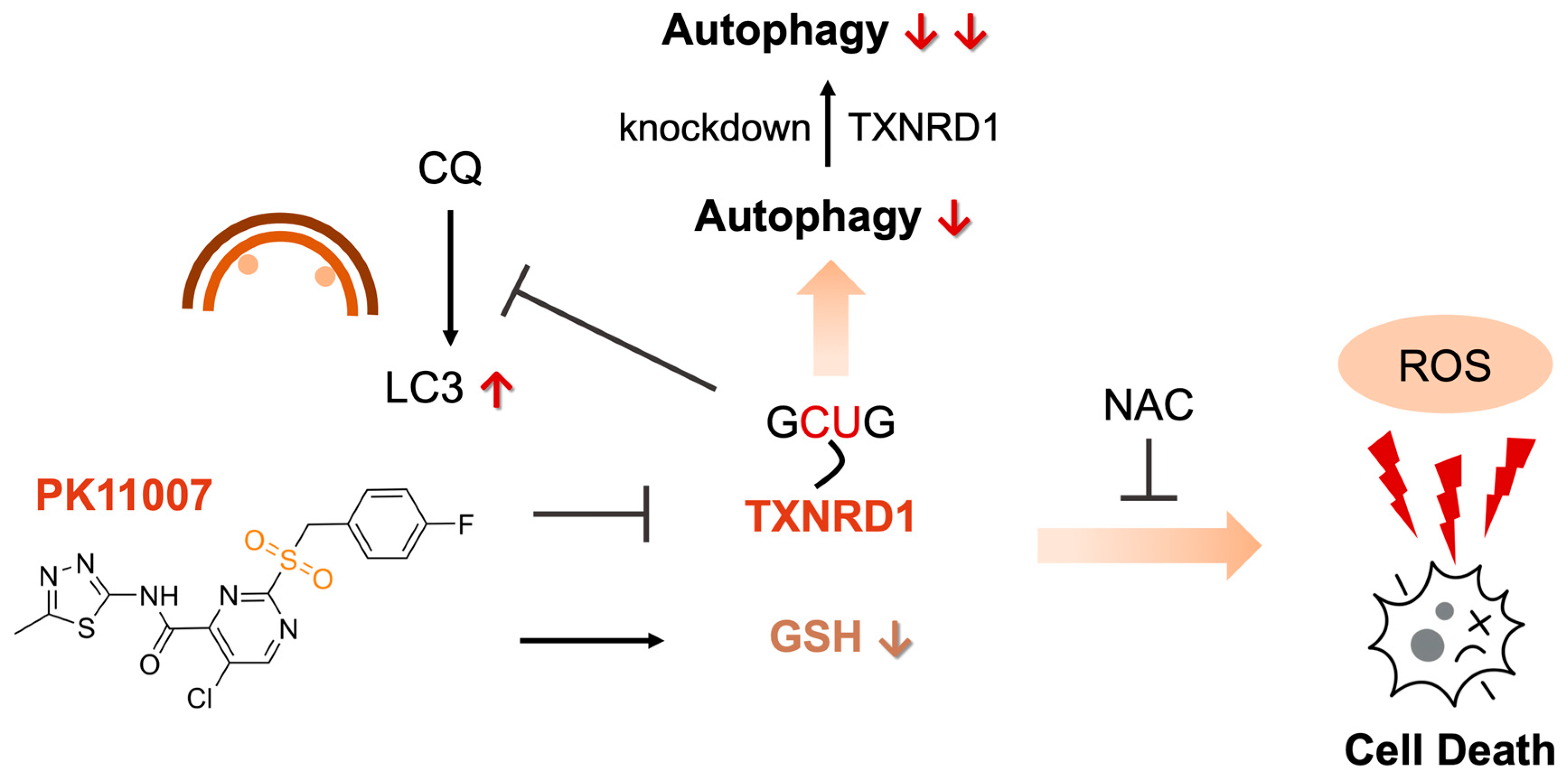
PK11007 Covalently Inhibits Thioredoxin Reductase 1 to Induce Oxidative Stress and Autophagy Impairment in NSCLC Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Key Resources
2.2. Cell Culture Conditions
2.3. Cell Viability Assay
2.4. Cell Proliferation Assay
2.5. Western Blotting
2.6. Cellular TXNRD Activity Determination
2.7. Recombinant TXNRD1 Activity Determination
- (1)
- DTNB reduction (10 nM wild-type TXNRD1 or 30–100 nM mutant TXNRD1, 2.5 mM DTNB, 300 μM NADPH in TE buffer, pH 7.5), monitoring the TNB− formation at 412 nm (εTNB = 13,600 M−1 cm−1) for 3 min.
- (2)
- 9,10-PQ/juglone reduction (30 nM TXNRD1, 30 μM 9,10-PQ/juglone, 200 μM NADPH), tracking NADPH oxidation at 340 nm (εNAPDH = 6200 M−1 cm−1) for 30 min.
