Internal Exposure to BTEX in Tropical Children: Does Exposure Speed Up Pubertal Development?
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Recruitment and Sample Collection
2.2. Physical Examination and Assessment of Puberty Status
2.3. Chemicals and Reagents
2.4. Sample Preparation and Instrumental Analysis
2.5. Quality Assurance and Quality Control
2.6. Statistical Analysis
3. Results
3.1. Demographic Characteristics of the Participants
3.2. Urinary Occurrence of BTEX Metabolites and 8-OHdG in Children
3.3. Relationship of 8-OHdG with BTEX Metabolites
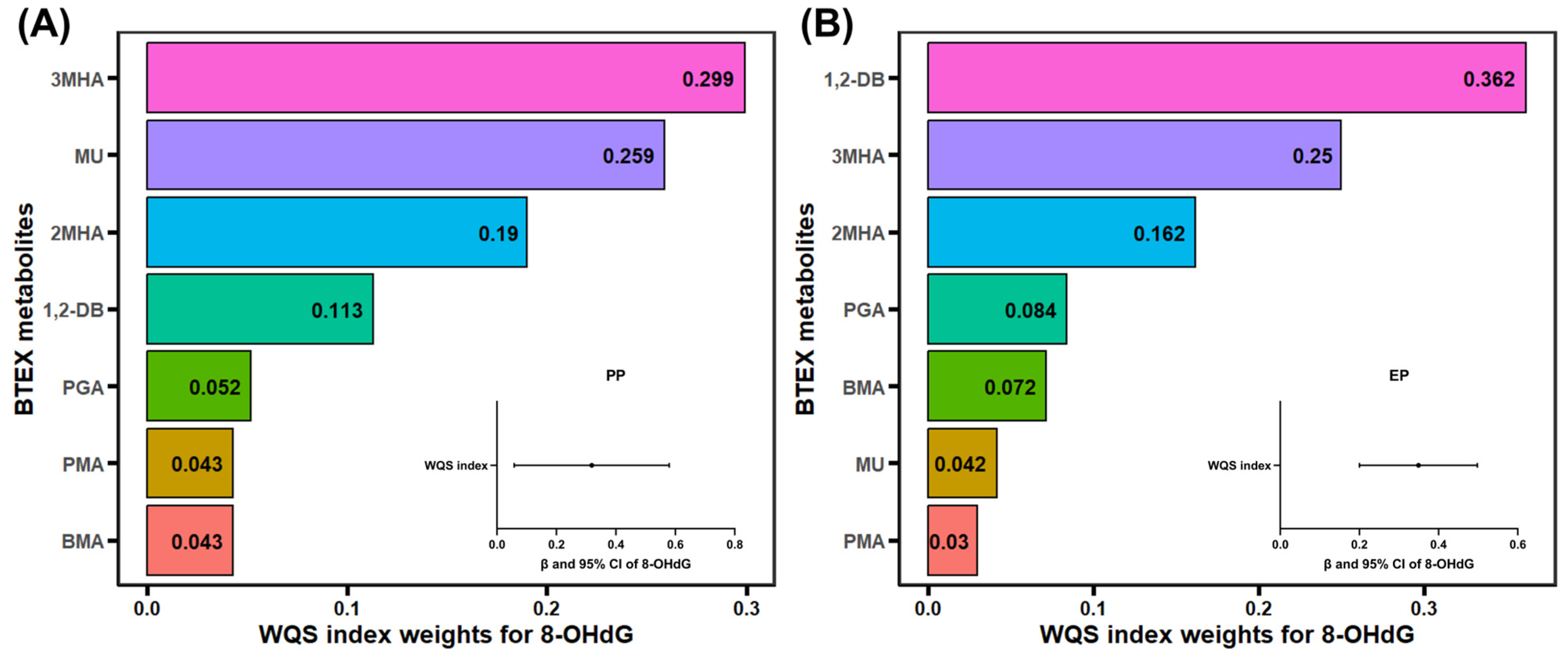
3.4. Association of BTEX Metabolites and Oxidative DNA Damage with Precocious Puberty
3.5. Association of BTEX Metabolites and Oxidative DNA Damage with Early Puberty
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, A.J.; Pal, V.K.; Kannan, K. A review of environmental occurrence, toxicity, biotransformation and biomonitoring of volatile organic compounds. Environ. Chem. Ecotoxicol. 2021, 3, 91–116. [Google Scholar] [CrossRef]
- Montero-Montoya, R.; Lopez-Vargas, R.; Arellano-Aguilar, O. Volatile organic compounds in air: Sources, distribution, exposure and associated illnesses in children. Ann. Glob. Health 2018, 84, 225–238. [Google Scholar] [CrossRef]
- Pal, V.K.; Li, A.J.; Zhu, H.; Kannan, K. Diurnal variability in urinary volatile organic compound metabolites and its association with oxidative stress biomarkers. Sci. Total Environ. 2022, 818, 151704. [Google Scholar] [CrossRef]
- Liu, R.; Wan, Y.; Zhu, B.; Liu, Q.; Wang, H.; Jiang, Q.; Feng, Y.; Zhu, K.; Zhao, S.; Xiang, Z.; et al. Association between urinary BTEX metabolites and dyslexic odds among school-aged children. Environ. Sci. Pollut. Res. 2024, 31, 31443–31454. [Google Scholar] [CrossRef] [PubMed]
- Riggs, D.W.; Malovichko, M.V.; Gao, H.; McGraw, K.E.; Taylor, B.S.; Krivokhizhina, T.; Rai, S.N.; Keith, R.J.; Bhatnagar, A.; Srivastava, S. Environmental exposure to volatile organic compounds is associated with endothelial injury. Toxicol. Appl. Pharmacol. 2022, 437, 115877. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, S.; Dales, R.E.; Liu, L.; Kauri, L.M.; Lemieux, C.L.; Hebbern, C.; Zhu, J. Residential exposure to volatile organic compounds and lung function: Results from a population-based cross-sectional survey. Environ. Pollut. 2014, 194, 145–151. [Google Scholar] [CrossRef]
- Yoon, H.I.; Hong, Y.C.; Cho, S.H.; Kim, H.; Kim, Y.H.; Sohn, J.R.; Kwon, M.; Park, S.H.; Cho, M.H.; Cheong, H.K. Exposure to volatile organic compounds and loss of pulmonary function in the elderly. Eur. Respir. J. 2010, 36, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Li, Z.; Lv, X.; Wu, P.; Tan, J.; Wu, Q.; Li, Y.; Jiang, W.; Pang, Q.; Wang, Y.; et al. Exposure to volatile organic compounds may be associated with oxidative DNA damage-mediated childhood asthma. Ecotoxicol. Environ. Saf. 2021, 210, 111864. [Google Scholar] [CrossRef]
