Abstract
The relationship between oxidative stress and cancer has been extensively studied and highlighted, along with its role in various aspects of angiogenesis. The modulation of oxidative levels and the adaptive mechanisms of oxidative stress in cancer systems are attractive research themes for developing anti-cancer strategies. Reactive oxygen species (ROS) are involved in various pathophysiological processes and play crucial roles in DNA damage and angiogenesis. Although cancer cells have developed various adaptive defense mechanisms against oxidative stress, excessive ROS production has been proposed as an anti-cancer strategy to induce cellular apoptosis. In particular, natural-source-based antioxidants have been identified as effective against cancers, and various delivery platforms have been developed to enhance their efficacy. In this review, we highlighted the anti-cancer components (plumbagin, quercetin, resveratrol, curcumin, xanthatin, carvacrol, telmisartan, and sulforaphane) that modulate ROS levels and the recent targeting platforms used to increase the application of anti-cancer drugs and the developed delivery platforms with diverse mechanisms of action. Further, we summarized the actual doses used and the effects of these drug candidates in various cancer systems. Overall, this review provides beneficial research themes for expanding cancer-targeting fields and addressing limited applications in diverse cancer types.
1. Modulation of Reactive Oxygen Species Levels to Treat Cancers
Cells possess multiple antioxidant defense systems, including superoxide dismutase, thioredoxin peroxidase, catalase, glutathione peroxidase, and antioxidants including glutathione, and peroxiredoxins [1]. Adaptive responses to oxidative stress allow cancer cells to maintain redox homeostasis and promote survival and proliferation. The redox balance is modulated by the ratio of reactive oxygen species (ROS) scavengers such as glutathione, oxidized components, and oxidative-stress-associated proteins. For instance, the ratio of reduced glutathione (GSH) to oxidized glutathione (GSSG), [GSH:GSSG] is considered a marker of oxidative stress. The GSH:GSSG ratio is the highest in the serum of patients with retinoblastoma [2]. Moreover, nuclear factor erythroid 2-related factor 2 (NRF2) is associated with elements of the antioxidant response in normal and cancer cells [3]. The depleted Kirsten rat sarcoma viral oncogene homolog (K-RAS) induces a downregulated NRF2-associated mechanism and dysregulates ROS levels in lung cancer cells [4]. Based on the examples of several reported ROS scavengers, the modulation of ROS is a promising strategy for cancer therapy.
Excessive ROS disrupts redox balance, proteins, lipids, and DNA, and subsequently triggers apoptotic pathways. Increased ROS levels have been implicated in various stages of cancer progression through increased cell proliferation and motility. For instance, ROS production is associated with the proliferation and migration of prostate cancer cells [5]. Blocking ROS production using the NADPH oxidase inhibitor diphenyliodonium [5] decreases the activity of matrix metalloproteinase (MMP) 9 and mitochondrial potential and subsequently decreases cell invasive activity in prostate cancer [5]. Further, administration of the ROS hydrogen peroxide (H2O2) reduces cell viability and causes simultaneous cell cycle arrest in Calu-6 and A549 lung cancer cells [6]. These cellular reactions are accompanied by the downregulation of Bcl-2 and procaspase-3 as well as the upregulation of caspase-3 and -8 [6]. Moreover, ROS stimulation induces cell death and necrosis through cell cycle G1 phase arrest and enhances caspase activity in lung cancer cells [6]. Several studies have investigated the modulation of redox balance to treat cancer cells and identified ROS-mediated anti-cancer agents as oxidative-stress-based strategies. Additionally, the regulation of ROS production has been suggested as an effective strategy to attenuate the proliferation and invasive activity of cancers.
Naturally derived compounds are suggested to avoid drug limitations and complications, such as toxic side effects. Several naturally derived and synthetic compounds are used for ROS production to induce cytotoxic effects. In this review, we selected bioactive ROS modulators, including polyphenols and alkaloids such as plumbagin, quercetin, resveratrol, curcumin, xanthatin, carvacrol, telmisartan, and sulforaphane, to reveal their diverse mechanisms of action in various cancers, summarize their actual doses and effects, and highlight the delivery platforms used to enhance their availability.
2. Methodology and Approach for Systemic Review
The literature search was based on online databases, such as PubMed, Scopus, and ClinicalTrials.gov, using the selected keywords cancer, antioxidants, selected natural compounds, oxidative stress, and reactive oxygen species. Initially, we screened articles based on their titles and abstracts to assess their relevance. We specifically considered compounds that demonstrated effects and potential applications across multiple cancer types. Thus, full-text articles of potentially relevant studies involving the selected compounds of plumbagin, quercetin, resveratrol, curcumin, xanthatin, carvacrol, telmisartan, and sulforaphane were thoroughly reviewed and summarized.
3. ROS Mechanism-Based Natural Compounds
3.1. Plumbagin
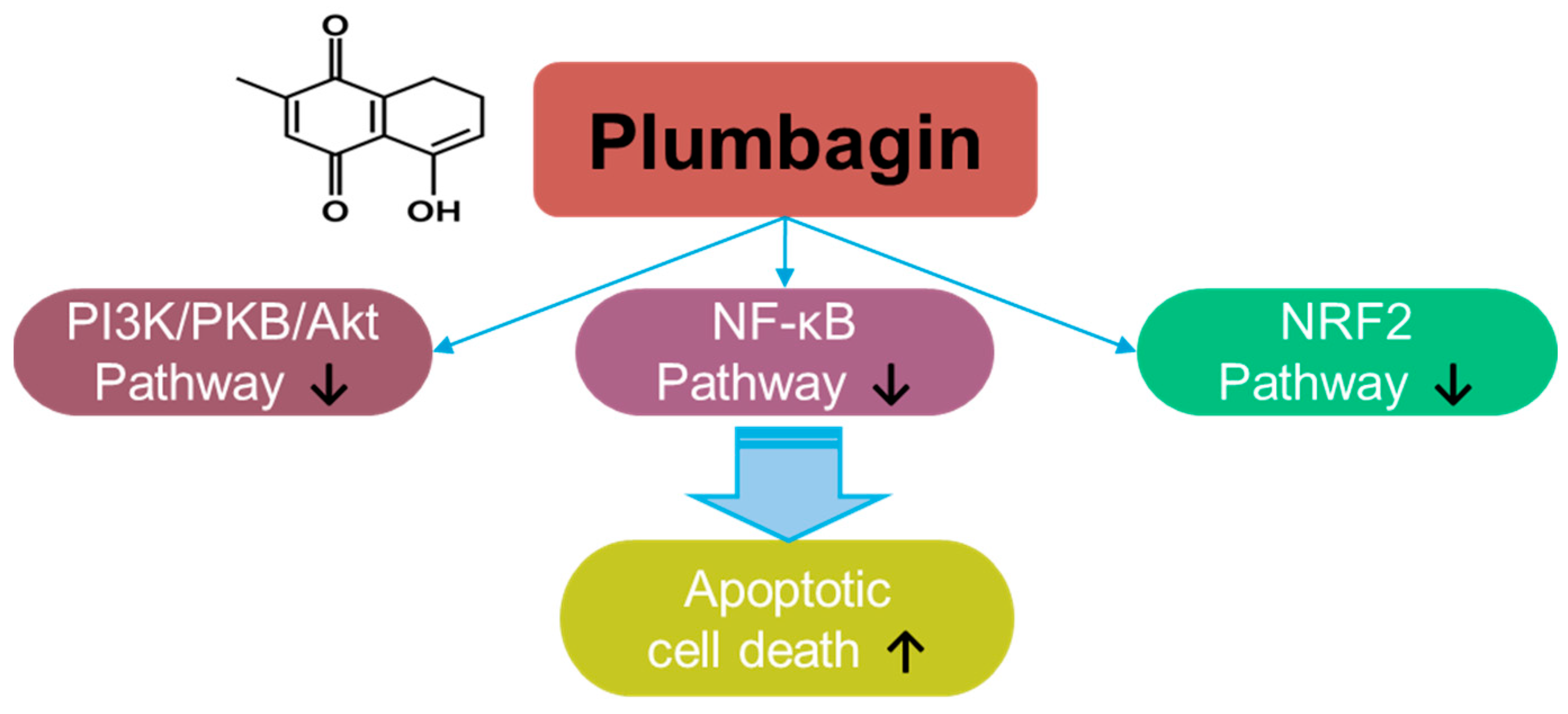
Plumbagin, a naphthoquinone compound, a hydroxy-1,4-naphthoquinone, derived from Plumbago zeylanica L., possesses antioxidant and anti-cancer properties [7] (Figure 1). The roles of plumbagin have been addressed in various cancers, including gastric cancer [8], colorectal cancer [9], lung cancer [10], melanoma [11], pancreatic cancer [12], hepatic cancer [13], oral squamous carcinoma [14,15], cervical carcinoma [16,17], glioma [18], and breast cancer [19,20,21]. Plumbagin treatment decreases the viability of prostate cancer cells by stimulating ROS production [22]. Further, plumbagin decreases cellular ROS metabolism-related genes, such as superoxide dismutase 2, with excessive elimination of glutathione [22]. Mechanistically, plumbagin suppresses IκB kinase α-mediated nuclear factor-κB (NF-κB) activation and subsequently inhibits the invasion of oncogene HER2-overexpressing breast cancer cells, such as the BT-474 and SK-BR-3 cell lines [23]. Plumbagin treatment also induces cell cycle arrest and apoptotic cell death by inhibiting the phosphoinositide 3-kinase (PI3K)/protein kinase B (PKB, Akt) pathway [24]. Moreover, plumbagin treatment inhibits epithelial-to-mesenchymal transition by inhibiting NRF2 [14]. More recently, combinational approaches of plumbagin with other compounds such as curcumin and cisplatin have been developed for breast cancer [25,26,27,28]. The neuroprotective effects of plumbagin in Parkinson’s disease have also been studied [29]. Therefore, the clinical use of plumbagin should be investigated in future studies.
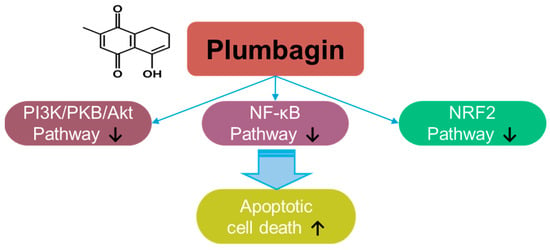
Figure 1.
Molecular structure and mechanisms of plumbagin.
3.2. Quercetin
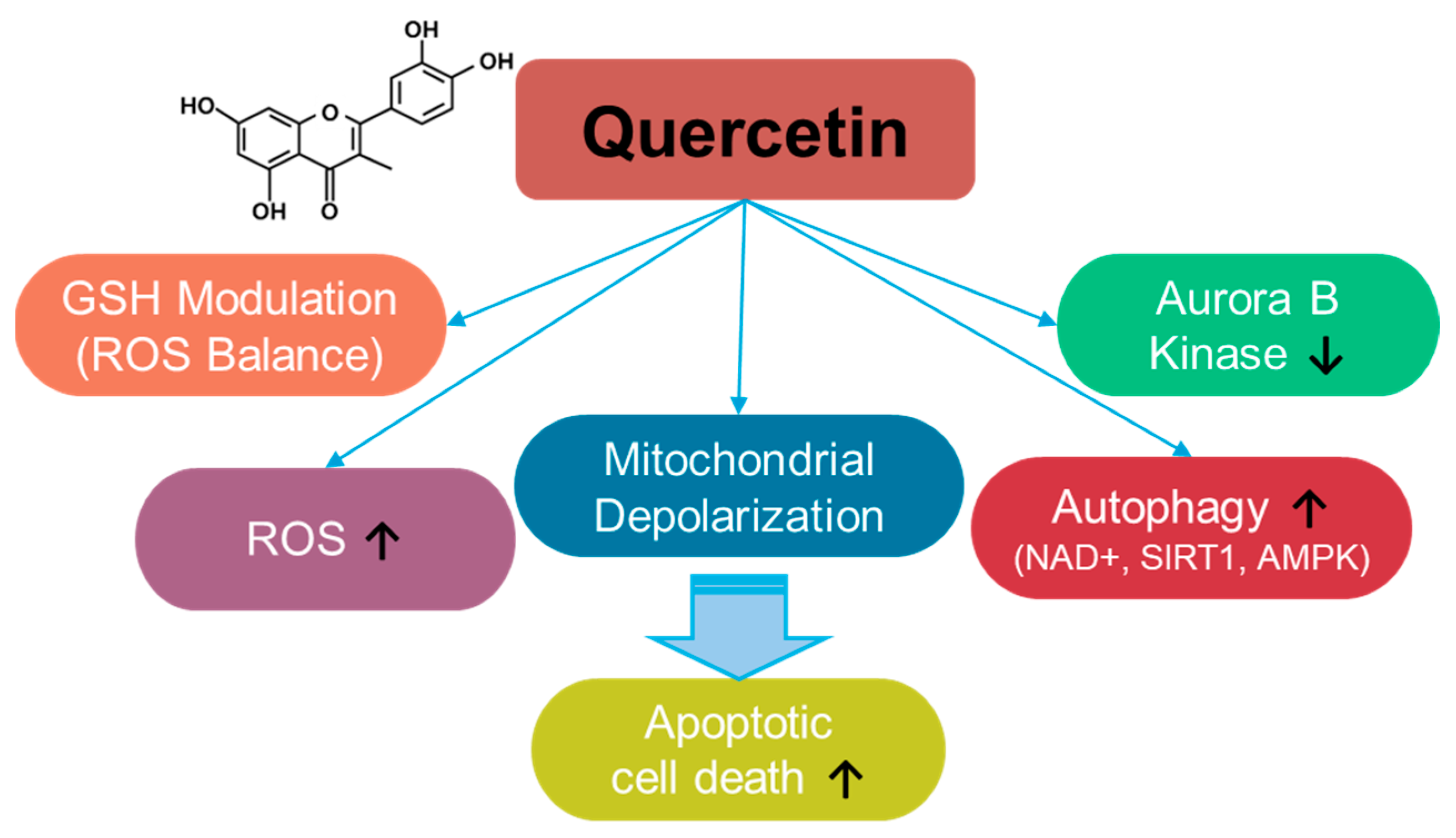
Quercetin is a fruit- and vegetable-based flavonoid [30] (Figure 2) that enhances ROS levels and modulates ROS balance by altering GSH levels [31,32,33,34]. Quercetin administration increases ROS production and induces apoptosis in breast cancer cells [35]. The depletion of GSH by quercetin administration induces mitochondrial depolarization and subsequently triggers apoptosis in SW872 liposarcoma cells [34]. Further, quercetin treatment induces autophagy through enhanced nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylases, sirtuin1 (SIRT1), and AMP-activated protein kinase (AMPK) signaling in A549 and H1299 lung cancer cells [36]. In addition, tumorigenesis-associated aurora B kinase is inhibited by quercetin treatment in A549 cells and in the A549–xenograft mouse model [36,37]. The anti-cancer roles of quercetin have been elucidated in multiple cancers, including osteosarcoma, ovarian, breast, prostate, and lung cancers, and have also been reviewed in various aspects [38,39,40,41,42,43,44,45,46,47,48,49]. The effects of quercetin on various cancer systems are summarized in Table 1. However, quercetin administration presents several challenges, such as limited solubility and stability. Therefore, to improve the accessibility and therapeutic outcomes of quercetin in cancer cells, carrier-conjugated approaches such as quercetin-conjugated silver nanoparticles, liposomes, and silica nanoparticles have been suggested [50,51]. Moreover, the combination of quercetin and sulforaphane to deplete intracellular glutathione enhances anti-cancer effects on HCT116 colorectal carcinoma cell–xenograft mouse models [52]. Although the current approaches to quercetin administration have the potential to treat cancers, several challenges remain, including a lack of clinical evidence, drug resistance, and toxicity, which should be addressed carefully.
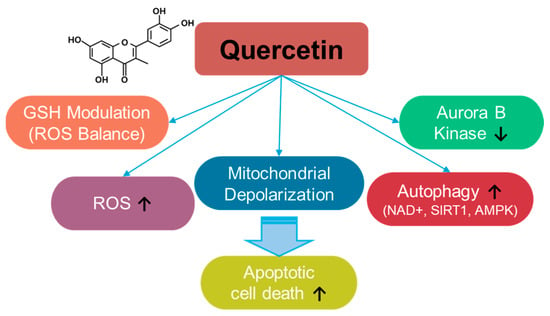
Figure 2.
Molecular structure and mechanisms of quercetin.

Table 1.
Anti-cancer effects of quercetin.
