Assessment of Antioxidant Potential of Carbon-Based Nanomaterials from Different Sources
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Carbon Dots (CDs)
Synthesis of Carbon Dots Derived from Citric Acid Precursor (CB-Ca)
Synthesis of Iron-Doped Carbon Dots (CB-Fe)
Synthesis of Carbon Dots Derived from Momordica charantia Leaves (CB-Mc)
2.2.2. Characterisation of CDs
- (a)
- Absorption characterisation using Ultraviolet-visible (UV-Vis) spectroscopy
- (b)
- Photoluminescence (PL) spectroscopy
- (c)
- Fourier Transform Infrared (FTIR) spectroscopy
- (d)
- X-ray Powder Diffraction (XRD)
- (e)
- High Resolution Transmission Electron Microscopy (HRTEM)
2.2.3. Antioxidant Assays
- (a)
- 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity
- (b)
- Hydrogen Peroxide Scavenging Effects
- (c)
- Ferric-Reducing Capability Assay
- (d)
- Total Antioxidant Capacity
3. Results
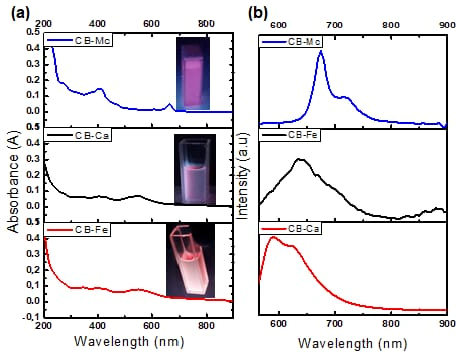
3.1. Optical Characterisation of CB-Ca, CB-Fe and CB-Mc
- (a)
- Absorption analysis
- (b)
- PL emission analysis
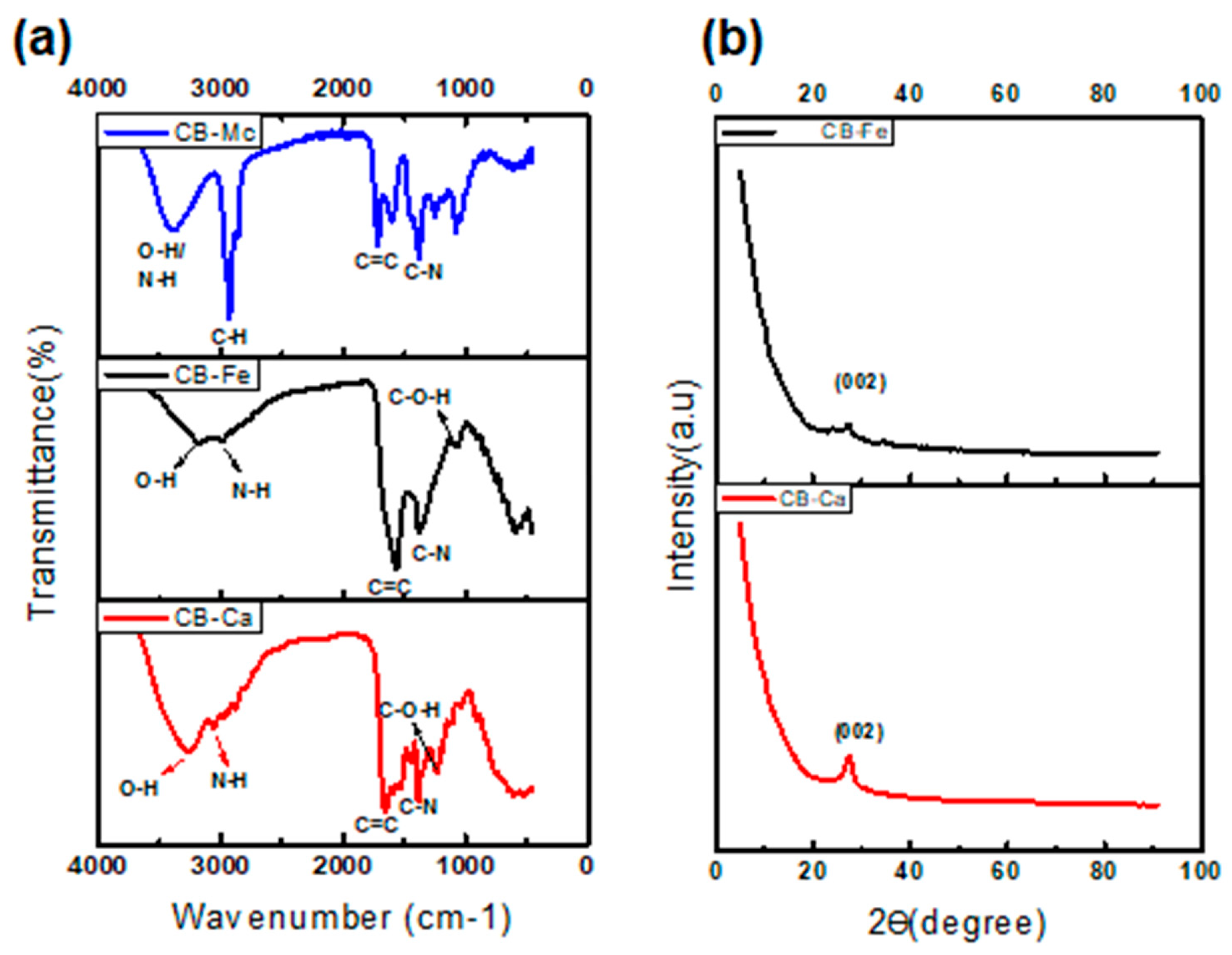
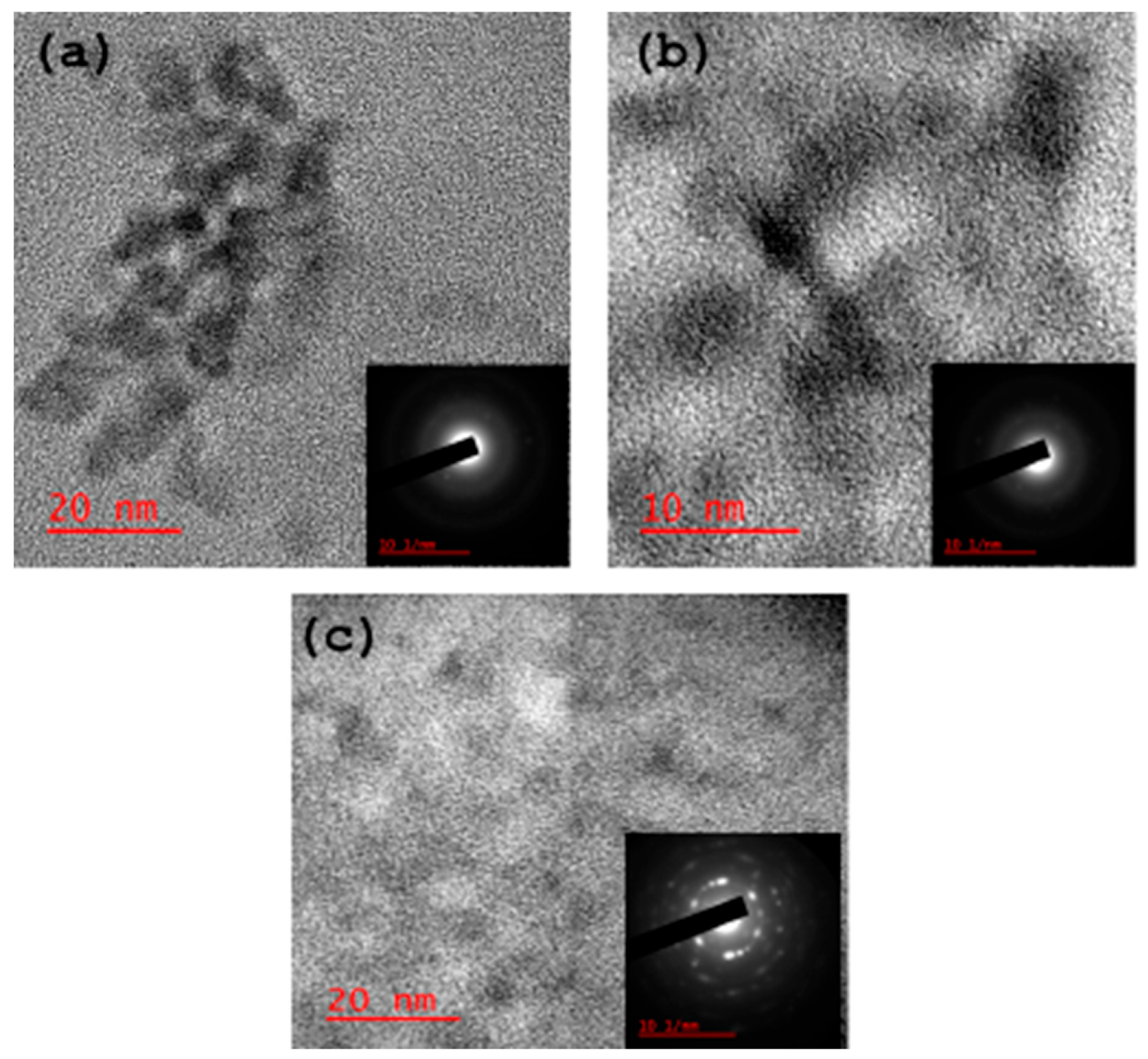
3.2. Structural Characterisation of CB-Ca, CB-Fe and CB-Mc
- (a)
- Fourier Transform Infrared (FTIR) spectra
- (b)
- X-Ray Diffraction (XRD) analysis
- (c)
- Dynamic Light Scattering (DLS)
- (d)
