The Influence of Sirtuin 6 on Chondrocyte Senescence in Osteoarthritis Under Aging: Focusing on Mitochondrial Dysfunction and Oxidative Stress
Abstract
1. Introduction
2. Chondrocyte Aging and OA
2.1. Inflammatory Response
2.2. ECM Degradation
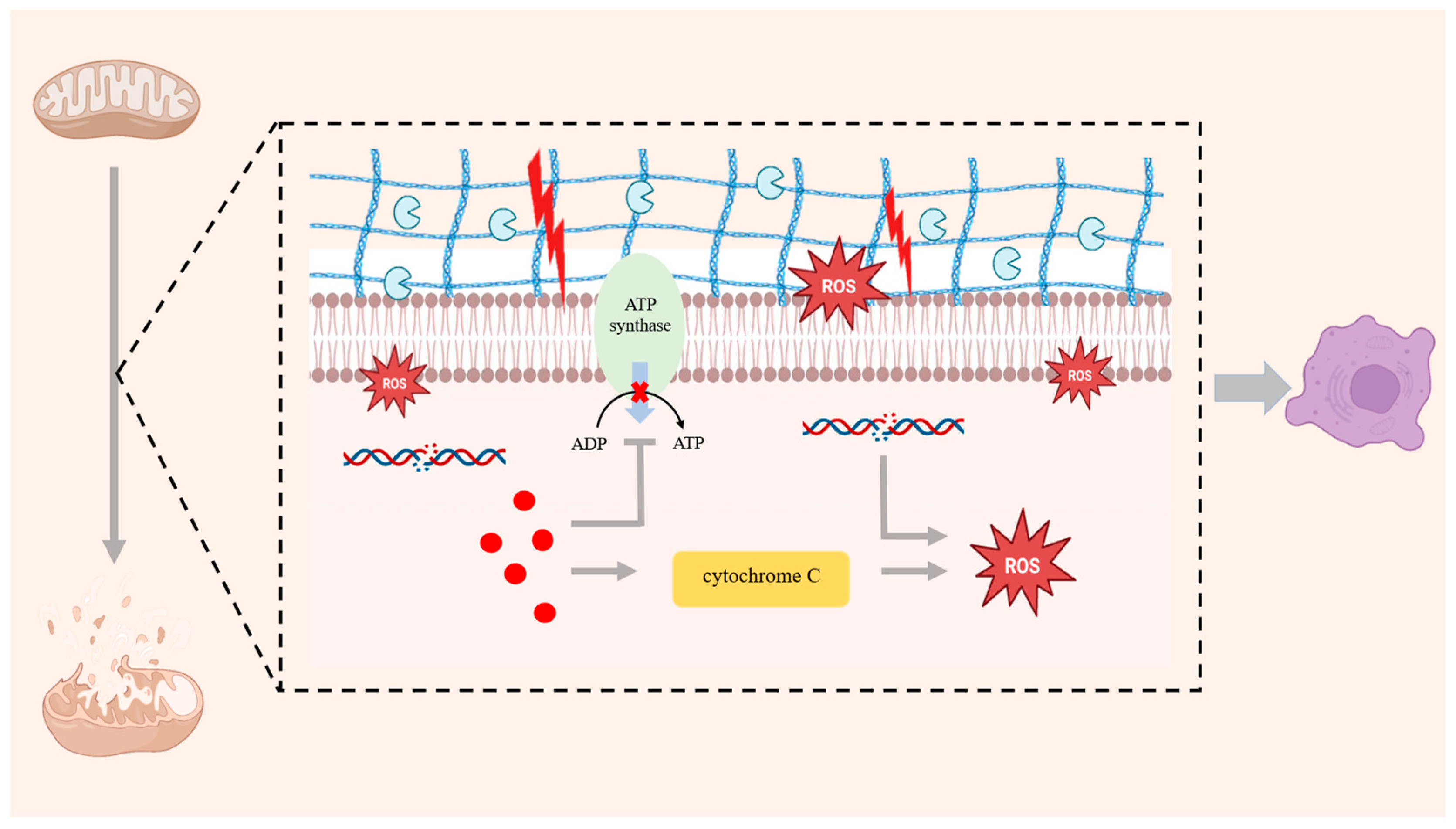
2.3. Mitochondrial Dysfunction and OS
2.3.1. Chondrocyte Senescence and Mitochondrial Dysfunction
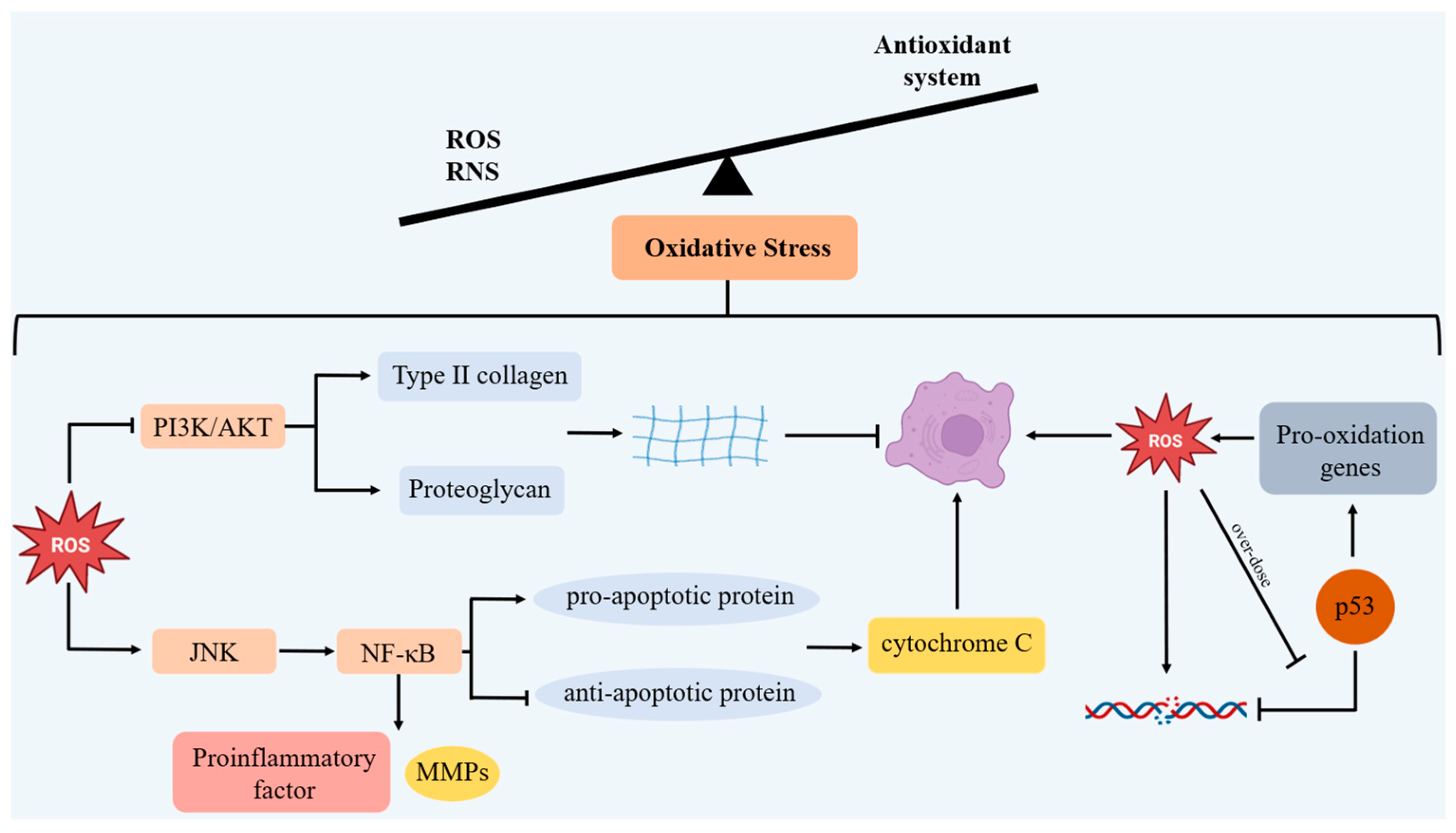
2.3.2. Chondrocyte Senescence and OS
2.4. Autophagy
3. SIRT6 and Mitochondrial Dysfunction
3.1. A Regulation of Number of Mitochondria and Integrity of the Mitochondrial Membranes
3.2. A Regulation of ROS in Mitochondria
3.3. A Regulation of Inflammation-Mediated Mitochondrial Damage
4. SRIT6 and OS
4.1. A Regulation of Antioxidant Enzymes
4.2. Inhibiting Excessive ROS Induced by Inflammatory Factors
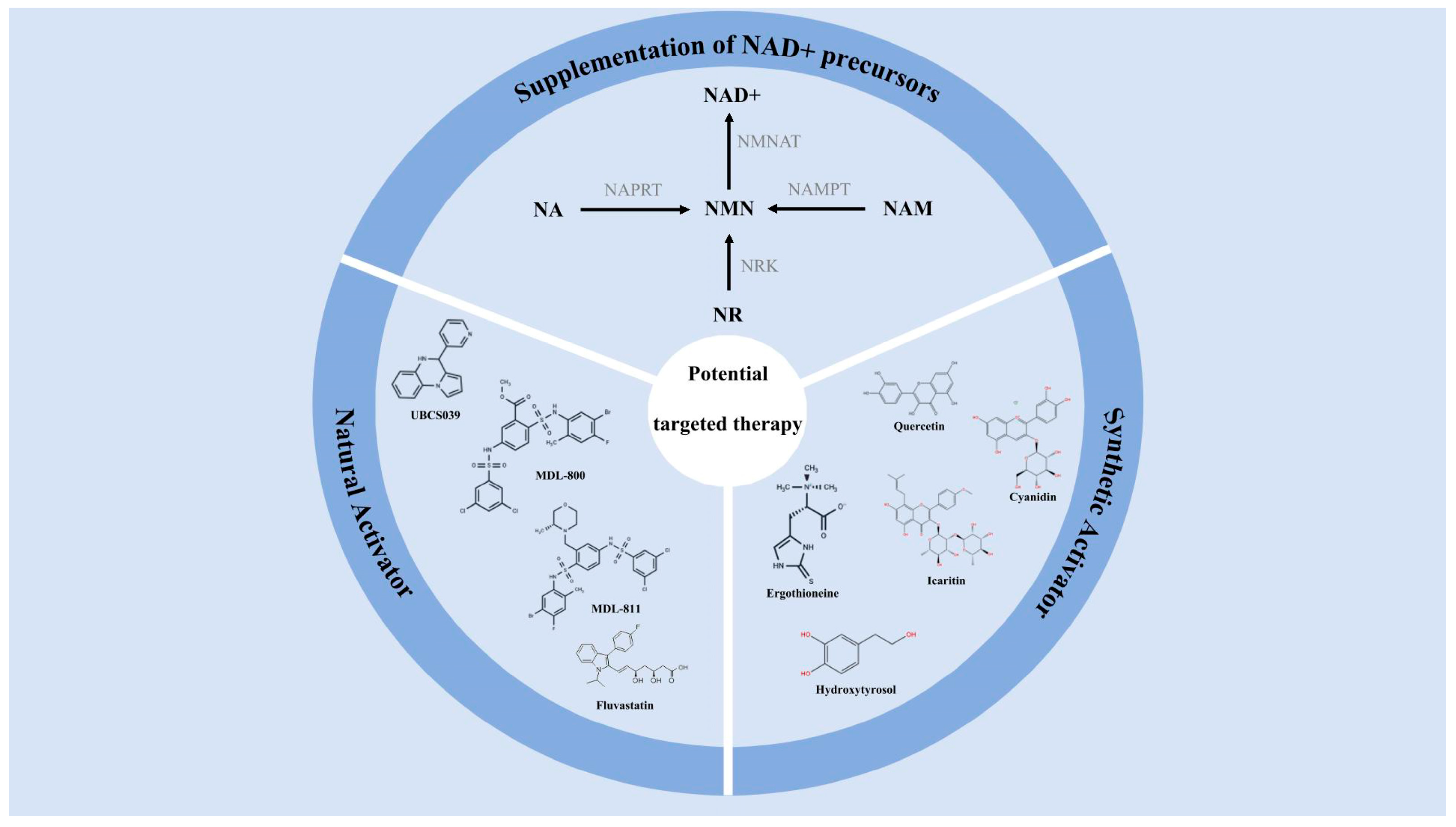
5. Potential Targeted Therapy of SIRT6
5.1. Supplementation of NAD+ Precursors
5.2. Selective Activator of SIRT6
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OA | Osteoarthritis |
| SIRT6 | Sirtuin6 |
| ROS | Reactive Oxygen Species |
| NAD+ | Nicotinamide adenine dinucleotide |
| SIRTs | Sirtuins |
| SASP | Senescence-associated secretory phenotype |
| MMPs | Matrix metalloproteinases |
| IL-1β | Interleukin—1β |
| iNOS | Inducible nitric oxide synthase |
| PLA2 | Phospholipase A2 |
| COX-2 | Cyclooxygenase-2 |
| PGE2 | ProstaglandinE2 |
| ECM | Extracellular matrix |
| Sox9 | SRY-box transcription factor 9 |
| ADAMTS | A Disintegrin and Metalloproteinase with Thrombospondin motifs |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| TNF-α | Tumor necrosis factor-α |
| IGF | Insulin-like growth factors |
| VEGF | Vascular endothelial growth factor |
| EVs | Extracellular vesicles |
| mtDNA | Mitochondrial DNA |
| MMP | Mitochondrial membrane potential |
| ATP | Adenosine triphosphate |
| OS | Oxidative stress |
| RNS | Reactive nitrogen species |
| mTOR | Mammalian target of rapamycin |
| PGC-1α | Peroxisome proliferators-activated receptor γ coactivator l alpha |
| NRF | Nuclear respiratory factor |
| TFAM | Mitochondrial transcription factor A |
| SIRT3 | Sirtuin 3 |
| SIRT4 | Sirtuin 4 |
| TFEB | Transcription factor EB |
| SOD2 | Superoxide dismutase 2 |
| Nrf2 | Nuclear Factor erythroid 2-Related Factor 2 |
| FOXO3a | Forkhead box protein O3 |
| HO-1 | Heme Oxygenase-1 |
| IL-15 | Interleukin-15 |
| STAT5 | Signal Transducer and Activator of Transcription 5 |
| JAK3 | Janus Kinase 3 |
| NR | Nicotinamide riboside |
| NAM | Nicotinamide |
| NA | Nicotinic acid |
| NMN | Nicotinamide mononucleotide |
| NMNAT | Nicotinamide mononucleotide adenylyltransferase |
| PRPP | 5-phosphoribosyl-1-pyrophosphate |
| NAPRT | Nicotinate phosphoribosyltransferase |
References
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Chen, X.; Tang, H.; Lin, J.; Zeng, R. Temporal trends in the disease burden of osteoarthritis from 1990 to 2019, and projections until 2030. PLoS ONE 2023, 18, e0288561. [Google Scholar] [CrossRef]
- Ji, Z.; Liu, G.H.; Qu, J. Mitochondrial sirtuins, key regulators of aging. Life Med. 2025, 4, lnaf019. [Google Scholar] [CrossRef]
- Michishita, E.; McCord, R.A.; Berber, E.; Kioi, M.; Padilla-Nash, H.; Damian, M.; Cheung, P.; Kusumoto, R.; Kawahara, T.L.; Barrett, J.C.; et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature 2008, 452, 492–496. [Google Scholar] [CrossRef]
- Liao, C.Y.; Kennedy, B.K. SIRT6, oxidative stress, and aging. Cell Res. 2016, 26, 143–144. [Google Scholar] [CrossRef]
- Ashraf, S.; Cha, B.H.; Kim, J.S.; Ahn, J.; Han, I.; Park, H.; Lee, S.H. Regulation of senescence associated signaling mechanisms in chondrocytes for cartilage tissue regeneration. Osteoarthr. Cartil. 2016, 24, 196–205. [Google Scholar] [CrossRef]
- Han, Z.; Wang, K.; Ding, S.; Zhang, M. Cross-talk of inflammation and cellular senescence: A new insight into the occurrence and progression of osteoarthritis. Bone Res. 2024, 12, 69. [Google Scholar] [CrossRef]
- Jacob, J.; Aggarwal, A.; Bhattacharyya, S.; Sahni, D.; Sharma, V.; Aggarwal, A. Fisetin and resveratrol exhibit senotherapeutic effects and suppress cellular senescence in osteoarthritic cartilage-derived chondrogenic progenitor cells. Eur. J. Pharmacol. 2025, 997, 177573. [Google Scholar] [CrossRef]
- Coryell, P.R.; Diekman, B.O.; Loeser, R.F. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat. Rev. Rheumatol. 2021, 17, 47–57. [Google Scholar] [CrossRef]
- Migliorini, P.; Italiani, P.; Pratesi, F.; Puxeddu, I.; Boraschi, D. The IL-1 family cytokines and receptors in autoimmune diseases. Autoimmun. Rev. 2020, 19, 102617. [Google Scholar] [CrossRef]
- Brom, M.; Saxer, F.; Mindeholm, L.; Schieker, M.; Conaghan, P.G. Is It Time to Revisit the Role of Interleukin-1 Inhibitors in Osteoarthritis? Diabetes Metab. Syndr. Obes. 2025, 18, 1753–1764. [Google Scholar] [CrossRef]
- Liu, L.; Luo, P.; Yang, M.; Wang, J.; Hou, W.; Xu, P. The role of oxidative stress in the development of knee osteoarthritis: A comprehensive research review. Front. Mol. Biosci. 2022, 9, 1001212. [Google Scholar] [CrossRef]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef]
- Guan, J.; Chen, C.; Wu, S.; Zhu, H. The Role of PGE2 in Age-related Diseases. Curr. Drug Targets 2025, 26, 757–769. [Google Scholar] [CrossRef]
- Jenei-Lanzl, Z.; Meurer, A.; Zaucke, F. Interleukin-1β signaling in osteoarthritis–chondrocytes in focus. Cell. Signal. 2019, 53, 212–223. [Google Scholar] [CrossRef]