3.3. Resveratrol
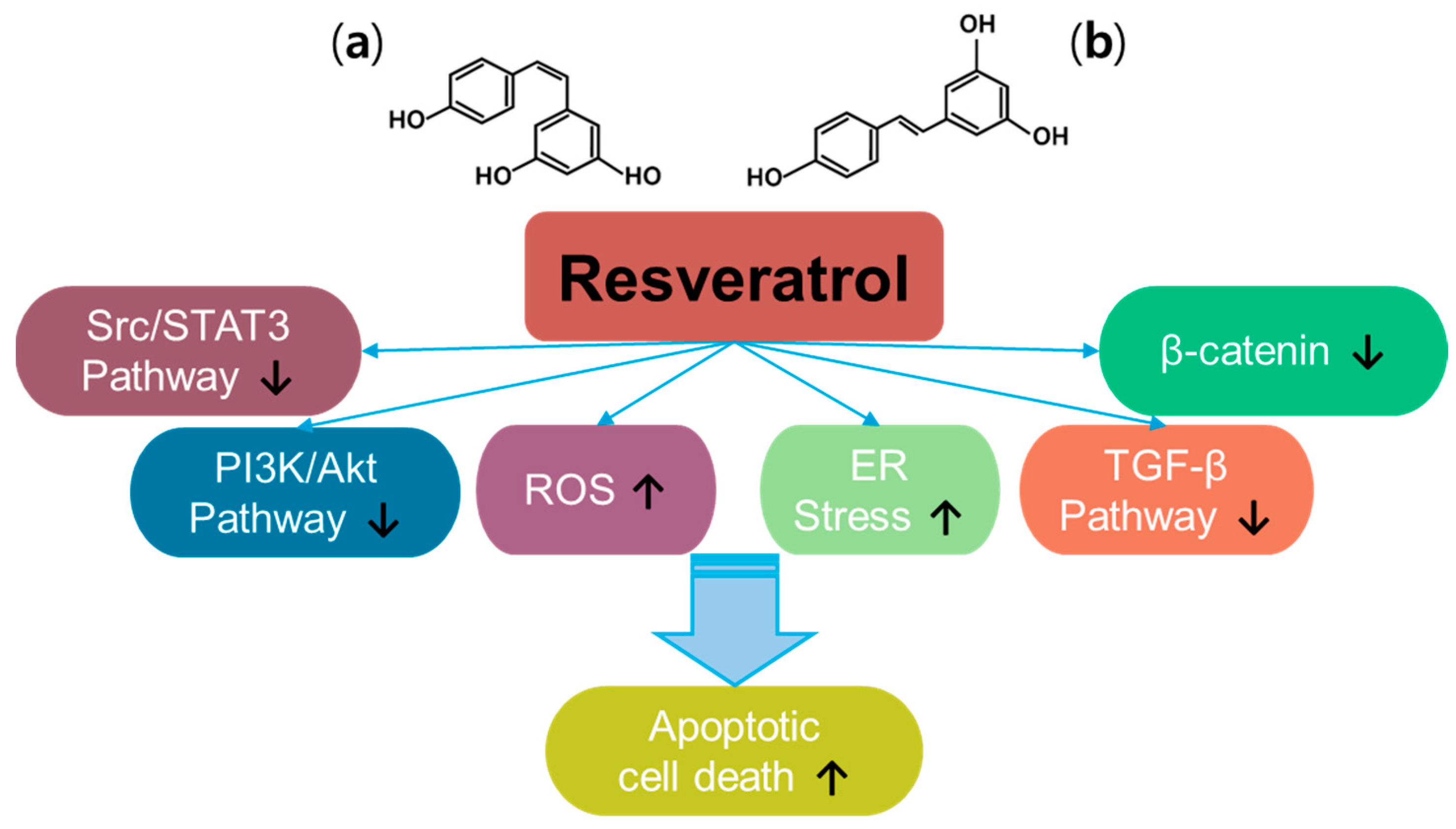
Resveratrol (trans-3, 5, 4′-trihydroxystilbene) is a plant-derived natural polyphenol product and a promising anti-cancer compound [54] (Figure 3). Resveratrol has two types of structures, the cis- and trans-forms [55]. Resveratrol has been examined in approximately 7000 articles (PubMed-based searches) that address its effects, especially against cancers, and has been reviewed extensively since its discovery [56,57,58,59]. Thus, resveratrol has been revealed to have multiple properties such as antioxidant, anti-inflammatory, and anti-cancer properties. With the different signaling pathways of resveratrol in cancers, resveratrol inhibits multiple intracellular mechanisms, such as β-catenin signaling, transforming growth factor (TGF)-β signaling, PI3K/Akt signaling, and Src/signal transducer and activator of transcription 3 (STAT3) signaling [57]. Further, resveratrol promotes ROS accumulation by regulating ROS-metabolism-associated enzymes to mediate oxidative stress and induces apoptosis in colon cancer [60]. More recently, resveratrol has been shown to induce endoplasmic reticulum (ER) stress and impair the regulation of oxidative mechanisms in A375 melanoma cells and metastatic cervical adenocarcinoma HeLa cells, respectively [61,62]. Although the beneficial effects of resveratrol have been addressed, renal toxicity in patients with myeloma and gastrointestinal side effects have been observed, indicating differences in properties depending on the cancer status or type [63,64,65,66]. Moreover, resveratrol possesses low solubility and rapid metabolism, which need to be addressed considering the pharmacological aspects of therapeutic drugs [55,57,67]. Further, resveratrol has a promising effect in increasing the sensitivity of cancers to chemo/radiotherapy [66]. Combined approaches of resveratrol and polyphenols such as epigallocatechin-3-gallate or thymoquinone (one of constituents in Nigella sativa black seed oil) induce synergistic anti-cancer effects in head and neck cancer models and hepatocellular carcinoma, respectively [68,69,70]. Although we did not fully highlight the anti-cancer effects of resveratrol and its delivery platforms for various cancer systems in this review, promising effects and wide applications in drug development are supported by extensive research.
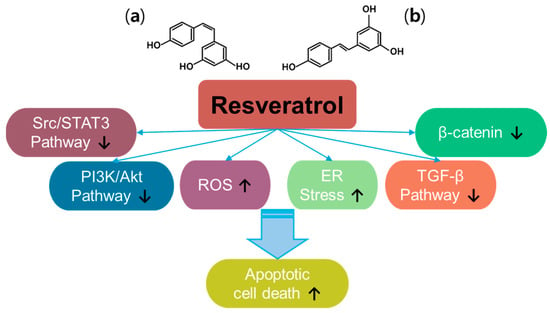
Figure 3.
Molecular structure and mechanisms of resveratrol; (a) cis-resveratrol; (b) trans-resveratrol.
3.4. Curcumin
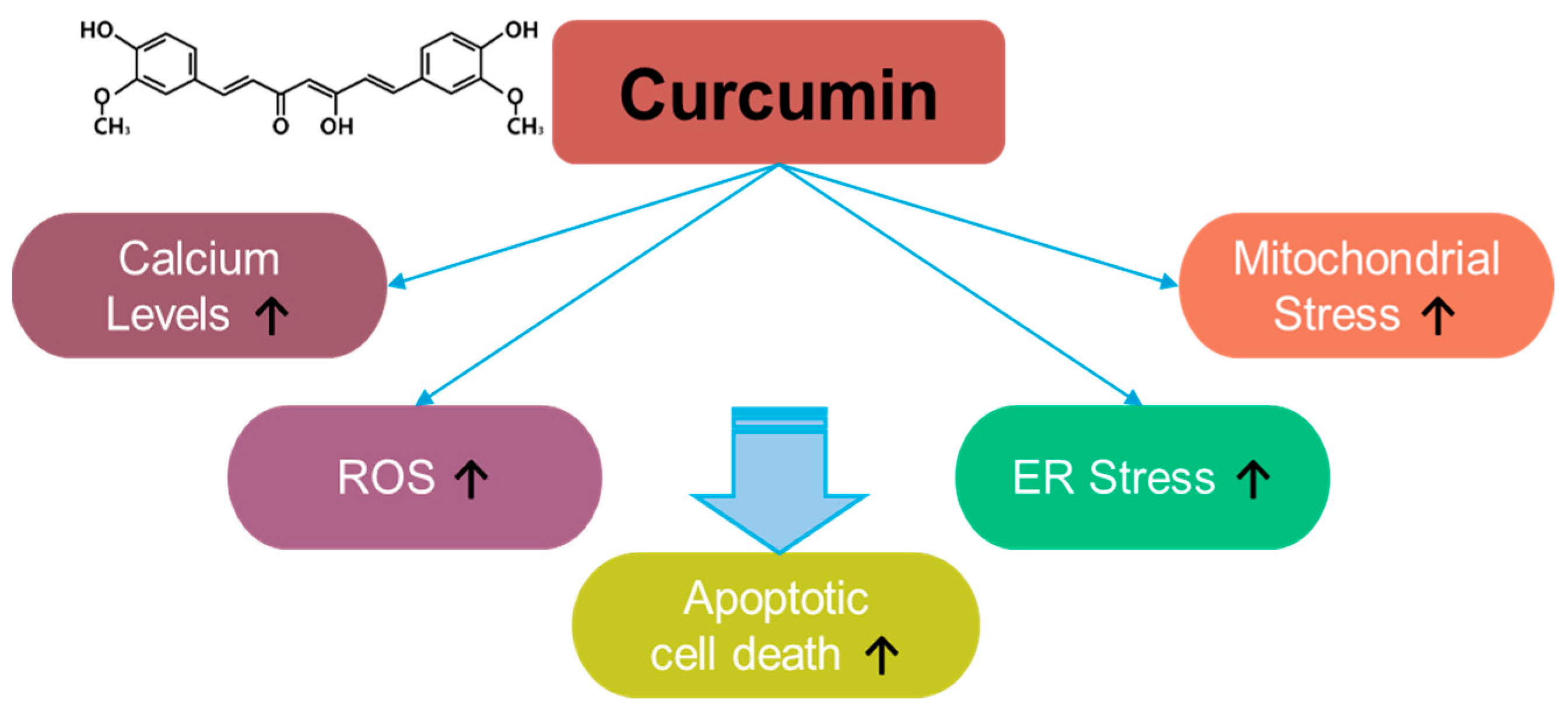
Curcumin, a component of Curcuma longa, exerts inhibitory effects on inflammation and oxidative stress [71] (Figure 4). For instance, curcumin induces apoptosis in rat histiocytoma AK-5 cells via ROS production [71]. Moreover, in the last two decades since its discovery, multiple studies have revealed the antioxidant role of curcumin in various cancers. Although curcumin exerts a different inhibitory effect on ROS generation in methylglyoxal-exposed hepatoma G2 cells [72], it is considered a strategic component against various cancers, including, liver, oral, cervical, colon and colorectal, lung, thyroid, gastric, bladder, pancreatic, ovarian, breast, and laryngeal cancers, as well as melanoma, nasopharyngeal carcinoma, osteosarcoma, leukemia, glioma, and head and neck squamous cell carcinoma. Curcumin exerts apoptotic effects in various cancers through ROS generation, calcium increase, and increases in ER and mitochondrial stress [73]. More recently, the combination of curcumin and thymoquinone reveals anti-cancer benefits in treating breast cancer [74]. The effective doses and effects of curcumin and its combinations in various cancers are summarized in Table 2.
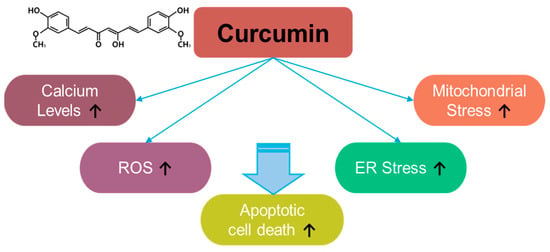
Figure 4.
Molecular structure and mechanisms of curcumin.

Table 2.
Anti-cancer effects of curcumin.
Although curcumin possesses effective anti-cancer properties, its pharmacological efficacy is low because of its instability and low solubility [111]. To enhance its therapeutic application, several delivery approaches, including kappa-carrageenan, nanoparticles, nanofibrous mats, halloysite nanotubes, liposomes, graphene-based nanoformulation-mediated delivery platforms, photodynamic therapy, and active metabolites, have been proposed and are summarized in Table 3.

Table 3.
Various delivery platforms of curcumin for different cancers.
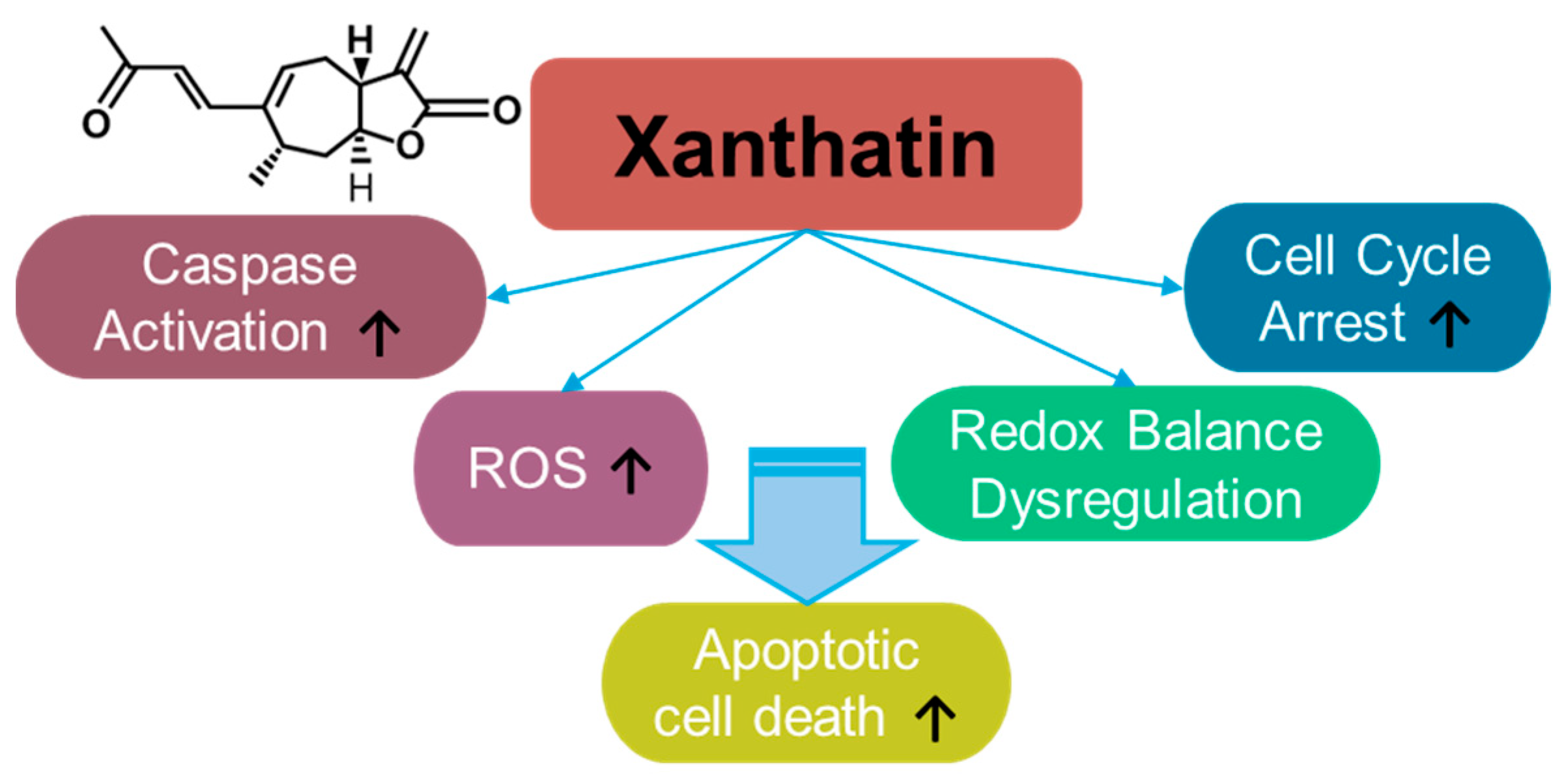
3.5. Xanthatin
Xanthatin is a sesquiterpene lactone (Figure 5) derived from Xanthiun strumarium L. and exerts cytotoxic effect on cancer cells [168,169]. Since the identification of the biological properties of xanthatin, its anti-cancer effects have been demonstrated in various cancer systems such as breast cancer [170,171,172,173], gastric carcinoma [174], lung cancer [175,176,177,178], melanoma [179], colon cancer [180,181,182,183], hepatocellular carcinoma [184,185,186], pancreatic cancer [187], and glioma [188,189]. Xanthatin induces cellular apoptosis through mitochondrial ROS accumulation and the dysregulation of redox balance [178]. Moreover, xanthatin induces caspase activation and cell cycle arrest [174]. The effective doses and effects of xanthatin on various cancers are summarized in Table 4. In addition to its effects on cancer, xanthatin derivatives have been considered as potential antifungal agents [190,191] and anti-asthmatic drugs [192]. More recently, the extracted fraction of Xanthium mongolicum showed anti-rheumatic activity by attenuating macrophage polarity [193].
