Abstract
The islet amyloid polypeptide (IAPP) is a 37-residue peptide hormone secreted by pancreatic β-cells that is known to aggregate into amyloid fibrils. These fibrils accumulate in the pancreatic islets of individuals afflicted with type 2 diabetes and are implicated in β-cell dysfunction. Metal ions such as copper and zinc are known to modulate IAPP fibrillization, yet the role of metal-induced oxidative modifications in this process remains largely unexplored. This study examines the non-enzymatic post-translational oxidation of IAPP and its effects on aggregation using the biologically relevant Cu/O2/ascorbate system. Mass spectrometry identified residues within the amyloidogenic region (residues 20–29) as the primary targets of oxidation. These oxidative modifications impaired the formation of cross-β-sheet amyloid fibrils and promoted the accumulation of amorphous aggregates. The H18A IAPP derivative, lacking the key metal-binding histidine, was also examined to assess the impact of sequence variation on oxidation and aggregation. Copper-mediated oxidation of H18A resulted in a broader distribution of oxidation sites and impacts fibril formation. These findings provide preliminary mechanistic insights into copper-induced oxidation and its impact on IAPP aggregation pathways.
1. Introduction
Type 2 diabetes (T2D) represents a major global public health crisis, ranking as the eighth leading cause of mortality and disability worldwide [1]. It is primarily characterized by chronic hyperglycemia driven by insulin resistance and progressive dysfunction of the pancreatic β-cells responsible for insulin biosynthesis. Moreover, the presence of amyloid plaques within the islets of Langerhans in the pancreas, observed in over 90% of patients, constitutes a prominent pathological hallmark of T2D [2,3]. These deposits are primarily composed of the islet amyloid polypeptide (IAPP), a 37-residue peptide hormone co-secreted with insulin by pancreatic β-cells. IAPP adopts primarily a disordered conformation under physiological conditions and plays key roles in glucose homeostasis, regulation of gastric emptying, and satiety [4]. Under pathological conditions, increased insulin demand leads to elevated local concentrations of IAPP, likely promoting its aggregation into amyloid fibrils in the vicinity of β-cells [5]. Albeit amyloid deposits can generate local inflammation, most experimental evidence indicates that small, soluble oligomeric intermediates of IAPP, are the most cytotoxic species, being able to disrupt plasma membrane integrity, to trigger β-cell apoptosis and to induce oxidative stress [6,7,8].
While an increased local concentration of IAPP associated with co-secretion of insulin can promote amyloid formation in the Langerhans islets [9,10], other molecular factors could also induce aggregation and tissue deposition. Among these, increased oxidative stress has emerged as a key contributor to T2D pathogenesis, disrupting pancreatic redox balance and exacerbating β-cell dysfunction [11,12,13]. Reactive oxygen species (ROS), including hydrogen peroxide, superoxide anion and hydroxyl radical, are central mediators of oxidative stress. These species cause DNA damage, lipid peroxidation, and induce non-enzymatic post-translational modifications (PTMs) of proteins, ultimately leading to β-cell impairment and apoptosis [14,15,16]. Notably, in T2D, IAPP has been shown to undergo non-enzymatic PTMs in response to oxidative stress. For instance, 4-hydroxynonenal (HNE), a product of lipid peroxidation, forms covalent adducts with histidine residues of IAPP, perturbing its conformation and enhancing its cytotoxicity [17]. A further notable modification involves the formation of advanced glycation end products (AGEs), which have been detected in IAPP and are tightly linked to oxidative stress. Methylglyoxal (MG), a highly reactive glucose-derived metabolite, interacts with residue Lys1 and leads to a glycated form that accelerates amyloid formation [18]. Additionally, AGEs amplify ROS production through the activation of their receptor (RAGE), forming a feedback loop that intensifies oxidative burden [19,20].
In parallel to these oxidative modifications, transition metals, potent modulators of oxidative stress, are also known to modulate polypeptide aggregation and IAPP interactions with polypeptide, as recently exemplified for Zn2+ and insulin [21]. Among them, copper has drawn increasing attention for its ability to alter IAPP structure and aggregation kinetics [22,23,24]. Remarkably, elevated levels of copper in the bloodstream have been consistently reported in both T2D patients and diabetic mouse models, suggesting a potential link between metal dysregulation and disease progression [25,26,27,28]. Copper not only impacts the structural dynamics of peptides but could also serve as a redox-active center. In the presence of physiologically reducing agents such as ascorbate, Cu(II) is reduced to Cu(I), which can react with molecular oxygen to produce ROS via redox cycling. This redox cycling has conceptual similarity to Fenton-like mechanism, in which copper reacts with hydrogen peroxide to generate highly reactive hydroxyl radicals (HO•) [29]. For example, the amyloid β (Aβ) peptide binds to Cu2+ and forms Cu–Aβ complex capable of catalyzing ROS production [30,31]. Moreover, it has been reported that copper binding accelerates aggregation and promotes the formation of toxic species of the Aβ peptide and Tau protein, both polypeptides being implicated in the progression of Alzheimer’s disease [31,32]. Similarly, it was reported that copper binds IAPP, predominantly through the imidazole Nδ1 of His18 residue, thereby inducing significant conformational rearrangements [33]. Upon binding to IAPP monomer, copper modulates the aggregation pathway mainly by interfering with the conformational transitions required for the formation of the organized cross-β-sheet quaternary structure. Notably, it has been shown that this interaction induces the formation of small aggregates [34,35].
Although pancreatic redox stress is well established as a central feature of T2D, and copper is known to both amplify ROS production and modulate the self-assembly of amyloidogenic polypeptides, the impact of metal-catalyzed oxidation on IAPP remains unexplored. In this context, the present study aimed at elucidating the effects of copper-mediated oxidation on IAPP self-assembly using the biologically relevant Cu/O2/ascorbate system. Oxidized residues were identified in the amyloidogenic region known to be between residues 20 and 29 [36]. These oxidative modifications significantly impaired the formation of the cross-β-sheet structure, promoting the formation of amorphous aggregates. To gain further insight into the oxidative mechanism and its impact on IAPP self-assembly, the H18A derivative, lacking the key metal-binding imidazole group, was also examined.
