Extraction Methods Shape the Phenolic Composition and Bioactivities of Defatted Moroccan Pistacia lentiscus L. Resin
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Plant Material
2.3. Extraction of Resin
2.3.1. Extraction Methods
- Ultrasound-Assisted Extraction (UAE): 20 g of powdered resin were extracted sequentially with 200 mL of each solvent in a WiseClean WUC-D22H ultrasonic bath (230 V, 50 Hz; frequency: 60 Hz; power: 500 W) at 40 °C for 15 min per solvent. Extraction time was selected according to commonly reported UAE protocols for phenolic compounds (typically 10–20 min) to ensure efficient recovery [26,27].
- Cold Maceration (CM): 20 g of powdered resin were mixed with 200 mL of solvent and stirred at ambient (room) temperature (100 rpm) in the dark for 24 h using a magnetic stirrer. A 24 h maceration period is commonly employed to ensure adequate recovery of phenolic compounds [29,30]. After filtration and solvent evaporation, the dried residue was subjected to the next solvent in the polarity sequence.
2.3.2. Extraction Yield
2.4. Phytochemical Analyses
2.4.1. Quantification of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.4.2. Phenolic Compound Profiling
- Autosampler (G4226A).
- Sampler thermostat (G1330B).
- Quaternary pump with degasser (G4204A; 1200 bar capacity).
- Thermostated column compartment (G1316A).
- 0 min: 5% B.
- 4 min: 20% B (linear increase from 0 to 4 min).
- 7 min: 90% B (linear increase from 4 to 7 min).
- 7–14 min: hold at 90% B.
- 14–15.1 min: decrease to 5% B.
- 15.1–20 min: re-equilibrate at 5% B.
2.5. Biological Activity Evaluation
2.5.1. Antioxidant Activity
- Acontrol is the absorbance of the radical solution without extract;
- Asample is the absorbance in the presence of extract.
2.5.2. Antimicrobial Activity
2.5.3. Cytotoxicity Evaluation via WST-8 Assay
2.6. Statistical Analysis
3. Results
3.1. Extraction Yields
3.2. Phytochemical Composition of the Resin Extracts
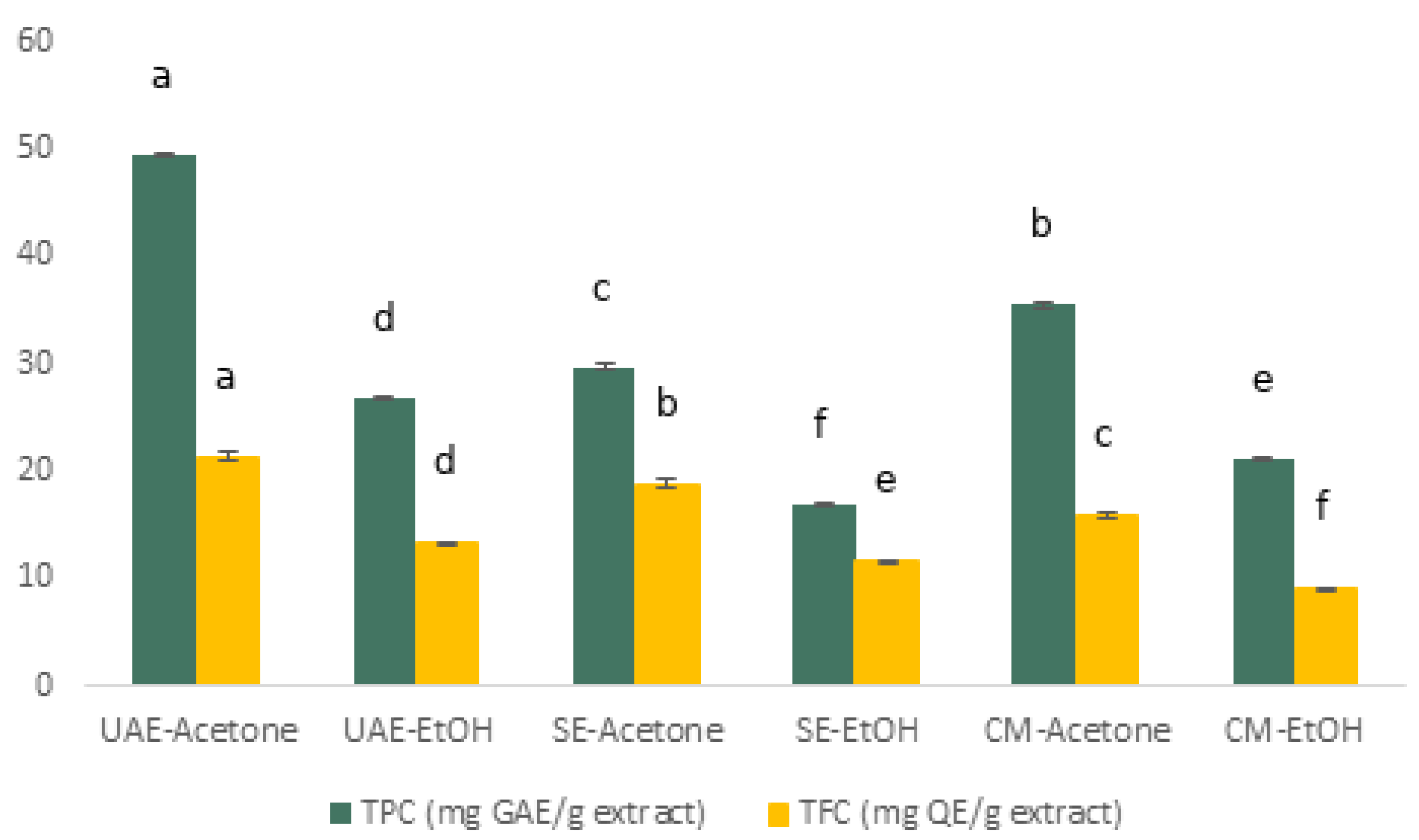
3.2.1. TPC and TFC
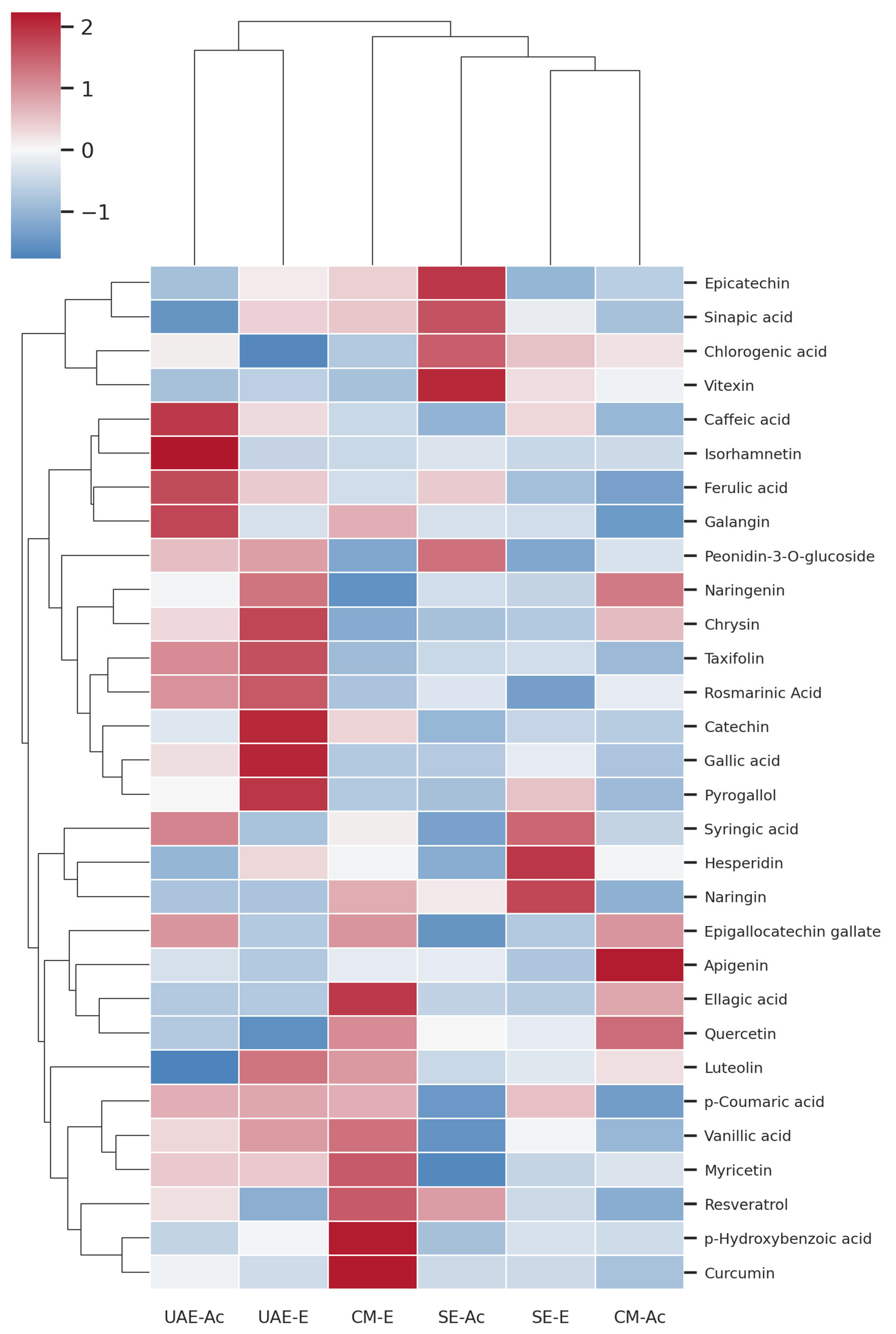
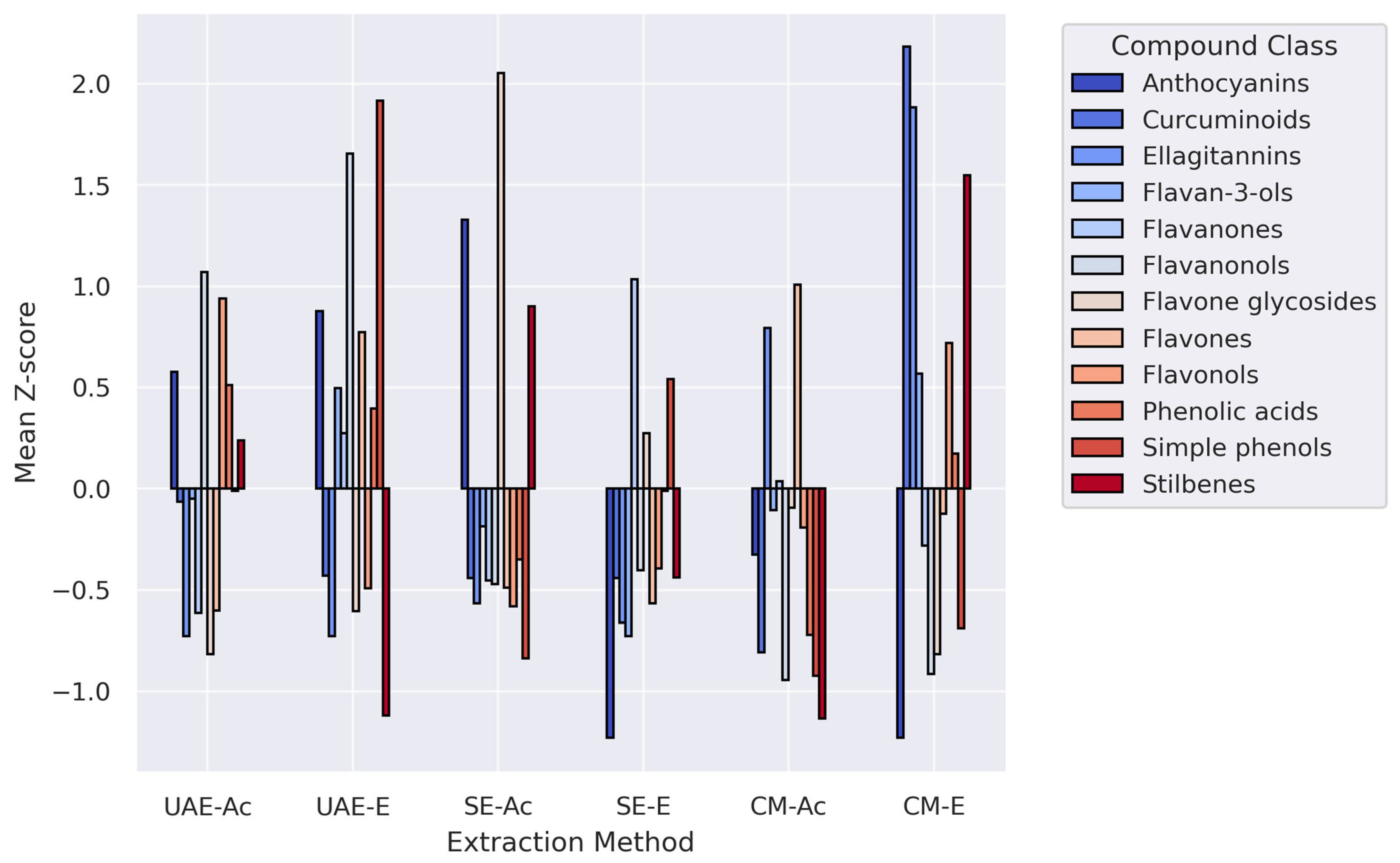
3.2.2. Phenolic Profiles
3.3. Biological Activity
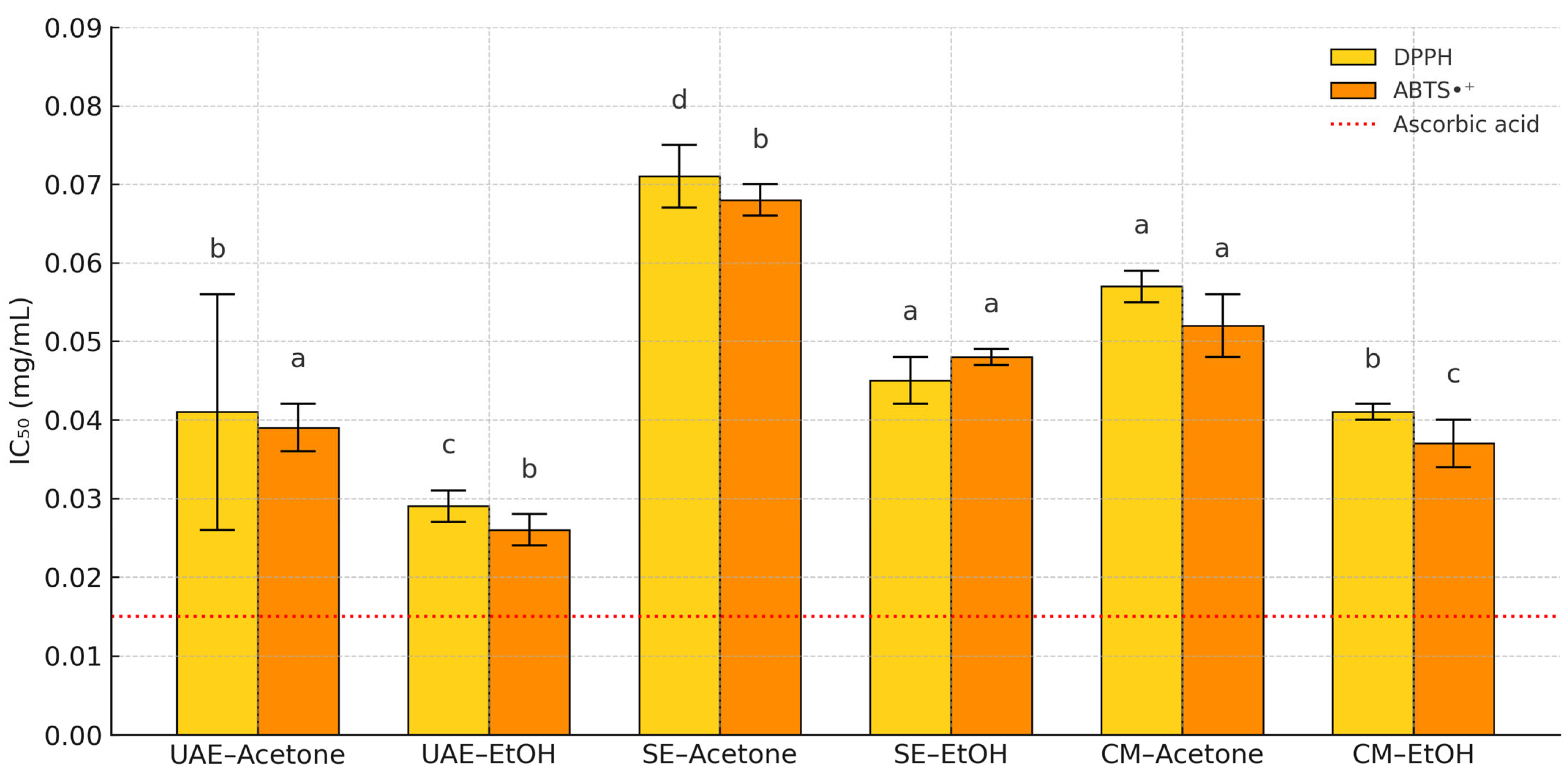
3.3.1. DPPH and ABTS•+ Scavenging Activity
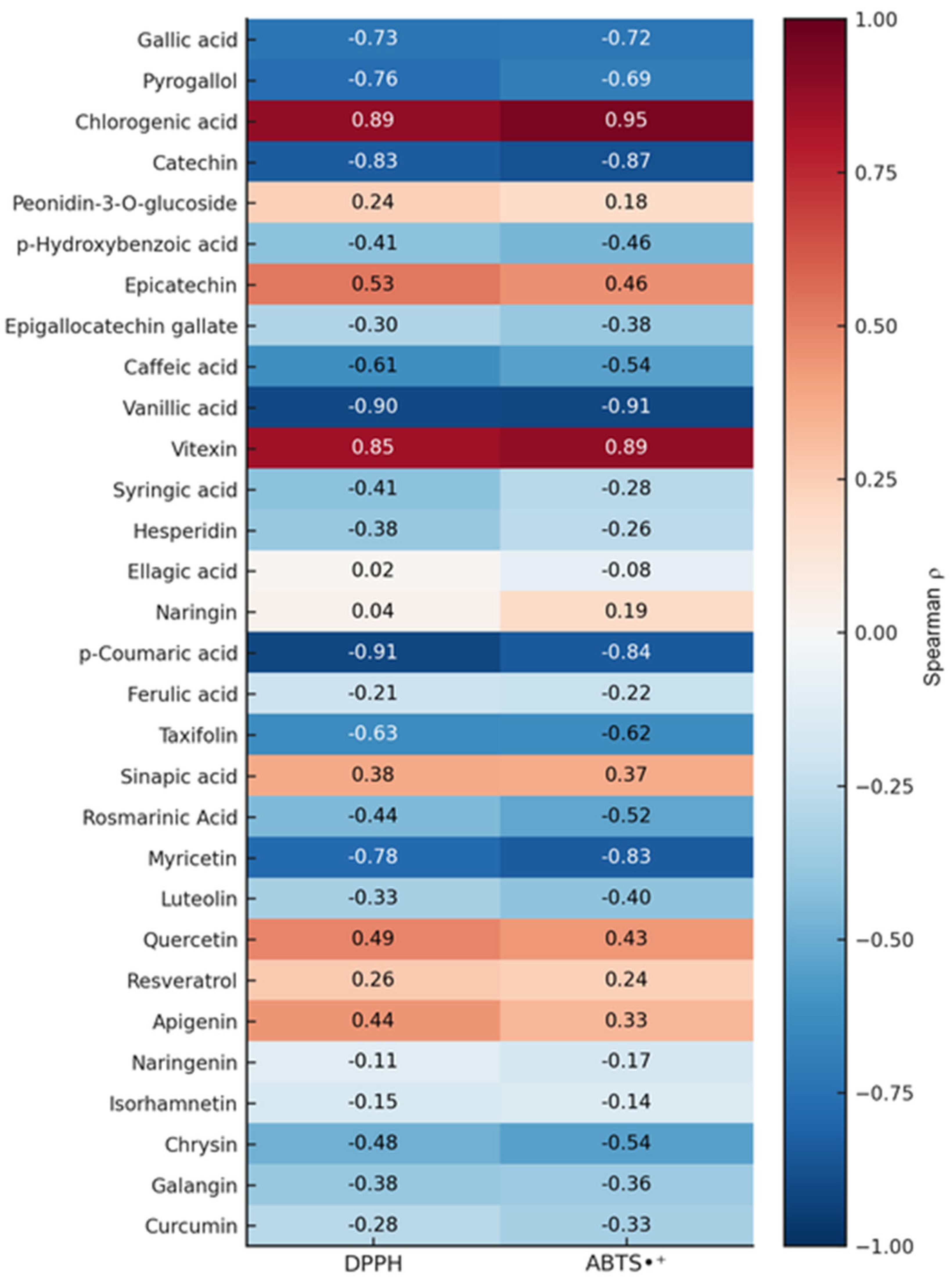
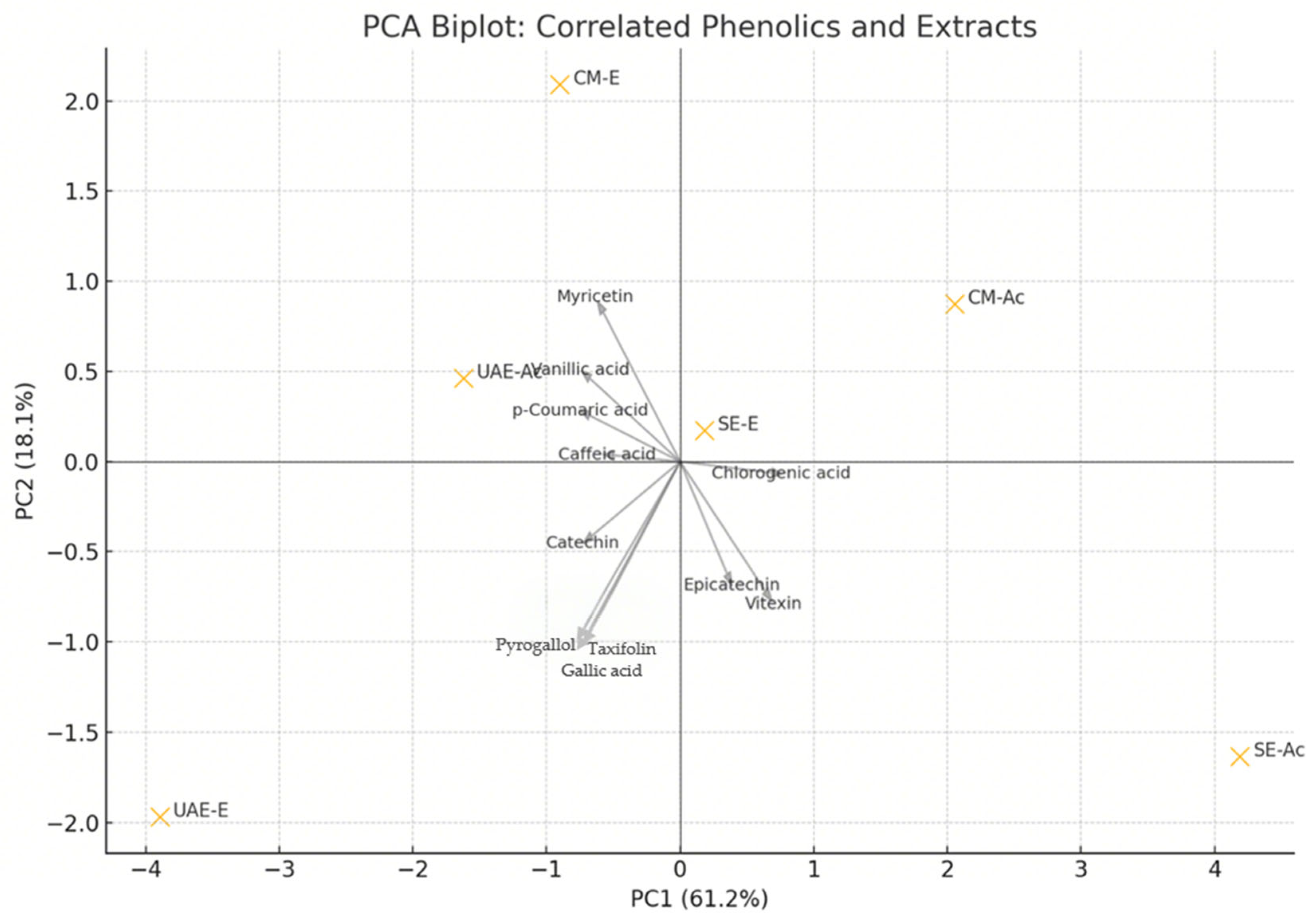
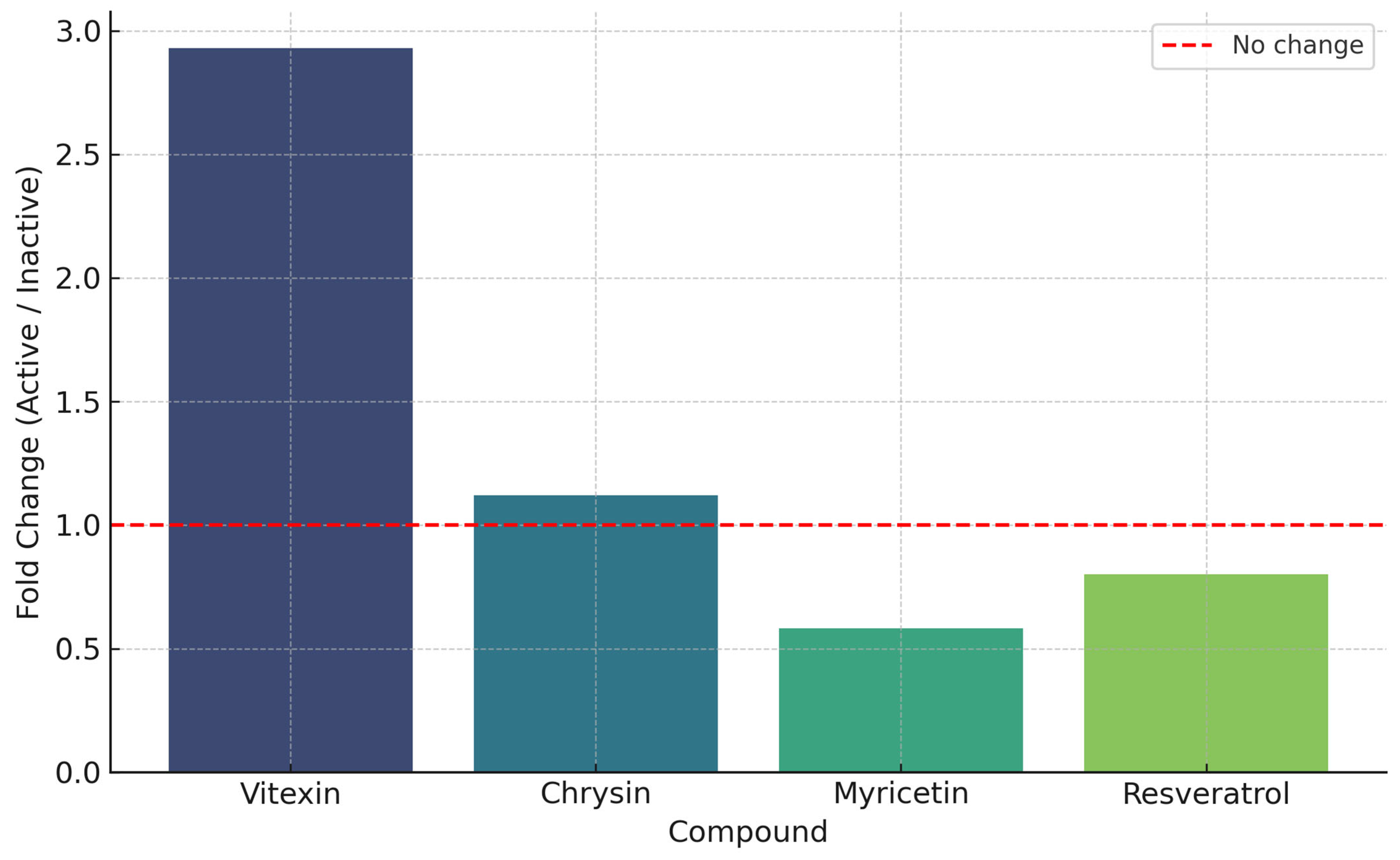
3.3.2. Contribution of Phenolic Compounds to the Radical Scavenging Activity
3.3.3. Antibacterial Activity
3.3.4. Antifungal Activity
3.3.5. Contribution of Phenolic Compounds to the Antifungal Activity
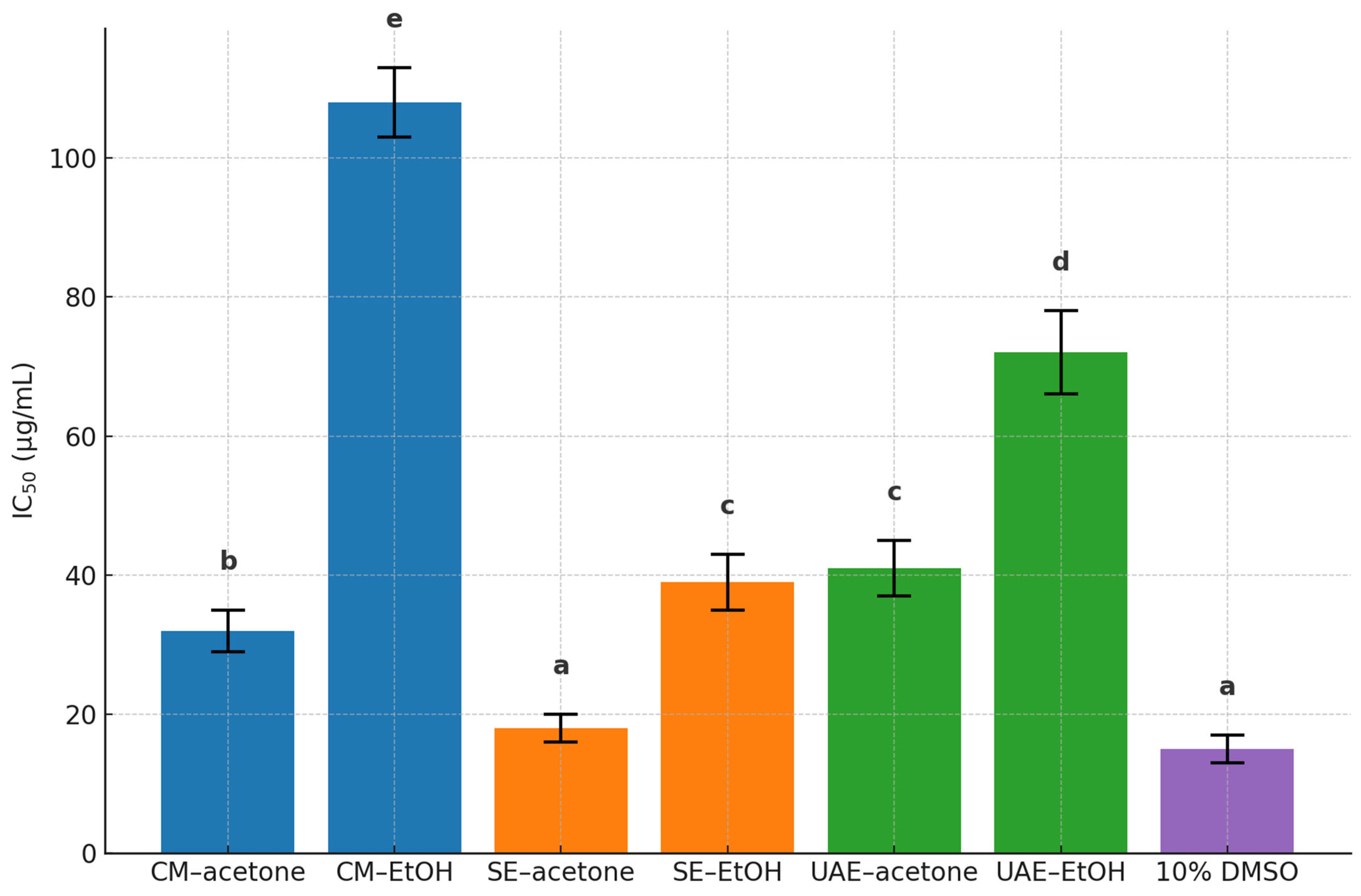
3.3.6. Cytotoxicity Evaluation
4. Discussion
4.1. Influence of Extraction Method and Solvent on Yield and Composition
