Schizochytrium Supplementation in Compound Feed: Effects on Growth, Metamorphosis, Intermediate Metabolism, and Intestinal Health of Bullfrogs (Lithobates catesbeianus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Diets, Bullfrog, and Sampling
2.3. Analytic Procedures
2.3.1. Analysis of Proximate Composition and Intestinal Histomorphometry
2.3.2. Analysis of Intestinal Biochemical Indicators and Microbiota
2.3.3. Analysis of Polymerase Chain Reaction (PCR)
2.4. Statistical Analysis
3. Results
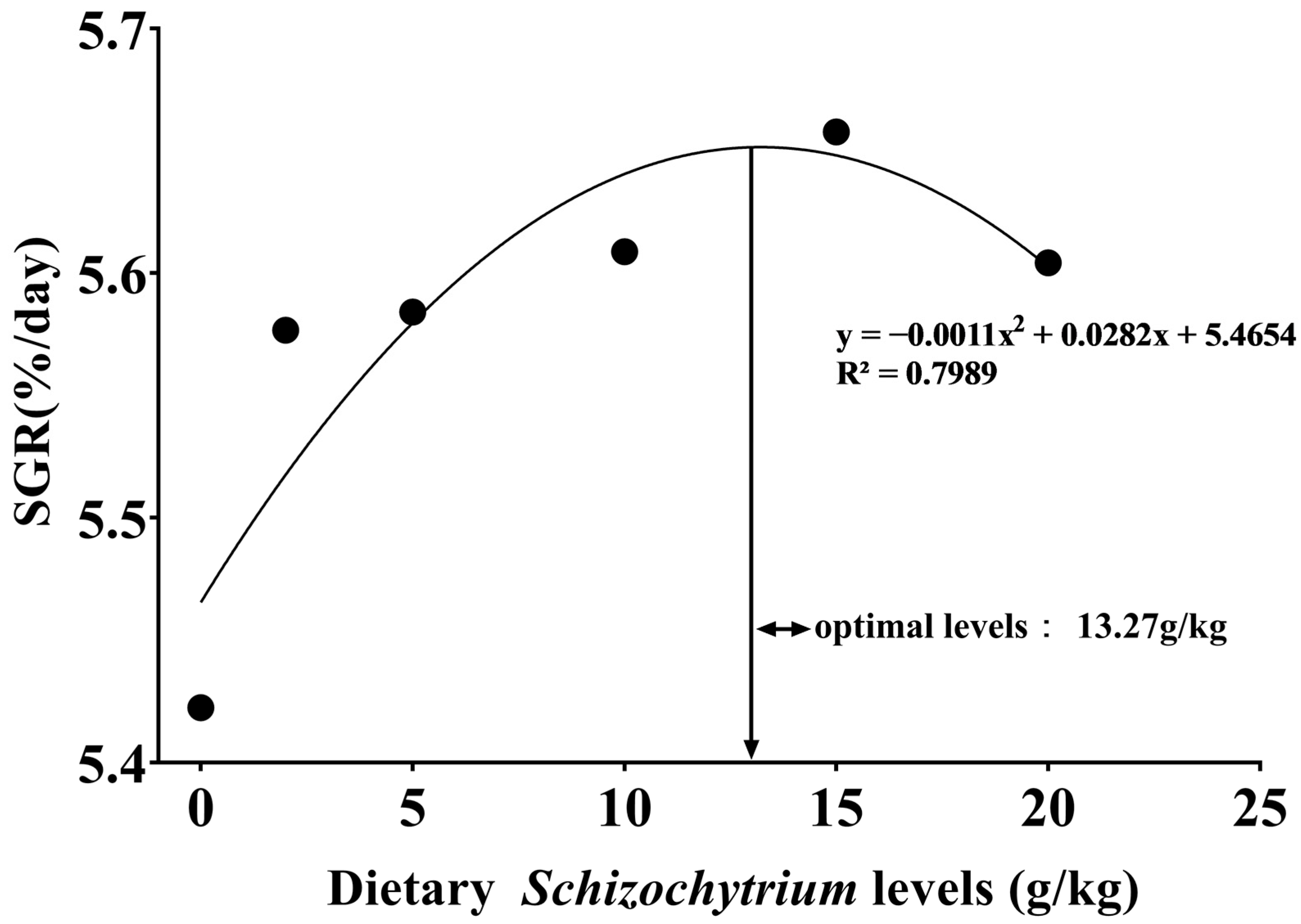
3.1. Growth Performance, Feed Utilization, and Metamorphosis Rate
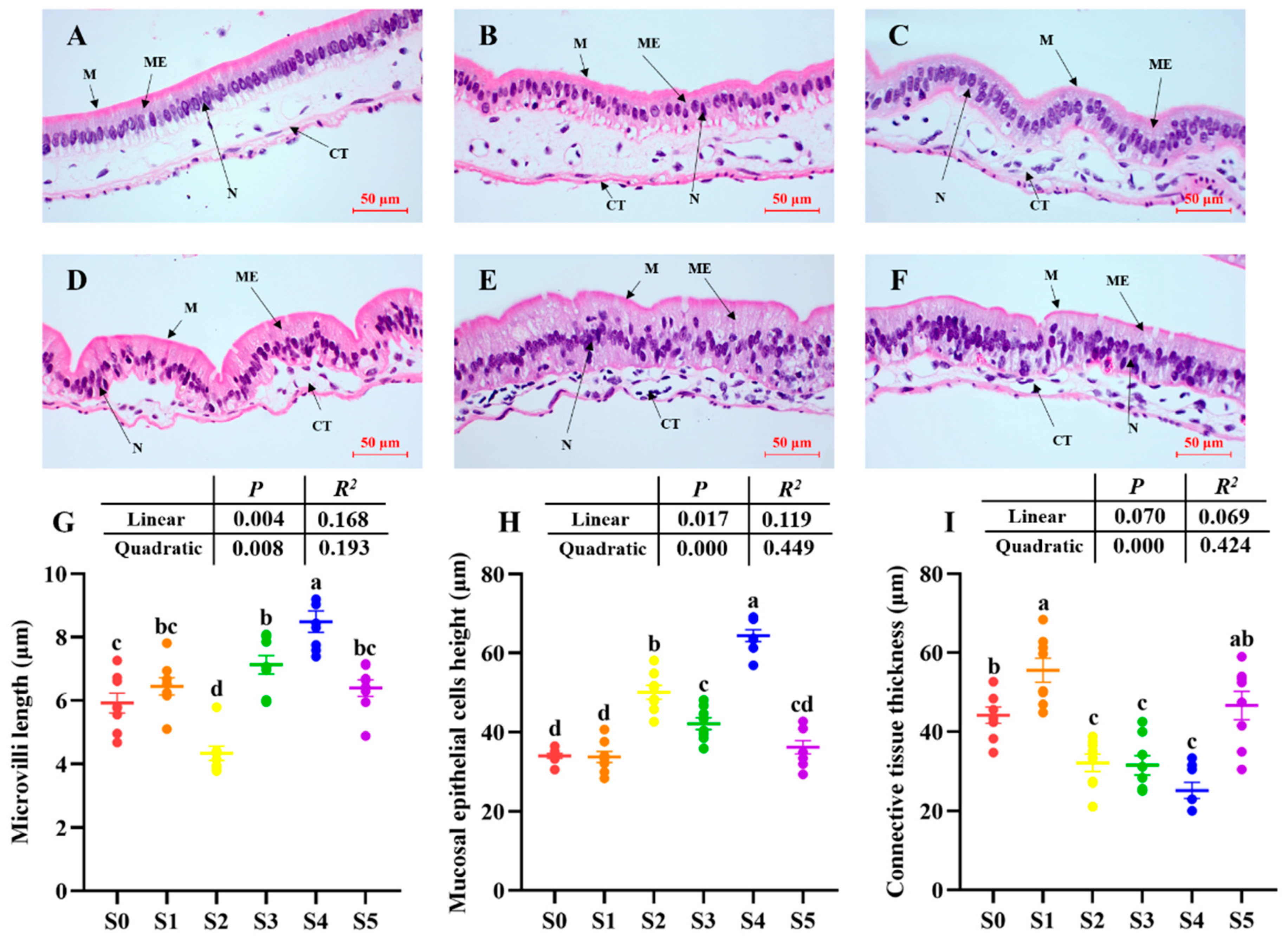
3.2. Intestinal Biochemical Indicators and Histomorphometry
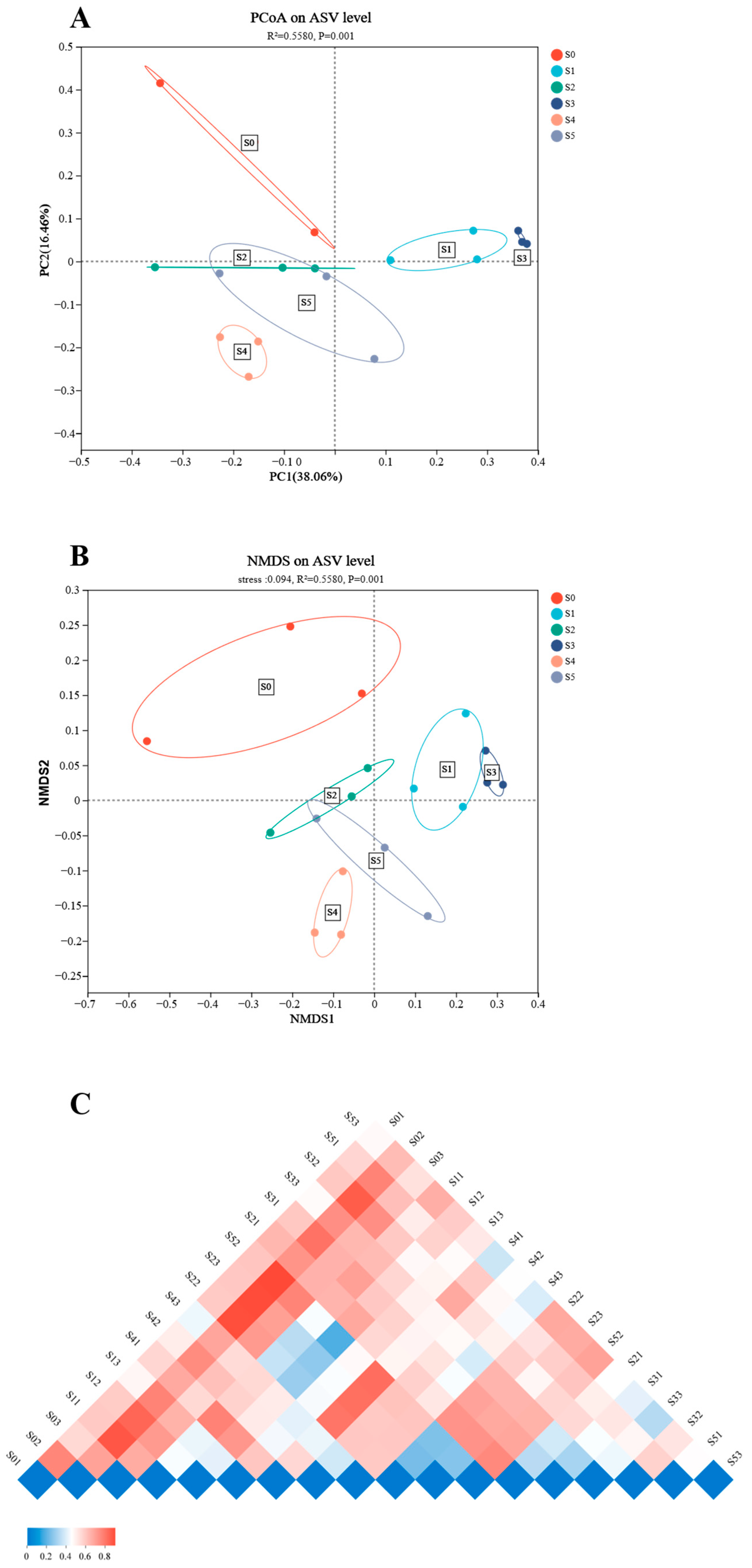
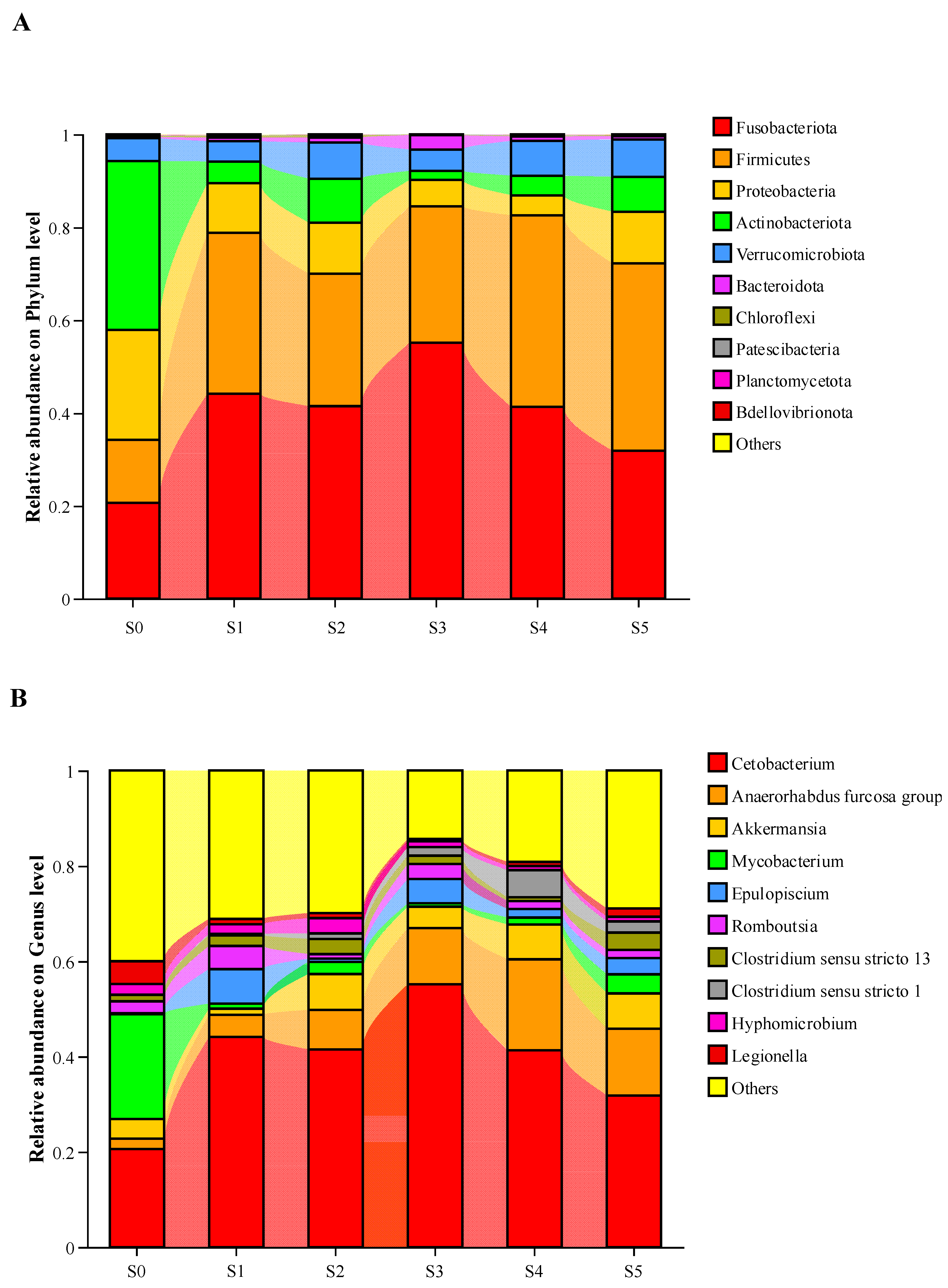
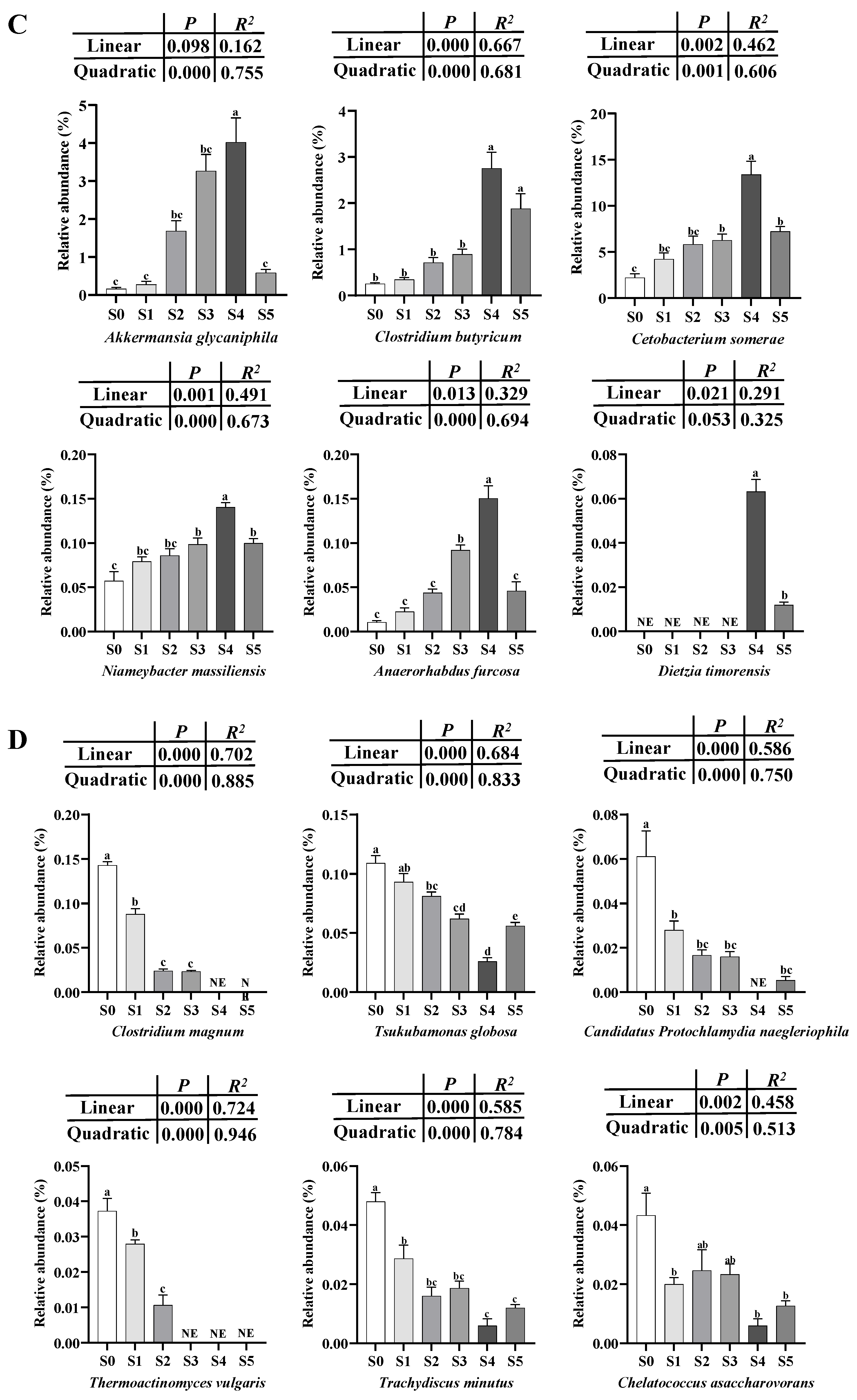
3.3. Analyses of Intestinal Microbiota
3.4. The Expression of Genes Related to Growth Performance, Energy Metabolism, and Immune Response
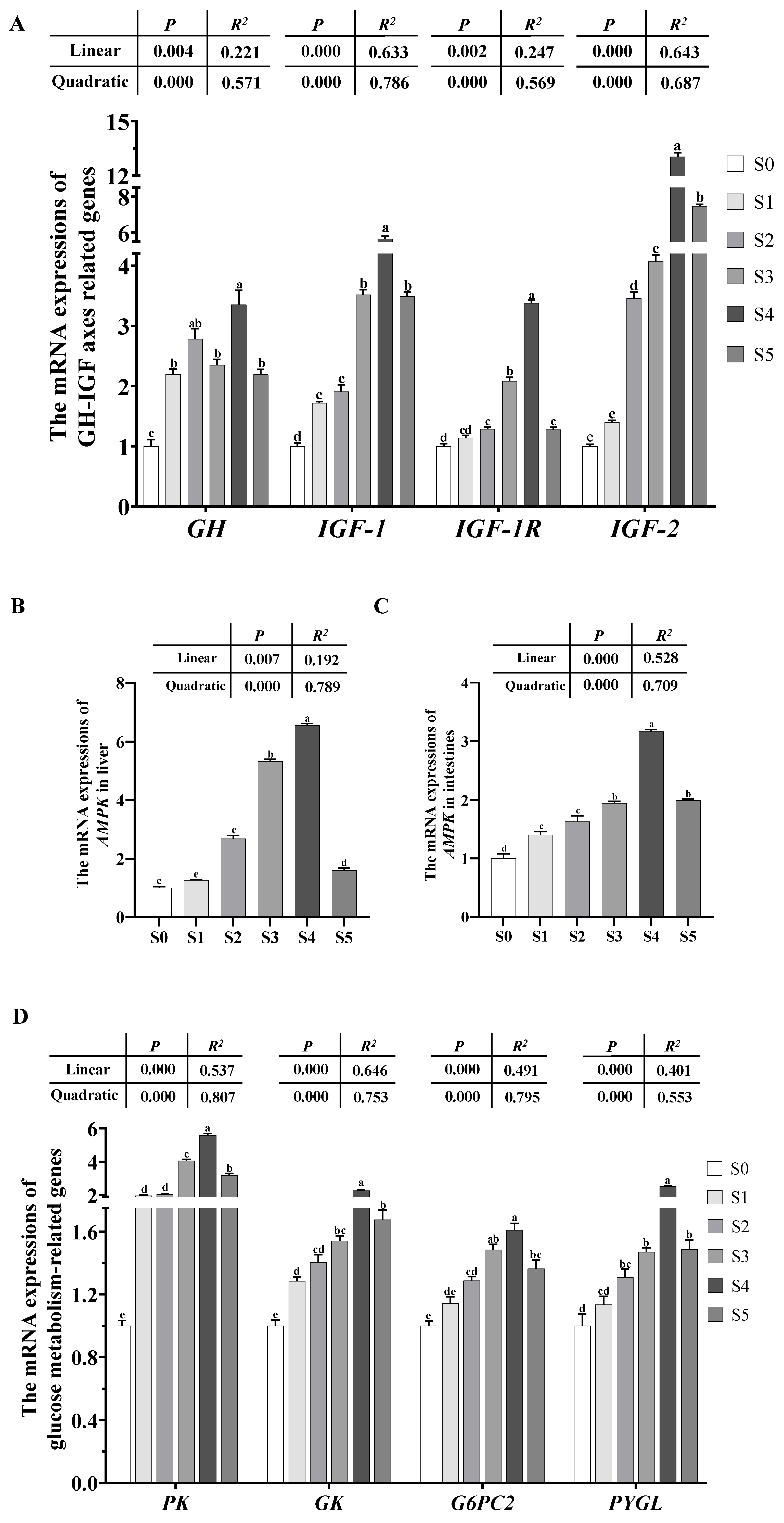
3.4.1. Expression of the Genes Involved in GH-IGF-1 Axes
3.4.2. Expression of the AMPK Gene
3.4.3. Expression of the Genes Involved in Glycolipid Metabolism
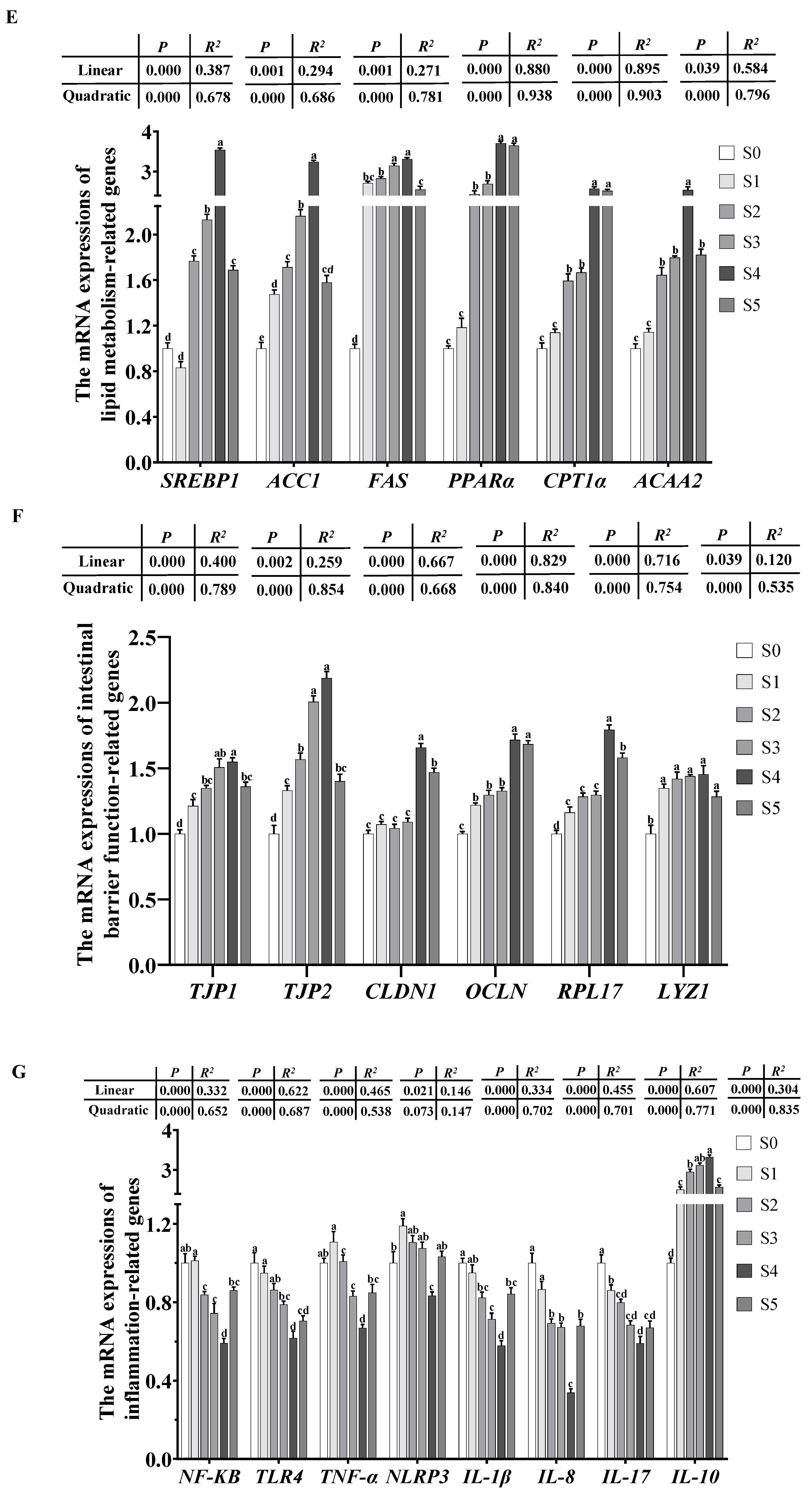
3.4.4. Expression of the Genes Involved in Intestinal Barrier Function
3.4.5. Expression of the Genes Involved in Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GH | Growth hormone |
| AMPK | Amp-activated protein kinase |
| IGF-1 | Insulin-like growth factor 1 |
| IGF-1R | Insulin-like growth factor 1 receptor |
| IGF-2 | Insulin-like growth factor 2 |
| PK | Pyruvate kinase |
| G6PC2 | Glucose-6-phosphatase catalytic, 2 |
| PYGL | Glycogen phosphorylase, liver |
| GK | Glycerol kinase |
| SREBP1 | Sterol-regulatory element binding protein 1 |
| ACC1 | Acetyl-coa carboxylase 1 |
| FAS | Fatty acid synthase |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| CPT1α | Carnitine palmitoyl transferase 1α |
| ACAA2 | Acetyl-coa acyltransferase 2 |
| LYZ1 | Lysozyme 1 |
| TJP1 | Tight junction protein 1 |
| TJP2 | Tight junction protein 2 |
| CLDN1 | Claudin 1 |
| OCLN | Occluding |
| RPL17 | Ribosomal protein L17 |
| NF-KB | Nuclear factor kappa B |
| TLR4 | Toll-like receptor 4 |
| NLRP3 | NACHT, LRR, and PYD domains-containing protein 3 |
| TNF-α | Tumor necrosis factor alpha |
| IL-1β | Interleukin 1β |
| IL-8 | Interleukin 8 |
| IL-10 | Interleukin 10 |
| IL-17 | Interleukin 17 |
| WGR | Weight gain rate |
| SGR | Specific growth rate |
| FCR | Feed conversion ratio |
| FI | Feed intake |
| CF | Condition factor |
| PPR | Post-premetamorphosis rate |
| MR | Metamorphosis rate |
| Na+, K+-ATPase | Sodium-potassium adenosine triphosphatase |
| AKP | Alkaline phosphatase |
| γ-GT | γ-glutamyl transferase |
| CK | Creatine kinase |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| T-AOC | Total antioxidant capacity |
| MDA | Malondialdehyde |
| ASV | Amplicon sequence variant |
| CT | Connective tissue |
| ME | Mucosal epithelial cells |
| M | Microvilli |
| N | Cell nucleus |
| PCoA | Principal co-ordinates analysis |
| NMDS | Non-metric multidimensional scaling |
References
- Mansano, C.F.M.; Stéfani, M.V.D.; Pereira, M.M.; Macente, B.I. Non-linear growth models for bullfrog tadpoles. Cienc. Agrotecnol. 2012, 36, 454–462. [Google Scholar] [CrossRef]
- Scott, D.E.; Casey, E.D.; Donovan, M.F.; Lynch, T.K. Amphibian lipid levels at metamorphosis correlate to post-metamorphic terrestrial survival. Oecologia 2007, 153, 521–532. [Google Scholar] [CrossRef]
- Stamper, C.; Downie, J.; Stevens, D.; Monaghan, P. The effects of perceived predation risk on pre-and post-metamorphic phenotypes in the common frog. J. Zool. 2009, 277, 205–213. [Google Scholar] [CrossRef]
