Melatonin-Mediated Nrf2 Activation as a Potential Therapeutic Strategy in Mutation-Driven Neurodegenerative Diseases
Abstract
1. Introduction
1.1. Aging and Neurodegenerative Diseases (NDs)
1.2. Nrf2 and NDs
1.3. Melatonin and Nrf2
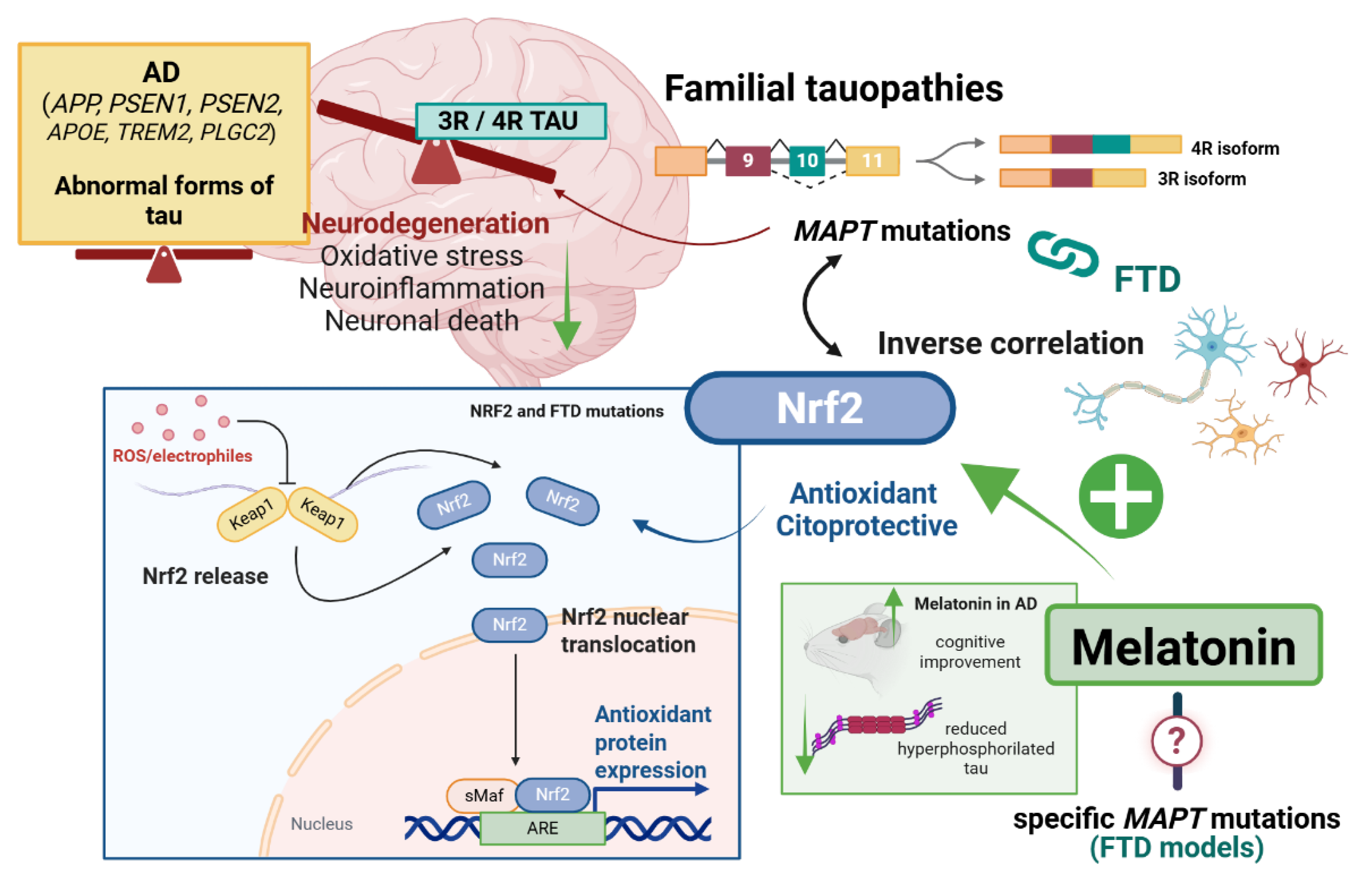
2. Familial Tauopathies and Nrf2/Melatonin Axis
3. PD and Nrf2/Melatonin Axis
4. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Keshavarz, M.; Xie, K.; Bano, D.; Ehninger, D. Aging—What It Is and How to Measure It. Mech. Ageing Dev. 2023, 213, 111837. [Google Scholar] [CrossRef]
- Li, S.; Vazquez, J.M.; Sudmant, P.H. The Evolution of Aging and Lifespan. Trends Genet. 2023, 39, 830–843. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxid. Med. Cell. Longev. 2019, 2019, 2105607. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.; Piekut, T.; Prendecki, M.; Sodel, A.; Kozubski, W.; Dorszewska, J. Mitochondrial and Nuclear DNA Oxidative Damage in Physiological and Pathological Aging. DNA Cell Biol. 2020, 39, 1410–1420. [Google Scholar] [CrossRef]
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 617588. [Google Scholar] [CrossRef]
- Stefanatos, R.; Sanz, A. The Role of Mitochondrial ROS in the Aging Brain. FEBS Lett. 2018, 592, 743–758. [Google Scholar] [CrossRef]
- Yin, F.; Boveris, A.; Cadenas, E. Mitochondrial Energy Metabolism and Redox Signaling in Brain Aging and Neurodegeneration. Antioxid. Redox Signal. 2014, 20, 353–371. [Google Scholar] [CrossRef]
- Wilson, D.M.; Cookson, M.R.; Bosch, L.V.D.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of Neurodegenerative Diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef]
- Buendia, I.; Michalska, P.; Navarro, E.; Gameiro, I.; Egea, J.; León, R. Nrf2-ARE Pathway: An Emerging Target against Oxidative Stress and Neuroinflammation in Neurodegenerative Diseases. Pharmacol. Ther. 2016, 157, 84–104. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Riera-Ponsati, L.; Kauppinen, S.; Klitgaard, H.; Erler, J.T.; Hansen, S.N. Targeting the NRF2 Pathway for Disease Modification in Neurodegenerative Diseases: Mechanisms and Therapeutic Implications. Front. Pharmacol. 2024, 15, 1437939. [Google Scholar] [CrossRef]
- Melzer, D.; Pilling, L.C.; Ferrucci, L. The Genetics of Human Ageing. Nat. Rev. Genet. 2020, 21, 88–101. [Google Scholar] [CrossRef]
- Azam, S.; Haque, M.E.; Balakrishnan, R.; Kim, I.-S.; Choi, D.-K. The Ageing Brain: Molecular and Cellular Basis of Neurodegeneration. Front. Cell Dev. Biol. 2021, 9, 683459. [Google Scholar] [CrossRef] [PubMed]
- Moskalev, A.; Guvatova, Z.; Lopes, I.D.A.; Beckett, C.W.; Kennedy, B.K.; De Magalhaes, J.P.; Makarov, A.A. Targeting Aging Mechanisms: Pharmacological Perspectives. Trends Endocrinol. Metab. TEM 2022, 33, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Effect of Antioxidants Supplementation on Aging and Longevity. BioMed Res. Int. 2014, 2014, 404680. [Google Scholar] [CrossRef] [PubMed]
- Minich, D.M.; Henning, M.; Darley, C.; Fahoum, M.; Schuler, C.B.; Frame, J. Is Melatonin the “Next Vitamin D”?: A Review of Emerging Science, Clinical Uses, Safety, and Dietary Supplements. Nutrients 2022, 14, 3934. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Esteras, N. Multitarget Effects of Nrf2 Signalling in the Brain: Common and Specific Functions in Different Cell Types. Antioxidants 2024, 13, 1502. [Google Scholar] [CrossRef]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Yu, C.; Xiao, J.-H. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxid. Med. Cell. Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef]
- Venci, J.V.; Gandhi, M.A. Dimethyl Fumarate (Tecfidera): A New Oral Agent for Multiple Sclerosis. Ann. Pharmacother. 2013, 47, 1697–1702. [Google Scholar] [CrossRef]
