Valorization of Hazelnut (Corylus avellana L.) Skin By-Product as a Multifunctional Ingredient for Cosmetic Emulsions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Microorganisms
2.2. Hazelnut Skin Biomass and Extraction Procedure
2.3. Hazelnut Skin Hydroalcoholic Extract (RHS-H) Characterization
2.3.1. Total Phenolic Content
2.3.2. Determination of Total Proanthocyanidin (Pas) Content (Vanillic Assay)
2.3.3. Thiolysis
2.3.4. Bleaching of the Radical 1,1-Diphenyl-2-picrylhydrazyl (DPPH Test)
2.3.5. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.3.6. UV Spectra and In Vitro Sun Protection Efficacy
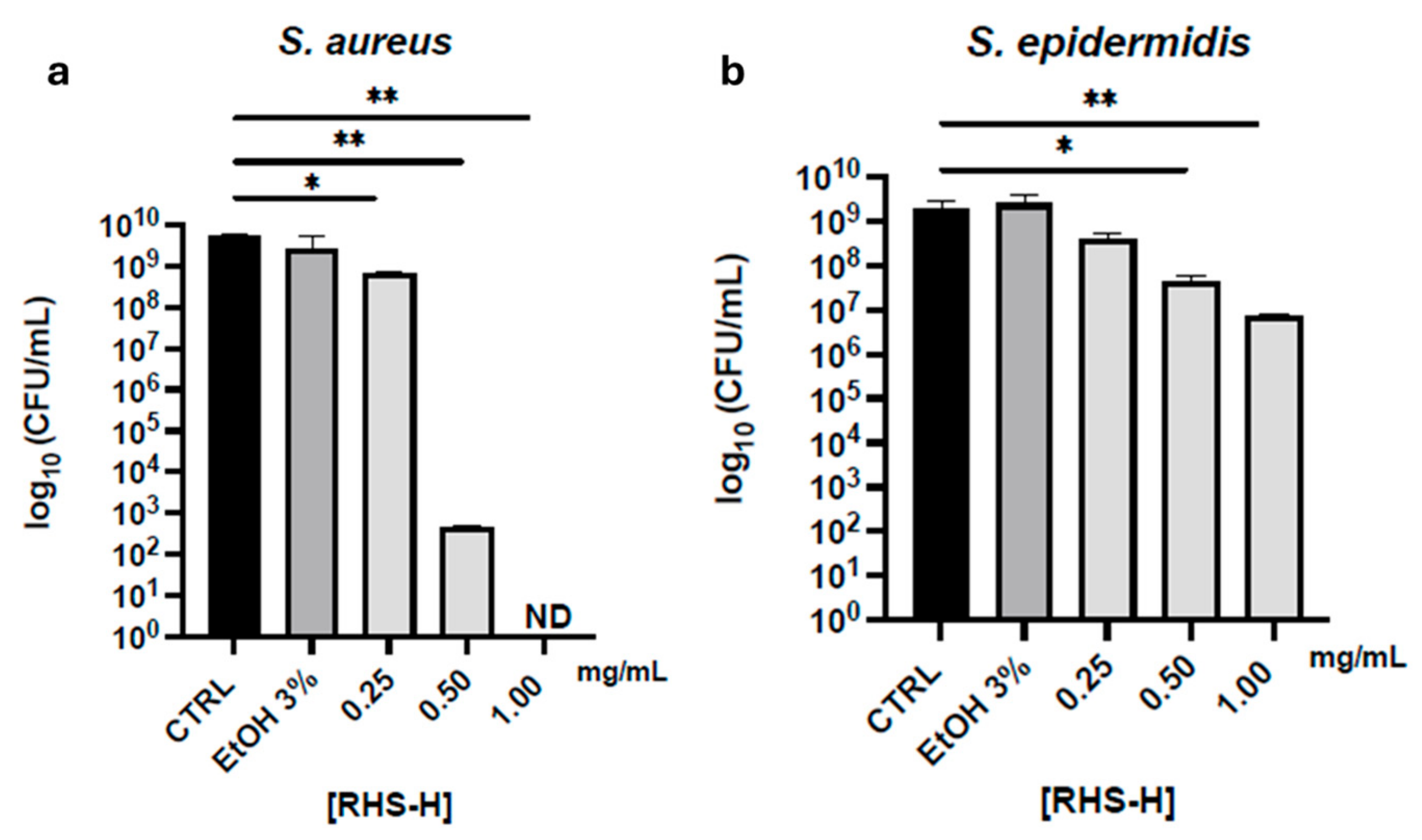
2.3.7. Determination of Minimum Bactericidal Concentration (MBC)
2.4. Development and Production of O/W Cosmetic Emulsions
2.5. Physico-Chemical Stability Tests
2.5.1. Stability Tests at 25 °C Up to 6 Months
2.5.2. Heat Shock Cycles
2.5.3. Centrifugation Stability Test
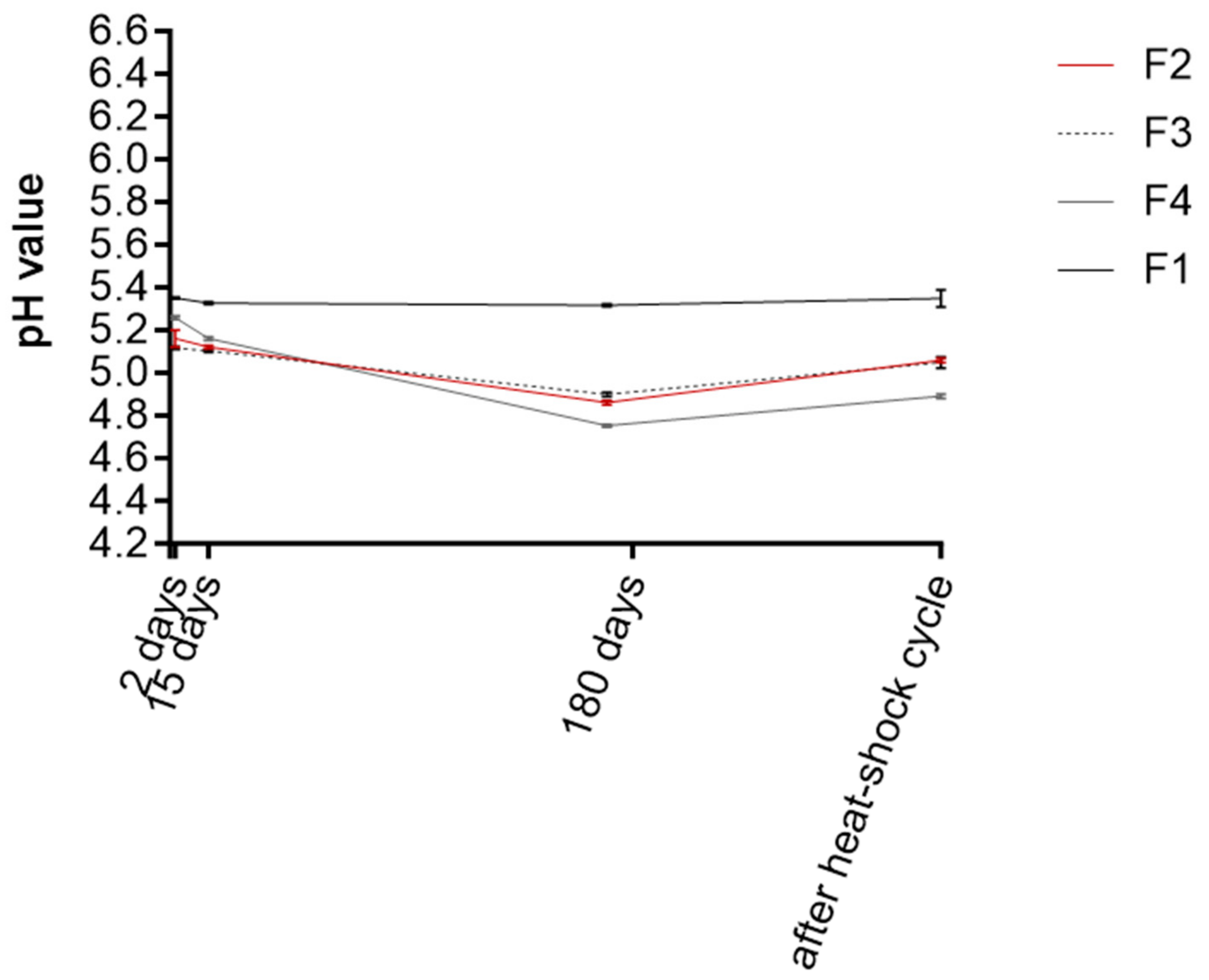
2.5.4. Organoleptic and pH Determination
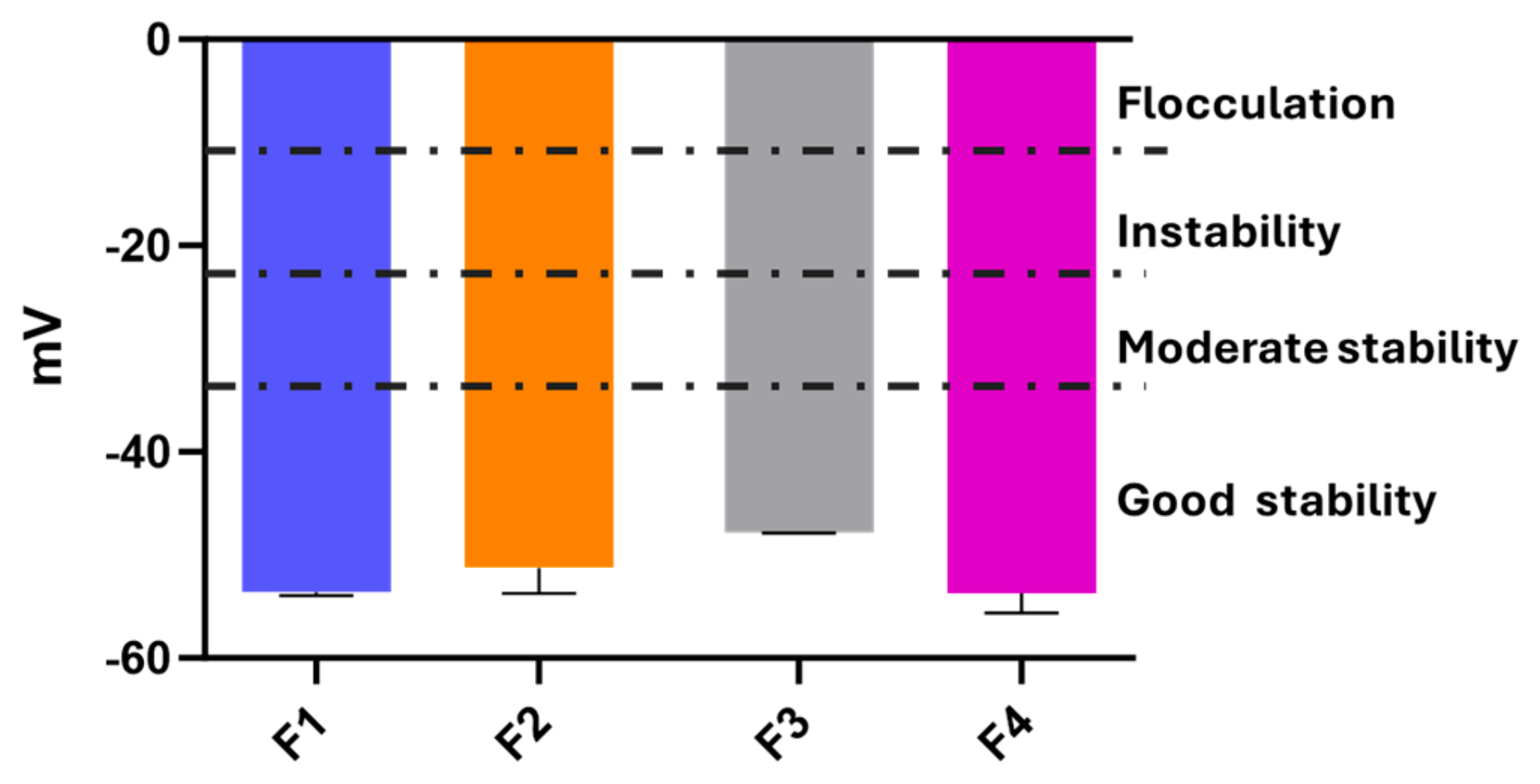
2.5.5. Zeta Potential
