Genus Veronica—Antioxidant, Cytotoxic and Antibacterial Activity of Phenolic Compounds from Wild and Cultivated Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation and Drying of Veronica Species
2.2. Reagents and Standards for LC/MS-MS and Other Analysis
2.3. Plant Preparation and Extraction
2.4. Chemical Identification of Phenolic Components
2.5. Antioxidant Activity of Veronica Extracts
2.5.1. The Measurement of the ORAC Values
2.5.2. The Measurement of the DPPH Radical Scavenging Activity
2.6. Antibacterial Activity
2.6.1. Disk Diffusion
2.6.2. Method of Diffusion by Agar Drilling
2.7. Cytotoxic Analysis
2.8. Statistical Analysis
3. Results
3.1. Phenolic Compounds
3.2. Antioxidant Activity
3.2.1. ORAC Activity
3.2.2. DPPH Activity
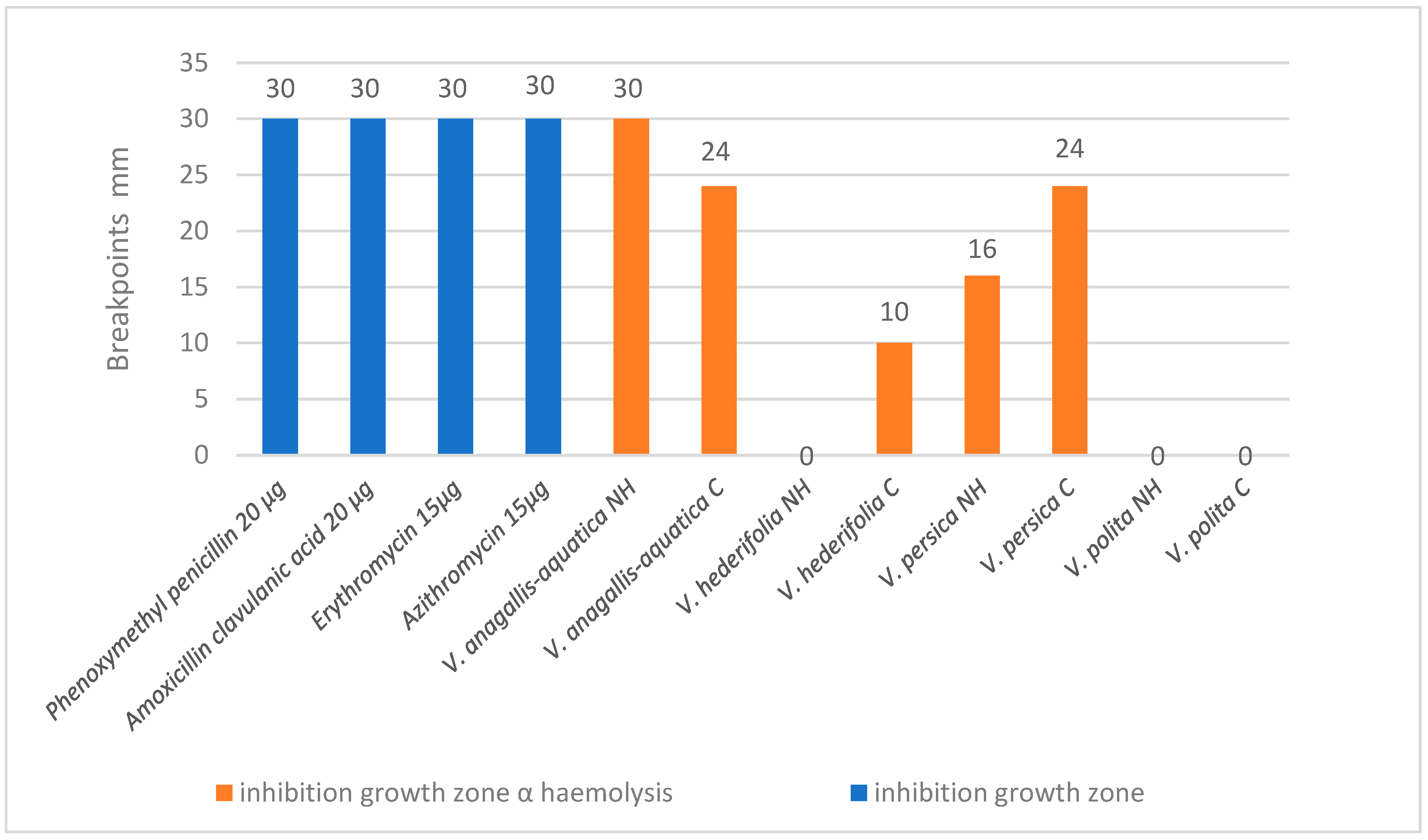
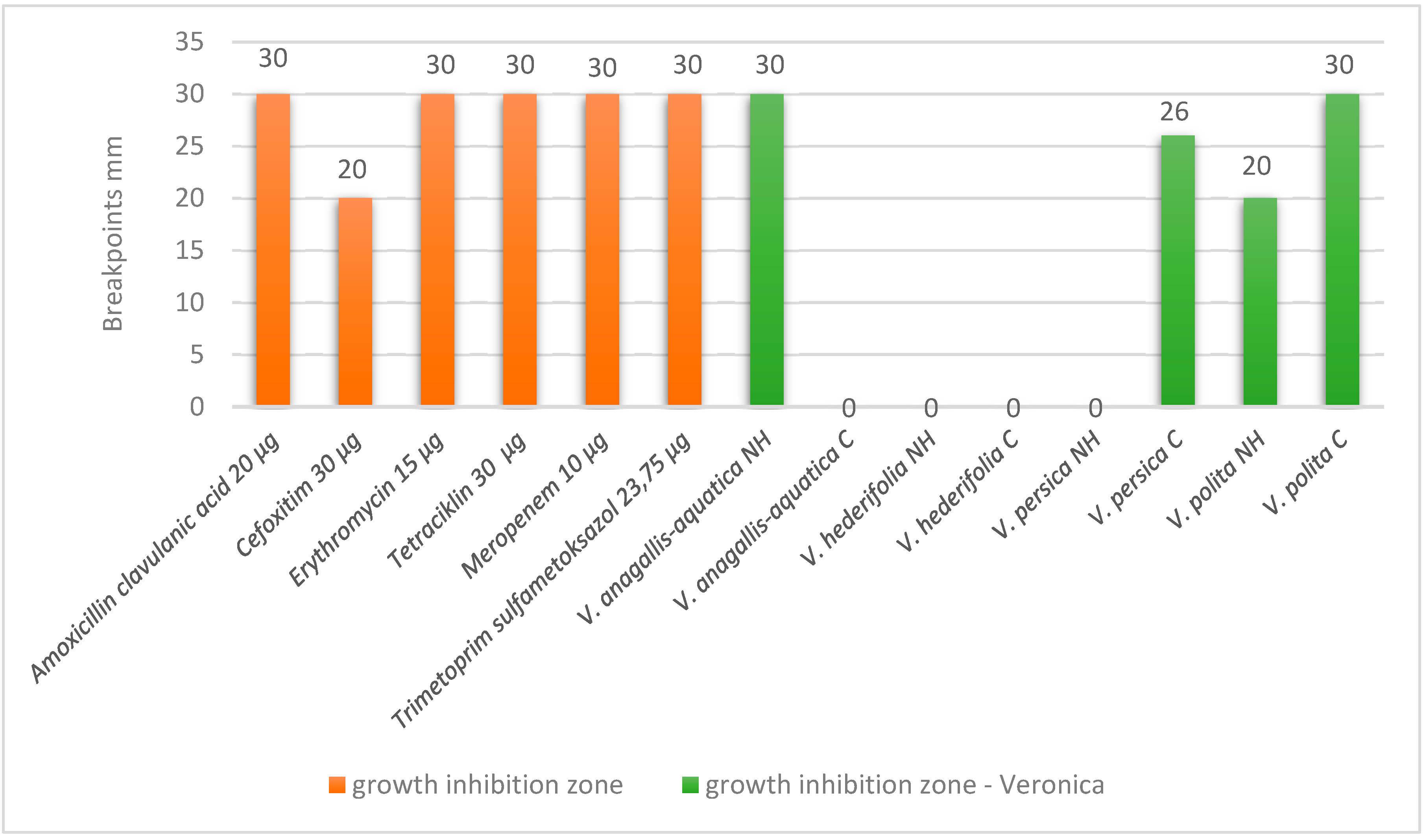
3.3. Antibacterial Activity
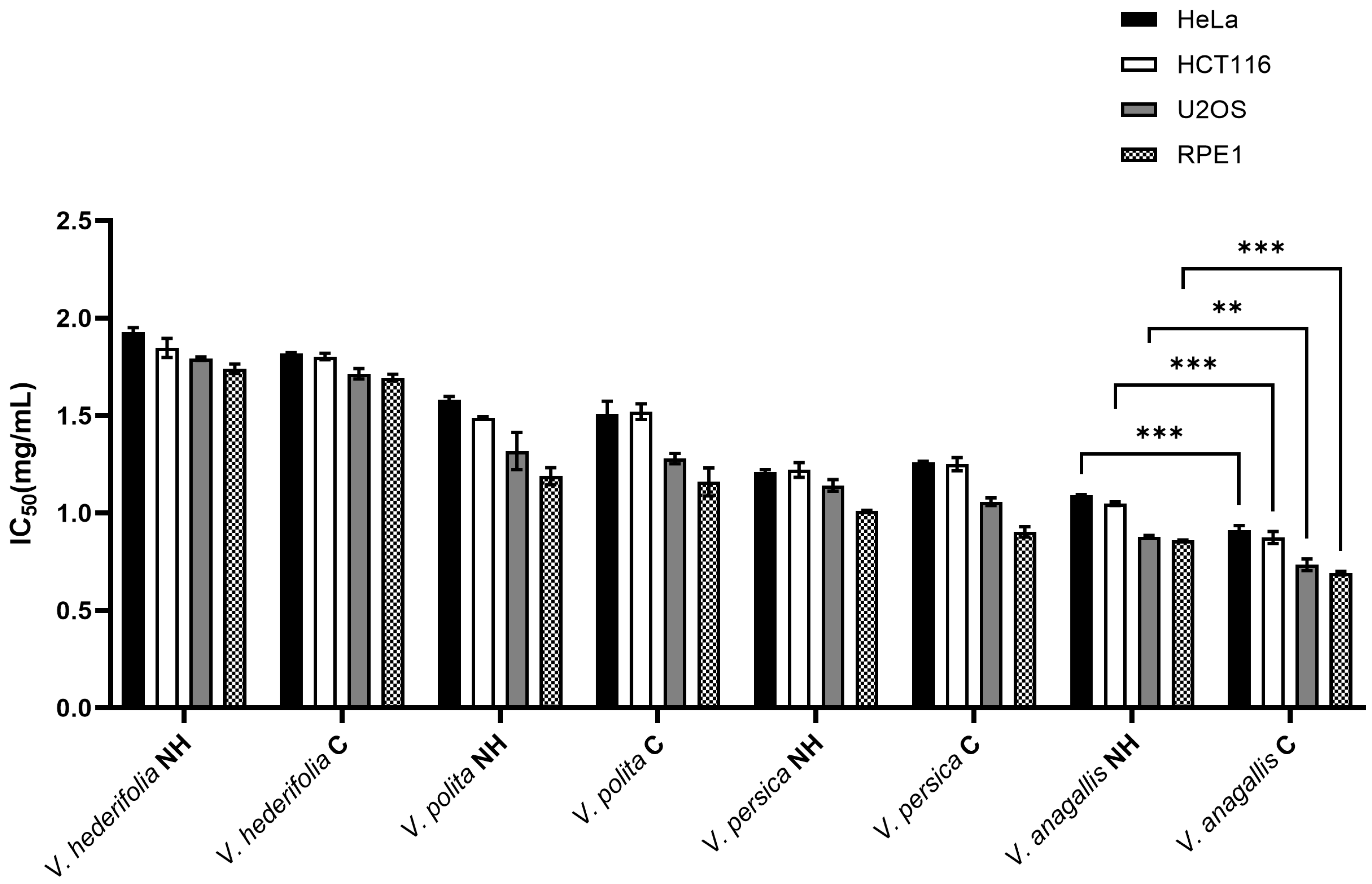
3.4. Cytotoxic Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salehi, B.; Shetty, M.S.; Anil Kumar, N.V.; Živković, J.; Calina, D.; Docea, A.O.; Emamzadeh-Yazdi, S.; Kılıç, C.S.; Goloshvili, T.; Nicola, S.; et al. Veronica Plants—Drifting from Farm to Traditional Healing, Food Application, and Phytopharmacology. Molecules 2019, 24, 2454. [Google Scholar] [CrossRef]
- Mocan, A.; Vodnar, D.C.; Vlase, L.; Crișan, O.; Gheldiu, A.M.; Crișan, G. Phytochemical Characterization of Veronica officinalis L., V. Teucrium L. and V. Orchidea crantz from Romania and Their Antioxidant and Antimicrobial Properties. Int. J. Mol. Sci. 2015, 16, 21109–21127. [Google Scholar] [CrossRef]