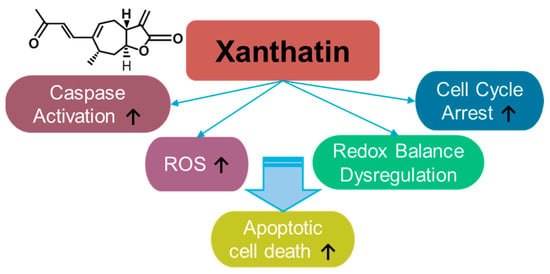
Figure 5.
Molecular structure and mechanisms of xanthatin.

Table 4.
Anti-cancer effects of xanthatin.
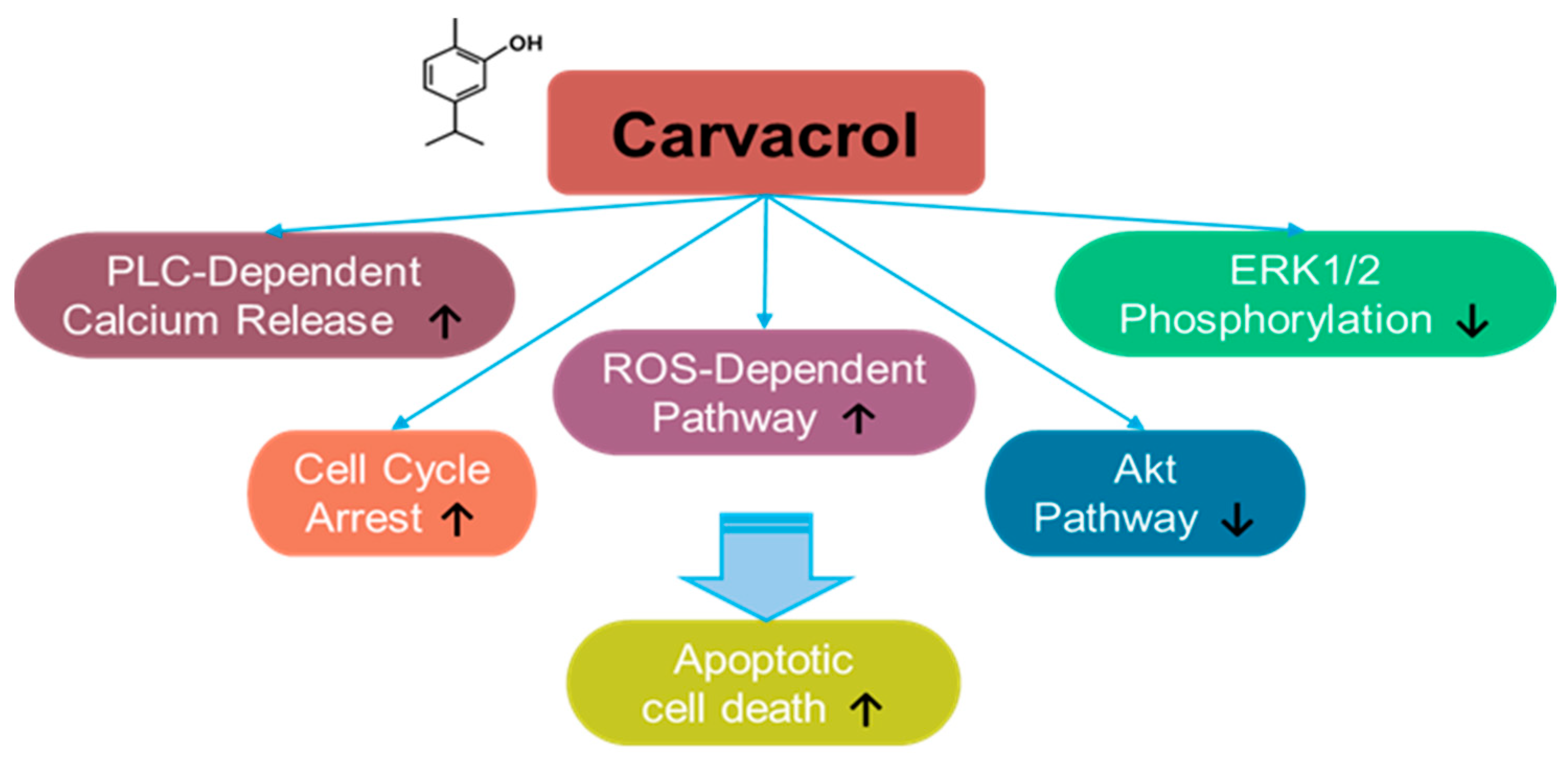
3.6. Carvacrol
Carvacrol is a monoterpene phenol component derived from natural aromatic or herbal plants, such as Carum copticum and Origanum vulgare [194,195,196,197,198] (Figure 6). Moreover, carvacrol is a major component of oregano essential oil, which is commonly used as a dietary supplement [194]. Carvacrol has been shown to exert a promising effect in cancer treatment, in addition to its anti-inflammatory effect. For instance, the effect of carvacrol on the cancer was addressed for the first time in hepatocarcinoma cells [195]. Carvacrol treatment inhibits ERK1/2 phosphorylation and induces apoptosis in HepG2 hepatic carcinoma cells [195]. The apoptotic effects of carvacrol have also been demonstrated in DBTRG-05MG and U87 human glioblastoma cells, OC2 oral cancer cells, and HOS and U2OS osteosarcoma cells through an increase in intracellular calcium and ROS generation [199,200,201,202]. Carvacrol-induced calcium increase is mediated by phospholipase C (PLC)-dependent calcium release from calcium stores and extracellular calcium in OC2 cells [200]. Further, the proliferation and migration of HCT 116 and LoVo colon cancer cells are inhibited by carvacrol treatment through cell cycle arrest and mitochondrial apoptosis [203].
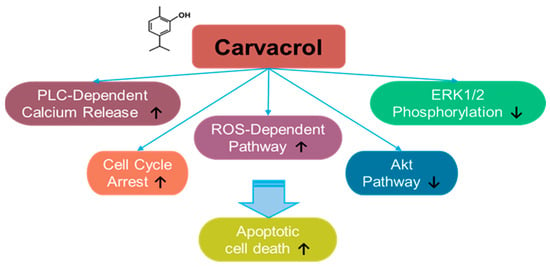
Figure 6.
Molecular structure and mechanisms of carvacrol.
The effect of carvacrol on plasma membrane calcium channels has been investigated in several cellular systems. Carvacrol treatment blocks the current of transient receptor potential melastatin 7 (TRPM7) in TRPM7-overexpressed HEK293 cells and the TRPM7-like current in U87 glioblastoma cells [202]. U87 glioblastoma cells overexpress TRPM7 compared with those in normal astrocytes [202]. Moreover, carvacrol treatment inhibits the proliferation and migration of glioblastoma cells by inhibiting the TRPM7-mediated mitogen-activated protein kinase (MAPK) and PI3K/Akt signaling pathways [202]. A similar approach has been used for prostate cancer. Carvacrol treatment inhibits TRPM7-like current and subsequently blocks the proliferation, migration, and invasion of PC-3 and DU145 prostate cancer cells [204]. In addition, carvacrol-mediated apoptosis was mediated by PLC-dependent calcium release, cell cycle arrest, and ROS-dependent pathways through inhibition of ERK1/2 and Akt pathways in prostate cancer PC-3 and DU145 cells [205,206,207]. In Tca-8113, SCC-25, and OC2 oral squamous cell carcinoma cells, carvacrol treatment inhibits migration and invasion by inhibiting cell cycle regulation and MMP signaling [200,208]. Recently, the anti-proliferative effect of carvacrol treatment on breast cancer cells has been demonstrated. Carvacrol treatment induces breast cancer cell apoptosis by modulating the mitochondrial apoptotic genes Bax and Bcl-2 in MCF-7 cells [209].
To develop the clinical use of carvacrol against partial solubility and pharmacological stability, recent studies have suggested conjugation- or nano-based techniques. For instance, carvacrol has been conjugated with several components, such as a copper–Schiff base complex in A549 lung cancer cells [210], triphenylantimony (V) complex in MCF-7 breast cancer cells [211], hydroxypropyl-β-cyclodextrin complex in HCT116 colorectal carcinoma cells [212], and selenium/chitosan/polyethylene glycol complex in U266 myeloma cells [213]. Moreover, carvacrol was loaded onto chitosan-based nanoparticles to improve drug efficacy in breast cancer MCF-7 and HeLa cells [214].
These findings indicate that carvacrol treatment mechanistically induces apoptotic signals through caspase activation and ROS-mediated mitochondrial dysregulation. A controversial study on the anti-cancer effects of carvacrol on cervical cancer indicated that co-treatment with carvacrol and cisplatin increased HeLa cervical cancer cell viability compared to that of HeLa cells treated with cisplatin alone [215]. Although we included this controversial study in this review, carvacrol-mediated cisplatin resistance needs to be verified in future studies.
The effective doses and effects of carvacrol on various cancers are summarized in Table 5. In this review, although we highlight the anti-cancer applications of carvacrol, its potential for application in non-cancer systems is also notable. Carvacrol possesses multiple targets. In addition to being a TRPM7 antagonist [202], carvacrol is also considered a TRP ankyrin 1 (TRPA1) agonist [216]. TRPA1 signaling has been studied in skin diseases such as psoriasis [217]. Moreover, the therapeutic effects of carvacrol on skin differentiation have been studied in TRP vanilloid 3 (TRPV3)-knockout mouse skin [218]. Therefore, detailed investigations on the effects of carvacrol on channelopathy-associated skin diseases, such as psoriasis, are warranted in future.

Table 5.
Anti-cancer effects of carvacrol.
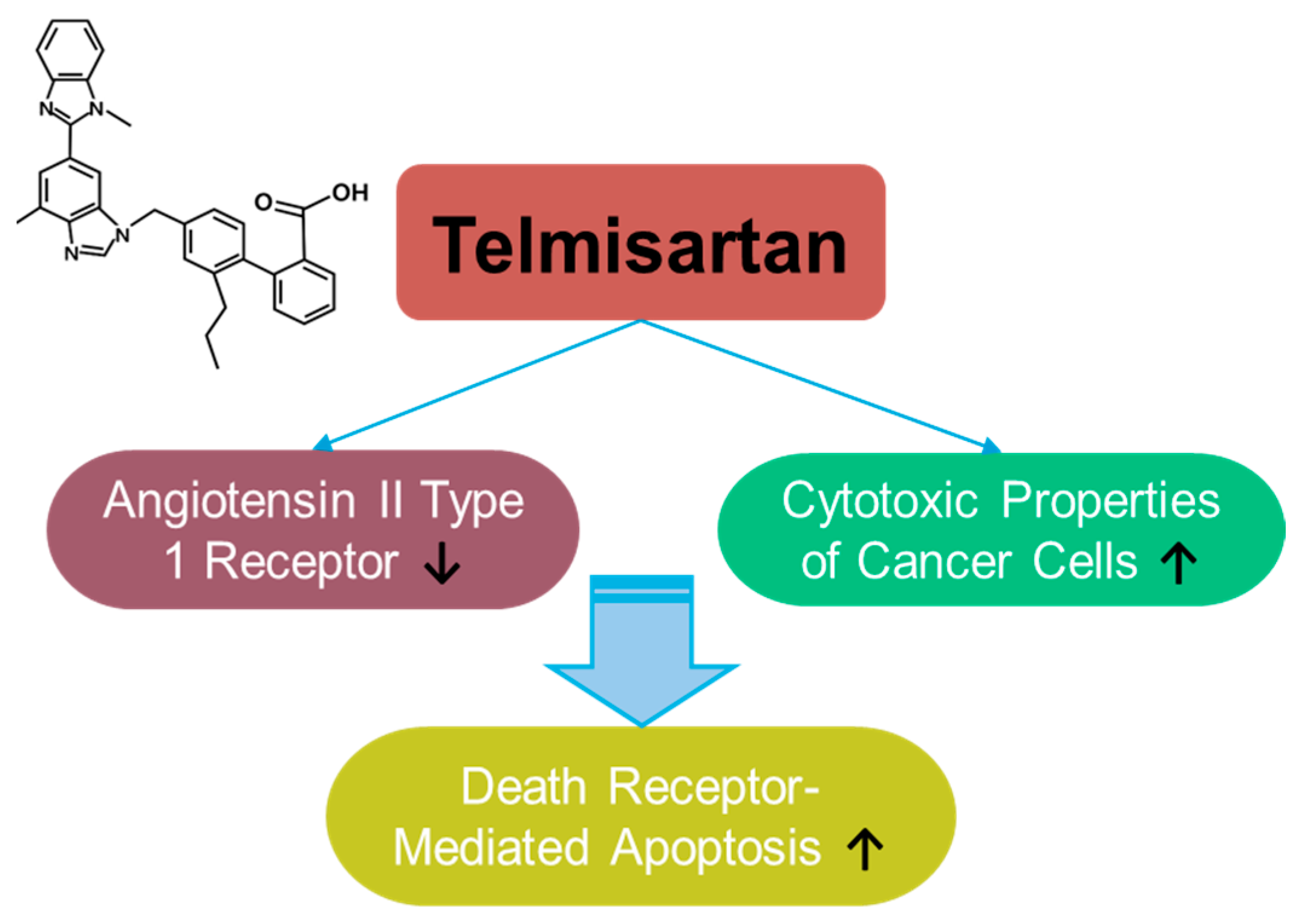
3.7. Telmisartan
Telmisartan is a selective blocker of the angiotensin II type 1 receptor, clinically approved in 1998 (Figure 7). Telmisartan has anti-inflammatory and antioxidant properties, as well as protective roles in hypertension [219,220,221,222] and cognitive impairment [223]. Although the anti-cancer effects of telmisartan have been reported in relatively few articles and have focused on liver, lung, and breast cancers [224,225,226,227,228], its wide usage in treating metabolic syndrome and its application in drug repositioning have been extensively reviewed [229,230,231]. Telmisartan enhances the cytotoxic properties of cancer cells by mediating death-receptor-mediated apoptosis in lung cancer cells [228]. As a delivery strategy, telmisartan was combined with a nanoparticle-mediated programmed drug release platform to permeate the deep breast tumor region [226]. Moreover, the modified structure of the telmisartan–Zn combination has been applied to improve anti-cancer properties through enhanced ROS-mediated cellular apoptosis in lung cancer cells [227]. Although telmisartan is a synthetic compound, the established safety profile of telmisartan possesses an attractive potential in drug repurposing strategies and combinational therapies. However, further investigations are required for the effective application of telmisartan based on the insufficient experimental evidence.
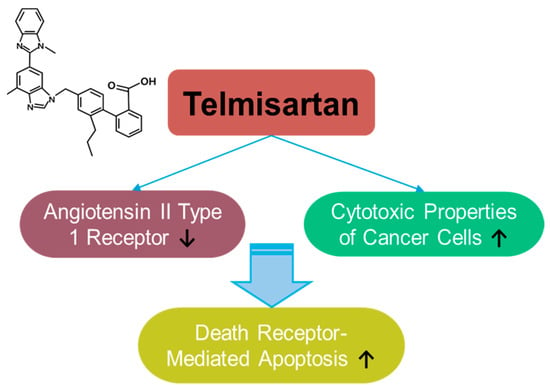
Figure 7.
Molecular structure and mechanisms of telmisartan.
