Stress Pathways in Chronic Kidney Disease: Linking Cortisol, Oxidative Stress, and Inflammation
Abstract
1. Introduction
2. Pathophysiological Links Between CKD and Stress Response
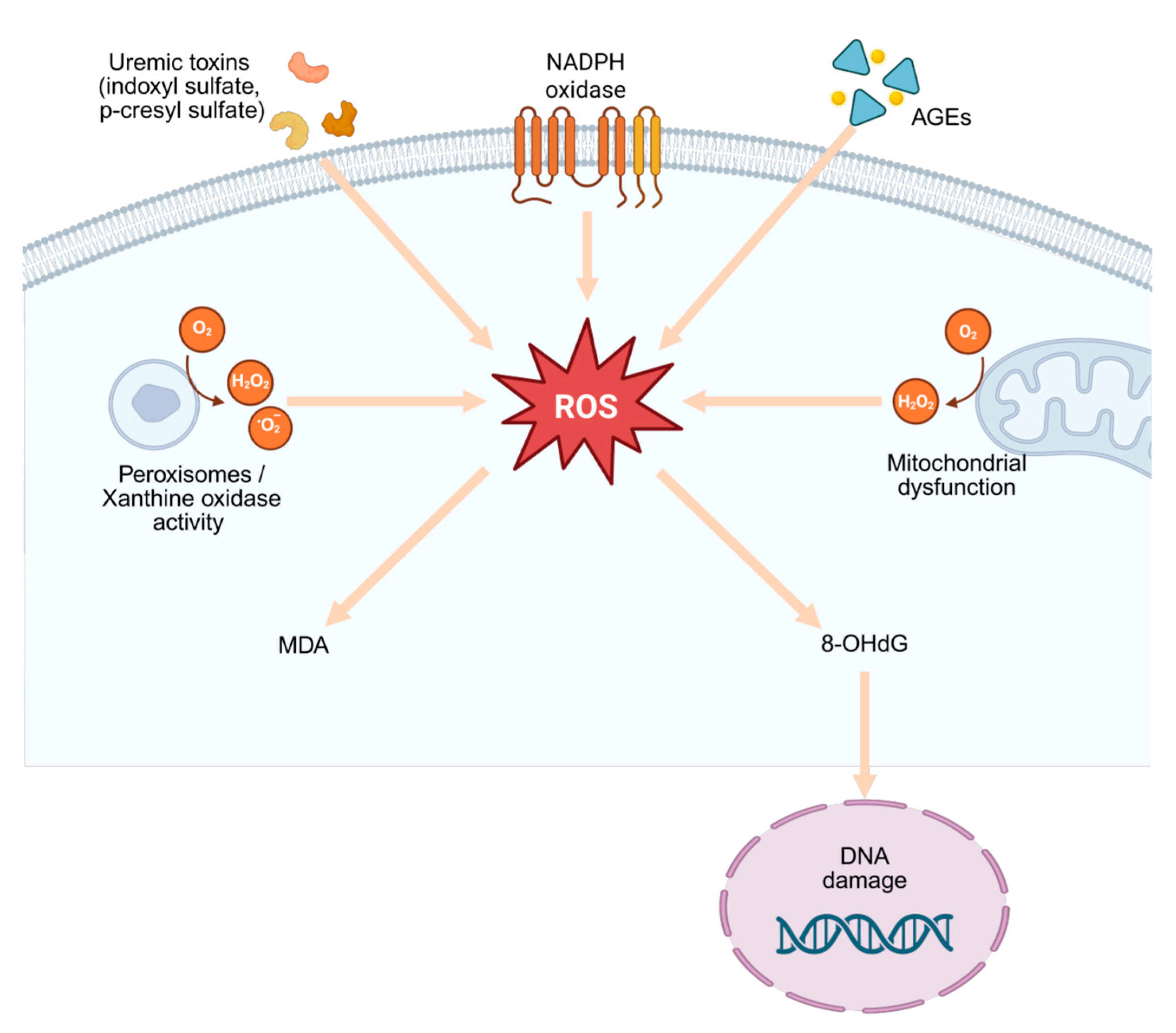
2.1. Oxidative Stress in CKD
| Aspect | Mechanism/Effect | References |
|---|---|---|
| ROS sources in CKD | Mitochondrial dysfunction, NOX4/NOX5, xanthine oxidase, myeloperoxidase, uncoupled eNOS; amplified under uremic conditions. | [11] |
| Antioxidant defense impairment | Depletion of enzymatic antioxidants (SOD, CAT, GPx) and non-enzymatic systems (GSH, vitamins). | [12] |
| Lipid peroxidation biomarkers | MDA, F2-isoprostanes ↑ with CKD stage, inversely correlate with eGFR. | [15] |
| Protein oxidation biomarkers | AOPPs and protein carbonyls ↑, associated with inflammation and renal fibrosis. | [16] |
| DNA oxidation biomarker | 8-OHdG reflects cumulative ROS burden, predicts progression and cardiovascular risk. | [17] |
| Composite indices | OBS: integrates diet and biomarkers, inversely associated with CKD incidence/progression. | [18] |
| Mitochondrial dysfunction | Impaired biogenesis, mitophagy, and dynamics drive ROS overproduction; activate NF-κB and NLRP3 inflammasome. | [19,20,21] |
| Phosphate-driven stress | Hyperphosphataemia activates NOX, causes endothelial injury, amplifies vascular damage. | [22] |
| Role of NOX isoforms | NOX4 has dual injury/protective role; endothelial NOX5 (human-specific) worsens diabetic kidney injury. | [23] |
| Mechanism-based therapies | Selective NOX4 inhibition preserves mitochondria and reduces injury. | [24] |
| Nutritional antioxidants | Polyphenol/flavonoid-rich diets show protective effects in CKD. | [25] |
| Targeting redox regulation | Nrf2 pathway activation restores cellular redox balance; context-dependent effects. | [27] |
| Clinical pharmacotherapy | SGLT2 inhibitors lower oxidative/inflammatory markers and improve renal outcomes. | [28] |
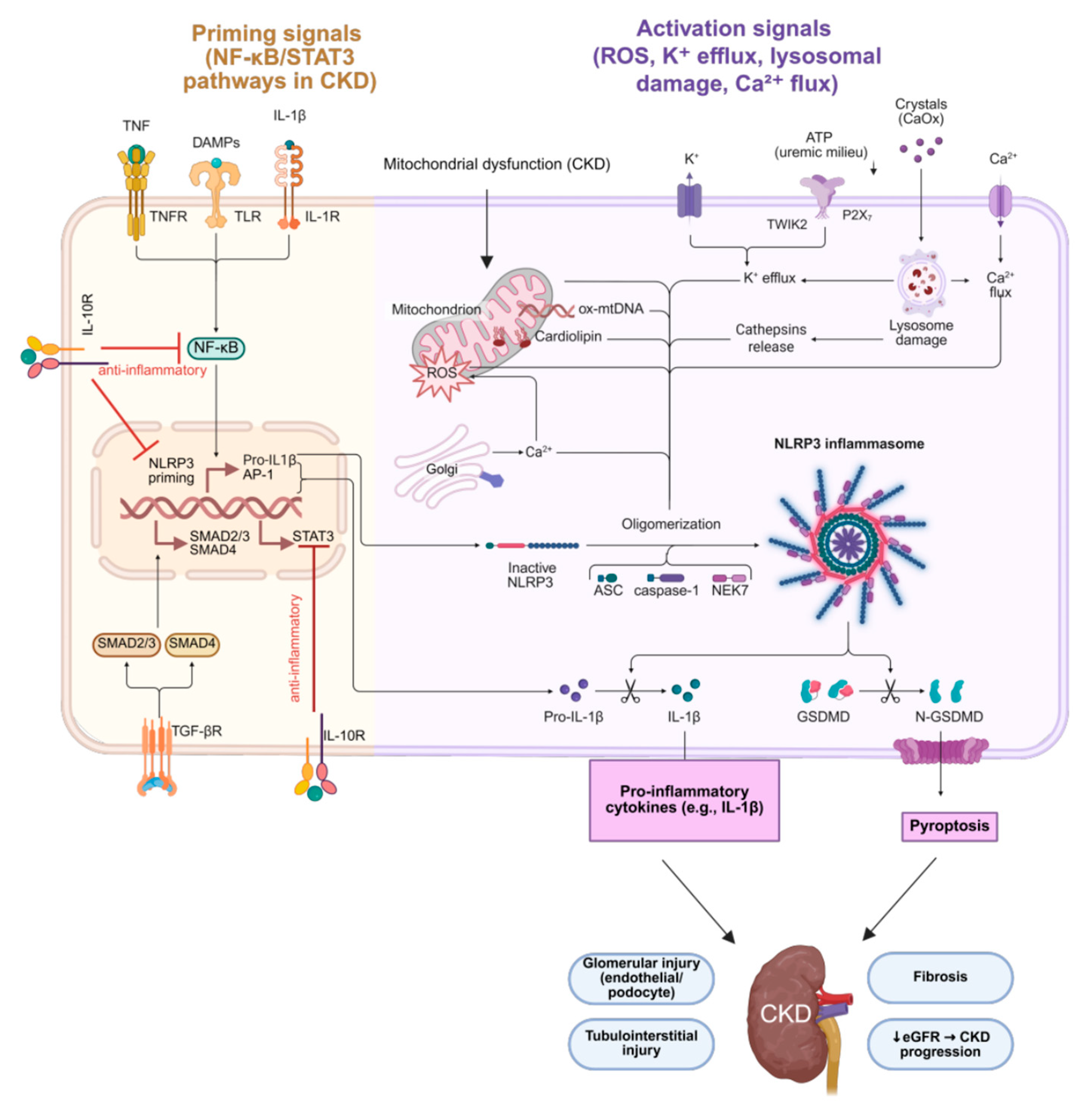
2.2. Inflammation and CKD
| Aspect | Mechanism/Effect | References |
|---|---|---|
| Chronic low-grade inflammation | Persistent systemic immune activation with elevated cytokines, driving fibrosis and cardiovascular comorbidities. | [29] |
| NLRP3 inflammasome | Activated in renal myeloid cells → ↑ IL-1β → amplifies inflammation and accelerates atherosclerosis. | [30] |
| Downstream signaling | JAK/STAT and NF-κB activation → adhesion molecules, chemokines, TGF-β (profibrotic). | [31] |
| AhR signaling | Mediates oxidative stress and inflammation; overactivation promotes glomerular injury and tubular apoptosis. | [32] |
| IL-6 | Central cytokine linking CKD, diabetes, and CVD; ↑ IL-6 and CRP correlate with low eGFR; therapeutic target. | [33,34,35] |
| TNF-α | Produced by immune, renal, and endothelial cells; drives IL-1β/IL-6 via NF-κB and MAPK; ↑ endothelial dysfunction and proteinuria. | [36,37] |
| Novel TNF-α mechanisms | Granulosa cell–derived TNF-α induces tubular apoptosis (PCOS-related); lin28a overexpression aggravates renal inflammation. | [38,39] |
| Gut microbiota dysbiosis | Endotoxin and metabolite translocation → TLR activation, oxidative stress, systemic inflammation. | [40] |
| IL-10 (anti-inflammatory) | Suppresses TNF-α, IL-1β, IL-6; reduced IL-10 in CKD linked with uncontrolled inflammation and fibrosis. | [43] |
2.3. HPA/PNEI Axis Dysregulation and CKD
2.4. Metabolic Risk Factors and CKD
2.4.1. Hypertension in CKD
2.4.2. Diabetes in CKD
2.4.3. Obesity in CKD
2.4.4. Dyslipidemia and Metabolic Syndrome in CKD
2.5. CKD and Accelerated Aging
2.6. The Relationship Between Diet, Cortisol, and CKD
3. Discussion
3.1. Integrated Mechanistic Perspective
3.2. Biomarkers and Clinical Relevance
3.3. Therapeutic Implications
3.4. Future Directions
3.5. Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| AhR | Aryl hydrocarbon receptor |
| AOPPs | Advanced oxidation protein products |
| BP | Blood pressure |
| CAT | Catalase |
| CKD | Chronic kidney disease |
| CRP | C-reactive protein |
| CVD | Cardiovascular disease |