- Umrao, A.; Pahuja, M.; Chatterjee, N.S. Safety and Efficacy of Omaveloxolone v/s Placebo for the Treatment of Friedreich’s Ataxia in Patients Aged More than 16 Years: A Systematic Review. Orphanet J. Rare Dis. 2024, 19, 495. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, C.P.; Glass, C.A.; Montgomery, M.B.; Lindl, K.A.; Ritson, G.P.; Chia, L.A.; Hamilton, R.L.; Chu, C.T.; Jordan-Sciutto, K.L. Expression of Nrf2 in Neurodegenerative Diseases. J. Neuropathol. Exp. Neurol. 2007, 66, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Kanninen, K.; Malm, T.M.; Jyrkkänen, H.-K.; Goldsteins, G.; Keksa-Goldsteine, V.; Tanila, H.; Yamamoto, M.; Ylä-Herttuala, S.; Levonen, A.-L.; Koistinaho, J. Nuclear Factor Erythroid 2-Related Factor 2 Protects against Beta Amyloid. Mol. Cell. Neurosci. 2008, 39, 302–313. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; Ulusoy, A.; Innamorato, N.G.; Sahin, G.; Rábano, A.; Kirik, D.; Cuadrado, A. α-Synuclein Expression and Nrf2 Deficiency Cooperate to Aggravate Protein Aggregation, Neuronal Death and Inflammation in Early-Stage Parkinson’s Disease. Hum. Mol. Genet. 2012, 21, 3173–3192. [Google Scholar] [CrossRef]
- van Muiswinkel, F.L.; de Vos, R.A.I.; Bol, J.G.J.M.; Andringa, G.; Steur, E.N.H.J.; Ross, D.; Siegel, D.; Drukarch, B. Expression of NAD(P)H:Quinone Oxidoreductase in the Normal and Parkinsonian Substantia Nigra. Neurobiol. Aging 2004, 25, 1253–1262. [Google Scholar] [CrossRef]
- Chen, P.-C.; Vargas, M.R.; Pani, A.K.; Smeyne, R.J.; Johnson, D.A.; Kan, Y.W.; Johnson, J.A. Nrf2-Mediated Neuroprotection in the MPTP Mouse Model of Parkinson’s Disease: Critical Role for the Astrocyte. Proc. Natl. Acad. Sci. USA 2009, 106, 2933–2938. [Google Scholar] [CrossRef] [PubMed]
- Innamorato, N.G.; Jazwa, A.; Rojo, A.I.; García, C.; Fernández-Ruiz, J.; Grochot–Przeczek, A.; Stachurska, A.; Jozkowicz, A.; Dulak, J.; Cuadrado, A. Different Susceptibility to the Parkinson’s Toxin MPTP in Mice Lacking the Redox Master Regulator Nrf2 or Its Target Gene Heme Oxygenase-1. PLoS ONE 2010, 5, e11838. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial Dysfunction and Oxidative Stress in Neurodegenerative Diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Buttari, B.; Tramutola, A.; Rojo, A.I.; Chondrogianni, N.; Saha, S.; Berry, A.; Giona, L.; Miranda, J.P.; Profumo, E.; Davinelli, S.; et al. Proteostasis Decline and Redox Imbalance in Age-Related Diseases: The Therapeutic Potential of NRF2. Biomolecules 2025, 15, 113. [Google Scholar] [CrossRef]
- Holmström, K.M.; Baird, L.; Zhang, Y.; Hargreaves, I.; Chalasani, A.; Land, J.M.; Stanyer, L.; Yamamoto, M.; Dinkova-Kostova, A.T.; Abramov, A.Y. Nrf2 Impacts Cellular Bioenergetics by Controlling Substrate Availability for Mitochondrial Respiration. Biol. Open 2013, 2, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The Emerging Role of Nrf2 in Mitochondrial Function. Free Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef]
- Villavicencio Tejo, F.; Quintanilla, R.A. Contribution of the Nrf2 Pathway on Oxidative Damage and Mitochondrial Failure in Parkinson and Alzheimer’s Disease. Antioxidants 2021, 10, 1069. [Google Scholar] [CrossRef]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.; Wang, X.-J.; Zhao, F.; Villeneuve, N.F.; Wu, T.; Jiang, T.; Sun, Z.; White, E.; Zhang, D.D. A Noncanonical Mechanism of Nrf2 Activation by Autophagy Deficiency: Direct Interaction between Keap1 and P62. Mol. Cell. Biol. 2010, 30, 3275–3285. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Kostov, R.V.; Dinkova-Kostova, A.T. The Multifaceted Role of Nrf2 in Mitochondrial Function. Curr. Opin. Toxicol. 2016, 1, 80–91. [Google Scholar] [CrossRef]
- Olney, N.T.; Spina, S.; Miller, B.L. Frontotemporal Dementia. Neurol. Clin. 2017, 35, 339–374. [Google Scholar] [CrossRef]
- Esteras, N.; Abramov, A.Y. Mitochondrial Calcium Deregulation in the Mechanism of Beta-Amyloid and Tau Pathology. Cells 2020, 9, 2135. [Google Scholar] [CrossRef]
- Flønes, I.H.; Tzoulis, C. Mitochondrial Respiratory Chain Dysfunction—A Hallmark Pathology of Idiopathic Parkinson’s Disease? Front. Cell Dev. Biol. 2022, 10, 874596. [Google Scholar] [CrossRef]
- Chen, C.; McDonald, D.; Blain, A.; Mossman, E.; Atkin, K.; Marusich, M.F.; Capaldi, R.; Bone, L.; Smith, A.; Filby, A.; et al. Parkinson’s Disease Neurons Exhibit Alterations in Mitochondrial Quality Control Proteins. npj Park. Dis. 2023, 9, 120. [Google Scholar] [CrossRef]
- Owens, L.; Buhr, E.; Tu, D.C.; Lamprecht, T.L.; Lee, J.; Van Gelder, R.N. Effect of Circadian Clock Gene Mutations on Nonvisual Photoreception in the Mouse. Investig. Ophthalmol. Vis. Sci. 2012, 53, 454–460. [Google Scholar] [CrossRef]
- Alberti, C. Melatonin: The first hormone isolated from the pineal body. Il Farm. Ed. Sci. 1958, 13, 604–605. [Google Scholar]
