumor response. Proc. Natl. Acad. Sci. USA 2025, 122, e2427254122. [Google Scholar] [CrossRef]
- Zhao, M.; Guo, W.; Wu, Y.; Yang, C.; Zhong, L.; Deng, G.; Zhu, Y.; Liu, W.; Gu, Y.; Lu, Y.; et al. SHP2 inhibition triggers anti-tumor immunity and synergizes with PD-1 blockade. Acta Pharm. Sin. B 2019, 9, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Noy, R.; Pollard, J.W. Tumor-associated macrophages: From mechanisms to therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; Liu, P.; Lv, D.; Shi, Y.; Tang, B.; Xu, J.; Zhong, T.; Xu, W.; Zhang, J.; et al. SHP2 deneddylation mediates tumor immunosuppression in colon cancer via the CD47/SIRPalpha axis. J. Clin. Investig. 2023, 133, e162870. [Google Scholar] [CrossRef]
- Achkova, D.; Maher, J. Role of the colony-stimulating factor (CSF)/CSF-1 receptor axis in cancer. Biochem. Soc. Trans. 2016, 44, 333–341. [Google Scholar] [CrossRef]
- Veillette, A.; Chen, J. SIRPalpha-CD47 Immune Checkpoint Blockade in Anticancer Therapy. Trends Immunol. 2018, 39, 173–184. [Google Scholar] [CrossRef]
- Xu, L.; Chen, Y.; Xiong, L.; Shen, Y.; Zhou, Z.; Wang, S.; Xu, X. A review of immune checkpoint inhibitor-associated myocarditis: Epidemiology, pathogenesis, and biomarkers. Hum. Vaccin. Immunother. 2025, 21, 2512645. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001, 291, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Palaskas, N.; Lopez-Mattei, J.; Durand, J.B.; Iliescu, C.; Deswal, A. Immune Checkpoint Inhibitor Myocarditis: Pathophysiological Characteristics, Diagnosis, and Treatment. J. Am. Heart Assoc. 2020, 9, e013757. [Google Scholar] [CrossRef]
- Tajiri, K.; Aonuma, K.; Sekine, I. Immune checkpoint inhibitor-related myocarditis. Jpn. J. Clin. Oncol. 2018, 48, 7–12. [Google Scholar] [CrossRef]
- Okazaki, T.; Tanaka, Y.; Nishio, R.; Mitsuiye, T.; Mizoguchi, A.; Wang, J.; Ishida, M.; Hiai, H.; Matsumori, A.; Minato, N.; et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med. 2003, 9, 1477–1483. [Google Scholar] [CrossRef]
- Lucas, J.A.; Menke, J.; Rabacal, W.A.; Schoen, F.J.; Sharpe, A.H.; Kelley, V.R. Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J. Immunol. 2008, 181, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Gotsman, I.; Grabie, N.; Dacosta, R.; Sukhova, G.; Sharpe, A.; Lichtman, A.H. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J. Clin. Investig. 2007, 117, 2974–2982. [Google Scholar] [CrossRef]
- Bu, D.X.; Tarrio, M.; Maganto-Garcia, E.; Stavrakis, G.; Tajima, G.; Lederer, J.; Jarolim, P.; Freeman, G.J.; Sharpe, A.H.; Lichtman, A.H. Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arter. Thromb. Vasc. Biol. 2011, 31, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Poels, K.; van Leent, M.M.T.; Boutros, C.; Tissot, H.; Roy, S.; Meerwaldt, A.E.; Toner, Y.C.A.; Reiche, M.E.; Kusters, P.J.H.; Malinova, T.; et al. Immune Checkpoint Inhibitor Therapy Aggravates T Cell-Driven Plaque Inflammation in Atherosclerosis. JACC Cardio Oncol. 2020, 2, 599–610. [Google Scholar] [CrossRef]
- Vuong, J.T.; Stein-Merlob, A.F.; Nayeri, A.; Sallam, T.; Neilan, T.G.; Yang, E.H. Immune Checkpoint Therapies and Atherosclerosis: Mechanisms and Clinical Implications: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 577–593. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Lu, S.; Zhang, C.; Ma, Z.; Su, R.; Li, Y.; Sun, T.; Li, Y.; Hong, M.; et al. Pairing of single-cell RNA analysis and T cell antigen receptor profiling indicates breakdown of T cell tolerance checkpoints in atherosclerosis. Nat. Cardiovasc. Res. 2023, 2, 290–306. [Google Scholar] [CrossRef]
