Antioxidant Properties of Albumin and Diseases Related to Obstetrics and Gynecology
Abstract
1. Introduction
2. Albumin’s Previously Known Characteristics, Functions, and Roles
3. Albumin’s Antioxidant Properties
3.1. Neutralization of Free Radicals by Albumin
3.2. Protection of Antioxidants by Albumin
3.3. Transport of Antioxidants by Albumin
3.4. Albumin Interaction with Agents
4. Albumin’s Role in Pathologies Related to Obstetrics and Gynecology
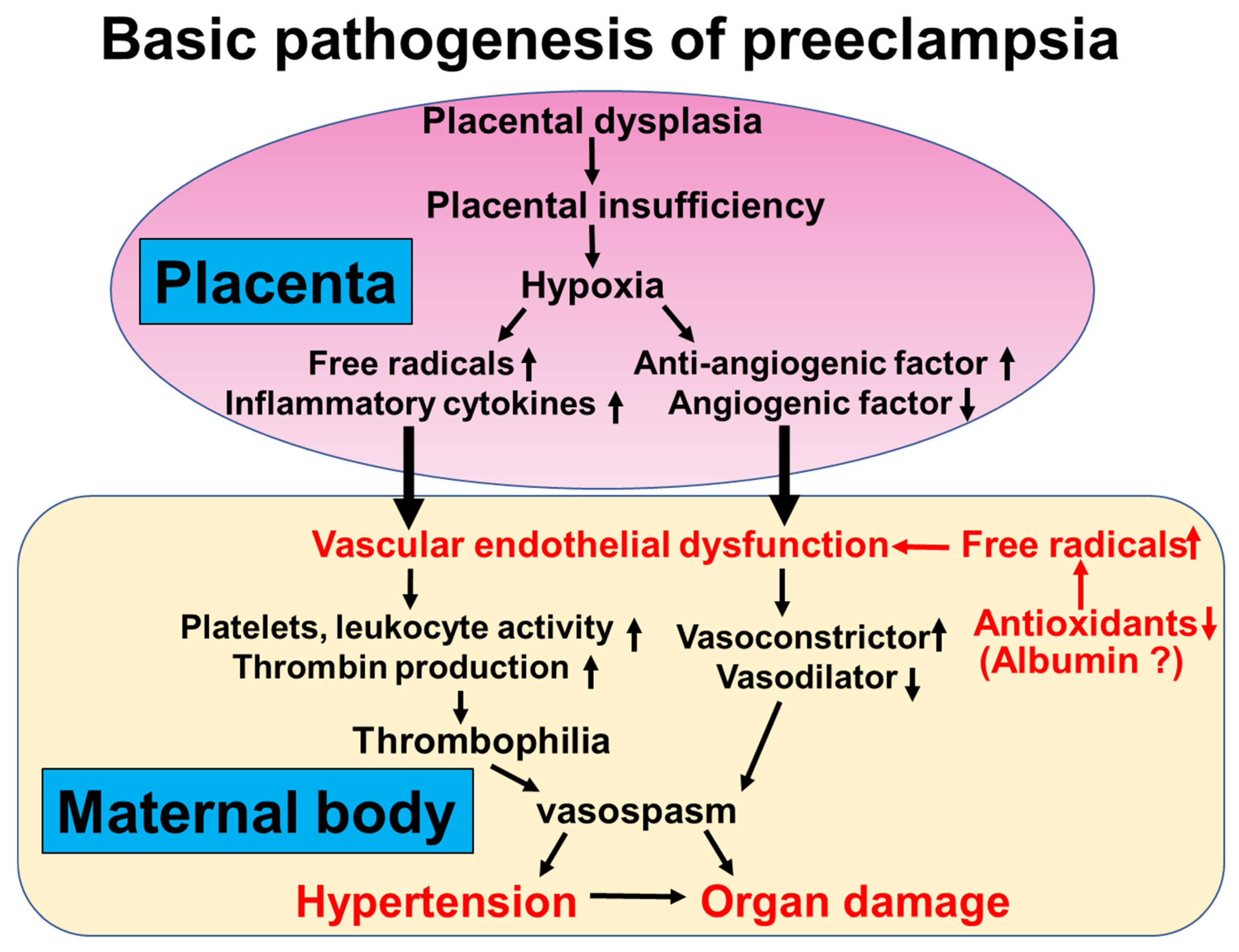
4.1. Vascular Endothelial Dysfunction in Patients with Preeclampsia
4.2. Oxidative Stress in Patients with Preeclampsia
4.3. Oxidative Stress in Combination with Vascular Endothelial Dysfunction in Patients with Preeclampsia
4.4. Antioxidant Effect of Albumin in Patients with Preeclampsia
4.5. Mechanistic Insight into Hypoalbuminemia in Patients with Preeclampsia
4.6. Mechanism Between Hypoalbuminemia and Inflammation in Patients with Preeclampsia
4.7. Relationship Between Women with Previous Preeclampsia and Future Development of Chronic Kidney Disease Development
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Petrs, T., Jr. All About Albumin: Biochemistry, Genetics and Medical Applications; Academic Press: London, UK; San Diego, CA, USA, 1996. [Google Scholar]
- Evans, T.W. Albumin as a drug—Biological effects of albumin unrelated to oncotic pressure. Aliment. Pharmacol. Ther. 2002, 16 (Suppl. 5), 6–11. [Google Scholar] [CrossRef]
- Mendez, C.M.; McClain, C.J.; Marsano, L.S. Albumin therapy in clinical practice. Nutr. Clin. Pract. 2005, 20, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Aspects Med. 2012, 33, 209–290. [Google Scholar] [CrossRef]
- di Masi, A.; Trezza, V.; Leboffe, L.; Ascenzi, P. Human plasma lipocalins and serum albumin: Plasma alternative carriers? J. Control Release 2016, 228, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Pstras, L.; Waniewski, J.; Lindholm, B. Transcapillary transport of water, small solutes and proteins during hemodialysis. Sci. Rep. 2020, 10, 18736. [Google Scholar] [CrossRef]
- Belinskaia, D.A.; Voronina, P.A.; Shmurak, V.I.; Vovk, M.A.; Batalova, A.A.; Jenkins, R.O.; Goncharov, N.V. The universal soldier: Enzymatic and non-enzymatic antioxidant functions of serum albumin. Antioxidants 2020, 9, 966. [Google Scholar] [CrossRef] [PubMed]
- Belinskaia, D.A.; Voronina, P.A.; Shmurak, V.I.; Jenkins, R.O.; Goncharov, N.V. Serum albumin in health and disease: Esterase, antioxidant, transporting and signaling properties. Int. J. Mol. Sci. 2021, 22, 10318. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A.A.; Manolis, T.A.; Melita, H.; Mikhailidis, D.P.; Manolis, A.S. Low serum albumin: A neglected predictor in patients with cardiovascular disease. Eur. J. Intern. Med. 2022, 102, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Watanabe, K.; Azma, T.; Feng, G.G.; Akahori, T.; Hayashi, H.; Sato, M.; Fujiwara, Y.; Wakatsuki, A. Human serum albumin and oxidative stress in preeclamptic women and the mechanism of albumin for stress reduction. Heliyon 2017, 3, e00369. [Google Scholar] [CrossRef]
- De Simone, G.; di Masi, A.; Ascenzi, P. Serum albumin: A multifaced enzyme. Int. J. Mol. Sci. 2021, 22, 10086. [Google Scholar] [CrossRef] [PubMed]
- Shojai, S.; Haeri Rohani, S.A.; Moosavi-Movahedi, A.A.; Habibi-Rezaei, M. Human serum albumin in neurodegeneration. Rev. Neurosci. 2022, 33, 803–817. [Google Scholar] [CrossRef]
- Li, X.; Yan, Y. Comparative Study of the Interactions between Ovalbumin and five Antioxidants by Spectroscopic Methods. J. Fluoresc. 2017, 27, 213–225. [Google Scholar] [CrossRef]
- Bae, M.J.; Ishii, T.; Minoda, K.; Kawada, Y.; Ichikawa, T.; Mori, T.; Kamihira, M.; Nakayama, T. Albumin stabilizes (-)-epigallocatechin gallate in human serum: Binding capacity and antioxidant property. Mol. Nutr. Food Res. 2009, 53, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Li, E.; Meng, X.; Tian, J.; Ran, X.; Zhang, Y.; Zang, Z.; Wang, W.; Li, B. Protective effects of bovine serum albumin on blueberry anthocyanins under illumination conditions and their mechanism analysis. Food Res. Int. 2019, 122, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Jing, H.; Chai, Z.; Zhao, G.; Stoll, S.; Ren, F.; Liu, F.; Leng, X. Design and characterization of protein-quercetin bioactive nanoparticles. J. Nanobiotechnol. 