Antioxidants Trolox and Methazolamide Protect Microvascular Endothelial Cells from Oxidative Damage Induced by Sporadic and Familial Forms of Oligomeric Amyloid-β
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthetic Peptides
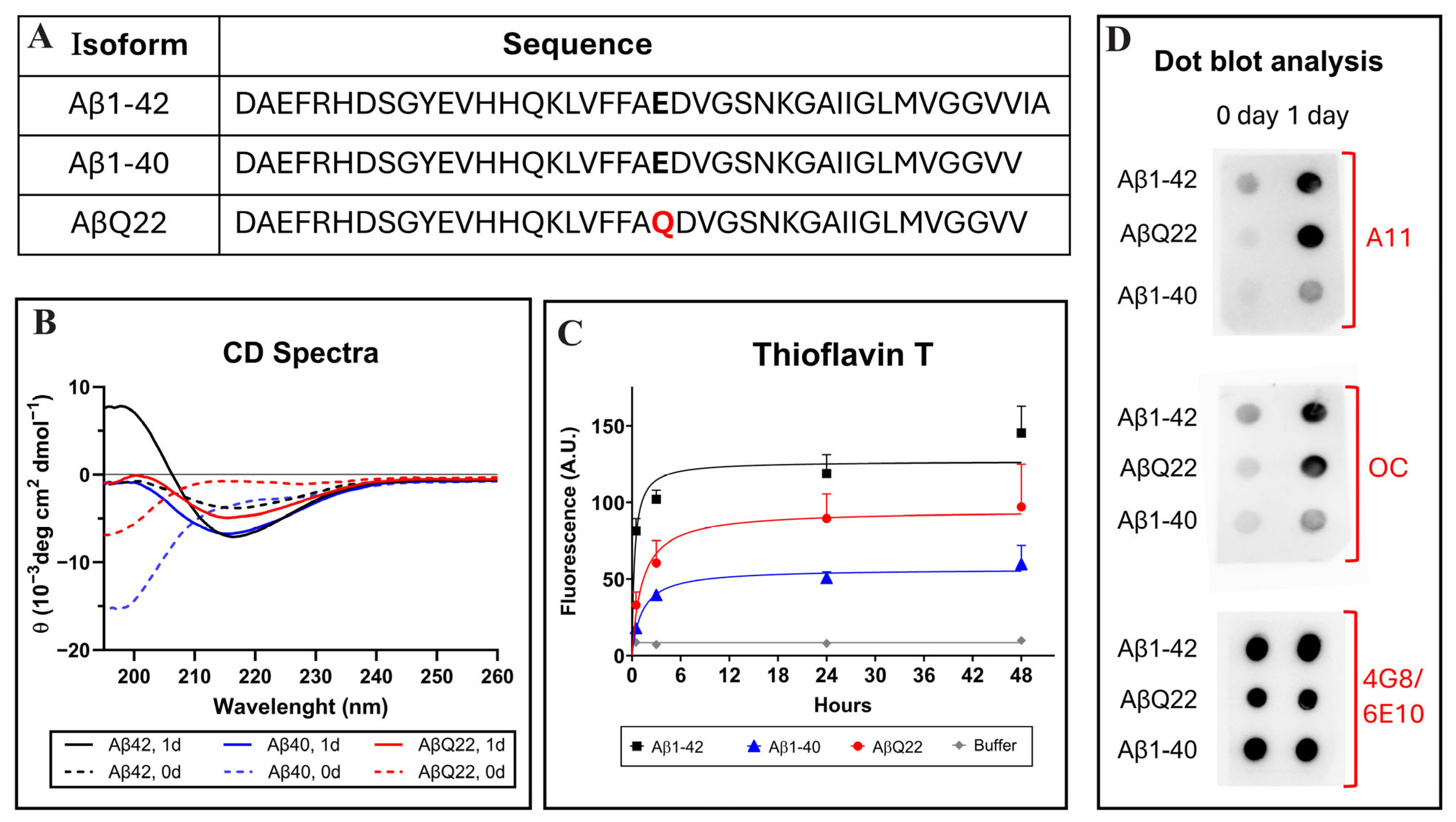
2.3. Thioflavin T Binding Assay
2.4. Circular Dichroism Spectroscopy
2.5. Assessment of Peptide Conformations by Dot Blot Analysis
2.6. Cell Culture Experiments
2.7. Detection of Reactive Oxygen Species
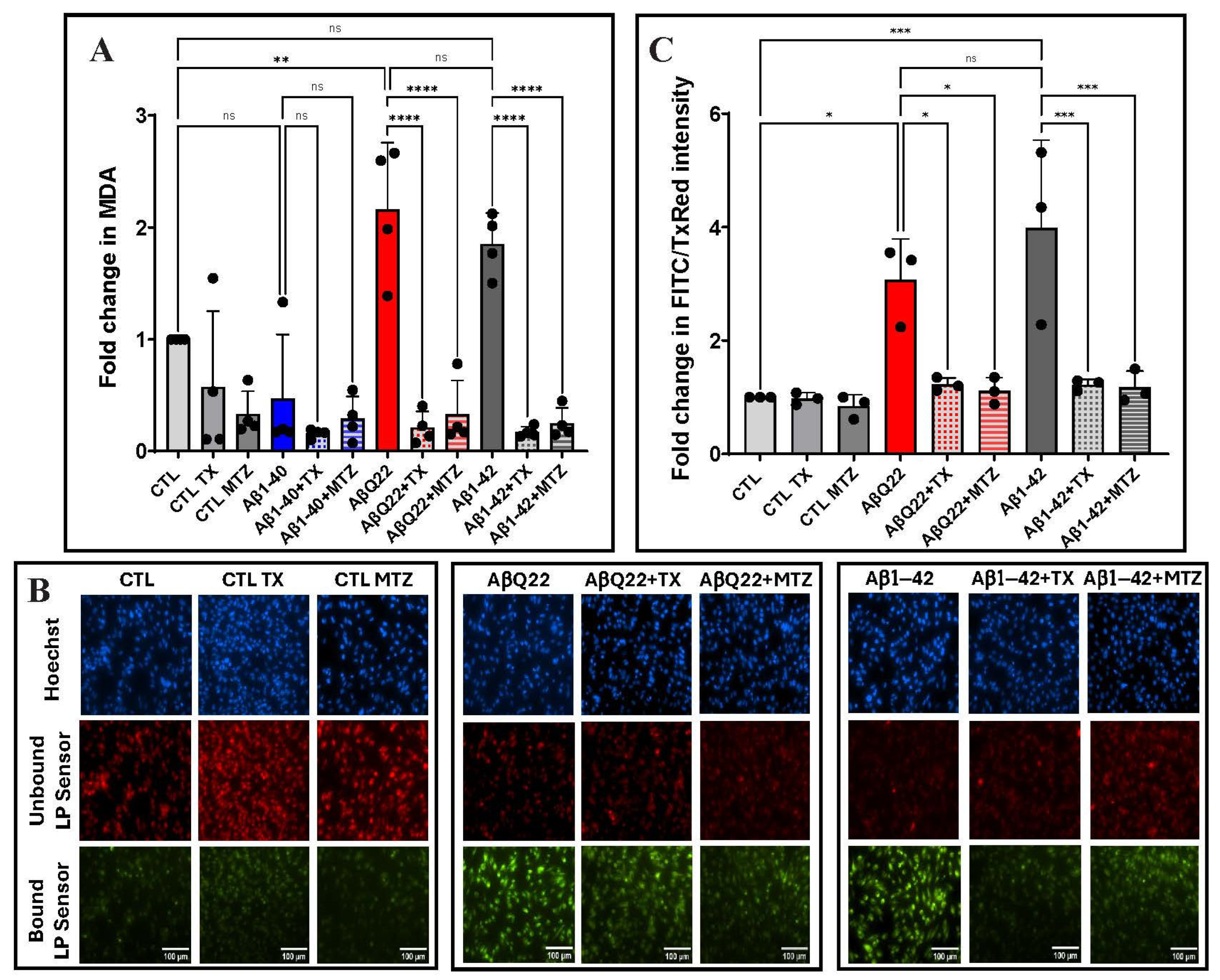
2.8. Detection of Lipid Peroxidation
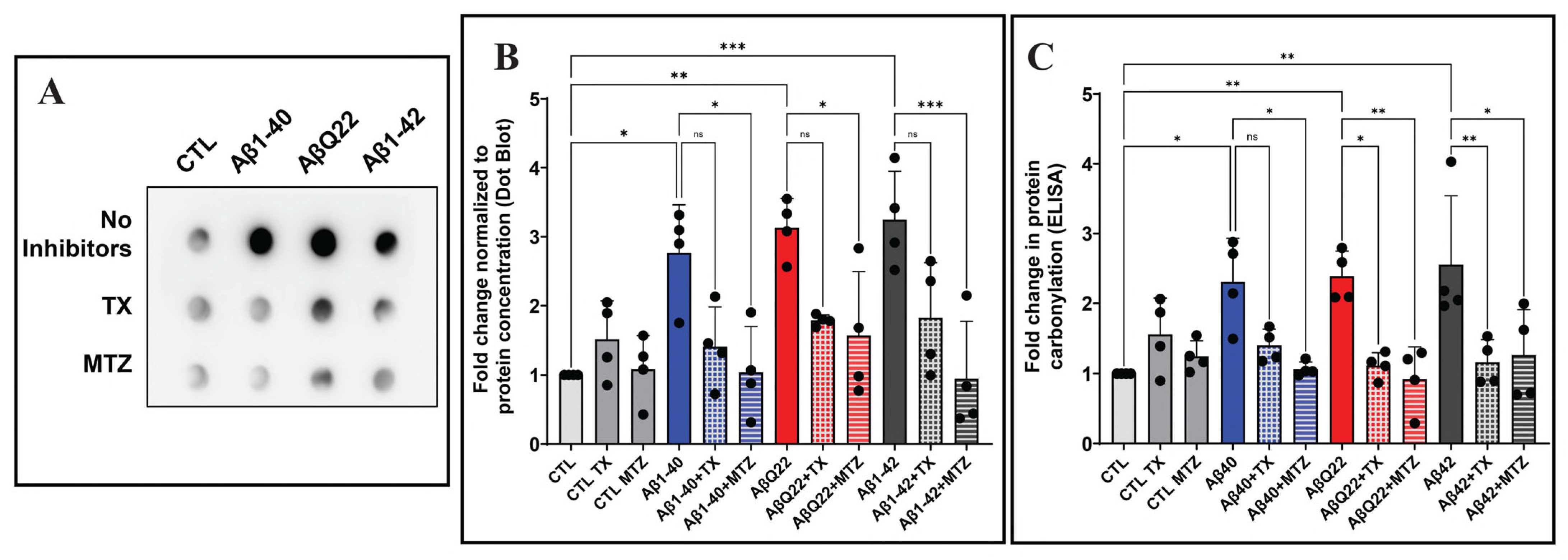
2.9. Detection of Protein Carbonylation
2.10. Statistical Analysis
3. Results
3.1. Structural and Aggregation Properties of Aβ1-42, Aβ1-40, and AβQ22 Dutch Variant
3.2. Aβ-Mediated ROS Generation in Cerebral Microvascular ECs and Protection by Antioxidants
3.3. Aβ-Mediated Lipid Peroxidation in Cerebral Microvascular ECs and Protection by Antioxidants
3.4. Aβ-Mediated Protein Carbonylation in Cerebral EC and Protection by Antioxidants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rostagno, A.; Holton, J.L.; Lashley, T.; Revesz, T.; Ghiso, J. Cerebral amyloidosis: Amyloid subunits, mutants and phenotypes. Cell. Mol. Life Sci. 2010, 67, 581–600. [Google Scholar] [CrossRef]
- Roher, A.E.; Lowenson, J.D.; Clarke, S.; Woods, A.S.; Cotter, R.J.; Gowing, E.; Ball, M.J. Beta-Amyloid-(1–42) is a major component of cerebrovascular amyloid deposits: Implications for the pathology of Alzheimer disease. Proc. Natl. Acad. Sci. USA 1993, 90, 10836–10840. [Google Scholar] [CrossRef]
- Biffi, A.; Greenberg, S.M. Cerebral amyloid angiopathy: A systematic review. J. Clin. Neurol. 2011, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.M.; Bacskai, B.J.; Hernandez-Guillamon, M.; Pruzin, J.; Sperling, R.; van Veluw, S.J. Cerebral amyloid angiopathy and Alzheimer disease—One peptide, two pathways. Nat. Rev. Neurol. 2020, 16, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Soontornniyomkij, V.; Choi, C.; Pomakian, J.; Vinters, H.V. High-definition characterization of cerebral β-amyloid angiopathy in Alzheimer’s disease. Hum. Pathol. 2010, 41, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Attems, J.; Lintner, F.; Jellinger, K.A. Amyloid beta peptide 1-42 highly correlates with capillary cerebral amyloid angiopathy and Alzheimer disease pathology. Acta Neuropathol. 2004, 107, 283–291. [Google Scholar] [CrossRef]
- Ghiso, J.; Fossati, S.; Rostagno, A. Amyloidosis associated with cerebral amyloid angiopathy: Cell signaling pathways elicited in cerebral endothelial cells. J. Alzheimers Dis. 2014, 42 (Suppl. S3), S167–S176. [Google Scholar] [CrossRef]
- Levy, E.; Carman, M.D.; Fernandez-Madrid, I.J.; Power, M.D.; Lieberburg, I.; van Duinen, S.G.; Bots, G.T.; Luyendijk, W.; Frangione, B. Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science 1990, 248, 1124–1126. [Google Scholar] [CrossRef]
- Monro, O.R.; Mackic, J.B.; Yamada, S.; Segal, M.B.; Ghiso, J.; Maurer, C.; Calero, M.; Frangione, B.; Zlokovic, B.V. Substitution at codon 22 reduces clearance of Alzheimer’s amyloid-beta peptide from the cerebrospinal fluid and prevents its transport from the central nervous system into blood. Neurobiol. Aging 2002, 23, 405–412. [Google Scholar] [CrossRef]
- Grinberg, L.T.; Korczyn, A.D.; Heinsen, H. Cerebral amyloid angiopathy impact on endothelium. Exp. Gerontol. 2012, 47, 838–842. [Google Scholar] [CrossRef]