2.6. Rheological Behavior Characterization
2.7. Evaluation of Free Radical Scavenging Activity of Formulations
2.8. Preservative Efficacy Testing (Challenge Test)
2.9. In Vitro Sun Protection Factor (SPF) Evaluation of the O/W Emulsions
2.10. Statistical Analyses
3. Results and Discussion
3.1. Production of RHS-H and Chemical Characterization
3.2. RHS-H Functional Characterization
3.2.1. Scavenging Activity Against DPPH and ABTS Radicals
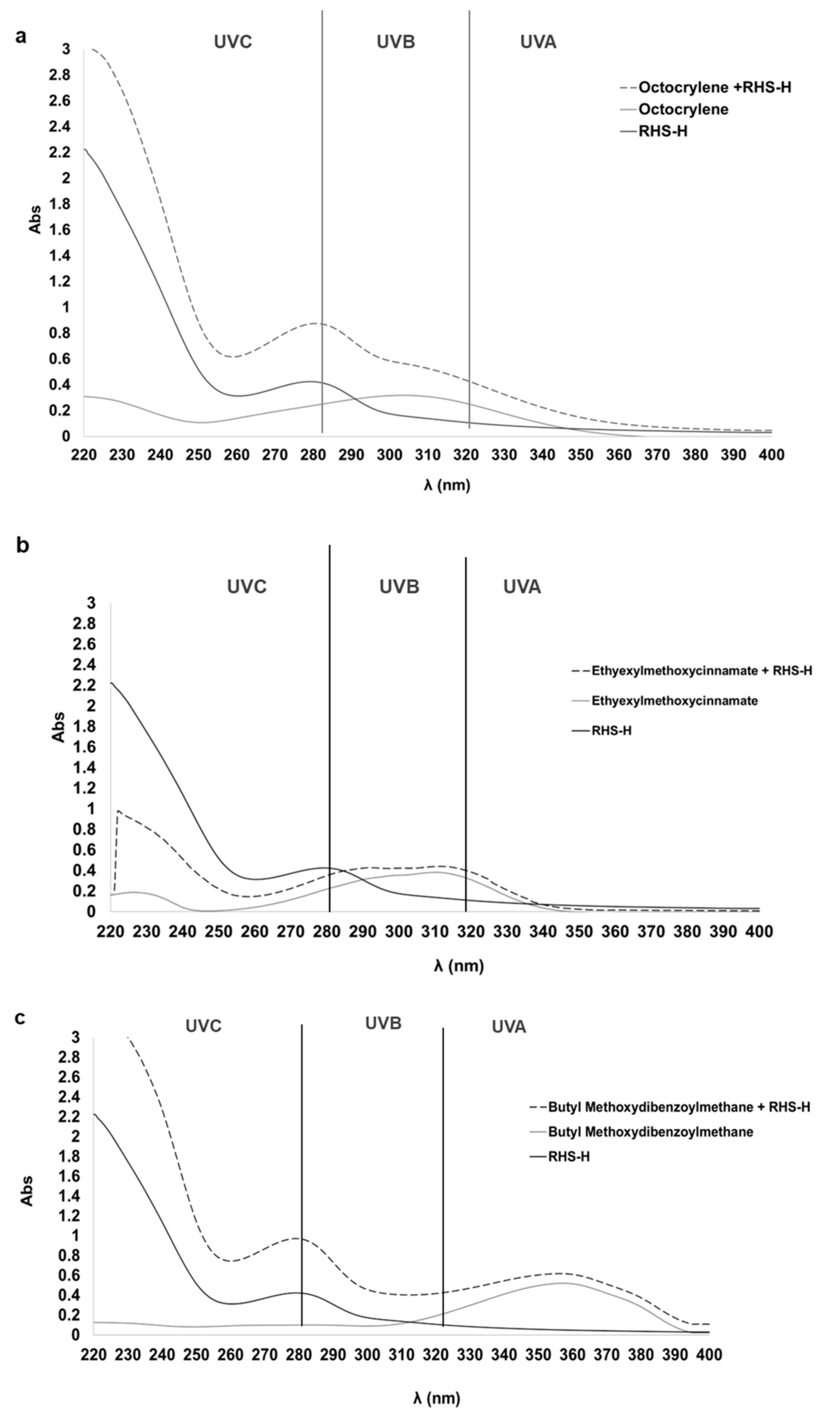
3.2.2. UV Absorbance and In Vitro SPF of Ingredients: RHS-H and Synthetic UV Filters
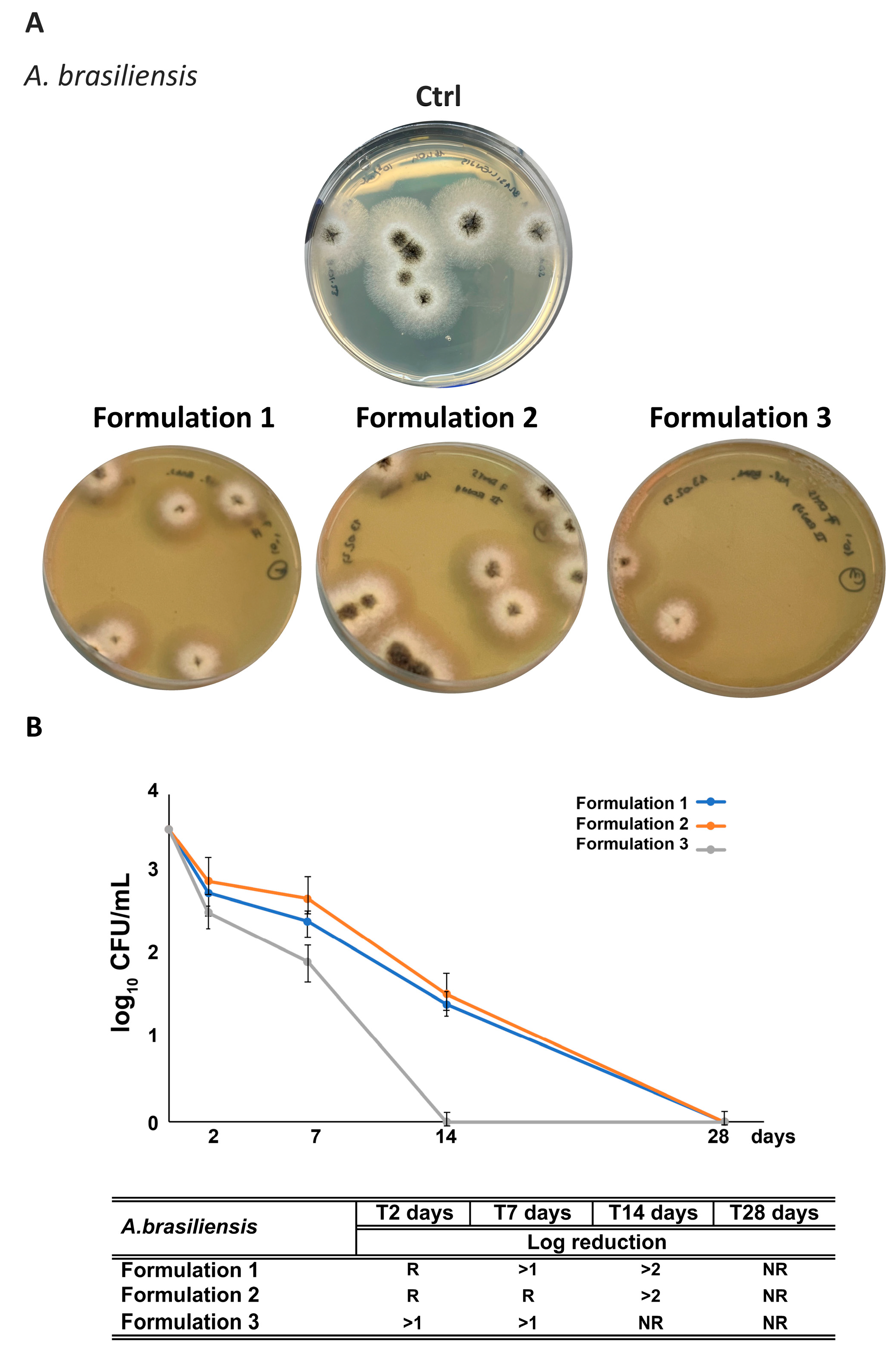
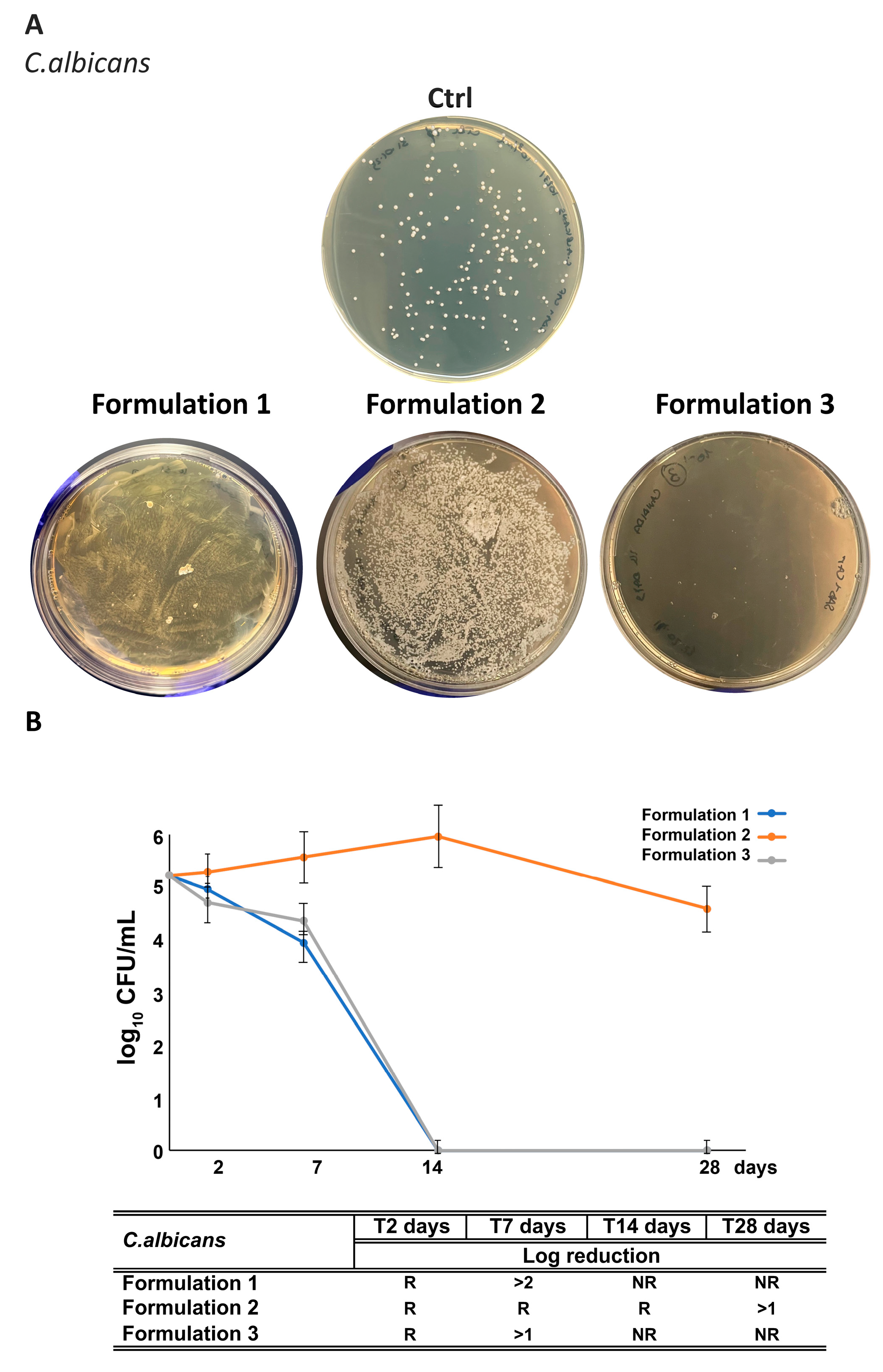
3.2.3. Antimicrobial Activity
3.3. Design and Development of O/W Cosmetic Emulsions
3.4. Stability Studies of O/W Emulsions F1–F4
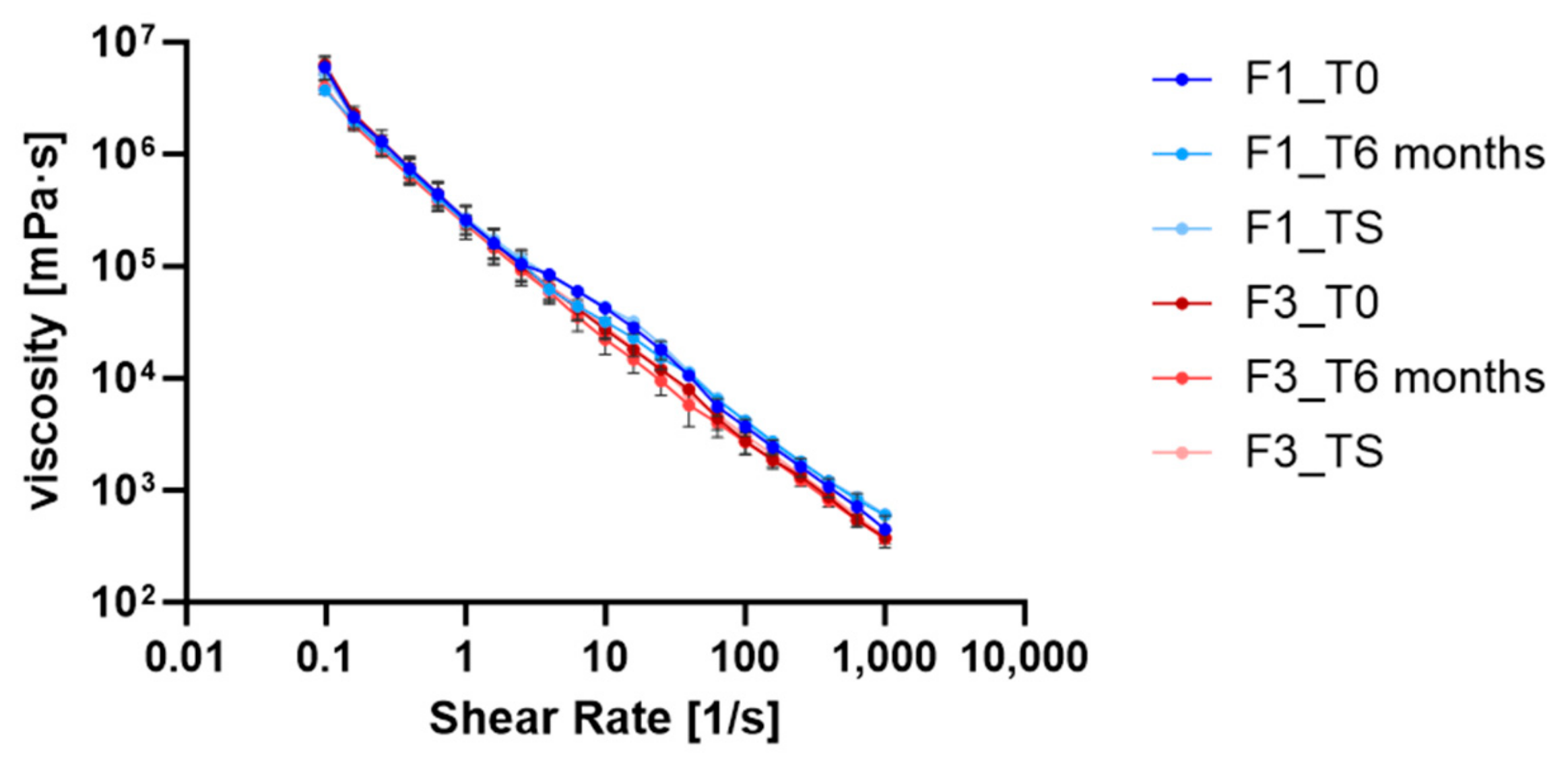
3.5. Rheological Behavior Analysis of O/W Emulsions
3.5.1. Flow Curve Analysis
3.5.2. Amplitude Sweep Test
3.5.3. Frequency Sweep Test
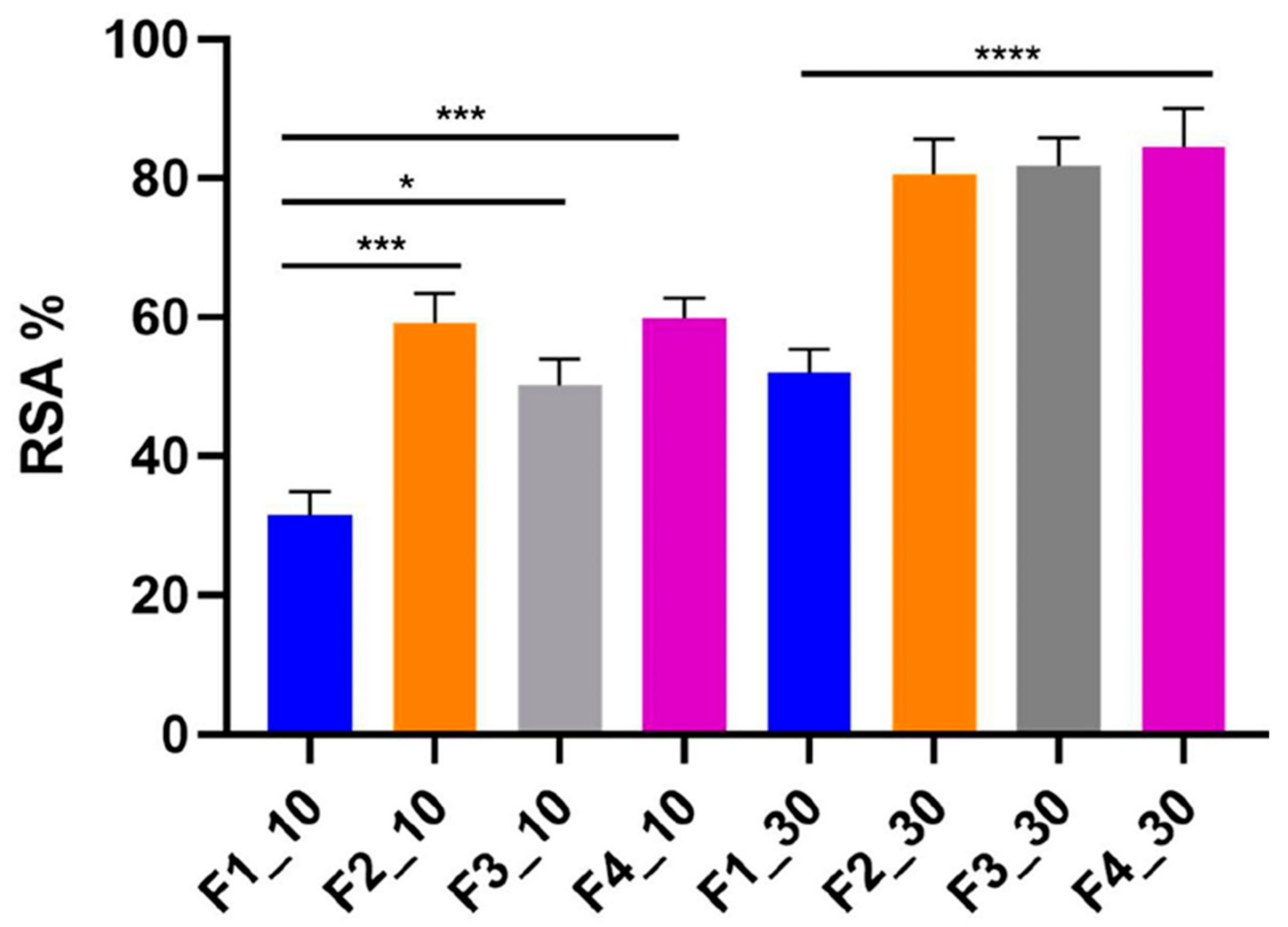
3.6. Evaluation of Antioxidant Activity of Emulsions F1–F4
