Exploring the Potential of Low-Temperature Vacuum Drying to Improve the Bioactive Compound Content and Health-Promoting Properties of Chilean Wild Murta
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation for Vacuum-Based Drying Processes
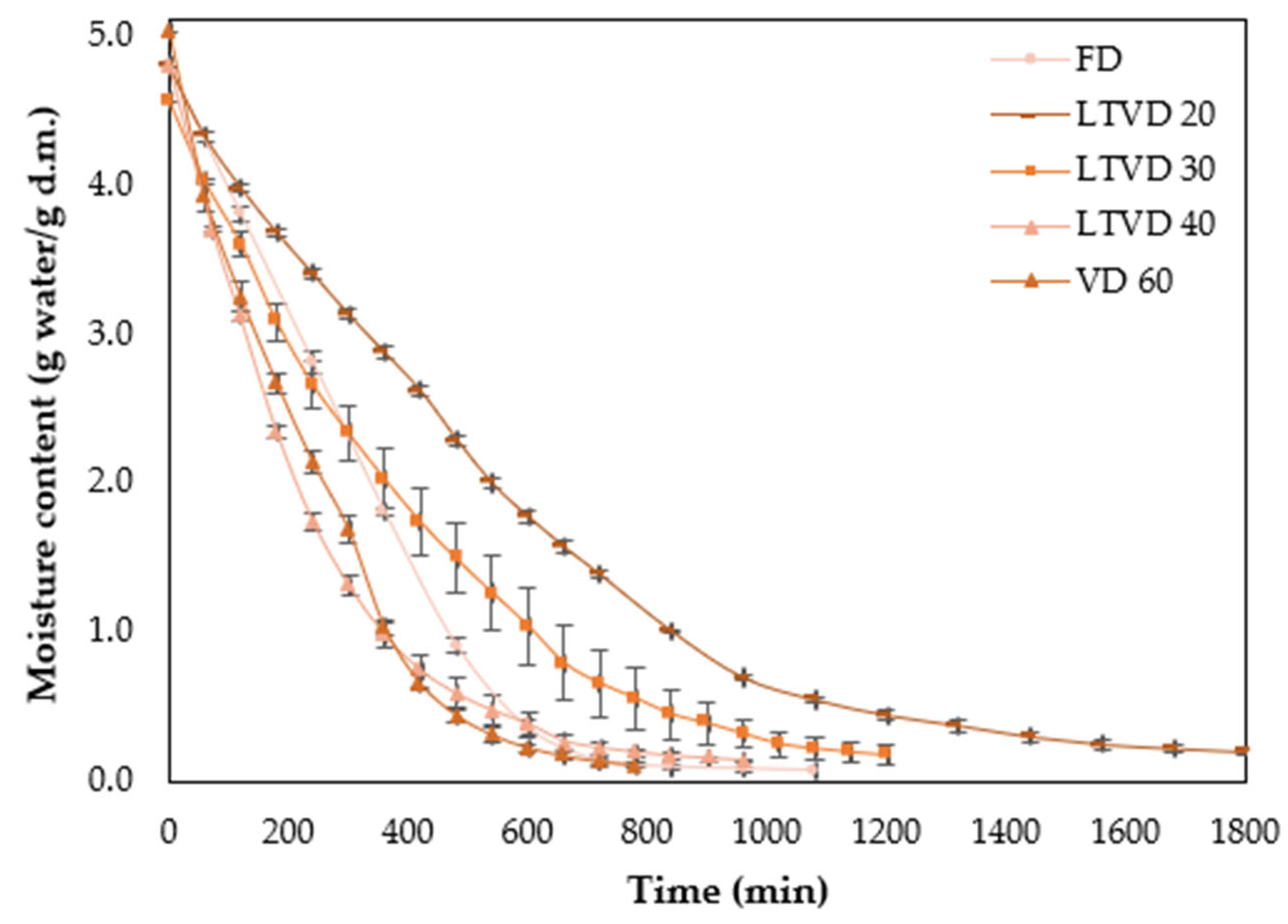
2.2. Vacuum-Based Drying Processes
2.3. Fatty Acids
2.4. Tocols
2.5. Extraction Procedure
2.6. Identification and Quantification of Individual Phenolic Compounds
2.7. Antioxidant and Anti-Inflammatory Assays
2.8. α-Glucosidase Inhibitory Activity Assay
2.9. Cytotoxicity Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Changes of Fatty Acids of Dried Murta Berry
3.2. Changes of Tocol Compounds of Dried Murta Berry
3.3. Changes in Bioactive Compounds of Dried Murta Berry
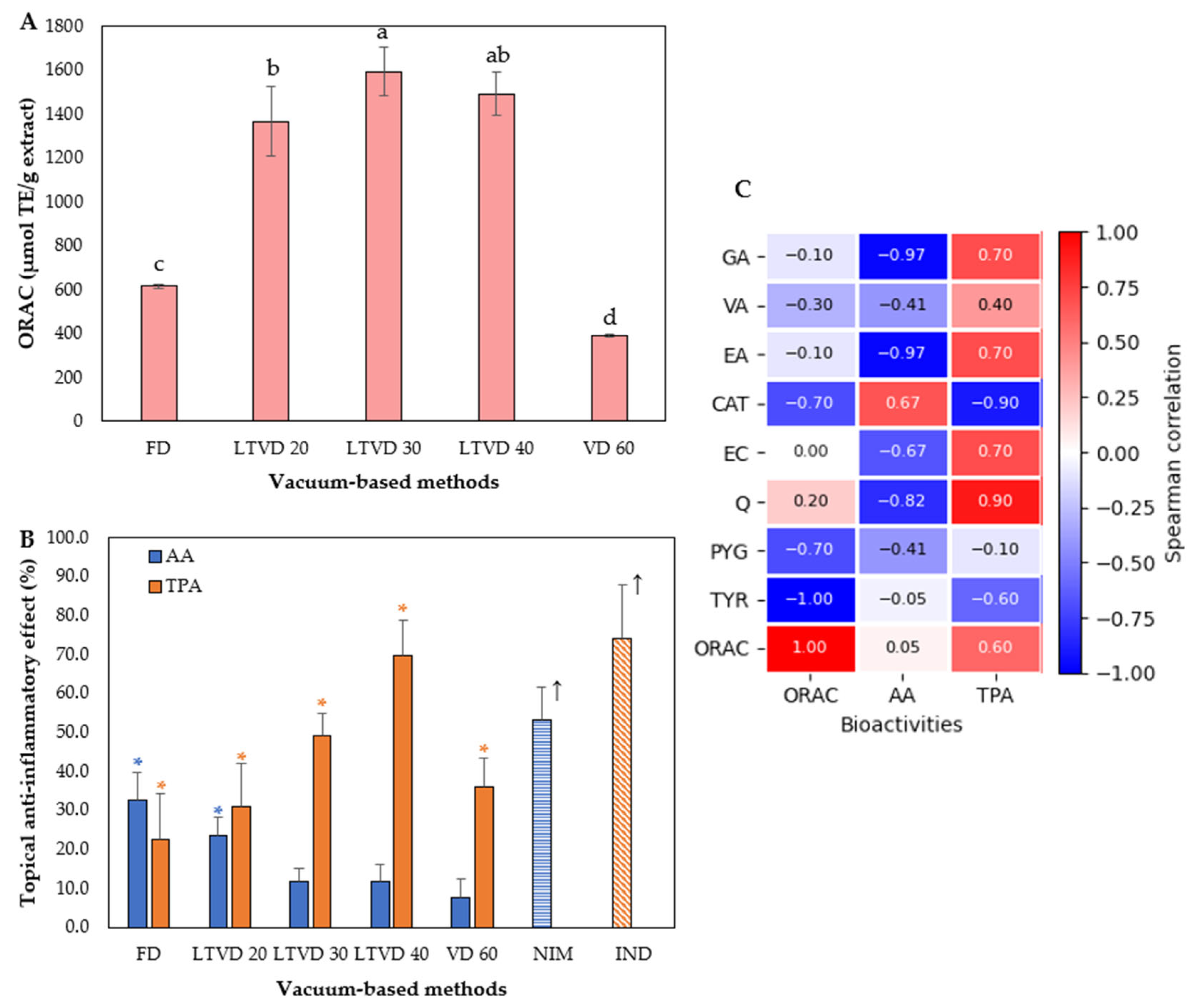
3.4. Antioxidant and Anti-Inflammatory Potential of Dried Murta
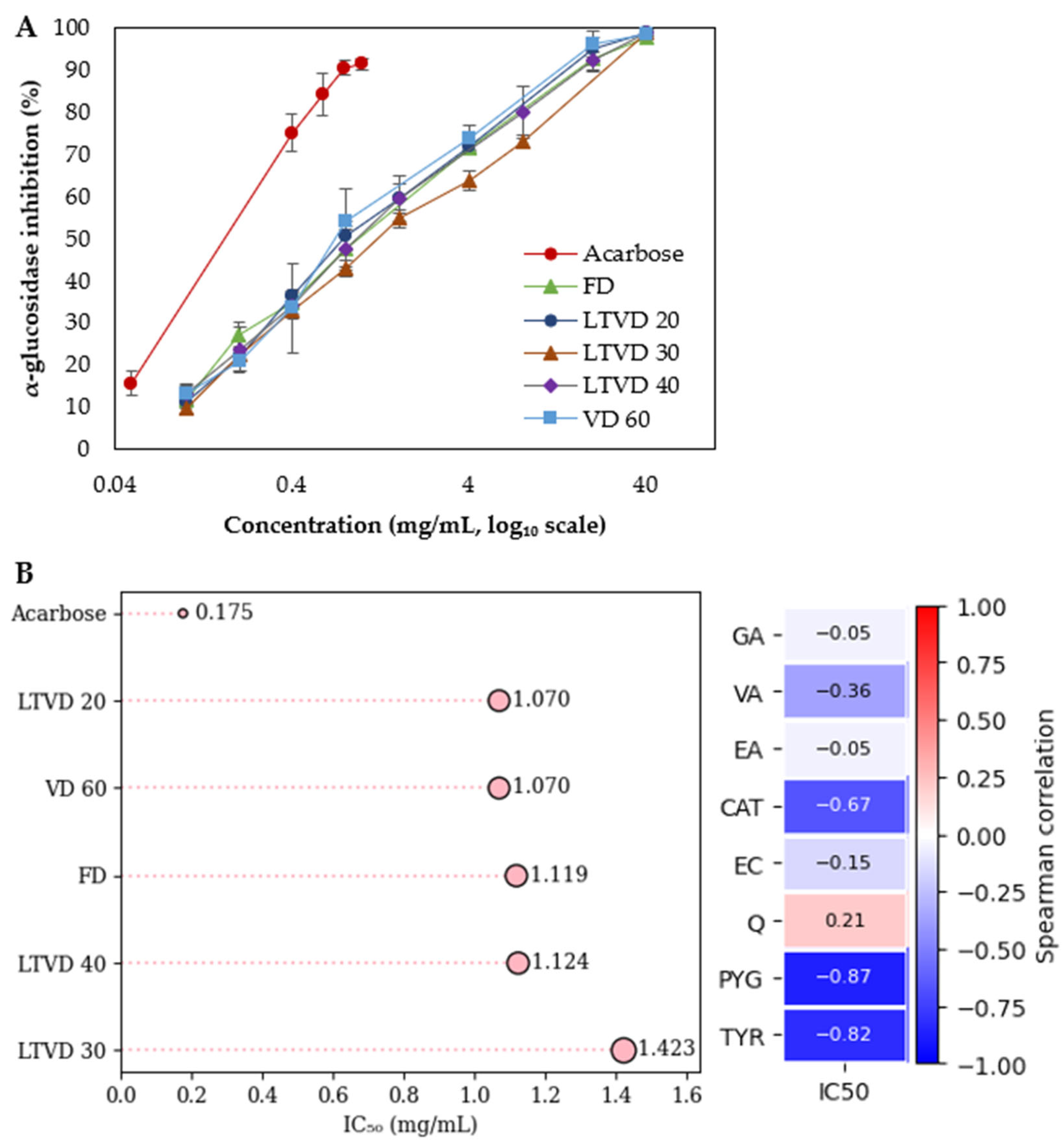
3.5. α-Glucosidase Inhibitory Activity of Dried Murta
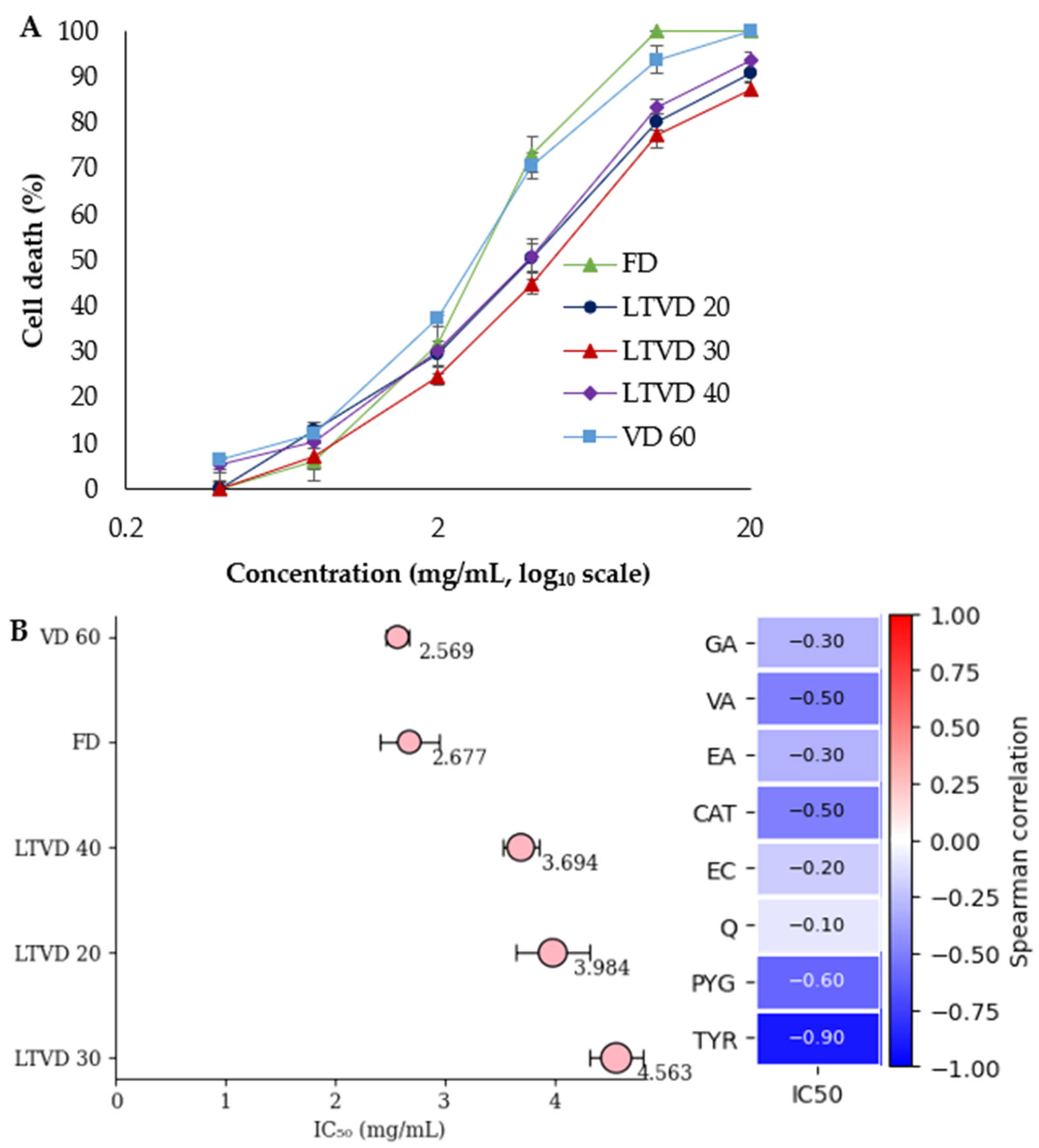
3.6. Cytotoxic Activity of Dried Murta Against Gastric Adenocarcinoma (AGS) Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuentes, L.; Figueroa, C.R.; Valdenegro, M.; Vinet, R. Patagonian berries: Healthy potential and the path to becoming functional foods. Foods 2019, 8, 289. [Google Scholar] [CrossRef] [PubMed]
- Fredes, C.; Parada, A.; Salinas, J.; Robert, P. Phytochemicals and traditional use of two southernmost Chilean berry fruits: Murta (Ugni molinae Turcz) and Calafate (Berberis buxifolia Lam.). Foods 2020, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Otero, C.; Klagges, C.; Morales, B.; Sotomayor, P.; Escobar, J.; Fuentes, J.A.; Moreno, A.A.; Llancalahuen, F.M.; Arratia-Perez, R.; Gordillo-Fuenzalida, F.; et al. Anti-inflammatory Chilean endemic plants. Pharmaceutics 2023, 15, 897. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Posada, A.C.; Porras, O.; Rincón-Cervera, M.A.; Frías, J.; Zielinski, A.A.F.; Bridi, R.; Arias-Santé, M.F.; Camargo, A.C. Antioxidant properties of phenolic extracts of murtilla pomace: First report on the importance of soluble and insoluble-bound compounds. Food Res. Int. 2024, 196, 115114. [Google Scholar] [CrossRef]
- Gomes-Lobo, C.J.; Luna, R.; Rodríguez-Machado, A.; Pérez-Correa, J.R.; Franco, W. Multiobjective optimization of Murta (Ugni molinae Turcz) juice lactic acid fermentation with monocultures and cocultures of Lactobacillus acidophilus and Lactiplantibacillus plantarum. Appl. Food Res. 2025, 5, 100996. [Google Scholar] [CrossRef]
