Ferroptosis Enhances T Lymphocyte Infiltration into Glioblastoma Spheroids
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture LN229 and Spheroid Generation
2.2. PD-L1 Knock-Down Cells
2.3. Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
2.4. Treatments
2.5. Viability Assay
2.6. Live-Cell Imaging (Incucyte)
2.7. Quantitative Real-Time PCR
2.8. Immunohistochemistry
2.9. ATP Release Assay
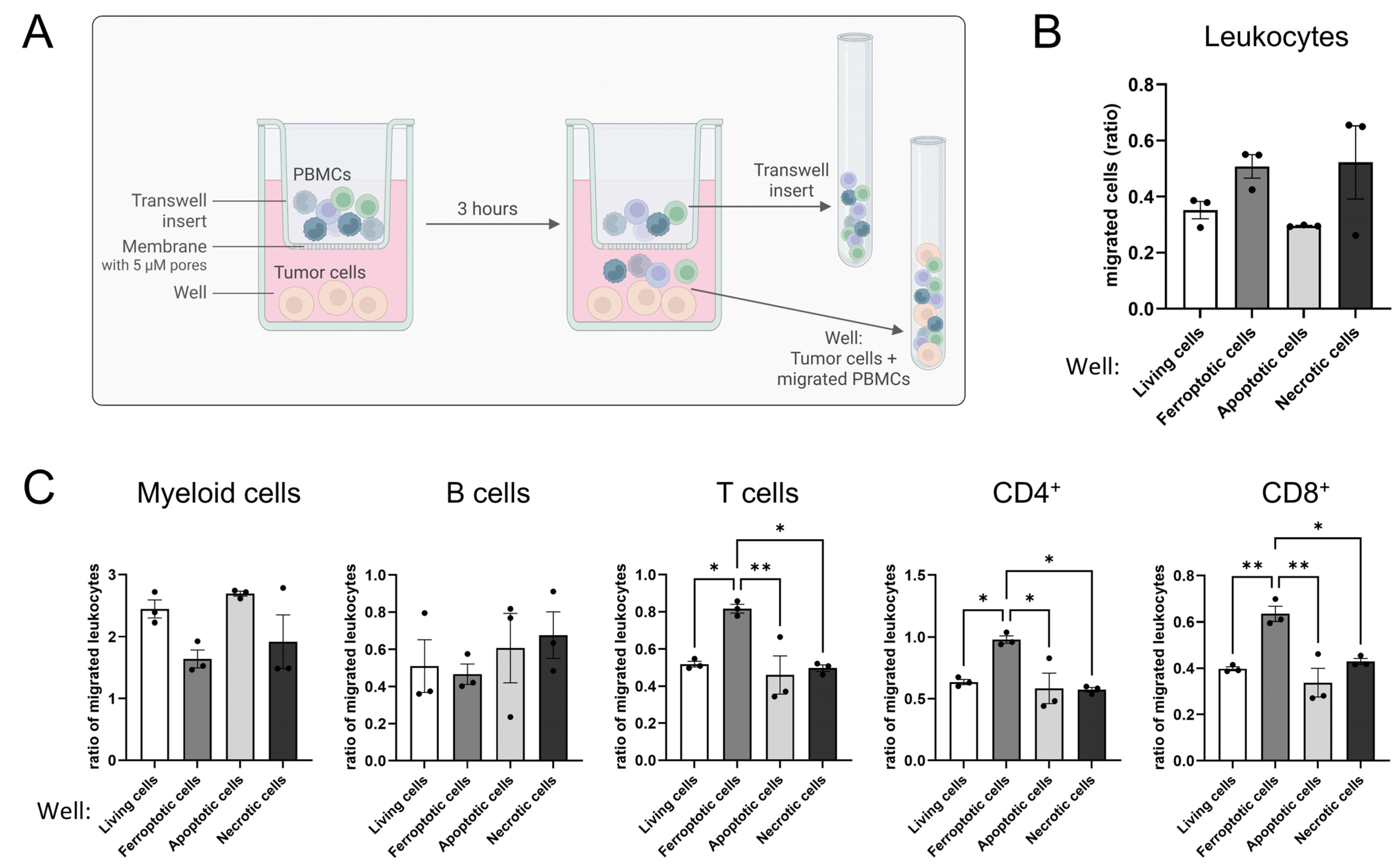
2.10. Spheroid Infiltration Assay
2.11. Migration Assay
2.12. Flow Cytometry and Imaging Flow Cytometry
2.13. T Cell Activation Assay of Migrated PBMCs
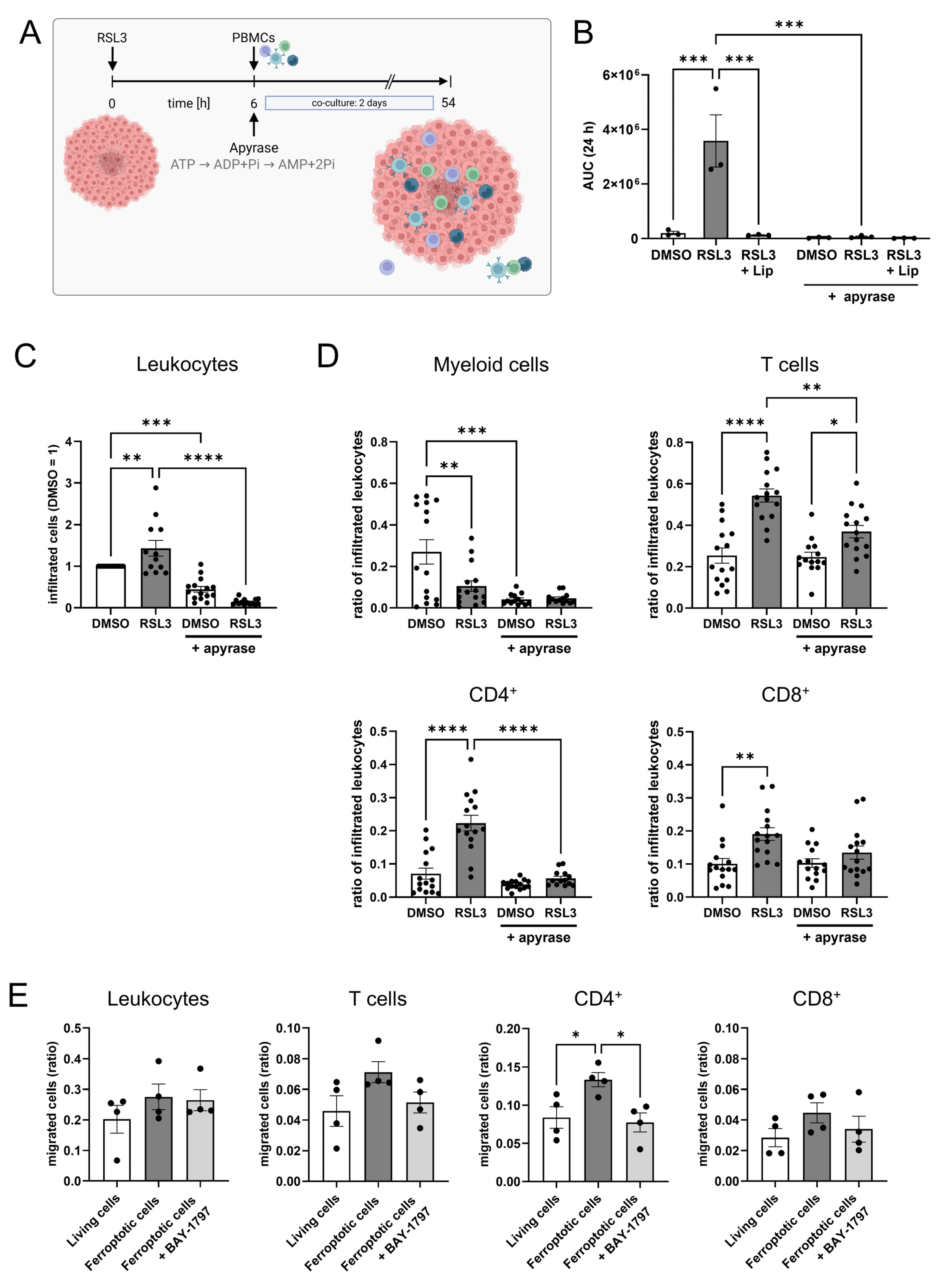
2.14. T Cell Activation Under Apyrase Treatment
2.15. T Cell Activation with PD-L1 Knock-Down Cells
2.16. LEGENDplexTM Multi-Analyte Flow Assay
2.17. Cytometric Bead Array (CBA)
2.18. Western Analysis
2.19. Data Presentation and Statistical Analysis
3. Results
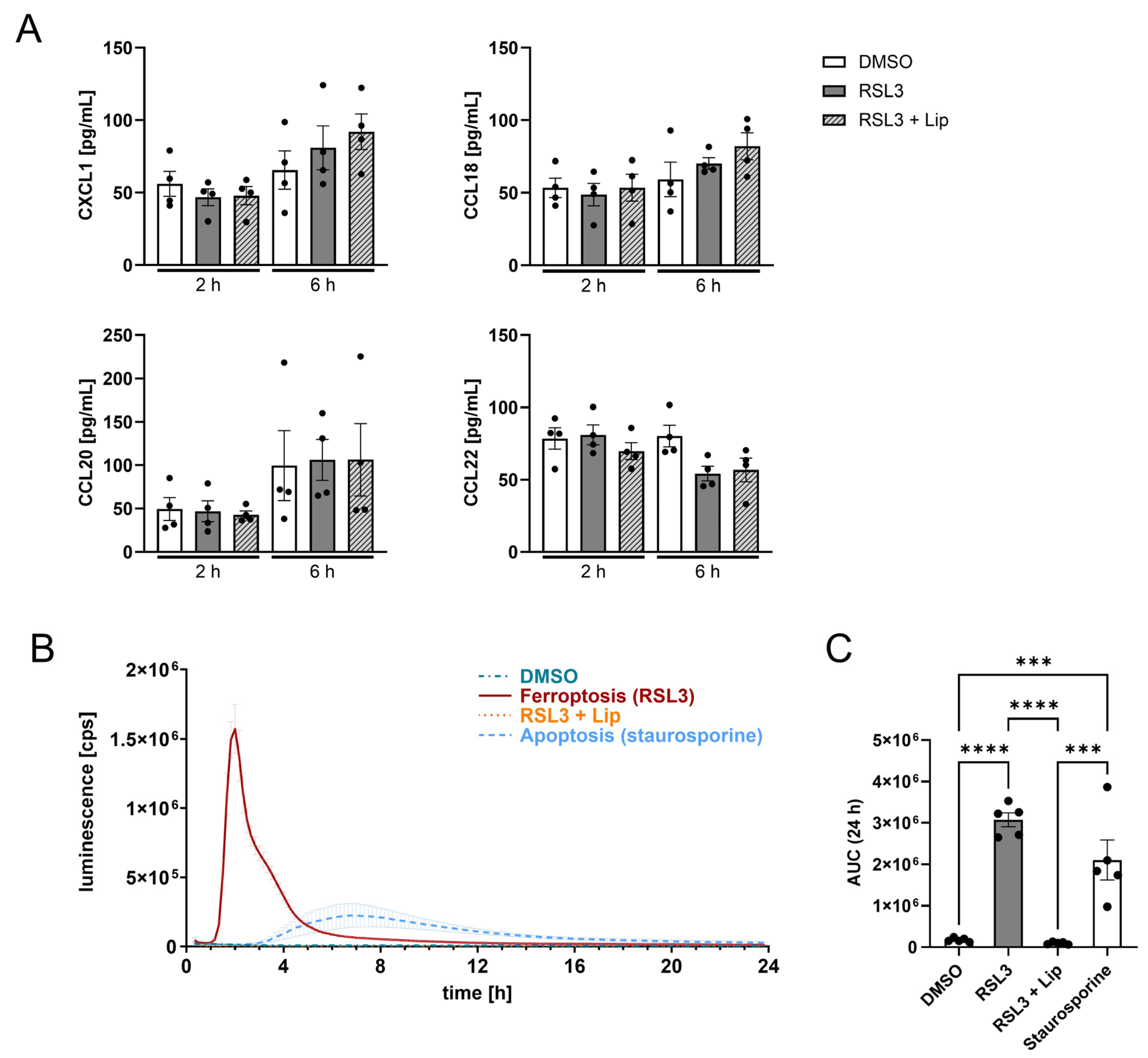
3.1. Glioblastoma LN229 Cells Undergo Ferroptosis upon RSL3 Treatment
3.2. Ferroptosis Facilitates T Cell Infiltration into Glioblastoma Spheroids
3.3. Ferroptotic Cells Attract T Cells More Efficiently than Other Forms of Cell Death
3.4. Death by Ferroptosis Releases More ATP than Apoptosis
3.5. ATP Causes T Cell Infiltration into Glioblastoma Spheroids
3.6. T Cell Activation Is Suppressed by Ferroptotic Cells
3.7. Apyrase Treatment Facilitates T Cell Responses and ATP Partially Inhibits T Cell Activation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxynonenal |
| ADP | Adenosine diphosphate |
| AMP | Adenosine monophosphate |
| ATP | Adenosine triphosphate |
| AUC | Area under the curve |
| CBA | Cytometric bead array |
| CCL | C-C motif chemokine ligand |
| CD | Cluster of differentiation |
| cDNA | Complementary deoxyribonucleic acid |
| CXCL | C-X-C motif chemokine ligand |
| DAMPs | Danger-associated molecular patterns |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| DMSO | Dimethyl sulfoxide |
| eATP | Extracellular adenosine triphosphate |
| EDTA | Ethylenediaminetetraacetic acid |
| FTH | ferritin heavy chain |