- Alvarez, R.; Real, M. Significance of initial weight of post-metamorphosis froglets for growth and fattening of Rana perezi Seoane, 1885, raised in captivity. Aquaculture 2006, 255, 429–435. [Google Scholar] [CrossRef]
- Cheng, R.B.; Ma, R.J.; Li, K.; Rong, H.; Lin, X.Z.; Wang, Z.K.; Yang, S.J.; Ma, Y. Agrobacterium tumefaciens mediated transformation of marine microalgae Schizochytrium. Microbiol. Res. 2012, 167, 179–186. [Google Scholar] [CrossRef]
- Shields, R.; Bell, G. Evaluation of a DHA-rich microalga (Schizochytruim sp.) as a diet enrichment for larval Atlantic halibut (Hippoglossus hippoglossus L.). Spec. Publ. Eur. Aquac. Soc. 1995, 24, 188–191. [Google Scholar]
- Wang, Y.; Li, M.; Filer, K.; Xue, Y.; Ai, Q.; Mai, K. Replacement of fish oil with a DHA-rich Schizochytrium meal on growth performance, activities of digestive enzyme and fatty acid profile of Pacific white shrimp (Litopenaeus vannamei) larvae. Aquacult. Nutr. 2017, 23, 1113–1120. [Google Scholar] [CrossRef]
- Xie, S.W.; Wei, D.; Tan, B.P.; Liu, Y.J.; Tian, L.X.; Niu, J. Schizochytrium limacinum supplementation in a low fish-meal diet improved immune response and intestinal health of juvenile Penaeus monodon. Front. Physiol. 2020, 11, 613. [Google Scholar] [CrossRef]
- Xie, J.; Fang, H.; Liao, S.; Guo, T.; Yin, P.; Liu, Y.; Tian, L.; Niu, J. Study on Schizochytrium sp. improving the growth performance and non-specific immunity of golden pompano (Trachinotus ovatus) while not affecting the antioxidant capacity. Fish Shellfish Immunol. 2019, 95, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Chen, S.; Wu, Y.; Ma, Y.; Qiao, H.; Fan, J.; Wu, H. Dietary supplementation with microalgae enhances the zebrafish growth performance by modulating immune status and gut microbiota. Appl. Microbiol. Biotechnol. 2022, 106, 773–788. [Google Scholar] [CrossRef]
- Sarker, P.K.; Kapuscinski, A.R.; Lanois, A.J.; Livesey, E.D.; Bernhard, K.P.; Coley, M.L. Towards sustainable aquafeeds: Complete substitution of fish oil with marine microalga Schizochytrium sp. improves growth and fatty acid deposition in juvenile nile tilapia (Oreochromis niloticus). PLoS ONE 2016, 11, e0156684. [Google Scholar] [CrossRef]
- Karataş, B. Effects of Chlorella sp. and Schizochytrium sp. extracts on growth indices, body composition, and gene expression profiles in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2025, 276, 111047. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Anderson, R.E. Metabolism in frog retinal pigment epithelium of docosahexaenoic and arachidonic acids derived from rod outer segment membranes. Exp. Eye Res. 1993, 57, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Santos, R.A.; Cohen-Cory, S. Impact of maternal n-3 polyunsaturated fatty acid deficiency on dendritic arbor morphology and connectivity of developing Xenopus laevis central neurons In Vivo. J. Neurosci. 2015, 35, 6079–6092. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Anderson, R.E. Differential incorporation of docosahexaenoic and arachidonic acids in frog retinal pigment epithelium. J. Lipid Res. 1993, 34, 1943–1955. [Google Scholar] [CrossRef]
- Hamasaki, K.; Suprayudi, M.A.; Takeuchi, T. Effects of dietary N-3HUFA on larval morphogenesis and metamorphosis to megalops in the seed production of the mud crab, Scylla serrata (Brachyura: Portunidae). Aquac. Sci. 2002, 50, 333–340. [Google Scholar] [CrossRef]
- Hamre, K.; Moren, M.; Solbakken, J.; Opstad, I.; Pittman, K. The impact of nutrition on metamorphosis in Atlantic halibut (Hippoglossus hippoglossus L.). Aquaculture 2005, 250, 555–565. [Google Scholar] [CrossRef]