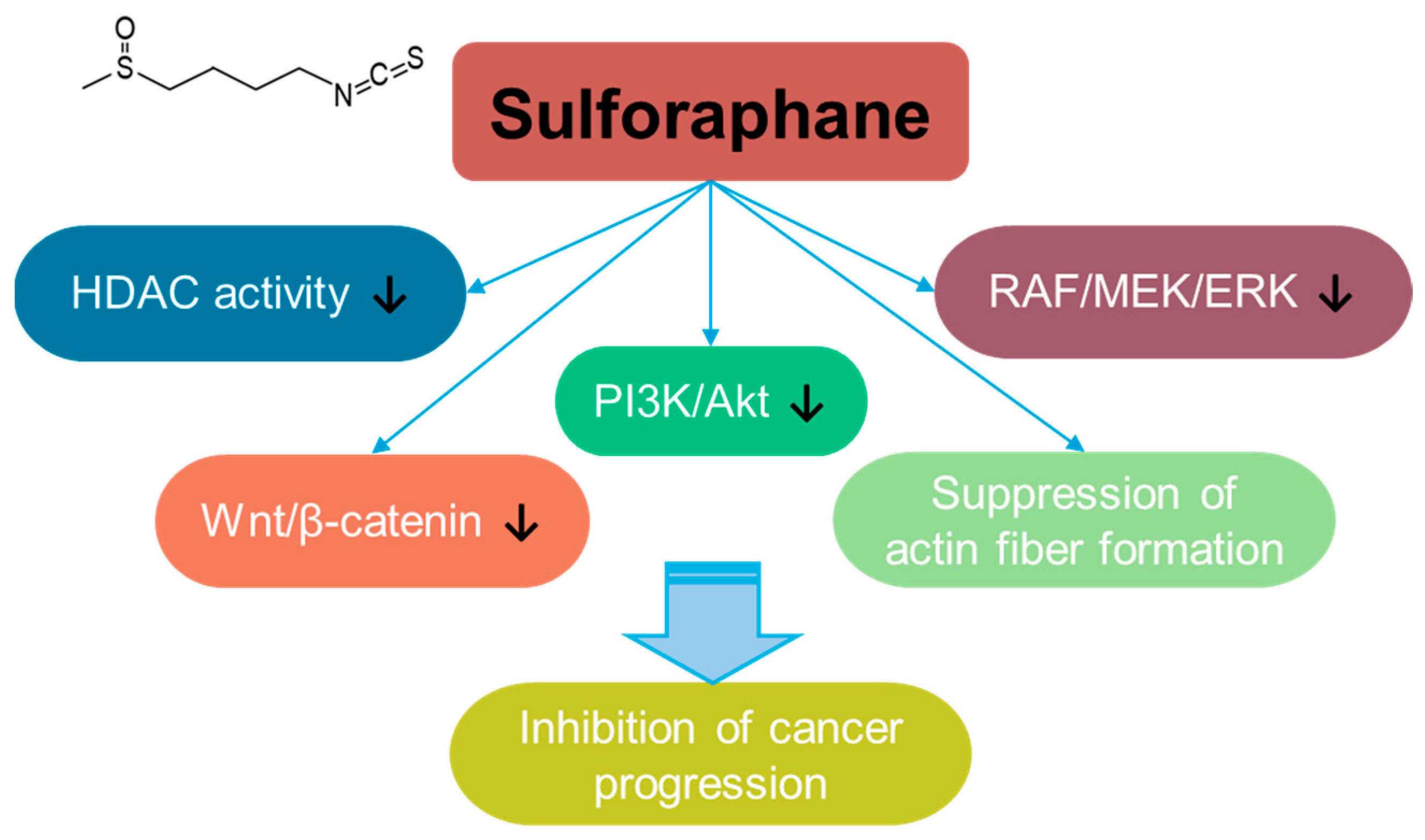
3.8. Sulforaphane
Sulforaphane is a bioactive isothiocyanate phytochemical that is isolated from cruciferous vegetables such as broccoli and kale [232] and extensively reviewed for its multifaceted effects such as its anti-angiogenesis, anti-bacterial, anti-aging, and anti-inflammatory properties [233,234,235]. Sulforaphane treatment suppresses breast cancer metastasis to inhibit actin fiber formation through the inhibition of the RAF/MEK/ERK pathway [236]. In addition, sulforaphane inhibits cancer progression through the inhibition of Wnt/β-catenin or PI3K/Akt signaling in colorectal cancer [237,238]. Sulforaphane also has an inhibitory effect on histone deacetylase (HDAC) activity [239], and its inhibitory effect on HDAC is addressed in breast, prostate, colon, and lung cancer cells [236,237,238,240] (Figure 8). Moreover, the co-administration of sulforaphane with other compounds, such as quercetin, reveals the potential of combinational therapy [52]. Although the anti-cancer roles of sulforaphane are addressed in several preclinical studies, a clinical approach and developmental strategies for sulforaphane should be required to verify its efficacy and safety.
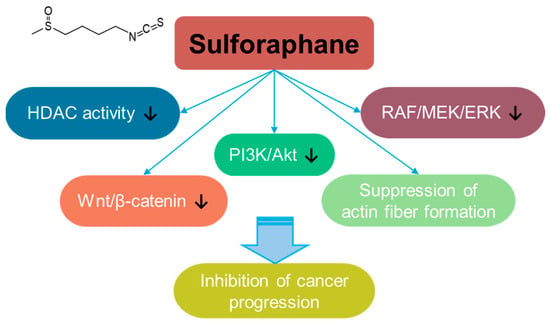
Figure 8.
Molecular structure and mechanisms of sulforaphane.
4. Conclusions and Perspectives
Understanding cancer-related oxidative mechanisms is an attractive strategy for overcoming cancer and developing effective treatments. In this review, ROS-based anti-cancer drugs were described along with the relevant delivery platforms developed to overcome common issues such as instability, efficacy, short half-life, and unwanted targeting. Over the past decades, advances in delivery systems have significantly enhanced the availability of various potential anti-cancer agents. Although enhancing ROS levels in cancer systems is an effective strategy, targeting cancer cells remains challenging. In addition, there are various hurdles to cancer treatment, such as the recognition of cancer-specific receptors or microenvironments, attenuation of adaptive response, disturbance of the immune system, complications of side effects, and budget limitations. To develop ROS-based cancer treatments, these hurdles must be addressed and verified in various cancers using both in vitro and in vivo experimental systems. Naturally derived anti-cancer compounds possess several beneficial effects, such as long-term use, positive immunomodulatory effects, reduced side effects, and the prevention of drug resistance, compared to other chemical compounds. However, among the compounds mentioned in this review, resveratrol and curcumin have been used in clinical studies against cancer, and telmisartan alone was clinically approved as an anti-hypertensive drug. Thus, this review highlights the challenging issues for basic cancer research on naturally derived anti-cancer compounds (Table 6) and emphasizes the importance of verifying their exact mechanisms of action in cancer systems for developing effective cancer treatment strategies and expanding the range of approved drugs.

Table 6.
Comparative anti-cancer effects of highlighted compounds.
Author Contributions
E.L., D.Y. and J.H.H. conceptualized and designed the study and acquired and interpreted the PubMed-based information; E.L. drew all the figures and tables; J.H.H. and D.Y. revised the manuscript critically for important intellectual content; J.H.H. contributed to funding acquisition and the final approval of the published version; and all authors are responsible for all aspects of the work with regard to the accuracy and integrity of the study. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Gachon University research fund of 2024 (GCU-202404120001: JHH) and by the National Research Foundation of Korea (NRF) funded by the Korean government (MSIT, 2022R1A2C1003890: JHH).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
All figures and tables were developed by the authors. We did not use generative AI or AI-assisted technologies.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Abbreviations
ROS: reactive oxygen species; DNA: deoxyribonucleic acid; GSH: reduced glutathione; GSSG: oxidized glutathione; NRF2: nuclear factor erythroid 2-related factor 2; K-RAS: Kirsten rat sarcoma viral oncogene homolog; NADPH: nicotinamide adenine dinucleotide phosphate.; MMP: matrix metalloproteinase; PI3K: phosphoinositide 3-kinase; PKB (Akt): protein kinase B; SIRT1: sirtuin 1 (NAD+-dependent deacetylase); AMPK: AMP-activated protein kinase; RAF: rapidly accelerated fibrosarcoma; MEK: mitogen-activated protein kinase kinase.
References
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxidative Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef] [PubMed]
- Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Adam, V.; Hubalek, J.; Trnkova, L.; Kruseova, J.; Eckschlager, T.; Kizek, R. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol. Lett. 2012, 4, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Gureev, A.P.; Popov, V.N.; Starkov, A.A. Crosstalk between the mTOR and Nrf2/ARE signaling pathways as a target in the improvement of long-term potentiation. Exp. Neurol. 2020, 328, 113285. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xiang, S.Y.; Kazi, A.; Sebti, S.M. The GTPase KRAS suppresses the p53 tumor suppressor by activating the NRF2-regulated antioxidant defense system in cancer cells. J. Biol. Chem. 2020, 295, 3055–3063. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Koul, S.; Khandrika, L.; Meacham, R.B.; Koul, H.K. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008, 68, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Park, W.H. Hydrogen peroxide inhibits the growth of lung cancer cells via the induction of cell death and G1-phase arrest. Oncol. Rep. 2018, 40, 1787–1794. [Google Scholar] [CrossRef]
- Juarez, P. Plant-derived anticancer agents: A promising treatment for bone metastasis. Bonekey Rep. 2014, 3, 599. [Google Scholar] [CrossRef] [PubMed]
- Joo, M.K.; Park, J.J.; Kim, S.H.; Yoo, H.S.; Lee, B.J.; Chun, H.J.; Lee, S.W.; Bak, Y.T. Antitumorigenic effect of plumbagin by induction of SH2-containing protein tyrosine phosphatase 1 in human gastric cancer cells. Int. J. Oncol. 2015, 46, 2380–2388. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.J.; Zhou, R.; Liang, X.L.; Kong, X.L.; Yang, B. Pharmacological targets and molecular mechanisms of plumbagin to treat colorectal cancer: A systematic pharmacology study. Eur. J. Pharmacol. 2020, 881, 173227. [Google Scholar] [CrossRef]
- Jiang, Z.B.; Xu, C.; Wang, W.J.; Zhang, Y.Z.; Huang, J.M.; Xie, Y.J.; Wang, Q.Q.; Fan, X.X.; Yao, X.J.; Xie, C.; et al. Plumbagin suppresses non-small cell lung cancer progression through downregulating ARF1 and by elevating CD8 T cells. Pharmacol. Res. 2021, 169, 105656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.R.; Zhang, A.J.; Gupte, A.A.; Hamilton, D.J. Plumbagin Elicits Cell-Specific Cytotoxic Effects and Metabolic Responses in Melanoma Cells. Pharmaceutics 2021, 13, 706. [Google Scholar] [CrossRef]
- Palanisamy, R.; Kahingalage, N.I.; Archibald, D.; Casari, I.; Falasca, M. Synergistic Anticancer Activity of Plumbagin and Xanthohumol Combination on Pancreatic Cancer Models. Int. J. Mol. Sci. 2024, 25, 2340. [Google Scholar] [CrossRef]
- Yao, L.Y.; Yan, D.; Jiang, B.Y.; Xue, Q.; Chen, X.; Huang, Q.T.; Qi, L.; Tang, D.L.; Chen, X.; Liu, J.B. Plumbagin is a novel GPX4 protein degrader that induces apoptosis in hepatocellular carcinoma cells. Free Radic. Biol. Med. 2023, 203, 1–10. [Google Scholar] [CrossRef]
- Pan, S.T.; Qin, Y.R.; Zhou, Z.W.; He, Z.X.; Zhang, X.J.; Yang, T.X.; Yang, Y.X.; Wang, D.; Zhou, S.F.; Qiu, J.X. Plumbagin suppresses epithelial to mesenchymal transition and stemness via inhibiting Nrf2-mediated signaling pathway in human tongue squamous cell carcinoma cells. Drug Des. Dev. Ther. 2015, 9, 5511–5551. [Google Scholar] [CrossRef]
- Chen, P.H.; Lu, H.K.; Renn, T.Y.; Chang, T.M.; Lee, C.J.; Tsao, Y.T.; Chuang, P.K.; Liu, J.F. Plumbagin Induces Reactive Oxygen Species and Endoplasmic Reticulum Stress-related Cell Apoptosis in Human Oral Squamous Cell Carcinoma. Anticancer Res. 2024, 44, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, S.; Sabanayagam, R.; Periyasamy, L.; Muruganantham, B.; Muthusami, S. Plumbagin as a preferential lead molecule to combat EGFR-driven matrix abundance and migration of cervical carcinoma cells. Med. Oncol. 2024, 41, 89. [Google Scholar] [CrossRef]
- Sidhu, H.; Capalash, N. Plumbagin downregulates UHRF1, p-Akt, MMP-2 and suppresses survival, growth and migration of cervical cancer CaSki cells. Toxicol. Vitr. 2023, 86, 105512. [Google Scholar] [CrossRef]
- Zhan, S.; Lu, L.; Pan, S.S.; Wei, X.Q.; Miao, R.R.; Liu, X.H.; Xue, M.; Lin, X.K.; Xu, H.L. Targeting NQO1/GPX4-mediated ferroptosis by plumbagin suppresses in vitro and in vivo glioma growth. Br. J. Cancer 2022, 127, 364–376. [Google Scholar] [CrossRef]
- De, U.; Son, J.Y.; Jeon, Y.; Ha, S.Y.; Park, Y.J.; Yoon, S.; Ha, K.T.; Choi, W.S.; Lee, B.M.; Kim, I.S.; et al. Plumbagin from a tropical pitcher plant (Blanco) induces apoptotic cell death via a p53-dependent pathway in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2019, 123, 492–500. [Google Scholar] [CrossRef]
- Pradubyat, N.; Sakunrangsit, N.; Mutirangura, A.; Ketchart, W. NADPH: Quinone oxidoreductase 1 (NQO1) mediated anti-cancer effects of plumbagin in endocrine resistant MCF7 breast cancer cells. Phytomedicine 2020, 66, 153133. [Google Scholar] [CrossRef] [PubMed]
- Jampasri, S.; Reabroi, S.; Tungmunnithum, D.; Parichatikanond, W.; Pinthong, D. Plumbagin Suppresses Breast Cancer Progression by Downregulating HIF-1α Expression via a PI3K/Akt/mTOR Independent Pathway under Hypoxic Condition. Molecules 2022, 27, 5716. [Google Scholar] [CrossRef]
- Powolny, A.A.; Singh, S.V. Plumbagin-induced Apoptosis in Human Prostate Cancer Cells is Associated with Modulation of Cellular Redox Status and Generation of Reactive Oxygen Species. Pharm. Res. 2008, 25, 2171–2180. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, J.; Chen, L.; Guo, Q.; Yang, B.; Zhang, W.; Kang, W. Anticancer Effects and Mechanisms of Action of Plumbagin: Review of Research Advances. Biomed. Res. Int. 2020, 2020, 6940953. [Google Scholar] [CrossRef]
- Li, Y.C.; He, S.M.; He, Z.X.; Li, M.H.; Yang, Y.X.; Pang, J.X.; Zhang, X.J.; Chow, K.V.; Zhou, Q.Y.; Duan, W.; et al. Plumbagin induces apoptotic and autophagic cell death through inhibition of the PI3K/Akt/mTOR pathway in human non-small cell lung cancer cells. Cancer Lett. 2014, 344, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.F.; Pan, S.T.; Zhou, X.M.; Ye, F.F.; Zhou, Q.; Shi, F.Z.; He, F.; Yu, H.; Qiu, J.X. Plumbagin Enhances the Anticancer Efficacy of Cisplatin by Increasing Intracellular ROS in Human Tongue Squamous Cell Carcinoma. Oxidative Med. Cell. Longev. 2020, 2020, 5649174. [Google Scholar] [CrossRef] [PubMed]
- Sameni, S.; Viswanathan, R.; Ng, G.Y.; Martinez-Lopez, W.; Hande, M.P. Telomerase Inhibition by MST-312 Sensitizes Breast Cancer Cells to the Anti-cancer Properties of Plumbagin. Genome Integr. 2023, 14, e20230002. [Google Scholar] [CrossRef]
- Ahmad, I.; Hoque, M.; Alam, S.S.M.; Zughaibi, T.A.; Tabrez, S. Curcumin and Plumbagin Synergistically Target the PI3K/Akt/mTOR Pathway: A Prospective Role in Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 6651. [Google Scholar] [CrossRef]
- Xin, Y.Q.; Jiang, Q.K.; Liu, C.S.; Qiu, J.X. Plumbagin has an inhibitory effect on the growth of TSCC PDX model and it enhances the anticancer efficacy of cisplatin. Aging 2023, 15, 12225–12250. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, M.; Wang, Q.; Xu, X.F.; Qin, P.F.; Huang, H.T.; Zhang, Y.T.; Zhou, Y.L.; Yan, J.G. Inhibition of the TLR/NF-B Signaling Pathway and Improvement of Autophagy Mediates Neuroprotective Effects of Plumbagin in Parkinson’s Disease. Oxidative Med. Cell. Longev. 2022, 2022, 1837278. [Google Scholar] [CrossRef]
- Formica, J.V.; Regelson, W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Jeong, J.H.; An, J.Y.; Kwon, Y.T.; Rhee, J.G.; Lee, Y.J. Effects of Low Dose Quercetin: Cancer Cell-Specific Inhibition of Cell Cycle Progression. J. Cell Biochem. 2009, 106, 73–82. [Google Scholar] [CrossRef]
- Lee, Y.K.; Hwang, J.T.; Kwon, D.Y.; Surh, Y.J.; Park, O.J. Induction of apoptosis by quercetin is mediated through AMPKα1/ASK1/p38 pathway. Cancer Lett. 2010, 292, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Cossarizza, A.; Ferraresi, R.; Troiano, L.; Roat, E.; Gibellini, L.; Bertoncelli, L.; Nasi, M.; Pinti, M. Simultaneous analysis of reactive oxygen species and reduced glutathione content in living cells by polychromatic flow cytometry. Nat. Protoc. 2009, 4, 1790–1797. [Google Scholar] [CrossRef]
- Gibellini, L.; Pinti, M.; Nasi, M.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cossarizza, A. Interfering with ROS Metabolism in Cancer Cells: The Potential Role of Quercetin. Cancers 2010, 2, 1288–1311. [Google Scholar] [CrossRef]
