Influence of Blueberry Mosaic Disease on Polyphenolic Profile and Antioxidant Capacity of Highbush Blueberry ‘Duke’ Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Leaf and Fruit Sampling
2.2. RT-PCR Analysis
2.3. Preparation of Blueberry Extracts
2.4. Determination of Total Anthocyanins, Flavonoids, Phenolics, and Antioxidant Capacity
2.5. UHPLC Q-ToF MS Analysis
2.6. Statistical Analysis
3. Results
3.1. BlMaV Detection
3.2. Quantification of Total Anthocyanins, Flavonoids, and Phenolics, and Determination of Antioxidative Capacity
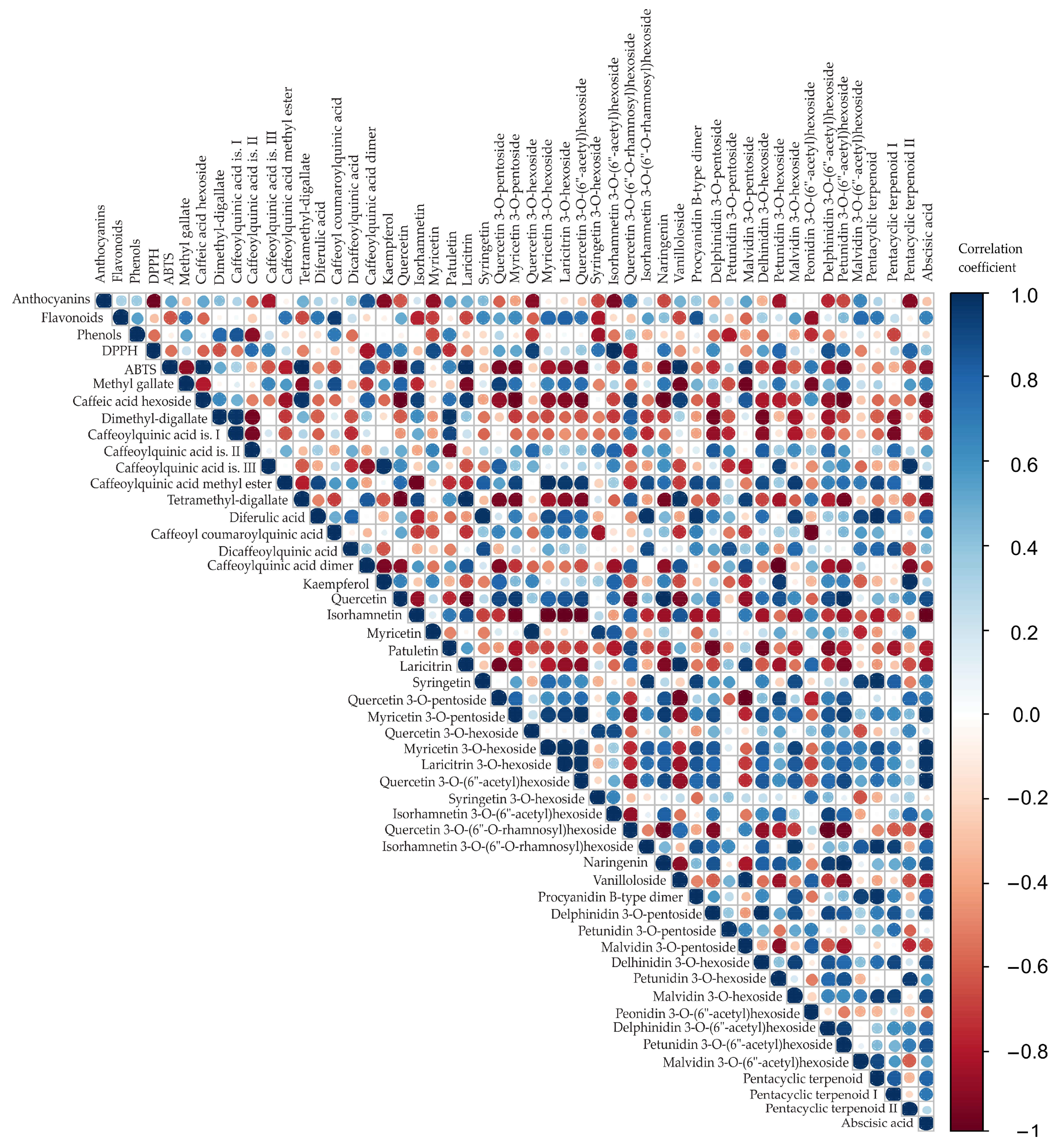
3.3. Identification of Phenolic Compounds
| No. | Compounds Name | RT (min) | Formula | Calculated Mass | m/z Exact Mass | mDa | Fragments (MS2) | Ref ** |
|---|---|---|---|---|---|---|---|---|
| Phenolic acids and derivatives | ||||||||
| 1 | Methyl gallate | 5.64 | C8H7O5− | 183.0293 | 183.0295 | 0.15 | 124.0159(100), 125.019, 106.0054 | [38] |
| 2 | Caffeic acid hexoside | 5.50 | C15H17O9− | 341.0873 | 341.0865 | −0.76 | 135.0444(100), 179.0338, 161.0228 | [39] |
| 3 | Dimethyl-digallate | 7.39 | C16H13O9− | 349.056 | 349.056 | 0.04 | 165.0183(100), 137.0236, 123.0081, 151.0028, 183.0297, 197.0445 | - |
| 4 | Caffeoylquinic acid is. I | 4.81 | C16H17O9− | 353.0873 | 353.0866 | −0.66 | 191.0549(100), 135.0443, 179.0339, 161.0231, 173.0450, 127.0398, 111.0441 | [40] |
| 5 | Caffeoylquinic acid is. II (Chlorogenic acid) * | 6.34 | C16H17O9− | 353.0873 | 353.0866 | −0.66 | 191.0550(100), 173.0446, 161.0235, 135.0444, 127.0395 | Std. |
| 6 | Caffeoylquinic acid is. III | 7.05 | C16H17O9− | 353.0873 | 353.0866 | −0.66 | 191.0550(100), 173.0435, 161.0235, 127.0395, 111.0442 | [40] |
| 7 | Caffeoylquinic acid methyl ester | 7.65 | C17H19O9− | 367.1029 | 367.1026 | −0.31 | 135.0446(100), 179.0342, 161.0234, 191.0551 | [41] |
| 8 | Tetramethyl-digallate | 9.07 | C18H17O9− | 377.0873 | 377.087 | −0.26 | 165.0184(100), 137.0231, 121.0186, 151.0034, 190.9980, 315.0131, 330.0345, 166.0219 | - |
| 9 | Diferulic acid | 11.56 | C20H17O8− | 385.0923 | 385.092 | −0.34 | 193.0491(100), 134.0366, 133.0302, 161.0230, 178.0253, 317.0350 | - |
| 10 | Caffeoyl coumaroylquinic acid | 8.77 | C25H23O11− | 499.124 | 499.1231 | –0.94 | 163.0392(100), 191.0550, 173.0442, 155.0341, 135.0446, 119.0495, 337.0907, 179.0337 | [42] |
| 11 | Dicaffeoylquinic acid | 8.18 | C25H23O12− | 515.119 | 515.1183 | −0.65 | 179.0339(100), 191.0550, 173.0445, 161.0233, 135.0444, 335.0766, 353.0860 | [42] |
| 12 | Caffeoylquinic acid dimer | 7.19 | C32H33O18− | 705.1667 | 705.1652 | −1.49 | 513.1014(100), 339.0483, 191.0545, 321.0375, 495.0926 | - |
| Flavonol aglycones | ||||||||
| 13 | Kaempferol * | 10.22 | C15H9O6− | 285.0399 | 285.0397 | −0.21 | 285.0390(100), 257.0425, 229.0488, 211.0404, 185.0587, 143.0528, 151.0064 | Std.; [43] |
| 14 | Quercetin * | 9.50 | C15H9O7− | 301.0348 | 301.0345 | −0.33 | 151.0029(100), 121.0290, 107.0135, 164.0109, 178.9975, 229.0487, 245.0438, 271.0234 | Std.; [43] |
