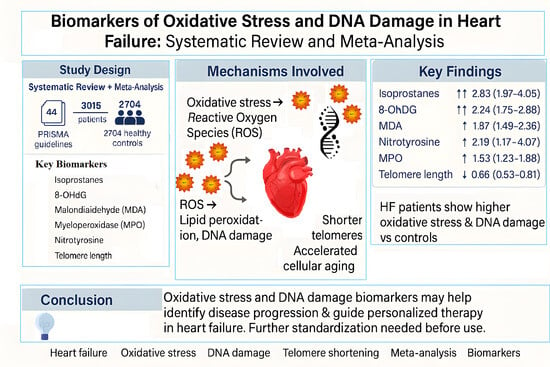
Oxidative Stress and DNA Damage Biomarkers in Heart Failure: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Study Outcome
2.3. Statistical Analysis
3. Results
3.1. Data Collection
3.2. Biomarkers of Oxidative Stress and DNA Damage in HF Patients
3.2.1. Meta-Analyses of Biomarkers of Oxidative Stress in HF Patients
| Study, Year | Population, Sex (M/F) | Mean, Age, Years (SD/IC) | HF Classification | HF Aethiology | Pharmacological Treatment | Sample | Biomarker | Results | p Value |
|---|---|---|---|---|---|---|---|---|---|
| 8-OHdG | |||||||||
| [26] | HF 78 (57 M/21 F) Controls 12 | HF 64 ± 14 | HFrEF | IHD, DCM, HCM | Diuretics 74%; ACEi 73%; BB 51%; anti-aldosterone agents 42%; digoxin 26%; CAs 10% and ARBs 16% | 8-OHdG | Blood sample | HF: 0.34 ± 0.54 Controls: 0.04 ± 0.07 | <0.05 |
| [27] | HF 194 (108 M/86 F) Controls 31 (20 M/11 F) | HF 57.1 ± 14.4 Controls 52.5 + 13.2 | HFrEF | DCM, MI | BB 80%, ACE-I 70%, 23% ARB, LDs 78%, 57% aldosterone antagonist, e 21% statin. | 8-OHdG | Urine sample | HF: 13.0 ± 5.7 Controls: 8.2 ± 1.9 | <0.0001 |
| [28] | HF 230 (140 M/90 F) Controls 42 (27 M/15 F) | HF 70.3 ± 12.8 | HFrEF and HFpEF | DCM, IHD, HVD, HHD, HVD | 73% ACEi; 51% BB; CCBs 19%; 67% Diuretics; Statins 16% | 8-OHdG | Blood sample | HF: 0.40 ± 0.24 Controls: 0.22 ± 0.09 ng/mL | <0.001 |
| [29] | HF 56 (42 M/14 F) Controls 20 | HF 53 ± 12 | HFrEF and HFpEF | DCM | 71% ACEi; ARBs 22%; CCBs 23%; Diuretics 55% | 8-OHdG | Blood sample | HF: 5.2 ± 2.9; Controls: 3.0 ± 1.5 | 0.0018 |
| [30] | HF 111 (M 52%/F 48%) Controls 30 | HF 57 ± 16 | HFrEF and HFpEF | 8-OHdG | Urine sample | HF: 14.6 ± 9.2, Controls: 6.8 ± 1.9 | <0.01 | ||
| [31] | 25 HF and 33 Controls | HF Mean 69 | HFrEF | CHD | 8-OHdG | Urine sample | HF: 33.7 ± 4.0 Controls: 12.6 ± 0.9 | <0.01 | |
| [32] | HF (24 M/8 F) Controls (8 M/6 F) | HF 46.6 ± 18.2 Controls 34.6 ± 6.9 | HFrEF | DCM | 84% Diuretics; ACEi/ARBs 94%; BB 78%; Digoxin 38% | 8-OHdG | Blood sample | HF: 0.75 ± 0.57 Controls: 0.23 ± 0.07 | 0.003 |
| Telomere | |||||||||
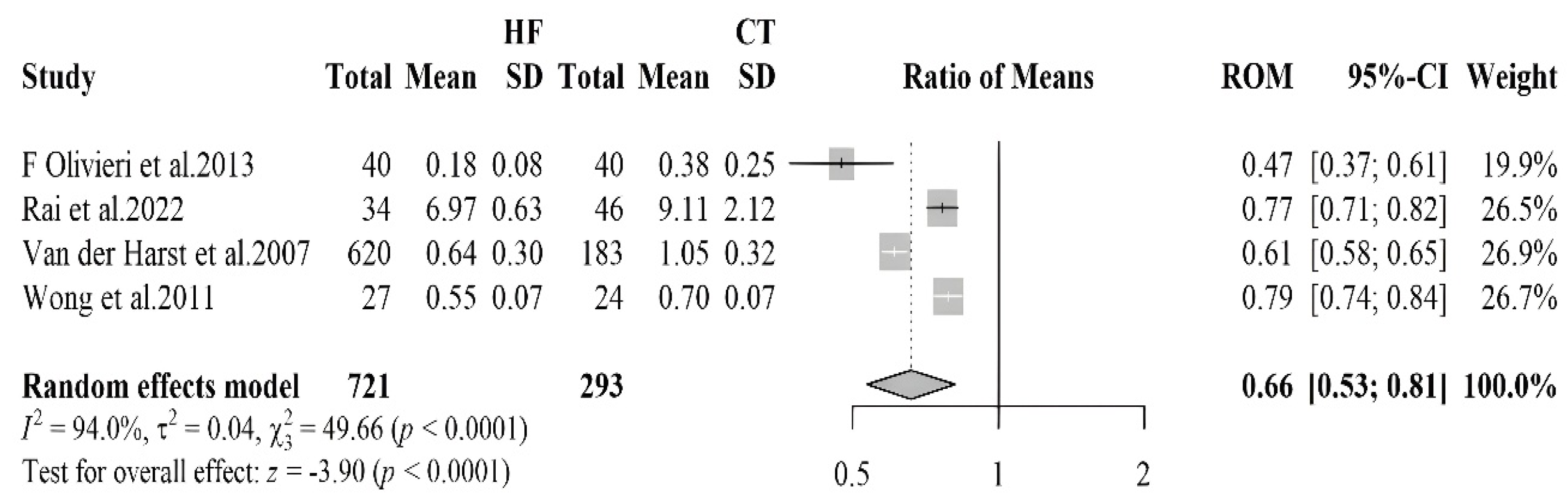
| [33] | HF 620 (493 M e 127 F) 183 Controls (145 M e 38 F) | HF 66.2 ± 8.9 Controls 66.2 ± 8.7 | HFrEF | ICHF and NICHF | 51% BB; 88% ACEi; 10% ARBs; 93% Diuretics | Telomere | Blood samples | HF: 0.64 ± 0.30 Controls: 1.05 ± 0.32 | <0.001 |
| [34] | HF 34 (24 M/10 F) Controls 46 (31 M/15 F) | HF 56 ± 9 Controls 46 ± 16 | HFrEF | IDCM | Telomere | Blood samples | IDCM: 6.97 ± 0.63 Controls: 9.11 ± 2.12 | <0.001 | |
| [35] | CHF 27 (23 M/4 F) Controls 24 (20 M/4 F) | HF 66 ± 6.6 Controls 69 ± 6.9 | HFrEF | 100% ACEI/ARB; 96% BB; 81% Diuretics; 88% Statins; 96% anticoagulants; 25% aldosterone antagonists | Telomere | Blood samples | HF: 0.55 ± 0.074 Controls: 0.70 ± 0.074 | 0.002 | |