- Gatesoupe, F. Live yeasts in the gut: Natural occurrence, dietary introduction, and their effects on fish health and development. Aquaculture 2007, 267, 20–30. [Google Scholar] [CrossRef]
- Kayama, H.; Okumura, R.; Takeda, K. Interaction between the microbiota, epithelia, and immune cells in the intestine. Annu. Rev. Immunol. 2020, 38, 23–48. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Zhou, T.; Wu, J.; Feng, F.; Wang, S.; Chi, Q.; Sha, Y.; Zha, S.; Shu, S.; et al. Gut microbiota-derived butyric acid regulates calcific aortic valve disease pathogenesis by modulating GAPDH lactylation and butyrylation. iMeta 2025, 4, e70048. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, W.B.; Li, H.Y.; Xu, W.N.; He, J.X.; Li, X.F.; Jiang, G.Z. Effects of dietary supplementation of fructooligosaccharide on growth performance, body composition, intestinal enzymes activities and histology of blunt snout bream (Megalobrama amblycephala) fingerlings. Aquacult. Nutr. 2013, 19, 886–894. [Google Scholar] [CrossRef]
- Li, X.; Rahimnejad, S.; Wang, L.; Lu, K.L.; Song, K.; Zhang, C.X. Substituting fish meal with housefly (Musca domestica) maggot meal in diets for bullfrog Rana (Lithobates) catesbeiana: Effects on growth, digestive enzymes activity, antioxidant capacity and gut health. Aquaculture 2019, 499, 295–305. [Google Scholar] [CrossRef]
- Li, M.H.; Robinson, E.H.; Tucker, C.S.; Manning, B.B.; Khoo, L. Effects of dried algae Schizochytrium sp., a rich source of docosahexaenoic acid, on growth, fatty acid composition, and sensory quality of channel catfish Ictalurus punctatus. Aquaculture 2009, 292, 232–236. [Google Scholar] [CrossRef]
- Liu, X.C.; Xiong, Y.; Zhong, H.C.; Zhang, C.X.; Wang, L.; Song, K.; Ma, R.J.; Li, X.S.; Lu, K.L. Effects of dietary supplementation with schizochytrium meal on growth performance, non-specific immunity and antioxidant capacity of Lateolabrax maculatus. Chin. J. Anim. Nutr. 2025, 37, 2576–2586. [Google Scholar]
- Mansano, C.; Macente, B.I.; Khan, K.U.; Fernandes, J.B.K.; Vanzela, L.S.; Américo-Pinheiro, J.H.P.; Frias, D.F.R.; De Stéfani, M.V. Importance of optimum water quality indices in successful frog culture practices. In Limnology—Some New Aspects of Inland Water Ecology; Gokce, D., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rhoads, J.M.; Chen, W.; Chu, P.; Berschneider, H.M.; Argenzio, R.A.; Paradiso, A.M. L-glutamine and L-asparagine stimulate Na+-H+ exchange in porcine jejunal enterocytes. Am. J. Physiol.-Gastrointest. Liver Physiol. 1994, 266, G828–G838. [Google Scholar] [CrossRef]
- Villanueva, J.; Vanacore, R.; Goicoechea, O.; Amthauer, R. Intestinal alkaline phosphatase of the fish Cyprinus carpio: Regional distribution and membrane association. J. Exp. Zool. 1997, 279, 347–355. [Google Scholar] [CrossRef]
- Wallimann, T.; Hemmer, W. Creatine kinase in non-muscle tissues and cells. Cell. Bioenerg. Role Coupled Creat. Kinases 1994, 133, 193–220. [Google Scholar] [CrossRef]
- Suzer, C.; Çoban, D.; Kamaci, H.O.; Saka, Ş.; Firat, K.; Otgucuoğlu, Ö.; Küçüksari, H. Lactobacillus spp. bacteria as probiotics in gilthead sea bream (Sparus aurata, L.) larvae: Effects on growth performance and digestive enzyme activities. Aquaculture 2008, 280, 140–145. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Chen, Y.Q.; Fisher, J.H.; Wang, M.H. Activation of the RON receptor tyrosine kinase by macrophage-stimulating protein inhibits inducible cyclooxygenase-2 expression in murine macrophages. J. Biol. Chem. 2002, 277, 38104–38110. [Google Scholar] [CrossRef] [PubMed]
