Roles of Autophagy and Oxidative Stress in Cardiovascular Disease
Abstract
1. Introduction
2. Mechanisms of Autophagy and Oxidative Stress
2.1. Autophagy Pathway and Selectivity
2.2. Core ATG Machinery and Regulatory Networks
2.3. Molecular Biology of Oxidative Stress
3. Relationship Between Autophagy and Oxidative Stress in Cardiovascular Disease
3.1. Atherosclerosis and Vascular Aging
3.2. Ischemia–Reperfusion (I/R) Injury
3.3. Hypertrophic Cardiomyopathy (HCM) and Heart Failure (HF)
3.4. Diabetic Cardiomyopathy (DCM) and Metabolic Syndrome
3.5. Arrhythmia and Electrophysiology
3.6. Cardiac Aging
3.7. Inflammation, Innate Immunity, and Noninfectious Injury
3.8. Fibroblast Activation and Matrix Remodeling
3.9. Therapeutic Modulation and Clinical Translation
4. Future Research Directions
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| •OH | Hydroxyl radical |
| 1O2 | Singlet oxygen |
| ABCA1 | ATP-binding cassette transporter A1 |
| ABCG1 | ATP-binding cassette transporter G1 |
| AGEs | Advanced glycation end products |
| AMBRA1 | Autophagy and beclin-1-regulated autophagy protein 1 |
| AMPK | AMP-activated protein kinase |
| APD | Action potential duration |
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| ATGs | Autophagy-related genes |
| ATP | Adenosine triphosphate |
| BAG3 | BCL2-associated athanogene 3 |
| BCL-2 | B cell lymphoma 2 |
| BNIP3 | BCL2/adenovirus E1B 19 kDa-interacting protein 3 |
| CaMKII | Ca2+/calmodulin-dependent protein kinase II |
| CASA | Chaperone-assisted selective autophagy |
| CMA | Chaperone-mediated autophagy |
| DAMPs | Damage-associated molecular patterns |
| DFCP1 | Double FYVE domain-containing protein 1 |
| DNMT3A | DNA (cytosine-5)-methyltransferase 3A |
| DRP1 | Dynamin-related protein 1 |
| EADs | Early afterdepolarizations |
| eNOS | Endothelial NO synthase |
| ER | Endoplasmic reticulum |
| ESCRT | Endosomal sorting complexes required for transport |
| FIP200 | Focal adhesion kinase family-interacting protein of 200 kDa |
| FUNDC1 | FUN14 domain-containing 1 |
| GABARAP | Gamma-aminobutyric acid receptor-associated protein |
| GPx | Glutathione peroxidase |
| GR | Glutathione reductase |
| GSH | Glutathione |
| GSSG | Glutathione disulfide |
| HOO• | Hydroperoxyl radical |
| HOPS | Homotypic fusion and vacuole protein sorting |
| I_Na,L | Late Na+ current |
| I/R | Ischemia–reperfusion |
| IDH | Isocitrate dehydrogenase |
| IRS | Insulin receptor substrate |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| KLF | Krüppel-like factor |
| LANDO | LC3-associated endocytosis |
| LAMPf2 | Lysosome-associated membrane protein 2 |
| LAP | LC3-associated phagocytosis |
| LC3 | Microtubule-associated protein light chain 3 |
| MCU | Mitochondrial calcium uniporter |
| MDH | Malate dehydrogenase |
| mETC | Mitochondrial electron transport chain |
| MFN | Mitofusin |
| MPT | Mitochondrial permeability transition |
| mPTP | Mitochondrial permeability transition pore |
| mtDNA | Mitochondrial DNA |
| mTORC1 | Mechanistic target of rapamycin complex 1 |
| mtROS | Mitochondrial ROS |
| N2O3 | Dinitrogen trioxide |
| NBR1 | Neighbor of BRCA1 gene 1 |
| NDP52 | Nuclear dot protein 52 kDa |
| NETosis | Neutrophil extracellular trap release |
| NIX | BCL2/adenovirus E1B 19 kDa protein-interacting protein 3-like |
| NLRP3 | NLR family pyrin domain-containing 3 |
| NNT | Nicotinamide nucleotide transhydrogenase |
| NO | Nitric oxide |
| NOX | NADPH oxidase |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| O2•− | Superoxide anions |
| ONOO− | Peroxynitrite |
| OPA1 | Optic atrophy 1 |
| OPTN | Optineurin |
| p62 | Sequestosome 1 |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PINK1 | PTEN-induced kinase 1 |
| PKC | Protein kinase C |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| Prx | Peroxiredoxin |
| RAPTOR | Regulatory-associated protein of mTOR |
| RET | Reverse electron transport |
| ROS | Reactive oxygen species |
| Rubicon | RUN domain- and cysteine-rich domain-containing beclin-1-interacting protein |
| RyR2 | Ryanodine receptor 2 |
| SERCA2a | Sarcoplasmic reticulum Ca2+ATPase 2a |
| SGLT2 | Sodium–glucose cotransporter-2 |
| SIRT | Sirtuin |
| SNARE | Soluble N-ethylmaleimide-sensitive factor attachment protein receptor |
| SODs | Superoxide dismutases |
| SOD1 | Cu/ZnSOD |
| SOD2 | MnSOD |
| SOX9 | SRY-box transcription factor 9 |
| STX17 | Syntaxin 17 |
| TET2 | Tet methylcytosine dioxygenase 2 |
| TFEB | Transcription factor EB |
| TGF-β | Transforming growth factor beta |
| TLRs | Toll-like receptors |
| TRPML1 | Transient receptor potential mucolipin 1 |
| Trx2 | Thioredoxin 2 |
| TrxR | Thioredoxin reductase |
| ULK1 | Uncoordinated-51-like kinase 1 |
| UVRAG | UV radiation resistance-associated gene |
| VAMP8 | Vesicle-associated membrane protein 8 |
| WIPI2 | WD repeat domain phosphoinositide-interacting protein 2 |
| ZDHHC13 | Zinc finger DHHC-type palmitoyltransferase 13 |
| ΔΨm | Mitochondrial membrane potential |
References
- De Duve, C. The lysosome. Sci. Am. 1963, 208, 64–72. [Google Scholar] [CrossRef]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cecconi, F.; Choi, A.M.; Chu, C.T.; Codogno, P.; Colombo, M.I.; et al. Molecular definitions of autophagy and related processes. EMBO J. 2017, 36, 1811–1836. [Google Scholar] [CrossRef] [PubMed]
- Ohsumi, Y. Historical landmarks of autophagy research. Cell Res. 2014, 24, 9–23. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)(1). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef]
- Gomez-Virgilio, L.; Silva-Lucero, M.D.; Flores-Morelos, D.S.; Gallardo-Nieto, J.; Lopez-Toledo, G.; Abarca-Fernandez, A.M.; Zacapala-Gomez, A.E.; Luna-Munoz, J.; Montiel-Sosa, F.; Soto-Rojas, L.O.; et al. Autophagy: A Key Regulator of Homeostasis and Disease: An Overview of Molecular Mechanisms and Modulators. Cells 2022, 11, 2262. [Google Scholar] [CrossRef]
- Levine, B.; Klionsky, D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell 2004, 6, 463–477. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Hickey, K.L.; Swarup, S.; Smith, I.R.; Paoli, J.C.; Miguel Whelan, E.; Paulo, J.A.; Harper, J.W. Proteome census upon nutrient stress reveals Golgiphagy membrane receptors. Nature 2023, 623, 167–174. [Google Scholar] [CrossRef]
- Boya, P.; Codogno, P.; Rodriguez-Muela, N. Autophagy in stem cells: Repair, remodelling and metabolic reprogramming. Development 2018, 145, 146506. [Google Scholar] [CrossRef]
- Germain, K.; So, R.W.L.; DiGiovanni, L.F.; Watts, J.C.; Bandsma, R.H.J.; Kim, P.K. Upregulated pexophagy limits the capacity of selective autophagy. Nat. Commun. 2024, 15, 375. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, H.; Tang, B.; Luo, Y.; Yang, Y.; Zhong, X.; Chen, S.; Xu, X.; Huang, S.; Liu, C. Macrophages in cardiovascular diseases: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Kapuy, O. Mechanism of Decision Making between Autophagy and Apoptosis Induction upon Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2024, 25, 4368. [Google Scholar] [CrossRef] [PubMed]
- Gouda, N.A.; Zhakupova, A.; Abdelaal, A.M.; Ahmad, F.; Elkamhawy, A. The interplay involving oxidative stress and autophagy: Mechanisms, implications, and therapeutic opportunities. Exp. Mol. Pathol. 2025, 143, 104989. [Google Scholar] [CrossRef]
- Yang, L.; Guo, C.; Zheng, Z.; Dong, Y.; Xie, Q.; Lv, Z.; Li, M.; Lu, Y.; Guo, X.; Deng, R.; et al. Stress dynamically modulates neuronal autophagy to gate depression onset. Nature 2025, 641, 427–437. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Interactions between reactive oxygen species and autophagy: Special issue: Death mechanisms in cellular homeostasis. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119041. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef]
- Chen, C.L.; Lin, Y.C. Autophagy Dysregulation in Metabolic Associated Fatty Liver Disease: A New Therapeutic Target. Int. J. Mol. Sci. 2022, 23, 10055. [Google Scholar] [CrossRef]
- Deretic, V. Autophagy in inflammation, infection, and immunometabolism. Immunity 2021, 54, 437–453. [Google Scholar] [CrossRef]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Cell Biol. 2023, 24, 560–575. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.; Guo, F.; Zhang, Y.; Peng, H.; Zhang, H.; Liu, Z.; Cao, C.; Xin, G.; Chen, Y.Y.; et al. Mitophagy in ischemic heart disease: Molecular mechanisms and clinical management. Cell Death Dis. 2024, 15, 934. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Aslkhodapasandhokmabad, H.; Henninger, N.; Demillard, L.J.; Nikanfar, M.; Nourazarian, A.; Ren, J. Targeting autophagy in neurodegenerative diseases: From molecular mechanisms to clinical therapeutics. Clin. Exp. Pharmacol. Physiol. 2021, 48, 943–953. [Google Scholar] [CrossRef]
- Palmer, J.E.; Wilson, N.; Son, S.M.; Obrocki, P.; Wrobel, L.; Rob, M.; Takla, M.; Korolchuk, V.I.; Rubinsztein, D.C. Autophagy, aging, and age-related neurodegeneration. Neuron 2025, 113, 29–48. [Google Scholar] [CrossRef]
- Morris, G.; Gevezova, M.; Sarafian, V.; Maes, M. Redox regulation of the immune response. Cell Mol. Immunol. 2022, 19, 1079–1101. [Google Scholar] [CrossRef]
- Kaushik, S.; Tasset, I.; Arias, E.; Pampliega, O.; Wong, E.; Martinez-Vicente, M.; Cuervo, A.M. Autophagy and the hallmarks of aging. Ageing Res. Rev. 2021, 72, 101468. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Casey, A.M.; Ryan, D.G.; Prag, H.A.; Chowdhury, S.R.; Marques, E.; Turner, K.; Gruszczyk, A.V.; Yang, M.; Wolf, D.M.; Miljkovic, J.L.; et al. Pro-inflammatory macrophages produce mitochondria-derived superoxide by reverse electron transport at complex I that regulates IL-1beta release during NLRP3 inflammasome activation. Nat. Metab. 2025, 7, 493–507. [Google Scholar] [CrossRef]
- Rimal, S.; Tantray, I.; Li, Y.; Pal Khaket, T.; Li, Y.; Bhurtel, S.; Li, W.; Zeng, C.; Lu, B. Reverse electron transfer is activated during aging and contributes to aging and age-related disease. EMBO Rep. 2023, 24, e55548. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Halliwell, B.; Adhikary, A.; Dingfelder, M.; Dizdaroglu, M. Hydroxyl radical is a significant player in oxidative DNA damage in vivo. Chem. Soc. Rev. 2021, 50, 8355–8360. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- Jose, E.; March-Steinman, W.; Wilson, B.A.; Shanks, L.; Parkinson, C.; Alvarado-Cruz, I.; Sweasy, J.B.; Paek, A.L. Temporal coordination of the transcription factor response to H2O2 stress. Nat. Commun. 2024, 15, 3440. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Tsuzuki, T.; Hidaka, M.; Ohno, M.; Nakatsu, Y.; Sekiguchi, M. Endogenous ROS production in early differentiation state suppresses endoderm differentiation via transient FOXC1 expression. Cell Death Discov. 2022, 8, 150. [Google Scholar] [CrossRef]