- Revesz, T.; Holton, J.L.; Lashley, T.; Plant, G.; Frangione, B.; Rostagno, A.; Ghiso, J. Genetics and molecular pathogenesis of sporadic and hereditary cerebral amyloid angiopathies. Acta Neuropathol. 2009, 118, 115–130. [Google Scholar] [CrossRef]
- Irizarry, B.; Davis, J.; Rajpoot, J.; Zhu, X.; Xu, F.; Smith, S.O.; Van Nostrand, W.E. Aβ40 Fibril Assembly on Human Cerebral Smooth Muscle Cells Impairs Cell Viability. Biochemistry 2025, 64, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Guo, S.; van Veluw, S.J.; Yu, Z.; Chan, S.J.; Takase, H.; Arai, K.; Ning, M.; Greenberg, S.M.; Lo, E.H.; et al. Effects of cerebral amyloid angiopathy on the brain vasculome. Aging Cell 2022, 21, e13503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Wang, J.; Zhang, Z.N.; Su, Q.; Guo, J.H. The relationship between amyloid-beta and brain capillary endothelial cells in Alzheimer’s disease. Neural. Regen. Res. 2022, 17, 2355–2363. [Google Scholar] [PubMed]
- Chen, J.; Xiang, P.; Duro-Castano, A.; Cai, H.; Guo, B.; Liu, X.; Yu, Y.; Lui, S.; Luo, K.; Ke, B.; et al. Rapid amyloid-β clearance and cognitive recovery through multivalent modulation of blood-brain barrier transport. Signal Transduct. Target. Ther. 2025, 10, 331. [Google Scholar] [CrossRef]
- Koizumi, K.; Wang, G.; Park, L. Endothelial Dysfunction and Amyloid-β-Induced Neurovascular Alterations. Cell. Mol. Neurobiol. 2016, 36, 155–165. [Google Scholar] [CrossRef]
- Parodi-Rullán, R.; Sone, J.Y.; Fossati, S. Endothelial Mitochondrial Dysfunction in Cerebral Amyloid Angiopathy and Alzheimer’s Disease. J. Alzheimers Dis. 2019, 72, 1019–1039. [Google Scholar] [CrossRef]
- Parodi-Rullán, R.M.; Javadov, S.; Fossati, S. Dissecting the Crosstalk between Endothelial Mitochondrial Damage, Vascular Inflammation, and Neurodegeneration in Cerebral Amyloid Angiopathy and Alzheimer’s Disease. Cells 2021, 10, 2903. [Google Scholar] [CrossRef]
- Kook, S.Y.; Hong, H.S.; Moon, M.; Ha, C.M.; Chang, S.; Mook-Jung, I. Aβ1-42-RAGE interaction disrupts tight junctions of the blood-brain barrier via Ca2+-calcineurin signaling. J. Neurosci. 2012, 32, 8845–8854. [Google Scholar] [CrossRef]
- Carrano, A.; Hoozemans, J.J.; van der Vies, S.M.; Rozemuller, A.J.; van Horssen, J.; de Vries, H.E. Amyloid Beta induces oxidative stress-mediated blood-brain barrier changes in capillary amyloid angiopathy. Antioxid. Redox Signal. 2011, 15, 1167–1178. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Wang, J.; He, L.; Lai, H.; Zhang, T.; Wang, X.; Li, W. Mitochondrial oxidative stress in brain microvascular endothelial cells: Triggering blood-brain barrier disruption. Mitochondrion 2023, 69, 71–82. [Google Scholar] [CrossRef]
- Petrushanko, I.Y.; Mitkevich, V.A.; Makarov, A.A. Effect of β-amyloid on blood-brain barrier properties and function. Biophys. Rev. 2023, 15, 183–197. [Google Scholar] [CrossRef]
- He, J.T.; Zhao, X.; Xu, L.; Mao, C.Y. Vascular Risk Factors and Alzheimer’s Disease: Blood-Brain Barrier Disruption, Metabolic Syndromes, and Molecular Links. J. Alzheimers Dis. 2020, 73, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, W.H.; Zhao, J.S.; Meng, F.Z.; Wang, H. Lutein protects against β-amyloid peptide-induced oxidative stress in cerebrovascular endothelial cells through modulation of Nrf-2 and NF-κb. Cell Biol. Toxicol. 