Mito-Genipin, a Novel Mitochondria-Targeted Genipin Derivative Modulates Oxidative Stress and Inflammation in Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry, Materials, and Instruments
2.1.1. Cell Cultures
2.1.2. Generation of UCP2 Knockout iPSC
2.1.3. Animals
2.1.4. Cell Death Analysis
2.1.5. MitoSOX Assay
2.1.6. TMRM Assay
2.1.7. Extracellular Flux Assay
2.1.8. Macrophage Activation
2.2. Fluorescence and Confocal Imaging
2.3. Gene Expression Analysis
2.4. Protein Extraction and Western Blotting
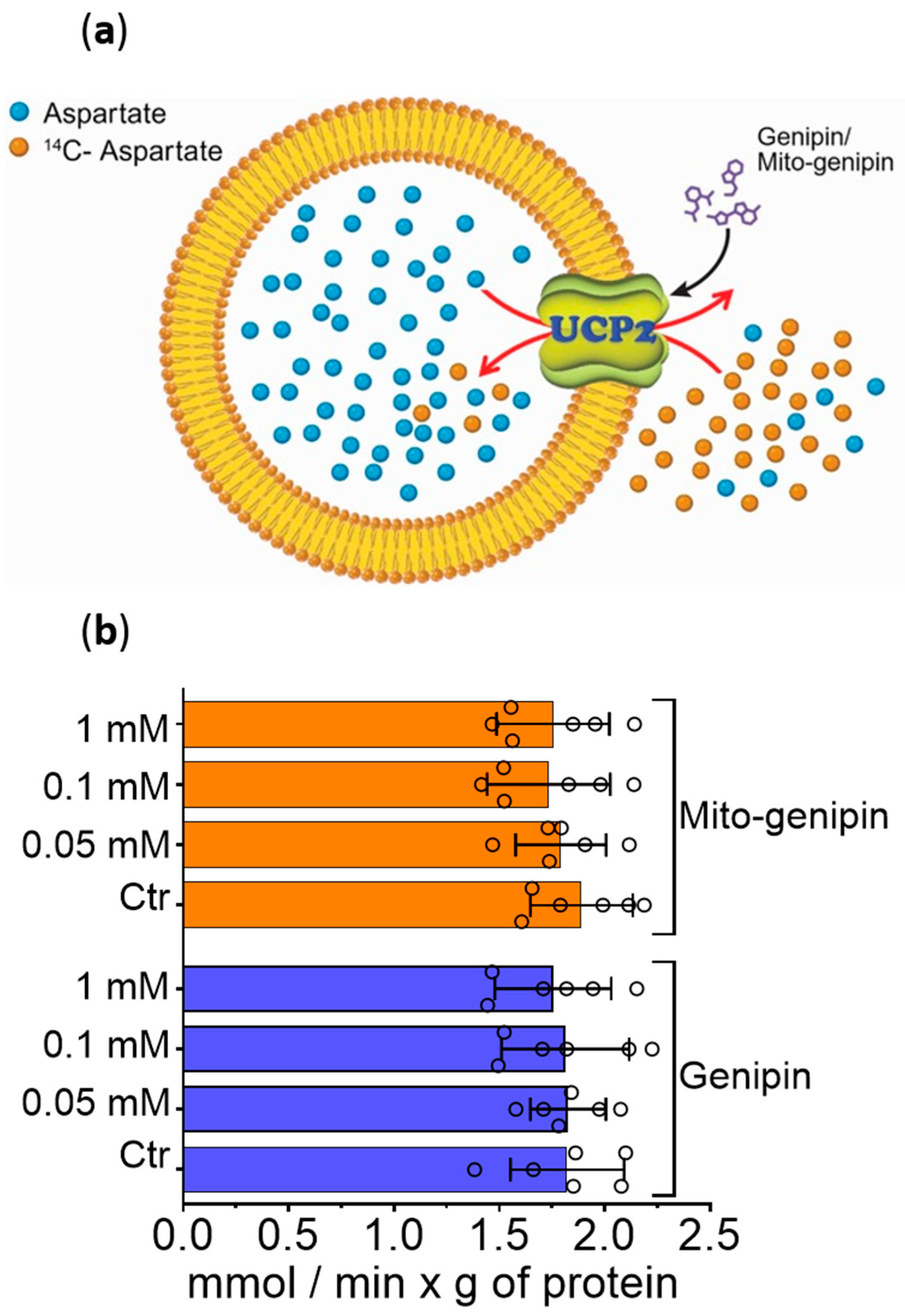
2.5. Reconstitution of Recombinant UCP2 into Liposomes and Transport Measurements
3. Results
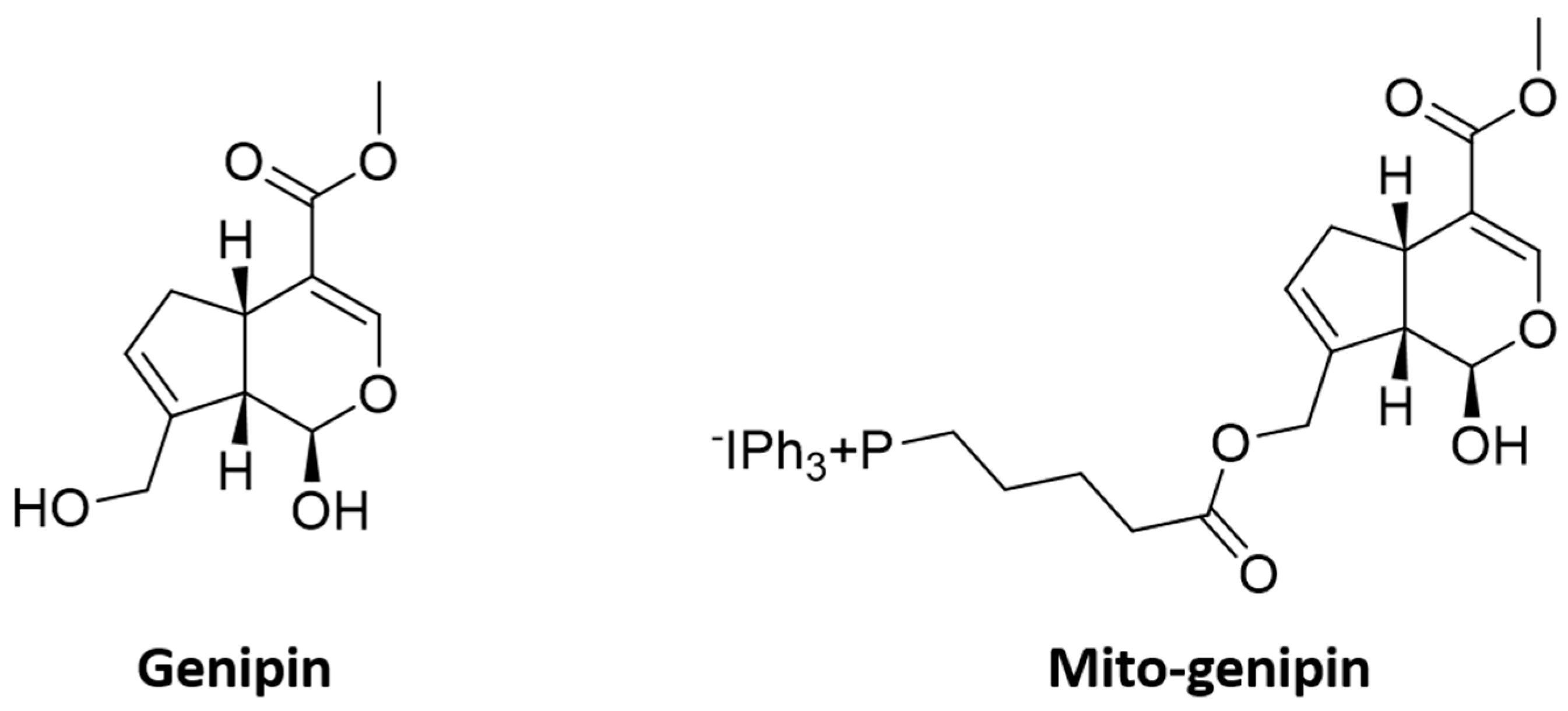
3.1. Synthesis of the Mitochondriotropic Analogue of Genipin, Mito-Genipin
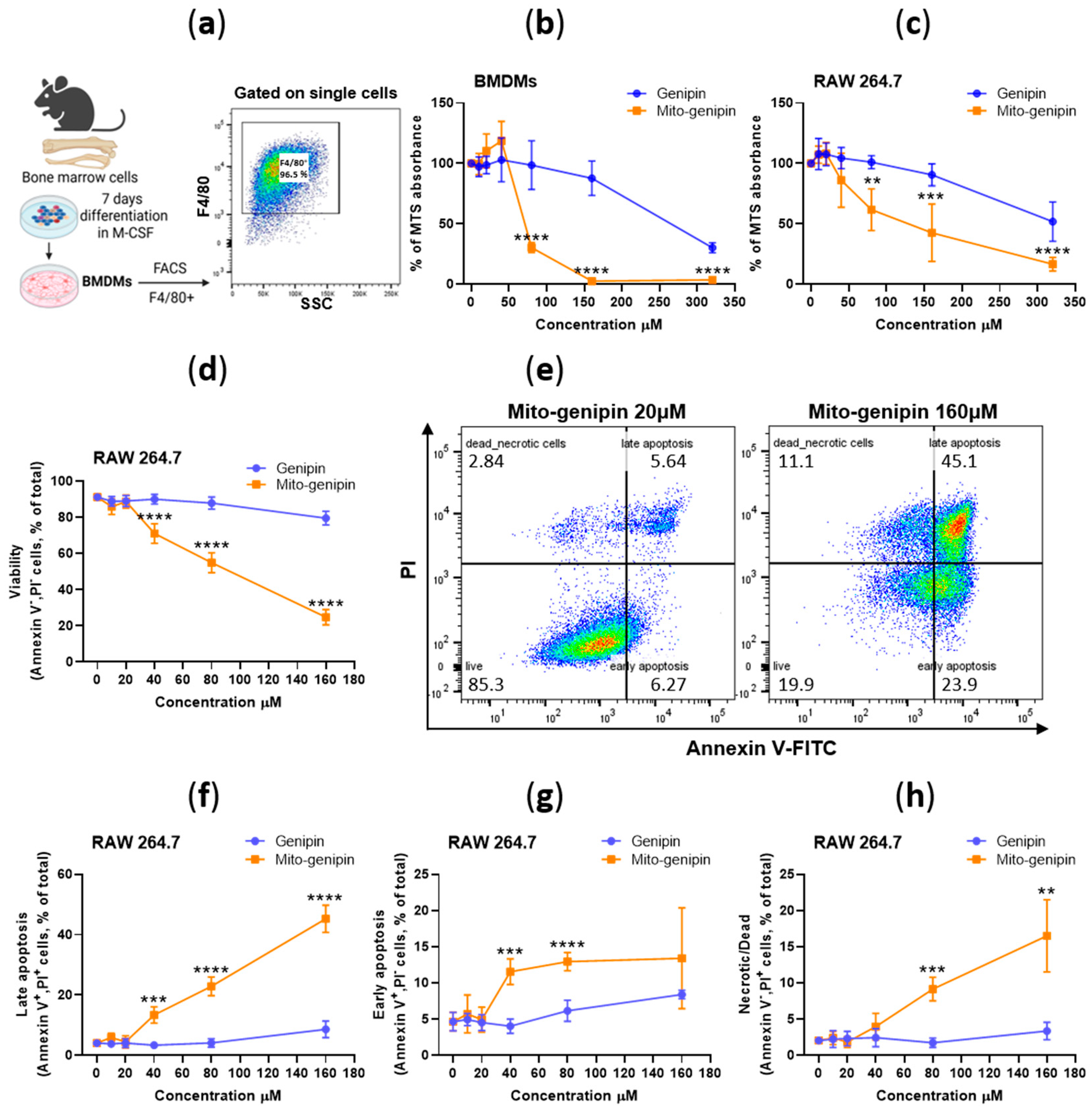
3.2. Assessment of Mito-Genipin Cytotoxicity in Macrophages
3.3. Mito-Genipin Efficiently Localizes to Mitochondria
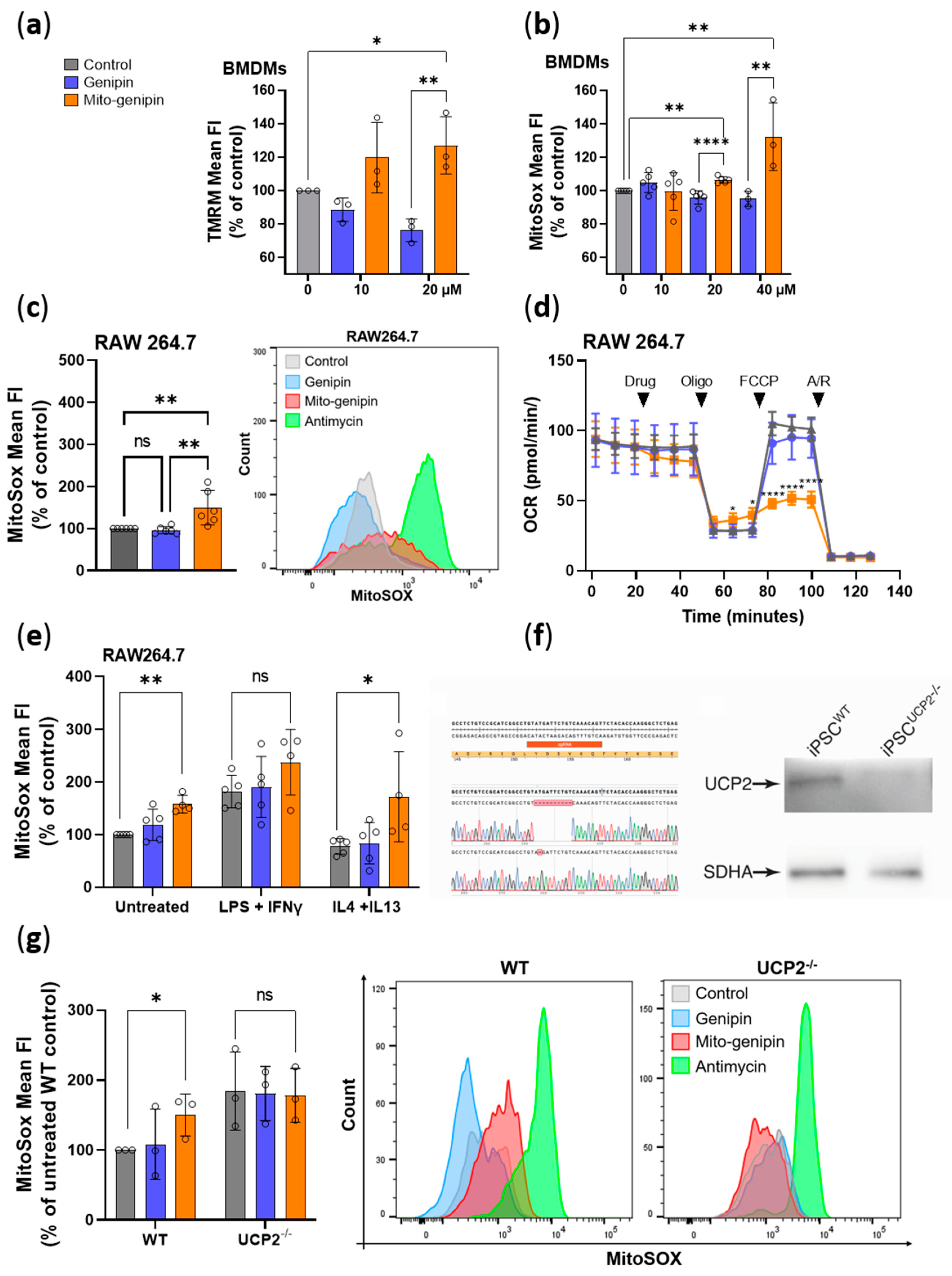
3.4. Mito-Genipin Induces Mitochondrial Dysfunction
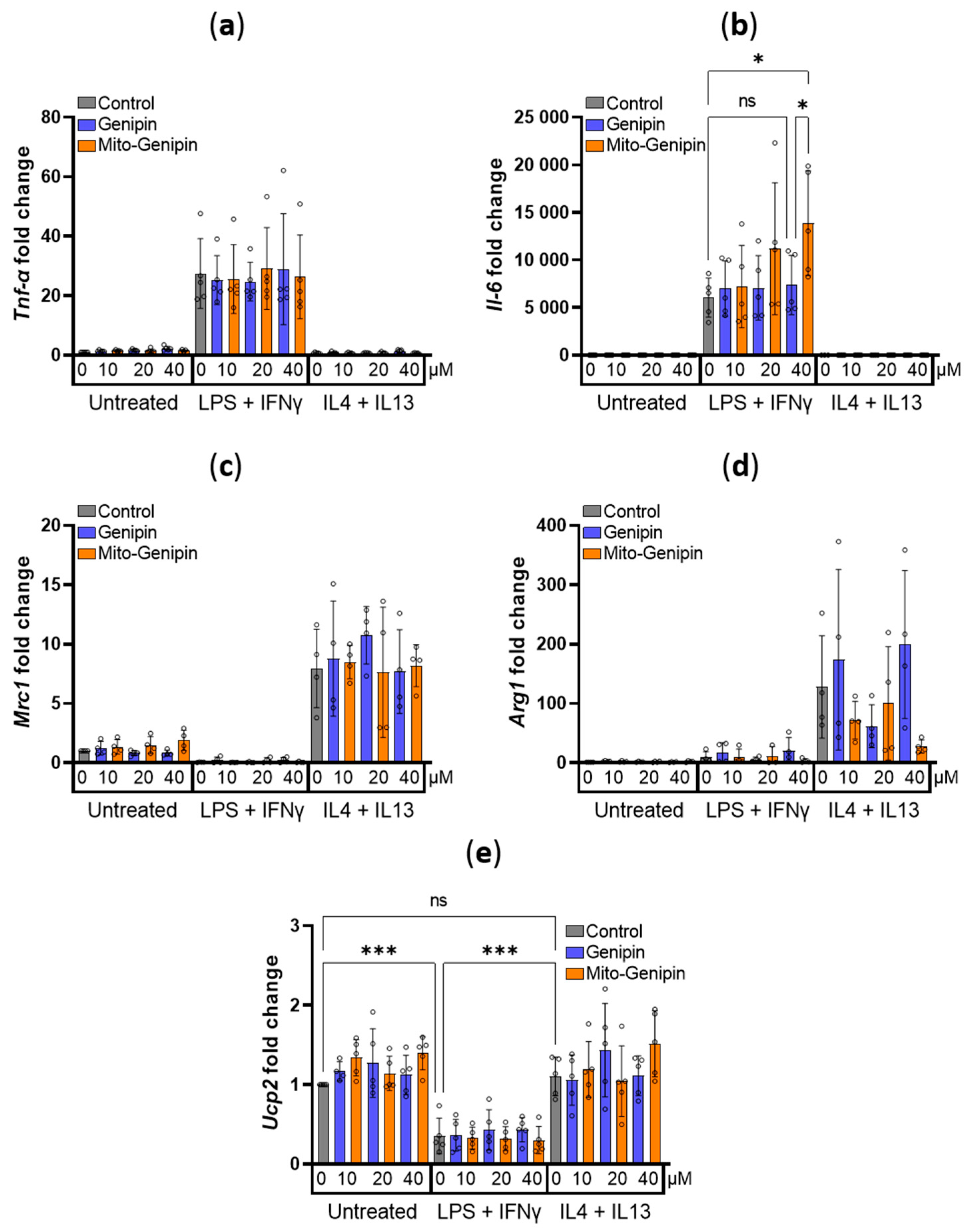
3.5. Impact of Mito-Genipin on Selected Marker Genes of Macrophage Polarization
3.6. Mito-Genipin Does Not Affect Metabolite Transport Activity Mediated by UCP2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Arg1 | Arginase 1 |
| BMDMs | Bone marrow-derived macrophages |
| IFNγ | Interferon gamma |
| IL-4 | Interleukin 4 |
| IL-6 | Interleukin 6 |
| IL-13 | Interleukin 13 |
| IMM | Inner mitochondrial membrane |
| LPS | Lipopolysaccharide |
| Mrc1 | Mannose receptor c-type 1 |
| ROS | Reactive oxygen species |
| TFN-α | Tumor necrosis factor alpha |
| TPP+ | Triphenylphosphonium |
| UCP2 | Uncoupling protein 2 |
References
- Zhang, C.Y.; Parton, L.E.; Ye, C.P.; Krauss, S.; Shen, R.; Lin, C.T.; Porco, J.A.; Lowell, B.B. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced β cell dysfunction in isolated pancreatic islets. Cell Metab. 2006, 3, 417–427. [Google Scholar] [CrossRef]
- Hirschenson, J.; Melgar-Bermudez, E.; Mailloux, R.J. The Uncoupling Proteins: A Systematic Review on the Mechanism Used in the Prevention of Oxidative Stress. Antioxidants 2022, 11, 322. [Google Scholar] [CrossRef]