| HEPES | N-2-Hydroxyethylpiperazine-N’-2-ethane sulphonic acid |
| HLA-DR | Human leucocyte antigen-DR |
| IFN | Interferon |
| IL | interleukin |
| Lip | Liproxstatin-1 |
| PBMCs | Peripheral blood mononuclear cells |
| PBS | Phosphate-buffered saline |
| PD-L1 | Programmed death-ligand 1 |
| (m)RNA | (messenger) Ribonucleic acid |
| RPMI | Roswell Park Memorial Institute |
| RSL3 | Ras-selective lethal small molecule 3 |
| RT-PCR | Reverse transcription polymerase chain reaction |
| SLC7A11 | Solute carrier family 7 member 11 |
| TBP | TATA box-binding protein |
| TFR | transferrin receptor |
| TNF | Tumor necrosis factor |
References
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Agmon, E.; Solon, J.; Bassereau, P.; Stockwell, B.R. Modeling the effects of lipid peroxidation during ferroptosis on membrane properties. Sci. Rep. 2018, 8, 5155. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Rock, K.L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 2008, 8, 279–289. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017, 53, 13–28. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, Y.; Tang, J.; Cao, M. Radiotherapy induced immunogenic cell death by remodeling tumor immune microenvironment. Front. Immunol. 2022, 13, 1074477. [Google Scholar] [CrossRef]
- Medina, C.B.; Mehrotra, P.; Arandjelovic, S.; Perry, J.S.A.; Guo, Y.; Morioka, S.; Barron, B.; Walk, S.F.; Ghesquière, B.; Krupnick, A.S.; et al. Metabolites released from apoptotic cells act as tissue messengers. Nature 2020, 580, 130–135. [Google Scholar] [CrossRef]
- Chekeni, F.B.; Elliott, M.R.; Sandilos, J.K.; Walk, S.F.; Kinchen, J.M.; Lazarowski, E.R.; Armstrong, A.J.; Penuela, S.; Laird, D.W.; Salvesen, G.S.; et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 2010, 467, 863–867. [Google Scholar] [CrossRef]
- Vénéreau, E.; Ceriotti, C.; Bianchi, M.E. DAMPs from Cell Death to New Life. Front. Immunol. 2015, 6, 422. [Google Scholar] [CrossRef]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef]