2.8. Glutathione Reductase Activity Assay
2.9. Mass Spectrometry Analysis
2.10. Real-Time PCR
2.11. Stable Cell Line Generation
2.12. Differential Scanning Fluorimetry (DSF) Assay
2.13. Human Cancer Cell Line Datasets
2.14. Statistical Analysis
3. Results
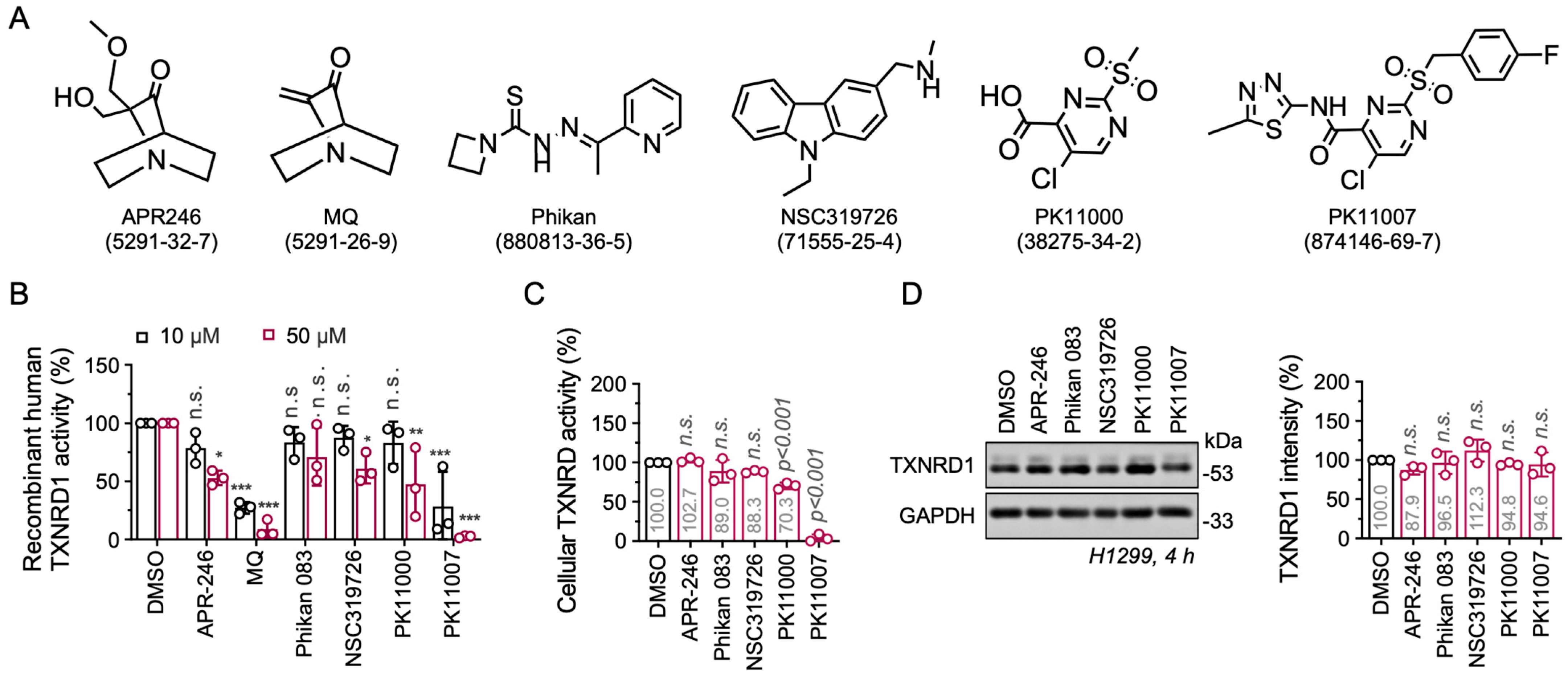
3.1. Electrophilic p53 Re-Activators MQ and PK11007 Inhibit Selenoprotein TXNRD1
3.2. PK11007 Irreversibly Inhibits TXNRD1 Activity
3.3. PK11007 Covalently Binds C-Terminal Redox-Active Residues of TXNRD1
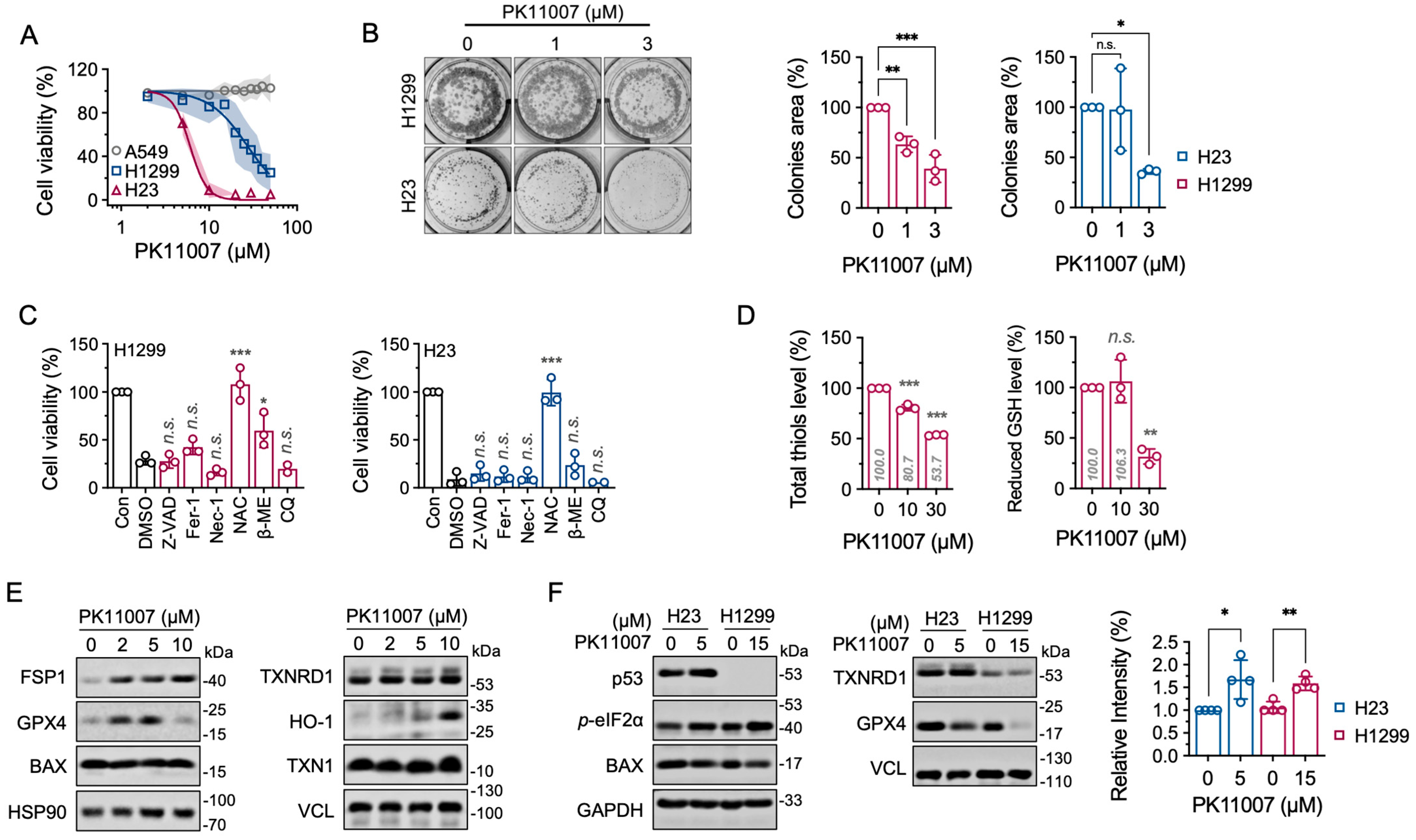
3.4. Inhibition of TXNRD1 by PK11007 Induces Cell Death in NSCLC Cells
3.5. Inhibition of TXNRD1 by PK11007 Disrupts Thiol Redox Homeostasis
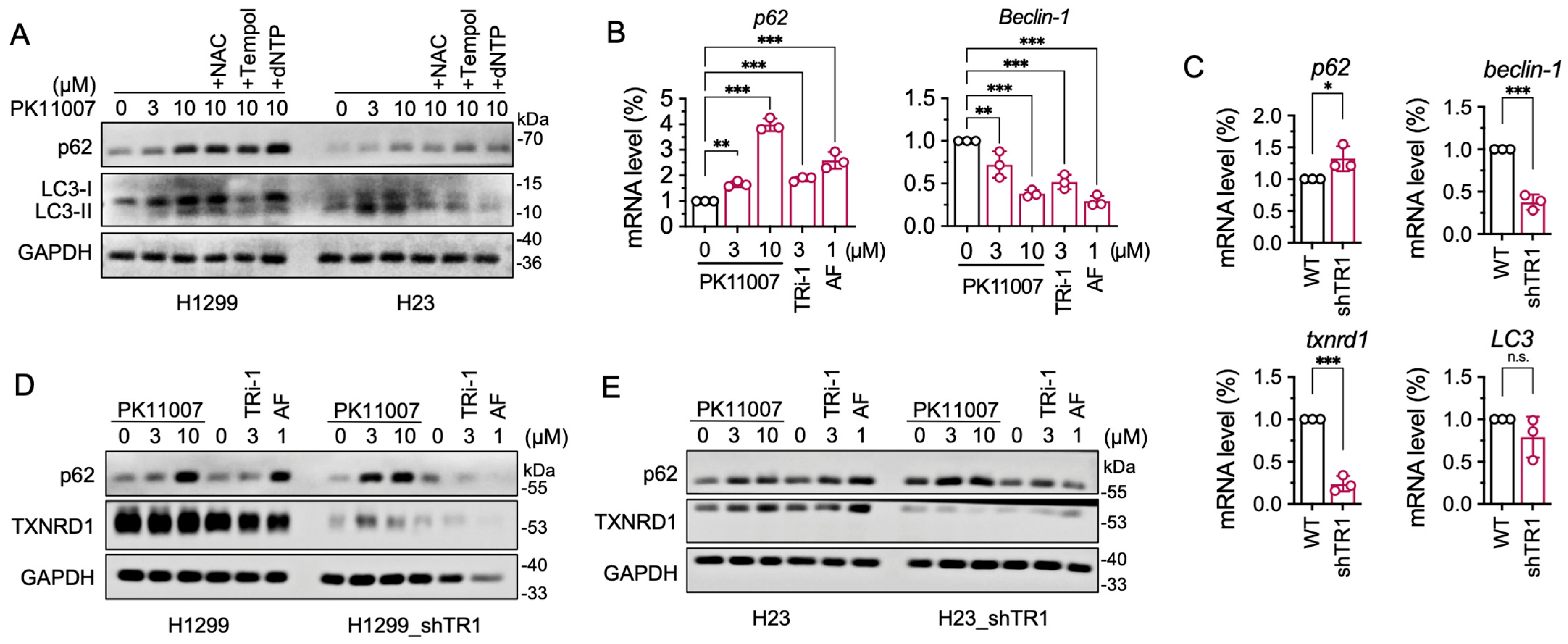
3.6. Inhibition of TXNRD1 by PK11007 Inhibits Autophagic Flux
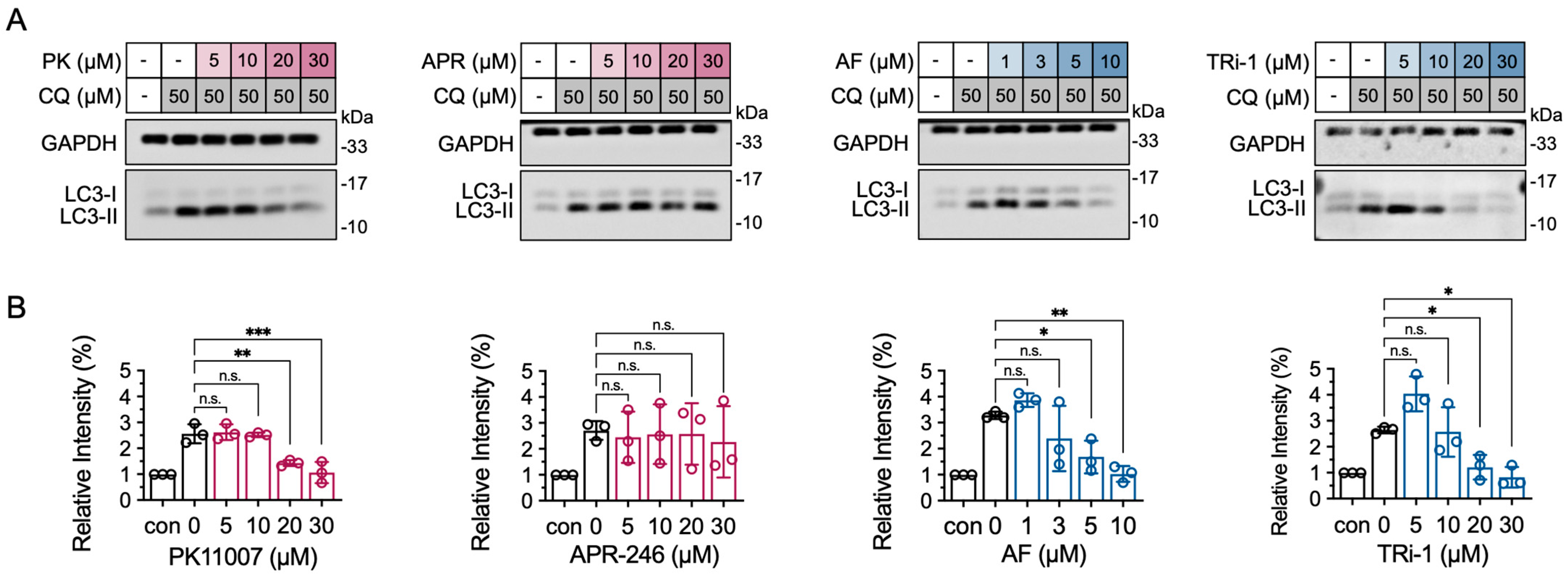
3.7. TXNRD1 Inhibition Reduces LC3-II Accumulation Induced by Chloroquine (CQ)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reich, H.J.; Hondal, R.J. Why Nature Chose Selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox Metabolism: ROS as Specific Molecular Regulators of Cell Signaling and Function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef] [PubMed]
- Arnér, E.S.J. Focus on Mammalian Thioredoxin Reductases--Important Selenoproteins with Versatile Functions. Biochim. Biophys. Acta 2009, 1790, 495–526. [Google Scholar] [CrossRef]
- Andor, A.; Mohanraj, M.; Pató, Z.A.; Úri, K.; Biri-Kovács, B.; Cheng, Q.; Arnér, E.S.J. TXNL1 Has Dual Functions as a Redox Active Thioredoxin-like Protein as Well as an ATP- and Redox-Independent Chaperone. Redox Biol. 2023, 67, 102897. [Google Scholar] [CrossRef] [PubMed]
- Martí-Andrés, P.; Finamor, I.; Torres-Cuevas, I.; Pérez, S.; Rius-Pérez, S.; Colino-Lage, H.; Guerrero-Gómez, D.; Morato, E.; Marina, A.; Michalska, P.; et al. TRP14 Is the Rate-Limiting Enzyme for Intracellular Cystine Reduction and Regulates Proteome Cysteinylation. EMBO J. 2024, 43, 2789–2812. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Sun, S.; Wang, G.; Liu, H.; Shi, W.; Zhang, Y.; Wang, Z.; Zhao, J.; Liu, H.; Yang, Z.; et al. Mutational Analysis of TXNRD1 Reveals the Essential Role of Trp114 in TRP14 Reduction and Identifies Key Determinants of Enzymatic Activity and Thermostability. Free Radic. Biol. Med. 2025, 238, 621–629. [Google Scholar] [CrossRef]
- Kritsiligkou, P.; Rand, J.D.; Weids, A.J.; Wang, X.; Kershaw, C.J.; Grant, C.M. Endoplasmic Reticulum (ER) Stress-Induced Reactive Oxygen Species (ROS) Are Detrimental for the Fitness of a Thioredoxin Reductase Mutant. J. Biol. Chem. 2018, 293, 11984–11995. [Google Scholar] [CrossRef]