- Nicholls, D.G. Mitochondrial proton leaks and uncoupling proteins. Biochim. et Biophys. Acta—Bioenerg. 2021, 1862, 148428. [Google Scholar] [CrossRef]
- Schulz, R.; Schlüter, K.D. Importance of Mitochondria in Cardiac Pathologies: Focus on Uncoupling Proteins and Monoamine Oxidases. Int. J. Mol. Sci. 2023, 24, 6459. [Google Scholar] [CrossRef] [PubMed]
- Rousset, S.; Alves-Guerra, M.C.; Mozo, J.; Miroux, B.; Cassard-Doulcier, A.M.; Bouillaud, F.; Ricquier, D. The Biology of Mitochondrial Uncoupling Proteins. Diabetes 2004, 53, S130–S135. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim. et Biophys. Acta—Bioenerg. 2018, 1859, 940–950. [Google Scholar] [CrossRef]
- Raho, S.; Capobianco, L.; Malivindi, R.; Vozza, A.; Piazzolla, C.; De Leonardis, F.; Gorgoglione, R.; Scarcia, P.; Pezzuto, F.; Agrimi, G.; et al. KRAS-regulated glutamine metabolism requires UCP2-mediated aspartate transport to support pancreatic cancer growth. Nat. Metab. 2020, 2, 1373–1381. [Google Scholar] [CrossRef]
- Vozza, A.; Parisi, G.; De Leonardis, F.; Lasorsa, F.M.; Castegna, A.; Amorese, D.; Marmo, R.; Calcagnile, V.M.; Palmieri, L.; Ricquier, D.; et al. UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc. Natl. Acad. Sci. USA 2014, 111, 960–965. [Google Scholar] [CrossRef] [PubMed]
- De Leonardis, F.; Ahmed, A.; Vozza, A.; Capobianco, L.; Riley, C.L.; Barile, S.N.; Di Molfetta, D.; Tiziani, S.; DiGiovanni, J.; Palmieri, L.; et al. Human mitochondrial uncoupling protein 3 functions as a metabolite transporter. FEBS Lett. 2024, 598, 338–346. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, R.; Zhang, Z.W.; He, X.Y.; Li, L.; Zhang, C.J.; Liu, Y.; Yu, H.T. Proliferation Inhibited by Genipin in Human Leukemia K562 Cells: Involvement of Uncoupling Protein 2 in Mitochondrial Damage. World J. Oncol. 2025, 16, 83–94. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, Y.S.; Jung, K.H.; Park, J.W.; Lee, K.H. Genipin enhances the antitumor effect of elesclomol in A549 lung cancer cells by blocking uncoupling protein-2 and stimulating reactive oxygen species production. Oncol. Lett. 2020, 20, 374. [Google Scholar] [CrossRef]