- Fuentes-Jorquera, N.; Villalva, M.; Pérez-Jiménez, J.; González-Miquel, M.; González, E.J.; Mariotti-Celis, M.S.; Pérez-Correa, J.R.; Canales, R.I. A sustainable approach to obtain polyphenols from Chilean wild murta, Ugni candollei B., and Ugni molinae T., using eutectic solvents and advanced extraction techniques. Innov. Food Sci. Emerg. Technol. 2025, 102, 104017. [Google Scholar] [CrossRef]
- López, J.; Vega-Gálvez, A.; Ah-Hen, K.S.; Rodríguez, A.; Quispe-Fuentes, I.; Delporte, C.; Valenzuela-Barra, G.; Arancibia, Y.; Zambrano, A. Evaluation of the antioxidant, anti-inflammatory, and anti-tumoral properties of bioactive compounds extracted from murta berries (Ugni molinae T.) dried by different methods. Front. Plant Sci. 2023, 14, 1095179. [Google Scholar] [CrossRef]
- Ordóñez, J.L.; Pérez, R.; Barriga, A.; Seguel, I.; Guzman, P.; Zúñiga, M.C.; Delporte, C. Comparative study of antioxidant and inhibitory activity on α-glucosidase and glycogen phosphorylase A of berry extracts from Ugni molinae genotypes. J. Berry Res. 2022, 12, 279–296. [Google Scholar] [CrossRef]
- Cabrera-Barjas, G.; Quezada, A.; Bernardo, Y.; Moncada, M.; Zúñiga, E.; Wilkens, M.; Giordano, A.; Nesic, A.; Delgado, N. Chemical composition and antibacterial activity of red murta (Ugni molinae Turcz.) seeds: An undervalued Chilean resource. J. Food Meas. Charact. 2020, 14, 1810–1821. [Google Scholar] [CrossRef]
- Avello-Lorca, M.; Pastene-Navarrete, E.; Barriga, A.; Bittner-Berner, M.; Ruiz-Ponce, E.; Becerra-Allende, J. Chemical properties and assessment of the antioxidant capacity of native species from the genus Ugni. Rev. Cubana Plantas Med. 2016, 21, 284–297. [Google Scholar]
- Jofré, I.; Pezoa, C.; Cuevas, M.; Scheuermann, E.; Freires, I.A.; Rosalen, P.L.; de Alencar, S.M.; Romero, F. Antioxidant and vasodilator activity of Ugni molinae Turcz. (Murtilla) and its modulatory mechanism in hypotensive response. Oxid. Med. Cell. Longev. 2016, 2016, 6513416. [Google Scholar] [CrossRef]
- Junqueira-Gonçalves, M.P.; Yáñez, L.; Morales, C.; Navarro, M.; Contreras, R.A.; Zúñiga, G.E. Isolation and characterization of phenolic compounds and anthocyanins from Murta (Ugni molinae Turcz.) fruits: Assessment of antioxidant and antibacterial activity. Molecules 2015, 20, 5698–5713. [Google Scholar] [CrossRef]
- Rubilar, M.; Jara, C.; Poo, Y.; Acevedo, F.; Gutierrez, C.; Sineiro, J.; Shene, C. Extracts of maqui (Aristotelia chilensis) and murta (Ugni molinae Turcz.): Sources of antioxidant compounds and α-glucosidase/α-amylase inhibitors. J. Agric. Food Chem. 2011, 59, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, M.C.; Delporte, C.; Backhouse, N.; Erazo, S.; Letelier, M.E.; Cassels, B.K.; Silva, X.; Alegría, S.; Negrete, R. Topical anti-inflammatory activity of 2α-hydroxy pentacyclic triterpene acids from the leaves of Ugni molinae. Bioorg. Med. Chem. 2006, 14, 5673–5677. [Google Scholar] [CrossRef]