- Phan-Chan-Du, A.; Hemmerlin, C.; Krikorian, D.; Sakarellos-Daitsiotis, M.; Tsikaris, V.; Sakarellos, C.; Marinou, M.; Thureau, A.; Cung, M.T.; Tzartos, S.J. Solution conformation of the antibody-bound tyrosine phosphorylation site of the nicotinic acetylcholine receptor β-subunit in its phosphorylated and nonphosphorylated states. Biochemistry 2003, 42, 7371–7380. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Q.; Zhao, C.R.; Lin, Y. Compare the effect of diet supplementation with uncoated or coated lysine on juvenile Jian Carp (Cyprinus carpio Var. Jian). Aquacult. Nutr. 2010, 13, 457–461. [Google Scholar] [CrossRef]
- Shu, Y.L.; Jiang, H.L.; Yuen, C.N.T.; Wang, W.C.; He, J.; Zhang, H.J.; Liu, G.X.; Wei, L.T.; Chen, L.G.; Wu, H.L. Microcystin-leucine arginine induces skin barrier damage and reduces resistance to pathogenic bacteria in Lithobates catesbeianus tadpoles. Ecotoxicol. Environ. Saf. 2022, 238, 113584. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Cao, D.; Yang, J.; Mao, H.; Sun, L.; Wang, C. Integrated multi-omics reveals the relationship between growth performance, rumen microbes and metabolic status of Hu sheep with different residual feed intakes. Anim. Nutr. 2024, 18, 284–295. [Google Scholar] [CrossRef]
- Kar, N.; Ghosh, K. Enzyme producing bacteria in the gastrointestinal tracts of Labeo rohita (Hamilton) and Channa punctatus (Bloch). Turk. J. Fish. Aquat. Sci. 2008, 8, 115–120. [Google Scholar] [CrossRef]
- Ramirez, R.F.; Dixon, B.A. Enzyme production by obligate intestinal anaerobic bacteria isolated from oscars (Astronotus ocellatus), angelfish (Pterophyllum scalare) and southern flounder (Paralichthys lethostigma). Aquaculture 2003, 227, 417–426. [Google Scholar] [CrossRef]
- Yu, C.X.; Zhang, P.; Liu, S.J.; Niu, Y.M.; Fu, L. SESN2 ablation weakens exercise benefits on resilience of gut microbiota following high-fat diet consumption in mice. Food Sci. Hum. Wellness 2023, 12, 1961–1968. [Google Scholar] [CrossRef]
- Zhang, J.H.; Meng, H.; Kong, X.C.; Cheng, X.Y.; Ma, T.; He, H.; Du, W.C.; Yang, S.G.; Li, S.Y.; Zhang, L.M. Combined effects of polyethylene and organic contaminant on zebrafish (Danio rerio): Accumulation of 9-Nitroanthracene, biomarkers and intestinal microbiota. Environ. Pollut. 2021, 277, 116767. [Google Scholar] [CrossRef]
- Orellana, L.H.; Francis, T.B.; Ferraro, M.A.; Hehemann, J.H.; Fuchs, B.M.; Amann, R.I. Verrucomicrobiota are specialist consumers of sulfated methyl pentoses during diatom blooms. ISME J. 2021, 16, 630–641. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef]
- Sweeney, T.E.; Morton, J.M. The Human Gut Microbiome: A Review of the Effect of Obesity and Surgically Induced Weight Loss. JAMA Surg. 2013, 148, 563–569. [Google Scholar] [CrossRef]
- Mathur, R.; Barlow, G.M. Obesity and the microbiome. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, Y.; Wang, K.; Chen, J.; Jin, K.; Peng, K.; Chen, X.; Liu, Z.; Ouyang, J.; Wang, Y.; et al. Effects of Litsea cubeba essential oil on growth performance, blood antioxidation, immune function, apparent digestibility of nutrients, and fecal microflora of pigs. Front. Pharmacol. 2023, 14, 1166022. [Google Scholar] [CrossRef] [PubMed]
- Iwaza, R.; Wasfy, R.M.; Dubourg, G.; Raoult, D.; Lagier, J.-C. Akkermansia muciniphila: The state of the art, 18 years after its first discovery. Front. Gastroenterol. 2022, 1, 1024393. [Google Scholar] [CrossRef]
- Meng, X.L.; Wu, S.K.; Hu, W.P.; Zhu, Z.X.; Yang, G.K.; Zhang, Y.M.; Qin, C.B.; Yang, L.P.; Nie, G.X. Clostridium butyricum improves immune responses and remodels the intestinal microbiota of common carp (Cyprinus carpio L.). Aquaculture 2021, 530, 735753. [Google Scholar] [CrossRef]