- Ge, H.; Zhang, F.; Duan, P.; Zhu, N.; Zhang, J.; Ye, F.; Shan, D.; Chen, H.; Lu, X.S.; Zhu, C.F.; et al. Mitochondrial Uncoupling Protein 2 in human cumulus cells is associated with regulating autophagy and apoptosis, maintaining gap junction integrity and progesterone synthesis. Mol. Cell Endocrinol. 2017, 443, 128–137. [Google Scholar] [CrossRef]
- Richard, D.; Clavel, S.; Huang, Q.; Sanchis, D.; Ricquier, D. Uncoupling protein 2 in the brain: Distribution and function. Biochem. Soc. Trans. 2001, 29, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Khanal, T.; Kim, H.G.; Do, M.T.; Choi, J.H.; Chung, Y.C.; Kim, H.S.; Park, Y.J.; Jeong, T.C.; Jeong, H.G. Genipin induces cyclooxygenase-2 expression via NADPH oxidase, MAPKs, AP-1, and NF-κB in RAW 264.7 cells. Food Chem. Toxicol. 2014, 64, 126–134. [Google Scholar] [CrossRef]
- Emre, Y.; Nübel, T. Uncoupling protein UCP2: When mitochondrial activity meets immunity. FEBS Lett. 2010, 584, 1437–1442. [Google Scholar] [CrossRef]
- Bai, Y.; Onuma, H.; Bai, X.; Medvedev, A.V.; Misukonis, M.; Weinberg, J.B.; Cao, W.; Robidoux, J.; Floering, L.M.; Daniel, K.W.; et al. Persistent Nuclear Factor-κB Activation in Ucp2−/− Mice Leads to Enhanced Nitric Oxide and Inflammatory Cytokine Production. J. Biol. Chem. 2005, 280, 19062–19069. [Google Scholar] [CrossRef] [PubMed]
- Emre, Y.; Hurtaud, C.; Nübel, T.; Criscuolo, F.; Ricquier, D.; Cassard-Doulcier, A.M. Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages. Biochem. J. 2007, 402, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Kizaki, T.; Suzuki, K.; Hitomi, Y.; Taniguchi, N.; Saitoh, D.; Watanabe, K.; Onoé, K.; Day, N.K.; Good, R.A.; Ohno, H. Uncoupling protein 2 plays an important role in nitric oxide production of lipopolysaccharide-stimulated macrophages. Proc. Natl. Acad. Sci. USA 2002, 99, 9392–9397. [Google Scholar] [CrossRef]
- Tan, H.Y.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The Reactive Oxygen Species in Macrophage Polarization: Reflecting Its Dual Role in Progression and Treatment of Human Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 2795090. [Google Scholar] [CrossRef]
