Thiol Isomerases: Enzymatic Mechanisms, Models of Oxidation, and Antagonism by Galloylated Polyphenols
Abstract
1. Introduction
2. Overview of Thiol Isomerase Family and Structural Diversity
- underscores its critical role in maintaining ER homeostasis and preventing the accumulation of misfolded proteins [9]. In addition, although PDI presumes a closed structural configuration in the reduced state compared to the open configuration in the oxidized state based on X-ray crystallography [40], atomic force microscopy [46], and single-molecule fluorescence resonance energy transfer (smFRET) studies [47,48] suggest a more dynamic structural configuration for PDI in specific redox states.
Function of Vascular Thiol Isomerases in Thrombotic Disorders
3. Chemical Mechanisms of Thiol Isomerase Oxidoreductase Activity
3.1. Oxidase
3.2. Isomerase
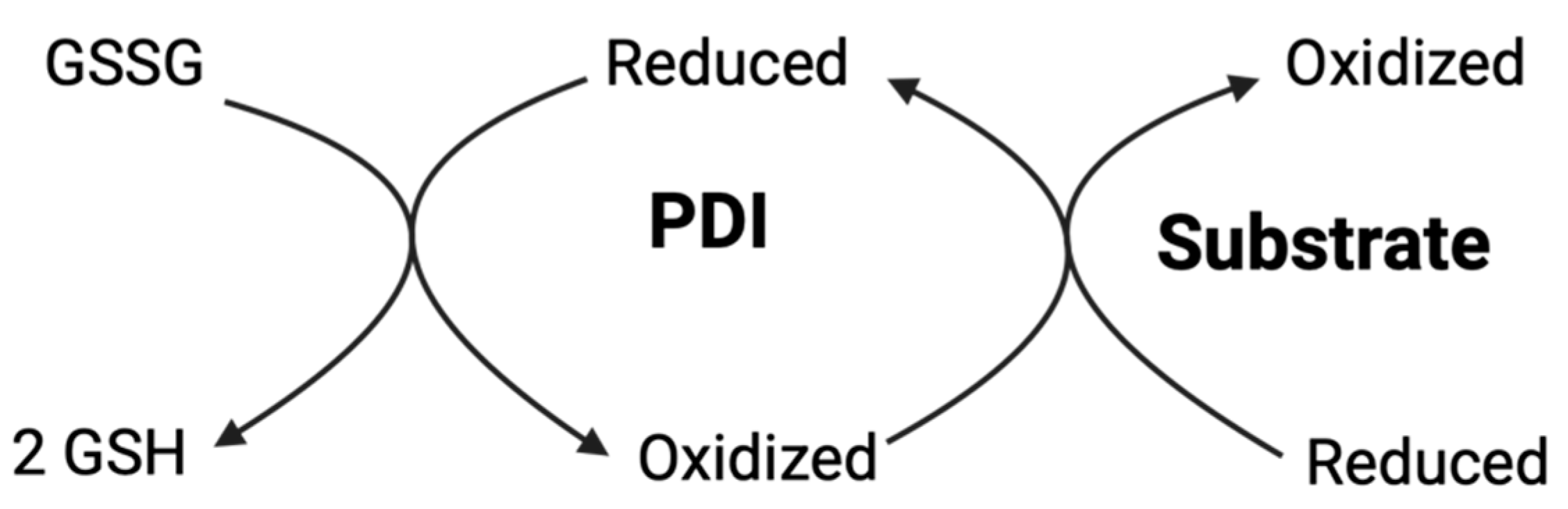
3.3. Reductase
3.4. Enzymatic Oxidation of Thiol Isomerases
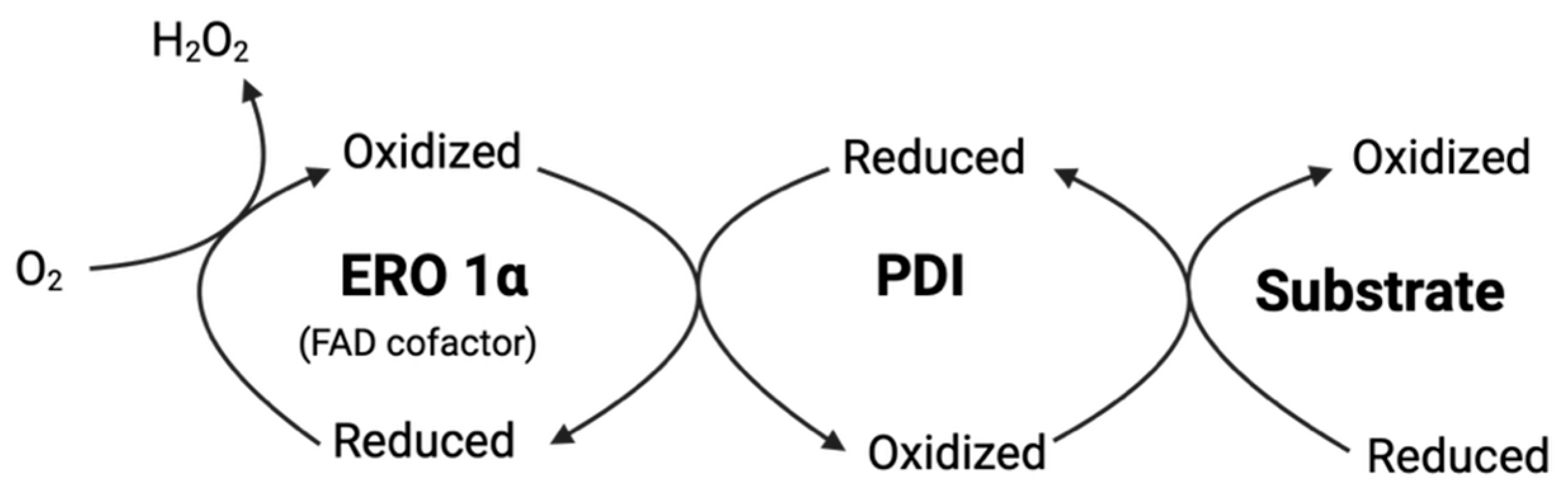
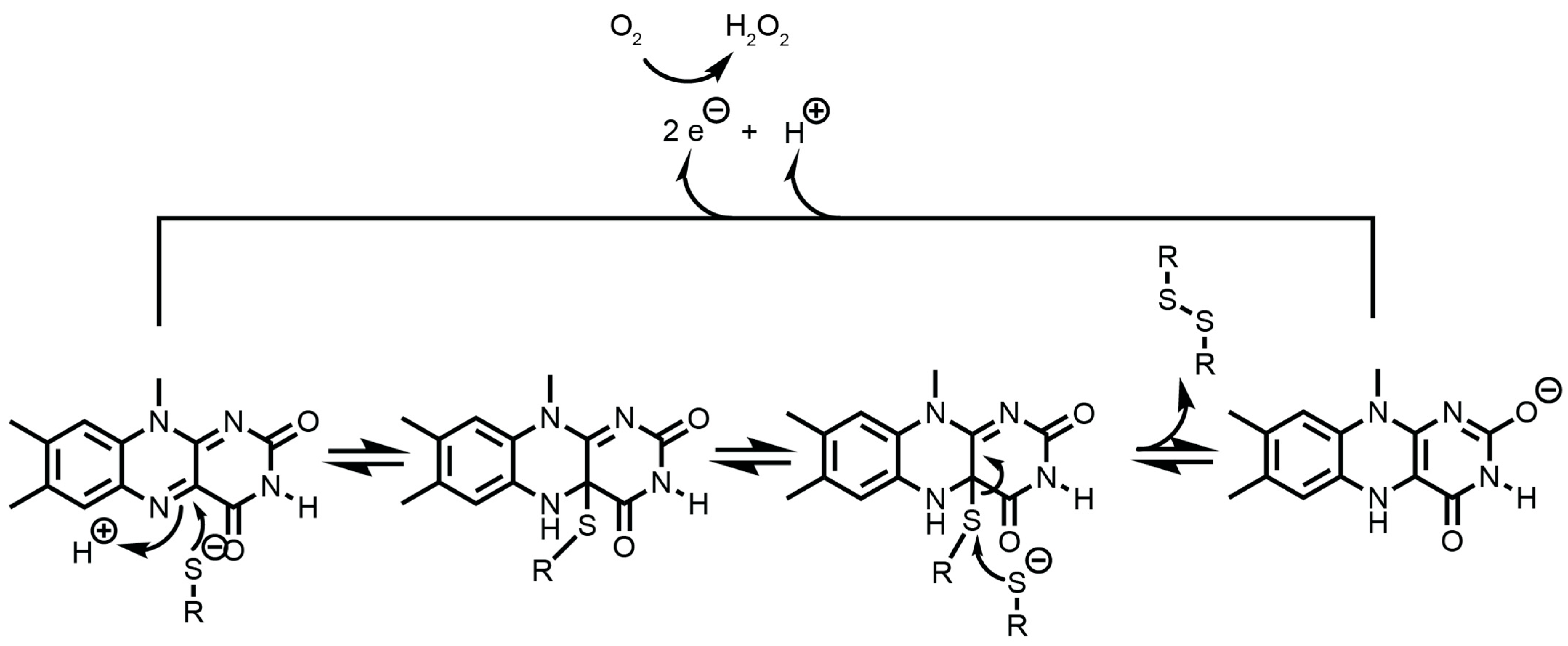
3.4.1. The Endoplasmic Reticulum Oxidoreductin 1 (ERO1)
3.4.2. Quiescin Sulfhydryl Oxidase
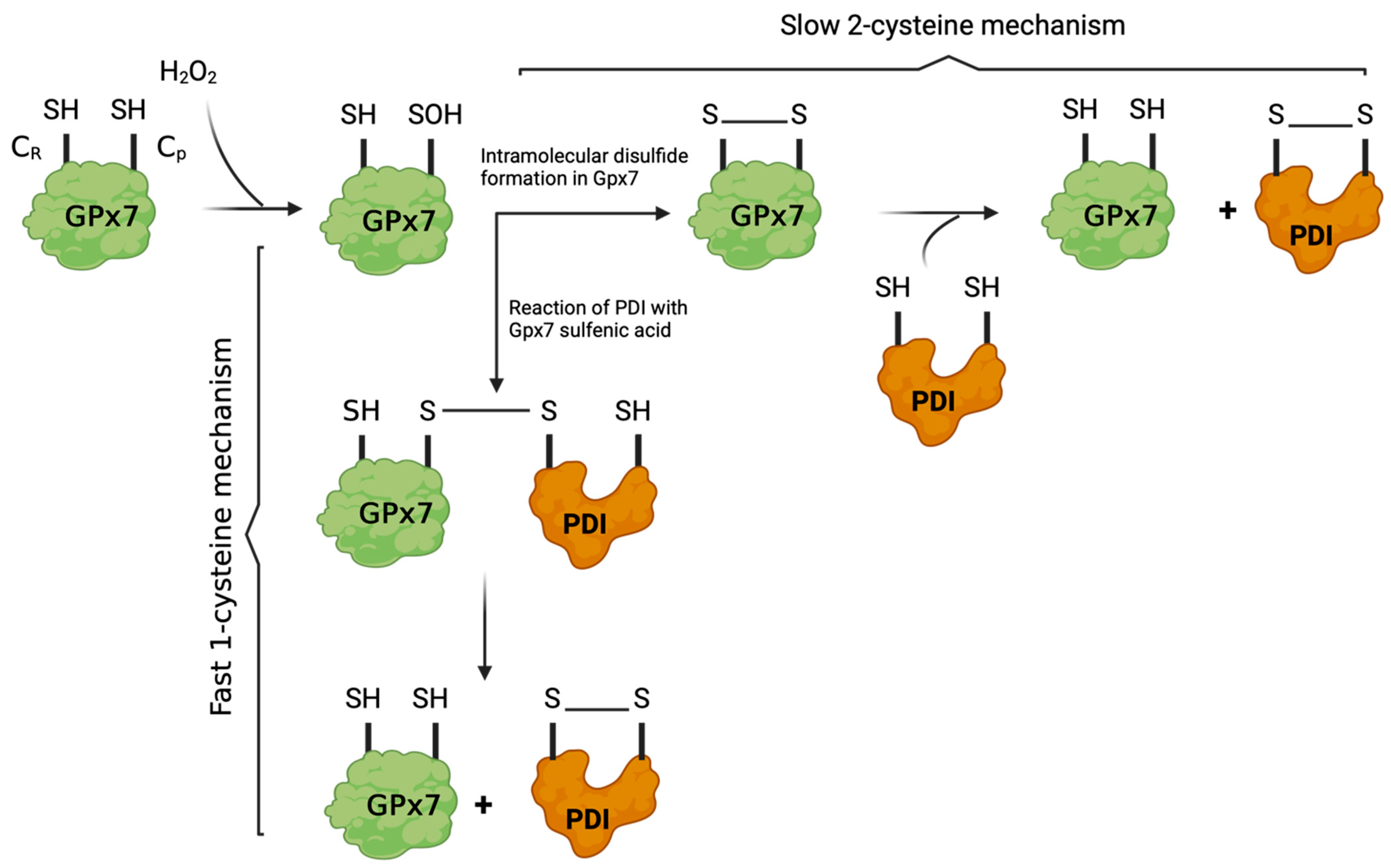
3.4.3. Glutathione Peroxidases
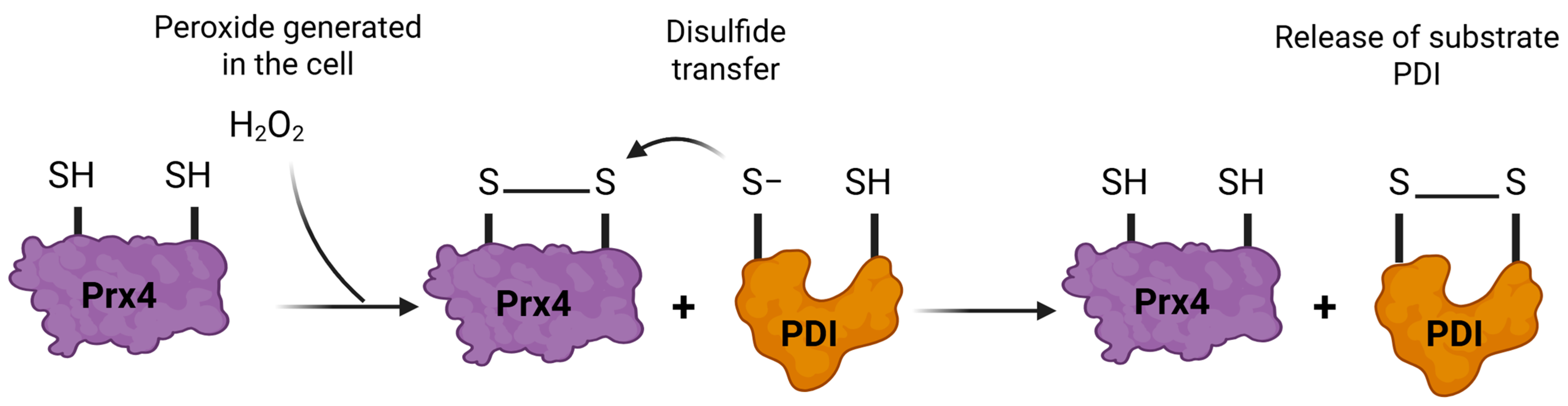
3.4.4. Peroxiredoxin 4
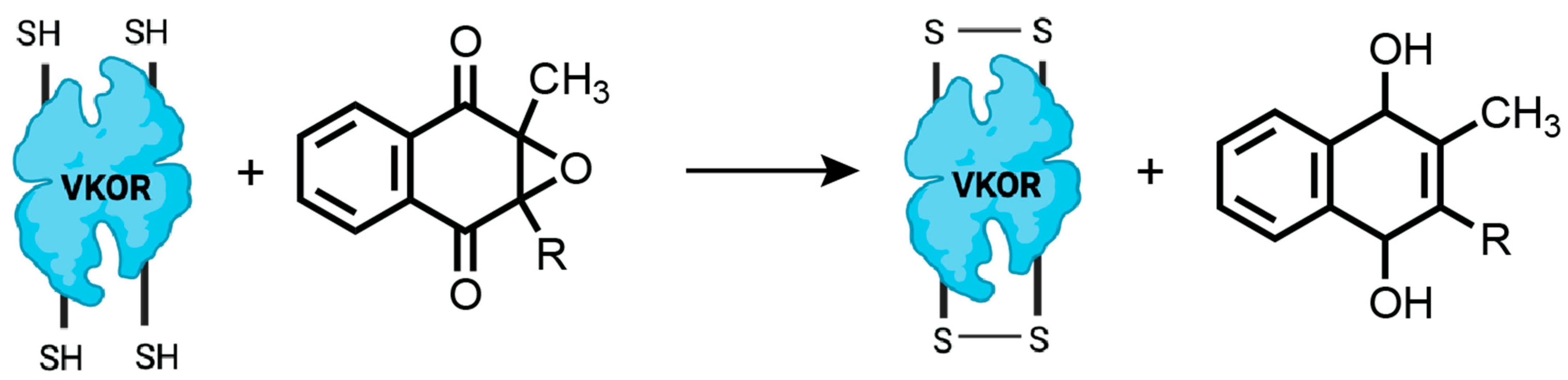
3.4.5. Vitamin K Epoxide Reductase (VKOR)
3.5. Chemical Oxidation of Thiol Isomerases
3.5.1. Hydrogen Peroxide (H2O2)
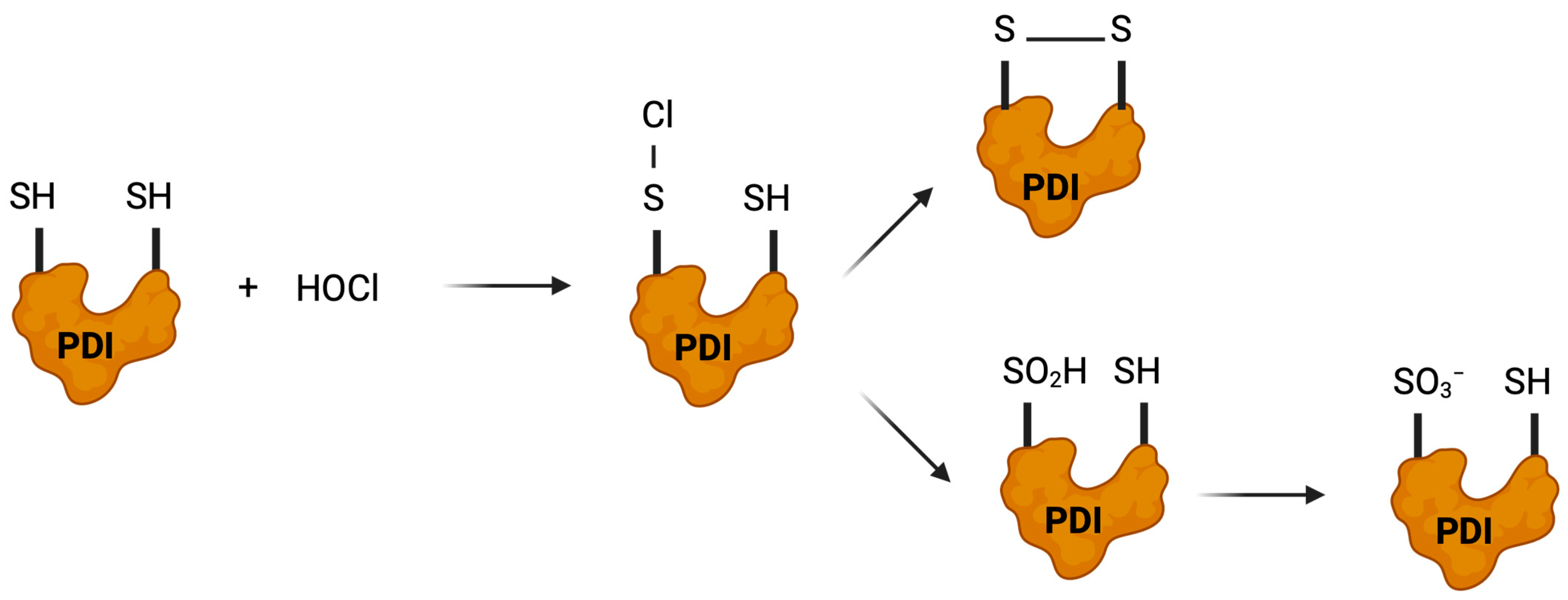
3.5.2. Hypochlorous Acid (HOCl)
3.5.3. Reactive Nitrogen Species: ONOO− and S-Nitrosation
3.5.4. Glutathione System and Thiol Redox Buffering
3.5.5. NADPH Oxidases (NOX) Derived Oxidative Species
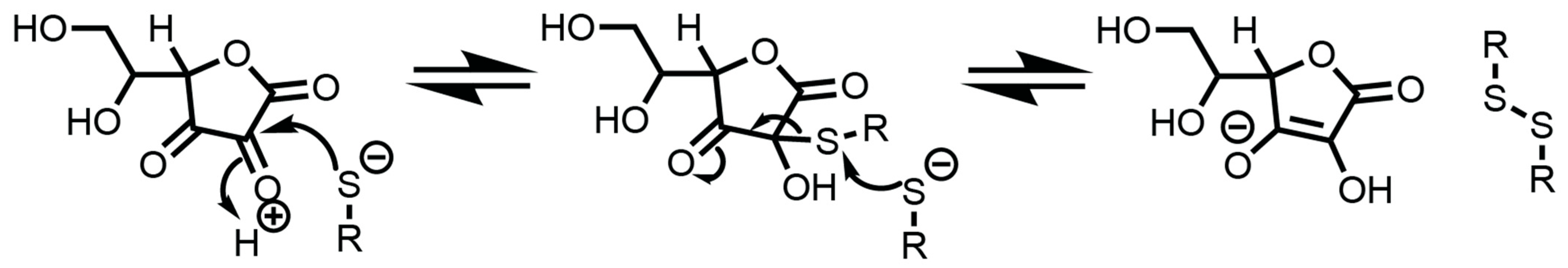
3.5.6. Dehydroascorbate (DHA)
3.5.7. Molecular Oxygen (O2) in the Presence of Transition Metals (Iron and Copper)
3.6. Implications for the Oxidase Activity of Thiol Isomerases
4. Natural Anti-Oxidative Galloylated Polyphenols Inhibit Thiol Isomerase Activity
4.1. Pharmacological Inhibitors of PDI
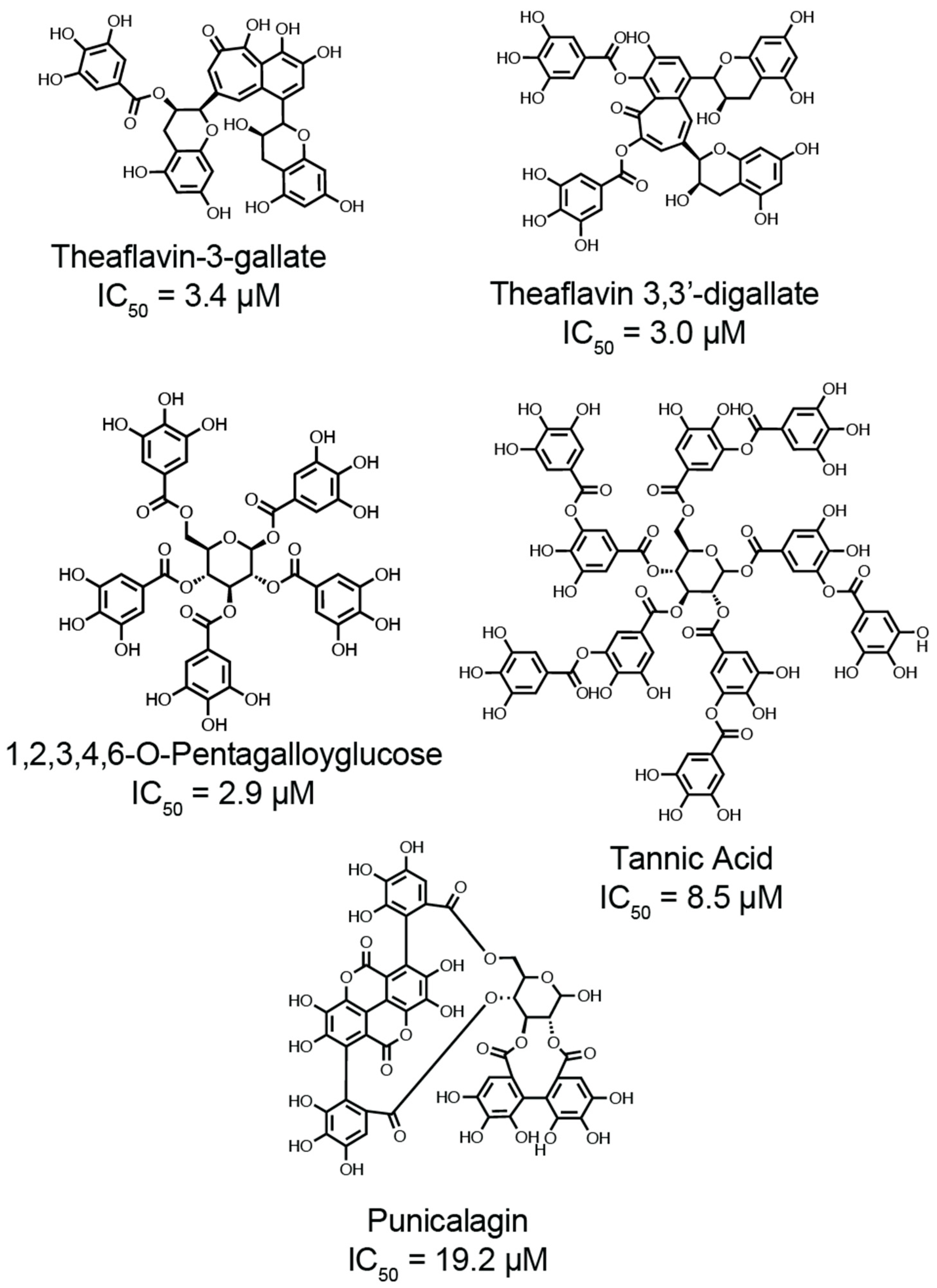
4.2. Galloylated Polyphenols as Thiol Protective Antioxidants
4.3. COVID-19-Associated Coagulopathy and Redox Enzymes
4.4. Discovery of Galloylated Polyphenols as Dual Inhibitors of Viral Protease and Thiol Isomerases
4.5. Targeting the Catalytic CXXC Motif of Thiol Isomerases
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anfinsen, C.B.; Haber, E.; Sela, M.; White, F.H., Jr. The kinetics of formation of native ribonuclease during oxidation of the reduced polypeptide chain. Proc. Natl. Acad. Sci. USA 1961, 47, 1309–1314. [Google Scholar] [CrossRef]
- Goldberger, R.F.; Epstein, C.J.; Anfinsen, C.B. Acceleration of reactivation of reduced bovine pancreatic ribonuclease by a microsomal system from rat liver. J. Biol. Chem. 1963, 238, 628–635. [Google Scholar] [CrossRef]
- Freedman, R.B.; Hirst, T.R.; Tuite, M.F. Protein disulphide isomerase: Building bridges in protein folding. Trends Biochem. Sci. 1994, 19, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Ellgaard, L.; Ruddock, L.W. The human protein disulphide isomerase family: Substrate interactions and functional properties. EMBO Rep. 2005, 6, 28–32. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, F.; Goldberger, R.F.; Steers, E., Jr.; Givol, D.; Anfinsen, B. Purification and properties of an enzyme from beef liver which catalyzes sulfhydryl-disulfide interchange in proteins. J. Biol. Chem. 1966, 241, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
- Appenzeller-Herzog, C.; Ellgaard, L. The human PDI family: Versatility packed into a single fold. Biochim. Biophys. Acta 2008, 1783, 535–548. [Google Scholar] [CrossRef]
- Kozlov, G.; Maattanen, P.; Thomas, D.Y.; Gehring, K. A structural overview of the PDI family of proteins. FEBS J. 2010, 277, 3924–3936. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Wang, C.C. Protein disulfide-isomerase, a folding catalyst and a redox-regulated chaperone. Free. Radic. Biol. Med. 2015, 83, 305–313. [Google Scholar] [CrossRef]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef]
- Flaumenhaft, R.; Furie, B.; Zwicker, J.I. Therapeutic implications of protein disulfide isomerase inhibition in thrombotic disease. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 16–23. [Google Scholar] [CrossRef]
- Gaspar, R.S.; Gibbins, J.M. Thiol Isomerases Orchestrate Thrombosis and Hemostasis. Antioxid. Redox Signal. 2021, 35, 1116–1133. [Google Scholar] [CrossRef]
- Essex, D.W.; Wang, L. Recent advances in vascular thiol isomerases and redox systems in platelet function and thrombosis. J. Thromb. Haemost. JTH 2024, 22, 1806–1818. [Google Scholar] [CrossRef]
- Oliveira, P.V.S.; Dalla Torre, M.; Debbas, V.; Orsi, A.; Laurindo, F.R.M.; Sitia, R. Transport of protein disulfide isomerase from the endoplasmic reticulum to the extracellular space without passage through the Golgi complex. J. Biol. Chem. 2024, 300, 107536. [Google Scholar] [CrossRef]
- Asquith, N.L.; Becker, I.C.; Scimone, M.T.; Boccia, T.; Camacho, V.; Barrachina, M.N.; Guo, S.; Freire, D.; Machlus, K.; Schulman, S.; et al. Targeting cargo to an unconventional secretory system within megakaryocytes allows the release of transgenic proteins from platelets. J. Thromb. Haemost. JTH 2024, 22, 3235–3248. [Google Scholar] [CrossRef]
- Theresa, L. Procoagulant activity of extracellular vesicles in plasma of patients with SARS-CoV-2 infection. EBioMedicine 2021, 68, 103411. [Google Scholar] [CrossRef] [PubMed]
- Jha, V.; Kumari, T.; Manickam, V.; Assar, Z.; Olson, K.L.; Min, J.K.; Cho, J. ERO1-PDI Redox Signaling in Health and Disease. Antioxid. Redox Signal 2021, 35, 1093–1115. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Yang, J.; Zhou, X.; Li, N.; Zhang, J. The Role of Protein Disulfide Isomerase Inhibitors in Cancer Therapy. ChemMedChem 2025, 20, e202400590. [Google Scholar] [CrossRef] [PubMed]
- Uehara, T.; Nakamura, T.; Yao, D.; Shi, Z.Q.; Gu, Z.; Ma, Y.; Masliah, E.; Nomura, Y.; Lipton, S.A. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 2006, 441, 513–517. [Google Scholar] [CrossRef]
- Parakh, S.; Shadfar, S.; Perri, E.R.; Ragagnin, A.M.G.; Piattoni, C.V.; Fogolin, M.B.; Yuan, K.C.; Shahheydari, H.; Don, E.K.; Thomas, C.J.; et al. The Redox Activity of Protein Disulfide Isomerase Inhibits ALS Phenotypes in Cellular and Zebrafish Models. iScience 2020, 23, 101097. [Google Scholar] [CrossRef]
- Zou, H.; Wen, C.; Peng, Z.; Shao, Y.; Hu, L.; Li, S.; Li, C.; Zhou, H.H. P4HB and PDIA3 are associated with tumor progression and therapeutic outcome of diffuse gliomas. Oncol. Rep. 2018, 39, 501–510. [Google Scholar] [CrossRef]
- Wise, R.; Duhachek-Muggy, S.; Qi, Y.; Zolkiewski, M.; Zolkiewska, A. Protein disulfide isomerases in the endoplasmic reticulum promote anchorage-independent growth of breast cancer cells. Breast Cancer Res. Treat. 2016, 157, 241–252. [Google Scholar] [CrossRef]