2017, 33, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, M.; Barzegari, A.; Letourneur, D.; Gueguen, V.; Pavon-Djavid, G. Oxidative Stress Regulation on Endothelial Cells by Hydrophilic Astaxanthin Complex: Chemical, Biological, and Molecular Antioxidant Activity Evaluation. Oxidative Med. Cell. Longev. 2017, 2017, 8073798. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, W.; Liu, X.; Wu, L.; He, G.; Li, P.; Guo, X.; Chen, Z.; Huang, Q. Apocynin protects endothelial cells from endoplasmic reticulum stress-induced apoptosis via IRE1α engagement. Mol. Cell. Biochem. 2018, 449, 257–265. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Rivas, F.; Poblete-Aro, C.; Pando, M.E.; Allel, M.J.; Fernandez, V.; Soto, A.; Nova, P.; Garcia-Diaz, D. Effects of Polyphenols in Aging and Neurodegeneration Associated with Oxidative Stress. Curr. Med. Chem. 2022, 29, 1045–1060. [Google Scholar] [CrossRef]
- Pasinetti, G.M.; Wang, J.; Ho, L.; Zhao, W.; Dubner, L. Roles of resveratrol and other grape-derived polyphenols in Alzheimer’s disease prevention and treatment. Biochim. Biophys. Acta 2015, 1852, 1202–1208. [Google Scholar] [CrossRef]
- Tahir, M.; Kang, M.H.; Park, T.J.; Ali, J.; Choe, K.; Park, J.S.; Kim, M.O. Multifaceted neuroprotective approach of Trolox in Alzheimer’s disease mouse model: Targeting Aβ pathology, neuroinflammation, oxidative stress, and synaptic dysfunction. Front. Cell. Neurosci. 2024, 18, 1453038. [Google Scholar] [CrossRef]
- Quintanilla, R.A.; Muñoz, F.J.; Metcalfe, M.J.; Hitschfeld, M.; Olivares, G.; Godoy, J.A.; Inestrosa, N.C. Trolox and 17β-Estradiol Protect against Amyloid β-Peptide Neurotoxicity by a Mechanism That Involves Modulation of the Wnt Signaling Pathway*. J. Biol. Chem. 2005, 280, 11615–11625. [Google Scholar] [CrossRef]
- Akhter, H.; Katre, A.; Li, L.; Liu, X.; Liu, R.M. Therapeutic potential and anti-amyloidosis mechanisms of tert-butylhydroquinone for Alzheimer’s disease. J. Alzheimers Dis. 2011, 26, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Koppal, T.; Subramaniam, R.; Drake, J.; Prasad, M.R.; Dhillon, H.; Butterfield, D.A. Vitamin E protects against Alzheimer’s amyloid peptide (25–35)-induced changes in neocortical synaptosomal membrane lipid structure and composition. Brain Res. 1998, 786, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, F.J.; Opazo, C.; Gil-Gómez, G.; Tapia, G.; Fernández, V.; Valverde, M.A.; Inestrosa, N.C. Vitamin E But Not 17β-Estradiol Protects against Vascular Toxicity Induced by β-Amyloid Wild Type and the Dutch Amyloid Variant. J. Neurosci. 2002, 22, 3081–3089. [Google Scholar] [CrossRef]
- Solesio, M.E.; Peixoto, P.M.; Debure, L.; Madamba, S.M.; de Leon, M.J.; Wisniewski, T.; Pavlov, E.V.; Fossati, S. Carbonic anhydrase inhibition selectively prevents amyloid β neurovascular mitochondrial toxicity. Aging Cell 2018, 17, e12787. [Google Scholar] [CrossRef]
- Fossati, S.; Giannoni, P.; Solesio, M.E.; Cocklin, S.L.; Cabrera, E.; Ghiso, J.; Rostagno, A. The carbonic anhydrase inhibitor methazolamide prevents amyloid beta-induced mitochondrial dysfunction and caspase activation protecting neuronal and glial cells in vitro and in the mouse brain. Neurobiol. Dis. 2016, 86, 29–40. [Google Scholar] [CrossRef]
- Wang, X.; Figueroa, B.E.; Stavrovskaya, I.G.; Zhang, Y.; Sirianni, A.C.; Zhu, S.; Day, A.L.; Kristal, B.S.; Friedlander, R.M. Methazolamide and Melatonin Inhibit Mitochondrial Cytochrome C Release and Are Neuroprotective in Experimental Models of Ischemic Injury. Stroke 2009, 40, 1877–1885. [Google Scholar] [CrossRef]