| 15 | Isorhamnetin * | 10.42 | C16H11O7− | 315.0505 | 315.0497 | −0.78 | 300.0261(100), 151.0035, 107.0141, 137.0233, 164.0108, 178.9993, 203.0324, 227.0339, 259.0225 | Std.; [44] |
| 16 | Myricetin * | 8.69 | C15H9O8− | 317.0297 | 317.0296 | −0.14 | 151.0029(100), 137.0237, 107.0135, 125.0239, 165.0182, 178.9977, 227.0338, 243.0280, 271.0233 | Std.; [43] |
| 17 | Patuletin | 9.06 | C16H11O8− | 331.0454 | 331.0453 | −0.09 | 243.0285(100), 299.0176, 271.0230, 255.0273, 227.0341, 215.0335, 199.0389, 183.0447, 171.0443, 143.0498 | - |
| 18 | Laricitrin | 9.58 | C16H11O8− | 331.0454 | 331.0453 | −0.09 | 151.0044(100), 316.0206, 299.0171, 271.0230, 259.0236, 178.9978, 164.0104, 287.0184, 136.0160, 107.0132 | [39] |
| 19 | Syringetin | 10.38 | C17H13O8− | 345.061 | 345.0602 | −0.84 | 315.0134(100), 287.0184, 330.0364, 345.0603, 301.0340, 259.0235, 271.0237, 203.0336, 151.0029 | [45] |
| Flavonol glycosides and acyl derivatives | ||||||||
| 20 | Quercetin 3-O-pentoside | 8.16 | C20H17O11− | 433.0771 | 433.0767 | −0.39 | 300.0259(100), 271.0234, 255.0289, 178.9989, 151.0032 | [18] |
| 21 | Myricetin 3-O-pentoside | 5.97 | C20H17O12− | 449.072 | 449.0714 | −0.60 | 449.0702(100), 299.0172, 317.0280, 271.0215, 190.9972 | [18] |
| 22 | Quercetin 3-O-hexoside | 7.89 | C21H19O12− | 463.0877 | 463.0871 | −0.55 | 300.0260(100), 301.0323, 463.0864, 271.0234, 255.0284, 151.0030, 178.9987 | [18] |
| 23 | Myricetin 3-O-hexoside | 7.44 | C21H19O13− | 479.0826 | 479.084 | 1.43 | 316.0207(100), 317.0261, 271.0234, 187.0184, 479.0810, 178.9980 | [18] |
| 24 | Laricitrin 3-O-hexoside | 8.06 | C22H21O13− | 493.0982 | 493.0977 | −0.52 | 330.0365(100), 331.0419, 315.0133, 300.0260, 287.0514, 178.9973, 151.0039, 433.0758 | [18] |
| 25 | Quercetin 3-O-(6″-acetyl)hexoside | 8.49 | C23H21O13− | 505.0982 | 505.0978 | −0.42 | 300.0262(100), 344.0518, 271.0234, 178.9974, 151.0025, 463.0861 | [31] |
| 26 | Syringetin 3-O-hexoside | 8.39 | C23H23O13− | 507.1139 | 507.1125 | −1.37 | 344.0521(100), 507.1112, 345.0574, 387.0699, 329.0300, 316.0569, 301.0403, 273.0381, 151.0031 | [18] |
| 27 | Isorhamnetin 3-O-(6″-acetyl)hexoside | 8.99 | C24H23O13− | 519.1139 | 519.113 | −0.87 | 314.0418(100), 519.1125, 315.0462, 299.0203, 285.0393, 271.0241, 257.0443, 243.0289, 151.0025, 357.0595 | - |
| 28 | Quercetin 3-O-(6″-O-rhamnosyl)hexoside (such as Rutin) * | 7.74 | C27H29O16− | 609.1456 | 609.145 | −0.56 | 300.0261(100), 609.1435, 301.0329, 271.0235, 151.003, 178.9975, 343.0431 | Std.; [18] |
| 29 | Isorhamnetin 3-O-(6″-O-rhamnosyl)hexoside (such as Narcissin) | 8.22 | C28H31O16− | 623.1612 | 623.1608 | −0.41 | 315.049(100), 623.1592, 314.0416, 300.0249, 271.0241, 151.0022, 357.0595 | [31] |
| Other phenolic compounds | ||||||||
| 30 | Naringenin * | 10.04 | C15H11O5− | 271.0606 | 271.0603 | −0.35 | 119.0499(100), 107.0132, 151.0024, 161.0590, 187.0388, 229.0458, 245.0477 | Std.; [46] |
| 31 | Vanilloloside | 3.95 | C14H19O8− | 315.108 | 315.108 | 0.01 | 123.0445(100), 153.0547, 124.0478 | - |