| [36] | 40 CHF (17 M e 23 F) Controls 40 (18 M e 22 F) | HF 82 (77–89) Controls 80 (76–85) | HFrEF | ICHF | Telomere | leukocytes | HF: 0.18 ± 0.08 Controls: 0.38 ±0.25 | <0.01 | |
| Malondialdehyde | |||||||||
| [37] | HF 58 (43 M/15 F) Controls 17 (12 M/7 F) | HFrEF | IDCM, IHD | 76% ACEI; 24% BB; 79% Diuretics; 58% Digoxin; 41% ASA; 15% Amiodarone | MDA | Blood sample | HF: 0.65 ± 0.10 Controls: 0.25 ± 0.05 | <0.005 | |
| [38] | HF 12 (10 M/2 F) Controls 6 (6 M) | HF (mean 52) Controls (mean 23) | HFrEF and HFpEF | HVD, HHD, DCM | 100% diuretics; 100% digitalis | MDA | Blood sample | HF: 3.7 ± 1.3 Controls: 1.9 ± 0.6 | <0.01 |
| [39] | HF 12 (M10/F2) Controls 4 (4 M) | DCM (54 ± 2) Controls (18, 25–27, 37) | HFrEF | DCM | MDA | Heart tissue | HF: 152 ± 38 Controls: 74 ± 6 | ||
| [40] | HF 109 (93 M/16 F) Controls 28 | HF (45.97 ± 10.8) | HFrEF | DCM | 94.5% BB, 92.66% ACEIs, 35.78% ARBs, 90.83% MRAs, 13.76% Amiodarone, LDs 60.55%, TDs 18.35%, OACs 44.95%, 63.30% digoxin | MDA | Blood sample | HF: 4.37 (3.68–5.78) Controls: 1.31 (1.14–1.41) | <0.001 |
| [41] | HF 10 (7 M/3 F) Controls 69 (40 M/29 F) | 61.6 ± 5.5 | HFrEF | 81.8% BB, 36.4% CCBs, 81.8% aspirin, 63.9% nitrates, 90.9% ACEi, 9.1% ARBs, 54.5% statins, 90.9% spironolactone, furosemide | MDA | Blood sample | HF: 8.5 ± 0.6 Controls: 7.3 ± 0.9 | <0.05 | |
| [42] | HF 30 (30 M) Controls 16 (14 M/2 F) | HF 63 ± 8.2 Controls 53.8 ± 15.7 | HFrEF | 76.7% ACEI, 87.7% nitrates, 63.3% diuretics, 73.3% digitalis, 3% hydralazine | MDA | Blood sample | HF: 2.65 ± 1.3 Controls: 1.45 ± 0.77 | <0.05 | |
| [43] | CHF 45 (37 M/8 F) Controls 45 (29 M/16 F) | HF 58 range (27–68) Controls 62 range (40–74) | Atherosclerosis | MDA | Blood sample | HF: 9 (IQR 7.9–10.2) Controls: 7.7 (IQR 6.9–9.2) | <0.01 | ||
| [44] | CHF 53 Controls 38 | HFrEF. HFpEF | IHD, HVD, IDC, CCHD | Diuretics, digoxin and vasodilators. | MDA | Blood sample | HF: 10.3 (IQR 9–12) Controls: 7.9 (IQR 7–9) | <0.001 | |
| [45] | HF 29 (19 M/10 F) Controls 15 (8 M/7 F) | HFrEF | IHD | MDA | Blood sample | HF: 16 ± 7.48 Controls: 8 ± 3.16 | <0.001 | ||
| [46] | CHF 30 (13 M/17 F) Controls 55 (30 M/25 F) | CHF 73.1 ± 7.4, Controls 80 ± 17.4 | HFrEF | Diuretics, BB, CAs, ACEi. | MDA | Blood sample | HF: 0.32 (0.21–0.52) Controls: 0.21 (0.17–0.25) | <0.001 | |
| [47] | HF 12 (9 M/3 F) Controls 25 (17 M/8 F) | HF 60.8 ± 4.6 Controls 56.0 ± 4.6 | HFrEF | IHD | MDA | Blood sample | HF: 5.54 ± 2.29 Controls: 1.49 ± 0.85 | <0.001 | |
| [48] | HF 29 (24 M/4 F) Controls 15 (10 M/5 F) | HF 61.9 ± 2.6 | HFrEF | IHD, DCM | ACEi 38%, ARBs 38%, Diuretics 86%, Spironolactone 38%, Digoxin 66%, BB 24% | MDA | Blood sample | HF: 3.71 ± 0.10; Controls: 2.69 ± 0.12 | <0.001 |
