Astaxanthin Alleviates Ochratoxin A (OTA)-Induced Spleen Dysfunction and Apoptosis in Broiler Chickens by Modulating the PTEN/PI3K/AKT Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Specialized Feed Preparation
2.2. Animal Research
2.3. Collection of Samples
2.4. Histopathology
2.5. TUNEL Apoptosis Analysis
2.6. Serum IgM and IgG Assays
2.7. Analysis of Splenic Oxidative Parameters
2.8. RNA Extraction and Real-Time Fluorescence Quantitative PCR
2.9. Western Blot Analysis
2.10. Statistical Analysis of Data
3. Results
3.1. Effect of AST on OTA-Induced Changes in the Spleen Index
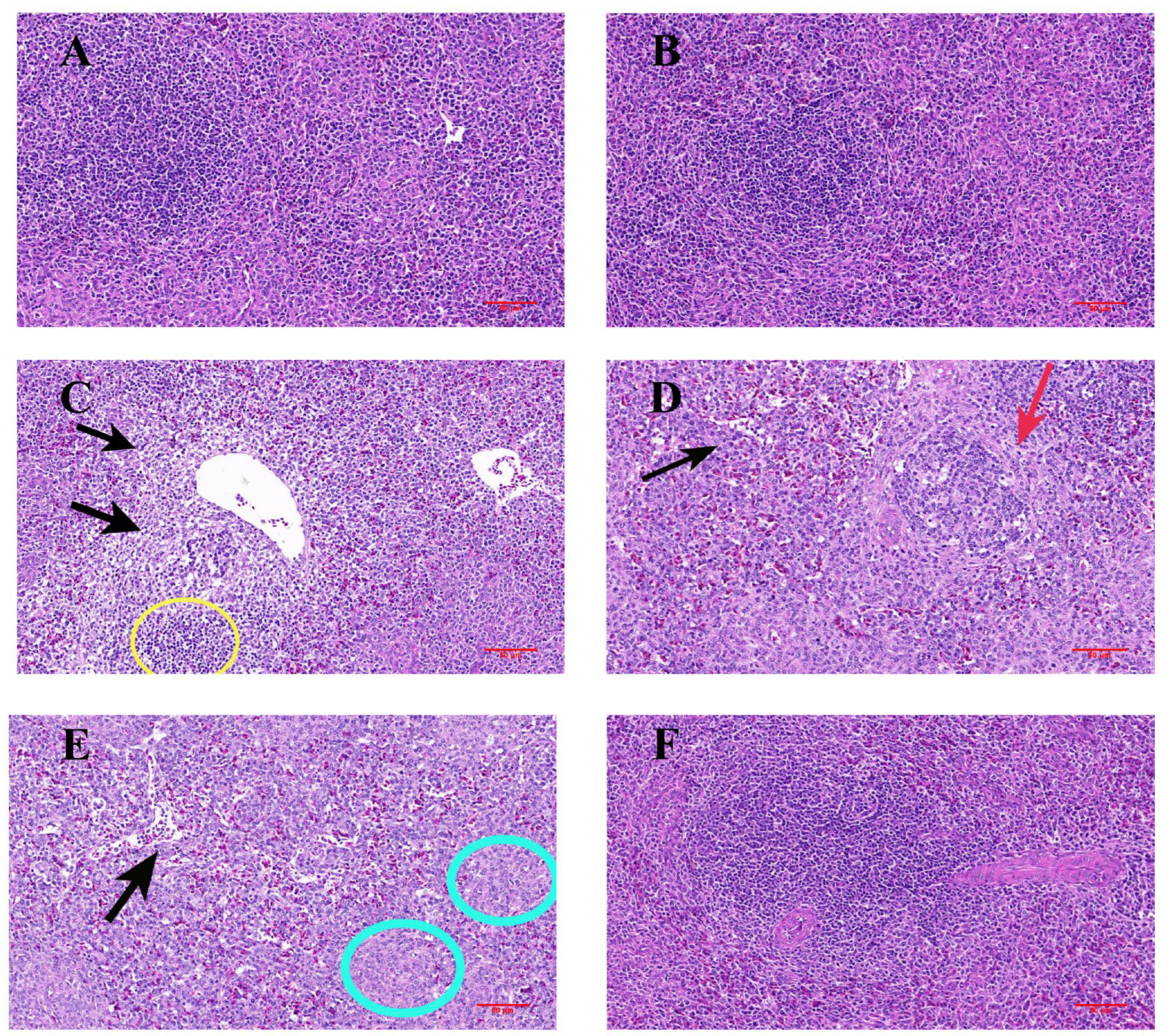
3.2. Histopathological Changes in the Spleen
3.3. Analysis of Apoptosis by TUNEL
3.4. Effect of AST on OTA-Induced Serum Immunoglobulins
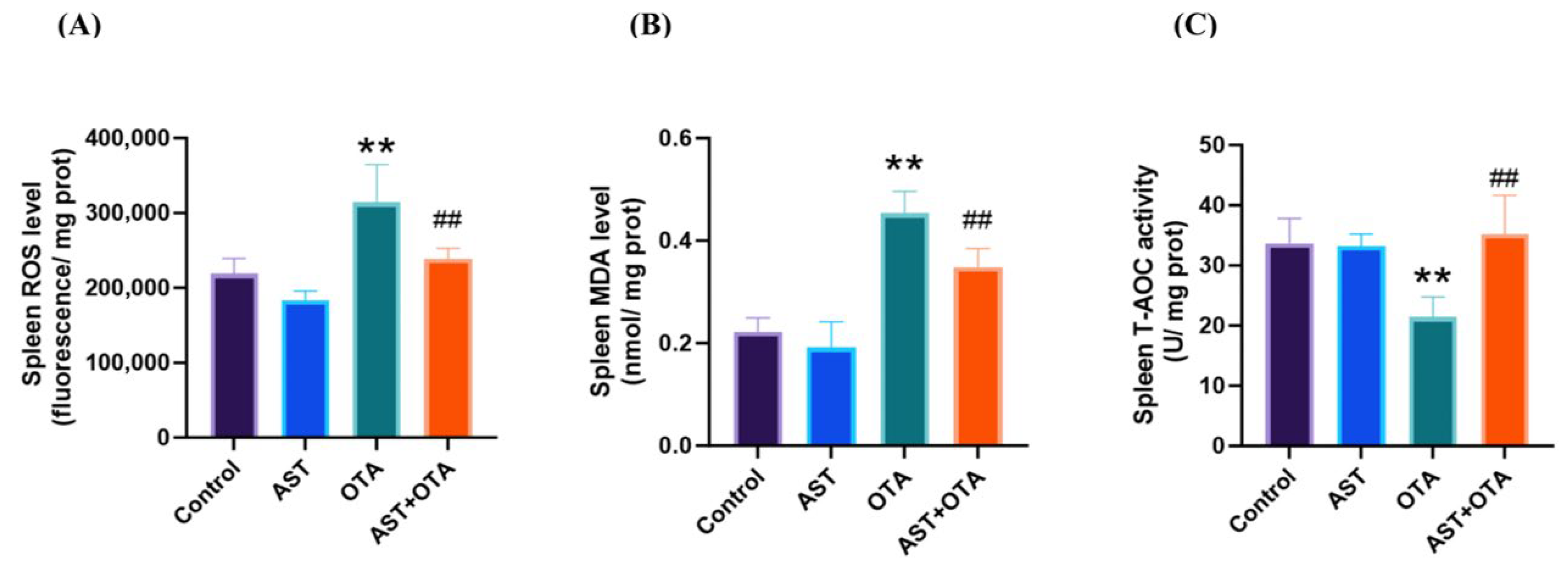
3.5. AST Mitigates the Effects of OTA-Induced Oxidative Stress
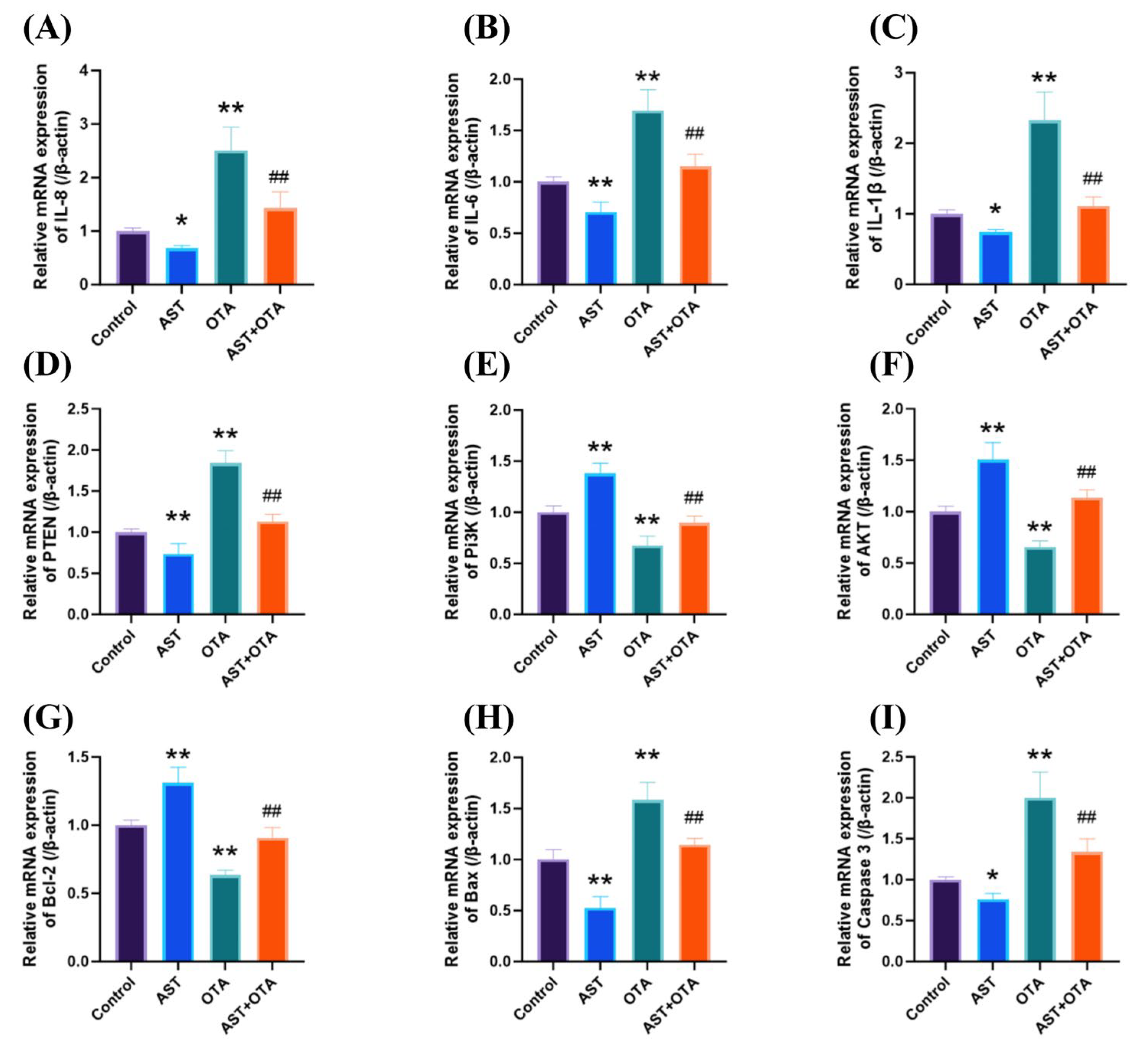
3.6. PTEN/PI3K/AKT (Ser473) Pathway, Apoptosis and Inflammatory Factor-Related Gene Expression
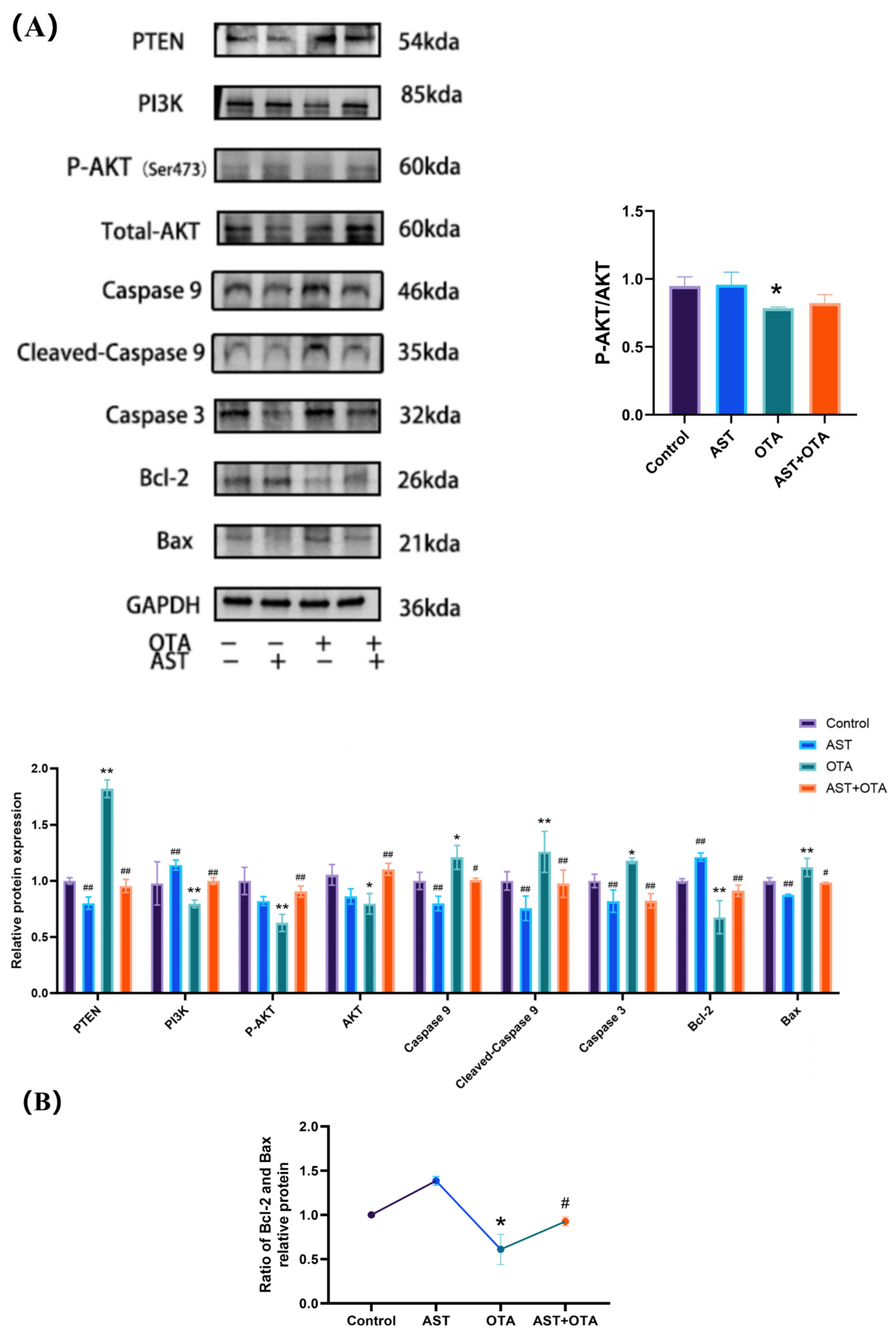
3.7. PTEN/PI3K/AKT (Ser473) Pathway and Apoptosis-Related Protein Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, G.; Li, E.; Gallo, A.; Perrone, G.; Varga, E.; Ma, J.; Yang, B.; Tai, B.; Xing, F. Impact of Environmental Factors on Ochratoxin A: From Natural Occurrence to Control Strategy. Environ. Pollut. 2023, 317, 120767. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hua, X.; Shi, J.; Jing, N.; Ji, T.; Lv, B.; Liu, L.; Chen, Y. Ochratoxin A: Occurrence and Recent Advances in Detoxification. Toxicon 2022, 210, 11–18. [Google Scholar] [CrossRef]
- Yang, Q.; Dhanasekaran, S.; Ngea, G.L.N.; Tian, S.; Li, B.; Zhang, H. Unveiling Ochratoxin a Controlling and Biodetoxification Molecular Mechanisms: Opportunities to Secure Foodstuffs from Ota Contamination. Food Chem. Toxicol. 2022, 169, 113437. [Google Scholar] [CrossRef]
- Ringot, D.; Chango, A.; Schneider, Y.-J.; Larondelle, Y. Toxicokinetics and Toxicodynamics of Ochratoxin a, an Update. Chem. Biol. Interact. 2006, 159, 18–46. [Google Scholar] [CrossRef] [PubMed]
