Chlorogenic Acid Alleviates the Detrimental Effects of Concurrent Hyperglycemia and Chronic Stress on Brain Homeostasis by Modulating Antioxidative Defense in Adult Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.1.1. Experimental Groups
2.1.2. Concurrent Exposure to CUMS+D
2.1.3. CGA Treatment
2.2. Fasting Blood Glucose Level Measurement and Brain Sample Extraction
2.3. Evaluation of Oxidative Stress
2.3.1. SOD Activity
2.3.2. Catalase Activity
2.3.3. GSH Content
2.4. Expression of Oxidative Stress-Sensitive Markers
2.4.1. Western Blotting
2.4.2. Immunostaining
2.4.3. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.5. Statistical Analysis
3. Results
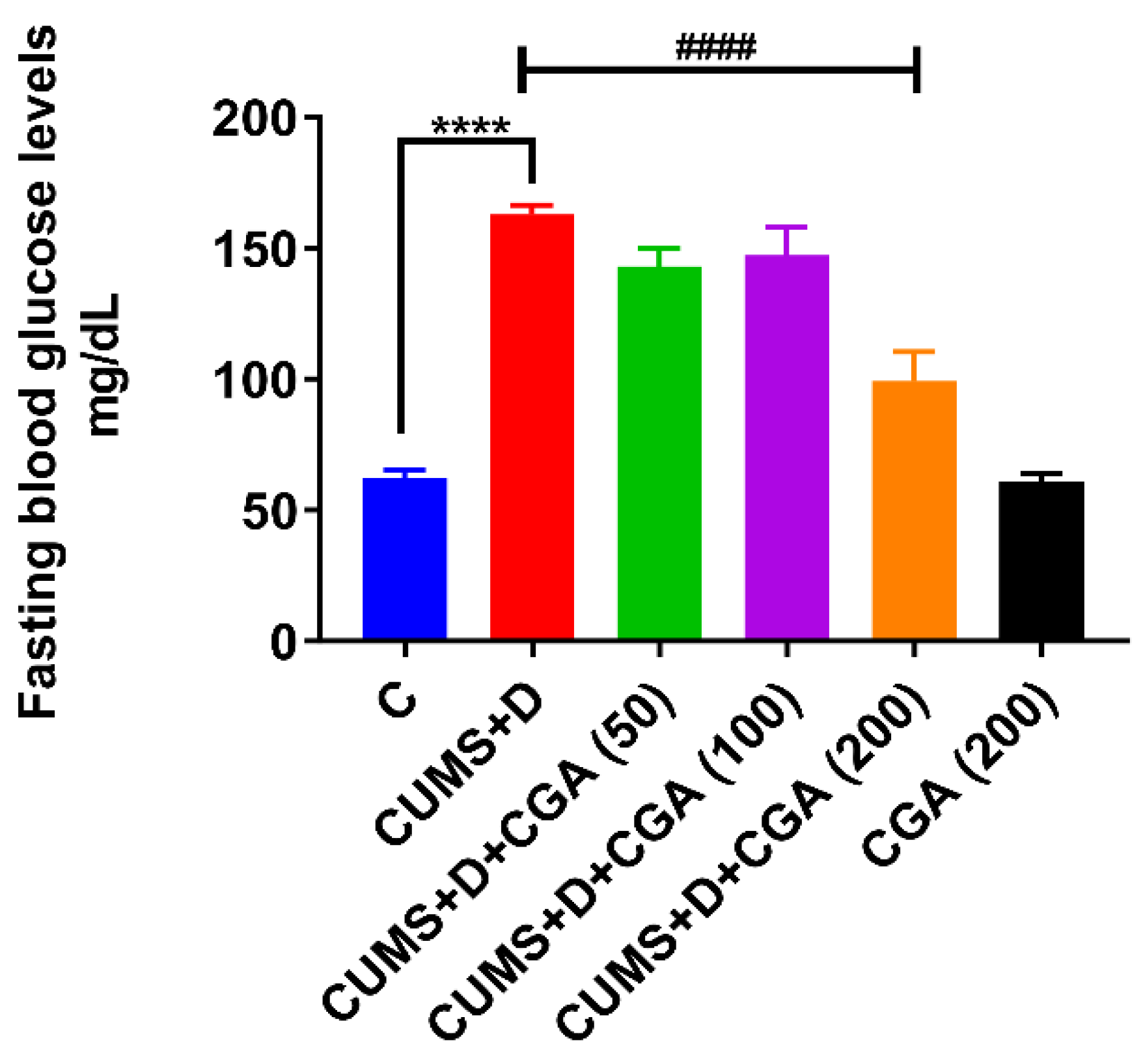
3.1. CGA Treatment Reversed CUMS+D Exposure-Induced Systemic Hyperglycemia
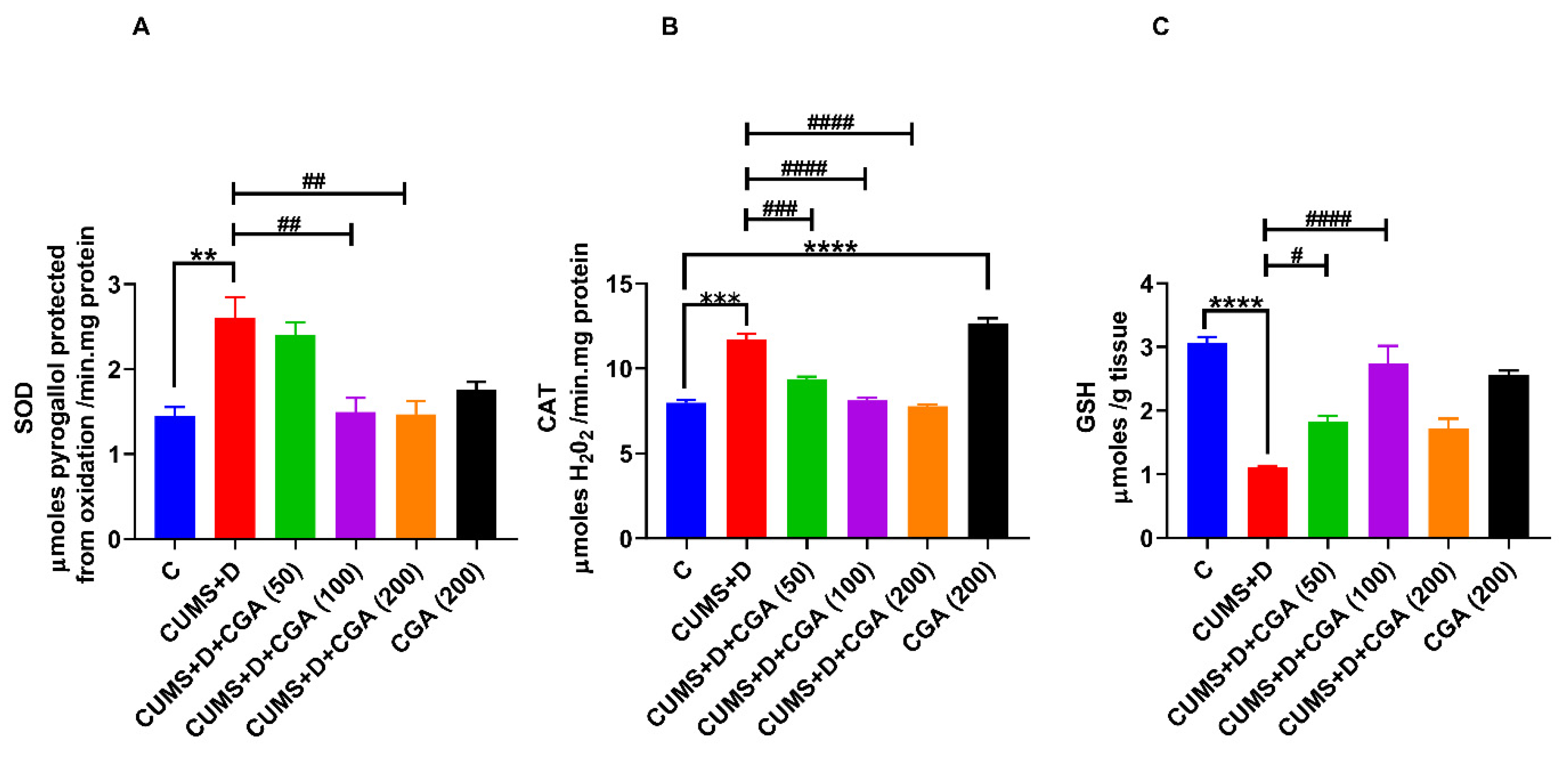
3.2. CGA Treatment Modulated Antioxidative Defense in the Brains of Fish Exposed to CUMS+D
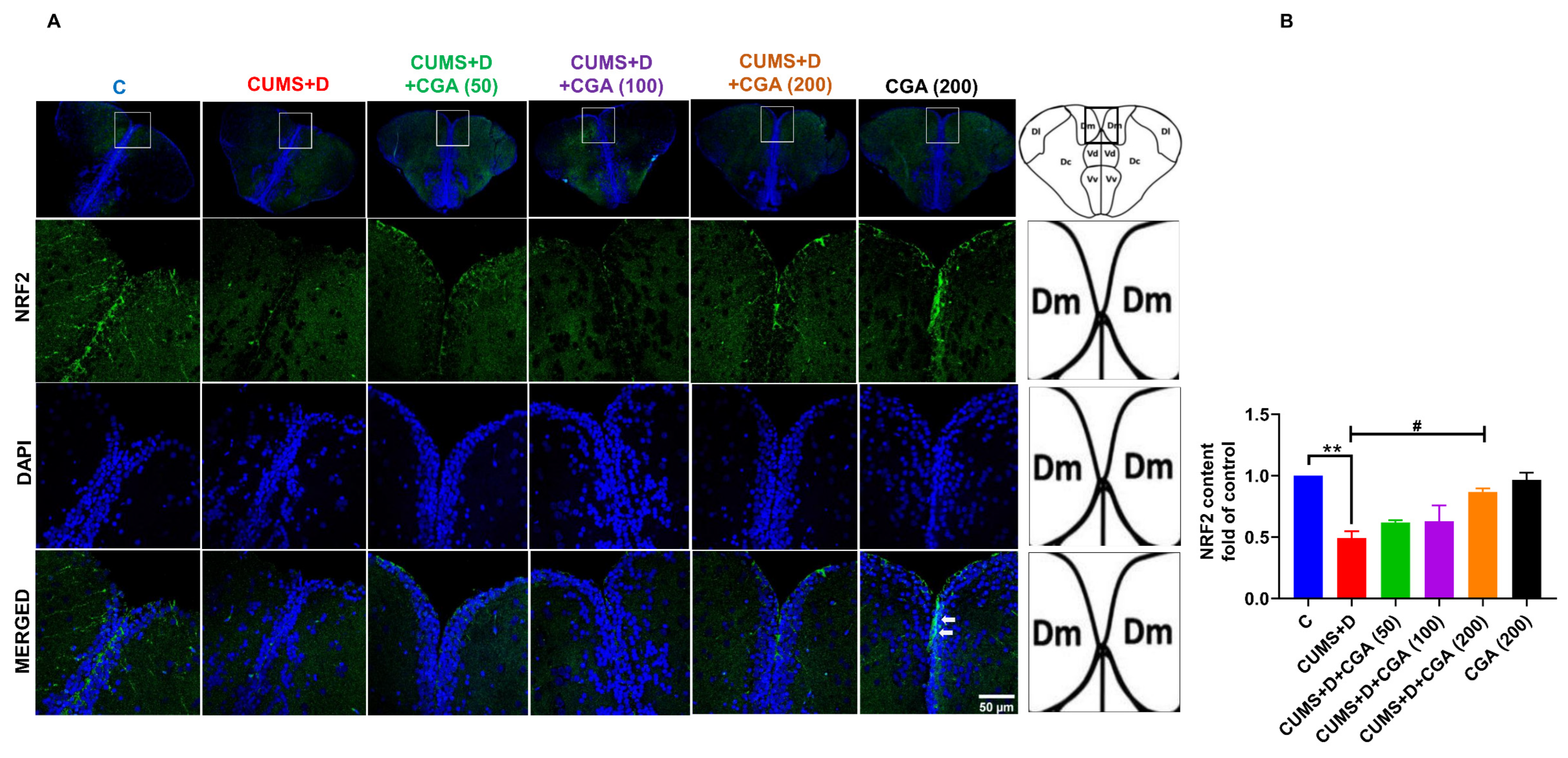
3.3. CGA Treatment Replenished NRF2 Protein Content and Normalized Keap1 mRNA Expression in the Brains of Fish Exposed to CUMS+D
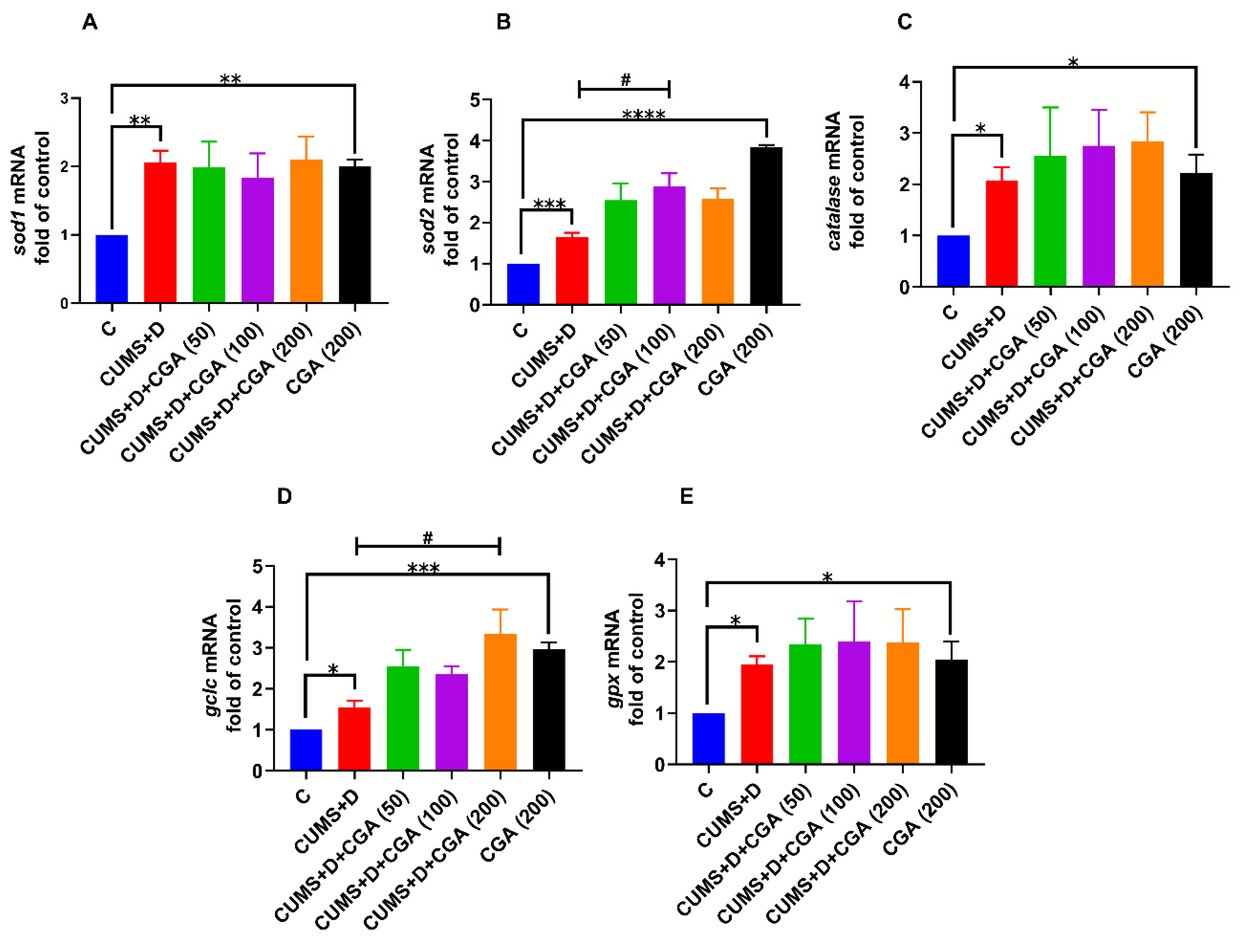
3.4. CGA Treatment Boosted the mRNA Expression Levels of NRF2-Target Antioxidant Genes in the Brains of Fish Exposed to CUMS+D
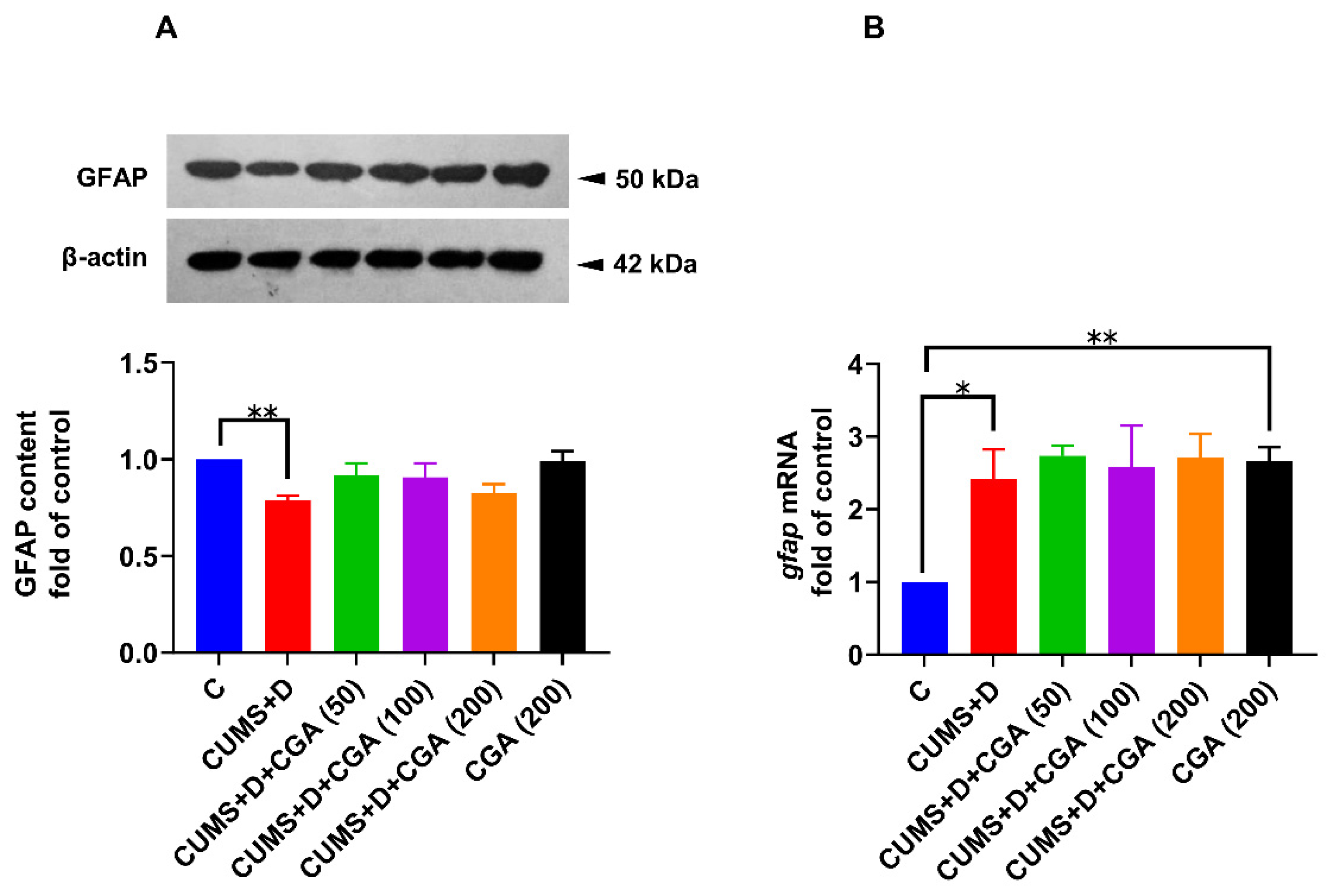
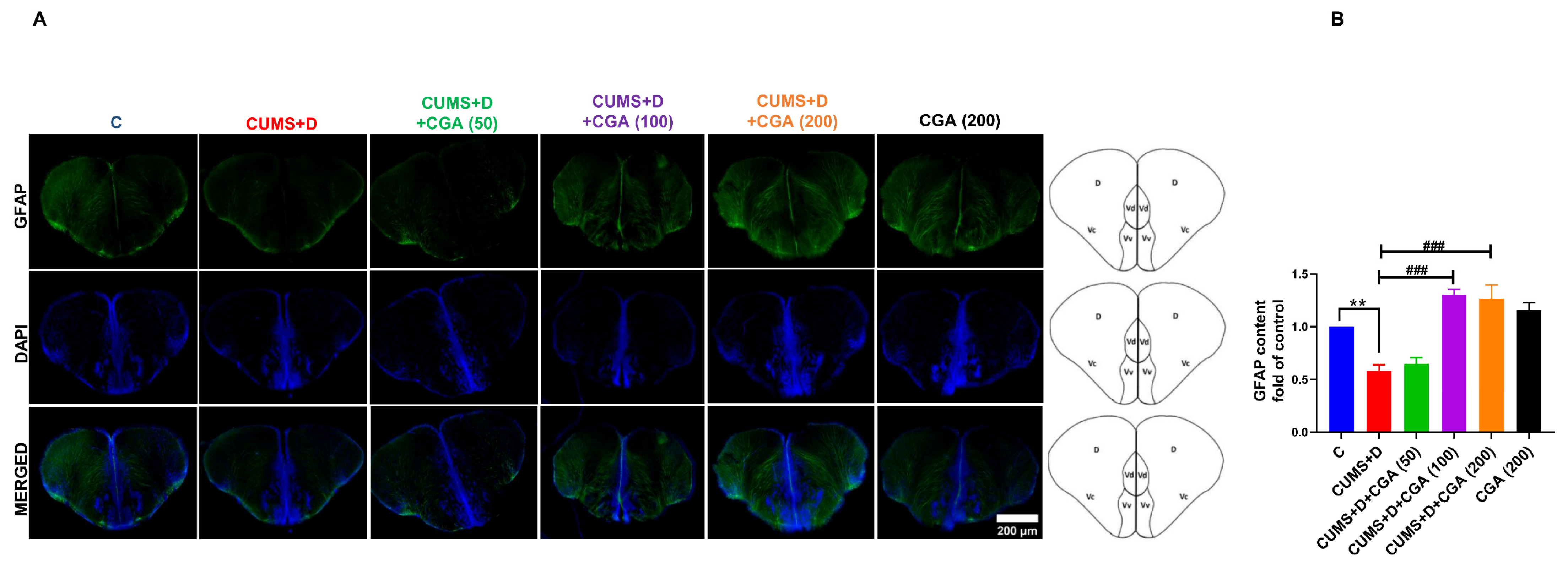
3.5. CGA Treatment Restored GFAP Protein Content in the Brains of Fish Exposed to CUMS+D
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C | Control group |
| CGA | Chlorogenic acid |
| CNS | Central nervous system |
| CUMS | Chronic unpredictable mild stress |
| D | Dextrose |
| DD | Diabetes distress |
| GCLC | Glutamate-cysteine ligase catalytic subunit |
| GFAP | Glial fibrillary acidic protein |
| GLAST | Glutamate–aspartate transporter |
| GLT-1 | Glutamate transporter-1 |
| GPX | Glutathione peroxidase |
| GSH | Glutathione |
| KEAP | Kelch-like ECH-associated protein 1 |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| S-100β | S100 calcium-binding protein B |
| SOD | Superoxide dismutase |
References
- Diabetes Federation 2021 IDF Diabetes Atlas, 10th ed.; Brussels, Belgium: 2021. Available online: https://www.diabetesatlas.org (accessed on 10 September 2025).
- Gupta, M.; Pandey, S.; Rumman, M.; Singh, B.; Mahdi, A.A. Molecular mechanisms underlying hyperglycemia-associated cognitive decline. IBRO Neurosci. Rep. 2022, 14, 57–63. [Google Scholar] [CrossRef]
- Fagherazzi, G. Technologies will not make diabetes disappear: How to integrate the concept of diabetes distress into care. Diabet. Epidemiol. Manag. 2023, 11, 100140. [Google Scholar] [CrossRef]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef] [PubMed]
- Singh Bhatti, J.; Sehrawat, A.; Mishra, J.; Singh Sidhu, I.; Shanker Navik, U.; Khullar, N.; Kumar, S.; Kaur Bhatti, G.; Hemachandra Reddy, P. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free. Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M.J.D. Molecular Mechanisms Regulating the KEAP1-NRFPathway. Mol. Cell. Biol. 2020, 10, e00099-20. [Google Scholar]
- Wang, B.; Zhu, S.; Guo, M.; Ma, R.D.; Tang, Y.L.; Nie, Y.X.; Gu, H.F. Artemisinin ameliorates cognitive decline by inhibiting hippocampal neuronal ferroptosis via Nrf2 activation in T2DM mice. Mol. Med. 2024, 30, 35. [Google Scholar] [CrossRef] [PubMed]