- Severino, A.; Campioni, M.; Straino, S.; Salloum, F.N.; Schmidt, N.; Herbrand, U.; Frede, S.; Toietta, G.; Di Rocco, G.; Bussani, R.; et al. Identification of protein disulfide isomerase as a cardiomyocyte survival factor in ischemic cardiomyopathy. J. Am. Coll. Cardiol. 2007, 50, 1029–1037. [Google Scholar] [CrossRef]
- Yang, M.; Silverstein, R.L. Targeting Cysteine Oxidation in Thrombotic Disorders. Antioxidants 2024, 13, 83. [Google Scholar] [CrossRef]
- Yang, M.; Flaumenhaft, R. Oxidative Cysteine Modification of Thiol Isomerases in Thrombotic Disease: A Hypothesis. Antioxid. Redox Signal 2021, 35, 1134–1155. [Google Scholar] [CrossRef] [PubMed]
- Jasuja, R.; Passam, F.H.; Kennedy, D.R.; Kim, S.H.; van Hessem, L.; Lin, L.; Bowley, S.R.; Joshi, S.S.; Dilks, J.R.; Furie, B.; et al. Protein disulfide isomerase inhibitors constitute a new class of antithrombotic agents. J. Clin. Investig. 2012, 122, 2104–2113. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Gopal, S.; Sharda, A.; Passam, F.; Bowley, S.R.; Stopa, J.; Xue, G.; Yuan, C.; Furie, B.C.; Flaumenhaft, R.; et al. Quercetin-3-rutinoside Inhibits Protein Disulfide Isomerase by Binding to Its b’x Domain. J. Biol. Chem. 2015, 290, 23543–23552. [Google Scholar] [CrossRef] [PubMed]
- Stopa, J.D.; Neuberg, D.; Puligandla, M.; Furie, B.; Flaumenhaft, R.; Zwicker, J.I. Protein disulfide isomerase inhibition blocks thrombin generation in humans by interfering with platelet factor V activation. JCI Insight 2017, 2, e89373. [Google Scholar] [CrossRef]
- Zwicker, J.I.; Schlechter, B.L.; Stopa, J.D.; Liebman, H.A.; Aggarwal, A.; Puligandla, M.; Caughey, T.; Bauer, K.A.; Kuemmerle, N.; Wong, E.; et al. Targeting protein disulfide isomerase with the flavonoid isoquercetin to improve hypercoagulability in advanced cancer. JCI Insight 2019, 4, e125851. [Google Scholar] [CrossRef]
- Chiu, J.; Hogg, P.J. Allosteric disulfides: Sophisticated molecular structures enabling flexible protein regulation. J. Biol. Chem. 2019, 294, 2949–2960. [Google Scholar] [CrossRef]
- Huang, D.; Jiang, Y.; Chen, W.; Yao, F.; Sun, L. Polyphenols with anti-proliferative activities from Penthorum chinense Pursh. Molecules 2014, 19, 11045–11055. [Google Scholar] [CrossRef]
- Zhang, T.T.; Xu, X.L.; Jiang, M.H.; Jiang, J.G. Hepatoprotective function of Penthorum chinense Pursh. Food Funct. 2013, 4, 1581–1585. [Google Scholar] [CrossRef]
- Yang, M.; Hancco Zirena, I.; Kennedy, Q.P.; Patel, A.; Merrill-Skoloff, G.; Sack, K.D.; Fulcidor, E.; Scartelli, C.; Guo, S.; Bekendam, R.H.; et al. Galloylated polyphenols represent a new class of antithrombotic agents with broad activity against thiol isomerases. J. Thromb. Haemost. JTH 2025, 23, 1850–1863. [Google Scholar] [CrossRef]
- Goszcz, K.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Bioactive polyphenols and cardiovascular disease: Chemical antagonists, pharmacological agents or xenobiotics that drive an adaptive response? Br. J. Pharmacol. 2017, 174, 1209–1225. [Google Scholar] [CrossRef]
- Anelli, T.; Sitia, R. Protein quality control in the early secretory pathway. EMBO J. 2008, 27, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Sharda, A.; Furie, B. Regulatory role of thiol isomerases in thrombus formation. Expert. Rev. Hematol. 2018, 11, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Jha, V.; Min, J.K.; Cho, J. Protein disulfide isomerase in cardiovascular disease. Exp. Mol. Med. 2020, 52, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.J.; Bulleid, N.J. Mechanisms of Disulfide Bond Formation in Nascent Polypeptides Entering the Secretory Pathway. Cells 2020, 9, 1994. [Google Scholar] [CrossRef]
- Edman, J.C.; Ellis, L.; Blacher, R.W.; Roth, R.A.; Rutter, W.J. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature 1985, 317, 267–270. [Google Scholar] [CrossRef]
- Fu, J.; Gao, J.; Liang, Z.; Yang, D. PDI-Regulated Disulfide Bond Formation in Protein Folding and Biomolecular Assembly. Molecules 2020, 26, 171. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Ren, J.; Fang, J.; Ke, H.; Gong, W.; Feng, W.; Wang, C.C. Structural insights into the redox-regulated dynamic conformations of human protein disulfide isomerase. Antioxid. Redox Signal 2013, 19, 36–45. [Google Scholar] [CrossRef]
- Wu, Y.; Essex, D.W. Vascular thiol isomerases in thrombosis: The yin and yang. J. Thromb. Haemost. JTH 2020, 18, 2790–2800. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Wallis, K.; Howard, M.J.; Haapalainen, A.M.; Salo, K.E.; Saaranen, M.J.; Sidhu, A.; Wierenga, R.K.; Freedman, R.B.; Ruddock, L.W.; et al. Alternative conformations of the x region of human protein disulphide-isomerase modulate exposure of the substrate binding b’ domain. J. Mol. Biol. 2008, 383, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, T.; Liu, Y.; Wang, X.; Zhang, J.; Wang, X.; Shi, G.; Lou, J.; Wang, L.; Wang, C.C.; et al. Phosphorylation switches protein disulfide isomerase activity to maintain proteostasis and attenuate ER stress. EMBO J. 2020, 39, e103841. [Google Scholar] [CrossRef] [PubMed]
- Romer, R.A.; Wells, S.A.; Emilio Jimenez-Roldan, J.; Bhattacharyya, M.; Vishweshwara, S.; Freedman, R.B. The flexibility and dynamics of protein disulfide isomerase. Proteins 2016, 84, 1776–1785. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.A.; Ramos, M.J. Theoretical insights into the mechanism for thiol/disulfide exchange. Chemistry 2004, 10, 257–266. [Google Scholar] [CrossRef]
- Okumura, M.; Noi, K.; Kanemura, S.; Kinoshita, M.; Saio, T.; Inoue, Y.; Hikima, T.; Akiyama, S.; Ogura, T.; Inaba, K. Dynamic assembly of protein disulfide isomerase in catalysis of oxidative folding. Nat. Chem. Biol. 2019, 15, 499–509. [Google Scholar] [CrossRef]
- Chinnaraj, M.; Barrios, D.A.; Frieden, C.; Heyduk, T.; Flaumenhaft, R.; Pozzi, N. Bioorthogonal Chemistry Enables Single-Molecule FRET Measurements of Catalytically Active Protein Disulfide Isomerase. Chembiochem 2021, 22, 134–138. [Google Scholar] [CrossRef]
- Chinnaraj, M.; Flaumenhaft, R.; Pozzi, N. Reduction of protein disulfide isomerase results in open conformations and stimulates dynamic exchange between structural ensembles. J. Biol. Chem. 2022, 298, 102217. [Google Scholar] [CrossRef]
- Hotchkiss, K.A.; Matthias, L.J.; Hogg, P.J. Exposure of the cryptic Arg-Gly-Asp sequence in thrombospondin-1 by protein disulfide isomerase. Biochim. Biophys. Acta 1998, 1388, 478–488. [Google Scholar] [CrossRef]
- Hotchkiss, K.A.; Chesterman, C.N.; Hogg, P.J. Catalysis of disulfide isomerization in thrombospondin 1 by protein disulfide isomerase. Biochemistry 1996, 35, 9761–9767. [Google Scholar] [CrossRef]