- High Resolution Transmission Electron Microscope (HRTEM)
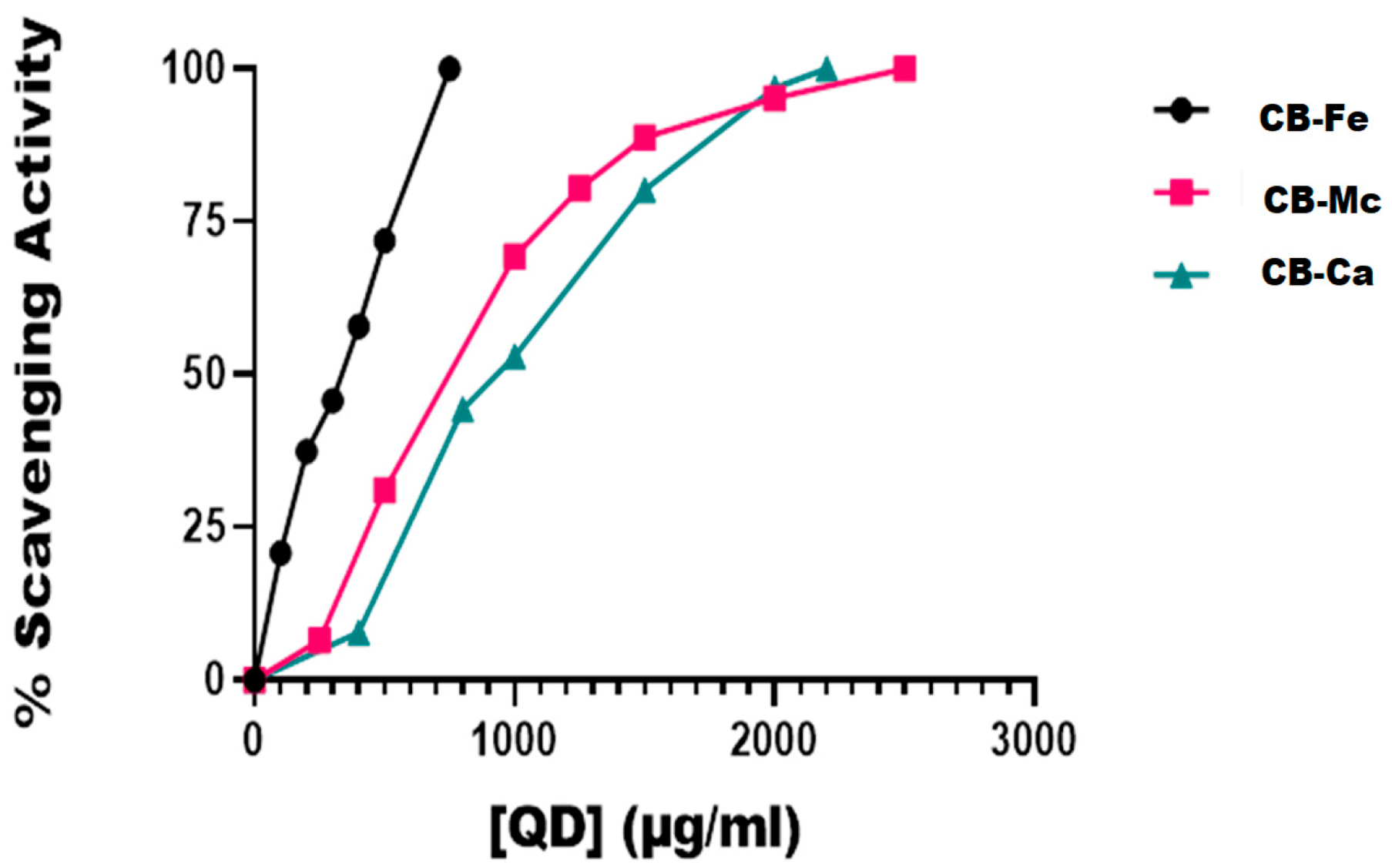
3.3. DPPH Radical Scavenging Effect
3.4. Hydrogen Peroxide Scavenging Activity
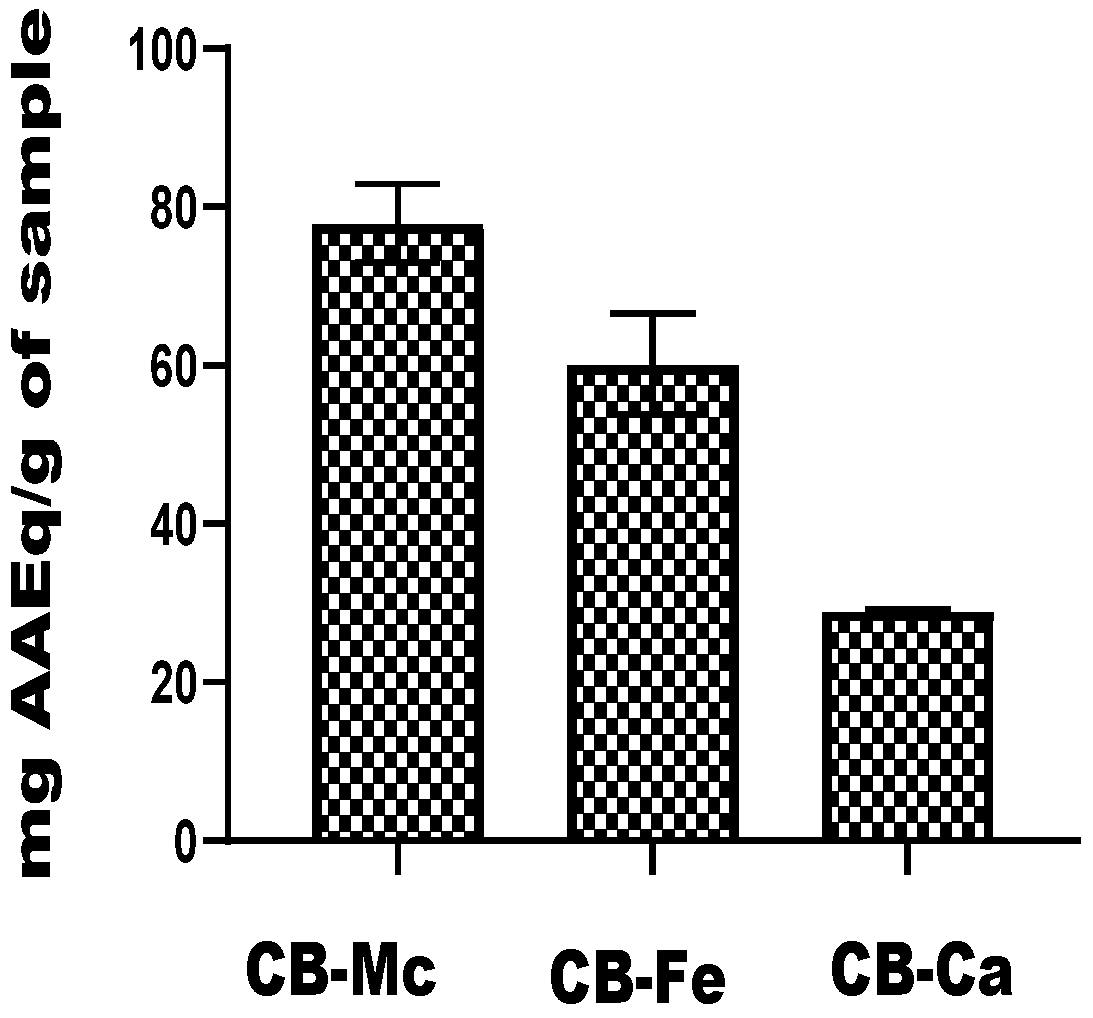
3.5. Ferric-Reducing Power Ability
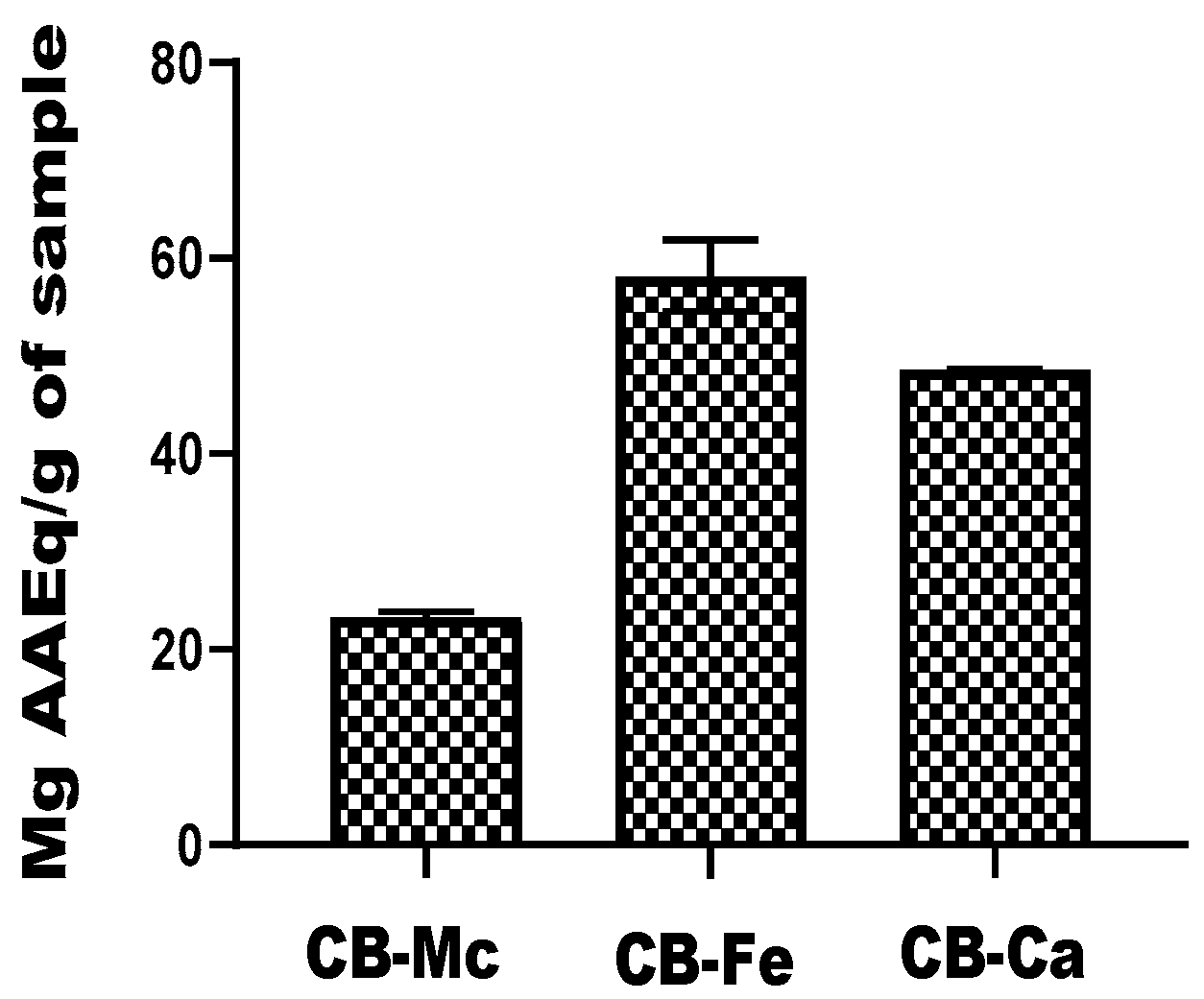
3.6. Total Antioxidant Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandal, M.; Sarkar, M.; Khan, A.; Biswas, M.; Masi, A.; Rakwal, R.; Agrawal, G.K.; Srivastava, A.; Sarkar, A. Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) in Plants—Maintenance of Structural Individuality and Functional Blend. Adv. Redox Res. 2022, 5, 100039. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox System in Health and Disease: The Latest Update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Al-Shehri, S.S. Reactive Oxygen and Nitrogen Species and Innate Immune Response. Biochimie 2021, 181, 52–64. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Huete-Acevedo, J.; Mas-Bargues, C.; Arnal-Forné, M.; Atencia-Rabadán, S.; Sanz-Ros, J.; Borrás, C. Role of Redox Homeostasis in the Communication Between Brain and Liver Through Extracellular Vesicles. Antioxidants 2024, 13, 1493. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human hHealth. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P.; Rahman, H.S. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Pal, D. Free Radicals, Natural Antioxidants, and Their Reaction Mechanisms. RSC Adv. 2015, 35, 27986–28006. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, P.; Zhang, H.; Zhu, Q.; Liao, C.; Jiang, G. Analysis, Occurrence, Toxicity and Environmental Health Risks of Synthetic Phenolic Antioxidants: A Review. Environ. Res. 2021, 201, 111531. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Akash, S.; Harun-Or-Rashid, M.; Ray, T.K.; Rahaman, M.S.; Islam, M.; Anika, F.; Hosain, M.K.; Aovi, F.I.; et al. Recent Advancements of Nanoparticles Application in Cancer and Neurodegenerative Disorders: At a Glance. Biomed. Pharmacother. 2022, 153, 113305. [Google Scholar] [CrossRef]
- Sudharsana, C.; Anvarsha, N.; Kalyani, P.; Sudharsana, C.; Anvarsha, N.; Kalyani, P. Carbon-Based Nanocomposites: A Comprehensive Review of Their Multifunctional Applications. In Nanocomposites—Properties, Preparations and Applications; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Speranza, G. Carbon Nanomaterials: Synthesis, Functionalization and Sensing Applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef]
- Rauti, R.; Musto, M.; Bosi, S.; Prato, M.; Ballerini, L. Properties and Behavior of Carbon Nanomaterials When Interfacing Neuronal Cells: How Far Have We Come? Carbon 2019, 143, 430–446. [Google Scholar] [CrossRef]