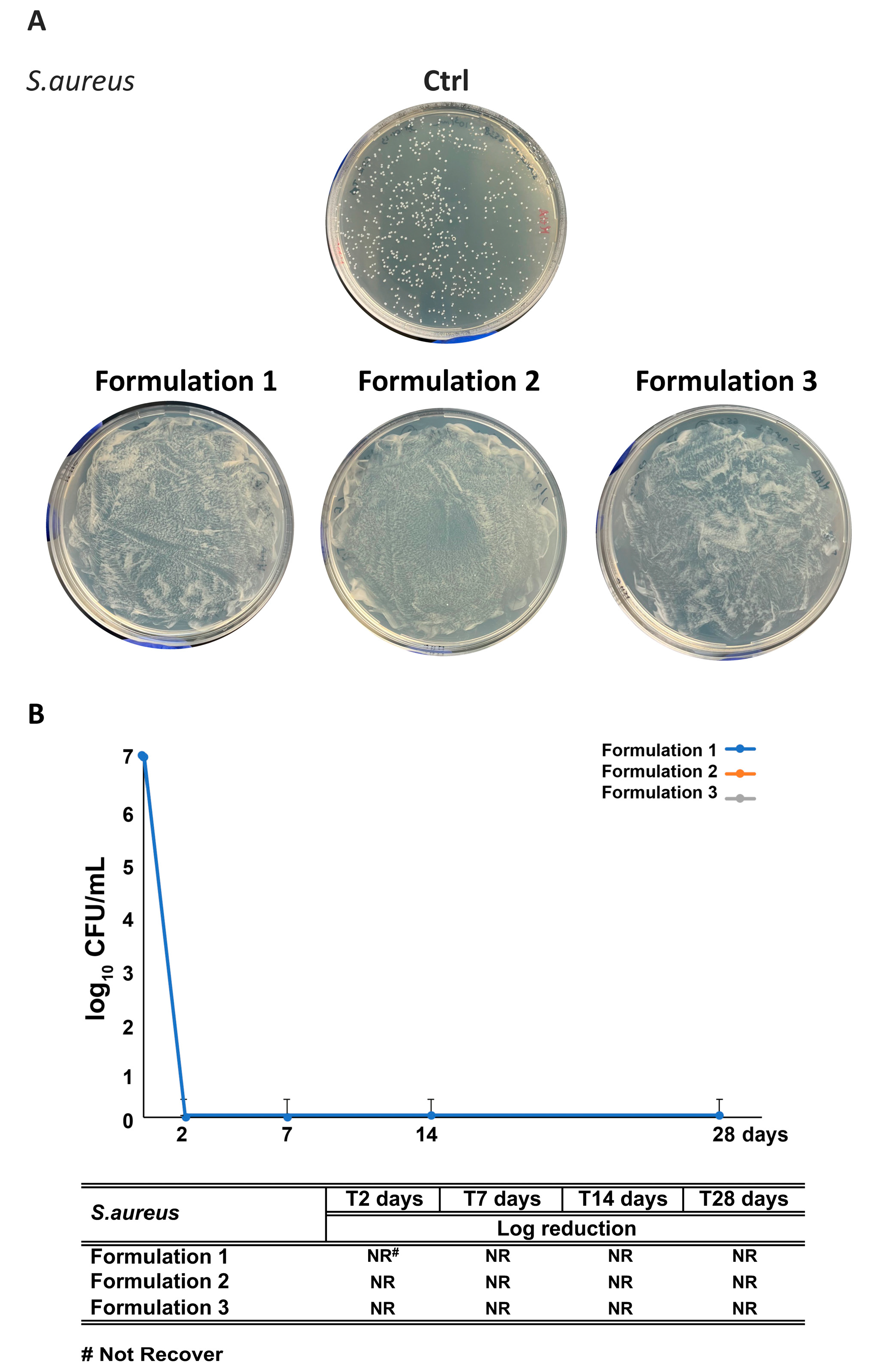
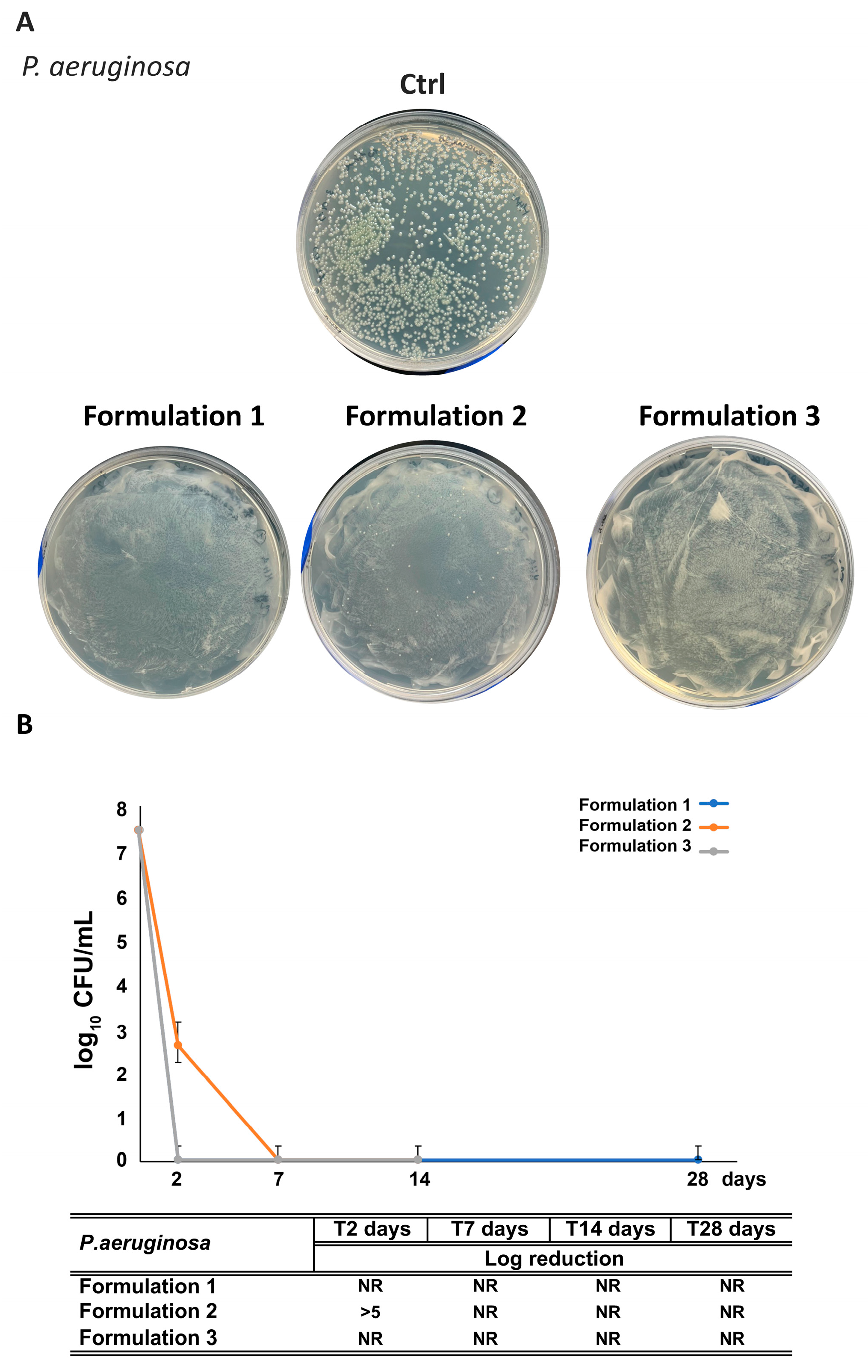
3.7. Assessment of RHS-H Preservative Performance in Developed O/W Emulsions: Challenge Test
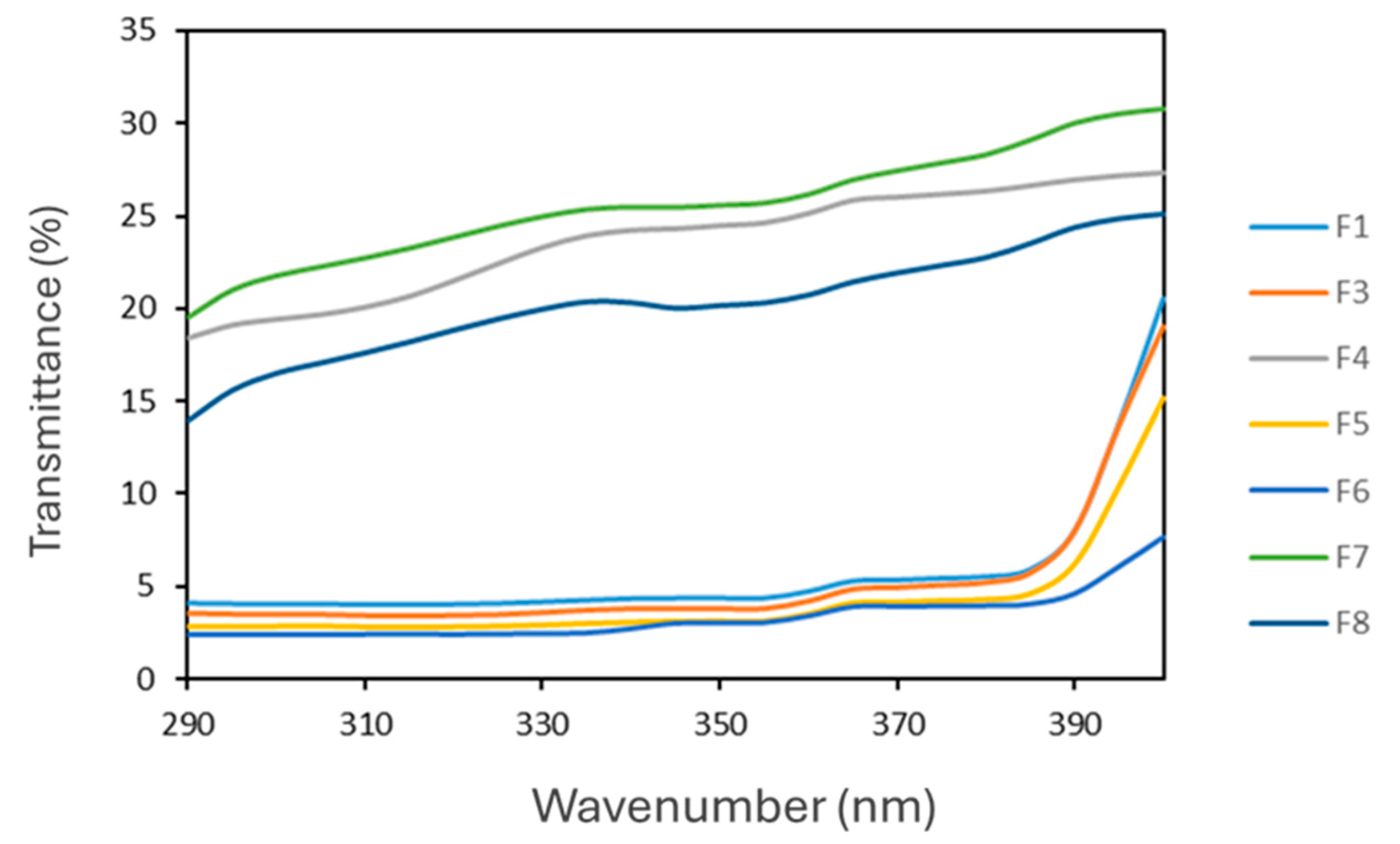
3.8. In Vitro Evaluation of SPF of O/W Cosmetic Formulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RHS | Roasted Hazelnut Skins |
| RHS-H | Roasted Hazelnut Skin Hydroalcoholic extract |
| GAE | Gallic Acid Equivalents |
| CE | Catechin Equivalents |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl radical |
| TEAC | Trolox Equivalent Antioxidant Capacity |
| UV | Ultraviolet |
| O/W | Oil in Water |
| SPF | Sun Protection Factor |
| ROS | Reactive Oxygen Species |
| DNA | Deoxyribonucleic acid |
| PBS | Phosphate-Buffered Saline |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| EDTA | Ethylenediaminetetraacetic acid |
| PLE | Pressurized Liquid Extraction |
| HPLC-UV-HRMS | High Performance Liquid Chromatography-Ultraviolet-High Resolution Mass Spectrometry |
| HPLC-UV | High Performance Liquid Chromatography-Ultraviolet |