- Better, J.; Estiri, M.; Wetstein, M.; Pervizaj-Oruqaj, L.; Malainou, C.; Ogungbemi-Alt, V.; Ferrero, M.R.; Langelage, M.; Kuznetsova, I.; Vazquez-Armendariz, A.I.; et al. Cell type–specific efferocytosis determines functional plasticity of alveolar macrophages. Sci. Immunol. 2025, 10, eadl3852. [Google Scholar] [CrossRef]
- Isali, I.; McClellan, P.; Shankar, E.; Gupta, S.; Jain, M.; Anderson, J.M.; Hijaz, A.; Akkus, O. Genipin guides and sustains the polarization of macrophages to the pro-regenerative M2 subtype via activation of the pSTAT6-PPAR-gamma pathway. Acta Biomater. 2021, 131, 198–210. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Y.; Zhang, L.; Xu, C.; Xu, H.; Chen, Z. Reprogramming Macrophage Polarization, Depleting ROS by Astaxanthin and Thioketal-Containing Polymers Delivering Rapamycin for Osteoarthritis Treatment. Adv. Sci. 2024, 11, e2305363. [Google Scholar] [CrossRef] [PubMed]
- Severin, F.; Urbani, A.; Varanita, T.; Bachmann, M.; Azzolini, M.; Martini, V.; Pizzi, M.; Tos, A.P.D.; Frezzato, F.; Mattarei, A.; et al. Pharmacological modulation of Kv1.3 potassium channel selectively triggers pathological B lymphocyte apoptosis in vivo in a genetic CLL model. J. Exp. Clin. Cancer Res. 2022, 41, 64. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.; Rossa, A.; Antoniazzi, G.; Biasutto, L.; Carrer, A.; Campagnaro, M.; Leanza, L.; Gonczi, M.; Csernoch, L.; Paradisi, C.; et al. Synthesis and cellular effects of a mitochondria-targeted inhibitor of the two-pore potassium channel TASK-3. Pharmacol. Res. 2021, 164, 105326. [Google Scholar] [CrossRef]
- Bachmann, M.; Rossa, A.; Varanita, T.; Fioretti, B.; Biasutto, L.; Milenkovic, S.; Checchetto, V.; Peruzzo, R.; Ahmad, S.A.; Patel, S.H.; et al. Pharmacological targeting of the mitochondrial calcium-dependent potassium channel KCa3.1 triggers cell death and reduces tumor growth and metastasis in vivo. Cell Death Dis. 2022, 13, 1055. [Google Scholar] [CrossRef]