- Mubarik, Y.; Boyetey, S.T.; Aikins, A.R.; Mutocheluh, M. Effect of Ochratoxin a (Ota) on the Immune System: A Systematic Review. Toxins 2025, 17, 256. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Pyo, M.C.; Shin, H.S.; Ryu, D.; Lee, K.-W. Renal Toxicity through Ahr, Pxr, and Nrf2 Signaling Pathway Activation of Ochratoxin a-Induced Oxidative Stress in Kidney Cells. Food Chem. Toxicol. 2018, 122, 59–68. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, B.; Dai, Y.; Li, H.; Xu, W. A Review: Epigenetic Mechanism in Ochratoxin A Toxicity Studies. Toxins 2017, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Akamo, A.J.; Rotimi, S.O.; Akinloye, D.I.; Ugbaja, R.N.; Adeleye, O.O.; Dosumu, O.A.; Eteng, O.E.; Amah, G.; Obijeku, A.; Cole, O.E. Naringin Prevents Cyclophosphamide-Induced Hepatotoxicity in Rats by Attenuating Oxidative Stress, Fibrosis, and Inflammation. Food Chem. Toxicol. 2021, 153, 112266. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.; Zhang, B.; Zhang, Y.; Xiao, Y.; An, Y.; Han, L.; Deng, H.; Yao, S.; Wang, H.; et al. Lonp1 and Sig-1r Contribute to the Counteraction of Ursolic Acid against Ochratoxin a-Induced Mitochondrial Apoptosis. Food Chem. Toxicol. 2023, 172, 113592. [Google Scholar] [CrossRef]
- Hou, L.; Gan, F.; Zhou, X.; Zhou, Y.; Qian, G.; Liu, Z.; Huang, K. Immunotoxicity of Ochratoxin A and Aflatoxin B1 in Combination Is associated with the Nuclear Factor Kappa B Signaling Pathway in 3d4/21 Cells. Chemosphere 2018, 199, 718–727. [Google Scholar] [CrossRef]
- Álvarez, L.; Gil, A.G.; Ezpeleta, O.; García-Jalón, J.A.; López de Cerain, A. Immunotoxic Effects of Ochratoxin a in Wistar Rats after Oral Administration. Food Chem. Toxicol. 2004, 42, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.E.; Taranu, I. Ochratoxin A and Its Effects on Immunity. Toxin Rev. 2015, 34, 11–20. [Google Scholar] [CrossRef]
- Khan, S.A.; Venancio, E.J.; Fernandes, E.V.; Hirooka, E.Y.; Oba, A.; Flaiban, K.K.; Itano, E.N. Low Doses of Ochratoxin-a Decrease Igy and Iga Production in Broiler Chicks. Toxins 2018, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, R.E.; Khalaf, A.A.A.; Elhady, M.A.; Ibrahim, M.A.; Hassanen, E.I.; Noshy, P.A. Quercetin Ameliorates Ochratoxin a-Induced Immunotoxicity in Broiler Chickens by Modulation of Pi3k/Akt Pathway. Chem. Biol. Interact. 2022, 351, 109720. [Google Scholar] [CrossRef]