- Hao, X.; Zhao, B.; Towers, M.; Liao, L.; Monteiro, E.L.; Xu, X.; Freeman, C.; Peng, H.; Tang, H.-Y.; Havas, A.; et al. TXNRD1 Drives the Innate Immune Response in Senescent Cells with Implications for Age-Associated Inflammation. Nat. Aging 2024, 4, 185–197. [Google Scholar] [CrossRef]
- Oka, S.-I.; Chin, A.; Park, J.Y.; Ikeda, S.; Mizushima, W.; Ralda, G.; Zhai, P.; Tong, M.; Byun, J.; Tang, F.; et al. Thioredoxin-1 Maintains Mitochondrial Function via Mechanistic Target of Rapamycin Signalling in the Heart. Cardiovasc. Res. 2020, 116, 1742–1755. [Google Scholar] [CrossRef]
- Dagnell, M.; Schmidt, E.E.; Arnér, E.S.J. The A to Z of Modulated Cell Patterning by Mammalian Thioredoxin Reductases. Free Radic. Biol. Med. 2018, 115, 484–496. [Google Scholar] [CrossRef]
- Johnson, F.D.; Ferrarone, J.; Liu, A.; Brandstädter, C.; Munuganti, R.; Farnsworth, D.A.; Lu, D.; Luu, J.; Sihota, T.; Jansen, S.; et al. Characterization of a Small Molecule Inhibitor of Disulfide Reductases That Induces Oxidative Stress and Lethality in Lung Cancer Cells. Cell Rep. 2022, 38, 110343. [Google Scholar] [CrossRef]
- McLoughlin, M.R.; Orlicky, D.J.; Prigge, J.R.; Krishna, P.; Talago, E.A.; Cavigli, I.R.; Eriksson, S.; Miller, C.G.; Kundert, J.A.; Sayin, V.I.; et al. TrxR1, Gsr, and Oxidative Stress Determine Hepatocellular Carcinoma Malignancy. Proc. Natl. Acad. Sci. USA 2019, 116, 11408–11417. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Sun, S.; Liu, H.; Meng, Y.; Ren, K.; Wang, G.; Liu, M.; Wu, J.; Zhang, Y.; Huang, H.; et al. Guiding Bar Motif of Thioredoxin Reductase 1 Modulates Enzymatic Activity and Inhibitor Binding by Communicating with the Co-Factor FAD and Regulating the Flexible C-Terminal Redox Motif. Redox Biol. 2024, 70, 103050. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Zou, L.; Wang, J.; Chasapis, C.T.; Peana, M. Thioredoxin Reductase as a Pharmacological Target. Pharmacol. Res. 2021, 174, 105854. [Google Scholar] [CrossRef]
- Yoo, M.-H.; Xu, X.-M.; Carlson, B.A.; Gladyshev, V.N.; Hatfield, D.L. Thioredoxin Reductase 1 Deficiency Reverses Tumor Phenotype and Tumorigenicity of Lung Carcinoma Cells. J. Biol. Chem. 2006, 281, 13005–13008. [Google Scholar] [CrossRef]
- Mandal, P.K.; Schneider, M.; Kölle, P.; Kuhlencordt, P.; Förster, H.; Beck, H.; Bornkamm, G.W.; Conrad, M. Loss of Thioredoxin Reductase 1 Renders Tumors Highly Susceptible to Pharmacologic Glutathione Deprivation. Cancer Res. 2010, 70, 9505–9514. [Google Scholar] [CrossRef]