- Yap, A.X.W.; You, Y.X.; Ajit Singh, D.K.; Mat, S.; Chong, C.P.; Mohamad Yahaya, N.H.; Maktar, J.F.; Abdul Rani, R.; Ooi, T.C.; Ismail, M.; et al. Efficacy of oral nutrition supplementation enriched with hydroxymethylbutyrate (HMB) and undenatured type-II collagen (UC-II) combined with exercise training on osteoarthritis-related outcomes among adults with knee osteoarthritis in Klang Valley of Malaysia: Study protocol for a randomised controlled trial. BMJ Open 2025, 15, e093885. [Google Scholar] [CrossRef]
- Cucchiarini, M.; Thurn, T.; Weimer, A.; Kohn, D.; Terwilliger, E.F.; Madry, H. Restoration of the extracellular matrix in human osteoarthritic articular cartilage by overexpression of the transcription factor SOX9. Arthritis Rheum. 2007, 56, 158–167. [Google Scholar] [CrossRef]
- Xu, Z.; Ke, T.; Zhang, Y.; Fu, C.; He, W. Agonism of GPR120 prevented IL-1β-induced reduction of extracellular matrix through SOX-9. Aging 2020, 12, 12074–12085. [Google Scholar] [CrossRef]
- Tabeian, H.; Betti, B.F.; Dos Santos Cirqueira, C.; de Vries, T.J.; Lobbezoo, F.; Ter Linde, A.V.; Zandieh-Doulabi, B.; Koenders, M.I.; Everts, V.; Bakker, A.D. IL-1β Damages Fibrocartilage and Upregulates MMP-13 Expression in Fibrochondrocytes in the Condyle of the Temporomandibular Joint. Int. J. Mol. Sci. 2019, 20, 2260. [Google Scholar] [CrossRef]
- Liao, Y.; Ren, Y.; Luo, X.; Mirando, A.J.; Long, J.T.; Leinroth, A.; Ji, R.R.; Hilton, M.J. Interleukin-6 signaling mediates cartilage degradation and pain in posttraumatic osteoarthritis in a sex-specific manner. Sci. Signal. 2022, 15, eabn7082. [Google Scholar] [CrossRef]
- Huang, B.; Lang, X.; Li, X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front. Oncol. 2022, 12, 1023177. [Google Scholar] [CrossRef]
- Srirangan, S.; Choy, E.H. The role of interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2010, 2, 247–256. [Google Scholar] [CrossRef]
- Bernhard, S.; Hug, S.; Stratmann, A.E.P.; Erber, M.; Vidoni, L.; Knapp, C.L.; Thomaß, B.D.; Fauler, M.; Nilsson, B.; Nilsson Ekdahl, K.; et al. Interleukin 8 Elicits Rapid Physiological Changes in Neutrophils That Are Altered by Inflammatory Conditions. J. Innate Immun. 2021, 13, 225–241. [Google Scholar] [CrossRef]
- Cecil, D.L.; Rose, D.M.; Terkeltaub, R.; Liu-Bryan, R. Role of interleukin-8 in PiT-1 expression and CXCR1-mediated inorganic phosphate uptake in chondrocytes. Arthritis Rheum. 2005, 52, 144–154. [Google Scholar] [CrossRef]
- Baud, V.; Karin, M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001, 11, 372–377. [Google Scholar] [CrossRef]
- Tu, P.; Guo, Y.; Zheng, S.; Pan, Y.; Wang, L.; Ma, Y. Research progress on signaling molecules involved in articular cartilage repair]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2019, 36, 343–348. [Google Scholar] [CrossRef]
- Mullen, L.M.; Best, S.M.; Ghose, S.; Wardale, J.; Rushton, N.; Cameron, R.E. Bioactive IGF-1 release from collagen-GAG scaffold to enhance cartilage repair in vitro. J. Mater. Sci. Mater. Med. 2015, 26, 5325. [Google Scholar] [CrossRef]
- Uchimura, T.; Foote, A.T.; Smith, E.L.; Matzkin, E.G.; Zeng, L. Insulin-Like Growth Factor II (IGF-II) Inhibits IL-1β-Induced Cartilage Matrix Loss and Promotes Cartilage Integrity in Experimental Osteoarthritis. J. Cell. Biochem. 2015, 116, 2858–2869. [Google Scholar] [CrossRef]
- Hu, K.; Olsen, B.R. Vascular endothelial growth factor control mechanisms in skeletal growth and repair. Dev. Dyn. 2017, 246, 227–234. [Google Scholar] [CrossRef]
- Liu, S.; Deng, Z.; Chen, K.; Jian, S.; Zhou, F.; Yang, Y.; Fu, Z.; Xie, H.; Xiong, J.; Zhu, W. Cartilage tissue engineering: From proinflammatory and anti-inflammatory cytokines to osteoarthritis treatments (Review). Mol. Med. Rep. 2022, 25, 99. [Google Scholar] [CrossRef]
- Mueller, M.B.; Tuan, R.S. Anabolic/Catabolic balance in pathogenesis of osteoarthritis: Identifying molecular targets. PM&R 2011, 3, S3–S11. [Google Scholar] [CrossRef]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef]
- Rowan, A.D.; Litherland, G.J.; Hui, W.; Milner, J.M. Metalloproteases as potential therapeutic targets in arthritis treatment. Expert Opin. Ther. Targets 2008, 12, 1–18. [Google Scholar] [CrossRef]
- Fu, P.J.; Zheng, S.Y.; Luo, Y.; Ren, Z.Q.; Li, Z.H.; Wang, Y.P.; Lu, B.B. Prg4 and Osteoarthritis: Functions, Regulatory Factors, and Treatment Strategies. Biomedicines 2025, 13, 693. [Google Scholar] [CrossRef]
- Nagase, H.; Kashiwagi, M. Aggrecanases and cartilage matrix degradation. Arthritis Res. Ther. 2003, 5, 94–103. [Google Scholar] [CrossRef]
- Li, Z.; Bi, R.; Zhu, S. The Dual Role of Small Extracellular Vesicles in Joint Osteoarthritis: Their Global and Non-Coding Regulatory RNA Molecule-Based Pathogenic and Therapeutic Effects. Biomolecules 2023, 13, 1606. [Google Scholar] [CrossRef]
- Miyaki, S. Cartilage/chondrocyte research and osteoarthritis. The role of microRNAs and extracellular vesicles in osteoarthritis pathogenesis. Clin. Calcium 2018, 28, 783–788. [Google Scholar]
- Zhou, Y.; Ming, J.; Li, Y.; Li, B.; Deng, M.; Ma, Y.; Chen, Z.; Zhang, Y.; Li, J.; Liu, S. Exosomes derived from miR-126-3p-overexpressing synovial fibroblasts suppress chondrocyte inflammation and cartilage degradation in a rat model of osteoarthritis. Cell Death Discov. 2021, 7, 37. [Google Scholar] [CrossRef]
- Jeon, O.H.; Wilson, D.R.; Clement, C.C.; Rathod, S.; Cherry, C.; Powell, B.; Lee, Z.; Khalil, A.M.; Green, J.J.; Campisi, J.; et al. Senescence cell-associated extracellular vesicles serve as osteoarthritis disease and therapeutic markers. JCI Insight 2019, 4, e125019. [Google Scholar] [CrossRef]