- Jordan, P.A.; Stevens, J.M.; Hubbard, G.P.; Barrett, N.E.; Sage, T.; Authi, K.S.; Gibbins, J.M. A role for the thiol isomerase protein ERP5 in platelet function. Blood 2005, 105, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Passam, F.H.; Lin, L.; Gopal, S.; Stopa, J.D.; Bellido-Martin, L.; Huang, M.; Furie, B.C.; Furie, B. Both platelet- and endothelial cell-derived ERp5 support thrombus formation in a laser-induced mouse model of thrombosis. Blood 2015, 125, 2276–2285. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, L.M.; Sandhar, G.K.; Sasikumar, P.; Schenk, M.P.; Stainer, A.R.; Sahli, K.A.; Flora, G.D.; Bicknell, A.B.; Gibbins, J.M. A humanized monoclonal antibody that inhibits platelet-surface ERp72 reveals a role for ERp72 in thrombosis. J. Thromb. Haemost. JTH 2018, 16, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wu, Y.; Zhou, J.; Chen, F.; Yang, A.; Essex, D.W. The transmembrane protein disulfide isomerase TMX1 negatively regulates platelet responses. Blood 2019, 133, 246–251. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Wang, H.J.; Chen, Y.C.; Chen, Y.; Chiu, J.; Li, L.; Wang, L.; Wang, J.; Tang, Z.; et al. Endoplasmic Reticulum Protein 72 Regulates Integrin Mac-1 Activity to Influence Neutrophil Recruitment. Arterioscler. Thromb. Vasc. Biol. 2024, 44, e82–e98. [Google Scholar] [CrossRef]
- Yan, X.; Lu, Y.; Lv, K.; Jiang, M.; Fang, C.; Wu, Y.; Yang, A. Endoplasmic reticulum protein 29 negatively regulates platelet functions and thrombosis in mice. Thromb. J. 2025, 23, 44. [Google Scholar] [CrossRef]
- Li, J.; Kim, K.; Jeong, S.Y.; Chiu, J.; Xiong, B.; Petukhov, P.A.; Dai, X.; Li, X.; Andrews, R.K.; Du, X.; et al. Platelet Protein Disulfide Isomerase Promotes Glycoprotein Ibalpha-Mediated Platelet-Neutrophil Interactions Under Thromboinflammatory Conditions. Circulation 2019, 139, 1300–1319. [Google Scholar] [CrossRef]
- Lippok, S.; Kolsek, K.; Lof, A.; Eggert, D.; Vanderlinden, W.; Muller, J.P.; Konig, G.; Obser, T.; Rohrs, K.; Schneppenheim, S.; et al. von Willebrand factor is dimerized by protein disulfide isomerase. Blood 2016, 127, 1183–1191. [Google Scholar] [CrossRef]
- Chen, V.M.; Ahamed, J.; Versteeg, H.H.; Berndt, M.C.; Ruf, W.; Hogg, P.J. Evidence for activation of tissue factor by an allosteric disulfide bond. Biochemistry 2006, 45, 12020–12028. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, Z.; Zhou, J.; Lu, Y.; Essex, D.W.; Wu, Y. Protein disulfide isomerase enhances tissue factor-dependent thrombin generation. Biochem. Biophys. Res. Commun. 2018, 501, 172–177. [Google Scholar] [CrossRef]
- Langer, F.; Spath, B.; Fischer, C.; Stolz, M.; Ayuk, F.A.; Kroger, N.; Bokemeyer, C.; Ruf, W. Rapid activation of monocyte tissue factor by antithymocyte globulin is dependent on complement and protein disulfide isomerase. Blood 2013, 121, 2324–2335. [Google Scholar] [CrossRef]
- Stopa, J.D.; Baker, K.M.; Grover, S.P.; Flaumenhaft, R.; Furie, B. Kinetic-based trapping by intervening sequence variants of the active sites of protein-disulfide isomerase identifies platelet protein substrates. J. Biol. Chem. 2017, 292, 9063–9074. [Google Scholar] [CrossRef]
- Lv, K.; Chen, S.; Xu, X.; Chiu, J.; Wang, H.J.; Han, Y.; Yang, X.; Bowley, S.R.; Wang, H.; Tang, Z.; et al. Protein disulfide isomerase cleaves allosteric disulfides in histidine-rich glycoprotein to regulate thrombosis. Nat. Commun. 2024, 15, 3129. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yuan, C.; Flaumenhaft, R.; Huang, M. Recent advances in vascular thiol isomerases: Insights into structures, functions in thrombosis and antithrombotic inhibitor development. Thromb. J. 2025, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chiu, J.; Chen, S.; Fang, C. Pathophysiological roles of cell surface and extracellular protein disulfide isomerase and their molecular mechanisms. Br. J. Pharmacol. 2021, 178, 2911–2930. [Google Scholar] [CrossRef] [PubMed]
- Givol, D.; Goldberger, R.F.; Anfinsen, C.B. Oxidation and Disulfide Interchange in the Reactivation of Reduced Ribonuclease. J. Biol. Chem. 1964, 239, PC3114–PC3116. [Google Scholar] [CrossRef]
- Butera, D.; Cook, K.M.; Chiu, J.; Wong, J.W.; Hogg, P.J. Control of blood proteins by functional disulfide bonds. Blood 2014, 123, 2000–2007. [Google Scholar] [CrossRef]
- Hatahet, F.; Ruddock, L.W. Protein disulfide isomerase: A critical evaluation of its function in disulfide bond formation. Antioxid. Redox Signal 2009, 11, 2807–2850. [Google Scholar] [CrossRef]
- Tian, G.; Kober, F.X.; Lewandrowski, U.; Sickmann, A.; Lennarz, W.J.; Schindelin, H. The catalytic activity of protein-disulfide isomerase requires a conformationally flexible molecule. J. Biol. Chem. 2008, 283, 33630–33640. [Google Scholar] [CrossRef]
- Cole, K.S.; Grandjean, J.M.D.; Chen, K.; Witt, C.H.; O’Day, J.; Shoulders, M.D.; Wiseman, R.L.; Weerapana, E. Characterization of an A-Site Selective Protein Disulfide Isomerase A1 Inhibitor. Biochemistry 2018, 57, 2035–2043. [Google Scholar] [CrossRef]
- Darby, N.; Creighton, T.E. Disulfide bonds in protein folding and stability. Methods Mol. Biol. 1995, 40, 219–252. [Google Scholar] [CrossRef]
- Kortemme, T.; Creighton, T.E. Ionisation of cysteine residues at the termini of model alpha-helical peptides. Relevance to unusual thiol pKa values in proteins of the thioredoxin family. J. Mol. Biol. 1995, 253, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Chivers, P.T.; Prehoda, K.E.; Raines, R.T. The CXXC motif: A rheostat in the active site. Biochemistry 1997, 36, 4061–4066. [Google Scholar] [CrossRef] [PubMed]
- Lappi, A.K.; Lensink, M.F.; Alanen, H.I.; Salo, K.E.; Lobell, M.; Juffer, A.H.; Ruddock, L.W. A conserved arginine plays a role in the catalytic cycle of the protein disulphide isomerases. J. Mol. Biol. 2004, 335, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Kortemme, T.; Darby, N.J.; Creighton, T.E. Electrostatic interactions in the active site of the N-terminal thioredoxin-like domain of protein disulfide isomerase. Biochemistry 1996, 35, 14503–14511. [Google Scholar] [CrossRef]
- Karala, A.R.; Lappi, A.K.; Ruddock, L.W. Modulation of an active-site cysteine pKa allows PDI to act as a catalyst of both disulfide bond formation and isomerization. J. Mol. Biol. 2010, 396, 883–892. [Google Scholar] [CrossRef]
- Garrido Ruiz, D.; Sandoval-Perez, A.; Rangarajan, A.V.; Gunderson, E.L.; Jacobson, M.P. Cysteine Oxidation in Proteins: Structure, Biophysics, and Simulation. Biochemistry 2022, 61, 2165–2176. [Google Scholar] [CrossRef]
- Roos, G.; Foloppe, N.; Messens, J. Understanding the pK(a) of redox cysteines: The key role of hydrogen bonding. Antioxid. Redox Signal 2013, 18, 94–127. [Google Scholar] [CrossRef]
- Woycechowsky, K.J.; Raines, R.T. The CXC motif: A functional mimic of protein disulfide isomerase. Biochemistry 2003, 42, 5387–5394. [Google Scholar] [CrossRef]
- Quan, S.; Schneider, I.; Pan, J.; Von Hacht, A.; Bardwell, J.C.A. The CXXC motif is more than a redox rheostat. J. Biol. Chem. 2007, 282, 28823–28833. [Google Scholar] [CrossRef]
- Frand, A.R.; Kaiser, C.A. Ero1p oxidizes protein disulfide isomerase in a pathway for disulfide bond formation in the endoplasmic reticulum. Mol. Cell 1999, 4, 469–477. [Google Scholar] [CrossRef]