| EC50 | Effective scavenger Concentration50 |
| TE | Trolox Equivalents |
| MBC | Minimum Bactericidal Concentration |
| MHB | Mueller–Hinton Broth |
| MHA | Mueller–Hinton Agar |
| ELS | Electrophoretic Light Scattering |
| LVR | Linear Viscoelastic Region |
| RSA | Radical Scavenging Activity |
| PAs | Proanthocyanidins |
| mDP | average Degree of Polymerization |
| CTRL | untreated control |
| EtOH | Ethanol |
| BHT | Butylated Hydroxytoluene |
| NR | No Recovery |
| R | Recovery |
| FDA | Food and Drug Administration |
References
- Piccinelli, A.L.; Pagano, I.; Esposito, T.; Mencherini, T.; Porta, A.; Petrone, A.M.; Gazzerro, P.; Picerno, P.; Sansone, F.; Rastrelli, L.; et al. HRMS Profile of a Hazelnut Skin Proanthocyanidin-Rich Fraction with Antioxidant and Anti-Candida Albicans Activities. J. Agric. Food Chem. 2016, 64, 585–595. [Google Scholar] [CrossRef]
- Spagnuolo, L.; Della Posta, S.; Fanali, C.; Dugo, L.; De Gara, L.; Gugliucci, A. Antioxidant and Antiglycation Effects of Polyphenol Compounds Extracted from Hazelnut Skin on Advanced Glycation End-Products (AGEs) Formation. Antioxidants 2021, 10, 424. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Lin, H.; Lin, Z. Hazelnut and Its By-Products: A Comprehensive Review of Nutrition, Phytochemical Profile, Extraction, Bioactivities and Applications. Food Chem. 2023, 413, 135576. [Google Scholar] [CrossRef] [PubMed]
- Frazzini, S.; Zuorro, A.; Panseri, S.; Pavlovic, R.; Sgoifo Rossi, C.A.; Rossi, L. Repurposing Hazelnut Waste Products for a Sustainable Economy: A Metabolomic Analysis of Cuticles and Shells to Highlight Their Antioxidant Potential and Inhibitory Activity against Verocytotoxic Escherichia coli. Sustainability 2023, 15, 3268. [Google Scholar] [CrossRef]
- Nie, F.; Liu, L.; Cui, J.; Zhao, Y.; Zhang, D.; Zhou, D.; Wu, J.; Li, B.; Wang, T.; Li, M.; et al. Oligomeric Proanthocyanidins: An Updated Review of Their Natural Sources, Synthesis, and Potentials. Antioxidants 2023, 12, 1004. [Google Scholar] [CrossRef] [PubMed]
- EC 1223/2009; Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products. European Union: Brussels, Belgium, 2009.