- Murao, A.; Aziz, M.; Wang, H.; Brenner, M.; Wang, P. Release mechanisms of major DAMPs. Apoptosis 2021, 26, 152–162. [Google Scholar] [CrossRef]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu. Rev. Pathol. 2020, 15, 493–518. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Krysko, D.V.; Conrad, M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer 2019, 19, 405–414. [Google Scholar] [CrossRef]
- Bell, H.N.; Stockwell, B.R.; Zou, W. Ironing out the role of ferroptosis in immunity. Immunity 2024, 57, 941–956. [Google Scholar] [CrossRef]
- Catanzaro, E.; Demuynck, R.; Naessens, F.; Galluzzi, L.; Krysko, D.V. Immunogenicity of ferroptosis in cancer: A matter of context? Trends Cancer 2024, 10, 407–416. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016–2020. Neuro Oncol. 2023, 25, iv1–iv99. [Google Scholar] [CrossRef]
- Roda, D.; Veiga, P.; Melo, J.B.; Carreira, I.M.; Ribeiro, I.P. Principles in the Management of Glioblastoma. Genes 2024, 15, 501. [Google Scholar] [CrossRef] [PubMed]
- Sipos, D.; Raposa, B.L.; Freihat, O.; Simon, M.; Mekis, N.; Cornacchione, P.; Kovács, Á. Glioblastoma: Clinical Presentation, Multidisciplinary Management, and Long-Term Outcomes. Cancers 2025, 17, 146. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, P.; Wang, L.; Wu, J.; Tian, Y.; Chen, C.; Li, D.; Yao, Z.; Chen, R.; Xiang, G.; Gong, J.; et al. Overcoming cold tumors: A combination strategy of immune checkpoint inhibitors. Front. Immunol. 2024, 15, 1344272. [Google Scholar] [CrossRef] [PubMed]
- Orrego, E.; Castaneda, C.A.; Castillo, M.; Bernabe, L.A.; Casavilca, S.; Chakravarti, A.; Meng, W.; Garcia-Corrochano, P.; Villa-Robles, M.R.; Zevallos, R.; et al. Distribution of tumor-infiltrating immune cells in glioblastoma. CNS Oncol. 2018, 7, CNS21. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, G.-R.; Mostafavi, S.; Bastan, S.; Ebrahimi, N.; Gharibvand, R.S.; Eskandari, N. Immunologic tumor microenvironment modulators for turning cold tumors hot. Cancer Commun. 2024, 44, 521–553. [Google Scholar] [CrossRef]