- Xu, H.; Hao, S.; Gan, F.; Wang, H.; Xu, J.; Liu, D.; Huang, K. In Vitro Immune Toxicity of Ochratoxin A in Porcine Alveolar Macrophages: A Role for the Ros-Relative Tlr4/Myd88 Signaling Pathway. Chem. Biol. Interact. 2017, 272, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yang, J.; Yang, Y.; Wang, X.; Chen, G.; Shi, A.; Lu, Y.; Jia, S.; Kang, X.; Lu, L. Rosmarinic Acid Exerts an Anticancer Effect on Osteosarcoma Cells by Inhibiting Dj-1 Via Regulation of the Pten-Pi3k-Akt Signaling Pathway. Phytomedicine 2020, 68, 153186. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Kang, M.; Chang, C.; Wei, H.; Zhang, C.; Chen, Y. Multiple Forms of Cell Death: A Focus on the Pi3k/Akt Pathway. J. Cell. Physiol. 2023, 238, 2026–2038. [Google Scholar] [CrossRef]
- Guo, H.; German, P.; Bai, S.; Barnes, S.; Guo, W.; Qi, X.; Lou, H.; Liang, J.; Jonasch, E.; Mills, G.B. The Pi3k/Akt Pathway and Renal Cell Carcinoma. J. Genet. Genom. 2015, 42, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Hung, M.-C. Physiological Regulation of Akt Activity and Stability. Am. J. Transl. Res. 2010, 2, 19. [Google Scholar]
- Li, G.; Xu, D.; Sun, J.; Zhao, S.; Zheng, D. Paclitaxel Inhibits Proliferation and Invasion and Promotes Apoptosis of Breast Cancer Cells by Blocking Activation of the Pi3k/Akt Signaling Pathway. Adv. Clin. Exp. Med. 2020, 29, 1337–1345. [Google Scholar] [CrossRef]
- Kumar, D.; Shankar, S.; Srivastava, R.K. Rottlerin induces autophagy and apoptosis in prostate cancer stem cells via PI3K/Akt/mTOR signaling pathway. Cancer Lett. 2014, 343, 179–189. [Google Scholar] [CrossRef]
- Song, Y.; Liu, W.; Zhao, Y.; Zang, J.; Gao, H. Ochratoxin a Induces Human Kidney Tubular Epithelial Cell Apoptosis through Regulating Lipid Raft/Pten/Akt Signaling Pathway. Environ. Toxicol. 2021, 36, 1880–1885. [Google Scholar] [CrossRef]
- Sorrenti, V.; Di Giacomo, C.; Acquaviva, R.; Barbagallo, I.; Bognanno, M.; Galvano, F. Toxicity of Ochratoxin A and Its Modulation by Antioxidants: A Review. Toxins 2013, 5, 1742–1766. [Google Scholar] [CrossRef]
- Longobardi, C.; Ferrara, G.; Andretta, E.; Montagnaro, S.; Damiano, S.; Ciarcia, R. Ochratoxin A and Kidney Oxidative Stress: The Role of Nutraceuticals in Veterinary Medicine—A Review. Toxins 2022, 14, 398. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.D.; Kasabe, P.J.; Dandge, P.B. Pharmaceutical and Nutraceutical Potential of Natural Bioactive Pigment: Astaxanthin. Nat. Prod. Bioprospecting 2022, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.X.; Xiong, F. Astaxanthin and Its Effects in Inflammatory Responses and Inflammation-Associated Diseases: Recent Advances and Future Directions. Molecules 2020, 25, 5342. [Google Scholar] [CrossRef]