| Isoprostanes | |||||||||
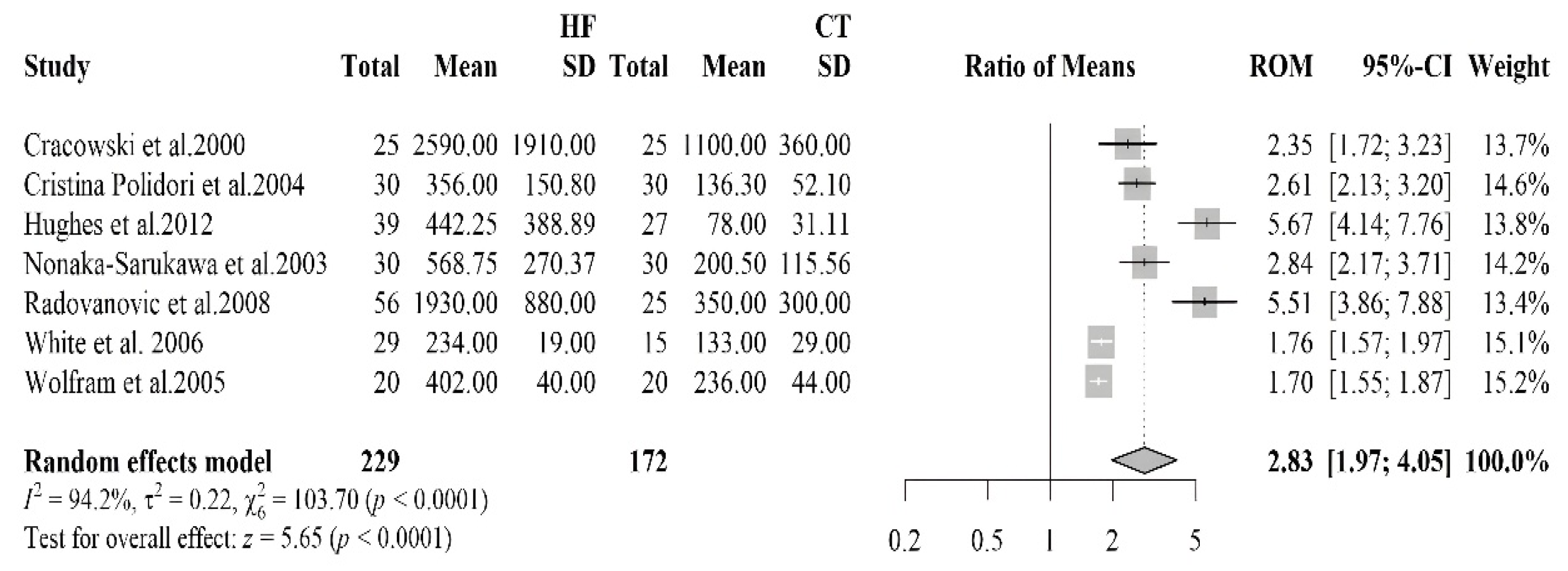
| [41] | HF 12 (9 M/3 F) Controls 25 (17 M/8 F) | HF 60.8 ± 4.6 Controls 56.0 ± 4.6 | HFrEF | IHD | Isoprostanes | Urine sample | HF: 1930 ± 880 Controls: 350 ± 300 | <0.001 | |
| [49] | HF 25 (22 M/3 F) Controls 25 (22 M/3 F) | HF 57 (27–75) | HFrEF | ICM, IDCM | ACEi 92%, LDs 88%, Digoxin 72%, Spironolactone 48%, BB 40%, ASA 40%, OAC 28%, Nitrates 4% | Isoprostaglandin F2α type III | Urine sample | HF: 2590 ± 1910 Controls: 1100 ± 360 | <0.0001 |
| [50] | CHF 39 (33 M/6 F) Controls 27 (21 M/6 F) | HF 66 ± 10 Controls 60 ± 10 | IHD, IDCM, VHD | ACEi/ARBs 22%, BB 79%, ASA 38%, Diuretics 77%, Digoxin 46%, Spironolactone 54%, Warfarin 41%, Statins 67% | Isoprostanes | Blood sample | HF: 449 (IQR: 173–698) Controls: 82 (IQR: 53–95) | <0.001 | |
| [51] | CHF 30 (14 M/16 F) Controls 30 (18 M/12 F) | HFrEF, HFpEF | Ischaemic origin | Diuretics, BB, CCBs, ACEi | Isoprostanes 8,12-iso-iPF2α-VI | Blood sample | CHF: 356.1 ± 150.8 Controls: 136.3 ± 52.1 | <0.0001 | |
| [52] | HF 20 (16 M/4 F) controls 20 (16 M/4 F) | HF 46 ± 7 controls 47 ± 6 | HFrEF | DCM | Antiplatelets, ACE inhibitors, Nitrates, α-receptor blockers, CCBs, NSAIDs, Diuretics | Isoprostanes 8-epi-PGF2a | Urine sample | HF: 402 ± 40 Controls 236 ± 44 | <0.001 |
| [53] | HF 15 (7 M/8 F) Controls 15 (8 M/7 F) | HFrEF | HVD, HHD, IDCM, CHD | Nitrates n = 6, Ca antagonists n = 6, BB n = 3, ACEi n = 15, Digoxin n = 13, Diuretics n = 23 | Isoprostane 15-F2t | urine sample | HF: 600 (IQR 355–720) Controls: 198 (IQR 125–281) | <0.001 | |
| [48] | HF 28 (24 M/4 F) controls 15 (10 M/5 F) | HF 61.9 ± 2.6 | HFrEF | IHD, IDCM | ACEi 38%, ARBs 38%, Diuretics 86%, Spironolactone 38%, Digoxin 66%, BB 24% | isoprostanes 8-epi-PGF2a | Blood sample | HF: 234 ± 19 Controls: 133 ± 29 | <0.01 |
| Myeloperoxidase | |||||||||
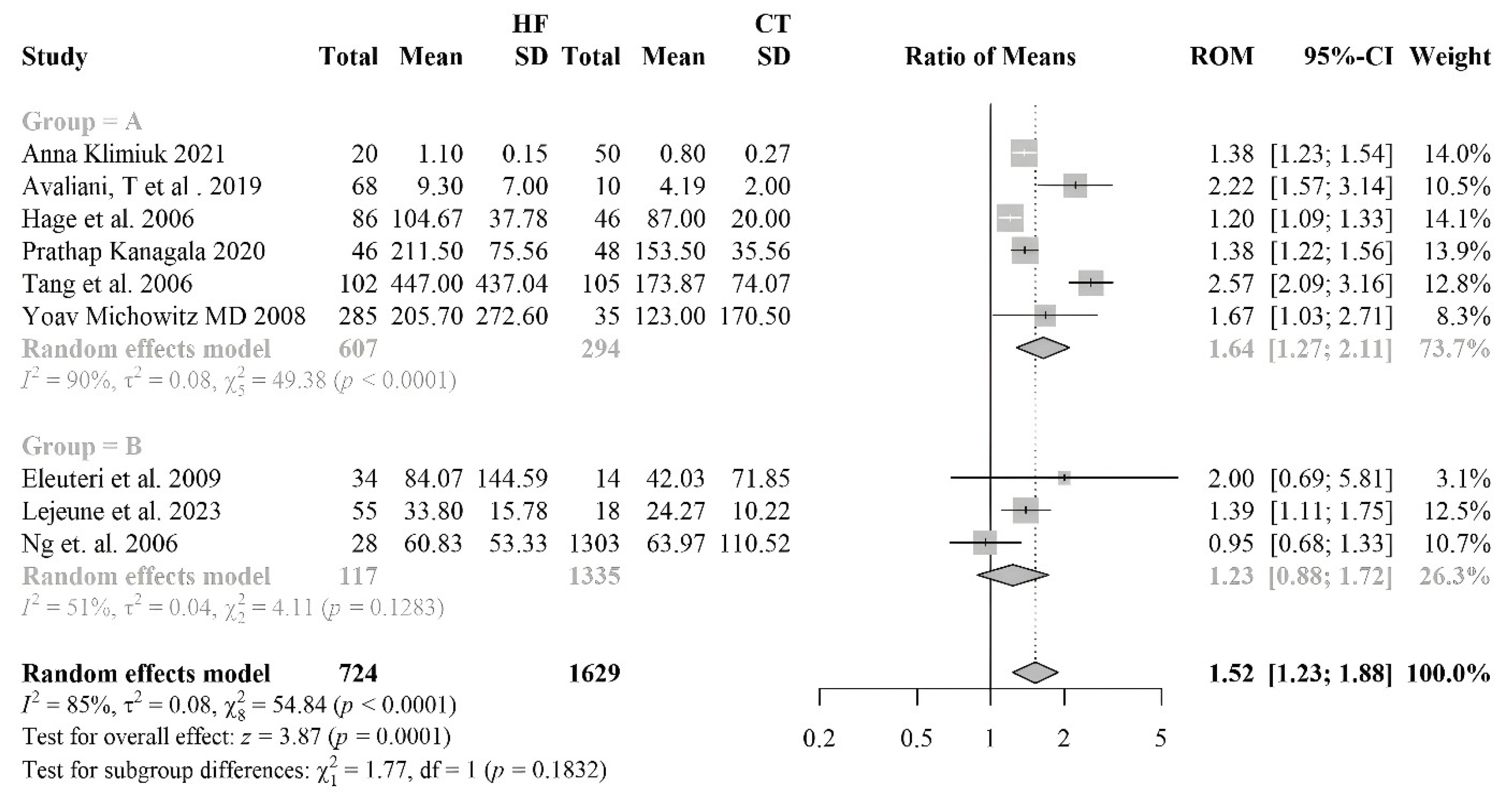
| [54] | HF 102 (58%M/42%F) controls 105 | HF 65 ± 14 Controls 44 ± 11 | HFrEF | IHD | MPO | Blood sample | HF: 212 (160–262) Controls: 153 (138–178) | <0.0001 | |
| [55] | HF 46 (23 M/23 F) control 48 (24 M/24 F) | HF 72 ± 8 controls 73 ± 5 | HFrEF | MPO | Blood sample | HF: 212 (160–262) Controls: 153 (138–178) | <0.05 | ||
| [56] | HF 86 (42 M/44 F) controls 46 | HF 73 (66–79) | HFrEF | ARBs 33%, ACEi 49%, Thiazide 16%, Potassium-sparing 21%, LDs 73%, CCBs 31%, BB 80%, Anticoags 55%, AP 34%, Statins 44%, Nitro 14%, Glucose-lowering 20% | MPO | Blood sample | HF: 101 (81–132) Controls: 86 (74–101) | <0.015 | |