| 32 | Procyanidin B-type dimer (such as Procyanidin B2) * | 6.04 | C30H25O12− | 577.1346 | 577.1335 | −1.10 | 289.0700(100), 407.0752, 125.0237, 137.0243, 161.0241, 245.0796, 273.0388, 339.0842, 381.0951, 425.0876, 451.1001 | Std.; [38] |
| Anthocyanins | ||||||||
| 33 | Delphinidin 3-O-pentoside | 6.47 | C20H19O11+ | 435.0927 | 435.0919 | −0.84 | 303.0488(100), 304.0523, 305.0543 | [21] |
| 34 | Petunidin 3-O-pentoside | 6.74 | C21H21O11+ | 449.1084 | 449.1075 | −0.89 | 317.0644(100), 318.068, 287.0535, 302.0409 | [21] |
| 35 | Malvidin 3-O-pentoside | 7.07 | C22H23O11+ | 463.124 | 463.1232 | −0.84 | 331.0802(100), 332.0835, 301.0695, 315.0488, 287.0534 | [21] |
| 36 | Delhinidin 3-O-hexoside | 6.19 | C21H21O12+ | 465.1033 | 465.1025 | −0.80 | 303.0488(100), 304.0522, 305.0543 | [21] |
| 37 | Petunidin 3-O-hexoside | 6.6 | C22H23O12+ | 479.119 | 479.1182 | −0.75 | 317.0645(100), 318.0677, 302.0409 | [21] |
| 38 | Malvidin 3-O-hexoside | 6.93 | C23H25O12+ | 493.1346 | 493.1338 | −0.8 | 331.0802(100), 332.0835, 315.0486, 287.0536 | [21] |
| 39 | Peonidin 3-O-(6″-acetyl)hexoside | 7.8 | C24H25O12+ | 505.1346 | 505.1343 | −0.30 | 301.0692(100), 302.0731, 303.0702, 286.0457 | [21] |
| 40 | Delphinidin 3-O-(6″-acetyl)hexoside | 7.32 | C23H23O13+ | 507.1139 | 507.1131 | −0.77 | 303.0487(100), 304.0521, 305.0547 | [21] |
| 41 | Petunidin 3-O-(6″-acetyl)hexoside | 7.48 | C24H25O13+ | 521.1295 | 521.1286 | −0.92 | 317.0643(100), 318.068, 302.0406 | [21] |
| 42 | Malvidin 3-O-(6″-acetyl)hexoside | 7.68 | C25H27O13+ | 535.1452 | 535.1444 | −0.77 | 331.0801(100), 332.0835, 315.0486 | [21] |
| Other compounds (Terpenoids) | ||||||||
| 43 | Pentacyclic terpenoid (like Maslinic or Pomolic acid) | 14.79 | C30H47O4− | 471.3474 | 471.3472 | −0.23 | 471.3460(100), 453.3342, 427.3566, 409.3457 | [19] |
| 44 | Pentacyclic terpenoid I (like Arjunolic, Euscaphic or Rotundic acid) | 12.58 | C30H47O5− | 487.3423 | 487.3415 | −0.85 | 487.3396(100), 469.3305, 437.3053, 425.3392, 405.3107, 393.3127, 443.3483 | [19] |
| 45 | Pentacyclic terpenoid II (like Arjunolic, Euscaphic or Rotundic acid) | 12.92 | C30H47O5− | 487.3423 | 487.3415 | −0.85 | 487.3405(100), 469.3288, 425.3394, 407.3258, 443.3536, 393.3111 | [19] |
| Other compounds (Plant hormone) | ||||||||
| 46 | Abscisic acid | 9.31 | C15H19O4− | 263.1283 | 263.1283 | −0.03 | 203.1068(100), 219.1372, 289.0910, 153.0911, 136.0521, 122.0367, 125.0605, 148.0525 | [47] |
3.4. Color Correlation Analysis
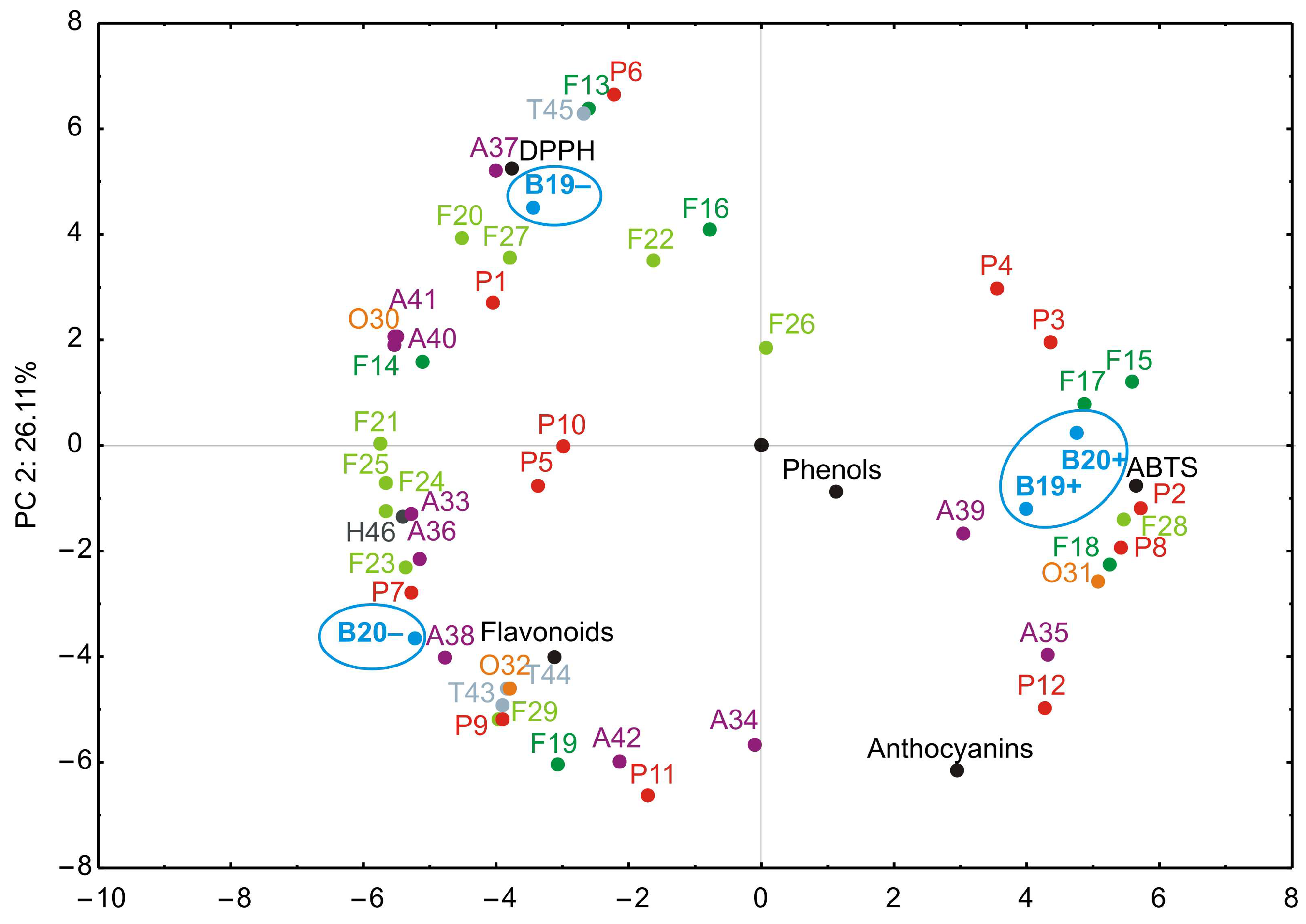
3.5. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BlMaV | Blueberry mosaic-associated ophiovirus |
| RT-PCR | Reverse transcription polymerase chain reaction |
| BSV, BlShV | Blueberry shock virus |
| BLMoV | Blueberry leaf mottle virus |
| BlScV | Blueberry scorch virus |
| BSSV | Blueberry shoestring virus |
| ToRSV | Tomato ringspot virus |
| TRSV | Tobacco ringspot virus |
| PRMV | Peach rosette mosaic virus |
| C3GE | Cyanidin-3-glucoside equivalents |
| CE | Catechin equivalents |
| GAE | Gallic acid equivalents |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| UHPLC Q-ToF MS | Ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry |
| PCA | Principal component analysis |
References
- Ma, L.; Sun, Z.; Zeng, Y.; Luo, M.; Yang, J. Molecular mechanism and health role of functional ingredients in blueberry for chronic disease in human beings. Int. J. Mol. Sci. 2018, 19, 2785. [Google Scholar] [CrossRef]
- FAOSTAT. 2025. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 7 July 2025).
- Leposavić, A.; Jevremović, D. Borovnica—Tehnologije Gajenja, Zaštite i Prerade; Naučno voćarsko društvo Srbije: Čačak, Serbia, 2020. [Google Scholar]
- Retamales, J.B.; Hancock, J.F. Blueberries, 2nd ed.; Cabi: Glasgow, UK, 2018. [Google Scholar]
- Saad, N.; Olmstead, J.W.; Jones, J.B.; Varsani, A.; Harmon, P.F. Known and new emerging viruses infecting blueberry. Plants 2021, 10, 2172. [Google Scholar] [CrossRef]
- Thekke-Veetil, T.; Ho, T.; Keller, K.E.; Martin, R.R.; Tzanetakis, I.E. A new ophiovirus is associated with blueberry mosaic disease. Virus Res. 2014, 189, 92–96. [Google Scholar] [CrossRef]
- Ramsdell, D.C.; Stretch, A.W. Blueberry mosaic. In Virus Diseases of Small Fruits; Converse, R.H., Ed.; US Government Printing Office, US Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 1987; pp. 119–120. [Google Scholar]
- Jevremović, D.; Vasilijević, B.; Leposavić, A.; Katanić, V. Molecular Characterization of Blueberry Mosaic-Associated Virus in Highbush Blueberries in Serbia. In Proceedings of the 6th International Scientific Conference: Modern Trends in Agricultural Production, Rural Development, Agroeconomy, Cooperatives and Environmental Protection, Vrnjačka Banja, Serbia, 27–28 June 2024; pp. 89–94. [Google Scholar]
- Jevremović, D.; Paunović, A.S.; Leposavić, A. Influence of blueberry mosaic associated virus on some fruit traits of highbush blueberry ‘Duke’. J. Mt. Agric. Balk. 2020, 23, 195–203. [Google Scholar]
- Li, R.; Mock, R.; Huang, Q.; Abad, J.; Hartung, J.; Kinard, G. A reliable and inexpensive method of nucleic acid extraction for the PCR-based detection of diverse plant pathogens. J. Virol. Methods 2008, 154, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Jevremović, D.; Leposavić, A.; Paunović, S. Incidence of viruses in highbush blueberry (Vaccinium corymbosum L.) in Serbia. Pestic. Fitomed. 2016, 31, 45–50. [Google Scholar] [CrossRef]
- Taghavi, T.; Patel, H.; Rafie, R. Comparing pH differential and methanol-based methods for anthocyanin assessments of strawberries. Food Sci. Nutr. 2022, 10, 2123–2131. [Google Scholar] [CrossRef]
- Ionescu, C.; Samide, A.; Tigae, C. Trend in detection of anthocyanins from fresh fruits and the influence of some factors on their stability impacting human health: Kinetic study assisted by UV–Vis spectrophotometry. Antioxidants 2025, 14, 227. [Google Scholar] [CrossRef]
- Belew, A.A.; Hanan, G.G.M.W.; Meshesha, D.S.; Akele, M.L. Evaluation of total phenolic, flavonoid contents, antioxidant and antibacterial activity of leaf extracts from Rhus vulgaris. Discov. Plants 2025, 2, 141. [Google Scholar] [CrossRef]