| eGFR | Estimated glomerular filtration rate |
| eNOS | Endothelial nitric oxide synthase |
| GLP-1RA | Glucagon-like peptide-1 receptor agonist |
| GPx | Glutathione peroxidase |
| GSH | Reduced glutathione |
| HPA axis | Hypothalamic–pituitary–adrenal axis |
| HDL | High-density lipoprotein |
| IL | Interleukin (IL-1β, IL-6, IL-10) |
| LDL | Low-density lipoprotein |
| MAPK | Mitogen-activated protein kinase |
| MDA | Malondialdehyde |
| MHY11 | Myosin heavy chain 11 |
| m6A | N6-methyladenosine (RNA modification) |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NOD-like receptor family pyrin domain-containing 3 inflammasome |
| NOX | NADPH oxidase (isoforms: NOX4, NOX5) |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| OBS | Oxidative balance score |
| PCOS | Polycystic ovary syndrome |
| PNEI | Psycho-neuro-endocrine-immune axis |
| PPARs | Peroxisome proliferator-activated receptors |
| RAAS | Renin–angiotensin–aldosterone system |
| ROS | Reactive oxygen species |
| RUNX2 | Runt-related transcription factor 2 |
| SASP | Senescence-associated secretory phenotype |
| SGLT2(i) | Sodium–glucose cotransporter-2 (inhibitors) |
| SII | Systemic immune-inflammation index |
| SIRT1 | Sirtuin 1 |
| SOD | Superoxide dismutase |
| SREBP-1 | Sterol regulatory element-binding protein 1 |
| TBARS | Thiobarbituric acid reactive substances |
| TGF-β | Transforming growth factor beta |
| Th1/Th17 | T helper 1/T helper 17 cells |
| TLR(s) | Toll-like receptor(s) |
| TNF-α | Tumor necrosis factor alpha |
References
- Kovesdy, C.P. Epidemiology of Chronic Kidney Disease: An Update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Mallamaci, F.; Tripepi, G. Risk Factors of Chronic Kidney Disease Progression: Between Old and New Concepts. J. Clin. Med. 2024, 13, 678. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Chertow, G.M.; Devarajan, P.; Levin, A.; Andreoli, S.P.; Bangalore, S.; Warady, B.A. Chronic Inflammation in Chronic Kidney Disease Progression: Role of Nrf2. Kidney Int. Rep. 2021, 6, 1775–1787. [Google Scholar] [CrossRef]
- Ebert, T.; Pawelzik, S.-C.; Witasp, A.; Arefin, S.; Hobson, S.; Kublickiene, K.; Shiels, P.G.; Bäck, M.; Stenvinkel, P. Inflammation and Premature Ageing in Chronic Kidney Disease. Toxins 2020, 12, 227. [Google Scholar] [CrossRef]
- Chen, Y.; Nie, Y.; Wu, J.; Li, C.; Zheng, L.; Zhu, B.; Min, Y.; Ling, T.; Liu, X. Association between Systemic Inflammatory Indicators with the Survival of Chronic Kidney Disease: A Prospective Study Based on NHANES. Front. Immunol. 2024, 15, 1365591. [Google Scholar] [CrossRef] [PubMed]
- Tsinari, A.; Roumeliotis, S.; Neofytou, I.E.; Varouktsi, G.; Veljkovic, A.; Stamou, A.; Leivaditis, K.; Liakopoulos, V. The Clinical Utility and Plausibility of Oxidative and Antioxidant Variables in Chronic and End-Stage Kidney Disease: A Review of the Literature. Int. J. Mol. Sci. 2025, 26, 3376. [Google Scholar] [CrossRef] [PubMed]
- Assani, M.Z.; Novac, M.B.; Dijmărescu, A.L.; Maiga, A.; Diallo, M.; Iancu, S.; Petrescu, R.; Stan, M.; Enache, D.; Mogoșanu, G.D. Intersecting Pathways of Inflammation, Oxidative Stress, and Atherogenesis in the Evaluation of CKD: Emerging Biomarkers PCSK9, EPHX2, AOPPs, and TBARSs. Life 2025, 15, 1287. [Google Scholar] [CrossRef]
- Dragoș, D.; Ionescu, R.; Matei, D.; Marinescu, A.; Tănăsescu, M.; Bălănescu, P.; Voiculescu, M. Oxidative Stress and Nutritional Antioxidants in Renal Disease. Antioxidants 2025, 14, 757. [Google Scholar] [CrossRef]
- Sagmeister, M.S.; Kerschbaum, J.; Kirsch, A.H.; Koller, L.; Kramar, R.; Rudnicki, M.; Mayer, G. Cortisol Excess in Chronic Kidney Disease—A Review of Changes and Impact on Mortality. Front. Endocrinol. 2023, 13, 1075809. [Google Scholar] [CrossRef]
- Bivolarska, A. Textbook of Medical Biochemistry, Part II (Functional Biochemistry); Lax Book: Plovdiv, Bulgaria, 2025. [Google Scholar]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Schmitt, R.; Melk, A. Molecular Mechanisms of Renal Aging. Kidney Int. 2017, 92, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Podkowińska, A.; Formanowicz, D. Chronic Kidney Disease as Oxidative Stress- and Inflammatory-Mediated Cardiovascular Disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef]