- Ge, Y.; Ge, Z.; Tian, F.; Tai, X.; Chen, D.; Sun, S.; Shi, Z.; Yin, J.; Wei, G.; Li, D.; et al. Sulforaphane Potentiates the Efficacy of Chemoradiotherapy in Glioblastoma by Selectively Targeting Thioredoxin Reductase 1. Cancer Lett. 2024, 611, 217429. [Google Scholar] [CrossRef]
- Chu, Y.; Nie, Q.; Zhou, X.; Yang, J.; Fang, J.; Zhang, J. Berberrubine as a Novel TrxR Inhibitor Enhances Cisplatin Sensitivity in the Treatment of Non-Small Cell Lung Cancer. Bioorg. Chem. 2025, 158, 108329. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Han, X.; Liu, R.; Fang, J. Targeting the Thioredoxin System for Cancer Therapy. Trends Pharmacol. Sci. 2017, 38, 794–808. [Google Scholar] [CrossRef]
- Gencheva, R.; Arnér, E.S.J. Thioredoxin Reductase Inhibition for Cancer Therapy. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Skos, L.; Schmidt, C.; Thomas, S.R.; Park, M.; Geiger, V.; Wenisch, D.; Bonsignore, R.; Del Favero, G.; Mohr, T.; Bileck, A.; et al. Gold-Templated Covalent Targeting of the CysSec-Dyad of Thioredoxin Reductase 1 in Cancer Cells. Cell Rep. Phys. Sci. 2024, 5, 102072. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, X.; Wang, L.; Zhang, R.; Pu, X.; Wu, S.; Li, L.; Tong, P.; Wang, J.; Meng, Q.H.; et al. Inhibition of Thioredoxin/Thioredoxin Reductase Induces Synthetic Lethality in Lung Cancers with Compromised Glutathione Homeostasis. Cancer Res. 2019, 79, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Cheff, D.M.; Huang, C.; Scholzen, K.C.; Gencheva, R.; Ronzetti, M.H.; Cheng, Q.; Hall, M.D.; Arnér, E.S.J. The Ferroptosis Inducing Compounds RSL3 and ML162 Are Not Direct Inhibitors of GPX4 but of TXNRD1. Redox Biol. 2023, 62, 102703. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, P.; Beusch, C.M.; Gencheva, R.; Cheng, Q.; Zubarev, R.; Arnér, E.S.J. Comprehensive Chemical Proteomics Analyses Reveal That the New TRi-1 and TRi-2 Compounds Are More Specific Thioredoxin Reductase 1 Inhibitors than Auranofin. Redox Biol. 2021, 48, 102184. [Google Scholar] [CrossRef]
- Stafford, W.C.; Peng, X.; Olofsson, M.H.; Zhang, X.; Luci, D.K.; Lu, L.; Cheng, Q.; Trésaugues, L.; Dexheimer, T.S.; Coussens, N.P.; et al. Irreversible Inhibition of Cytosolic Thioredoxin Reductase 1 as a Mechanistic Basis for Anticancer Therapy. Sci. Transl. Med. 2018, 10, eaaf7444. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, Y.; Xu, W.; Yang, R.; Yang, Y.; Guo, J.; Ma, Q.; Ma, K.; Zhang, J.; Xu, J. Plumbagin Reduction by Thioredoxin Reductase 1 Possesses Synergy Effects with GLUT1 Inhibitor on KEAP1-Mutant NSCLC Cells. Biomed. Pharmacother. 2022, 146, 112546. [Google Scholar] [CrossRef]