- Liang, H.; Li, M.; Chen, J.; Zhou, W.H.; Xia, D.M.; Ding, Q.W.; Yang, Y.L.; Zhang, Z.; Ran, C.; Zhou, Z.G. The intestinal microbiome and Cetobacterium somerae inhibit viral infection through TLR2-type I IFN signaling axis in zebrafish. Microbiome 2024, 12, 244. [Google Scholar] [CrossRef] [PubMed]
- Turgut, S.; Erken, H.A.; Erken, G.; Ayada, C.; Genc, O.; Turgut, G. The effects of docosahexaenoic acid supplementation and exercise on growth hormone and insulin-like growth factor I serum levels during chronic hypoxia in rats. J. Basic. Clin. Physiol. Pharmacol. 2011, 22, 103–107. [Google Scholar] [CrossRef]
- Butler, A.A.; Roith, D.L. Control of growth by the somatropic axis: Growth hormone and the insulin-like growth factors have related and independent roles. Annu. Rev. Physiol. 2001, 63, 141–164. [Google Scholar] [CrossRef]
- Xu, C.; Liu, W.B.; Zhang, D.D.; Wang, K.Z.; Xia, S.L.; Li, X.F. Molecular characterization of AMP-activated protein kinase α2 from herbivorous fish Megalobrama amblycephala and responsiveness to glucose loading and dietary carbohydrate levels. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2017, 208, 24–34. [Google Scholar] [CrossRef]
- Manzi, L.; Costantini, L.; Molinari, R.; Merendino, N. Effect of dietary ω-3 polyunsaturated fatty acid DHA on glycolytic enzymes and Warburg phenotypes in cancer. Biomed. Res. Int. 2015, 2015, 137097. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, Y.W.; Kim, S.G. AMPK-mediated GSK3β inhibition by isoliquiritigenin contributes to protecting mitochondria against iron-catalyzed oxidative stress. Biochem. Pharmacol. 2010, 79, 1352–1362. [Google Scholar] [CrossRef]
- Xu, C.; Liu, W.B.; Zhang, D.D.; Cao, X.F.; Shi, H.J.; Li, X.F. Interactions between dietary carbohydrate and metformin: Implications on energy sensing, insulin signaling pathway, glycolipid metabolism and glucose tolerance in blunt snout bream Megalobrama amblycephala. Aquaculture 2018, 483, 183–195. [Google Scholar] [CrossRef]
- Lee, W.J.; Kim, M.; Park, H.-S.; Kim, H.S.; Jeon, M.J.; Oh, K.S.; Koh, E.H.; Won, J.C.; Kim, M.-S.; Oh, G.T.; et al. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARα and PGC-1. Biochem. Biophys. Res. Commun. 2006, 340, 291–295. [Google Scholar] [CrossRef]
- Huang, C.W.; Chen, Y.J.; Mersmann, H.; Ding, S.-T. DHA up-regulated expression of lipogenic and endoplasmic reticulum stress related genes in pig adipose tissues. FASEB J. 2015, 29, 568.520. [Google Scholar] [CrossRef]
- Harmon, R.C.; Terneus, M.V.; Kiningham, K.K.; Valentovic, M. Time-dependent effect of p-aminophenol (PAP) toxicity in renal slices and development of oxidative stress. Toxicol. Appl. Pharmacol. 2005, 209, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Niklasson, L. Intestinal Mucosal Immunology of Salmonids Response to Stress and Infection and Crosstalk with the Physical Barrier. Doctoral Thesis, Gothenburg University, Gothenburg, Sweden, 2013. [Google Scholar]
- Yang, W.R.; Liao, T.T.; Bao, Z.Q.; Zhou, C.Q.; Luo, H.Y.; Lu, C.; Pan, M.H.; Wang, X.Z. Role of AMPK in the expression of tight junction proteins in heat-treated porcine Sertoli cells. Theriogenology 2018, 121, 42–52. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; You, Q.; He, S.; Meng, Q.Q.; Gao, J.; Wu, X.D.; Shen, Y.; Sun, Y.; Wu, X.F. Activating AMPK to restore tight junction assembly in intestinal epithelium and to attenuate experimental colitis by metformin. Front. Pharmacol. 2018, 9, 761. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.L.; Feng, L.; Jiang, W.D.; Liu, Y.; Jiang, J.; Li, S.H.; Tang, L.; Zhang, Y.A.; Kuang, S.Y.; Zhou, X.Q. Dietary tryptophan modulates intestinal immune response, barrier function, antioxidant status and gene expression of TOR and Nrf2 in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2014, 40, 275–287. [Google Scholar] [CrossRef]