- Rathee, P.; Sehrawat, R.; Rathee, P.; Khatkar, A.; Akkol, E.K.; Khatkar, S.; Redhu, N.; Türkcanoğlu, G.; Sobarzo-Sánchez, E. Polyphenols: Natural Preservatives with Promising Applications in Food, Cosmetics and Pharma Industries; Problems and Toxicity Associated with Synthetic Preservatives; Impact of Misleading Advertisements; Recent Trends in Preservation and Legislation. Materials 2023, 16, 4793. [Google Scholar] [CrossRef]
- Jesus, A.; Sousa, E.; Cruz, M.T.; Cidade, H.; Lobo, J.M.S.; Almeida, I.F. UV Filters: Challenges and Prospects. Pharmaceuticals 2022, 15, 263. [Google Scholar] [CrossRef]
- Pourzand, C.; Albieri-Borges, A.; Raczek, N.N. Shedding a New Light on Skin Aging, Iron-and Redox-Homeostasis and Emerging Natural Antioxidants. Antioxidants 2022, 11, 471. [Google Scholar] [CrossRef]
- Masaki, H. Role of Antioxidants in the Skin: Anti-Aging Effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Petruk, G.; Del Giudice, R.; Rigano, M.M.; Monti, D.M. Antioxidants from Plants Protect against Skin Photoaging. Oxidative Med. Cell. Longev. 2018, 2018, 1454936. [Google Scholar] [CrossRef]
- Ngoc, L.T.N.; van Tran, V.; Moon, J.Y.; Chae, M.; Park, D.; Lee, Y.C. Recent Trends of Sunscreen Cosmetic: An Update Review. Cosmetics 2019, 6, 64. [Google Scholar] [CrossRef]
- Saewan, N.; Jimtaisong, A. Natural Products as Photoprotection. J. Cosmet. Dermatol. 2015, 14, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.J.; Pedrosa, S.S.; Pintado, M.; Oliveira, A.L.S.; Madureira, A.R. New Natural and Sustainable Cosmetic Preservative Based on Sugarcane Straw Extract. Molecules 2024, 29, 3928. [Google Scholar] [CrossRef]
- Silvério, L.A.L.; Coco, J.C.; de Macedo, L.M.; dos Santos, É.M.; Sueiro, A.C.; Ataide, J.A.; Tavares, G.D.; Paiva-Santos, A.C.; Mazzola, P.G. Natural Product-Based Excipients for Topical Green Formulations. Sustain. Chem. Pharm. 2023, 33, 1426. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Gomez-Molina, M.; Albaladejo-Marico, L.; Yepes-Molina, L.; Nicolas-Espinosa, J.; Navarro-León, E.; Garcia-Ibañez, P.; Carvajal, M. Exploring Phenolic Compounds in Crop By-Products for Cosmetic Efficacy. Int. J. Mol. Sci. 2024, 25, 5884. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.S.; Romero-Díez, R.; Álvarez, A.; Bronze, M.R.; Rodríguez-Rojo, S.; Mato, R.B.; Cocero, M.J.; Matias, A.A. Polyphenol-Rich Extracts Obtained from Winemakingwaste Streams as Natural Ingredients with Cosmeceutical Potential. Antioxidants 2019, 8, 355. [Google Scholar] [CrossRef]
- Farhan, M. The Promising Role of Polyphenols in Skin Disorders. Molecules 2024, 29, 865. [Google Scholar] [CrossRef]
- Gǎlbǎu, C.Ş.; Irimie, M.; Neculau, A.E.; Dima, L.; Pogačnik da Silva, L.; Vârciu, M.; Badea, M. The Potential of Plant Extracts Used in Cosmetic Product Applications—Antioxidants Delivery and Mechanism of Actions. Antioxidants 2024, 13, 1425. [Google Scholar] [CrossRef]
- Esposito, T.; Silva, N.H.C.S.; Almeida, A.; Silvestre, A.J.D.; Piccinelli, A.; Aquino, R.P.; Sansone, F.; Mencherini, T.; Vilela, C.; Freire, C.S.R. Valorisation of Chestnut Spiny Burs and Roasted Hazelnut Skins Extracts as Bioactive Additives for Packaging Films. Ind. Crops Prod. 2020, 151, 112491. [Google Scholar] [CrossRef]
- Butler, L.G.; Price, M.L.; Brotherton, J.E. Vanillin Assay for Proanthocyanidins (Condensed Tannins): Modification of the Solvent for Estimation of the Degree of Polymerization. J. Agric. Food Chem. 1982, 30, 1087–1089. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.; Hammerstone, J.F.; Beecher, G.; Cunningham, D.; Vannozzi, S.; Prior, R.L. Fractionation of Polymeric Procyanidins from Lowbush Blueberry and Quantification of Procyanidins in Selected Foods with an Optimized Normal-Phase HPLC-MS Fluorescent Detection Method. J. Agric. Food Chem. 2002, 50, 4852–4860. [Google Scholar] [CrossRef] [PubMed]
- Esposito, T.; Celano, R.; Pane, C.; Piccinelli, A.L.; Sansone, F.; Picerno, P.; Zaccardelli, M.; Aquino, R.P.; Mencherini, T. Chestnut (Castanea sativa Miller.) Burs Extracts and Functional Compounds: Uhplc-Uv-Hrms Profiling, Antioxidant Activity, and Inhibitory Effects on Phytopathogenic Fungi. Molecules 2019, 24, 302. [Google Scholar] [CrossRef] [PubMed]
- Reis Mansur, M.C.P.P.; Leitão, S.G.; Cerqueira-Coutinho, C.; Vermelho, A.B.; Silva, R.S.; Presgrave, O.A.F.; Leitão, Á.A.C.; Leitão, G.G.; Ricci-Júnior, E.; Santos, E.P. In Vitro and in Vivo Evaluation of Efficacy and Safety of Photoprotective Formulations Containing Antioxidant Extracts. Rev. Bras. Farmacogn. 2016, 26, 251–258. [Google Scholar] [CrossRef]
- Sayre, R.M.; Agin, P.P.; Levee, G.J.; Maruiwe, E. A comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef]
- Esposito, T.; Mencherini, T.; Sansone, F.; Auriemma, G.; Gazzerro, P.; Puca, R.V.; Iandoli, R.; Aquino, R.P. Development, Characterization, and Clinical Investigation of a New Topical Emulsion System Containing a Castanea Sativa Spiny Burs Active Extract. Pharmaceutics 2021, 13, 1634. [Google Scholar] [CrossRef]
- Serra, M.; Botelho, C.; Almeida, H.; Casas, A.; Teixeira, J.A.; Barros, A.N. Stable and Functional Cosmetic Creams Enriched with Grape Stem Extract: A Sustainable Skincare Strategy. Antioxidants 2025, 14, 784. [Google Scholar] [CrossRef]
- Caruso, C.; Porta, A.; Tosco, A.; Eletto, D.; Pacente, L.; Bartollino, S.; Costagliola, C. A Novel Vitamin E TPGS-Based Formulation Enhances Chlorhexidine Bioavailability in Corneal Layers. Pharmaceutics 2020, 12, 642. [Google Scholar] [CrossRef]
- Diffey, B.L.; Robson, J. A New Substrate to Measure Sunscreen Protection Factors throughout the Ultraviolet Spectrum. J. Soc. Cosmet. Chem. 1989, 40, 127–133. [Google Scholar]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Bencresciuto, G.F.; Carnevale, M.; Paris, E.; Gallucci, F.; Santangelo, E.; Migliori, C.A. A Sustainable Alternative for Cosmetic Applications: NADES Extraction of Bioactive Compounds from Hazelnut By-Products. Sustainability 2025, 17, 1516. [Google Scholar] [CrossRef]
- Ku, C.S.; Mun, S.P. Characterization of Proanthocyanidin in Hot Water Extract Isolated from Pinus Radiata Bark. Wood Sci. Technol. 2007, 41, 235–247. [Google Scholar] [CrossRef]