- Chen, P.; Zeng, L.; Wang, T.; He, J.; Xiong, S.; Chen, G.; Wang, Q.; Chen, H.; Xie, J. The communication role of extracellular vesicles in the osteoarthritis microenvironment. Front. Immunol. 2025, 16, 1549833. [Google Scholar] [CrossRef]
- Maldonado, M.; Nam, J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed Res. Int. 2013, 2013, 284873. [Google Scholar] [CrossRef]
- Yin, H.; Li, M.; Tian, G.; Ma, Y.; Ning, C.; Yan, Z.; Wu, J.; Ge, Q.; Sui, X.; Liu, S.; et al. The role of extracellular vesicles in osteoarthritis treatment via microenvironment regulation. Biomater. Res. 2022, 26, 52. [Google Scholar] [CrossRef]
- Sukhikh, S.; Noskova, S.; Ivanova, S.; Ulrikh, E.; Izgaryshev, A.; Babich, O. Chondroprotection and Molecular Mechanism of Action of Phytonutraceuticals on Osteoarthritis. Molecules 2021, 26, 2391. [Google Scholar] [CrossRef]
- Mobasheri, A.; Matta, C.; Giles, W.; Choi, H.; Ivanavicius, S. The interplay between inflammatory mediators and mechanotransduction is mediated by ion channels in articular chondrocytes: Functional consequences in osteoarthritis. Phys. Life Rev. 2024, 49, 123–126. [Google Scholar] [CrossRef]
- Duong, V.; Abdel Shaheed, C.; Ferreira, M.L.; Narayan, S.W.; Venkatesha, V.; Hunter, D.J.; Zhu, J.; Atukorala, I.; Kobayashi, S.; Goh, S.L.; et al. Risk factors for the development of knee osteoarthritis across the lifespan: A systematic review and meta-analysis. Osteoarthr. Cartil. 2025, 33, 1162–1179. [Google Scholar] [CrossRef]
- Servin-Vences, M.R.; Richardson, J.; Lewin, G.R.; Poole, K. Mechanoelectrical transduction in chondrocytes. Clin. Exp. Pharmacol. Physiol. 2018, 45, 481–488. [Google Scholar] [CrossRef]
- Gao, W.; Hasan, H.; Anderson, D.E.; Lee, W. The Role of Mechanically-Activated Ion Channels Piezo1, Piezo2, and TRPV4 in Chondrocyte Mechanotransduction and Mechano-Therapeutics for Osteoarthritis. Front. Cell Dev. Biol. 2022, 10, 885224. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, L.; Liu, Z.; Geng, B.; Teng, Y.; Liu, X.; Yi, Q.; Yu, D.; Chen, X.; Zhao, D.; et al. Mechanosensory and mechanotransductive processes mediated by ion channels in articular chondrocytes: Potential therapeutic targets for osteoarthritis. Channels 2021, 15, 339–359. [Google Scholar] [CrossRef]
- Guan, M.; Han, X.; Liao, B.; Han, W.; Chen, L.; Zhang, B.; Peng, X.; Tian, Y.; Xiao, G.; Li, X.; et al. LIPUS Promotes Calcium Oscillation and Enhances Calcium Dependent Autophagy of Chondrocytes to Alleviate Osteoarthritis. Adv. Sci. 2025, 12, e2413930. [Google Scholar] [CrossRef]
- Matta, C.; Takács, R.; Ducza, L.; Ebeid, R.A.; Choi, H.; Mobasheri, A. Ion channels involved in inflammation and pain in osteoarthritis and related musculoskeletal disorders. Am. J. Physiol. Cell Physiol. 2023, 325, C257–C271. [Google Scholar] [CrossRef]
- Tan, S.; Sun, Y.; Li, S.; Wu, H.; Ding, Y. The impact of mitochondrial dysfunction on osteoarthritis cartilage: Current insights and emerging mitochondria-targeted therapies. Bone Res. 2025, 13, 77. [Google Scholar] [CrossRef]
- Akhmedov, A.T.; Marín-García, J. Mitochondrial DNA maintenance: An appraisal. Mol. Cell. Biochem. 2015, 409, 283–305. [Google Scholar] [CrossRef]
- Mobasheri, A.; Rayman, M.P.; Gualillo, O.; Sellam, J.; van der Kraan, P.; Fearon, U. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2017, 13, 302–311. [Google Scholar] [CrossRef]
- Saberi, M.; Zhang, X.; Mobasheri, A. Targeting mitochondrial dysfunction with small molecules in intervertebral disc aging and degeneration. Geroscience 2021, 43, 517–537. [Google Scholar] [CrossRef] [PubMed]
- Staunton, C.A.; Lewis, R.; Barrett-Jolley, R. Ion channels and osteoarthritic pain: Potential for novel analgesics. Curr. Pain Headache Rep. 2013, 17, 378. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X. Regulation of pain neurotransmitters and chondrocytes metabolism mediated by voltage-gated ion channels: A narrative review. Heliyon 2023, 9, e17989. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Song, D.; Ni, J.; Ding, M.; Huang, J.; Yan, M. AMPK: Implications in osteoarthritis and therapeutic targets. Am. J. Transl. Res. 2020, 12, 7670–7681. [Google Scholar]
- Minguzzi, M.; Cetrullo, S.; D’Adamo, S.; Silvestri, Y.; Flamigni, F.; Borzì, R.M. Emerging Players at the Intersection of Chondrocyte Loss of Maturational Arrest, Oxidative Stress, Senescence and Low-Grade Inflammation in Osteoarthritis. Oxidative Med. Cell Longev. 2018, 2018, 3075293. [Google Scholar] [CrossRef]
- Choi, M.C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-κB Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef]
- Otero, M.; Plumb, D.A.; Tsuchimochi, K.; Dragomir, C.L.; Hashimoto, K.; Peng, H.; Olivotto, E.; Bevilacqua, M.; Tan, L.; Yang, Z.; et al. E74-like factor 3 (ELF3) impacts on matrix metalloproteinase 13 (MMP13) transcriptional control in articular chondrocytes under proinflammatory stress. J. Biol. Chem. 2012, 287, 3559–3572. [Google Scholar] [CrossRef]
- Qi, Z.; Zhu, J.; Cai, W.; Lou, C.; Li, Z. The role and intervention of mitochondrial metabolism in osteoarthritis. Mol. Cell. Biochem. 2024, 479, 1513–1524. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Hu, J.; Wang, C.; Wang, H.; Zhong, H.; Zhang, Z.; Wen, G.; Wang, L.; Dong, M.; Tian, Y. Mitochondria-targeted NAD+/O2 co-delivery interpenetrating network hydrogel for respiratory chain restoration and osteoarthritis therapy. J. Control. Release 2025, 385, 113975. [Google Scholar] [CrossRef]