4.2. Relationship Between Composition and Biological Activity
4.3. Strategic Considerations for Extraction Optimization
- Antioxidant applications (nutraceuticals, cosmetics): UAE–EtOH is the most suitable strategy, yielding high levels of electron-donating phenolics with strong radical-scavenging activity. The method also maintains eco-efficiency and minimizes thermal degradation.
- Antifungal applications: SE–acetone and SE–EtOH provided enhanced efficacy against G. candidum and A. niger, respectively, consistent with the enrichment of moderately polar compounds such as vitexin and chrysin. UAE–EtOH was effective against R. glutinis, offering a greener alternative with comparable results.
- Cytotoxic applications (anticancer research): SE–acetone exhibited the highest potency against MIA PaCa-2 cells, consistent with its enrichment in chlorogenic acid and vitexin. This method appears particularly effective for recovering phenolics that are strongly matrix-associated or less accessible to milder solvents.
4.4. Novelty of This Study in Context of Previous Research
- Defatting-enabled enrichment—facilitated recovery of low-abundance phenolics.
- Polarity–proticity-guided sequential extraction—produced distinct and complementary profiles.
- Compound-specific bioactivity associations—linked activity to gallic acid, vitexin, and others.
- Bioactivity-driven extraction strategies—identified optimal solvent–method combinations.
4.5. Limitations and Perspectives for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UAE | Ultrasound-Assisted Extraction |
| SE | Soxhlet Extraction |
| CM | Cold Maceration |
| TPC | Total Phenolic Content |
| TFC | Total Flavonoid Content |
| UHPLC–ESI–MS/MS | Ultra-High-Performance Liquid Chromatography Coupled with Electrospray Ionization Tandem Mass Spectrometry |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| ABTS•+ | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| PCA | Principal Component Analysis |
References
- Mezni, F.; Roig, A.; Scotti-Saintagne, C.; Hamrouni, L.; Fady, B.; Khaldi, A. Genetic Diversity and Population Structuring of Pistacia lentiscus L. across Mediterranean Region. Ann. For. Res. 2024, 67, 19–30. [Google Scholar] [CrossRef]
- Pachi, V.K.; Mikropoulou, E.V.; Gkiouvetidis, P.; Siafakas, K.; Argyropoulou, A.; Angelis, A.; Mitakou, S.; Halabalaki, M. Traditional Uses, Phytochemistry and Pharmacology of Chios Mastic Gum (Pistacia lentiscus var. Chia, Anacardiaceae): A Review. J. Ethnopharmacol. 2020, 254, 112485. [Google Scholar] [CrossRef]
- Soulaidopoulos, S.; Tsiogka, A.; Chrysohoou, C.; Lazarou, E.; Aznaouridis, K.; Doundoulakis, I.; Tyrovola, D.; Tousoulis, D.; Tsioufis, K.; Vlachopoulos, C.; et al. Overview of Chios Mastic Gum (Pistacia lentiscus) Effects on Human Health. Nutrients 2022, 14, 590. [Google Scholar] [CrossRef] [PubMed]