- Zeb, A.; Rahman, F. Phenolic profile, total bioactive contents, and antioxidant activity of pear fruits. Food Chem. Adv. 2024, 5, 100780. [Google Scholar] [CrossRef]
- Ding, Y.; Morozova, K.; Imperiale, S.; Angeli, L.; Asma, U.; Ferrentino, G.; Scampicchio, M. HPLC-Triple detector (Coulometric array, diode array and mass spectrometer) for the analysis of antioxidants in officinal plants. LWT-Food Sci. Technol. 2022, 162, 113456. [Google Scholar] [CrossRef]
- Miletić, N.; Jevremović, D.; Mitić, M.; Popović, B.; Petković, M. Influence of D and Rec strains of plum pox virus on phenolic profile and antioxidant capacity of fresh plum fruits of ‘Čačanska Lepotica’ cultivar. Span. J. Agric. Res. 2022, 20, e1005. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Masuero, D.; Palmieri, L.; Mattivi, F. Identification and quantification of flavonol glycosides in cultivated blueberry cultivars. J. Food Compos. Anal. 2012, 25, 9–16. [Google Scholar] [CrossRef]
- Tu, P.C.; Liang, Y.C.; Huang, G.J.; Lin, M.K.; Kao, M.C.; Lu, T.L.; Sung, P.J.; Kuo, Y.H. Cytotoxic and anti-inflammatory terpenoids from the whole plant of Vaccinium emarginatum. Planta Med. 2020, 86, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Ruales, J.; Moreno, D.A.; Barrio, D.A.; Stinco, C.M.; Martínez-Cifuentes, G.; Meléndez-Martínez, A.J.; García-Ruiz, A. Characterization of Andean blueberry in bioactive compounds, evaluation of biological properties, and in vitro bioaccessibility. Foods 2020, 9, 1483. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.S.; Nguyen, H.P.; Shen, S.; Schug, K.A. General method for extraction of blueberry anthocyanins and identification using high performance liquid chromatography–electrospray ionization-ion trap-time of flight-mass spectrometry. J. Chromatogr. A 2009, 1216, 4728–4735. [Google Scholar] [CrossRef]
- Dragović-Uzelac, V.; Savić, Z.; Brala, A.; Levaj, B.; Bursać Kovačević, D.; Biško, A. Evaluation of phenolic content and antioxidant capacity of blueberry cultivars (Vaccinium corymbosum L.) grown in the Northwest Croatia. Food Technol. Biotechnol. 2010, 48, 214–221. [Google Scholar]
- Okan, O.T.; Deniz, I.; Yayli, N.; Şat, I.G.; Öz, M.; Hatipoglu Serdar, G. Antioxidant activity, sugar content and phenolic profiling of blueberries cultivars: A comprehensive comparison. Not. Bot. Horti. Agrobo. 2018, 46, 639–652. [Google Scholar] [CrossRef]
- Shibata, Y.; Ohara, K.; Matsumoto, K.; Hasegawa, T.; Akimoto, M. Total anthocyanin content, total phenolic content, and antioxidant activity of various blueberry cultivars grown in Togane, Chiba Prefecture, Japan. J. Nutr. Sci. Vitaminol. 2021, 67, 201–209. [Google Scholar] [CrossRef]
- Rochat, B. Proposed Confidence Scale and ID Score in the Identification of Known-Unknown Compounds Using High Resolution MS Data. J. Am. Soc. Mass Spectrom. 2017, 28, 709–723. [Google Scholar] [CrossRef]