- Wang, L.; Sun, S.; Liu, H.; Zhang, Q.; Meng, Y.; Sun, F.; Zhang, J.; Liu, H.; Xu, W.; Ye, Z.; et al. Thioredoxin Reductase Inhibition and Glutathione Depletion Mediated by Glaucocalyxin A Promote Intracellular Disulfide Stress in Gastric Cancer Cells. FEBS J. 2024, 291, 5276–5289. [Google Scholar] [CrossRef]
- Synnott, N.C.; Bauer, M.R.; Madden, S.; Murray, A.; Klinger, R.; O’Donovan, N.; O’Connor, D.; Gallagher, W.M.; Crown, J.; Fersht, A.R.; et al. Mutant P53 as a Therapeutic Target for the Treatment of Triple-Negative Breast Cancer: Preclinical Investigation with the Anti-P53 Drug, PK11007. Cancer Lett. 2018, 414, 99–106. [Google Scholar] [CrossRef]
- Bauer, M.R.; Joerger, A.C.; Fersht, A.R. 2-Sulfonylpyrimidines: Mild Alkylating Agents with Anticancer Activity toward P53-Compromised Cells. Proc. Natl. Acad. Sci. USA 2016, 113, E5271–E5280. [Google Scholar] [CrossRef]
- Ceder, S.; Eriksson, S.E.; Cheteh, E.H.; Dawar, S.; Corrales Benitez, M.; Bykov, V.J.N.; Fujihara, K.M.; Grandin, M.; Li, X.; Ramm, S.; et al. A Thiol-Bound Drug Reservoir Enhances APR-246-Induced Mutant P53 Tumor Cell Death. EMBO Mol. Med. 2021, 13, e10852. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, M.-Q.-Z.; Conserva, F.; Hosny, G.; Selivanova, G.; Bykov, V.J.N.; Arnér, E.S.J.; Wiman, K.G. APR-246/PRIMA-1MET Inhibits Thioredoxin Reductase 1 and Converts the Enzyme to a Dedicated NADPH Oxidase. Cell Death Dis. 2013, 4, e881. [Google Scholar] [CrossRef]
- Liu, D.S.; Duong, C.P.; Haupt, S.; Montgomery, K.G.; House, C.M.; Azar, W.J.; Pearson, H.B.; Fisher, O.M.; Read, M.; Guerra, G.R.; et al. Inhibiting the System xC-/Glutathione Axis Selectively Targets Cancers with Mutant-P53 Accumulation. Nat. Commun. 2017, 8, 14844. [Google Scholar] [CrossRef] [PubMed]
- Hedström, E.; Eriksson, S.; Zawacka-Pankau, J.; Arnér, E.S.J.; Selivanova, G. P53-Dependent Inhibition of TrxR1 Contributes to the Tumor-Specific Induction of Apoptosis by RITA. Cell Cycle 2009, 8, 3584–3591. [Google Scholar] [CrossRef]
- Lu, J.; Chew, E.-H.; Holmgren, A. Targeting Thioredoxin Reductase Is a Basis for Cancer Therapy by Arsenic Trioxide. Proc. Natl. Acad. Sci. USA 2007, 104, 12288–12293. [Google Scholar] [CrossRef]
- Xu, J.; Croitoru, V.; Rutishauser, D.; Cheng, Q.; Arnér, E.S.J. Wobble Decoding by the Escherichia Coli Selenocysteine Insertion Machinery. Nucleic Acids Res. 2013, 41, 9800–9811. [Google Scholar] [CrossRef]