- Verma, Y.; Rana, S.V.S. Endocrinal toxicity of industrial solvents—A mini review. Indian J. Exp. Biol. 2009, 47, 537–549. [Google Scholar]
- Bahadar, H.; Mostafalou, S.; Abdollahi, M. Current understandings and perspectives on non-cancer health effects of benzene: A global concern. Toxicol. Appl. Pharmacol. 2014, 276, 83–94. [Google Scholar] [CrossRef]
- Svensson, B.G.; Nise, G.; Erfurth, E.M.; Olsson, H. Neuroendocrine effects in printing workers exposed to toluene. Br. J. Ind. Med. 1992, 49, 402–408. [Google Scholar] [CrossRef][Green Version]
- Wei, C.; Cao, L.; Zhou, Y.; Zhang, W.; Zhang, P.; Wang, M.; Xiong, M.; Deng, C.; Xiong, Q.; Liu, W.; et al. Multiple statistical models reveal specific volatile organic compounds affect sex hormones in American adult male: NHANES 2013–2016. Front. Endocrinol. 2023, 13, 1076664. [Google Scholar] [CrossRef]
- Wei, C.; Pan, Y.; Zhang, W.; He, Q.; Chen, Z.; Zhang, Y. Comprehensive analysis between volatile organic compound (VOC) exposure and female sex hormones: A cross-sectional study from NHANES 2013–2016. Environ. Sci. Pollut. Res. 2023, 30, 95828–95839. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Fuller, R.; Fisher, S.; Suk, W.A.; Sly, P.; Chiles, T.C.; Bose-O’Reilly, S. Pollution and children’s health. Sci. Total Environ. 2019, 650, 2389–2394. [Google Scholar] [CrossRef]
- Di Pietro, G.; Forcucci, F.; Chiarelli, F. Endocrine disruptor chemicals and children’s health. Int. J. Mol. Sci. 2023, 24, 2671. [Google Scholar] [CrossRef]
- Cheuiche, A.V.; da Silveira, L.G.; Pedroso de Paula, L.C.; Siqueira Lucena, I.R.; Silveiro, S.P. Diagnosis and management of precocious sexual maturation: An updated review. Eur. J. Pediatr. 2021, 180, 3073–3087. [Google Scholar] [CrossRef] [PubMed]
- Farello, G.; Altieri, C.; Cutini, M.; Pozzobon, G.; Verrotti, A. Review of the literature on current changes in the timing of pubertal development and the incomplete forms of early puberty. Front. Pediatr. 2019, 7, 147. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Ding, G.; Tian, Y.; Zhou, Z.; Wang, X.; Shen, L.; Huang, H. Association between bisphenol a exposure and idiopathic central precocious puberty (ICPP) among school-aged girls in Shanghai, China. Environ. Int. 2018, 115, 410–416. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kwon, A.; Jung, M.K.; Kim, K.E.; Suh, J.; Chae, H.W.; Kim, D.H.; Ha, S.; Seo, G.H.; Kim, H.-S. Incidence and prevalence of central precocious puberty in Korea: An epidemiologic study based on a national database. J. Pediatr. 2019, 208, 221–228. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, T.; Li, X.; Pan, D.; Lai, X.; Chen, Y.; Wang, X.; Yu, X.; Fu, S.; Huang, S.; et al. Prevalence of precocious puberty among Chinese children: A school population-based study. Endocrine 2021, 72, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ge, A.; Xie, H.; Li, W.; Qin, Y.; Yang, W.; Wang, D.; Gu, W.; Wang, X. Effects of ambient air pollution on precocious puberty: A case-crossover analysis in Nanjing, China. J. Clin. Med. 2023, 12, 282. [Google Scholar] [CrossRef]