- Zeng, M.; Zou, Y.; Shi, Z.; Wang, J.; Yang, Y.; Bai, Y.; Ping, A.; Zhang, P.; Chen, Y.; Tao, H.; et al. A broad-spectrum broth rapidly and completely repairing the sublethal injuries of Escherichia coli caused by freezing and lactic acid alone or in combination for accurate enumeration. LWT 2024, 201, 116219. [Google Scholar] [CrossRef]
- Salminen, A.; Hyttinen, J.M.; Kaarniranta, K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: Impact on healthspan and lifespan. J. Mol. Med. 2011, 89, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lai, J.; Yang, L.; Ruan, G.; Chaugai, S.; Ning, Q.; Chen, C.; Wang, D.W. Trimetazidine prevents macrophage-mediated septic myocardial dysfunction via activation of the histone deacetylase sirtuin 1. Br. J. Pharmacol. 2016, 173, 545–561. [Google Scholar] [CrossRef] [PubMed]

| Groups | ||||||
|---|---|---|---|---|---|---|
| Items | S0 | S1 | S2 | S3 | S4 | S5 |
| Formulation (%) | ||||||
| Fish meal | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Chicken meal | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 |
| Dephenolized cottonseed protein | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Rapeseed meal | 16.0 | 16.0 | 16.0 | 16.0 | 16.0 | 16.0 |
| Soybean meal | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 |
| Corn starch | 14.5 | 14.5 | 14.5 | 14.5 | 14.5 | 14.5 |
| Schizochytrium (g/kg) | - | 2.0 | 5.0 | 10.0 | 15.0 | 20.0 |
| Fish oil | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Soybean oil | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Calcium biphosphate | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Choline chloride | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Premix * | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| DL-lysine | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| DL-methionine | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Proximate analysis (% dry matter basis) | ||||||
| Moisture | 8.14 | 7.87 | 8.03 | 7.93 | 8.01 | 7.90 |
| Crude protein | 29.17 | 28.14 | 28.9 | 29.16 | 28.77 | 29.13 |
| Crude lipid | 4.23 | 4.53 | 4.51 | 4.43 | 4.49 | 4.52 |
| Ash | 15.53 | 15.01 | 14.95 | 14.90 | 15.17 | 15.01 |
| Target Genes | Forward (5′-3) | Reverse (5′-3) | Accession Numbers or References |
|---|---|---|---|
| GH | TAGACCTCCTCCGCTTCTCC | ACCGTAGTTCCGGACGTTTC | AY251538.1 |
| AMPK | GGAGGTGCTCAGCTGCTTGT | AATGAATCGGGCGGGCTTGT | PIO34006.1 |
| IGF-1 | CAGTTTGTATGTGGAGACAGAGGCT | CAGTACATCTCTAGCCTCCTCAGA | XM040344286.1 |
| IGF-1R | TCCGTCTGTTAGGCGTTGT | AGGGTTGTTCTCGGCATCT | KF819507 |
| IGF-2 | TGCCACTGCATTGCCATCTCT | TCTATTTGCCTCACCCACCGC | XM040328472.1 |
| PK | TCCTTCATTCGCAAGGCAGC | TGATATTCTTTCCTTTCTCCCCGA | XM040343042.1 |
| G6PC2 | CTGCTCCAGTCATTGAACAGTTTC | CCCCATAGCGTGACCTGAAG | LH203031 |
| PYGL | CTCGAGTGCTCTATCCCAATGAT | ACCACAAAGTACTCCTGCTTCAGA | LH181312 |
| GK | AACGCTTTGAGCCACAGATTAAT | CTGCTTTTTTCCATCGAGCAT | LH193866 |
| SREBP1 | CCACCGCCTGCACCAA | CACTCAGCGCCATGTTGATG | LH362962 |
| ACC1 | GTTAAAGCTGCCATCCTCACTGT | TGTCCGTCTGGCTAAGATGGT | LH212450 |
| FAS | CCTCCACGCCAGAACAAGAT | GATATTTTTATGAGTGGACATTGTATCGA | LH228595 |
| PPARα | CCCGACATTCGATGTTTAGAGATT | CCAGCCCATCTTCTATCACCTT | LH193621 |
| CPT1α | TGATTGGCAAAATCAAAGAACATC | AATGCTCTGACCCTGGTGAGA | LH022414 |
| ACAA2 | TGTTAGCAGGAGGTAAAAGGAAAGTC | ATGCGTTCAACAGTTTTTGGAA | LH119205 |
| LYZ1 | ATGGAAAGACAGCAGGAG | TCCAGAAGTATACGATCC | [25] |
| TJP1 | TACGAGAAAGTGGTGCTC | CCTCACTCACAAGACTTG | [25] |
| TJP2 | GACCGTAGAAGTGCATAC | CCTGATCTCTTCCATAGC | [25] |
