Influence of Cutting Styles on Antioxidant Capabilities of Fresh-Cut Cauliflower by Regulating ROS Metabolism and Antioxidant Enzyme Activity
Abstract
1. Introduction
2. Materials and Methods
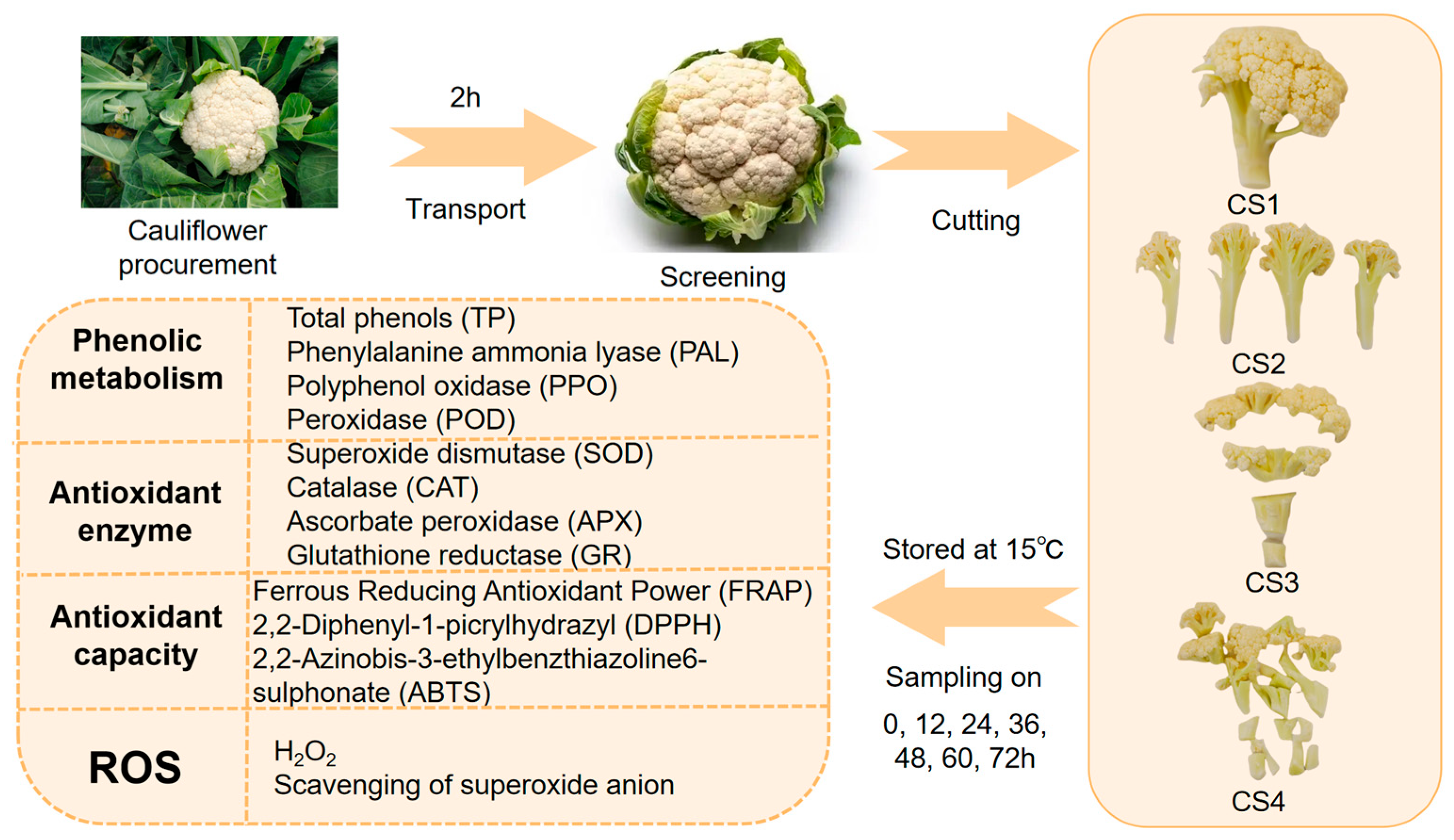
2.1. Materials and Treatments
2.2. Total Phenol (TP) Content and Phenylalanine Ammonia-Lyase (PAL) Activity Measurement
2.3. Polyphenol Oxidase (PPO) and Peroxidase (POD) Activity Measurement
2.4. Reactive Oxygen Species (ROS) Measurement
2.5. Enzymes Activity Measurement
2.5.1. Superoxide Dismutase (SOD) and Catalase (CAT) Activity Measurement
2.5.2. Glutathione Reductase (GR) and Ascorbate Peroxidase (APX) Activity
2.6. Antioxidant Capabilities Measurement
2.7. Cauliflower Weight Loss and Color Measurement
2.8. Sensory Analysis
2.9. Statistics
3. Results
3.1. Total Phenol (TP) Content and Phenylalanine Ammonia-Lyase (PAL) Activity
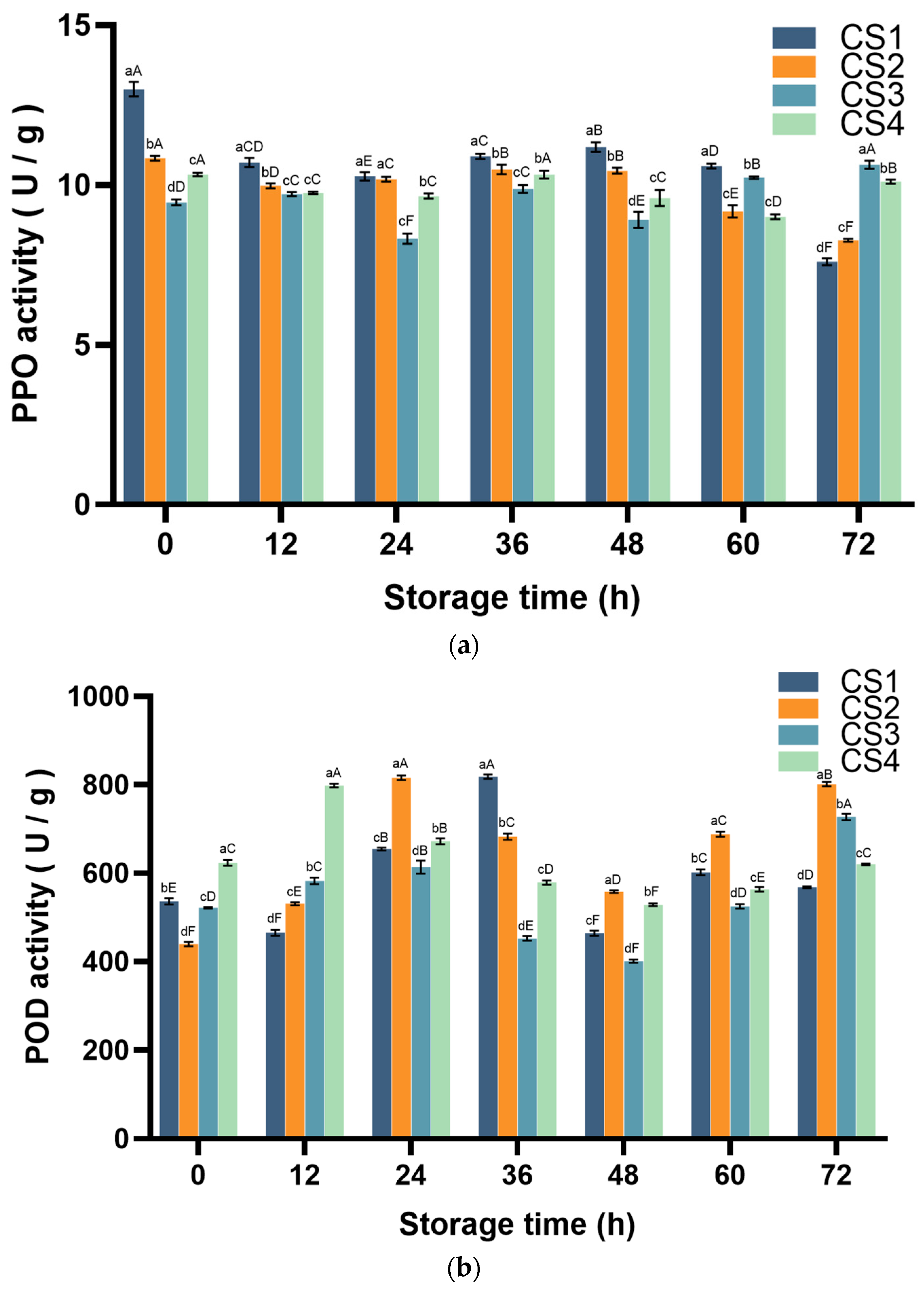
3.2. Polyphenol Oxidase (PPO) and Peroxidase (POD) Activity
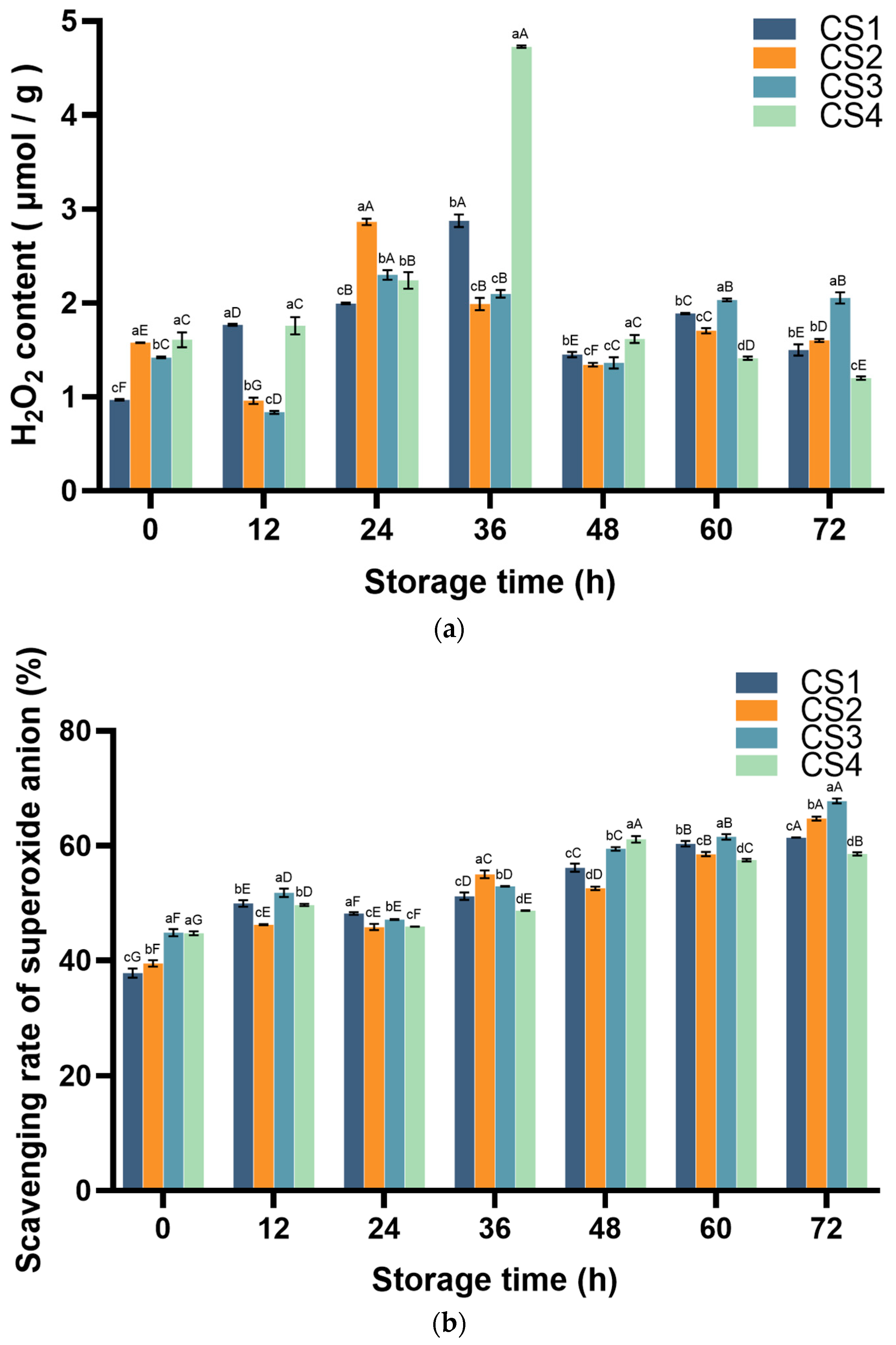
3.3. Reactive Oxygen Species (ROS)
3.4. Enzymes Activity
3.4.1. Superoxide Dismutase (SOD) and Catalase (CAT) Activity
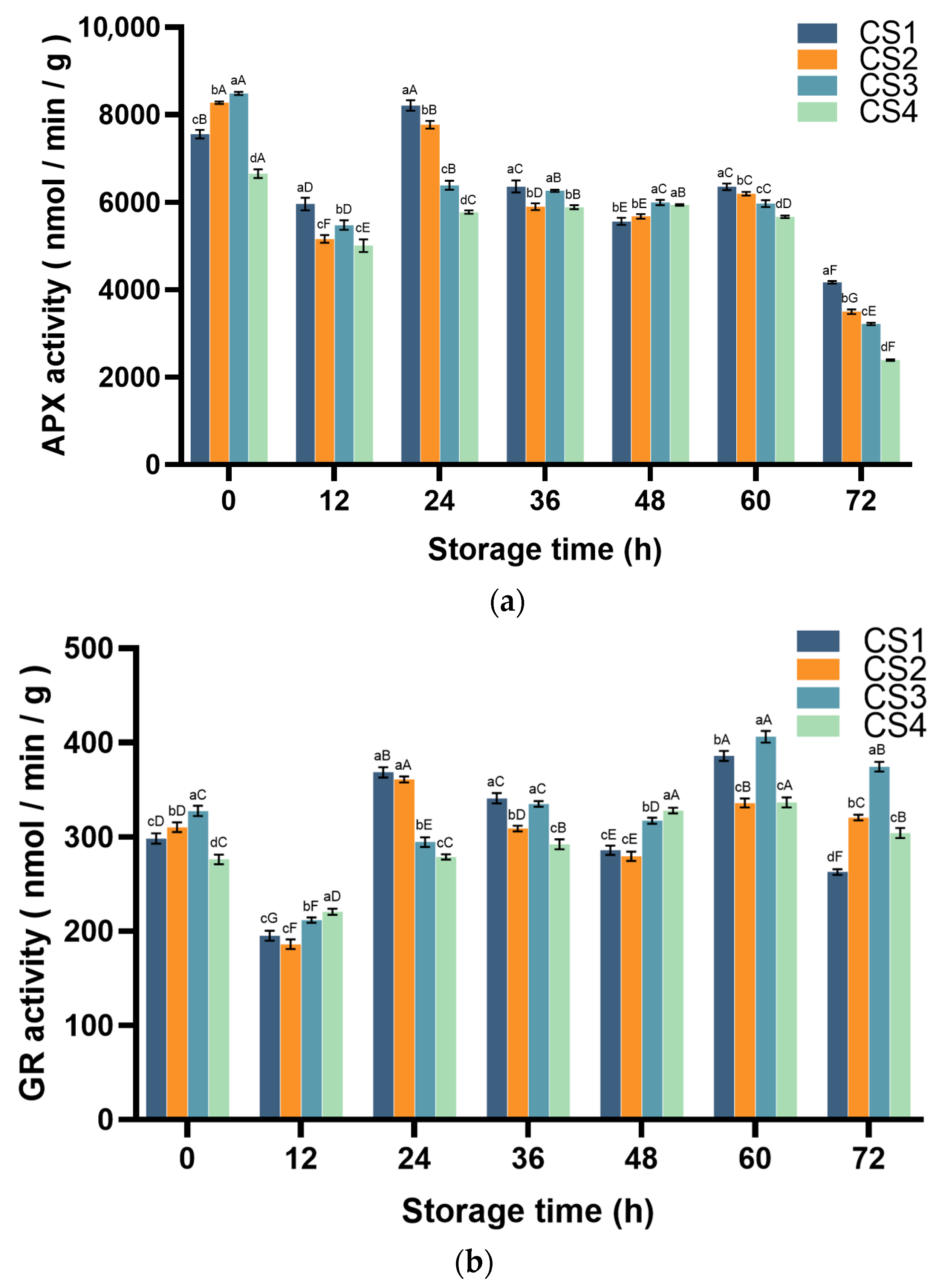
3.4.2. Ascorbate Peroxidase (APX) and Glutathione Reductase (GR) Activity
3.5. Antioxidant Capabilities
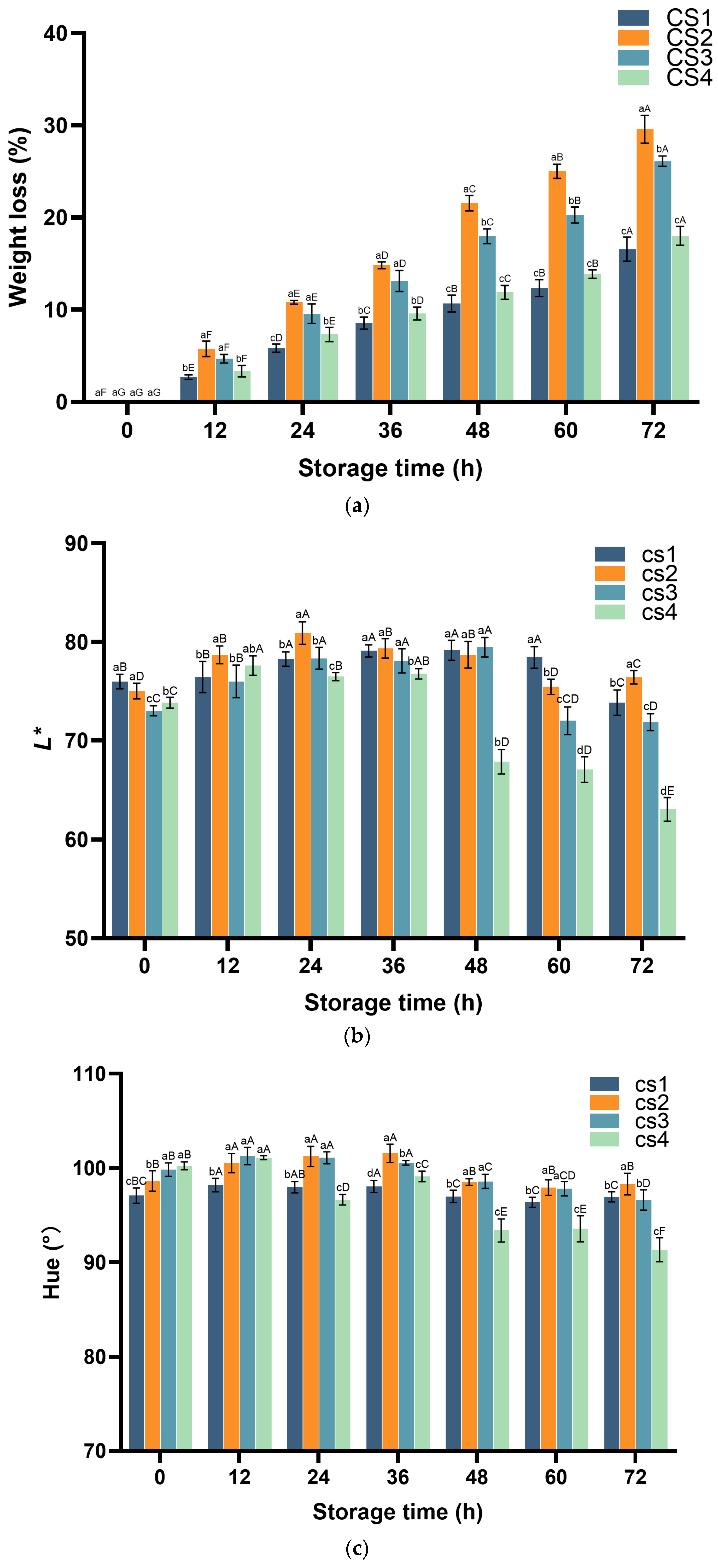
3.6. Cauliflower Weight Loss and Color
3.7. Sensory Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Volden, J.; Bengtsson, G.B.; Wicklund, T. Glucosinolates, l-ascorbic acid, total phenols, anthocyanins, antioxidant capacities and colour in cauliflower (Brassica oleracea L. ssp. botrytis); effects of long-term freezer storage. Food Chem. 2009, 112, 967–976. [Google Scholar] [CrossRef]
- Wang, J.; Hu, W.; Guan, Y.; Yu, J.; Zhao, M. Research progress on fresh-cut cauliflower preservation technology. Sci. Technol. Food Ind. 2020, 41, 327–332. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations of Database. 27 February 2025. Available online: https://www.fao.org/faostat/zh/#data/QCL (accessed on 1 June 2025).