- Lerner, A.; Case, J.; Takahashi, Y.; Lee, T.; Mori, W. Isolation of Melatonin, the Pineal Gland Factor That Lightens Melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Mineiro, R.; Rodrigues Cardoso, M.; Catarina Duarte, A.; Santos, C.; Cipolla-Neto, J.; Gaspar do Amaral, F.; Costa, D.; Quintela, T. Melatonin and Brain Barriers: The Protection Conferred by Melatonin to the Blood-Brain Barrier and Blood-Cerebrospinal Fluid Barrier. Front. Neuroendocrinol. 2024, 75, 101158. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal Melatonin: Sources, Regulation, and Potential Functions. Cell. Mol. Life Sci. CMLS 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Conti, A.; Conconi, S.; Hertens, E.; Skwarlo-Sonta, K.; Markowska, M.; Maestroni, J.M. Evidence for Melatonin Synthesis in Mouse and Human Bone Marrow Cells. J. Pineal Res. 2000, 28, 193–202. [Google Scholar] [CrossRef]
- Han, Y.; DeMorrow, S.; Invernizzi, P.; Jing, Q.; Glaser, S.; Renzi, A.; Meng, F.; Venter, J.; Bernuzzi, F.; White, M.; et al. Melatonin Exerts by an Autocrine Loop Antiproliferative Effects in Cholangiocarcinoma; Its Synthesis Is Reduced Favoring Cholangiocarcinoma Growth. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G623–G633. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Bernatoniene, J. Molecular Mechanisms of Melatonin-Mediated Cell Protection and Signaling in Health and Disease. Pharmaceutics 2021, 13, 129. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding Expectations. Physiology 2014, 29, 325–333. [Google Scholar] [CrossRef]
- Deng, Y.; Zhu, J.; Mi, C.; Xu, B.; Jiao, C.; Li, Y.; Xu, D.; Liu, W.; Xu, Z. Melatonin Antagonizes Mn-Induced Oxidative Injury Through the Activation of Keap1–Nrf2–ARE Signaling Pathway in the Striatum of Mice. Neurotox. Res. 2015, 27, 156–171. [Google Scholar] [CrossRef]
- Galvani, F.; Cammarota, M.; Vacondio, F.; Rivara, S.; Boscia, F. Protective Activity of Melatonin Combinations and Melatonin-Based Hybrid Molecules in Neurodegenerative Diseases. J. Pineal Res. 2024, 76, e70008. [Google Scholar] [CrossRef]
- Wang, X.; Sirianni, A.; Pei, Z.; Cormier, K.; Smith, K.; Jiang, J.; Zhou, S.; Wang, H.; Zhao, R.; Yano, H.; et al. The Melatonin MT1 Receptor Axis Modulates Mutant Huntingtin-Mediated Toxicity. J. Neurosci. 2011, 31, 14496–14507. [Google Scholar] [CrossRef] [PubMed]
- Vriend, J.; Reiter, R.J. The Keap1-Nrf2-Antioxidant Response Element Pathway: A Review of Its Regulation by Melatonin and the Proteasome. Mol. Cell. Endocrinol. 2015, 401, 213–220. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Jou, M.-J.; Acuna-Castroviejo, D. Melatonin Mitigates Mitochondrial Meltdown: Interactions with SIRT3. Int. J. Mol. Sci. 2018, 19, 2439. [Google Scholar] [CrossRef]
- Wang, C.-F.; Song, C.-Y.; Wang, X.; Huang, L.-Y.; Ding, M.; Yang, H.; Wang, P.; Xu, L.-L.; Xie, Z.-H.; Bi, J.-Z. Protective Effects of Melatonin on Mitochondrial Biogenesis and Mitochondrial Structure and Function in the HEK293-APPswe Cell Model of Alzheimer’s Disease. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3542–3550. [Google Scholar] [CrossRef]
- Carloni, S.; Nasoni, M.G.; Casabianca, A.; Orlandi, C.; Capobianco, L.; Iaconisi, G.N.; Cerioni, L.; Burattini, S.; Benedetti, S.; Reiter, R.J.; et al. Melatonin Reduces Mito-Inflammation in Ischaemic Hippocampal HT22 Cells and Modulates the cGAS–STING Cytosolic DNA Sensing Pathway and FGF21 Release. J. Cell. Mol. Med. 2024, 28, e70285. [Google Scholar] [CrossRef]
- Jauhari, A.; Monek, A.C.; Suofu, Y.; Amygdalos, O.R.; Singh, T.; Baranov, S.V.; Carlisle, D.L.; Friedlander, R.M. Melatonin Deficits Result in Pathologic Metabolic Reprogramming in Differentiated Neurons. J. Pineal Res. 2025, 77, e70037. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, K.S.; Sindhu, K.M.; Mohanakumar, K.P. Melatonin Protects against Rotenone-Induced Oxidative Stress in a Hemiparkinsonian Rat Model. J. Pineal Res. 2007, 42, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.F.D.; Rösler, T.W.; Höglinger, G.U. Exploring the Neuroprotective Potential of Nrf2-Pathway Activators against Annonacin Toxicity. Sci. Rep. 2024, 14, 20123. [Google Scholar] [CrossRef]