- An, X.; Yu, W.; Liu, J.; Tang, D.; Yang, L.; Chen, X. Oxidative cell death in cancer: Mechanisms and therapeutic opportunities. Cell Death Dis. 2024, 15, 556. [Google Scholar] [CrossRef]
- Agostini, F.; Bisaglia, M.; Plotegher, N. Linking ROS Levels to Autophagy: The Key Role of AMPK. Antioxidants 2023, 12, 1406. [Google Scholar] [CrossRef]
- Pathak, T.; Benson, J.C.; Tang, P.W.; Trebak, M.; Hempel, N. Crosstalk between calcium and reactive oxygen species signaling in cancer revisited. Cell Calcium 2025, 127, 103014. [Google Scholar] [CrossRef]
- Sies, H.; Mailloux, R.J.; Jakob, U. Fundamentals of redox regulation in biology. Nat. Rev. Mol. Cell Biol. 2024, 25, 701–719. [Google Scholar] [CrossRef]
- Jackson, B.T.; Finley, L.W.S. Metabolic regulation of the hallmarks of stem cell biology. Cell Stem Cell 2024, 31, 161–180. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.Y.; Liu, Y.J.; Feng, J.; Wu, Y.; Shen, H.M.; Lu, G.D. Full-coverage regulations of autophagy by ROS: From induction to maturation. Autophagy 2022, 18, 1240–1255. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Wen, W.; Shi, Z.; Gu, Q.; Ahammed, G.J.; Cao, K.; Shah Jahan, M.; Shu, S.; Wang, J.; et al. Hydrogen peroxide mediates spermidine-induced autophagy to alleviate salt stress in cucumber. Autophagy 2021, 17, 2876–2890. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Kuruvilla, J.; Tan, E.K. Mitophagy and reactive oxygen species interplay in Parkinson’s disease. NPJ Parkinsons Dis. 2022, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, Y.; Tsuji, D.; Itoh, K. Oxidative Stress Impairs Autophagy via Inhibition of Lysosomal Transport of VAMP8. Biol. Pharm. Bull. 2022, 45, 1609–1615. [Google Scholar] [CrossRef]
- Li, B.; Ming, H.; Qin, S.; Nice, E.C.; Dong, J.; Du, Z.; Huang, C. Redox regulation: Mechanisms, biology and therapeutic targets in diseases. Signal Transduct. Target. Ther. 2025, 10, 72. [Google Scholar] [CrossRef]
- Botanska, B.; Dovinova, I.; Barancik, M. The Interplay between Autophagy and Redox Signaling in Cardiovascular Diseases. Cells 2022, 11, 1203. [Google Scholar] [CrossRef]
- Wang, S.; Long, H.; Hou, L.; Feng, B.; Ma, Z.; Wu, Y.; Zeng, Y.; Cai, J.; Zhang, D.W.; Zhao, G. The mitophagy pathway and its implications in human diseases. Signal Transduct. Target. Ther. 2023, 8, 304. [Google Scholar] [CrossRef]
- Jeong, S.J.; Oh, G.T. Unbalanced Redox With Autophagy in Cardiovascular Disease. J. Lipid Atheroscler. 2023, 12, 132–151. [Google Scholar] [CrossRef]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct. Target. Ther. 2023, 8, 375. [Google Scholar] [CrossRef]
- Barnaba, C.; Broadbent, D.G.; Kaminsky, E.G.; Perez, G.I.; Schmidt, J.C. AMPK regulates phagophore-to-autophagosome maturation. J. Cell Biol. 2024, 223, 202309145. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, H.; Xu, X.; Chen, H.; Qi, B. Association between circulating cell-free mitochondrial DNA and inflammation factors in noninfectious diseases: A systematic review. PLoS ONE 2024, 19, e0289338. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Zheng, L.; Zhou, J.; Wang, P.; Wang, L.; Zhang, Y.; Man, Z.S.; Chen, Y.H.; Gu, F.; Niu, G.P. Evaluation of plasma LC3B+ extracellular vesicles as a potential novel diagnostic marker for hepatocellular carcinoma. Int. Immunopharmacol. 2022, 108, 108760. [Google Scholar] [CrossRef] [PubMed]
- Dergilev, K.; Gureenkov, A.; Parfyonova, Y. Autophagy as a Guardian of Vascular Niche Homeostasis. Int. J. Mol. Sci. 2024, 25, 10097. [Google Scholar] [CrossRef] [PubMed]
- Wai, K.W.; Low, L.E.; Goh, B.H.; Yap, W.H. Nrf2 Connects Cellular Autophagy and Vascular Senescence in Atherosclerosis: A Mini-Review. J. Lipid Atheroscler. 2024, 13, 292–305. [Google Scholar] [CrossRef]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Petroni, G.; Amaravadi, R.K.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cadwell, K.; Cecconi, F.; Choi, A.M.K.; et al. Autophagy in major human diseases. EMBO J. 2021, 40, e108863. [Google Scholar] [CrossRef]
- Adriaenssens, E.; Ferrari, L.; Martens, S. Orchestration of selective autophagy by cargo receptors. Curr. Biol. 2022, 32, R1357–R1371. [Google Scholar] [CrossRef]
- Kuchitsu, Y.; Taguchi, T. Lysosomal microautophagy: An emerging dimension in mammalian autophagy. Trends Cell Biol. 2024, 34, 606–616. [Google Scholar] [CrossRef]
- Sakai, Y.; Oku, M. ATG and ESCRT control multiple modes of microautophagy. FEBS Lett. 2024, 598, 48–58. [Google Scholar] [CrossRef]
- Yao, R.; Shen, J. Chaperone-mediated autophagy: Molecular mechanisms, biological functions, and diseases. MedComm 2023, 4, e347. [Google Scholar] [CrossRef]
- Xu, D.D.; Du, L.L. Fission Yeast Autophagy Machinery. Cells 2022, 11, 1086. [Google Scholar] [CrossRef]
- Jung, C.H.; Seo, M.; Otto, N.M.; Kim, D.H. ULK1 inhibits the kinase activity of mTORC1 and cell proliferation. Autophagy 2011, 7, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Stenmark, H. Autophagosome Biogenesis. Cells 2023, 12, 668. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Tabata, K.; Imai, K.; Fukuda, K.; Yamamoto, K.; Kunugi, H.; Fujita, T.; Kaminishi, T.; Tischer, C.; Neumann, B.; Reither, S.; et al. Palmitoylation of ULK1 by ZDHHC13 plays a crucial role in autophagy. Nat. Commun. 2024, 15, 7194. [Google Scholar] [CrossRef]
- Chen, M.; Nguyen, T.N.; Ren, X.; Khuu, G.; Cook, A.S.I.; Zhao, Y.; Yildiz, A.; Lazarou, M.; Hurley, J.H. Structure and activation of the human autophagy-initiating ULK1C:PI3KC3-C1 supercomplex. Nat. Struct. Mol. Biol. 2025, 123, 345a. [Google Scholar] [CrossRef]
- Javed, R.; Mari, M.; Trosdal, E.; Lopes Alberto Duque, T.; Paddar, M.; Allers, L.; Akepati, P.; Mudd, M.H.; Reggiori, F.; Deretic, V. ATG9A controls all stages of autophagosome biogenesis. Autophagy 2025, 21, 1859–1861. [Google Scholar] [CrossRef]
- Nahse, V.; Raiborg, C.; Tan, K.W.; Mork, S.; Torgersen, M.L.; Wenzel, E.M.; Nager, M.; Salo, V.T.; Johansen, T.; Ikonen, E.; et al. ATPase activity of DFCP1 controls selective autophagy. Nat. Commun. 2023, 14, 4051. [Google Scholar] [CrossRef]
- Nahse, V.; Stenmark, H.; Schink, K.O. Omegasomes control formation, expansion, and closure of autophagosomes. Bioessays 2024, 46, e2400038. [Google Scholar] [CrossRef]
- Jian, F.; Wang, S.; Tian, R.; Wang, Y.; Li, C.; Li, Y.; Wang, S.; Fang, C.; Ma, C.; Rong, Y. The STX17-SNAP47-VAMP7/VAMP8 complex is the default SNARE complex mediating autophagosome-lysosome fusion. Cell Res. 2024, 34, 151–168. [Google Scholar] [CrossRef]
- Song, X.; Xi, Y.; Dai, M.; Li, T.; Du, S.; Zhu, Y.; Li, M.; Li, Y.; Liu, S.; Ding, X.; et al. STING guides the STX17-SNAP29-VAMP8 complex assembly to control autophagy. Cell Insight 2024, 3, 100147. [Google Scholar] [CrossRef]
- Metlagel, Z.; Otomo, C.; Takaesu, G.; Otomo, T. Structural basis of ATG3 recognition by the autophagic ubiquitin-like protein ATG12. Proc. Natl. Acad. Sci. USA 2013, 110, 18844–18849. [Google Scholar] [CrossRef]
- Agrotis, A.; von Chamier, L.; Oliver, H.; Kiso, K.; Singh, T.; Ketteler, R. Human ATG4 autophagy proteases counteract attachment of ubiquitin-like LC3/GABARAP proteins to other cellular proteins. J. Biol. Chem. 2019, 294, 12610–12621. [Google Scholar] [CrossRef]
- Wu, M.Y.; Li, Z.W.; Lu, J.H. Molecular Modulators and Receptors of Selective Autophagy: Disease Implication and Identification Strategies. Int. J. Biol. Sci. 2024, 20, 751–764. [Google Scholar] [CrossRef] [PubMed]
- North, B.J.; Fracchiolla, D.; Ragusa, M.J.; Martens, S.; Shoemaker, C.J. The rapidly expanding role of LC3-interacting regions in autophagy. J. Cell Biol. 2025, 224, 202504076. [Google Scholar] [CrossRef] [PubMed]
- Valverde, D.P.; Yu, S.; Boggavarapu, V.; Kumar, N.; Lees, J.A.; Walz, T.; Reinisch, K.M.; Melia, T.J. ATG2 transports lipids to promote autophagosome biogenesis. J. Cell Biol. 2019, 218, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Dooley, H.C.; Razi, M.; Polson, H.E.; Girardin, S.E.; Wilson, M.I.; Tooze, S.A. WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12-5-16L1. Mol. Cell 2014, 55, 238–252. [Google Scholar] [CrossRef]
- Maeda, S.; Yamamoto, H.; Kinch, L.N.; Garza, C.M.; Takahashi, S.; Otomo, C.; Grishin, N.V.; Forli, S.; Mizushima, N.; Otomo, T. Structure, lipid scrambling activity and role in autophagosome formation of ATG9A. Nat. Struct. Mol. Biol. 2020, 27, 1194–1201. [Google Scholar] [CrossRef]
- Lin, X.; Liang, L.; Liao, S.; Li, Y.; Zhou, Y. Progress on multifunctional transmembrane protein ATG9A. Cell Commun. Signal 2025, 23, 314. [Google Scholar] [CrossRef]
- Zhou, C.; Ma, K.; Gao, R.; Mu, C.; Chen, L.; Liu, Q.; Luo, Q.; Feng, D.; Zhu, Y.; Chen, Q. Regulation of mATG9 trafficking by Src- and ULK1-mediated phosphorylation in basal and starvation-induced autophagy. Cell Res. 2017, 27, 184–201. [Google Scholar] [CrossRef]
- Javed, R.; Mari, M.; Trosdal, E.; Duque, T.; Paddar, M.A.; Allers, L.; Mudd, M.H.; Claude-Taupin, A.; Akepati, P.R.; Hendrix, E.; et al. ATG9A facilitates the closure of mammalian autophagosomes. J. Cell Biol. 2025, 224, e202404047. [Google Scholar] [CrossRef]
- Takahashi, Y.; He, H.; Tang, Z.; Hattori, T.; Liu, Y.; Young, M.M.; Serfass, J.M.; Chen, L.; Gebru, M.; Chen, C.; et al. An autophagy assay reveals the ESCRT-III component CHMP2A as a regulator of phagophore closure. Nat. Commun. 2018, 9, 2855. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Dancourt, J.; Shteyn, V.; Puente, G.; Fong, W.M.; Nag, S.; Bewersdorf, J.; Yamamoto, A.; Antonny, B.; Melia, T.J. Lipidation of the LC3/GABARAP family of autophagy proteins relies on a membrane-curvature-sensing domain in Atg3. Nat. Cell Biol. 2014, 16, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Nakatogawa, H.; Ishii, J.; Asai, E.; Ohsumi, Y. Atg4 recycles inappropriately lipidated Atg8 to promote autophagosome biogenesis. Autophagy 2012, 8, 177–186. [Google Scholar] [CrossRef]
- Menzies, F.M.; Moreau, K.; Puri, C.; Renna, M.; Rubinsztein, D.C. Measurement of autophagic activity in mammalian cells. Curr. Protoc. Cell Biol. 2012, 54, 15–16. [Google Scholar] [CrossRef]