- Smith, R.A.J.; Hartley, R.C.; Murphy, M.P. Mitochondria-targeted small molecule therapeutics and probes. Antioxid. Redox Signal. 2011, 15, 3021–3038. [Google Scholar] [CrossRef]
- Toda, G.; Yamauchi, T.; Kadowaki, T.; Ueki, K. Preparation and culture of bone marrow-derived macrophages from mice for functional analysis. STAR Protoc. 2021, 2, 100246. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, A.; Bräuer, A.U.; Smorodchenko, A.; Goyn, J.; Hilse, K.E.; Shabalina, I.G.; Infante-Duarte, C.; Pohl, E.E. Quantification of uncoupling protein 2 reveals its main expression in immune cells and selective up-regulation during t-cell proliferation. PLoS ONE 2012, 7, e41406. [Google Scholar] [CrossRef]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef]
- Cubisino, S.A.M.; Milenkovic, S.; Conti-Nibali, S.; Musso, N.; Bonacci, P.; De Pinto, V.; Ceccarelli, M.; Reina, S. Electrophysiological properties and structural prediction of the SARS-CoV-2 viroprotein E. Front. Mol. Biosci. 2024, 11, 1334819. [Google Scholar] [CrossRef]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Hou, J.; Ding, Y.; Zhang, T.; Zhang, Y.; Wang, J.; Shi, C.; Fu, W.; Cai, Z. The hydroxyl at position C1 of genipin is the active inhibitory group that affects mitochondrial uncoupling protein 2 in Panc-1 cells. PLoS ONE 2016, 11, e0147026. [Google Scholar] [CrossRef]
- Wahba, M.I. A comprehensive review on genipin: An efficient natural cross-linker for biopolymers. Polym. Bull. 2024, 81, 14251–14305. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Larsen, J.; Krasieva, T.B.; Lyubovitsky, J.G. Effect of genipin crosslinking on the optical spectral properties and structures of collagen hydrogels. ACS Appl. Mater. Interfaces 2011, 3, 2579–2584. [Google Scholar] [CrossRef]
- Matcham, S.; Novakovic, K. Fluorescence imaging in genipin crosslinked chitosan-poly(vinyl pyrrolidone) hydrogels. Polymers 2016, 8, 385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mao, L.; Liu, J.; Liu, T. Self-fluorescent drug delivery vector based on genipin-crosslinked polyethylenimine conjugated globin nanoparticle. Mater. Sci. Eng. C 2017, 71, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Leanza, L.; Romio, M.; Becker, K.A.; Azzolini, M.; Trentin, L.; Managò, A.; Venturini, E.; Zaccagnino, A.; Mattarei, A.; Carraretto, L.; et al. Direct Pharmacological Targeting of a Mitochondrial Ion Channel Selectively Kills Tumor Cells In Vivo. Cancer Cell 2017, 31, 516–531.e10. [Google Scholar] [CrossRef]
- Lang, L.; Zheng, D.; Jiang, Q.; Meng, T.; Ma, X.; Yang, Y. Uncoupling protein 2 modulates polarization and metabolism of human primary macrophages via glycolysis and the NF-κB pathway. Exp. Ther. Med. 2023, 26, 583. [Google Scholar] [CrossRef]
- Cho, Y.S. Genipin, an Inhibitor of UCP2 as a Promising New Anticancer Agent: A Review of the Literature. Int. J. Mol. Sci. 2022, 23, 5637. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, J.; Zhang, X. Inhibition of uncoupling protein 2 by genipin reduces insulin-stimulated glucose uptake in 3T3-L1 adipocytes. Arch. Biochem. Biophys. 2009, 486, 88–93. [Google Scholar] [CrossRef]
- Kleih, M.; Böpple, K.; Dong, M.; Gaißler, A.; Heine, S.; Olayioye, M.A.; Aulitzky, W.E.; Essmann, F. Direct impact of cisplatin on mitochondria induces ROS production that dictates cell fate of ovarian cancer cells. Cell Death Dis. 2019, 10, 851. [Google Scholar] [CrossRef]
- Dalla Pozza, E.; Fiorini, C.; Dando, I.; Menegazzi, M.; Sgarbossa, A.; Costanzo, C.; Palmieri, M.; Donadelli, M. Role of mitochondrial uncoupling protein 2 in cancer cell resistance to gemcitabine. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 1856–1863. [Google Scholar] [CrossRef]
- Yu, S.X.; Du, C.T.; Chen, W.; Lei, Q.Q.; Li, N.; Qi, S.; Zhang, X.J.; Hu, G.Q.; Deng, X.M.; Han, W.Y.; et al. Genipin inhibits NLRP3 and NLRC4 inflammasome activation via autophagy suppression. Sci. Rep. 2015, 5, 17935. [Google Scholar] [CrossRef]
- Kreiter, J.; Rupprecht, A.; Zimmermann, L.; Moschinger, M.; Rokitskaya, T.I.; Antonenko, Y.N.; Gille, L.; Fedorova, M.; Pohl, E.E. Molecular Mechanisms Responsible for Pharmacological Effects of Genipin on Mitochondrial Proteins. Biophys. J. 2019, 117, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- Zinovkin, R.A.; Zamyatnin, A.A. Mitochondria-Targeted Drugs. Curr. Mol. Pharmacol. 2018, 12, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Donadelli, M.; Dando, I.; Fiorini, C.; Palmieri, M. UCP2, a mitochondrial protein regulated at multiple levels. Cell. Mol. Life Sci. 2014, 71, 1171–1190. [Google Scholar] [CrossRef]