- Goity, L.E.; Queupil, M.-J.; Jara, D.; Alegría, S.E.; Peña, M.; Barriga, A.; Aguirre, M.C.; Delporte, C. An HPLC-UV and HPLC-ESI-MS based method for identification of anti-inflammatory triterpenoids from the extracts of Ugni molinae. Bol. Latinoam. Caribe Plantas Med. Aromat. 2013, 12, 108–116. [Google Scholar]
- Arancibia-Radich, J.; Peña-Cerda, M.; Jara, D.; Valenzuela-Bustamante, P.; Goity, L.; Valenzuela-Barra, G.; Silva, X.; Garrido, G.; Delporte, C.; Seguel, I. Comparative study of anti-inflammatory activity and qualitative-quantitative composition of triterpenoids from ten genotypes of Ugni molinae. Bol. Latinoam. Caribe Plant. Med. Aromat. 2016, 15, 274–287. [Google Scholar]
- Schmeda-Hirschmann, G.; Jiménez-Aspee, F.; Theoduloz, C.; Ladio, A. Patagonian berries as native food and medicine. J. Ethnopharmacol. 2019, 241, 111979. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, Y.; Huang, L.; Zhang, C.; Xie, P. Low Temperature Vacuum Drying of natural gardenia yellow pigment. Dry. Technol. 2011, 29, 1132–1139. [Google Scholar] [CrossRef]
- Do, L.T.K.; Vu, L.T.K.; Phan, D.T.A.; Dzung, N.T. Mathematical modeling and optimization of low-temperature vacuum drying for banana. Carpathian J. Food Sci. Technol. 2021, 13, 47–61. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, K.; Wang, Y.; Dai, B.; Liu, S.; Li, Y. Moisture diffusion and shrinkage characteristics of broad bean during low-temperature vacuum drying. Int. J. Food Prop. 2020, 23, 2217–2230. [Google Scholar] [CrossRef]
- Caro-Corrales, J.J.; Zazueta-Niebla, J.A.; Ordorica-Falomir, C.A.; Zazueta-Morales, J.J.; Gutiérrez-López, G.F.; Alamilla-Beltrán, L. Controlled low-temperature vacuum dehydration and tunnel drying: A comparative study. Int. J. Food Prop. 2005, 8, 529–542. [Google Scholar] [CrossRef]
- López, J.; Shun Ah-Hen, K.; Vega-Gálvez, A.; Morales, A.; García-Segovia, P.; Uribe, E. Effects of drying methods on quality attributes of murta (Ugni molinae Turcz) berries: Bioactivity, nutritional aspects, texture profile, microstructure and functional properties. J. Food Process Eng. 2017, 40, e12511. [Google Scholar] [CrossRef]
- López, J.; Vega-Gálvez, A.; Rodríguez, A.; Stucken, K.; Barraza, C.; Aguilera, L.E. Relationship between antimicrobial activity, phenolic profile and antioxidant capacity of murta (Ugni molinae Turcz) extracts prepared by different drying methods. J. Berry Res. 2019, 9, 587–601. [Google Scholar] [CrossRef]