- Guan, Y.; Hu, W.; Jiang, A.; Xu, Y.; Yu, J.; Zhao, M.; Ji, Y.; Feng, K.; Sarengaowa; Yang, X. Influence of cut type on quality, antioxidant substances and antioxidant activity of fresh-cut broccoli. Int. J. Food Sci. Technol. 2020, 55, 3019–3030. [Google Scholar] [CrossRef]
- Hu, W.; Sarengaowa; Guan, Y.; Feng, K. Biosynthesis of Phenolic Compounds and Antioxidant Activity in Fresh-Cut Fruits and Vegetables. Front. Microbiol. 2022, 13, 906069. [Google Scholar] [CrossRef]
- Berno, N.D.; Tezotto-Uliana, J.V.; dos Santos Dias, C.T.; Kluge, R.A. Storage temperature and type of cut affect the biochemical and physiological characteristics of fresh-cut purple onions. Postharvest Biol. Technol. 2014, 93, 91–96. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Martínez-Hernández, G.B.; del C. Rodríguez, S.; Cao, C.-M.; Cisneros-Zevallos, L. Plants as Biofactories: Physiological Role of Reactive Oxygen Species on the Accumulation of Phenolic Antioxidants in Carrot Tissue under Wounding and Hyperoxia Stress. J. Agric. Food Chem. 2011, 59, 6583–6593. [Google Scholar] [CrossRef]
- Wang, J.; Guan, Y.; Wang, Y.; Yuan, N.; Chen, J.; Chen, Y.; Chen, T.; Hu, W. Effects of limonene treatment on physiological metabolism, taste and flavor of fresh-cut cauliflower. J. Future Foods 2022, 43, 248–256. [Google Scholar] [CrossRef]
- Hu, W.; Guan, Y.; Ji, Y.; Yang, X. Effect of cutting styles on quality, antioxidant activity, membrane lipid peroxidation, and browning in fresh-cut potatoes. Food Biosci. 2021, 44, 101435. [Google Scholar] [CrossRef]
- Shi, C.; Shen, S.-W.; Sun, Y.; Liu, E.-Q.; Gong, H.; Ni, Z.-Z.; He, Y. Effect of cutting styles on physicochemical properties and antioxidant capacity of fresh-cut kiwifruits (Actinidia spp.). J. Food Meas. Charact. 2024, 18, 550–559. [Google Scholar] [CrossRef]