- Ferroni, L.; Zago, M.; Patergnani, S.; Campbell, S.E.; Hébert, L.; Nielsen, M.; Scarpa, C.; Bassetto, F.; Pinton, P.; Zavan, B. Fluorescent Light Energy (FLE) Acts on Mitochondrial Physiology Improving Wound Healing. J. Clin. Med. 2020, 9, 559. [Google Scholar] [CrossRef]
- Lyu, Y.; Wang, T.; Huang, S.; Zhang, Z. Mitochondrial Damage-Associated Molecular Patterns and Metabolism in the Regulation of Innate Immunity. J. Innate Immun. 2023, 15, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis*. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016, 22, 146–153. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef]
- Yudoh, K.; van Trieu, N.; Nakamura, H.; Hongo-Masuko, K.; Kato, T.; Nishioka, K. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: Oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res. Ther. 2005, 7, R380–R391. [Google Scholar] [CrossRef]
- Kan, S.; Duan, M.; Liu, Y.; Wang, C.; Xie, J. Role of Mitochondria in Physiology of Chondrocytes and Diseases of Osteoarthritis and Rheumatoid Arthritis. Cartilage 2021, 13, 1102s–1121s. [Google Scholar] [CrossRef] [PubMed]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta 2016, 1862, 576–591. [Google Scholar] [CrossRef]
- Ramasamy, T.S.; Yee, Y.M.; Khan, I.M. Chondrocyte Aging: The Molecular Determinants and Therapeutic Opportunities. Front. Cell Dev. Biol. 2021, 9, 625497. [Google Scholar] [CrossRef]
- Huang, K.; Cai, H. The role of chondrocyte senescence in osteoarthritis pathogenesis and therapeutic implications. Exp. Gerontol. 2025, 208, 112828. [Google Scholar] [CrossRef]
- Zahan, O.M.; Serban, O.; Gherman, C.; Fodor, D. The evaluation of oxidative stress in osteoarthritis. Med. Pharm. Rep. 2020, 93, 12–22. [Google Scholar] [CrossRef]
- Chen, N.Y.; Lu, K.; Yuan, J.M.; Li, X.J.; Gu, Z.Y.; Pan, C.X.; Mo, D.L.; Su, G.F. 3-Arylamino-quinoxaline-2-carboxamides inhibit the PI3K/Akt/mTOR signaling pathways to activate P53 and induce apoptosis. Bioorganic Chem. 2021, 114, 105101. [Google Scholar] [CrossRef]
- Xu, J.; Yi, Y.; Li, L.; Zhang, W.; Wang, J. Osteopontin induces vascular endothelial growth factor expression in articular cartilage through PI3K/AKT and ERK1/2 signaling. Mol. Med. Rep. 2015, 12, 4708–4712. [Google Scholar] [CrossRef]
- Campbell, K.J.; Tait, S.W.G. Targeting BCL-2 regulated apoptosis in cancer. Open Biol. 2018, 8, 180002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Z.; Liu, M.Q.; Chen, H.W.; Wu, Z.L.; Gao, Y.C.; Ma, Z.J.; He, X.G.; Kang, X.W. NF-κB signalling pathways in nucleus pulposus cell function and intervertebral disc degeneration. Cell Prolif. 2021, 54, e13057. [Google Scholar] [CrossRef] [PubMed]
- Cheresh, P.; Kim, S.J.; Tulasiram, S.; Kamp, D.W. Oxidative stress and pulmonary fibrosis. Biochim. Biophys. Acta 2013, 1832, 1028–1040. [Google Scholar] [CrossRef]
- Liu, B.; Chen, Y.; St Clair, D.K. ROS and p53: A versatile partnership. Free Radic. Biol. Med. 2008, 44, 1529–1535. [Google Scholar] [CrossRef]
- Duan, R.; Xie, H.; Liu, Z.Z. The Role of Autophagy in Osteoarthritis. Front. Cell Dev. Biol. 2020, 8, 608388. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Caramés, B.; Olmer, M.; Kiosses, W.B.; Lotz, M.K. The relationship of autophagy defects to cartilage damage during joint aging in a mouse model. Arthritis Rheumatol. 2015, 67, 1568–1576. [Google Scholar] [CrossRef]
- Hill, S.M.; Wrobel, L.; Rubinsztein, D.C. Correction to: Post-translational modifications of Beclin 1 provide multiple strategies for autophagy regulation. Cell Death Differ. 2019, 26, 2810. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, T.; Sun, X.; Nie, M. Autophagy and apoptosis: Regulatory factors of chondrocyte phenotype transition in osteoarthritis. Hum. Cell 2023, 36, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Andrei, C.; Mihai, D.P.; Nitulescu, G.M.; Nitulescu, G.; Zanfirescu, A. Modulating Autophagy in Osteoarthritis: Exploring Emerging Therapeutic Drug Targets. Int. J. Mol. Sci. 2024, 25, 13695. [Google Scholar] [CrossRef]
- Huang, W.; Ao, P.; Li, J.; Wu, T.; Xu, L.; Deng, Z.; Chen, W.; Yin, C.; Cheng, X. Autophagy Protects Advanced Glycation End Product-Induced Apoptosis and Expression of MMP-3 and MMP-13 in Rat Chondrocytes. Biomed Res. Int. 2017, 2017, 6341919. [Google Scholar] [CrossRef]
- Ryan, P.J.; Uranga, S.; Stanelle, S.T.; Lewis, M.H.; O’Reilly, C.L.; Cardin, J.M.; Deaver, J.W.; Morton, A.B.; Fluckey, J.D. The autophagy inhibitor NSC185058 suppresses mTORC1-mediated protein anabolism in cultured skeletal muscle. Sci. Rep. 2024, 14, 8094. [Google Scholar] [CrossRef]
- Caramés, B.; Hasegawa, A.; Taniguchi, N.; Miyaki, S.; Blanco, F.J.; Lotz, M. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann. Rheum. Dis. 2012, 71, 575–581. [Google Scholar] [CrossRef]
- Cejka, D.; Hayer, S.; Niederreiter, B.; Sieghart, W.; Fuereder, T.; Zwerina, J.; Schett, G. Mammalian target of rapamycin signaling is crucial for joint destruction in experimental arthritis and is activated in osteoclasts from patients with rheumatoid arthritis. Arthritis Rheum. 2010, 62, 2294–2302. [Google Scholar] [CrossRef]
- Li, Y.S.; Zhang, F.J.; Zeng, C.; Luo, W.; Xiao, W.F.; Gao, S.G.; Lei, G.H. Autophagy in osteoarthritis. Jt. Bone Spine 2016, 83, 143–148. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, H.; Guan, H.; Wu, C.; Qi, W.; Yang, L.; Yin, J.; Zhang, H.; Liu, L.; Lu, Y.; et al. PDZK1 protects against mechanical overload-induced chondrocyte senescence and osteoarthritis by targeting mitochondrial function. Bone Res. 2024, 12, 41. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, L.; Duan, X.; Niu, Y.; Li, M.; Yun, L.; Sun, H.; Ma, Y.; Guo, Y. Sirtuins mediate mitochondrial quality control mechanisms: A novel therapeutic target for osteoporosis. Front. Endocrinol. 2023, 14, 1281213. [Google Scholar] [CrossRef]
