Cancer-Related Malnutrition and Oxidative Stress in Colorectal Cancer Surgery: A Narrative Review of Pathophysiology and Postoperative Outcomes
Abstract
1. Introduction
Literature Search Approach
2. Malnutrition in Colorectal Cancer—Clinical Relevance
3. Oxidative Stress in Colorectal Cancer: Mechanisms and Clinical Impact
3.1. Definition and Molecular Basis
3.2. Oxidative Stress in Gastrointestinal Cancers
3.3. Oxidative Stress and Surgical Outcomes
3.4. Biomarkers of Oxidative Stress in Colorectal Cancer Patients
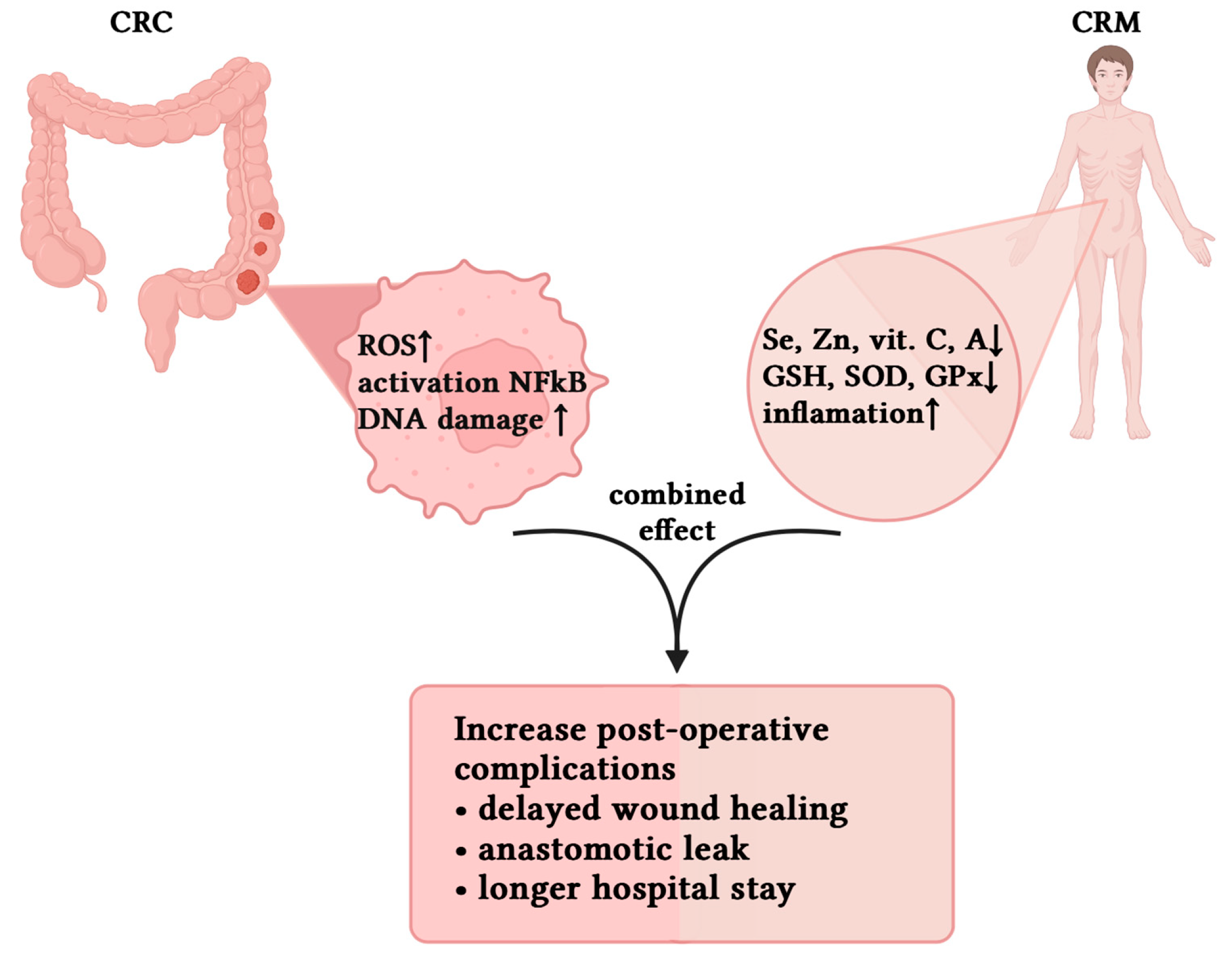
4. Pathophysiological Interactions Between Cancer-Related Malnutrition and Oxidative Stress
4.1. Overview of the Relationship Between CRM and OS
4.2. Deficient Antioxidant Defenses in CRM
4.3. Metabolic Derangements and Oxidative Load
4.4. Perioperative Clinical Implications
5. Perioperative Interventions in Colorectal Cancer: Nutrition and Redox Modulation
5.1. Nutritional Interventions: Evidence and Recommendations
5.2. Modulating Oxidative Stress—Current Concepts and Challenges
The Antioxidant Paradox: Risks of Overcorrection
5.3. Combined Strategies and Individualized Perioperative Care
6. Summary and Future Directions
6.1. Key Conclusions
6.2. Clinical Implications
6.3. Research Gaps and Future Perspectives
7. Limitations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Wang, M.; Xiao, Y.; Miao, J.; Zhang, X.; Liu, M.; Zhu, L.; Liu, H.; Shen, X.; Wang, J.; Xie, B.; et al. Oxidative Stress and Inflammation: Drivers of Tumorigenesis and Therapeutic Opportunities. Antioxidants 2025, 14, 735. [Google Scholar] [CrossRef]
- Bardelčíková, A.; Šoltys, J.; Mojžiš, J. Oxidative Stress, Inflammation and Colorectal Cancer: An Overview. Antioxidants 2023, 12, 901. [Google Scholar] [CrossRef]
- Shivshankar, S.; Patil, P.S.; Deodhar, K.; Budukh, A.M. Epidemiology of colorectal cancer: A review with special emphasis on India. Indian. J. Gastroenterol. 2025, 44, 142–153. [Google Scholar] [CrossRef]
- Li, X.; Xiao, X.; Wu, Z.; Li, A.; Wang, W.; Lin, R. Global, regional, and national burden of early-onset colorectal cancer and projection to 2050: An analysis based on the Global Burden of Disease Study 2021. Public Health 2025, 238, 245–253. [Google Scholar] [CrossRef]
- Sung, H.; Siegel, R.L.; Laversanne, M.; Jiang, C.; Morgan, E.; Zahwe, M.; Cao, Y.; Bray, F.; Jemal, A. Colorectal cancer incidence trends in younger versus older adults: An analysis of population-based cancer registry data. Lancet Oncol. 2025, 26, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Colorectal Cancer. (n.d.) Available online: https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer?utm_source=chatgpt.com (accessed on 9 October 2025).
- Liang, X.; Weng, J.; You, Z.; Wang, Y.; Wen, J.; Xia, Z.; Huang, S.; Luo, P.; Cheng, Q. Oxidative stress in cancer: From tumor and microenvironment remodeling to therapeutic frontiers. Mol. Cancer 2025, 24, 219. [Google Scholar] [CrossRef]
- Irani, J.L.; Hedrick, T.L.; Miller, T.E.; Lee, L.; Steinhagen, E.; Shogan, B.D.; Goldberg, J.E.; Feingold, D.L.; Lightner, A.L.; Paquette, I.M. Clinical Practice Guidelines for Enhanced Recovery After Colon and Rectal Surgery From the American Society of Colon and Rectal Surgeons and the Society of American Gastrointestinal and Endoscopic Surgeons. Dis. Colon. Rectum 2023, 66, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.G.; et al. ESPEN Guideline ESPEN practical guideline: Clinical nutrition in surgery. Clin. Nutr. 2021, 40, 4745–4761. [Google Scholar] [CrossRef]