| CLDN1 | TTGTCCCTCCTGTCTGTCATCT | ACTGGAGAGGTGAAGTAGTGG | XM040320982.1 |
| OCLN | TGCAACGATCGTGTACAC | GTGAATAGAACTGGTTGC | [25] |
| RPL17 | CAAACAGTGGGACTGGACACA | TTCTTCAGCATGTGCAGGAGGAAT | XM040336449.1 |
| NF-KB | AGGAACAAGATCATCATAGAGGAC | TCAGAAAGAGGGGCCAAGTG | XM040329914.1 |
| TLR4 | ACACCTTCAAGGCTCGCTAC | ATGATGGGTCGCCATCTTCC | XM040322748.1 |
| NLRP3 | ATCACCACAAGGTCACTGGC | TGATTGGCCAGAGCACACAGC | XM040352091.1 |
| TNF-α | ACAGACTTGATGGACCTC | AGTTCCAGCTTCGATGTG | [25] |
| IL-1β | TCATTCGGGACAGCAGGCAGAA | GCTTCACTGGCACGGTTGTTCT | [14] |
| IL-8 | ACCCTTACCCTCTTCCTGCT | TCAGCTTCACACACTGGCA | XM040335899.1 |
| IL-10 | GGAAGTTGTCAGCGGGCTAT | GCCCTCTTGTGCATGTGGTA | XM040339663.1 |
| IL-17 | TGATAGTCACGCACTGAGTCCG | ATGTTCACCAGCCAGTCAATGC | [14] |
| β-actin | CATCCTTCTTGGGTATGGAATCA | TGGCATACAGGTCCTTACGGATA | AB094353 |
| Groups | Polynomial Contrasts (p, R2) | |||||||
|---|---|---|---|---|---|---|---|---|
| Items | S0 | S1 | S2 | S3 | S4 | S5 | Linear | Quadratic |
| Initial weight (g) | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.524, 0.026 | 0.707, 0.045 |
| Final weight (g) | 4.69 ± 0.02 e | 5.34 ± 0.03 d | 5.41 ± 0.05 c,d | 5.66 ± 0.06 b | 6.15 ± 0.06 a | 5.58 ± 0.05 b,c | 0.001, 0.522 | 0.000, 0.826 |
| WGR † (%) | 13,066.15 ± 148.16 c | 15,028.36 ± 197.53 b | 15,127.35 ± 85.10 b | 15,469.35 ± 64.80 b | 16,173.45 ± 202.30 a | 15,405.86 ± 83.80 b | 0.002, 0.474 | 0.000, 0.775 |
| SGR § (%/day) | 5.42 ± 0.01 c | 5.58 ± 0.01 b | 5.58 ± 0.01 b | 5.61 ± 0.00 a,b | 5.66 ± 0.01 a | 5.60 ± 0.01 b | 0.002, 0.467 | 0.000, 0.768 |
| FCR ¶ | 2.55 ± 0.04 a | 2.51 ± 0.05 a | 2.47 ± 0.04 a,b | 2.44 ± 0.03 a,b | 2.24 ± 0.05 c | 2.31 ± 0.02 b,c | 0.000, 0.654 | 0.000, 0.670 |
| FI (g/bullfrog tadpoles/d) # | 0.13 ± 0.00 c | 0.15 ± 0.00 a,b | 0.15 ± 0.00 a,b | 0.15 ± 0.00 a | 0.15 ± 0.00 a | 0.14 ± 0.00 b | 0.151, 0.124 | 0.000, 0.697 |
| CF ∫ | 0.96 ± 0.02 | 0.97 ± 0.03 | 0.92 ± 0.03 | 0.98 ± 0.02 | 0.98 ± 0.04 | 0.91 ± 0.03 | 0.725, 0.008 | 0.195, 0.196 |
| PPR ‽ (%) | 79.17 ± 2.20 b | 80.83 ± 3.00 a,b | 82.50 ± 3.82 a,b | 86.67 ± 3.33 a,b | 93.33 ± 1.67 a | 85.00 ± 1.44 a,b | 0.017, 0.307 | 0.011, 0.452 |
| MR1 ↑ (%) | 8.33 ± 2.20 b | 10.00 ± 1.44 b | 13.33 ± 0.83 b | 17.50 ± 2.89 a,b | 23.33 ± 1.67 a | 17.50 ± 2.50 a,b | 0.001, 0.533 | 0.000, 0.679 |
| Proximate analysis (% dry matter basis) | ||||||||
| Moisture | 80.16 ± 2.64 | 76.69 ± 2.90 | 77.02 ± 1.64 | 75.23 ± 2.49 | 76.62 ± 1.53 | 79.15 ± 1.88 | 0.882, 0.001 | 0.224, 0.181 |
| Crude protein | 76.53 ± 0.91 c | 81.84 ± 0.21 a,b | 80.68 ± 1.03 b,c | 84.02 ± 0.08 a,b | 84.56 ± 1.46 a,b | 85.19 ± 0.78 a | 0.000, 0.627 | 0.000, 0.712 |
| Crude lipid | 14.56 ± 0.14 a,b | 14.4 ± 0.22 b | 14.51 ± 0.21 b | 15.91 ± 0.33 a | 15.03 ± 0.15 a,b | 15.5 ± 0.52 a,b | 0.015, 0.317 | 0.034, 0.362 |
| Ash | 12.37 ± 0.51 | 12.08 ± 0.85 | 12.98 ± 0.39 | 11.9 ± 1.54 | 12.3 ± 1.34 | 12.93 ± 1.63 | 0.783, 0.005 | 0.907, 0.013 |
| Groups | Polynomial Contrasts (p, R2) | |||||||
|---|---|---|---|---|---|---|---|---|
| Items | S0 | S1 | S2 | S3 | S4 | S5 | Linear | Quadratic |
| Amylase (U/mg protein) | 0.24 ± 0.01 d | 0.27 ± 0.00 c,d | 0.28 ± 0.01 c | 0.29 ± 0.01 c | 0.38 ± 0.01 a | 0.33 ± 0.01 b | 0.000, 0.563 | 0.000, 0.601 |
| Lipase (U/g protein) | 8.20 ± 0.30 e | 11.17 ± 0.39 d | 11.65 ± 0.12 d | 13.38 ± 0.14 c | 19.6 ± 0.25 a | 15.8 ± 0.34 b | 0.000, 0.852 | 0.000, 0.806 |
| Protease (U/mg protein) | 1.03 ± 0.03 e | 1.36 ± 0.04 d | 1.72 ± 0.03 c | 1.99 ± 0.03 b | 2.46 ± 0.03 a | 2.01 ± 0.02 b | 0.000, 0.686 | 0.000, 0.919 |
| Na+, K+-ATPase (U/g protein) | 44.89 ± 1.81 d | 59.55 ± 2.78 cd | 78.19 ± 2.77 c | 121.00 ± 5.03 b | 159.59 ± 12.80 a | 143.56 ± 3.67 a,b | 0.000, 0.767 | 0.000, 0.827 |