- Subba, R.; Fasciolo, G.; Geremia, E.; Muscari Tomajoli, M.T.; Petito, A.; Carrella, S.; Mondal, A.C.; Napolitano, G.; Venditti, P. Simultaneous induction of systemic hyperglycaemia and stress impairs brain redox homeostasis in the adult zebrafish. Arch. Biochem. Biophys. 2024, 759, 110101. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Meyer, C.J.; Block, G.A.; Chertow, G.M.; Shiels, P.G. Understanding the role of the cytoprotective transcription factor nuclear factor erythroid 2-related factor 2-lessons from evolution, the animal kingdom and rare progeroid syndromes. Nephrol. Dial. Transplant. 2020, 35, 2036–2045. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Qin, L.; Wang, J.; Wu, X.; Song, L.; Zhang, Y.; Gong, M.; Wang, Y.; Li, B. Antidepressant effects of 70% ethanolic extract of Lonicerae japonicae flos and it contained chlorogenic acid via upregulation of BDNF-TrkB pathway in the hippocampus of mice. Brain Res. Bull. 2023, 204, 110796. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, X.; Guo, K.; Zhou, F.; Yang, H. Use of Chlorogenic Acid against Diabetes Mellitus and Its Complications. J. Immunol. Res. 2020, 2020, 9680508. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Butt, A.; Li, B.; Illes, P.; Zorec, R.; Semyanov, A.; Tang, Y.; Sofroniew, M.V. Astrocytes in human central nervous system diseases: A frontier for new therapies. Signal Transduct. Target. Ther. 2023, 8, 396. [Google Scholar] [CrossRef] [PubMed]
- Dossi, E.; Vasile, F.; Rouach, N. Human astrocytes in the diseased brain. Brain Res. Bull. 2018, 136, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Ayuob, N.N.; Firgany, A.E.D.L.; El-Mansy, A.A.; Ali, S. Can Ocimum basilicum relieve chronic unpredictable mild stress-induced depression in mice? Exp. Mol. Pathol. 2017, 103, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Aten, S.; Du, Y.; Taylor, O.; Dye, C.; Collins, K.; Thomas, M.; Kiyoshi, C.; Zhou, M. Chronic Stress Impairs the Structure and Function of Astrocyte Networks in an Animal Model of Depression. Neurochem. Res. 2023, 48, 1191–1210. [Google Scholar] [CrossRef]
- Meng, F.; Fu, J.; Zhang, L.; Guo, M.; Zhuang, P.; Yin, Q.; Zhang, Y. Function and therapeutic value of astrocytes in diabetic cognitive impairment. Neurochem. Int. 2023, 169, 105591. [Google Scholar] [CrossRef]
- Dorsemans, A.C.; Lefebvre d’hellencourt, C.; Ait-Arsa, I.; Jestin, E.; Meilhac, O.; Diotel, N. Acute and chronic models of hyperglycemia in zebrafish: A method to assess the impact of hyperglycemia on neurogenesis and the biodistribution of radiolabeled molecules. J. Vis. Exp. 2017, 26, 55203. [Google Scholar]
- Girotti, M.; Bulin, S.E.; Carreno, F.R. Effects of chronic stress on cognitive function-From neurobiology to intervention. Neurobiol. Stress 2024, 33, 100670. [Google Scholar] [CrossRef]
- Swathi, M.; Manjusha, S.; Isatrin, J.V.; Gururaj, A. Prevalence and correlates of stress, anxiety, and depression in patients with chronic diseases: A cross-sectional study. Middle East Curr. Psychiatr. 2023, 30, 66. [Google Scholar]
- Kinkel, M.D.; Eames, S.C.; Philipson, L.H.; Prince, V.E. Intraperitoneal injection into adult zebrafish. J. Vis. Exp. 2010, 42, 2126. [Google Scholar]