- Dovene, A.K.; Wang, L.; Bokhary, S.U.F.; Madebo, M.P.; Zheng, Y.; Jin, P. Effect of Cutting Styles on Quality and Antioxidant Activity of Stored Fresh-Cut Sweet Potato (Ipomoea batatas L.) Cultivars. Foods 2019, 8, 674. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Hu, W.; Wang, L.; Yang, B. Different Cutting Methods Affect the Quality of Fresh-Cut Cucumbers by Regulating ROS Metabolism. Horticulturae 2023, 9, 514. [Google Scholar] [CrossRef]
- Torres-Contreras, A.M.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Stability of Bioactive Compounds in Broccoli as Affected by Cutting Styles and Storage Time. Molecules 2017, 22, 636. [Google Scholar] [CrossRef]
- Fernando Reyes, L.; Emilio Villarreal, J.; Cisneros-Zevallos, L. The increase in antioxidant capacity after wounding depends on the type of fruit or vegetable tissue. Food Chem. 2007, 101, 1254–1262. [Google Scholar] [CrossRef]
- Li, J.; Biao, P.; Zhou, F. Characterization of POD and PPO in Cichlidium spp. J. Chin. Inst. Food Sci. Technol. 2006, 6, 72–76. [Google Scholar] [CrossRef]
- Gaowa, S.; Feng, K.; Li, Y.; Long, Y.; Hu, W. Effect of Alginate-Based Edible Coating Containing Thyme Essential Oil on Quality and Microbial Safety of Fresh-Cut Potatoes. Horticulturae 2023, 9, 543. [Google Scholar] [CrossRef]
- Boumail, A.; Salmieri, S.; St-Yves, F.; Lauzon, M.; Lacroix, M. Effect of antimicrobial coatings on microbiological, sensorial and physico-chemical properties of pre-cut cauliflowers. Postharvest Biol. Technol. 2016, 116, 1–7. [Google Scholar] [CrossRef]
- Harich, M.; Maherani, B.; Salmieri, S.; Lacroix, M. Evaluation of antibacterial activity of two natural bio-preservatives formulations on freshness and sensory quality of ready to eat (RTE) foods. Food Control 2018, 85, 29–41. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Miguel, M.G.C.; Faleiro, M.L.; Antunes, M.D.C. The influence of edible coatings enriched with citral and eugenol on the raspberry storage ability, nutritional and sensory quality. Food Packag. Shelf Life 2016, 9, 20–28. [Google Scholar] [CrossRef]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The Chemistry Behind the Folin-Ciocalteu Method for the Estimation of (Poly)phenol Content in Food: Total Phenolic Intake in a Mediterranean Dietary Pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Velioglu, Y.S. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- Cass, C.L.; Peraldi, A.; Dowd, P.F.; Mottiar, Y.; Santoro, N.; Karlen, S.D.; Bukhman, Y.V.; Foster, C.E.; Thrower, N.; Bruno, L.C.; et al. Effects of Phenylalanine Ammonia Lyase (PAL) knockdown on cell wall composition, biomass digestibility, and biotic and abiotic stress responses in Brachypodium. J. Exp. Bot. 2015, 66, 4317–4335. [Google Scholar] [CrossRef]
- Babaoğlu Aydaş, S.; Ozturk, S.; Aslım, B. Phenylalanine ammonia lyase (PAL) enzyme activity and antioxidant properties of some cyanobacteria isolates. Food Chem. 2013, 136, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Hussain, P.R.; Wani, A.M.; Meena, R.S.; Dar, M.A. Gamma irradiation induced enhancement of phenylalanine ammonia-lyase (PAL) and antioxidant activity in peach (Prunus persica Bausch, Cv. Elberta). Radiat. Phys. Chem. 2010, 79, 982–989. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B. Tissue and method specificities of phenylalanine ammonia-lyase assay. J. Plant Physiol. 2012, 169, 1317–1320. [Google Scholar] [CrossRef]
- Yingsanga, P.; Srilaong, V.; Kanlayanarat, S.; Noichinda, S.; McGlasson, W.B. Relationship between browning and related enzymes (PAL, PPO and POD) in rambutan fruit (Nephelium lappaceum Linn.) cvs. Rongrien and See-Chompoo. Postharvest Biol. Technol. 2008, 50, 164–168. [Google Scholar] [CrossRef]
- Zou, J.; Gao, J.; Wang, Y. Synthesis of highly active H2O2-sensitized sulfated titania nanoparticles with a response to visible light. J. Photochem. Photobiol. A Chem. 2009, 202, 128–135. [Google Scholar] [CrossRef]
