Aspirin Eugenol Ester Alleviates Vascular Endothelial Ferroptosis by Enhancing Antioxidant Ability and Inhibiting the JNK/c-Jun/NCOA4/FTH Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
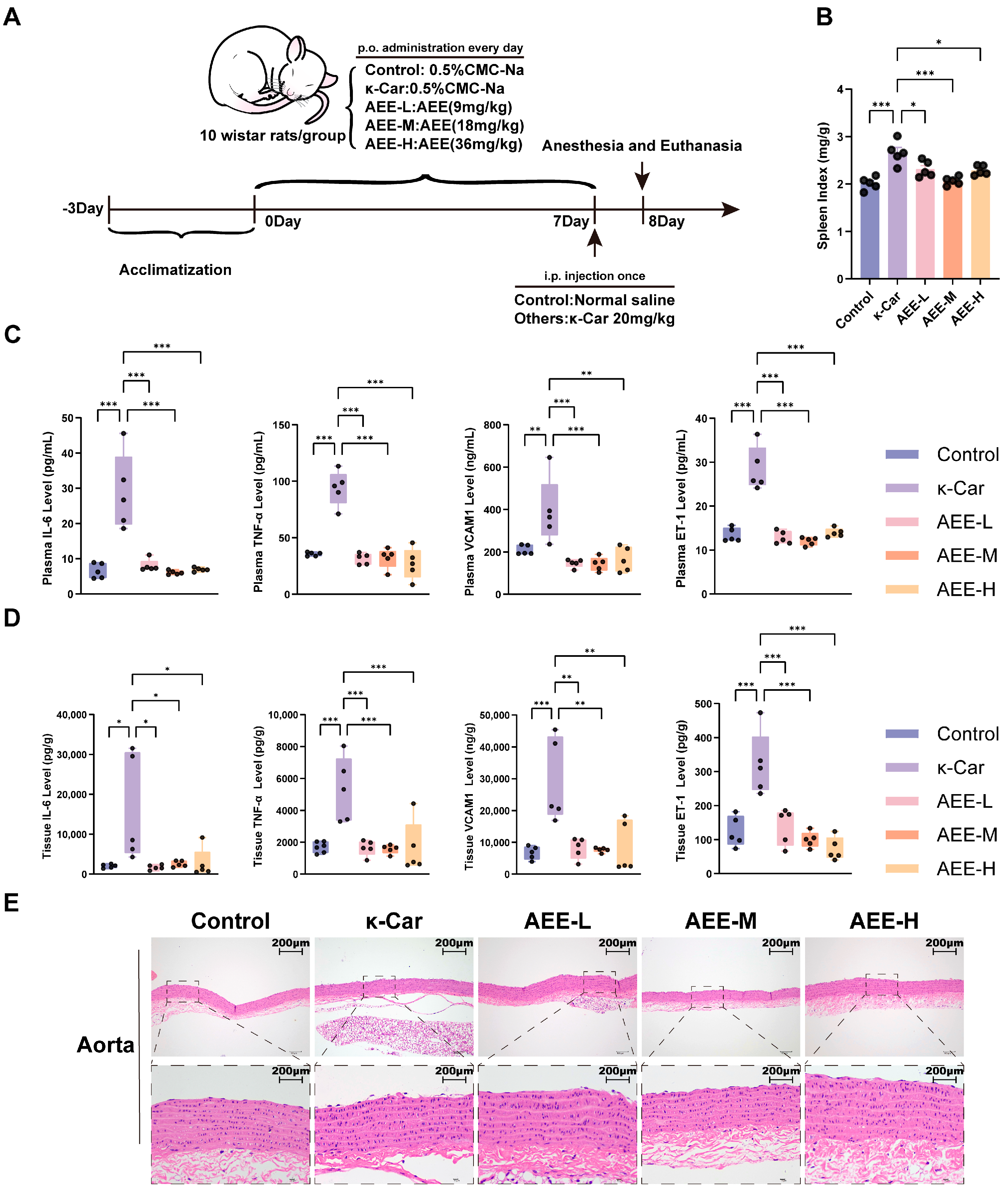
2.2. Animal Experiments
2.3. Hematoxylin–Eosin (HE) Staining
2.4. Immunohistochemistry
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Cell Culture
2.7. Cell Viability Assay
2.8. Cell Experiment Design
2.9. Lactate Dehydrogenase (LDH) Release Rate Assay
2.10. GSH Level Measurement
2.11. MDA Level Measurement
2.12. Fe2+ Level Measurement
2.13. Lipid Peroxide (LPO) Level Measurement
2.14. RT-qPCR
2.15. Transcriptome Analysis
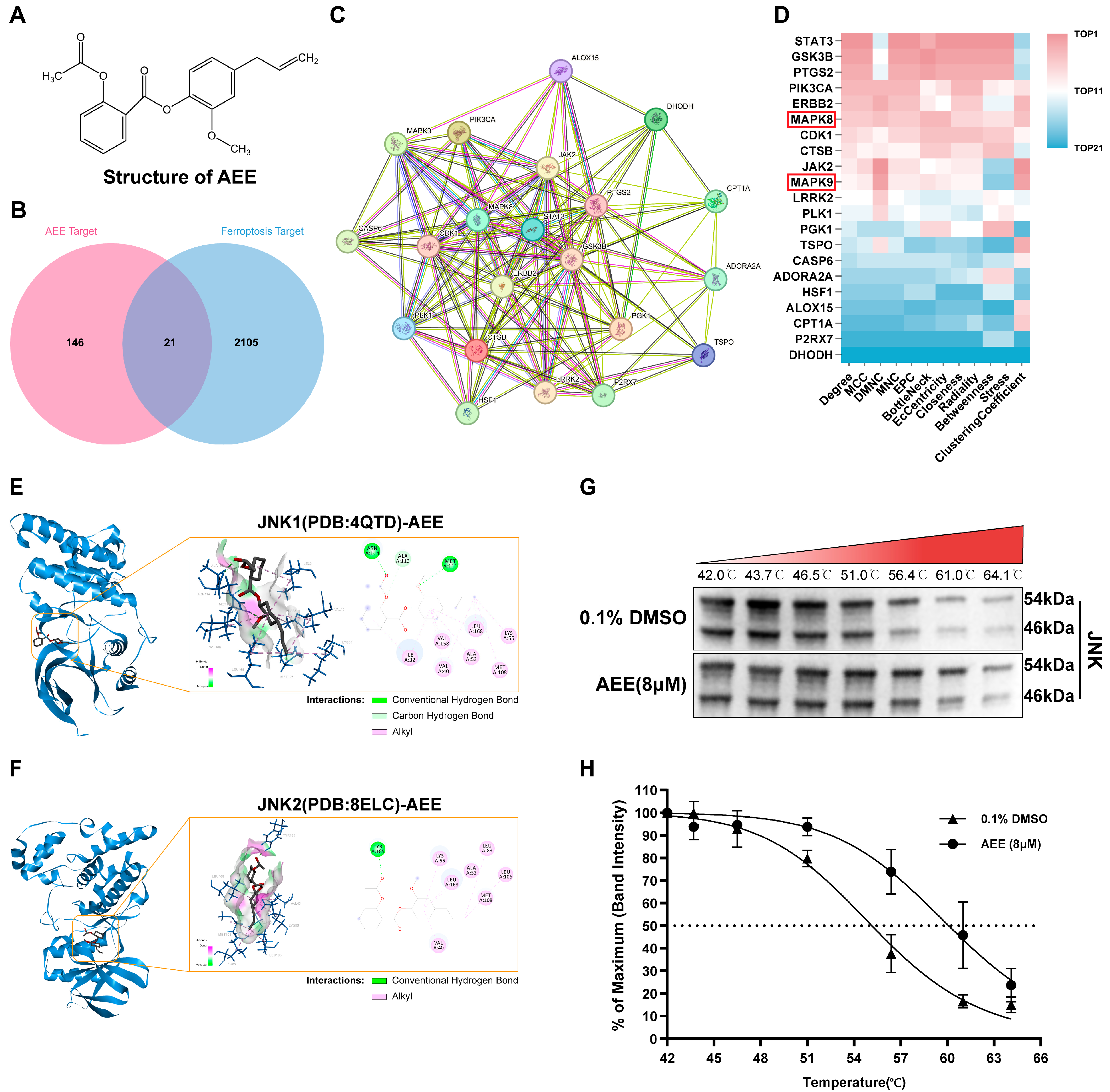
2.16. Network Pharmacology Analysis
2.17. Molecular Docking
2.18. Immunofluorescence Analysis
2.19. Cellular Thermal Shift Assay (CETSA)
2.20. Western Blot
2.21. Statistical Analysis
3. Results
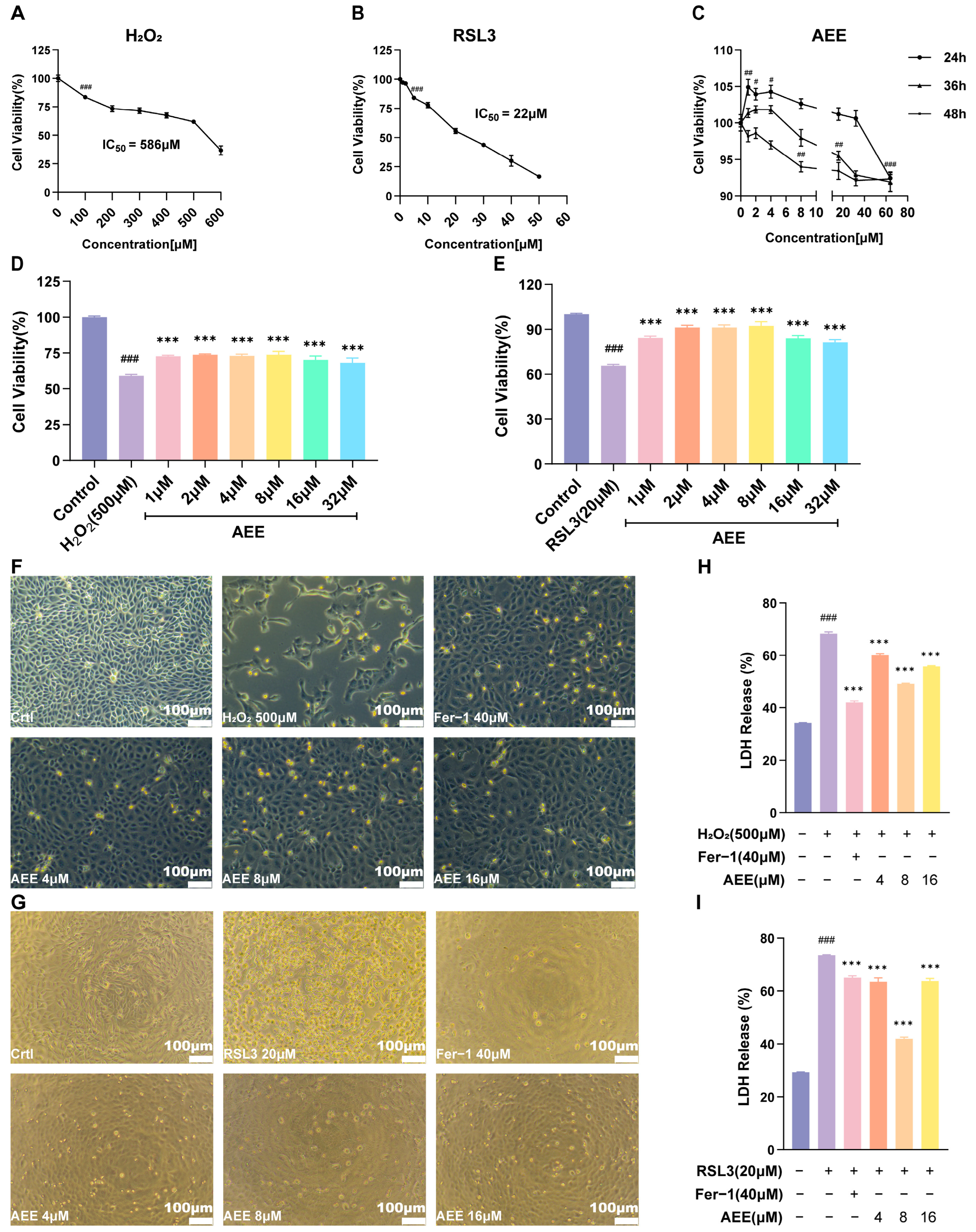
3.1. AEE Attenuates H2O2 or RSL3-Induced BAEC Damage
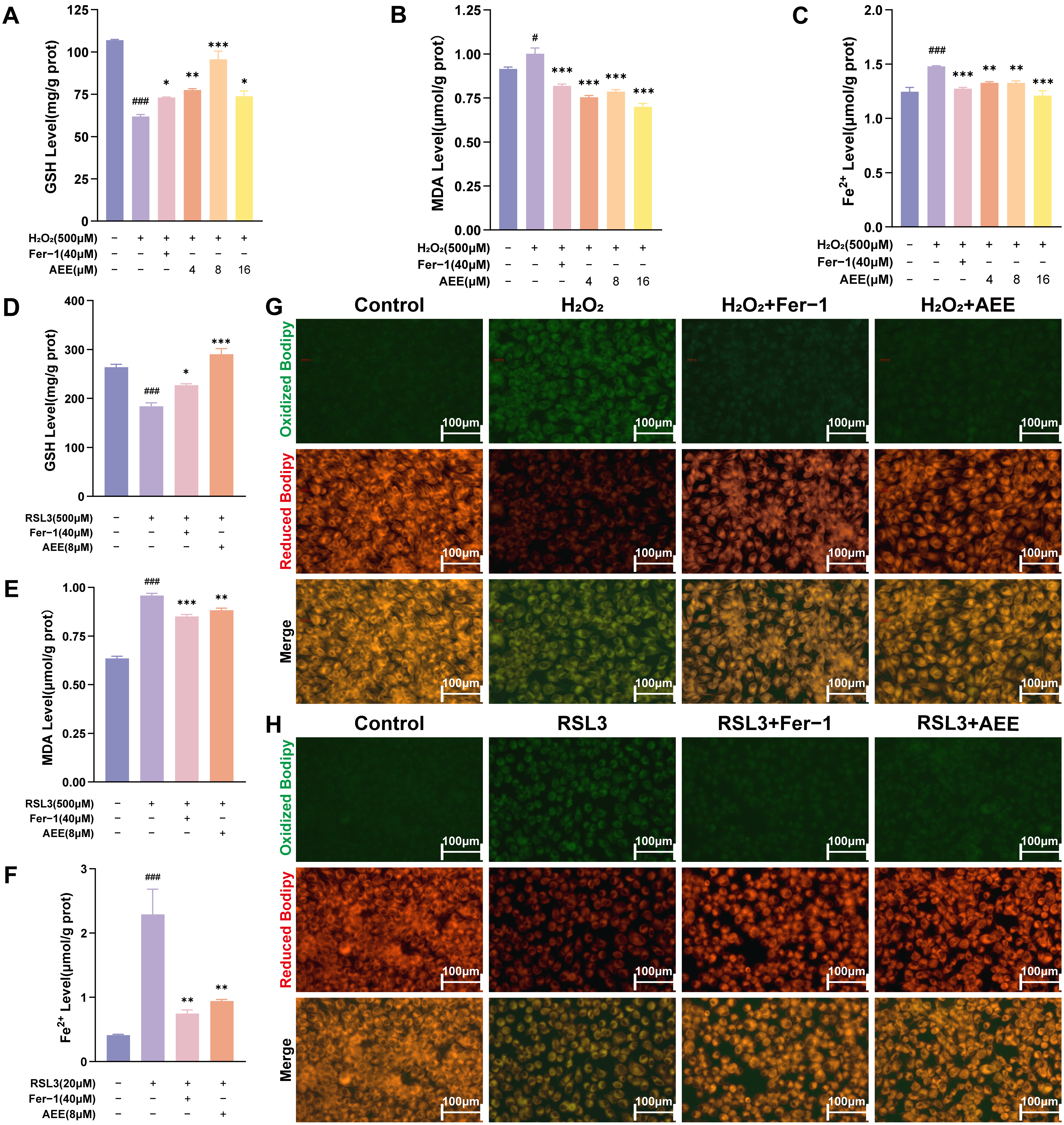
3.2. AEE Inhibited H2O2 or RSL3-Induced Ferroptosis in BAECs
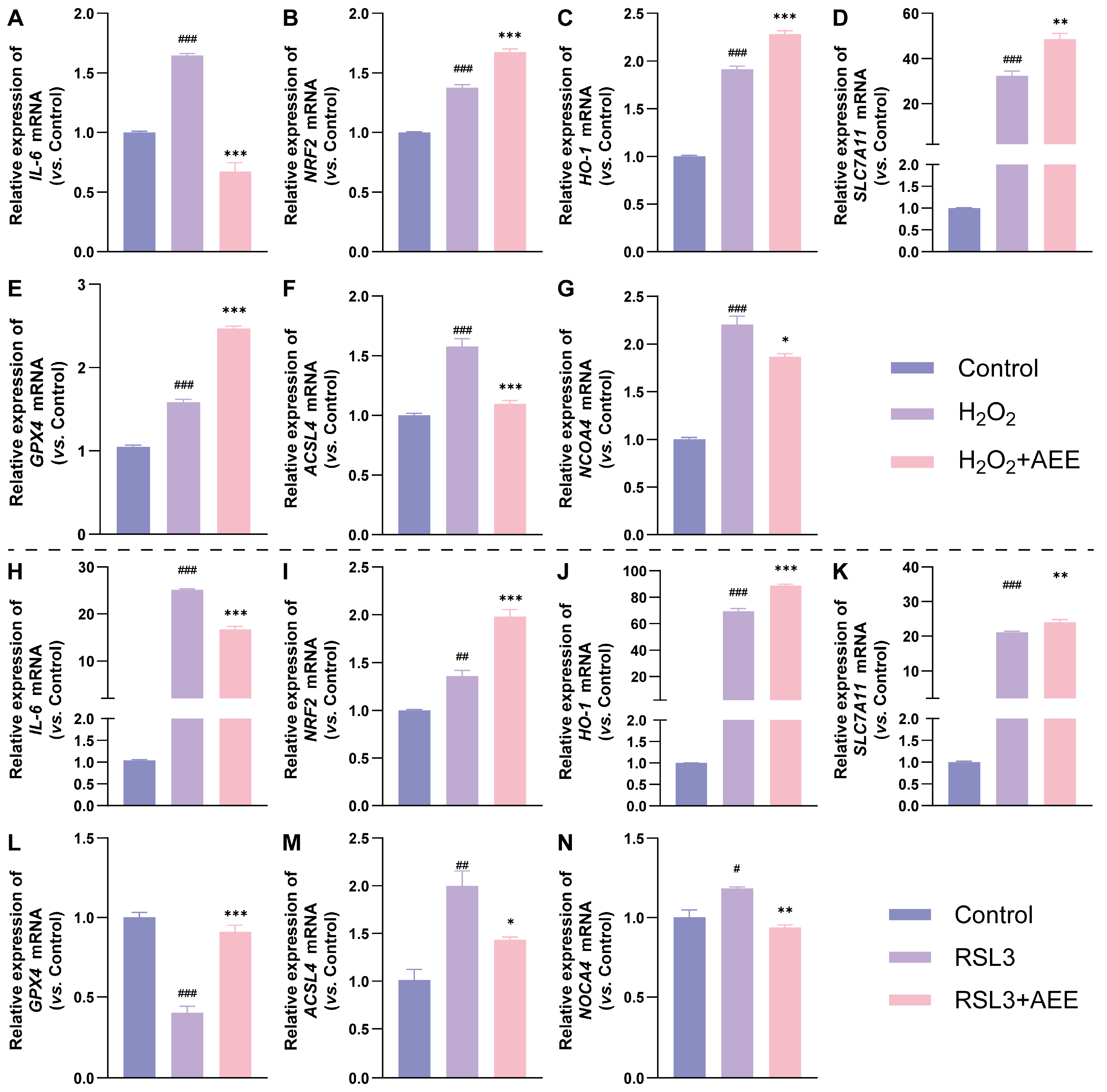
3.3. AEE Enhanced Antioxidant Gene Expression and Inhibited Ferroptosis-Related Gene Expression in BAECs
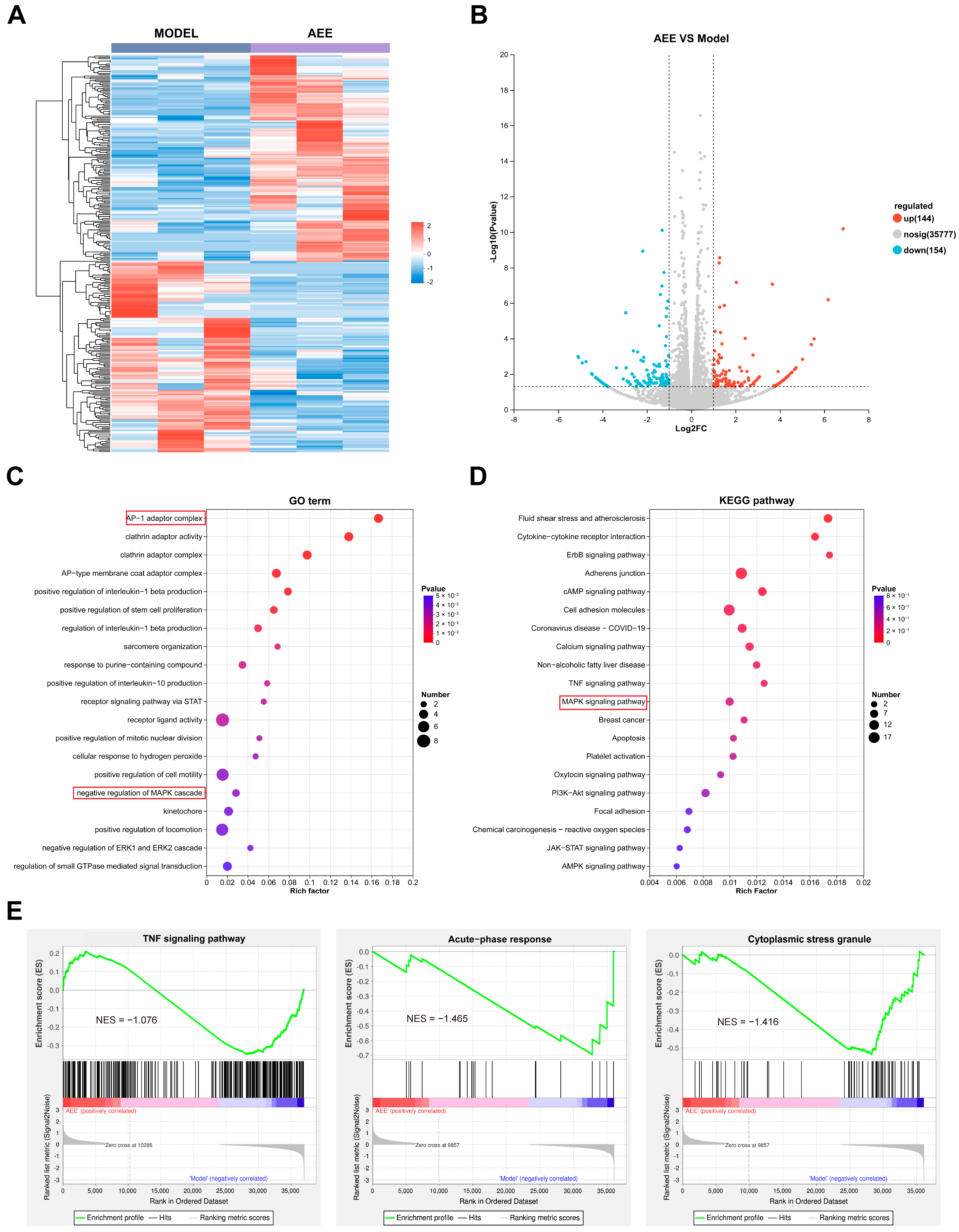
3.4. Transcriptome Analysis Results
3.5. JNK Was a Key Protein Target for AEE Attenuating Ferroptosis
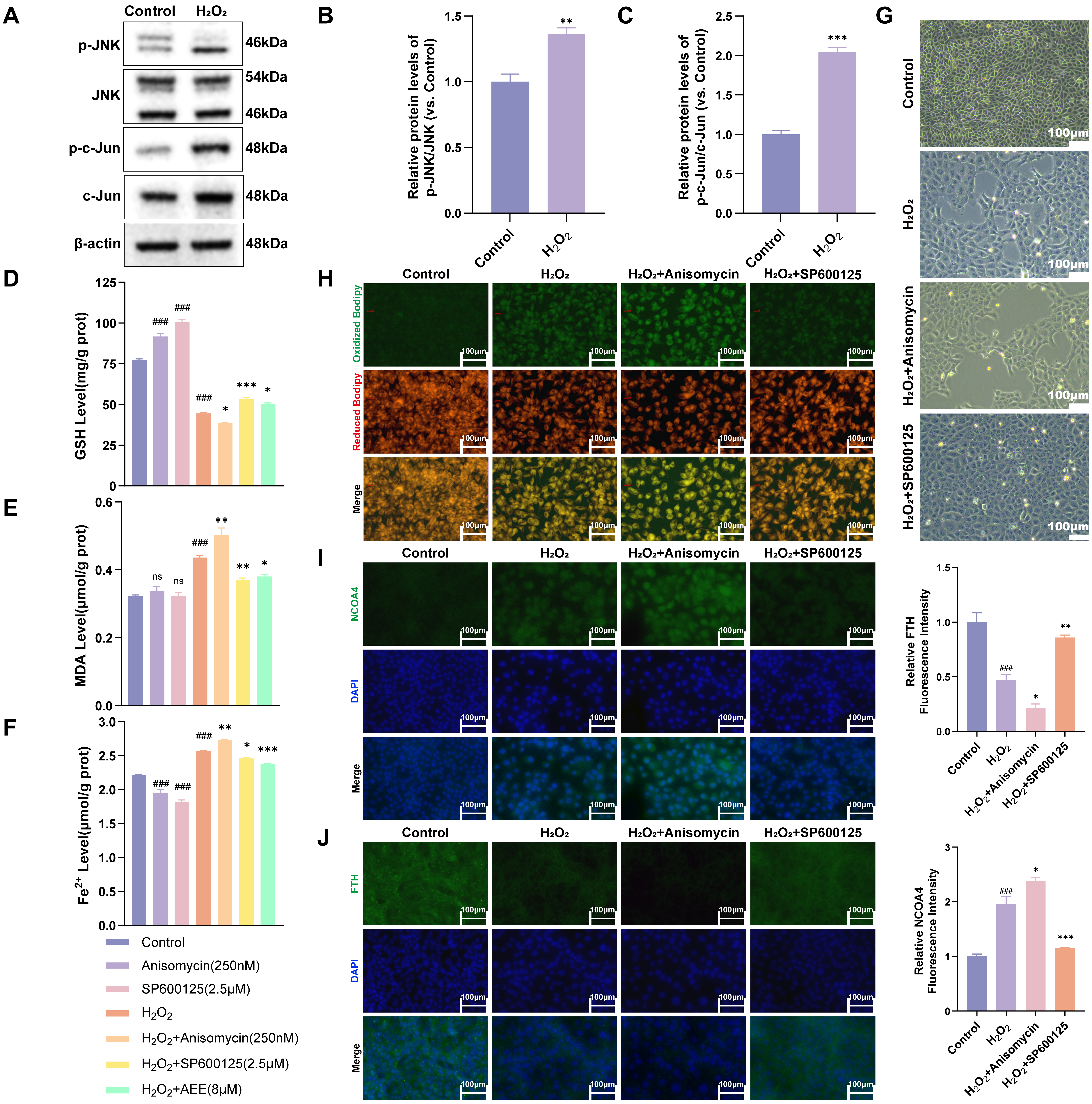
3.6. H2O2 Induced Ferroautophagy via Activated JNK/c-Jun Phosphorylation
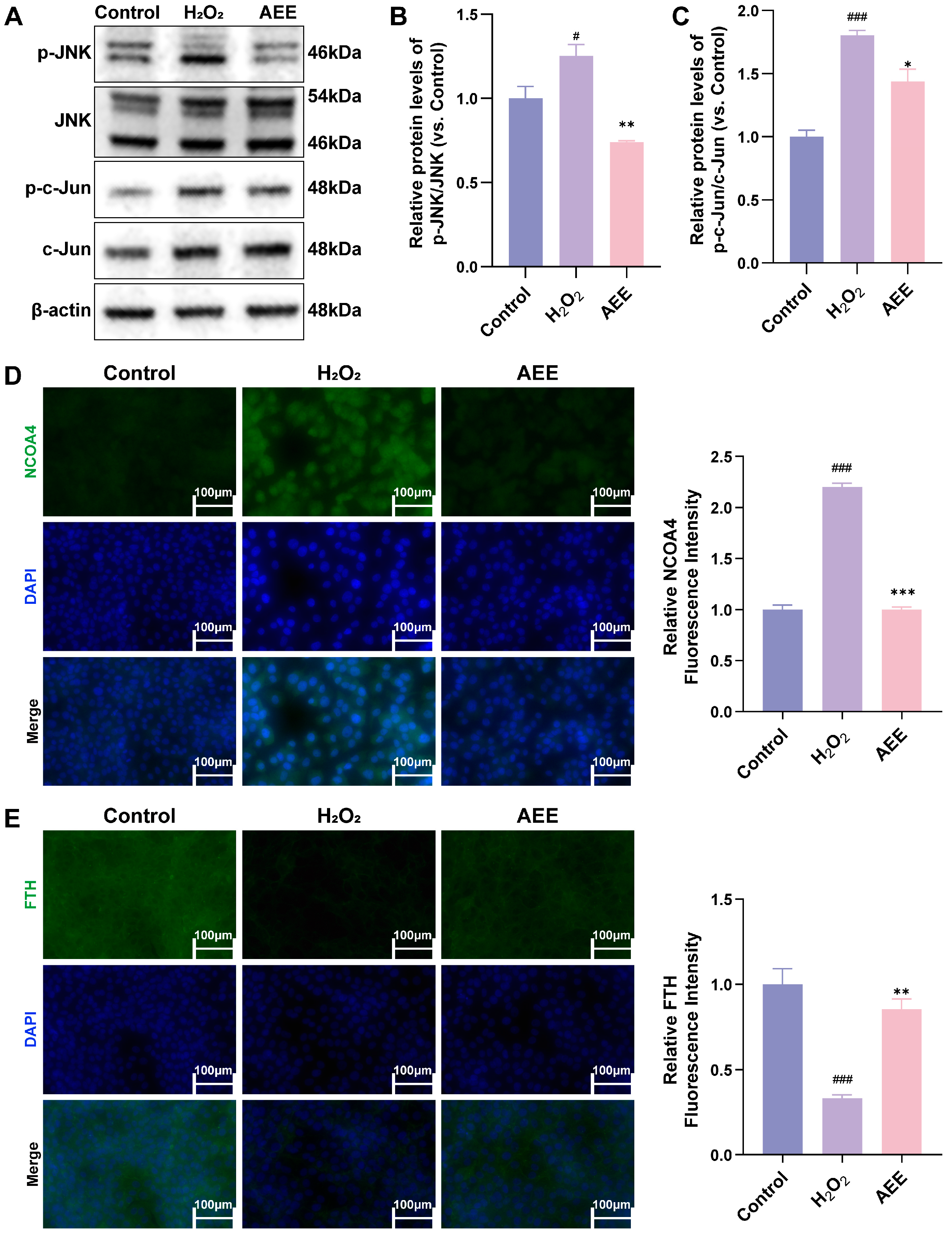
3.7. AEE Attenuates Ferroautophagy via Inhibiting JNK/c-Jun Phosphorylation in H2O2-Induced BAECs
3.8. AEE Alleviates Endothelial Damage in a κ-Car-Induced Rat Aortic Vascular Endothelial Damage Model
3.9. AEE Enhanced Antioxidant Capacity and Attenuated Ferroptosis in Rat Aorta
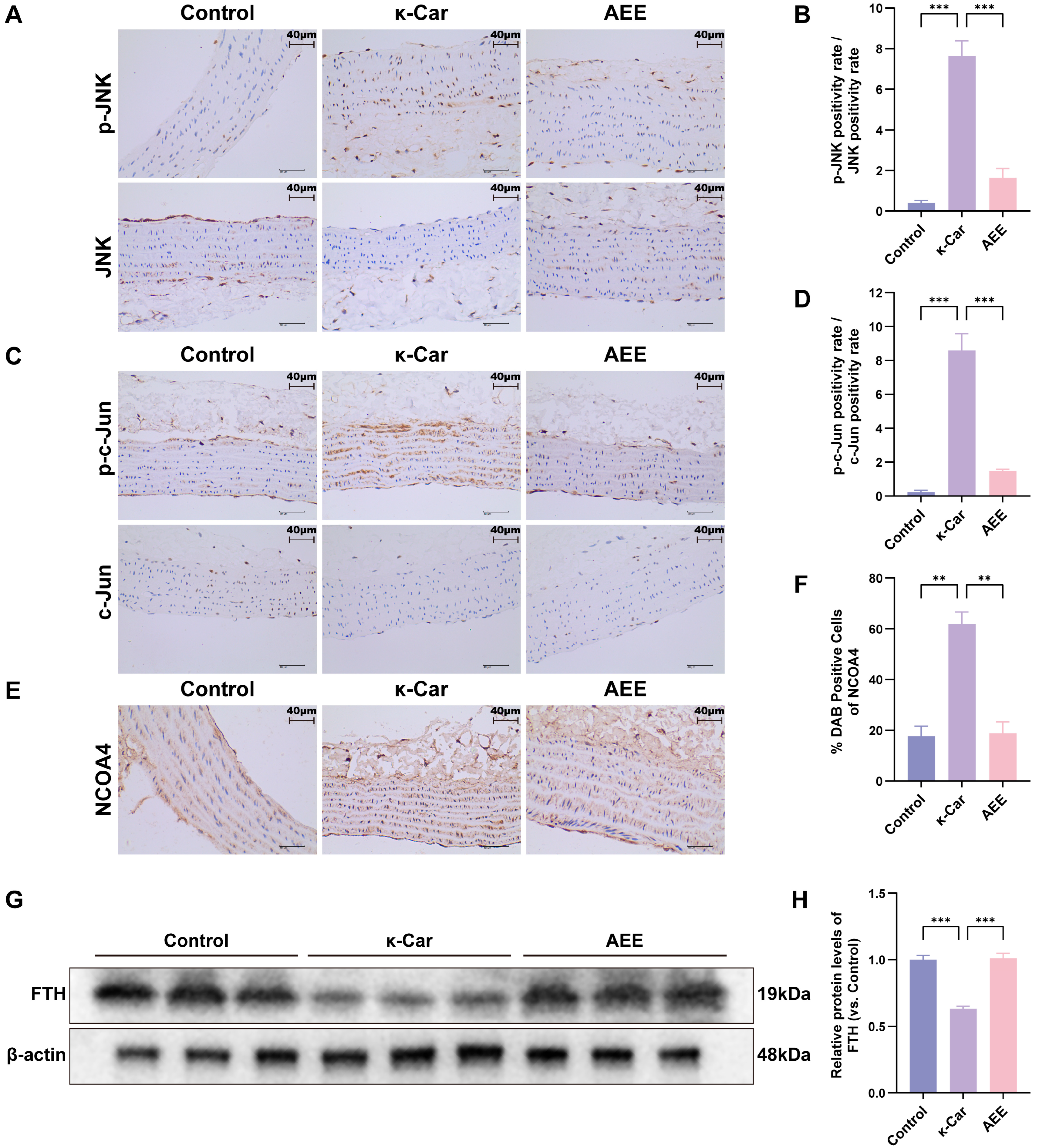
3.10. AEE Attenuates κ-Car-Induced Ferroautophagy of Rat Aorta via Inhibiting JNK/c-Jun Phosphorylation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VECs | Vascular Endothelial Cells |
| ROS | Reactive Oxygen Species |
| FTH | Ferritin Heavy Chain |
| NCOA4 | Nuclear Receptor Coactivator 4 |
| ·OH | Hydroxyl Radical |
| PUFAs | Polyunsaturated Fatty Acids |
| Fe2+ | Ferrous Ion |
| AEE | Aspirin Eugenol Ester |
| H2O2 | Hydrogen Peroxide |
| HUVECs | Human Umbilical Vein Endothelial Cells |
| GSH | Glutathione |
| SOD | Superoxide Dismutase |
| MDA | Malondialdehyde |
| BAECs | Bovine Aortic Endothelial Cells |
| Fer-1 | Ferrostatin-1 |
| κ-Car | Carrageenan |
| CMC-Na | Sodium Carboxymethyl Cellulose |
| SPF | Specific Pathogen-Free |
| HE | Hematoxylin–Eosin |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| VCAM1 | Vascular Cell Adhesion Molecule 1 |
| TNF-α | Tumor Necrosis Factor Alpha |
| IL-6 | Interleukin 6 |
| ET-1 | Endothelin 1 |
| FBS | Fetal Bovine Serum |
| LDH | Lactate Dehydrogenase |
| LPO | Lipid Peroxide |
| DEGs | Differentially Expressed Genes |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| PPI | Protein–Protein Interaction |
| PDB | Protein Data Bank |
| MAPK | Mitogen Activated Protein Kinase |
| JNK | c-Jun N-terminal Kinase |
| CETSA | Cellular Thermal Shift Assay |
| Tagg | Aggregation Temperature |