- Arnér, E.S.; Holmgren, A. Measurement of Thioredoxin and Thioredoxin Reductase. Curr. Protoc. Toxicol. 2001, 24, 7.4.1–7.4.14. [Google Scholar] [CrossRef]
- Xu, J.; Arnér, E.S.J. Pyrroloquinoline Quinone Modulates the Kinetic Parameters of the Mammalian Selenoprotein Thioredoxin Reductase 1 and Is an Inhibitor of Glutathione Reductase. Biochem. Pharmacol. 2012, 83, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, D.; Cui, W.; Ji, M.; Qian, X.; Zhong, L. Molecular Bases of Thioredoxin and Thioredoxin Reductase-Mediated Prooxidant Actions of (-)-Epigallocatechin-3-Gallate. Free Radic. Biol. Med. 2010, 49, 2010–2018. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liu, H.; Shi, W.; Zhou, H.; Wu, H.; Xu, W.; Xu, J. Protocol for Assaying Irreversible Inhibitors of Thioredoxin Reductase 1. STAR Protoc. 2024, 5, 103235. [Google Scholar] [CrossRef]
- Wu, T.; Hornsby, M.; Zhu, L.; Yu, J.C.; Shokat, K.M.; Gestwicki, J.E. Protocol for Performing and Optimizing Differential Scanning Fluorimetry Experiments. STAR Protoc. 2023, 4, 102688. [Google Scholar] [CrossRef]
- Peuget, S.; Zhou, X.; Selivanova, G. Translating P53-Based Therapies for Cancer into the Clinic. Nat. Rev. Cancer 2024, 24, 192–215. [Google Scholar] [CrossRef]
- Sun, S.; Xu, W.; Zhang, Y.; Yang, Y.; Ma, Q.; Xu, J. Menadione Inhibits Thioredoxin Reductase 1 via Arylation at the Sec498 Residue and Enhances Both NADPH Oxidation and Superoxide Production in Sec498 to Cys498 Substitution. Free Radic. Biol. Med. 2021, 172, 482–489. [Google Scholar] [CrossRef]
- Xu, J.; Cheng, Q.; Arnér, E.S.J. Details in the Catalytic Mechanism of Mammalian Thioredoxin Reductase 1 Revealed Using Point Mutations and Juglone-Coupled Enzyme Activities. Free Radic. Biol. Med. 2016, 94, 110–120. [Google Scholar] [CrossRef]
- Pérez-Pérez, M.E.; Zaffagnini, M.; Marchand, C.H.; Crespo, J.L.; Lemaire, S.D. The Yeast Autophagy Protease Atg4 Is Regulated by Thioredoxin. Autophagy 2014, 10, 1953–1964. [Google Scholar] [CrossRef]
- Swamynathan, M.M.; Kuang, S.; Watrud, K.E.; Doherty, M.R.; Gineste, C.; Mathew, G.; Gong, G.Q.; Cox, H.; Cheng, E.; Reiss, D.; et al. Dietary Pro-Oxidant Therapy by a Vitamin K Precursor Targets PI 3-Kinase VPS34 Function. Science 2024, 386, eadk9167. [Google Scholar] [CrossRef]
- Prasad, C.B.; Oo, A.; Liu, Y.; Qiu, Z.; Zhong, Y.; Li, N.; Singh, D.; Xin, X.; Cho, Y.-J.; Li, Z.; et al. The Thioredoxin System Determines CHK1 Inhibitor Sensitivity via Redox-Mediated Regulation of Ribonucleotide Reductase Activity. Nat. Commun. 2024, 15, 4667. [Google Scholar] [CrossRef]