- Fisher, M.M.; Eugster, E.A. What is in our environment that effects puberty? Reprod. Toxicol. 2014, 44, 7–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Y.; Shi, H.; Jiang, X.; Zhao, Y.; Fang, X.; Xie, C. Could exposure to phthalates speed up or delay pubertal onset and development? A 1.5-year follow-up of a school-based population. Environ. Int. 2015, 83, 41–49. [Google Scholar] [CrossRef]
- Tang, J.; Chen, Y.; Xue, P.; Chen, Y.; Kong, H.; Lin, C.; Wang, X.; Liu, S. Exposure to synthetic steroid hormones and precocious puberty in girls: A case-control study. Ecotoxicol. Environ. Saf. 2024, 283, 116814. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, P.; Li, J.; Zhao, Y.; Huang, Y.; Leung, K.S.-Y.; Shi, H.; Zhang, Y. Mixed exposure to phthalates and organic UV filters affects Children’s pubertal development in a gender-specific manner. Chemosphere 2023, 320, 138073. [Google Scholar] [CrossRef]
- Alwis, K.U.; Blount, B.C.; Britt, A.S.; Patel, D.; Ashley, D.L. Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS). Anal. Chim. Acta 2012, 750, 152–160. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 1969, 44, 291–303. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 1970, 45, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Carrico, C.; Gennings, C.; Wheeler, D.C.; Factor-Litvak, P. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J. Agric. Biol. Environ. Stat. 2015, 20, 100–120. [Google Scholar] [CrossRef] [PubMed]
- Protano, C.; Guidotti, M.; Manini, P.; Petyx, M.; La Torre, G.; Vitali, M. Benzene exposure in childhood: Role of living environments and assessment of available tools. Environ. Int. 2010, 36, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Boyle, E.B.; Viet, S.M.; Wright, D.J.; Merrill, L.S.; Alwis, K.U.; Blount, B.C.; Mortensen, M.E.; Moye, J., Jr.; Dellarco, M. Assessment of exposure to VOCs among pregnant women in the national children’s study. Int. J. Environ. Res. Public Health 2016, 13, 376. [Google Scholar] [CrossRef]
- Jain, R.B. Levels of selected urinary metabolites of volatile organic compounds among children aged 6–11 years. Environ. Res. 2015, 142, 461–470. [Google Scholar] [CrossRef]
- El-Metwally, D.; Chain, K.; Stefanak, M.P.; Alwis, U.; Blount, B.C.; LaKind, J.S.; Bearer, C.F. Urinary metabolites of volatile organic compounds of infants in the neonatal intensive care unit. Pediatr. Res. 2018, 83, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Caron-Beaudoin, E.; Valter, N.; Chevrier, J.; Ayotte, P.; Frohlich, K.; Verner, M.-A. Gestational exposure to volatile organic compounds (VOCs) in Northeastern British Columbia, Canada: A pilot study. Environ. Int. 2018, 110, 131–138. [Google Scholar] [CrossRef]