- Omran, B.; Baek, K.H. Nanoantioxidants: Pioneer Types, Advantages, Limitations, and Future Insights. Molecules 2021, 26, 7031. [Google Scholar] [CrossRef]
- Tang, N.; Ding, Z.; Zhang, J.; Cai, Y.; Bao, X. Recent Advances of Antioxidant Low-Dimensional Carbon Materials for Biomedical Applications. Front. Bioeng. Biotechnol. 2023, 11, 1121477. [Google Scholar] [CrossRef]
- Knez, E.; Kadac-Czapska, K.; Grembecka, M. Evaluation of Spectrophotometric Methods for Assessing Antioxidant Potential in Plant Food Samples—A Critical Approach. Appl. Sci. 2025, 15, 5925. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants: A Comprehensive Review. Arch. Toxicol. 2025, 99, 1893. [Google Scholar] [CrossRef]
- Dutta, A.K. Green Biosynthesis of Carbon-Based Nanomaterials for Antioxidant, Anti-Bacterial, and Anti-Cancer Applications. In Sustainable Chemistry and Pioneering Green Engineering Solutions; IGI Global Scientific Publishing: Hershey, PA, USA, 2026; pp. 117–142. [Google Scholar]
- Dechsri, K.; Suwanchawalit, C.; Pengnam, S.; Pornpitchanarong, C.; Opanasopit, P.; Apirakaramwong, A. Antioxidant Activity of Gallic Acid Carbon-Based Nanomaterials. Sci. Eng. Health Stud. 2024, 18, 24050019. [Google Scholar] [CrossRef]