- Xiao, H.S.; He, W.J.; Fu, W.Q.; Cao, H.Y.; Fan, Z.N. A spectrophotometer method testing oxygen radicals. Prog. Biochem. Biophys. 1999, 26, 180–182. [Google Scholar]
- Lin, S.; Wu, X. Exploration of the conditions of SOD detection by the tetrazolium blue (NBT) method. Strait Pharm. J. 2009, 21, 43–45. [Google Scholar] [CrossRef]
- Ma, Y.; Ren, X.; Liang, C. Exogenous Ca2+ enhances antioxidant defense in rice to simulated acid rain by regulating ascorbate peroxidase and glutathione reductase. Planta 2021, 254, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, C.; Song, J.; Ji, X.; Wang, W. Cytidine-gold nanoclusters as peroxidase mimetic for colorimetric detection of glutathione (GSH), glutathione disulfide (GSSG) and glutathione reductase (GR). Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2021, 250, 119316. [Google Scholar] [CrossRef]
- Ishikawa, T.; Shigeoka, S. Recent Advances in Ascorbate Biosynthesis and the Physiological Significance of Ascorbate Peroxidase in Photosynthesizing Organisms. Biosci. Biotechnol. Biochem. 2008, 72, 1143–1154. [Google Scholar] [CrossRef]
- Hobl, E.-L.; Jilma, B.; Ebner, J.; Schmid, R.W. Simultaneous determination of acetylsalicylic acid and salicylic acid in human plasma by isocratic high-pressure liquid chromatography with post-column hydrolysis and fluorescence detection. Biomed. Chromatogr. 2013, 27, 695–698. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Tian, X.; Schaich, K.M. Effects of Molecular Structure on Kinetics and Dynamics of the Trolox Equivalent Antioxidant Capacity Assay with ABTS. J. Agric. Food Chem. 2013, 61, 5511–5519. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Oh, G.; La, I.-J.; Lee, D.-S.; Chae, J.-W.; Im, J.-H.; Park, S.W.; Fu, X.; Lim, J.-S.; Kim, M.-H.; Seong, Y.-S.; et al. Assessment of Bioactive Compounds and Antioxidant Activity of Barley Sprouts. Separations 2025, 12, 68. [Google Scholar] [CrossRef]