- Sevier, C.S.; Kaiser, C.A. Formation and transfer of disulphide bonds in living cells. Nat. Rev. Mol. Cell Biol. 2002, 3, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Hudson, D.A.; Gannon, S.A.; Thorpe, C. Oxidative protein folding: From thiol-disulfide exchange reactions to the redox poise of the endoplasmic reticulum. Free. Radic. Biol. Med. 2015, 80, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Araki, K.; Nagata, K. Functional in vitro analysis of the ERO1 protein and protein-disulfide isomerase pathway. J. Biol. Chem. 2011, 286, 32705–32712. [Google Scholar] [CrossRef] [PubMed]
- Araki, K.; Iemura, S.; Kamiya, Y.; Ron, D.; Kato, K.; Natsume, T.; Nagata, K. Ero1-alpha and PDIs constitute a hierarchical electron transfer network of endoplasmic reticulum oxidoreductases. J. Cell Biol. 2013, 202, 861–874. [Google Scholar] [CrossRef]
- Kemmink, J.; Darby, N.J.; Dijkstra, K.; Nilges, M.; Creighton, T.E. Structure determination of the N-terminal thioredoxin-like domain of protein disulfide isomerase using multidimensional heteronuclear 13C/15N NMR spectroscopy. Biochemistry 1996, 35, 7684–7691. [Google Scholar] [CrossRef]
- Caba, C.; Ali Khan, H.; Auld, J.; Ushioda, R.; Araki, K.; Nagata, K.; Mutus, B. Conserved Residues Lys(57) and Lys(401) of Protein Disulfide Isomerase Maintain an Active Site Conformation for Optimal Activity: Implications for Post-Translational Regulation. Front. Mol. Biosci. 2018, 5, 18. [Google Scholar] [CrossRef]
- Klappa, P.; Ruddock, L.W.; Darby, N.J.; Freedman, R.B. The b’ domain provides the principal peptide-binding site of protein disulfide isomerase but all domains contribute to binding of misfolded proteins. EMBO J. 1998, 17, 927–935. [Google Scholar] [CrossRef]
- Kim, S.; Sideris, D.P.; Sevier, C.S.; Kaiser, C.A. Balanced Ero1 activation and inactivation establishes ER redox homeostasis. J. Cell Biol. 2012, 196, 713–725. [Google Scholar] [CrossRef]
- Poole, L.B. The basics of thiols and cysteines in redox biology and chemistry. Free. Radic. Biol. Med. 2015, 80, 148–157. [Google Scholar] [CrossRef]
- Appenzeller-Herzog, C.; Riemer, J.; Zito, E.; Chin, K.T.; Ron, D.; Spiess, M.; Ellgaard, L. Disulphide production by Ero1alpha-PDI relay is rapid and effectively regulated. EMBO J. 2010, 29, 3318–3329. [Google Scholar] [CrossRef] [PubMed]
- Zito, E.; Melo, E.P.; Yang, Y.; Wahlander, A.; Neubert, T.A.; Ron, D. Oxidative protein folding by an endoplasmic reticulum-localized peroxiredoxin. Mol. Cell 2010, 40, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Weissman, J.S.; Kim, P.S. Efficient catalysis of disulphide bond rearrangements by protein disulphide isomerase. Nature 1993, 365, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Rutkevich, L.A.; Cohen-Doyle, M.F.; Brockmeier, U.; Williams, D.B. Functional relationship between protein disulfide isomerase family members during the oxidative folding of human secretory proteins. Mol. Biol. Cell 2010, 21, 3093–3105. [Google Scholar] [CrossRef]
- Puig, A.; Gilbert, H.F. Protein disulfide isomerase exhibits chaperone and anti-chaperone activity in the oxidative refolding of lysozyme. J. Biol. Chem. 1994, 269, 7764–7771. [Google Scholar] [CrossRef]
- Oliver, J.D.; Roderick, H.L.; Llewellyn, D.H.; High, S. ERp57 functions as a subunit of specific complexes formed with the ER lectins calreticulin and calnexin. Mol. Biol. Cell 1999, 10, 2573–2582. [Google Scholar] [CrossRef]
- Kozlov, G.; Maattanen, P.; Schrag, J.D.; Pollock, S.; Cygler, M.; Nagar, B.; Thomas, D.Y.; Gehring, K. Crystal structure of the bb’ domains of the protein disulfide isomerase ERp57. Structure 2006, 14, 1331–1339. [Google Scholar] [CrossRef]
- Walker, K.W.; Lyles, M.M.; Gilbert, H.F. Catalysis of oxidative protein folding by mutants of protein disulfide isomerase with a single active-site cysteine. Biochemistry 1996, 35, 1972–1980. [Google Scholar] [CrossRef]
- Wilkinson, B.; Gilbert, H.F. Protein disulfide isomerase. Biochim. Biophys. Acta 2004, 1699, 35–44. [Google Scholar] [CrossRef]
- Kadokura, H.; Beckwith, J. Mechanisms of oxidative protein folding in the bacterial cell envelope. Antioxid. Redox Signal 2010, 13, 1231–1246. [Google Scholar] [CrossRef]
- Pirneskoski, A.; Klappa, P.; Lobell, M.; Williamson, R.A.; Byrne, L.; Alanen, H.I.; Salo, K.E.; Kivirikko, K.I.; Freedman, R.B.; Ruddock, L.W. Molecular characterization of the principal substrate binding site of the ubiquitous folding catalyst protein disulfide isomerase. J. Biol. Chem. 2004, 279, 10374–10381. [Google Scholar] [CrossRef]
- Denisov, A.Y.; Maattanen, P.; Dabrowski, C.; Kozlov, G.; Thomas, D.Y.; Gehring, K. Solution structure of the bb’ domains of human protein disulfide isomerase. FEBS J. 2009, 276, 1440–1449. [Google Scholar] [CrossRef]
- Walker, K.W.; Gilbert, H.F. Scanning and escape during protein-disulfide isomerase-assisted protein folding. J. Biol. Chem. 1997, 272, 8845–8848. [Google Scholar] [CrossRef] [PubMed]
- Grubb, S.; Guo, L.; Fisher, E.A.; Brodsky, J.L. Protein disulfide isomerases contribute differentially to the endoplasmic reticulum-associated degradation of apolipoprotein B and other substrates. Mol. Biol. Cell 2012, 23, 520–532. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Cunningham, C.N.; Manickam, N.; Liu, M.; Arvan, P.; Tsai, B. PDI reductase acts on Akita mutant proinsulin to initiate retrotranslocation along the Hrd1/Sel1L-p97 axis. Mol. Biol. Cell 2015, 26, 3413–3423. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Ito, S.; Noi, K.; Inoue, M.; Ushioda, R.; Kato, Y.; Nagata, K.; Inaba, K. Mechanistic characterization of disulfide bond reduction of an ERAD substrate mediated by cooperation between ERdj5 and BiP. J. Biol. Chem. 2023, 299, 105274. [Google Scholar] [CrossRef]
- Sevier, C.S.; Kaiser, C.A. Ero1 and redox homeostasis in the endoplasmic reticulum. Biochim. Biophys. Acta 2008, 1783, 549–556. [Google Scholar] [CrossRef]
- Moilanen, A.; Korhonen, K.; Saaranen, M.J.; Ruddock, L.W. Molecular analysis of human Ero1 reveals novel regulatory mechanisms for oxidative protein folding. Life Sci. Alliance 2018, 1, e201800090. [Google Scholar] [CrossRef]
- Konno, T.; Pinho Melo, E.; Lopes, C.; Mehmeti, I.; Lenzen, S.; Ron, D.; Avezov, E. ERO1-independent production of H2O2 within the endoplasmic reticulum fuels Prdx4-mediated oxidative protein folding. J. Cell Biol. 2015, 211, 253–259. [Google Scholar] [CrossRef]
- Inaba, K.; Masui, S.; Iida, H.; Vavassori, S.; Sitia, R.; Suzuki, M. Crystal structures of human Ero1alpha reveal the mechanisms of regulated and targeted oxidation of PDI. EMBO J. 2010, 29, 3330–3343. [Google Scholar] [CrossRef]
- Masui, S.; Vavassori, S.; Fagioli, C.; Sitia, R.; Inaba, K. Molecular bases of cyclic and specific disulfide interchange between human ERO1alpha protein and protein-disulfide isomerase (PDI). J. Biol. Chem. 2011, 286, 16261–16271. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthi, S.; Jessop, C.E.; Willer, M.; Stirling, C.J.; Bulleid, N.J. Intracellular catalysis of disulfide bond formation by the human sulfhydryl oxidase, QSOX1. Biochem. J. 2007, 404, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Heckler, E.J.; Rancy, P.C.; Kodali, V.K.; Thorpe, C. Generating disulfides with the Quiescin-sulfhydryl oxidases. Biochim. Biophys. Acta 2008, 1783, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Reznik, N.; Fass, D. Disulfide bond formation and redox regulation in the Golgi apparatus. FEBS Lett. 2022, 596, 2859–2872. [Google Scholar] [CrossRef]