- Chien, S.Y.; Wu, Y.C.; Chung, J.G.; Yang, J.S.; Lu, H.F.; Tsou, M.F.; Wood, W.G.; Kuo, S.J.; Chen, D.R. Quercetin-induced apoptosis acts through mitochondrial- and caspase-3-dependent pathways in human breast cancer MDA-MB-231 cells. Hum. Exp. Toxicol. 2009, 28, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.J.; Ding, H.; Tang, X.; Liang, M.L.; Li, S.; Zhang, J.; Cao, J. Quercetin induces pro-apoptotic autophagy via SIRT1/AMPK signaling pathway in human lung cancer cell lines A549 and H1299 in vitro. Thorac. Cancer 2021, 12, 1415–1422. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Ma, P.J.E.; Peng, D.; Wang, Y.; Wang, D.J.; Chen, X.Z.; Zhang, X.J.; Song, Y.R. Quercetin suppresses lung cancer growth by targeting Aurora B kinase. Cancer Med. 2016, 5, 3156–3165. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef] [PubMed]
- Shafabakhsh, R.; Asemi, Z. Quercetin: A natural compound for ovarian cancer treatment. J. Ovarian Res. 2019, 12, 31202269. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, M.; Yousefi, B.; Velaei, K.; Safa, A. A review on anti-cancer properties of Quercetin in breast cancer. Life Sci. 2020, 248, 117463. [Google Scholar] [CrossRef]
- Kasiri, N.; Rahmati, M.; Ahmadi, L.; Eskandari, N.; Motedayyen, H. Therapeutic potential of quercetin on human breast cancer in different dimensions. Inflammopharmacology 2020, 28, 39–62. [Google Scholar] [CrossRef]
- Dana, P.M.; Sadoughi, F.; Asemi, Z.; Yousefi, B. Anti-cancer properties of quercetin in osteosarcoma. Cancer Cell Int. 2021, 21, 349. [Google Scholar] [CrossRef]
- Karimian, A.; Majidinia, M.; Moliani, A.; Alemi, F.; Asemi, Z.; Yousefi, B.; Naghibi, A.F. The modulatory effects of two bioflavonoids, quercetin and thymoquinone on the expression levels of DNA damage and repair genes in human breast, lung and prostate cancer cell lines. Pathol. Res. Pract. 2022, 240, 154143. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, W.; Yang, Z.Y.; Zhou, X.S.; Gong, C.; Zhang, T.R.; Wei, X.; Ma, D.; Ye, F.; Gao, Q.L. Quercetin induces protective autophagy and apoptosis through ER stress via the p-STAT3/Bcl-2 axis in ovarian cancer. Apoptosis 2017, 22, 544–557. [Google Scholar] [CrossRef]
- Gao, X.; Wang, B.L.; Wei, X.W.; Men, K.; Zheng, F.J.; Zhou, Y.F.; Zheng, Y.; Gou, M.L.; Huang, M.J.; Guo, G.; et al. Anticancer effect and mechanism of polymer micelle-encapsulated quercetin on ovarian cancer. Nanoscale 2012, 4, 7883. [Google Scholar] [CrossRef]
- Ren, M.X.; Deng, X.H.; Ai, F.; Yuan, G.Y.; Song, H.Y. Effect of quercetin on the proliferation of the human ovarian cancer cell line SKOV-3. Exp. Ther. Med. 2015, 10, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Berndt, K.; Campanile, C.; Muff, R.; Strehler, E.; Born, W.; Fuchs, B. Evaluation of Quercetin as a Potential Drug in Osteosarcoma Treatment. Anticancer Res. 2013, 33, 1297–1306. [Google Scholar]
- Wu, B.; Zeng, W.; Ouyang, W.; Xu, Q.; Chen, J.; Wang, B.; Zhang, X. Quercetin induced NUPR1-dependent autophagic cell death by disturbing reactive oxygen species homeostasis in osteosarcoma cells. J. Clin. Biochem. Nutr. 2020, 67, 137–145. [Google Scholar] [CrossRef]
- Xie, X.B.; Yin, J.Q.; Jia, Q.; Wang, J.; Zou, C.Y.; Brewer, K.J.; Colombo, C.; Wang, Y.F.; Huang, G.; Shen, J.N. Quercetin induces apoptosis in the methotrexate-resistant osteosarcoma cell line U2-OS/MTX300 via mitochondrial dysfunction and dephosphorylation of Akt. Oncol. Rep. 2011, 26, 687–693. [Google Scholar] [CrossRef]
- Vinayak, M.; Maurya, A.K. Quercetin Loaded Nanoparticles in Targeting Cancer: Recent Development. Anti-Cancer Agents Med. Chem. 2019, 19, 1560–1576. [Google Scholar] [CrossRef] [PubMed]
- Aghapour, F.; Moghadamnia, A.A.; Nicolini, A.; Kani, S.N.M.; Barari, L.; Morakabati, P.; Rezazadeh, L.; Kazemi, S. Quercetin conjugated with silica nanoparticles inhibits tumor growth in MCF-7 breast cancer cell lines. Biochem. Biophys. Res. Commun. 2018, 500, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jang, C.H.; Kim, Y.; Oh, J.; Kim, J.S. Quercetin-Induced Glutathione Depletion Sensitizes Colorectal Cancer Cells to Oxaliplatin. Foods 2023, 12, 1733. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.P.; Gao, N.; Zhang, Z.; Chen, G.; Budhraja, A.; Ke, Z.J.; Son, Y.O.; Wang, X.; Luo, J.; Shi, X.L. Quercetin Induces Tumor-Selective Apoptosis through Downregulation of Mcl-1 and Activation of Bax. Clin. Cancer Res. 2010, 16, 5679–5691. [Google Scholar] [CrossRef] [PubMed]
- Clément, M.V.; Hirpara, J.L.; Chawdhury, S.H.; Pervaiz, S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood 1998, 92, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Benfante, V.; Di Raimondo, D.; Salvaggio, G.; Tuttolomondo, A.; Comelli, A. Recent Developments in Nanoparticle Formulations for Resveratrol Encapsulation as an Anticancer Agent. Pharmaceuticals 2024, 17, 126. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. 2018, 58, 1428–1447. [Google Scholar] [CrossRef]
- Ren, B.X.; Kwah, M.X.Y.; Liu, C.L.; Ma, Z.W.; Shanmugam, M.K.; Ding, L.W.; Xiang, X.Q.; Ho, P.C.L.; Wang, L.Z.; Ong, P.S.; et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Brockmueller, A.; Sajeev, A.; Koklesova, L.; Samuel, S.M.; Kubatka, P.; Büsselberg, D.; Kunnumakkara, A.B.; Shakibaei, M. Resveratrol as sensitizer in colorectal cancer plasticity. Cancer Metastasis Rev. 2023, 43, 55–85. [Google Scholar] [CrossRef]
- Wang, P.; Shang, R.Z.; Ma, Y.; Wang, D.; Zhao, W.T.; Chen, F.; Hu, X.S.; Zhao, X.Y. Targeting microbiota-host interactions with resveratrol on cancer: Effects and potential mechanisms of action. Crit. Rev. Food Sci. 2024, 64, 311–333. [Google Scholar] [CrossRef]
- Santandreu, F.M.; Valle, A.; Oliver, J.; Roca, P. Resveratrol Potentiates the Cytotoxic Oxidative Stress Induced by Chemotherapy in Human Colon Cancer Cells. Cell Physiol. Biochem. 2011, 28, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.R.; Kim, S.M.; Hwang, K.A.; Kang, J.H.; Choi, K.C. Resveratrol induced reactive oxygen species and endoplasmic reticulum stress-mediated apoptosis, and cell cycle arrest in the A375SM malignant melanoma cell line. Int. J. Mol. Med. 2018, 42, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Enríquez, S.; Pacheco-Velázquez, S.C.; Marín-Hernández, A.; Gallardo-Pérez, J.C.; Robledo-Cadena, D.X.; Hernández-Reséndiz, I.; García-García, J.D.; Belmont-Díaz, J.; López-Marure, R.; Hernández-Esquivel, L.; et al. Resveratrol inhibits cancer cell proliferation by impairing oxidative phosphorylation and inducing oxidative stress. Toxicol. Appl. Pharm. 2019, 370, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Vasilinin, G.; Sen, A.; Schinas, A.M.; Piccirilli, G.; et al. Repeat Dose Study of the Cancer Chemopreventive Agent Resveratrol in Healthy Volunteers: Safety, Pharmacokinetics, and Effect on the Insulin-like Growth Factor Axis. Cancer Res. 2010, 70, 9003–9011. [Google Scholar] [CrossRef] [PubMed]
- la Porte, C.; Voduc, N.; Zhang, G.J.; Seguin, I.; Tardiff, D.; Singhal, N.; Cameron, D.W. Steady-State Pharmacokinetics and Tolerability of -Resveratrol 2000 mg Twice Daily with Food, Quercetin and Alcohol (Ethanol) in Healthy Human Subjects. Clin. Pharmacokinet. 2010, 49, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Popat, R.; Plesner, T.; Davies, F.; Cook, G.; Cook, M.; Elliott, P.; Jacobson, E.; Gumbleton, T.; Oakervee, H.; Cavenagh, J. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br. J. Haematol. 2013, 160, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.D.; Chen, K.; Cheng, L.; Yan, B.; Qian, W.K.; Cao, J.Y.; Li, J.; Wu, E.X.; Ma, Q.Y.; Yang, W. Resveratrol and cancer treatment: Updates. Ann. N. Y. Acad. Sci. 2017, 1403, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Bioavailability of resveratrol. Resveratrol Health 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Wang, D.; Nannapaneni, S.; Lamichhane, R.; Chen, Z.G.; Shin, D.M. Combination of resveratrol and green tea epigallocatechin gallate induces synergistic apoptosis and inhibits tumor growth in vivo in head and neck cancer models. Oncol. Rep. 2021, 45, 87. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef]
- Ismail, N.; Abdel, Y.; Ahmed, A.A.E.; El-Maraghy, N.N. Novel combination of thymoquinone and resveratrol enhances anticancer effect on hepatocellular carcinoma cell line. Future J. Pharm. Sci. 2018, 4, 41–46. [Google Scholar] [CrossRef]
- Bhaumik, S.; Anjum, R.; Rangaraj, N.; Pardhasaradhi, B.V.V.; Khar, A. Curcumin mediated apoptosis in AK-5 tumor cells involves the production of reactive oxygen intermediates. FEBS Lett. 1999, 456, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.H.; Wu, H.J.; Hsuuw, Y.D. Curcumin inhibits ROS formation and apoptosis in methylglyoxal-treated human hepatoma G2 cells. Ann. N. Y. Acad. Sci. 2005, 1042, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Liczbinski, P.; Michalowicz, J.; Bukowska, B. Molecular mechanism of curcumin action in signaling pathways: Review of the latest research. Phytother. Res. 2020, 34, 1992–2005. [Google Scholar] [CrossRef] [PubMed]
- El-Far, A.H.; Saddiq, A.A.; Mohamed, S.A.; Almaghrabi, O.A.; Mousa, S.A. Curcumin and Thymoquinone Combination Attenuates Breast Cancer Cell Lines’ Progression. Integr. Cancer Ther. 2022, 21, 15347354221099537. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, A.; Guler, E.M. Curcumin induce DNA damage and apoptosis through generation of reactive oxygen species and reducing mitochondrial membrane potential in melanoma cancer cells. Cell Mol. Biol. 2017, 63, 97–105. [Google Scholar] [CrossRef]
- Liao, W.; Xiang, W.; Wang, F.F.; Wang, R.; Ding, Y. Curcumin inhibited growth of human melanoma A375 cells via inciting oxidative stress. Biomed. Pharmacother. 2017, 95, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Heo, J.Y.; Kim, D.S.; Choi, Y.S.; Kim, S.; Nam, H.S.; Lee, S.H.; Cho, M.K. Curcumin Enhances the Anticancer Effects of Binimetinib on Melanoma Cells by Inducing Mitochondrial Dysfunction and Cell Apoptosis with Necroptosis. Ann. Dermatol. 2023, 35, 217–228. [Google Scholar] [CrossRef]
- Fontes, S.S.; Nogueira, M.L.; Dias, R.B.; Rocha, C.A.G.; Soares, M.B.P.; Vannier-Santos, M.A.; Bezerra, D.P. Combination Therapy of Curcumin and Disulfiram Synergistically Inhibits the Growth of B16-F10 Melanoma Cells by Inducing Oxidative Stress. Biomolecules 2022, 12, 1600. [Google Scholar] [CrossRef] [PubMed]
- Li, P.M.; Li, Y.L.; Liu, B.; Wang, W.J.; Wang, Y.Z.; Li, Z. Curcumin Inhibits MHCC97H Liver Cancer Cells by Activating ROS/TLR-4/Caspase Signaling Pathway. Asian Pac. J. Cancer Prev. 2014, 15, 2329–2334. [Google Scholar] [CrossRef]
- Li, W.F.; Gong, Y.X.; Li, H.F.; Sun, F.L.; Li, W.L.; Chen, D.Q.; Xie, D.P.; Ren, C.X.; Guo, X.Y.; Wang, Z.Y.; et al. Curcumin Activates ROS Signaling to Promote Pyroptosis in Hepatocellular Carcinoma HepG2 Cells. Vivo 2021, 35, 249–257. [Google Scholar] [CrossRef]
- Chen, J.P.; Li, L.; Su, J.Y.; Li, B.; Zhang, X.; Chen, T.F. Proteomic Analysis of G2/M Arrest Triggered by Natural Borneol/Curcumin in HepG2 Cells, the Importance of the Reactive Oxygen Species-p53 Pathway. J. Agric. Food Chem. 2015, 63, 6440–6449. [Google Scholar] [CrossRef]
- Lee, H.M.; Patel, V.; Shyur, L.F.; Lee, W.L. Copper supplementation amplifies the anti-tumor effect of curcumin in oral cancer cells. Phytomedicine 2016, 23, 1535–1544. [Google Scholar] [CrossRef]
- Kim, B.; Kim, H.S.; Jung, E.J.; Lee, J.Y.; Tsang, B.K.; Lim, J.M.; Song, Y.S. Curcumin induces ER stress-mediated apoptosis through selective generation of reactive oxygen species in cervical cancer cells. Mol. Carcinog. 2016, 55, 918–928. [Google Scholar] [CrossRef]
- Kumar, D.; Basu, S.; Parija, L.; Rout, D.; Manna, S.; Dandapat, J.; Debata, P.R. Curcumin and Ellagic acid synergistically induce ROS generation, DNA damage, p53 accumulation and apoptosis in HeLa cervical carcinoma cells. Biomed. Pharmacother. 2016, 81, 31–37. [Google Scholar] [CrossRef]
- Rana, C.; Piplani, H.; Vaish, V.; Nehru, B.; Sanyal, S.N. Downregulation of PI3-K/Akt/PTEN pathway and activation of mitochondrial intrinsic apoptosis by Diclofenac and Curcumin in colon cancer. Mol. Cell Biochem. 2015, 402, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Sankpal, U.T.; Nagaraju, G.P.; Gottipolu, S.R.; Hurtado, M.; Jordan, C.G.; Simecka, J.W.; Shoji, M.; El-Rayes, B.; Basha, R. Combination of Tolfenamic acid and curcumin induces colon cancer cell growth inhibition through modulating specific transcription factors and reactive oxygen species. Oncotarget 2016, 7, 3186–3200. [Google Scholar] [CrossRef]
- Huang, Y.F.; Zhu, D.J.; Chen, X.W.; Chen, Q.K.; Luo, Z.T.; Liu, C.C.; Wang, G.X.; Zhang, W.J.; Liao, N.Z. Curcumin enhances the effects of irinotecan on colorectal cancer cells through the generation of reactive oxygen species and activation of the endoplasmic reticulum stress pathway. Oncotarget 2017, 8, 40264–40275. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Wang, Y.Y.; Xu, K.D.; Lu, G.H.; Ying, Z.; Wu, L.J.; Zhan, J.W.; Fang, R.; Wu, Y.Q.; Zhou, J.Y. Curcumin induces apoptosis in human lung adenocarcinoma A549 cells through a reactive oxygen species-dependent mitochondrial signaling pathway. Oncol. Rep. 2010, 23, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.G.; Duan, X.Y.; Cai, H.; Wang, L.; Li, M.; Qu, J.K.; Li, W.J.; Wang, Y.H.; Wang, J.S. Curcumin inhibits the invasion of lung cancer cells by modulating the PKCα/Nox-2/ROS/ATF-2/MMP-9 signaling pathway. Oncol. Rep. 2015, 34, 691–698. [Google Scholar] [CrossRef]
- Hosseinzadehdehkordi, M.; Adelinik, A.; Tashakor, A. Dual effect of curcumin targets reactive oxygen species, adenosine triphosphate contents and intermediate steps of mitochondria-mediated apoptosis in lung cancer cell lines. Eur. J. Pharmacol. 2015, 769, 203–210. [Google Scholar] [CrossRef]