- Ottria, R.; Xynomilakis, O.; Casati, S.; Abbiati, E.; Maconi, G.; Ciuffreda, P. Chios Mastic Gum: Chemical Profile and Pharmacological Properties in Inflammatory Bowel Disease: From the Past to the Future. Int. J. Mol. Sci. 2023, 24, 12038. [Google Scholar] [CrossRef] [PubMed]
- Floris, S.; Di Petrillo, A.; Pintus, F.; Delogu, G.L. Pistacia lentiscus: Phytochemistry and Antidiabetic Properties. Nutrients 2024, 16, 1638. [Google Scholar] [CrossRef]
- Svingou, D.; Mikropoulou, E.V.; Pachi, V.K.; Smyrnioudis, I.; Halabalaki, M. Chios Mastic Gum: A Validated Method towards Authentication. J. Food Compos. Anal. 2023, 115, 104997. [Google Scholar] [CrossRef]
- Tabanca, N.; Nalbantsoy, A.; Kendra, P.E.; Demirci, F.; Demirci, B. Chemical Characterization and Biological Activity of the Mastic Gum Essential Oils of Pistacia lentiscus var. Chia from Turkey. Molecules 2020, 25, 2136. [Google Scholar] [CrossRef]
- Sarikahya, N.B.; Okkali, G.S.; Coven, F.O.; Isen, F.; Goren, A.C.; Nalbantsoy, A. Chemical Characteristics and Biological Activity Screening of Pistacia lentiscus Mastic Gum and Leaves from Türkiye. J. Sci. Food Agric. 2024, 104, 1691–1701. [Google Scholar] [CrossRef]
- Kilic-Buyukkurt, O.; Guclu, G.; Kelebek, H.; Selli, S. Characterization of the Key Odorants of Mastic Gum (Pistacia lentiscus var. Chia) from Two Different Countries. Appl. Sci. 2025, 15, 5329. [Google Scholar] [CrossRef]
- Bouta, W.; Bouda, S.; El Rasafi, T.; Hansali, M.; Haddioui, A. Morphological Diversity in Wild Populations of Mastic Tree, Pistacia lentiscus L. (Anacardiaceae) in Morocco. Phytomorphology 2021, 71, 17–24. [Google Scholar]
- Beraich, A.; Dikici, B.; El Farissi, H.; Batovska, D.; Nikolova, K.; Belbachir, Y.; Choukoud, A.; Bentouhami, N.E.; Asehraou, A.; Talhaoui, A. The Moroccan Meska Horra: A Natural Candidate for Food and Therapeutic Applications. Foods 2025, 14, 1158. [Google Scholar] [CrossRef]
- Bouakline, H.; Bouknana, S.; Merzouki, M.; Ziani, I.; Challioui, A.; Bnouham, M.; Tahani, A.; El Bachiri, A. The Phenolic Content of Pistacia lentiscus Leaf Extract and Its Antioxidant and Antidiabetic Properties. Sci. World J. 2024, 2024, 1998870. [Google Scholar] [CrossRef]
- Bendada, M.; Hadini, A.; El Asri, O.; Taarabt, Y.; Nazih, A.; Andich, K.; El Bekkaye, K.; Chaabane, K. Evaluation of Phytochemical Content and in Vitro Antioxidant Activities of Pistacia lentiscus L. Leaves Extracts. Scientifica 2024, 2024, 9999175. [Google Scholar] [CrossRef]