- Wu, C.; Zheng, P.; Ma, L.; Xu, C.; Hu, L.; Yang, Z.; Fei, F.; Shen, Z.; Zhang, X.; Wu, Z.; et al. Protein Tyrosine Phosphatase SHP2 in Macrophages Acts as an Antiatherosclerotic Regulator in Mice. Arter. Thromb. Vasc. Biol. 2024, 44, 202–217. [Google Scholar] [CrossRef]
- Yan, M.; Zhang, X.; Chen, A.; Gu, W.; Liu, J.; Ren, X.; Zhang, J.; Wu, X.; Place, A.T.; Minshall, R.D.; et al. Endothelial cell SHP-2 negatively regulates neutrophil adhesion and promotes transmigration by enhancing ICAM-1-VE-cadherin interaction. FASEB J. 2017, 31, 4759–4769. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, J.; Qi, T.; Huang, Y.; Lu, Y.; Zhan, T.; Gong, H.; Zhu, Z.; Shi, Y.; Zhou, J.; et al. SHP2 protects endothelial cell barrier through suppressing VE-cadherin internalization regulated by MET-ARF1. FASEB J. 2019, 33, 1124–1137. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.R.; Witting, P.K.; Drummond, G.R. Redox control of endothelial function and dysfunction: Molecular mechanisms and therapeutic opportunities. Antioxid. Redox. Signal. 2008, 10, 1713–1765. [Google Scholar] [CrossRef]
- Pichavaram, P.; Mani, A.M.; Singh, N.K.; Rao, G.N. Cholesterol crystals promote endothelial cell and monocyte interactions via H(2)O(2)-mediated PP2A inhibition, NFkappaB activation and ICAM1 and VCAM1 expression. Redox. Biol. 2019, 24, 101180. [Google Scholar] [CrossRef]
- Didion, S.P. Cellular and Oxidative Mechanisms Associated with Interleukin-6 Signaling in the Vasculature. Int. J. Mol. Sci. 2017, 18, 2563. [Google Scholar] [CrossRef]
- Liu, P.; Li, Y.; Li, M.; Zhou, H.; Zhang, H.; Zhang, Y.; Xu, J.; Xu, Y.; Zhang, J.; Xia, B.; et al. Endothelial Shp2 deficiency controls alternative activation of macrophage preventing radiation-induced lung injury through notch signaling. iScience 2022, 25, 103867. [Google Scholar] [CrossRef]
- Chen, J.; Cao, Z.; Guan, J. SHP2 inhibitor PHPS1 protects against atherosclerosis by inhibiting smooth muscle cell proliferation. BMC Cardiovasc. Disord. 2018, 18, 72. [Google Scholar] [CrossRef]
- Mattoon, D.R.; Lamothe, B.; Lax, I.; Schlessinger, J. The docking protein Gab1 is the primary mediator of EGF-stimulated activation of the PI-3K/Akt cell survival pathway. BMC Biol. 2004, 2, 24. [Google Scholar] [CrossRef]
- Ushio-Fukai, M. Redox signaling in angiogenesis: Role of NADPH oxidase. Cardiovasc. Res. 2006, 71, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Ruess, D.A.; Heynen, G.J.; Ciecielski, K.J.; Ai, J.; Berninger, A.; Kabacaoglu, D.; Gorgulu, K.; Dantes, Z.; Wormann, S.M.; Diakopoulos, K.N.; et al. Mutant KRAS-driven cancers depend on PTPN11/SHP2 phosphatase. Nat. Med. 2018, 24, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Richards, C.E.; Elamin, Y.Y.; Carr, A.; Gately, K.; Rafee, S.; Cremona, M.; Hanrahan, E.; Smyth, R.; Ryan, D.; Morgan, R.K.; et al. Protein Tyrosine Phosphatase Non-Receptor 11 (PTPN11/Shp2) as a Driver Oncogene and a Novel Therapeutic Target in Non-Small Cell Lung Cancer (NSCLC). Int. J. Mol. Sci. 2023, 24, 10545. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Coad, J.; Ducatman, B.; Agazie, Y.M. SHP2 is up-regulated in breast cancer cells and in infiltrating ductal carcinoma of the breast, implying its involvement in breast oncogenesis. Histopathology 2008, 53, 389–402. [Google Scholar] [CrossRef]
- Tartaglia, M.; Niemeyer, C.M.; Fragale, A.; Song, X.; Buechner, J.; Jung, A.; Hahlen, K.; Hasle, H.; Licht, J.D.; Gelb, B.D. Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat. Genet. 2003, 34, 148–150. [Google Scholar] [CrossRef]