2. Materials and Methods
2.1. Peptide Synthesis, Purification, Characterization and Monomerization
IAPP and its analogs were synthesized by Fmoc solid-phase peptide synthesis on a Rink Amide AM resin, as previously described [37]. Crude peptides were purified by preparative HPLC using a C18 column and a linear gradient of acetonitrile in water containing 0.6% (v/v) trifluoroacetic acid (TFA). Fractions were analyzed by high-resolution mass spectrometry using an Agilent 1200 series HPLC coupled to an electrospray time-of-flight mass spectrometer (ESI-TOF) (Agilent Technologies, Palo Alto, CA, USA). Fractions containing the desired peptide with a purity above 95% were pooled and lyophilized. Peptide cyclization was performed by oxidation in 100% dimethyl sulfoxide (DMSO) at room temperature overnight to form the C2–C7 disulfide bridge. The solution was then diluted and subjected to a second round of HPLC purification. The solution was filtered through a 0.22 µm hydrophilic polyvinylidene difluoride (PVDF) membrane and sonicated for 30 min prior to lyophilization. Aliquots of monomerized peptide were prepared by dissolving the lyophilized powder a second time in 100% hexafluoro-2-propanol (HFIP) at a concentration of 1 mg/mL. The solution was then sonicated for 1 h, aliquoted and lyophilized. Monomerized samples were stored in a dried state at −80 °C for a maximum of 4 weeks.
2.2. Metal-Catalyzed Oxidation of Peptides and Proteolytic Digestion
Cu(II)-catalyzed oxidation of peptides was performed by incubating the peptide (60 µM) with CuSO4 (50 µM) and ascorbate (500 µM) in 50 mM phosphate buffer (pH 7.4) for 30 min at room temperature. These molar ratios were selected according to previous studies using the amyloid β-peptide [30,38,39]. Ammonium bicarbonate buffer (0.1 M, pH 8.0) and α-chymotrypsin (0.05 µg/µL in 0.1% formic acid) were added to obtain a chymotrypsin/peptide ratio of 1:40 (w/w). Digestion was performed at 37 °C for 4 h in a Thermomixer (Eppendorf), with mixing for 2 h at 650 rpm. The digestion was quenched by adding 700 µL of water. The resulting solution was purified using a 1 cm3 (30 mg) Oasis HLB solid-phase extraction cartridge and eluted with two additions of 500 µL of 100% methanol. Extracts were then evaporated under vacuum and stored at −30 °C until further use.
2.3. HPLC-MS/MS Analysis of Peptide Samples
Dried samples were reconstituted in 10% acetonitrile containing 0.2% formic acid. Chromatographic separation was performed using a Shimadzu Nexera UHPLC system (Columbia, MD, USA) equipped with an autosampler maintained at 15 °C. Samples (20 µL) were injected onto an Aeris PEPTIDE XB-C18 column (100 mm × 2.1 mm, 1.7 µm) fitted with a SecurityGuard UHPLC C18-peptide cartridge (Phenomenex, Torrance, CA, USA). Separation was achieved at a flow rate of 300 µL/min using a linear gradient with mobile phase A (0.1% formic acid in water) and mobile phase B (0.1% formic acid in acetonitrile). The gradient program was as follows: 5% B (0–1 min), ramped to 25% B at 10 min, to 50% B at 11 min, then to 85% B at 11 min, and held at 85% B until 14 min. Mass spectrometry analysis was carried out on a high-resolution TripleTOF 5600 Q-TOF instrument (Sciex, Concord, ON, Canada) equipped with a DuoSpray ion source, operated in full-scan positive mode. Ion source parameters were set as: temperature 500 °C, ion spray voltage 5 kV, GS1 and GS2 at 50 psi, and declustering potential at 80 V. Information-dependent acquisition (IDA) was employed for data acquisition over an m/z range of 120–1250, with dynamic background subtraction and collision energy of 30 ± 10 V (cycle time: 1.05 s). The top 10 most intense precursor ions were selected for collision-induced dissociation (CID), and MS/MS spectra were acquired over an m/z range of 80–1300. Data processing was conducted using the PEAKS software v13 (Bioinformatics Solution Inc., Waterloo, ON, Canada). Oxidized peptides were identified based on specific mass shifts and their absence in control digests. Extracted ion chromatograms (XICs) for native and oxidized chymotryptic peptides were generated using PeakView v2.2 and MasterView v1.1 (Sciex), with a mass tolerance of 0.02 Da to ensure high mass accuracy and confident peptide identification.
2.4. Aggregation Kinetics by Thioflavin T Fluorescence
Peptide solutions were prepared by dissolving the lyophilized peptides at a concentration of 50 µM in 50 mM phosphate buffer (pH 7.4). Final peptide concentrations were 12.5 µM and 25 µM and thioflavin T (ThT) concentration was fixed at 40 µM. Aggregation assays were performed at 25 °C without stirring, in sealed, black-wall, clear-bottom 96-well nonbinding surface plates, with a final volume of 100 µL per well. ThT fluorescence was measured every 10 min over a 24-h period using a SpectraMax i3 multimode plate reader (Molecular Devices, San Jose, CA, USA) with excitation at 440 nm and emission at 485 nm. For each experiment, data from triplicate wells were averaged, baseline-corrected by subtracting the corresponding control signal, and plotted as fluorescence intensity in relation to time. The resulting data were fitted to a sigmoidal Boltzmann model given in Equation (1), where Y0 and Ymax are, respectively, the initial and maximum ThT fluorescence value, t1/2 is the time required to reach half of the fluorescence intensity, and k is the elongation rate [40,41].
Lag time and final ThT fluorescence data from at least four independent experiments were averaged and reported as mean ± standard deviation (SD). Statistical analysis was performed with Prism version 6.0 software using Student t-test, with a threshold of p < 0.05.
2.5. Fluorescence Spectroscopy
Peptides were dissolved in 50 mM phosphate buffer (pH 7.4) at a concentration of 60 µM and incubated at room temperature under quiescent conditions for 72 h. ThT and anilino-8-naphthalenesulfonate (ANS) were added to the peptide samples at final concentrations of 40 µM and 60 µM, respectively. Excitation wavelengths were set at 355 nm for ANS and 440 nm for ThT. Emission spectra were recorded from 385 to 585 nm for ANS and from 450 to 550 nm for ThT. Fluorescence measurements were performed in a 10 mm quartz cuvette using a PTI QuantaMaster 400 spectrofluorometer (Edison, NJ, USA). For each measurement, a blank sample containing the fluorophore and buffer was used as a control.
2.6. Atomic Force Microscopy
After the desired time of incubation, peptide solutions at a concentration of 60 µM in 50 mM phosphate buffer (pH 7.4) were diluted in 1% acetic acid to reach a concentration of 15 μM and immediately applied on freshly cleaved mica. Acetic acid solution was added to the IAPP samples just prior deposition on the mica to promote adsorption of the peptide aggregates to the negatively charged surface, a procedure not affecting the morphology and stability of the obtained aggregates [42]. The surface was then washed twice with deionized water and air-dried overnight. Samples were analyzed using a Veeco/Bruker Multimode atomic force microscope operated in ScanAsyst-Air mode with a silicon tip (tip radius: 2–12 nm; force constant: 0.4 N/m) mounted on a nitride lever. Images were acquired at a scan rate of 0.9 Hz with a resolution of 1024 scans/min and a scan frame of 10 μm × 10 μm.