- Delgobo, M.; Gonçalves, R.M.; Delazeri, M.A.; Falchetti, M.; Zandoná, A.; Nascimento das Neves, R.; Almeida, K.; Fagundes, A.C.; Gelain, D.P.; Fracasso, J.I.; et al. Thioredoxin Reductase-1 Levels Are Associated with NRF2 Pathway Activation and Tumor Recurrence in Non-Small Cell Lung Cancer. Free Radic. Biol. Med. 2021, 177, 58–71. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Cao, M.; Fu, Z.; Wang, H.; Zhang, S.; Zhu, K.; Hou, Z.; Cui, J.; Yue, P.; et al. Thioredoxin Facilitates Hepatocellular Carcinoma Stemness and Metastasis by Increasing BACH1 Stability to Activate the AKT/mTOR Pathway. FASEB J. 2023, 37, e22943. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Nie, L.; Zhang, Y.; Yan, Y.; Wang, C.; Colic, M.; Olszewski, K.; Horbath, A.; Chen, X.; Lei, G.; et al. Actin Cytoskeleton Vulnerability to Disulfide Stress Mediates Disulfidptosis. Nat. Cell Biol. 2023, 25, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Dirks, K.; Kim, S.Y.; Qiu, Z.; Gao, Y.; Sun, D.; Peruggia, G.; Sallavanti, J.; Li, W. Inhibition of Thioredoxin Reductase 1 Sensitizes Glucose-Starved Glioblastoma Cells to Disulfidptosis. Cell Death Differ. 2025, 32, 598–612. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Zhang, C.; Ni, S.; Ma, M.; Zhang, X.; Sang, W.; Lv, T.; Qian, Z.; Yi, C.; Yu, B. NFATc1-Mediated Expression of SLC7A11 Drives Sensitivity to TXNRD1 Inhibitors in Osteoclast Precursors. Redox Biol. 2023, 63, 102711. [Google Scholar] [CrossRef]
- Llabani, E.; Hicklin, R.W.; Lee, H.Y.; Motika, S.E.; Crawford, L.A.; Weerapana, E.; Hergenrother, P.J. Diverse Compounds from Pleuromutilin Lead to a Thioredoxin Inhibitor and Inducer of Ferroptosis. Nat. Chem. 2019, 11, 521–532. [Google Scholar] [CrossRef]
- Dos Santos, A.F.; Fazeli, G.; Xavier da Silva, T.N.; Friedmann Angeli, J.P. Ferroptosis: Mechanisms and Implications for Cancer Development and Therapy Response. Trends Cell Biol. 2023, 33, 1062–1076. [Google Scholar] [CrossRef]
- Saitoh, M.; Nishitoh, H.; Fujii, M.; Takeda, K.; Tobiume, K.; Sawada, Y.; Kawabata, M.; Miyazono, K.; Ichijo, H. Mammalian Thioredoxin Is a Direct Inhibitor of Apoptosis Signal-Regulating Kinase (ASK) 1. EMBO J. 1998, 17, 2596–2606. [Google Scholar] [CrossRef]
- Reynoso, E.; Liu, H.; Li, L.; Yuan, A.L.; Chen, S.; Wang, Z. Thioredoxin-1 Actively Maintains the Pseudokinase MLKL in a Reduced State to Suppress Disulfide Bond-Dependent MLKL Polymer Formation and Necroptosis. J. Biol. Chem. 2017, 292, 17514–17524. [Google Scholar] [CrossRef]