- Sreedhar, A.; Zhao, Y. Uncoupling protein 2 and metabolic diseases. Mitochondrion 2017, 34, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Alves-Guerra, M.C.; Rousset, S.; Pecqueur, C.; Mallat, Z.; Blanc, J.; Tedgui, A.; Bouillaud, F.; Cassard-Doulcier, A.M.; Ricquier, D.; Miroux, B. Bone Marrow Transplantation Reveals the in Vivo Expression of the Mitochondrial Uncoupling Protein 2 in Immune and Nonimmune Cells during Inflammation. J. Biol. Chem. 2003, 278, 42307–42312. [Google Scholar] [CrossRef]
- van Dierendonck, X.A.M.H.; Sancerni, T.; Alves-Guerra, M.C.; Stienstra, R. The role of uncoupling protein 2 in macrophages and its impact on obesity-induced adipose tissue inflammation and insulin resistance. J. Biol. Chem. 2020, 295, 17535–17548. [Google Scholar] [CrossRef]
- Berardi, M.J.; Shih, W.M.; Harrison, S.C.; Chou, J.J. Mitochondrial uncoupling protein 2 structure determined by NMR molecular fragment searching. Nature 2011, 476, 109–113. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, W.; Zhang, J.; Huang, T.; Hong, Y.; Zhao, T.; Liu, M.; Chen, Q.; Yang, Y.; Wang, S.; et al. UCP2 inhibition eliminates pancreatic β cell autoinflammation in T2DM with islet-mitochondrial sequential targeting nanomedicines. Nat. Commun. 2025, 16, 6840. [Google Scholar] [CrossRef]
- Touyama, R.; Inoue, K.; Takeda, Y.; Yatsuzuka, M.; Ikumoto, T.; Moritome, N.; Shingu, T.; Yokoi, T.; Inouye, H. Studies on the blue pigments produced from genipin and methylamine. II. On the formation mechanisms of brownish-red intermediates leading to the blue pigment formation. Chem. Pharm. Bull. 1994, 42, 1571–1578. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Luo, W.; Cai, T.; Li, Z.; Liu, Y.; Gao, W.; Wan, Q.; Wang, X.; Wang, J.; et al. Regeneration of skeletal system with genipin crosslinked biomaterials. J. Tissue Eng. 2020, 11, 2041731420974861. [Google Scholar] [CrossRef] [PubMed]