- Wang, C.J.; Song, X.G.; Shang, M.; Zou, W.; Zhang, M.P.; Wei, H.Y.; Shao, H. Curcumin exerts cytotoxicity dependent on reactive oxygen species accumulation in non-small-cell lung cancer cells. Future Oncol. 2019, 15, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.F.; Huang, Y.H.; Chiu, L.Y.; Cherng, S.H.; Sheu, G.T.; Yang, T.Y. Curcumin Induces Apoptosis of Chemoresistant Lung Cancer Cells via ROS-Regulated p38 MAPK Phosphorylation. Int. J. Mol. Sci. 2022, 23, 8248. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Ma, Y.G.; Xue, Y.X.; Liu, Y.Y.; Xie, H.; Qiu, G.R. Curcumin induces small cell lung cancer NCI-H446 cell apoptosis via the reactive oxygen species-mediated mitochondrial pathway and not the cell death receptor pathway. DNA Cell Biol. 2012, 31, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Wu, S.Y.; Ip, S.W.; Wu, P.P.; Yu, C.S.; Yang, J.S.; Chen, P.Y.; Wu, S.H.; Chung, J.G. Apoptotic death in curcumin-treated NPC-TW 076 human nasopharyngeal carcinoma cells is mediated through the ROS, mitochondrial depolarization and caspase-3-dependent signaling responses. Int. J. Oncol. 2011, 39, 319–328. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, L.; Cheng, X.; Lin, X.F.; Lu, R.R.; Bao, J.D.; Yu, H.X. Curcumin inhibits hypoxia-induced migration in K1 papillary thyroid cancer cells. Exp. Biol. Med. 2015, 240, 925–935. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, X.J.; Xue, W.H.; Zhao, S.F.; Zhang, X.; Pei, J.Y. Curcumin Induced Human Gastric Cancer BGC-823 Cells Apoptosis by ROS-Mediated ASK1-MKK4-JNK Stress Signaling Pathway. Int. J. Mol. Sci. 2014, 15, 15754–15765. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Chen, X.W.; Du, Z.Y.; Li, G.F.; Chen, M.Y.; Chen, X.; Liang, G.; Chen, T.K. Curcumin suppresses gastric tumor cell growth via ROS-mediated DNA polymerase γ depletion disrupting cellular bioenergetics. J. Exp. Clin. Cancer Res. 2017, 36, 47. [Google Scholar] [CrossRef]
- Zou, P.; Zhang, J.R.; Xia, Y.Q.; Kanchana, K.; Guo, G.L.; Chen, W.B.; Huang, Y.; Wang, Z.; Yang, S.L.; Liang, G. ROS generation mediates the anti-cancer effects of WZ35 via activating JNK and ER stress apoptotic pathways in gastric cancer. Oncotarget 2015, 6, 5860–5876. [Google Scholar] [CrossRef]
- Tong, R.Y.; Wu, X.; Liu, Y.; Liu, Y.; Zhou, J.G.; Jiang, X.Y.; Zhang, L.; He, X.Y.; Ma, L.B. Curcumin-Induced DNA Demethylation in Human Gastric Cancer Cells Is Mediated by the DNA-Damage Response Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 2543504. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.Q.; Xing, J.C.; Yu, X.C. Curcumin induces osteosarcoma MG63 cells apoptosis via ROS/Cyto-C/Caspase-3 pathway. Tumor Biol. 2014, 35, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Li, H.Y.; Yan, J.Y.; Liu, Y. Flavonoid compound breviscapine suppresses human osteosarcoma Saos-2 progression property and induces apoptosis by regulating mitochondria-dependent pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22633. [Google Scholar] [CrossRef]
- Sharma, V.; Jha, A.K.; Kumar, A.; Bhatnagar, A.; Narayan, G.; Kaur, J. Curcumin-Mediated Reversal of Gene Promoter Methylation: Implication in Anti-Neoplastic Action against Acute Lymphoid Leukaemia Cell Line. Folia Biol. 2015, 61, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Papiez, M.A.; Krzysciak, W.; Szade, K.; Bukowska-Straková, K.; Kozakowska, M.; Hajduk, K.; Bystrowska, B.; Dulak, J.; Jozkowicz, A. Curcumin enhances the cytogenotoxic effect of etoposide in leukemia cells through induction of reactive oxygen species. Drug Des. Dev. Ther. 2016, 10, 557–570. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakamae, I.; Morimoto, T.; Shima, H.; Shionyu, M.; Fujiki, H.; Yoneda-Kato, N.; Yokoyama, T.; Kanaya, S.; Kakiuchi, K.; Shirai, T.; et al. Curcumin Derivatives Verify the Essentiality of ROS Upregulation in Tumor Suppression. Molecules 2019, 24, 4067. [Google Scholar] [CrossRef]
- Altundag, E.M.; Yilmaz, A.M.; Koçtürk, S.; Taga, Y.; Yalçin, A.S. Synergistic Induction of Apoptosis by Quercetin and Curcumin in Chronic Myeloid Leukemia (K562) Cells. Nutr. Cancer 2018, 70, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Park, B.H.; Lim, J.E.; Jeon, H.G.; Seo, S.I.; Lee, H.M.; Choi, H.Y.; Jeon, S.S.; Jeong, B.C. Curcumin potentiates antitumor activity of cisplatin in bladder cancer cell lines via ROS-mediated activation of ERK1/2. Oncotarget 2016, 7, 63870–63886. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Liu, J.B.; Zhang, L.; Xiao, X.; Li, W. Curcumin inhibits HO-induced invasion and migration of human pancreatic cancer via suppression of the ERK/NF-κB pathway. Oncol. Rep. 2016, 36, 2245–2251. [Google Scholar] [CrossRef]
- Cai, Y.Y.; Lin, W.P.; Li, A.P.; Xu, J.Y. Combined Effects of Curcumin and Triptolide on an Ovarian Cancer Cell Line. Asian Pac. J. Cancer Prev. 2013, 14, 4267–4271. [Google Scholar] [CrossRef] [PubMed]
- Moghtaderi, H.; Sepehri, H.; Attari, F. Combination of arabinogalactan and curcumin induces apoptosis in breast cancer cells in vitro and inhibits tumor growth via overexpression of p53 level in vivo. Biomed. Pharmacother. 2017, 88, 582–594. [Google Scholar] [CrossRef]
- Passos, C.L.A.; Polinati, R.M.; Ferreira, C.; dos Santos, N.A.N.; Lima, D.G.V.; da Silva, J.L.; Fialho, E. Curcumin and melphalan cotreatment induces cell cycle arrest and apoptosis in MDA-MB-231 breast cancer cells. Sci. Rep. 2023, 13, 13446. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Liu, Q.; Yang, L.N.; Zhu, R.R.; Yu, C.Z.; Wang, S.L. Rational Design of Multifunctional Dendritic Mesoporous Silica Nanoparticles to Load Curcumin and Enhance Efficacy for Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2016, 8, 26511–26523. [Google Scholar] [CrossRef]
- Patel, P.B.; Thakkar, V.R.; Patel, J.S. Cellular Effect of Curcumin and Citral Combination on Breast Cancer Cells: Induction of Apoptosis and Cell Cycle Arrest. J. Breast Cancer 2015, 18, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Xu, Y.J.; Zhang, J.F.; Zhang, Y.X.; He, W.; Ju, J.L.; Wu, Y.H.; Wang, Y.L. The effect of resveratrol, curcumin and quercetin combination on immuno-suppression of tumor microenvironment for breast tumor-bearing mice. Sci. Rep. 2023, 13, 13278. [Google Scholar] [CrossRef] [PubMed]
- Kütük, S.G.; Gökçe, G.; Kütük, M.; Cila, H.E.S.G.; Naziroglu, M. Curcumin enhances cisplatin-induced human laryngeal squamous cancer cell death through activation of TRPM2 channel and mitochondrial oxidative stress. Sci. Rep. 2019, 9, 17784. [Google Scholar] [CrossRef]
- Seo, S.U.; Kim, T.H.; Kim, D.E.; Min, K.J.; Kwon, T.K. NOX4-mediated ROS production induces apoptotic cell death via down-regulation of c-FLIP and Mcl-1 expression in combined treatment with thioridazine and curcumin. Redox Biol. 2017, 13, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Gersey, Z.C.; Rodriguez, G.A.; Barbarite, E.; Sanchez, A.; Walters, W.M.; Ohaeto, K.C.; Komotar, R.J.; Graham, R.M. Curcumin decreases malignant characteristics of glioblastoma stem cells via induction of reactive oxygen species. BMC Cancer 2017, 17, 99. [Google Scholar] [CrossRef]
- Sathuvan, M.; Thangam, R.; Gajendiran, M.; Vivek, R.; Balasubramanian, S.; Nagaraj, S.; Gunasekaran, P.; Madhan, B.; Rengasamy, R. kappa-Carrageenan: An effective drug carrier to deliver curcumin in cancer cells and to induce apoptosis. Carbohydr. Polym. 2017, 160, 184–193. [Google Scholar] [CrossRef]
- Sun, M.D.; Zhang, Y.; He, Y.; Xiong, M.H.; Huang, H.Y.; Pei, S.C.; Liao, J.F.; Wang, Y.S.; Shao, D. Green synthesis of carrier-free curcumin nanodrugs for light-activated breast cancer photodynamic therapy. Colloids Surf. B 2019, 180, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.C.; de Matos, R.P.A.; Primo, F.L.; Tedesco, A.C.; Rahal, P.; Calmon, M.F. Effect of curcumin-nanoemulsion associated with photodynamic therapy in breast adenocarcinoma cell line. Bioorg. Med. Chem. 2019, 27, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.Q.; Xing, L.; Cui, P.F.; Qiao, J.B.; He, Y.J.; Chen, B.A.; Jin, L.; Jiang, H.L. Curcumin-coordinated nanoparticles with improved stability for reactive oxygen species-responsive drug delivery in lung cancer therapy. Int. J. Nanomed. 2017, 12, 855–869. [Google Scholar] [CrossRef]
- Han, X.; Deng, S.; Wang, N.; Liu, Y.; Yang, X. Inhibitory effects and molecular mechanisms of tetrahydrocurcumin against human breast cancer MCF-7 cells. Food Nutr. Res. 2016, 60, 30616. [Google Scholar] [CrossRef]
- Yoysungnoen, B.; Bhattarakosol, P.; Changtam, C.; Patumraj, S. Effects of Tetrahydrocurcumin on Tumor Growth and Cellular Signaling in Cervical Cancer Xenografts in Nude Mice. Biomed. Res. Int. 2016, 2016, 391748. [Google Scholar] [CrossRef]
- Song, G.Q.; Lu, H.H.; Chen, F.; Wang, Y.M.; Fan, W.B.; Shao, W.F.; Lu, H.Q.; Lin, B. Tetrahydrocurcumin-induced autophagy via suppression of PI3K/Akt/mTOR in non-small cell lung carcinoma cells. Mol. Med. Rep. 2018, 17, 5964–5969. [Google Scholar] [CrossRef] [PubMed]
- Kalashnikova, I.; Mazar, J.; Neal, C.J.; Rosado, A.L.; Das, S.; Westmoreland, T.J.; Seal, S. Nanoparticle delivery of curcumin induces cellular hypoxia and ROS-mediated apoptosis modulation of Bcl-2/Bax in human neuroblastoma. Nanoscale 2017, 9, 10375–10387. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Bandyopadhyay, A.; Chakraborty, A.; Sarkar, K. Enhancement of anticancer activity and drug delivery of chitosan-curcumin nanoparticle via molecular docking and simulation analysis. Carbohydr. Polym. 2018, 182, 188–198. [Google Scholar] [CrossRef]
- Jaiswal, S.; Mishra, P. Co-delivery of curcumin and serratiopeptidase in HeLa and MCF-7 cells through nanoparticles show improved anti-cancer activity. Mat. Sci. Eng. C Mater. 2018, 92, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.H.; Yu, K.H.; Huang, Y.C.; Lee, C.I. EGFR-targeted photodynamic therapy by curcumin-encapsulated chitosan/TPP nanoparticles. Int. J. Nanomed. 2018, 13, 903–916. [Google Scholar] [CrossRef]
- Kumari, P.; Paul, M.; Bobde, Y.; Soniya, K.; Rompicharla, S.V.K.; Ghosh, B.; Biswas, S. Albumin-based lipoprotein nanoparticles for improved delivery and anticancer activity of curcumin for cancer treatment. Nanomedicine 2020, 15, 2851–2868. [Google Scholar] [CrossRef] [PubMed]
- Mansourizadeh, F.; Alberti, D.; Bitonto, V.; Tripepi, M.; Sepehri, H.; Khoee, S.; Crich, S.G. Efficient synergistic combination effect of Quercetin with Curcumin on breast cancer cell apoptosis through their loading into Apo ferritin cavity. Colloids Surf. B 2020, 191, 110982. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.G.; Jiao, W.Q.; Yang, H.; Liu, J.; Liu, D.H. Phenylboronic acid-conjugated chitosan nanoparticles for high loading and efficient delivery of curcumin. Carbohydr. Polym. 2021, 256, 117497. [Google Scholar] [CrossRef]
- Askar, M.A.; El Shawi, O.E.; Abou Zaid, O.A.R.; Mansour, N.A.; Hanafy, A.M. Breast cancer suppression by curcumin-naringenin-magnetic-nano-particles: In vitro and in vivo studies. Tumour Biol. 2021, 43, 225–247. [Google Scholar] [CrossRef]
- Ghosh, S.; Dutta, S.; Sarkar, A.; Kundu, M.; Sil, P.C. Targeted delivery of curcumin in breast cancer cells via hyaluronic acid modified mesoporous silica nanoparticle to enhance anticancer efficiency. Colloids Surf. B 2021, 197, 111404. [Google Scholar] [CrossRef]
- Zhu, X.J.; Yu, Z.J.; Feng, L.B.; Deng, L.; Fang, Z.B.; Liu, Z.L.; Li, Y.; Wu, X.R.; Qin, L.Y.; Guo, R.; et al. Chitosan-based nanoparticle co-delivery of docetaxel and curcumin ameliorates anti-tumor chemoimmunotherapy in lung cancer. Carbohydr. Polym. 2021, 268, 118237. [Google Scholar] [CrossRef] [PubMed]
- Lotocki, V.; Yazdani, H.; Zhang, Q.; Gran, E.R.; Nyrko, A.; Maysinger, D.; Kakkar, A. Miktoarm Star Polymers with Environment-Selective ROS/GSH Responsive Locations: From Modular Synthesis to Tuned Drug Release through Micellar Partial Corona Shedding and/or Core Disassembly. Macromol. Biosci. 2021, 21, e2000305. [Google Scholar] [CrossRef]
- Zhang, H.Y.; van Os, W.L.; Tian, X.B.; Zu, G.Y.; Ribovski, L.; Bron, R.; Bussmann, J.; Kros, A.; Liu, Y.; Zuhorn, I.S. Development of curcumin-loaded zein nanoparticles for transport across the blood-brain barrier and inhibition of glioblastoma cell growth. Biomater. Sci. 2021, 9, 7092–7103. [Google Scholar] [CrossRef]
- Karimi, F.; Shaabani, E.; Martínez-Rovira, I.; Yousef, I.; Ghahremani, M.H.; Kharrazi, S. Infrared microspectroscopy studies on the protective effect of curcumin coated gold nanoparticles against HO-induced oxidative stress in human neuroblastoma SK-N-SH cells. Analyst 2021, 146, 6902–6916. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.G.; Hu, X.L.; Cai, M.R.; Wang, K.X.; Peng, H.Y.; Bai, J.; Xv, Y.; Fu, T.T.; Dong, X.; Ni, J.; et al. Preparation, Characterization, and In Vitro Release of Curcumin-Loaded IRMOF-10 Nanoparticles and Investigation of Their Pro-Apoptotic Effects on Human Hepatoma HepG2 Cells. Molecules 2022, 27, 3940. [Google Scholar] [CrossRef] [PubMed]