- Wade, A.G.; Farmer, M.; Harari, G.; Fund, N.; Laudon, M.; Nir, T.; Frydman-Marom, A.; Zisapel, N. Add-on Prolonged-Release Melatonin for Cognitive Function and Sleep in Mild to Moderate Alzheimer’s Disease: A 6-Month, Randomized, Placebo-Controlled, Multicenter Trial. Clin. Interv. Aging 2014, 9, 947–961. [Google Scholar] [CrossRef]
- Savage, R.A.; Zafar, N.; Yohannan, S.; Miller, J.-M.M. Melatonin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- DeMuro, R.L.; Nafziger, A.N.; Blask, D.E.; Menhinick, A.M.; Bertino, J.S., Jr. The Absolute Bioavailability of Oral Melatonin. J. Clin. Pharmacol. 2000, 40, 781–784. [Google Scholar] [CrossRef]
- Grigg-Damberger, M.M.; Ianakieva, D. Poor Quality Control of Over-the-Counter Melatonin: What They Say Is Often Not What You Get. J. Clin. Sleep Med. 2017, 13, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.L.; Siedlak, S.L.; Raina, A.K.; Bautista, J.M.; Smith, M.A.; Perry, G. Increased Neuronal Glucose-6-Phosphate Dehydrogenase and Sulfhydryl Levels Indicate Reductive Compensation to Oxidative Stress in Alzheimer Disease. Arch. Biochem. Biophys. 1999, 370, 236–239. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, M.; Zhang, Z.; Wang, R.; Wang, D.; Sang, Z.; Zhao, P.; Liu, X.; Zhu, X.; Liang, G.; et al. The Ameliorative Effects of Melatonin against BDE-47-Induced Hippocampal Neuronal Ferroptosis and Cognitive Dysfunction through Nrf2-Chaperone-Mediated Autophagy of ACSL4 Degradation. Ecotoxicol. Environ. Saf. 2025, 290, 117542. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Jin, X.; Chen, L.; Yang, Y.; Tan, R.; Jiang, C. Adverse Effects of Nrf2 in Different Organs and the Related Diseases. Antioxid. Redox Signal. 2025, 42, 973–985. [Google Scholar] [CrossRef]
- Strang, K.H.; Golde, T.E.; Giasson, B.I. MAPT Mutations, Tauopathy, and Mechanisms of Neurodegeneration. Lab. Investig. 2019, 99, 912–928. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, I.; Schellenberg, G.D. Regulation of Tau Isoform Expression and Dementia. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2005, 1739, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Onyike, C.U.; Diehl-Schmid, J. The Epidemiology of Frontotemporal Dementia. Int. Rev. Psychiatry 2013, 25, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Campbell, M.R.; Lacher, S.E.; Cho, H.-Y.; Wan, M.; Crowl, C.L.; Chorley, B.N.; Bond, G.L.; Kleeberger, S.R.; Slattery, M.; et al. A Polymorphic Antioxidant Response Element Links NRF2/sMAF Binding to Enhanced MAPT Expression and Reduced Risk of Parkinsonian Disorders. Cell Rep. 2016, 15, 830–842. [Google Scholar] [CrossRef]
- Dong, H.; Wang, H.; Wang, L. Inhibition of MAPT Enhances the Effect of Bexarotene and Attenuates the Damage after Traumatic Brain Injury Using in Vivo and in Vitro Experiments. Folia Neuropathol. 2020, 58, 253–264. [Google Scholar] [CrossRef]
- Pajares, M.; Jiménez-Moreno, N.; García-Yagüe, Á.J.; Escoll, M.; de Ceballos, M.L.; Leuven, F.V.; Rábano, A.; Yamamoto, M.; Rojo, A.I.; Cuadrado, A. Transcription Factor NFE2L2/NRF2 Is a Regulator of Macroautophagy Genes. Autophagy 2016, 12, 1902–1916. [Google Scholar] [CrossRef]
- Jo, C.; Gundemir, S.; Pritchard, S.; Jin, Y.N.; Rahman, I.; Johnson, G.V.W. Nrf2 Reduces Levels of Phosphorylated Tau Protein by Inducing Autophagy Adaptor Protein NDP52. Nat. Commun. 2014, 5, 3496. [Google Scholar] [CrossRef]
- Cuadrado, A.; Kügler, S.; Lastres-Becker, I. Pharmacological Targeting of GSK-3 and NRF2 Provides Neuroprotection in a Preclinical Model of Tauopathy. Redox Biol. 2018, 14, 522–534. [Google Scholar] [CrossRef]
- Riordan, R.; Rong, W.; Yu, Z.; Ross, G.; Valerio, J.; Dimas-Muñoz, J.; Heredia, V.; Magnusson, K.; Galvan, V.; Perez, V.I. Effect of Nrf2 Loss on Senescence and Cognition of Tau-Based P301S Mice. GeroScience 2023, 45, 1451–1469. [Google Scholar] [CrossRef]
- Mohamed, A.S.; ElKaffas, M.; Metwally, K.; Abdelfattah, M.; Elsery, E.A.; Elshazly, A.; Gomaa, H.E.; Alsayed, A.; El-Desouky, S.; El-Gamal, R.; et al. Impairment of Nrf2 Signaling in the Hippocampus of P301S Tauopathy Mice Model Aligns with the Cognitive Impairment and the Associated Neuroinflammation. J. Inflamm. 2024, 21, 29. [Google Scholar] [CrossRef]
- Xie, J.-Z.; Zhang, Y.; Li, S.-H.; Wei, H.; Yu, H.-L.; Zhou, Q.-Z.; Wei, L.-Y.; Ke, D.; Wang, Q.; Yang, Y.; et al. P301S-hTau Acetylates KEAP1 to Trigger Synaptic Toxicity via Inhibiting NRF2/ARE Pathway: A Novel Mechanism Underlying hTau-induced Synaptic Toxicities. Clin. Transl. Med. 2022, 12, e1003. [Google Scholar] [CrossRef] [PubMed]