- Hadian, K.; Stockwell, B.R. The Therapeutic Potential of Targeting Regulated Non-Apoptotic Cell Death. Nat. Rev. Drug Discov. 2023, 22, 723–742. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Olzmann, J.A. The Cell Biology of Ferroptosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 424–442. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Antholine, W.E.; Myers, J.M.; Kalyanaraman, B.; Arnér, E.S.J.; Myers, C.R. The Selenium-Independent Inherent pro-Oxidant NADPH Oxidase Activity of Mammalian Thioredoxin Reductase and Its Selenium-Dependent Direct Peroxidase Activities. J. Biol. Chem. 2010, 285, 21708–21723. [Google Scholar] [CrossRef] [PubMed]
- Anestål, K.; Prast-Nielsen, S.; Cenas, N.; Arnér, E.S.J. Cell Death by SecTRAPs: Thioredoxin Reductase as a Prooxidant Killer of Cells. PLoS ONE 2008, 3, e1846. [Google Scholar] [CrossRef]
- Nagakannan, P.; Iqbal, M.A.; Yeung, A.; Thliveris, J.A.; Rastegar, M.; Ghavami, S.; Eftekharpour, E. Perturbation of Redox Balance after Thioredoxin Reductase Deficiency Interrupts Autophagy-Lysosomal Degradation Pathway and Enhances Cell Death in Nutritionally Stressed SH-SY5Y Cells. Free Radic. Biol. Med. 2016, 101, 53–70. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Sun, S.; Liu, H.; Li, T.; Xu, Y.; Yang, R.; Liu, H.; He, L.; Xu, W.; Guan, S.; et al. PK11007 Covalently Inhibits Thioredoxin Reductase 1 to Induce Oxidative Stress and Autophagy Impairment in NSCLC Cells. Antioxidants 2025, 14, 1222. https://doi.org/10.3390/antiox14101222
Zhou H, Sun S, Liu H, Li T, Xu Y, Yang R, Liu H, He L, Xu W, Guan S, et al. PK11007 Covalently Inhibits Thioredoxin Reductase 1 to Induce Oxidative Stress and Autophagy Impairment in NSCLC Cells. Antioxidants. 2025; 14(10):1222. https://doi.org/10.3390/antiox14101222
Chicago/Turabian StyleZhou, Hanziyi, Shibo Sun, Haowen Liu, Tong Li, Yiran Xu, Rui Yang, Haiyan Liu, Leiyu He, Weiping Xu, Shui Guan, and et al. 2025. "PK11007 Covalently Inhibits Thioredoxin Reductase 1 to Induce Oxidative Stress and Autophagy Impairment in NSCLC Cells" Antioxidants 14, no. 10: 1222. https://doi.org/10.3390/antiox14101222
APA StyleZhou, H., Sun, S., Liu, H., Li, T., Xu, Y., Yang, R., Liu, H., He, L., Xu, W., Guan, S., & Xu, J. (2025). PK11007 Covalently Inhibits Thioredoxin Reductase 1 to Induce Oxidative Stress and Autophagy Impairment in NSCLC Cells. Antioxidants, 14(10), 1222. https://doi.org/10.3390/antiox14101222