- Collymore, C. Anesthesia, Analgesia, and Euthanasia of the Laboratory Zebrafish. In The Zebrafish in Biomedical Research: Biology, Husbandry, Diseases, and Research Applications; Cartner, S., Eisen, J.S., Farmer, S.F., Guillemin, K.J., Kent, M.L., Sanders, G.E., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 403–413. [Google Scholar]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Claiborne, A.L. Catalase activity. In Handbook Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1984; pp. 283–284. [Google Scholar]
- Jollow, D.J.; Mitchell, J.R.; Zampaglione, N.; Gillette, J.R. Bromobenzene-Induced Liver Necrosis. Protective Role of Glutathione and Evidence for 3,4-Bromobenzene Oxide as the Hepatotoxic Metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.K.; Mondal, A.C. TrkB receptor antagonism inhibits stab injury induced proliferative response in adult zebrafish (Danio rerio) brain. Neurosci. Lett. 2018, 672, 28–33. [Google Scholar] [CrossRef]
- Pérez Gutiérrez, R.M.; Muñiz-Ramirez, A.; Garcia-Campoy, A.H.; Mota Flores, J.M. Evaluation of the Antidiabetic Potential of Extracts of Urtica dioica, Apium graveolens, and Zingiber officinale in Mice, Zebrafish, and Pancreatic β-Cell. Plants 2021, 10, 1438. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, A.E.; Olthof, M.R.; Meeuse, J.C.; Seebus, E.; Heine, R.J.; Van Dam, R.M. Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on glucose tolerance. Diabetes Care 2009, 32, 1023–1025. [Google Scholar] [CrossRef]
- Ong, K.W.; Hsu, A.; Tan, B.K. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: A contributor to the beneficial effects of coffee on diabetes. PLoS ONE 2012, 7, e32718. [Google Scholar] [CrossRef]
- Kumar Ramalingam, P.; Chandrasekaran, M.; Gupta, P.; Nelamangala Umesh, V.; Bharadwaj, T.; Krishna, N.B.; Lalitha, R.; Gunaseelan, G.S. In Silico Screening of Chlorogenic Acids from Plant Sources against Human Translocase-I to Identify Competitive Inhibitors to Treat Diabetes. ACS Omega 2024, 9, 6561–6568. [Google Scholar] [CrossRef]
- Singh, A.K.; Rana, H.K.; Singh, V.; Yadav, T.C.; Varadwaj, P.; Pandey, A.K. Evaluation of antidiabetic activity of dietary phenolic compound chlorogenic acid in streptozotocin induced diabetic rats: Molecular docking, molecular dynamics, in silico toxicity, in vitro and in vivo studies. Comput. Biol. Med. 2021, 134, 104462. [Google Scholar] [CrossRef]
- Peixoto, J.A.B.; Andrade, N.; Machado, S.; Costa, A.S.; Oliveira, M.B.P.; Martel, F.; Alves, R.C.J.F. Green/roasted coffee and silverskin extracts inhibit sugar absorption by human intestinal epithelial (Caco-2) cells by decreasing GLUT2 gene expression. Foods 2022, 11, 3902. [Google Scholar] [CrossRef]
- Nam, Y.H.; Hong, B.N.; Rodriguez, I.; Ji, M.G.; Kim, K.; Kim, U.J.; Kang, T.H. Synergistic Potentials of Coffee on Injured Pancreatic Islets and Insulin Action via KATP Channel Blocking in Zebrafish. J. Agric. Food. Chem. 2015, 63, 5612–5621. [Google Scholar] [CrossRef]
- Tabassum, N.; Tai, H.; Jung, D.W.; Williams, D.R. Fishing for Nature’s Hits: Establishment of the Zebrafish as a Model for Screening Antidiabetic Natural Products. Evid.-Based Complement. Altern. Med. 2015, 2015, 287847. [Google Scholar] [CrossRef]