- Sun, Y.; Meyers, B.A.; Czako, B.; Leonard, P.; Mseeh, F.; Harris, A.L.; Wu, Q.; Johnson, S.; Parker, C.A.; Cross, J.B.; et al. Allosteric SHP2 Inhibitor, IACS-13909, Overcomes EGFR-Dependent and EGFR-Independent Resistance Mechanisms toward Osimertinib. Cancer Res. 2020, 80, 4840–4853. [Google Scholar] [CrossRef]
- Kano, H.; Ichihara, E.; Watanabe, H.; Nishii, K.; Ando, C.; Nakasuka, T.; Ninomiya, K.; Kato, Y.; Kubo, T.; Rai, K.; et al. SHP2 Inhibition Enhances the Effects of Tyrosine Kinase Inhibitors in Preclinical Models of Treatment-naive ALK-, ROS1-, or EGFR-altered Non-small Cell Lung Cancer. Mol. Cancer Ther. 2021, 20, 1653–1662. [Google Scholar] [CrossRef]
- Quintana, E.; Schulze, C.J.; Myers, D.R.; Choy, T.J.; Mordec, K.; Wildes, D.; Shifrin, N.T.; Belwafa, A.; Koltun, E.S.; Gill, A.L.; et al. Allosteric Inhibition of SHP2 Stimulates Antitumor Immunity by Transforming the Immunosuppressive Environment. Cancer Res. 2020, 80, 2889–2902. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Willard, D.; Rudolph, J. Redox regulation of SH2-domain-containing protein tyrosine phosphatases by two backdoor cysteines. Biochemistry 2009, 48, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Pouremamali, F.; Pouremamali, A.; Dadashpour, M.; Soozangar, N.; Jeddi, F. An update of Nrf2 activators and inhibitors in cancer prevention/promotion. Cell Commun. Signal. 2022, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Syahputra, E.W.; Lee, H.; Cho, H.; Park, H.J.; Park, K.S.; Hwang, D. PROTAC Delivery Strategies for Overcoming Physicochemical Properties and Physiological Barriers in Targeted Protein Degradation. Pharmaceutics 2025, 17, 501. [Google Scholar] [CrossRef]
- Fallatah, M.M.; Alradwan, I.; Alfayez, N.; Aodah, A.H.; Alkhrayef, M.; Majrashi, M.; Jamous, Y.F. Nanoparticles for Cancer Immunotherapy: Innovations and Challenges. Pharmaceuticals 2025, 18, 1086. [Google Scholar] [CrossRef]
| Reference | SHP2 Effect | Signaling Pathways Regulated | Cellular Localization | ROS Involvement | Phosphatase Activity | Scaffold/Structural Role |
|---|---|---|---|---|---|---|
| Christofides et al., 2023, Nat Immunol [5] | Negative regulator of myeloid differentiation via PD-L1–SHP2; deletion boosts anti-tumor myelopoiesis | PD-1/ITSM → SHP2; GM-CSF/GM-CSFR; HOXA10/IRF8 axis | Plasma membrane (PD-1 complex), cytosol | Not assessed | Implicated/required (genetic loss phenocopies SHP2 absence) | PD-1 ITSM docking; GM-CSFR/LYN complex assembly |
| Wei et al., 2022, JMCB [32] | Negative regulator of AKT in ovary | PI3K/AKT (FSH/H2O2 context) | Cytosol; membrane-proximal | Upstream (H2O2 modulates SHP2 → p85) | Required (for AKT restraint) | PI3K p85 interaction |
| Guo et al., 2017, Nat Commun [35] | Negative regulator of inflammasome | NLRP3 via ANT1 mitochondrial homeostasis | Mitochondria | Downstream (limits mROS) | Required | ANT1-linked complex |
| Wang et al., 2021, Sci Rep [41] | SHP2 blockade ↑ anti-tumor immunity | RTK/MAPK, PD-1-related programs | Plasma membrane; cytosol | Not assessed | Inhibited (drug) | RTK/PD-1 complexes perturbed |
| Lan et al., 2015, EMBO J [42] | Suppresses senescence (pro-tumor) | Src–FAK–MEK/ERK → Skp2/AurA/DIII | Cytosol; membrane (functional) | Not assessed | Not directly tested | Src/FAK/MEK axis organization |
| Marasco et al., 2020, Sci Adv [52] | PD-1 directly activates SHP2 | PD-1/ITIM/ITSM → SHP2; TCR inhibition | Plasma membrane (immune synapse) | Not assessed | Required | PD-1 tail ITIM/ITSM docking (structural) |
| Panchal et al., 2022, JACI [56] | Allosteric inhibition restores T cell function (SAP deficiency) | PD-1/SHP2-mediated negative signaling; TCR/SLAM | Immune synapse | Not assessed | Inhibited (drug) | PD-1/SAP pathway docking curtailed |