3. Results and Discussion
3.1. Site-Specific Oxidation of IAPP Induced by Copper Redox Cycling
Previous studies have identified His18 and adjacent residues as key sites for Cu(II) binding to IAPP [33,34,43]. Consequently, these residues could also be susceptible to copper-catalyzed oxidation. For the detection of site-specific oxidative modifications, IAPP was incubated with ascorbic acid and copper for 30 min then digested with chymotrypsin prior to analysis by LC-MS/MS. This approach follows the model proposed by Cheignon and colleagues, who investigated the effects of Aβ metal-catalyzed oxidation on ROS production [30]. Table 1 lists the oxidized residues identified, along with their corresponding monoisotopic masses and respective retention times. The digested peptides corresponding to missing cleavages and other modifications are presented in Table S1.

Table 1.
Theoretical and experimental monoisotopic m/z values, mass accuracies, and retention times are presented for chymotryptic IAPP peptides in both their non-oxidized and oxidized forms. Detected oxidative modifications are indicated in parentheses, where −2, +16, and +32, respectively, correspond to the loss of two hydrogen atoms, the addition of one oxygen atom, and the addition of two oxygen atoms.
High-resolution extracted ion chromatograms were acquired with a mass accuracy of ±5 ppm. Representative MS/MS spectra are provided in the Supplementary Information (Figure S1), and a summary of the detected residue modifications is shown in Figure 1E.
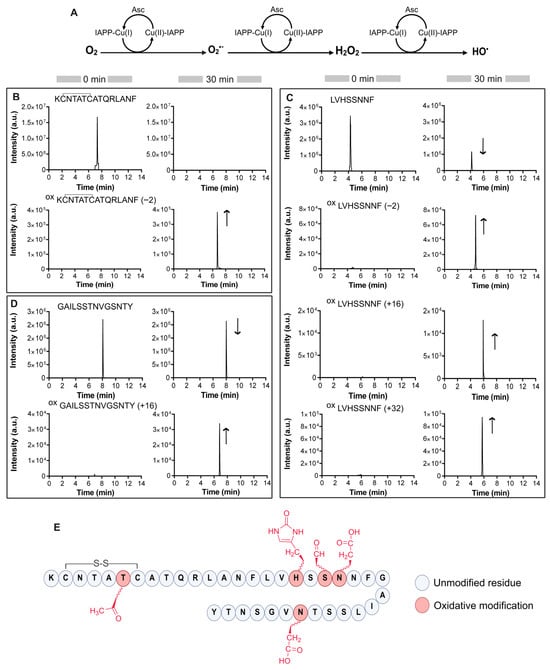
Figure 1.
Identification of site-specific IAPP oxidation. (A) Generation of ROS catalyzed by copper in the presence of IAPP peptide. Asc refers to ascorbate. (B–D) Extracted ion chromatograms of chymotryptic IAPP: (B) 1KCNTATCATQRLANF15, (C) 16LVHSSNNF23 and (D) 24GAILSSTNVGSNTY37 prior and after 30 min oxidation reaction. The direction of the arrows reflects changes in chromatographic peak intensities. (E) Summary of Cu-mediated oxidative modifications on IAPP. Oxidative sites identified by MS/MS analysis are highlighted in red.
Among the detected oxidative modifications, IAPP(1–15) fragment displayed a unique mass shift of −2 Da with a prominent ion detected at m/z 547.2610 (Figure 1B). This result suggests the loss of two hydrogen atoms, consistent with the dehydrogenation of a threonine side chain, converting the hydroxyl group into a ketone [44].
For the chymotryptic peptide IAPP(16–23), which contains a histidine residue, three different oxidized species were detected (Figure 1C). The first ion, observed at m/z 458.2201, corresponds to a −2 Da mass shift, indicative of the oxidation of serine hydroxyl group to 3-oxoalanine, also known as formylglycine [45]. Additional ions detected at m/z 467.2253 and 475.2228 exhibit mass shifts of +16 Da and +32 Da, respectively. These products correspond to oxidative modifications, with the +16 Da ion indicative of histidine oxidation to 2-oxo-histidine [46]. The +32 Da shift suggests the incorporation of two oxygen atoms, likely reflecting concurrent oxidation of histidine and asparagine, the latter being converted to aspartate [47]. The relative signal intensities indicate that co-oxidation involving histidine and asparagine, is more prevalent than histidine and serine oxidation alone, as evidenced by peak intensities of approximately 105 versus 104, respectively.
As for the third chymotryptic peptide IAPP24–37, LC-MS analysis revealed a single peak at m/z 700.3391 corresponding to a +16 Da mass shift. The MS/MS spectra revealed the oxidation of an asparagine residue to aspartate (Figure 1D and Figure S1). Additionally, given that IAPP is naturally amidated at its C-terminus, we investigated the potential deamidation of Tyr37. This modification was observed in both oxidized and non-oxidized forms of the peptide, suggesting that it is not a consequence of ROS-mediated oxidation (Table S1), but rather results from spontaneous hydrolysis occurring during sample preparation [48]. Finally, no oxidative modifications were detected in the control sample containing unmodified chymotryptic IAPP under the conditions tested.
3.2. Cu(II)-Mediated Oxidation Impacts the Kinetics of Amyloid Self-Assembly
It has been previously demonstrated that oxidative modifications influence the self-assembly behavior of amyloidogenic polypeptides, such as Aβ [49,50] and α-synuclein [51]. Thus, the impact of copper-mediated oxidation on IAPP aggregation into amyloid fibrils was assessed. Amyloid fibril formation was monitored using the fluorescence of ThT, a dye that exhibits enhanced signal upon interaction with cross-β-sheet quaternary structures [52]. Figure 2 displays the aggregation kinetics of IAPP in absence or in presence of, respectively, copper, ascorbic acid, and both reagents that induce IAPP oxidation.
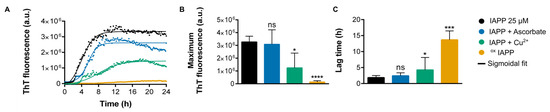
Figure 2.
Assessment of IAPP amyloid self-assembly in the presence or absence of Cu(II) and ascorbic acid. (A) Kinetics of IAPP amyloid formation at 25 µM monitored by ThT. (B) ThT fluorescence intensity at endpoint. (C) Lag time determined from sigmoidal fit. (B,C). Data were collected from at least four independent experiments performed in triplicates. p < 0.05 (*); p < 0.0005 (***); p < 0.0001 (****); ns: non-significant.