- Pinto, M.P.; Jacobsen, B.M.; Horwitz, K.B. An immunohistochemical method to study breast cancer cell subpopulations and their growth regulation by hormones in three-dimensional cultures. Front. Endocrinol. 2011, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, S.; Guo, Q.; Kessel, S.; Rubinoff, I.; Chan, L.L.-Y.; Li, P.; Liu, Y.; Qiu, J.; Zhou, C. Optical Coherence Tomography Detects Necrotic Regions and Volumetrically Quantifies Multicellular Tumor Spheroids. Cancer Res. 2017, 77, 6011–6020. [Google Scholar] [CrossRef]
- Erlichman, C.; Tannock, I.F. Growth and characterization of multicellular tumor spheroids of human bladder carcinoma origin. Vitr. Cell. Dev. Biol. 1986, 22, 449–456. [Google Scholar] [CrossRef]
- Däster, S.; Amatruda, N.; Calabrese, D.; Ivanek, R.; Turrini, E.; Droeser, R.A.; Zajac, P.; Fimognari, C.; Spagnoli, G.C.; Iezzi, G.; et al. Induction of hypoxia and necrosis in multicellular tumor spheroids is associated with resistance to chemotherapy treatment. Oncotarget 2017, 8, 1725–1736. [Google Scholar] [CrossRef]
- Battaglia, A.M.; Chirillo, R.; Aversa, I.; Sacco, A.; Costanzo, F.; Biamonte, F. Ferroptosis and Cancer: Mitochondria Meet the “Iron Maiden” Cell Death. Cells 2020, 9, 1505. [Google Scholar] [CrossRef]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P.; et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef]
- Kronlage, M.; Song, J.; Sorokin, L.; Isfort, K.; Schwerdtle, T.; Leipziger, J.; Robaye, B.; Conley, P.B.; Kim, H.-C.; Sargin, S.; et al. Autocrine Purinergic Receptor Signaling Is Essential for Macrophage Chemotaxis. Sci. Signal. 2010, 3, ra55. [Google Scholar] [CrossRef]
- Schumacher, T.; Schreiber, R. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
- Hung, K.; Hayashi, R.; Lafond-Walker, A.; Lowenstein, C.; Pardoll, D.; Levitsky, H. The Central Role of CD4+ T Cells in the Antitumor Immune Response. J. Exp. Med. 1998, 188, 2357–2368. [Google Scholar] [CrossRef]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef] [PubMed]
- Berndt, C.; Alborzinia, H.; Amen, V.S.; Ayton, S.; Barayeu, U.; Bartelt, A.; Bayir, H.; Bebber, C.M.; Birsoy, K.; Böttcher, J.P.; et al. Ferroptosis in health and disease. Redox Biol. 2024, 75, 103211. [Google Scholar] [CrossRef] [PubMed]