- Sarang, S.S.; Yoshida, T.; Cadet, R.; Valeras, A.S.; Jensen, R.V.; Gullans, S.R. Discovery of molecular mechanisms of neuroprotection using cell-based bioassays and oligonucleotide arrays. Physiol. Genom. 2002, 11, 45–52. [Google Scholar] [CrossRef]
- Anzovino, A.; Canepa, E.; Alves, M.; Lemon, N.L.; Carare, R.O.; Fossati, S. Amyloid Beta Oligomers Activate Death Receptors and Mitochondria-Mediated Apoptotic Pathways in Cerebral Vascular Smooth Muscle Cells; Protective Effects of Carbonic Anhydrase Inhibitors. Cells 2023, 12, 2840. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Rostagno, A.; Ghiso, J. Alzheimer’s Disease Pathogenic Mechanisms: Linking Redox Homeostasis and Mitochondria-Associated Metabolic Pathways Through Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2). Antioxidants 2025, 14, 812. [Google Scholar] [CrossRef]
- Sotolongo, K.; Ghiso, J.; Rostagno, A. Nrf2 activation through the PI3K/GSK-3 axis protects neuronal cells from Aβ-mediated oxidative and metabolic damage. Alzheimers Res. Ther. 2020, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Fossati, S.; Cam, J.; Meyerson, J.; Mezhericher, E.; Romero, I.A.; Couraud, P.O.; Weksler, B.B.; Ghiso, J.; Rostagno, A. Differential activation of mitochondrial apoptotic pathways by vasculotropic amyloid-beta variants in cells composing the cerebral vessel walls. FASEB J. 2010, 24, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Allan Butterfield, D.; Castegna, A.; Lauderback, C.M.; Drake, J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. Neurobiol. Aging 2002, 23, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Perluigi, M.; Di Domenico, F.; Butterfield, D.A. Oxidative damage in neurodegeneration: Roles in the pathogenesis and progression of Alzheimer disease. Physiol. Rev. 2024, 104, 103–197. [Google Scholar] [CrossRef]
- Solito, R.; Corti, F.; Fossati, S.; Mezhericher, E.; Donnini, S.; Ghiso, J.; Giachetti, A.; Rostagno, A.; Ziche, M. Dutch and Arctic mutant peptides of beta amyloid(1-40) differentially affect the FGF-2 pathway in brain endothelium. Exp. Cell Res. 2009, 315, 385–395. [Google Scholar] [CrossRef]
- Rostagno, A.; Cabrera, E.; Lashley, T.; Ghiso, J. N-terminally truncated Aβ4-x proteoforms and their relevance for Alzheimer’s pathophysiology. Transl. Neurodegener. 2022, 11, 30. [Google Scholar] [CrossRef]
- Viana, R.J.; Nunes, A.F.; Castro, R.E.; Ramalho, R.M.; Meyerson, J.; Fossati, S.; Ghiso, J.; Rostagno, A.; Rodrigues, C.M. Tauroursodeoxycholic acid prevents E22Q Alzheimer’s Abeta toxicity in human cerebral endothelial cells. Cell. Mol. Life Sci. 2009, 66, 1094–1104. [Google Scholar] [CrossRef]
- Kayed, R.; Head, E.; Sarsoza, F.; Saing, T.; Cotman, C.W.; Necula, M.; Margol, L.; Wu, J.; Breydo, L.; Thompson, J.L.; et al. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol. Neurodegener. 2007, 2, 18. [Google Scholar] [CrossRef]
- Kayed, R.; Head, E.; Thompson, J.L.; McIntire, T.M.; Milton, S.C.; Cotman, C.W.; Glabe, C.G. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 2003, 300, 486–489. [Google Scholar] [CrossRef]
- Weksler, B.; Romero, I.A.; Couraud, P.O. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 2013, 10, 16. [Google Scholar] [CrossRef]
- Parodi-Rullán, R.; Ghiso, J.; Cabrera, E.; Rostagno, A.; Fossati, S. Alzheimer’s amyloid β heterogeneous species differentially affect brain endothelial cell viability, blood-brain barrier integrity, and angiogenesis. Aging Cell 2020, 19, e13258. [Google Scholar] [CrossRef]