The addition of 10 molar eq. of ascorbic acid did not significantly affect the kinetics of IAPP self-assembly, as the ThT sigmoidal profile remained comparable to the control conditions. Under neutral pH conditions, IAPP efficiently forms amyloid fibrils in vitro, even at low micromolar concentrations [10]. In contrast, introducing 1 molar eq. of copper led to a clear delay in fibrillization, indicating that copper interacts with IAPP proteospecies and modulates the aggregation pathway (Figure 2A). This delay in fibril formation in the presence of copper is consistent with previous findings reported [23] and supports the modulatory effect of copper on IAPP aggregation. Most notably, the presence of both copper and ascorbic acid, which readily induces the oxidation of IAPP as confirmed by mass spectrometry (Figure 1), significantly prolonged the lag phase before fibril formation, extending it to approximately 12 h, three times longer than the 3-h lag phase observed for the non-oxidized counterpart (Figure 2C and Table S2). Moreover, the final ThT fluorescence was decreased significantly under oxidative conditions, suggesting a lower content of structures with a well-defined cross-β-sheet architecture (Figure 2C and Table S2).
3.3. Copper-Induced Oxidation Promotes the Formation of Amorphous Aggregates
To gain further insights on the effects of oxidation on amyloid formation observed with ThT kinetics, the resulting supramolecular organization was analyzed (Figure 3). To do so, IAPP was incubated under fully quiescent conditions (pH 7.4) in phosphate buffer for 72 h in presence, or not, of copper and/or ascorbic acid. Considering that the recurrent displacements of the microplate within the fluorimeter induce significant agitation (Figure 2), which promotes amyloid formation by mass transport and fibril fragmentation [40], self-assembly under fully quiescent conditions was evaluated at an IAPP concentration of 60 μM, compared to 25 μM for the ThT kinetics assay. ThT fluorescence spectra recorded for oxidized IAPP showed markedly reduced signal, suggesting a structural organization that is less compatible with strong dye binding compared to the non-oxidized species (Figure 3A). In parallel, ANS fluorescence spectra recorded for oxidized IAPP exhibit a reduced signal accompanied by a red shift, with a fluorescence maximum at 528 nm compared to approximately 470 nm for the non-oxidized peptide (Figure 3B). These results suggest that oxidized IAPP displays lower surface-accessible hydrophobicity and follows a different aggregation mechanism than the non-oxidized species [53].
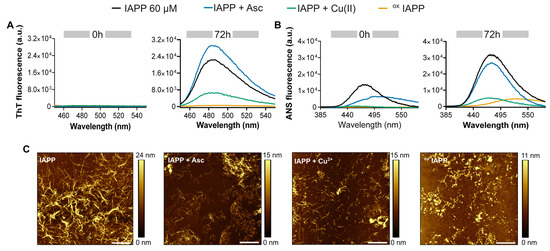
Figure 3.
Analysis of the effect of oxidation on IAPP assemblies. (A–C) IAPP was incubated at 60 µM at room temperature for 72 h in the presence of Cu(II) (50 µM) and/or ascorbic acid (500 µM). The mixture was analyzed by: (A) ThT fluorescence with excitation at 440 nm, (B) ANS fluorescence with excitation at 355 nm, and (C) AFM: Scale bar is 2 µm and each image is 10 μm × 10 μm.
Figure 3C presents the morphological characterization of the aggregates by atomic force microscopy (AFM). IAPP incubated in the presence of ascorbic acid assembled into long, well-defined fibrils that resemble the twisted and flat ribbon-like structures characteristic of amyloid quaternary assemblies [54]. Conversely, samples incubated with copper alone exhibited shorter fibrils, whereas oxidized IAPP predominantly formed amorphous, non-fibrillar aggregates. Similar morphologies were observed for IAPP incubated for shorter period, i.e., 24 h (Figure S2). These morphological differences align with the altered dye-binding properties observed by fluorescence spectroscopy. Unfortunately, circular dichroism spectroscopy, which is commonly employed to probe the secondary conformational shift in IAPP associated with its aggregation [55], could not be used because of the interference of the oxidative mixture with the CD signal at shorter wavelength.
3.4. Oxidized IAPP Aggregates Do Not Seed Amyloid Formation
Considering the divergence in the supramolecular organization of the aggregated structures obtained for oxidized IAPP, we evaluated their capacity to induce the aggregation of unmodified IAPP with a seeding experiment. Seeding, similar to a prion-like effect, has long attracted interest, as it represents a hallmark of pathological processes involved in protein misfolding disorders [56]. Amyloid seeds were prepared by incubating either oxidized, or non-oxidized IAPP, for 72 h at room temperature without agitation. Next, the aggregation kinetics of 25 µM IAPP, or oxidized IAPP, in presence or in absence of these preformed assemblies, were monitored by ThT fluorescence over 24 h. As anticipated, the addition of 10% preformed unmodified IAPP fibrils markedly accelerated IAPP amyloid self-assembly, with the quasi-disappearance of the lag-phase (Figure 4A,B). In contrast, seeding unmodified IAPP with pre-aggregated oxidized IAPP had no significant effect on the aggregation kinetics of IAPP, either under oxidation conditions or not (Figure 4A,B).
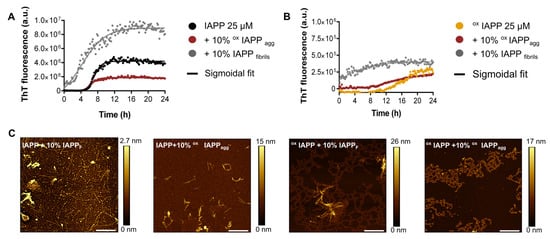
Figure 4.
Evaluation of the seeding capacity of oxidized IAPP aggregates. (A) Aggregation kinetics of 25 µM IAPP in the presence, or absence, of 10% preformed IAPP fibrils or oxidized IAPP aggregates. (B) Aggregation kinetics of 25 µM oxidized IAPP in the presence or absence of 10% preformed IAPP fibrils or oxidized IAPP aggregates. (A,B) Peptides were incubated at 25 µM in 50 mM phosphate buffer, pH 7.4, with or without seeds, and ThT fluorescence was measured using an excitation wavelength of 440 nm. Curves were fitted using a sigmoidal growth model. (C) AFM imaging after 72 h of incubation. Scale bar is 2 µm and each image is 10 μm × 10 μm.
The seeding effect of pre-assembled fibrils was further investigated by analyzing the final fibril morphology using AFM (Figure 4C). The AFM images of unmodified IAPP seeded with preformed IAPP fibrils revealed predominantly long, twisted, and homogeneous fibrils (Figure 3C). In contrast, seeding unmodified IAPP with preformed oxidized IAPP aggregates resulted in shorter fibrils with heterogeneous sizes. Moreover, the assembly of oxidized IAPP, i.e., incubated with Cu and ascorbic acid, in the presence of 10% IAPP fibrils or oxidized IAPP aggregates mainly led to the formation of amorphous aggregates. These observations suggest that under oxidative conditions, IAPP self-assembles into mainly amorphous aggregates, even in presence of well-defined amyloid seeds. A plausible hypothesis is that oxidative modifications induced by copper alter specific molecular interactions within IAPP, thereby delaying or disrupting the critical steps required for the ordered formation of amyloid self-assembly.