- Ito, F.; Sono, Y.; Ito, T. Measurement and Clinical Significance of Lipid Peroxidation as a Biomarker of Oxidative Stress: Oxidative Stress in Diabetes, Atherosclerosis, and Chronic Inflammation. Antioxidants 2019, 8, 72. [Google Scholar] [CrossRef]
- Vodošek Hojs, N.; Bevc, S.; Ekart, R.; Hojs, R. Oxidative Stress Markers in Chronic Kidney Disease with Emphasis on Diabetic Nephropathy. Antioxidants 2020, 9, 925. [Google Scholar] [CrossRef]
- Di Minno, A.; Turnu, L.; Porro, B.; Squellerio, I.; Cavalca, V.; Tremoli, E.; Di Minno, M.N. 8-Hydroxy-2-Deoxyguanosine Levels and Cardiovascular Disease: A Systematic Review and Meta-Analysis of the Literature. Antioxid. Redox Signal. 2016, 24, 548–555. [Google Scholar] [CrossRef]
- Yin, Y.; Zhao, C.; Niu, Y.; Qi, J.; Zhang, Y.; Lu, B. Associations between Oxidative Balance Score and Chronic Kidney Disease Events in US Adults: A Population-Based Study. Sci. Rep. 2024, 14, 13743. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pang, Y.; Fan, X. Mitochondria in Oxidative Stress, Inflammation and Aging: From Mechanisms to Therapeutic Advances. Signal Transduct. Target. Ther. 2025, 10, 190. [Google Scholar] [CrossRef]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression through Oxidative Stress. Front. Physiol. 2021, 12, 627837. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wang, G.; Shi, X. Advances in the Progression and Prognosis Biomarkers of Chronic Kidney Disease. Front. Pharmacol. 2021, 12, 785375. [Google Scholar] [CrossRef]
- Lacerda-Abreu, M.A.; Meyer-Fernandes, J.R. Hyperphosphataemia and NADPH Oxidase Regulation in Pathophysiological Processes: Implications for Oxidative Stress and Disease Progression. Antioxidants 2025, 14, 461. [Google Scholar] [CrossRef]
- Jha, J.C.; Banal, C.; Okabe, J.; Gray, S.P.; Hettige, T.; Chow, B.S.M.; Thallas-Bonke, V.; De Vos, L.; Holterman, C.E.; Coughlan, M.T.; et al. NADPH Oxidase Nox5 Accelerates Renal Injury in Diabetic Nephropathy. Diabetes 2017, 66, 2691–2703. [Google Scholar] [CrossRef]
- Schiffer, T.A.; Carvalho, L.R.R.A.; Guimaraes, D.; Boeder, A.; Wikström, P.; Carlström, M. Specific NOX4 Inhibition Preserves Mitochondrial Function and Dampens Kidney Dysfunction Following Ischemia–Reperfusion-Induced Kidney Injury. Antioxidants 2024, 13, 489. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-L.; Lin, J.-H.; Hammes, H.-P.; Zhang, C. Flavonoids in Treatment of Chronic Kidney Disease. Molecules 2022, 27, 2365. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Chopra, H.; Goyal, R.; Jin, S.; Dong, Z.; Das, T.; Bhattacharya, T. Therapeutic Effect of Targeted Antioxidant Natural Products. Discov. Nano 2024, 19, 144. [Google Scholar] [CrossRef]
- Lim, T.S.T.; Ng, K.H.; Zhang, Y. NRF2 Dysregulation and Therapeutic Insights Across Chronic Kidney Diseases. Int. J. Mol. Sci. 2025, 26, 7471. [Google Scholar] [CrossRef]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.M.; Albulescu, L.; Necula, L.G.; Petrescu, D.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef]
- Islamuddin, M.; Qin, X. Renal Macrophages and NLRP3 Inflammasomes in Kidney Diseases and Therapeutics. Cell Death Discov. 2024, 10, 229. [Google Scholar] [CrossRef]
- Li, G.; Yang, H.; Zhang, D.; Zhang, Y.; Liu, B.; Wang, Y.; Zhou, H.; Xu, Z.-X.; Wang, Y. The Role of Macrophages in Fibrosis of Chronic Kidney Disease. Biomed. Pharmacother. 2024, 177, 117079. [Google Scholar] [CrossRef]
- Mo, Y.; Lu, Z.; Wang, L.; Ji, C.; Zou, C.; Liu, X. The Aryl Hydrocarbon Receptor in Chronic Kidney Disease: Friend or Foe? Front. Cell Dev. Biol. 2020, 8, 589752. [Google Scholar] [CrossRef] [PubMed]
- Kreiner, F.F.; Kraaijenhof, J.M.; von Herrath, M.; Hovingh, G.K.K.; von Scholten, B.J. Interleukin 6 in Diabetes, Chronic Kidney Disease, and Cardiovascular Disease: Mechanisms and Therapeutic Perspectives. Expert Rev. Clin. Immunol. 2022, 18, 377–389. [Google Scholar] [CrossRef]