- Ba, Q.; Raghavan, G.; Kiselyov, K.; Yang, G. Whole-Cell Scale Dynamic Organization of Lysosomes Revealed by Spatial Statistical Analysis. Cell Rep. 2018, 23, 3591–3606. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Zhang, H. Autophagosome maturation: An epic journey from the ER to lysosomes. J. Cell Biol. 2019, 218, 757–770. [Google Scholar] [CrossRef]
- Cason, S.E.; Carman, P.J.; Van Duyne, C.; Goldsmith, J.; Dominguez, R.; Holzbaur, E.L.F. Sequential dynein effectors regulate axonal autophagosome motility in a maturation-dependent pathway. J. Cell Biol. 2021, 220, 202010179. [Google Scholar] [CrossRef]
- Wang, L.; Diao, J. VAMP8 phosphorylation regulates lysosome dynamics during autophagy. Autophagy Rep. 2022, 1, 79–82. [Google Scholar] [CrossRef]
- Zheng, D.; Tong, M.; Zhang, S.; Pan, Y.; Zhao, Y.; Zhong, Q.; Liu, X. Human YKT6 forms priming complex with STX17 and SNAP29 to facilitate autophagosome-lysosome fusion. Cell Rep. 2024, 43, 113760. [Google Scholar] [CrossRef]
- Saji, T.; Endo, M.; Okada, Y.; Minami, Y.; Nishita, M. KIF1C facilitates retrograde transport of lysosomes through Hook3 and dynein. Commun. Biol. 2024, 7, 1305. [Google Scholar] [CrossRef]
- Shin, H.R.; Zoncu, R. The Lysosome at the Intersection of Cellular Growth and Destruction. Dev. Cell 2020, 54, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, X. Lysosome biogenesis: Regulation and functions. J. Cell Biol. 2021, 220, 202102001. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Garcia Arencibia, M.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P.; et al. TFEB links autophagy to lysosomal biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Han, T.; Zhan, Y.A. Mechanism and role of mitophagy in the development of severe infection. Cell Death Discov. 2024, 10, 88. [Google Scholar] [CrossRef]
- Kablak-Ziembicka, A.; Badacz, R.; Okarski, M.; Wawak, M.; Przewlocki, T.; Podolec, J. Cardiac microRNAs: Diagnostic and therapeutic potential. Arch. Med. Sci. 2023, 19, 1360–1381. [Google Scholar] [CrossRef]
- Amaravadi, R.K.; Kimmelman, A.C.; Debnath, J. Targeting Autophagy in Cancer: Recent Advances and Future Directions. Cancer Discov. 2019, 9, 1167–1181. [Google Scholar] [CrossRef]
- Ondaro, J.; Hernandez-Eguiazu, H.; Garciandia-Arcelus, M.; Loera-Valencia, R.; Rodriguez-Gomez, L.; Jimenez-Zuniga, A.; Goikolea, J.; Rodriguez-Rodriguez, P.; Ruiz-Martinez, J.; Moreno, F.; et al. Defects of Nutrient Signaling and Autophagy in Neurodegeneration. Front. Cell Dev. Biol. 2022, 10, 836196. [Google Scholar] [CrossRef]
- Kench, U.; Sologova, S.; Smolyarchuk, E.; Prassolov, V.; Spirin, P. Pharmaceutical Agents for Targeting Autophagy and Their Applications in Clinics. Pharmaceuticals 2024, 17, 1355. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Okoye, C.N.; Koren, S.A.; Wojtovich, A.P. Mitochondrial complex I ROS production and redox signaling in hypoxia. Redox Biol. 2023, 67, 102926. [Google Scholar] [CrossRef]
- Goncalves, R.L.S.; Watson, M.A.; Wong, H.S.; Orr, A.L.; Brand, M.D. The use of site-specific suppressors to measure the relative contributions of different mitochondrial sites to skeletal muscle superoxide and hydrogen peroxide production. Redox Biol. 2020, 28, 101341. [Google Scholar] [CrossRef]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and Antioxidants: Physiological and Pathological Role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Eleutherio, E.C.A.; Silva Magalhaes, R.S.; de Araujo Brasil, A.; Monteiro Neto, J.R.; de Holanda Paranhos, L. SOD1, more than just an antioxidant. Arch. Biochem. Biophys. 2021, 697, 108701. [Google Scholar] [CrossRef] [PubMed]
- Azadmanesh, J.; Slobodnik, K.; Struble, L.R.; Lutz, W.E.; Coates, L.; Weiss, K.L.; Myles, D.A.A.; Kroll, T.; Borgstahl, G.E.O. Revealing the atomic and electronic mechanism of human manganese superoxide dismutase product inhibition. Nat. Commun. 2024, 15, 5973. [Google Scholar] [CrossRef]
- Thomas, C.; Mackey, M.M.; Diaz, A.A.; Cox, D.P. Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: Implications for diseases associated with iron accumulation. Redox Rep. 2009, 14, 102–108. [Google Scholar] [CrossRef]
- Saraev, D.D.; Pratt, D.A. Reactions of lipid hydroperoxides and how they may contribute to ferroptosis sensitivity. Curr. Opin. Chem. Biol. 2024, 81, 102478. [Google Scholar] [CrossRef]
- Ferrer-Sueta, G.; Campolo, N.; Trujillo, M.; Bartesaghi, S.; Carballal, S.; Romero, N.; Alvarez, B.; Radi, R. Biochemistry of Peroxynitrite and Protein Tyrosine Nitration. Chem. Rev. 2018, 118, 1338–1408. [Google Scholar] [CrossRef]
- Prolo, C.; Piacenza, L.; Radi, R. Peroxynitrite: A multifaceted oxidizing and nitrating metabolite. Curr. Opin. Chem. Biol. 2024, 80, 102459. [Google Scholar] [CrossRef]
- Griswold-Prenner, I.; Kashyap, A.K.; Mazhar, S.; Hall, Z.W.; Fazelinia, H.; Ischiropoulos, H. Unveiling the human nitroproteome: Protein tyrosine nitration in cell signaling and cancer. J. Biol. Chem. 2023, 299, 105038. [Google Scholar] [CrossRef]
- Dent, M.R.; DeMartino, A.W. Nitric oxide and thiols: Chemical biology, signalling paradigms and vascular therapeutic potential. Br. J. Pharmacol. 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Moller, M.N.; Vitturi, D.A. The chemical biology of dinitrogen trioxide. Redox Biochem. Chem. 2024, 8, 100026. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Turano, H.; Haddad, L.A.; Netto, L.E.S. Human mitochondrial peroxiredoxin Prdx3 is dually localized in the intermembrane space and matrix subcompartments. Redox Biol. 2024, 78, 103436. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, G.; Mastrogiovanni, M.; Zeida, A.; Viera, N.; Radi, R.; Reyes, A.M.; Trujillo, M. Mitochondrial Peroxiredoxin 3 Is Rapidly Oxidized and Hyperoxidized by Fatty Acid Hydroperoxides. Antioxidants 2023, 12, 408. [Google Scholar] [CrossRef]
- Handy, D.E.; Loscalzo, J. The role of glutathione peroxidase-1 in health and disease. Free Radic. Biol. Med. 2022, 188, 146–161. [Google Scholar] [CrossRef]
- Kameritsch, P.; Singer, M.; Nuernbergk, C.; Rios, N.; Reyes, A.M.; Schmidt, K.; Kirsch, J.; Schneider, H.; Muller, S.; Pogoda, K.; et al. The mitochondrial thioredoxin reductase system (TrxR2) in vascular endothelium controls peroxynitrite levels and tissue integrity. Proc. Natl. Acad. Sci. USA 2021, 118, 1921828118. [Google Scholar] [CrossRef]
- Anwar, S.; Alrumaihi, F.; Sarwar, T.; Babiker, A.Y.; Khan, A.A.; Prabhu, S.V.; Rahmani, A.H. Exploring Therapeutic Potential of Catalase: Strategies in Disease Prevention and Management. Biomolecules 2024, 14, 697. [Google Scholar] [CrossRef]
- Chen, T.H.; Wang, H.C.; Chang, C.J.; Lee, S.Y. Mitochondrial Glutathione in Cellular Redox Homeostasis and Disease Manifestation. Int. J. Mol. Sci. 2024, 25, 1314. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Wang, J.; Guo, X.; Song, Y.; Fu, K.; Gao, Z.; Liu, D.; He, W.; Yang, L.L. Energy metabolism in health and diseases. Signal Transduct. Target. Ther. 2025, 10, 69. [Google Scholar] [CrossRef]
- Francisco, A.; Figueira, T.R.; Castilho, R.F. Mitochondrial NAD(P)+ Transhydrogenase: From Molecular Features to Physiology and Disease. Antioxid. Redox Signal 2022, 36, 864–884. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S. NADPH-The Forgotten Reducing Equivalent. Cold Spring Harb. Perspect. Biol. 2021, 13, 40550. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Azad, M.B.; Gibson, S.B. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009, 16, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Scherz-Shouval, R.; Shvets, E.; Fass, E.; Shorer, H.; Gil, L.; Elazar, Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007, 26, 1749–1760. [Google Scholar] [CrossRef]
- Sharma, P.; Kaushal, N.; Saleth, L.R.; Ghavami, S.; Dhingra, S.; Kaur, P. Oxidative stress-induced apoptosis and autophagy: Balancing the contrary forces in spermatogenesis. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166742. [Google Scholar] [CrossRef]
- Carretero-Fernandez, M.; Cabrera-Serrano, A.J.; Sanchez-Maldonado, J.M.; Ruiz-Duran, L.; Jimenez-Romera, F.; Garcia-Verdejo, F.J.; Gonzalez-Olmedo, C.; Cardus, A.; Diaz-Beltran, L.; Gutierrez-Bautista, J.F.; et al. Autophagy and oxidative stress in solid tumors: Mechanisms and therapeutic opportunities. Crit. Rev. Oncol. Hematol. 2025, 212, 104820. [Google Scholar] [CrossRef]
- Russell, R.C.; Tian, Y.; Yuan, H.; Park, H.W.; Chang, Y.Y.; Kim, J.; Kim, H.; Neufeld, T.P.; Dillin, A.; Guan, K.L. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 2013, 15, 741–750. [Google Scholar] [CrossRef]
- Medina, D.L.; Di Paola, S.; Peluso, I.; Armani, A.; De Stefani, D.; Venditti, R.; Montefusco, S.; Scotto-Rosato, A.; Prezioso, C.; Forrester, A.; et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 2015, 17, 288–299. [Google Scholar] [CrossRef]
- Hamasaki, M.; Furuta, N.; Matsuda, A.; Nezu, A.; Yamamoto, A.; Fujita, N.; Oomori, H.; Noda, T.; Haraguchi, T.; Hiraoka, Y.; et al. Autophagosomes form at ER-mitochondria contact sites. Nature 2013, 495, 389–393. [Google Scholar] [CrossRef]
- Ji, C.; Zhang, Z.; Li, Z.; She, X.; Wang, X.; Li, B.; Xu, X.; Song, D.; Zhang, D. Mitochondria-Associated Endoplasmic Reticulum Membranes: Inextricably Linked with Autophagy Process. Oxid. Med. Cell Longev. 2022, 2022, 7086807. [Google Scholar] [CrossRef]
- Jimenez-Loygorri, J.I.; Villarejo-Zori, B.; Viedma-Poyatos, A.; Zapata-Munoz, J.; Benitez-Fernandez, R.; Frutos-Lison, M.D.; Tomas-Barberan, F.A.; Espin, J.C.; Area-Gomez, E.; Gomez-Duran, A.; et al. Mitophagy curtails cytosolic mtDNA-dependent activation of cGAS/STING inflammation during aging. Nat. Commun. 2024, 15, 830. [Google Scholar] [CrossRef]
- Zhong, W.; Rao, Z.; Xu, J.; Sun, Y.; Hu, H.; Wang, P.; Xia, Y.; Pan, X.; Tang, W.; Chen, Z.; et al. Defective mitophagy in aged macrophages promotes mitochondrial DNA cytosolic leakage to activate STING signaling during liver sterile inflammation. Aging Cell 2022, 21, e13622. [Google Scholar] [CrossRef]
- Yoshii, A.; McMillen, T.S.; Wang, Y.; Zhou, B.; Chen, H.; Banerjee, D.; Herrero, M.; Wang, P.; Muraoka, N.; Wang, W.; et al. Blunted Cardiac Mitophagy in Response to Metabolic Stress Contributes to HFpEF. Circ. Res. 2024, 135, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Ma, X.; Liu, H.; Song, J.; Wei, T.; Zhang, R.; Zhan, X.; Li, H.; Zhou, J. Inhibition of PDGFRbeta alleviates endothelial cell apoptotic injury caused by DRP-1 overexpression and mitochondria fusion failure after mitophagy. Cell Death Dis. 2023, 14, 756. [Google Scholar] [CrossRef] [PubMed]