3.5. Substitution of IAPP His18 by Ala Alters the Oxidation Pattern
Previous studies have demonstrated that the histidine imidazole sidechain plays a critical role for copper coordination and subsequent oxidation [57,58]. Thus, the histidine 18 of IAPP was substituted by an alanine to assess the impact of a change in copper coordination (loosely bound copper) on IAPP oxidation using the same UPLC-MS/MS workflow applied to the wild-type (WT) peptide. Figure 5 presents the extracted ion chromatograms of chymotryptic peptides from the H18A derivative before and after 30 min copper-mediated oxidation. Representative MS/MS spectra are provided in the Supplementary Information (Figure S3). Compared to WT IAPP, chromatograms revealed an increased diversity of oxidized peptide signals and complexity for IAPP H18A, in agreement with a metal-catalyzed oxidation driven by a loosely bound copper. It is worth mentioning that the H18A substitution, in contrast to the S20G mutation that is associated with early-onset diabetes by increasing amyloidogenicity [59,60], is not a naturally occurring mutation. It is simply used in this study as a chemical tool to probe the contribution of His sidechain on copper-mediated oxidation.
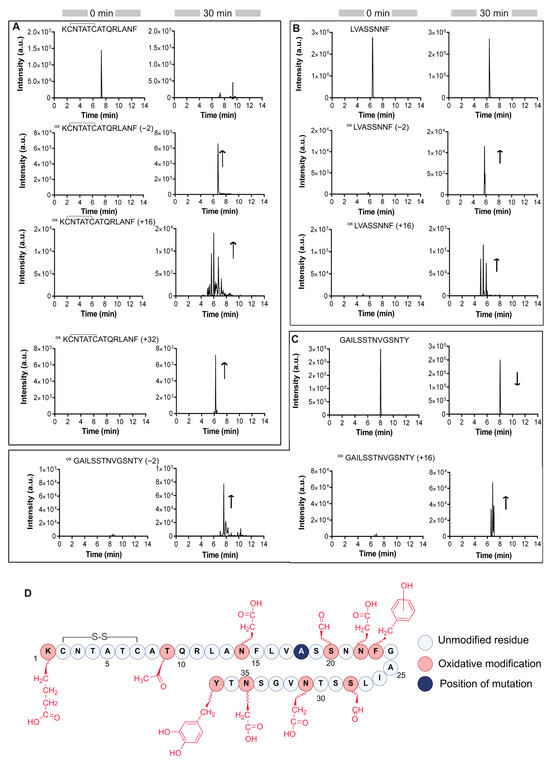
Figure 5.
Trace chromatograms of chymotryptic IAPP H18A before and after 30 min oxidation using Cu/O2/Asc/ system at room temperature. (A) 1KCNTATCATQRLANF15, (B) 16LVHSSNNF23 and (C) 24GAILSSTNVGSNTY37. The arrows specify the trend of chromatographic peaks. Mass accuracy and retention time assigned to each peak are presented in Table S3. (D) Summary of Cu-catalyzed oxidative modifications on [H18A]IAPP. The His-to-Ala substitution is highlighted in dark blue, and oxidative modification sites identified by MS/MS analysis are shown in red.
Analysis of the first chymotryptic peptide from [H18A]IAPP(1–15) revealed a noticeable increase in the number of the oxidation products compared to its WT counterpart. Specifically, the extracted ion chromatogram corresponding to a +16 Da mass shift, indicative of the addition of a single oxygen atom, displayed several distinct peaks with identical m/z values but different retention times (Table S3). This pattern indicates the presence of positional isomers arising from oxidation at multiple sites. A similar multiplicity of oxidized species was also observed for the [H18A]IAPP(16–23) and [H18A]IAPP(24–37) peptides. Figure 5 provides an overview of the oxidized residues identified by MS/MS analysis (see also Figure S3). In addition to oxidative modifications previously observed in certain residues of WT IAPP, MS/MS analysis of oxidized [H18A]IAPP revealed novel oxidative reactions, including the oxidation of lysine to aminoadipic acid, phenylalanine to tyrosine, and tyrosine to L-DOPA [61,62,63].
3.6. Oxidation of [H18A]IAPP Modulates Amyloid Formation
Next, the impact of the widespread oxidation of IAPP H18A on its ability to self-assemble was assessed using ThT fluorescence and AFM imaging. In comparison to WT IAPP, the H18A derivative displayed accelerated aggregation kinetics, with no detectable lag phase. Notably, the presence of copper did not alter the rate of aggregation, which remained comparable to that observed in its absence. This suggests that the absence of histidine at position 18 eliminates the primary copper-binding site, thereby changing the copper coordination sphere and its associated modulatory effects on IAPP aggregation [64]. Consequently, the aggregation kinetics of [H18A]IAPP remained unaffected by copper, in contrast to WT IAPP where Cu(II)–His18 interactions significantly alter the aggregation pathway.
Furthermore, oxidized [H18A]IAPP rapidly formed ThT-positive fibrils, as evidenced by AFM imaging. After 72 h of fully quiescent incubation, short fibrils were clearly visible for oxidized [H18A]IAPP (Figure 6), whereas WT IAPP predominantly formed amorphous aggregates under similar conditions (Figure 3). These observations suggest that substituting histidine with alanine at position 18 promotes fibril formation and bypasses copper-induced modulation of aggregation. The absence of this key metal-binding residue appears to promote organized fibrillization, despite the heterogeneous nature of oxidative modifications.
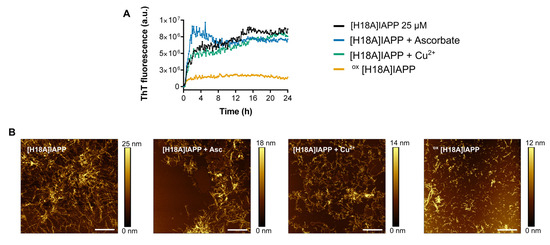
Figure 6.
(A) Self-assembly of [H18A]IAPP (25 µM) monitored by ThT fluorescence in the presence of copper (25 µM), and/or ascorbate (250 µM), in phosphate buffer (pH 7.4). (B) AFM images of [H18A]IAPP (60 µM) in the presence, or absence, of copper and ascorbate, after pre-incubation under quiescent conditions for 72 h prior to analysis. Scale bar is 2 µm and each image is 10 μm × 10 μm.