- Kuang, H.; Li, Y.; Jiang, W.; Wu, P.; Tan, J.; Zhang, H.; Pang, Q.; Ma, S.; An, T.; Fan, R. Simultaneous determination of urinary 31 metabolites of VOCs, 8-hydroxy-2′-deoxyguanosine, and trans-3′-hydroxycotinine by UPLC-MS/MS: 13C- and 15N-labeled isotoped internal standards are more effective on reduction of matrix effect. Anal. Bioanal. Chem. 2019, 411, 7841–7855. [Google Scholar] [CrossRef] [PubMed]
- Keith, R.J.; Fetterman, J.L.; Orimoloye, O.A.; Dardari, Z.; Lorkiewicz, P.K.; Hamburg, N.M.; DeFilippis, A.P.; Blaha, M.J.; Bhatnagar, A. Characterization of volatile organic compound metabolites in cigarette smokers, electronic nicotine device users, dual users, and nonusers of tobacco. Nicotine Tob. Res. 2020, 22, 264–272. [Google Scholar] [CrossRef]
- Song, W.; Han, Q.; Wan, Y.; Qian, X.; Wei, M.; Jiang, Y.; Wang, Q. Repeated measurements of 21 urinary metabolites of volatile organic compounds and their associations with three selected oxidative stress biomarkers in 0–7-year-old healthy children from south and central China. Chemosphere 2022, 287, 132065. [Google Scholar] [CrossRef]
- Kuang, H.; Li, Y.; Li, L.; Ma, S.; An, T.; Fan, R. Four-year population exposure study: Implications for the effectiveness of e-waste control and biomarkers of e-waste pollution. Sci. Total Environ. 2022, 842, 156595. [Google Scholar] [CrossRef]
- Lee, I.; Park, H.; Kim, M.J.; Kim, S.; Choi, S.; Park, J.; Cho, Y.H.; Hong, S.; Yoo, J.; Cheon, G.J.; et al. Exposure to polycyclic aromatic hydrocarbons and volatile organic compounds is associated with a risk of obesity and diabetes mellitus among Korean adults: Korean National Environmental Health Survey (KoNEHS) 2015–2017. Int. J. Hyg. Environ. Health 2022, 240, 113886. [Google Scholar] [CrossRef]
- Qin, N.; Zhu, Y.; Zhong, Y.; Tian, J.; Li, J.; Chen, L.; Fan, R.; Wei, F. External exposure to btex, internal biomarker response, and health risk assessment of nonoccupational populations near a coking plant in southwest China. Int. J. Environ. Res. Public Health 2022, 19, 847. [Google Scholar] [CrossRef]
- Yan, M.; Zhu, H.; Luo, H.; Zhang, T.; Sun, H.; Kannan, K. Daily exposure to environmental volatile organic compounds triggers oxidative damage: Evidence from a large-scale survey in China. Environ. Sci. Technol. 2023, 57, 20501–20509. [Google Scholar] [CrossRef]
- Shi, H.; Liang, S.; Wang, Z.; Lv, Q.; Zhang, Q.; Li, M. Non-targeted analysis of odor components and hazardous volatiles in children’s raincoats. Ecotoxicol. Environ. Saf. 2025, 296, 118220. [Google Scholar] [CrossRef]
- Xu, W.; Xing, Q.; Pan, L.; Wang, Z.; Cao, X.; Yan, W.; Xie, W.; Meng, X.; Wu, X. Characterization, source apportionment, and risk assessment of ambient volatile organic compounds in urban and background regions of Hainan Island, China. Atmos. Environ. 2024, 316, 120167. [Google Scholar] [CrossRef]
- IARC Some Industrial Chemicals. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 77. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Some-Industrial-Chemicals-2000 (accessed on 11 January 2025).