- Pescatore, L.A.; Gamarra, L.F.; Liberman, M. Multifaceted Mechanisms of Vascular Calcification in Aging. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Musunuru, K. A common coding variant in BAG3 protects from heart failure. Nat. Cardiovasc. Res. 2023, 2, 609–610. [Google Scholar] [CrossRef]
- Ramos, V.M.; Serna, J.D.C.; Vilas-Boas, E.A.; Cabral-Costa, J.V.; Cunha, F.M.; Kataura, T.; Korolchuk, V.I.; Kowaltowski, A.J. Mitochondrial sodium/calcium exchanger (NCLX) regulates basal and starvation-induced autophagy through calcium signaling. FASEB J. 2024, 38, e23454. [Google Scholar] [CrossRef]
- Zhang, J.; Peng, Y.; Fu, W.; Wang, R.; Cao, J.; Li, S.; Tian, X.; Li, Z.; Hua, C.; Zhai, Y.; et al. PLEKHM2 deficiency induces impaired mitochondrial clearance and elevated ROS levels in human iPSC-derived cardiomyocytes. Cell Death Discov. 2024, 10, 142. [Google Scholar] [CrossRef]
- Martin, T.G.; Myers, V.D.; Dubey, P.; Dubey, S.; Perez, E.; Moravec, C.S.; Willis, M.S.; Feldman, A.M.; Kirk, J.A. Cardiomyocyte contractile impairment in heart failure results from reduced BAG3-mediated sarcomeric protein turnover. Nat. Commun. 2021, 12, 2942. [Google Scholar] [CrossRef]
- Tam, E.; Song, E.; Noskovicova, N.; Hinz, B.; Xu, A.; Sweeney, G. Autophagy deficiency exacerbated hypoxia-reoxygenation induced inflammation and cell death via a mitochondrial DNA/STING/IRF3 pathway. Life Sci. 2024, 358, 123173. [Google Scholar] [CrossRef]
- Hu, X.; Li, D.; Chen, W.; Kuang, H.; Yang, D.; Gong, Z.; Long, Y.; Liu, G.; Wang, K.; Xia, M.; et al. Sodium Glucose Transporter 2 Inhibitor Protects Against Heart Failure With Preserved Ejection Fraction: Preclinical “2-Hit” Model Reveals Autophagy Enhancement Via AMP-Activated Protein Kinase/Mammalian Target of Rapamycin Complex 1 Pathway. J. Am. Heart Assoc. 2025, 14, e040093. [Google Scholar] [CrossRef]
- Bielawska, M.; Warszynska, M.; Stefanska, M.; Blyszczuk, P. Autophagy in Heart Failure: Insights into Mechanisms and Therapeutic Implications. J. Cardiovasc. Dev. Dis. 2023, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, Y.; Yan, L.; Tan, M.; Jin, Y.; Yin, Y.; Han, L.; Ma, X.; Li, Y.; Yang, T.; et al. Corosolic acid attenuates cardiac ischemia/reperfusion injury through the PHB2/PINK1/parkin/mitophagy pathway. iScience 2024, 27, 110448. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Lee, D.H.; Kim, D.H. Redefining the role of AMPK in autophagy and the energy stress response. Nat. Commun. 2023, 14, 2994. [Google Scholar] [CrossRef]
- Janaszak-Jasiecka, A.; Ploska, A.; Wieronska, J.M.; Dobrucki, L.W.; Kalinowski, L. Endothelial dysfunction due to eNOS uncoupling: Molecular mechanisms as potential therapeutic targets. Cell Mol. Biol. Lett. 2023, 28, 21. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-kappaB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Hu, M.; Ladowski, J.M.; Xu, H. The Role of Autophagy in Vascular Endothelial Cell Health and Physiology. Cells 2024, 13, 825. [Google Scholar] [CrossRef]
- Nivoit, P.; Mathivet, T.; Wu, J.; Salemkour, Y.; Sankar, D.S.; Baudrie, V.; Bourreau, J.; Guihot, A.L.; Vessieres, E.; Lemitre, M.; et al. Autophagy protein 5 controls flow-dependent endothelial functions. Cell Mol. Life Sci. 2023, 80, 210. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Pratico, D.; Lin, L.; Mantzoros, C.S.; Bahijri, S.; Tuomilehto, J.; Ren, J. Inflammation in atherosclerosis: Pathophysiology and mechanisms. Cell Death Dis. 2024, 15, 817. [Google Scholar] [CrossRef]
- Rajamaki, K.; Lappalainen, J.; Oorni, K.; Valimaki, E.; Matikainen, S.; Kovanen, P.T.; Eklund, K.K. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: A novel link between cholesterol metabolism and inflammation. PLoS ONE 2010, 5, e11765. [Google Scholar] [CrossRef]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nunez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef]
- Ouimet, M.; Franklin, V.; Mak, E.; Liao, X.; Tabas, I.; Marcel, Y.L. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab. 2011, 13, 655–667. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, S.N.; Yuan, S.T.; Lei, X.; Sun, X.; Xing, L.; Li, H.J.; He, C.X.; Qin, W.; Zhao, D.; et al. Multiple functions of autophagy in vascular calcification. Cell Biosci. 2021, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Liu, J.X.; Zheng, X.; Yan, X.; Zhao, P.; Yin, C.; Li, W.; Song, Z. Sox9 mediates autophagy-dependent vascular smooth muscle cell phenotypic modulation and transplant arteriosclerosis. iScience 2022, 25, 105161. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Ding, J.; Xie, S.; Huang, L.; Zhang, H.; Chen, X.; Ren, X.; Zhou, S.; He, H.; Ma, W.; et al. Myocardin/microRNA-30a/Beclin1 signaling controls the phenotypic modulation of vascular smooth muscle cells by regulating autophagy. Cell Death Dis. 2022, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Scalabrin, S.; Cagnin, S. Cardiovascular diseases in the elderly: Possibilities for modulating autophagy using non-coding RNAs. Front. Cell Dev. Biol. 2025, 13, 1520850. [Google Scholar] [CrossRef]
- Im, G.B.; Melero-Martin, J.M. Mitochondrial transfer in endothelial cells and vascular health. Trends Cell Biol. 2025, S0962-8924. [Google Scholar] [CrossRef]
- Joshi, D.; Coon, B.G.; Chakraborty, R.; Deng, H.; Yang, Z.; Babar, M.U.; Fernandez-Tussy, P.; Meredith, E.; Attanasio, J.; Joshi, N.; et al. Endothelial gamma-protocadherins inhibit KLF2 and KLF4 to promote atherosclerosis. Nat. Cardiovasc. Res. 2024, 3, 1035–1048. [Google Scholar] [CrossRef]
- Bharath, L.P.; Cho, J.M.; Park, S.K.; Ruan, T.; Li, Y.; Mueller, R.; Bean, T.; Reese, V.; Richardson, R.S.; Cai, J.; et al. Endothelial Cell Autophagy Maintains Shear Stress-Induced Nitric Oxide Generation via Glycolysis-Dependent Purinergic Signaling to Endothelial Nitric Oxide Synthase. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1646–1656. [Google Scholar] [CrossRef]
- Grootaert, M.O.J.; Moulis, M.; Roth, L.; Martinet, W.; Vindis, C.; Bennett, M.R.; De Meyer, G.R.Y. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc. Res. 2018, 114, 622–634. [Google Scholar] [CrossRef]
- Lai, Z.; Wang, C.; Liu, X.; Sun, H.; Guo, Z.; Shao, J.; Li, K.; Chen, J.; Wang, J.; Lei, X.; et al. Characterization of the proteome of stable and unstable carotid atherosclerotic plaques using data-independent acquisition mass spectrometry. J. Transl. Med. 2024, 22, 247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Z.; Zhang, W.; Zhang, L. MicroRNAs play an essential role in autophagy regulation in various disease phenotypes. Biofactors 2019, 45, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Ortuno-Sahagun, D.; Enterria-Rosales, J.; Izquierdo, V.; Grinan-Ferre, C.; Pallas, M.; Gonzalez-Castillo, C. The Role of the miR-17-92 Cluster in Autophagy and Atherosclerosis Supports Its Link to Lysosomal Storage Diseases. Cells 2022, 11, 2991. [Google Scholar] [CrossRef] [PubMed]
- Diez-Diez, M.; Ramos-Neble, B.L.; de la Barrera, J.; Silla-Castro, J.C.; Quintas, A.; Vazquez, E.; Rey-Martin, M.A.; Izzi, B.; Sanchez-Garcia, L.; Garcia-Lunar, I.; et al. Unidirectional association of clonal hematopoiesis with atherosclerosis development. Nat. Med. 2024, 30, 2857–2866. [Google Scholar] [CrossRef]
- Fuster, J.J.; MacLauchlan, S.; Zuriaga, M.A.; Polackal, M.N.; Ostriker, A.C.; Chakraborty, R.; Wu, C.L.; Sano, S.; Muralidharan, S.; Rius, C.; et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017, 355, 842–847. [Google Scholar] [CrossRef]
- Oren, O.; Small, A.M.; Libby, P. Clonal hematopoiesis and atherosclerosis. J. Clin. Investig. 2024, 134, 180066. [Google Scholar] [CrossRef]
- Madrigal-Matute, J.; de Bruijn, J.; van Kuijk, K.; Riascos-Bernal, D.F.; Diaz, A.; Tasset, I.; Martin-Segura, A.; Gijbels, M.J.J.; Sander, B.; Kaushik, S.; et al. Protective role of chaperone-mediated autophagy against atherosclerosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2121133119. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef]
- Duan, Q.; Yang, W.; Zhu, X.; Feng, Z.; Song, J.; Xu, X.; Kong, M.; Mao, J.; Shen, J.; Deng, Y.; et al. Deptor protects against myocardial ischemia-reperfusion injury by regulating the mTOR signaling and autophagy. Cell Death Discov. 2024, 10, 508. [Google Scholar] [CrossRef]
- Al-Salam, S.; Hashmi, S.; Jagadeesh, G.S.; Sudhadevi, M.; Awwad, A.; Nemmar, A. Early Cardiac Ischemia-Reperfusion Injury: Interactions of Autophagy with Galectin-3 and Oxidative Stress. Biomedicines 2024, 12, 2474. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijevic, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, S.; Sadoshima, J. Yin and Yang of NADPH Oxidases in Myocardial Ischemia-Reperfusion. Antioxidants 2022, 11, 1069. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, H.; Zhang, S.; Zhou, G.; Zhang, D.; Yang, Q.; Liu, Y.; Huang, X. Research Progress on the Mechanism of Lysosome in Myocardial Ischemia-Reperfusion Injury Based on Autophagy. Rev. Cardiovasc. Med. 2024, 25, 113. [Google Scholar] [CrossRef]
- Martin, J.L.; Costa, A.S.H.; Gruszczyk, A.V.; Beach, T.E.; Allen, F.M.; Prag, H.A.; Hinchy, E.C.; Mahbubani, K.; Hamed, M.; Tronci, L.; et al. Succinate accumulation drives ischaemia-reperfusion injury during organ transplantation. Nat. Metab. 2019, 1, 966–974. [Google Scholar] [CrossRef]
- Tabata Fukushima, C.; Dancil, I.S.; Clary, H.; Shah, N.; Nadtochiy, S.M.; Brookes, P.S. Reactive oxygen species generation by reverse electron transfer at mitochondrial complex I under simulated early reperfusion conditions. Redox Biol. 2024, 70, 103047. [Google Scholar] [CrossRef]
- He, W.; McCarroll, C.S.; Nather, K.; Ford, K.; Mangion, K.; Riddell, A.; O’Toole, D.; Zaeri, A.; Corcoran, D.; Carrick, D.; et al. Inhibition of myocardial cathepsin-L release during reperfusion following myocardial infarction improves cardiac function and reduces infarct size. Cardiovasc. Res. 2022, 118, 1535–1547. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, W.; Liao, Y.; Wang, W.; Deng, X.; Wang, C.; Shi, W. Autophagy: A double-edged sword in ischemia-reperfusion injury. Cell Mol. Biol. Lett. 2025, 30, 42. [Google Scholar] [CrossRef]
- Nishida, K.; Kyoi, S.; Yamaguchi, O.; Sadoshima, J.; Otsu, K. The role of autophagy in the heart. Cell Death Differ. 2009, 16, 31–38. [Google Scholar] [CrossRef]