- Kenny, O.; O’Beirne, D. Antioxidant phytochemicals in fresh-cut carrot disks as affected by peeling method. Postharvest Biol. Technol. 2010, 58, 247–253. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Aguiló-Aguayo, I.; Martín-Belloso, O.; Soliva-Fortuny, R. Effects of pulsed light treatments on quality and antioxidant properties of fresh-cut mushrooms (Agaricus bisporus). Postharvest Biol. Technol. 2010, 56, 216–222. [Google Scholar] [CrossRef]
- Faliarizao, N.T.; Siddiq, M.; Dolan, K.D. Total phenolics, antioxidant, and physical properties of red chili peppers (Capsicum annum L.) as affected by drying methods. Int. J. Food Prop. 2025, 28, 2492823. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Su, N.; Liu, W.; Gao, M.; Li, R.; Li, Y.; Bi, Y. L-Phenylalanine preharvest spraying effectively enhances the reactive oxygen metabolism and antioxidant capacity in postharvest broccoli. Plant Physiol. Biochem. 2025, 227, 110185. [Google Scholar] [CrossRef]
- Favela-González, K.M.; Hernández-Almanza, A.Y.; De la Fuente-Salcido, N.M. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: A review. J. Food Biochem. 2020, 44, e13414. [Google Scholar] [CrossRef]
- Guan, Y.; Hu, W.; Jiang, A.; Xu, Y.; Sa, R.; Feng, K.; Zhao, M.; Yu, J.; Ji, Y.; Hou, M.; et al. Effect of Methyl Jasmonate on Phenolic Accumulation in Wounded Broccoli. Molecules 2019, 24, 3537. [Google Scholar] [CrossRef]
- Marszałek, K.; Woźniak, Ł.; Barba, F.J.; Skąpska, S.; Lorenzo, J.M.; Zambon, A.; Spilimbergo, S. Enzymatic, physicochemical, nutritional and phytochemical profile changes of apple (Golden delicious L.) juice under supercritical carbon dioxide and long-term cold storage. Food Chem. 2018, 268, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Saito, K.; Yonekura-Sakakibara, K.; Nakabayashi, R.; Higashi, Y.; Yamazaki, M.; Tohge, T.; Fernie, A.R. The flavonoid biosynthetic pathway in Arabidopsis: Structural and genetic diversity. Plant Physiol. Biochem. 