- Ivanović, S.; Avramović, N.; Dojčinović, B.; Trifunović, S.; Novaković, M.; Tešević, V.; Mandić, B. Chemical Composition, Total Phenols and Flavonoids Contents and Antioxidant Activity as Nutritive Potential of Roasted Hazelnut Skins (Corylus avellana L.). Foods 2020, 9, 430. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Tsatalas, P.; Galanakis, I.M. Phenols from Olive Mill Wastewater and Other Natural Antioxidants as UV Filters in Sunscreens. Environ. Technol. Innov. 2018, 9, 160–168. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Tsatalas, P.; Galanakis, I.M. Implementation of Phenols Recovered from Olive Mill Wastewater as UV Booster in Cosmetics. Ind. Crops Prod. 2018, 111, 30–37. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M.; Strzępek-Gomółka, M.; Antosiewicz, B. Characterization of Cistus × incanus L. and Cistus ladanifer L. Extracts as Potential Multifunctional Antioxidant Ingredients for Skin Protecting Cosmetics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liao, H.; Dai, Y.; Qi, Y.; Zou, Z. Characterization and Anti-Ultraviolet Radiation Activity of Proanthocyanidin-Rich Extracts from Cinnamomum camphora by Ultrasonic-Assisted Method. Molecules 2024, 29, 796. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Gomes, S.M.; Santos, L. A Novel Approach in Skin Care: By-Product Extracts as Natural UV Filters and an Alternative to Synthetic Ones. Molecules 2023, 28, 2037. [Google Scholar] [CrossRef]
- Era, B.; Floris, S.; Sogos, V.; Porcedda, C.; Piras, A.; Medda, R.; Fais, A.; Pintus, F. Anti-Aging Potential of Extracts from Washingtonia filifera Seeds. Plants 2021, 10, 151. [Google Scholar] [CrossRef]
- Krishna, S.; Miller, L.S. Host-Pathogen Interactions between the Skin and Staphylococcus Aureus. Curr. Opin. Microbiol. 2012, 15, 28–35. [Google Scholar] [CrossRef]
- Severn, M.M.; Horswill, A.R. Staphylococcus Epidermidis and Its Dual Lifestyle in Skin Health and Infection. Nat. Rev. Microbiol. 2023, 21, 97–111. [Google Scholar] [CrossRef]
- Di Michele, A.; Pagano, C.; Allegrini, A.; Blasi, F.; Cossignani, L.; Di Raimo, E.; Faieta, M.; Oliva, E.; Pittia, P.; Primavilla, S.; et al. Hazelnut Shells as Source of Active Ingredients: Extracts Preparation and Characterization. Molecules 2021, 26, 6607. [Google Scholar] [CrossRef]
- Harfoush, A.; Swaidan, A.; Khazaal, S.; Salem Sokhn, E.; Grimi, N.; Debs, E.; Louka, N.; El Darra, N. From Spent Black and Green Tea to Potential Health Boosters: Optimization of Polyphenol Extraction and Assessment of Their Antioxidant and Antibacterial Activities. Antioxidants 2024, 13, 1588. [Google Scholar] [CrossRef] [PubMed]
- Nitulescu, G.; Lupuliasa, D.; Adam-Dima, I.; Nitulescu, G.M. Ultraviolet Filters for Cosmetic Applications. Cosmetics 2023, 10, 101. [Google Scholar] [CrossRef]
- Pinto, D.; Lameirão, F.; Delerue-Matos, C.; Rodrigues, F.; Costa, P. Characterization and Stability of a Formulation Containing Antioxidants-Enriched Castanea sativa Shells Extract. Cosmetics 2021, 8, 49. [Google Scholar] [CrossRef]
- Tang, Z.; Du, Q. Mechanism of Action of Preservatives in Cosmetics. J. Dermatol. Sci. Cosmet. Technol. 2024, 1, 100054. [Google Scholar] [CrossRef]
- Singh, S.; Lohani, A.; Mishra, A.K.; Verma, A. Formulation and Evaluation of Carrot Seed Oil-Based Cosmetic Emulsions. J. Cosmet. Laser Ther. 2019, 21, 99–107. [Google Scholar] [CrossRef]
- Mirhosseini, H.; Tan, C.P.; Hamid, N.S.A.; Yusof, S. Effect of Arabic Gum, Xanthan Gum and Orange Oil Contents on ζ-Potential, Conductivity, Stability, Size Index and PH of Orange Beverage Emulsion. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 47–56. [Google Scholar] [CrossRef]
- Saharudin, S.H.; Ahmad, Z. Role of Xanthan Gum on Physicochemical and Rheological Properties of Rice Bran Oil Emulsion. Int. Food Res. J. 2016, 23, 1361. [Google Scholar]
- de Oliveira Paulo, L.A.; Fernandes, R.N.; Simiqueli, A.A.; Rocha, F.; dos Santos Dias, M.M.; Minim, V.P.R.; Minim, L.A.; Vidigal, M.C.T.R. Baru Oil (Dipteryx alata Vog.) Applied in the Formation of O/W Nanoemulsions: A Study of Physical-Chemical, Rheological and Interfacial Properties. Food Res. Int. 2023, 170, 112961. [Google Scholar] [CrossRef]
- Danila, A.; Ibanescu, S.A.; Zaharia, C.; Muresan, E.I.; Popescu, A.; Danu, M.; Rotaru, V. Eco-Friendly O/W Emulsions with Potential Application in Skincare Products. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125969. [Google Scholar] [CrossRef]
- Huynh, A.; Garcia, A.G.; Young, L.K.; Szoboszlai, M.; Liberatore, M.W.; Baki, G. Measurements Meet Perceptions: Rheology–Texture–Sensory Relations When Using Green, Bio-Derived Emollients in Cosmetic Emulsions. Int. J. Cosmet. Sci. 2021, 43, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Cefali, L.C.; Ataide, J.A.; Fernandes, A.R.; Sousa, I.M.D.O.; Gonçalves, F.C.D.S.; Eberlin, S.; Dávila, J.L.; Jozala, A.F.; Chaud, M.V.; Sanchez-Lopez, E.; et al. Flavonoid-Enriched Plant-Extract-Loaded Emulsion: A Novel Phytocosmetic Sunscreen Formulation with Antioxidant Properties. Antioxidants 2019, 8, 443. [Google Scholar] [CrossRef]
- López-Hortas, L.; Falqué, E.; Domínguez, H.; Torres, M.D. Microwave Hydrodiffusion and Gravity versus Conventional Distillation for Acacia Dealbata Flowers. Recovery of Bioactive Extracts for Cosmetic Purposes. J. Clean. Prod. 2020, 274, 123143. [Google Scholar] [CrossRef]
- Ibănescu, C.; Danu, M.; Nanu, A.; Lungu, M.; Simionescu, B.C. Stability of Disperse Systems Estimated Using Rheological Oscillatory Shear Tests. Rev. Roum. Chim 2010, 55, 933–940. [Google Scholar]
- Turcov, D.; Barna, A.S.; Blaga, A.C.; Ibanescu, C.; Danu, M.; Trifan, A.; Zbranca, A.; Suteu, D. Dermatocosmetic Emulsions Based on Resveratrol, Ferulic Acid and Saffron (Crocus sativus) Extract to Combat Skin Oxidative Stress-Trigger Factor of Some Potential Malignant Effects: Stability Studies and Rheological Properties. Pharmaceutics 2022, 14, 2376. [Google Scholar] [CrossRef]
- Adejokun, D.A.; Dodou, K. Quantitative Sensory Interpretation of Rheological Parameters of a Cream Formulation. Cosmetics 2020, 7, 2. [Google Scholar] [CrossRef]
- Mieles-Gómez, L.; Lastra-Ripoll, S.E.; Torregroza-Fuentes, E.; Quintana, S.E.; García-Zapateiro, L.A. Rheological and Microstructural Properties of Oil-in-Water Emulsion Gels Containing Natural Plant Extracts Stabilized with Carboxymethyl Cellulose/Mango (Mangifera indica) Starch. Fluids 2021, 6, 312. [Google Scholar] [CrossRef]
- Irato, P.; Santovito, G. Enzymatic and Non-Enzymatic Molecules with Antioxidant Function. Antioxidants 2021, 10, 579. [Google Scholar] [CrossRef]
- Vilela, F.M.P.; Oliveira, F.M.; Vicentini, F.T.M.C.; Casagrande, R.; Verri, W.A.; Cunha, T.M.; Fonseca, M.J.V. Commercial Sunscreen Formulations: UVB Irradiation Stability and Effect on UVB Irradiation-Induced Skin Oxidative Stress and Inflammation. J. Photochem. Photobiol. B 2016, 163, 413–420. [Google Scholar] [CrossRef]