- AOCS. Official Method Ce 2-66. Preparation of Methyl Esters of Fatty Acids. In Official Methods and Recommended Practices of the AOCS, 4th ed.; AOCS Press: Champaign, IL, USA, 1993. [Google Scholar]
- AOCS. Official Method Ce 8-89. Determination of Tocopherols and Tocotrienols in Vegetable Oils and Fats by HPLC. In Official Methods and Recommended Practices of the AOCS, 4th ed.; AOCS Press: Champaign, IL, USA, 1993. [Google Scholar]
- Bravo, S.; Inostroza, K.; Lorenzo, J.M.; Sepúlveda, G.; Domínguez, R.; Scheuermann, E.; Paz, E.A.; Quiñones, J.; Santos, E.M.; Andrés, S.C.; et al. Influence of Murta (Ugni molinae Turcz) powder on the frankfurters quality. Appl. Sci. 2021, 11, 8610. [Google Scholar] [CrossRef]
- Lenaerts, S.; Van der Borght, M.; Callens, A.; Van Campenhout, L. Suitability of microwave drying for mealworms (Tenebrio molitor) as alternative to freeze drying: Impact on nutritional quality and colour. Food Chem. 2018, 254, 129–136. [Google Scholar] [CrossRef]
- Lei, D.; Liu, Y.; Xie, Y.; Sun, W.; Guo, J.; Xu, C. Combined radio frequency heating and pulsed vacuum technology to enhance drying characteristics and heat-mass transfer in peanut pod drying. Innov. Food Sci. Emerg. Technol. 2025, 102, 103976. [Google Scholar] [CrossRef]
- Zheng, Y.; Oellig, C.; Zhu, L.; Bauer, V.; Vetter, W.; Zhang, Y. Revealing the key aroma codes and (furan) fatty acids in fresh red goji berries and the impacts of the hot-air drying process. Food Chem. 2025, 484, 144336. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.A.M.; Al Juhaimi, F.Y.; Osman, M.A.; Al Maiman, S.A.; Hassan, A.B.; Alqah, H.A.S.; Babiker, E.E.; Ghafoor, K. Effect of oven roasting treatment on the antioxidant activity, phenolic compounds, fatty acids, minerals, and protein profile of Samh (Mesembryanthemum forsskalei Hochst) seeds. LWT Food Sci. Technol. 2020, 131, 109825. [Google Scholar] [CrossRef]
- Nisha; Sharma, N.; Mohite, A.M. Retaining bioactive components in fish mint leaves (Houttuynia cordata): Effects of recirculating, vacuum, and through-flow drying methods. Food Humanit. 2025, 5, 100656. [Google Scholar] [CrossRef]
- Caballero-Gutiérrez, B.L.; Márquez-Cardozo, C.J.; Ciro-Velásquez, H.J.; Cortés-Rodríguez, M. Pumpkin powder with co-products seeds and peel: Effect on the physicochemical and functional properties by spray drying. Appl. Food Res. 2025, 5, 100879. [Google Scholar] [CrossRef]