- Prodhan, M.J.A.; Al Mahmud, M.; Saha, S.K.; Salauddin, S.M.; Ali, M. Association of Inflammatory Marker C-Reactive Protein and Interleukin-6 with Stages 3–5 of Chronic Kidney Disease. Saudi J. Med. 2024, 9, 344–353. [Google Scholar] [CrossRef]
- Chen, W.; Yuan, H.; Cao, W.; Wang, T.; Chen, W.; Yu, H.; Fu, Y.; Jiang, B.; Zhou, H.; Guo, H.; et al. Blocking Interleukin-6 Trans-Signaling Protects Against Renal Fibrosis by Suppressing STAT3 Activation. Theranostics 2019, 9, 3980–3991. [Google Scholar] [CrossRef]
- Mehaffey, E.; Majid, D.S.A. Tumor Necrosis Factor-α, Kidney Function, and Hypertension. Am. J. Physiol. Ren. Physiol. 2017, 313, F1005–F1008. [Google Scholar] [CrossRef]
- Baaten, C.C.F.M.J.; Vondenhoff, S.; Noels, H. Endothelial Cell Dysfunction and Increased Cardiovascular Risk in Patients With Chronic Kidney Disease. Circ. Res. 2023, 132, 970–992. [Google Scholar] [CrossRef]
- Ye, H.Y.; Song, Y.L.; Ye, W.T.; Fang, S.S.; Liu, X.X.; Zhang, W.W.; Zhuang, G.Z.; Wang, J.Y. Serum Granulosa Cell-Derived TNF-α Promotes Inflammation and Apoptosis of Renal Tubular Cells and PCOS-Related Kidney Injury Through NF-κB Signaling. Acta Pharmacol. Sin. 2023, 44, 2432–2444. [Google Scholar] [CrossRef]
- Futorian, A.; Armon, L.; Waldman Ben-Asher, H.; Levi, R.; Meidan, E.; Davidovits, M.; Goldstein, R.S. Nephron-Specific Lin28A Overexpression Triggers Severe Inflammatory Response and Kidney Damage. Int. J. Biol. Sci. 2024, 20, 4044–4054. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Shan, Q.Y.; Wu, X.; Miao, H.; Zhao, Y.Y. Gut Microbiota Regulates Oxidative Stress and Inflammation: A Double-Edged Sword in Renal Fibrosis. Cell Mol. Life Sci. 2024, 81, 480. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Sun, W.; Liu, B.; Zhang, Y.; He, X.; Chen, L.; Yang, J. Chk2 Modulates Bmi1-Deficiency-Induced Renal Aging and Fibrosis via Oxidative Stress, DNA Damage, and p53/TGFβ1-Induced Epithelial-Mesenchymal Transition. Int. J. Biol. Sci. 2024, 20, 2008–2026. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Jiang, F.; Xu, X.J.; Liu, Y.; Chen, Q.; Li, S.; Zhou, H. Inhibition of IGF2BP1 Attenuates Renal Injury and Inflammation by Alleviating m6A Modifications and E2F1/MIF Pathway. Int. J. Biol. Sci. 2023, 19, 593–609. [Google Scholar] [CrossRef]
- Prionas, A.; Hamaoui, K.; Vanezis, K.; Reebye, V.; Habib, N.; Papalois, V. The Effect of Interleukin-10 Immunotherapy on Renal Ischemia-Reperfusion Injury: A Systematic Review and Meta-Analysis of Preclinical Studies. Int. J. Mol. Sci. 2024, 25, 6231. [Google Scholar] [CrossRef]
- Cardoso, E.M.; Arregger, A.L.; Budd, D.; Zucchini, A.E.; Contreras, L.N. Dynamics of Salivary Cortisol in Chronic Kidney Disease Patients at Stages 1 through 4. Clin. Endocrinol. 2016, 85, 313–319. [Google Scholar] [CrossRef]
- Boswell, L.; Vega-Beyhart, A.; Blasco, M.; White, A.; Patel, N.; Fernandez, R.; Smith, J.; Clements, J. Hair Cortisol and Changes in Cortisol Dynamics in Chronic Kidney Disease. Front. Endocrinol. 2024, 15, 1282564. [Google Scholar] [CrossRef]
- Sagmeister, M.S.; Taylor, A.E.; Fenton, A.; Wall, N.A.; Chanouzas, D.; Nightingale, P.G.; Ferro, C.J.; Arlt, W.; Cockwell, P.; Hardy, R.S.; et al. Glucocorticoid Activation by 11β-Hydroxysteroid Dehydrogenase Enzymes in Relation to Inflammation and Glycaemic Control in Chronic Kidney Disease: A Cross-Sectional Study. Clin. Endocrinol. 2019, 90, 241–249. [Google Scholar] [CrossRef]
- Jang, K.W.; Hur, J.; Lee, D.W.; Kim, S.R. Metabolic Syndrome, Kidney-Related Adiposity, and Kidney Microcirculation: Unraveling the Damage. Biomedicines 2024, 12, 2706. [Google Scholar] [CrossRef]
- Amasi-Hartoonian, N.; Sforzini, L.; Cattaneo, A.; Pariante, C.M. Cause or Consequence? Understanding the Role of Cortisol in the Increased Inflammation Observed in Depression. Curr. Opin. Endocr. Metab. Res. 2022, 24, 100356. [Google Scholar] [CrossRef]
- Ameer, O.Z. Hypertension in Chronic Kidney Disease: What Lies behind the Scene. Front. Pharmacol. 2022, 13, 949260. [Google Scholar] [CrossRef] [PubMed]