- Adewale, I.O.; Adebiyi, V.G.; Famutimi, O.G.; Dada, O.V. Kinetics of Trypsin Inhibition by Methanolic and Solvent-Partitioned Fractions of Two Medicinal Plants—Momordica charantia and Xylopia aethiopica. S. Afr. J. Bot. 2023, 152, 174–181. [Google Scholar] [CrossRef]
- Liu, Z.; Gong, J.; Huang, W.; Lu, F.; Dong, H. The Effect of Momordica charantia in the Treatment of Diabetes mellitus: A Review. Evid. Based Complement. Altern. Med. 2021, 2021, 3796265. [Google Scholar] [CrossRef] [PubMed]
- Oyelere, S.F.; Ajayi, O.H.; Ayoade, T.E.; Santana Pereira, G.B.; Dayo Owoyemi, B.C.; Ilesanmi, A.O.; Akinyemi, O.A. A Detailed Review on the Phytochemical Profiles and Anti-Diabetic Mechanisms of Momordica charantia. Heliyon 2022, 8, e09253. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.T.M.; Rahman, M.M.; Sharif, M.; Khan, T.A.; Islam, S.N. Bioactive Polyphenolic Compounds and Antioxidant Potentials of Two Leafy Vegetables in Bangladesh: The Momordica charantia and the Ipomoea aquatica. Food Prod. Process. Nutr. 2024, 6, 35. [Google Scholar] [CrossRef]
- Masha, S.; Samuel Oluwafemi, O. Cost-Effective Synthesis of Red-Emitting Carbon-Based Quantum Dots and Its Photothermal Profiling. Mater. Lett. 2022, 323, 132590. [Google Scholar] [CrossRef]