| NRF2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| HO-1 | Heme Oxygenase-1 |
| SLC7A11 | Solute Carrier Family 7 Member 11 |
| GPX4 | Glutathione Peroxidase 4 |
| ACSL4 | Long Chain Fatty Acid CoA Ligase 4 |
| PLOOHs | Phospholipid Hydroperoxides |
References
- Ziveri, J.; Le Guennec, L.; Souza, I.d.S.; Barnier, J.-P.; Walter, S.M.; Diallo, Y.; Smail, Y.; Le Seac’h, E.; Bouzinba-Segard, H.; Faure, C.; et al. Angiopoietin-like 4 protects against endothelial dysfunction during bacterial sepsis. Nat. Microbiol. 2024, 9, 2434–2447. [Google Scholar] [CrossRef] [PubMed]
- Valerie, E.R.; Nandakumar, P.; Lorraine, M.S. Role of endothelial cells in bovine mammary gland health and disease. Anim. Health Res. Rev. 2015, 16, 135–149. [Google Scholar] [CrossRef]
- Walker, C.C.F.; Sordillo, L.M.; Contreras, G.A. Anandamide Alters Barrier Integrity of Bovine Vascular Endothelial Cells during Endotoxin Challenge. Antioxidants 2022, 11, 1461. [Google Scholar] [CrossRef]
- Tamargo, I.A.; Baek, K.I.; Kim, Y.; Park, C.; Jo, H. Flow-induced reprogramming of endothelial cells in atherosclerosis. Nat. Rev. Cardiol. 2023, 20, 738–753. [Google Scholar] [CrossRef]
- Liberale, L.; Puspitasari, Y.M.; Ministrini, S.; Akhmedov, A.; Kraler, S.; Bonetti, N.R.; Beer, G.; Vukolic, A.; Bongiovanni, D.; Han, J.; et al. Corrigendum to: JCAD promotes arterial thrombosis through PI3K/Akt modulation: A translational study. Eur. Heart J. 2023, 44, 1843. [Google Scholar] [CrossRef]
- Yue, K.M.; Pu, X.D.; Loor, J.J.; Jiang, Q.M.; Dong, J.H.; Shen, T.Y.; Li, G.J.; Gao, W.W.; Lei, L.; Du, X.L.; et al. Impaired autophagy aggravates oxidative stress in mammary gland of dairy cows with clinical ketosis. J. Dairy Sci. 2022, 105, 6030–6040. [Google Scholar] [CrossRef]
- Song, Y.X.; Loor, J.J.; Li, C.Y.; Liang, Y.S.; Li, N.; Shu, X.; Yang, Y.C.; Feng, X.C.; Du, X.L.; Wang, Z.; et al. Enhanced mitochondrial dysfunction and oxidative stress in the mammary gland of cows with clinical ketosis. J. Dairy Sci. 2021, 104, 6909–6918. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.Y.; Zhang, Z.H.; Wang, J.Q.; Sun, Z.W.; Qaed, E.; Chi, X.M.; Wang, J.; Jamalat, Y.; Geng, Z.H.; Tang, Z.Y.; et al. Protective effects of phosphocreatine on human vascular endothelial cells against hydrogen peroxide-induced apoptosis and in the hyperlipidemic rat model. Chem. Biol. Interact. 2023, 383, 110683. [Google Scholar] [CrossRef]
- Yao, H.; Sun, J.Y.; Wei, J.; Zhang, X.; Chen, B.; Lin, Y.J. Kaempferol Protects Blood Vessels From Damage Induced by Oxidative Stress and Inflammation in Association With the Nrf2/HO-1 Signaling Pathway. Front. Pharmacol. 2020, 11, 1118. [Google Scholar] [CrossRef]
- Peng, C.W.; Li, H.L.; Mao, Q.L.; Tang, K.Y.; Sun, M.; Ai, Q.D.; Yang, Y.T.; Liu, F. Quercetin inhibits hydrogen peroxide-induced cleavage of heat shock protein 90 to prevent glutathione peroxidase 4 degradation via chaperone-mediated autophagy. Phytomedicine 2025, 136, 156286. [Google Scholar] [CrossRef]
- Wang, B.Q.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.M.; Peng, Z.Y. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef]