- Finck, B.N.; Kelly, D.P. PGC-1 coactivators: Inducible regulators of energy metabolism in health and disease. J. Clin. Investig. 2006, 116, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Han, C.Y.; Moon, Y.J.; Lee, J.H.; Bae, E.J.; Park, B.H. Sirt6 reprograms myofibers to oxidative type through CREB-dependent Sox6 suppression. Nat. Commun. 2022, 13, 1808. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yang, Q.; Yang, Y.; Gao, Z.; Ma, Y.; Zhang, L.; Liang, W.; Ding, G. Sirt6 Suppresses High Glucose-Induced Mitochondrial Dysfunction and Apoptosis in Podocytes through AMPK Activation. Int. J. Biol. Sci. 2019, 15, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Matsushita, T.; Matsuzaki, T.; Takayama, K.; Matsumoto, T.; Kuroda, R.; Kurosaka, M. Depletion of SIRT6 causes cellular senescence, DNA damage, and telomere dysfunction in human chondrocytes. Osteoarthr. Cartil. 2015, 23, 1412–1420. [Google Scholar] [CrossRef]
- Wei, W.; Li, T.; Chen, J.; Fan, Z.; Gao, F.; Yu, Z.; Jiang, Y. SIRT3/6: An amazing challenge and opportunity in the fight against fibrosis and aging. Cell. Mol. Life Sci. 2024, 81, 69. [Google Scholar] [CrossRef]
- Smirnov, D.; Eremenko, E.; Stein, D.; Kaluski, S.; Jasinska, W.; Cosentino, C.; Martinez-Pastor, B.; Brotman, Y.; Mostoslavsky, R.; Khrameeva, E.; et al. SIRT6 is a key regulator of mitochondrial function in the brain. Cell Death Dis. 2023, 14, 35. [Google Scholar] [CrossRef]
- Gordon, S.; Akopyan, G.; Garban, H.; Bonavida, B. Transcription factor YY1: Structure, function, and therapeutic implications in cancer biology. Oncogene 2006, 25, 1125–1142. [Google Scholar] [CrossRef]
- Kawamura, K.; Higuchi, T.; Fujiwara, S. YAF2-Mediated YY1-Sirtuin6 Interactions Responsible for Mitochondrial Downregulation in Aging Tunicates. Mol. Cell. Biol. 2021, 41, e0004721. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Q.; Zhao, D.; Lian, F.; Li, X.; Qi, W. The impact of oxidative stress-induced mitochondrial dysfunction on diabetic microvascular complications. Front. Endocrinol. 2023, 14, 1112363. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845–1863. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, D.; Chen, S.; Liu, Y.; Shi, J.; Zhang, J.; Wen, P.; Wang, Z.; Li, J.; Guo, W.; et al. Sirt6 opposes glycochenodeoxycholate-induced apoptosis of biliary epithelial cells through the AMPK/PGC-1α pathway. Cell Biosci. 2020, 10, 43. [Google Scholar] [CrossRef]
- Zheng, G.; Zhan, Y.; Li, X.; Pan, Z.; Zheng, F.; Zhang, Z.; Zhou, Y.; Wu, Y.; Wang, X.; Gao, W.; et al. TFEB, a potential therapeutic target for osteoarthritis via autophagy regulation. Cell Death Dis. 2018, 9, 858. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yang, J.; Wu, Q.; Wang, X. The Role and Molecular Pathways of SIRT6 in Senescence and Age-related Diseases. Adv. Biol. 2025, 9, e2400469. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.A.; Kapustina, M.; Bolduc, J.A.; Pike, J.F.W.; Diekman, B.O.; Mix, K.; Chubinskaya, S.; Eroglu, E.; Michel, T.; Poole, L.B.; et al. Sirtuin 6 (SIRT6) regulates redox homeostasis and signaling events in human articular chondrocytes. Free Radic. Biol. Med. 2021, 166, 90–103. [Google Scholar] [CrossRef]
- Kanfi, Y.; Naiman, S.; Amir, G.; Peshti, V.; Zinman, G.; Nahum, L.; Bar-Joseph, Z.; Cohen, H.Y. The sirtuin SIRT6 regulates lifespan in male mice. Nature 2012, 483, 218–221. [Google Scholar] [CrossRef]
- He, Y.; Wu, Z.; Xu, L.; Xu, K.; Chen, Z.; Ran, J.; Wu, L. The role of SIRT3-mediated mitochondrial homeostasis in osteoarthritis. Cell. Mol. Life Sci. 2020, 77, 3729–3743. [Google Scholar] [CrossRef]
- Pirinen, E.; Lo Sasso, G.; Auwerx, J. Mitochondrial sirtuins and metabolic homeostasis. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 759–770. [Google Scholar] [CrossRef]
- Pan, H.; Guan, D.; Liu, X.; Li, J.; Wang, L.; Wu, J.; Zhou, J.; Zhang, W.; Ren, R.; Zhang, W.; et al. SIRT6 safeguards human mesenchymal stem cells from oxidative stress by coactivating NRF2. Cell Res. 2016, 26, 190–205. [Google Scholar] [CrossRef]
- Xia, W.; Xiao, J.; Tong, C.; Lu, J.; Tu, Y.; Li, S.; Ni, L.; Shi, Y.; Luo, P.; Zhang, X.; et al. Orientin inhibits inflammation in chondrocytes and attenuates osteoarthritis through Nrf2/NF-κB and SIRT6/NF-κB pathway. J. Orthop. Res. 2023, 41, 2405–2417. [Google Scholar] [CrossRef]
- Wood, M.; Rymarchyk, S.; Zheng, S.; Cen, Y. Trichostatin A inhibits deacetylation of histone H3 and p53 by SIRT6. Arch. Biochem. Biophys. 2018, 638, 8–17. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, L.; Wang, Y.; Li, W.; Lin, Y.; Yu, D.; Zhang, L.; Li, F.; Pan, Z. Overexpression of Sirtuin 6 suppresses cellular senescence and NF-κB mediated inflammatory responses in osteoarthritis development. Sci. Rep. 2015, 5, 17602. [Google Scholar] [CrossRef]
- Collins, J.A.; Kim, C.J.; Coleman, A.; Little, A.; Perez, M.M.; Clarke, E.J.; Diekman, B.; Peffers, M.J.; Chubinskaya, S.; Tomlinson, R.E.; et al. Cartilage-specific Sirt6 deficiency represses IGF-1 and enhances osteoarthritis severity in mice. Ann. Rheum. Dis. 2023, 82, 1464–1473. [Google Scholar] [CrossRef]
- Chang, A.R.; Ferrer, C.M.; Mostoslavsky, R. SIRT6, a Mammalian Deacylase with Multitasking Abilities. Physiol. Rev. 2020, 100, 145–169. [Google Scholar] [CrossRef]