| [57] | HF 55 (19 M/36 F) controls 18 | HF 80 ± 8.7 | HFrEF | ICM | LDs 76%, Mineralocorticoid receptor antagonists 33%, BB 62%, ACEi/ARBs 78%, Statins 64% | MPO | Blood sample | HF: 34.7 (22.7–44.0) Controls: 22.6 (18.2–32.0) | <0.026 |
| [58] | HF 28 (22 M/6 F) Controls 1303 (730 M/573 F) | HF 68 (51–80) Controls 63 (45–80) | HFpEF | IHD | ACEi 88%, LDs 70%, Other diuretics 12%, BB 67%, Aldosterone antagonists 33%, CCBs 19% | MPO | Blood sample | HF: 49.5 (30.5–102.5) Controls: 27.3 (7.7–156.9) | <0.0001 |
| [59] | HF 23 (20 M/3 F) Controls 14 (14 M) | HF 68 (51–80) Controls 63 (45–80) | HFrEF | ICM, IHD | MPO | Blood sample | HF: 33.6 (11.7–206.9) Controls: 18.3 (5.4–102.4) | <0.02 | |
| [60] | HF 285 (215 M/70 F) controls 35 | HF 71.2 ± 11.3 | HFrEF, HFpEF | IHD | ACEi/ARBs 82.8%, BB 69.8%, Digoxin 23.8%, ASA 67.7%, Spironolactone 54%, Diuretics 82.1%, Statins 62.4% | MPO | Blood sample | HF: 205.7 ± 272.6 Controls: 123± 170.5 | =0.01 |
| [61] | HF 68 (45 M/23 F) controls 10 | HF 64.3 ± 13.4 | HFrEF | IHD | MPO | Blood sample | HF: 9.3 ± 7 Controls: 4.19 ± 2 | <0.007 | |
| [62] | HF 27 (14 M/13 F) Control 40 (29 M/11 F) | HF 64 (49–85) Control 66 (42–87) | HFrEF | Diuretics 51.85%, ACE 48.15, Cardiac glycosides 12.5%, Organic nitrate 4.17%, Statins 48.15% | MPO | Blood sample | HF: 1.1 (1.0–1.2) Controls: 0.80 (0.62–0.98) | p ˂ 0.05 | |
| Nitrotirosine | |||||||||
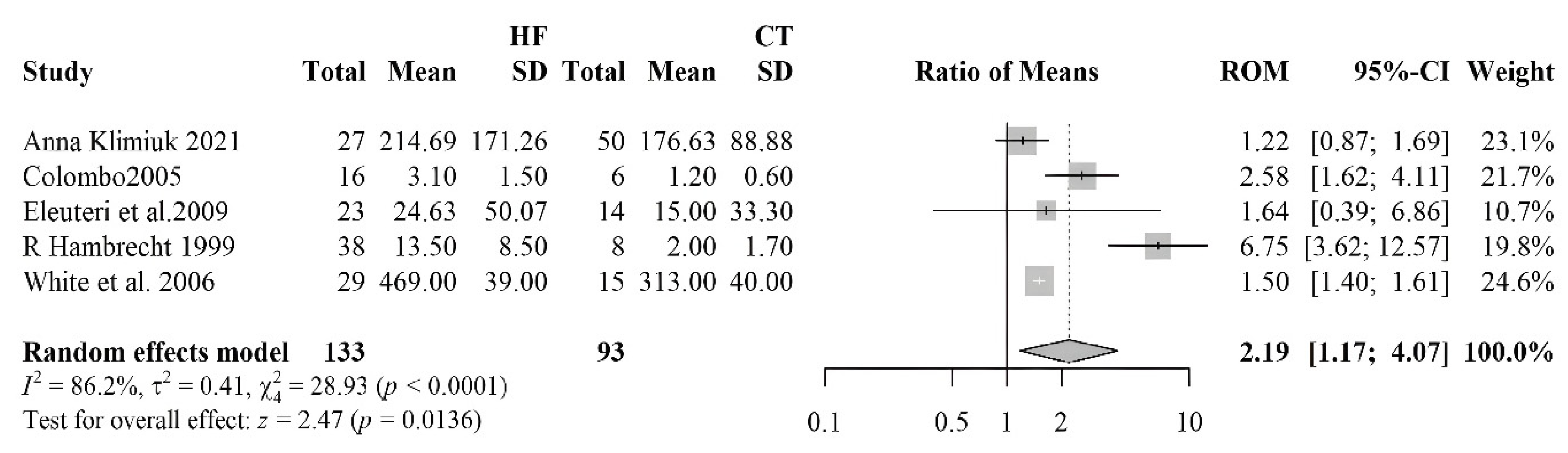
| [59] | HF 23 (20 M/3 F) Controls 14 (14 M) | HFrEF | ICM, IHD | Nitrotirosine | Blood sample | HF: 6.3 (0.0–67.6) Controls: 0.0 (0.0–45.0) | <0.02 | ||
| [48] | HF 28 (24 M/4 F) 15 Controls (10 M/5 F) | HF 61.9 ± 2.6 | HFrEF | IHD, IDCM | ACEi 38%, ARBS 38%, Diuretics 86%, Spironolactone 38%, Digoxin 66%, BB 24% | Nitrotirosine | Blood sample | HF: 469 ± 39 Controls: 313 ± 40 | <0.05 |
| [62] | HF 27 (14 M/13 F) Controls 50 (29 M/21 F) | HF 64 (49–85) Controls 66 (42–87) | HFrEF | Diuretics 51.85%, ACE 48.15, Cardiac glycosides 12.5%, Organic nitrate 4.17%, Statins 48.15% | Nitrotirosine | Blood sample | HF: 206.6 (154.6–307.2) Controls: 181.1 (114.4–234.4) | =0.0005 | |
| [63] | 38 HF and 8 Controls | HF 55.5 ± 10.0 Controls 47.6 ± 13.0 | HFrEF | IHD, DCM | ACEi 89.5%, digitalis 84.2%, diuretic 89.5%, and nitrates 23.6% | Nitrotirosine | Muscle tissue | HF: 13.5 ± 8.5 Controls: 2.0 ± 1.7 | <0.001 |
3.2.2. 8-Hydroxy-2′-Deoxyguanosine
3.2.3. Telomere Length
3.2.4. Malondialdehyde
3.2.5. Isoprostane
3.2.6. Nitrotyrosine
3.2.7. Myeloperoxydase
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| AAs | aldosterone antagonists |
| ACEi | angiotensin-converting enzyme inhibitors |
| AP | antiplatelets |
| ARBs | angiotensin receptor blockers |
| ASA | acetylsalicylic acid (aspirin) |
| BB | beta-blockers |
| BNP | B-type natriuretic peptide |
| CAT | catalase |