- Gu, S.; Tan, J.; Li, Q.; Liu, S.; Ma, J.; Zheng, Y.; Liu, J.; Bi, W.; Sha, P.; Li, X.; et al. Downregulation of LAPTM4B Contributes to the Impairment of the Autophagic Flux via Unopposed Activation of mTORC1 Signaling During Myocardial Ischemia/Reperfusion Injury. Circ. Res. 2020, 127, e148–e165. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Z.; Wang, L.; Jiang, T.; Dong, D.; Sun, M. The PINK1/Parkin signaling pathway-mediated mitophagy: A forgotten protagonist in myocardial ischemia/reperfusion injury. Pharmacol. Res. 2024, 209, 107466. [Google Scholar] [CrossRef]
- Liu, R.; Xu, C.; Zhang, W.; Cao, Y.; Ye, J.; Li, B.; Jia, S.; Weng, L.; Liu, Y.; Liu, L.; et al. FUNDC1-mediated mitophagy and HIF1alpha activation drives pulmonary hypertension during hypoxia. Cell Death Dis. 2022, 13, 634. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, H.; Song, J.; Wang, T.; Dong, Y.; Zhan, A.; Li, Y.; Liang, G. AMPK Activation Alleviates Myocardial Ischemia-Reperfusion Injury by Regulating Drp1-Mediated Mitochondrial Dynamics. Front. Pharmacol. 2022, 13, 862204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.H.; Ruze, A.; Zhao, L.; Li, Q.L.; Tang, J.; Xiefukaiti, N.; Gai, M.T.; Deng, A.X.; Shan, X.F.; Gao, X.M. The role and mechanisms of microvascular damage in the ischemic myocardium. Cell Mol. Life Sci. 2023, 80, 341. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hu, J.; Wang, Y.; Gan, T.; Ding, Y.; Wang, X.; Xu, Q.; Xiong, J.; Xiong, N.; Lu, S.; et al. 9-PAHSA ameliorates microvascular damage during cardiac ischaemia/reperfusion injury by promoting LKB1/AMPK/ULK1-mediated autophagy-dependent STING degradation. Phytomedicine 2025, 136, 156241. [Google Scholar] [CrossRef]
- Zhou, D.; Yang, Y.; Han, R.; He, J.; Liu, D.; Xia, W.; Cai, Y.; Perek, B.; Xia, Z. Ferroptosis and its Potential Determinant Role in Myocardial Susceptibility to Ischemia/Reperfusion Injury in Diabetes. Rev. Cardiovasc. Med. 2024, 25, 360. [Google Scholar] [CrossRef]
- Adameova, A.; Horvath, C.; Abdul-Ghani, S.; Varga, Z.V.; Suleiman, M.S.; Dhalla, N.S. Interplay of Oxidative Stress and Necrosis-like Cell Death in Cardiac Ischemia/Reperfusion Injury: A Focus on Necroptosis. Biomedicines 2022, 10, 127. [Google Scholar] [CrossRef]
- de Waha, S.; Patel, M.R.; Granger, C.B.; Ohman, E.M.; Maehara, A.; Eitel, I.; Ben-Yehuda, O.; Jenkins, P.; Thiele, H.; Stone, G.W. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: An individual patient data pooled analysis from seven randomized trials. Eur. Heart J. 2017, 38, 3502–3510. [Google Scholar] [CrossRef]
- Prag, H.A.; Aksentijevic, D.; Dannhorn, A.; Giles, A.V.; Mulvey, J.F.; Sauchanka, O.; Du, L.; Bates, G.; Reinhold, J.; Kula-Alwar, D.; et al. Ischemia-Selective Cardioprotection by Malonate for Ischemia/Reperfusion Injury. Circ. Res. 2022, 131, 528–541. [Google Scholar] [CrossRef]
- Figueroa-Juarez, E. Uncovering the origin of oxidative damage in ischaemia-reperfusion injury in the heart. Nat. Rev. Endocrinol. 2023, 19, 560. [Google Scholar] [CrossRef] [PubMed]
- Stiermaier, T.; Thiele, H.; Eitel, I. Coronary Microvascular Obstruction: Key Factor in the Prognosis of ST-Segment-Elevation Myocardial Infarction. Circ. Cardiovasc. Imaging 2017, 10, e006568. [Google Scholar] [CrossRef] [PubMed]
- Garcia-de la Cruz, D.D.; Juarez-Rojop, I.E.; Tovilla-Zarate, C.A.; Nicolini, H.; Genis-Mendoza, A.D. Circulating Cell-Free Mitochondrial DNA in Plasma of Individuals with Schizophrenia and Cognitive Deficit in Mexican Population. Neuropsychiatr. Dis. Treat. 2024, 20, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.; Li, X.; Zhang, J.; Han, Y.; Sun, W.; Ren, D.; Tong, Q.; Li, J. Substrate metabolism regulated by Sestrin2-mTORC1 alleviates pressure overload-induced cardiac hypertrophy in aged heart. Redox Biol. 2020, 36, 101637. [Google Scholar] [CrossRef]
- Egan, D.; Kim, J.; Shaw, R.J.; Guan, K.L. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 2011, 7, 643–644. [Google Scholar] [CrossRef]
- Zhao, M.; Klionsky, D.J. AMPK-dependent phosphorylation of ULK1 induces autophagy. Cell Metab. 2011, 13, 119–120. [Google Scholar] [CrossRef]
- Titus, A.S.; Sung, E.A.; Zablocki, D.; Sadoshima, J. Mitophagy for cardioprotection. Basic Res. Cardiol. 2023, 118, 42. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Sun, S.J.; Cao, W.X.; Lan, X.T.; Ni, M.; Fu, H.; Li, D.J.; Wang, P.; Shen, F.M. Excessive ROS production and enhanced autophagy contribute to myocardial injury induced by branched-chain amino acids: Roles for the AMPK-ULK1 signaling pathway and alpha7nAChR. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 165980. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Liu, C.X.; Liu, H.X.; Wen, S.Y. The Emerging Roles of Vacuolar-Type ATPase-Dependent Lysosomal Acidification in Cardiovascular Disease. Biomolecules 2025, 15, 525. [Google Scholar] [CrossRef]
- Hernandez-Resendiz, S.; Prakash, A.; Loo, S.J.; Semenzato, M.; Chinda, K.; Crespo-Avilan, G.E.; Dam, L.C.; Lu, S.; Scorrano, L.; Hausenloy, D.J. Targeting mitochondrial shape: At the heart of cardioprotection. Basic Res. Cardiol. 2023, 118, 49. [Google Scholar] [CrossRef]
- Vasquez-Trincado, C.; Garcia-Carvajal, I.; Pennanen, C.; Parra, V.; Hill, J.A.; Rothermel, B.A.; Lavandero, S. Mitochondrial dynamics, mitophagy and cardiovascular disease. J. Physiol. 2016, 594, 509–525. [Google Scholar] [CrossRef]
- Miotto, M.C.; Reiken, S.; Wronska, A.; Yuan, Q.; Dridi, H.; Liu, Y.; Weninger, G.; Tchagou, C.; Marks, A.R. Structural basis for ryanodine receptor type 2 leak in heart failure and arrhythmogenic disorders. Nat. Commun. 2024, 15, 8080. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef] [PubMed]
- Caporizzo, M.A.; Prosser, B.L. The microtubule cytoskeleton in cardiac mechanics and heart failure. Nat. Rev. Cardiol. 2022, 19, 364–378. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, G.; Fumarulo, I.; Caffe, A.; Chiatto, M.; Montone, R.A.; Aspromonte, N. Microvascular Dysfunction across the Spectrum of Heart Failure Pathology: Pathophysiology, Clinical Features and Therapeutic Implications. Int. J. Mol. Sci. 2024, 25, 7628. [Google Scholar] [CrossRef] [PubMed]
- Hahn, V.S.; Selvaraj, S.; Sharma, K.; Shah, S.H. Towards Metabolomic-Based Precision Approaches for Classifying and Treating Heart Failure. JACC Basic Transl. Sci. 2024, 9, 1144–1158. [Google Scholar] [CrossRef]
- Rosano, G.M.C.; Vitale, C.; Spoletini, I. Precision Cardiology: Phenotype-targeted Therapies for HFmrEF and HFpEF. Int. J. Heart Fail. 2024, 6, 47–55. [Google Scholar] [CrossRef]
- Hong, K.N.; Eshraghian, E.A.; Arad, M.; Argiro, A.; Brambatti, M.; Bui, Q.; Caspi, O.; de Frutos, F.; Greenberg, B.; Ho, C.Y.; et al. International Consensus on Differential Diagnosis and Management of Patients With Danon Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2023, 82, 1628–1647. [Google Scholar] [CrossRef]
- Chi, C.; Leonard, A.; Knight, W.E.; Beussman, K.M.; Zhao, Y.; Cao, Y.; Londono, P.; Aune, E.; Trembley, M.A.; Small, E.M.; et al. LAMP-2B regulates human cardiomyocyte function by mediating autophagosome-lysosome fusion. Proc. Natl. Acad. Sci. USA 2019, 116, 556–565. [Google Scholar] [CrossRef]
- Nishino, I.; Fu, J.; Tanji, K.; Yamada, T.; Shimojo, S.; Koori, T.; Mora, M.; Riggs, J.E.; Oh, S.J.; Koga, Y.; et al. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease). Nature 2000, 406, 906–910. [Google Scholar] [CrossRef]
- Yerabandi, N.; Kouznetsova, V.L.; Kesari, S.; Tsigelny, I.F. The role of BAG3 in dilated cardiomyopathy and its association with Charcot-Marie-Tooth disease type 2. Acta Myol. 2022, 41, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Liu, S.; Gao, M.; Wang, W.; Chen, K.; Huang, L.; Liu, Y. Diabetic vascular diseases: Molecular mechanisms and therapeutic strategies. Signal Transduct. Target. Ther. 2023, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, J.; Huang, H.; Zhan, Q.; Wang, F.; Chen, Z.; Lu, X.; Sun, G. Post-translational modifications in diabetic cardiomyopathy. J. Cell Mol. Med. 2024, 28, e18158. [Google Scholar] [CrossRef] [PubMed]
- Paneque, A.; Fortus, H.; Zheng, J.; Werlen, G.; Jacinto, E. The Hexosamine Biosynthesis Pathway: Regulation and Function. Genes 2023, 14, 933. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, C.; Jiang, H. Novel target for treating Alzheimer’s Diseases: Crosstalk between the Nrf2 pathway and autophagy. Ageing Res. Rev. 2021, 65, 101207. [Google Scholar] [CrossRef]
- Shan, C.; Wang, Y.; Wang, Y. The Crosstalk between Autophagy and Nrf2 Signaling in Cancer: From Biology to Clinical Applications. Int. J. Biol. Sci. 2024, 20, 6181–6206. [Google Scholar] [CrossRef]
- Park, K.; Lim, H.; Kim, J.; Hwang, Y.; Lee, Y.S.; Bae, S.H.; Kim, H.; Kim, H.; Kang, S.W.; Kim, J.Y.; et al. Lysosomal Ca2+-mediated TFEB activation modulates mitophagy and functional adaptation of pancreatic beta-cells to metabolic stress. Nat. Commun. 2022, 13, 1300. [Google Scholar] [CrossRef]
- Yan, X.; Yang, L.; Fu, X.; Luo, X.; Wang, C.; Xie, Q.P.; OuYang, F. Transcription factor EB, a promising therapeutic target in cardiovascular disease. PeerJ 2024, 12, e18209. [Google Scholar] [CrossRef]
- Rudokas, M.W.; McKay, M.; Toksoy, Z.; Eisen, J.N.; Bogner, M.; Young, L.H.; Akar, F.G. Mitochondrial network remodeling of the diabetic heart: Implications to ischemia related cardiac dysfunction. Cardiovasc. Diabetol. 2024, 23, 261. [Google Scholar] [CrossRef]
- Kaludercic, N.; Di Lisa, F. Mitochondrial ROS Formation in the Pathogenesis of Diabetic Cardiomyopathy. Front. Cardiovasc. Med. 2020, 7, 12. [Google Scholar] [CrossRef]
- Giraldo-Gonzalez, G.C.; Roman-Gonzalez, A.; Canas, F.; Garcia, A. Molecular Mechanisms of Type 2 Diabetes-Related Heart Disease and Therapeutic Insights. Int. J. Mol. Sci. 2025, 26, 4548. [Google Scholar] [CrossRef]
- Panwar, A.; Malik, S.O.; Adib, M.; Lopaschuk, G.D. Cardiac energy metabolism in diabetes: Emerging therapeutic targets and clinical implications. Am. J. Physiol. Heart Circ. Physiol. 2025, 328, H1089–H1112. [Google Scholar] [CrossRef]
- Dubois, M.; Boulghobra, D.; Rochebloine, G.; Pallot, F.; Yehya, M.; Bornard, I.; Gayrard, S.; Coste, F.; Walther, G.; Meyer, G.; et al. Hyperglycemia triggers RyR2-dependent alterations of mitochondrial calcium homeostasis in response to cardiac ischemia-reperfusion: Key role of DRP1 activation. Redox Biol. 2024, 70, 103044. [Google Scholar] [CrossRef]