- Campos-Sandoval, J.A.; Gómez-García, M.C.; Santos-Jiménez, J.d.L.; Matés, J.M.; Alonso, F.J.; Márquez, J. Antioxidant responses related to temozolomide resistance in glioblastoma. Neurochem. Int. 2021, 149, 105136. [Google Scholar] [CrossRef]
- Carvalho, S.M.; Mansur, A.A.P.; Da Silveira, I.B.; Pires, T.F.S.; Victória, H.F.V.; Krambrock, K.; Leite, M.F.; Mansur, H.S. Nanozymes with Peroxidase-like Activity for Ferroptosis-Driven Biocatalytic Nanotherapeutics of Glioblastoma Cancer: 2D and 3D Spheroids Models. Pharmaceutics 2023, 15, 1702. [Google Scholar] [CrossRef]
- Ghosal, J.; Sinchana, V.K.; Chakrabarty, S. Ferroptosis meets microRNAs: A new frontier in anti-cancer therapy. Free Radic. Biol. Med. 2025, 226, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Shirahama, H.; Tani, Y.; Tsukahara, S.; Okamoto, Y.; Hasebe, A.; Noda, T.; Ando, S.; Ushijima, M.; Matsuura, M.; Tomida, A. Induction of stearoyl-CoA desaturase confers cell density-dependent ferroptosis resistance in melanoma. J. Cell. Biochem. 2024, 125, e30542. [Google Scholar] [CrossRef]
- Seiler, A.; Schneider, M.; Förster, H.; Roth, S.; Wirth, E.K.; Culmsee, C.; Plesnila, N.; Kremmer, E.; Rådmark, O.; Wurst, W.; et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008, 8, 237–248. [Google Scholar] [CrossRef]
- Roeck, B.F.; Lotfipour Nasudivar, S.; Vorndran, M.R.H.; Schueller, L.; Yapici, F.I.; Rübsam, M.; von Karstedt, S.; Niessen, C.M.; Garcia-Saez, A.J. Ferroptosis spreads to neighboring cells via plasma membrane contacts. Nat. Commun. 2025, 16, 2951. [Google Scholar] [CrossRef]
- Linkermann, A.; Skouta, R.; Himmerkus, N.; Mulay, S.R.; Dewitz, C.; de Zen, F.; Prokai, A.; Zuchtriegel, G.; Krombach, F.; Welz, P.-S.; et al. Synchronized renal tubular cell death involves ferroptosis. Proc. Natl. Acad. Sci. USA 2014, 111, 16836–16841. [Google Scholar] [CrossRef] [PubMed]
- Riegman, M.; Bradbury, M.S.; Overholtzer, M. Population Dynamics in Cell Death: Mechanisms of Propagation. Trends Cancer 2019, 5, 558–568. [Google Scholar] [CrossRef]