- Rebouças, E.L.; da Silva, A.W.; Rodrigues, M.C.; Ferreira, M.K.A.; Mendes, F.R.S.; Marinho, M.M.; Marinho, E.M.; Pereira, L.R.; Araújo, J.I.F.; da Silva, J.Y.G.; et al. Antinociceptive, anti-inflammatory and hypoglycemic activities of the ethanolic Turnera subulate Sm. flower extract in adult zebrafish (Danio rerio). J. Biomol. Struct. Dyn. 2022, 40, 13062–13074. [Google Scholar] [CrossRef] [PubMed]
- Ghaddar, B.; Veeren, B.; Rondeau, P.; Bringart, M.; Lefebvre d’Hellencourt, C.; Meilhac, O.; Bascands, J.L.; Diotel, N. Impaired brain homeostasis and neurogenesis in diet-induced overweight zebrafish: A preventive role from A. borbonica extract. Sci. Rep. 2020, 10, 14496. [Google Scholar] [CrossRef] [PubMed]
- Uthaiah, C.A.; Devaru, N.C.; Shivakumar, N.H.; R, R.; Madhunapantula, S.R.V. Vitamin D Mitigates Hyperglycemia-Induced Cognition Decline in Danio rerio (Zebrafish) through the Activation of Antioxidant Mechanisms. Antioxidants 2022, 11, 2114. [Google Scholar] [CrossRef] [PubMed]
- Zimath, P.L.; Dalmagro, A.P.; da Silva, L.M.; Malheiros, A.; de Souza, M.M. Myrsinoic acid B from Myrsine coriacea reverses depressive-like behavior and brain oxidative stress in streptozotocin-diabetic rats. Chem. Biol. Interact. 2021, 347, 109603. [Google Scholar] [CrossRef]
- Ajiboye, B.O.; Famusiwa, C.D.; Amuda, M.O.; Afolabi, S.O.; Ayotunde, B.T.; Adejumo, A.A.; Akindele, A.F.I.; Oyinloye, B.E.; Owolabi, O.V.; Genovese, C.; et al. Attenuation of PI3K/AKT signaling pathway by Ocimum gratissimum leaf flavonoid-rich extracts in streptozotocin-induced diabetic male rats. Biochem. Biophys. Rep. 2024, 38, 101735. [Google Scholar] [CrossRef]
- Geddie, H.; Cairns, M.; Smith, L.; van Wyk, M.; Beselaar, L.; Truter, N.; Rautenbach, F.; Marnewick, J.L.; Joseph, D.E.; Essop, M.F. The impact of chronic stress on intracellular redox balance: A systems level analysis. Physiol. Rep. 2023, 11, e15640. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, W.; Wang, J.; Wu, S.; Linghu, T.; Ge, J.; Zhang, R. Effects of MMP8 inhibitors on chronic unpredictable mild stress-induced neuroinflammation and depressive-like behavior: Exploring the underlying molecular mechanisms. Psychopharmacology, 2025; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Marcon, M.; Mocelin, R.; Sachett, A.; Siebel, A.M.; Herrmann, A.P.; Piato, A. Enriched environment prevents oxidative stress in zebrafish submitted to unpredictable chronic stress. PeerJ 2018, 6, e5136. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Hemmati, M.A.; Nasimi, F.; Pakdel, R.; Jamialahmadi, T.; Sahebkar, A. Empagliflozin alleviates diabetes-induced cognitive impairments by lowering nicotinamide adenine dinucleotide phosphate oxidase-4 expression and potentiating the antioxidant defense system in brain tissue of diabetic rats. Behav. Brain Res. 2024, 460, 114830. [Google Scholar] [CrossRef]