- Andras, D.; Lazar, A.M.; Crețoiu, D.; Berghea, F.; Georgescu, D.E.; Grigorean, V.; Iacoban, S.R.; Mastalier, B. Analyzing postoperative complications in colorectal cancer surgery: A systematic review enhanced by artificial intelligence. Front. Surg. 2024, 11, 1452223. [Google Scholar] [CrossRef] [PubMed]
- Jelska, A.; Polecka, A.; Zahorodnii, A.; Olszewska, E. The Role of Oxidative Stress and the Potential Therapeutic Benefits of Aronia melanocarpa Supplementation in Obstructive Sleep Apnea Syndrome: A Comprehensive Literature Review. Antioxidants 2024, 13, 1300. [Google Scholar] [CrossRef]
- Qin, X.; Sun, J.; Liu, M.; Zhang, L.; Yin, Q.; Chen, S. The effects of oral nutritional supplements interventions on nutritional status in patients undergoing colorectal cancer surgery: A systematic review. Int. J. Nurs. Pract. 2024, 30, e13226. [Google Scholar] [CrossRef]
- Salehi, S.S.; Mirmiranpour, H.; Rabizadeh, S.; Esteghamati, A.; Tomasello, G.; Alibakhshi, A.; Najafi, N.; Rajab, A.; Nakhjavani, M. Improvement in Redox Homeostasis after Cytoreductive Surgery in Colorectal Adenocarcinoma. Oxidative Med. Cell. Longev. 2021, 2021, 8864905. [Google Scholar] [CrossRef]
- Leimkühler, M.; Bourgonje, A.R.; van Goor, H.; Campmans-Kuijpers, M.J.E.; de Bock, G.H.; van Leeuwen, B.L. Oxidative Stress Predicts Post-Surgery Complications in Gastrointestinal Cancer Patients. Ann. Surg. Oncol. 2022, 29, 4540–4547. [Google Scholar] [CrossRef] [PubMed]
- Ngoc Anh, L.T.; Kien, T.G.; Tuan, N.V.; Tuong, T.T.A.; Ko, J.; Dan, P.T.; Cho, J.; Tap, N.V. Malnutrition in Colorectal Cancer Patients: Association with the Lack of Eating Motivation and Inappropriate Diet. Asian Pac. J. Cancer Prev. APJCP 2025, 26, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Escribano, C.; Moreno, F.A.; Pérez-López, M.; Cunha-Pérez, C.; Belenguer-Varea, Á.; Peredo, D.C.; González, F.J.B.; Tarazona-Santabalbina, F.J. Malnutrition and Increased Risk of Adverse Outcomes in Elderly Patients Undergoing Elective Colorectal Cancer Surgery: A Case-Control Study Nested in a Cohort. Nutrients 2022, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Knight, S.R.; Ghosh, D.; Kingsley, P.A.; Lapitan, M.C.; Parreno-Sacdalan, M.D.; Sundar, S.; Qureshi, A.U.; Valparaiso, A.P.; Pius, R.; et al. Impact of malnutrition on early outcomes after cancer surgery: An international, multicentre, prospective cohort study. Lancet Glob. Health 2023, 11, e341–e349. [Google Scholar] [CrossRef]
- Bossi, P.; Delrio, P.; Mascheroni, A.; Zanetti, M. The Spectrum of Malnutrition/Cachexia/Sarcopenia in Oncology According to Different Cancer Types and Settings: A Narrative Review. Nutrients 2021, 13, 1980. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Shen, N.; Wen, J.; Chen, C.; Chen, X.; Zhang, W.; Garijo, P.D.; Wei, M.Y.K.; Chen, W.; Xue, X.; Sun, X. The relationship between GLIM-malnutrition, post-operative complications and long-term prognosis in elderly patients undergoing colorectal cancer surgery. J. Gastrointest. Oncol. 2023, 14, 2134–2145. [Google Scholar] [CrossRef]