2011, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Hao, R.; Wu, X.; Li, Q.; Leng, X.; Jing, H. Bovine serum albumin nanoparticle promotes the stability of quercetin in simulated intestinal fluid. J. Agric. Food Chem. 2011, 59, 6292–6298. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: From basic research to clinical aplication. Am. J. Med. 1991, 91, 31S–38S. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Whiteman, M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Albumin-an important extracellular antioxidant? Biochem. Pharmacol. 1988, 37, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Clerici, M.; Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. Redox albuminomics: Oxidized albumin in human diseases. Antioxid. Redox Signal 2012, 17, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Chuang, V.T.; Maruyama, T.; Otagiri, M. Redox properties of serum albumin. Biochim. Biophys. Acta 2013, 1830, 5465–5472. [Google Scholar] [CrossRef]
- Carballal, S.; Alvarez, B.; Turell, L.; Botti, H.; Freeman, B.A.; Radi, R. Sulfenic acid in human serum albumin. Amino Acids 2007, 32, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Peter, T. Serum albumin. In The Plasma Proteins Structure, Function, and Genetic Control; Putnam, F.W., Ed.; Academic Press: Bloomington, IN, USA, 1975; pp. 133–181. [Google Scholar]
- van der Vusse, G.J. Albumin as fatty acid transporter. Drug Metab. Pharmacokinet. 2009, 24, 300–307. [Google Scholar] [CrossRef]
- Rothschild, M.A.; Oratz, M.; Schreiber, S.S. Serum albumin. Hepatology 1988, 8, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Campos Munoz, A.; Jain, N.K.; Gupta, M. Albumin Colloid. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Eckart, A.; Struja, T.; Kutz, A.; Baumgartner, A.; Baumgartner, T.; Zurfluh, S.; Neeser, O.; Huber, A.; Stanga, Z.; Mueller, B.; et al. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: A prospective study. Am. J. Med. 2020, 133, 713–722.e7. [Google Scholar] [CrossRef] [PubMed]
- Sheinenzon, A.; Shehadeh, M.; Michelis, R.; Shaoul, E.; Ronen, O. Serum albumin levels and inflammation. Int. J. Biol. Macromol. 2021, 184, 857–862. [Google Scholar] [CrossRef]
- Sudlow, G.; Birkett, D.J.; Wade, D.N. The characterization of two specific drug binding sites on human serum albumin. Mol. Pharmacol. 1975, 11, 824–832. [Google Scholar] [PubMed]
- Carter, D.C.; Ho, J.X. Structure of serum albumin. Adv. Protein Chem. 1994, 45, 153–203. [Google Scholar]
- di Masi, A.; Leboffe, L.; Polticelli, F.; Tonon, F.; Zennaro, C.; Caterino, M.; Stano, P.; Fischer, S.; Hägele, M.; Müller, M.; et al. Human serum albumin is an essential component of the host defense mechanism against Clostridium difficile intoxication. J. Infect. Dis. 2018, 218, 1424–1435. [Google Scholar] [CrossRef]
- Alinovskaya, L.I.; Sedykh, S.E.; Ivanisenko, N.V.; Soboleva, S.E.; Nevinsky, G.A. How human serum albumin recognizes DNA and RNA. Biol. Chem. 2018, 399, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Soboleva, S.E.; Guschina, T.A.; Nevinsky, G.A. Human serum and milk albumins are metal-dependent DNases. IUBMB Life 2018, 70, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Vita, G.M.; De Simone, G.; Leboffe, L.; Montagnani, F.; Mariotti, D.; Di Bella, S.; Luzzati, R.; Gori, A.; Ascenzi, P.; di Masi, A. Human serum albumin binds streptolysin O (SLO) toxin produced by Group A streptococcus and inhibits its cytotoxic and hemolytic effects. Front. Immunol. 2020, 11, 507092. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, E.M.; Edwards, T. Update on albumin therapy in critical illness. Vet. Clin. North. Am. Small Anim. Pract. 2020, 50, 1289–1305. [Google Scholar] [CrossRef] [PubMed]
- Lundsgaard-Hansen, P. Physiology and pathophysiology of colloid osmotic pressure and albumin metabolism. Curr. Stud. Hematol. Blood Transfus. 1986, 53, 1–17. [Google Scholar]
- Hankins, J. The role of albumin in fluid and electrolyte balance. J. Infus. Nurs. 2006, 29, 260–265. [Google Scholar] [CrossRef]
- Bihari, S.; Bannard-Smith, J.; Bellomo, R. Albumin as a drug: Its biological effects beyond volume expansion. Crit. Care Resusc. 2020, 22, 257–265. [Google Scholar] [CrossRef]