- Albach, D.C.; Grayer, R.J.; Jensen, S.R.; Özgökce, F.; Veitch, N.C. Acylated Flavone Glycosides from Veronica. Phytochemistry 2003, 64, 1295–1301. [Google Scholar] [CrossRef]
- Xue, H.; Chen, K.X.; Zhang, L.Q.; Li, Y.M. Review of the Ethnopharmacology, Phytochemistry, and Pharmacology of the Genus Veronica. Am. J. Chin. Med. 2019, 47, 1193–1221. [Google Scholar] [CrossRef]
- Taskova, R.M.; Kokubun, T.; Ryan, K.G.; Garnock-Jones, P.J.; Jensen, S.R. Phenylethanoid and Iridoid Glycosides in the New Zealand Snow Hebes (Veronica, Plantaginaceae). Chem. Pharm. Bull. 2010, 58, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Marchenko, A.; Kintya, P.; Wyrzykiewicz, B.; Gorincioi, E. Steroidal Glycosides from Veronica chamaedrys L. Part I. The Structures of Chamaedrosides C, C1, C2, E, E1and E 2. Nat. Prod. Commun. 2012, 7, 565–568. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Dias, M.I.; Živković, J.; Stojkovic, D.; Soković, M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic Profiling of Veronica Spp. Grown in Mountain, Urban and Sandy Soil Environments. Food Chem. 2014, 163, 275–283. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Escribano-Bailon, M.T.; Lattanzio, V. International Conference on Polyphenols (24th: 2008: Salamanca, S). In Recent Advances in Polyphenol Research; Wiley-Blackwell: Hoboken, NJ, USA, 2010; Volume 2, ISBN 9781405158374. [Google Scholar]
- Živković, J.; Ćebović, T.; Maksimović, Z. In Vivo and in Vitro Antioxidant Effects of Three Veronica Species. Cent. Eur. J. Biol. 2012, 7, 559–568. [Google Scholar] [CrossRef]