| CCBs | calcium channel blockers |
| CHD | congenital heart disease |
| CHF | congestive heart failure |
| CVD | cardiovascular disease |
| DCM | dilated cardiomyopathy |
| EF | ejection fraction |
| GPx | glutathione peroxidase |
| GSH | glutathione |
| HF | heart failure |
| HFmrEF | mid-range or mildly reduced ejection fraction |
| HFpEF | preserved ejection fraction |
| HFrEF | reduced ejection fraction |
| HHD | hypertensive heart disease |
| HVD | heart valve disease |
| ICHF | ischaemic congestive heart failure |
| ICM | ischaemic cardiomyopathy |
| IDCM | idiopathic dilated cardiomyopathy |
| MDA | malondialdehyde |
| MI | myocardial infarction |
| MPO | myeloperoxidase |
| MRAs | mineralocorticoid receptor antagonists |
| NAD+ | nicotinamide adenine dinucleotide |
| NICHF | non-ischaemic congestive heart failure |
| NOS | nitric oxide synthase |
| NSAIDs | non-steroidal anti-inflammatory drugs. |
| NT-proBNP | N-terminal proBNP |
| NYHA | New York Heart Association |
| OACs | oral anticoagulants |
| RNS | reactive nitrogen species |
| ROM | ratio of means |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
References
- Bozkurt, B.; Fonarow, G.C.; Goldberg, L.R.; Guglin, M.; Josephson, R.A.; Forman, D.E.; Lin, G.; Lindenfeld, J.; O’Connor, C.; Panjrath, G.; et al. Cardiac Rehabilitation for Patients with Heart Failure: JACC Expert Panel. J. Am. Coll. Cardiol. 2021, 77, 1454–1469. [Google Scholar] [CrossRef]
- Simmonds, S.J.; Cuijpers, I.; Heymans, S.; Jones, E.A.V. Cellular and Molecular Differences between HFpEF and HFrEF: A Step Ahead in an Improved Pathological Understanding. Cells 2020, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global Burden of Heart Failure: A Comprehensive and Updated Review of Epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Schwinger, R.H.G. Pathophysiology of Heart Failure. Cardiovasc. Diagn. Ther. 2021, 11, 263–276. [Google Scholar] [CrossRef]
- Ng, M.L.; Ang, X.; Yap, K.Y.; Ng, J.J.; Goh, E.C.H.; Khoo, B.B.J.; Richards, A.M.; Drum, C.L. Novel Oxidative Stress Biomarkers with Risk Prognosis Values in Heart Failure. Biomedicines 2023, 11, 917. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Sawyer, D.B.; Colucci, W.S. Mitochondrial Oxidative Stress in Heart Failure: “Oxygen Wastage” Revisited. Circ. Res. 2000, 86, 119–120. [Google Scholar] [CrossRef]
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating Oxidative Stress in Heart Failure: Past, Present and Future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From Imbalance to Impairment: The Central Role of Reactive Oxygen Species in Oxidative Stress-Induced Disorders and Therapeutic Exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2010.