- Subbiah, V.; Zhong, B.; Nawaz, M.A.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Screening of phenolic compounds in Australian grown berries by LC-ESI-QTOF-MS/MS and determination of their antioxidant potential. Antioxidants 2021, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, B.; Dong, K.; Li, J.; Li, Y.; Sun, H. Identification and quantification of anthocyanins of 62 blueberry cultivars via UPLC-MS. Biotechnol. Biotec. Eq. 2022, 36, 587–597. [Google Scholar] [CrossRef]
- Bergmann, A.R.; Siebeneichler, T.J.; Oliveira Fischer, L.; Holz, Í.R.; Rombaldi, C.V.; Santos Oliveira, B.A.; Oliveira Fischer, D.L.; Silva, C.S.; Helbig, E. Physicochemical characterization, phenolic composition and antioxidant activity of genotypes and commercial cultivars of blueberry fruits. Cienc. Rural 2023, 53, e20220450. [Google Scholar] [CrossRef]
- Araniti, F.; Baron, G.; Ferrario, G.; Pesenti, M.; Vedova, L.D.; Prinsi, B.; Sacchi, G.A.; Aldini, G.; Espen, L. Chemical profiling and antioxidant potential of berries from six blueberry genotypes harvested in the Italian Alps in 2020: A comparative biochemical pilot study. Agronomy 2025, 15, 262. [Google Scholar] [CrossRef]
- Zhang, Q.; Zang, H.; Guo, X.; Li, S.; Xin, X.; Li, Y. A systematic study on composition and antioxidant of 6 varieties of highbush blueberries by 3 soil matrixes in China. Food Chem. 2025, 472, 142974. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. HPLC–MSn identification and quantification of flavonol glycosides in 28 wild and cultivated berry species. Food Chem. 2012, 135, 2138–2146. [Google Scholar] [CrossRef]
- Chansriniyom, C.; Nooin, R.; Nuengchamnong, N.; Wongwanakul, R.; Petpiroon, N.; Srinuanchai, W.; Chantarasuwan, B.; Pitchakarn, P.; Temviriyanukul, P.; Nuchuchua, O. Tandem mass spectrometry of aqueous extract from Ficus dubia sap and its cell-based assessments for use as a skin antioxidant. Sci. Rep. 2021, 11, 16899. [Google Scholar] [CrossRef]
- Ancillotti, C.; Ciofi, L.; Rossini, D.; Chiuminatto, U.; Stahl-Zeng, J.; Orlandini, S.; Furlanetto, S.; Bubba, M.D. Liquid chromatographic/electrospray ionization quadrupole/time of flight tandem mass spectrometric study of polyphenolic composition of different Vaccinium berry species and their comparative evaluation. Anal. Bioanal. Chem. 2017, 409, 1347–1368. [Google Scholar] [CrossRef]
- Kim, J.H.; Kwon, R.H.; Kim, S.A.; Na, H.; Cho, J.Y.; Kim, H.W. Characterization of anthocyanins including acetylated glycosides from highbush blueberry (Vaccinium corymbosum L.) cultivated in Korea based on UPLC-DAD-QToF/MS and UPLC-Qtrap-MS/MS. Foods 2025, 14, 188. [Google Scholar] [CrossRef]
- Neto, C.C. Ursolic acid and other pentacyclic triterpenoids: Anticancer activities and occurrence in berries. In Berries and Cancer Prevention; Stoner, G.D., Seeram, N.P., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2011; pp. 41–49. [Google Scholar] [CrossRef]
- Similie, D.; Minda, D.; Bora, L.; Kroškins, V.; Lugiņina, J.; Turks, M.; Dehelean, C.A.; Danciu, C. An update on pentacyclic triterpenoids ursolic and oleanolic acids and related derivatives as anticancer candidates. Antioxidants 2024, 13, 952. [Google Scholar] [CrossRef]
- Jaakola, L. Phenolic compounds in Vaccinium spp.: Diversity, biosynthesis, and molecular regulation. Acta Hortic. 2023, 1357, 1–8. [Google Scholar] [CrossRef]