- Coppock, D.L.; Thorpe, C. Multidomain flavin-dependent sulfhydryl oxidases. Antioxid. Redox Signal 2006, 8, 300–311. [Google Scholar] [CrossRef]
- Kodali, V.K.; Thorpe, C. Oxidative protein folding and the Quiescin-sulfhydryl oxidase family of flavoproteins. Antioxid. Redox Signal 2010, 13, 1217–1230. [Google Scholar] [CrossRef]
- Alon, A.; Grossman, I.; Gat, Y.; Kodali, V.K.; DiMaio, F.; Mehlman, T.; Haran, G.; Baker, D.; Thorpe, C.; Fass, D. The dynamic disulphide relay of quiescin sulphydryl oxidase. Nature 2012, 488, 414–418. [Google Scholar] [CrossRef]
- Grossman, I.; Yuval Aviram, H.; Armony, G.; Horovitz, A.; Hofmann, H.; Haran, G.; Fass, D. Single-molecule spectroscopy exposes hidden states in an enzymatic electron relay. Nat. Commun. 2015, 6, 8624. [Google Scholar] [CrossRef]
- Fass, D.; Thorpe, C. Chemistry and Enzymology of Disulfide Cross-Linking in Proteins. Chem. Rev. 2018, 118, 1169–1198. [Google Scholar] [CrossRef]
- Rutkevich, L.A.; Williams, D.B. Vitamin K epoxide reductase contributes to protein disulfide formation and redox homeostasis within the endoplasmic reticulum. Mol. Biol. Cell 2012, 23, 2017–2027. [Google Scholar] [CrossRef]
- Toppo, S.; Vanin, S.; Bosello, V.; Tosatto, S.C. Evolutionary and structural insights into the multifaceted glutathione peroxidase (Gpx) superfamily. Antioxid. Redox Signal 2008, 10, 1501–1514. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Saaranen, M.J.; Karala, A.R.; Lappi, A.K.; Wang, L.; Raykhel, I.B.; Alanen, H.I.; Salo, K.E.; Wang, C.C.; Ruddock, L.W. Two endoplasmic reticulum PDI peroxidases increase the efficiency of the use of peroxide during disulfide bond formation. J. Mol. Biol. 2011, 406, 503–515. [Google Scholar] [CrossRef]
- Kanemura, S.; Sofia, E.F.; Hirai, N.; Okumura, M.; Kadokura, H.; Inaba, K. Characterization of the endoplasmic reticulum-resident peroxidases GPx7 and GPx8 shows the higher oxidative activity of GPx7 and its linkage to oxidative protein folding. J. Biol. Chem. 2020, 295, 12772–12785. [Google Scholar] [CrossRef] [PubMed]
- Bassot, A.; Chen, J.; Simmen, T. Post-Translational Modification of Cysteines: A Key Determinant of Endoplasmic Reticulum-Mitochondria Contacts (MERCs). Contact 2021, 4, 25152564211001213. [Google Scholar] [CrossRef]
- Bulleid, N.J.; Ellgaard, L. Multiple ways to make disulfides. Trends Biochem. Sci. 2011, 36, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L.; Niu, Y.; Sitia, R.; Wang, C.C. Glutathione peroxidase 7 utilizes hydrogen peroxide generated by Ero1alpha to promote oxidative protein folding. Antioxid. Redox Signal 2014, 20, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Bosello-Travain, V.; Conrad, M.; Cozza, G.; Negro, A.; Quartesan, S.; Rossetto, M.; Roveri, A.; Toppo, S.; Ursini, F.; Zaccarin, M.; et al. Protein disulfide isomerase and glutathione are alternative substrates in the one Cys catalytic cycle of glutathione peroxidase 7. Biochim. Biophys. Acta 2013, 1830, 3846–3857. [Google Scholar] [CrossRef]
- Tosatto, S.C.; Bosello, V.; Fogolari, F.; Mauri, P.; Roveri, A.; Toppo, S.; Flohe, L.; Ursini, F.; Maiorino, M. The catalytic site of glutathione peroxidases. Antioxid. Redox Signal 2008, 10, 1515–1526. [Google Scholar] [CrossRef]
- Elko, E.A.; Manuel, A.M.; White, S.; Zito, E.; van der Vliet, A.; Anathy, V.; Janssen-Heininger, Y.M.W. Oxidation of peroxiredoxin-4 induces oligomerization and promotes interaction with proteins governing protein folding and endoplasmic reticulum stress. J. Biol. Chem. 2021, 296, 100665. [Google Scholar] [CrossRef]
- Zito, E. PRDX4, an endoplasmic reticulum-localized peroxiredoxin at the crossroads between enzymatic oxidative protein folding and nonenzymatic protein oxidation. Antioxid. Redox Signal 2013, 18, 1666–1674. [Google Scholar] [CrossRef]
- Sato, Y.; Kojima, R.; Okumura, M.; Hagiwara, M.; Masui, S.; Maegawa, K.; Saiki, M.; Horibe, T.; Suzuki, M.; Inaba, K. Synergistic cooperation of PDI family members in peroxiredoxin 4-driven oxidative protein folding. Sci. Rep. 2013, 3, 2456. [Google Scholar] [CrossRef]
- Schulman, S.; Wang, B.; Li, W.; Rapoport, T.A. Vitamin K epoxide reductase prefers ER membrane-anchored thioredoxin-like redox partners. Proc. Natl. Acad. Sci. USA 2010, 107, 15027–15032. [Google Scholar] [CrossRef]
- Stolyarchuk, M.; Botnari, M.; Tchertanov, L. Vitamin K Epoxide Reductase Complex-Protein Disulphide Isomerase Assemblies in the Thiol-Disulphide Exchange Reactions: Portrayal of Precursor-to-Successor Complexes. Int. J. Mol. Sci. 2024, 25, 4135. [Google Scholar] [CrossRef]
- Shen, G.; Cui, W.; Cao, Q.; Gao, M.; Liu, H.; Su, G.; Gross, M.L.; Li, W. The catalytic mechanism of vitamin K epoxide reduction in a cellular environment. J. Biol. Chem. 2021, 296, 100145. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef]
- Peixoto, A.S.; Geyer, R.R.; Iqbal, A.; Truzzi, D.R.; Soares Moretti, A.I.; Laurindo, F.R.M.; Augusto, O. Peroxynitrite preferentially oxidizes the dithiol redox motifs of protein-disulfide isomerase. J. Biol. Chem. 2018, 293, 1450–1465. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Carroll, K.S. Cysteine-mediated redox signaling: Chemistry, biology, and tools for discovery. Chem. Rev. 2013, 113, 4633–4679. [Google Scholar] [CrossRef]
- Poole, L.B.; Nelson, K.J. Discovering mechanisms of signaling-mediated cysteine oxidation. Curr. Opin. Chem. Biol. 2008, 12, 18–24. [Google Scholar] [CrossRef]
- Gupta, V.; Carroll, K.S. Sulfenic acid chemistry, detection and cellular lifetime. Biochim. Biophys. Acta 2014, 1840, 847–875. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Pattison, D.I.; Davies, M.J. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids 2003, 25, 259–274. [Google Scholar] [CrossRef]
- Pullar, J.M.; Vissers, M.C.; Winterbourn, C.C. Living with a killer: The effects of hypochlorous acid on mammalian cells. IUBMB Life 2000, 50, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Summers, F.A.; Forsman Quigley, A.; Hawkins, C.L. Identification of proteins susceptible to thiol oxidation in endothelial cells exposed to hypochlorous acid and N-chloramines. Biochem. Biophys. Res. Commun. 2012, 425, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Flouda, K.; Gammelgaard, B.; Davies, M.J.; Hawkins, C.L. Modulation of hypochlorous acid (HOCl) induced damage to vascular smooth muscle cells by thiocyanate and selenium analogues. Redox Biol. 2021, 41, 101873. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Davies, M.J.; Hawkins, C.L. Role of thiocyanate in the modulation of myeloperoxidase-derived oxidant induced damage to macrophages. Redox Biol. 2020, 36, 101666. [Google Scholar] [CrossRef]