2013, 72, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Yue, J. Effects of cutting method and methyl jasmonate pretreatment on the quality and antioxidant activity of fresh-cut pineapple. Food Sci. 2017, 39, 258–263. [Google Scholar]
- Mayer, A.M. Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry 2006, 67, 2318–2331. [Google Scholar] [CrossRef]
- Tilley, A.; McHenry, M.P.; McHenry, J.A.; Solah, V.; Bayliss, K. Enzymatic browning: The role of substrates in polyphenol oxidase mediated browning. Curr. Res. Food Sci. 2023, 7, 100623. [Google Scholar] [CrossRef]
- Valadez-Carmona, L.; Plazola-Jacinto, C.P.; Hernández-Ortega, M.; Hernández-Navarro, M.D.; Villarreal, F.; Necoechea-Mondragón, H.; Ortiz-Moreno, A.; Ceballos-Reyes, G. Effects of microwaves, hot air and freeze-drying on the phenolic compounds, antioxidant capacity, enzyme activity and microstructure of cacao pod husks (Theobroma cacao L.). Innov. Food Sci. Emerg. Technol. 2017, 41, 378–386. [Google Scholar] [CrossRef]
- Osae, R.; Zhou, C.; Xu, B.; Tchabo, W.; Tahir, H.E.; Mustapha, A.T.; Ma, H. Effects of ultrasound, osmotic dehydration, and osmosonication pretreatments on bioactive compounds, chemical characterization, enzyme inactivation, color, and antioxidant activity of dried ginger slices. J. Food Biochem. 2019, 43, e12832. [Google Scholar] [CrossRef]
- Hu, W.; Guan, Y.; Wang, Y.; Yuan, N. Effect of Wounding Intensity on Edible Quality by Regulating Physiological and ROS Metabolism in Fresh-Cut Pumpkins. Horticulturae 2023, 9, 512. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Xu, Z.; Li, F.; Liu, H.; Li, L.; Liao, Y.; Zhao, Y. Physiological and transcriptomic insights into low temperature-induced suppression of internal browning in postharvest pineapple (Ananas comosus L.). Postharvest Biol. Technol. 2025, 230, 113811. [Google Scholar] [CrossRef]