- Živković, J.; Barreira, J.C.M.; Stojković, D.; Ćebović, T.; Santos-Buelga, C.; Maksimović, Z.; Ferreira, I.C.F.R. Phenolic Profile, Antibacterial, Antimutagenic and Antitumour Evaluation of Veronica urticifolia Jacq. J. Funct. Foods 2014, 9, 192–201. [Google Scholar] [CrossRef]
- Vitaglione, P.; Morisco, F.; Caporaso, N.; Fogliano, V. Dietary Antioxidant Compounds and Liver Health. Crit. Rev. Food Sci. Nutr. 2004, 44, 575–586. [Google Scholar] [CrossRef]
- Pokorný, J. Are Natural Antioxidants Better—And Safer—Than Synthetic Antioxidants? Eur. J. Lipid Sci. Technol. 2007, 109, 629–642. [Google Scholar] [CrossRef]
- Lobiuc, A.; Pavăl, N.E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.C.; Amăriucăi-Mantu, D.; Stoleru, V. Future Antimicrobials: Natural and Functionalized Phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; Ramakrishna, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef] [PubMed]
- Muluneh, M.G. Impact of Climate Change on Biodiversity and Food Security: A Global Perspective—A Review Article. Agric Food Secur 2021, 10, 36. [Google Scholar] [CrossRef]
- Vrca, I.; Orhanović, S.; Pezelj, I.; Sušić, K.; Dunkić, V.; Kremer, D.; Nazlić, M. Identification of Phenolic Compounds Present in Three Speedwell (Veronica L.) Species and Their Antioxidant Potential. Antioxidants 2024, 13, 738. [Google Scholar] [CrossRef] [PubMed]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reyes, A.S.; dos Santos, T.C.; Fit, C.S.; Leitão, S.G. Screening of Brazilian Plant Extracts for Antioxidant Activity by the Use of DPPH Free Radical Method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Yen, G.-C.; Duh, P.-D. Scavenging Effect of Methanolic Extracts of Peanut Hulls on Free-Radical and Active-Oxygen Species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar] [CrossRef]