- Tapias, V.; Jainuddin, S.; Ahuja, M.; Stack, C.; Elipenahli, C.; Vignisse, J.; Gerges, M.; Starkova, N.; Xu, H.; Starkov, A.A.; et al. Benfotiamine Treatment Activates the Nrf2/ARE Pathway and Is Neuroprotective in a Transgenic Mouse Model of Tauopathy. Hum. Mol. Genet. 2018, 27, 2874–2892. [Google Scholar] [CrossRef] [PubMed]
- Castro-Sánchez, S.; García-Yagüe, Á.J.; Kügler, S.; Lastres-Becker, I. CX3CR1-Deficient Microglia Shows Impaired Signalling of the Transcription Factor NRF2: Implications in Tauopathies. Redox Biol. 2019, 22, 101118. [Google Scholar] [CrossRef]
- Jiwaji, Z.; Tiwari, S.S.; Avilés-Reyes, R.X.; Hooley, M.; Hampton, D.; Torvell, M.; Johnson, D.A.; McQueen, J.; Baxter, P.; Sabari-Sankar, K.; et al. Reactive Astrocytes Acquire Neuroprotective as Well as Deleterious Signatures in Response to Tau and Aß Pathology. Nat. Commun. 2022, 13, 135. [Google Scholar] [CrossRef]
- Swieten, J.V.; Spillantini, M.G. Hereditary Frontotemporal Dementia Caused by Tau Gene Mutations. Brain Pathol. 2007, 17, 63–73. [Google Scholar] [CrossRef]
- Schwab, K.; Melis, V.; Harrington, C.R.; Wischik, C.M.; Magbagbeolu, M.; Theuring, F.; Riedel, G. Proteomic Analysis of Hydromethylthionine in the Line 66 Model of Frontotemporal Dementia Demonstrates Actions on Tau-Dependent and Tau-Independent Networks. Cells 2021, 10, 2162. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-H.; Chen, M.C.; Cheng, I.H. Antroquinonol Lowers Brain Amyloid-β Levels and Improves Spatial Learning and Memory in a Transgenic Mouse Model of Alzheimer’s Disease. Sci. Rep. 2015, 5, 15067. [Google Scholar] [CrossRef] [PubMed]
- Francesca, F.; Caitlin, A.; Sarah, L.; Robyn, G.L. Antroquinonol Administration in Animal Preclinical Studies for Alzheimer’s Disease (AD): A New Avenue for Modifying Progression of AD Pathophysiology. Brain Behav. Immun. Health 2022, 21, 100435. [Google Scholar] [CrossRef]
- Kim, J. Pre-Clinical Neuroprotective Evidences and Plausible Mechanisms of Sulforaphane in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 2929. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.E.; Luchsinger, J.A.; Cirio, R.; Chen, H.; Franchino-Elder, J.; Hirsch, J.A.; Bettendorff, L.; Chen, Z.; Flowers, S.A.; Gerber, L.M.; et al. Benfotiamine and Cognitive Decline in Alzheimer’s Disease: Results of a Randomized Placebo-Controlled Phase IIa Clinical Trial. J. Alzheimers Dis. 2020, 78, 989–1010. [Google Scholar] [CrossRef]
- Masahiro, S.; Fukunaga, K.; Kawahata, I. Use of Melatonin for the Treatment of Dementia: Addressing Core Symptoms and Behavioral Challenges. J. Clin. Basic Psychosom. 2023, 1, 1174. [Google Scholar] [CrossRef]
- Zhang, Z.; Xue, P.; Bendlin, B.B.; Zetterberg, H.; Felice, F.D.; Tan, X.; Benedict, C. Melatonin: A Potential Nighttime Guardian against Alzheimer’s. Mol. Psychiatry 2025, 30, 237–250. [Google Scholar] [CrossRef]
- Das, R.; Balmik, A.A.; Chinnathambi, S. Melatonin Reduces GSK3β-Mediated Tau Phosphorylation, Enhances Nrf2 Nuclear Translocation and Anti-Inflammation. ASN Neuro 2020, 12, 1759091420981204. [Google Scholar] [CrossRef]
- Sotolongo, K.; Ghiso, J.; Rostagno, A. Nrf2 Activation through the PI3K/GSK-3 Axis Protects Neuronal Cells from Aβ-Mediated Oxidative and Metabolic Damage. Alzheimers Res. Ther. 2020, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.; Shah, M.; Shah, S.A.; Habib, S.H.; Ehtesham, E.; Ahmed, N. Melatonin Rescues Pregnant Female Mice and Their Juvenile Offspring from High Fat Diet-Induced Alzheimer Disease Neuropathy. Heliyon 2024, 10, e36921. [Google Scholar] [CrossRef] [PubMed]
- Gáll, Z.; Boros, B.; Kelemen, K.; Urkon, M.; Zolcseak, I.; Márton, K.; Kolcsar, M. Melatonin Improves Cognitive Dysfunction and Decreases Gliosis in the Streptozotocin-Induced Rat Model of Sporadic Alzheimer’s Disease. Front. Pharmacol. 2024, 15, 1447757. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cheng, M.; Guo, J.; Cao, D.; Luo, J.; Wan, Y.; Fang, Y.; Jin, Y.; Xie, S.-S.; Liu, J. Dual Functional Antioxidant and Butyrylcholinesterase Inhibitors for the Treatment of Alzheimer’s Disease: Design, Synthesis and Evaluation of Novel Melatonin-Alkylbenzylamine Hybrids. Bioorg. Med. Chem. 2023, 78, 117146. [Google Scholar] [CrossRef]