- Valle, M.L.; Anderson, Y.T.; Grimsey, N.; Zastre, J. Thiamine insufficiency induces Hypoxia Inducible Factor-1α as an upstream mediator for neurotoxicity and AD-like pathology. Mol. Cell. Neurosci. 2022, 123, 103785. [Google Scholar] [CrossRef] [PubMed]
- Foret, M.K.; Orciani, C.; Welikovitch, L.A.; Huang, C.; Cuello, A.C.; Do Carmo, S. Early oxidative stress and DNA damage in Aβ-burdened hippocampal neurons in an Alzheimer’s-like transgenic rat model. Commun. Biol. 2024, 7, 861. [Google Scholar]
- Andreyev, A.Y.; Kushnareva, Y.E.; Starkov, A.A. Mitochondrial metabolism of reactive oxygen species. Biochemistry 2005, 70, 200–214. [Google Scholar] [PubMed]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef]
- Patel, D.A.; Henry, J.E.; Good, T.A. Attenuation of beta-amyloid-induced toxicity by sialic-acid-conjugated dendrimers: Role of sialic acid attachment. Brain Res. 2007, 1161, 95–105. [Google Scholar] [CrossRef]
- Ju, T.C.; Chen, S.D.; Liu, C.C.; Yang, D.I. Protective effects of S-nitrosoglutathione against amyloid beta-peptide neurotoxicity. Free Radic. Biol. Med. 2005, 38, 938–949. [Google Scholar]
- Meijer, M.; Hendriks, H.S.; Heusinkveld, H.J.; Langeveld, W.T.; Westerink, R.H.S. Comparison of plate reader-based methods with fluorescence microscopy for measurements of intracellular calcium levels for the assessment of in vitro neurotoxicity. NeuroToxicology 2014, 45, 31–37. [Google Scholar] [CrossRef]
- Heusinkveld, H.J.; Westerink, R.H.S. Caveats and limitations of plate reader-based high-throughput kinetic measurements of intracellular calcium levels. Toxicol. Appl. Pharmacol. 2011, 255, 1–8. [Google Scholar]
- Wojtala, A.; Bonora, M.; Malinska, D.; Pinton, P.; Duszynski, J.; Wieckowski, M.R. Methods to monitor ROS production by fluorescence microscopy and fluorometry. Methods Enzymol. 2014, 542, 243–262. [Google Scholar] [PubMed]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, A.; García-Vicente, R.; Morales, M.L.; Ortiz-Ruiz, A.; Martínez-López, J.; Linares, M. Protein Carbonylation and Lipid Peroxidation in Hematological Malignancies. Antioxidants 2020, 9, 1212. [Google Scholar] [CrossRef]
- König, J.; Jung, T.; Grune, T. Protein Carbonylation in Aging and Senescence. In Protein Carbonylation: Principles, Analysis, and Biological Implications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 272–290. [Google Scholar]
- Revesz, T.; Holton, J.L.; Lashley, T.; Plant, G.; Rostagno, A.; Ghiso, J.; Frangione, B. Sporadic and familial cerebral amyloid angiopathies. Brain Pathol. 2002, 12, 343–357. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef]
- Iadecola, C. The pathobiology of vascular dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef]
- Zampar, S.; Klafki, H.W.; Sritharen, K.; Bayer, T.A.; Wiltfang, J.; Rostagno, A.; Ghiso, J.; Miles, L.A.; Wirths, O. N-terminal heterogeneity of parenchymal and vascular amyloid-β deposits in Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2020, 46, 673–685. [Google Scholar] [CrossRef]
- Golde, T.E.; Eckman, C.B.; Younkin, S.G. Biochemical detection of Abeta isoforms: Implications for pathogenesis, diagnosis, and treatment of Alzheimer’s disease. Biochim. Biophys. Acta 2000, 1502, 172–187. [Google Scholar] [CrossRef]