- Matuschek, E.; Brown, D.F.J.; Kahlmeter, G. Development of the EUCAST Disk Diffusion Antimicrobial Susceptibility Testing Method and Its Implementation in Routine Microbiology Laboratories. Clin. Microbiol. Infect. 2014, 20, O255–O266. [Google Scholar] [CrossRef] [PubMed]
- Koneman, E.W.; Winn, W.C.; Allen, S.D.; Janda, W.M.; Schreckenberger, P.C.; Procop, G.W.; Woods, G.L. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; ISBN 0781730147. [Google Scholar]
- Nazlić, M.; Dunkić, V.; Dželalija, M.; Maravić, A.; Mandić, M.; Srečec, S.; Vrca, I.; Vuko, E.; Kremer, D. Evaluation of Antiphytoviral and Antibacterial Activity of Essential Oil and Hydrosol Extracts from Five Veronica Species. Agriculture 2023, 13, 1517. [Google Scholar] [CrossRef]
- Çelik, E.; Yuvali, G.; Meysun, I.A. Essential Oil Composition and Antibacterial Activity of Some Plant Species. J. Appl. Biol. Sci. 2010, 4, 45–48. [Google Scholar]
- Hemeg, H.A.; Moussa, I.M.; Ibrahim, S.; Dawoud, T.M.; Alhaji, J.H.; Mubarak, A.S.; Kabli, S.A.; Alsubki, R.A.; Tawfik, A.M.; Marouf, S.A. Antimicrobial Effect of Different Herbal Plant Extracts against Different Microbial Population. Saudi J. Biol. Sci. 2020, 27, 3221–3227. [Google Scholar] [CrossRef]
- EUCAST Disk Diffusion Method for Antimicrobial Susceptibility Testing Antimicrobial Susceptibility Testing EUCAST Disk Diffusion Method. 2025. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2025_manuals/Manual_v_13.0_EUCAST_Disk_Test_2025.pdf (accessed on 15 January 2025).