- Levitt, D.G.; Levitt, M.D. Human serum albumin homeostasis: A new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int. J. Gen. Med. 2016, 9, 229–255. [Google Scholar] [CrossRef]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and clinical significance. JPEN J. Parenter. Enter. Nutral 2019, 43, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Khosravifarsani, M.; Monfared, A.S.; Pouramir, M.; Zabihi, E. Effects of Fenton reaction on human serum albumin: An in vitro study. Electron. Phys. 2016, 8, 2970–2976. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, Z.; Ali, R. Reactive oxygen species damaged human serum albumin in patients with type 1 diabetes mellitus: Biochemical and immunological studies. Life Sci. 2006, 79, 2320–2328. [Google Scholar] [CrossRef] [PubMed]
- Jurasekova, Z.; Tinti, A.; Torreggiani, A. Use of Raman spectroscopy for the identification of radical-mediated damages in human serum albumin. Anal. Bioanal. Chem. 2011, 400, 2921–2931. [Google Scholar] [CrossRef] [PubMed]
- Montero, G.; Arriagada, F.; Günther, G.; Bollo, S.; Mura, F.; Berríos, E.; Morales, J. Phytoestrogen coumestrol: Antioxidant capacity and its loading in albumin nanoparticles. Int. J. Pharm. 2019, 562, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, Z.; Ahmad, R.; Rasheed, N.; Ali, R. Enhanced recognition of reactive oxygen species damaged human serum albumin by circulating systemic lupus erythematosus autoantibodies. Autoimmunity 2007, 40, 512–520. [Google Scholar] [CrossRef]
- Rasheed, Z.; Khan, M.W.; Ali, R. Hydroxyl radical modification of human serum albumin generated cross reactive antibodies. Autoimmunity 2006, 39, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.J.; Blindauer, C.A.; Berezenko, S.; Sleep, D.; Tooth, D.; Sadler, P.J. Role of Tyr84 in controlling the reactivity of Cys34 of human albumin. FEBS J. 2005, 272, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Arenas, A.; Vasquez, R.; López-Alarcón, C.; Lissi, E.; Silva, E. Oxidative damage of lysozyme and human serum albumin and their mixtures: A comparison of photosensitized and peroxyl radical promoted processes. Protein J. 2011, 30, 359–365. [Google Scholar] [CrossRef]
- Roy, D.; Quiles, J.; Gaze, D.C.; Collinson, P.; Kaski, J.C.; Baxter, G.F. Role of reactive oxygen species on the formation of the novel diagnostic marker ischemia modified albumin. Heart 2006, 92, 113–114. [Google Scholar] [CrossRef]
- Challier, C.; Beassoni, P.; Boetsch, C.; García, N.A.; Biasutti, M.A.; Criado, S. Interaction between human serum albumin and antidiabetic compounds and its influence on the O2((1)Δg)-mediated degradation of the protein. J. Photochem. Photobiol. B 2015, 142, 20–28. [Google Scholar] [CrossRef]
- Rey, F.E.; Cifuentes, M.E.; Kiarash, A.; Quinn, M.T.; Pagano, P.J. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular 02− and systolic blood pressure in mice. Circ. Res. 2001, 89, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Saitou, T.; Watanabe, K.; Kinoshita, H.; Iwasaki, A.; Owaki, Y.; Matsushita, H.; Wakatsuki, A. Hypoalbuminemia is related to endothelial dysfunction resulting from oxidative stress in parturients with preeclampsia. Nagoya J. Med. Sci. 2021, 83, 741–748. [Google Scholar]
- Asai, A.; Hatayama, N.; Kamiya, K.; Yamauchi, M.; Kinashi, H.; Yamaguchi, M.; Katsuno, T.; Nobata, H.; Watanabe, K.; Wakatsuki, A.; et al. Roles of glomerular endothelial hyaluronan in the development of proteinuria. Physiol. Rep. 2021, 9, e15019. [Google Scholar] [CrossRef] [PubMed]
- Sitar, M.E.; Aydin, S.; Cakatay, U. Human serum albumin and its relation with oxidative stress. Clin. Lab. 2013, 59, 945–952. [Google Scholar] [CrossRef]
- Bourdon, E.; Loreau, N.; Lagrost, L.; Blache, D. Differential effects of cysteine and methionine residues in the antioxidant activity of human serum albumin. Free Radic. Res. 2005, 39, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.; Loonis, M.; Dangles, O. Inhibition of the peroxidation of linoleic acid by the flavonoid quercetin within their complex with human serum albumin. Free Radic. Biol. Med. 2007, 43, 241–252. [Google Scholar] [CrossRef] [PubMed]
- van de Langerijt, T.M.; O’Mahony, J.A.; Crowley, S.V. Structural, binding and functional properties of milk protein-polyphenol systems:A review. Molecules 2023, 28, 2288. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, J.; Sadler, P.J.; Tucker, A. 1H NMR of albumin in human blood plasma: Drug binding and redox reactions at Cys34. FEBS Lett. 1995, 376, 1–5. [Google Scholar] [CrossRef]
