Evaluation of the Anticancer Activity of Medicinal Plants Predominantly Accumulating Ellagic Acid Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Chemicals
2.3. Sample Preparation
2.3.1. Extraction Using Organic Solvents
2.3.2. Exchange of Extract Solvent for Anticancer In Vitro Testing
2.4. Spectrophotometric Analysis
2.5. HPLC UV-VIS System Analysis
2.6. HPLC-ED System Analysis
2.7. Medicinal Plant Extract Effect on Cell Viability
2.7.1. Cell Lines
2.7.2. MTS Cell Proliferation Assay
2.8. Statistical Analysis
3. Results
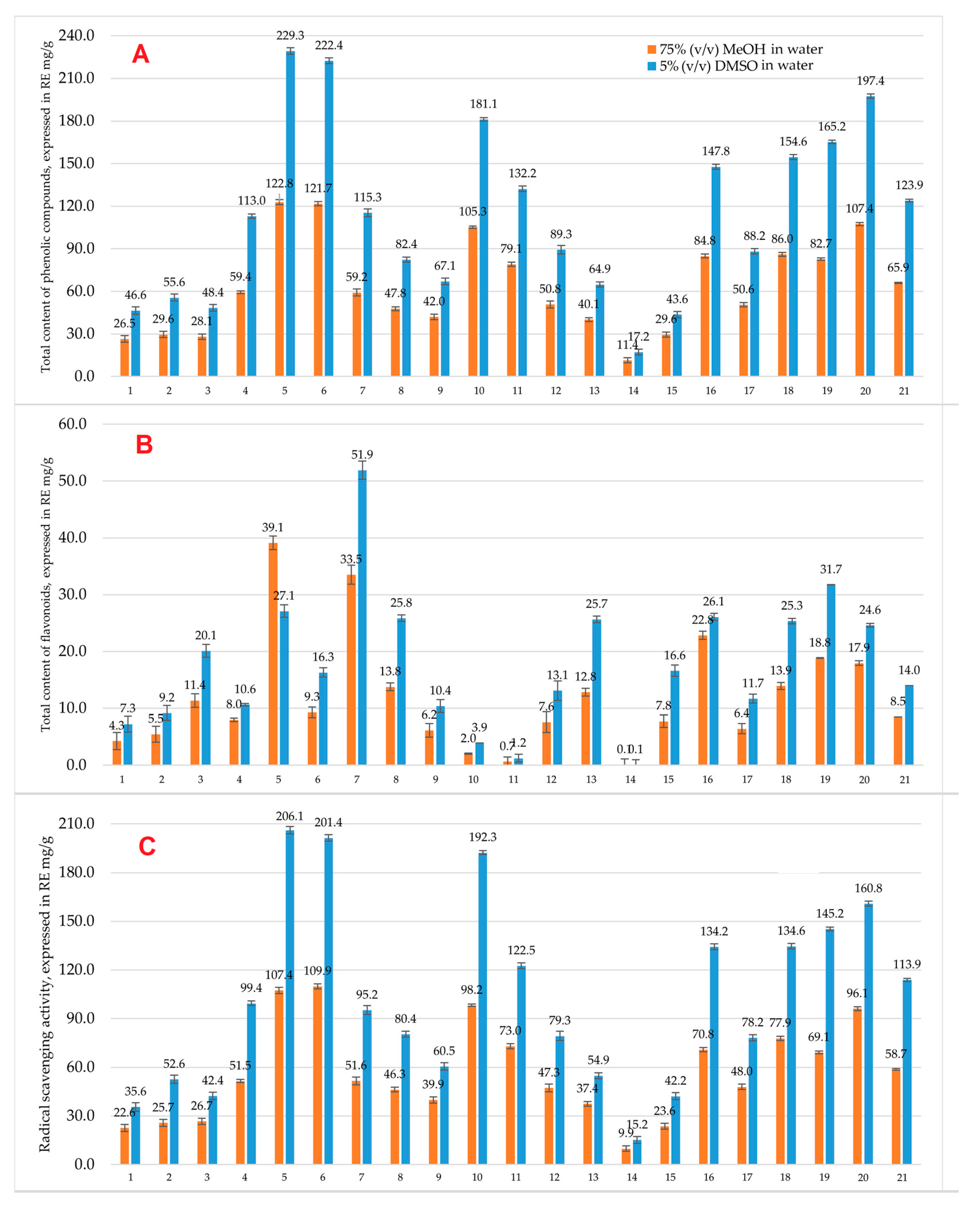
3.1. Determination of Total Phenolic Content, Flavonoid Content, and Antioxidant Activity by Spectrometric Methods
3.2. HPLC Analysis
3.2.1. 75% (v/v) Methanol in Water Extracts
3.2.2. Extracts After Solvent Exchange to 5% (v/v) DMSO in Water
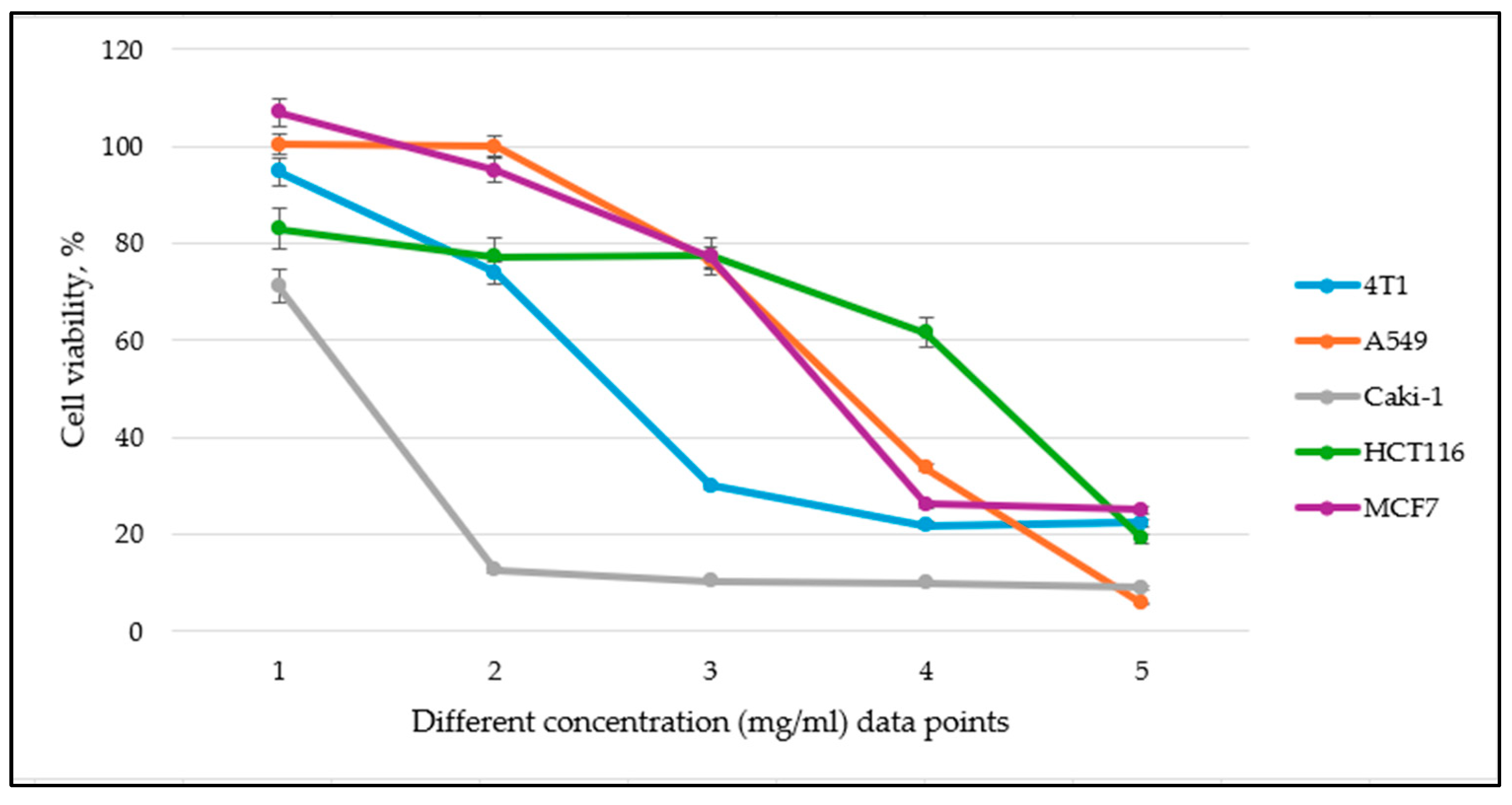
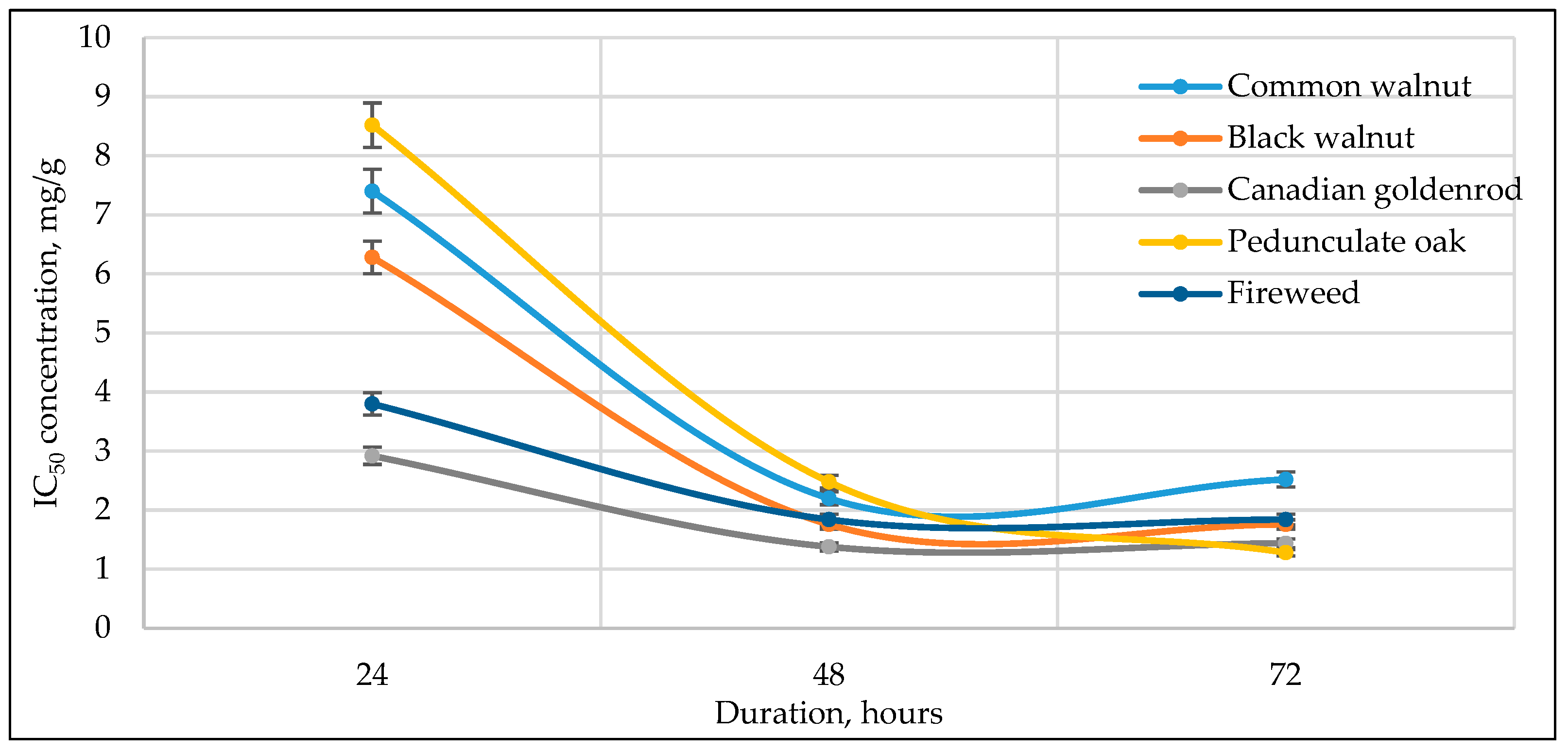
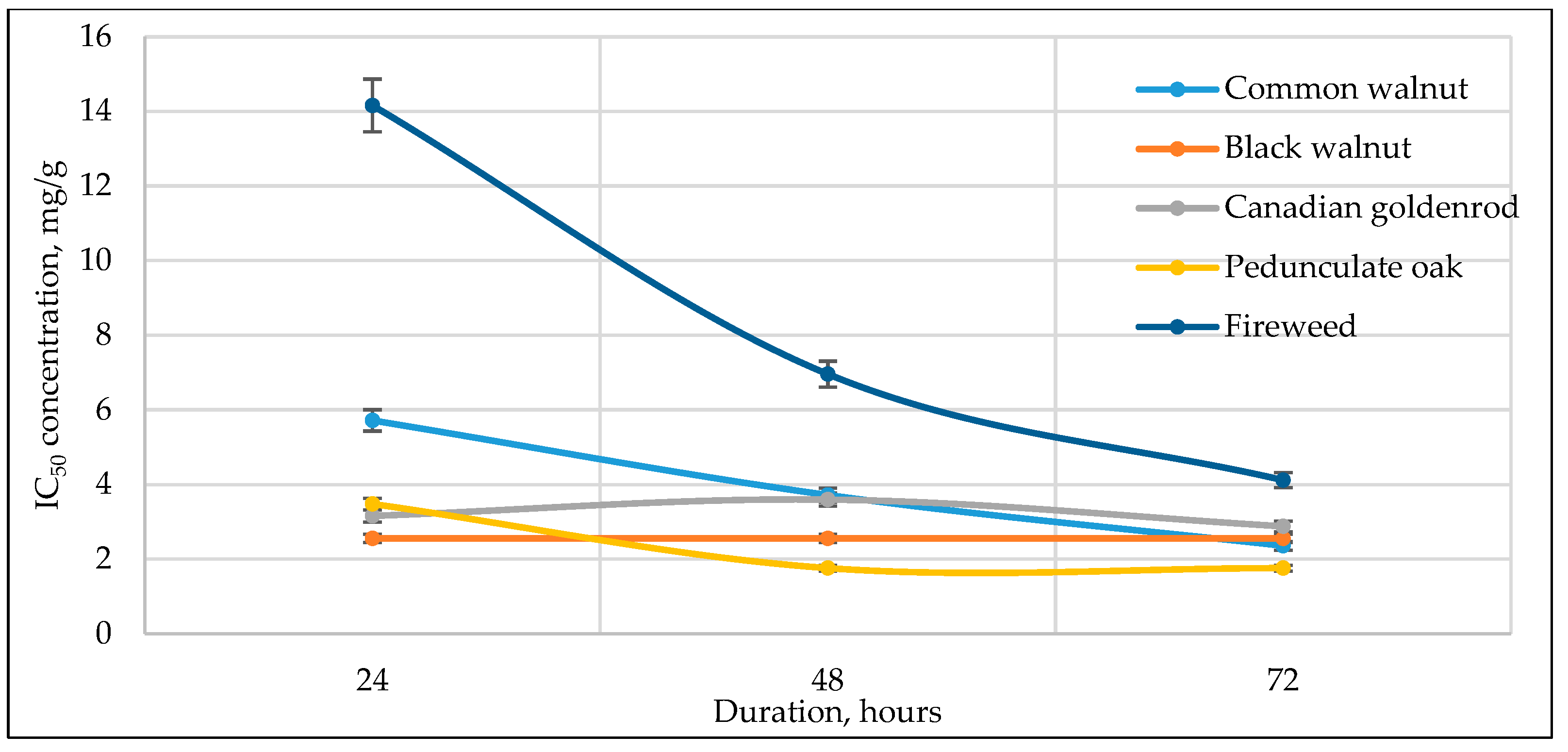
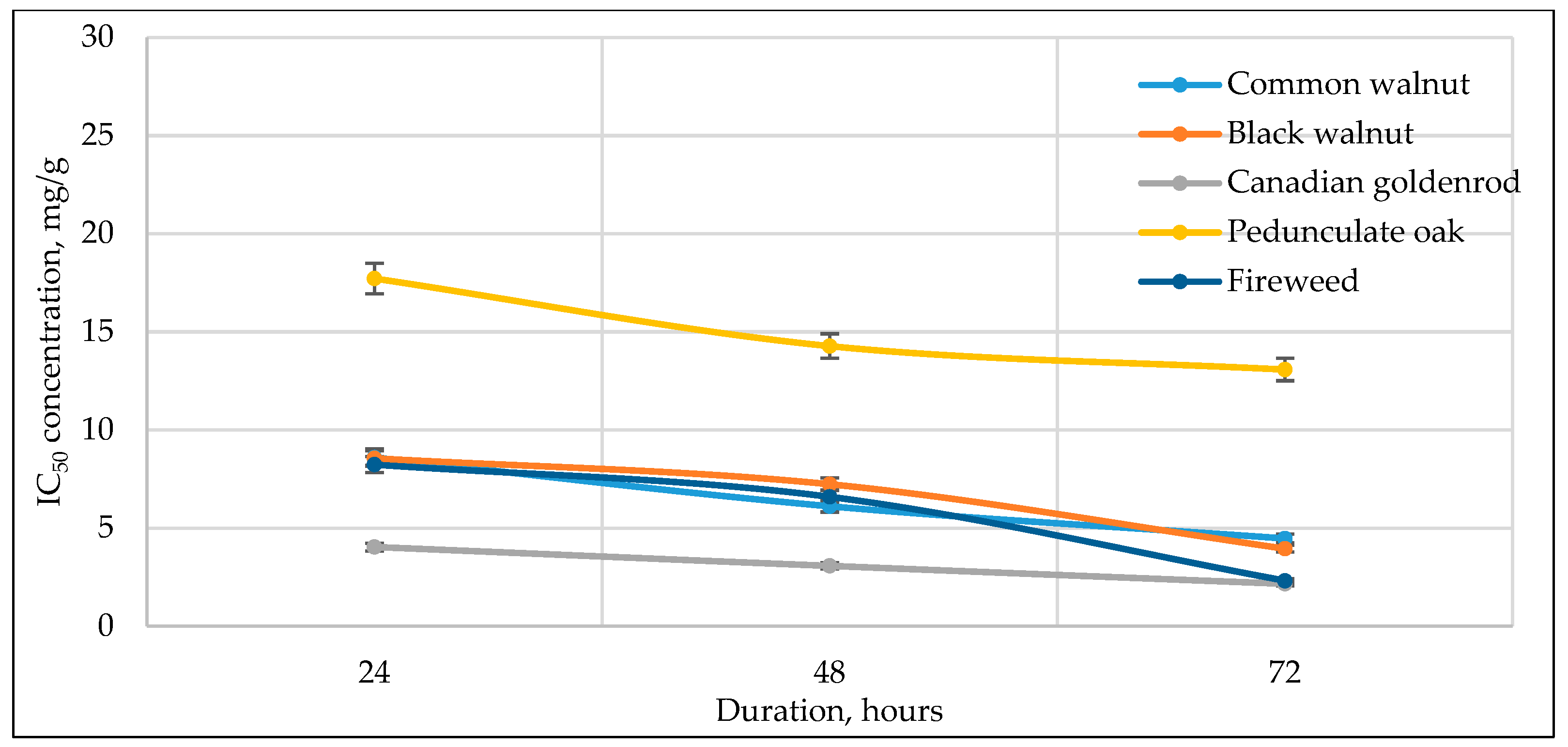
3.3. Cell Viability to Medicinal Plant Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| DNA | Deoxyribonucleic acid |
| DMSO | Dimethyl sulfoxide |
| UV-VIS | Ultraviolet–visible |
| HPLC-ED | High-performance liquid chromatography–electrochemical detection |
| HPLC | High-performance liquid chromatography |
| MTS | 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| RPMI | Roswell Park Memorial Institute |
| DMEM | Dulbecco’s Modified Eagle Medium |
| RSD | Relative standard deviation |
| RE | Rutin equivalents |
| IC50 | Half-maximal inhibitory concentration |
| FBS | Fetal bovine serum |
| RSA | Radical scavenging activity |
| TPC | Total phenolic content |
| TFC | Total flavonoid content |
| n.s. | No significant correlation observed (p ≥ 0.05) |
| 4T1 | Breast cancer cell line derived from mammary gland tissue of a mouse |
| A549 | Adenocarcinoma human alveolar basal epithelial cell line |
| Caki-1 | Human kidney carcinoma cell line |
| HCT116 | Human colon cancer cell line |
| MCF7 | Human breast cancer cell line |
| HEK-293 | Healthy human embryonic kidney cell line |
| WHO | World Health Organization |
| KUBG | Kaunas Botanical Garden Herbarium |
References
- Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 4 November 2025).