- Ju, M.; Kim, Y.; Seo, K.W. Role of nutrition in wound healing and nutritional recommendations for promotion of wound healing: A narrative review. Ann. Clin. Nutr. Metab. 2023, 15, 67–71. [Google Scholar] [CrossRef]
- Lee, D.U.; Fan, G.H.; Hastie, D.J.; Addonizio, E.A.; Suh, J.; Prakasam, V.N.; Karagozian, R. The clinical impact of malnutrition on the postoperative outcomes of patients undergoing colorectal resection surgery for colon or rectal cancer: Propensity score matched analysis of 2011–2017 US hospitals. Surg. Oncol. 2021, 38, 101587. [Google Scholar] [CrossRef]
- Song, H.N.; Wang, W.B.; Luo, X.; Huang, D.D.; Ruan, X.J.; Xing, C.G.; Chen, W.Z.; Dong, Q.T.; Chen, X.L. Effect of GLIM-defined malnutrition on postoperative clinical outcomes in patients with colorectal cancer. Jpn. J. Clin. Oncol. 2022, 52, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Hamaker, M.E.; Oosterlaan, F.; van Huis, L.H.; Thielen, N.; Vondeling, A.; van den Bos, F. Nutritional status and interventions for patients with cancer—A systematic review. J. Geriatr. Oncol. 2021, 12, 6–21. [Google Scholar] [CrossRef]
- Ford, K.L.; Prado, C.M.; Weimann, A.; Schuetz, P.; Lobo, D.N. Unresolved issues in perioperative nutrition: A narrative review. Clin. Nutr. 2022, 41, 1578–1590. [Google Scholar] [CrossRef]
- Cui, J.; Liu, Y.; Li, F.; Zhuo, W.; Huang, L.; Xu, C.; Li, W.; Qin, J.; Sun, X.; Tian, H.; et al. Evidence-based guideline on immunonutrition in patients with cancer. Precis. Nutr. 2023, 2, e00031. [Google Scholar] [CrossRef]
- Ogilvie, J.; Mittal, R.; Sangster, W.; Parker, J.; Lim, K.; Kyriakakis, R.; Luchtefeld, M. Preoperative Immuno-Nutrition and Complications After Colorectal Surgery: Results of a 2-Year Prospective Study. J. Surg. Res. 2023, 289, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, J.; Wang, Y.; Deng, F.; Deng, Z. Oxidative Stress: Signaling Pathways, Biological Functions, and Disease. MedComm 2025, 6, e70268. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323. [Google Scholar] [CrossRef]
- Ezema, B.O.; Eze, C.N.; Ayoka, T.O.; Nnadi, C.O. Antioxidant-enzyme Interaction in Non-communicable Diseases. J. Explor. Res. Pharmacol. 2024, 9, 262–275. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free. Radic. Biol. Med. 2010, 49, 1603. [Google Scholar] [CrossRef] [PubMed]
- Tsounis, D.; Villiotou, V.; Melpidou, A.; Pantsiou, C.; Argyrou, A.; Giannopoulou, C.; Grigoratou, A.; Rontogianni, D.; Mantzaris, G.J.; Papatheodoridis, G. Oxidative imbalance increases the risk for colonic polyp and colorectal cancer development. World J. Gastrointest. Oncol. 2022, 14, 2208–2223. [Google Scholar] [CrossRef]
- Gu, H.; Li, B.; Xiang, L.; Xu, Z.; Tang, Y.; Zhu, Z.; Jiang, Y.; Peng, L.; He, H.; Wang, Y. Association between oxidative stress exposure and colorectal cancer risk in 98,395 participants: Results from a prospective study. Front. Nutr. 2023, 10, 1284066. [Google Scholar] [CrossRef]
- Hu, M.; Yuan, L.; Zhu, J. The Dual Role of NRF2 in Colorectal Cancer: Targeting NRF2 as a Potential Therapeutic Approach. J. Inflamm. Res. 2024, 17, 5985–6004. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Jeong, S.; Park, S.; Nam, S.; Chung, J.W.; Kim, K.O.; An, J.; Kim, J.H. Significance of 8-OHdG Expression as a Predictor of Survival in Colorectal Cancer. Cancers 2023, 15, 4613. [Google Scholar] [CrossRef]