- Pradhan, L.K.; Sahoo, P.K.; Chauhan, N.R.; Das, S.K. Temporal exposure to chronic unpredictable stress induces precocious neurobehavioral deficits by distorting neuromorphology and glutathione biosynthesis in zebrafish brain. Behav. Brain Res. 2022, 418, 113672. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, J.-S.; Jang, H.-J.; Kim, S.-M.; Chang, M.S.; Park, S.H.; Kim, K.S.; Bae, J.; Park, J.-W.; Lee, B.J. Chlorogenic acid ameliorates brain damage and edema by inhibiting matrix metalloproteinase-2 and 9 in a rat model of focal cerebral ischemia. Eur. J. Pharmacol. 2012, 689, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, A.T.; Santin, J.R.; Machado, I.D.; de Oliveira e Silva, A.M.; de Melo, I.L.P.; Mancini-Filho, J.; Farsky, S.H. Antiulcerogenic activity of chlorogenic acid in different models of gastric ulcer. Naunyn. Schmiedebergs Arch. Pharmacol. 2013, 386, 5–14. [Google Scholar] [CrossRef]
- Orhan, S.; Turkmen, R.; Demirel, H.H.; Akosman, M.S.; Turkmen, T.; Fırat, F. Chlorogenic acid mitigates potassium dichromate-induced acute hepato-nephrotoxicity by attenuating the NF-κB signalling pathway. Mol. Biol. Rep. 2024, 51, 798. [Google Scholar] [CrossRef]
- Metwally, D.M.; Alajmi, R.A.; El-Khadragy, M.F.; Yehia, H.M.; Al-Megrin, W.A.; Akabawy, A.M.A.; Amin, H.K.; Abdel Moneim, A.E. Chlorogenic acid confers robust neuroprotection against arsenite toxicity in mice by reversing oxidative stress, inflammation, and apoptosis. J. Funct. Foods 2020, 75, 104202. [Google Scholar] [CrossRef]
- Singh, S.S.; Rai, S.N.; Birla, H.; Zahra, W.; Rathore, A.S.; Dilnashin, H.; Singh, R.; Singh, S.P. Neuroprotective Effect of Chlorogenic Acid on Mitochondrial Dysfunction-Mediated Apoptotic Death of da Neurons in a Parkinsonian Mouse Model. Oxid. Med. Cell. Longev. 2020, 2020, 6571484. [Google Scholar] [CrossRef]
- He, L.; Sun, Y. The potential role of Keap1-Nrf2 pathway in the pathogenesis of Alzheimer’s disease, type 2 diabetes, and type 2 diabetes-related Alzheimer’s disease. Metab. Brain Dis. 2021, 36, 1469–1479. [Google Scholar] [CrossRef]
- Alizade, S.; Faramarzi, M.; Banitalebi, E.; Saghaei, E. Effect of resistance and endurance training with ursolic acid on oxidative stress and cognitive impairment in hippocampal tissue in HFD/STZ-induced aged diabetic rats. Iran. J. Basic. Med. Sci. 2023, 26, 1449–1459. [Google Scholar]
- Zhao, Q.; Zhang, F.; Yu, Z.; Guo, S.; Liu, N.; Jiang, Y.; Lo, E.H.; Xu, Y.; Wang, X. HDAC3 inhibition prevents blood-brain barrier permeability through Nrf2 activation in type 2 diabetes male mice. J. Neuroinflamm. 2019, 16, 103. [Google Scholar] [CrossRef]
- Yu, Z.; Lin, L.; Jiang, Y.; Chin, I.; Wang, X.; Li, X.; Lo, E.H.; Wang, X. Recombinant FGF21 Protects Against Blood-Brain Barrier Leakage Through Nrf2 Upregulation in Type 2 Diabetes Mice. Mol. Neurobiol. 2019, 56, 2314–2327. [Google Scholar] [CrossRef]
- Yao, J.; Peng, S.; Xu, J.; Fang, J. Reversing ROS-mediated neurotoxicity by chlorogenic acid involves its direct antioxidant activity and activation of Nrf2-ARE signaling pathway. BioFactors 2019, 45, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.W.; Boo, Y.C. Siegesbeckiae herba extract and chlorogenic acid ameliorate the death of hacat keratinocytes exposed to airborne particulate matter by mitigating oxidative stress. Antioxidants 2021, 10, 1762. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Gu, X.; Gao, J.; Wang, Z.; Liu, G.; Barkema, H.W.; Han, B. Chlorogenic acid promotes the Nrf2/HO-1 anti-oxidative pathway by activating p21 Waf1/Cip1 to resist dexamethasone-induced apoptosis in osteoblastic cells. Free. Radic. Biol. Med. 2019, 137, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Zheng, Z.; Shi, L.; Jin, Y.; Ji, L. Natural polyphenol chlorogenic acid protects against acetaminophen-induced hepatotoxicity by activating erk/nrf2 antioxidative pathway. Toxicol. Sci. 2018, 162, 99–112. [Google Scholar] [CrossRef]