- Chu, D.T.; Nguyen, T.T.; Le Bao Tien, N.; Tran, D.K.; Jeong, J.H.; Anh, P.G.; Thanh, V.V.; Truong, D.T.; Dinh, T.C. Recent progress of stem cell therapy in cancer treatment: Molecular mechanisms and potential applications. Cells 2020, 9, 563. [Google Scholar] [CrossRef]
- Ghemrawi, R.; Abuamer, L.; Kremesh, S.; Hussien, G.; Ahmed, R.; Mousa, W.; Khoder, G.; Khair, M. Revolutionizing cancer treatment: Recent advances in immunotherapy. Biomedicines 2024, 12, 2158. [Google Scholar] [CrossRef]
- Ismail, T.; Calcabrini, C.; Diaz, A.R.; Fimognari, C.; Turrini, E.; Catanzaro, E.; Akhtar, S.; Sestili, P. Ellagitannins in cancer chemoprevention and therapy. Toxins 2016, 8, 151. [Google Scholar] [CrossRef]
- Maruška, A.; Ugenskienė, R.; Raulinaitytė, D.; Juozaitytė, E.; Kaškonienė, V.; Drevinskas, T.; Stelmakienė, A.; Akuneca, I.; Makaravičius, T.; Tiso, N.; et al. Analysis of anti-proliferative effect of Chamerion angustifolium water extracts and its fractions on several breast cancer cell lines. Adv. Med. Sci. 2017, 62, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Paul, S.; Walia, Y.K.; Kumar, A.; Singhal, P. Therapeutic potential of medical plants: A review. J. Biol. Chem. Chron. 2015, 1, 46–54. [Google Scholar]
- Chaachouay, N.; Zidane, L. Plant-derived natural products: A source for drug discovery and development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Fatima, N.; Baqri, S.S.R.; Alsulimani, A.; Fagoonee, S.; Slama, P.; Kesari, K.K.; Roychoudhry, S.; Haque, S. Phytochemicals from Indian ethnomedicines: Promising prospects for the management of oxidative stress and cancer. Antioxidants 2021, 10, 1606. [Google Scholar] [CrossRef]
- Liaudanskas, M.; Žvikas, V.; Petrikaitė, V. The potential of dietary antioxidants from a series of plant extracts as anticancer agents against melanoma, glioblastoma, and breast cancer. Antioxidants 2021, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hsu, Y.Y.; Tang, J.Y.; Cheng, Y.B.; Chuang, Y.T.; Jeng, J.H.; Yen, C.H.; Chang, H.W. Methanol extracts of Commelina plant inhibits oral cancer cell proliferation. Antioxidants 2022, 11, 1813. [Google Scholar] [CrossRef] [PubMed]
- Wehbe, N.; Badran, A.; Baydoun, S.; Al-Sawalmih, A.; Maresca, M.; Baydoun, E.; Mesmar, J.E. The antioxidant potential and anticancer activity of Halodule uninervis ethanolic extract against triple-negative breast cancer cells. Antioxidants 2024, 13, 726. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Natural Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Nguyen, M.M.; Karboune, S. Combinatorial interactions of essential oils enriched with individual polyphenols, polyphenol mixes and plant extracts: Multi-antioxidant systems. Antioxidants 2023, 12, 486. [Google Scholar] [CrossRef] [PubMed]
- Armonavičius, D.; Stankevičius, M.; Maruška, A. Extraction of bioactive compounds and influence of storage conditions of raw material Chamaenerion angustifolium (L.) Holub using different strategies. Molecules 2024, 29, 5530. [Google Scholar] [CrossRef] [PubMed]
- Stankevičius, M.; Akuneca, I.; Jãkobsone, I.; Maruška, A. Comparative analysis of radical scavenging and antioxidant activity of phenolic compounds present in everyday use spice plants by means of spectrometric and chromatographic methods. J. Sep. Sci. 2011, 34, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Lee, J.E.; Jayakody, J.T.M.; Kim, J.; Jeong, J.W.; Choi, K.M.; Kim, T.S.; Seo, C.; Azimi, I.; Hyun, J.; Ryu, B. The influence of solvent choice on the extraction of bioactive compounds from Asteraceae: A comparative review. Foods 2024, 13, 31–51. [Google Scholar] [CrossRef]
- Tourabi, M.; Metouekel, A.; Ghouizi, A.E.L.; Jeddi, M.; Nouioura, G.; Laaroussi, H.; Hosen, M.E.; Benbrahim, K.F.; Bourhia, M.; Salamatullah, A.M.; et al. Efficacy of various extraction solvents on phytochemical composition, and biological properties of Mentha longifolia L. leaf extracts. Sci. Rep. 2023, 13, 18028. [Google Scholar] [CrossRef]
- Herranz-López, M.; Losada-Echeberría, M.; Barrajón-Catalán, E. The multitarget activity of natural extracts on cancer: Synergy and xenohormesis. Medicines 2018, 6, 6. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Kulkarni, G.T.; Nagpal, K. Unlocking antioxidant-anticancer synergy: An exploration of therapeutic bioactives from methanolic extracts of Rubus ellipticus and Boerhavia diffusa using HeLA cell line. Assay Drug Dev. Technol. 2024, 22, 361–372. [Google Scholar] [CrossRef]
- Li, W.Y.; Chan, S.W.; Guo, D.J.; Yu, P.H.F. Correlation between antioxidative power and anticancer activity in herbs from traditional Chinese medicine formulae with anticancer therapeutic effect. Pharm. Biol. 2007, 45, 541–546. [Google Scholar] [CrossRef]
- Muchtaridi, M.; Az-Zahra, F.; Wongso, H.; Setyawati, L.U.; Novitasari, D.; Ikram, E.H.K. Molecular mechanism of natural food antioxidants to regulate ROS in treating cancer: A review. Antioxidants 2024, 13, 207. [Google Scholar] [CrossRef] [PubMed]
- Sonboli, A.; Mojarrad, M.; Ebrahimi, S.N.; Enayat, S. Free radical scavenging activity and total phenolic content of methanolic extracts from male inflorescence of Salix aegyptica grown in Iran. Iran J. Pharm. Res. 2010, 9, 293–296. [Google Scholar]
- Arena, M.E.; Postemsky, P. Accumulation patterns of phenolic compounds during fruit growth and ripening of Berberis buxifolia, a native Patagonian species. N. Z. J. Bot. 2012, 50, 15–28. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Gupta, M.; Sasmal, S.; Majumdar, S.; Mukherjee, A. HPLC profiles of standard phenolic compounds present in medical plants. Int. J. Pharmacogn. Phytochem. Res. 2012, 4, 162–167. [Google Scholar]
- Dobes, J.; Zitka, O.; Sochor, J.; Adam, V.; Kizek, R. Electrochemical tools for determination of phenolic compounds in plants. Int. J. Electrochem. Sci. 2013, 8, 4520–4542. [Google Scholar] [CrossRef]
- Lin, H.-H.; Chen, J.-H.; Huang, C.-C.; Wang, C.-J. Apoptotic effect of 3,4-dihydroxybenzoic acid on human gastric carcinoma cells involving JNK/p38 MAPK signaling activation. Int. J. Cancer 2007, 120, 2306–2316. [Google Scholar] [CrossRef]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef]
- Chauhan, A.; Yadav, M.; Chauhan, R.; Basniwal, R.K.; Pathak, V.M.; Ranjan, A.; Kapardar, R.K.; Srivastav, R.; Tuli, H.S.; Ramniwas, S.; et al. Exploring the Potential of Ellagic Acid in Gastrointestinal Cancer Prevention: Recent Advances and Future Directions. Oncol. Ther. 2024, 12, 685–699. [Google Scholar] [CrossRef]
- Ho, K.-V.; Roy, A.; Foote, S.; Vo, P.H.; Lall, N.; Lin, C.-H. Profiling anticancer and antioxidant activities of phenolic compounds present in black walnuts (Juglans nigra) using a high-throughput screening approach. Molecules 2020, 25, 4516. [Google Scholar] [CrossRef]