- Sugiyama, S.; Kugiyama, K.; Aikawa, M.; Nakamura, S.; Ogawa, H.; Libby, P. Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression: Involvement of myeloperoxidase-mediated oxidant in plaque erosion and thrombogenesis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1309–1314. [Google Scholar] [CrossRef]
- Stocker, R.; Huang, A.; Jeranian, E.; Hou, J.Y.; Wu, T.T.; Thomas, S.R.; Keaney, J.F., Jr. Hypochlorous acid impairs endothelium-derived nitric oxide bioactivity through a superoxide-dependent mechanism. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2028–2033. [Google Scholar] [CrossRef]
- Belcastro, E.; Gaucher, C.; Corti, A.; Leroy, P.; Lartaud, I.; Pompella, A. Regulation of protein function by S-nitrosation and S-glutathionylation: Processes and targets in cardiovascular pathophysiology. Biol. Chem. 2017, 398, 1267–1293. [Google Scholar] [CrossRef]
- Trujillo, M.; Ferrer-Sueta, G.; Radi, R. Peroxynitrite detoxification and its biologic implications. Antioxid. Redox Signal 2008, 10, 1607–1620. [Google Scholar] [CrossRef]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Bartesaghi, S.; Radi, R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 2018, 14, 618–625. [Google Scholar] [CrossRef]
- Ferrer-Sueta, G.; Radi, R. Chemical biology of peroxynitrite: Kinetics, diffusion, and radicals. ACS Chem. Biol. 2009, 4, 161–177. [Google Scholar] [CrossRef]
- Alvarez, B.; Ferrer-Sueta, G.; Freeman, B.A.; Radi, R. Kinetics of peroxynitrite reaction with amino acids and human serum albumin. J. Biol. Chem. 1999, 274, 842–848. [Google Scholar] [CrossRef]
- Benhar, M.; Forrester, M.T.; Stamler, J.S. Protein denitrosylation: Enzymatic mechanisms and cellular functions. Nat. Rev. Mol. Cell Biol. 2009, 10, 721–732. [Google Scholar] [CrossRef]
- Bekendam, R.H.; Iyu, D.; Passam, F.; Stopa, J.D.; De Ceunynck, K.; Muse, O.; Bendapudi, P.K.; Garnier, C.L.; Gopal, S.; Crescence, L.; et al. Protein disulfide isomerase regulation by nitric oxide maintains vascular quiescence and controls thrombus formation. J. Thromb. Haemost. JTH 2018, 16, 2322–2335. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Sinskey, A.J.; Lodish, H.F. Oxidized redox state of glutathione in the endoplasmic reticulum. Science 1992, 257, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Neves, R.P.P.; Fernandes, P.A.; Ramos, M.J. Mechanistic insights on the reduction of glutathione disulfide by protein disulfide isomerase. Proc. Natl. Acad. Sci. USA 2017, 114, E4724–E4733. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef]
- Fuentes, E.; Gibbins, J.M.; Holbrook, L.M.; Palomo, I. NADPH oxidase 2 (NOX2): A key target of oxidative stress-mediated platelet activation and thrombosis. Trends Cardiovasc. Med. 2018, 28, 429–434. [Google Scholar] [CrossRef]
- Yang, M.; Li, W.; Harberg, C.; Chen, W.; Yue, H.; Ferreira, R.B.; Wynia-Smith, S.L.; Carroll, K.S.; Zielonka, J.; Flaumenhaft, R.; et al. Cysteine sulfenylation by CD36 signaling promotes arterial thrombosis in dyslipidemia. Blood Adv. 2020, 4, 4494–4507. [Google Scholar] [CrossRef]
- Yang, M.; Chiu, J.; Scartelli, C.; Ponzar, N.; Patel, S.; Patel, A.; Ferreira, R.B.; Keyes, R.F.; Carroll, K.; Pozzi, N.; et al. Sulfenylation links oxidative stress to protein disulfide isomerase oxidase activity and thrombus formation. J. Thromb. Haemost. JTH 2023, 21, 2137–2150. [Google Scholar] [CrossRef]
- Nagarkoti, S.; Kim, Y.M.; Ash, D.; Das, A.; Vitriol, E.; Read, T.A.; Youn, S.W.; Sudhahar, V.; McMenamin, M.; Hou, Y.; et al. Protein disulfide isomerase A1 as a novel redox sensor in VEGFR2 signaling and angiogenesis. Angiogenesis 2023, 26, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Bechor, E.; Dahan, I.; Fradin, T.; Berdichevsky, Y.; Zahavi, A.; Federman Gross, A.; Rafalowski, M.; Pick, E. The dehydrogenase region of the NADPH oxidase component Nox2 acts as a protein disulfide isomerase (PDI) resembling PDIA3 with a role in the binding of the activator protein p67 (phox.). Front. Chem. 2015, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Saaranen, M.J.; Karala, A.R.; Lappi, A.K.; Ruddock, L.W. The role of dehydroascorbate in disulfide bond formation. Antioxid. Redox Signal 2010, 12, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Eben, S.S.; Imlay, J.A. Evidence that protein thiols are not primary targets of intracellular reactive oxygen species in growing Escherichia coli. Front. Microbiol. 2023, 14, 1305973. [Google Scholar] [CrossRef]
- Tyson, E.L.; Ament, M.S.; Yoon, T.P. Transition metal photoredox catalysis of radical thiol-ene reactions. J. Org. Chem. 2013, 78, 2046–2050. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Peskin, A.V.; Parsons-Mair, H.N. Thiol oxidase activity of copper, zinc superoxide dismutase. J. Biol. Chem. 2002, 277, 1906–1911. [Google Scholar] [CrossRef]
- Ngamchuea, K.; Batchelor-McAuley, C.; Compton, R.G. The Copper(II)-Catalyzed Oxidation of Glutathione. Chemistry 2016, 22, 15937–15944. [Google Scholar] [CrossRef]
- Hippeli, S.; Elstner, E.F. Transition metal ion-catalyzed oxygen activation during pathogenic processes. FEBS Lett. 1999, 443, 1–7. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.M.; Jones, D.P. Redox compartmentalization in eukaryotic cells. Biochim. Biophys. Acta 2008, 1780, 1273–1290. [Google Scholar] [CrossRef] [PubMed]
- Raturi, A.; Mutus, B. Characterization of redox state and reductase activity of protein disulfide isomerase under different redox environments using a sensitive fluorescent assay. Free. Radic. Biol. Med. 2007, 43, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, A. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J. Biol. Chem. 1979, 254, 9627–9632. [Google Scholar] [CrossRef]
- Watanabe, M.M.; Laurindo, F.R.; Fernandes, D.C. Methods of measuring protein disulfide isomerase activity: A critical overview. Front. Chem. 2014, 2, 73. [Google Scholar] [CrossRef]
- Creighton, T.E. Intermediates in the refolding of reduced ribonuclease A. J. Mol. Biol. 1979, 129, 411–431. [Google Scholar] [CrossRef]
- Lyles, M.M.; Gilbert, H.F. Catalysis of the oxidative folding of ribonuclease A by protein disulfide isomerase: Dependence of the rate on the composition of the redox buffer. Biochemistry 1991, 30, 613–619. [Google Scholar] [CrossRef]
- Rothwarf, D.M.; Li, Y.J.; Scheraga, H.A. Regeneration of bovine pancreatic ribonuclease A: Identification of two nativelike three-disulfide intermediates involved in separate pathways. Biochemistry 1998, 37, 3760–3766. [Google Scholar] [CrossRef]
- Freedman, R.B.; Klappa, P.; Ruddock, L.W. Model peptide substrates and ligands in analysis of action of mammalian protein disulfide-isomerase. Methods Enzymol. 2002, 348, 342–354. [Google Scholar] [CrossRef]
- Ruddock, L.W.; Hirst, T.R.; Freedman, R.B. pH-dependence of the dithiol-oxidizing activity of DsbA (a periplasmic protein thiol:disulphide oxidoreductase) and protein disulphide-isomerase: Studies with a novel simple peptide substrate. Biochem. J. 1996, 315 Pt 3, 1001–1005. [Google Scholar] [CrossRef]