- González, A.; Richards, A.M.; de Boer, R.A.; Thum, T.; Arfsten, H.; Hülsmann, M.; Falcao-Pires, I.; Díez, J.; Foo, R.S.Y.; Chan, M.Y.; et al. Cardiac Remodelling—Part 1: From Cells and Tissues to Circulating Biomarkers. A Review from the Study Group on Biomarkers of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2022, 24, 927–943. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Canty, J.M.; Das, S.; DeFilippi, C.R.; Gintant, G.A.; Gutstein, D.E.; Jaffe, A.; Kaushik, E.P.; Leptak, C.; Mehta, C.; et al. Gaining Efficiency in Clinical Trials With Cardiac Biomarkers. J. Am. Coll. Cardiol. 2021, 77, 1922–1933. [Google Scholar] [CrossRef]
- Wei, Y.; Giunta, S.; Xia, S. Hypoxia in Aging and Aging-Related Diseases: Mechanism and Therapeutic Strategies. Int. J. Mol. Sci. 2022, 23, 8165. [Google Scholar] [CrossRef]
- Triantafyllis, A.S.; Sfantou, D.; Karapedi, E.; Peteinaki, K.; Kotoulas, S.C.; Saad, R.; Fountoulakis, P.N.; Tsamakis, K.; Tsiptsios, D.; Rallidis, L.; et al. Coronary Implications of COVID-19. Med. Princ. Pract. 2025, 34, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Giacconi, R.; Maggi, F.; Macera, L.; Pistello, M.; Provinciali, M.; Giannecchini, S.; Martelli, F.; Spezia, P.G.; Mariani, E.; Galeazzi, R.; et al. Torquetenovirus (TTV) Load Is Associated with Mortality in Italian Elderly Subjects. Exp. Gerontol. 2018, 112, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Milani, F.; De Iure, A.; Proietti, S.; Limongi, D.; Prezioso, C.; Checconi, P.; Zagà, V.; Novazzi, F.; Maggi, F.; et al. Effect of Cigarette Smoking on Clinical and Molecular Endpoints in COPD Patients. Int. J. Mol. Sci. 2024, 25, 5834. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Milani, F.; Limongi, D.; Prezioso, C.; Novazzi, F.; Ferrante, F.D.; Maggi, F.; Antonelli, G.; Bonassi, S. The Effect of Torque Teno Virus (TTV) Infection on Clinical Outcomes, Genomic Integrity, and Mortality in COPD Patients. Mech. Ageing Dev. 2025, 224, 112024. [Google Scholar] [CrossRef]
- Russo, P.; Milani, F.; Vitiello, L.; Antonelli, G.; Maggi, F.; Limongi, L.; Bonassi, S.; Rosano, G. TTV as Marker of Cardivascular Disease. San Raffaele University: Roma, Italy, 2026; manuscript in preparation. [Google Scholar]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 1–31. [Google Scholar] [CrossRef]
- Omari Shekaftik, S.; Nasirzadeh, N. 8-Hydroxy-2′-Deoxyguanosine (8-OHdG) as a Biomarker of Oxidative DNA Damage Induced by Occupational Exposure to Nanomaterials: A Systematic Review. Nanotoxicology 2021, 15, 850–864. [Google Scholar] [CrossRef]
- Ndrepepa, G. Myeloperoxidase—A Bridge Linking Inflammation and Oxidative Stress with Cardiovascular Disease. Clin. Chim. Acta 2019, 493, 36–51. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Rouhi, L.; Cheedipudi, S.M.; Cathcart, B.; Gurha, P.; Marian, A.J. Cytosolic DNA Sensing Protein Pathway Is Activated in Human Hearts with Dilated Cardiomyopathy. J. Cardiovasc. Aging 2023, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Tubić Vukajlović, J.; Simić, I.; Smiljanić, Z.; Grujičić, D.; Milošević-Djordjević, O. Genome Instability in Peripheral Blood Lymphocytes of Patients with Heart Failure and Reduced Ejection Fraction. Mutagenesis 2023, 38, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Mondal, N.K.; Sorensen, E.; Hiivala, N.; Feller, E.; Griffith, B.; Wu, Z.J. Oxidative Stress, DNA Damage and Repair in Heart Failure Patients after Implantation of Continuous Flow Left Ventricular Assist Devices. Int. J. Med. Sci. 2013, 10, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.; Roselló-Lletí, E.; García de Burgos, F.; Bertomeu, V.; Payá, R.; Cortés, R.; Martínez-Dolz, L.; Jordán, A.; Pérez-Boscá, J.L.; Salvador, A.; et al. 8-hydroxy-2′-deoxyguanosine and lipid peroxidation in patients with heart failure. Rev. Esp. Cardiol. 2006, 59, 1140–1145. [Google Scholar] [CrossRef]
- Kobayashi, S.; Susa, T.; Tanaka, T.; Wada, Y.; Okuda, S.; Doi, M.; Nao, T.; Yoshiga, Y.; Yamada, J.; Okamura, T.; et al. Urinary 8-Hydroxy-2′-Deoxyguanosine Reflects Symptomatic Status and Severity of Systolic Dysfunction in Patients with Chronic Heart Failure. Eur. J. Heart Fail. 2011, 13, 29–36. [Google Scholar] [CrossRef]
- Suzuki, S.; Shishido, T.; Ishino, M.; Katoh, S.; Sasaki, T.; Nishiyama, S.; Miyashita, T.; Miyamoto, T.; Nitobe, J.; Watanabe, T.; et al. 8-Hydroxy-2′-Deoxyguanosine Is a Prognostic Mediator for Cardiac Event. Eur. J. Clin. Invest. 2011, 41, 759–766. [Google Scholar] [CrossRef]
- Kono, Y.; Nakamura, K.; Kimura, H.; Nishii, N.; Watanabe, A.; Banba, K.; Miura, A.; Nagase, S.; Sakuragi, S.; Kusano, K.F.; et al. Elevated Levels of Oxidative DNA Damage in Serum and Myocardium of Patients With Heart Failure. Circ. J. 2006, 70, 1001–1005. [Google Scholar] [CrossRef]
- Susa, T.; Kobayashi, S.; Kawamura, S.; Ito, S.; Tanaka, T.; Yoshiga, Y.; Ueyama, T.; Okamura, T.; Ohkusa, T.; Yano, M.; et al. Abstract 1703: Urinary 8-Hydroxy-2′-Deoxyguanosine Level Reflects Symptomatic Status and Severity of Systolic Dysfunction in Patients with Chronic Heart Failure. Circulation 2007, 116, II_358. [Google Scholar]
- Nagayoshi, Y.; Kawano, H.; Hokamaki, J.; Uemura, T.; Soejima, H.; Kaikita, K.; Sugiyama, S.; Yamabe, H.; Shioji, I.; Sasaki, S.; et al. Differences in Oxidative Stress Markers Based on the Aetiology of Heart Failure: Comparison of Oxidative Stress in Patients with and without Coronary Artery Disease. Free Radic. Res. 2009, 43, 1159–1166. [Google Scholar] [CrossRef]