- Gordanian, B.; Behbahani, M.; Carapetian, J.; Fazilati, M. In vitro evaluation of cytotoxic activity of flower, leaf, stem and root extracts of five Artemisia species. Res. Pharm. Sci. 2014, 9, 91–96. [Google Scholar] [PubMed]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in cancer prevention: New insights (Review). Int. J. Funct. Nutr. 2020, 1, 9. [Google Scholar] [CrossRef]
- Carvalho, A.J.S.; Ishikawa, T.; Gouvêa, C.M.C.P. Aqueous extract of Plinia edulis leaves: Antioxidant activity and cytotoxicity to human breast cancer MCF-7 cell line. South Afr. J. Bot. 2012, 81, 1–7. [Google Scholar] [CrossRef]
- Basu, P.; Maier, C. Phytoestrogens and breast cancer: In vitro anticancer activities of isoflavones, lignans, coumestans, stilbenes and their analogs and derivatives. Biomed. Pharmacother. 2018, 107, 1648–1666. [Google Scholar] [CrossRef]
- Đurović, S.; Kojić, I.; Radić, D.; Smyatskaya, Y.A.; Bazarnova, J.G.; Filip, S.; Tosti, T. Chemical Constituents of Stinging Nettle (Urtica dioica L.): A Comprehensive Review on Phenolic and Polyphenolic Compounds and Their Bioactivity. Int. J. Mol. Sci. 2024, 25, 3430. [Google Scholar] [CrossRef]
- Papoutsi, Z.; Kassi, E.; Chinou, I.; Halabalki, M.; Skaltsounis, L.A.; Moutsatsou, P. Walnut extract (Juglans regia L.) and its component ellagic acid exhibit anti-inflammatory activity in human aorta endothelial cells and osteoblastic activity in the cell line KS483. Br. J. Nutr. 2008, 99, 715–722. [Google Scholar] [CrossRef]
- Chrzanowski, G.; Leszczynksi, B.; Czerniewicz, P.; Sytykiewicz, H.; Matok, H.; Krzyżanowski, R. Phenolic acids of walnut (Juglans regia L.). Herba Pol. 2011, 57, 22–29. [Google Scholar]
- Ho, K.V.; Schreiber, K.L.; Vu, D.C.; Rottinghaus, S.M.; Jackson, D.E.; Brown, C.R.; Lei, Z.; Sumner, L.W.; Coggeshall, M.V.; Lin, C.H. Black Walnut (Juglans nigra) Extracts Inhibit Proinflammatory Cytokine Production from Lipopolysaccharide-Stimulated Human Promonocytic Cell Line U-937. Front Pharmacol. 2019, 10, 1059. [Google Scholar] [CrossRef]
- Vu, D.C.; Nguyen, T.H.D.; Ho, T.L. An overview of phytochemicals and potential health-promoting properties of black walnut. RSC Adv. 2020, 10, 33378–33388. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Mattar, M.A.; Al-Yafrasi, M.A.; El-Ansary, D.O.; El-Abedin, T.K.Z.; Yessoufou, K. Polyphenol Profile and Pharmaceutical Potential of Quercus spp. Bark Extracts. Plants 2019, 8, 486. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Paduch, R.; Wiater, A.; Dudek, A.; Pleszczyńska, M.; Tomczykowa, M.; Granica, S.; Polak, P.; Tomczyk, M. In Vitro Antiproliferative and Antioxidant Effects of Extracts from Rubus caesius Leaves and Their Quality Evaluation. Evid.-Based Complement. Altern. Med. 2016, 2016, 5698685. [Google Scholar] [CrossRef]
- Neagu, E.; Paun, G.; Albu, C.; Eremia, S.A.-M.V.; Radu, G.L. Artemisia abrotanum and Symphytum officinale Polyphenolic Compounds-Rich Extracts with Potential Application in Diabetes Management. Metabolites 2023, 13, 354. [Google Scholar] [CrossRef]
- Bimbiraitė-Survilienė, K.; Stankevičius, M.; Šuštauskaitė, S.; Gęgotek, A.; Maruška, A.; Skrzydlewska, E.; Barsteigienė, Z.; Akuneca, I.; Ragažinskienė, O.; Lukošius, A. Evaluation of chemical compositions, radical scaveging and antitumor activities of Satureja hortensis L. herb extracts. Antioxidants 2021, 10, 53. [Google Scholar] [CrossRef]
- Lee, O.W.; Austin, S.; Gamma, M.; Cheff, D.M.; Lee, T.D.; Wilson, K.M.; Johnson, J.; Travers, J.; Braisted, J.C.; Guha, R.; et al. Cytotoxic profiling of annotated and diverse chemical libraries using quantitative high-throughput screening. SLAS Discov. 2020, 25, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Karaklajic-Stajic, Z.; Tomic, J.; Pesakovic, M.; Paunovic, S.M.; Stampar, F.; Mikulic-Petkovsek, M.; Grohar, M.C.; Hudina, M.; Jakopic, J. Black Queens of Fruits: Chemical Composition of Blackberry (Rubus subg. rubus Watson) and Black Currant (Ribes nigrum L.) Cultivars Selected in Serbia. Foods 2023, 12, 2775. [Google Scholar] [CrossRef]
- Lasinskas, M.; Jarienė, E.; Vaitkevičienė, N.; Kulaitienė, J.; Adamavičienė, A.; Hallmann, E. The Impact of Solid-Phase Fermentation on Flavonoids, Phenolic Acids, Tannins and Antioxidant Activity in Chamerion angustifolium (L.) Holub (Fireweed) Leaves. Plants 2023, 12, 277. [Google Scholar] [CrossRef]
- Verma, G.; Mishra, M. Development and optimization of UV-VIS spectroscopy—A review. World J. Pharm. Res. 2018, 7, 1170–1180. [Google Scholar]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical sensors and their applications: A review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Ivanauskas, L.; Uminska, K.; Gudžinskas, Z.; Heinrich, M.; Georgiyants, V.; Kozurak, A.; Mykhailenko, O. Phenological Variations in the Content of Polyphenols and Triterpenoids in Epilobium angustifolium Herb Originating from Ukraine. Plants 2023, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Shanaida, M.; Palamar, O.; Holembiovska, O. Chromatographic Profile of Polyphenols in the Agastache foeniculum (Pursh) Kuntze Herb: Evaluation of Optimal Extraction Efficiency. Biomed. Pharmacol. J. 2024, 17, 63–69. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Głód, D.; Kula, M.; Majdan, M.; Hałasa, R.; Matkowski, A.; Kozłowska, W.; Kawiak, A. Chemical composition and biological activity of Rubus idaeus shoots--a traditional herbal remedy of Eastern Europe. BMC Complement. Altern. Med. 2014, 14, 480. [Google Scholar] [CrossRef] [PubMed]
- Fursenco, C.; Calalb, T.; Uncu, L.; Dinu, M.; Ancuceanu, R. Solidago virgaurea L.: A Review of Its Ethnomedicinal Uses, Phytochemistry, and Pharmacological Activities. Biomolecules 2020, 10, 1619. [Google Scholar] [CrossRef]
- Peñarrieta, J.M.; Alvarado, J.A.; Bergenståhl, J.A.; Ákesson, B. Total Antioxidant Capacity and Content of Phenolic Compounds in Wild Strawberries (Fragaria vesca) Collected in Bolivia. Int. J. Fruit Sci. 2009, 9, 344–359. [Google Scholar] [CrossRef]
- Tomas, M. Effect of dietary fiber addition on the content and in vitro bioaccessibility of antioxidants in red raspberry puree. Food Chem. 2022, 1, 131897. [Google Scholar] [CrossRef]
- Cyboran, S.; Bonarska-Kujawa, D.; Pruchnik, H.; Żyłka, R.; Oszmiański, J.; Kleszczyńska, H. Phenolic content and biological activity of extracts of blackcurrant fruit and leaves. Food Res. Int. 2014, 65 (Pt A), 47–58. [Google Scholar] [CrossRef]
- Wu, Q.; Naeem, A.; Zou, J.; Yu, C.; Wang, Y.; Chen, J.; Ping, Y. Isolation of Phenolic Compounds from Raspberry Based on Molecular Imprinting Techniques and Investigation of Their Anti-Alzheimer’s Disease Properties. Molecules 2022, 27, 6893. [Google Scholar] [CrossRef]
- Li, Y.-K.; Zhao, Q.-J.; Hu, J.; Zou, Z.; He, X.-Y.; Yuan, H.-B.; Shi, X.-Y. Two New Quinoline Alkaloid Mannopyranosides from Solidago canadensis. Helv. Chim. Acta 2009, 92, 928–931. [Google Scholar] [CrossRef]