- Lv, Y.H.; Wu, M.Y.; Wang, Z.; Wang, J.Q. Ferroptosis: From regulation of lipid peroxidation to the treatment of diseases. Cell Biol. Toxicol. 2023, 39, 827–851. [Google Scholar] [CrossRef]
- Mancias, J.D.; Wang, X.X.; Gygi, S.P.; Harper, J.W.; Kimmelman, A.C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 2014, 509, 105–109. [Google Scholar] [CrossRef]
- Bogdan, A.R.; Miyazawa, M.; Hashimoto, K.; Tsuji, Y. Regulators of Iron Homeostasis: New Players in Metabolism, Cell Death, and Disease. Trends Biochem. Sci. 2016, 41, 274–286. [Google Scholar] [CrossRef]
- Liu, Z.B.; Zhou, S.F.; Wang, F.; Xie, H.B.; Zhang, J.X.; Wu, C.H.; Xu, D.X.; Zhu, Q.X. C5b-9 promotes ferritinophagy leading to ferroptosis in renal tubular epithelial cells of trichloroethylene-sensitized mice. Sci. Total Environ. 2024, 923, 171378. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, Y.; Shi, W.; Lu, L.; Wei, J.L.; Wang, J.H.; Zhang, H.; Pu, Y.P.; Yin, L.H. Angiotensin II Induces Vascular Endothelial Dysfunction by Promoting Lipid Peroxidation-Mediated Ferroptosis via CD36. Biomolecules 2024, 14, 1456. [Google Scholar] [CrossRef]
- Zhao, L.L.; Yang, N.; Song, Y.Q.; Si, H.L.; Qin, Q.; Guo, Z.G. Effect of iron overload on endothelial cell calcification and its mechanism. Ann. Transl. Med. 2021, 9, 1658. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.D.; Liu, J.; Piao, H.L.; Zhu, Z.C.; Wei, R.; Liu, K.X. ROS-triggered endothelial cell death mechanisms: Focus on pyroptosis, parthanatos, and ferroptosis. Front. Immunol. 2022, 13, 1039241. [Google Scholar] [CrossRef]
- He, X.; Zheng, X.H.; Xie, W.D. Isopropyl 3-(3,4-Dihydroxyphenyl)-2-hydroxypropanoate Alleviates Palmitic Acid-Induced Vascular Aging in HUVEC Cells through ROS/Ferroptosis Pathway. Int. J. Mol. Sci. 2024, 25, 9278. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.L.; Zhen, W.R.; Bai, D.Y.; Hu, X.D.; Zhang, H.J.; Zhang, R.L.; Ito, K.; Zhang, Y.; Zhang, B.K.; Ma, Y.B. Effects of Aspirin Eugenol Ester on Liver Oxidative Damage and Energy Metabolism in Immune-Stressed Broilers. Antioxidants 2024, 13, 341. [Google Scholar] [CrossRef]
- Tao, Q.; Fan, L.P.; Feng, J.; Zhang, Z.J.; Liu, X.W.; Qin, Z.; Li, J.Y.; Yang, Y.J. Platelet Proteomics and Tissue Metabolomics Investigation for the Mechanism of Aspirin Eugenol Ester on Preventive Thrombosis Mechanism in a Rat Thrombosis Model. Int. J. Mol. Sci. 2024, 25, 10747. [Google Scholar] [CrossRef]
- Huang, M.Z.; Yang, Y.J.; Liu, X.W.; Qin, Z.; Li, J.Y. Aspirin eugenol ester attenuates oxidative injury of vascular endothelial cells by regulating NOS and Nrf2 signalling pathways. Brit. J. Pharmacol. 2019, 176, 906–918. [Google Scholar] [CrossRef]
- Zhang, Z.D.; Yang, Y.J.; Liu, X.W.; Qin, Z.; Li, S.H.; Li, J.Y. Aspirin eugenol ester ameliorates paraquat-induced oxidative damage through ROS/p38-MAPK-mediated mitochondrial apoptosis pathway. Toxicology 2021, 453, 152721. [Google Scholar] [CrossRef]
- Sun, L.; Li, X.F.; Zhang, J.; Pei, J.C.; Zhang, J.H.; Wang, Y.H.; Lin, F.; Zhao, G.A. SAL protects endothelial cells from H2O2-induced endothelial dysfunction: Regulation of inflammation and autophagy by EZH2. Int. Immunopharmacol. 2024, 142, 113060. [Google Scholar] [CrossRef] [PubMed]