| Gavrieli et al., 2003, BBRC [18] | Negative (BTLA recruits SHP2) | BTLA/ITIM → SHP2; TCR inhibition | Plasma membrane (immune synapse) | Not assessed | Required | BTLA phosphotyrosine motifs |
| Yokosuka et al., 2012, J Exp Med [57] | Negative (PD-1 microclusters) | PD-1 microclusters–SHP2 → TCR inhibition | PD-1 clustered ITIM/ITSM platform | Not assessed | Required | PD-1 clustered ITIM/ITSM platform |
| Zhao et al., 2019, Acta Pharm Sin B [61] | SHP2 inhibition triggers immunity; synergy with PD-1 blockade | PD-1-related programs; RTK/MAPK | Cytosol; membrane | Not assessed | Inhibited (drug) | RTK immune complexes |
| Wu et al., 2024, ATVB [77] | Repressor of macrophage inflammatory activation | ROS/NLRP3 inflammasome restraint | Cytosol; mitochondria (macrophage) | Yes (limits ROS–NLRP3) | Required | Not specified |
| Yan et al., 2017, FASEB J [78] | Negative regulator of neutrophil adhesion; promotes transmigration | ICAM-1–VE-cadherin junction (conditional) | Endothelial junctions | Not assessed | Loss-of-function assays | Junctional complex dependent |
| Liu et al., 2022, iScience [83] | Endothelial SHP2 deletion exacerbates RILI via macrophage reprogramming | Notch signaling; alternate (via macrophage reprogramming) | Endothelium | Not assessed | Required | Not specified (specific case) |
| Reference | SHP2 Effect | Signaling Pathways Regulated | Cellular Localization | ROS Involvement | Phosphatase Activity | Scaffold/Structural Role |
|---|---|---|---|---|---|---|
| Huang et al., 2002, JBC [12] | Positive (required for Elk-1 activation) | GAB1 → ERK → Elk1 | Cytosol; plasma membrane | Not assessed | Required | GAB1 docking platform |
| Noguchi et al., 1994, Mol Cell Biol [24] | Positive (insulin Ras activation) | IR → IRS-1 → RAS/ERK | Plasma membrane; cytosol | Not assessed | Required | IRS-1/IR complex docking |
| Kandadi et al., 2010, Acta Pharmacol Sin [25] | Positive (migration/proliferation) | PDGF → ERK | Cytosol; plasma membrane | Not assessed | Required | PDGFR-Grb2 complex |
| Yu et al., 2014, Biochemistry [28] | GOF (LEOPARD) enhances activity | RAS/ERK hyperactivation | Cytosol; plasma membrane | Not assessed | Enhanced (mutants relieve autoinhibition) | Structural focus (no specific scaffold) |
| Wu et al., 2001, Oncogene [30] | Positive (growth-factor PI3K/AKT) | PI3K/AKT (and ERK interplay) | Membrane-proximal; cytosol | Not assessed | Required | GAB1, IRS-1 implied |
| Kan et al., 2024, JCI Insight [33] | GOF (E76K) activates complexes I and III | OXPHOS/ETC activation (metabolic) | Mitochondria (liquid–liquid phase separation/LLPS condensates) | Downstream (↑ mtROS) | Not required (LLPS-dependent) | LLPS-mediated ETC complex assembly |
| Bentires-Alj et al., 2004, Cancer Res [44] | GOF mutations (oncogenic) | Constitutive RAS/MAPK | Cytosol; plasma membrane | Not assessed | Required | Not scaffold-specific |
| Xu et al., 2013, PLoS One [45] | GOF → ↑ ROS; MPN | RAS/MAPK + ROS | Cytosol; mitochondria-linked | Yes (↑ ROS downstream of GOF) | Required | Not scaffold-specific |
| De Rocca-Serra-Nedelec et al., 2012, PNAS [46] | GOF hyperactivates ERK; IGF-1 | GH → ERK hyperactivation; inhibition of IGF-1 secretion | Cytosol; plasma membrane | Not assessed | Required | GH receptor complex |
| Schneberger et al., 2014, Carcinogenesis [47] | GOF E76K drives lung tumors | ERK/MAPK hyperactivation | Cytosol; plasma membrane | Not assessed | Required | Not scaffold-specific |