- Hu, W.; Luo, Q.; Ma, X.; Wu, K.; Liu, J.; Chen, Y.; Xiong, S.; Wang, J.; Sadler, P.J.; Wang, F. Arene control over thiolate to sulfinate oxidation in albumin by organometallic ruthenium anticancer complexes. Chemistry 2009, 15, 6586–6594. [Google Scholar] [CrossRef]
- Hayakawa, A.; Kuwata, K.; Era, S.; Sogami, M.; Shimonaka, H.; Yamamoto, M.; Dohi, S.; Hirose, H. Alteration of redox state of human serum albumin in patients under anesthesia and invasive surgery. J. Chromatogr. B Biomed. Sci. Appl. 1997, 698, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Era, S.; Kuwata, K.; Imai, H.; Nakamura, K.; Hayashi, T.; Sogami, M. Age-related change in redox state of human serum albumin. Biochim. Biophys. Acta 1995, 1247, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Carballal, S.; Radi, R.; Kirk, M.C.; Barnes, S.; Freeman, B.A.; Alvarez, B. Sulfenic acid formation in human serum albumin by hydrogen peroxide and peroxynitrite. Biochemistry 2003, 42, 9906–9914. [Google Scholar] [CrossRef] [PubMed]
- Soejima, A.; Kaneda, F.; Manno, S.; Matsuzawa, N.; Kouji, H.; Nagasawa, T.; Era, S.; Takakuwa, Y. Useful markers for detecting decreased serum antioxidant activity inhemodialysis patients. Am. J. Kidney Dis. 2002, 39, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Kurano, M.; Yasukawa, K.; Ikeda, H.; Aoki, J.; Yatomi, Y. Redox state of albumin affects its lipid mediator binding characteristics. Free Radic. Res. 2019, 53, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Eicosanoids. Essays Biochem. 2020, 64, 423–441. [Google Scholar]
- Magee, L.A.; Brown, M.A.; Hall, D.R.; Gupte, S.; Hennessy, A.; Karumanchi, S.A.; Kenny, L.C.; McCarthy, F.; Myers, J.; Poon, L.C.; et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022, 27, 148–169. [Google Scholar] [PubMed]
- Roberts, J.M.; Taylor, R.N.; Musci, T.J.; Rodgers, G.M.; Hubel, C.A.; McLaughlin, M.K. Preeclampsia: An endothelial cell disorder. Am. J. Obstet. Gynecol. 1989, 161, 1200–1204. [Google Scholar] [CrossRef]
- Goulopoulou, S.; Davidge, S.T. Molecular mechanisms of maternal vascular dysfunction in preeclampsia. Trends Mol. Med. 2015, 21, 88–97. [Google Scholar] [CrossRef]
- Okatani, Y.; Watanabe, K.; Nakano, Y.; Sagara, Y. Relaxant effect of nitric oxide and prostacyclin on serotonin-induced vasocontraction of human umbilical artery. Acta Obstet. Gynecol. Scand. 1996, 75, 108–112. [Google Scholar] [CrossRef]
- Watanabe, K.; Okatani, Y.; Sagara, Y. Potentiating effect of hydrogen peroxide on the serotonin-induced vasocontraction in human umbilical artery. Acta Obstet. Gynecol. Scand. 1996, 75, 783–789. [Google Scholar] [CrossRef]
- Okatani, Y.; Watanabe, K.; Sagara, Y. Effect of nitric oxide, prostacyclin, and thromboxane on the vasospastic action of hydrogen peroxide on human umbilical artery. Acta Obstet. Gynecol. Scand. 1997, 76, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Mori, T.; Iwasaki, A.; Kimura, C.; Matsushita, H.; Shinohara, K.; Wakatsuki, A. Increased oxygen free radical production during pregnancy may impair vascular reactivity in preeclamptic women. Hypertens. Res. 2013, 36, 356–360. [Google Scholar] [CrossRef]
- Mori, T.; Watanabe, K.; Iwasaki, A.; Kimura, C.; Matsushita, H.; Shinohara, K.; Wakatsuki, A. Differences in vascular reactivity between pregnant women with chronic hypertension and preeclampsia. Hypertens. Res. 2014, 37, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Rubanyi, G.M.; Romero, J.C.; Vanhoutte, P.M. Flow-induced release of endothelium-derived relaxing factor. Am. J. Physiol. 1986, 250, H1145–H1149. [Google Scholar] [CrossRef]
- Wisdom, S.J.; Wilson, R.; Mckillop, J.H.; Walker, J.J. Antioxidant system in normal pregnancy and pregnancy induced hypertension. Am. J. Obstet. Gynecol. 1991, 165, 1701–1704. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best. Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Sibai, B.; Dekker, G.; Kupferminc, M. Pre-eclampsia. Lancet 2005, 365, 785–799. [Google Scholar] [CrossRef]
- Baumwell, S.; Karumanchi, S.A. Pre-eclampsia: Clinical manifestations and molecular mechanisms. Nephron Clin. Pract. 2007, 106, c72–c81. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Dhingra, R.; Arya, D.S.; Kalaivani, M.; Bhatla, N.; Kumar, R. Role of oxidative stress markers and antioxidants in the placental of preeclamptic patients. J. Obstet. Gynaecol. Res. 2010, 36, 1189–1194. [Google Scholar] [CrossRef]