- Cabrera, E.; Mathews, P.; Mezhericher, E.; Beach, T.G.; Deng, J.; Neubert, T.A.; Rostagno, A.; Ghiso, J. Aβ truncated species: Implications for brain clearance mechanisms and amyloid plaque deposition. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 208–225. [Google Scholar]
- Viola, K.L.; Bicca, M.A.; Bebenek, A.M.; Kranz, D.L.; Nandwana, V.; Waters, E.A.; Haney, C.R.; Lee, M.; Gupta, A.; Brahmbhatt, Z.; et al. The Therapeutic and Diagnostic Potential of Amyloid β Oligomers Selective Antibodies to Treat Alzheimer’s Disease. Front. Neurosci. 2022, 15, 768646. [Google Scholar] [CrossRef]
- Hector, A.; Brouillette, J. Hyperactivity Induced by Soluble Amyloid-β Oligomers in the Early Stages of Alzheimer’s Disease. Front. Mol. Neurosci. 2021, 13, 600084. [Google Scholar] [CrossRef] [PubMed]
- Viola, K.L.; Klein, W.L. Amyloid β oligomers in Alzheimer’s disease pathogenesis, treatment, and diagnosis. Acta Neuropathol. 2015, 129, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Araki, W. Aβ Oligomer Toxicity-Reducing Therapy for the Prevention of Alzheimer’s Disease: Importance of the Nrf2 and PPARγ Pathways. Cells 2023, 12, 1386. [Google Scholar] [CrossRef]
- Blömeke, L.; Rehn, F.; Kraemer-Schulien, V.; Kutzsche, J.; Pils, M.; Bujnicki, T.; Lewczuk, P.; Kornhuber, J.; Freiesleben, S.D.; Schneider, L.S.; et al. Aβ oligomers peak in early stages of Alzheimer’s disease preceding tau pathology. Alzheimers Dement. 2024, 16, e12589. [Google Scholar] [CrossRef]
- Zhao, H.; Fan, M.; Zhang, J.; Gao, Y.; Chen, L.; Huang, L. Amyloid beta-induced mitochondrial dysfunction and endothelial permeability in cerebral microvascular endothelial cells: The protective role of dexmedetomidine. Brain Res. Bull. 2025, 220, 111137. [Google Scholar] [CrossRef]
- Wang, H.L.; Zhang, C.L.; Qiu, Y.M.; Chen, A.Q.; Li, Y.N.; Hu, B. Dysfunction of the Blood-brain Barrier in Cerebral Microbleeds: From Bedside to Bench. Aging Dis. 2021, 12, 1898–1919. [Google Scholar] [CrossRef]
- Harper, J.D.; Wong, S.S.; Lieber, C.M.; Lansbury, P.T. Observation of metastable Abeta amyloid protofibrils by atomic force microscopy. Chem. Biol. 1997, 4, 119–125. [Google Scholar] [CrossRef]
- Walsh, D.M.; Lomakin, A.; Benedek, G.B.; Condron, M.M.; Teplow, D.B. Amyloid beta-protein fibrillogenesis. Detection of a protofibrillar intermediate. J. Biol. Chem. 1997, 272, 22364–22372. [Google Scholar] [CrossRef]
- Soreghan, B.; Kosmoski, J.; Glabe, C. Surfactant properties of Alzheimer’s A beta peptides and the mechanism of amyloid aggregation. J. Biol. Chem. 1994, 269, 28551–28554. [Google Scholar] [CrossRef]
- Lomakin, A.; Teplow, D.B.; Kirschner, D.A.; Benedek, G.B. Kinetic theory of fibrillogenesis of amyloid beta-protein. Proc. Natl. Acad. Sci. USA 1997, 94, 7847–7942. [Google Scholar] [CrossRef]
- Lambert, M.P.; Barlow, A.K.; Chromy, B.A.; Edwards, C.; Freed, R.; Liosatos, M.; Morgan, T.E.; Rozovsky, I.; Trommer, B.; Viola, K.L.; et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. USA 1998, 95, 6448–6453. [Google Scholar] [CrossRef]
- Bucciantini, M.; Giannoni, E.; Chiti, F.; Baroni, F.; Formigli, L.; Zurdo, J.; Taddei, N.; Ramponi, G.; Dobson, C.M.; Stefani, M. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 2002, 416, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Price, J.L.; McKeel, D.W., Jr.; Buckles, V.D.; Roe, C.M.; Xiong, C.; Grundman, M.; Hansen, L.A.; Petersen, R.C.; Parisi, J.E.; Dickson, D.W.; et al. Neuropathology of nondemented aging: Presumptive evidence for preclinical Alzheimer disease. Neurobiol. Aging 2009, 30, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, P.; Polidori, M.C.; Metastasio, A.; Mariani, E.; Mattioli, P.; Cherubini, A.; Catani, M.; Cecchetti, R.; Senin, U.; Mecocci, P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol. Aging 2003, 24, 915–919. [Google Scholar] [CrossRef]