- Nakano, H.; Murai, S.; Moriwaki, K. Regulation of the release of damage-associated molecular patterns from necroptotic cells. Biochem. J. 2022, 479, 677–685. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, P.; You, J.; Kim, E.H. Role of Damage-Associated Molecular Pattern/Cell Death Pathways in Vaccine-Induced Immunity. Viruses 2021, 13, 2340. [Google Scholar] [CrossRef]
- Dosch, M.; Gerber, J.; Jebbawi, F.; Beldi, G. Mechanisms of ATP Release by Inflammatory Cells. Int. J. Mol. Sci. 2018, 19, 1222. [Google Scholar] [CrossRef]
- Trabanelli, S.; Ocadlíková, D.; Gulinelli, S.; Curti, A.; Salvestrini, V.; Vieira, R.d.P.; Idzko, M.; Di Virgilio, F.; Ferrari, D.; Lemoli, R.M. Extracellular ATP exerts opposite effects on activated and regulatory CD4+ T cells via purinergic P2 receptor activation. J. Immunol. 2012, 189, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.-L.; Cai, Y.-Q.; Zhu, M.-C.; Xing, L.-J.; Wang, X. The yin and yang functions of extracellular ATP and adenosine in tumor immunity. Cancer Cell Int. 2020, 20, 110. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Carvalho, I.; Banuelos, A.; Da Borges Silva, H. Tissue- and temporal-specific roles of extracellular ATP on T cell metabolism and function. Immunometabolism 2023, 5, e00025. [Google Scholar] [CrossRef]
- Aswad, F.; Dennert, G. P2X7 receptor expression levels determine lethal effects of a purine based danger signal in T lymphocytes. Cell. Immunol. 2006, 243, 58–65. [Google Scholar] [CrossRef]
- Aswad, F.; Kawamura, H.; Dennert, G. High Sensitivity of CD4+CD25+ Regulatory T Cells to Extracellular Metabolites Nicotinamide Adenine Dinucleotide and ATP: A Role for P2X7 Receptors. J. Immunol. 2005, 175, 3075–3083. [Google Scholar] [CrossRef]
- Del Prete, A.; Schioppa, T.; Tiberio, L.; Stabile, H.; Sozzani, S. Leukocyte trafficking in tumor microenvironment. Curr. Opin. Pharmacol. 2017, 35, 40–47. [Google Scholar] [CrossRef]
- Verma, N.K.; Wong, B.H.S.; Poh, Z.S.; Udayakumar, A.; Verma, R.; Goh, R.K.J.; Duggan, S.P.; Shelat, V.G.; Chandy, K.G.; Grigoropoulos, N.F. Obstacles for T-lymphocytes in the tumour microenvironment: Therapeutic challenges, advances and opportunities beyond immune checkpoint. EBioMedicine 2022, 83, 104216. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sun, Z.; Chen, L. Memory T cells: Strategies for optimizing tumor immunotherapy. Protein Cell 2020, 11, 549–564. [Google Scholar] [CrossRef]