- Zhao, H.; Feng, L.; Zhong, W.; Zhen, H.; Chi, Q.; Wang, X. Hyperphosphorylation of Tau Due to the Interference of Protein Phosphatase Methylesterase-1 Overexpression by MiR-125b-5p in Melatonin Receptor Knockout Mice. Int. J. Mol. Sci. 2021, 22, 11850. [Google Scholar] [CrossRef]
- Zhu, L.; Gong, Y.; Lju, H.; Sun, G.; Zhang, Q.; Qian, Z. Mechanisms of Melatonin Binding and Destabilizing the Protofilament and Filament of Tau R3–R4 Domains Revealed by Molecular Dynamics Simulation. Phys. Chem. Chem. Phys. 2021, 23, 20615–20626. [Google Scholar] [CrossRef]
- Rong, K.; Zheng, H.; Yang, R.; Liu, X.; Li, L.; Chen, N.; Zhao, G.; Gong, C.; Deng, Y. Melatonin and Its Metabolite N (1)-acetyl- N (1)-formyl-5-methoxykynuramine Improve Learning and Memory Impairment Related to Alzheimer’s Disease in Rats. J. Biochem. Mol. Toxicol. 2020, 34, e22430. [Google Scholar] [CrossRef]
- Bourdenx, M.; Koulakiotis, N.S.; Sanoudou, D.; Bezard, E.; Dehay, B.; Tsarbopoulos, A. Protein Aggregation and Neurodegeneration in Prototypical Neurodegenerative Diseases: Examples of Amyloidopathies, Tauopathies and Synucleinopathies. Prog. Neurobiol. 2017, 155, 171–193. [Google Scholar] [CrossRef]
- Clausen, L.; Okarmus, J.; Voutsinos, V.; Meyer, M.; Lindorff-Larsen, K.; Hartmann-Petersen, R. PRKN-Linked Familial Parkinson’s Disease: Cellular and Molecular Mechanisms of Disease-Linked Variants. Cell. Mol. Life Sci. 2024, 81, 223. [Google Scholar] [CrossRef]
- Vizziello, M.; Borellini, L.; Franco, G.; Ardolino, G. Disruption of Mitochondrial Homeostasis: The Role of PINK1 in Parkinson’s Disease. Cells 2021, 10, 3022. [Google Scholar] [CrossRef]
- Rui, Q.; Ni, H.; Li, D.; Gao, R.; Chen, G. The Role of LRRK2 in Neurodegeneration of Parkinson Disease. Curr. Neuropharmacol. 2018, 16, 1348–1357. [Google Scholar] [CrossRef]
- Repici, M.; Giorgini, F. DJ-1 in Parkinson’s Disease: Clinical Insights and Therapeutic Perspectives. J. Clin. Med. 2019, 8, 1377. [Google Scholar] [CrossRef]
- Siddiqui, I.J.; Pervaiz, N.; Abbasi, A.A. The Parkinson Disease Gene SNCA: Evolutionary and Structural Insights with Pathological Implication. Sci. Rep. 2016, 6, 24475. [Google Scholar] [CrossRef] [PubMed]
- Anandhan, A.; Chen, W.; Nguyen, N.; Madhavan, L.; Dodson, M.; Zhang, D.D. α-Syn Overexpression, NRF2 Suppression, and Enhanced Ferroptosis Create a Vicious Cycle of Neuronal Loss in Parkinson’s Disease. Free Radic. Biol. Med. 2022, 192, 130–140. [Google Scholar] [CrossRef]
- Czaniecki, C.; Ryan, T.; Stykel, M.G.; Drolet, J.; Heide, J.; Hallam, R.; Wood, S.; Coackley, C.; Sherriff, K.; Bailey, C.D.C.; et al. Axonal Pathology in hPSC-Based Models of Parkinson’s Disease Results from Loss of Nrf2 Transcriptional Activity at the Map1b Gene Locus. Proc. Natl. Acad. Sci. USA 2019, 116, 14280–14289. [Google Scholar] [CrossRef] [PubMed]
- Anandhan, A.; Nguyen, N.; Syal, A.; Dreher, L.A.; Dodson, M.; Zhang, D.D.; Madhavan, L. NRF2 Loss Accentuates Parkinsonian Pathology and Behavioral Dysfunction in Human α-Synuclein Overexpressing Mice. Aging Dis. 2021, 12, 964–982, Erratum in Aging Dis. 2024, 15, 951–952. [Google Scholar] [CrossRef] [PubMed]
- LaPak, K.M.; Saeidi, S.; Bok, I.; Wamsley, N.T.; Plutzer, I.B.; Bhatt, D.P.; Luo, J.; Ashrafi, G.; Ben Major, M. Proximity Proteomic Analysis of the NRF Family Reveals the Parkinson’s Disease Protein ZNF746/PARIS as a Co-Complexed Repressor of NRF2. Sci. Signal. 2023, 16, eadi9018. [Google Scholar] [CrossRef]
- Sheng, X.-J.; Tu, H.-J.; Chien, W.-L.; Kang, K.-H.; Lu, D.-H.; Liou, H.-H.; Lee, M.-J.; Fu, W.-M. Antagonism of Proteasome Inhibitor-Induced Heme Oxygenase-1 Expression by PINK1 Mutation. PLoS ONE 2017, 12, e0183076. [Google Scholar] [CrossRef]
- Murata, H.; Takamatsu, H.; Liu, S.; Kataoka, K.; Huh, N.-H.; Sakaguchi, M. NRF2 Regulates PINK1 Expression under Oxidative Stress Conditions. PLoS ONE 2015, 10, e0142438. [Google Scholar] [CrossRef]
- Kawakami, F.; Imai, M.; Tamaki, S.; Ohta, E.; Kawashima, R.; Maekawa, T.; Kurosaki, Y.; Ohba, K.; Ichikawa, T. Nrf2 Expression Is Decreased in LRRK2 Transgenic Mouse Brain and LRRK2 Overexpressing SH-SY5Y Cells. Biol. Pharm. Bull. 2023, 46, 123–127. [Google Scholar] [CrossRef]