- Dorian, M.C.; Chuying, H.; Karoline, S.; Radosveta, G.; Michael, R.; Qing, C.; Matthew, D.H.; Elias, S.J.A. The ferroptosis inducing compounds RSL3 and ML162 are not direct inhibitors of GPX4 but of TXNRD1. Redox Biol. 2023, 62, 102703. [Google Scholar] [CrossRef]
- Antoine, D.; Olivier, M.; Vincent, Z. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Joanna, S.A.; Ada, H. Searching Online Mendelian Inheritance in Man (OMIM): A Knowledgebase of Human Genes and Genetic Phenotypes. Curr. Protoc. Bioinform. 2017, 58, 1–2. [Google Scholar] [CrossRef]
- Safran, M.; Dalah, I.; Alexander, J.; Rosen, N.; Stein, T.I.; Shmoish, M.; Nativ, N.; Bahir, I.; Doniger, T.; Krug, H.; et al. GeneCards Version 3: The human gene integrator. Database 2010, 5, baq020. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Yuan, X.Q.; Du, Q.S.; Zhang, Z.Y.; Shi, X.L.; Bao, J.K.; Ning, Y.P.; Peng, L. FerrDb V2: Update of the manually curated database of ferroptosis regulators and ferroptosis-disease associations. Nucleic Acids Res. 2023, 51, D571–D582. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Lill, M.A.; Danielson, M.L. Computer-aided drug design platform using PyMOL. J. Comput. Aid. Mol. Des. 2011, 25, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. Software News and Update AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Hsin, K.Y.; Ghosh, S.; Kitano, H. Combining Machine Learning Systems and Multiple Docking Simulation Packages to Improve Docking Prediction Reliability for Network Pharmacology. PLoS ONE 2013, 8, 83922. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.T.; Shen, J.L.; Liu, X.L.; Cui, J.H.; Liu, J.Y.; Peng, D.Q.; Jin, Y.C. β-Sitosterol Suppresses Lipopolysaccharide-Induced Inflammation and Lipogenesis Disorder in Bovine Mammary Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 14644. [Google Scholar] [CrossRef]
- Djulbegovic, M.B.; Uversky, V.N. Ferroptosis—An iron- and disorder-dependent programmed cell death. Int. J. Biol. Macromol. 2019, 135, 1052–1069. [Google Scholar] [CrossRef]
- Huang, M.Z.; Zhang, Z.D.; Yang, Y.J.; Liu, X.W.; Qin, Z.; Li, J.Y. Aspirin Eugenol Ester Protects Vascular Endothelium From Oxidative Injury by the Apoptosis Signal Regulating Kinase-1 Pathway. Front. Pharmacol. 2020, 11, 588755. [Google Scholar] [CrossRef]
- Fang, Z.Y.; Liu, G.W.; Zhu, M.Y.; Wang, S.; Jiang, Q.M.; Loor, J.J.; Yu, H.; Hao, X.; Chen, M.; Gao, W.W.; et al. Low abundance of mitophagy markers is associated with reactive oxygen species overproduction in cows with fatty liver and causes reactive oxygen species overproduction and lipid accumulation in calf hepatocytes. J. Dairy Sci. 2022, 105, 7829–7841. [Google Scholar] [CrossRef]
- Querio, G.; Antoniotti, S.; Foglietta, F.; Bertea, C.M.; Canaparo, R.; Gallo, M.P.; Levi, R. Chamazulene Attenuates ROS Levels in Bovine Aortic Endothelial Cells Exposed to High Glucose Concentrations and Hydrogen Peroxide. Front. Physiol. 2018, 9, 246. [Google Scholar] [CrossRef]