- Hasan, H.T.; Kadhim, E.J. Phytochemical Investigation and Pharmacological Activity of Solidago canadensis L. against H1N1 Virus, involving the Separation and Identification of Three New Compounds. Int. J. Drug Deliv. Technol. 2023, 13, 180–192. [Google Scholar] [CrossRef]
- Hasan, H.T.; Kadhim, E.J. Phytochemical investigation of Solidago canadensis L. and its activity against Colorectal Adenocarcinoma and acute monocytic leukemia. Int. J. Health Sci. 2022, 6, 211–231. [Google Scholar] [CrossRef]
- Nguyen, S.; Nguyen, H.; Truong, K. Comparative cytotoxic effects of methanol, ethanol and DMSO on human cancer cell lines. Biomed. Res. Ther. 2020, 7, 3855–3859. [Google Scholar] [CrossRef]
- Vadeikienė, R.; Jakštys, B.; Ugenskienė, R.; Šatkauskas, S.; Juozaitytė, E. Systematic optimization of gene electrotransfer protocol using hard-to-transfect UT-7 cell line as model. Biomedicines 2022, 10, 2687. [Google Scholar] [CrossRef]
- Khalef, L.; Lydia, R.; Filicia, K.; Moussa, B. Cell viability and cytotoxicity assays: Biochemical elements and cellular compartments. Cell Biochem. Funct. 2024, 42, e4007. [Google Scholar] [CrossRef]
- Nunes, V.A.; Gozzo, A.J.; Juliano, M.A.; César, M.C.; Sampaio, M.U.; Sampaio, C.A.M.; Araújo, M.S. Antioxidant dietary deficiency caspase activation in chick skeletal cells. Braz. J. Med. Biol. Res. 2003, 36, 1047–1053. [Google Scholar] [CrossRef]
- Oyewole, A.O.; Birch-Machin, M.A. Mitochondria-targeted antioxidants. FASEB J. 2015, 29, 4766–4771. [Google Scholar] [CrossRef]


| No | Plant Name | Collection Date | Raw Material | Vegetation Stage |
|---|---|---|---|---|
| 1 | Stinging nettle (U. dioica L.) | 5 June 2022 | Aerial part (Herba) | Flower bud development |
| 2 | Common walnut (J. regia L.) | 30 September 2022 | Pericarp | Ripening |
| 3 | 22 July 2022 | Leaves | Ripening | |
| 4 | 25 July 2022 | Bark | Ripening | |
| 5 | Black walnut (J. nigra L.) | 6 October 2022 | Pericarp | Ripening |
| 6 | 20 October 2022 | Fruit | Ripening | |
| 7 | Canadian goldenrod (S. canadensis L.) | 10 August 2022 | Aerial part (Herba) | Beginning of the flowering |
| 8 | European goldenrod (S. virgaurea L.) | 17 August 2022 | Aerial part (Herba) | Massive flowering |
| 9 | Pedunculate oak (Q. robur L.) | 21 June 2022 | Leaves | Ripening |
| 10 | 10 September 2022 | Fruit | Ripening | |
| 11 | 6 June 2022 | Bark | Ripening | |
| 12 | European dewberry (R. caesius L.) | 30 June 2022 | Leaves | Massive flowering |
| 13 | Wild strawberry (F. vesca L.) | 30 June 2022 | Leaves | Massive flowering |
| 14 | Common comfrey (S. officinale L.) | 30 June 2022 | Roots | The end of vegetation |
| 15 | Blackcurrant (R. nigrum L.) | 5 June 2022 | Leaves | Ripening |
| 16 | Raspberry (R. idaeus L.) | 30 June 2022 | Leaves | Intensive growth |
| 17 | Fireweed (C.angustifolium L.) | 13 June 2022 | Aerial part (Herba) | Intensive growth |
| 18 | 20 June 2022 | Aerial part (Herba) | Flower bud development | |
| 19 | 28 June 2022 | Aerial part (Herba) | Beginning of the flowering | |
| 20 | 2 July 2022 | Aerial part (Herba) | Massive flowering | |
| 21 | 14 July 2022 | Aerial part (Herba) | The end of the flowering |
| No | Medicinal Plant Name and Part | Compound | HPLC UV-VIS | HPLC-ED | ||
|---|---|---|---|---|---|---|
| CRE, mg/g * | Total Peak Area, mV ** | CRE, mg/g * | Total Peak Area, µCoulombs *** | |||
| 1 | Stinging nettle (U. dioica L.). Aerial part (Herba) | 3,4-dihydroxybenzoic acid | 0.03 ± 0.001 | 5155.5 ± 54.7 | 0.12 ± 0.004 | 2692.0 ± 56.9 |
| Chlorogenic acid | 0.21 ± 0.01 | 0.08 ± 0.003 | ||||
| 2 | Common walnut (J. regia L.). Pericarp | Gallic acid | 0.05 ± 0.001 | 3453.2 ± 79.7 | 0.09 ± 0.004 | 1052.8 ± 34.7 |
| 3,4-dihydroxybenzoic acid | 0.03 ± 0.001 | 0.08 ± 0.003 | ||||
| Chlorogenic acid | 0.07 ± 0.002 | 0.08 ± 0.003 | ||||
| Ferulic acid | 0.03 ± 0.001 | 0.12 ± 0.005 | ||||
| Rutin | 0.03 ± 0.001 | 0.19 ± 0.008 | ||||
| 3 | Common walnut (J. regia L.). Leaves | Gallic acid | 0.03 ± 0.001 | 6385.1 ± 58.9 | 0.15 ± 0.006 | 2268.8 ± 88.6 |
| 3,4-dihydroxybenzoic acid | 0.08 ± 0.003 | 0.16 ± 0.007 | ||||
| Vanillic acid | 0.03 ± 0.001 | 0.08 ± 0.003 | ||||
| 2-hydroxycinammic acid | 0.07 ± 0.003 | 0.23 ± 0.01 | ||||
| Ellagic acid | 0.08 ± 0.003 | 0.08 ± 0.001 | ||||
| 4 | Common walnut (J. regia L.). Bark | Gallic acid | 0.03 ± 0.001 | 5370.7 ± 89.8 | 0.37 ± 0.015 | 3008.6 ± 120.7 |
| 3,4-dihydroxybenzoic acid | 0.03 ± 0.001 | 0.12 ± 0.005 | ||||
| Vanillic acid | 0.04 ± 0.001 | 0.16 ± 0.007 | ||||
| Ferulic acid | 0.03 ± 0.001 | 0.10 ± 0.003 | ||||
| 5 | Black walnut (J. nigra L.). Pericarp | Gallic acid | 0.18 ± 0.007 | 18,726.4 ± 178.4 | 0.11 ± 0.004 | 7473.6 ± 241.8 |
| 3,4-dihydroxybenzoic acid | 0.12 ± 0.002 | 0.09 ± 0.003 | ||||
| Caffeic acid | 0.44 ± 0.012 | 0.12 ± 0.005 | ||||
| Ferulic acid | 0.24 ± 0.01 | 0.32 ± 0.01 | ||||
| trans-sinapic acid | 0.22 ± 0.01 | 0.36 ± 0.01 | ||||
| 6 | Black walnut (J. nigra L.). Fruit | Gallic acid | 0.06 ± 0.002 | 3979.3 ± 44.8 | 0.08 ± 0.001 | 2381.9 ± 59.3 |
| 3,4-dihydroxybenzoic acid | 0.04 ± 0.001 | 0.08 ± 0.001 | ||||
| Caffeic acid | 0.08 ± 0.002 | 0.12 ± 0.004 | ||||
| Ferulic acid | 0.10 ± 0.002 | 0.12 ± 0.005 | ||||
| trans-sinapic acid | 0.08 ± 0.002 | 0.14 ± 0.006 | ||||
| 7 | Canadian goldenrod (S. canadensis L.). Aerial part (Herba) | 3,4-dihydroxybenzoic acid | 0.03 ± 0.001 | 22,017.6 ± 218.7 | 0.09 ± 0.004 | 6102.6 ± 183.6 |
| Vanillic acid | 0.18 ± 0.008 | 0.15 ± 0.006 | ||||
| 2-hydroxycinammic acid | 0.04 ± 0.001 | 0.11 ± 0.004 | ||||
| Rutin | 0.04 ± 0.001 | 0.10 ± 0.004 | ||||
| trans-cinnamic acid | 0.77 ± 0.02 | - | ||||
| 8 | European goldenrod (S. virgaurea L.). Aerial part (Herba) | Chlorogenic acid | 0.19 ± 0.01 | 14,739.5 ± 118.2 | 0.08 ± 0.001 | 2983.4 ± 114.8 |
| Ferulic acid | 0.06 ± 0.001 | 0.09 ± 0.002 | ||||
| Salicylic acid | 0.05 ± 0.002 | 0.08 ± 0.003 | ||||
| Rutin | 0.79 ± 0.03 | 0.78 ± 0.02 | ||||
| 9 | Pedunculate oak (Q. robur L.). Leaves | Gallic acid | 0.20 ± 0.009 | 6367.3 ± 58.9 | 0.39 ± 0.01 | 3991.5 ± 148.7 |
| Caffeic acid | 0.03 ± 0.001 | 0.09 ± 0.002 | ||||
| Ellagic acid | 0.18 ± 0.008 | 0.11 ± 0.004 | ||||
| 10 | Pedunculate oak (Q. robur L.). Fruit | Gallic acid | 0.17 ± 0.002 | 8201.3 ± 108.9 | 0.10 ± 0.003 | 6958.8 ± 241.9 |
| 3,4-dihydroxybenzoic acid | 0.04 ± 0.001 | 0.14 ± 0.006 | ||||
| Chlorogenic acid | 0.03 ± 0.001 | 0.21 ± 0.01 | ||||
| Ferulic acid | 0.09 ± 0.003 | 0.12 ± 0.005 | ||||
| 2-hydroxycinammic acid | 0.06 ± 0.002 | 0.10 ± 0.004 | ||||
| trans-cinnamic acid | 0.10 ± 0.004 | - | ||||
| Ellagic acid | 0.35 ± 0.01 | 0.44 ± 0.02 | ||||
| 11 | Pedunculate oak (Q. robur L.). Bark | Gallic acid | 0.06 ± 0.001 | 3745.2 ± 57.8 | 0.85 ± 0.03 | 2863.6 ± 112.5 |
| 2-hydroxycinammic acid | 0.04 ± 0.001 | - | ||||
| Ellagic acid | 0.20 ± 0.009 | 0.11 ± 0.004 | ||||