- Wu, Y.; Matsui, M.S.; Chen, J.Z.S.; Jin, X.; Shu, C.M.; Jin, G.Y.; Dong, G.H.; Wang, Y.K.; Gao, X.H.; Chen, H.D.; et al. Antioxidants Add Protection to a Broad-Spectrum Sunscreen. Clin. Exp. Dermatol. 2011, 36, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.M.; Falé, Z.; Santos, L. Sustainability in Skin Care: Incorporation of Avocado Peel Extracts in Topical Formulations. Molecules 2022, 27, 1782. [Google Scholar] [CrossRef] [PubMed]
- Karioti, A.; Furlan, C.; Vincieri, F.F.; Bilia, A.R. Analysis of the Constituents and Quality Control of Viola Odorata Aqueous Preparations by HPLC-DAD and HPLC-ESI-MS. Anal. Bioanal. Chem. 2011, 399, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Nostro, A.; Cannatelli, M.A.; Morelli, I.; Cioni, P.L.; Bader, A.; Marino, A.; Alonzo, V. Preservative Properties of Calamintha Officinalis Essential Oil with and without EDTA. Lett. Appl. Microbiol. 2002, 35, 385–389. [Google Scholar] [CrossRef]
- Boukhira, S.; Balouiri, M.; El Mansouri, L.; El Youbi, A.E.H.; Bouarfa, M.; Lebtar, S.; Ouhammou, A.; Bousta, D. Development of Natural Preservative from Silene Vulgaris Extract in Topical Formulation under a Challenge Test and Its Stability Study. J. Appl. Pharm. Sci. 2017, 7, 142–148. [Google Scholar] [CrossRef]
- de Melo, R.S.; Reis, S.A.G.B.; Guimarães, A.L.; Silva, N.D.D.S.; Rocha, J.M.; El Aouad, N.; Almeida, J.R.G. da S. Phytocosmetic Emulsion Containing Extract of Morus nigra L. (Moraceae): Development, Stability Study, Antioxidant and Antibacterial Activities. Cosmetics 2022, 9, 39. [Google Scholar] [CrossRef]
- Verma, A.; Zanoletti, A.; Kareem, K.Y.; Adelodun, B.; Kumar, P.; Ajibade, F.O.; Silva, L.F.O.; Phillips, A.J.; Kartheeswaran, T.; Bontempi, E.; et al. Skin Protection from Solar Ultraviolet Radiation Using Natural Compounds: A Review. Environ. Chem. Lett. 2024, 22, 273–295. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. About Suncare Products. In Chemistry Research Summaries; Nova Science Publisher Inc.: Hauppauge, NY, USA, 2016; Volume 2, pp. 45–47. ISBN 9781622576623. [Google Scholar]
- Hübner, A.A.; Sarruf, F.D.; Oliveira, C.A.; Neto, A.V.; Fischer, D.C.H.; Kato, E.T.M.; Lourenço, F.R.; Baby, A.R.; Bacchi, E.M. Safety and Photoprotective Efficacy of a Sunscreen System Based on Grape Pomace (Vitis vinifera L.) Phenolics from Winemaking. Pharmaceutics 2020, 12, 1148. [Google Scholar] [CrossRef]
- Morocho-Jácome, A.L.; Freire, T.B.; de Oliveira, A.C.; de Almeida, T.S.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. In Vivo SPF from Multifunctional Sunscreen Systems Developed with Natural Compounds—A Review. J. Cosmet. Dermatol. 2021, 20, 729–737. [Google Scholar] [CrossRef]




| Wavelength (λ, nm) | EE × I Normalized |
|---|---|
| 290 | 0.0150 |
| 295 | 0.0817 |
| 300 | 0.2874 |
| 305 | 0.3278 |
| 310 | 0.1864 |
| 315 | 0.0839 |
| 320 | 0.0180 |
| Total | 1 |
| Ingredient (INCI Name) | Function | F1 | F2 | F3 | F4 |
|---|---|---|---|---|---|
| Concentration % (w/w) | |||||
| Aqueous phase (A) | |||||
| Aqua | Solvent | *qs. 100 | qs. 100 | qs. 100 | qs. 100 |
| Glycerin | Humectant | 4.00 | 3.00 | 3.00 | 3.00 |
| Xanthan Gum | Thickening agent | 0.25 | 0.25 | 0.25 | 0.25 |
| Disodium EDTA | Chelating agent | 0.10 | 0.10 | 0.10 | 0.10 |
| Oil phase (B) | |||||
| Butyrospermum parkii | Skin conditioning/ Consistency regulator | 3.00 | 3.00 | 3.00 | 4.00 |
| Glyceryl Stearate (and) Peg-100 Stearate 1 | Emulsifier | 5.00 | 5.00 | 5.00 | 5.00 |
| Cetearyl Alcohol | Emulsion stabilising | 3.00 | 3.00 | 3.00 | 3.00 |
| Ethylhexyl Stearate | Emollient | 2.00 | 2.00 | 2.00 | 2.00 |
| Persea Gratissima Oil | Skin conditioning | 1.00 | 1.00 | 1.00 | 3.00 |
| Dimethicone | Antifoaming | 0.50 | 0.50 | 0.50 | 0.50 |
| Caprylic/Capric Triglyceride | Skin conditioning | 3.00 | 3.00 | 3.00 | 3.00 |
| Dicaprylyl Ether | Emollient | 1.00 | 1.00 | 1.00 | 1.00 |
| C12-15 Alkyl Benzoate | Solubilizer for UV filters/Emollient | 8.00 | 8.00 | 8.00 | 8.00 |
| Ethylhexyl Methoxycinnamate | UV-B filter | 8.00 | 8.00 | 8.00 | - |
| Butyl Methoxydibenzoylmethane | UV-A filter | 4.70 | 4.70 | 4.70 | - |
| Octocrylene Butylhydroxytoluene | UV-B filter Antioxidant | 8.00 0.01 | 8.00 0.01 | 8.00 0.01 | - 0.01 |
| Phase C | |||||
| Phenoxyethanol (and) Ethylhexylglycerin | Synthetic Preservative | 1.00 | - | 0.50 | 1.00 |
| Phase D | |||||
| RHS-H | Roasted Hazelnut skin hydroalcoholic extract | - | 0.20 | 0.20 | 0.20 |
| Glycerin | Humectant | - | 1.00 | 1.00 | 1.00 |
| Aqua | Solvent | - | 1.00 | 1.00 | 1.00 |
| Sunscreen Ingredient (Concentration) | UV-Filter Booster | In Vitro SPF (Mansur Equation) |
|---|---|---|
| RHS-H (0.1 mg/mL) | - | 3.63 ± 0.11 |
| Octocrylene (0.01 mg/mL) | - | 4.90 ± 0.12 |
| Ethylhexyl Methoxycinnamate (0.005 mg/mL) | - | 3.88 ± 0.49 |
| Butyl Methoxydibenzoylmethane (0.01 mg/mL) | - | 3.98 ± 0.11 |
| Octocrylene (0.01 mg/mL) | RHS-H (0.1 mg/mL) | 9.13 ± 0.91 |
| Ethylhexyl Methoxycinnamate (0.005 mg/mL) | RHS-H (0.1 mg/mL) | 9.73 ± 0.57 |
| Butyl Methoxydibenzoylmethane (0.01 mg/mL) | RHS-H (0.1 mg/mL) | 7.33 ± 0.58 |
| Emulsion System | SPF | UVA/UVB | Cλ |
|---|---|---|---|
| F1 | 27.7 ± 1.2 | 0.82 | 385 |
| F3 | 30.3 ± 1.7 | 0.81 | 385 |
| F4 | 5.2 ± 1.3 | 0.78 | 388 |
| F5 | 33.9 ± 1.2 | 0.81 | 397 |
| F6 | 40.1 ± 2.3 | 0.83 | 395 |
| F7 | 4.3 ± 0.5 | 0.79 | 391 |
| F8 | 5.6 ± 0.2 | 0.78 | 391 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mencherini, T.; Esposito, T.; Sansone, F.; Eletto, D.; Pannetta, M.; Gordobil, O.; Piccinelli, A.L.; Ferniani, C.; Auriemma, G.; Rastrelli, L.; et al. Valorization of Hazelnut (Corylus avellana L.) Skin By-Product as a Multifunctional Ingredient for Cosmetic Emulsions. Antioxidants 2025, 14, 1199. https://doi.org/10.3390/antiox14101199
Mencherini T, Esposito T, Sansone F, Eletto D, Pannetta M, Gordobil O, Piccinelli AL, Ferniani C, Auriemma G, Rastrelli L, et al. Valorization of Hazelnut (Corylus avellana L.) Skin By-Product as a Multifunctional Ingredient for Cosmetic Emulsions. Antioxidants. 2025; 14(10):1199. https://doi.org/10.3390/antiox14101199
Chicago/Turabian StyleMencherini, Teresa, Tiziana Esposito, Francesca Sansone, Daniela Eletto, Martina Pannetta, Oihana Gordobil, Anna Lisa Piccinelli, Carlo Ferniani, Giulia Auriemma, Luca Rastrelli, and et al. 2025. "Valorization of Hazelnut (Corylus avellana L.) Skin By-Product as a Multifunctional Ingredient for Cosmetic Emulsions" Antioxidants 14, no. 10: 1199. https://doi.org/10.3390/antiox14101199
APA StyleMencherini, T., Esposito, T., Sansone, F., Eletto, D., Pannetta, M., Gordobil, O., Piccinelli, A. L., Ferniani, C., Auriemma, G., Rastrelli, L., & Aquino, R. P. (2025). Valorization of Hazelnut (Corylus avellana L.) Skin By-Product as a Multifunctional Ingredient for Cosmetic Emulsions. Antioxidants, 14(10), 1199. https://doi.org/10.3390/antiox14101199