- Aneesh, P.; Ajeeshkumar, K.; Lekshmi, R.K.; Anandan, R.; Ravishankar, C.; Mathew, S. Bioactivities of Astaxanthin from Natural Sources, Augmenting Its Biomedical Potential: A Review. Trends Food Sci. Technol. 2022, 125, 81–90. [Google Scholar] [CrossRef]
- Oslan, S.N.H.; Tan, J.S.; Oslan, S.N.; Matanjun, P.; Mokhtar, R.A.M.; Shapawi, R.; Huda, N. Haematococcus Pluvialis as a Potential Source of Astaxanthin with Diverse Applications in Industrial Sectors: Current Research and Future Directions. Molecules 2021, 26, 6470. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, S.; Yang, J.; Qin, C.; Jin, B.; Liang, Z.; Yang, S.; Li, L.; Long, M. Protective Effects of Astaxanthin on Ochratoxin A-Induced Liver Injury: Effects of Endoplasmic Reticulum Stress and Mitochondrial Fission–Fusion Balance. Toxins 2024, 16, 68. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Li, L.; Xu, W.; Wang, M.; Jiao, D.; Yao, B.; Xu, K.; Chen, Y.; Yang, S.; Long, M. Astaxanthin Protects Ochratoxin a-Induced Oxidative Stress and Apoptosis in the Heart Via the Nrf2 Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 7639109. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, M.; Cui, G.; Li, L.; Jiao, D.; Yao, B.; Xu, K.; Chen, Y.; Long, M.; Yang, S. Astaxanthin Protects Ota-Induced Lung Injury in Mice through the Nrf2/Nf-Κb Pathway. Toxins 2019, 11, 540. [Google Scholar] [CrossRef] [PubMed]
- Dhanshetty, M.; Banerjee, K. Simultaneous Direct Analysis of Aflatoxins and Ochratoxin a in Cereals and Their Processed Products by Ultra-High Performance Liquid Chromatography with Fluorescence Detection. J. AOAC Int. 2019, 102, 1666–1672. [Google Scholar] [CrossRef]
- Fan, R.; Tian, W.; Jin, B.; Sun, Y.; Long, M.; Yang, S.; Li, P. The Effect of Astaxanthin on Ochratoxin a-Induced Intestinal Injury in Chickens through Ripk1/Ripk3/Mlkl Pathway. Antioxidants 2025, 14, 915. [Google Scholar] [CrossRef]
- Hosseindoust, A.; Oh, S.M.; Ko, H.S.; Jeon, S.M.; Ha, S.H.; Jang, A.; Son, J.S.; Kim, G.Y.; Kang, H.K.; Kim, J.S. Muscle Antioxidant Activity and Meat Quality Are Altered by Supplementation of Astaxanthin in Broilers Exposed to High Temperature. Antioxidants 2020, 9, 1032. [Google Scholar] [CrossRef]