- Arendshorst, W.J.; Vendrov, A.E.; Kumar, N.; Ganesh, S.K.; Madamanchi, N.R. Oxidative Stress in Kidney Injury and Hypertension. Antioxidants 2024, 13, 1454. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Ullah, M.M.; Collet, J.A.; Mehrotra, P. T Helper 17 Cells in the Pathophysiology of Acute and Chronic Kidney Disease. Kidney Res. Clin. Pract. 2021, 40, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.M.; Sagmeister, M.S.; Fenton, C.G.; Seabright, A.P.; Lai, Y.-C.; Jones, S.W.; Filer, A.; Cooper, M.S.; Lavery, G.G.; Raza, K.; et al. Global Deletion of 11β-HSD1 Prevents Muscle Wasting Associated with Glucocorticoid Therapy in Polyarthritis. Int. J. Mol. Sci. 2021, 22, 7828. [Google Scholar] [CrossRef]
- Gianotti, L.; Loviselli, E.; De Marchi, S.; Tassone, F. The Stress Axis in Obesity and Diabetes Mellitus: An Update. Endocrines 2021, 2, 334–347. [Google Scholar] [CrossRef]
- Yu, P.; Meng, X.; Kan, R.; Wang, Z.; Yu, X. Association between Metabolic Scores for Visceral Fat and Chronic Kidney Disease: A Cross-Sectional Study. Front. Endocrinol. 2022, 13, 1052736. [Google Scholar] [CrossRef]
- Wang, J.H.; Lin, Y.L.; Hsu, B.G. Endothelial Dysfunction in Chronic Kidney Disease: Mechanisms, Biomarkers, Diagnostics, and Therapeutic Strategies. Tzu Chi Med. J. 2025, 37, 125–134. [Google Scholar] [CrossRef]
- de la Puente-Aldea, J.; Lopez-Llanos, O.; Horrillo, D.; Marcos-Sanchez, H.; Sanz-Ballesteros, S.; Franco, R.; Jaisser, F.; Senovilla, L.; Palacios-Ramirez, R. Mineralocorticoid Receptor and Sleep Quality in Chronic Kidney Disease. Int. J. Mol. Sci. 2024, 25, 12320. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.F.; Hsu, P.T.; Yang, K.L. The Mediating Effect of Sleep Quality and Fatigue between Depression and Renal Function in Nondialysis Chronic Kidney Disease: A Cross-Sectional Study. BMC Nephrol. 2022, 23, 126. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Gamboa, J.L.; Shibao, C.A.; Cornelius, D.C.; Steppan, J.; Luther, J.M.; Ikizler, T.A.; Hatzoglou, A.; Roberts, L.J.; Shah, A.; et al. Exercise Modulates Sympathetic and Vascular Function in Older Adults with Chronic Kidney Disease. JCI Insight 2023, 8, e164221. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.; Kovesdy, C.P.; Clase, C.M.; Sood, M.M.; Pecoits-Filho, R. Aldosterone, Mineralocorticoid Receptor Activation, and CKD: A Review of Evolving Treatment Paradigms. Am. J. Kidney Dis. 2022, 80, 658–666. [Google Scholar] [CrossRef]
- Nowak, K.L.; Kakkar, R.; Devalaraja, M.; Lo, L.; Park, W.; Gobburu, J.; Kling, D.; Davidson, M.; Chonchol, M. A Phase 1 Randomized Dose-Escalation Study of a Human Monoclonal Antibody to IL-6 in CKD. Kidney360 2020, 2, 224–235. [Google Scholar] [CrossRef]
- Wada, Y.; Jensen, C.; Meyer, A.S.P.; Zonoozi, A.A.M.; Honda, H. Efficacy and Safety of Interleukin-6 Inhibition with Ziltivekimab in Patients at High Risk of Atherosclerotic Events in Japan (RESCUE-2): A Randomized, Double-Blind, Placebo-Controlled, Phase 2 Trial. J. Cardiol. 2023, 82, 279–285. [Google Scholar] [CrossRef]
- Arazi, H.; Mohabbat, M.; Saidie, P.; Falahati, A.; Suzuki, K. Effects of Different Types of Exercise on Kidney Diseases. Sports 2022, 10, 42. [Google Scholar] [CrossRef]
- Correa, H.L.; Rosa, T.S.; Santos, R.L.; Mestrinho, V.M.; Aquino, T.S.; Santos, W.O.; Neves, R.P.; Deus, L.A.; Reis, A.L.; Barbosa, J.M.; et al. The Impact of Different Exercise Modalities on Chronic Kidney Disease: An Umbrella Review of Meta-Analyses. Front. Physiol. 2025, 15, 1444976. [Google Scholar] [CrossRef]
- De Bhailis, Á.M.; Kalra, P.A. Hypertension and the kidneys. Br. J. Hosp. Med. 2022, 83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Cao, W.; Wu, L.; Zhang, X.; Zhou, J.; Wang, J.; Yang, Z.; Su, H.; Liu, Y.; Wilcox, C.S.; Hou, F.F. Sympathetic Overactivity in CKD Disrupts Buffering of Neurotransmission by Endothelium-Derived Hyperpolarizing Factor and Enhances Vasoconstriction. J. Am. Soc. Nephrol. 2020, 31, 2312–2325. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. Diabetes and Kidney Disease. IDF, 2023. Available online: https://diabetesatlas.org/atlas/diabetes-and-kidney-disease/ (accessed on 24 June 2025).