- Watanabe, E.; Matsuda, N.; Shiga, T.; Kajimoto, K.; Ajiro, Y.; Kawarai, H.; Kasanuki, H.; Kawana, M. Significance of 8-Hydroxy-2′-Deoxyguanosine Levels in Patients With Idiopathic Dilated Cardiomyopathy. J. Card. Fail. 2006, 12, 527–532. [Google Scholar] [CrossRef]
- van der Harst, P.; van der Steege, G.; de Boer, R.A.; Voors, A.A.; Hall, A.S.; Mulder, M.J.; van Gilst, W.H.; van Veldhuisen, D.J. MERIT-HF Study Group Telomere Length of Circulating Leukocytes Is Decreased in Patients with Chronic Heart Failure. J. Am. Coll. Cardiol. 2007, 49, 1459–1464. [Google Scholar] [CrossRef]
- Rai, S.; Badarinath, A.R.S.; George, A.; Sitaraman, S.; Bronson, S.C.; Anandt, S.; Babu, K.T.; Moses, A.; Saraswathy, R.; Hande, M.P. Association of Telomere Length with Diabetes Mellitus and Idiopathic Dilated Cardiomyopathy in a South Indian Population: A Pilot Study. Mutat. Res. Toxicol. Environ. Mutagen. 2022, 874–875, 503439. [Google Scholar] [CrossRef]
- Wong, L.S.M.; Huzen, J.; de Boer, R.A.; van Gilst, W.H.; van Veldhuisen, D.J.; van der Harst, P. Telomere Length of Circulating Leukocyte Subpopulations and Buccal Cells in Patients with Ischemic Heart Failure and Their Offspring. PLoS ONE 2011, 6, e23118. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, F.; Antonicelli, R.; Recchioni, R.; Mariotti, S.; Marcheselli, F.; Lisa, R.; Spazzafumo, L.; Galeazzi, R.; Caraceni, D.; Testa, R.; et al. Telomere/Telomerase System Impairment in Circulating Angiogenic Cells of Geriatric Patients with Heart Failure. Int. J. Cardiol. 2013, 164, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Keith, M.; Geranmayegan, A.; Sole, M.J.; Kurian, R.; Robinson, A.; Omran, A.S.; Jeejeebhoy, K.N. Increased Oxidative Stress in Patients with Congestive Heart Failure. J. Am. Coll. Cardiol. 1998, 31, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Ikeda, H.; Haramaki, N.; Yoshida, N.; Imaizumi, T. Oxidative Stress Is Related to Exercise Intolerance in Patients with Heart Failure. Am. Heart J. 1998, 135, 115–120. [Google Scholar] [CrossRef]
- Rochette, L.; Tatou, E.; Maupoil, V.; Zeller, M.; Cottin, Y.; Jazayeri, S.; Brenot, R.; Girard, C.; David, M.; Vergely, C. Atrial and Vascular Oxidative Stress in Patients with Heart Failure. Cell. Physiol. Biochem. 2011, 27, 497–502. [Google Scholar] [CrossRef]
- Wojciechowska, C.; Romuk, E.; Tomasik, A.; Skrzep-Poloczek, B.; Nowalany-Kozielska, E.; Birkner, E.; Jacheć, W. Oxidative Stress Markers and C-Reactive Protein Are Related to Severity of Heart Failure in Patients with Dilated Cardiomyopathy. Mediators Inflamm. 2014, 2014, 147040. [Google Scholar] [CrossRef]
- Radovanovic, S.; Savic-Radojevic, A.; Pljesa-Ercegovac, M.; Djukic, T.; Suvakov, S.; Krotin, M.; Simic, D.V.; Matic, M.; Radojicic, Z.; Pekmezovic, T.; et al. Markers of Oxidative Damage and Antioxidant Enzyme Activities as Predictors of Morbidity and Mortality in Patients With Chronic Heart Failure. J. Card. Fail. 2012, 18, 493–501. [Google Scholar] [CrossRef]
- Díaz-Vélez, C.R.; García-Castiñeiras, S.; Mendoza-Ramos, E.; Hernández-López, E. Increased Malondialdehyde in Peripheral Blood of Patients with Congestive Heart Failure. Am. Heart J. 1996, 131, 146–152. [Google Scholar] [CrossRef]
- Belch, J.J.; Bridges, A.B.; Scott, N.; Chopra, M. Oxygen Free Radicals and Congestive Heart Failure. Heart 1991, 65, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Mcmurray, J.; Chopra, M.; Abdullah, I.; Smith, W.E.; Dargie, H.J. Evidence of Oxidative Stress in Chronic Heart Failure in Humans. Eur. Heart J. 1993, 14, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.; McLay, J.; Chopra, M.; Bridges, A.; Belch, J.J.F. Evidence for Enhanced Free Radical Activity in Chronic Congestive Heart Failure Secondary to Coronary Artery Disease. Am. J. Cardiol. 1990, 65, 1261–1262. [Google Scholar] [CrossRef]
- Polidori, M.C.; Savino, K.; Alunni, G.; Freddio, M.; Senin, U.; Sies, H.; Stahl, W.; Mecocci, P. Plasma Lipophilic Antioxidants and Malondialdehyde in Congestive Heart Failure Patients: Relationship to Disease Severity. Free Radic. Biol. Med. 2002, 32, 148–152. [Google Scholar] [CrossRef]
- Radovanovic, S.; Krotin, M.; Simic, D.V.; Mimic-Oka, J.; Savic-Radojevic, A.; Pljesa-Ercegovac, M.; Matic, M.; Ninkovic, N.; Ivanovic, B.; Simic, T. Markers of Oxidative Damage in Chronic Heart Failure: Role in Disease Progression. Redox Rep. 2008, 13, 109–116. [Google Scholar] [CrossRef]
- White, M.; Ducharme, A.; Ibrahim, R.; Whittom, L.; Lavoie, J.; Guertin, M.-C.; Racine, N.; He, Y.; Yao, G.; Rouleau, J.L.; et al. Increased Systemic Inflammation and Oxidative Stress in Patients with Worsening Congestive Heart Failure: Improvement after Short-Term Inotropic Support. Clin. Sci. 2006, 110, 483–489. [Google Scholar] [CrossRef]
- Cracowski, J.L. Increased Formation of F2-Isoprostanes in Patients with Severe Heart Failure. Heart 2000, 84, 439–440. [Google Scholar] [CrossRef]
- Hughes, C.M.; Woodside, J.V.; McGartland, C.; Roberts, M.J.; Nicholls, D.P.; McKeown, P.P. Nutritional Intake and Oxidative Stress in Chronic Heart Failure. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 376–382. [Google Scholar] [CrossRef]
- Cristina Polidori, M.; Praticó, D.; Savino, K.; Rokach, J.; Stahl, W.; Mecocci, P. Increased F2 Isoprostane Plasma Levels in Patients with Congestive Heart Failure Are Correlated with Antioxidant Status and Disease Severity. J. Card. Fail. 2004, 10, 334–338. [Google Scholar] [CrossRef]
- Wolfram, R.; Oguogho, A.; Palumbo, B.; Sinzinger, H. Enhanced Oxidative Stress in Coronary Heart Disease and Chronic Heart Failure as Indicated by an Increased 8-Epi-PGF2α. Eur. J. Heart Fail. 2005, 7, 167–172. [Google Scholar] [CrossRef]