| 12 | European dewberry (R. caesius L.). Leaves | Gallic acid | 0.07 ± 0.003 | 14,023.4 ± 55.6 | 0.09 ± 0.002 | 7712.3 ± 249.5 |
| 3,4-dihydroxybenzoic acid | 0.04 ± 0.001 | 0.31 ± 0.01 | ||||
| 2-hydroxycinammic acid | 0.06 ± 0.001 | 0.14 ± 0.004 | ||||
| trans-cinnamic acid | 0.22 ± 0.009 | - | ||||
| 13 | Wild strawberry (F. vesca L.). Leaves | trans-p-coumaric acid | 0.42 ± 0.02 | 15,863.1 ± 115.8 | 0.47 ± 0.02 | 8999.5 ± 238.6 |
| 2-hydroxycinammic acid | 0.11 ± 0.004 | 0.09 ± 0.002 | ||||
| Ellagic acid | 0.32 ± 0.01 | 1.11 ± 0.03 | ||||
| 14 | Common comfrey (S. officinale L.). Roots | 3,4-dihydroxybenzoic acid | 0.03 ± 0.001 | 3581.1 ± 22.8 | 0.11 ± 0.001 | 2138.6 ± 86.9 |
| 15 | Blackcurrant (R. nigrum L.). Leaves | Gallic acid | 0.03 ± 0.001 | 6906.2 ± 69.4 | 0.09 ± 0.001 | 3527.0 ± 127.6 |
| 3,4-dihydroxybenzoic acid | 0.06 ± 0.002 | 0.10 ± 0.003 | ||||
| Vanillic acid | 0.06 ± 0.002 | 0.08 ± 0.001 | ||||
| Caffeic acid | 0.03 ± 0.001 | 0.08 ± 0.002 | ||||
| 16 | Raspberry (R. idaeus L.). Leaves | 3,4-dihydroxybenzoic acid | 0.04 ± 0.001 | 11,668.8 ± 241.8 | 0.38 ± 0.01 | 5556.35 ± 187.6 |
| trans-p-coumaric acid | 0.03 ± 0.001 | 0.14 ± 0.006 | ||||
| trans-cinnamic acid | 0.25 ± 0.01 | - | ||||
| 17 | Fireweed (C. angustifolium L.). Aerial part (Herba) | Gallic acid | 0.12 ± 0.005 | 24,617.3 ± 254.2 | 0.36 ± 0.01 | 9789.5 ± 358.4 |
| Oenothein B | 1.29 ± 0.04 | 3.94 ± 0.18 | ||||
| 3,4-dihydroxybenzoic acid | 0.43 ± 0.02 | 0.10 ± 0.003 | ||||
| Ellagic acid | 0.08 ± 0.01 | 0.20 ± 0.01 | ||||
| 18 | Fireweed (C. angustifolium L.). Aerial part (Herba) | Gallic acid | 0.03 ± 0.001 | 23,545.9 ± 199.8 | 0.68 ± 0.03 | 14,456.4 ± 542.8 |
| Oenothein B | 1.16 ± 0.04 | 5.54 ± 0.21 | ||||
| 3,4-dihydroxybenzoic acid | 0.30 ± 0.01 | 0.10 ± 0.004 | ||||
| trans-p-coumaric acid | 0.03 ± 0.001 | 0.17 ± 0.007 | ||||
| Ferulic acid | 0.04 ± 0.001 | 0.11 ± 0.004 | ||||
| trans-sinapic acid | 0.03 ± 0.001 | 0.12 ± 0.004 | ||||
| 2-hydroxycinammic acid | 0.11 ± 0.002 | 0.19 ± 0.009 | ||||
| Ellagic acid | 0.11 ± 0.004 | 0.24 ± 0.01 | ||||
| 19 | Fireweed (C. angustifolium L.). Aerial part (Herba) | Gallic acid | 0.11 ± 0.003 | 28,101.1 ± 395.7 | 0.29 ± 0.008 | 10,354.0 ± 213.6 |
| Oenothein B | 1.40 ± 0.06 | 3.92 ± 0.18 | ||||
| 3,4-dihydroxybenzoic acid | 0.38 ± 0.012 | 0.10 ± 0.003 | ||||
| Ferulic acid | 0.03 ± 0.001 | 0.09 ± 0.004 | ||||
| Ellagic acid | 0.08 ± 0.003 | 0.23 ± 0.011 | ||||
| 20 | Fireweed (C. angustifolium L.). Aerial part (Herba) | Gallic acid | 0.05 ± 0.002 | 17,783.2 ± 248.9 | 0.20 ± 0.008 | 11,219.1 ± 413.9 |
| Oenothein B | 0.96 ± 0.01 | 4.18 ± 0.16 | ||||
| 3,4-dihydroxybenzoic acid | 0.20 ± 0.009 | 0.09 ± 0.003 | ||||
| Ferulic acid | 0.03 ± 0.001 | 0.10 ± 0.003 | ||||
| 2-hydroxycinammic acid | 0.04 ± 0.001 | 0.16 ± 0.008 | ||||
| Ellagic acid | 0.04 ± 0.001 | 0.24 ± 0.01 | ||||
| 21 | Fireweed (C. angustifolium L.). Aerial part (Herba) | Gallic acid | 0.11 ± 0.04 | 22,338.4 ± 269.9 | 0.23 ± 0.01 | 9970.7 ± 397.6 |
| Oenothein B | 1.20 ± 0.04 | 4.17 ± 0.19 | ||||
| 3,4-dihydroxybenzoic acid | 0.27 ± 0.008 | 0.10 ± 0.004 | ||||
| Ellagic acid | 0.06 ± 0.002 | 0.17 ± 0.007 | ||||
| No | Medicinal Plant Name and Part | Compound | HPLC UV-VIS | HPLC-ED | ||
|---|---|---|---|---|---|---|
| CRE, mg/g * | Total Peak Area, mV ** | CRE, mg/g * | Total Peak Area, µCoulombs *** | |||
| 1 | Stinging nettle (U. dioica L.). Aerial part (Herba) | 3,4-dihydroxybenzoic acid | 0.04 ± 0.001 | 5697.7 ± 121.4 | 0.11 ± 0.004 | 1424.9 ± 54.8 |
| Chlorogenic acid | 0.22 ± 0.01 | 0.37 ± 0.01 | ||||
| Caffeic acid | 0.03 ± 0.001 | - | ||||
| 2 | Common walnut (J. regia L.). Pericarp | Gallic acid | 0.04 ± 0.001 | 3639.2 ± 88.4 | 0.22 ± 0.01 | 2056.6 ± 63.7 |
| 3,4-dihydroxybenzoic acid | 0.03 ± 0.001 | 0.25 ± 0.01 | ||||
| Chlorogenic acid | 0.07 ± 0.002 | 0.10 ± 0.004 | ||||
| Ferulic acid | 0.04 ± 0.001 | 0.34 ± 0.01 | ||||
| Rutin | 0.03 ± 0.001 | 0.11 ± 0.003 | ||||
| 3 | Common walnut (J. regia L.). Leaves | Gallic acid | 0.03 ± 0.001 | 5629.9 ± 112.5 | 0.22 ± 0.01 | 5234.2 ± 169.7 |
| 3,4-dihydroxybenzoic acid | 0.04 ± 0.001 | 0.25 ± 0.01 | ||||
| Vanillic acid | 0.05 ± 0.001 | 0.10 ± 0.004 | ||||
| 2-hydroxycinammic acid | 0.06 ± 0.002 | 0.34 ± 0.01 | ||||
| Ellagic acid | 0.16 ± 0.003 | 0.11 ± 0.003 | ||||
| 4 | Common walnut (J. regia L.). Bark | Gallic acid | 0.03 ± 0.001 | 3495.1 ± 86.7 | 0.67 ± 0.03 | 6701.4 ± 239.7 |
| 3,4-dihydroxybenzoic acid | 0.03 ± 0.001 | 0.09 ± 0.002 | ||||
| Vanillic acid | 0.04 ± 0.001 | 0.16 ± 0.007 | ||||
| Ferulic acid | 0.03 ± 0.001 | 0.44 ± 0.02 | ||||
| 5 | Black walnut (J. nigra L.). Pericarp | Gallic acid | 0.29 ± 0.01 | 15,526.3 ± 224.6 | 0.12 ± 0.004 | 10,489.1 ± 351.8 |
| 3,4-dihydroxybenzoic acid | 0.15 ± 0.004 | 0.10 ± 0.003 | ||||
| Caffeic acid | 0.43 ± 0.01 | 0.15 ± 0.006 | ||||
| Ferulic acid | 0.05 ± 0.001 | 0.25 ± 0.01 | ||||
| trans-sinapic acid | 0.07 ± 0.002 | 0.36 ± 0.01 | ||||
| trans-cinnamic acid | 0.27 ± 0.012 | - | ||||
| 6 | Black walnut (J. nigra L.). Fruit | Gallic acid | 0.03 ± 0.001 | 3331.0 ± 105.8 | 0.09 ± 0.003 | 5001.7 ± 198.6 |
| 3,4-dihydroxybenzoic acid | 0.03 ± 0.001 | 0.09 ± 0.004 | ||||
| Caffeic acid | 0.06 ± 0.002 | 0.19 ± 0.008 | ||||
| Ferulic acid | 0.07 ± 0.003 | 0.09 ± 0.003 | ||||
| trans-sinapic acid | 0.06 ± 0.002 | 0.17 ± 0.007 | ||||
| trans-cinnamic acid | 0.14 ± 0.05 | - | ||||
| 7 | Canadian goldenrod (S. canadensis L.). Aerial part (Herba) | 3,4-dihydroxybenzoic acid | 0.03 ± 0.001 | 40,509.1 ± 458.8 | 0.13 ± 0.005 | 19,574.8 ± 589.6 |
| Vanillic acid | 0.43 ± 0.01 | 0.15 ± 0.006 | ||||
| 2-hydroxycinammic acid | 0.08 ± 0.03 | 3.51 ± 0.15 | ||||
| Rutin | 0.11 ± 0.03 | 0.11 ± 0.001 | ||||
| trans-cinnamic acid | 1.40 ± 0.05 | 0.15 ± 0.006 | ||||
| 8 | European goldenrod (S. virgaurea L.). Aerial part (Herba) | Chlorogenic acid | 0.12 ± 0.004 | 9250.8 ± 115.8 | 0.09 ± 0.001 | 7126.5 ± 234.7 |
| 2-hydroxycinammic acid | 0.03 ± 0.001 | 0.13 ± 0.005 | ||||
| Rutin | 0.05 ± 0.001 | 0.20 ± 0.008 | ||||
| 9 | Pedunculate oak (Q. robur L.). Leaves | Gallic acid | 0.25 ± 0.01 | 13,425.9 ± 182.6 | 0.46 ± 0.02 | 8125.2 ± 203.6 |
| Caffeic acid | 0.03 ± 0.001 | 0.10 ± 0.003 | ||||
| Ellagic acid | 0.05 ± 0.001 | 0.13 ± 0.005 | ||||
| 10 | Pedunculate oak (Q. robur L.). Fruit | Gallic acid | 0.17 ± 0.006 | 23,021.3 ± 259.3 | 0.14 ± 0.006 | 9266.2 ± 396.4 |
| 3,4-dihydroxybenzoic acid | 0.04 ± 0.001 | 0.15 ± 0.006 | ||||
| Chlorogenic acid | 0.03 ± 0.001 | 0.34 ± 0.01 | ||||
| Ferulic acid | 0.09 ± 0.003 | 0.14 ± 0.006 | ||||
| 2-hydroxycinammic acid | 0.06 ± 0.002 | 0.10 ± 0.004 | ||||
| trans-cinnamic acid | 0.10 ± 0.004 | - | ||||
| Ellagic acid | 0.35 ± 0.01 | 0.74 ± 0.03 | ||||
| 11 | Pedunculate oak (Q. robur L.). Bark | Gallic acid | 0.15 ± 0.006 | 8810.5 ± 148.9 | 2.51 ± 0.12 | 7868.8 ± 267.8 |
| Ellagic acid | 0.29 ± 0.01 | 0.15 ± 0.006 | ||||