- Zhu, Q.; Zhou, S.; Wen, Z.; Li, H.; Huang, B.; Chen, Y.; Li, X.; Lin, H.; Wang, Y.; Ge, R.-S. Xylene delays the development of Leydig cells in pubertal rats by inducing reactive oxidative species. Toxicology 2021, 454, 152740. [Google Scholar] [CrossRef]
- Suaidi, N.A.; Alshawsh, M.A.; Hoe, S.-Z.; Mokhtar, M.H.; Zin, S.R.M. Toxicological effects of technical xylene mixtures on the female reproductive system: A systematic review. Toxics 2022, 10, 235. [Google Scholar] [CrossRef]
- Lian, X.; Guo, J.; Wang, Y.; Wang, S.; Li, J. Association between volatile organic compound exposure and sex hormones in adolescents: The mediating role of serum albumin. Toxics 2024, 12, 438. [Google Scholar] [CrossRef] [PubMed]
- Harrath, A.H.; Alrezaki, A.; Jalouli, M.; Aldawood, N.; Aldahmash, W.; Mansour, L.; Alwasel, S. Ethylbenzene exposure disrupts ovarian function in Wistar rats via altering folliculogenesis and steroidogenesis-related markers and activating autophagy and apoptosis. Ecotoxicol. Environ. Saf. 2022, 229, 113081. [Google Scholar] [CrossRef] [PubMed]
- Staessen, J.A.; Nawrot, T.; Den Hond, E.; Thijs, L.; Fagard, R.; Hoppenbrouwers, K.; Koppen, G.; Nelen, V.; Schoeters, G.; Vanderschueren, D.; et al. Renal function, cytogenetic measurements, and sexual development in adolescents in relation to environmental pollutants: A feasibility study of biomarkers. Lancet 2001, 357, 1660–1669. [Google Scholar] [CrossRef]
- Webb, E.; Bushkin-Bedient, S.; Cheng, A.; Kassotis, C.D.; Balise, V.; Nagel, S.C. Developmental and reproductive effects of chemicals associated with unconventional oil and natural gas operations. Rev. Environ. Health 2014, 29, 307–318. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Harrath, A.H. Influence of oil-related environmental pollutants on female reproduction. Reprod. Toxicol. 2017, 71, 142–145. [Google Scholar] [CrossRef]
- Plunk, E.C.; Richards, S.M. Endocrine-disrupting air pollutants and their effects on the hypothalamus-pituitary-gonadal axis. Int. J. Mol. Sci. 2020, 21, 9191. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef]
- Alviggi, C.; Guadagni, R.; Conforti, A.; Coppola, G.; Picarelli, S.; De Rosa, P.; Vallone, R.; Strina, I.; Pagano, T.; Mollo, A.; et al. Association between intrafollicular concentration of benzene and outcome of controlled ovarian stimulation in IVF/ICSI cycles: A pilot study. J. Ovarian Res. 2014, 7, 67. [Google Scholar] [CrossRef]
- Arnold, S.M.; Angerer, J.; Boogaard, P.J.; Hughes, M.F.; O’Lone, R.B.; Robison, S.H.; Schnatter, A.R. The use of biomonitoring data in exposure and human health risk assessment: Benzene case study. Crit. Rev. Toxicol. 2013, 43, 119–153. [Google Scholar] [CrossRef]