- Vasiljević, M.; Milićević, D.; Pleadin, J.; Tolimir, N.; Trailović, S.; Resanović, R.; Trailović, J. Effect of Modified Clinoptilolite to Counteract the Deleterious Effects of Ochratoxin A on Egg Production and Quality. Braz. J. Poult. Sci. 2022, 24, eRBCA-2021-1495. [Google Scholar] [CrossRef]
- Bulikowski, W.; Borzęcki, A.; Skorupski, R.; Trocka, K.; Lingas, W. Calcium Concentration in the Skin of Male Rats Exposed to High Doses of Ochratoxin A (Ota). Med. Pr. 2005, 56, 363–366. [Google Scholar]
- Chen, Y.; Zhao, S.; Jiao, D.; Yao, B.; Yang, S.; Li, P.; Long, M. Astaxanthin Alleviates Ochratoxin A-Induced Cecum Injury and Inflammation in Mice by Regulating the Diversity of Cecal Microbiota and Tlr4/Myd88/Nf-Κb Signaling Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 8894491. [Google Scholar] [CrossRef] [PubMed]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide Contamination of Food-Crops with Mycotoxins: Validity of the Widely Cited ‘Fao Estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Nalle, C.; Angi, A.; Supit, M.; Ambarwati, S. Aflatoxin and Ochratoxin a Contamination in Corn Grains and Sago (Putak Meal) from Different Sources for Poultry in West Timor, Indonesia. Int. J. Poult. Sci. 2019, 18, 353–360. [Google Scholar] [CrossRef]
- Akinmusire, O.O.; El-Yuguda, A.-D.; Musa, J.A.; Oyedele, O.A.; Sulyok, M.; Somorin, Y.M.; Ezekiel, C.N.; Krska, R. Mycotoxins in Poultry Feed and Feed Ingredients in Nigeria. Mycotoxin Res. 2019, 35, 149–155. [Google Scholar] [CrossRef]
- Gan, F.; Hou, L.; Zhou, Y.; Liu, Y.; Huang, D.; Chen, X.; Huang, K. Effects of Ochratoxin A on Er Stress, Mapk Signaling Pathway and Autophagy of Kidney and Spleen in Pigs. Environ. Toxicol. 2017, 32, 2277–2286. [Google Scholar] [CrossRef]
- Elhady, M.A.; Khalaf, A.A.A.; Ibrahim, M.A.; Hassanen, E.I.; Abdelrahman, R.E.; Noshy, P.A. Protective Effects of Bacillus Subtilis Fermentation Extract against Ochratoxin A-Induced Nephrotoxicity and Immunotoxicity in Broiler Chickens. J. Vet. Res. 2022, 66, 167. [Google Scholar] [CrossRef] [PubMed]