4. Conclusions
This study revealed that copper-catalyzed oxidation provides an alternative pathway that precludes the formation of well-ordered IAPP fibrils by promoting the accumulation of amorphous aggregates. These results shed light on the interrelation between oxidative modifications and amyloid self-assembly, highlighting how redox-active metals such as copper can modulate the aggregation outcome of amyloidogenic peptides. The identification of oxidized residues within the amyloidogenic core region further emphasizes the critical role of site-specific modifications in altering aggregation behavior. Moreover, the H18A substitution, which led to loosely bound copper, promoted distinct oxidation patterns and enabled fibril formation even under oxidative conditions. Together, these findings revealed that the presence or absence of a key copper-binding residue, such as histidine, can dramatically alter the conformational ensemble of IAPP under oxidative stress. An important open question, however, is whether the amorphous aggregates produced by copper-mediated oxidation are intrinsically toxic to pancreatic β-cells and thus contribute to the progression of type 2 diabetes, or whether this oxidative pathway instead represents a cellular defense mechanism designed to limit amyloid-driven toxicity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox14111269/s1; Table S1: Mass spectrometry data of miss-cleaved IAPP peptides; Figure S1: Annotated MS/MS spectra of native and oxidized IAPP; Table S2: Parameters from sigmoidal fits using Boltzmann equation for fitted curves of IAPP; Figure S2: AFM images obtained after pre-incubation under quiescent conditions for 24 h; Table S3: Mass spectrometry data of [H18A]IAPP peptides; Figure S3: Annotated MS/MS spectra of native and oxidized [H18A]IAPP.
Author Contributions
Conceptualization, O.A., F.C. and S.B.; methodology, O.A., L.P. and P.T.N.; validation, O.A., F.C. and S.B.; formal analysis, O.A.; investigation, O.A.; data curation, O.A., F.C. and S.B.; writing—original draft preparation, O.A., F.C. and S.B.; writing—review and editing, O.A., F.C. and S.B.; visualization, O.A., F.C. and S.B.; supervision, F.C. and S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC, RGPIN-2025-06834) to S.B. O.A. acknowledges funding by the NanoX Graduate School of Research (project ANR-17-EURE-0009). S.B. holds the Canada Research Chair in Chemistry of Biological Nanoassemblies.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article or Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors acknowledge Leanne Ohlund for her technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, Regional, and National Burden of Diabetes from 1990 to 2021, with Projections of Prevalence to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234, Erratum in Lancet 2025, 405, 202. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Abad-Jiménez, Z.; Martínez de Marañón, A.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef]
- Clark, A.; Saad, M.F.; Nezzer, T.; Uren, C.; Knowler, W.C.; Bennett, P.H.; Turner, R.C. Islet Amyloid Polypeptide in Diabetic and Non-Diabetic Pima Indians. Diabetologia 1990, 33, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Cao, P.; Noor, H.; Ridgway, Z.; Tu, L.-H.; Wang, H.; Wong, A.G.; Zhang, X.; Abedini, A.; Schmidt, A.M.; et al. Islet Amyloid Polypeptide: Structure, Function, and Pathophysiology. J. Diabetes Res. 2016, 2016, 2798269. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; White, K.; Terry, C. Linking hIAPP Misfolding and Aggregation with Type 2 Diabetes Mellitus: A Structural Perspective. Biosci. Rep. 2022, 42, BSR20211297. [Google Scholar] [CrossRef]
- Gurlo, T.; Ryazantsev, S.; Huang, C.; Yeh, M.W.; Reber, H.A.; Hines, O.J.; O’Brien, T.D.; Glabe, C.G.; Butler, P.C. Evidence for Proteotoxicity in β Cells in Type 2 Diabetes: Toxic Islet Amyloid Polypeptide Oligomers Form Intracellularly in the Secretory Pathway. Am. J. Pathol. 2010, 176, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Raleigh, D.; Zhang, X.; Hastoy, B.; Clark, A. The β-Cell Assassin: IAPP Cytotoxicity. J. Mol. Endocrinol. 2017, 59, R121–R140. [Google Scholar] [CrossRef]
- Bishoyi, A.K.; Roham, P.H.; Rachineni, K.; Save, S.; Hazari, M.A.; Sharma, S.; Kumar, A. Human Islet Amyloid Polypeptide (hIAPP)—A Curse in Type II Diabetes Mellitus: Insights from Structure and Toxicity Studies. Biol. Chem. 2021, 402, 133–153. [Google Scholar] [CrossRef]
- Paulsson, J.F.; Westermark, G.T. Aberrant Processing of Human Proislet Amyloid Polypeptide Results in Increased Amyloid Formation. Diabetes 2005, 54, 2117–2125. [Google Scholar] [CrossRef]
- Khemtemourian, L.; Antoniciello, F.; Sahoo, B.R.; Decossas, M.; Lecomte, S.; Ramamoorthy, A. Investigation of the Effects of Two Major Secretory Granules Components, Insulin and Zinc, on Human-IAPP Amyloid Aggregation and Membrane Damage. Chem. Phys. Lipids 2021, 237, 105083. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes Mellitus and Oxidative Stress—A Concise Review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef]
- Eguchi, N.; Vaziri, N.D.; Dafoe, D.C.; Ichii, H. The Role of Oxidative Stress in Pancreatic β Cell Dysfunction in Diabetes. Int. J. Mol. Sci. 2021, 22, 1509. [Google Scholar] [CrossRef]
- Renaudin, X. Chapter Three—Reactive Oxygen Species and DNA Damage Response in Cancer. In International Review of Cell and Molecular Biology; Weyemi, U., Galluzzi, L., Eds.; Chromatin and Genomic Instability in Cancer; Academic Press: Cambridge, MA, USA, 2021; Volume 364, pp. 139–161. [Google Scholar]
- Ma, Z.A. The Role of Peroxidation of Mitochondrial Membrane Phospholipids in Pancreatic β-Cell Failure. Curr. Diabetes Rev. 2012, 8, 69–75. [Google Scholar] [CrossRef]