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin Microbiol Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Fredotović, Ž.; Soldo, B.; Šprung, M.; Marijanović, Z.; Jerković, I.; Puizina, J. Comparison of Organosulfur and Amino Acid Composition between Triploid Onion Allium Cornutum Clementi Ex Visiani, 1842, and Common Onion Allium cepa L., and Evidences for Antiproliferative Activity of Their Extracts. Plants 2020, 9, 98. [Google Scholar] [CrossRef]
- Mathews, A.; Arbal, A.V.; Kaarunya, A.; Jha, P.K.; Le-Bail, A.; Rawson, A. Conventional vs Modern Extraction Techniques in the Food Industry. Extr. Process. Food Ind. 2024, 97–146. [Google Scholar] [CrossRef]
- Vrca, I.; Jukić, D.; Radić, J.; Anđelić, I. Phenolic Compounds in Edible Tropaeolum majus L. Leaves and Its In Vitro Digestion. Analytica 2025, 6, 14. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A Comprehensive Review of Ultrasonic Assisted Extraction (UAE) for Bioactive Components: Principles, Advantages, Equipment, and Combined Technologies. Ultrason. Sonochem 2023, 101, 106646. [Google Scholar] [CrossRef] [PubMed]
- Mnayer, D.; Fabiano-Tixier, A.-S.; Petitcolas, E.; Ruiz, K.; Hamieh, T.; Chemat, F. Extraction of Green Absolute from Thyme Using Ultrasound and Sunflower Oil. Resour.-Effic. Technol. 2017, 3, 12–21. [Google Scholar] [CrossRef]
- Beara, I.; Živković, J.; Lesjak, M.; Ristić, J.; Šavikin, K.; Maksimović, Z.; Janković, T. Phenolic Profile and Anti-Inflammatory Activity of Three Veronica Species. Ind. Crops Prod. 2015, 63, 276–280. [Google Scholar] [CrossRef]
- Kwak, J.H.; Kim, H.J.; Lee, K.H.; Kang, S.C.; Zee, O.P. Antioxidative Iridoid Glycosides and Phenolic Compounds from Veronica Peregrina. Arch. Pharm. Res. 2009, 32, 207–213. [Google Scholar] [CrossRef]
- Stojković, D.S.; Živković, J.; Soković, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Janković, T.; Maksimović, Z. Antibacterial Activity of Veronica montana L. Extract and of Protocatechuic Acid Incorporated in a Food System. Food Chem. Toxicol. 2013, 55, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.D.; Kennedy, J.A. Plant Metabolism and the Environment: Implications for Managing Phenolics. Crit. Rev. Food Sci. Nutr. 2010, 50, 620–643. [Google Scholar] [CrossRef]
- Graham, J.G.; Quinn, M.L.; Fabricant, D.S.; Farnsworth, N.R. Plants Used against Cancer—An Extension of the Work of Jonathan Hartwell. J. Ethnopharmacol. 2000, 73, 347–377. [Google Scholar] [CrossRef] [PubMed]
- Su, B.N.; Zhu, Q.X.; Jia, Z.J. Aquaticol, a Novel Bis-Sesquiterpene from Veronica Anagallis-Aquatica. Tetrahedron Lett. 1999, 40, 357–358. [Google Scholar] [CrossRef]
- Tomassini, L.; Brkic, D.; Serafini, M.; Nicoletti, M. Constituents of Veronica Hederifolia and Veronica Polita. Fitoterapia 1995, 66, 382. [Google Scholar]
- Nazlić, M.; Fredotović, Ž.; Vuko, E.; Fabijanić, L.; Kremer, D.; Stabentheiner, E.; Ruščić, M.; Dunkić, V. Wild Species Veronica officinalis L. and Veronica saturejoides Vis. Ssp. Saturejoides—Biological Potential of Free Volatiles. Horticulturae 2021, 7, 295. [Google Scholar] [CrossRef]
- Nazlić, M.; Fredotović, Ž.; Vuko, E.; Vuletić, N.; Ljubenkov, I.; Kremer, D.; Jurišić Grubešić, R.; Stabentheiner, E.; Randić, M.; Dunkić, V. Free Volatile Compounds of Veronica austriaca Ssp. Jacquinii (Baumg.) Eb. Fisch. and Their Biological Activity. Plants 2021, 10, 2529. [Google Scholar] [CrossRef]