- Hayani, M.; Benabbouha, T.; Naceiri Mrabti, N.; Eljebri, S.; Bouskout, M.; Hashem, A.; Zair, T. Phytochemical Profiling, In Silico Molecular Docking for Antioxidant Activity, and Corrosion Inhibition Properties of Pistacia lentiscus L. Extract. Green Chem. Lett. Rev. 2025, 18, 2525113. [Google Scholar] [CrossRef]
- El Allaoui, H.; Haboubi, K.; El Ahmadi, K.; Bouhrim, M.; El Abdouni, A.; Eto, B.; Shahat, A.A.; Herqash, R.N.; El Bestrioui, M.; Zouaoui, Z.; et al. Comprehensive Assessment of Antioxidant, Antidiabetic, and Anti-Glycation Properties of Pistacia lentiscus L. Leaves. Front. Pharmacol. 2025, 16, 1551841. [Google Scholar] [CrossRef] [PubMed]
- Thode, J.M.; Harris, D.P.; Wan, C.; Leonard, B.M. Synthesis of Metastable Ternary Pd-W and Pd-Mo Transition Metal Carbide Nanomaterials. Molecules 2021, 26, 6650. [Google Scholar] [CrossRef] [PubMed]
- Bastos, K.V.L.D.S.; de Souza, A.B.; Tomé, A.C.; Souza, F.D.M. New Strategies for the Extraction of Antioxidants from Fruits and Their By-Products: A Systematic Review. Plants 2025, 14, 755. [Google Scholar] [CrossRef]
- Santos, F.; Soares, C.; Morais, S.L.; Neves, C.; Grosso, C.; Ramalhosa, M.J.; Vieira, M.; Delerue-Matos, C.; Domingues, V.F. Optimized Extraction Protocols for Bioactive Antioxidants from Commercial Seaweeds in Portugal: A Comparative Study of Techniques. Foods 2025, 14, 453. [Google Scholar] [CrossRef]
- Beraich, A.; El Farissi, H.; Cacciola, F.; El-Shazly, M.; Yahyaoui, M.I.; Yousra, B.; Talhaoui, A. Exploring the Healing Power of Pistacia lentiscus Stems: Insights into Extraction Methods, Polyphenolic Composition, and Health-Promoting Activities. Int. J. Environ. Health Res. 2025, 35, 439–452. [Google Scholar] [CrossRef]
- Rahman, H.S. Phytochemical Analysis and Antioxidant and Anticancer Activities of Mastic Gum Resin from Pistacia atlantica Subspecies kurdica. OncoTargets Ther. 2018, 11, 4559–4572. [Google Scholar] [CrossRef]
- Tahmourespour, A.; Aminzadeh, A.; Salehifard, I. Anti-Adherence and Anti-Bacterial Activities of Pistacia atlantica Resin Extract against Strongly Adherent Streptococcus mutans Strains. Dent. Res. J. 2022, 19, 36. [Google Scholar] [CrossRef]
- Usman, I.; Hussain, M.; Imran, A.; Afzaal, M.; Saeed, F.; Javed, M.; Saewan, S.A. Traditional and Innovative Approaches for the Extraction of Bioactive Compounds. Int. J. Food Prop. 2022, 25, 1215–1233. [Google Scholar] [CrossRef]
- Mungwari, C.P.; King’ondu, C.K.; Sigauke, P.; Obadele, B.A. Conventional and Modern Techniques for Bioactive Compounds Recovery from Plants: Review. Sci. Afr. 2025, 27, e02509. [Google Scholar] [CrossRef]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and Characterization of Phenolic Compounds and Their Potential Antioxidant Activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef]