- Hansson, S.R.; Nääv, Å.; Erlandsson, L. Oxidative stress in preeclampsia and the role of free fetal hemoglobin. Front. Physiol. 2014, 5, 516. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Benian, A.; Madazli, R.; Uludag, S.; Uzun, H.; Kaya, S. Plasma malondialdehyde, superoxide dismutase, sE-selectin, fibronectin, endothelin-1 and nitric oxide levels in women with preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 113, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Agarwal, A.; Sharma, R.K. The role of placental oxidative stress and lipid peroxidation in preeclampsia. Obstet. Gynecol. Surv. 2005, 60, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Borrego, E.; Proverbio, T.; Marín, R.; Proverbio, F. Lipid peroxidation and Ca-ATPase activity of basal plasma membranes of syncytiotrophoblast from normotensive pregnant women. Gynecol. Obstet. Investig. 2006, 61, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Kimura, C.; Watanabe, K.; Iwasaki, A.; Mori, T.; Matsushita, H.; Shinohara, K.; Wakatsuki, A. The severity of hypoxic changes and oxidative DNA damage in the placenta of early-onset preeclamptic women and fetal growth restriction. J. Matern. Fetal Neonatal Med. 2013, 26, 491–496. [Google Scholar] [CrossRef]
- Fujimaki, A.; Watanabe, K.; Mori, T.; Kimura, C.; Shinohara, K.; Wakatsuki, A. Placentaloxidative DNA damage and its repair in preeclamptic women with fetal growth restriction. Placenta 2011, 32, 367–372. [Google Scholar] [CrossRef]
- Wang, Y.; Walsh, S.W.; Kay, H.H. Placental lipid peroxides and thromboxane are increased and prostacyclin is decreased in women with preeclampsia. Am. J. Obstet. Gynecol. 1992, 167, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Poranen, A.K.; Ekblad, U.; Uotila, P.; Ahotupa, M. Lipid peroxidation and antioxidants in normal and pre-eclamptic pregnancies. Placenta 1996, 17, 401–405. [Google Scholar] [CrossRef]
- Wang, Y.; Walsh, S.W. Placental mitochondria as a source of oxidative stress in preeclampsia. Placenta 1998, 19, 581–586. [Google Scholar] [CrossRef]
- Gurjit, K.; Soumya, M.; Alka, S.; Rajendra, P. Alterations in lipid peroxidation and antioxidant status in pregnancy with preeclampsia. Mol. Cell Biochem. 2008, 313, 37–44. [Google Scholar]
- Watanabe, K.; Mori, T.; Iwasaki, A.; Kimura, C.; Matsushita, H.; Shinohara, K.; Wakatsuki, A. Increased oxidant generation in the metabolism of hypoxanthine to uric acid and endothelial dysfunction in early- and late-onset preeclamptic women. J. Matern. Fetal Neonatal Med. 2012, 25, 2662–2666. [Google Scholar] [CrossRef]
- Watanabe, K.; Iwasaki, A.; Mori, T.; Kimura, C.; Matsushita, H.; Shinohara, K.; Wakatsuki, A. Oxidative stress in the fetus of preeclamptic women with fetal growth restriction. Hypertens. Res. Pregnancy 2013, 2, 98–102. [Google Scholar] [CrossRef][Green Version]
- Burton, G.J.; Jauniaux, E. Placental oxidative stress: From miscarriage to preeclampsia. J. Soc. Gynecol. Investig. 2004, 11, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; D’Angelo, G.; Manti, S.; Arrigo, T.; Barberi, I.; Reiter, R.J.; Gitto, E. Oxidative stress-mediated aging during the fetal and perinatal periods. Oxid. Med. Cell Longev. 2014, 2014, 358375. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Vázquez, C.M.; Mate, A.; Sobrevia, L.; Marín, R. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165354. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Boeldt, D.S.; Bird, I.M. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J. Endocrinol. 2017, 232, R27–R44. [Google Scholar] [CrossRef] [PubMed]
- Tranquilli, A.L.; Dekker, G.; Magee, L.; Roberts, J.; Sibai, B.M.; Steyn, W.; Zeeman, G.G.; Brown, M.A. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISHHP. Pregnancy Hypertens. 2014, 4, 97–104. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Baczyk, D.; Kingdom, J.C. Endothelial dysfunction in severe preeclampsia is mediated by soluble factors, rather than extracellular vesicles. Sci. Rep. 2017, 19, 5887. [Google Scholar] [CrossRef]
- Wakatsuki, A.; Okatani, Y. Melatonin protects against the free radical-induced impairment of nitric oxide production in the human umbilical artery. J. Pineal Res. 2000, 28, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Wakatsuki, A.; Okatani, Y.; Ikenoue, N.; Shinohara, K.; Watanabe, K.; Fukaya, T. Melatonin protects against oxidized low-density lipoprotein-induced inhibition of nitric oxide production in human umbilical artery. J. Pineal Res. 2001, 31, 281–288. [Google Scholar] [CrossRef]