- Lin, Y.C.; Chang, Y.H.; Yang, S.Y.; Wu, K.D.; Chu, T.S. Update of pathophysiology and management of diabetic kidney disease. J. Formos. Med. Assoc. 2018, 117, 662–675. [Google Scholar] [CrossRef]
- Gupta, S.; Dominguez, M.; Golestaneh, L. Diabetic Kidney Disease: An Update. Med. Clin. N. Am. 2023, 107, 689–705. [Google Scholar] [CrossRef]
- Holliday, M.W., Jr.; Frost, L.; Navaneethan, S.D. Emerging Evidence for Glucagon-Like Peptide-1 Agonists in Slowing Chronic Kidney Disease Progression. Curr. Opin. Nephrol. Hypertens. 2024, 33, 331–336. [Google Scholar] [CrossRef]
- Taal, M.W.; Selby, N.M. Glucagon-like Peptide-1 Receptor Agonists: New Evidence of Kidney and Cardiovascular Protection from the FLOW and SELECT Trials. Am. J. Kidney Dis. 2025, 85, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.J.; Han, J.; Montez-Rath, M.E.; Chertow, G.M. Comparative Effectiveness of Sodium-Glucose Cotransporter-2 Inhibitors versus Glucagon-Like Peptide-1 Receptor Agonists in Patients with Type 2 Diabetes and Mild/Moderate Chronic Kidney Disease. Diabetes Obes. Metab. 2024, 26, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wang, Y.; Zhao, X.; Cui, H.; Han, M.; Ren, X.; Gang, X.; Wang, G. Obesity and Chronic Kidney Disease. Am. J. Physiol. Endocrinol. Metab. 2023, 324, E24–E41. [Google Scholar] [CrossRef]
- D’Agati, V.D.; Chagnac, A.; de Vries, A.P.; Levi, M.; Porrini, E.; Herman-Edelstein, M.; Praga, M. Obesity-related glomerulopathy: Clinical and pathologic characteristics and pathogenesis. Nat. Rev. Nephrol. 2016, 12, 453–471. [Google Scholar] [CrossRef]
- Stasi, A.; Cosola, C.; Caggiano, G.; Cimmarusti, M.T.; Palieri, R.; Acquaviva, P.M.; Rana, G.; Gesualdo, L. Obesity-related chronic kidney disease: Principal mechanisms and new approaches in nutritional management. Front. Nutr. 2022, 9, 925619. [Google Scholar] [CrossRef]
- Conley, M.M.; Mayr, H.L.; Hepburn, K.S.; Holland, J.J.; Mudge, D.W.; Tonges, T.J.; Modderman, R.S.; Gerzina, S.A.; Johnson, D.W.; Viecelli, A.K.; et al. Low Energy Diets for Obesity and CKD (SLOW-CKD Randomized Feasibility Study). Kidney Int. Rep. 2025, 10, 2153–2164. [Google Scholar] [CrossRef] [PubMed]
- Parvathareddy, V.P.; Ella, K.M.; Shah, M.; Navaneethan, S.D. Treatment Options for Managing Obesity in Chronic Kidney Disease. Curr. Opin. Nephrol. Hypertens. 2021, 30, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Taber-Hight, E.; Gilmore, A.; Friedman, A.N. Anti-Obesity Pharmacotherapy in Adults with Chronic Kidney Disease. Kidney Int. 2024, 105, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.H.; Kim, S.W. Dyslipidemia in Patients with Chronic Kidney Disease: An Updated Overview. Diabetes Metab. J. 2023, 47, 612–629. [Google Scholar] [CrossRef]
- Hoca, E.; Mermer, H.B.; Kula, A.C.; Ahbab, S.; Ataoglu, H.E. The Effects of Chronic Kidney Disease Stages on Dyslipidemia, Cardiovascular Disease Prevalence and Mortality. North. Clin. Istanb. 2025, 12, 95–102. [Google Scholar] [CrossRef]
- Yim, H.E.; Yoo, K.H. Obesity and Chronic Kidney Disease: Prevalence, Mechanism, and Management. Clin. Exp. Pediatr. 2021, 64, 511–518. [Google Scholar] [CrossRef]
- Xu, W.; Zhu, Y.; Wang, S.; Liu, J.; Li, H. From Adipose to Ailing Kidneys: The Role of Lipid Metabolism in Obesity-Related Chronic Kidney Disease. Antioxidants 2024, 13, 1540. [Google Scholar] [CrossRef]
- Tonelli, M.; Wanner, C. Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members. Lipid Management in Chronic Kidney Disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2013 Clinical Practice Guideline. Ann. Intern. Med. 2014, 160, 182. [Google Scholar] [CrossRef]
- Zhang, Y.; Ou, G.; Peng, L.; Pan, J.; Zhang, S.; Shi, J. Genetic Association Analysis of Lipid-Lowering Drug Target Genes in Chronic Kidney Disease. Front. Endocrinol. 2025, 15, 1434145. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Fontanesi, F.; Merscher, S.; Fornoni, A. The Vicious Cycle of Renal Lipotoxicity and Mitochondrial Dysfunction. Front. Physiol. 2020, 11, 732. [Google Scholar] [CrossRef] [PubMed]
- Glassock, R.J.; Rule, A.D. Aging and the Kidneys: Anatomy, Physiology and Consequences for Defining Chronic Kidney Disease. Nephron 2016, 134, 25–29. [Google Scholar] [CrossRef]