- Zahoor-ul-Hassan; Zargham Khan, M.; Kashif Saleemi, M.; Khan, A.; Javed, I.; Hussain, A. Immunological Status of White Leghorn Chicks Hatched from Eggs Inoculated with Ochratoxin A (Ota). J. Immunotoxicol. 2011, 8, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Zahoor-ul-Hassan; Khan, M.Z.; Khan, A.; Javed, I.; Saleemi, M.K. Immunological Status of the Progeny of Breeder Hens Kept on Ochratoxin A (Ota)-Contaminated Feed. J. Immunotoxicol. 2011, 8, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zeid, S.M.; Aljuaydi, S.H.; AbuBakr, H.O.; Tahoun, E.A.; Di Cerbo, A.; Alagawany, M.; Khalil, S.R.; Farag, M.R. Astaxanthin Mitigates Thiacloprid-Induced Liver Injury and Immunotoxicity in Male Rats. Mar. Drugs 2021, 19, 525. [Google Scholar] [CrossRef]
- Okai, Y.; Higashi-Okai, K. Possible Immunomodulating Activities of Carotenoids in In Vitro Cell Culture Experiments. Int. J. Immunopharmacol. 1996, 18, 753–758. [Google Scholar] [CrossRef]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin Decreased Oxidative Stress and Inflammation and Enhanced Immune Response in Humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense against Oxidative Stress: Antioxidant Enzymes, Nanomaterials with Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Sies, H. What Is Oxidative Stress? In Oxidative Stress and Vascular Disease; Springer: Berlin/Heidelberg, Germany, 1985; pp. 1–8. [Google Scholar]
- Li, Q.; Dong, Z.; Lian, W.; Cui, J.; Wang, J.; Shen, H.; Liu, W.; Yang, J.; Zhang, X.; Cui, H. Ochratoxin a Causes Mitochondrial Dysfunction, Apoptotic and Autophagic Cell Death and Also Induces Mitochondrial Biogenesis in Human Gastric Epithelium Cells. Arch. Toxicol. 2019, 93, 1141–1155. [Google Scholar] [CrossRef]
- Kang, W.; Suzuki, M.; Saito, T.; Miyado, K. Emerging Role of Tca Cycle-Related Enzymes in Human Diseases. Int. J. Mol. Sci. 2021, 22, 13057. [Google Scholar] [CrossRef]
- Lee, J.; Cho, Y.S.; Jung, H.; Choi, I. Pharmacological Regulation of Oxidative Stress in Stem Cells. Oxidative Med. Cell. Longev. 2018, 2018, 4081890. [Google Scholar] [CrossRef]
- Lauridsen, C. From Oxidative Stress to Inflammation: Redox Balance and Immune System. Poult. Sci. 2019, 98, 4240–4246. [Google Scholar] [CrossRef]
- Meng, Z.; Yan, C.; Deng, Q.; Gao, D.-f.; Niu, X.-l. Curcumin Inhibits Lps-Induced Inflammation in Rat Vascular Smooth Muscle Cells in Vitro Via Ros-Relative Tlr4-Mapk/Nf-Κb Pathways. Acta Pharmacol. Sin. 2013, 34, 901–911. [Google Scholar] [CrossRef]