- Nakayama, A.; Odake, J.; Kanke, A.; Sakatsume, M.; Kasama, T.; Shiba, K. Redox state of urinary albumin in patients with IgA nephropathy. Rinsho Byori Jpn. J. Clin. Pathol. 2011, 59, 1013–1018. [Google Scholar]
- Anraku, M.; Kragh-Hansen, U.; Maruyama, T.; Otagiri, M. Glycative and oxidative modification of human serum albumin: Conformational and functional changes. In Human Serum Albumin (HSA): Functional Structure, Synthesis and Therapeutic Uses, 1st ed.; Stoke, T., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2015; pp. 91–112. [Google Scholar]
- Terawaki, H.; Yoshimura, K.; Hasegawa, T.; Matsuyama, Y.; Negawa, T.; Yamada, K.; Matsushima, M.; Nakayama, M.; Hosoya, T.; Era, S. Oxidative stress is enhanced in correlation with renal dysfunction: Examination with the redox state of albumin. Kidney Int. 2004, 66, 1988–1993. [Google Scholar] [CrossRef] [PubMed]
- Oettl, K.; Marsche, G. Redox state of human serum albumin in terms of cysteine-34 in health and disease. Cellulases 2010, 474, 181–195. [Google Scholar]
- Magzal, F.; Sela, S.; Szuchman-Sapir, A.; Tamir, S.; Michelis, R.; Kristal, B. In vivo oxidized albumin—A pro-inflammatory agent in hypoalbuminemia. PLoS ONE 2017, 12, e0177799. [Google Scholar] [CrossRef]
- Soejima, A.; Matsuzawa, N.; Hayashi, T.; Kimura, R.; Ootsuka, T.; Fukuoka, K.; Yamada, A.; Nagasawa, T.; Era, S. Alteration of redox state of human serum albumin before and after hemodialysis. Blood Purif. 2004, 22, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, S.M.; Araos, P.; Reyes, J.; Gravez, B.; Barrera-Chimal, J.; Amador, C.A. Oxidized albumin as a mediator of kidney disease. Antioxidants 2021, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Okamoto, T.; Saitou, T.; Iwasaki, A.; Matsushita, H.; Takeuchi, K.; Asai, A.; Ito, Y.; Hara, M.; Wakatsuki, A. Increased urinary albumin leakage is related to injuries of glomerular glycocalyx and podocytes, and associated with tubular dysfunction in preeclampsia. Pregnancy Hypertens. 2023, 32, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sean Eardley, K.S.; Cockwell, P. Macrophages and progressive tubulointerstitial disease. Kidney Int. 2005, 68, 437–455. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Iturbe, B.; Johnson, R.J.; Herrera-Acosta, J. Tubulointerstitial damage and progression of renal failure. Kidney Int. Suppl. 2005, 99, S82–S86. [Google Scholar] [CrossRef] [PubMed]
- Abboud, H.; Labreuche, J.; Meseguer, E.; Lavallee, P.C.; Simon, O.; Olivot, J.M.; Mazighi, M.; Dehoux, M.; Benessiano, J.; Steg, P.G.; et al. Ischemia-modified albumin in acute stroke. Cerebrovasc. Dis. 2007, 23, 216–220. [Google Scholar] [CrossRef]
- Ma, S.G.; Wei, C.L.; Hong, B.; Yu, W.N. Ischemia dified albumin in type 2 diabetic patients with and without peripheral arterial disease. Clinics 2011, 66, 1677–1680. [Google Scholar] [PubMed]
- Vyakaranam, S.; Bhongir, A.V.; Patlolla, D.; Chintapally, R. Maternal serum ischemia modified albumin as a marker for hypertensive disorders of pregnancy: A pilot study. Int. J. Reprod. Contracept. Obstet. Gynecol. 2015, 4, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Onat, T.; Aydoğan Kırmızı, D.; Başer, E.; Ercan, M.; Demir Çaltekin, M.; Yalcın, S.; Kara, M.; Esinler, D.; Yalvaç, E.S. The relationship between oxidative stress and preeclampsia. The serum ischemia-modified albumin levels and thiol/disulfide homeostasis. Turk. J. Obstet. Gynecol. 2020, 17, 102–107. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, J.M.; Pai, V.R.; Harish, S.; Shriyan, C.; D’Souza, N. IMA and IMAR in serum and saliva of preeclampsia: A preliminary study. Hypertens. Pregnancy 2014, 33, 440–448. [Google Scholar] [CrossRef]
- D’Souza, J.M.P.; Harish, S.; Pai, V.R.; Shriyan, C. Increased oxidatively modified forms of albumin in association with decreased total antioxidant activity in different types of hypertensive disorders of pregnancy. Indian. J. Clin. Biochem. 2017, 32, 200–206. [Google Scholar] [CrossRef]
- Huang, Q.T.; Zhong, M.; Tian, J.W.; Hou, F.F. Higher plasma AOPP is associated with increased proteinuria excretion and decreased glomerular filtration rate in pre-eclamptic women. Pregnancy Hypertens. 2013, 3, 16–20. [Google Scholar] [CrossRef]