- Edo, G.I.; Nwachukwu, S.C.; Ali, B.M.A.; Yousif, E.; Jikah, A.N.; Zainulabdeen, K.; Ekokotu, H.A.; Isoje, E.F.; Igbuku, U.A.; Opiti, R.A.; et al. A Review on the Composition, Extraction and Applications of Phenolic Compounds. Ecol. Front. 2025, 45, 7–23. [Google Scholar] [CrossRef]
- González-Silva, N.; Nolasco-González, Y.; Aguilar-Hernández, G.; Sáyago-Ayerdi, S.G.; Villagrán, Z.; Acosta, J.L.; Montalvo-González, E.; Anaya-Esparza, L.M. Ultrasound-Assisted Extraction of Phenolic Compounds from Psidium cattleianum Leaves: Optimization Using the Response Surface Methodology. Molecules 2022, 27, 3557. [Google Scholar] [CrossRef] [PubMed]
- Tsiaka, T.; Lantzouraki, D.Z.; Polychronaki, G.; Sotiroudis, G.; Kritsi, E.; Sinanoglou, V.J.; Kalogianni, D.P.; Zoumpoulakis, P. Optimization of Ultrasound- and Microwave-Assisted Extraction for the Determination of Phenolic Compounds in Peach Byproducts. Molecules 2023, 28, 518. [Google Scholar] [CrossRef]
- Alibekov, R.S.; Mustapa Kamal, S.M.; Taip, F.S.; Sulaiman, A.; Azimov, A.M.; Urazbayeva, K.A. Recovery of Phenolic Compounds from Jackfruit Seeds Using Subcritical Water Extraction. Foods 2023, 12, 3296. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of Extraction Solvent on Total Phenol Content, Total Flavonoid Content, and Antioxidant Activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Nortjie, E.; Basitere, M.; Moyo, D.; Nyamukamba, P. Extraction Methods, Quantitative and Qualitative Phytochemical Screening of Medicinal Plants for Antimicrobial Textiles: A Review. Plants 2022, 11, 2011. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin; Springer: Berlin, Germany, 2017; Volume 1601, pp. 1–17. [Google Scholar] [CrossRef]
- Thosar, N.; Basak, S.; Bahadure, R.N.; Rajurkar, M. Antimicrobial Efficacy of Five Essential Oils against Oral Pathogens: An in Vitro Study. Eur. J. Dent. 2013, 7 (Suppl. S1), S071–S077. [Google Scholar] [CrossRef]
- Fadairo, O.S.; Nandasiri, R.; Nguyen, T.; Eskin, N.A.M.; Aluko, R.E.; Scanlon, M.G. Improved Extraction Efficiency and Antioxidant Activity of Defatted Canola Meal Extract Phenolic Compounds Obtained from Air-Fried Seeds. Antioxidants 2022, 11, 2411. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A Comprehensive Review of Ultrasonic Assisted Extraction (UAE) for Bioactive Components: Principles, Advantages, Equipment, and Combined Technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef] [PubMed]
- Goswami, M.J.; Dutta, U.; Kakati, D. Ultrasound-Assisted Extraction for Food, Pharmacy, and Biotech Industries. In Ultrasound: Advances in Research and Applications; Springer Nature: Cham, Switzerland, 2024; pp. 103–128. [Google Scholar] [CrossRef]