- Bang, E.; Kim, D.H.; Chung, H.Y. Protease-Activated Receptor 2 Induces Ros-Mediated Inflammation through Akt-Mediated Nf-Κb and Foxo6 Modulation During Skin Photoaging. Redox Biol. 2021, 44, 102022. [Google Scholar] [CrossRef]
- Morris, G.; Gevezova, M.; Sarafian, V.; Maes, M. Redox Regulation of the Immune Response. Cell. Mol. Immunol. 2022, 19, 1079–1101. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Pittet, M.J. The Spleen in Local and Systemic Regulation of Immunity. Immunity 2013, 39, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Wang, F.; Yang, Y.; Pan, S.; Wang, J.; Xiao, N.; Wang, X.; Ma, Z.; Xu, X.; Dong, Z. Monotropein Alleviates Septic Acute Liver Injury by Restricting Oxidative Stress, Inflammation, and Apoptosis Via the Akt (Ser473)/Gsk3β (Ser9)/Fyn/Nrf2 Pathway. Int. Immunopharmacol. 2024, 142, 113178. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Y.; Zhang, W.; Zhang, J.; Zhang, Y.; Xu, P. Effect of Fgf-21 on Implant Bone Defects through Hepatocyte Growth Factor (Hgf)-Mediated Pi3k/Akt Signaling Pathway. Biomed. Pharmacother. 2019, 109, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Wu, J.; Liu, R.; Wang, S.; Luo, J.; Yang, Y.; Qin, Y.; Li, T.; Zheng, X.; Song, J. A Novel Compound Dbz Ameliorates Neuroinflammation in Lps-Stimulated Microglia and Ischemic Stroke Rats: Role of Akt (Ser473)/Gsk3β (Ser9)-Mediated Nrf2 Activation. Redox Biol. 2020, 36, 101644. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, J.; Wei, F.; Gu, Q.; Tian, M.; Lv, H.-B. Tert-Butylhydroquinone Protects the Retina from Oxidative Stress in Stz-Induced Diabetic Rats Via the Pi3k/Akt/Enos Pathway. Eur. J. Pharmacol. 2022, 935, 175297. [Google Scholar] [CrossRef]
- Feng, Y.; Hua, X.; Niu, R.; Du, Y.; Shi, C.; Zhou, R.; Chen, F.-H. Ros Play an Important Role in Atpr Inducing Differentiation and Inhibiting Proliferation of Leukemia Cells by Regulating the Pten/Pi3k/Akt Signaling Pathway. Biol. Res. 2019, 52, 26. [Google Scholar] [CrossRef]
- Li, K.; Cao, Z.; Guo, Y.; Tong, C.; Yang, S.; Long, M.; Li, P.; He, J. Selenium Yeast Alleviates Ochratoxin a-Induced Apoptosis and Oxidative Stress Via Modulation of the Pi3k/Akt and Nrf2/Keap1 Signaling Pathways in the Kidneys of Chickens. Oxidative Med. Cell. Longev. 2020, 2020, 4048706. [Google Scholar] [CrossRef]
- Li, P.; Li, K.; Zou, C.; Tong, C.; Sun, L.; Cao, Z.; Yang, S.; Lyu, Q. Selenium Yeast Alleviates Ochratoxin A-Induced Hepatotoxicity Via Modulation of the Pi3k/Akt and Nrf2/Keap1 Signaling Pathways in Chickens. Toxins 2020, 12, 143. [Google Scholar] [CrossRef]