- van Rijn, B.B.; Franx, A.; Sikkema, J.M.; van Rijn, H.J.; Bruinse, H.W.; Voorbij, H.A. Ischemia modified albumin in normal pregnancy and preeclampsia. Hypertens. Pregnancy 2008, 27, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Gafsou, B.; Lefèvre, G.; Hennache, B.; Houfflin Debarge, V.; Ducloy-Bouthors, A.S. Maternal serum ischemia-modified albumin: A biomarker to distinguish between normal pregnancy and preeclampsia? Hypertens. Pregnancy 2010, 29, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Üstün, Y.; Engin-Üstün, Y.; Öztürk, O.; Alanbay, I.; Yaman, H. Ischemia modified albumin as an oxidative stress marker in preeclampsia. J. Matern. Fetal Neonatal Med. 2011, 24, 418–421. [Google Scholar] [CrossRef]
- Afrose, D.; Chen, H.; Ranashinghe, A.; Liu, C.C.; Henessy, A.; Hansbro, P.M.; McClements, L. The diagnostic potential of oxidative stress biomarkers for preeclampsia: Systematic review and meta-analysis. Biol. Sex. Differ. 2022, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Watanabe, K.; Banno, T.; Saitou, T.; Sugiura, K.; Iwasaki, A.; Matsushita, H.; Wakatsuki, A. Amount of proteinuria as associated with severity classification of pregnant women with preeclampsia. Pregnancy Hypertens. 2022, 29, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Sánchez, J.C.; Peña-Alzate, S.; Peña, R.B.; Agudelo-Jaramillo, B.; Cadavid-Jaramillo, A.P.; Chaouat, G.; Maldonado-Estrada, J.G. Sera from early-onset, severely preeclamptic women directly modulate HLA-E expression in the EA. hy296 endothelial cell line. J. Reprod. Immunol. 2014, 104–105, 68–79. [Google Scholar] [CrossRef]
- Betteridge, K.B.; Arkill, K.P.; Neal, C.R.; Harper, S.J.; Foster, R.R.; Satchell, S.C.; Bates, D.O.; Salmon, A.H.J. Sialic acids regulate microvessel permeability, revealed by novel in vivo studies of endothelial glycocalyx structure and function. J. Physiol. 2017, 595, 5015–5035. [Google Scholar] [CrossRef]
- Butler, M.J.; Ramnath, R.; Kadoya, H.; Desposito, D.; Riquier-Brison, A.; Ferguson, J.K.; Onions, K.L.; Ogier, A.S.; ElHegni, H.; Coward, R.J.; et al. Aldosterone induces albuminuria via matrix metalloproteinase-dependent damage of the endothelial glycocalyx. Kidney Int. 2019, 95, 94–107. [Google Scholar] [CrossRef]
- Curry, F.E. Layer upon layer: The functional consequences of disrupting the glycocalyx-endothelial barrier in vivo and in vitro. Cardiovasc. Res. 2017, 113, 559–561. [Google Scholar] [CrossRef]
- Curry, F.E.; Michel, C.C. The endothelial glycocalyx: Barrier functions versus red cell hemodynamics: A model of steady state ultrafiltration through a bi-layer formed by a porous outer layer and more selective membrane-associated inner layer. Biorheology 2019, 56, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, K.; Mundel, P. The role of podocytes in glomerular pathobiology. Clin. Exp. Nephrol. 2003, 7, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Chen, Y.; Sun, W.; Liu, S. Association between hypertensive disorders during pregnancy and the subsequent risk of end-stage renal disease: A population-based follow-up study. J. Obstet. Gynaecol. Can. 2018, 40, 1129–1138. [Google Scholar] [CrossRef]
- Oishi, M.; Iino, K.; Tanaka, K.; Ishihara, K.; Yokoyama, Y.; Takahashi, I.; Mizunuma, H. Hypertensive disorders of pregnancy increase the risk for chronic kidney disease: A population-based retrospective study. Clin. Exp. Hypertens. 2017, 39, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, D.C.; Cottrell, J.; Amaral, L.M.; LaMarca, B. Inflammatory mediators: A causal link to hypertension during preeclampsia. Br. J. Pharmacol. 2019, 176, 1914–1921. [Google Scholar] [CrossRef] [PubMed]
- Schuster, J.; Cheng, S.B.; Padbury, J.; Sharma, S. Placental extracellular vesicles and pre-eclampsia. Am. J. Reprod. Immunol. 2021, 85, e13297. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Romero, R.; Yeo, L.; Gomez-Lopez, N.; Chaemsaithong, P.; Jaovisidha, A.; Gotsch, F.; Erez, O. The etiology of preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S844–S866. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal immunological adaptation during normal pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ye, Y.; Zhang, J.; Ruan, C.C.; Gao, P.J. Immune imbalance is associated with the development of preeclampsia. Medicine 2019, 98, e15080. [Google Scholar] [CrossRef] [PubMed]
- Visser, N.; van Rijn, B.B.; Rijkers, G.T.; Franx, A.; Bruinse, H.W. Inflammatory changes in preeclampsia: Current understanding of the maternal innate and adaptive immune response. Obstet. Gynecol. Surv. 2007, 62, 191–201. [Google Scholar] [CrossRef]