- Bigambo, F.M.; Sun, H.; Yan, W.; Wu, D.; Xia, Y.; Wang, X.; Wang, X. Association between phenols exposure and earlier puberty in children: A systematic review and meta-analysis. Environ. Res. 2020, 190, 110056. [Google Scholar] [CrossRef] [PubMed]
- Ozen, S.; Darcan, S. Effects of environmental endocrine disruptors on pubertal development. J. Clin Res. Pediatr. Endocrinol. 2011, 3, 1–6. [Google Scholar] [CrossRef]
- Liu, G.; Guo, J.; Zhang, X.; Lu, Y.; Miao, J.; Xue, H. Obesity is a risk factor for central precocious puberty: A case-control study. BMC Pediatr. 2021, 21, 509. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Qian, H.; Yang, J.; Hu, Y. The association analysis between exposure to volatile organic chemicals and obesity in the general USA population: A cross-sectional study from NHANES program. Chemosphere 2023, 315, 137738. [Google Scholar] [CrossRef]
- Faber, W.D.; Roberts, L.S.G.; Stump, D.G.; Tardif, R.; Krishnan, K.; Tort, M.; Dimond, S.; Dutton, D.; Moran, E.; Lawrence, W. Two generation reproduction study of ethylbenzene by inhalation in Crl-CD rats. Birth Defects Res. Part B-Dev. Reprod. Toxicol. 2006, 77, 10–21. [Google Scholar] [CrossRef]
- Nguyen, H.D. Exposure to mixed chemicals elevated triiodothyronine (T3) and follicle-stimulating hormone (FSH) levels: Epidemiology and in silico toxicogenomic involvement. Environ. Sci. Pollut. Res. 2023, 30, 88803–88823. [Google Scholar] [CrossRef]
- Borgert, C.J. Hypothesis-driven weight of evidence evaluation indicates styrene lacks endocrine disruption potential. Crit. Rev. Toxicol. 2023, 53, 53–68. [Google Scholar] [CrossRef] [PubMed]




| Characteristic | PP (n = 61) | Non-PP (n = 48) | p | EP (n = 185) | Non-EP (n = 179) | p |
|---|---|---|---|---|---|---|
| Age (years), mean ± SD | 6.89 ± 0.61 | 6.92 ± 0.61 | 0.790 a | 8.36 ± 0.49 | 8.39 ± 0.50 | 0.649 |
| BMI (kg/m2), mean ± SD | 17.7 ± 3.10 | 16.0 ± 2.88 | 0.001 | 17.5 ± 2.97 | 15.5 ± 2.07 | 0.000 |
| Sex, n (%) | 0.841 b | 0.371 | ||||
| Male | 13 (21.3) | 11 (22.9) | 11 (5.9) | 7 (3.9) | ||
| Female | 48 (78.7) | 37 (77.1) | 174 (94.1) | 172 (96.1) | ||
| Birth weight, n (%) | 0.344 | 0.513 | ||||
| <2500 g | 4 (6.6) | 7 (14.6) | 11 (5.9) | 13 (7.3) | ||
| 2500–4000 g | 52 (85.2) | 36 (75.0) | 159 (85.9) | 146 (81.6) | ||
| ≥4000 g | 5 (8.2) | 5 (10.4) | 15 (8.1) | 20 (11.2) | ||
| Mode of delivery, n (%) | 0.012 | 0.183 | ||||
| Natural birth | 30 (49.2) | 35 (72.9) | 102 (55.1) | 111 (62.0) | ||
| Cesarean section | 31 (50.8) | 13 (27.1) | 83 (44.9) | 68 (38.0) | ||
| Feeding style during the first 4 months, n (%) | 0.758 | 0.387 | ||||