- Janion, K.; Szczepańska, E.; Nowakowska-zajdel, E.; Strzelczyk, J.; Copija, A. Selected Oxidative Stress Markers in Colorectal Cancer Patients in Relation to Primary Tumor Location-A Preliminary Research. Medicina 2020, 56, 47. [Google Scholar] [CrossRef]
- Rašić, I.; Holjan, S.; Papović, V.; Glavaš, S.; Mulabdić, A.; Rašić, A. Evaluation of serum levels of malondialdehyde and endogenous non-enzymatic antioxidants in relation to colorectal cancer stage and intestinal wall infiltration. J. Health Sci. 2021, 11, 142–148. [Google Scholar] [CrossRef]
- Berechet, B.M.; Orășan, O.H.; Negrean, V.; Para, I.; Chiș, I.C.; Sporiș, N.D.; Cozma, A.; Sitar-Tăuț, A.V.; Clichici, S.V. From Adenoma to Carcinoma: Oxidative Stress and Lipidomic Profile in Colorectal Cancer Patients. J. Mind Med. Sci. 2025, 12, 16. [Google Scholar] [CrossRef]
- Boakye, D.; Jansen, L.; Schöttker, B.; Jansen, E.H.J.M.; Schneider, M.; Halama, N.; Gào, X.; Chang-Claude, J.; Hoffmeister, M.; Brenner, H. Blood markers of oxidative stress are strongly associated with poorer prognosis in colorectal cancer patients. Int. J. Cancer 2020, 147, 2373–2386. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Yu, W.; Liu, J.; Tang, D.; Yang, L.; Chen, X. Oxidative cell death in cancer: Mechanisms and therapeutic opportunities. Cell Death Dis. 2024, 15, 556. [Google Scholar] [CrossRef]
- Toro-Pérez, J.; Rodrigo, R. Contribution of oxidative stress in the mechanisms of postoperative complications and multiple organ dysfunction syndrome. Redox Rep. Commun. Free Radic. Res. 2021, 26, 35. [Google Scholar] [CrossRef]
- Sawai, K.; Goi, T.; Kimura, Y.; Koneri, K. Reduction of Blood Oxidative Stress Following Colorectal Cancer Resection. Cancers 2024, 16, 3550. [Google Scholar] [CrossRef]
- Ukaegbu, K.; Allen, E.; Svoboda, K.K.H. Reactive Oxygen Species and Antioxidants in Wound Healing: Mechanisms and Therapeutic Potential. Int. Wound J. 2025, 22, e70330. [Google Scholar] [CrossRef]
- Xu, H.; Yang, J.; Wei, Z.; Bao, S.; Liu, Z. Oxidative stress in vascular surgical diseases: Mechanisms, impacts and therapeutic perspectives. Front. Pharmacol. 2025, 16, 1527684. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wu, H.; Yang, Y.; Jiang, Y.; Yuan, J.; Tong, Q. Oxidative Stress Level as a Predictor of Anastomotic Leakage after Rectal Surgery. Mediat. Inflamm. 2021, 2021, 9968642. [Google Scholar] [CrossRef] [PubMed]
- Sawai, K.; Goi, T.; Sakamoto, S.; Matsunaka, T.; Maegawa, N.; Koneri, K. Oxidative Stress as a Biomarker for Predicting the Prognosis of Patients with Colorectal Cancer. Oncology 2022, 100, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Zedan, A.; Elshiekh, E.; Omar, M.I.; Raafat, M.; Khallaf, S.M.; Atta, H.; Hussien, M.T. Laparoscopic versus Open Complete Mesocolic Excision for Right Colon Cancer. Int. J. Surg. Oncol. 2021, 2021, 8859879. [Google Scholar] [CrossRef]
- Cui, M.; Liu, S. Meta-analysis of the effect of laparoscopic surgery and open surgery on long-term quality of life in patients with colorectal cancer. Medicine 2023, 102, e34922. [Google Scholar] [CrossRef]
- Shi, W.; Lou, J.; Zhang, X.; Ji, Y.; Weng, X.; Du, J. Adipose tissue alleviates the stress response by releasing adiponectin during laparoscopic surgery in patients with colorectal cancer. Lipids Health Dis. 2021, 20, 166. [Google Scholar] [CrossRef]