- Opichka, M.A.; Rappelt, M.W.; Gutterman, D.D.; Grobe, J.L.; McIntosh, J.J. Vascular dysfunction in preeclampsia. Cells 2021, 10, 3055. [Google Scholar] [CrossRef] [PubMed]
- Gounden, V.; Vashisht, R.; Jialal, I. Hypoalbuminemia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Fleck, A.; Raines, G.; Hawker, F.; Trotter, J.; Wallace, P.I.; Ledingham, I.M.; Calman, K.C. Increased vascular permeability: A major cause of hypoalbuminaemia in disease and injury. Lancet 1985, 1, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Oian, P.; Maltau, J.M.; Noddeland, H.; Fadnes, H.O. Transcapillary fluid balance in pre-eclampsia. Br. J. Obstet. Gynaecol. 1986, 93, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Rolnik, D.L.; Wright, D.; Poon, L.C.; O’Gorman, N.; Syngelaki, A.; de Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N. Engl. J. Med. 2017, 377, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Chaemsaithong, P.; Cuenca-Gomez, D.; Plana, M.N.; Gil, M.M.; Poon, L.C. Does low-dose aspirin initiated before 11 weeks’ gestation reduce the rate of preeclampsia? Am. J. Obstet. Gynecol. 2020, 222, 437–450. [Google Scholar] [CrossRef]
- Nguyen-Hoang, L.; Dinh, L.T.; Tai, A.S.T.; Nguyen, D.A.; Pooh, R.K.; Shiozaki, A.; Zheng, M.; Hu, Y.; Li, B.; Kusuma, A.; et al. Implementation of First-Trimester Screening and Prevention of Preeclampsia: A Stepped Wedge Cluster-Randomized Trial in Asia. Circulation. 2024, 150, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Vahedian-Azimi, A.; Karimi, L.; Reiner, Ž.; Makvandi, S.; Sahebkar, A. Effects of statins on preeclampsia: A systematic review. Pregnancy Hypertens. 2021, 23, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.I.A.; Azis, M.A.; Riu, D.S.; Wawengkang, E.; Ernawati, E.; Bachnas, M.A.; Sulistyowati, S.; Dachlan, E.G.; Mose, J.C.; Dekker, G. INOVASIA Study: A Multicenter Randomized Clinical Trial of Pravastatin to Prevent Preeclampsia in High-Risk Patients. Am. J. Perinatol. 2024, 41, 1203–1211. [Google Scholar] [CrossRef]

| Direct Effects | ||
|---|---|---|
| Target Oxidants | Roles | References |
| Hydroxyl radical | Stabilization, Neutralization | [8,11,16,24,25,43,44,45,46,47,48,49,50,51,52] |
| Peroxyl radicals | Inactivation | [8,11,16,25,51] |
| Singlet oxygen | Inactivation | [53] |
| Superoxide | NADPH oxidase inhibition | [8,10,11,25,52,54,55,56,57] |
| Indirect Effects | ||
| Target Substances | Roles | References |
| L-ascorbic acid | Stabilization, Protection | [13] |
| α-Tocopherol | Stabilization, Protection | [11,13] |
| Procyanidin B3 | Stabilization, Protection | [13] |
| β-Carotene | Stabilization, Protection | [13] |
| Astaxanthin | Stabilization, Protection | [13] |
| (-)-Epigallocatechin gallate | Stabilization, Protection | [14] |
| Anthocyanins | Stabilization, Protection | [15] |
| Fatty acids, Bilirubin, Bile acids, Calcium, drugs, and Tryptophan, etc. | Binding to prevent peroxidation and reactive oxygen species formation of the substances | [8,11,16,43,53] |
| Metals, including Cu2+, Fe2+, Fe3+ | Binding to prevent hydroxyl radical production | [8,11,16,43,44,58,59] |
| Quercetin and Catechin | Binding to enhance antioxidative effects of the substances | [17,18,60,61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, K.; Kinoshita, H.; Okamoto, T.; Sugiura, K.; Kawashima, S.; Kimura, T. Antioxidant Properties of Albumin and Diseases Related to Obstetrics and Gynecology. Antioxidants 2025, 14, 55. https://doi.org/10.3390/antiox14010055
Watanabe K, Kinoshita H, Okamoto T, Sugiura K, Kawashima S, Kimura T. Antioxidant Properties of Albumin and Diseases Related to Obstetrics and Gynecology. Antioxidants. 2025; 14(1):55. https://doi.org/10.3390/antiox14010055
Chicago/Turabian StyleWatanabe, Kazushi, Hiroyuki Kinoshita, Tomohito Okamoto, Kazumasa Sugiura, Shingo Kawashima, and Tetsuro Kimura. 2025. "Antioxidant Properties of Albumin and Diseases Related to Obstetrics and Gynecology" Antioxidants 14, no. 1: 55. https://doi.org/10.3390/antiox14010055
APA StyleWatanabe, K., Kinoshita, H., Okamoto, T., Sugiura, K., Kawashima, S., & Kimura, T. (2025). Antioxidant Properties of Albumin and Diseases Related to Obstetrics and Gynecology. Antioxidants, 14(1), 55. https://doi.org/10.3390/antiox14010055