- Sohal, R.S. Role of Oxidative Stress and Protein Oxidation in the Aging Process. Free Radic. Biol. Med. 2002, 33, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Babych, M.; Nguyen, P.T.; Côté-Cyr, M.; Kihal, N.; Quittot, N.; Golizeh, M.; Sleno, L.; Bourgault, S. Site-Specific Alkylation of the Islet Amyloid Polypeptide Accelerates Self-Assembly and Potentiates Perturbation of Lipid Membranes. Biochemistry 2021, 60, 2285–2299. [Google Scholar] [CrossRef]
- Milordini, G.; Zacco, E.; Percival, M.; Puglisi, R.; Dal Piaz, F.; Temussi, P.; Pastore, A. The Role of Glycation on the Aggregation Properties of IAPP. Front. Mol. Biosci. 2020, 7, 104. [Google Scholar] [CrossRef]
- Coughlan, M.T.; Thorburn, D.R.; Penfold, S.A.; Laskowski, A.; Harcourt, B.E.; Sourris, K.C.; Tan, A.L.Y.; Fukami, K.; Thallas-Bonke, V.; Nawroth, P.P.; et al. RAGE-Induced Cytosolic ROS Promote Mitochondrial Superoxide Generation in Diabetes. J. Am. Soc. Nephrol. 2009, 20, 742. [Google Scholar] [CrossRef] [PubMed]
- Abedini, A.; Cao, P.; Plesner, A.; Zhang, J.; He, M.; Derk, J.; Patil, S.A.; Rosario, R.; Lonier, J.; Song, F.; et al. RAGE Binds Preamyloid IAPP Intermediates and Mediates Pancreatic β Cell Proteotoxicity. J. Clin. Investig. 2018, 128, 682–698. [Google Scholar] [CrossRef] [PubMed]
- McCalpin, S.D.; Khemtemourian, L.; Suladze, S.; Ivanova, M.I.; Reif, B.; Ramamoorthy, A. Zinc and pH Modulate the Ability of Insulin to Inhibit Aggregation of Islet Amyloid Polypeptide. Commun. Biol. 2024, 7, 776. [Google Scholar] [CrossRef]
- Tomasello, M.F.; Sinopoli, A.; Pappalardo, G. On the Environmental Factors Affecting the Structural and Cytotoxic Properties of IAPP Peptides. J. Diabetes Res. 2015, 2015, 918573. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Meng, F.; Lu, T.; Wang, C.; Li, F. Regulation of Divalent Metal Ions to the Aggregation and Membrane Damage of Human Islet Amyloid Polypeptide Oligomers. RSC Adv. 2021, 11, 12815–12825. [Google Scholar] [CrossRef]
- Poulson, B.G.; Szczepski, K.; Lachowicz, J.I.; Jaremko, L.; Emwas, A.H.; Jaremko, M. Aggregation of Biologically Important Peptides and Proteins: Inhibition or Acceleration Depending on Protein and Metal Ion Concentrations. RSC Adv. 2020, 10, 215–227. [Google Scholar] [CrossRef]
- Lowe, J.; Taveira-da-Silva, R.; Hilário-Souza, E. Dissecting Copper Homeostasis in Diabetes Mellitus. IUBMB Life 2017, 69, 255–262. [Google Scholar] [CrossRef]
- Tanaka, A.; Kaneto, H.; Miyatsuka, T.; Yamamoto, K.; Yoshiuchi, K.; Yamasaki, Y.; Shimomura, I.; Matsuoka, T.; Matsuhisa, M. Role of Copper Ion in the Pathogenesis of Type 2 Diabetes. Endocr. J. 2009, 56, 699–706. [Google Scholar] [CrossRef]
- Qiu, Q.; Zhang, F.; Zhu, W.; Wu, J.; Liang, M. Copper in Diabetes Mellitus: A Meta-Analysis and Systematic Review of Plasma and Serum Studies. Biol. Trace Elem. Res. 2017, 177, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; Chen, Y.; Xu, Y.; Jiang, X.; Liu, B.; Wang, Y. Associations between Whole Blood Trace Elements Concentrations and HbA1c Levels in Patients with Type 2 Diabetes. Biometals 2022, 35, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.N.; Xing, G.; Miller, C.J.; Waite, T.D. Fenton-like Copper Redox Chemistry Revisited: Hydrogen Peroxide and Superoxide Mediation of Copper-Catalyzed Oxidant Production. J. Catal. 2013, 301, 54–64. [Google Scholar] [CrossRef]
- Cheignon, C.; Faller, P.; Testemale, D.; Hureau, C.; Collin, F. Metal-Catalyzed Oxidation of Aβ and the Resulting Reorganization of Cu Binding Sites Promote ROS Production. Metallomics 2016, 8, 1081–1089. [Google Scholar] [CrossRef]
- Faller, P.; Hureau, C.; La Penna, G. Metal Ions and Intrinsically Disordered Proteins and Peptides: From Cu/Zn Amyloid-β to General Principles. Acc. Chem. Res. 2014, 47, 2252–2259. [Google Scholar] [CrossRef]
- Ahmadi, S.; Zhu, S.; Sharma, R.; Wilson, D.J.; Kraatz, H.-B. Interaction of Metal Ions with Tau Protein. The Case for a Metal-Mediated Tau Aggregation. J. Inorg. Biochem. 2019, 194, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, C.; Cortés-Mejía, R.; Miotto, M.C.; Binolfi, A.; Fernández, C.O.; del Campo, J.M.; Quintanar, L. Copper Coordination Features of Human Islet Amyloid Polypeptide: The Type 2 Diabetes Peptide. Inorg. Chem. 2016, 55, 10727–10740. [Google Scholar] [CrossRef] [PubMed]
- Rivillas-Acevedo, L.; Sánchez-López, C.; Amero, C.; Quintanar, L. Structural Basis for the Inhibition of Truncated Islet Amyloid Polypeptide Aggregation by Cu(II): Insights into the Bioinorganic Chemistry of Type II Diabetes. Inorg. Chem. 2015, 54, 3788–3796. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, X.; Wang, Y.; Zheng, W.; Chen, T. Cu(II) Inhibits hIAPP Fibrillation and Promotes hIAPP-Induced Beta Cell Apoptosis through Induction of ROS-Mediated Mitochondrial Dysfunction. J. Inorg. Biochem. 2014, 140, 143–152. [Google Scholar] [CrossRef]
- Westermark, P.; Engström, U.; Johnson, K.H.; Westermark, G.T.; Betsholtz, C. Islet Amyloid Polypeptide: Pinpointing Amino Acid Residues Linked to Amyloid Fibril Formation. Proc. Natl. Acad. Sci. USA 1990, 87, 5036–5040. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Zottig, X.; Sebastiao, M.; Arnold, A.A.; Marcotte, I.; Bourgault, S. Identification of Transmissible Proteotoxic Oligomer-like Fibrils That Expand Conformational Diversity of Amyloid Assemblies. Commun. Biol. 2021, 4, 939. [Google Scholar] [CrossRef]
- Cheignon, C.; Hureau, C.; Collin, F. Real-Time Evolution of Aβ40 Metal-Catalyzed Oxidation Reveals Asp1 as the Main Target and a Dependence on Metal Binding Site. Inorganica Chim. Acta 2018, 472, 111–118. [Google Scholar] [CrossRef]
- Seal, M.; Dey, S.G. Active-Site Environment of Copper-Bound Human Amylin Relevant to Type 2 Diabetes. Inorg. Chem. 2018, 57, 129–138. [Google Scholar] [CrossRef]