- Deftereos, I.; Kiss, N.; Isenring, E.; Carter, V.M.; Yeung, J.M. A systematic review of the effect of preoperative nutrition support on nutritional status and treatment outcomes in upper gastrointestinal cancer resection. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2020, 46, 1423–1434. [Google Scholar] [CrossRef]
- Tejchman, K.; Kotfis, K.; Sieńko, J. Biomarkers and Mechanisms of Oxidative Stress—Last 20 Years of Research with an Emphasis on Kidney Damage and Renal Transplantation. Int. J. Mol. Sci. 2021, 22, 8010. [Google Scholar] [CrossRef]
- Krzystek-Korpacka, M.; Kempiński, R.; Bromke, M.A.; Neubauer, K. Oxidative Stress Markers in Inflammatory Bowel Diseases: Systematic Review. Diagnostics 2020, 10, 601. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-León, D.; Monzó-Beltrán, L.; Pérez-Sánchez, L.; Naranjo-Morillo, E.; Gómez-Abril, S.Á.; Estañ-Capell, N.; Bañuls, C.; Sáez, G. Oxidative Stress and DNA Damage Markers in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 11664. [Google Scholar] [CrossRef]
- Gryszczyńska, B.; Budzyń, M.; Formanowicz, D.; Formanowicz, P.; Krasiński, Z.; Majewska, N.; Iskra, M.; Kasprzak, M.P. Advanced Oxidation Protein Products and Carbonylated Proteins Levels in Endovascular and Open Repair of an Abdominal Aortic Aneurysm: The Effect of Pre-, Intra-, and Postoperative Treatment. BioMed Res. Int. 2019, 2019, 7976043. [Google Scholar] [CrossRef] [PubMed]
- Ajebli, M.; Meretsky, C.R.; Akdad, M.; Amssayef, A.; Hebi, M. The Role of Dietary Vitamins and Antioxidants in Preventing Colorectal Cancer: A Systematic Review. Cureus 2024, 16, e64277. [Google Scholar] [CrossRef]
- Ahmadzadeh, A.; Khodayar, M.J.; Salehcheh, M.; Khorasgani, Z.N.; Matin, M. Evaluation of the Total Oxidant Status to the Antioxidant Capacity Ratio as a Valuable Biomarker in Breast Cancer Patients. Rep. Biochem. Mol. Biol. 2023, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Zowczak-Drabarczyk, M.; Białecki, J.; Grzelak, T.; Michalik, M.; Formanowicz, D. Selenium, Zinc, and Plasma Total Antioxidant Status and the Risk of Colorectal Adenoma and Cancer. Metabolites 2024, 14, 486. [Google Scholar] [CrossRef]
- Fan, Y.; Feng, Y.; Liu, W. Role of micronutrition in patients with oral cancer and nutritional intervention strategies. Frontiers in Nutrition 2025, 12, 1616344. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Z.; Gong, P.; Yao, W.; Ba, Q.; Wang, H. Review on the health-promoting effect of adequate selenium status. Front. Nutr. 2023, 10, 1136458. [Google Scholar] [CrossRef]
- Ciancarelli, I.; Morone, G.; Iosa, M.; Cerasa, A.; Calabrò, R.S.; Iolascon, G.; Gimigliano, F.; Tonin, P.; Tozzi Ciancarelli, M.G. Influence of Oxidative Stress and Inflammation on Nutritional Status and Neural Plasticity: New Perspectives on Post-Stroke Neurorehabilitative Outcome. Nutrients 2022, 15, 108. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and nutritional antioxidants in human diseases. Front. Physiol. 2018, 9, 360203. [Google Scholar] [CrossRef]
- Marini, H.R.; Facchini, B.A.; di Francia, R.; Freni, J.; Puzzolo, D.; Montella, L.; Facchini, G.; Ottaiano, A.; Berretta, M.; Minutoli, L. Glutathione: Lights and Shadows in Cancer Patients. Biomedicines 2023, 11, 2226. [Google Scholar] [CrossRef] [PubMed]