| Breastfeeding | 39 (63.9) | 31 (64.6) | 97 (52.4) | 102 (57.0) | ||
| Mixed feeding | 9 (14.8) | 5 (10.4) | 35 (18.9) | 37 (20.7) | ||
| Artificial feeding | 13 (21.3) | 12 (25.0) | 53 (28.6) | 40 (22.3) | ||
| The only child, n (%) | 0.024 | 0.001 | ||||
| No | 44 (72.1) | 43 (89.6) | 118 (63.8) | 142 (79.3) | ||
| Yes | 17 (27.9) | 5 (10.4) | 67 (36.2) | 37 (20.7) | ||
| Parental education level c, n (%) | 0.003 | 0.675 | ||||
| Below high school | 7 (11.5) | 17 (35.4) | 33 (17.8) | 35 (19.6) | ||
| High school or above | 54 (88.5) | 31 (64.6) | 152 (82.2) | 144 (80.4) | ||
| Frequency of supplementing vitamin D | 0.251 | 0.002 | ||||
| 0 | 49 (80.3) | 37 (77.1) | 156 (84.3) | 127 (70.9) | ||
| Less than twice a week | 9 (14.8) | 10 (20.8) | 18 (9.7) | 41 (22.9) | ||
| 3–6 times a week | 3 (4.9) | 0 (0.0) | 10 (5.4) | 7 (3.9) | ||
| ≥7 times a week | 0 (0.0) | 1 (2.1) | 1 (0.5) | 4 (2.2) |
| Analyte | PMA | 1,2-DB | MU | BMA | PGA | 2MHA | 3MHA | 8-OHdG | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 109) | PP (n = 61) | DF | 96.7% | 96.7% | 100% | 96.7% | 100% | 100% | 100% | 100% |
| GM | 7.09 | 60.1 | 52.5 | 3.71 | 418 | 51.3 | 44.9 | 6.21 | ||
| Median | 8.69 | 85.3 | 47.4 | 3.74 | 496 | 39.3 | 42.5 | 5.31 | ||
| Non-PP (n = 48) | DF | 93.8% | 87.5% | 100% | 95.8% | 97.9% | 97.9% | 100% | 100% | |
| GM | 6.56 | 29.7 | 67.6 | 4.12 | 406 | 46.8 | 42.9 | 5.71 | ||
| Median | 7.84 | 42.4 | 60.0 | 4.30 | 468 | 47.9 | 39.9 | 6.02 | ||
| p a | 0.784 | 0.015 | 0.046 | 0.550 | 0.840 | 0.705 | 0.913 | 0.462 | ||
| Girl (n = 85) | PP (n = 48) | GM | 6.81 | 55.9 | 55.2 | 4.30 | 428 | 50.0 | 44.5 | 6.19 |
| Median | 8.95 | 83.6 | 54.1 | 3.90 | 496 | 40.7 | 43.6 | 5.50 | ||
| Non-PP (n = 37) | GM | 6.76 | 24.2 | 71.0 | 5.54 | 462 | 53.4 | 41.2 | 4.95 | |
| Median | 7.95 | 35.5 | 61.2 | 5.12 | 455 | 48.4 | 35.2 | 5.57 | ||
| p | 0.958 | 0.020 | 0.079 | 0.300 | 0.936 | 0.451 | 0.703 | 0.832 | ||
| Boy (n = 24) | PP (n = 13) | GM | 8.22 | 78.8 | 43.8 | 2.15 | 384 | 56.2 | 46.4 | 6.29 |
| Median | 5.43 | 88.2 | 42.0 | 2.46 | 379 | 34.5 | 28.0 | 5.26 | ||
| Non-PP (n = 11) | GM | 5.90 | 59.1 | 57.5 | 1.53 | 264 | 30.1 | 49.5 | 9.19 | |
| Median | 6.52 | 69.5 | 53.0 | 2.42 | 600 | 42.3 | 52.9 | 9.02 | ||
| p | 0.733 | 0.424 | 0.252 | 0.955 | 1.000 | 0.569 | 0.494 | 0.134 |
| Analyte | PMA | 1,2-DB | MU | BMA | PGA | 2MHA | 3MHA | 8-OHdG | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 364) | EP (n = 185) | DF | 97.3% | 100% | 99.5% | 96.8% | 98.4% | 99.5% | 100% | 100% |
| GM | 5.74 | 75.0 | 48.2 | 4.12 | 284 | 39.7 | 37.7 | 6.10 | ||
| Median | 6.35 | 62.0 | 42.7 | 4.60 | 348 | 36.8 | 32.8 | 5.58 | ||