- Tanriover, C.; Copur, S.; Mutlu, A.; Peltek, I.B.; Galassi, A.; Ciceri, P.; Cozzolino, M.; Kanbay, M. Early Aging and Premature Vascular Aging in Chronic Kidney Disease. Clin. Kidney J. 2023, 16, 1751–1765. [Google Scholar] [CrossRef]
- Siracusa, C.; Carabetta, N.; Morano, M.B.; Manica, M.; Strangio, A.; Sabatino, J.; Leo, I.; Castagna, A.; Cianflone, E.; Torella, D.; et al. Understanding Vascular Calcification in Chronic Kidney Disease: Pathogenesis and Therapeutic Implications. Int. J. Mol. Sci. 2024, 25, 13096. [Google Scholar] [CrossRef]
- Arefin, S.; Mudrovcic, N.; Hobson, S.; Pietrocola, F.; Ebert, T.; Ward, L.J.; Witasp, A.; Hernandez, L.; Wennberg, L.; Lundgren, T.; et al. Early Vascular Aging in Chronic Kidney Disease: Focus on Microvascular Maintenance, Senescence Signature and Potential Therapeutics. Transl. Res. 2025, 275, 32–47. [Google Scholar] [CrossRef]
- Almalki, W.H.; Almujri, S.S. Oxidative Stress and Senescence in Aging Kidneys: The Protective Role of SIRT1. EXCLI J. 2024, 23, 1030–1067. [Google Scholar] [CrossRef]
- Levstek, T.; Trebušak Podkrajšek, K. Telomere Attrition in Chronic Kidney Diseases. Antioxidants 2023, 12, 579. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Chu, Q.; Lv, H.; Li, J.; Yi, F. Cellular Senescence in Kidney Diseases. Chin. Med. J. 2025, 138, 2234–2242. [Google Scholar] [CrossRef]
- Docherty, M.H.; O’Sullivan, E.D.; Bonventre, J.V.; Ferenbach, D.A. Cellular Senescence in the Kidney. J. Am. Soc. Nephrol. 2019, 30, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-C.; Wang, P.-C.; Hsiung, T.; Fan, Y.-H.; Wu, J.-T.; Kan, W.-C.; Shiao, C.-C. Sarcopenia in Chronic Kidney Disease: A Narrative Review from Pathophysiology to Therapeutic Approaches. Biomedicines 2025, 13, 352. [Google Scholar] [CrossRef]
- Lee, C.; Kim, H.J.; Chang, T.I.; Kang, E.W.; Joo, Y.S.; Kim, H.W.; Park, J.T.; Yoo, T.H.; Kang, S.W.; Han, S.H. Synergic Association of Diabetes Mellitus and Chronic Kidney Disease with Muscle Loss and Cachexia: Results of a 16-Year Longitudinal Follow-Up of a Community-Based Prospective Cohort Study. Aging 2021, 13, 21941–21961. [Google Scholar] [CrossRef]
- Li, J.; Tu, H.; Zhang, Y.; Yang, S.; Yu, P.; Liu, J. Risks of All-Cause Mortality in Adults With Chronic Kidney Disease With Sarcopenia or Obesity: A Population-Based Study. J. Cachexia Sarcopenia Muscle 2025, 16, e13828. [Google Scholar] [CrossRef]
- Di Polito, N.; Stylianakis, A.A.; Richardson, R.; Baker, K.D. Real-World Intake of Dietary Sugars Is Associated with Reduced Cortisol Reactivity Following an Acute Physiological Stressor. Nutrients 2023, 15, 209. [Google Scholar] [CrossRef]
- He, X.; Zhang, X.; Si, C.; Feng, Y.; Zhu, Q.; Li, S.; Shu, L. Ultra-Processed Food Consumption and Chronic Kidney Disease Risk: A Systematic Review and Dose-Response Meta-Analysis. Front. Nutr. 2024, 11, 1359229. [Google Scholar] [CrossRef]
- Alufer, L.; Tsaban, G.; Rinott, E.; Kaplan, A.; Meir, A.Y.; Zelicha, H.; Ceglarek, U.; Isermann, B.; Blüher, M.; Stumvoll, M.; et al. Long-Term Green-Mediterranean Diet May Favor Fasting Morning Cortisol Stress Hormone; the DIRECT-PLUS Clinical Trial. Front. Endocrinol. 2023, 14, 1243910. [Google Scholar] [CrossRef] [PubMed]
- Podadera-Herreros, A.; Arenas-de Larriva, A.P.; Gutierrez-Mariscal, F.M.; Alcala-Diaz, J.F.; Ojeda-Rodriguez, A.; Rodriguez-Cantalejo, F.; Cardelo, M.P.; Rodriguez-Cano, D.; Torres-Peña, J.D.; Luque, R.M.; et al. Mediterranean Diet as a Strategy for Preserving Kidney Function in Patients with Coronary Heart Disease with Type 2 Diabetes and Obesity: A Secondary Analysis of CORDIOPREV Randomized Controlled Trial. Nutr. Diabetes 2024, 14, 27. [Google Scholar] [CrossRef]
- Pradhan, N.; Kerner, J.; Campos, L.A.; Dobre, M. Personalized Nutrition in Chronic Kidney Disease. Biomedicines 2025, 13, 647. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Borelli, G.; Gessaroli, E.; Ruotolo, C.; Bin, S.; Papalia, G.; Patella, G.; Liberti, M.E.; Baraldi, O.; Zaza, G.; et al. Individualized Diets in Patients with Kidney Disease and Kidney Transplants: A Narrative Review. Life 2025, 15, 896. [Google Scholar] [CrossRef] [PubMed]
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-Supplemented Vegetarian Very Low-Protein Diet and CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 2164–2176. [Google Scholar] [CrossRef]