- Sethi, S.; Rathod, V.K. Recent Advances in Ultrasound-Assisted Extraction of Natural Products Using Novel Solvents: A Mini-Review. Curr. Opin. Chem. Eng. 2025, 48, 101132. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Boateng, I.D. Recent Advances in Combined Avant-Garde Technologies (Thermal–Thermal, Non-Thermal–Non-Thermal, and Thermal–Non-Thermal Matrix) to Extract Polyphenols from Agro-Byproducts. J. Food Drug Anal. 2023, 31, 552–582. [Google Scholar] [CrossRef]
- Irakli, M.; Skendi, A.; Bouloumpasi, E.; Christaki, S.; Biliaderis, C.G.; Chatzopoulou, P. Sustainable Recovery of Phenolic Compounds from Distilled Rosemary By-Product Using Green Extraction Methods. Molecules 2023, 28, 6669. [Google Scholar] [CrossRef]
- Cannas, M.; Conte, P.; Piga, A.; Del Caro, A. Green Recovery Optimization of Phenolic Compounds from “Spinoso Sardo” Globe Artichoke By-Products Using Response Surface Methodology. Front. Sustain. Food Syst. 2023, 7, 1215809. [Google Scholar] [CrossRef]
- El Mannoubi, I. Impact of Different Solvents on Extraction Yield, Phenolic Composition, In Vitro Antioxidant and Antibacterial Activities of Deseeded Opuntia stricta Fruit. J. Umm Al Qura Univ. Appl. Sci. 2023, 9, 176–184. [Google Scholar] [CrossRef]
- Herrera-Pool, E.; Ramos-Díaz, A.L.; Lizardi-Jiménez, M.A.; Pech-Cohuo, S.; Ayora-Talavera, T.; Cuevas-Bernardino, J.C.; García-Cruz, U.; Pacheco, N. Effect of Solvent Polarity on the Ultrasound-Assisted Extraction and Antioxidant Activity of Phenolic Compounds from Habanero Pepper Leaves (Capsicum chinense). Ultrason. Sonochem. 2021, 76, 105658. [Google Scholar] [CrossRef]
- Xiang, Z.; Liu, L.; Xu, Z.; Kong, Q.; Feng, S.; Chen, T.; Zhou, L.; Yang, H.; Xiao, Y.; Ding, C. Solvent Effects on the Phenolic Compounds and Antioxidant Activity Associated with Camellia polyodonta Flower Extracts. ACS Omega 2024, 9, 27192–27203. [Google Scholar] [CrossRef]
- Ghaffar, N.; Perveen, A. Solvent Polarity Effects on Extraction Yield, Phenolic Content, and Antioxidant Properties of Malvaceae Family Seeds: A Comparative Study. N. Zealand J. Bot. 2024, 63, 627–637. [Google Scholar] [CrossRef]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the Extraction Method on the Recovery of Bioactive Phenolic Compounds from Food Industry By-Products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef]
- Lee, J.E.; Jayakody, J.T.M.; Kim, J.I.; Jeong, J.W.; Choi, K.M.; Kim, T.S.; Seo, C.; Azimi, I.; Hyun, J.M.; Ryu, B.M. The Influence of Solvent Choice on the Extraction of Bioactive Compounds from Asteraceae: A Comparative Review. Foods 2024, 13, 3151. [Google Scholar] [CrossRef]