- Unnisa, A.; Greig, N.H.; Kamal, M.A. Inhibition of Caspase 3 and Caspase 9 Mediated Apoptosis: A Multimodal Therapeutic Target in Traumatic Brain Injury. Curr. Neuropharmacol. 2023, 21, 1001–1012. [Google Scholar] [CrossRef] [PubMed]



| Name | Sense Strand/Sense Primer (5′–3′) | Antisense Strand/Antisense Primer (5′–3′) | Accession No. |
|---|---|---|---|
| PTEN | ACTCACTCTTGGCGAAGGAA | CTCCTGCTCCACCAACACTA | XM_015278701.2 |
| PI3K | CTTCGGATGTTGCCTTACGG | GACACAGTAGCCAGCACAAG | NM_001004410.1 |
| AKT | CCTTTTGTGGACCCTTCTGC | AGAAAATACCGTGGCCTCCA | NM_205055.1 |
| Bcl-2 | TTCAAGCGAAAACAGGGTGG | CTCTGAGCACATGGAAAGCC | NM_205339.2 |
| Bax | CACCTTTGTCTCACCTGTGC | GATGGCAGTGATGAGCATGG | XM_015290060.2 |
| Caspase3 | TTGAAGCAGACAGTGGACCA | GTTCAAGTTTCCTGGCGTGT | NM_204725.1 |
| IL-8 | GGCTTGCTAGGGGAAATGA | AGCTGACTCTGACTAGGAAACTGT | NM_204608 |
| IL-6 | CAAGGTGACGGAGGAGGAC | TGGCGAGGAGGGATTTCT | NM_001277996 |
| IL-1β | ACGTGGCAGCTTTTGAAGAT | GCGGTGGTTTTGTAACAGTG | XM_46931582 |
| β-actin | CCCACACCCCTGTGATGAAA | TAGAACTTTGGGGGCGTTCG | NM_205518.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Sang, W.; Li, P.; Yang, S. Astaxanthin Alleviates Ochratoxin A (OTA)-Induced Spleen Dysfunction and Apoptosis in Broiler Chickens by Modulating the PTEN/PI3K/AKT Signaling Pathway. Antioxidants 2025, 14, 1160. https://doi.org/10.3390/antiox14101160
Cheng Z, Sang W, Li P, Yang S. Astaxanthin Alleviates Ochratoxin A (OTA)-Induced Spleen Dysfunction and Apoptosis in Broiler Chickens by Modulating the PTEN/PI3K/AKT Signaling Pathway. Antioxidants. 2025; 14(10):1160. https://doi.org/10.3390/antiox14101160
Chicago/Turabian StyleCheng, Zhibi, Weilun Sang, Peng Li, and Shuhua Yang. 2025. "Astaxanthin Alleviates Ochratoxin A (OTA)-Induced Spleen Dysfunction and Apoptosis in Broiler Chickens by Modulating the PTEN/PI3K/AKT Signaling Pathway" Antioxidants 14, no. 10: 1160. https://doi.org/10.3390/antiox14101160
APA StyleCheng, Z., Sang, W., Li, P., & Yang, S. (2025). Astaxanthin Alleviates Ochratoxin A (OTA)-Induced Spleen Dysfunction and Apoptosis in Broiler Chickens by Modulating the PTEN/PI3K/AKT Signaling Pathway. Antioxidants, 14(10), 1160. https://doi.org/10.3390/antiox14101160