| Fedida et al., 2018, Cancer Discov [48] | SHP2 enables adaptive resistance; inhibits feedback reactivation | RTK → RAS → ERK feedback regulation | Cytosol; plasma membrane | Not assessed | Required for reactivation prevention | GAB1/SOS-centered feedback node |
| Feng et al., 2021, ATVB [81] | Endothelial deletion ↓ VEGF/angiogenic signaling | VEGF–ERK engagement ↓ by SHP2 deletion | Cytosol; membrane-proximal | Yes (enhances NOX signaling) | Required | VEGFR2/Tie2 adaptor complex |
| Mattoon et al., 2004, BMC Biol [85] | Positive, anti-apoptotic | PI3K/AKT → caspase-3 suppression | Cytosol; membrane-proximal | Not assessed | Required | GAB1 |
| Ushio-Fukai, 2006, Cardiovasc Res [86] | SHP2 → VEGF signaling → angiogenesis | VEGF/MAPK cascade | Endothelium (NOX microdomain) | Yes (enhances ROS) | Required | p47phox/NOX2 interactions |
| Reference | SHP2 Effect | Signaling Pathways Regulated | Cellular Localization | ROS Involvement | Phosphatase Activity | Scaffold/Structural Role |
|---|---|---|---|---|---|---|
| Xu et al., 2002, Exp Cell Res [7] | Context-dependent; mapping partners | Adhesion/immune: PZR, PECAM-1; growth: SHC, GAB1, IRS1 | Cytosol; plasma membrane | Not assessed | Not directly tested | PZR, PECAM-1, SHC, GAB1, IRS1 as SHP2-binding scaffolds |
| Rota et al., 2018, Cell Rep [58] | Dispensable for PD-1 signaling/exhaustion in vivo | PD-1/TCR pathways (murine) | T cell membrane/cytosol (physiologic) | Not assessed | Not required (in vivo) | Not essential scaffold in that context |
| Foster et al., 2025, PNAS [60] | Dual SHP1/2 deletion → CD4+ ICOS, poor anti-tumor | TCR survival/apoptosis circuits | T cell compartments | Not assessed | Abolished (both PTPs) | Redundant checkpoint scaffolding removed |
| Canmann et al., 2022, Front Immunol [59] | GOF (constitutive) → ↑ memory T cell formation AND ↓ acute T cell activation | TCR/MAPK transcriptional programs | T cell compartments | Not assessed | Enhanced (constitutive) | Not primary (functional outcome focus) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López Moreno, S.F.; Lenz, S.A.; Casso-Chapa, B.; Paniagua-Bojorges, A.; Kim, J.H.; Palaskas, N.L.; Nead, K.T.; Samanthapudi, V.S.K.; Mejia, G.; Hoang, O.; et al. SHP2: A Redox-Sensitive Regulator Linking Immune Checkpoint Inhibitor Therapy to Cancer Treatment and Vascular Risk. Antioxidants 2025, 14, 1388. https://doi.org/10.3390/antiox14121388
López Moreno SF, Lenz SA, Casso-Chapa B, Paniagua-Bojorges A, Kim JH, Palaskas NL, Nead KT, Samanthapudi VSK, Mejia G, Hoang O, et al. SHP2: A Redox-Sensitive Regulator Linking Immune Checkpoint Inhibitor Therapy to Cancer Treatment and Vascular Risk. Antioxidants. 2025; 14(12):1388. https://doi.org/10.3390/antiox14121388
Chicago/Turabian StyleLópez Moreno, Silvia Fernanda, Stefania Assunto Lenz, Bernardo Casso-Chapa, Angelica Paniagua-Bojorges, Jung Hyun Kim, Nicolas L. Palaskas, Kevin T. Nead, Venkata S. K. Samanthapudi, Gilbert Mejia, Oanh Hoang, and et al. 2025. "SHP2: A Redox-Sensitive Regulator Linking Immune Checkpoint Inhibitor Therapy to Cancer Treatment and Vascular Risk" Antioxidants 14, no. 12: 1388. https://doi.org/10.3390/antiox14121388
APA StyleLópez Moreno, S. F., Lenz, S. A., Casso-Chapa, B., Paniagua-Bojorges, A., Kim, J. H., Palaskas, N. L., Nead, K. T., Samanthapudi, V. S. K., Mejia, G., Hoang, O., Lee, J., Lin, S. H., Herrmann, J., Wang, G., Yusuf, S. W., Iliescu, C. A., Beinart, N. I., Manisty, C., Ushio-Fukai, M., ... Abe, J.-i. (2025). SHP2: A Redox-Sensitive Regulator Linking Immune Checkpoint Inhibitor Therapy to Cancer Treatment and Vascular Risk. Antioxidants, 14(12), 1388. https://doi.org/10.3390/antiox14121388