- Tourabi, M.; Faiz, K.; Ezzouggari, R.; Louasté, B.; Merzouki, M.; Dauelbait, M.; Bourhia, M.; Almaary, K.S.; Siddique, F.; Lyoussi, B.; et al. Optimization of Extraction Process and Solvent Polarities to Enhance the Recovery of Phytochemical Compounds, Nutritional Content, and Biofunctional Properties of Mentha longifolia L. Extracts. Bioresour. Bioprocess. 2025, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Alavi Rafiee, S.; Farhoosh, R.; Sharif, A. Antioxidant Activity of Gallic Acid as Affected by an Extra Carboxyl Group than Pyrogallol in Various Oxidative Environments. Eur. J. Lipid Sci. Technol. 2018, 120, 1800319. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant Properties of Catechins: Comparison with Other Antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gülçin, İ.; Alwasel, S.H. Antioxidant Activity of Taxifolin: An Activity–Structure Relationship. J. Enzyme Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef]
- Marinho, L.F.; Sganzerla, W.G.; Ferreira, V.C.; Jimenez Moreno, J.A.; Rostagno, M.A.; Forster-Carneiro, T. Advances in Green Extraction Methods, Biological Properties, and Applications of Betanin and Vitexin: An Updated Review and Bibliometric Analysis. Biocatal. Agric. Biotechnol. 2023, 51, 102744. [Google Scholar] [CrossRef]
- Adesina, A.F.; Adewuyi, A.; Otuechere, C.A. Exploratory Studies on Chrysin via Antioxidant, Antimicrobial, ADMET, PASS and Molecular Docking Evaluations. Pharmacol. Res. Mod. Chin. Med. 2024, 11, 100413. [Google Scholar] [CrossRef]
- Al Aboody, M.S.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Milia, E.P.; Sardellitti, L.; Eick, S. Antimicrobial Efficiency of Pistacia lentiscus L. Derivates against Oral Biofilm-Associated Diseases—A Narrative Review. Microorganisms 2023, 11, 1378. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, L.L.; Xue, N.N.; Li, C.; Guo, H.H.; Ren, T.K.; Zhan, Y.; Li, W.B.; Zhang, J.; Chen, X.G.; et al. Chlorogenic Acid Effectively Treats Cancers through Induction of Cancer Cell Differentiation. Theranostics 2019, 9, 6745–6763. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Molecular Targets of Vitexin and Isovitexin in Cancer Therapy: A Critical Review. Ann. N. Y. Acad. Sci. 2017, 1401, 102–113. [Google Scholar] [CrossRef]
- Johnson, J.B.; Kazak, A.; Gallini, N.; Rudenko, M.; Naiker, M. Prediction of Antioxidant Capacity in Faba Bean from Individual Phenolic Constituents. Chem. Pap. 2024, 78, 4285–4294. [Google Scholar] [CrossRef]
- Bernal-Gallardo, J.O.; Mena-Violante, H.G.; Luna-Suárez, S. Study of the Phenolic Compounds and Biological Activities of the Wild Fruits of Vaccinium leucanthum Schltdl. Horticulturae 2024, 10, 1091. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications—A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunne, P.J. CHEM21 Selection Guide of Classical- and Less Classical-Solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- López-Bascón, M.A.; Luque de Castro, M.D. Soxhlet Extraction. In Liquid-Phase Extraction; Poole, C.F., Ed.; Handbooks in Separation Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 327–354. [Google Scholar] [CrossRef]