- Marsden, S.B. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Marrassini, C.; Peralta, I.; Anesini, C. Comparative Study of the Polyphenol Content-Related Anti-Inflammatory and Antioxidant Activities of Two Urera aurantiaca Specimens from Different Geographical Areas. Chin. Med. 2018, 13, 22. [Google Scholar] [CrossRef]
- Ruch, R.J.; Cheng, S.; Klaunig, J.E. Prevention of Cytotoxicity and Inhibition of Intercellular Communication by Antioxidant Catechins Isolated from Chinese Green Tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef]
- Tantary, S.; Masood, A.; Bhat, A.H.; Dar, K.B.; Zargar, M.A.; Ganie, S.A. In Vitro Antioxidant and RBC Membrane Stabilization Activity of Euphorbia wallichii. Free Radic. Antioxid. 2016, 7, 13–22. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Baptista, P.; Vilas-Boas, M.; Barros, L. Free-Radical Scavenging Capacity and Reducing Power of Wild Edible Mushrooms from Northeast Portugal: Individual Cap and Stipe Activity. Food Chem. 2007, 100, 1511–1516. [Google Scholar] [CrossRef]
- Jan, S.; Khan, M.R.; Rashid, U.; Bokhari, J. Assessment of Antioxidant Potential, Total Phenolics and Flavonoids of Different Solvent Fractions of Monotheca buxifolia Fruit. Osong Public Heal. Res. Perspect. 2013, 4, 246–254. [Google Scholar] [CrossRef]
- Bailon-Ruiz, S.J.; Cedeno-Mattei, Y.; Alamo-Nole, L. Fe-Doped ZnS Quantum Dot Photocatalysts for the Degradation of Cefalexin in Water. Micro 2025, 5, 31. [Google Scholar] [CrossRef]