| AKP (U/g protein) | 55.28 ± 0.18 c | 54.10 ± 0.78 b,c | 60.34 ± 0.75 b,c | 63.28 ± 0.91 a,b | 68.83 ± 0.57 a | 60.34 ± 0.85 b,c | 0.002, 0.446 | 0.000, 0.766 |
| γ-GT (U/g protein) | 79.18 ± 4.85 c | 76.79 ± 1.66 c | 99.78 ± 3.53 b | 122.77 ± 4.97 a | 140.00 ± 5.37 a | 73.09 ± 3.04 c | 0.251, 0.081 | 0.000, 0.709 |
| CK (U/mg protein) | 1.47 ± 0.08 c | 1.85 ± 0.02 b | 1.95 ± 0.03 a,b | 1.99 ± 0.02 a,b | 2.12 ± 0.03 a | 2.06 ± 0.04 a | 0.000, 0.476 | 0.000, 0.638 |
| SOD (U/mg protein) | 555.75 ± 5.33 c | 576.85 ± 17.78 b,c | 600.13 ± 15.64 b,c | 629.39 ± 9.12 a,b | 669.74 ± 11.9 a | 580.32 ± 7.12 b,c | 0.064, 0.202 | 0.000, 0.666 |
| CAT (U/mg protein) | 10.35 ± 0.17 d | 11.32 ± 0.35 c | 11.89 ± 0.16 c | 12.92 ± 0.16 b | 14.01 ± 0.07 a | 11.35 ± 0.07 c | 0.000, 0.215 | 0.000, 0.703 |
| T-AOC (mmol/g protein) | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.703, 0.009 | 0.762, 0.036 |
| MDA (nmol/mg protein) | 4.59 ± 0.05 a | 4.39 ± 0.08 a,b | 4.28 ± 0.04 b,c | 4.17 ± 0.07 b,c | 3.68 ± 0.05 d | 4.10 ± 0.06 c | 0.000, 0.461 | 0.000, 0.765 |
| Groups | Polynomial Contrasts (p, R2) | |||||||
|---|---|---|---|---|---|---|---|---|
| Items | S0 | S1 | S2 | S3 | S4 | S5 | Linear | Quadratic |
| ASV | 248.67 ± 3.84 a,b | 271.33 ± 38.92 a,b | 182.00 ± 25.32 b | 216.67 ± 19.97 a,b | 352.00 ± 31.34 a | 234.00 ± 41.14 a,b | 0.457, 0.035 | 0.761, 0.036 |
| Ace | 250.33 ± 3.18 a,b | 274.33 ± 39.96 a,b | 182.33 ± 25.12 b | 216.67 ± 19.97 a,b | 353.67 ± 31.86 a | 235.00 ± 41.04 a,b | 0.474, 0.033 | 0.774, 0.034 |
| Chao1 | 250.33 ± 3.18 a,b | 274.33 ± 39.96 a,b | 182.33 ± 25.12 b | 216.67 ± 19.97 a,b | 353.67 ± 31.86 a | 235.00 ± 41.04 a,b | 0.474, 0.033 | 0.774, 0.034 |
| Shannon | 2.98 ± 0.12 | 2.34 ± 0.31 | 2.21 ± 0.34 | 2.46 ± 0.18 | 3.31 ± 0.13 | 2.62 ± 0.55 | 0.483, 0.031 | 0.699, 0.047 |
| Simpson | 0.14 ± 0.04 | 0.28 ± 0.07 | 0.28 ± 0.07 | 0.18 ± 0.01 | 0.10 ± 0.02 | 0.22 ± 0.13 | 0.600, 0.018 | 0.874, 0.018 |
| Shannoneven | 0.54 ± 0.02 | 0.42 ± 0.04 | 0.42 ± 0.05 b | 0.46 ± 0.03 | 0.56 ± 0.02 a | 0.48 ± 0.09 | 0.541, 0.024 | 0.730, 0.041 |
| Simpsoneven | 0.03 ± 0.01 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.387, 0.047 | 0.527, 0.082 |
| Phylogenetic diversity | 28.64 ± 0.11 | 31.04 ± 5.40 | 21.61 ± 2.32 | 25.54 ± 1.52 | 35.86 ± 3.03 | 26.28 ± 3.72 | 0.719, 0.008 | 0.918, 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, H.; He, Y.; Song, Y.; Liang, J.; Li, W.; Xu, C.; Yang, H. Schizochytrium Supplementation in Compound Feed: Effects on Growth, Metamorphosis, Intermediate Metabolism, and Intestinal Health of Bullfrogs (Lithobates catesbeianus). Antioxidants 2025, 14, 1208. https://doi.org/10.3390/antiox14101208
Ding H, He Y, Song Y, Liang J, Li W, Xu C, Yang H. Schizochytrium Supplementation in Compound Feed: Effects on Growth, Metamorphosis, Intermediate Metabolism, and Intestinal Health of Bullfrogs (Lithobates catesbeianus). Antioxidants. 2025; 14(10):1208. https://doi.org/10.3390/antiox14101208
Chicago/Turabian StyleDing, Hao, Yinglin He, Yujian Song, Jingjing Liang, Woxing Li, Chao Xu, and Huirong Yang. 2025. "Schizochytrium Supplementation in Compound Feed: Effects on Growth, Metamorphosis, Intermediate Metabolism, and Intestinal Health of Bullfrogs (Lithobates catesbeianus)" Antioxidants 14, no. 10: 1208. https://doi.org/10.3390/antiox14101208
APA StyleDing, H., He, Y., Song, Y., Liang, J., Li, W., Xu, C., & Yang, H. (2025). Schizochytrium Supplementation in Compound Feed: Effects on Growth, Metamorphosis, Intermediate Metabolism, and Intestinal Health of Bullfrogs (Lithobates catesbeianus). Antioxidants, 14(10), 1208. https://doi.org/10.3390/antiox14101208