- Zhang, X.L.; Liu, X.M.; Wan, F.C.; You, W.; Tan, X.W.; Sheng, Q.K.; Li, C.H.; Hu, Z.R.; Liu, G.F.; Zhao, H.B. Protective effect of resveratrol against hydrogen peroxide-induced oxidative stress in bovine skeletal muscle cells. Meat Sci. 2022, 185, 108724. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Y.; Zhao, D.; Yang, S.; Zhang, S.; Wei, Z.; Wang, Y.; Qian, K.; Zhao, B.C.; Zhu, Y.; et al. LongShengZhi Capsule reduces carrageenan-induced thrombosis by for reducing activation of platelets and endothelial cells. Pharmacol. Res. 2019, 144, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Yan, X.J.; Mai, T.Y.; Wang, F.; Xu, W.F. λ-Carrageenan oligosaccharides elicit reactive oxygen species production resulting in mitochondrial-dependent apoptosis in human umbilical vein endothelial cells. Int. J. Mol. Med. 2009, 24, 801–806. [Google Scholar] [CrossRef]
- Chen, X.Y.; Jiang, T.Z.; Li, Y.T.; Zhang, Y.F.; Chen, J.Q.; Zhao, X.; Yang, H. Carrageenan-ferrocene-eicosapentaenoic acid composite hydrogel induce ferroptosis and apoptosis for anti-tumor recurrence and metastasis. Int. J. Biol. Macromol. 2024, 276, 133942. [Google Scholar] [CrossRef] [PubMed]
- Cristina, B.; Gabriela Adriana, F.; Luminiţa, D.; Bianca, M.; Ioana, B.; Diana, O.; Mara, F.; Pompei, B.; Monica, P.; Alina, T.; et al. Viburnum opulus fruit extract-capped gold nanoparticles attenuated oxidative stress and acute inflammation in carrageenan-induced paw edema model. Green Chem. Lett. Rev. 2022, 15, 320–336. [Google Scholar] [CrossRef]
- Lovatt, M.; Adnan, K.; Kocaba, V.; Dirisamer, M.; Peh, G.S.L.; Mehta, J.S. Peroxiredoxin-1 regulates lipid peroxidation in corneal endothelial cells. Redox Biol. 2020, 30, 101417. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, J.; Wang, B.; Xu, G.; Yang, X.; Zou, Z.; Yu, C. Ferritinophagy is involved in the zinc oxide nanoparticles-induced ferroptosis of vascular endothelial cells. Autophagy 2021, 17, 4266–4285. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, Y.; Li, M.Q.; Wang, X.B.; Peng, X.; Sun, H.W.; Zhang, L.Z. FeC2O4•2H2O enables sustainable conversion of hydrogen peroxide to hydroxyl radical for promoted mineralization and detoxification of sulfadimidine. J. Hazard. Mater. 2022, 436, 129049. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.J.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Li, C.; Sun, G.C.; Chen, B.L.; Xu, L.; Ye, Y.F.; He, J.Y.; Bao, Z.Y.; Zhao, P.Z.; Miao, Z.; Zhao, L.; et al. Nuclear receptor coactivator 4-mediated ferritinophagy contributes to cerebral ischemia-induced ferroptosis in ischemic stroke. Pharmacol. Res. 2021, 174, 105933. [Google Scholar] [CrossRef]
- Liao, P.; Wang, W.M.; Wang, W.C.; Kryczek, I.; Li, X.; Bian, Y.J.; Sell, A.; Wei, S.; Grove, S.; Johnson, J.K.; et al. CD8+ T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell 2022, 40, 365–378. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, J.; Liu, J.H.; Zhang, L.; Xu, Z.G.; Kang, Y.J.; Xue, P. Manganese-Based Redox Homeostasis Disruptor for Inducing Intense Ferroptosis/Apoptosis Through xCT Inhibition And Oxidative Stress Injury. Adv. Healthc. Mater. 2023, 12, 2301453. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Palte, M.J.; Deik, A.A.; Li, H.X.; Eaton, J.K.; Wang, W.Y.; Tseng, Y.Y.; Deasy, R.; Kost-Alimova, M.; Dancík, V.; et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat. Commun. 2019, 10, 1617. [Google Scholar] [CrossRef]