| 12 | European dewberry (R. caesius L.). Leaves | Gallic acid | 0.08 ± 0.003 | 17,983.0 ± 248.9 | 0.09 ± 0.001 | 17,376.2 ± 593.6 |
| 3,4-dihydroxybenzoic acid | 0.07 ± 0.002 | 0.46 ± 0.02 | ||||
| trans-cinnamic acid | 0.19 ± 0.005 | - | ||||
| 13 | Wild strawberry (F. vesca L.). Leaves | trans-p-coumaric acid | 0.51 ± 0.02 | 16,822.8 ± 238.5 | 0.71 ± 0.03 | 21,042.0 ± 637.9 |
| 2-hydroxycinammic acid | 0.10 ± 0.03 | 0.08 ± 0.003 | ||||
| Ellagic acid | 0.26 ± 0.01 | 1.36 ± 0.05 | ||||
| 14 | Common comfrey (S. officinale L.). Roots | 3,4-dihydroxybenzoic acid | 0.03 ± 0.001 | 3101.6 ± 115.4 | 0.16 ± 0.007 | 4235.8 ± 157.2 |
| 15 | Blackcurrant (R. nigrum L.). Leaves | Gallic acid | 0.11 ± 0.003 | 11603.7 ± 193.7 | 0.09 ± 0.003 | 7149.9 ± 217.9 |
| 3,4-dihydroxybenzoic acid | 0.03 ± 0.001 | 0.16 ± 0.007 | ||||
| Vanillic acid | 0.09 ± 0.003 | 0.09 ± 0.003 | ||||
| Caffeic acid | 0.03 ± 0.001 | 0.09 ± 0.004 | ||||
| 16 | Raspberry (R. idaeus L.). Leaves | Gallic acid | 0.04 ± 0.001 | 12,810.1 ± 358.6 | 0.09 ± 0.003 | 8135.1 ± 364.8 |
| 3,4-dihydroxybenzoic acid | 0.04 ± 0.001 | 0.16 ± 0.007 | ||||
| Vanillic acid | 0.04 ± 0.001 | 0.09 ± 0.003 | ||||
| trans-p-coumaric acid | 0.03 ± 0.001 | 0.09 ± 0.004 | ||||
| trans-cinnamic acid | 0.25 ± 0.01 | - | ||||
| 17 | Fireweed (C. angustifolium L.). Aerial part (Herba) | Gallic acid | 0.17 ± 0.005 | 60,522.1 ± 589.6 | 0.18 ± 0.008 | 29,890.5 ± 893.6 |
| Oenothein B | 3.42 ± 0.12 | 11.90 ± 0.13 | ||||
| 3,4-dihydroxybenzoic acid | 1.19 ± 0.05 | 0.23 ± 0.01 | ||||
| Ellagic acid | 0.09 ± 0.002 | 0.17 ± 0.004 | ||||
| 18 | Fireweed (C. angustifolium L.). Aerial part (Herba) | Gallic acid | 0.13 ± 0.004 | 54,315.7 ± 598.3 | 0.37 ± 0.012 | 31,719.4 ± 963.8 |
| Oenothein B | 3.19 ± 0.11 | 12.01 ± 0.21 | ||||
| 3,4-dihydroxybenzoic acid | 1.03 ± 0.04 | 0.27 ± 0.01 | ||||
| Ellagic acid | 0.08 ± 0.002 | 0.21 ± 0.01 | ||||
| 19 | Fireweed (C. angustifolium L.). Aerial part (Herba) | Gallic acid | 0.15 ± 0.006 | 53,762.1 ± 482.6 | 0.17 ± 0.008 | 24,974.0 ± 679.8 |
| Oenothein B | 3.26 ± 0.12 | 9.77 ± 0.18 | ||||
| 3,4-dihydroxybenzoic acid | 0.90 ± 0.01 | 0.19 ± 0.009 | ||||
| Ellagic acid | 0.12 ± 0.004 | 0.10 ± 0.004 | ||||
| 20 | Fireweed (C. angustifolium L.). Aerial part (Herba) | Gallic acid | 0.13 ± 0.002 | 58,664.3 ± 369.4 | 0.21 ± 0.01 | 25,122.9 ± 896.3 |
| Oenothein B | 3.19 ± 0.13 | 9.80 ± 0.16 | ||||
| 3,4-dihydroxybenzoic acid | 0.84 ± 0.03 | 0.19 ± 0.006 | ||||
| 2-hydroxycinammic acid | 0.03 ± 0.001 | 0.25 ± 0.01 | ||||
| Ellagic acid | 0.12 ± 0.03 | 0.14 ± 0.005 | ||||
| 21 | Fireweed (C. angustifolium L.). Aerial part (Herba) | Gallic acid | 0.12 ± 0.05 | 43,218.0 ± 449.6 | 0.28 ± 0.012 | 33,813.0 ± 967.8 |
| Oenothein B | 2.99 ± 0.11 | 13.71 ± 0.19 | ||||
| 3,4-dihydroxybenzoic acid | 0.64 ± 0.02 | 0.19 ± 0.008 | ||||
| Ellagic acid | 0.11 ± 0.004 | 0.16 ± 0.007 | ||||
| No | Plant Name | RSA, Expressed in RE mg/g * | Incubation Period | Half-Maximal Inhibitory Concentration (IC50), RE mg/g | ||||
|---|---|---|---|---|---|---|---|---|
| 4T1 | A549 | Caki-1 | HCT116 | MCF7 | ||||
| 1 | Stinging nettle (U. dioica L.) | 35.6 ± 2.2 | 24 h | NA ** | 9.24 ± 0.02 | 13.84 ± 0.01 | NA | NA |
| 48 h | 9.88 ± 0.04 | 5.76 ± 0.04 | 8.20 ± 0.05 | ND *** | NA | |||
| 72 h | 5.52 ± 0.05 | 0.36 ± 0.01 | 5.12 ± 0.02 | 7.20 ± 0.02 | NA | |||
| 2 | Common walnut (J. regia L.) | 52.6 ± 2.1 | 24 h | 8.56 ± 0.02 | 3.40 ± 0.04 | 1.60 ± 0.02 | 19.92 ± 0.06 | 7.76 ± 0.02 |
| 48 h | 2.88 ± 0.04 | 2.24 ± 0.02 | 2.00 ± 0.05 | 6.96 ± 0.09 | 6.72 ± 0.21 | |||
| 72 h | 2.24 ± 0.02 | 2.08 ± 0.04 | 1.48 ± 0.05 | 4.32 ± 0.02 | 4.92 ± 0.05 | |||
| 3 | Common walnut (J. regia L.) | 42.4 ± 1.0 | 24 h | 12.44 ± 0.05 | 5.28 ± 0.08 | 5.08 ± 0.08 | 21.60 ± 0.08 | 10.28 ± 0.02 |
| 48 h | 5.44 ± 0.08 | 5.40 ± 0.05 | 3.00 ± 0.01 | 7.52 ± 0.01 | 4.72 ± 0.01 | |||
| 72 h | 3.12 ± 0.10 | 4.36 ± 0.04 | 2.00 ± 0.02 | 8.48 ± 0.08 | 4.56 ± 0.06 | |||
| 4 | Common walnut (J. regia L.) | 99.4 ± 1.9 | 24 h | 7.40 ± 0.07 | 1.36 ± 0.02 | 5.72 ± 0.07 | ND | 8.60 ± 0.05 |
| 48 h | 2.20 ± 0.04 | 1.20 ± 0.02 | 3.72 ± 0.09 | 9.16 ± 0.08 | 6.12 ± 0.02 | |||
| 72 h | 2.52 ± 0.06 | 0.40 ± 0.01 | 2.36 ± 0.10 | 5.92 ± 0.11 | 4.48 ± 0.11 | |||
| 5 | Black walnut (J. nigra L.) | 206.1 ± 1.4 | 24 h | 6.28 ± 0.02 | 1.65 ± 0.05 | 2.56 ± 0.11 | 5.28 ± 0.05 | 8.56 ± 0.05 |
| 48 h | 1.76 ± 0.01 | 1.45 ± 0.02 | 2.56 ± 0.15 | 2.56 ± 0.02 | 7.24 ± 0.01 | |||
| 72 h | 1.76 ± 0.02 | 0.52 ± 0.02 | 2.56 ± 0.06 | 2.56 ± 0.06 | 3.96 ± 0.06 | |||
| 6 | Black walnut (J. nigra L.) | 24 h | 9.48 ± 0.05 | 1.60 ± 0.05 | 3.16 ± 0.05 | 13.08 ± 0.02 | 11.76 ± 0.02 | |
| 201.4 ± 2.5 | 48 h | 2.48 ± 0.02 | 1.28 ± 0.01 | 1.56 ± 0.01 | 4.92 ± 0.07 | 9.04 ± 0.06 | ||
| 72 h | 1.72 ± 0.05 | 0.64 ± 0.07 | 1.56 ± 0.02 | 2.96 ± 0.08 | 8.44 ± 0.11 | |||
| 7 | Canadian goldenrod (S. canadensis L.) | 95.2 ± 2.4 | 24 h | 2.92 ± 0.05 | 2.20 ± 0.05 | 3.16 ± 0.01 | 6.96 ± 0.01 | 4.04 ± 0.01 |
| 48 h | 1.38 ± 0.07 | 2.20 ± 0.06 | 3.60 ± 0.06 | 4.20 ± 0.01 | 3.08 ± 0.06 | |||
| 72 h | 1.44 ± 0.02 | 1.32 ± 0.08 | 2.88 ± 0.01 | 4.96 ± 0.02 | 2.16 ± 0.01 | |||
| 8 | European goldenrod (S. virgaurea L.) | 80.4 ± 1.4 | 24 h | 6.96 ± 0.05 | 5.00 ± 0.02 | 6.52 ± 0.07 | 14.96 ± 0.06 | ND |
| 48 h | 5.64 ± 0.06 | 3.80 ± 0.08 | 4.40 ± 0.07 | 13.52 ± 0.05 | ND | |||
| 72 h | 2.52 ± 0.01 | 4.40 ± 0.02 | 3.40 ± 0.01 | 12.68 ± 0.01 | ND | |||
| 9 | Pedunculate oak (Q. robur L.) | 60.5 ± 1.9 | 24 h | 5.08 ± 0.06 | 1.04 ± 0.03 | 4.48 ± 0.02 | ND | 13.80 ± 0.06 |
| 48 h | 1.56 ± 0.02 | 1.16 ± 0.02 | 2.56 ± 0.06 | 15.40 ± 0.02 | 10.76 ± 0.11 | |||
| 72 h | 0.84 ± 0.02 | 0.44 ± 0.02 | 1.64 ± 0.06 | 11.04 ± 0.06 | 8.32 ± 0.12 | |||
| 10 | Pedunculate oak (Q. robur L.) | 192.3 ± 4.6 | 24 h | 8.52 ± 0.01 | 1.24 ± 0.01 | 3.48 ± 0.02 | 24.40 ± 0.12 | 17.72 ± 0.06 |
| 48 h | 2.48 ± 0.06 | 0.92 ± 0.07 | 1.76 ± 0.07 | 12.48 ± 0.06 | 14.28 ± 0.15 | |||
| 72 h | 1.28 ± 0.02 | 0.44 ± 0.07 | 1.76 ± 0.06 | 6.36 ± 0.06 | 13.08 ± 0.06 | |||
| 11 | Pedunculate oak (Q. robur L.) | 122.5 ± 1.5 | 24 h | 6.52 ± 0.01 | 1.44 ± 0.08 | 5.28 ± 0.01 | 32.88 ± 0.24 | 15.44 ± 0.06 |
| 48 h | 2.24 ± 0.05 | 1.48 ± 0.05 | 3.84 ± 0.06 | 21.04 ± 0.06 | 12.32 ± 0.07 | |||
| 72 h | 1.00 ± 0.10 | 0.52 ± 0.07 | 1.84 ± 0.02 | 16.20 ± 0.22 | 9.96 ± 0.01 | |||
| 12 | European dewberry (R. caesius L.) | 79.3 ± 2.5 | 24 h | 9.96 ± 0.11 | 7.60 ± 0.09 | 7.64 ± 0.06 | 19.84 ± 0.06 | 14.28 ± 0.08 |
| 48 h | 2.96 ± 0.06 | 2.00 ± 0.02 | 3.56 ± 0.02 | 7.16 ± 0.02 | 12.08 ± 0.11 | |||
| 72 h | 1.08 ± 0.02 | 0.52 ± 0.02 | 2.04 ± 0.06 | 5.64 ± 0.06 | 11.24 ± 0.06 | |||
| 13 | Wild strawberry (F. vesca L.) | 54.9 ± 1.4 | 24 h | 14.76 ± 0.10 | 3.60 ± 0.06 | 8.16 ± 0.07 | 29.68 ± 0.01 | ND |
| 48 h | 3.15 ± 0.15 | 3.28 ± 0.03 | 6.16 ± 0.01 | 18.04 ± 0.01 | ND | |||
| 72 h | 1.40 ± 0.01 | 0.88 ± 0.02 | 2.12 ± 0.06 | 7.52 ± 0.06 | ND | |||