- Lal, P.; Kawakami, K.J. Integrated behavioral, genetic and brain circuit visualization methods to unravel functional anatomy of zebrafish amygdala. Front. Neuroanat. 2022, 16, 837527. [Google Scholar] [CrossRef]
- Wullimann, M.F. Good heavens! Finally a landslide analysis of basal ganglia circuitry in teleosts. Cell Rep. 2024, 43, 113915. [Google Scholar] [CrossRef]
- Kumar, S.; Chowdhury, S.; Razdan, A.; Kumari, D.; Purty, R.S.; Ram, H.; Kumar, P.; Nayak, P.; Shukla, S.D. Downregulation of Candidate Gene Expression and Neuroprotection by Piperine in Streptozotocin-Induced Hyperglycemia and Memory Impairment in Rats. Front. Pharmacol. 2021, 11, 595471. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Q.; Shen, L.; Guo, K.; Zhou, X. Chlorogenic acid improves glucose tolerance, lipid metabolism, inflammation and microbiota composition in diabetic db/db mice. Front. Endocrinol. 2022, 13, 1042044. [Google Scholar] [CrossRef]
- Feng, Y.; Chu, A.; Luo, Q.; Wu, M.; Shi, X.; Chen, Y. The Protective Effect of Astaxanthin on Cognitive Function via Inhibition of Oxidative Stress and Inflammation in the Brains of Chronic T2DM Rats. Front. Pharmacol. 2018, 9, 748. [Google Scholar] [CrossRef]
- Li, C.; Ge, H.; Huang, J.; Si, L.; Sun, L.; Wu, L.; Xiao, L.; Xie, Y.; Wang, G. Resveratrol alleviates depression-like behaviors by inhibiting ferroptosis via AKT/NRF2 pathway. Brain Res. Bull. 2025, 220, 111136. [Google Scholar] [CrossRef]
- Storz, P. Forkhead homeobox type O transcription factors in the responses to oxidative stress. Antioxid. Redox Signal. 2011, 14, 593–605. [Google Scholar] [CrossRef]
- Kwak, M.K.; Itoh, K.; Yamamoto, M.; Kensler, T.W. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: Role of antioxidant response element-like sequences in the nrf2 promoter. Mol. Cell. Biol. 2002, 22, 2883–2892. [Google Scholar] [CrossRef]
- Schmidt, R.; Strähle, U.; Scholpp, S. Neurogenesis in zebrafish-from embryo to adult. Neural Dev. 2013, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Jurisch-Yaksi, N.; Yaksi, E.; Kizil, C. Radial glia in the zebrafish brain: Functional, structural, and physiological comparison with the mammalian glia. Glia 2020, 68, 2451–2470. [Google Scholar] [CrossRef] [PubMed]
- Li, L.F.; Yang, J.; Ma, S.P.; Qu, R. Magnolol treatment reversed the glial pathology in an unpredictable chronic mild stress-induced rat model of depression. Eur. J. Pharmacol. 2013, 711, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Tang, Y.Y.; Zhu, W.W.; Li, C.; Zhang, P.; Li, R.Q.; Chen, Y.J.; Zou, W.; Tang, X.Q. PI3K/AKT pathway mediates the antidepressant- and anxiolytic-like roles of hydrogen sulfide in streptozotocin-induced diabetic rats via promoting hippocampal neurogenesis. NeuroToxicology 2021, 85, 201–208. [Google Scholar] [CrossRef]