- Liu, X.; Ren, S.; Li, Z.; Hao, D.; Zhao, X.; Zhang, Z.; Liu, D. Sirt6 mediates antioxidative functions by increasing Nrf2 abundance. Exp. Cell Res. 2023, 422, 113409. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Chen, L.; Wang, X.; Ma, X. SIRT6 inhibits endoplasmic reticulum stress-mediated ferroptosis by activating Nrf2/HO-1 signaling to alleviate osteoarthritis. Inflamm. Res. 2025, 74, 35. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.L.; Kensler, T.W.; et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.W.; Jiang, Q.Y.; Meng, N.; Xiao, L.; Zhang, Q.; Chen, Y.X.; Liu, L.J.; Wang, L. Sirt6 promotes DNA damage repair in osteoarthritis chondrocytes by activating the Keap1/Nrf2/HO-1 signaling pathway. Cell Cycle 2024, 23, 205–217. [Google Scholar] [CrossRef]
- Jiang, H.; Khan, S.; Wang, Y.; Charron, G.; He, B.; Sebastian, C.; Du, J.; Kim, R.; Ge, E.; Mostoslavsky, R.; et al. SIRT6 regulates TNF-α secretion through hydrolysis of long-chain fatty acyl lysine. Nature 2013, 496, 110–113. [Google Scholar] [CrossRef]
- Krolopp, J.E.; Thornton, S.M.; Abbott, M.J. IL-15 Activates the Jak3/STAT3 Signaling Pathway to Mediate Glucose Uptake in Skeletal Muscle Cells. Front. Physiol. 2016, 7, 626. [Google Scholar] [CrossRef]
- Tóthová, Z.; Tomc, J.; Debeljak, N.; Solár, P. STAT5 as a Key Protein of Erythropoietin Signalization. Int. J. Mol. Sci. 2021, 22, 7109. [Google Scholar] [CrossRef]
- Régnier, P.; Le Joncour, A.; Maciejewski-Duval, A.; Desbois, A.C.; Comarmond, C.; Rosenzwajg, M.; Klatzmann, D.; Cacoub, P.; Saadoun, D. Targeting JAK/STAT pathway in Takayasu’s arteritis. Ann. Rheum. Dis. 2020, 79, 951–959. [Google Scholar] [CrossRef]
- Ji, M.L.; Jiang, H.; Li, Z.; Geng, R.; Hu, J.Z.; Lin, Y.C.; Lu, J. Sirt6 attenuates chondrocyte senescence and osteoarthritis progression. Nat. Commun. 2022, 13, 7658. [Google Scholar] [CrossRef]
- Sun, K.; Wu, Y.; Zeng, Y.; Xu, J.; Wu, L.; Li, M.; Shen, B. The role of the sirtuin family in cartilage and osteoarthritis: Molecular mechanisms and therapeutic targets. Arthritis Res. Ther. 2022, 24, 286. [Google Scholar] [CrossRef]
- Chen, J.; Chen, S.; Cai, D.; Wang, Q.; Qin, J. The role of Sirt6 in osteoarthritis and its effect on macrophage polarization. Bioengineered 2022, 13, 9677–9689. [Google Scholar] [CrossRef] [PubMed]
- Makarov, M.V.; Migaud, M.E. Syntheses and chemical properties of β-nicotinamide riboside and its analogues and derivatives. Beilstein J. Org. Chem. 2019, 15, 401–430. [Google Scholar] [CrossRef] [PubMed]
- Bogan, K.L.; Brenner, C. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu. Rev. Nutr. 2008, 28, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Cai, X.; Liang, Y.; Wang, M.; Yang, W. Roles of NAD+ and Its Metabolites Regulated Calcium Channels in Cancer. Molecules 2020, 25, 4826. [Google Scholar] [CrossRef]
- Fang, E.F.; Lautrup, S.; Hou, Y.; Demarest, T.G.; Croteau, D.L.; Mattson, M.P.; Bohr, V.A. NAD+ in Aging: Molecular Mechanisms and Translational Implications. Trends Mol. Med. 2017, 23, 899–916. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.F.; Yoshida, S.; Stein, L.R.; Grozio, A.; Kubota, S.; Sasaki, Y.; Redpath, P.; Migaud, M.E.; Apte, R.S.; Uchida, K.; et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016, 24, 795–806. [Google Scholar] [CrossRef]
- Feuz, M.B.; Meyer-Ficca, M.L.; Meyer, R.G. Beyond Pellagra-Research Models and Strategies Addressing the Enduring Clinical Relevance of NAD Deficiency in Aging and Disease. Cells 2023, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, M.S.; Caffa, I.; Monacelli, F.; Nencioni, A. Inhibitors of NAD+ Production in Cancer Treatment: State of the Art and Perspectives. Int. J. Mol. Sci. 2024, 25, 2092. [Google Scholar] [CrossRef] [PubMed]
- Shade, C. The Science Behind NMN-A Stable, Reliable NAD+Activator and Anti-Aging Molecule. Integr. Med. 2020, 19, 12–14. [Google Scholar]
- Revollo, J.R.; Grimm, A.A.; Imai, S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J. Biol. Chem. 2004, 279, 50754–50763. [Google Scholar] [CrossRef]
- Ummarino, S.; Mozzon, M.; Zamporlini, F.; Amici, A.; Mazzola, F.; Orsomando, G.; Ruggieri, S.; Raffaelli, N. Simultaneous quantitation of nicotinamide riboside, nicotinamide mononucleotide and nicotinamide adenine dinucleotide in milk by a novel enzyme-coupled assay. Food Chem. 2017, 221, 161–168. [Google Scholar] [CrossRef]
- You, W.; Zheng, W.; Weiss, S.; Chua, K.F.; Steegborn, C. Structural basis for the activation and inhibition of Sirtuin 6 by quercetin and its derivatives. Sci. Rep. 2019, 9, 19176. [Google Scholar] [CrossRef]
- Das, D.; Banerjee, A.; Mukherjee, S.; Maji, B.K. Quercetin inhibits NF-kB and JAK/STAT signaling via modulating TLR in thymocytes and splenocytes during MSG-induced immunotoxicity: An in vitro approach. Mol. Biol. Rep. 2024, 51, 277. [Google Scholar] [CrossRef]
- Akter, R.; Afrose, A.; Rahman, M.R.; Chowdhury, R.; Nirzhor, S.S.R.; Khan, R.I.; Kabir, M.T. A Comprehensive Analysis into the Therapeutic Application of Natural Products as SIRT6 Modulators in Alzheimer’s Disease, Aging, Cancer, Inflammation, and Diabetes. Int. J. Mol. Sci. 2021, 22, 4180. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.S.; Lee, E.G.; Jeon, H.S.; Chae, H.J.; Park, S.J.; Lee, Y.C.; Yoo, W.H. Quercetin inhibits IL-1β-induced proliferation and production of MMPs, COX-2, and PGE2 by rheumatoid synovial fibroblast. Inflammation 2012, 35, 1585–1594. [Google Scholar] [CrossRef]