- Li, X.; Bi, X. Integrated Control of Fatty Acid Metabolism in Heart Failure. Metabolites 2023, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Ghazal, R.; Wang, M.; Liu, D.; Tschumperlin, D.J.; Pereira, N.L. Cardiac Fibrosis in the Multi-Omics Era: Implications for Heart Failure. Circ. Res. 2025, 136, 773–802. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, M.; Ying, X.; Yuan, J.; Wang, C.; Zhang, W.; Tian, S.; Yan, X. Caloric restriction increases the resistance of aged heart to myocardial ischemia/reperfusion injury via modulating AMPK-SIRT(1)-PGC(1a) energy metabolism pathway. Sci. Rep. 2023, 13, 2045. [Google Scholar] [CrossRef]
- Shah, I.A.; Ishaq, S.; Lee, S.D.; Wu, B.T. Effects of Exercise Training on Cardiac Mitochondrial Functions in Diabetic Heart: A Systematic Review. Int. J. Mol. Sci. 2024, 26, 8. [Google Scholar] [CrossRef]
- Baartscheer, A.; Schumacher, C.A.; Wust, R.C.; Fiolet, J.W.; Stienen, G.J.; Coronel, R.; Zuurbier, C.J. Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia 2017, 60, 568–573. [Google Scholar] [CrossRef]
- Trum, M.; Riechel, J.; Lebek, S.; Pabel, S.; Sossalla, S.T.; Hirt, S.; Arzt, M.; Maier, L.S.; Wagner, S. Empagliflozin inhibits Na+/H+ exchanger activity in human atrial cardiomyocytes. ESC Heart Fail. 2020, 7, 4429–4437. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Belohlavek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Matsuura, T.R.; Puchalska, P.; Crawford, P.A.; Kelly, D.P. Ketones and the Heart: Metabolic Principles and Therapeutic Implications. Circ. Res. 2023, 132, 882–898. [Google Scholar] [CrossRef]
- Gomora-Garcia, J.C.; Montiel, T.; Huttenrauch, M.; Salcido-Gomez, A.; Garcia-Velazquez, L.; Ramiro-Cortes, Y.; Gomora, J.C.; Castro-Obregon, S.; Massieu, L. Effect of the Ketone Body, D-beta-Hydroxybutyrate, on Sirtuin2-Mediated Regulation of Mitochondrial Quality Control and the Autophagy-Lysosomal Pathway. Cells 2023, 12, 486. [Google Scholar] [CrossRef]
- Szrok-Jurga, S.; Czumaj, A.; Turyn, J.; Hebanowska, A.; Swierczynski, J.; Sledzinski, T.; Stelmanska, E. The Physiological and Pathological Role of Acyl-CoA Oxidation. Int. J. Mol. Sci. 2023, 24, 14857. [Google Scholar] [CrossRef] [PubMed]
- Lamb, R.J.; Griffiths, K.; Lip, G.Y.H.; Sorokin, V.; Frenneaux, M.P.; Feelisch, M.; Madhani, M. ALDH2 polymorphism and myocardial infarction: From alcohol metabolism to redox regulation. Pharmacol. Ther. 2024, 259, 108666. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, X.; Chen, Z.; Xu, J.; Zhao, R.; Liu, Y.; Wen, Y.; Chen, L. Aldehyde dehydrogenase 2 alleviates mitochondrial dysfunction by promoting PGC-1alpha-mediated biogenesis in acute kidney injury. Cell Death Dis. 2023, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hernandez-Ochoa, E.O.; Viswanathan, M.C.; Blum, I.D.; Do, D.C.; Granger, J.M.; Murphy, K.R.; Wei, A.C.; Aja, S.; Liu, N.; et al. CaMKII oxidation is a critical performance/disease trade-off acquired at the dawn of vertebrate evolution. Nat. Commun. 2021, 12, 3175. [Google Scholar] [CrossRef]
- Chiang, D.Y.; Lahiri, S.; Wang, G.; Karch, J.; Wang, M.C.; Jung, S.Y.; Heck, A.J.R.; Scholten, A.; Wehrens, X.H.T. Phosphorylation-Dependent Interactome of Ryanodine Receptor Type 2 in the Heart. Proteomes 2021, 9, 27. [Google Scholar] [CrossRef]
- Haugsten Hansen, M.; Sadredini, M.; Hasic, A.; Anderson, M.E.; Sjaastad, I.; Korseberg Stokke, M. CaMKII and reactive oxygen species contribute to early reperfusion arrhythmias, but oxidation of CaMKIIdelta at methionines 281/282 is not a determining factor. J. Mol. Cell Cardiol. 2023, 175, 49–61. [Google Scholar] [CrossRef]
- Erickson, J.R.; Joiner, M.L.; Guan, X.; Kutschke, W.; Yang, J.; Oddis, C.V.; Bartlett, R.K.; Lowe, J.S.; O’Donnell, S.E.; Aykin-Burns, N.; et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 2008, 133, 462–474. [Google Scholar] [CrossRef]
- Hamilton, S.; Terentyev, D. ER stress and calcium-dependent arrhythmias. Front. Physiol. 2022, 13, 1041940. [Google Scholar] [CrossRef]
- Murphy, E.; Liu, J.C. Mitochondrial calcium and reactive oxygen species in cardiovascular disease. Cardiovasc. Res. 2023, 119, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.; Terentyeva, R.; Clements, R.T.; Belevych, A.E.; Terentyev, D. Sarcoplasmic reticulum-mitochondria communication; implications for cardiac arrhythmia. J. Mol. Cell Cardiol. 2021, 156, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Glynn, P.; Musa, H.; Wu, X.; Unudurthi, S.D.; Little, S.; Qian, L.; Wright, P.J.; Radwanski, P.B.; Gyorke, S.; Mohler, P.J.; et al. Voltage-Gated Sodium Channel Phosphorylation at Ser571 Regulates Late Current, Arrhythmia, and Cardiac Function In Vivo. Circulation 2015, 132, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Howard, T.; Greer-Short, A.; Satroplus, T.; Patel, N.; Nassal, D.; Mohler, P.J.; Hund, T.J. CaMKII-dependent late Na+ current increases electrical dispersion and arrhythmia in ischemia-reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H794–H801. [Google Scholar] [CrossRef]
- Horvath, B.; Szentandrassy, N.; Almassy, J.; Dienes, C.; Kovacs, Z.M.; Nanasi, P.P.; Banyasz, T. Late Sodium Current of the Heart: Where Do We Stand and Where Are We Going? Pharmaceuticals 2022, 15, 231. [Google Scholar] [CrossRef]
- Di Diego, J.M.; Cordeiro, J.M.; Goodrow, R.J.; Fish, J.M.; Zygmunt, A.C.; Perez, G.J.; Scornik, F.S.; Antzelevitch, C. Ionic and cellular basis for the predominance of the Brugada syndrome phenotype in males. Circulation 2002, 106, 2004–2011. [Google Scholar] [CrossRef]
- Trum, M.; Islam, M.M.T.; Lebek, S.; Baier, M.; Hegner, P.; Eaton, P.; Maier, L.S.; Wagner, S. Inhibition of cardiac potassium currents by oxidation-activated protein kinase A contributes to early afterdepolarizations in the heart. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H1347–H1357. [Google Scholar] [CrossRef]
- Orfali, R.; Gamal El-Din, A.H.; Karthick, V.; Lamis, E.; Xiao, V.; Ramanishka, A.; Alwatban, A.; Alkhamees, O.; Alaseem, A.; Nam, Y.W.; et al. Modulation of Redox-Sensitive Cardiac Ion Channels. Antioxidants 2025, 14, 836. [Google Scholar] [CrossRef]
- Kistamas, K.; Hezso, T.; Horvath, B.; Nanasi, P.P. Late sodium current and calcium homeostasis in arrhythmogenesis. Channels 2021, 15, 1–19. [Google Scholar] [CrossRef]
- Shiferaw, Y.; Aistrup, G.L.; Wasserstrom, J.A. Intracellular Ca2+ waves, afterdepolarizations, and triggered arrhythmias. Cardiovasc. Res. 2012, 95, 265–268. [Google Scholar] [CrossRef]
- Pun, R.; Kim, M.H.; North, B.J. Role of Connexin 43 phosphorylation on Serine-368 by PKC in cardiac function and disease. Front. Cardiovasc. Med. 2022, 9, 1080131. [Google Scholar] [CrossRef]
- Fong, J.T.; Kells, R.M.; Gumpert, A.M.; Marzillier, J.Y.; Davidson, M.W.; Falk, M.M. Internalized gap junctions are degraded by autophagy. Autophagy 2012, 8, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Beardslee, M.A.; Laing, J.G.; Beyer, E.C.; Saffitz, J.E. Rapid turnover of connexin43 in the adult rat heart. Circ. Res. 1998, 83, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Sykora, M.; Szeiffova Bacova, B.; Andelova, K.; Egan Benova, T.; Martiskova, A.; Kurahara, L.H.; Hirano, K.; Tribulova, N. Connexin43, A Promising Target to Reduce Cardiac Arrhythmia Burden in Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2024, 25, 3275. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, E.; Girao, H.; Yuste, A.; Patel, B.; Marques, C.; Spray, D.C.; Pereira, P.; Cuervo, A.M. Autophagy modulates dynamics of connexins at the plasma membrane in a ubiquitin-dependent manner. Mol. Biol. Cell 2012, 23, 2156–2169. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Guo, X.; Li, Y.; Ogurlu, R.; Lu, F.; Prondzynski, M.; de la Serna Buzon, S.; Ma, Q.; Zhang, D.; et al. Increased Reactive Oxygen Species-Mediated Ca2+/Calmodulin-Dependent Protein Kinase II Activation Contributes to Calcium Handling Abnormalities and Impaired Contraction in Barth Syndrome. Circulation 2021, 143, 1894–1911. [Google Scholar] [CrossRef]
- Hudgins, E.C.; Bonar, A.M.; Nguyen, T.; Fancher, I.S. Targeting Lipid-Ion Channel Interactions in Cardiovascular Disease. Front. Cardiovasc. Med. 2022, 9, 876634. [Google Scholar] [CrossRef]
- Cs Szabo, B.; Szabo, M.; Nagy, P.; Varga, Z.; Panyi, G.; Kovacs, T.; Zakany, F. Novel insights into the modulation of the voltage-gated potassium channel K(V)1.3 activation gating by membrane ceramides. J. Lipid Res. 2024, 65, 100596. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Chiong, M.; Lavandero, S.; Klionsky, D.J.; Ren, J. Mitophagy in cardiovascular diseases: Molecular mechanisms, pathogenesis, and treatment. Trends Mol. Med. 2022, 28, 836–849. [Google Scholar] [CrossRef]
- Forte, M.; D’Ambrosio, L.; Schiattarella, G.G.; Salerno, N.; Perrone, M.A.; Loffredo, F.S.; Bertero, E.; Pilichou, K.; Manno, G.; Valenti, V.; et al. Mitophagy modulation for the treatment of cardiovascular diseases. Eur. J. Clin. Investig. 2024, 54, e14199. [Google Scholar] [CrossRef]
- Belardinelli, L.; Liu, G.; Smith-Maxwell, C.; Wang, W.Q.; El-Bizri, N.; Hirakawa, R.; Karpinski, S.; Li, C.H.; Hu, L.; Li, X.J.; et al. A novel, potent, and selective inhibitor of cardiac late sodium current suppresses experimental arrhythmias. J. Pharmacol. Exp. Ther. 2013, 344, 23–32. [Google Scholar] [CrossRef]
- Mitchell, W.; Pharaoh, G.; Tyshkovskiy, A.; Campbell, M.; Marcinek, D.J.; Gladyshev, V.N. The Mitochondria-Targeted Peptide Therapeutic Elamipretide Improves Cardiac and Skeletal Muscle Function During Aging Without Detectable Changes in Tissue Epigenetic or Transcriptomic Age. Aging Cell 2025, 24, e70026. [Google Scholar] [CrossRef]
- Rouhana, S.; Virsolvy, A.; Fares, N.; Richard, S.; Thireau, J. Ranolazine: An Old Drug with Emerging Potential; Lessons from Pre-Clinical and Clinical Investigations for Possible Repositioning. Pharmaceuticals 2021, 15, 31. [Google Scholar] [CrossRef]
- Rakoubian, A.; Khinchin, J.; Yarbro, J.; Kobayashi, S.; Liang, Q. Isoform-specific roles of AMP-activated protein kinase in cardiac physiology and pathophysiology. Front. Cardiovasc. Med. 2025, 12, 1638515. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.X.; Finkel, T. Lysosomes in senescence and aging. EMBO Rep. 2023, 24, e57265. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Xu, S.C.; Deng, W.; Tang, Q. Metabolic landscape in cardiac aging: Insights into molecular biology and therapeutic implications. Signal Transduct. Target. Ther. 2023, 8, 114. [Google Scholar] [CrossRef]
- Vakka, A.; Warren, J.S.; Drosatos, K. Cardiovascular aging: From cellular and molecular changes to therapeutic interventions. J. Cardiovasc. Aging 2023, 3, 23. [Google Scholar] [CrossRef]