- Moreira, P.I.; Zhu, X.; Wang, X.; Lee, H.G.; Nunomura, A.; Petersen, R.B.; Perry, G.; Smith, M.A. Mitochondria: A therapeutic target in neurodegeneration. Biochim. Biophys. Acta 2010, 1802, 212–220. [Google Scholar] [CrossRef]
- Carvalho, C.; Correia, S.C.; Santos, R.X.; Cardoso, S.; Moreira, P.I.; Clark, T.A.; Zhu, X.; Smith, M.A.; Perry, G. Role of mitochondrial-mediated signaling pathways in Alzheimer disease and hypoxia. J. Bioenerg. Biomembr. 2009, 41, 433–440. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, B. Oxidative Stress and the Pathogenesis of Alzheimer′s Disease. Oxidative Med. Cell. Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef]
- Santos, R.X.; Correia, S.C.; Zhu, X.; Lee, H.G.; Petersen, R.B.; Nunomura, A.; Smith, M.A.; Perry, G.; Moreira, P.I. Nuclear and mitochondrial DNA oxidation in Alzheimer’s disease. Free Radic. Res. 2012, 46, 565–576. [Google Scholar] [CrossRef]
- Siegel, S.J.; Bieschke, J.; Powers, E.T.; Kelly, J.W. The oxidative stress metabolite 4-hydroxynonenal promotes Alzheimer protofibril formation. Biochemistry 2007, 46, 1503–1510. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Gonos, E.S.; Kapetanou, M.; Sereikaite, J.; Bartosz, G.; Naparło, K.; Grzesik, M.; Sadowska-Bartosz, I. Origin and pathophysiology of protein carbonylation, nitration and chlorination in age-related brain diseases and aging. Aging 2018, 10, 868–901. [Google Scholar] [CrossRef] [PubMed]
- Akagawa, M. Protein carbonylation: Molecular mechanisms, biological implications, and analytical approaches. Free Radic. Res. 2021, 55, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; Boyd-Kimball, D.; Poon, H.F.; Cai, J.; Pierce, W.M.; Klein, J.B.; Merchant, M.; Markesbery, W.R.; Butterfield, D.A. Redox proteomics identification of oxidized proteins in Alzheimer’s disease hippocampus and cerebellum: An approach to understand pathological and biochemical alterations in AD. Neurobiol. Aging 2006, 27, 1564–1576. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.N.; Schmitt, F.A.; Scheff, S.W.; Ding, Q.; Chen, Q.; Butterfield, D.A.; Markesbery, W.R. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology 2005, 64, 1152–1156. [Google Scholar] [CrossRef]
- Bizzozero, O.A. Protein Carbonylation in Neurodegenerative and Demyelinating CNS Diseases. In Handbook of Neurochemistry and Molecular Neurobiology: Brain and Spinal Cord Trauma; Lajtha, A., Banik, N., Ray, S.K., Eds.; Springer: Boston, MA, USA, 2009; pp. 543–562. [Google Scholar]
- Hensley, K.; Hall, N.; Subramaniam, R.; Cole, P.; Harris, M.; Aksenov, M.; Aksenova, M.; Gabbita, S.P.; Wu, J.F.; Carney, J.M.; et al. Brain Regional Correspondence Between Alzheimer’s Disease Histopathology and Biomarkers of Protein Oxidation. J. Neurochem. 1995, 65, 2146–2156. [Google Scholar] [CrossRef]