- Kim, J.; Daadi, E.W.; Daadi, E.S.; Oh, T.; Deleidi, M.; Daadi, M.M. LRRK2 Attenuates Antioxidant Response in Familial Parkinson’s Disease Derived Neural Stem Cells. Cells 2023, 12, 2550. [Google Scholar] [CrossRef]
- Banerjee, R.; Raj, A.; Potdar, C.; Pal, P.K.; Yadav, R.; Kamble, N.; Holla, V.; Datta, I. Astrocytes Differentiated from LRRK2-I1371V Parkinson’s-Disease-Induced Pluripotent Stem Cells Exhibit Similar Yield but Cell-Intrinsic Dysfunction in Glutamate Uptake and Metabolism, ATP Generation, and Nrf2-Mediated Glutathione Machinery. Cells 2023, 12, 1592. [Google Scholar] [CrossRef]
- Weindel, C.G.; Ellzey, L.M.; Coleman, A.K.; Patrick, K.L.; Watson, R.O. LRRK2 Kinase Activity Restricts NRF2-Dependent Mitochondrial Protection in Microglia. BioRxiv Prepr. Serv. Biol. 2024. [Google Scholar] [CrossRef]
- Lind-Holm Mogensen, F.; Scafidi, A.; Poli, A.; Michelucci, A. PARK7/DJ-1 in Microglia: Implications in Parkinson’s Disease and Relevance as a Therapeutic Target. J. Neuroinflamm. 2023, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Helgueta, S.; Heurtaux, T.; Sciortino, A.; Gui, Y.; Ohnmacht, J.; Mencke, P.; Boussaad, I.; Halder, R.; Garcia, P.; Krüger, R.; et al. Park7 Deletion Leads to Sex-Specific Transcriptome Changes Involving NRF2-CYP1B1 Axis in Mouse Midbrain Astrocytes. npj Park. Dis. 2025, 11, 8. [Google Scholar] [CrossRef]
- Brunialti, E.; Villa, A.; Toffoli, M.; Lucas Del Pozo, S.; Rizzi, N.; Meda, C.; Maggi, A.; Schapira, A.H.V.; Ciana, P. Sex-Specific Microglial Responses to Glucocerebrosidase Inhibition: Relevance to GBA1-Linked Parkinson’s Disease. Cells 2023, 12, 343. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Casado, M.E.; Lima, E.; García, J.A.; Doerrier, C.; Aranda, P.; Sayed, R.K.; Guerra-Librero, A.; Escames, G.; López, L.C.; Acuña-Castroviejo, D. Melatonin Rescues Zebrafish Embryos from the Parkinsonian Phenotype Restoring the Parkin/PINK1/DJ-1/MUL1 Network. J. Pineal Res. 2016, 61, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Martínez, P.; Fernández-Martínez, J.; Ramírez-Casas, Y.; Rodríguez-Santana, C.; Rusanova, I.; Escames, G.; Acuña-Castroviejo, D. Chronodisruption and Loss of Melatonin Rhythm, Associated with Alterations in Daily Motor Activity and Mitochondrial Dynamics in Parkinsonian Zebrafish, Are Corrected by Melatonin Treatment. Antioxidants 2023, 12, 954. [Google Scholar] [CrossRef]
- Anichtchik, O.; Diekmann, H.; Fleming, A.; Roach, A.; Goldsmith, P.; Rubinsztein, D.C. Loss of PINK1 Function Affects Development and Results in Neurodegeneration in Zebrafish. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 8199–8207. [Google Scholar] [CrossRef]
- Pourhanifeh, M.H.; Hosseinzadeh, A.; Koosha, F.; Reiter, R.J.; Mehrzadi, S. Therapeutic Effects of Melatonin in the Regulation of Ferroptosis: A Review of Current Evidence. Curr. Drug Targets 2024, 25, 543–557. [Google Scholar] [CrossRef]
- Lv, Q.-K.; Tao, K.-X.; Yao, X.-Y.; Pang, M.-Z.; Cao, B.-E.; Liu, C.-F.; Wang, F. Melatonin MT1 Receptors Regulate the Sirt1/Nrf2/Ho-1/Gpx4 Pathway to Prevent α-Synuclein-Induced Ferroptosis in Parkinson’s Disease. J. Pineal Res. 2024, 76, e12948. [Google Scholar] [CrossRef]
- Su, L.-Y.; Li, H.; Lv, L.; Feng, Y.-M.; Li, G.-D.; Luo, R.; Zhou, H.-J.; Lei, X.-G.; Ma, L.; Li, J.-L.; et al. Melatonin Attenuates MPTP-Induced Neurotoxicity via Preventing CDK5-Mediated Autophagy and SNCA/α-Synuclein Aggregation. Autophagy 2015, 11, 1745–1759. [Google Scholar] [CrossRef]
- Brito-Armas, J.M.; Baekelandt, V.; Castro-Hernández, J.R.; González-Hernández, T.; Rodríguez, M.; Castro, R. Melatonin Prevents Dopaminergic Cell Loss Induced by Lentiviral Vectors Expressing A30P Mutant Alpha-Synuclein. Histol. Histopathol. 2013, 28, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Dao, J.-J.; Zhang, W.; Liu, C.; Li, Q.; Qiao, C.-M.; Cui, C.; Shen, Y.-Q.; Chen, S.-X.; Zhao, W.-J. Targeted ErbB4 Receptor Activation Prevents D-Galactose-Induced Neuronal Senescence via Inhibiting Ferroptosis Pathway. Front. Pharmacol. 2025, 16, 1528604. [Google Scholar] [CrossRef]
- Biswal, L.; Sardoiwala, M.N.; Kushwaha, A.C.; Mukherjee, S.; Karmakar, S. Melatonin-Loaded Nanoparticles Augment Mitophagy to Retard Parkinson’s Disease. ACS Appl. Mater. Interfaces 2024, 16, 8417–8429. [Google Scholar] [CrossRef]