- Zhang, Q.; Siyuan, Z.; Xing, C.; Ruxiu, L. SIRT3 regulates mitochondrial function: A promising star target for cardiovascular disease therapy. Biomed. Pharmacother. 2024, 170, 116004. [Google Scholar] [CrossRef]
- Lu, Y.; An, L.; Taylor, M.R.G.; Chen, Q.M. Nrf2 signaling in heart failure: Expression of Nrf2, Keap1, antioxidant, and detoxification genes in dilated or ischemic cardiomyopathy. Physiol. Genomics 2022, 54, 115–127. [Google Scholar] [CrossRef]
- Ribeiro, A.S.F.; Zerolo, B.E.; Lopez-Espuela, F.; Sanchez, R.; Fernandes, V.S. Cardiac System during the Aging Process. Aging Dis. 2023, 14, 1105–1122. [Google Scholar] [CrossRef]
- Vahle, B.; Heilmann, L.; Schauer, A.; Augstein, A.; Jarabo, M.P.; Barthel, P.; Mangner, N.; Labeit, S.; Bowen, T.S.; Linke, A.; et al. Modulation of Titin and Contraction-Regulating Proteins in a Rat Model of Heart Failure with Preserved Ejection Fraction: Limb vs. Diaphragmatic Muscle. Int. J. Mol. Sci. 2024, 25, 6618. [Google Scholar] [CrossRef]
- Newman, L.E.; Shadel, G.S. Mitochondrial DNA Release in Innate Immune Signaling. Annu. Rev. Biochem. 2023, 92, 299–332. [Google Scholar] [CrossRef]
- Dasgupta, N.; Arnold, R.; Equey, A.; Gandhi, A.; Adams, P.D. The role of the dynamic epigenetic landscape in senescence: Orchestrating SASP expression. NPJ Aging 2024, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.F.; Hussain, S.; Mirzoyev, S.A.; Edwards, W.D.; Maleszewski, J.J.; Redfield, M.M. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015, 131, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Erlich, A.T.; Brownlee, D.M.; Beyfuss, K.; Hood, D.A. Exercise induces TFEB expression and activity in skeletal muscle in a PGC-1alpha-dependent manner. Am. J. Physiol. Cell Physiol. 2018, 314, C62–C72. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, X.; Zhu, Y.; Li, Z.; Zhu, Y.T.; Wu, J.C.; Qin, Z.H.; Xiang, M.; Lin, F. Exercise activates lysosomal function in the brain through AMPK-SIRT1-TFEB pathway. CNS Neurosci. Ther. 2019, 25, 796–807. [Google Scholar] [CrossRef]
- Ghosh, R.; Gillaspie, J.J.; Campbell, K.S.; Symons, J.D.; Boudina, S.; Pattison, J.S. Chaperone-mediated autophagy protects cardiomyocytes against hypoxic-cell death. Am. J. Physiol. Cell Physiol. 2022, 323, C1555–C1575. [Google Scholar] [CrossRef]
- Barcena, M.L.; Aslam, M.; Norman, K.; Ott, C.; Ladilov, Y. Role of AMPK and Sirtuins in Aging Heart: Basic and Translational Aspects. Aging Dis. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Lu, H.; Sun, J.; Hamblin, M.H.; Chen, Y.E.; Fan, Y. Transcription factor EB regulates cardiovascular homeostasis. EBioMedicine 2021, 63, 103207. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Y.; Wang, S.; Fan, Y.; Ye, Y.; Jing, Y.; Liu, Z.; Yang, S.; Xiong, M.; Yang, K.; et al. Single-nucleus transcriptomics reveals a gatekeeper role for FOXP1 in primate cardiac aging. Protein Cell 2023, 14, 279–293. [Google Scholar] [CrossRef]
- Ciutac, A.M.; Pana, T.; Dawson, D.; Myint, P.K. Sex-related differences in heart failure patients: Physiological mechanisms of cardiovascular ageing and evidence-based sex-specific medical therapies. Ther. Adv. Cardiovasc. Dis. 2025, 19, 17539447241309673. [Google Scholar] [CrossRef]
- Ministrini, S.; Puspitasari, Y.M.; Beer, G.; Liberale, L.; Montecucco, F.; Camici, G.G. Sirtuin 1 in Endothelial Dysfunction and Cardiovascular Aging. Front. Physiol. 2021, 12, 733696. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, J.; Luan, Y.; Li, X.; Meng, X.; Liao, W.; Tang, J.; Wang, Z. cGAS-STING, inflammasomes and pyroptosis: An overview of crosstalk mechanism of activation and regulation. Cell Commun. Signal 2024, 22, 22. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.S.; Chung, J.H. Molecular mechanisms of mitochondrial DNA release and activation of the cGAS-STING pathway. Exp. Mol. Med. 2023, 55, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef]
- Onomoto, K.; Onoguchi, K.; Yoneyama, M. Regulation of RIG-I-like receptor-mediated signaling: Interaction between host and viral factors. Cell Mol. Immunol. 2021, 18, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Basit, A.; Cho, M.G.; Kim, E.Y.; Kwon, D.; Kang, S.J.; Lee, J.H. The cGAS/STING/TBK1/IRF3 innate immunity pathway maintains chromosomal stability through regulation of p21 levels. Exp. Mol. Med. 2020, 52, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Biasizzo, M.; Kopitar-Jerala, N. Interplay Between NLRP3 Inflammasome and Autophagy. Front. Immunol. 2020, 11, 591803. [Google Scholar] [CrossRef]
- Paik, S.; Kim, J.K.; Silwal, P.; Sasakawa, C.; Jo, E.K. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell Mol. Immunol. 2021, 18, 1141–1160. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, H.; Li, H.; Mou, H.; Yinwang, E.; Xue, Y.; Wang, S.; Zhang, Y.; Wang, Z.; Chen, T.; et al. Cancer cells reprogram to metastatic state through the acquisition of platelet mitochondria. Cell Rep. 2023, 42, 113147. [Google Scholar] [CrossRef]
- Lou, J.; Zhang, J.; Deng, Q.; Chen, X. Neutrophil extracellular traps mediate neuro-immunothrombosis. Neural Regen. Res. 2024, 19, 1734–1740. [Google Scholar] [CrossRef]
- Heckmann, B.L.; Boada-Romero, E.; Cunha, L.D.; Magne, J.; Green, D.R. LC3-Associated Phagocytosis and Inflammation. J. Mol. Biol. 2017, 429, 3561–3576. [Google Scholar] [CrossRef]
- Siapoush, S.; Rezaei, R.; Alavifard, H.; Hatami, B.; Zali, M.R.; Vosough, M.; Lorzadeh, S.; Los, M.J.; Baghaei, K.; Ghavami, S. Therapeutic implications of targeting autophagy and TGF-beta crosstalk for the treatment of liver fibrosis. Life Sci. 2023, 329, 121894. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, K.; Jo, T.; Nagira, D.; Konaka, H.; Park, J.H.; Yoshimura, S.I.; Ninomiya, A.; Sugihara, F.; Hirayama, T.; Itotagawa, E.; et al. The lysosomal Ragulator complex activates NLRP3 inflammasome in vivo via HDAC6. EMBO J. 2023, 42, e111389. [Google Scholar] [CrossRef] [PubMed]
- Martin-Salgado, M.; Ochoa-Echeverria, A.; Merida, I. Diacylglycerol kinases: A look into the future of immunotherapy. Adv. Biol. Regul. 2024, 91, 100999. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, J.; Lin, P.; Wan, L.; Qu, Y.; Cao, L.; Wang, L. A review of the mechanisms of abnormal ceramide metabolism in type 2 diabetes mellitus, Alzheimer’s disease, and their co-morbidities. Front. Pharmacol. 2024, 15, 1348410. [Google Scholar] [CrossRef] [PubMed]
- Kazanietz, M.G.; Cooke, M. Protein kinase C signaling “in” and “to” the nucleus: Master kinases in transcriptional regulation. J. Biol. Chem. 2024, 300, 105692. [Google Scholar] [CrossRef]
- Otoda, T.; Aihara, K.I.; Takayama, T. Lysosomal Stress in Cardiovascular Diseases: Therapeutic Potential of Cardiovascular Drugs and Future Directions. Biomedicines 2025, 13, 1053. [Google Scholar] [CrossRef]
- Balka, K.R.; Venkatraman, R.; Saunders, T.L.; Shoppee, A.; Pang, E.S.; Magill, Z.; Homman-Ludiye, J.; Huang, C.; Lane, R.M.; York, H.M.; et al. Termination of STING responses is mediated via ESCRT-dependent degradation. EMBO J. 2023, 42, e112712. [Google Scholar] [CrossRef]
- Marchi, S.; Bittremieux, M.; Missiroli, S.; Morganti, C.; Patergnani, S.; Sbano, L.; Rimessi, A.; Kerkhofs, M.; Parys, J.B.; Bultynck, G.; et al. Endoplasmic Reticulum-Mitochondria Communication Through Ca2+ Signaling: The Importance of Mitochondria-Associated Membranes (MAMs). Adv. Exp. Med. Biol. 2017, 997, 49–67. [Google Scholar] [CrossRef]
- Beretta, M.; Santos, C.X.; Molenaar, C.; Hafstad, A.D.; Miller, C.C.; Revazian, A.; Betteridge, K.; Schroder, K.; Streckfuss-Bomeke, K.; Doroshow, J.H.; et al. Nox4 regulates InsP3 receptor-dependent Ca2+ release into mitochondria to promote cell survival. EMBO J. 2020, 39, e103530. [Google Scholar] [CrossRef]
- Barazzuol, L.; Giamogante, F.; Brini, M.; Cali, T. PINK1/Parkin Mediated Mitophagy, Ca2+ Signalling, and ER-Mitochondria Contacts in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1772. [Google Scholar] [CrossRef] [PubMed]
- Pena-Martinez, C.; Rickman, A.D.; Heckmann, B.L. Beyond autophagy: LC3-associated phagocytosis and endocytosis. Sci. Adv. 2022, 8, eabn1702. [Google Scholar] [CrossRef] [PubMed]
- Hooper, K.M.; Jacquin, E.; Li, T.; Goodwin, J.M.; Brumell, J.H.; Durgan, J.; Florey, O. V-ATPase is a universal regulator of LC3-associated phagocytosis and non-canonical autophagy. J. Cell Biol. 2022, 221, 202105112. [Google Scholar] [CrossRef] [PubMed]
- Magne, J.; Green, D.R. LC3-associated endocytosis and the functions of Rubicon and ATG16L1. Sci. Adv. 2022, 8, eabo5600. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Li, H.E.; Hughes, R.N.; Watson, G.D.R.; Gallegos, D.; West, A.E.; Kim, I.H.; Yin, H.H. A striatal interneuron circuit for continuous target pursuit. Nat. Commun. 2019, 10, 2715. [Google Scholar] [CrossRef]
- Ferreira, A.V.; Dominguez-Andres, J.; Merlo Pich, L.M.; Joosten, L.A.B.; Netea, M.G. Metabolic Regulation in the Induction of Trained Immunity. Semin. Immunopathol. 2024, 46, 7. [Google Scholar] [CrossRef]
- Vuscan, P.; Kischkel, B.; Joosten, L.A.B.; Netea, M.G. Trained immunity: General and emerging concepts. Immunol. Rev. 2024, 323, 164–185. [Google Scholar] [CrossRef]
- Gupta, S.S.; Zeglinski, M.R.; Rattan, S.G.; Landry, N.M.; Ghavami, S.; Wigle, J.T.; Klonisch, T.; Halayko, A.J.; Dixon, I.M. Inhibition of autophagy inhibits the conversion of cardiac fibroblasts to cardiac myofibroblasts. Oncotarget 2016, 7, 78516–78531. [Google Scholar] [CrossRef]
- Migneault, F.; Hebert, M.J. Autophagy, tissue repair, and fibrosis: A delicate balance. Matrix Biol. 2021, 100–101, 182–196. [Google Scholar] [CrossRef]
- Saadat, S.; Noureddini, M.; Mahjoubin-Tehran, M.; Nazemi, S.; Shojaie, L.; Aschner, M.; Maleki, B.; Abbasi-Kolli, M.; Rajabi Moghadam, H.; Alani, B.; et al. Pivotal Role of TGF-beta/Smad Signaling in Cardiac Fibrosis: Non-coding RNAs as Effectual Players. Front. Cardiovasc. Med. 2020, 7, 588347. [Google Scholar] [CrossRef]
- Jain, M.; Rivera, S.; Monclus, E.A.; Synenki, L.; Zirk, A.; Eisenbart, J.; Feghali-Bostwick, C.; Mutlu, G.M.; Budinger, G.R.; Chandel, N.S. Mitochondrial reactive oxygen species regulate transforming growth factor-beta signaling. J. Biol. Chem. 2013, 288, 770–777. [Google Scholar] [CrossRef]
- Tian, K.; Yu, M.; Jiang, M.; Gao, Z.; Zheng, D.; Shi, W.; Cheng, D.; Zhao, X. Lysosomal Acidification: A New Perspective on the Pathogenesis and Treatment of Pulmonary Fibrosis. Compr. Physiol. 2025, 15, e70023. [Google Scholar] [CrossRef] [PubMed]