- Ghoreyshi, N.; Ghahremanloo, A.; Javid, H.; Tabrizi, M.H.; Hashemy, S.I. Effect of folic acid-linked chitosan-coated PLGA-based curcumin nanoparticles on the redox system of glioblastoma cancer cells. Phytochem. Anal. 2023, 34, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Bolouri Ebrahimi, S.S.; Jafari, S.; Basiri, A.; Yazdani, J.; Sharifi, S.; Abdolahinia, E.D. Co-Delivery of Cisplatin and Curcumin Using Mesoporous Silica Nanoparticles to Improve their Anticancer Effects. Pharm. Nanotechnol. 2023, 11, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Zhu, X.Y.; Ruan, H.J.; Yang, J.; Wei, W.; Wu, Y.; Zhou, L.Z.; Jiang, H.J.; Ji, M.H.; Chen, J. Curcumin-stabilized silver nanoparticles encapsulated in biocompatible electrospun nanofibrous scaffold for sustained eradication of drug-resistant bacteria. J. Hazard. Mater. 2023, 452, 131290. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Kouhsoltani, M.; Pourreza, K.; Sharifi, S.; Abdolahinia, E.D. Preparation, Characterization, and Evaluation of the Anticancer Effect of Mesoporous Silica Nanoparticles Containing Rutin and Curcumin. Pharm. Nanotechnol. 2024, 12, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.M.; Wang, Y.L.; Su, W.H.; Chen, L.W.; Liu, Y.Z.; Yang, Y.W.; Fei, Y.Y.; Ma, J.; Chen, Y.; Mi, L. Enhancing the photodynamic effect of curcumin through modification with TiO2 nanoparticles and cationic polymers. J. Photochem. Photobiol. B 2024, 252, 112851. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, Z.H.; Shen, H.; Jian, Z.Y.; Li, J.S.; Chen, Y.J.; Shen, Y.; Dai, X.Y. Topically applicated curcumin/gelatin-blended nanofibrous mat inhibits pancreatic adenocarcinoma by increasing ROS production and endoplasmic reticulum stress mediated apoptosis. J. Nanobiotechnol. 2020, 18, 126. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.X.; Chang, Y.Z.; Yang, J.; You, Y.Y.; He, R.; Chen, T.F.; Zhou, C.R. Functionalized halloysite nanotube by chitosan grafting for drug delivery of curcumin to achieve enhanced anticancer efficacy. J. Mater. Chem. B 2016, 4, 2253–2263. [Google Scholar] [CrossRef]
- Nyankson, E.; Awuzah, D.; Tiburu, E.K.; Efavi, J.K.; Agyei-Tuffour, B.; Paemka, L. Curcumin loaded Ag-TiO-halloysite nanotubes platform for combined chemo-photodynamic therapy treatment of cancer cells. RSC Adv. 2022, 12, 33108–33123. [Google Scholar] [CrossRef]
- Mahmud, M.; Piwoni, A.; Filiczak, N.; Janicka, M.; Gubernator, J. Long-Circulating Curcumin-Loaded Liposome Formulations with High Incorporation Efficiency, Stability and Anticancer Activity towards Pancreatic Adenocarcinoma Cell Lines. PLoS ONE 2016, 11, e0167787. [Google Scholar] [CrossRef] [PubMed]
- Sesarman, A.; Tefas, L.; Sylvester, B.; Licarete, E.; Rauca, V.; Luput, L.; Patras, L.; Banciu, M.; Porfire, A. Anti-angiogenic and anti-inflammatory effects of long-circulating liposomes co-encapsulating curcumin and doxorubicin on C26 murine colon cancer cells. Pharmacol. Rep. 2018, 70, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Z.; Peng, L.; Liu, A.C.; Ji, J.B.; Zhao, L.X.; Zhai, G.X. The enhanced effect of tetrahydrocurcumin on radiosensitivity of glioma cells. J. Pharm. Pharmacol. 2018, 70, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, B.A.; Reddy, A.S.; Sangubotla, R.; Hong, J.W.; Kim, S. Ruthenium(II)-curcumin liposome nanoparticles: Synthesis, characterization, and their effects against cervical cancer. Colloids Surf. B 2021, 204, 111773. [Google Scholar] [CrossRef]
- Prathyusha, E.; Prabakaran, A.; Ahmed, H.; Dethe, M.R.; Agrawal, M.; Gangipangi, V.; Sudhagar, S.; Krishna, K.V.; Dubey, S.K.; Pemmaraju, D.B.; et al. Investigation of ROS generating capacity of curcumin-loaded liposomes and its cytotoxicity on MCF-7 cell lines using photodynamic therapy. Photodiagnosis Photodyn. Ther. 2022, 40, 103091. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Zhang, J.W.; Yang, L.Y.; Qiao, P.P.; Lu, D.; Yu, Y.F. Curcumin-loaded graphene oxide quantum dots enhance otoprotective effects via blocking cuproptosis. Front. Bioeng. Biotechnol. 2023, 11, 1183197. [Google Scholar] [CrossRef] [PubMed]
- Ellerkamp, V.; Bortel, N.; Schmid, E.; Kirchner, B.; Armeanu-Ebinger, S.; Fuchs, J. Photodynamic Therapy Potentiates the Effects of Curcumin on Pediatric Epithelial Liver Tumor Cells. Anticancer Res. 2016, 36, 3363–3372. [Google Scholar]
- Chen, C.Y.; Yang, W.L.; Kuo, S.Y. Cytotoxic Activity and Cell Cycle Analysis of Hexahydrocurcumin on SW 480 Human Colorectal Cancer Cells. Nat. Prod. Commun. 2011, 6, 1671–1672. [Google Scholar] [CrossRef] [PubMed]
- Srimuangwong, K.; Tocharus, C.; Chintana, P.Y.; Suksamrarn, A.; Tocharus, J. Hexahydrocurcumin enhances inhibitory effect of 5-fluorouracil on HT-29 human colon cancer cells. World J. Gastroenterol. 2012, 18, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Zhang, Z.B.; Lin, G.S.; Luo, D.D.; Chen, H.B.; Yang, H.M.; Liang, J.L.; Liu, Y.H.; Xie, J.H.; Su, Z.R.; et al. Tetrahydrocurcumin is more effective than curcumin in inducing the apoptosis of H22 cells via regulation of a mitochondrial apoptosis pathway in ascites tumor-bearing mice. Food Funct. 2017, 8, 3120–3129. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zou, J.; Yan, L.; Du, W.; Zhang, Y.; Sun, H.; Lu, P.; Geng, S.; Gu, R.; et al. Tetrahydrocurcumin induces mesenchymal-epithelial transition and suppresses angiogenesis by targeting HIF-1alpha and autophagy in human osteosarcoma. Oncotarget 2017, 8, 91134–91149. [Google Scholar] [CrossRef]
- Yoysungnoen, B.; Bhattarakosol, P.; Patumraj, S.; Changtam, C. Effects of Tetrahydrocurcumin on Hypoxia-Inducible Factor-1α and Vascular Endothelial Growth Factor Expression in Cervical Cancer Cell-Induced Angiogenesis in Nude Mice. Biomed. Res. Int. 2015, 2015, 391748. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, P.; Huang, Z.P.; Chen, X.R.; Yan, X.; Zhai, J.Q.; Ma, Y. Evaluation of curcumin-mediated photodynamic therapy on the reverse of multidrug resistance in tumor cells. RSC Adv. 2020, 10, 298–306. [Google Scholar] [CrossRef]
- Yadav, P.; Zhang, C.; Whittaker, A.K.; Kailasam, K.; Shanavas, A. Magnetic and Photocatalytic Curcumin Bound Carbon Nitride Nanohybrids for Enhanced Glioma Cell Death. ACS Biomater. Sci. Eng. 2019, 5, 6590–6601. [Google Scholar] [CrossRef] [PubMed]
- Kuang, G.Z.; Zhang, Q.F.; He, S.S.; Liu, Y. Curcumin-loaded PEGylated mesoporous silica nanoparticles for effective photodynamic therapy. RSC Adv. 2020, 10, 24624–24630. [Google Scholar] [CrossRef] [PubMed]
- Musib, D.; Pal, M.; Raza, M.K.; Roy, M. Photo-physical, theoretical and photo-cytotoxic evaluation of a new class of lanthanide(III)-curcumin/diketone complexes for PDT application. Dalton Trans. 2020, 49, 10786–10798. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Latest Innovations and Nanotechnologies with Curcumin as a Nature-Inspired Photosensitizer Applied in the Photodynamic Therapy of Cancer. Pharmaceutics 2021, 13, 1562. [Google Scholar] [CrossRef] [PubMed]
- Raschpichler, M.; Preis, E.; Pinnapireddy, S.R.; Baghdan, E.; Pourasghar, M.; Schneider, M.; Bakowsky, U. Photodynamic inactivation of circulating tumor cells: An innovative approach against metastatic cancer. Eur. J. Pharm. Biopharm. 2020, 157, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Altube, M.J.; Caputo, E.N.; Rivero, M.N.; Gutiérrez, M.L.; Romero, E.L. Photodynamic Therapy with Nebulized Nanocurcumin on A549 Cells, Model Vessels, Macrophages and Beyond. Pharmaceutics 2022, 14, 2637. [Google Scholar] [CrossRef]
- Magar, T.B.T.; Mallik, S.K.; Gurung, P.; Lim, J.; Kim, Y.T.; Shrestha, R.; Kim, Y.W. Chlorin E6-Curcumin-Mediated Photodynamic Therapy Promotes an Anti-Photoaging Effect in UVB-Irradiated Fibroblasts. Int. J. Mol. Sci. 2023, 24, 13468. [Google Scholar] [CrossRef] [PubMed]
- Alkahtani, S.; AL-Johani, N.S.; Alarifi, S.; Afzal, M. Cytotoxicity Mechanisms of Blue-Light-Activated Curcumin in T98G Cell Line: Inducing Apoptosis through ROS-Dependent Downregulation of MMP Pathways. Int. J. Mol. Sci. 2023, 24, 3842. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Singh, V.; Kushwaha, R.; Dolui, D.; Rai, R.; Dhar, P.; Dutta, A.; Koch, B.; Banerjee, S. Polypyridyl Co-Curcumin Complexes as Photoactivated Anticancer and Antibacterial Agents. Chembiochem 2023, 24, e202300033. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.S.; Park, S.H.; Choi, S.U.; Lee, C.O.; Kim, S.K.; Kim, Y.K.; Kim, S.H.; Ryu, S.Y. Two cytotoxic sesquiterpene lactones from the leaves of and their inhibitory activity on farnesyltransferase. Planta Med. 2003, 69, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Erosa, I.; Huang, Y.G.; Hickie, R.A.; Sutherland, R.G.; Barl, B. Xanthatin and xanthinosin from the burs of Xanthium strumarium L. as potential anticancer agents. Can. J. Physiol. Pharm. 2007, 85, 1160–1172. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Matsuo, K.; Yaji, K.; Okajima-Miyazaki, S.; Harada, M.; Miyoshi, H.; Okamoto, Y.; Amamoto, T.; Shindo, M.; Omiecinski, C.J.; et al. (-)-Xanthatin Selectively Induces GADD45γ and Stimulates Caspase-Independent Cell Death in Human Breast Cancer MDA-MB-231 Cells. Chem. Res. Toxicol. 2011, 24, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Noguchi, M.; Matsuo, K.; Yamaguchi, Y.; Kudo, T.; Nishimura, H.; Okamoto, Y.; Amamoto, T.; Shindo, M.; Omiecinski, C.J.; et al. (-)-Xanthatin up-regulation of the GADD45γ tumor suppressor gene in MDA-MB-231 breast cancer cells: Role of topoisomerase IIα inhibition and reactive oxygen species. Toxicology 2013, 305, 1–9. [Google Scholar] [CrossRef]
- Takeda, S.; Nishimura, H.; Koyachi, K.; Matsumoto, K.; Yoshida, K.; Okamoto, Y.; Amamoto, T.; Shindo, M.; Aramaki, H. (-)-Xanthatin induces the prolonged expression of c-Fos through an -acetyl-L-cysteine (NAC)-sensitive mechanism in human breast cancer MDA-MB-231 cells. J. Toxicol. Sci. 2013, 38, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yu, J.; Pei, C.G.; Li, Y.Y.; Tu, P.; Gao, G.P.; Shao, Y. Xanthatin, a novel potent inhibitor of VEGFR2 signaling, inhibits angiogenesis and tumor growth in breast cancer cells. Int. J. Clin. Exp. Pathol. 2015, 8, 10355–10364. [Google Scholar] [PubMed]
- Zhang, L.; Tao, L.; Ruan, J.S.; Li, W.D.; Wu, Y.; Yan, L.G.; Zhang, F.; Fan, F.T.; Zheng, S.Z.; Wang, A.Y.; et al. Xanthatin Induces G2/M Cell Cycle Arrest and Apoptosis in Human Gastric Carcinoma MKN-45 Cells. Planta Med. 2012, 78, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ruan, J.S.; Yan, L.G.; Li, W.D.; Wu, Y.; Tao, L.; Zhang, F.; Zheng, S.Z.; Wang, A.Y.; Lu, Y. Xanthatin Induces Cell Cycle Arrest at G2/M Checkpoint and Apoptosis via Disrupting NF-κB Pathway in A549 Non-Small-Cell Lung Cancer Cells. Molecules 2012, 17, 3736–3750. [Google Scholar] [CrossRef]
- Tao, L.; Cao, Y.Z.; Wei, Z.H.; Jia, Q.; Yu, S.Y.; Zhong, J.Q.; Wang, A.Y.; Woodgett, J.R.; Lu, Y. Xanthatin triggers Chk1-mediated DNA damage response and destabilizes Cdc25C lysosomal degradation in lung cancer cells. Toxicol. Appl. Pharm. 2017, 337, 85–94. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.; Guan, H.M.; Zhou, J.Y.; Gao, Y. Xanthatin synergizes with cisplatin to suppress homologous recombination through JAK2/STAT4/BARD1 axis in human NSCLC cells. J. Cell Mol. Med. 2021, 25, 1688–1699. [Google Scholar] [CrossRef]
- Xie, Y.B.; Zhu, X.Y.; Liu, P.; Liu, Y.X.; Geng, Y.D.; Zhang, L. Xanthatin inhibits non-small cell lung cancer proliferation by breaking the redox balance. Drug Dev. Res. 2022, 83, 1176–1189. [Google Scholar] [CrossRef] [PubMed]
- Li, W.D.; Wu, Y.; Zhang, L.; Yan, L.G.; Yin, F.Z.; Ruan, J.S.; Chen, Z.P.; Yang, G.M.; Yan, C.P.; Zhao, D.; et al. Characterization of xanthatin: Anticancer properties and mechanisms of inhibited murine melanoma and. Phytomedicine 2013, 20, 865–873. [Google Scholar] [CrossRef]
- Geng, Y.D.; Zhang, L.; Wang, G.Y.; Feng, X.J.; Chen, Z.L.; Jiang, L.; Shen, A.Z. Xanthatin mediates G/M cell cycle arrest, autophagy and apoptosis via ROS/XIAP signaling in human colon cancer cells. Nat. Prod. Res. 2020, 34, 2616–2620. [Google Scholar] [CrossRef]
- Piloto-Ferrer, J.; Sánchez-Lamar, A.; Francisco, M.; González, M.L.; Merino, N.; Aparicio, G.; Pérez, C.; Rodeiro, I.; Lopes, M.T.P. xanthatins induces mitotic arrest and apoptosis in CT26WT colon carcinoma cells. Phytomedicine 2019, 57, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Liu, P.; Xie, Y.B.; Liu, Y.X.; Chen, Z.L.; Geng, Y.D.; Zhang, L. Xanthatin inhibits human colon cancer cells progression via mTOR signaling mediated energy metabolism alteration. Drug Dev. Res. 2022, 83, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.D.; Li, L.L.; Liu, P.; Chen, Z.L.; Shen, A.Z.; Zhang, L. TMT-Based Quantitative Proteomic Analysis Identified Proteins and Signaling Pathways Involved in the Response to Xanthatin Treatment in Human HT-29 Colon Cancer Cells. Anti-Cancer Agents Med. Chem. 2022, 22, 887–896. [Google Scholar] [CrossRef]
- Shi, T.L.; Zhang, L.; Cheng, Q.Y.; Yu, J.S.; Liu, J.; Shen, Y.J.; Feng, X.J.; Shen, Y.X. Xanthatin induces apoptosis by activating endoplasmic reticulum stress in hepatoma cells. Eur. J. Pharmacol. 2019, 843, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.Y.; Zhang, H.; Zhao, L.; Tan, S.; Ren, Q.C.; Wang, L.; Shen, X.F. A new xanthatin analogue 1β-hydroxyl-5α-chloro-8-epi-xanthatin induces apoptosis through ROS-mediated ERK/p38 MAPK activation and JAK2/STAT3 inhibition in human hepatocellular carcinoma. Biochimie 2018, 152, 43–52. [Google Scholar] [CrossRef]
- Fang, X.Y.; Zhang, H.; Zhao, L.; Tan, S.; Ren, Q.C.; Wang, L.; Shen, X.F. Corrigendum to “A new xanthatin analogue 1beta-hydroxyl-5alpha-chloro-8-epi-xanthatin induces apoptosis through ROS-mediated ERK/p38 MAPK activation and JAK2/STAT3 inhibition in human hepatocellular carcinoma” [Biochimie 152C (2018) 43–52]. Biochimie 2020, 171–172, 21–22. [Google Scholar] [CrossRef]