- Cocetta, G.; Rossoni, M.; Gardana, C.; Mignani, I.; Ferrante, A.; Spinardi, A. Methyl jasmonate affects phenolic metabolism and gene expression in blueberry (Vaccinium corymbosum). Physiol. Plant. 2015, 153, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, M.; Wu, L.; Wang, F.; Li, L.; Zhang, S.; Sun, B. Qualitative and quantitative analysis of phenolic compounds in blueberries and protective effects on hydrogen peroxide-induced cell injury. J. Sep. Sci. 2021, 44, 2837–2855. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, V.; Kajdzanoska, M.; Gjamovski, V.; Stefova, M. Separation, characterization and quantification of phenolic compounds in blueberries and red and black currants by HPLC–DAD–ESI-MSn. J. Agric. Food Chem. 2011, 59, 4009–4018. [Google Scholar] [CrossRef]
- Wan, C.; Yuan, T.; Cirello, A.L.; Seeram, N.P. Antioxidant and α-glucosidase inhibitory phenolics isolated from highbush blueberry flowers. Food Chem. 2012, 135, 1929–1937. [Google Scholar] [CrossRef]
- Wang, L.J.; Wu, J.; Wang, H.X.; Li, S.S.; Zheng, X.C.; Du, H.; Xu, Y.J.; Wang, L.S. Composition of phenolic compounds and antioxidant activity in the leaves of blueberry cultivars. J. Funct. Foods 2015, 16, 295–304. [Google Scholar] [CrossRef]
- Häkkinen, S.; Heinonen, M.; Kärenlampi, S.; Mykkänen, H.; Ruuskanen, J.; Törrönen, R. Screening of selected flavonoids and phenolic acids in 19 berries. Food Res. Int. 1999, 32, 345–353. [Google Scholar] [CrossRef]
- Cvetković, M.; Kočić, M.; Dabić Zagorac, D.; Ćirić, I.; Natić, M.; Hajder, Đ.; Životić, A.; Fotirić Akšić, M. When is the right moment to pick blueberries? Variation in agronomic and chemical properties of blueberry (Vaccinium corymbosum) cultivars at different harvest times. Metabolites 2022, 12, 798. [Google Scholar] [CrossRef]
- Sun, J.; Lin, L.Z.; Chen, P. Study of the mass spectrometric behaviors of anthocyanins in negative ionization mode and its applications for characterization of anthocyanins and non-anthocyanin polyphenols. Rapid Commun. Mass Spectrom. 2012, 26, 1123–1133. [Google Scholar] [CrossRef]
- Oliva, E.; Viteritti, E.; Fanti, F.; Eugelio, F.; Pepe, A.; Palmieri, S.; Sergi, M.; Compagnone, D. Targeted and semi-untargeted determination of phenolic compounds in plant matrices by high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2021, 1651, 462315. [Google Scholar] [CrossRef]
- Oh, H.D.; Yu, D.J.; Chung, S.W.; Chea, S.; Lee, H.J. Abscisic acid stimulates anthocyanin accumulation in ‘Jersey’ highbush blueberry fruits during ripening. Food Chem. 2018, 244, 403–407. [Google Scholar] [CrossRef]
- Petković, M.; Lukyanov, A.; Đurović, I.; Miletić, N. A novel method for analyzing the kinetics of convective/IR bread drying (CIRD) with sensor technology. Appl. Sci. 2025, 15, 4964. [Google Scholar] [CrossRef]
- Ramaroson, M.L.; Koutouan, C.; Helesbeux, J.J.; Le Clerc, V.; Hamama, L.; Geoffriau, E.; Briard, M. Role of phenylpropanoids and flavonoids in plant resistance to pests and diseases. Molecules 2022, 27, 8371. [Google Scholar] [CrossRef]