- Santana, I.; Castelo-Branco, V.N.; Guimarães, B.M.; Silva, L.O.; Peixoto, V.O.D.S.; Cabrale, L.M.C.; Freitas, S.P.; Torres, A.G. Hass avocado (Persea americana Mill.) oil enriched in phenolic compounds and tocopherols by expeller-pressing the unpeeled microwave dried fruit. Food Chem. 2019, 286, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Jorquera, N.; Canales, R.I.; Pérez-Correa, J.R.; Pérez-Jiménez, J.; Mariotti-Celis, M.S. Differential extraction and preliminary identification of polyphenols from Ugni candollei (white murta) berries. Antioxidants 2024, 13, 623. [Google Scholar] [CrossRef]
- D’Almeida, C.T.S.; Sales, A.C.A.; Xavier, A.A.O.; Mameri, H.; Ferreira, M.S.L.; Tavares, G.M. β-Lactoglobulin and sorghum phenolic compounds molecular binding: Interaction mechanism and thermal stability impact. Food Chem. 2025, 478, 143632. [Google Scholar] [CrossRef] [PubMed]
- Curtasu, M.V.; Nørskov, N.P. Comprehensive quantification of flavonoids and salicylic acid representative of Salix spp. using microLiquid Chromatography–Triple Quadrupole Mass Spectrometry: The importance of drying procedures and extraction solvent when performing classical solid–liquid extraction. J. Chromatogr. A 2023, 1705, 464139. [Google Scholar] [PubMed]
- Valadez-Carmona, L.; Plazola-Jacinto, C.P.; Hernández-Ortega, M.; Hernández-Navarro, M.D.; Villarreal, F.; Necoechea-Mondragón, H.; Ortiz-Moreno, A.; Ceballos-Reyes, G. Effects of microwaves, hot air and freeze-drying on the phenolic compounds, antioxidant capacity, enzyme activity and microstructure of cacao pod husks (Theobroma cacao L.). Innov. Food Sci. Emerg. Technol. 2017, 41, 378–386. [Google Scholar] [CrossRef]
- Speisky, H.; López-Alarcón, C.; Gómez, M.; Fuentes, J.; Sandoval-Acuña, C. First web-based database on total phenolics and oxygen radical absorbance capacity (ORAC) of fruits produced and consumed within the South Andes region of South America. J. Agric. Food Chem. 2012, 60, 8851–8859. [Google Scholar] [CrossRef]
- Alburquenque, A.; Busch, C.; Gómez-Lillo, G.; Gamboa, A.; Perez, C.; Fuentes, N.C.; Gotteland, M.; Abugoch, L.; Tapia, C. Obtainment of flavonoid-enriched fractions from maqui (Aristotelia chilensis) and murta (Ugni molinae) extracts via preparative HPLC and evaluation of their anti-inflammatory effects in cell-based assays. Antioxidants 2025, 14, 600. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Chu, L.; Wei, Y.; Wang, D.; Cai, S.; Zhou, F.; Ji, B. Relationship between the structures of flavonoids and oxygen radical absorbance capacity values: A quantum chemical analysis. J. Phys. Chem. A 2013, 117, 1784–1794. [Google Scholar] [CrossRef]