- Ghasemi-Tarie, R.; Kiasalari, Z.; Fakour, M.; Khorasani, M.; Keshtkar, S.; Baluchnejadmojarad, T.; Roghani, M. Nobiletin prevents amyloid β(1-40)-induced cognitive impairment via inhibition of neuroinflammation and oxidative/nitrosative stress. Metab. Brain Dis. 2022, 37, 1337–1349. [Google Scholar] [CrossRef]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.J.; Won, Y.S.; Kim, E.K.; Park, S.I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Moi, P.; Chan, K.; Asunis, I.; Cao, A.; Kan, Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA 1994, 91, 9926–9930. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Sherratt, P.J.; Huang, H.C.; Yang, C.S.; Pickett, C.B. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. J. Biol. Chem. 2003, 278, 4536–4541. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Kang, M.I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Pritam, P.; Deka, R.; Bhardwaj, A.; Srivastava, R.; Kumar, D.; Jha, A.K.; Jha, N.K.; Villa, C.; Jha, S.K. Antioxidants in Alzheimer’s Disease: Current Therapeutic Significance and Future Prospects. Biology 2022, 11, 212. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X. Antioxidant therapies for Alzheimer’s disease. Oxidative Med. Cell. Longev. 2012, 2012, 472932. [Google Scholar] [CrossRef]
- Suzen, S.; Tucci, P.; Profumo, E.; Buttari, B.; Saso, L. A Pivotal Role of Nrf2 in Neurodegenerative Disorders: A New Way for Therapeutic Strategies. Pharmaceuticals 2022, 15, 692. [Google Scholar] [CrossRef]
- Lisk, C.; McCord, J.; Bose, S.; Sullivan, T.; Loomis, Z.; Nozik-Grayck, E.; Schroeder, T.; Hamilton, K.; Irwin, D.C. Nrf2 activation: A potential strategy for the prevention of acute mountain sickness. Free Radic. Biol. Med. 2013, 63, 264–273. [Google Scholar] [CrossRef]
- Lúcio, M.; Nunes, C.; Gaspar, D.; Ferreira, H.; Lima, J.L.F.C.; Reis, S. Antioxidant Activity of Vitamin E and Trolox: Understanding of the Factors that Govern Lipid Peroxidation Studies In Vitro. Food Biophys. 2009, 4, 312–320. [Google Scholar] [CrossRef]
- Bono, S.; Feligioni, M.; Corbo, M. Impaired antioxidant KEAP1-NRF2 system in amyotrophic lateral sclerosis: NRF2 activation as a potential therapeutic strategy. Mol. Neurodegener. 2021, 16, 71. [Google Scholar] [CrossRef]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxidative Med. Cell. Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valle, M.L.; Getaneh, B.; William, C.; Ghiso, J.; Rostagno, A. Antioxidants Trolox and Methazolamide Protect Microvascular Endothelial Cells from Oxidative Damage Induced by Sporadic and Familial Forms of Oligomeric Amyloid-β. Antioxidants 2025, 14, 1375. https://doi.org/10.3390/antiox14111375
Valle ML, Getaneh B, William C, Ghiso J, Rostagno A. Antioxidants Trolox and Methazolamide Protect Microvascular Endothelial Cells from Oxidative Damage Induced by Sporadic and Familial Forms of Oligomeric Amyloid-β. Antioxidants. 2025; 14(11):1375. https://doi.org/10.3390/antiox14111375
Chicago/Turabian StyleValle, Maria Luisa, Bitseat Getaneh, Christopher William, Jorge Ghiso, and Agueda Rostagno. 2025. "Antioxidants Trolox and Methazolamide Protect Microvascular Endothelial Cells from Oxidative Damage Induced by Sporadic and Familial Forms of Oligomeric Amyloid-β" Antioxidants 14, no. 11: 1375. https://doi.org/10.3390/antiox14111375
APA StyleValle, M. L., Getaneh, B., William, C., Ghiso, J., & Rostagno, A. (2025). Antioxidants Trolox and Methazolamide Protect Microvascular Endothelial Cells from Oxidative Damage Induced by Sporadic and Familial Forms of Oligomeric Amyloid-β. Antioxidants, 14(11), 1375. https://doi.org/10.3390/antiox14111375