- Mohadjerani, M.; Asadollahi, S. Veronica crista-galli Steven and Veronica persica Poir. as Anticancer and Antioxidant Plants in-Vitro. Trends Phytochem. Res. (TPR) Trends Phytochem. Res. 2019, 3, 61–66. [Google Scholar]
- Saracoglu, I.; Oztunca, F.H.; Nagatsu, A.; Harput, U.S. Iridoid Content and Biological Activities of Veronica cuneifolia Subsp. Cuneifolia and V. Cymbalaria. Pharm. Biol. 2011, 49, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Vrca, I.; Čikeš Čulić, V.; Lozić, M.; Dunkić, N.; Kremer, D.; Ruščić, M.; Nazlić, M.; Dunkić, V. Isolation of Volatile Compounds by Microwave-Assisted Extraction from Six Veronica Species and Testing of Their Antiproliferative and Apoptotic Activities. Plants 2023, 12, 3244. [Google Scholar] [CrossRef] [PubMed]
- Harput, U.S.; Saracoglu, I.; Inoue, M.; Ogihara, Y. Anti-Inflammatory and Cytotoxic Activities of Five Veronica Species. Biol. Pharm. Bull. 2002, 25, 483–486. [Google Scholar] [CrossRef]
- Moreno-Escobar, J.A.; Bazalda, S.; Villarreal, M.L.; Bonilla-Barbosa, J.R.; Mendoza, S.; Rodríguez-López, V. Cytotoxic and Antioxidant Activities of Selected Lamiales Species from Mexico. Pharm. Biol. 2011, 49, 1243–1248. [Google Scholar] [CrossRef]
- Feng, K.; Jiang, R.; Sun, L.W. Studies on Anti-Tumor Activity in Vitro of the Flavonoid Extract in Round Leaf Speedwell. In Proceedings of the First International Conference on Cellular, Molecular Biology, Biophysics and Bioengineering, Lushan, China, 17 September 2010. [Google Scholar]
- Andueza, A.; García-Garzón, A.; Ruiz De Galarreta, M.; Ansorena, E.; Iraburu, M.J.; López-Zabalza, M.J.; Martínez-Irujo, J.J. Oxidation Pathways Underlying the Pro-Oxidant Effects of Apigenin. Free Radic. Biol. Med. 2015, 87, 169–180. [Google Scholar] [CrossRef]
- Benali, T.; Bakrim, S.; Ghchime, R.; Benkhaira, N.; El Omari, N.; Balahbib, A.; Taha, D.; Zengin, G.; Hasan, M.M.; Bibi, S.; et al. Pharmacological Insights into the Multifaceted Biological Properties of Quinic Acid. Biotechnol. Genet. Eng. Rev. 2024, 40, 3408–3437. [Google Scholar] [CrossRef]
- Nikolova, M. Screening of Radical Scavenging Activity and Polyphenol Content of Bulgarian Plant Species. Pharmacogn. Res. 2011, 3, 256. [Google Scholar] [CrossRef]
- Ninfali, P.; Mea, G.; Giorgini, S.; Rocchi, M.; Bacchiocca, M. Antioxidant Capacity of Vegetables, Spices and Dressings Relevant to Nutrition. Br. J. Nutr. 2005, 93, 257–266. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant Activity and Phenolic Compounds in Selected Herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Wojcikowski, K.; Stevenson, L.; Leach, D.; Wohlmuth, H.; Gobe, G. Antioxidant Capacity of 55 Medicinal Herbs Traditionally Used to Treat the Urinary System: A Comparison Using a Sequential Three-Solvent Extraction Process. J. Altern. Complement. Med. 2007, 13, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Horozić, E.; Mekić, L.; Cipurković, S.; Kolarević, L.; Husejnagić, D.; Ibišević, M.; Karić, E.; Cilović Kozarević, E.; Pođanin, M.; Brekalo-Lazarević, S.; et al. Cytotoxic, Antibacterial and Antioxidant Activity of the Methanolic Extract of Speedwells (Veronica officinalis L.). Technol. Acta 2025, 17, 7–12. [Google Scholar] [CrossRef]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological Activities of the Natural Antioxidant Oleuropein: Exceeding the Expectation—A Mini-Review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- Moghadam, A.; Larsen, H. Importance of Listeria monocytogenes in Food Safety: A Review of Its Prevalence, Detection, and Antibiotic Resistance. Iran. J. Vet. Res. 2019, 20, 241–254. [Google Scholar]