- Tamminen, J.; Holappa, J.; Vladimirovich Gradov, D.; Koiranen, T. Scaling Up Continuous Ultrasound-Assisted Extractor for Plant Extracts by Using Spinach Leaves as a Test Material. Ultrason. Sonochem. 2022, 90, 106171. [Google Scholar] [CrossRef]
- Gaudêncio, S.P.; Bayram, E.; Lukić Bilela, L.; Cueto, M.; Díaz-Marrero, A.R.; Haznedaroğlu, B.Z.; Jimenez, C.; Mandalakis, M.; Pereira, F.; Reyes, F.; et al. Advanced Methods for Natural Products Discovery: Bioactivity Screening, Dereplication, Metabolomics Profiling, Genomic Sequencing, Databases and Informatic Tools, and Structure Elucidation. Mar. Drugs 2023, 21, 308. [Google Scholar] [CrossRef] [PubMed]
- Sabotič, J.; Bayram, E.; Ezra, D.; Gaudêncio, S.P.; Haznedaroğlu, B.Z.; Janež, N.; Ktari, L.; Luganini, A.; Mandalakis, M.; Safarik, I.; et al. A Guide to the Use of Bioassays in Exploration of Natural Resources. Biotechnol. Adv. 2024, 71, 108307. [Google Scholar] [CrossRef] [PubMed]
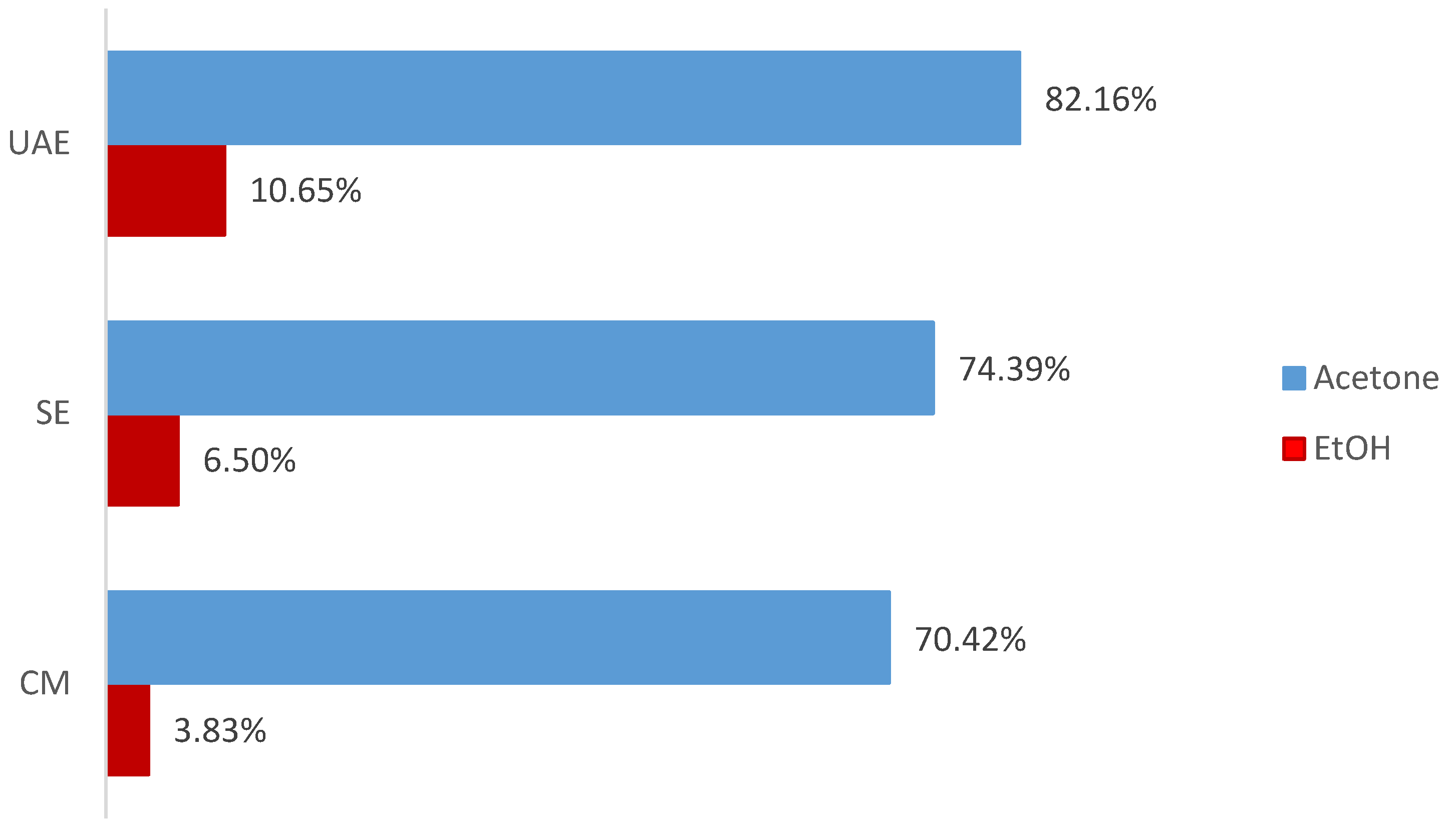
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beraich, A.; Batovska, D.; Nikolova, K.; Dikici, B.; Gören, G.; Belbachir, Y.; Taibi, M.; Elbouzidi, A.; Mincheva, I.; Panova, N.; et al. Extraction Methods Shape the Phenolic Composition and Bioactivities of Defatted Moroccan Pistacia lentiscus L. Resin. Antioxidants 2025, 14, 1207. https://doi.org/10.3390/antiox14101207
Beraich A, Batovska D, Nikolova K, Dikici B, Gören G, Belbachir Y, Taibi M, Elbouzidi A, Mincheva I, Panova N, et al. Extraction Methods Shape the Phenolic Composition and Bioactivities of Defatted Moroccan Pistacia lentiscus L. Resin. Antioxidants. 2025; 14(10):1207. https://doi.org/10.3390/antiox14101207
Chicago/Turabian StyleBeraich, Abdessamad, Daniela Batovska, Krastena Nikolova, Burak Dikici, Göksen Gören, Yousra Belbachir, Mohamed Taibi, Amine Elbouzidi, Irena Mincheva, Natalina Panova, and et al. 2025. "Extraction Methods Shape the Phenolic Composition and Bioactivities of Defatted Moroccan Pistacia lentiscus L. Resin" Antioxidants 14, no. 10: 1207. https://doi.org/10.3390/antiox14101207
APA StyleBeraich, A., Batovska, D., Nikolova, K., Dikici, B., Gören, G., Belbachir, Y., Taibi, M., Elbouzidi, A., Mincheva, I., Panova, N., Tahani, A., Asehraou, A., & Talhaoui, A. (2025). Extraction Methods Shape the Phenolic Composition and Bioactivities of Defatted Moroccan Pistacia lentiscus L. Resin. Antioxidants, 14(10), 1207. https://doi.org/10.3390/antiox14101207