- Skroza, D.; Šimat, V.; Vrdoljak, L.; Jolić, N.; Skelin, A.; Čagalj, M.; Frleta, R.; Generalić Mekinić, I. Investigation of antioxidant synergisms and antagonisms among phenolic acids in the model matrices using FRAP and ORAC methods. Antioxidants 2022, 11, 1784. [Google Scholar] [CrossRef]
- Sakurai, S.; Kawakami, Y.; Kuroki, M.; Gotoh, H. Structure–antioxidant activity (oxygen radical absorbance capacity) relationships of phenolic compounds. Struct. Chem. 2022, 33, 1055–1062. [Google Scholar] [CrossRef]
- Chatterjee, A.; Bhattacharya, S.; Sur, R. Alginate/chitosan/diallyl disulfide nanoparticles: Synthesis, characterization and their anti-inflammatory efficacy on TPA-induced acute mouse ear inflammation. Carbohydr. Polym. Technol. Appl. 2023, 6, 100362. [Google Scholar] [CrossRef]
- Vivesh, V.; Kumari, P.; Singh, P. A rationale approach in developing proline based small peptide as U-shaped analogues of arachidonic acid: Dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase for developing anti-inflammatory agents. Bioorg. Chem. 2025, 160, 108452. [Google Scholar] [CrossRef]
- Pérez-González, M.Z.; Juárez-Vázquez, M.d.C.; Sánchez-Ramos, M.; Moreno-Villalba, L.; Jiménez-Arellanes, M.A. Anti-inflammatory, antioxidant, and acute toxicity of Brugmansia arborea extracts from wild plants and shoots obtained by indirect organogenesis. A thermographic assay to anti-inflammatory evaluation. Biomed. Pharmacother. 2025, 186, 117972. [Google Scholar] [CrossRef] [PubMed]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the antioxidant and anti-inflammatory activities of selected plant compounds and their metal ion complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Valko, R.; Liska, J.; Nepovimova, E.; Kuca, K.; Valko, M. Flavonoids and their role in oxidative stress, inflammation, and human diseases. Chem. Biol. Interact. 2025, 413, 111489. [Google Scholar] [CrossRef]
- Sutherland, B.A.; Rahman, R.M.A.; Appleton, I. Mechanisms of action of green tea catechins, with a focus on ischemia-induced neurodegeneration. J. Nutr. Biochem. 2006, 17, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Beiza, N.; Pérez-Correa, J.R.; Franco, W. Fermentation of murta (Ugni molinae) juice: Effect on antioxidant activity and control of enzymes associated with glucose assimilation. Int. J. Mol. Sci. 2023, 24, 15197. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Zhang, C.; Ma, L.; Wei, T.; Zhao, Y.; Peng, X. Comparative study of inhibition mechanisms of structurally different flavonoid compounds on α-glucosidase and synergistic effect with acarbose. Food Chem. 2021, 347, 129056. [Google Scholar] [CrossRef]