- Sebastiao, M.; Quittot, N.; Bourgault, S. Thioflavin T Fluorescence to Analyse Amyloid Formation Kinetics: Measurement Frequency as a Factor Explaining Irreproducibility. Anal. Biochem. 2017, 532, 83–86. [Google Scholar] [CrossRef]
- Shoffner, S.K.; Schnell, S. Estimation of the Lag Time in a Subsequent Monomer Addition Model for Fibril Elongation. Phys. Chem. Chem. Phys. 2016, 18, 21259–21268. [Google Scholar] [CrossRef]
- Kihal, N.; Archambault, M.J.; Babych, M.; Nazemi, A.; Bourgault, S. Probing the Molecular Determinants of the Activation of Toll-like Receptor 2/6 by Amyloid Nanostructures through Directed Peptide Self-Assembly. Soft Matter 2024, 20, 7821–7831. [Google Scholar] [CrossRef]
- Opazo, C.; Barría, M.I.; Ruiz, F.H.; Inestrosa, N.C. Copper Reduction by Copper Binding Proteins and Its Relation to Neurodegenerative Diseases. Biometals 2003, 16, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, M.; Robinson, W.E. Bioactive Dehydrotyrosyl and Dehydrodopyl Compounds of Marine Origin. Mar. Drugs 2010, 8, 2906–2935. [Google Scholar] [CrossRef] [PubMed]
- Appel, M.J.; Bertozzi, C.R. Formylglycine, a Post-Translationally Generated Residue with Unique Catalytic Capabilities and Biotechnology Applications. ACS Chem. Biol. 2015, 10, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Schöneich, C. Mechanisms of Metal-Catalyzed Oxidation of Histidine to 2-Oxo-Histidine in Peptides and Proteins. J. Pharm. Biomed. Anal. 2000, 21, 1093–1097. [Google Scholar] [CrossRef]
- Irudayanathan, F.J.; Zarzar, J.; Lin, J.; Izadi, S. Deciphering Deamidation and Isomerization in Therapeutic Proteins: Effect of Neighboring Residue. MAbs 2022, 14, 2143006. [Google Scholar] [CrossRef]
- Hao, P.; Adav, S.S.; Gallart-Palau, X.; Sze, S.K. Recent Advances in Mass Spectrometric Analysis of Protein Deamidation. Mass Spectrom. Rev. 2017, 36, 677–692. [Google Scholar] [CrossRef]
- Cheignon, C.; Collin, F.; Sabater, L.; Hureau, C. Oxidative Damages on the Alzheimer’s Related-Aβ Peptide Alters Its Ability to Assemble. Antioxidants 2023, 12, 472. [Google Scholar] [CrossRef]
- Hou, L.; Kang, I.; Marchant, R.E.; Zagorski, M.G. Methionine 35 Oxidation Reduces Fibril Assembly of the Amyloid Aβ-(1–42) Peptide of Alzheimer’s Disease. J. Biol. Chem. 2002, 277, 40173–40176. [Google Scholar] [CrossRef]
- Glaser, C.B.; Yamin, G.; Uversky, V.N.; Fink, A.L. Methionine Oxidation, α-Synuclein and Parkinson’s Disease. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2005, 1703, 157–169. [Google Scholar] [CrossRef]
- Khurana, R.; Coleman, C.; Ionescu-Zanetti, C.; Carter, S.A.; Krishna, V.; Grover, R.K.; Roy, R.; Singh, S. Mechanism of Thioflavin T Binding to Amyloid Fibrils. J. Struct. Biol. 2005, 151, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Kanti Mal, D.; Jonnalgadda, P.N.; Chakraborty, G. Aggregation Assisted Turn-on Response of ANS Dye towards Protamine. New J. Chem. 2023, 47, 2107–2116. [Google Scholar] [CrossRef]
- Luca, S.; Yau, W.-M.; Leapman, R.; Tycko, R. Peptide Conformation and Supramolecular Organization in Amylin Fibrils: Constraints from Solid-State NMR. Biochemistry 2007, 46, 13505–13522. [Google Scholar] [CrossRef]
- Identification of a Hinge Residue Controlling Islet Amyloid Polypeptide Self-Assembly and Cytotoxicity—Journal of Biological Chemistry. Available online: https://www.jbc.org/article/S0021-9258 (accessed on 12 October 2025).
- Ren, B.; Zhang, Y.; Zhang, M.; Liu, Y.; Zhang, D.; Gong, X.; Feng, Z.; Tang, J.; Chang, Y.; Zheng, J. Fundamentals of Cross-Seeding of Amyloid Proteins: An Introduction. J. Mater. Chem. B 2019, 7, 7267–7282. [Google Scholar] [CrossRef]
- Magrì, A.; Pietropaolo, A.; Tabbì, G.; La Mendola, D.; Rizzarelli, E. From Peptide Fragments to Whole Protein: Copper(II) Load and Coordination Features of IAPP. Chem. Eur. J. 2017, 23, 17898–17902. [Google Scholar] [CrossRef]
- Magrì, A.; La Mendola, D.; Nicoletti, V.G.; Pappalardo, G.; Rizzarelli, E. New Insight in Copper-Ion Binding to Human Islet Amyloid: The Contribution of Metal-Complex Speciation To Reveal the Polypeptide Toxicity. Chem. Eur. J. 2016, 22, 13287–13300. [Google Scholar] [CrossRef]
- Missense Mutation of Amylin Gene (S20G) in Japanese NIDDM Patients | Diabetes | American Diabetes Association. Available online: https://diabetesjournals.org/diabetes/article/45/9/1279/9564/Missense-Mutation-of-Amylin-Gene-S20G-in-Japanese (accessed on 12 October 2025).
- S20G Mutant Amylin Exhibits Increased in Vitro Amyloidogenicity and Increased Intracellular Cytotoxicity Compared to Wild-Type Amylin—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0002944010648481 (accessed on 12 October 2025).
- Fan, X.; Zhang, J.; Theves, M.; Strauch, C.; Nemet, I.; Liu, X.; Qian, J.; Giblin, F.J.; Monnier, V.M. Mechanism of Lysine Oxidation in Human Lens Crystallins during Aging and in Diabetes. J. Biol. Chem. 2009, 284, 34618–34627. [Google Scholar] [CrossRef]
- Woolf, L.I.; Jakubovič, A.; Chan-Henry, E. The Non-Enzymic Hydroxylation of Phenylalanine to Tyrosine by 2-Amino-4-Hydroxy-6,7-Dimethyl-5,6,7,8-Tetrahydropteridine. Biochem. J. 1971, 125, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, P.; Bergès, J.; Houée-Lévin, C. Toward Understanding the Protein Oxidation Processes: •OH Addition on Tyrosine, Phenylalanine, or Methionine? Int. J. Quantum Chem. 2011, 111, 1143–1151. [Google Scholar] [CrossRef]
- Roy, D.; Maity, N.C.; Kumar, S.; Maity, A.; Ratha, B.N.; Biswas, R.; Maiti, N.C.; Mandal, A.K.; Bhunia, A. Modulatory Role of Copper on hIAPP Aggregation and Toxicity in Presence of Insulin. Int. J. Biol. Macromol. 2023, 241, 124470. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).