- Nonaka-Sarukawa, M. Increased Urinary 15-F2t-Isoprostane Concentrations in Patients with Non-Ischaemic Congestive Heart Failure: A Marker of Oxidative Stress. Heart 2003, 89, 871–874. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Brennan, M.-L.; Philip, K.; Tong, W.; Mann, S.; Van Lente, F.; Hazen, S.L. Plasma Myeloperoxidase Levels in Patients With Chronic Heart Failure. Am. J. Cardiol. 2006, 98, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Kanagala, P.; Arnold, J.R.; Singh, A.; Chan, D.C.S.; Cheng, A.S.H.; Khan, J.N.; Gulsin, G.S.; Yang, J.; Zhao, L.; Gupta, P.; et al. Characterizing Heart Failure with Preserved and Reduced Ejection Fraction: An Imaging and Plasma Biomarker Approach. PLoS ONE 2020, 15, e0232280. [Google Scholar] [CrossRef] [PubMed]
- Hage, C.; Michaëlsson, E.; Kull, B.; Miliotis, T.; Svedlund, S.; Linde, C.; Donal, E.; Daubert, J.-C.; Gan, L.-M.; Lund, L.H. Myeloperoxidase and Related Biomarkers Are Suggestive Footprints of Endothelial Microvascular Inflammation in HFpEF Patients. ESC Heart Fail. 2020, 7, 1534–1546. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, S.; Ginion, A.; Menghoum, N.; Vancraeynest, D.; Pasquet, A.; Gerber, B.L.; Horman, S.; Beauloye, C.; Pouleur, A.-C. Association of Plasma Myeloperoxidase with Inflammation and Diabetic Status in HFpEF. Rev. Cardiovasc. Med. 2023, 24, 56. [Google Scholar] [CrossRef]
- Ng, L.L.; Pathik, B.; Loke, I.W.; Squire, I.B.; Davies, J.E. Myeloperoxidase and C-Reactive Protein Augment the Specificity of B-Type Natriuretic Peptide in Community Screening for Systolic Heart Failure. Am. Heart J. 2006, 152, 94–101. [Google Scholar] [CrossRef]
- Eleuteri, E.; Di Stefano, A.; Ricciardolo, F.L.; Magno, F.; Gnemmi, I.; Colombo, M.; Anzalone, R.; Cappello, F.; La Rocca, G.; Genta, F.T.; et al. Increased Nitrotyrosine Plasma Levels in Relation to Systemic Markers of Inflammation and Myeloperoxidase in Chronic Heart Failure. Int. J. Cardiol. 2009, 135, 386–390. [Google Scholar] [CrossRef]
- Michowitz, Y.; Kisil, S.; Guzner-Gur, H.; Rubinstein, A.; Wexler, D.; Sheps, D.; Keren, G.; George, J. Usefulness of Serum Myeloperoxidase in Prediction of Mortality in Patients with Severe Heart Failure. Hypertension 2008, 173, 60–67. [Google Scholar]
- Avaliani, T.; Talakvadze, T.; Tabagari, S. Influence of nutritional state on outcome in patients with chronic heart failure. Georgian Med News 2019, 288, 61–66. [Google Scholar]
- Klimiuk, A.; Zalewska, A.; Knapp, M.; Sawicki, R.; Ładny, J.R.; Maciejczyk, M. Salivary Gland Dysfunction in Patients with Chronic Heart Failure Is Aggravated by Nitrosative Stress, as Well as Oxidation and Glycation of Proteins. Biomolecules 2021, 11, 119. [Google Scholar] [CrossRef]
- Hambrecht, R.; Adams, V.; Gielen, S.; Linke, A.; Möbius-Winkler, S.; Yu, J.; Niebauer, J.; Jiang, H.; Fiehn, E.; Schuler, G. Exercise Intolerance in Patients with Chronic Heart Failure and Increased Expression of Inducible Nitric Oxide Synthase in the Skeletal Muscle. J. Am. Coll. Cardiol. 1999, 33, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Colombo, P.C.; Banchs, J.E.; Celaj, S.; Talreja, A.; Lachmann, J.; Malla, S.; DuBois, N.B.; Ashton, A.W.; Latif, F.; Jorde, U.P.; et al. Endothelial Cell Activation in Patients With Decompensated Heart Failure. Circulation 2005, 111, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Meijers, W.C.; Bayes-Genis, A.; Mebazaa, A.; Bauersachs, J.; Cleland, J.G.F.; Coats, A.J.S.; Januzzi, J.L.; Maisel, A.S.; McDonald, K.; Mueller, T.; et al. Circulating Heart Failure Biomarkers beyond Natriuretic Peptides: Review from the Biomarker Study Group of the Heart Failure Association (HFA), European Society of Cardiology (ESC). Eur. J. Heart Fail. 2021, 23, 1610–1632. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC)Developed with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Fenech, M.; Knasmueller, S.; Knudsen, L.E.; Kirsch-Volders, M.; Deo, P.; Franzke, B.; Stopper, H.; Andreassi, M.-G.; Bolognesi, C.; Dhillon, V.S.; et al. “Micronuclei and Disease” Special Issue: Aims, Scope, and Synthesis of Outcomes. Mutat. Res. Mutat. Res. 2021, 788, 108384. [Google Scholar] [CrossRef]




| Biomarker | Studies Included (n) | HF Patients (n) | Controls (n) |
|---|---|---|---|
| 8-OHdG | 7 | 625 | 182 |
| MDA | 12 | 583 | 335 |
| Telomere | 4 | 721 | 293 |
| Isoprostanes | 7 | 229 | 172 |
| MPO | 9 | 724 | 1629 |
| Nitrotyrosine | 5 | 133 | 93 |
| Total | 44 | 3015 | 2704 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milani, F.; Porreca, A.; Rosano, G.; Vitiello, L.; Volterrani, M.; Russo, P.; Bonassi, S. Oxidative Stress and DNA Damage Biomarkers in Heart Failure: A Systematic Review and Meta-Analysis. Antioxidants 2025, 14, 1249. https://doi.org/10.3390/antiox14101249
Milani F, Porreca A, Rosano G, Vitiello L, Volterrani M, Russo P, Bonassi S. Oxidative Stress and DNA Damage Biomarkers in Heart Failure: A Systematic Review and Meta-Analysis. Antioxidants. 2025; 14(10):1249. https://doi.org/10.3390/antiox14101249
Chicago/Turabian StyleMilani, Francesca, Annamaria Porreca, Giuseppe Rosano, Laura Vitiello, Maurizio Volterrani, Patrizia Russo, and Stefano Bonassi. 2025. "Oxidative Stress and DNA Damage Biomarkers in Heart Failure: A Systematic Review and Meta-Analysis" Antioxidants 14, no. 10: 1249. https://doi.org/10.3390/antiox14101249
APA StyleMilani, F., Porreca, A., Rosano, G., Vitiello, L., Volterrani, M., Russo, P., & Bonassi, S. (2025). Oxidative Stress and DNA Damage Biomarkers in Heart Failure: A Systematic Review and Meta-Analysis. Antioxidants, 14(10), 1249. https://doi.org/10.3390/antiox14101249