- Avello, M.; Pastene, E.; Torres, E. Identification of water-soluble compounds contained in aqueous extracts and fractions obtained from leaves of Ugni molinae to determine their effect on the viability of human gastric cancer cells. J. Chil. Chem. Soc. 2020, 65, 4849. [Google Scholar] [CrossRef]
| Fatty Acids | Abbreviation | Vacuum-Based Methods | ||||
|---|---|---|---|---|---|---|
| (%) | FD | LTVD 20 | LTVD 30 | LTVD 40 | VD 60 | |
| Palmitic acid | C16:0 | 12.75 ± 0.21 a | 5.58 ± 0.03 c | 5.78 ± 0.22 c | 6.59 ± 0.18 b | 5.80 ± 0.11 c |
| Stearic acid | C18:0 | 2.14 ± 0.20 a | 2.16 ± 0.00 a | 2.02 ± 0.04 a | 2.12 ± 0.12 a | 2.19 ± 0.11 a |
| Oleic acid | C18:1n-9 | 13.02 ± 0.49 a | 7.09 ± 0.00 c | 7.02 ± 0.01 c | 12.23 ± 0.17 b | 7.21 ± 0.14 c |
| Vaccenic acid | C18:1n-7 | 0.75 ± 0.02 a | 0.25 ± 0.00 b | 0.23 ± 0.02 b | 0.79 ± 0.00 a | 0.28 ± 0.07 b |
| Linoleic acid | C18:2n-6 | 69.00 ± 0.18 c | 82.65 ± 0.10 a | 83.26 ± 0.15 a | 75.77 ± 0.44 b | 83.04 ± 0.20 a |
| α-Linolenic acid | C18:3n-3 | 1.77 ± 0.24 a | 1.10 ± 0.01 b | 1.02 ± 0.02 b | 1.93 ± 0.11 a | 0.94 ± 0.02 b |
| Arachidic acid | C20:0 | ND | 0.53 ± 0.04 a | 0.50 ± 0.02 bc | 0.58 ± 0.07 a | 0.40 ± 0.03 c |
| Eicosenoic acid | C20:1n-9 | ND | 0.17 ± 0.00 b | 0.16 ± 0.02 b | 0.25 ± 0.01 a | 0.14 ± 0.01 b |
| Behenic acid | C22:0 | 0.56 ± 0.03 a | 0.15 ± 0.00 b | ND | ND | ND |
| SFAs | 15.45 | 8.42 | 8.30 | 9.29 | 8.39 | |
| MUFAs | 13.77 | 7.51 | 7.41 | 13.27 | 7.63 | |
| PUFAs | 70.77 | 83.75 | 84.28 | 77.70 | 83.98 | |
| PUFA/SFA | 4.58 | 9.95 | 10.15 | 8.36 | 10.01 | |
| Tocopherols | Abbreviation | Vacuum-Based Methods | ||||
|---|---|---|---|---|---|---|
| (μg/g of Oil) | FD | LTVD 20 | LTVD 30 | LTVD 40 | VD 60 | |
| α-Tocopherol | α-TP | 7.86 ± 1.14 e | 624.61 ± 2.47 a | 355.28 ± 4.65 c | 134.89 ± 3.71 d | 393.37 ± 7.38 b |
| β-Tocopherol | β-TP | 6.11 ± 0.22 d | 51.69 ± 2.59 b | 64.62 ± 0.49 a | 35.33 ± 0.07 c | 49.50 ± 0.50 b |
| γ-Tocopherol | γ-TP | 24.99 ± 0.11 e | 129.47 ± 0.77 b | 170.90 ± 1.25 a | 89.79 ± 1.57 d | 116.12 ± 2.33 c |
| δ-Tocopherol | δ-TP | 8.21 ± 0.22 e | 14.04 ± 0.01 d | 38.28 ± 0.16 a | 19.39 ± 0.01 b | 17.24 ± 0.21 c |
| α-Tocotrienol | α-TT | 7.65 ± 0.21 d | 24.42 ± 1.79 a | 15.68 ± 0.20 b | 8.93 ± 0.89 d | 13.47 ± 0.09 c |
| β-Tocotrienol | β-TT | ND | ND | 3.19 ± 0.13 a | ND | ND |
| γ-Tocotrienol | γ-TT | ND | ND | 7.24 ± 0.12 a | ND | ND |
| Phenolic Compounds | Abbreviation | Vacuum-Based Methods | ||||
|---|---|---|---|---|---|---|