- Rhee, C.M.; Wang, A.Y.; Biruete, A.; Kistler, B.; Kovesdy, C.P.; Zarantonello, D.; Ko, G.J.; Piccoli, G.B.; Garibotto, G.; Brunori, G.; et al. Nutritional and Dietary Management of Chronic Kidney Disease Under Conservative and Preservative Kidney Care Without Dialysis. J. Ren. Nutr. 2023, 33, S56–S66. [Google Scholar] [CrossRef] [PubMed]
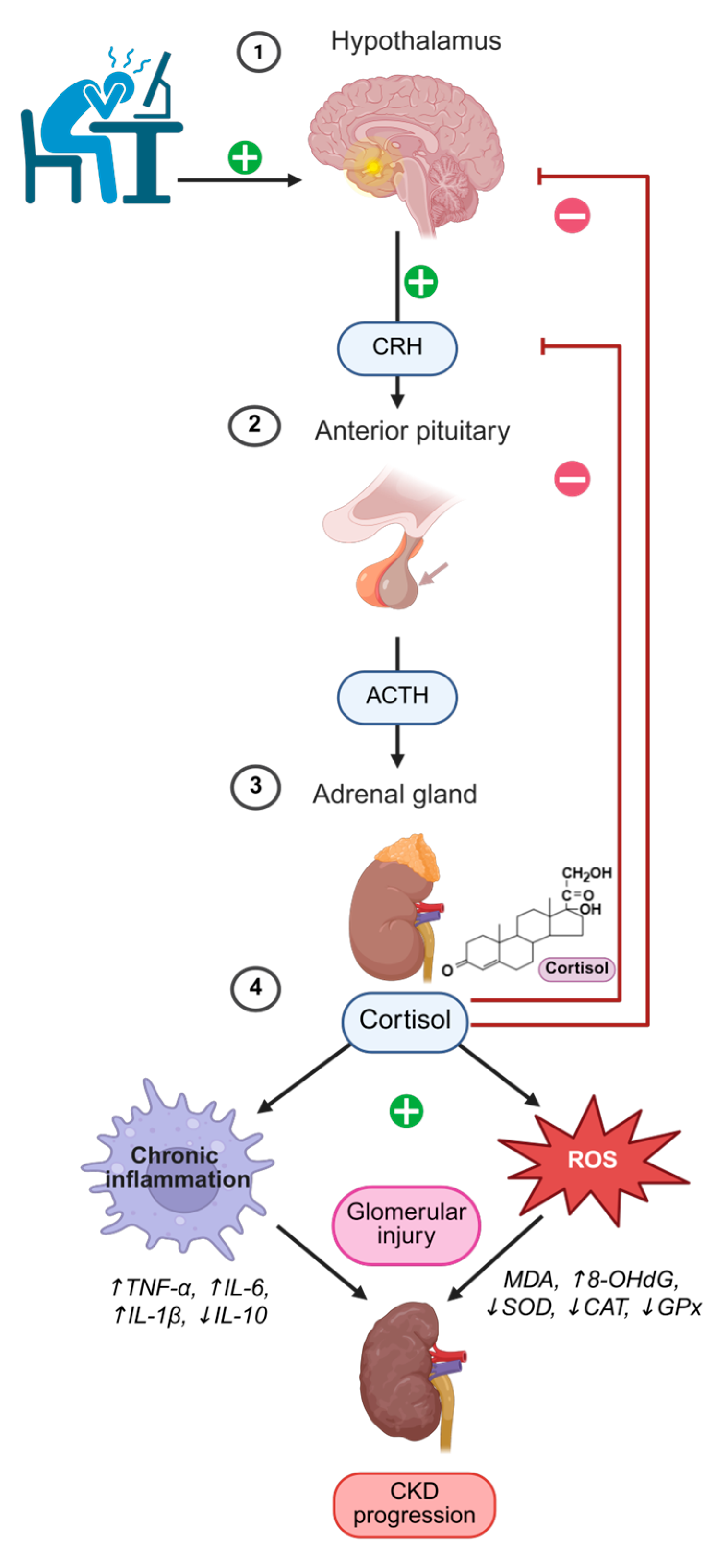
| Aspect | Mechanism/Effect | References |
|---|---|---|
| Cortisol secretion and circadian rhythm | Normally peaks in the morning and declines in the evening; in CKD the rhythm is blunted/phase-shifted with reduced morning peak and elevated evening levels. | [44] |
| Altered cortisol metabolism | Reduced renal clearance and increased 11β-HSD1 activity enhance local cortisol regeneration and impair HPA feedback. | [45] |
| Metabolic and cardiovascular effects | Elevated cortisol promotes visceral adiposity, insulin resistance, hypertension, and dyslipidemia—key risk factors for CKD. | [46,52,53,54] |
| Glucocorticoid resistance and inflammation | Chronic cortisol excess induces immune cell resistance, paradoxically enhancing IL-6, TNF-α, and CRP production. | [45,47] |
| PNEI crosstalk (SNS and RAAS) | Sympathetic overactivation increases RAAS signaling, blood pressure, and fibrosis; NADPH oxidases drive oxidative stress. | [9,48,49] |
| Immune imbalance | Cortisol and catecholamine excess alter T-cell differentiation and macrophage polarization, favoring Th1/Th17 and M1 phenotypes, sustaining inflammation. | [50,51] |
| Psychological/behavioral aspects | Dysregulated stress hormones linked with sleep disturbances and depression, further destabilizing HPA regulation. | [56,57] |
| Therapeutic implications | MR antagonists mitigate cortisol/aldosterone-driven injury; IL-6 inhibition and structured exercise improve vascular function, inflammation, and stress response. | [58,59,60,61,62,63] |
| Clinical relevance | Monitoring cortisol dynamics and HPA activity may allow early detection of maladaptive stress responses and guide personalized CKD interventions. | [9,44] |
| Dietary Pattern/Intervention | Effect on Cortisol/HPA Axis and CKD Outcomes | References |
|---|---|---|
| High intake of refined sugars and ultra-processed foods | Reduced cortisol reactivity, insulin resistance, systemic inflammation; ↑ CKD risk. | [96,97] |
| Mediterranean-style and polyphenol-rich diets | Lower fasting morning cortisol, improved metabolic parameters and kidney function. | [98,99] |
| Medical nutrition therapy (protein, sodium, phosphorus, potassium, calcium control) | Alleviates renal workload, improves metabolic balance in CKD. | [100] |
| Mediterranean and plant-based diets in CKD and transplant patients | Improve glycemic control, lipid profile, BP; indirectly modulate cortisol and systemic stress. | [101] |
| Vegetarian very low-protein diet + ketoanalogues (CKD stages 3–4) | Slows CKD progression, improves metabolic acidosis. | [102] |
| Plant-based/low-protein individualized diets | Reduce dietary acid load and phosphorus burden; stabilize symptoms, support metabolic health; indirect effects on HPA axis. | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motrenikova, M.; Boyanov, K.; Bojinova, N.; Bivolarska, A. Stress Pathways in Chronic Kidney Disease: Linking Cortisol, Oxidative Stress, and Inflammation. Antioxidants 2025, 14, 1259. https://doi.org/10.3390/antiox14101259
Motrenikova M, Boyanov K, Bojinova N, Bivolarska A. Stress Pathways in Chronic Kidney Disease: Linking Cortisol, Oxidative Stress, and Inflammation. Antioxidants. 2025; 14(10):1259. https://doi.org/10.3390/antiox14101259
Chicago/Turabian StyleMotrenikova, Maria, Krasimir Boyanov, Neli Bojinova, and Anelia Bivolarska. 2025. "Stress Pathways in Chronic Kidney Disease: Linking Cortisol, Oxidative Stress, and Inflammation" Antioxidants 14, no. 10: 1259. https://doi.org/10.3390/antiox14101259
APA StyleMotrenikova, M., Boyanov, K., Bojinova, N., & Bivolarska, A. (2025). Stress Pathways in Chronic Kidney Disease: Linking Cortisol, Oxidative Stress, and Inflammation. Antioxidants, 14(10), 1259. https://doi.org/10.3390/antiox14101259