- Morgan, B.; Sobotta, M.C.; Dick, T.P. Measuring E(GSH) and H2O2 with roGFP2-based redox probes. Free. Radic. Biol. Med. 2011, 51, 1943–1951. [Google Scholar] [CrossRef]
- Xu, S.; Sankar, S.; Neamati, N. Protein disulfide isomerase: A promising target for cancer therapy. Drug Discov. Today 2014, 19, 222–240. [Google Scholar] [CrossRef]
- Krajewski, D.; Polukort, S.H.; Gelzinis, J.; Rovatti, J.; Kaczenski, E.; Galinski, C.; Pantos, M.; Shah, N.N.; Schneider, S.S.; Kennedy, D.R.; et al. Protein Disulfide Isomerases Regulate IgE-Mediated Mast Cell Responses and Their Inhibition Confers Protective Effects During Food Allergy. Front. Immunol. 2020, 11, 606837. [Google Scholar] [CrossRef]
- Pierre, A.S.; Gavriel, N.; Guilbard, M.; Ogier-Denis, E.; Chevet, E.; Delom, F.; Igbaria, A. Modulation of Protein Disulfide Isomerase Functions by Localization: The Example of the Anterior Gradient Family. Antioxid. Redox Signal 2024, 41, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Yuen, K.L.; Molony, R.D.; Silvers, C.R.; Akash, M.M.H.; Messing, E.M.; Lee, Y.F. Protein disulfide isomerase-enriched extracellular vesicles from bladder cancer cells support tumor survival and malignant transformation in the bladder. Oncogene 2025, 44, 2158–2169. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Lin, Y.; Detwiler, T.C. Protein disulfide isomerase activity is released by activated platelets. Blood 1992, 79, 2226–2228. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Furie, B.C.; Coughlin, S.R.; Furie, B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J. Clin. Investig. 2008, 118, 1123–1131. [Google Scholar] [CrossRef]
- Schulman, S.; Bendapudi, P.; Sharda, A.; Chen, V.; Bellido-Martin, L.; Jasuja, R.; Furie, B.C.; Flaumenhaft, R.; Furie, B. Extracellular Thiol Isomerases and Their Role in Thrombus Formation. Antioxid. Redox Signal 2016, 24, 1–15. [Google Scholar] [CrossRef]
- Rahman, N.S.A.; Zahari, S.; Syafruddin, S.E.; Firdaus-Raih, M.; Low, T.Y.; Mohtar, M.A. Functions and mechanisms of protein disulfide isomerase family in cancer emergence. Cell Biosci. 2022, 12, 129. [Google Scholar] [CrossRef]
- Xu, S.; Butkevich, A.N.; Yamada, R.; Zhou, Y.; Debnath, B.; Duncan, R.; Zandi, E.; Petasis, N.A.; Neamati, N. Discovery of an orally active small-molecule irreversible inhibitor of protein disulfide isomerase for ovarian cancer treatment. Proc. Natl. Acad. Sci. USA 2012, 109, 16348–16353. [Google Scholar] [CrossRef]
- Stojak, M.; Milczarek, M.; Kurpinska, A.; Suraj-Prazmowska, J.; Kaczara, P.; Wojnar-Lason, K.; Banach, J.; Stachowicz-Suhs, M.; Rossowska, J.; Kalvins, I.; et al. Protein Disulphide Isomerase A1 Is Involved in the Regulation of Breast Cancer Cell Adhesion and Transmigration via Lung Microvascular Endothelial Cells. Cancers 2020, 12, 2850. [Google Scholar] [CrossRef]
- Bekendam, R.H.; Bendapudi, P.K.; Lin, L.; Nag, P.P.; Pu, J.; Kennedy, D.R.; Feldenzer, A.; Chiu, J.; Cook, K.M.; Furie, B.; et al. A substrate-driven allosteric switch that enhances PDI catalytic activity. Nat. Commun. 2016, 7, 12579. [Google Scholar] [CrossRef]
- Khan, M.M.; Simizu, S.; Lai, N.S.; Kawatani, M.; Shimizu, T.; Osada, H. Discovery of a small molecule PDI inhibitor that inhibits reduction of HIV-1 envelope glycoprotein gp120. ACS Chem. Biol. 2011, 6, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Pace, N.J.; Brown, D.R.; Weerapana, E. 1,3,5-Triazine as a modular scaffold for covalent inhibitors with streamlined target identification. J. Am. Chem. Soc. 2013, 135, 2497–2500. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.H.; Zhu, Q.; Gao, G.W.; Zhou, C.C.; Li, D.W. Preparation, characterization and potential application of monoclonal antibody 18A4 against AGR2. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2010, 26, 49–51. [Google Scholar] [PubMed]
- Rao, M.J.; Zheng, B. The Role of Polyphenols in Abiotic Stress Tolerance and Their Antioxidant Properties to Scavenge Reactive Oxygen Species and Free Radicals. Antioxidants 2025, 14, 74. [Google Scholar] [CrossRef]
- Andres, C.M.C.; Perez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Perez-Lebena, E. Antioxidant Metabolism Pathways in Vitamins, Polyphenols, and Selenium: Parallels and Divergences. Int. J. Mol. Sci. 2024, 25, 2600. [Google Scholar] [CrossRef]
- Stiller, A.; Garrison, K.; Gurdyumov, K.; Kenner, J.; Yasmin, F.; Yates, P.; Song, B.H. From Fighting Critters to Saving Lives: Polyphenols in Plant Defense and Human Health. Int. J. Mol. Sci. 2021, 22, 8995. [Google Scholar] [CrossRef]
- Kappen, J.; Rashan, L.; Franke, K.; Wessjohann, L.A. Profiling and Bioactivity of Polyphenols from the Omani Medicinal Plant Terminalia dhofarica (syn. Anogeissus dhofarica). Molecules 2025, 30, 952. [Google Scholar] [CrossRef]
- Dewick, P.M. The Shikimate Pathway: Aromatic Amino Acids and Phenylpropanoids. In Medicine Natural Products: A Biosynthetic Approach, 3rd ed.; Wiley, A John Wiley and Sons, Ltd.: Chichester, UK; West Sussex, UK, 2009; pp. 137–186. [Google Scholar]
- Ding, L.; Wang, H.P.; Zhao, J.Y.; Zhao, X.; Sha, Y.; Qin, L.Q.; Hidayat, K. Coffee and tea consumption and cardiovascular disease and all-cause and cause-specific mortality in individuals with diabetes mellitus: A meta-analysis of prospective observational studies. Front. Nutr. 2025, 12, 1570644. [Google Scholar] [CrossRef]
- Masselli, E.; Pozzi, G.; Vaccarezza, M.; Mirandola, P.; Galli, D.; Vitale, M.; Carubbi, C.; Gobbi, G. ROS in Platelet Biology: Functional Aspects and Methodological Insights. Int. J. Mol. Sci. 2020, 21, 4866. [Google Scholar] [CrossRef]
- Liao, X.; Zhuang, X.; Liang, C.; Li, J.; Flaumenhaft, R.; Yuan, C.; Huang, M. Flavonoids as Protein Disulfide Isomerase Inhibitors: Key Molecular and Structural Features for the Interaction. J. Agric. Food Chem. 2022, 70, 4475–4483. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Y.; Liu, P.; Zhou, Y.; Jiang, L.; Yuan, C.; Huang, M. Orally delivered rutin in lipid-based nano-formulation exerts strong antithrombotic effects by protein disulfide isomerase inhibition. Drug Deliv. 2022, 29, 1824–1835. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A.; Aatif, M.; Muteeb, G.; Khan, K.; Siddiqui, F.A. Dietary Polyphenols, Plant Metabolites, and Allergic Disorders: A Comprehensive Review. Pharmaceuticals 2024, 17, 670. [Google Scholar] [CrossRef]
- Scott, M.B.; Styring, A.K.; McCullagh, J.S.O. Polyphenols: Bioavailability, Microbiome Interactions and Cellular Effects on Health in Humans and Animals. Pathogens 2022, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Cosme, F.; Aires, A.; Pinto, T.; Oliveira, I.; Vilela, A.; Goncalves, B. A Comprehensive Review of Bioactive Tannins in Foods and Beverages: Functional Properties, Health Benefits, and Sensory Qualities. Molecules 2025, 30, 800. [Google Scholar] [CrossRef] [PubMed]