- Harris, K.M.; Clements, M.A.; Kwilasz, A.J.; Watkins, L.R. T cell transgressions: Tales of T cell form and function in diverse disease states. Int. Rev. Immunol. 2022, 41, 475–516. [Google Scholar] [CrossRef]
- Gomez, S.; Tabernacki, T.; Kobyra, J.; Roberts, P.; Chiappinelli, K.B. Combining epigenetic and immune therapy to overcome cancer resistance. Semin. Cancer Biol. 2020, 65, 99–113. [Google Scholar] [CrossRef]
- Wang, C.M.; Ploia, C.; Anselmi, F.; Sarukhan, A.; Viola, A. Adenosine triphosphate acts as a paracrine signaling molecule to reduce the motility of T cells. EMBO J. 2014, 33, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-H.; Lin, C.-Y.; Chen, C.-Y.; Hsueh, C.-W.; Chang, Y.-W.; Wang, C.-C.; Chu, P.-Y.; Tai, S.-K.; Yang, M.-H. Ferroptosis Signature Shapes the Immune Profiles to Enhance the Response to Immune Checkpoint Inhibitors in Head and Neck Cancer. Adv. Sci. 2023, 10, e2204514. [Google Scholar] [CrossRef]
- Desterke, C.; Xiang, Y.; Elhage, R.; Duruel, C.; Chang, Y.; Hamaï, A. Ferroptosis Inducers Upregulate PD-L1 in Recurrent Triple-Negative Breast Cancer. Cancers 2023, 16, 155. [Google Scholar] [CrossRef] [PubMed]
- Kythreotou, A.; Siddique, A.; Mauri, F.A.; Bower, M.; Pinato, D.J. PD-L1. J. Clin. Pathol. 2018, 71, 189–194. [Google Scholar] [CrossRef]




| Target | Accession | Forward | Reverse |
|---|---|---|---|
| TBP | NM_003194.5 | GCATCACTGTTTCTTGGCGT | CGCTGGAACTCGTCTCACTA |
| TFR | NM_003234.4 | GAGCGTCGGGATATCGGGT | CAGGATGAAGGGAGGACACG |
| FTH | NM_002032.3 | TGACAAAAATGACCCCCATT | CAGGGTGTGCTTGTCAAAGA |
| SLC7A11 | NM_014331.4 | GGTCCATTACCAGCTTTTGTACG | AATGTAGCGTCCAAATGCCAG |
| PD-L1 | NM_014143.4/.2 | TGGCATTTGCTGAACGCATTT | TGCAGCCAGGTCTAATTGTTTT |
| Target | Article Description | Art. No. | Supplier |
|---|---|---|---|
| CD4 | BD HorizonTM Customs BB630-P2 Mouse anti-human CD4 | Custom-made | BD Bioscience |