- Wang, H.; Yan, Y.; Pathak, J.L.; Hong, W.; Zeng, J.; Qian, D.; Hao, B.; Li, H.; Gu, J.; Jaspers, R.T.; et al. Quercetin prevents osteoarthritis progression possibly via regulation of local and systemic inflammatory cascades. J. Cell. Mol. Med. 2023, 27, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; vd Gaag, M.; Mengelers, M.J.; van Trijp, J.M.; de Vries, J.H.; Katan, M.B. Absorption and disposition kinetics of the dietary antioxidant quercetin in man. Free Radic. Biol. Med. 1996, 21, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Galvano, F.; La Fauci, L.; Lazzarino, G.; Fogliano, V.; Ritieni, A.; Ciappellano, S.; Battistini, N.C.; Tavazzi, B.; Galvano, G. Cyanidins: Metabolism and biological properties. J. Nutr. Biochem. 2004, 15, 2–11. [Google Scholar] [CrossRef]
- Fiorentino, F.; Mai, A.; Rotili, D. Emerging Therapeutic Potential of SIRT6 Modulators. J. Med. Chem. 2021, 64, 9732–9758. [Google Scholar] [CrossRef]
- Rahnasto-Rilla, M.; Tyni, J.; Huovinen, M.; Jarho, E.; Kulikowicz, T.; Ravichandran, S.; Bohr, V.A.; Ferrucci, L.; Lahtela-Kakkonen, M.; Moaddel, R. Natural polyphenols as sirtuin 6 modulators. Sci. Rep. 2018, 8, 4163. [Google Scholar] [CrossRef]
- Shozib, H.B.; Islam, M.M.; Mahmud, S.A.S.; Bari, M.N.; Akter, N.; Jahan, S.; Hosen, S.; Hossain, M.N.; Nabi, A.; Siddiquee, M.A.; et al. Application of Cyanidin-3-Glucosides as a functional food ingredient in rice-based bakery products. Saudi J. Biol. Sci. 2021, 28, 7472–7480. [Google Scholar] [CrossRef]
- Sato, S.; Saika, A.; Koshiyama, T.; Higashiyama, Y.; Fukuoka, T.; Morita, T. Biosynthesis of ergothioneine: Current state, achievements, and perspectives. Appl. Microbiol. Biotechnol. 2025, 109, 93. [Google Scholar] [CrossRef]
- Cheng, J.; Keuthan, C.J.; Esumi, N. The many faces of SIRT6 in the retina and retinal pigment epithelium. Front. Cell Dev. Biol. 2023, 11, 1244765. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, J.; Miao, Z.; Sun, Y.; Dong, M.; Lin, Y.; Wu, Y.; Sun, Z. Ergothioneine inhibits the progression of osteoarthritis via the Sirt6/NF-κB axis both in vitro and in vivo. Int. Immunopharmacol. 2023, 119, 110211. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Cheah, I.K.; Tang, R.M.Y. Ergothioneine—A diet-derived antioxidant with therapeutic potential. FEBS Lett. 2018, 592, 3357–3366. [Google Scholar] [CrossRef]
- Tian, X.; Thorne, J.L.; Moore, J.B. Ergothioneine: An underrecognised dietary micronutrient required for healthy ageing? Br. J. Nutr. 2023, 129, 104–114. [Google Scholar] [CrossRef]
- Zhu, X.; Wen, S.; Gul, H.; Xu, P.; Yang, Y.; Liao, X.; Ye, Y.; Xu, Z.; Zhang, X.; Wu, L. Exploring regulatory network of icariin synthesis in Herba Epimedii through integrated omics analysis. Front. Plant Sci. 2024, 15, 1409601. [Google Scholar] [CrossRef]
- Gao, J.; He, Y.; Shi, F.; Hou, F.; Wu, X.; Yi, Y.; Zhang, Y.; Gong, Q. Activation of Sirt6 by icariside II alleviates depressive behaviors in mice with poststroke depression by modulating microbiota-gut-brain axis. J. Adv. Res. 2025; in press. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; He, L.; Wang, Q.; Wang, L.; Yuan, T.; Xiao, Y.; Fan, Y.; Zhang, X. Icariin conjugated hyaluronic acid/collagen hydrogel for osteochondral interface restoration. Acta Biomater. 2018, 74, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; He, X.W.; Jiang, J.G.; Xu, X.L. Hydroxytyrosol and its potential therapeutic effects. J. Agric. Food Chem. 2014, 62, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Velotti, F.; Bernini, R. Hydroxytyrosol Interference with Inflammaging via Modulation of Inflammation and Autophagy. Nutrients 2023, 15, 1774. [Google Scholar] [CrossRef]
- Zhi, L.Q.; Yao, S.X.; Liu, H.L.; Li, M.; Duan, N.; Ma, J.B. Hydroxytyrosol inhibits the inflammatory response of osteoarthritis chondrocytes via SIRT6-mediated autophagy. Mol. Med. Rep. 2018, 17, 4035–4042. [Google Scholar] [CrossRef]
- Campus, M.; Corrias, F.; Angioni, A.; Arru, N.; Sedda, P.; Addis, M.; Fiori, M.; Paba, A.; Chessa, L.; Comunian, R. Efficacy of a Native Microbial Starter in Promoting Table Olive Fermentation: An Industrial-Scale Trial at Controlled and Ambient Temperature. Foods 2025, 14, 2159. [Google Scholar] [CrossRef]
- You, W.; Rotili, D.; Li, T.M.; Kambach, C.; Meleshin, M.; Schutkowski, M.; Chua, K.F.; Mai, A.; Steegborn, C. Structural Basis of Sirtuin 6 Activation by Synthetic Small Molecules. Angew. Chem. Int. Ed. Engl. 2017, 56, 1007–1011. [Google Scholar] [CrossRef]
- Jiao, F.; Zhang, Z.; Hu, H.; Zhang, Y.; Xiong, Y. SIRT6 Activator UBCS039 Inhibits Thioacetamide-Induced Hepatic Injury In Vitro and In Vivo. Front. Pharmacol. 2022, 13, 837544. [Google Scholar] [CrossRef] [PubMed]
- Iachettini, S.; Trisciuoglio, D.; Rotili, D.; Lucidi, A.; Salvati, E.; Zizza, P.; Di Leo, L.; Del Bufalo, D.; Ciriolo, M.R.; Leonetti, C.; et al. Pharmacological activation of SIRT6 triggers lethal autophagy in human cancer cells. Cell Death Dis. 2018, 9, 996. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, J.; Deng, W.; Chen, Y.; Shang, J.; Song, K.; Zhang, L.; Wang, C.; Lu, S.; Yang, X.; et al. Identification of a cellularly active SIRT6 allosteric activator. Nat. Chem. Biol. 2018, 14, 1118–1126. [Google Scholar] [CrossRef]
- Copp, M.E.; Shine, J.; Brown, H.L.; Nimmala, K.R.; Hansen, O.B.; Chubinskaya, S.; Collins, J.A.; Loeser, R.F.; Diekman, B.O. Sirtuin 6 activation rescues the age-related decline in DNA damage repair in primary human chondrocytes. Aging 2023, 15, 13628–13645. [Google Scholar] [CrossRef]