- Kȩpa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wasik, T.J. Antimicrobial Potential of Caffeic Acid against Staphylococcus aureus Clinical Strains. BioMed Res. Int. 2018, 2018, 7413504. [Google Scholar] [CrossRef]
- Almuhanna, Y.; Alshalani, A.; AlSudais, H.; Alanazi, F.; Alissa, M.; Asad, M.; Joseph, B. Antibacterial, Antibiofilm, and Wound Healing Activities of Rutin and Quercetin and Their Interaction with Gentamicin on Excision Wounds in Diabetic Mice. Biology 2024, 13, 676. [Google Scholar] [CrossRef] [PubMed]
- Ercan, L.; Dogru, M. Antioxidant and Antimicrobial Capacity of Quinic Acid. Bitlis Eren Üniversitesi Fen. Bilim. Derg. 2022, 11, 1018–1025. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. P-Coumaric Acid Kills Bacteria through Dual Damage Mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Elagdi, C.; Bouaouda, K.; Rahhal, R.; Hsaine, M.; Badri, W.; Fougrach, H.; Hajjouji, H. EL Phenolic Compounds, Antioxidant and Antibacterial Activities of the Methanolic Extracts of Euphorbia resinifera and Euphorbia echinus. Sci. Afr. 2023, 21, e01779. [Google Scholar] [CrossRef]
- Darabpour, E.; Motamedi, H.; Nejad, S.M.S. Antimicrobial Properties of Teucrium Polium against Some Clinical Pathogens. Asian Pac. J. Trop. Med. 2010, 3, 124–127. [Google Scholar] [CrossRef]
- Dulger, B.; Ugurlu, E. Evaluation of Antimicrobial Activity of Some Endemic Scrophulariaceae Members from Turkey. Pharm. Biol. 2005, 43, 275–279. [Google Scholar] [CrossRef][Green Version]

| Taxa | Locality | Latitude | Longitude | Altitude a.s.l. (m) | Voucher No. |
|---|---|---|---|---|---|
| V. anagallis-aquatica L. [17] * | Split | 43°31′44.7″ N | 16°28′45.8″ E | 22 | CROVeS-01-2022 |
| V. persica Poir. [17] * | Hvar | 43°09′42.8″ N | 16°40′37.6″ E | 18 | CROVeS-22-2022 |
| V. polita Fr. [17] * | Hvar | 43°10′42.3″ N | 16°36′43.6″ E | 38 | CROVeS-23-2022 |
| V. hederifolia L. | Zaton Obrovački | 44°13′00.1″ N | 15°40′49.91″ E | 135 | CROVeS-24-2022 |
| Extracts | V. anagallis-aquatica | V. hederifolia | V. persica | V. polita | ||||
|---|---|---|---|---|---|---|---|---|
| Extraction yield (%) | ||||||||
| 80%-ethanol | NH | C | NH | C | NH | C | NH | C |
| 32 [17] | 27 | 32 | 34 | 18 [17] | 24 | 14 [17] | 19 | |
| V. anagallis-aquatica | V. hederifolia | V. persica | V. polita | |||||
|---|---|---|---|---|---|---|---|---|
| Compound | 80% EtOH Extract (NH) * | 80% EtOH Extract (C) | 80% EtOH Extract (NH) | 80% EtOH Extract (C) | 80% EtOH Extract (NH) * | 80% EtOH Extract (C) | 80% EtOH Extract (NH) * | 80% EtOH Extract (C) |
| p-hydroxybenzoic acid | 5.0 ± 0.36 | 2.3 ± 0.25 | 0.14 ± 0.010 | 0.05 ± 0.000 | 2.9 ± 0.16 | 0.48 ± 0.020 | 0.54 ± 0.020 | 0.13 ± 0.010 |
| Protocatechuic acid | 3.9 ± 0.13 | 2.1 ± 0.29 | 0.36 ± 0.010 | 0.12 ± 0.010 | 4.8 ± 0.37 | 2.97 ± 0.43 | 0.24 ± 0.004 | 0.11 ± 0.010 |
| Gentisic acid | 3.9 ± 0.20 | 2.0 ± 0.31 | 0.36 ± 0.030 | 0.13 ± 0.010 | 4.9 ± 0.24 | 2.86 ± 0.38 | 0.27 ± 0.010 | 0.09 ± 0.000 |
| Vanillic acid | 3.0 ± 0.18 | 0.62 ± 0.080 | 1.31 ± 0.05 | 0.41 ± 0.050 | 2 ± 0.2 | 0.46 ± 0.030 | 0.36 ± 0.040 | 0.15 ± 0.010 |
| Gallic acid | 0.38 ± 0.010 | 0.19 ± 0.000 | n.d. | n.d. | 1.1 ± 0.08 | 0.17 ± 0.000 | 0.18 ± 0.002 | n.d. |
| Syringic acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| p-coumaric acid | 1.3 ± 0.01 | 0.24 ± 0.020 | 0.06 ± 0.000 | 0.04 ± 0.000 | 0.64 ± 0.020 | 0.13 ± 0.000 | 1.7 ± 0.08 a | 0.10 ± 0.000 b |
| o-coumaric acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.09 ± 0.001 | n.d. |