- Lin, C.-H.; Huang, J.-Y.; Ching, C.-H.; Chuang, J.-I. Melatonin Reduces the Neuronal Loss, Downregulation of Dopamine Transporter, and Upregulation of D2 Receptor in Rotenone-Induced Parkinsonian Rats. J. Pineal Res. 2008, 44, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, M.Z.; Andrabi, S.S.; Salman, M.; Tabassum, H.; Shaquiquzzaman, M.; Parveen, S.; Parvez, S. Melatonin Improves Behavioral and Biochemical Outcomes in a Rotenone-Induced Rat Model of Parkinson’s Disease. J. Environ. Pathol. Toxicol. Oncol. Off. Organ. Int. Soc. Environ. Toxicol. Cancer 2018, 37, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Sato, K.; Nagamine, H.; Kanatani, M.; Horikoshi, Y.; Nakaso, K. Cytoprotective Effect of Melatonin against MPP+ Toxicity in SH-SY5Y Cells: Role Sharing of Two Types of Antioxidative Activities of Melatonin. Biochem. Biophys. Res. Commun. 2025, 742, 151074. [Google Scholar] [CrossRef] [PubMed]
- Michalska, P.; Buendia, I.; Duarte, P.; FernandezMendivil, C.; Negredo, P.; Cuadrado, A.; López, M.G.; Leon, R. Melatonin-Sulforaphane Hybrid ITH12674 Attenuates Glial Response in Vivo by Blocking LPS Binding to MD2 and Receptor Oligomerization. Pharmacol. Res. 2020, 152, 104597. [Google Scholar] [CrossRef]
- Pachón-Angona, I.; Martin, H.; Chhor, S.; Oset-Gasque, M.-J.; Refouvelet, B.; Marco-Contelles, J.; Ismaili, L. Synthesis of New Ferulic/Lipoic/Comenic Acid-Melatonin Hybrids as Antioxidants and Nrf2 Activators via Ugi Reaction. Future Med. Chem. 2019, 11, 3097–3108. [Google Scholar] [CrossRef]
- Duarte, P.; Michalska, P.; Crisman, E.; Cuadrado, A.; León, R. Novel Series of Dual NRF2 Inducers and Selective MAO-B Inhibitors for the Treatment of Parkinson’s Disease. Antioxidants 2022, 11, 247. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-L.; Wei, X.-J.; Zhang, T.; Sun, T. Molecular Mechanisms of Melatonin-Induced Alleviation of Synaptic Dysfunction and Neuroinflammation in Parkinson’s Disease: A Review. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 5070–5082. [Google Scholar] [CrossRef]
- Yousef, O.; Abouelmagd, M.E.; Khaddam, H.; Shbani, A.; Yousef, R.; Meshref, M.; Hanafi, I. The Effectiveness of Melatonin for Sleep Disturbances in Parkinson’ Disease: Systematic Review and Meta-Analysis. J. Sleep Res. 2025, e70097. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Daga, V.; Singer, C. Reevaluating Melatonin’s Efficacy in Alzheimer’s Disease: A 20th-Anniversary Updated Literature Review of Sleep Disturbances and Treatment Trials. Alzheimers Dement. 2024, 20, e095541. [Google Scholar] [CrossRef]
- López-Caneda, C.H.; Antón-Fuente, S.; Pérez-Haro, M.J.; Sánchez-Franco, C.M.; Alvarez-Rodríguez, E.; Aguado-Valcarcel, M.; Marcos-Bobillo, M.; Torrente-Carballido, M.; González-Suárez, I. Beyond Efficacy: Persistence, NEDA, and Therapeutic Decision-Making in First-Line Multiple Sclerosis Treatment. Neurol. Ther. 2025; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Dayalan Naidu, S.; Dinkova-Kostova, A.T. Omaveloxolone (SkyclarysTM) for Patients with Friedreich’s Ataxia. Trends Pharmacol. Sci. 2023, 44, 394–395. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Íñigo-Catalina, L.; Ortiz-Cabello, M.; Navarro, E.; Esteras, N.; Rancan, L.; Paredes, S.D. Melatonin-Mediated Nrf2 Activation as a Potential Therapeutic Strategy in Mutation-Driven Neurodegenerative Diseases. Antioxidants 2025, 14, 1190. https://doi.org/10.3390/antiox14101190
Íñigo-Catalina L, Ortiz-Cabello M, Navarro E, Esteras N, Rancan L, Paredes SD. Melatonin-Mediated Nrf2 Activation as a Potential Therapeutic Strategy in Mutation-Driven Neurodegenerative Diseases. Antioxidants. 2025; 14(10):1190. https://doi.org/10.3390/antiox14101190
Chicago/Turabian StyleÍñigo-Catalina, Lucía, María Ortiz-Cabello, Elisa Navarro, Noemí Esteras, Lisa Rancan, and Sergio D. Paredes. 2025. "Melatonin-Mediated Nrf2 Activation as a Potential Therapeutic Strategy in Mutation-Driven Neurodegenerative Diseases" Antioxidants 14, no. 10: 1190. https://doi.org/10.3390/antiox14101190
APA StyleÍñigo-Catalina, L., Ortiz-Cabello, M., Navarro, E., Esteras, N., Rancan, L., & Paredes, S. D. (2025). Melatonin-Mediated Nrf2 Activation as a Potential Therapeutic Strategy in Mutation-Driven Neurodegenerative Diseases. Antioxidants, 14(10), 1190. https://doi.org/10.3390/antiox14101190