- Zahra, K.F.; Lefter, R.; Ali, A.; Abdellah, E.C.; Trus, C.; Ciobica, A.; Timofte, D. The Involvement of the Oxidative Stress Status in Cancer Pathology: A Double View on the Role of the Antioxidants. Oxidative Med. Cell. Longev. 2021, 2021, 9965916. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, M.; Zhang, X.; Liu, T.; Liu, C.; Wang, Z.; Tang, M.; Song, C.; Zhang, X.; Cong, M.; et al. Characteristics of malnutrition in malignant cancer patients. Precis. Nutr. 2024, 3, e00082. [Google Scholar] [CrossRef]
- Hegde, M.; Daimary, U.D.; Girisa, S.; Kumar, A.; Kunnumakkara, A.B. Tumor cell anabolism and host tissue catabolism-energetic inefficiency during cancer cachexia. Exp. Biol. Med. 2022, 247, 713–733. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gong, C.; Ye, Y.; Liao, L. Prognostic role of global leadership initiative on malnutrition in colorectal cancer patients: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2025, 69, 87–95. [Google Scholar] [CrossRef]
- Reber, E.; Schönenberger, K.A.; Vasiloglou, M.F.; Stanga, Z. Nutritional Risk Screening in Cancer Patients: The First Step Toward Better Clinical Outcome. Front. Nutr. 2021, 8, 603936. [Google Scholar] [CrossRef]
- Wang, M.; Guo, Q.; Liu, H.; Liu, M.; Tang, C.; Wu, J.; Feng, G.; Wu, W. GLIM criteria using NRS-2002 and MUST as the first step adequately diagnose the malnutrition in Crohn’s disease inpatients: A retrospective study. Front. Nutr. 2023, 9, 1059191. [Google Scholar] [CrossRef]
- Bıçaklı, D.H. Individualized nutritional management for cancer patients: A key component of treatment. Clin. Sci. Nutr. 2025, 7, 31–140. [Google Scholar] [CrossRef]
- Sánchez-Guillén, L.; Arroyo, A. Immunonutrition in patients with colon cancer. Immunotherapy 2020, 12, 5–8. [Google Scholar] [CrossRef]
- Wong, C.S.; Zaman, S.; Siddiraju, K.; Sellvaraj, A.; Ghattas, T.; Tryliskyy, Y. Effects of enteral immunonutrition in laparoscopic versus open resections in colorectal cancer surgery: A meta-analysis of randomised controlled trials. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2025, 51, 109488. [Google Scholar] [CrossRef]
- Ambrosio, M.R.; Spagnoli, L.; Perotti, B.; Petrelli, F.; Caini, S.; Saieva, C.; Usai, S.; Bianchini, M.; Cavazzana, A.; Arganini, M.; et al. Paving the Path for Immune Enhancing Nutrition in Colon Cancer: Modulation of Tumor Microenvironment and Optimization of Outcomes and Costs. Cancers 2023, 15, 437. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, G.; Jin, X.; Wang, J.; Yu, S. Pre-Operative Immunonutrition Enhances Postoperative Outcomes and Elevates Tumor-Infiltrating Lymphocyte Counts in Colorectal Cancer Patients: A Meta-Analysis of Randomized Controlled Trials. Nutr. Cancer 2024, 76, 499–512. [Google Scholar] [CrossRef] [PubMed]
- McKechnie, T.; Kazi, T.; Jessani, G.; Shi, V.; Sne, N.; Doumouras, A.; Hong, D.; Eskicioglu, C. The use of preoperative enteral immunonutrition in patients undergoing elective colorectal cancer surgery: A systematic review and meta-analysis. Color. Dis. 2025, 27, e70061. [Google Scholar] [CrossRef] [PubMed]
- Barchielli, G.; Capperucci, A.; Tanini, D. The Role of Selenium in Pathologies: An Updated Review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef]
- Friedman, J.; Thiele, R. Enhanced Recovery After Surgery (ERAS) and Immunonutrition: An Evidence-Based Approach. Pract. Gastroenterol. 2020, 25, 24–37. [Google Scholar]