- Liu, P.F.; Feng, Y.T.; Li, H.W.; Chen, X.; Wang, G.S.; Xu, S.Y.; Li, Y.L.; Zhao, L. Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cell. Mol. Biol. Lett. 2020, 25, 10. [Google Scholar] [CrossRef]
- Chang, Y.Q.; Li, S.L.; Guo, W.N.; Yang, Y.Q.; Zhang, W.G.; Zhang, Q.; He, Y.M.; Yi, X.L.; Cui, T.T.; An, Y.W.; et al. Simvastatin Protects Human Melanocytes from H2O2-Induced Oxidative Stress by Activating Nrf2. J. Investig. Dermatol. 2017, 137, 1286–1296. [Google Scholar] [CrossRef]
- Yongjun, Z.; Hua, W.; Mengmei, W.; Yang, Y.; Mengyue, G.; Hui, Z.; Shuying, D. Dexmedetomidine alleviates ferroptosis following hepatic ischemia-reperfusion injury by upregulating Nrf2/GPx4-dependent antioxidant responses. Biomed. Pharmacother. 2023, 169, 115915. [Google Scholar] [CrossRef]
- Pan, M.L.; Wang, Z.; Wang, Y.Y.; Jiang, X.Q.; Fan, Y.L.; Gong, F.H.; Sun, Y.P.; Wang, D.Z. Celastrol alleviated acute kidney injury by inhibition of ferroptosis through Nrf2/GPX4 pathway. Biomed. Pharmacother. 2023, 166, 115333. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhang, P.; Liu, J.L.; Wang, R.L.; Kaufman, R.J.; Yaden, B.C.; Karin, M. ATF4 suppresses hepatocarcinogenesis by inducing SLC7A11 (xCT) to block stress-related ferroptosis. J. Hepatol. 2023, 79, 362–377. [Google Scholar] [CrossRef]
- Li, J.Y.; Wang, X.F.; Zhou, R.R.; Cheng, F.; Tang, X.Y.; Lao, J.; Xu, L.B.; He, W.; Wan, D.; Zeng, H.L.; et al. Polygonatum cyrtonema Hua Polysaccharides Protect BV2 Microglia Relief Oxidative Stress and Ferroptosis by Regulating NRF2/HO-1. Molecules 2022, 27, 7088. [Google Scholar] [CrossRef]
- Yan, R.H.; Lin, B.Y.; Jin, W.W.; Tang, L.; Hu, S.M.; Cai, R. NRF2, a Superstar of Ferroptosis. Antioxidants 2023, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.M.; Chen, H.; Zhang, J.M.; Tian, R.; Xie, C.X.; Sun, Q.B.; Wang, H.; Shi, T.J.; Guo, D.Q.; Wang, Y.; et al. Qishen granule alleviates doxorubicin-induced cardiotoxicity by suppressing ferroptosis via nuclear erythroid factor 2-related factor 2 (Nrf2) pathway. J. Ethnopharmacol. 2024, 335, 118604. [Google Scholar] [CrossRef]
- Yu, X.; Wang, S.; Wang, X.; Li, Y.; Dai, Z. Melatonin improves stroke by inhibiting autophagy-dependent ferroptosis mediated by NCOA4 binding to FTH1. Exp. Neurol. 2024, 379, 114868. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Y.; Jiang, P.Y.; Zhao, L.Y.; Fei, X.H.; Tang, Y.X.; Luo, Y.; Gong, H.; Wang, X.Q.; Li, X.; Li, S.; et al. Ligustilide inhibits Purkinje cell ferritinophagy via the ULK1/NCOA4 pathway to attenuate valproic acid-induced autistic features. Phytomedicine 2024, 126, 155443. [Google Scholar] [CrossRef]
- Shin, D.; Kim, E.H.; Lee, J.; Roh, J.L. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free. Radic. Biol. Med. 2018, 129, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.Y.; Zuo, Z.F.; Di, X.W.; Huang, Y.; Gong, G.; Xu, B.; Wang, L.L.; Zhang, X.Y.; Liang, Z.; Hou, Y.; et al. Salidroside attenuates HALI via IL-17A-mediated ferroptosis of alveolar epithelial cells by regulating Act1-TRAF6-p38 MAPK pathway. Cell Commun. Signal. 2022, 20, 183. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Cao, P.P.; Yang, S.S.; Lin, F.; Wang, J. Prolonged exposure to environmental levels of microcystin-LR triggers ferroptosis in brain via the activation of Erk/MAPK signaling pathway. Ecotoxicol. Environ. Saf. 2023, 267, 115651. [Google Scholar] [CrossRef]