| Caffeic acid | 7.5 ± 0.06 | 0.69 ± 0.120 | 0.44 ± 0.020 | 0.11 ± 0.010 | 3.9 ± 0.04 | 1.84 ± 0.28 | 1.2 ± 0.04 a | 0.13 ± 0.010 b |
| Ferulic acid | 0.54 ± 0.020 | 0.14 ± 0.000 | 0.08 ± 0.000 | 0.02 ± 0.000 | 0.30 ± 0.010 | 0.19 ± 0.01 | 0.14. ± 0.000 | 0.10 ± 0.000 |
| Chlorogenic acid | 3.0 ± 0.01 | 1.3 ± 0.20 | 0.11 ± 0.000 a | 0.08 ± 0.000 a | 0.26 ± 0.010 | 4.62 ± 1.42 | 0.40 ± 0.010 | 0.13 ± 0.000 |
| Quinic acid | n.d. | n.d. | n.d. | n.d. | n.d. | 3.44 ± 0.55 | n.d. | n.d. |
| Sinapic acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Rosmarinic acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Cinnamic acid | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Epicatechin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Catechin | n.d. | n.d. | 0.36 ± 0.000 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Resveratrol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Astringin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| EGCG (Epigallocatechin gallate) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Hesperetin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Quercetin | 3.2 ± 0.22 | n.d. | 0.52 ± 0.000 | n.d. | 2.2 ± 0.13 | n.d. | 1.2 ± 0.02 | n.d. |
| Myricetin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Apigenin | 950 ± 22 a | 113 ± 5 b | 46 ± 5.1 | 10 ± 3.1 | 661 ± 26 a | 6.4 ± 0.51 b | 48 ± 1.8 a | 0.81 ± 0.060 b |
| Naringenin | 0.64 ± 0.030 | n.d. | 0.08 ± 0.000 | n.d. | 0.56 ± 0.020 | n.d. | 0.22 ± 0.010 | n.d. |
| Rutin | 3.5 ± 0.17 | 1.8 ± 0.05 | 2.4 ± 0.32 | 0.26 ± 0.010 | 1.0 ± 0.07 | 5.1 ± 1.10 | 0.59 ± 0.030 | n.d. |
| Species | Natural Habitat (NH) | Cultivated (C) |
|---|---|---|
| V. hederifolia | 1700 ± 100 aB | 2000 ± 35 aB |
| V. polita | 1300 ± 200 *bB | 3000 ± 100 aA |
| V. persica | 3100 ± 270 *aA | 3200 ± 190 aA |
| V. anagallis-aquatica | 2700 ± 370 *aA | 3100 ± 120 aA |
| Species | Natural Habitat (NH) | Cultivated (C) |
|---|---|---|
| V. hederifolia | 341 ± 25 bB | 240 ± 20 aB |
| V. polita | 474 ± 2 *bC | 72 ± 3 aA |
| V. persica | 121 ± 11 *bA | 67 ± 5 aA |
| V. anagallis-aquatica | 127 ± 17 *bA | 65 ± 8 aA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrca, I.; Mikrut, A.; Fredotović, Ž.; Akrap, K.; Kremer, D.; Orhanović, S.; Bačić, K.; Dunkić, V.; Nazlić, M. Genus Veronica—Antioxidant, Cytotoxic and Antibacterial Activity of Phenolic Compounds from Wild and Cultivated Species. Antioxidants 2025, 14, 1308. https://doi.org/10.3390/antiox14111308
Vrca I, Mikrut A, Fredotović Ž, Akrap K, Kremer D, Orhanović S, Bačić K, Dunkić V, Nazlić M. Genus Veronica—Antioxidant, Cytotoxic and Antibacterial Activity of Phenolic Compounds from Wild and Cultivated Species. Antioxidants. 2025; 14(11):1308. https://doi.org/10.3390/antiox14111308
Chicago/Turabian StyleVrca, Ivana, Antonija Mikrut, Željana Fredotović, Karla Akrap, Dario Kremer, Stjepan Orhanović, Katarina Bačić, Valerija Dunkić, and Marija Nazlić. 2025. "Genus Veronica—Antioxidant, Cytotoxic and Antibacterial Activity of Phenolic Compounds from Wild and Cultivated Species" Antioxidants 14, no. 11: 1308. https://doi.org/10.3390/antiox14111308
APA StyleVrca, I., Mikrut, A., Fredotović, Ž., Akrap, K., Kremer, D., Orhanović, S., Bačić, K., Dunkić, V., & Nazlić, M. (2025). Genus Veronica—Antioxidant, Cytotoxic and Antibacterial Activity of Phenolic Compounds from Wild and Cultivated Species. Antioxidants, 14(11), 1308. https://doi.org/10.3390/antiox14111308