- Åberg, C. Kinetics of nanoparticle uptake into and distribution in human cells. Nanoscale Adv. 2021, 3, 2196–2212. [Google Scholar] [CrossRef]
- Sadeghi, S.; Checchetto, V.; Varanita, T. A Comprehensive Pan-Cancer Analysis of the Mitochondrial Uncoupling Protein UCP2, with a Focus on Sex and Gender-Related Aspects. Cell Physiol. Biochem. 2024, 58, 630–653. [Google Scholar] [PubMed]
- Li, J.; Jiang, R.; Cong, X.; Zhao, Y. UCP2 gene polymorphisms in obesity and diabetes, and the role of UCP2 in cancer. FEBS Lett. 2019, 593, 2525–2534. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angi, B.; Di Molfetta, D.; Pendin, D.; Antoniazzi, G.; Flora, C.A.; De Leonardis, F.; Buono, M.; Fiermonte, G.; Szabo, I.; Mattarei, A.; et al. Mito-Genipin, a Novel Mitochondria-Targeted Genipin Derivative Modulates Oxidative Stress and Inflammation in Macrophages. Antioxidants 2025, 14, 1281. https://doi.org/10.3390/antiox14111281
Angi B, Di Molfetta D, Pendin D, Antoniazzi G, Flora CA, De Leonardis F, Buono M, Fiermonte G, Szabo I, Mattarei A, et al. Mito-Genipin, a Novel Mitochondria-Targeted Genipin Derivative Modulates Oxidative Stress and Inflammation in Macrophages. Antioxidants. 2025; 14(11):1281. https://doi.org/10.3390/antiox14111281
Chicago/Turabian StyleAngi, Beatrice, Daria Di Molfetta, Diana Pendin, Giuseppe Antoniazzi, Carlo Alberto Flora, Francesco De Leonardis, Martina Buono, Giuseppe Fiermonte, Ildiko Szabo, Andrea Mattarei, and et al. 2025. "Mito-Genipin, a Novel Mitochondria-Targeted Genipin Derivative Modulates Oxidative Stress and Inflammation in Macrophages" Antioxidants 14, no. 11: 1281. https://doi.org/10.3390/antiox14111281
APA StyleAngi, B., Di Molfetta, D., Pendin, D., Antoniazzi, G., Flora, C. A., De Leonardis, F., Buono, M., Fiermonte, G., Szabo, I., Mattarei, A., & Varanita, T. (2025). Mito-Genipin, a Novel Mitochondria-Targeted Genipin Derivative Modulates Oxidative Stress and Inflammation in Macrophages. Antioxidants, 14(11), 1281. https://doi.org/10.3390/antiox14111281