- Zhao, L.; Miao, H.; Quan, M.Q.; Wang, S.H.; Zhang, Y.; Zhou, H.K.; Zhang, X.L.; Lin, Z.H.; Piao, J.J. β-Lapachone induces ferroptosis of colorectal cancer cells via NCOA4-mediated ferritinophagy by activating JNK pathway. Chem. Biol. Interact. 2024, 389, 110866. [Google Scholar] [CrossRef]
- Feng, L.M.; Wang, C.; Zhang, C.; Zhang, W.L.; Zhu, W.M.; He, Y.; Xia, Z.H.; Song, W.T. p38 MAPK inhibitor SB202190 suppresses ferroptosis in the glutamate-induced retinal excitotoxicity glaucoma model. Neural Regen. Res. 2024, 19, 2299–2309. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Hou, L.C.; Guo, Z.; Wang, G.C.; Guo, J.C.; Xu, J.T.; Zhang, X.; Guo, F.J. JNK-JUN-NCOA4 axis contributes to chondrocyte ferroptosis and aggravates osteoarthritis via ferritinophagy. Free Radic. Biol. Med. 2023, 200, 87–101. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ling, Y.H.; Zhang, Z.Y.; Mertens, R.T.; Cao, Q.; Xu, X.T.; Guo, K.; Shi, Q.; Zhang, X.L.; Huo, L.X.; et al. Butyrate reverses ferroptosis resistance in colorectal cancer by inducing c-Fos-dependent xCT suppression. Redox Biol. 2023, 65, 102822. [Google Scholar] [CrossRef]
- Shu, G.J.; Chen, K.; Li, J.Y.; Liu, B.; Chen, X.; Wang, J.; Hu, X.S.; Lu, W.X.; Huang, H.R.; Zhang, S.S. Galangin alleviated Doxorubicin-induced cardiotoxicity by inhibiting ferroptosis through GSTP1/JNK pathway. Phytomedicine 2024, 134, 155989. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Ou, Y.; Zhang, C.; Chen, H.; Yue, H.; Yang, Z.J.; Zhong, X.F.; Hu, W.H.; Sun, P. Seratrodast, a thromboxane A2 receptor antagonist, inhibits neuronal ferroptosis by promoting GPX4 expression and suppressing JNK phosphorylation. Brain Res. 2022, 1795, 148073. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.Y.; Hu, X.Y.; Sun, S.W.; Su, B.; Cai, J.Y.; Jiang, J.H. Oridonin promotes RSL3-induced ferroptosis in breast cancer cells by regulating the oxidative stress signaling pathway JNK/Nrf2/HO-1. Eur. J. Pharmacol. 2024, 974, 176620. [Google Scholar] [CrossRef]
- Fuhrmann, D.C.; Mondorf, A.; Beifuss, J.; Jung, M.; Brüne, B. Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol. 2020, 36, 101670. [Google Scholar] [CrossRef]
- Shen, D.S.; Yang, Y.J.; Kong, X.J.; Ma, N.; Liu, X.W.; Li, S.H.; Jiao, Z.H.; Qin, Z.; Huang, M.Z.; Li, J.Y. Aspirin eugenol ester inhibits agonist-induced platelet aggregation by regulating PI3K/Akt, MAPK and Sirt 1/CD40L pathways. Eur. J. Pharmacol. 2019, 852, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Kon, N.; Li, T.Y.; Wang, S.J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef]
- Lv, C.Y.; Zhou, L.Y.; Meng, Y.K.; Yuan, H.T.; Geng, J. PKD knockdown mitigates Ang II-induced cardiac hypertrophy and ferroptosis via the JNK/P53 signaling pathway. Cell. Signal. 2024, 113, 110974. [Google Scholar] [CrossRef]

| Gene Name | NCBI ID | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|---|
| GPX4 (Bovine) | NM_174770.4 | TCCTCATTGACAAGAACGGCTGTG | TAGCACGGCAGGTCCTTCTCTATC |
| NRF2 (Bovine) | NM_001011678.2 | TCAGCCAGCACAACACATACCATC | ACGGGAATGTCTCTGCCAAAAGC |
| HO-1 (Bovine) | NM_001014912.1 | CCGCTACCTGGGAGACCTGTC | ACTTGGTGGCACTGGCGATATTG |