- Masha, S.; Oluwafemi, O.S. Synthesis of Blue and Green Emitting Carbon-Based Quantum Dots (CBQDs) and Their Cell Viability against Colon and Bladder Cancer Cell Lines. Mater. Lett. 2021, 283, 128790. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P. Vitamin C—Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Carr, A.C.; Tveden-Nyborg, P. The Pharmacology of Vitamin C. Pharmacol. Rev. 2025, 77, 100043. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.T.; Chowdhury, Z.Z.; Simarani, K.; Basirun, W.J.; Badruddin, I.A.; Hussien, M.; Alrobei, H.; Kamangar, S. Nanoantioxidants: The Fourth Generation of Antioxidants—Recent Research Roadmap and Future Perspectives. Coatings 2022, 12, 1568. [Google Scholar] [CrossRef]
- Cheng, C.; Li, M.; Shi, C.; Hu, S.; Chen, M.; Cheng, Y.; Cao, Q.; Wang, X.; Kong, X.; He, Z.; et al. Hierarchical All-Carbon Nanozyme Architectures for Enhanced Intelligent Rutin Detection. Chem. Eng. J. 2025, 514, 163289. [Google Scholar] [CrossRef]
| Quantum Dots | Size (nm) | Zeta Potential (mV) |
|---|---|---|
| CB-Ca | 3.98 ± 0.047 | −27 |
| CB-Fe | 4.60 ± 0.053 | +31 |
| CB-Mc | 5.99 ± 0.084 | −24 |
| Quantum Dots | H2O2 IC50 (µg/mL) |
|---|---|
| CB-Fe | 251.3 ± 22.82 |
| CB-Mc | 1501 ± 149.7 |
| CB-Ca | 84.2 ± 11.87 |