| (mg/100 g of Sample) | FD | LTVD 20 | LTVD 30 | LTVD 40 | VD 60 | |
| Gallic acid | GA | 1.90 ± 0.19 d | 2.06 ± 0.20 d | 2.48 ± 0.18 c | 3.05 ± 0.09 b | 3.74 ± 0.20 a |
| Vanillic acid | VA | 9.48 ± 0.38 d | 11.35 ± 0.63 c | 9.32 ± 0.45 d | 15.95 ± 0.25 a | 14.76 ± 0.69 b |
| Ellagic acid | EA | 0.83 ± 0.07 d | 1.07 ± 0.01 c | 1.23 ± 0.06 b | 1.62 ± 0.05 a | 1.70 ± 0.09 a |
| Trans Cinnamic acid | TCA | NQ | NQ | NQ | NQ | NQ |
| Catechin | CAT | 46.50 ± 0.21 a | 37.81 ± 0.41 b | 12.97 ± 0.28 d | 27.91 ± 0.88 c | 37.03 ± 0.84 b |
| Epicatechin | EC | 25.41 ± 0.59 c | 39.21 ± 1.47 b | 38.67 ± 0.38 b | 50.23 ± 0.32 a | 39.52 ± 0.22 b |
| Quercetin | Q | 2.53 ± 0.05 e | 5.24 ± 0.32 d | 6.85 ± 0.37 c | 10.41 ± 0.17 a | 7.34 ± 0.09 b |
| Pyrogallol | PYG | 39.30 ± 1.32 d | 59.44 ± 1.87 b | 30.34 ± 1.66 e | 48.86 ± 0.55 c | 66.69 ± 2.31 a |
| Tyrosol | TYR | 10.88 ± 0.71 b | 10.19 ± 0.24 b | 4.70 ± 0.08 d | 9.13 ± 0.27 c | 13.55 ± 0.11 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vega-Galvez, A.; Pasten, A.; Uribe, E.; Mejias, N.; Corco, I.; Poblete, J.; Ortiz-Viedma, J.; Valenzuela-Barra, G.; Acevedo-Hernández, J.; Toledo, T. Exploring the Potential of Low-Temperature Vacuum Drying to Improve the Bioactive Compound Content and Health-Promoting Properties of Chilean Wild Murta. Antioxidants 2025, 14, 1201. https://doi.org/10.3390/antiox14101201
Vega-Galvez A, Pasten A, Uribe E, Mejias N, Corco I, Poblete J, Ortiz-Viedma J, Valenzuela-Barra G, Acevedo-Hernández J, Toledo T. Exploring the Potential of Low-Temperature Vacuum Drying to Improve the Bioactive Compound Content and Health-Promoting Properties of Chilean Wild Murta. Antioxidants. 2025; 14(10):1201. https://doi.org/10.3390/antiox14101201
Chicago/Turabian StyleVega-Galvez, Antonio, Alexis Pasten, Elsa Uribe, Nicol Mejias, Isadora Corco, Jacqueline Poblete, Jaime Ortiz-Viedma, Gabriela Valenzuela-Barra, Javier Acevedo-Hernández, and Tamar Toledo. 2025. "Exploring the Potential of Low-Temperature Vacuum Drying to Improve the Bioactive Compound Content and Health-Promoting Properties of Chilean Wild Murta" Antioxidants 14, no. 10: 1201. https://doi.org/10.3390/antiox14101201
APA StyleVega-Galvez, A., Pasten, A., Uribe, E., Mejias, N., Corco, I., Poblete, J., Ortiz-Viedma, J., Valenzuela-Barra, G., Acevedo-Hernández, J., & Toledo, T. (2025). Exploring the Potential of Low-Temperature Vacuum Drying to Improve the Bioactive Compound Content and Health-Promoting Properties of Chilean Wild Murta. Antioxidants, 14(10), 1201. https://doi.org/10.3390/antiox14101201