| IL-6 (Bovine) | NM_173923.2 | TGAGTGTGAAAGCAGCAAGGAGAC | CAAGCAAATCGCCTGATTGAACCC |
| ACSL4 (Bovine) | XM_024988727.2 | ATAGACATCCCTGGAGCAGACACC | TTATTCACTCGGCGGTTCACTTCG |
| SLC7A11 (Bovine) | XM_024977578.2 | TTCAAGGTGCCGCTGTTCATCC | TAGGCAGGAATCCCAGTCAGAGTG |
| NCOA4 (Bovine) | NM_001075868.1 | GGCTGCTGGAGTTGCTGATTC | TTCCTTGTTGGGACATCCTTCTTTG |
| GAPDH (Bovine) | NM_001034034.2 | CGGCACAGTCAAGGCAGAGAAC | CCACATACTCAGCACCAGCATCAC |
| GPX4 (Rat) | NM_174770.4 | CCAGCAACAGCCACGAGTTCC | CACACGCAACCCCTGTACTTATCC |
| NRF2 (Rat) | NM_001399173.1 | TGCTCAACCGCTTGCTGTATGC | TCATCCGCCACTCATTCCTCTCC |
| HO-1 (Rat) | NM_012580.2 | CCGCCTTCCTGCTCAACATTG | TCTGTGAGGGACTCTGGTCTTTG |
| IL-6 (Rat) | NM_012589.2 | CTTCCAGCCAGTTGCCTTCTTG | TGGTCTGTTGTGGGTGGTATCC |
| SLC7A11 (Rat) | NM_001107673.3 | CTTTCAAGGTGCCTCTGTTCATCC | CAGTCAAGGTGATAAGGAAGCCAAC |
| ACSL4 (Rat) | NM_001431649.1 | TCACCATTGTATTGCTGCCTGTCC | CGGGTTTGTCTGAAGTGGGCTTAG |
| NCOA4 (Rat) | NM_001034007.1 | TGGCTCCTTAACAGTCATCAACAAG | AGAACTGGTGCTACAATGGCTATTAC |
| FTH (Rat) | NM_012848.2 | TGCCATCAACCGCCAGATCAAC | AAGTTCTTCAGGGCCACATCATCC |
| β-actin (Rat) | NM_031144.3 | GCTGTGCTATGTTGCCCTAGACTTC | GGAACCGCTCATTGCCGATAGTG |
| Compound | AEE | SP600125 | |||
|---|---|---|---|---|---|
| Protein | PDB | Binding Energy (kcal/mol) | pKi | Binding Energy (kcal/mol) | pKi |
| JNK1 (MAPK8) | 4QTD | −7.62 ± 0.13 | 5.59 ± 0.10 | −8.98 ± 0.13 | 6.60 ± 0.07 |
| 4L7F | −7.58 ± 0.16 | 5.56 ± 0.12 | −8.54 ± 0.21 | 6.26 ± 0.15 | |
| 3ELJ | −7.34 ± 0.23 | 5.38 ± 0.17 | −8.98 ± 0.16 | 6.58 ± 0.12 | |
| 4AWI | −7.02 ± 0.13 | 5.23 ± 0.17 | −7.92 ± 0.16 | 5.81 ± 0.12 | |
| JNK2 (MAPK9) | 8ELC | −7.80 ± 0.12 | 5.72 ± 0.09 | −8.34 ± 0.75 | 6.11 ± 0.55 |
| 7N8T | −7.40 ± 0.16 | 5.42 ± 0.12 | −9.10 ± 0.27 | 6.67 ± 0.20 | |
| 3NPC | −7.28 ± 0.08 | 5.34 ± 0.06 | −8.26 ± 0.23 | 6.05 ± 0.17 | |
| 3E7O | −7.26 ± 0.11 | 5.32 ± 0.08 | −8.84 ± 0.15 | 6.48 ± 0.11 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Tao, Q.; Zhang, Z.-J.; Yu, Q.-F.; Yang, Y.-J.; Li, J.-Y. Aspirin Eugenol Ester Alleviates Vascular Endothelial Ferroptosis by Enhancing Antioxidant Ability and Inhibiting the JNK/c-Jun/NCOA4/FTH Signaling Pathway. Antioxidants 2025, 14, 1220. https://doi.org/10.3390/antiox14101220
Feng J, Tao Q, Zhang Z-J, Yu Q-F, Yang Y-J, Li J-Y. Aspirin Eugenol Ester Alleviates Vascular Endothelial Ferroptosis by Enhancing Antioxidant Ability and Inhibiting the JNK/c-Jun/NCOA4/FTH Signaling Pathway. Antioxidants. 2025; 14(10):1220. https://doi.org/10.3390/antiox14101220
Chicago/Turabian StyleFeng, Ji, Qi Tao, Zhi-Jie Zhang, Qin-Fang Yu, Ya-Jun Yang, and Jian-Yong Li. 2025. "Aspirin Eugenol Ester Alleviates Vascular Endothelial Ferroptosis by Enhancing Antioxidant Ability and Inhibiting the JNK/c-Jun/NCOA4/FTH Signaling Pathway" Antioxidants 14, no. 10: 1220. https://doi.org/10.3390/antiox14101220
APA StyleFeng, J., Tao, Q., Zhang, Z.-J., Yu, Q.-F., Yang, Y.-J., & Li, J.-Y. (2025). Aspirin Eugenol Ester Alleviates Vascular Endothelial Ferroptosis by Enhancing Antioxidant Ability and Inhibiting the JNK/c-Jun/NCOA4/FTH Signaling Pathway. Antioxidants, 14(10), 1220. https://doi.org/10.3390/antiox14101220