| 14 | Common Comfrey (S. officinale L.) | 15.2 ± 1.7 | 24 h | 6.72 ± 0.02 | 6.00 ± 0.07 | 24.08 ± 0.16 | ND | ND |
| 48 h | 5.46 ± 0.05 | 6.20 ± 0.03 | 21.40 ± 0.01 | ND | ND | |||
| 72 h | 1.72 ± 0.06 | 3.76 ± 0.07 | 6.64 ± 0.06 | ND | ND | |||
| 15 | Blackcurrant (R. nigrum L.) | 42.2 ± 1.6 | 24 h | 3.48 ± 0.11 | 1.60 ± 0.01 | 3.32 ± 0.06 | ND | 11.16 ± 0.02 |
| 48 h | 1.36 ± 0.05 | 1.88 ± 0.01 | 2.72 ± 0.01 | ND | 10.44 ± 0.07 | |||
| 72 h | 1.16 ± 0.01 | 0.52 ± 0.01 | 2.12 ± 0.06 | ND | 7.00 ± 0.01 | |||
| 16 | Raspberry (R. idaeus L.) | 134.2 ± 1.3 | 24 h | 11.48 ± 0.05 | 3.20 ± 0.02 | 10.56 ± 0.06 | 21.20 ± 0.02 | ND |
| 48 h | 2.44 ± 0.02 | 2.68 ± 0.05 | 4.80 ± 0.02 | 6.52 ± 0.09 | 11.48 ± 0.16 | |||
| 72 h | 1.08 ± 0.06 | 1.00 ± 0.01 | 2.96 ± 0.10 | 6.16 ± 0.06 | 8.56 ± 0.12 | |||
| 17 | Fireweed (C. angustifolium L.) | 78.2 ± 0.9 | 24 h | 12.20 ± 0.02 | 2.40 ± 0.02 | 12.36 ± 0.01 | 21.44 ± 0.01 | 21.80 ± 0.44 |
| 48 h | 1.76 ± 0.06 | 2.40 ± 0.06 | 6.48 ± 0.15 | 8.28 ± 0.06 | 13.32 ± 0.06 | |||
| 72 h | 0.92 ± 0.01 | 1.04 ± 0.01 | 3.24 ± 0.01 | 4.88 ± 0.21 | 11.56 ± 0.07 | |||
| 18 | Fireweed (C. angustifolium L.) | 134.6 ± 1.1 | 24 h | 10.64 ± 0.13 | 1.80 ± 0.06 | 9.32 ± 0.06 | 11.68 ± 0.06 | 17.12 ± 0.11 |
| 48 h | 1.84 ± 0.01 | 1.64 ± 0.06 | 5.56 ± 0.06 | 6.12 ± 0.06 | 11.88 ± 0.07 | |||
| 72 h | 0.28 ± 0.01 | 0.76 ± 0.01 | 2.08 ± 0.09 | 2.52 ± 0.05 | 9.40 ± 0.06 | |||
| 19 | Fireweed (C. angustifolium L.) | 145.2 ± 0.5 | 24 h | 10.04 ± 0.01 | 7.20 ± 0.05 | 10.76 ± 0.02 | 20.52 ± 0.06 | 20.96 ± 0.06 |
| 48 h | 2.28 ± 0.06 | 7.00 ± 0.02 | 6.36 ± 0.09 | 8.64 ± 0.09 | 14.32 ± 0.22 | |||
| 72 h | 1.76 ± 0.06 | 6.52 ± 0.05 | 3.84 ± 0.08 | 9.56 ± 0.05 | 12.60 ± 0.06 | |||
| 20 | Fireweed (C. angustifolium L.) | 160.8 ± 3.9 | 24 h | 3.80 ± 0.07 | 2.80 ± 0.02 | 14.16 ± 0.06 | 9.88 ± 0.05 | 8.24 ± 0.15 |
| 48 h | 1.84 ± 0.02 | 2.40 ± 0.06 | 6.96 ± 0.15 | 3.92 ± 0.09 | 6.60 ± 0.16 | |||
| 72 h | 1.84 ± 0.06 | 1.04 ± 0.02 | 4.12 ± 0.01 | 5.48 ± 0.10 | 2.32 ± 0.06 | |||
| 21 | Fireweed (C. angustifolium L.) | 113.9 ± 2.5 | 24 h | 11.44 ± 0.02 | 2.00 ± 0.05 | 12.56 ± 0.06 | 15.20 ± 0.12 | 15.44 ± 0.02 |
| 48 h | 1.28 ± 0.06 | 2.00 ± 0.06 | 6.12 ± 0.06 | 5.76 ± 0.05 | 8.28 ± 0.06 | |||
| 72 h | 1.12 ± 0.07 | 0.40 ± 0.02 | 2.68 ± 0.06 | 5.16 ± 0.01 | 5.28 ± 0.11 | |||
| Variables | TPC * | TFC ** | RSA *** | HPLC UV-VIS | HPLC-ED | IC50 (4T1) | IC50 (A549) | IC50 (Caki-1) | IC50 (HCT116) | IC50 (MCF7) |
|---|---|---|---|---|---|---|---|---|---|---|
| TPC * | 1 | r = 0.407 p = 0.007 | r = 0.993 p < 0.001 | r = 0.435 p = 0.004 | r = 0.449 p = 0.003 | r = −0.471, p = 0.031 | n.s. **** | n.s. | n.s. | r = 0.449, p = 0.041 |
| TFC ** | dup. ***** | 1 | r = 0.355 p = 0.021 | r = 0.475 p = 0.001 | r = 0.418 p = 0.006 | n.s. | n.s. | n.s. | n.s. | n.s. |
| RSA *** | dup. | dup. | 1 | r = 0.397 p = 0.009 | r = 0.416 p = 0.006 | r = −0.468, p = 0.032 | r = −0.434, p = 0.049 | n.s. | n.s. | r = 0.488, p = 0.025 |
| HPLC UV-VIS | dup. | dup. | dup. | 1 | r = 0.905 p < 0.001 | r = −0.442, p = 0.045 | n.s. | n.s. | n.s. | r = 0.521, p = 0.015 |
| HPLC-ED | dup. | dup. | dup. | dup. | 1 | r = −0.486, p = 0.025 | n.s. | n.s. | n.s. | r = 0.480, p = 0.028 |
| r = −0.500, p = 0.021 | ||||||||||
| IC50 (4T1) | dup. | n.s. | dup. | dup. | dup. | 1 | r = 0.661, p = 0.001 | n.s. | r = 0.673, p < 0.001 | r = −0.624, p = 0.003 |
| dup. | r = −0.537, p = 0.012 | |||||||||
| IC50 (A549) | n.s. | n.s. | dup. | n.s. | n.s. | dup. | 1 | r = 0.467, p = 0.033 | n.s. | n.s. |
| r = 0.602, p = 0.004 | ||||||||||
| IC50 (Caki-1) | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | dup. | 1 | n.s. | r = −0.464, p = 0.034 |
| dup. | ||||||||||
| IC50 (HCT116) | n.s. | n.s. | n.s. | n.s. | n.s. | dup. | n.s. | n.s. | 1 | n.s. |
| IC50 (MCF7) | dup. | n.s. | dup. | dup. | dup. | dup. | n.s. | dup. | n.s. | 1 |
| dup. |
| No | Plant Name | Plant Part | Incubation Period | Millions of Cancer Cells Killed Per 1 g (×106) of Medicinal Plant | ||||
|---|---|---|---|---|---|---|---|---|
| 4T1 | A549 | Caki-1 | HCT116 | MCF7 | ||||
| 1 | Canadian goldenrod (S. canadensis L.) | Aerial part (Herba) | 24 h | 12.33–15.07 | - | - | - | 8.91–10.89 |
| 2 | Pedunculate oak (Q. robur L.) | Leaves | - | 34.62–42.31 | - | - | - | |
| 3 | Common walnut (J. regia L.) | Pericarp | 22.50–27.50 | - | ||||
| 4 | Black walnut (J. nigra L.) | Pericarp | - | - | - | 6.82–8.33 | - | |
| 5 | Fireweed (C.angustifolium L.) | Aerial part (Herba) | 48 h | 25.31–37.81 | - | - | - | - |
| 6 | Pedunculate oak (Q. robur L.) | Fruit | - | 35.22–52.61 | - | - | - | |
| 7 | Black walnut (J. nigra L.) | Fruit | - | - | 20.77–31.03 | - | - | |
| 8 | Black walnut (J. nigra L.) | Pericarp | - | - | - | 12.66–18.91 | - | |
| 9 | Canadian goldenrod (S. canadensis L.) | Aerial part (Herba) | - | - | - | - | 10.52–15.71 | |
| 10 | Fireweed (C.angustifolium L.) | Aerial part (Herba) | 72 h | 104.14–190.14 | - | - | 11.57–21.13 | - |
| 11 | Stinging nettle (U. dioica L.) | Aerial part (Herba) | - | 81.00–147.89 | - | - | - | |
| 12 | Common walnut (J. regia L.) | Pericarp | - | - | 19.70–35.97 | - | - | |
| 13 | Canadian goldenrod (S. canadensis L.) | Aerial part (Herba) | - | - | - | - | 13.50–24.65 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armonavičius, D.; Maruška, A.; Jakštys, B.; Stankevičius, M.; Drevinskas, T.; Bimbiraitė-Survilienė, K.; Čaplikaitė, M.; Ihara, H.; Takafuji, M.; Skrzydlewska, E.; et al. Evaluation of the Anticancer Activity of Medicinal Plants Predominantly Accumulating Ellagic Acid Compounds. Antioxidants 2025, 14, 1339. https://doi.org/10.3390/antiox14111339
Armonavičius D, Maruška A, Jakštys B, Stankevičius M, Drevinskas T, Bimbiraitė-Survilienė K, Čaplikaitė M, Ihara H, Takafuji M, Skrzydlewska E, et al. Evaluation of the Anticancer Activity of Medicinal Plants Predominantly Accumulating Ellagic Acid Compounds. Antioxidants. 2025; 14(11):1339. https://doi.org/10.3390/antiox14111339
Chicago/Turabian StyleArmonavičius, Domantas, Audrius Maruška, Baltramiejus Jakštys, Mantas Stankevičius, Tomas Drevinskas, Kristina Bimbiraitė-Survilienė, Modesta Čaplikaitė, Hirotaka Ihara, Makoto Takafuji, Elżbieta Skrzydlewska, and et al. 2025. "Evaluation of the Anticancer Activity of Medicinal Plants Predominantly Accumulating Ellagic Acid Compounds" Antioxidants 14, no. 11: 1339. https://doi.org/10.3390/antiox14111339
APA StyleArmonavičius, D., Maruška, A., Jakštys, B., Stankevičius, M., Drevinskas, T., Bimbiraitė-Survilienė, K., Čaplikaitė, M., Ihara, H., Takafuji, M., Skrzydlewska, E., Ragažinskienė, O., Kuwahara, Y., Nagaoka, S., Kaškonienė, V., Šatkauskas, S., & Kanopka, A. (2025). Evaluation of the Anticancer Activity of Medicinal Plants Predominantly Accumulating Ellagic Acid Compounds. Antioxidants, 14(11), 1339. https://doi.org/10.3390/antiox14111339