- Li, J.F.; Hu, W.Y.; Chang, H.X.; Bao, J.H.; Kong, X.X.; Ma, H.; Li, Y.F. Astrocytes underlie a faster-onset antidepressant effect of hypidone hydrochloride (YL-0919). Front. Pharmacol. 2023, 14, 1175938. [Google Scholar] [CrossRef]
- Rappeneau, V.; Blaker, A.; Petro, J.R.; Yamamoto, B.K.; Shimamoto, A. Disruption of the Glutamate-Glutamine Cycle Involving Astrocytes in an Animal Model of Depression for Males and Females. Front. Behav. Neurosci. 2016, 10, 231. [Google Scholar] [CrossRef]
- Imbe, H.; Kimura, A.; Donishi, T.; Kaneoke, Y. Chronic restraint stress decreases glial fibrillary acidic protein and glutamate transporter in the periaqueductal gray matter. Neuroscience 2012, 223, 209–218. [Google Scholar] [CrossRef]
- Wen, G.; Zhan, X.; Xu, X.; Xia, X.; Jiang, S.; Ren, X.; Ren, W.; Lou, H.; Lu, L.; Hermenean, A.; et al. Ketamine Improves the Glymphatic Pathway by Reducing the Pyroptosis of Hippocampal Astrocytes in the Chronic Unpredictable Mild Stress Model. Mol. Neurobiol. 2024, 61, 2049–2062. [Google Scholar] [CrossRef]
- Nagayach, A.; Patro, N.; Patro, I. Astrocytic and microglial response in experimentally induced diabetic rat brain. Metab. Brain Dis. 2014, 29, 747–761. [Google Scholar] [CrossRef]
- Jiao, X.H.; Wan, J.; Wu, W.F.; Ma, L.H.; Chen, C.; Dong, W.; Liu, Y.Q.; Jin, C.H.; Sun, A.; Zhou, Y.; et al. GLT-1 downregulation in hippocampal astrocytes induced by type 2 diabetes contributes to postoperative cognitive dysfunction in adult mice. CNS Neurosci. Ther. 2024, 30, e70024. [Google Scholar] [CrossRef]
- Tsai, S.F.; Wu, H.T.; Chen, P.C.; Chen, Y.W.; Yu, M.; Wang, T.F.; Wu, S.Y.; Tzeng, S.F.; Kuo, Y.M. High-fat diet suppresses the astrocytic process arborization and downregulates the glial glutamate transporters in the hippocampus of mice. Brain Res. 2018, 1700, 66–77. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, C.; Huang, J.; Tang, X.; Liu, C.; Huang, K.; Xu, J.; Guo, G.; Tong, A.; Zhou, L. The role of astrocytes in oxidative stress of central nervous system: A mixed blessing. Cell Prolif. 2020, 53, e12781. [Google Scholar] [CrossRef]
- Wang, J.J.; Li, Y.J.; Lai, K.; Zhong, Q.M.; Demin, K.A.; Kalueff, A.V.; Song, C. High-glucose/high-cholesterol diet in zebrafish evokes diabetic and affective pathogenesis: The role of peripheral and central inflammation, microglia and apoptosis. Prog. Neuro-Psychopharmacol. Biol. Psych. 2020, 96, 109752. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, B.P.; Zhang, Y.P.; Peng, Z.; Wang, J.; Collier, A.D.; Echevarria, D.J.; Savelieva, K.V.; Lawrence, R.F.; Rex, C.S.; et al. Modeling consequences of prolonged strong unpredictable stress in zebrafish: Complex effects on behavior and physiology. Prog. Neuro-Psychopharmacol. Biol. Psych. 2018, 81, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Abd El Wahab, M.G.; Ayuob, N.N.; Suliaman, M. The antidepressant-like effect of Ocimum basilicum in an animal model of depression. Biotech. Histochem. 2017, 92, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Codeluppi, S.A.; Chatterjee, D.; Prevot, T.D.; Bansal, Y.; Misquitta, K.A.; Sibille, E.; Banasr, M. Chronic Stress Alters Astrocyte Morphology in Mouse Prefrontal Cortex. Int. J. Neuropsychopharmacol. 2021, 24, 842–853. [Google Scholar] [CrossRef]