- Roark, K.M.; Iffland, P.H., 2nd. Rapamycin for longevity: The pros, the cons, and future perspectives. Front. Aging 2025, 6, 1628187. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Cho, G.W.; Kong, Y.; Li, D.L.; Altamirano, F.; Luo, X.; Morales, C.R.; Jiang, N.; Schiattarella, G.G.; May, H.I.; et al. Activation of Autophagic Flux Blunts Cardiac Ischemia/Reperfusion Injury. Circ. Res. 2021, 129, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.H.; Luo, Y.X.; Yao, X.Q. Exercise-driven cellular autophagy: A bridge to systematic wellness. J. Adv. Res. 2025, 76, 271–291. [Google Scholar] [CrossRef]
- Goel, S.; Singh, R.; Singh, V.; Singh, H.; Kumari, P.; Chopra, H.; Sharma, R.; Nepovimova, E.; Valis, M.; Kuca, K.; et al. Metformin: Activation of 5′ AMP-activated protein kinase and its emerging potential beyond anti-hyperglycemic action. Front. Genet. 2022, 13, 1022739. [Google Scholar] [CrossRef]
- Bensalem, J.; Teong, X.T.; Hattersley, K.J.; Hein, L.K.; Fourrier, C.; Dang, L.V.P.; Singh, S.; Liu, K.; Wittert, G.A.; Hutchison, A.T.; et al. Intermittent time-restricted eating may increase autophagic flux in humans: An exploratory analysis. J. Physiol. 2025, 603, 3019–3032. [Google Scholar] [CrossRef]
- Moradi, N.; Sanfrancesco, V.C.; Champsi, S.; Hood, D.A. Regulation of lysosomes in skeletal muscle during exercise, disuse and aging. Free Radic. Biol. Med. 2024, 225, 323–332. [Google Scholar] [CrossRef]
- Papini, N.; Giussani, P.; Tringali, C. Metformin Lysosomal Targeting: A Novel Aspect to Be Investigated for Metformin Repurposing in Neurodegenerative Diseases? Int. J. Mol. Sci. 2024, 25, 8884. [Google Scholar] [CrossRef]
- Feng, X.; Cai, W.; Li, Q.; Zhao, L.; Meng, Y.; Xu, H. Activation of lysosomal Ca2+ channels mitigates mitochondrial damage and oxidative stress. J. Cell Biol. 2025, 224, 3104. [Google Scholar] [CrossRef]
- Parker, A.M.; Lees, J.G.; Murray, A.J.; Velagic, A.; Lim, S.Y.; De Blasio, M.J.; Ritchie, R.H. Precision Medicine: Therapeutically Targeting Mitochondrial Alterations in Heart Failure. JACC Basic Transl. Sci. 2025, 10, 101345. [Google Scholar] [CrossRef]
- Hofer, S.J.; Daskalaki, I.; Bergmann, M.; Friscic, J.; Zimmermann, A.; Mueller, M.I.; Abdellatif, M.; Nicastro, R.; Masser, S.; Durand, S.; et al. Spermidine is essential for fasting-mediated autophagy and longevity. Nat. Cell Biol. 2024, 26, 1571–1584. [Google Scholar] [CrossRef] [PubMed]
- Carollo, C.; Sorce, A.; Cirafici, E.; Mule, G.; Caimi, G. Sirtuins and Resveratrol in Cardiorenal Diseases: A Narrative Review of Mechanisms and Therapeutic Potential. Nutrients 2025, 17, 1212. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ma, J.; Lu, L.; Xiong, X.; Shao, Y.; Ren, J.; Yang, J.; Liu, J. Melatonin Restores Autophagic Flux by Activating the Sirt3/TFEB Signaling Pathway to Attenuate Doxorubicin-Induced Cardiomyopathy. Antioxidants 2023, 12, 1716. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Fu, Q.; Yang, Q.; Yang, Y.; Li, R.; Yang, X.; Yang, Q.; Li, S.; Tian, J.; Liu, H. Different types of cell death and their interactions in myocardial ischemia-reperfusion injury. Cell Death Discov. 2025, 11, 87. [Google Scholar] [CrossRef]
- Oberle, C.; Huai, J.; Reinheckel, T.; Tacke, M.; Rassner, M.; Ekert, P.G.; Buellesbach, J.; Borner, C. Lysosomal membrane permeabilization and cathepsin release is a Bax/Bak-dependent, amplifying event of apoptosis in fibroblasts and monocytes. Cell Death Differ. 2010, 17, 1167–1178. [Google Scholar] [CrossRef]
- Liu, Y.; Shoji-Kawata, S.; Sumpter, R.M., Jr.; Wei, Y.; Ginet, V.; Zhang, L.; Posner, B.; Tran, K.A.; Green, D.R.; Xavier, R.J.; et al. Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc. Natl. Acad. Sci. USA 2013, 110, 20364–20371. [Google Scholar] [CrossRef]
- Schmeisser, K.; Parker, J.A. Pleiotropic Effects of mTOR and Autophagy During Development and Aging. Front. Cell Dev. Biol. 2019, 7, 192. [Google Scholar] [CrossRef]
- Bensalem, J.; Hattersley, K.J.; Hein, L.K.; Teong, X.T.; Carosi, J.M.; Hassiotis, S.; Grose, R.H.; Fourrier, C.; Heilbronn, L.K.; Sargeant, T.J. Measurement of autophagic flux in humans: An optimized method for blood samples. Autophagy 2021, 17, 3238–3255. [Google Scholar] [CrossRef]
- Ginet, V.; Puyal, J.; Truttmann, A.C. Autophagy-related proteins measured in umbilical blood cord samples from human newborns: What can we learn from? Pediatr. Res. 2024, 96, 1120–1122. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y.; Xu, B.; Zhu, X.; Mou, J.; Xie, J.; Che, Z.; Zuo, L.; Li, J.; Jia, H.; et al. Recent advances in the roles of extracellular vesicles in cardiovascular diseases: Pathophysiological mechanisms, biomarkers, and cell-free therapeutic strategy. Mol. Med. 2025, 31, 169. [Google Scholar] [CrossRef] [PubMed]
- Blumer, V.; Januzzi, J.L., Jr.; Lindenfeld, J.; Solomon, S.D.; Psotka, M.A.; Carson, P.E.; Bristow, M.R.; Abraham, W.T.; Gandotra, C.; Saville, B.R.; et al. Heart Failure Drug Development Over the Eras: From the Heart Failure Collaboratory. JACC Heart Fail. 2024, 12, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Peng, P.; Xiao, F.; Tian, J.; He, X.; Lu, S.; Xiao, H.; He, M.; Wei, Q. Plasma SQSTM1/p62 act as a biomarker for steroid-induced osteonecrosis of the femoral head. Sci. Rep. 2024, 14, 24932. [Google Scholar] [CrossRef] [PubMed]
- Mengozzi, A.; Armenia, S.; De Biase, N.; Punta, L.D.; Cappelli, F.; Duranti, E.; Nannipieri, V.; Remollino, R.; Trico, D.; Virdis, A.; et al. Circulating mitochondrial DNA signature in cardiometabolic patients. Cardiovasc. Diabetol. 2025, 24, 106. [Google Scholar] [CrossRef]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Tao, M.; Dhaliwal, S.; Ghosalkar, D.; Sheng, S.; Dianati-Maleki, N.; Tam, E.; Rahman, T.; Mann, N.; Kort, S. Utility of native T1 mapping and myocardial extracellular volume fraction in patients with nonischemic dilated cardiomyopathy: A systematic review and meta-analysis. Int. J. Cardiol. Heart Vasc. 2024, 51, 101339. [Google Scholar] [CrossRef]
- Mihos, C.G.; Liu, J.E.; Anderson, K.M.; Pernetz, M.A.; O’Driscoll, J.M.; Aurigemma, G.P.; Ujueta, F.; Wessly, P.; American Heart Association Council on Peripheral Vascular Disease; Council on Cardiovascular and Stroke Nursing and Council on Clinical Cardiology; et al. Speckle-Tracking Strain Echocardiography for the Assessment of Left Ventricular Structure and Function: A Scientific Statement From the American Heart Association. Circulation 2025, 152, e96–e109. [Google Scholar] [CrossRef]
- Weng, H.; Zou, W.; Tian, F.; Xie, H.; Liu, A.; Liu, W.; Liu, Y.; Zhou, N.; Cai, X.; Wu, J.; et al. Inhalable cardiac targeting peptide modified nanomedicine prevents pressure overload heart failure in male mice. Nat. Commun. 2024, 15, 6058. [Google Scholar] [CrossRef]
- Tu, M.; Tan, V.P.; Yu, J.D.; Tripathi, R.; Bigham, Z.; Barlow, M.; Smith, J.M.; Brown, J.H.; Miyamoto, S. RhoA signaling increases mitophagy and protects cardiomyocytes against ischemia by stabilizing PINK1 protein and recruiting Parkin to mitochondria. Cell Death Differ. 2022, 29, 2472–2486. [Google Scholar] [CrossRef]
- Wang, B.; Nie, J.; Wu, L.; Hu, Y.; Wen, Z.; Dong, L.; Zou, M.H.; Chen, C.; Wang, D.W. AMPKalpha2 Protects Against the Development of Heart Failure by Enhancing Mitophagy via PINK1 Phosphorylation. Circ. Res. 2018, 122, 712–729. [Google Scholar] [CrossRef]
- Kim, Y.; Landstrom, A.P.; Shah, S.H.; Wu, J.C.; Seidman, C.E.; American Heart, A. Gene Therapy in Cardiovascular Disease: Recent Advances and Future Directions in Science: A Science Advisory From the American Heart Association. Circulation 2024, 150, e471–e480. [Google Scholar] [CrossRef]
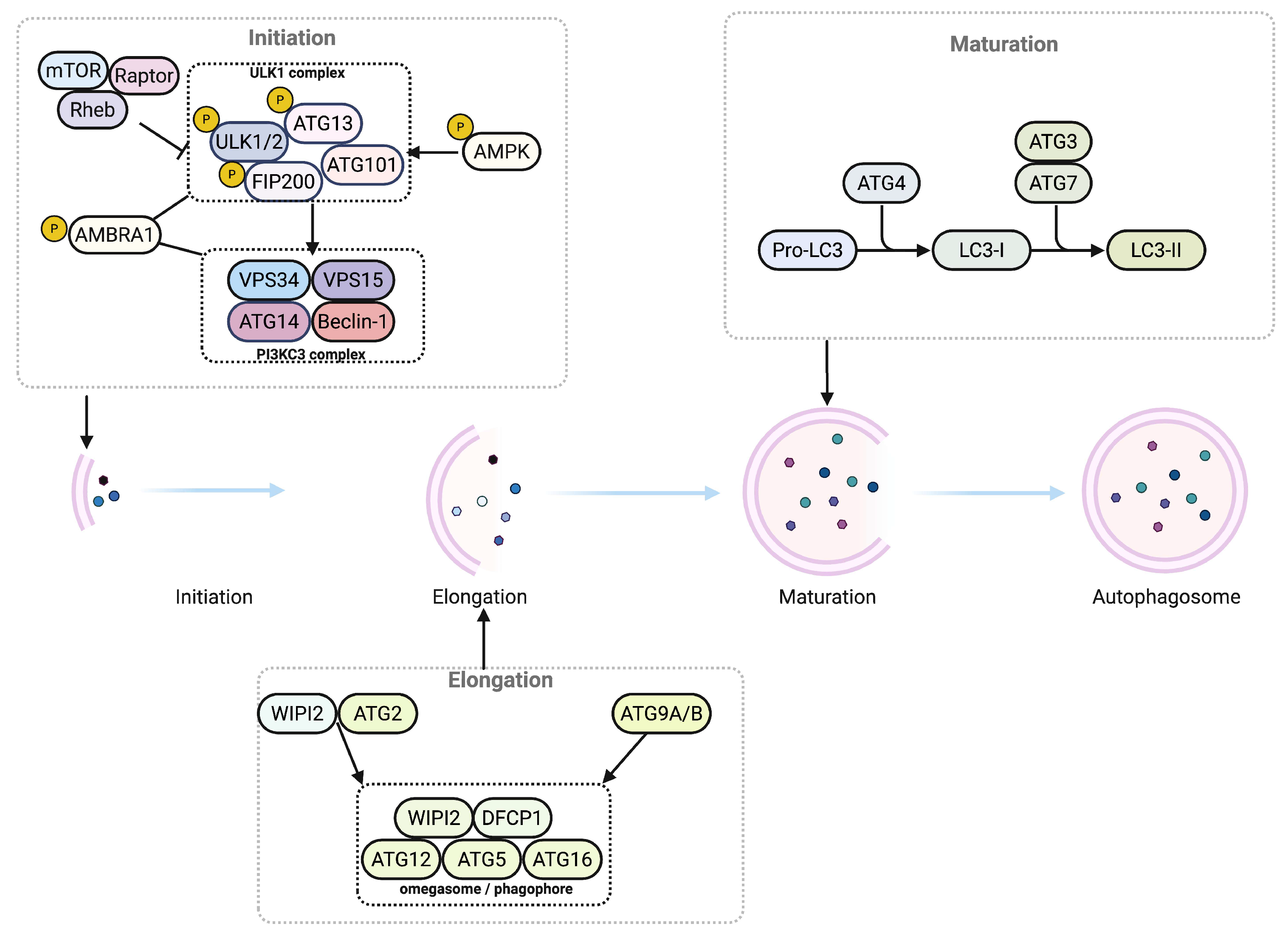
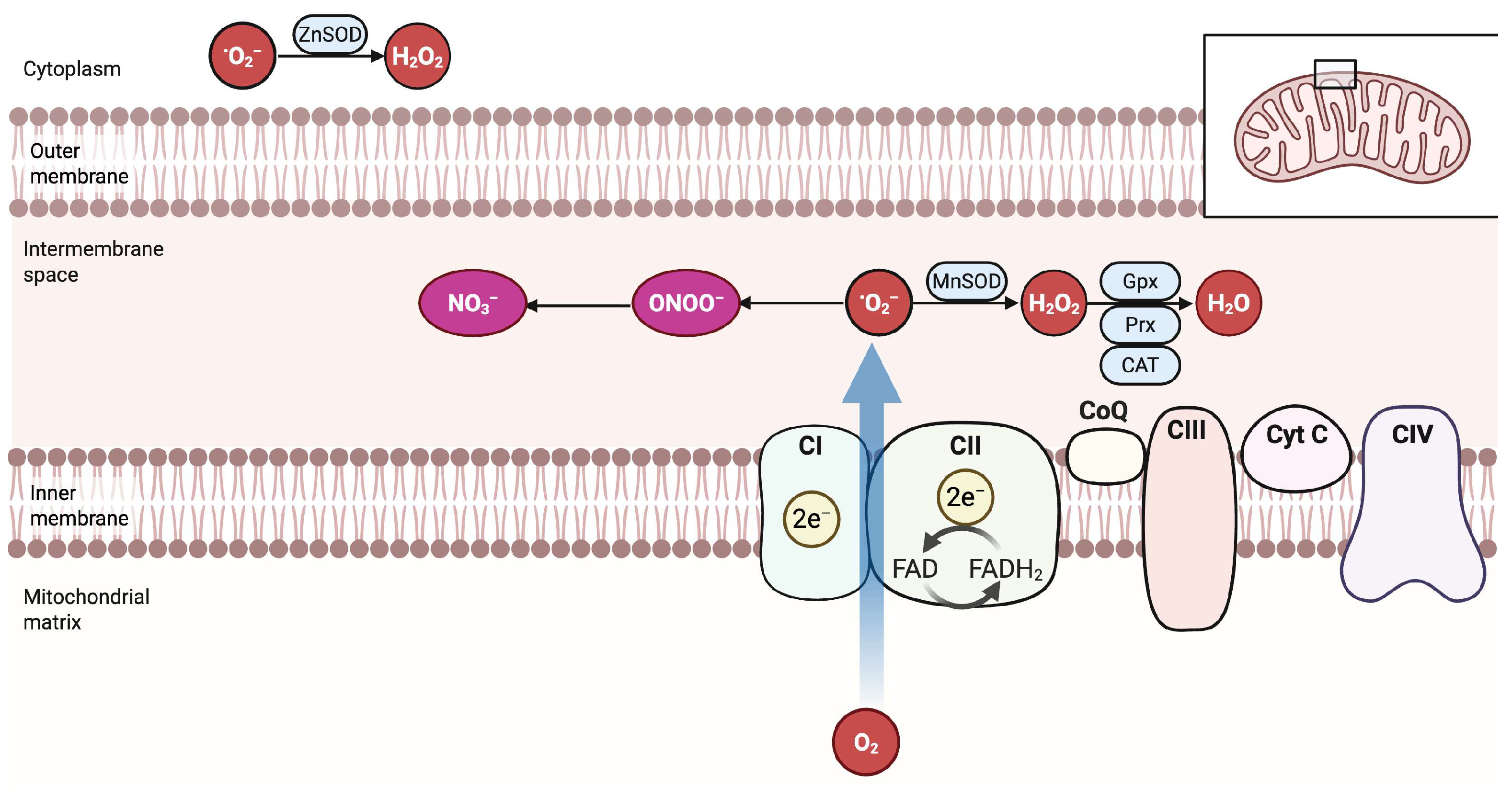

| Term | Operational Definition | Representative Measurement |
|---|---|---|
| Autophagic flux | Net turnover of autophagic cargo from autophagosome formation to lysosomal degradation | LC3-II turnover with lysosomal inhibitors such as bafilomycin A1 or chloroquine, p62 or SQSTM1 degradation, tandem mRFP GFP LC3 reporter, electron microscopy quantifying autophagosomes and autolysosomes |
| Mitophagy | Selective autophagic clearance of mitochondria | PINK1 stabilization and Parkin recruitment, mt-Keima or mito QC reporters, LC3 colocalization with mitochondrial markers, loss of mitochondrial proteins during flux blockade |
| Lysosomal competence | Capacity of lysosomes to acidify and degrade cargo | Lysosomal pH probes, such as LysoSensor or LysoTracker, with calibration; cathepsin activity assays; DQ BSA degradation; TFEB nuclear localization as an indirect marker |
| TFEB activation | Induction of lysosome and autophagy gene programs by TFEB | Nuclear TFEB localization, expression of CLEAR network targets, reporter assays |
| Mitochondrial ROS (mtROS) | Reactive oxygen species generated within mitochondria | MitoSOX with appropriate controls, targeted redox probes such as roGFP Orp1, electron paramagnetic resonance when available |
| Mitochondrial membrane potential (ΔΨm) | Electrical potential across the inner mitochondrial membrane | TMRM or TMRE in non-quench mode with calibration, JC 1 with caution |
| Autophagosome–lysosome fusion | Fusion of LC3-positive autophagosomes with LAMP1- or LAMP2-positive lysosomes | STX17 SNAP29 VAMP8 assays, LC3 and LAMP co localization, tandem mRFP GFP LC3 quench analysis |
| Lysosomal membrane permeabilization | Loss of lysosomal integrity with cathepsin release | Galectin 3 puncta, acridine orange relocation, cathepsin activity in cytosol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, H.R.; Singh, M.K.; Han, S.; Ranbhise, J.S.; Ha, J.; Kim, S.S.; Kang, I. Roles of Autophagy and Oxidative Stress in Cardiovascular Disease. Antioxidants 2025, 14, 1263. https://doi.org/10.3390/antiox14101263
Yun HR, Singh MK, Han S, Ranbhise JS, Ha J, Kim SS, Kang I. Roles of Autophagy and Oxidative Stress in Cardiovascular Disease. Antioxidants. 2025; 14(10):1263. https://doi.org/10.3390/antiox14101263
Chicago/Turabian StyleYun, Hyeong Rok, Manish Kumar Singh, Sunhee Han, Jyotsna S. Ranbhise, Joohun Ha, Sung Soo Kim, and Insug Kang. 2025. "Roles of Autophagy and Oxidative Stress in Cardiovascular Disease" Antioxidants 14, no. 10: 1263. https://doi.org/10.3390/antiox14101263
APA StyleYun, H. R., Singh, M. K., Han, S., Ranbhise, J. S., Ha, J., Kim, S. S., & Kang, I. (2025). Roles of Autophagy and Oxidative Stress in Cardiovascular Disease. Antioxidants, 14(10), 1263. https://doi.org/10.3390/antiox14101263