- Shang, J.; Zhu, Z.; Chen, Y.; Song, J.; Huang, Y.; Song, K.; Zhong, J.; Xu, X.; Wei, J.; Wang, C.; et al. Small-molecule activating SIRT6 elicits therapeutic effects and synergistically promotes anti-tumor activity of vitamin D3 in colorectal cancer. Theranostics 2020, 10, 5845–5864. [Google Scholar] [CrossRef]
- He, T.; Shang, J.; Gao, C.; Guan, X.; Chen, Y.; Zhu, L.; Zhang, L.; Zhang, C.; Zhang, J.; Pang, T. A novel SIRT6 activator ameliorates neuroinflammation and ischemic brain injury via EZH2/FOXC1 axis. Acta Pharm. Sin. B 2021, 11, 708–726. [Google Scholar] [CrossRef]
- You, W.; Steegborn, C. Structural Basis for Activation of Human Sirtuin 6 by Fluvastatin. ACS Med. Chem. Lett. 2020, 11, 2285–2289. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, P.; Ge, J.; Li, H. SIRT6 in Aging, Metabolism, Inflammation and Cardiovascular Diseases. Aging Dis. 2022, 13, 1787–1822. [Google Scholar] [CrossRef] [PubMed]
- Riegger, J.; Maurer, S.; Pulasani, S.; Brenner, R.E. Simvastatin and fluvastatin attenuate trauma-induced cell death and catabolism in human cartilage. Front. Bioeng. Biotechnol. 2022, 10, 965302. [Google Scholar] [CrossRef]



| NAD+ Precursors | Metabolic Pathways | Mechanism | Application | Ref. |
|---|---|---|---|---|
| NR | Salvage pathway | Convert to NMN through NRK enzyme | Traditional NAD+ supplements | [115,116,117,118] |
| NAM | Salvage pathway | Convert to NMN through NAMPT | [119,120] | |
| NA | Preiss–Handler pathway | Convert to NMN through NAPRT | [121] | |
| NMN | Salvage pathway | Generate NAD+ through NMNAT | Repair DNA; Slow down aging; Regulate metabolism; Traditional NAD+ supplements | [122,123,124] |
| Activators | Sources | Mechanisms | Evidence Level | Effects in OA Models | Ref. | |
|---|---|---|---|---|---|---|
| Natural Activator | Quercetin | Onions, apples, broccoli, berries, green tea, red wine, cocoa, and vegetable juices | Regulate Nrf2; Reduce IL-1β, TNF-α and IL-6 | Low | 20 µM quercetin reduced MMP13 mRNA by 35–45% and COX-2 protein by 30%; Rat oral administration of 50 mg kg−1 of quercetin reduced the immunoreactive area of MMP-13 in cartilage by approximately 40%, and decreased COX-2 by about 30% | [139,140,141,142,143,144] |
| Inhibit JAK/STAT; Reduce inflammatory mediators | ||||||
| Reduce MMP-13; Inhibit ECM degradation; Protect chondrocytes | ||||||
| Cyanidin | Blackberry, blackcurrant, black rice and purple corn | Upregulate FOXO3a; Downregulate Twist1 and GLUT1; Reduce excessive ROS production | Low | 460 µM cyanidin can stimulate the deacetylation activity of SIRT6 by up to 55 times | [145,146,147,148] | |
| Ergothioneine | Mushrooms, fermented soy products, fermented rice bran and spirulina | Regulate NF-κB; Inhibit the inflammatory response | Medium | 50 mg/kg ergothioneine decreased Mankin score by 32% and reduced the area of subchondral bone sclerosis by 28% | [149,150,151,152,153] | |
| Icaritin | Herba Epimedii | Regulate NF-κB; Exert anti-inflammatory effects | Low | In an animal experimental model, it was confirmed that the expression of type II collagen was upregulated and cartilage repair was significantly improved | [154,155,156] | |
| Hydroxytyrosol | Tea, olive oil and olives | Inhibit MMPs; Reduce inflammatory responses and cartilage degradation | Low | An in vitro mouse experiment showed 50 µM hydroxytyrosol led to a 51% reduction in MMP-13 | [157,158,159,160] | |
| Eliminate free radicals; Promote autophagy; Prevent oxidative damage to chondrocytes | ||||||
| Synthetic Activator | UBCS039 | Artificially synthesized | Inhibit NF-κB; Reduce inflammatory factors | Low | 40 µM UBCS039 (in vitro) or 20 mg kg−1 (in vivo) can reduce main inflammatory indicators (IL-1β and TNF-α) by 55–62% | [161,162,163] |
| Increase ATP; Prevent aggravation of mitochondrial dysfunction | ||||||
| MDL-800 | Inhibit inflammatory factors; Promote synthesis of cartilage matrix; Inhibit cell aging | Medium | MDL-800 resulted in a 2.1-point decrease in the Mankin score of DMM mice after 8 weeks, a 48% reduction in the proportion of p16INK4a-positive chondrocytes, and a 52% decrease in the fluorescence intensity of γH2AX | [126,164,165] | ||
| Reduce DNA damage; Inhibit cell aging | ||||||
| MDL-811 | Reduce TNF-α, IL-1β and IL-6; Alleviate inflammatory response | Low | An animal experiment showed that MDL-811 can reduce TNF-α by 70% and IL-1β by 60% | [166,167] | ||
| Fluvastatin | Block NF-κB; Downregulate MMP-13 and ADAMTS | Low | 10 µM fluvastatin reduced mortality rate of chondrocytes and downregulated MMPs | [168,169,170] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Wu, W. The Influence of Sirtuin 6 on Chondrocyte Senescence in Osteoarthritis Under Aging: Focusing on Mitochondrial Dysfunction and Oxidative Stress. Antioxidants 2025, 14, 1228. https://doi.org/10.3390/antiox14101228
Zhao H, Wu W. The Influence of Sirtuin 6 on Chondrocyte Senescence in Osteoarthritis Under Aging: Focusing on Mitochondrial Dysfunction and Oxidative Stress. Antioxidants. 2025; 14(10):1228. https://doi.org/10.3390/antiox14101228
Chicago/Turabian StyleZhao, Huiying, and Wei Wu. 2025. "The Influence of Sirtuin 6 on Chondrocyte Senescence in Osteoarthritis Under Aging: Focusing on Mitochondrial Dysfunction and Oxidative Stress" Antioxidants 14, no. 10: 1228. https://doi.org/10.3390/antiox14101228
APA StyleZhao, H., & Wu, W. (2025). The Influence of Sirtuin 6 on Chondrocyte Senescence in Osteoarthritis Under Aging: Focusing on Mitochondrial Dysfunction and Oxidative Stress. Antioxidants, 14(10), 1228. https://doi.org/10.3390/antiox14101228