| CD3 | BD HorizonTM BUV805 Mouse anti-human CD3 | 612893 | BD Bioscience |
| CD8 | BD HorizonTM BV650 Mouse anti-human CD8 | 563821 | BD Bioscience |
| CD20 | BD PharmingenTM APC-H7 Mouse anti-human CD20 | 560853 | BD Bioscience |
| CD25 | BD PharmingenTM PE-CyTM7 Mouse Anti-human CD25 | 557741 | BD Bioscience |
| CD33 | BD HorizonTM BV510 Mouse anti-human CD33 | 563257 | BD Bioscience |
| CD45 | BD OptiBuildTM BUV661 Mouse anti-human CD45 | 750178 | BD Bioscience |
| CD56 | Brilliant Violet 605TM anti-human CD56 (NCAM) | 318334 | BioLegend |
| CD123 | PE/Cyanine5 anti-human CD123 | 306008 | BioLegend |
| CD127 | BD HorizonTM BB700 Mouse anti-human CD127 | 566398 | BD Bioscience |
| CD274 | APC anti-human CD274 (B7-H1, PD-L1) | 329707 | BioLegend |
| HLA-DR | APC/FireTM 750 anti-human HLA-DR | 307658 | BioLegend |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwantes, A.; Shadid, Y.; Guerrero Ruiz, V.M.; Aliraj, B.; Wickert, A.; Palmer, M.A.; Meyer, S.P.; Weigert, A.; Brüne, B.; Fuhrmann, D.C. Ferroptosis Enhances T Lymphocyte Infiltration into Glioblastoma Spheroids. Antioxidants 2025, 14, 1373. https://doi.org/10.3390/antiox14111373
Schwantes A, Shadid Y, Guerrero Ruiz VM, Aliraj B, Wickert A, Palmer MA, Meyer SP, Weigert A, Brüne B, Fuhrmann DC. Ferroptosis Enhances T Lymphocyte Infiltration into Glioblastoma Spheroids. Antioxidants. 2025; 14(11):1373. https://doi.org/10.3390/antiox14111373
Chicago/Turabian StyleSchwantes, Anna, Yara Shadid, Vanesa Maria Guerrero Ruiz, Blerina Aliraj, Anja Wickert, Megan A. Palmer, Sofie P. Meyer, Andreas Weigert, Bernhard Brüne, and Dominik C. Fuhrmann. 2025. "Ferroptosis Enhances T Lymphocyte Infiltration into Glioblastoma Spheroids" Antioxidants 14, no. 11: 1373. https://doi.org/10.3390/antiox14111373
APA StyleSchwantes, A., Shadid, Y., Guerrero Ruiz, V. M., Aliraj, B., Wickert, A., Palmer, M. A., Meyer, S. P., Weigert, A., Brüne, B., & Fuhrmann, D. C. (2025). Ferroptosis Enhances T Lymphocyte Infiltration into Glioblastoma Spheroids. Antioxidants, 14(11), 1373. https://doi.org/10.3390/antiox14111373