- Rettew, A.; Garrahy, I.; Rahimian, S.; Brown, R.; Sangha, N. COVID-19 Coagulopathy. Life 2024, 14, 953. [Google Scholar] [CrossRef] [PubMed]
- Obeagu, E.I.; Obeagu, G.U. Thromboinflammation in COVID-19: Unraveling the interplay of coagulation and inflammation. Medicine 2024, 103, e38922. [Google Scholar] [CrossRef]
- Gando, S.; Akiyama, T. Disseminated intravascular coagulation is associated with poor prognosis in patients with COVID-19. Sci. Rep. 2024, 14, 12443. [Google Scholar] [CrossRef]
- Sofizan, N.; Rahman, A.; Soon, L.P.; Ly, C.K.; Abdullah, N.Z.B. Autopsy findings in COVID-19 infection-related death: A systematic review. Egypt. J. Forensic Sci. 2022, 12, 22. [Google Scholar] [CrossRef]
- Paramo, J.A.; Marcos-Jubilar, M. Immunothrombosis: A key mechanism in the COVID-19 pandemic. Med. Clin. 2024, 163, 517–521. [Google Scholar] [CrossRef]
- Almskog, L.M.; Agren, A. Thromboinflammation vs. immunothrombosis: Strategies for overcoming anticoagulant resistance in COVID-19 and other hyperinflammatory diseases. Is ROTEM helpful or not? Front. Immunol. 2025, 16, 1599639. [Google Scholar] [CrossRef] [PubMed]
- Wolszczak-Biedrzycka, B.; Dorf, J.; Matowicka-Karna, J.; Dymicka-Piekarska, V.; Wojewodzka-Zelezniakowicz, M.; Zukowski, P.; Zalewska, A.; Dabrowski, L.; Maciejczyk, M. Redox Biomarkers—An Effective Tool for Diagnosing COVID-19 Patients and Convalescents. J. Inflamm. Res. 2024, 17, 2589–2607. [Google Scholar] [CrossRef] [PubMed]
- Bowley, S.R.; Fang, C.; Merrill-Skoloff, G.; Furie, B.C.; Furie, B. Protein disulfide isomerase secretion following vascular injury initiates a regulatory pathway for thrombus formation. Nat. Commun. 2017, 8, 14151. [Google Scholar] [CrossRef] [PubMed]
- Sharda, A.V.; Bogue, T.; Barr, A.; Mendez, L.M.; Flaumenhaft, R.; Zwicker, J.I. Circulating Protein Disulfide Isomerase Is Associated with Increased Risk of Thrombosis in JAK2-Mutated Myeloproliferative Neoplasms. Clin. Cancer Res. 2021, 27, 5708–5717. [Google Scholar] [CrossRef]
- Jing, H.; Wu, X.; Xiang, M.; Liu, L.; Novakovic, V.A.; Shi, J. Pathophysiological mechanisms of thrombosis in acute and long COVID-19. Front. Immunol. 2022, 13, 992384. [Google Scholar] [CrossRef]
- Gevorgyan, S.; Khachatryan, H.; Shavina, A.; Gharaghani, S.; Zakaryan, H. Targeting SARS-CoV-2 main protease: A comprehensive approach using advanced virtual screening, molecular dynamics, and in vitro validation. Virol. J. 2024, 21, 330. [Google Scholar] [CrossRef]
- Pelesz, A.; Rafa-Zablocka, K.; Kaczara, P.; Chlopicki, S.; Przyborowski, K. Protein disulfide isomerase 1 (PDIA1) regulates platelet-derived extracellular vesicle release. Thromb. Res. 2025, 245, 109209. [Google Scholar] [CrossRef]
- Khan, A.B.; Siddiqui, U.; Fatima, S.; Rehman, A.A.; Jairajpuri, M.A. Naringin binds to protein disulfide isomerase to inhibit its activity and modulate the blood coagulation rates: Implications in controlling thrombosis. Int. J. Biol. Macromol. 2023, 252, 126241. [Google Scholar] [CrossRef]
- Tuculeanu, G.; Barbu, E.C.; Lazar, M.; Chitu-Tisu, C.E.; Moisa, E.; Negoita, S.I.; Ion, D.A. Coagulation Disorders in Sepsis and COVID-19-Two Sides of the Same Coin? A Review of Inflammation-Coagulation Crosstalk in Bacterial Sepsis and COVID-19. J. Clin. Med. 2023, 12, 601. [Google Scholar] [CrossRef]
- Ullrich, S.; Nitsche, C. The SARS-CoV-2 main protease as drug target. Bioorg Med. Chem. Lett. 2020, 30, 127377. [Google Scholar] [CrossRef]
- Ferreira, J.C.; Fadl, S.; Villanueva, A.J.; Rabeh, W.M. Catalytic Dyad Residues His41 and Cys145 Impact the Catalytic Activity and Overall Conformational Fold of the Main SARS-CoV-2 Protease 3-Chymotrypsin-Like Protease. Front. Chem. 2021, 9, 692168. [Google Scholar] [CrossRef]
- Lin, L.; Chen, D.Y.; Scartelli, C.; Xie, H.; Merrill-Skoloff, G.; Yang, M.; Sun, L.; Saeed, M.; Flaumenhaft, R. Plant flavonoid inhibition of SARS-CoV-2 main protease and viral replication. iScience 2023, 26, 107602. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.F.; Wang, W.J.; Chen, C.Y.; Chang, W.C.; Hsueh, P.R.; Peng, S.L.; Wu, C.S.; Chen, Y.; Huang, H.Y.; Shen, W.J.; et al. The natural tannins oligomeric proanthocyanidins and punicalagin are potent inhibitors of infection by SARS-CoV-2. Elife 2023, 12, e84899. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, Y.; Wang, L.; Rauova, L.; Hayes, V.M.; Poncz, M.; Essex, D.W. The C-terminal CGHC motif of protein disulfide isomerase supports thrombosis. J. Clin. Investig. 2015, 125, 4391–4406. [Google Scholar] [CrossRef] [PubMed]

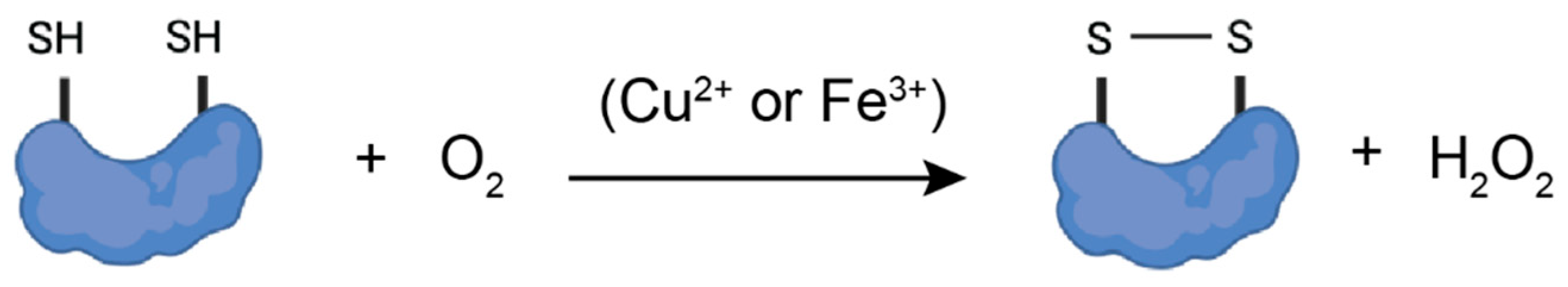

| Thiol Isomerase | Gene Name | Amino Acids | Molecular Mass (kD) | Domain Structure | CXXC Motif |
|---|---|---|---|---|---|
| PDI | P4HB | 508 | 57.1 | a-b-b′-a′ | 53CGHC56; 397CGHC400 |
| PDIp | PDIA2 | 525 | 58.2 | a-b-b′-a′ | 71CGHC74; 418CTHC421 |
| PDIr | PDIA5 | 519 | 59.5 | a0-a-b-b′ | 182CSMC185; 305CGHC308; 426CPHC429 |
| PDILT | PDILT | 584 | 66.6 | a0-a-b-b′ | 72SKQS75; 417SKKC420 |
| ERp5 | PDIA6 | 440 | 48.1 | a-a-b | 55CGHC58; 190CGHC193 |
| ERp18 | TXNDC12 | 172 | 19.2 | a | 66CGAC69 |
| ERp27 | ERP27 | 273 | 30.5 | b-b′ | ---- |
| ERp29 | ERP29 | 261 | 29 | b-b′ | ---- |
| ERp44 | ERP44 | 406 | 46.9 | a-b-b′ | 58CRFS60 |
| ERp46 | TXNDC5 | 432 | 47.6 | a-b-b′ | 89CGHC92; 217CGHC220; 350CGHC353 |
| ERp57 | PDIA3 | 505 | 56.8 | a-b-b′-a′ | 57CGHC60; 406CGHC409 |
| ERp72 | PDIA4 | 645 | 72.9 | a0-a-b-b′-a | 91CGHC94; 206CGHC209; 555CGHC558 |
| ERp90 | TXNDC16 | 825 | 93.5 | a-a-a-b-b′ | 84CX8C93; 216CX9C226; 449CX6C456 |
| ERdj5 | DNAJC10 | 793 | 91.1 | a0-b-a-a-a | 158CSHC161; 480CPPC483; 588CHPC591; 700CGPC703 |
| TMX1 | TMX1 | 280 | 31.7 | a | 56CPAC59 |
| TMX2 | TMX2 | 296 | 34 | b | 167SNDC170 |
| TMX3 | TMX3 | 454 | 51.8 | a-b-b′ | 53CGHC56 |
| TMX4 | TMX4 | 349 | 38.9 | a | 64CPSC67 |
| TMX5 | TXNDC15 | 360 | 39.6 | b′ | 220CRFS223 |
| AGR2 | AGR2 | 175 | 19.9 | a | 81CPHS84 |
| AGR3 | AGR3 | 166 | 19.1 | a | 71CQYS74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owegie, O.C.; Kennedy, Q.P.; Davizon-Castillo, P.; Yang, M. Thiol Isomerases: Enzymatic Mechanisms, Models of Oxidation, and Antagonism by Galloylated Polyphenols. Antioxidants 2025, 14, 1193. https://doi.org/10.3390/antiox14101193
Owegie OC, Kennedy QP, Davizon-Castillo P, Yang M. Thiol Isomerases: Enzymatic Mechanisms, Models of Oxidation, and Antagonism by Galloylated Polyphenols. Antioxidants. 2025; 14(10):1193. https://doi.org/10.3390/antiox14101193
Chicago/Turabian StyleOwegie, Osamede C., Quinn P. Kennedy, Pavel Davizon-Castillo, and Moua Yang. 2025. "Thiol Isomerases: Enzymatic Mechanisms, Models of Oxidation, and Antagonism by Galloylated Polyphenols" Antioxidants 14, no. 10: 1193. https://doi.org/10.3390/antiox14101193
APA StyleOwegie, O. C., Kennedy, Q. P., Davizon-Castillo, P., & Yang, M. (2025). Thiol Isomerases: Enzymatic Mechanisms, Models of Oxidation, and Antagonism by Galloylated Polyphenols. Antioxidants, 14(10), 1193. https://doi.org/10.3390/antiox14101193