| Non-EP (n = 179) | DF | 99.4% | 96.1% | 100% | 99.4% | 99.4% | 100% | 99.4% | 100% | |
| GM | 5.63 | 44.3 | 52.7 | 4.46 | 381 | 45 | 34.7 | 6.31 | ||
| Median | 5.90 | 48.1 | 48.5 | 4.31 | 462 | 39.5 | 32.5 | 5.90 | ||
| p a | 0.779 | 0.002 | 0.386 | 0.709 | 0.003 | 0.107 | 0.500 | 0.129 | ||
| Girl (n = 346) | EP (n = 174) | GM | 5.61 | 75.02 | 47.2 | 4.02 | 285 | 39.6 | 37.4 | 5.95 |
| Median | 6.22 | 61.3 | 42.7 | 4.55 | 354 | 37.2 | 33.2 | 5.51 | ||
| Non-EP (n = 172) | GM | 5.71 | 44.5 | 53.1 | 4.45 | 382 | 45.1 | 34.4 | 6.26 | |
| Median | 5.96 | 48.5 | 49.2 | 4.30 | 462 | 40.8 | 32.5 | 5.87 | ||
| p | 0.979 | 0.004 | 0.271 | 0.822 | 0.005 | 0.121 | 0.442 | 0.105 | ||
| Boy (n = 18) | EP (n = 11) | GM | 8.38 | 74.4 | 66.7 | 5.88 | 271 | 42.1 | 42.4 | 8.99 |
| Median | 6.93 | 80.6 | 55.7 | 5.84 | 286 | 28.1 | 26.2 | 7.48 | ||
| Non-EP (n = 7) | GM | 3.97 | 39.9 | 43.7 | 4.60 | 360 | 42.1 | 42.2 | 3.97 | |
| Median | 4.89 | 29.6 | 35.5 | 5.65 | 417 | 35.6 | 27.6 | 4.89 | ||
| p | 0.151 | 0.246 | 0.659 | 0.536 | 0.479 | 0.659 | 0.724 | 0.930 |
| Analyte | Parental Education Level | |||
|---|---|---|---|---|
| Below High School (n = 68) | p a | High School or Above (n = 296) | p | |
| PMA | 3.66 (1.32, 10.2) | 0.013 | 0.81 (0.61, 1.08) | 0.152 |
| 1,2-DB | 1.55 (0.86, 2.81) | 0.147 | 1.35 (1.06, 1.74) | 0.014 |
| MU | 0.54 (0.20, 1.44) | 0.220 | 0.92 (0.66, 1.28) | 0.626 |
| BMA | 0.90 (0.54, 1.50) | 0.677 | 0.98 (0.76, 1.26) | 0.867 |
| PGA | 0.45 (0.18, 1.16) | 0.098 | 0.74 (0.56, 0.97) | 0.032 |
| 2MHA | 0.98 (0.20, 4.76) | 0.983 | 0.76 (0.46, 1.23) | 0.260 |
| 3MHA | 0.65 (0.17, 2.48) | 0.531 | 1.42 (0.89, 2.26) | 0.142 |
| 8-OHdG | 1.43 (0.27, 7.69) | 0.675 | 0.69 (0.39, 1.24) | 0.214 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Zhou, Q.; Wang, D.; Luan, Y.-L.; Guo, Y. Internal Exposure to BTEX in Tropical Children: Does Exposure Speed Up Pubertal Development? Antioxidants 2025, 14, 1164. https://doi.org/10.3390/antiox14101164
Lu Y, Zhou Q, Wang D, Luan Y-L, Guo Y. Internal Exposure to BTEX in Tropical Children: Does Exposure Speed Up Pubertal Development? Antioxidants. 2025; 14(10):1164. https://doi.org/10.3390/antiox14101164
Chicago/Turabian StyleLu, Yao, Qin Zhou, Dan Wang, Yu-Ling Luan, and Ying Guo. 2025. "Internal Exposure to BTEX in Tropical Children: Does Exposure Speed Up Pubertal Development?" Antioxidants 14, no. 10: 1164. https://doi.org/10.3390/antiox14101164
APA StyleLu, Y., Zhou, Q., Wang, D., Luan, Y.-L., & Guo, Y. (2025). Internal Exposure to BTEX in Tropical Children: Does Exposure Speed Up Pubertal Development? Antioxidants, 14(10), 1164. https://doi.org/10.3390/antiox14101164