- Fathi, A. Oral Nutrition Supplements in Enhanced Recovery After Surgery (ERAS): The Pandora’s Box for Prehabilitation Practice. Biomed. J. Sci. Tech. Res. 2021, 38, 30031–30040. [Google Scholar] [CrossRef]
- Gustafsson, U.O.; Rockall, T.A.; Wexner, S.; How, K.Y.; Emile, S.; Marchuk, A.; Fawcett, W.J.; Sioson, M.; Riedel, B.; Chahal, R.; et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations 2025. Surgery 2025, 184, 109397. [Google Scholar] [CrossRef]
- Stevens, J.L.; McKenna, H.; Gurusamy, K.S.; Van Schoor, J.; Grocott, M.P.W.; Jell, G.; Martin, D. Perioperative antioxidants for adults undergoing elective non-cardiac surgery. Cochrane Database Syst. Rev. 2018, 2018, CD013174. [Google Scholar] [CrossRef]
- Pedersen, S.S.; Fabritius, M.L.; Kongebro, E.K.; Meyhoff, C.S. Antioxidant treatment to reduce mortality and serious adverse events in adult surgical patients: A systematic review with meta-analysis and trial sequential analysis. Acta Anaesthesiol. Scand. 2021, 65, 438–450. [Google Scholar] [CrossRef]
- Bekhet, O.H.; Eid, M.E. The interplay between reactive oxygen species and antioxidants in cancer progression and therapy: A narrative review. Transl. Cancer Res. 2021, 10, 4196–4206. [Google Scholar] [CrossRef]
- Wu, K.; El Zowalaty, A.E.; Sayin, V.I.; Papagiannakopoulos, T. The pleiotropic functions of reactive oxygen species in cancer. Nat. Cancer 2024, 5, 384–399. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, W.; Yang, L.; Wang, T.; Li, C.; Yu, J.; Zhang, P.; Yin, Y.; Li, R.; Tao, K. Nrf2 inhibition increases sensitivity to chemotherapy of colorectal cancer by promoting ferroptosis and pyroptosis. Sci. Rep. 2023, 13, 14359. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Qiao, X.; Bergö, M.O. Effects of antioxidants on cancer progression. EMBO Mol. Med. 2025, 17, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Kizilarslanoglu, M.C. Effects of nutrition and physical exercise intervention in palliative cancer patients. Clin. Nutr. 2019, 38, 1474. [Google Scholar] [CrossRef]
| Oxidative Damage | Antioxidative Capacity | ||
|---|---|---|---|
| Enzymatic Defenses | |||
| Malondialdehyde (MDA) | Byproduct of lipid peroxidation. Associated with oxidative damage to cell membranes [38,53]. | Superoxide Dismutase (SOD) | An antioxidant that catalyzes the dismutation of superoxide radicals into oxygen and hydrogen peroxide [31,54]. |
| 4-Hydroxynonenal (4-HNE) | Reactive lipid peroxidation product that forms protein and DNA adducts [53]. | Catalase (CAT) | An enzyme that decomposes hydrogen peroxide into water and oxygen, preventing oxidative damage [51,54,55]. |
| 8-Hydroxy-2′-deoxyguanosine (8-OHdG): | A marker of ROS-induced DNA damage, detectable in plasma and tumor tissue, correlated with cancer progression and recurrence risk [53]. | Glutathione Peroxidase (GPx) | A selenium-containing enzyme that reduces hydrogen peroxide and lipid hydroperoxides using glutathione [51,54]. |
| Advanced Oxidation Protein Products (AOPP) | Product of protein oxidation, elevated in CRC patients and linked to disease severity [53,56]. | Non-enzymatic antioxidants | |
| Glutathione (GSH) | Antioxidant tripeptide that neutralizes free radicals and maintains intracellular redox balance [51,54]. | ||
| Vitamins C, E, and A | neutralize free radicals, protect cell membranes from lipid peroxidation, and support immune function and tissue repair. Key non-enzymatic antioxidants [51,57]. | ||