| Ascorbic acid | 24.2 ± 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Famutimi, O.G.; Masha, S.; Maluleke, R.; Ncapayi, V.; Lebepe, T.C.; Mgedle, N.; Kungwa, C.M.; Fanoro, O.T.; Adewale, I.O.; Oluwafemi, O.S. Assessment of Antioxidant Potential of Carbon-Based Nanomaterials from Different Sources. Antioxidants 2025, 14, 1227. https://doi.org/10.3390/antiox14101227
Famutimi OG, Masha S, Maluleke R, Ncapayi V, Lebepe TC, Mgedle N, Kungwa CM, Fanoro OT, Adewale IO, Oluwafemi OS. Assessment of Antioxidant Potential of Carbon-Based Nanomaterials from Different Sources. Antioxidants. 2025; 14(10):1227. https://doi.org/10.3390/antiox14101227
Chicago/Turabian StyleFamutimi, Oladoyin Grace, Sam Masha, Rodney Maluleke, Vuyelwa Ncapayi, Thabang Calvin Lebepe, Nande Mgedle, Cynthia Mutendu Kungwa, Olufunto Tolulope Fanoro, Isaac Olusanjo Adewale, and Oluwatobi Samuel Oluwafemi. 2025. "Assessment of Antioxidant Potential of Carbon-Based Nanomaterials from Different Sources" Antioxidants 14, no. 10: 1227. https://doi.org/10.3390/antiox14101227
APA StyleFamutimi, O. G., Masha, S., Maluleke, R., Ncapayi, V., Lebepe, T. C., Mgedle, N., Kungwa, C. M., Fanoro, O. T., Adewale, I. O., & Oluwafemi, O. S. (2025). Assessment of Antioxidant Potential of Carbon-Based Nanomaterials from Different Sources. Antioxidants, 14(10), 1227. https://doi.org/10.3390/antiox14101227