- Mishra, J.; Srivastava, R.; Trivedi, P.K.; Verma, P.C. Effect of virus infection on the secondary metabolite production and phytohormone biosynthesis in plants. 3 Biotech 2020, 10, 547. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Király, L.; Al-Mansori, A.N.A.; Younes, H.A.; Zeid, A.; Elsharkawy, M.M.; Behiry, S.I. Defense responses and metabolic changes involving phenylpropanoid pathway and PR genes in squash (Cucurbita pepo L.) following Cucumber mosaic virus infection. Plants 2022, 11, 1908. [Google Scholar] [CrossRef]

| Viral Status | Harvest Year | |
|---|---|---|
| 2019 | 2020 | |
| BlMaV− | B19− | B20− |
| BlMaV+ | B19+ | B20+ |
| Month | Average Air Temperature (°C) | Average Precipitation (mm) | ||
|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | |
| April | 11.7 | 10.5 | 166.8 | 70.0 |
| May | 13.1 | 14.1 | 110.2 | 197.2 |
| June | 20.4 | 17.7 | 328.4 | 231.0 |
| July | 20.1 | 19.4 | 107.2 | 17.4 |
| August | 21.3 | 20.6 | 71.2 | 46.6 |
| September | 16.5 | 17.2 | 2.4 | 49.6 |
| October | 12.4 | 11.8 | 5.4 | 152.6 |
| ANOVA | Blueberry Samples | ||||
|---|---|---|---|---|---|
| B19− | B19+ | B20− | B20+ | ||
| Anthocyanins (mg/100 g fw) | ** | 112.06 ± 5.02 b | 125.05 ± 7.09 ab | 129.57 ± 5.51 a | 134.34 ± 2.89 a |
| Flavonoids (mg/100 g fw) | *** | 106.73 ± 2.45 b | 101.45 ± 1.60 c | 112.05 ± 0.52 a | 108.68 ± 1.81 ab |
| Phenols (mg/100 g fw) | ns | 325.26 ± 4.16 | 322.61 ± 6.49 | 324.64 ± 9.67 | 328.15 ± 4.49 |
| Antioxidant capacity (DPPH, μmol TE/100 g fw) | ns | 77.03 ± 4.23 | 74.14 ± 3.99 | 73.34 ± 2.78 | 70.51 ± 6.28 |
| Antioxidant capacity (ABTS, mmol TE/100 g fw) | ns | 2.41 ± 0.09 | 2.50 ± 0.10 | 2.42 ± 0.07 | 2.48 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miletić, N.; Milinčić, D.D.; Pešić, M.B.; Lončar, B.; Petković, M.; Vasilijević, B.; Jevremović, D. Influence of Blueberry Mosaic Disease on Polyphenolic Profile and Antioxidant Capacity of Highbush Blueberry ‘Duke’ Fruits. Antioxidants 2025, 14, 1302. https://doi.org/10.3390/antiox14111302
Miletić N, Milinčić DD, Pešić MB, Lončar B, Petković M, Vasilijević B, Jevremović D. Influence of Blueberry Mosaic Disease on Polyphenolic Profile and Antioxidant Capacity of Highbush Blueberry ‘Duke’ Fruits. Antioxidants. 2025; 14(11):1302. https://doi.org/10.3390/antiox14111302
Chicago/Turabian StyleMiletić, Nemanja, Danijel D. Milinčić, Mirjana B. Pešić, Biljana Lončar, Marko Petković, Bojana Vasilijević, and Darko Jevremović. 2025. "Influence of Blueberry Mosaic Disease on Polyphenolic Profile and Antioxidant Capacity of Highbush Blueberry ‘Duke’ Fruits" Antioxidants 14, no. 11: 1302. https://doi.org/10.3390/antiox14111302
APA StyleMiletić, N., Milinčić, D. D., Pešić, M. B., Lončar, B., Petković, M., Vasilijević, B., & Jevremović, D. (2025). Influence of Blueberry Mosaic Disease on Polyphenolic Profile and Antioxidant Capacity of Highbush Blueberry ‘Duke’ Fruits. Antioxidants, 14(11), 1302. https://doi.org/10.3390/antiox14111302