- Jiao, Y.; Wu, J.; Yu, W.; Jiao, W.; Wang, X. Enhanced sulforaphane accumulation in fresh-cut broccoli via exogenous glutathione-mediated modulation of the AsA-GSH cycle. Postharvest Biol. Technol. 2025, 229, 113717. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Kato, M.; Yamawaki, K.; Asai, T.; Nishikawa, F.; Ikoma, Y.; Matsumoto, H. Effect of 1-methylcyclopropene on the expression of genes for ascorbate metabolism in postharvest broccoli. Postharvest Biol. Technol. 2010, 58, 121–128. [Google Scholar] [CrossRef][Green Version]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Q.; Li, B.; Wang, J.; Shi, M.; Wang, J.; Chen, Y.; Zhang, Y.; Sarengaowa; Xiao, Y.; Feng, K. Influence of Cutting Styles on Antioxidant Capabilities of Fresh-Cut Cauliflower by Regulating ROS Metabolism and Antioxidant Enzyme Activity. Antioxidants 2025, 14, 1188. https://doi.org/10.3390/antiox14101188
Guo Q, Li B, Wang J, Shi M, Wang J, Chen Y, Zhang Y, Sarengaowa, Xiao Y, Feng K. Influence of Cutting Styles on Antioxidant Capabilities of Fresh-Cut Cauliflower by Regulating ROS Metabolism and Antioxidant Enzyme Activity. Antioxidants. 2025; 14(10):1188. https://doi.org/10.3390/antiox14101188
Chicago/Turabian StyleGuo, Qihan, Bingheng Li, Jiarui Wang, Minke Shi, Jiayu Wang, Yan Chen, Yunjie Zhang, Sarengaowa, Ying Xiao, and Ke Feng. 2025. "Influence of Cutting Styles on Antioxidant Capabilities of Fresh-Cut Cauliflower by Regulating ROS Metabolism and Antioxidant Enzyme Activity" Antioxidants 14, no. 10: 1188. https://doi.org/10.3390/antiox14101188
APA StyleGuo, Q., Li, B., Wang, J., Shi, M., Wang, J., Chen, Y., Zhang, Y., Sarengaowa, Xiao, Y., & Feng, K. (2025). Influence of Cutting Styles on Antioxidant Capabilities of Fresh-Cut Cauliflower by Regulating ROS Metabolism and Antioxidant Enzyme Activity. Antioxidants, 14(10), 1188. https://doi.org/10.3390/antiox14101188