- Geng, Y.D.; Liu, P.; Xie, Y.B.; Liu, Y.X.; Zhang, X.E.; Hou, X.C.; Zhang, L. Xanthatin suppresses pancreatic cancer cell growth via the ROS/RBL1 signaling pathway: In vitro and in vivo insights. Phytomedicine 2023, 119, 155004. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Q.; Zhu, T.; Huang, X.J.; Xu, W.S.; Di, Z.M.; Ma, Y.Y.; Xue, M.; Bi, S.X.; Shen, Y.J.; Yu, Y.Q.; et al. Xanthatin suppresses proliferation and tumorigenicity of glioma cells through autophagy inhibition via activation of the PI3K-Akt-mTOR pathway. Pharmacol. Res. Perspect. 2023, 11, e01041. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Y.; Di, Z.M.; Cao, Q.; Xu, W.S.; Bi, S.X.; Yu, J.S.; Shen, Y.J.; Yu, Y.Q.; Shen, Y.X.; Feng, L.J. Xanthatin induces glioma cell apoptosis and inhibits tumor growth via activating endoplasmic reticulum stress-dependent CHOP pathway. Acta Pharmacol. Sin. 2020, 41, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.Y.; Song, L.L.; Liang, J.; Wei, S.Q.; Li, Y.; Zhang, Y.; Hao, X.J.; Cao, H.; Yang, C. Synthesis and in vitro antifungal activity of new Michael-type amino derivatives of xanthatin, a natural sesquiterpene lactone from Xanthium strumarium L. Bioorg. Med. Chem. Lett. 2022, 55, 128481. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, Y.; Zhang, Y.; Hu, Q.; Liu, Y.; Li, Y.F.; Shi, H.C.; Song, L.L.; Cao, H.; Hao, X.J.; et al. Natural Sesquiterpene Lactone as Source of Discovery of Novel Fungicidal Candidates: Structural Modification and Antifungal Activity Evaluation of Xanthatin Derived from Xanthium strumarium L. J. Agric. Food Chem. 2023, 71, 11239–11251. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, S.W.; Jiang, B.P.; Wang, J.W.; Ge, X.J.; Wu, B.R.; Zhang, S.; Wang, D.S. Sesquiterpene lactones from Patrin alleviate asthma by modulating the Th1/Th2 balance in a murine model. Phytomedicine 2022, 99, 154032. [Google Scholar] [CrossRef]
- Han, J.; Wang, J.W.; Wang, Y.C.; Zhu, Z.Q.; Zhang, S.W.; Wu, B.R.; Meng, M.S.; Zhao, J.N.; Wang, D.S. Sesquiterpene lactones-enriched fractions from Kitag alleviate RA by regulating M1 macrophage polarization NF-κB and MAPK signaling pathway. Front. Pharmacol. 2023, 14, 1104153. [Google Scholar] [CrossRef]
- Crocoll, C.; Asbach, J.; Novak, J.; Gershenzon, J.; Degenhardt, J. Terpene synthases of oregano (Origanum vulgare L.) and their roles in the pathway and regulation of terpene biosynthesis. Plant Mol. Biol. 2010, 73, 587–603. [Google Scholar] [CrossRef]
- Yin, Q.H.; Yan, F.X.; Zu, X.Y.; Wu, Y.H.; Wu, X.P.; Liao, M.C.; Deng, S.W.; Yin, L.L.; Zhuang, Y.Z. Anti-proliferative and pro-apoptotic effect of carvacrol on human hepatocellular carcinoma cell line HepG-2. Cytotechnology 2012, 64, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Avola, R.; Granata, G.; Geraci, C.; Napoli, E.; Graziano, A.C.E.; Cardile, V. Oregano (Origanum vulgare L.) essential oil provides anti-inflammatory activity and facilitates wound healing in a human keratinocytes cell model. Food Chem. Toxicol. 2020, 144, 111586. [Google Scholar] [CrossRef]
- Singh, J.; Luqman, S.; Meena, A. Carvacrol as a Prospective Regulator of Cancer Targets/Signalling Pathways. Curr. Mol. Pharmacol. 2023, 16, 542–558. [Google Scholar] [CrossRef]
- Kim, H.J.; Hong, J.H. Multiplicative Effects of Essential Oils and Other Active Components on Skin Tissue and Skin Cancers. Int. J. Mol. Sci. 2024, 25, 5397. [Google Scholar] [CrossRef]
- Liang, W.Z.; Lu, C.H. Carvacrol-induced [Ca] rise and apoptosis in human glioblastoma cells. Life Sci. 2012, 90, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.Z.; Chou, C.T.; Lu, T.; Chi, C.C.; Tseng, L.L.; Pan, C.C.; Lin, K.L.; Kuo, C.C.; Jan, C.R. The mechanism of carvacrol-evoked [Ca2+]i rises and non-Ca2+-triggered cell death in OC2 human oral cancer cells. Toxicology 2013, 303, 152–161. [Google Scholar] [CrossRef]
- Chiu, K.W.; Chen, H.Y.; Chen, C.L.; Hsieh, C.P.; Huang, Y.F. Attenuation of Endoplasmic Reticulum Stress Enhances Carvacrol-Induced Apoptosis in Osteosarcoma Cell Lines. Life 2023, 13, 744. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Barszczyk, A.; Turlova, E.; Deurloo, M.; Liu, B.; Yang, B.B.; Rutka, J.T.; Feng, Z.P.; Sun, H.S. Inhibition of TRPM7 by carvacrol suppresses glioblastoma cell proliferation, migration and invasion. Oncotarget 2015, 6, 16321–16340. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Li, X.L.; Cao, Y.G.; Qi, H.P.; Li, L.; Zhang, Q.H.; Sun, H.L. Carvacrol inhibits proliferation and induces apoptosis in human colon cancer cells. Anti-Cancer Drug 2015, 26, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wu, J.Y.; Lu, M.H.; Shi, Z.; Na, N.; Di, J.M. Carvacrol Alleviates Prostate Cancer Cell Proliferation, Migration, and Invasion through Regulation of PI3K/Akt and MAPK Signaling Pathways. Oxidative Med. Cell. Longev. 2016, 2016, 1469693. [Google Scholar] [CrossRef]
- Horng, C.T.; Chou, C.T.; Sun, T.K.; Liang, W.Z.; Kuo, C.C.; Wang, J.L.; Shieh, P.; Jan, C.R. Effect of Carvacrol on Ca(2)(+) Movement and Viability in PC3 Human Prostate Cancer Cells. Chin. J. Physiol. 2017, 60, 275–283. [Google Scholar] [CrossRef]
- Khan, F.; Khan, I.; Farooqui, A.; Ansari, I.A. Carvacrol Induces Reactive Oxygen Species (ROS)-mediated Apoptosis Along with Cell Cycle Arrest at G/G in Human Prostate Cancer Cells. Nutr. Cancer 2017, 69, 1075–1087. [Google Scholar] [CrossRef]
- Heidarian, E.; Keloushadi, M. Antiproliferative and Anti-invasion Effects of Carvacrol on PC3 Human Prostate Cancer Cells through Reducing pSTAT3, pAKT, and pERK1/2 Signaling Proteins. Int. J. Prev. Med. 2019, 10, 156. [Google Scholar] [CrossRef]
- Dai, W.; Sun, C.; Huang, S.; Zhou, Q. Carvacrol suppresses proliferation and invasion in human oral squamous cell carcinoma. Onco Targets Ther. 2016, 9, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Moradipour, A.; Dariushnejad, H.; Ahmadizadeh, C.; Lashgarian, H.E. Dietary flavonoid carvacrol triggers the apoptosis of human breast cancer MCF-7 cells via the p53/Bax/Bcl-2 axis. Med. Oncol. 2022, 40, 46. [Google Scholar] [CrossRef]
- Bansal, A.; Saleh-E-In, M.M.; Kar, P.; Roy, A.; Sharma, N.R. Synthesis of Carvacrol Derivatives as Potential New Anticancer Agent against Lung Cancer. Molecules 2022, 27, 4597. [Google Scholar] [CrossRef]
- Kapetana, M.; Banti, C.N.; Papachristodoulou, C.; Psycharis, V.; Raptopoulou, C.P.; Hadjikakou, S.K. Conjugation of triphenylantimony(V) with carvacrol against human breast cancer cells. J. Biol. Inorg. Chem. 2022, 27, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, M.I.; Mercader-Ros, M.T.; Pérez-Garrido, A.; Pérez-Sánchez, H.; Pellicer, J.A.; Lucas-Abellán, C.; Montoro-García, S.; Yáñez-Gascón, M.J.; Gil-Izquierdo, A.; Núñez-Delicado, E.; et al. Carvacrol and HP-β-Cyclodextrin Complexes: Extensive Characterization and Potential Cytotoxic Effect in Human Colorectal Carcinoma Cells. Pharmaceutics 2022, 14, 2638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Zhao, J.; Chinnathambi, A.; Meganathan, V.; Gu, X.Z. Anti-cancer potential of selenium-chitosan-polyethylene glycol-carvacrol nanocomposites in multiple myeloma U266 cells. J. Biochem. Mol. Toxicol. 2023, 37, e23424. [Google Scholar] [CrossRef] [PubMed]
- Akhlaq, A.; Ashraf, M.; Omer, M.O.; Altaf, I. Carvacrol-Fabricated Chitosan Nanoparticle Synergistic Potential with Topoisomerase Inhibitors on Breast and Cervical Cancer Cells. ACS Omega 2023, 8, 31826–31838. [Google Scholar] [CrossRef] [PubMed]
- Potocnjak, I.; Gobin, I.; Domitrovic, R. Carvacrol induces cytotoxicity in human cervical cancer cells but causes cisplatin resistance: Involvement of MEK-ERK activation. Phytother. Res. 2018, 32, 1090–1097. [Google Scholar] [CrossRef]
- Mukaiyama, M.; Usui, T.; Nagumo, Y. Non-electrophilic TRPA1 agonists, menthol, carvacrol and clotrimazole, open epithelial tight junctions via TRPA1 activation. J. Biochem. 2020, 168, 407–415. [Google Scholar] [CrossRef]
- Maglie, R.; Souza Monteiro de Araujo, D.; Antiga, E.; Geppetti, P.; Nassini, R.; De Logu, F. The Role of TRPA1 in Skin Physiology and Pathology. Int. J. Mol. Sci. 2021, 22, 3065. [Google Scholar] [CrossRef]
- Wang, Y.J.; Li, H.; Xue, C.; Chen, H.; Xue, Y.N.; Zhao, F.; Cao, Z.Y.; Zhu, M.X. TRPV3 enhances skin keratinocyte proliferation through EGFR-dependent signaling pathways. Cell Biol. Toxicol. 2021, 37, 313–330. [Google Scholar] [CrossRef]
- Timmermans, P.B.M.W.M. Angiotensin II receptor antagonists: An emerging new class of cardiovascular therapeutics. Hypertens. Res.-Clin. E 1999, 22, 147–153. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, Q.; Xie, Y.Q.; Bai, G.; Liu, H.W.; Chen, Q.; Duan, H.J.; Wang, L.S.; Xu, H.; Sun, Y.X.; et al. Telmisartan loading thermosensitive hydrogel repairs gut epithelial barrier for alleviating inflammatory bowel disease. Colloids Surf. B 2024, 236, 113799. [Google Scholar] [CrossRef] [PubMed]
- Li, X.R.; Lan, Y.H.; Wang, Y.; Nie, M.H.; Lu, Y.H.; Zhao, E.Y. Telmisartan suppresses cardiac hypertrophy by inhibiting cardiomyocyte apoptosis via the NFAT/ANP/BNP signaling pathway. Mol. Med. Rep. 2017, 15, 2574–2582. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Xu, T.T.; Zhang, S.J.; Cai, Y.; Min, S.D.; Zhao, Z.; Lu, C.Q.; Wang, Y.C.; Ju, S.H. Telmisartan ameliorates cardiac fibrosis and diastolic function in cardiorenal heart failure with preserved ejection fraction. Exp. Biol. Med. 2021, 246, 2511–2521. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.X.; Wei, B.; Cao, H.M.; Duan, R.; Deng, Y.; Lian, H.W.; Zhang, Y.D.; Jiang, T. Telmisartan Alleviates Alzheimer’s Disease-Related Neuropathologies and Cognitive Impairments. J. Alzheimers Dis. 2023, 94, 919–933. [Google Scholar] [CrossRef]
- Oura, K.; Tadokoro, T.; Fujihara, S.; Morishita, A.; Chiyo, T.; Samukawa, E.; Yamana, Y.; Fujita, K.; Sakamoto, T.; Nomura, T.; et al. Telmisartan inhibits hepatocellular carcinoma cell proliferation in vitro by inducing cell cycle arrest. Oncol. Rep. 2017, 38, 2825–2835. [Google Scholar] [CrossRef]
- Kumar, U.; Aich, J.; Devarajan, S. Exploring the repurposing potential of telmisartan drug in breast cancer: An in-silico and in-vitro approach. Anticancer Drugs 2023, 34, 1094–1103. [Google Scholar] [CrossRef]
- Fang, T.X.; Zhang, J.; Zuo, T.T.; Wu, G.Y.; Xu, Y.X.; Yang, Y.F.; Yang, J.; Shen, Q. Chemo-Photothermal Combination Cancer Therapy with ROS Scavenging, Extracellular Matrix Depletion, and Tumor Immune Activation by Telmisartan and Diselenide-Paclitaxel Prodrug Loaded Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 31292–31308. [Google Scholar] [CrossRef] [PubMed]
- Martínez, V.R.; Aguirre, M.V.; Todaro, J.S.; Ferrer, E.G.; Williams, P.A.M. Improvement of the Anticancer Activities of Telmisartan by Zn(II) Complexation and Mechanisms of Action. Biol. Trace Elem. Res. 2020, 197, 454–463. [Google Scholar] [CrossRef]
- Rasheduzzaman, M.; Moon, J.H.; Lee, J.H.; Nazim, U.M.; Park, S.Y. Telmisartan generates ROS-dependent upregulation of death receptor 5 to sensitize TRAIL in lung cancer via inhibition of autophagy flux. Int. J. Biochem. Cell B 2018, 102, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Ihara, M.; Saito, S. Drug Repositioning for Alzheimer’s Disease: Finding Hidden Clues in Old Drugs. J. Alzheimers Dis. 2020, 74, 1013–1028. [Google Scholar] [CrossRef]
- Chatterjee, B.; Thakur, S.S. ACE2 as a potential therapeutic target for pandemic COVID-19. RSC Adv. 2020, 10, 39808–39813. [Google Scholar] [CrossRef] [PubMed]
- Imenshahidi, M.; Roohbakhsh, A.; Hosseinzadeh, H. Effects of telmisartan on metabolic syndrome components: A comprehensive review. Biomed. Pharmacother. 2024, 171, 116169. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.; McDougal, A.; Wang, F.; Safe, S. Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis 1998, 19, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Juge, N.; Mithen, R.F.; Traka, M. Molecular basis for chemoprevention by sulforaphane: A comprehensive review. Cell Mol. Life Sci. 2007, 64, 1105–1127. [Google Scholar] [CrossRef]
- Tomczyk, J.; Olejnik, A. Sulforaphane—A possible agent in prevention and therapy of cancer. Postep. Hig Med Dosw (Online) 2010, 64, 590–603. [Google Scholar]
- Otoo, R.A.; Allen, A.R. Sulforaphane’s Multifaceted Potential: From Neuroprotection to Anticancer Action. Molecules 2023, 28, 6902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, Q.; Li, N.; Xu, M.; Miyamoto, T.; Liu, J. Sulforaphane suppresses metastasis of triple-negative breast cancer cells by targeting the RAF/MEK/ERK pathway. npj Breast Cancer 2022, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Bernkopf, D.B.; Daum, G.; Bruckner, M.; Behrens, J. Sulforaphane inhibits growth and blocks Wnt/beta-catenin signaling of colorectal cancer cells. Oncotarget 2018, 9, 33982–33994. [Google Scholar] [CrossRef]
- Liu, F.; Lv, R.B.; Liu, Y.; Hao, Q.; Liu, S.J.; Zheng, Y.Y.; Li, C.; Zhu, C.; Wang, M. Salinomycin and Sulforaphane Exerted Synergistic Antiproliferative and Proapoptotic Effects on Colorectal Cancer Cells by Inhibiting the PI3K/Akt Signaling Pathway in vitro and in vivo. Onco Targets Ther. 2020, 13, 4957–4969. [Google Scholar] [CrossRef]
- Myzak, M.C.; Karplus, P.A.; Chung, F.L.; Dashwood, R.H. A novel mechanism of chemoprotection by sulforaphane: Inhibition of histone deacetylase. Cancer Res. 2004, 64, 5767–5774. [Google Scholar] [CrossRef]
- Nian, H.; Delage, B.; Ho, E.; Dashwood, R.H. Modulation of histone deacetylase activity by dietary isothiocyanates and allyl sulfides: Studies with sulforaphane and garlic organosulfur compounds. Environ. Mol. Mutagen. 2009, 50, 213–221. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).