| Study (Author, Year) | Study Design/Population | CRM or OS Parameters Assessed | Key Findings |
|---|---|---|---|
| Riad et al., 2023 [18] | Prospective multicenter cohort study (n = 5709) | Clinical diagnosis of malnutrition | Malnutrition was associated with a twofold increase 30-day postoperative mortality and increased complications |
| Lee et al., 2021 [23] | Retrospective cohort, CRC patients from US database (n = 11357) | Diagnosed malnutrition | Malnourished patients had a higher risk of complications, prolonged stay, and mortality |
| Shen et al., 2023 [21] | Prospective cohort, elderly CRC patients (n = 385) | GLIM-defined malnutrition | Malnutrition significantly increased risk of postoperative complications and lowered long-term survival |
| Song et al., 2022 [24] | Prospective cohort, CRC patients (n = 918) | GLIM criteria | Malnourished patients had significantly worse short-term surgical outcomes |
| Martínez-Escribano et al., 2022 [17] | Case–control study in elderly CRC patients | Clinical nutritional status | Malnutrition increased risk of surgical complications and adverse discharge outcomes |
| Leimkühler et al., 2022 [15] | Observational study, gastrointestinal cancer incl. CRC (n = 81) | Serum free thiols (OS marker) | Low preoperative serum thiol levels, reflecting increased systemic oxidative stress, were predictive of postoperative complications and prolonged hospital stay |
| Sawai et al., 2022 [48] | Retrospective study, CRC patients (n = 163) | d-ROMs (Derivatives-reactive oxygen metabolites) | High oxidative stress levels were associated with worse prognosis in CRC, independent of tumor stage |
| Boakye et al., 2020 [41] | Prospective cohort, CRC patients (n > 3300) | d-ROMs and total thiol level | High oxidative stress levels were strongly associated with poorer prognosis |
| Kang et al., 2023 [37] | Retrospective cohort study CRC patients (n > 564) | 8-OHdG levels in tumor tissue | Low tumor expression of 8-OHdG was significantly associated with poorer 5-year event-free and disease-specific survival |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahorodnii, A.; Jelska, A.; Głuszyńska, P.; Razak Hady, H. Cancer-Related Malnutrition and Oxidative Stress in Colorectal Cancer Surgery: A Narrative Review of Pathophysiology and Postoperative Outcomes. Antioxidants 2025, 14, 1289. https://doi.org/10.3390/antiox14111289
Zahorodnii A, Jelska A, Głuszyńska P, Razak Hady H. Cancer-Related Malnutrition and Oxidative Stress in Colorectal Cancer Surgery: A Narrative Review of Pathophysiology and Postoperative Outcomes. Antioxidants. 2025; 14(11):1289. https://doi.org/10.3390/antiox14111289
Chicago/Turabian StyleZahorodnii, Andrii, Alicja Jelska, Paulina Głuszyńska, and Hady Razak Hady. 2025. "Cancer-Related Malnutrition and Oxidative Stress in Colorectal Cancer Surgery: A Narrative Review of Pathophysiology and Postoperative Outcomes" Antioxidants 14, no. 11: 1289. https://doi.org/10.3390/antiox14111289
APA StyleZahorodnii, A., Jelska, A., Głuszyńska, P., & Razak Hady, H. (2025). Cancer-Related Malnutrition and Oxidative Stress in Colorectal Cancer Surgery: A Narrative Review of Pathophysiology and Postoperative Outcomes. Antioxidants, 14(11), 1289. https://doi.org/10.3390/antiox14111289