- Abdel Fattah, S.; Waly, H.; El-enein, A.A.; kamel, A.; Labib, H. Mesenchymal stem cells versus curcumin in enhancing the alterations in the cerebellar cortex of streptozocin-induced diabetic albino rats. The role of GFAP, PLC and α-synuclein. J. Chem. Neuroanat. 2020, 109, 101842. [Google Scholar] [CrossRef]
- Calkins, M.J.; Vargas, M.R.; Johnson, D.A.; Johnson, J.A.J.T.S. Astrocyte-specific overexpression of Nrf2 protects striatal neurons from mitochondrial complex II inhibition. Toxicol Sci. 2010, 115, 557–568. [Google Scholar] [CrossRef]
- Vargas, M.R.; Johnson, D.A.; Sirkis, D.W.; Messing, A.; Johnson, J.A. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J. Neurosci. 2008, 28, 13574–13581. [Google Scholar] [CrossRef]
- Miyazaki, I.; Isooka, N.; Wada, K.; Kikuoka, R.; Kitamura, Y.; Asanuma, M. Effects of enteric environmental modification by coffee components on neurodegeneration in Rotenone-Treated Mice. Cells 2019, 8, 221. [Google Scholar] [CrossRef]


| Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Accession ID | |
|---|---|---|---|
| nrf2 | AACGAGTTCTCCCTTCAGCA | ATTTTGTCGCCGATTTTGTC | BC152659 |
| keap1 | TGGATAACTACCTCTATGCCGT | CCTTGGTTAAATCCACCTAACAC | NM_182864 |
| sod1 | CAATGCTAACTTTGTCAGGCCA | CCTTCCCCAAGTCATCCTCC | BC165134 |
| sod2 | CTTGGGATAGATGTCTGGG | GTGGTCTGATTAATTGTGCG | XM_057353025 |
| catalase | AACCAACAACCCTCCAGACAG | TCCGCTCTCGGTCAAAATGG | NM_130912 |
| gpx | AGATGTCATTCCTGCACACG | AAGGAGAAGCTTCCTCAGCC | AY216589 |
| gclc | AACCGACACCCAAAGATTCAGCACT | CCATCATCCTCTGGAAACACCTCC | XM_031693913 |
| gfap | GGATGCAGCCAATCGTAAT | TTCCAGGTCACAGGTCAG | AH012040 |
| β-actin | CGAGCAGGAGATGGGAACC | CAACGGAAACGCTCATTGC | BC045846 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subba, R.; Fasciolo, G.; Petito, A.; Geremia, E.; Muscari Tomajoli, M.T.; Mondal, A.C.; Napolitano, G.; Venditti, P. Chlorogenic Acid Alleviates the Detrimental Effects of Concurrent Hyperglycemia and Chronic Stress on Brain Homeostasis by Modulating Antioxidative Defense in Adult Zebrafish. Antioxidants 2025, 14, 1386. https://doi.org/10.3390/antiox14121386
Subba R, Fasciolo G, Petito A, Geremia E, Muscari Tomajoli MT, Mondal AC, Napolitano G, Venditti P. Chlorogenic Acid Alleviates the Detrimental Effects of Concurrent Hyperglycemia and Chronic Stress on Brain Homeostasis by Modulating Antioxidative Defense in Adult Zebrafish. Antioxidants. 2025; 14(12):1386. https://doi.org/10.3390/antiox14121386
Chicago/Turabian StyleSubba, Rhea, Gianluca Fasciolo, Adriana Petito, Eugenio Geremia, Maria Teresa Muscari Tomajoli, Amal Chandra Mondal, Gaetana Napolitano, and Paola Venditti. 2025. "Chlorogenic Acid Alleviates the Detrimental Effects of Concurrent Hyperglycemia and Chronic Stress on Brain Homeostasis by Modulating Antioxidative Defense in Adult Zebrafish" Antioxidants 14, no. 12: 1386. https://doi.org/10.3390/antiox14121386
APA StyleSubba, R., Fasciolo, G., Petito, A., Geremia, E., Muscari Tomajoli, M. T., Mondal, A. C., Napolitano, G., & Venditti, P. (2025). Chlorogenic Acid Alleviates the Detrimental Effects of Concurrent Hyperglycemia and Chronic Stress on Brain Homeostasis by Modulating Antioxidative Defense in Adult Zebrafish. Antioxidants, 14(12), 1386. https://doi.org/10.3390/antiox14121386











