The Liver-Protective Effects of the Essential Oil from Amomum villosum in Tilapia (Oreochromis niloticus): Antioxidant, Transcriptomic, and Metabolomic Modulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Histopathology Analysis
2.3. Analysis of Antioxidant Capacity and Inflammatory Indicators
2.4. Transcriptome Analysis
2.5. Analysis of Hepatobiliary Disease Key Target Genes and Molecular Docking of EOA
2.6. Metabolomic Analysis
3. Results
3.1. Liver Histopathology Alterations by Dietary EOA
3.2. Analysis of Antioxidant Capacity and Inflammatory Indicators
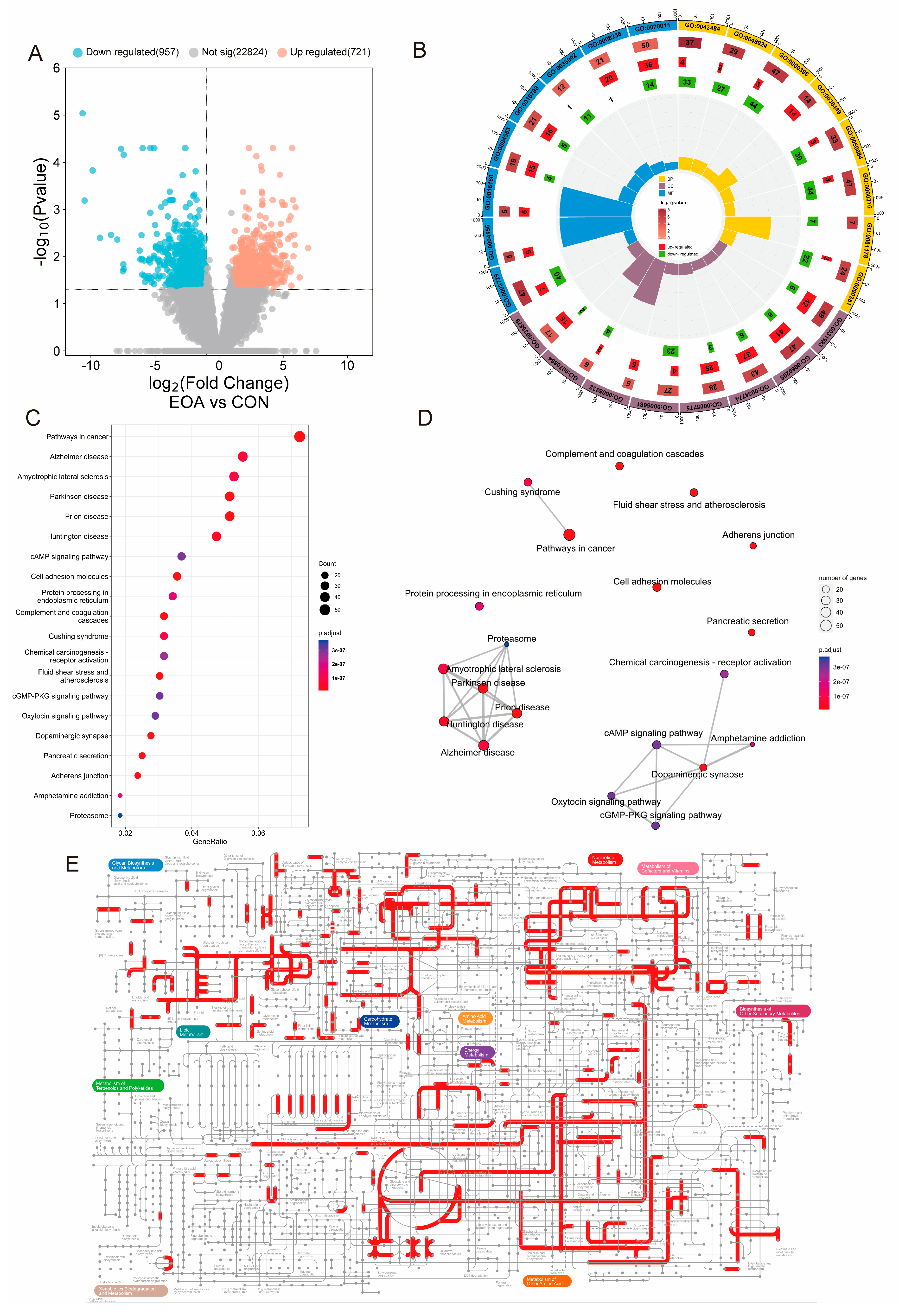
3.3. Transcriptome Analysis
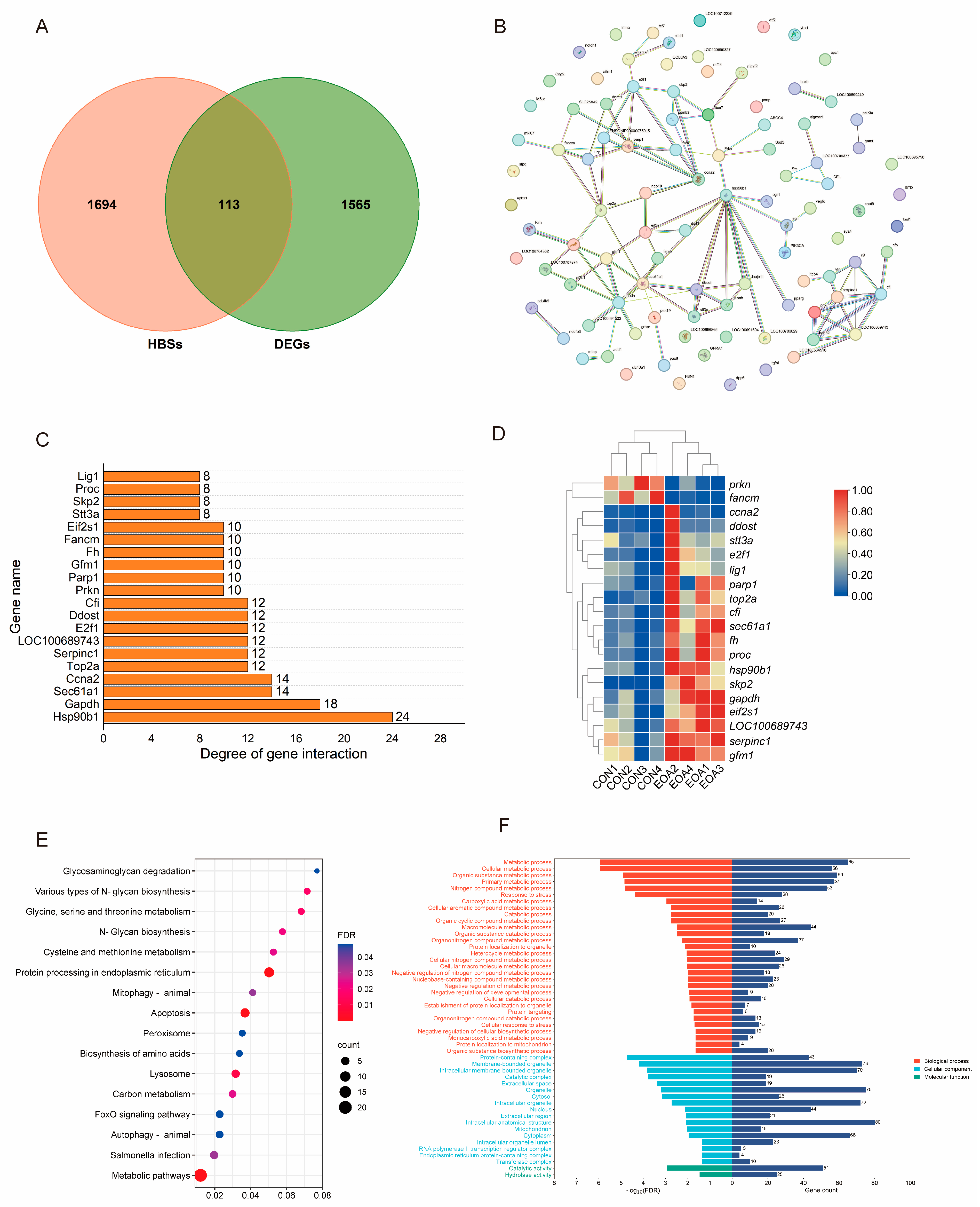
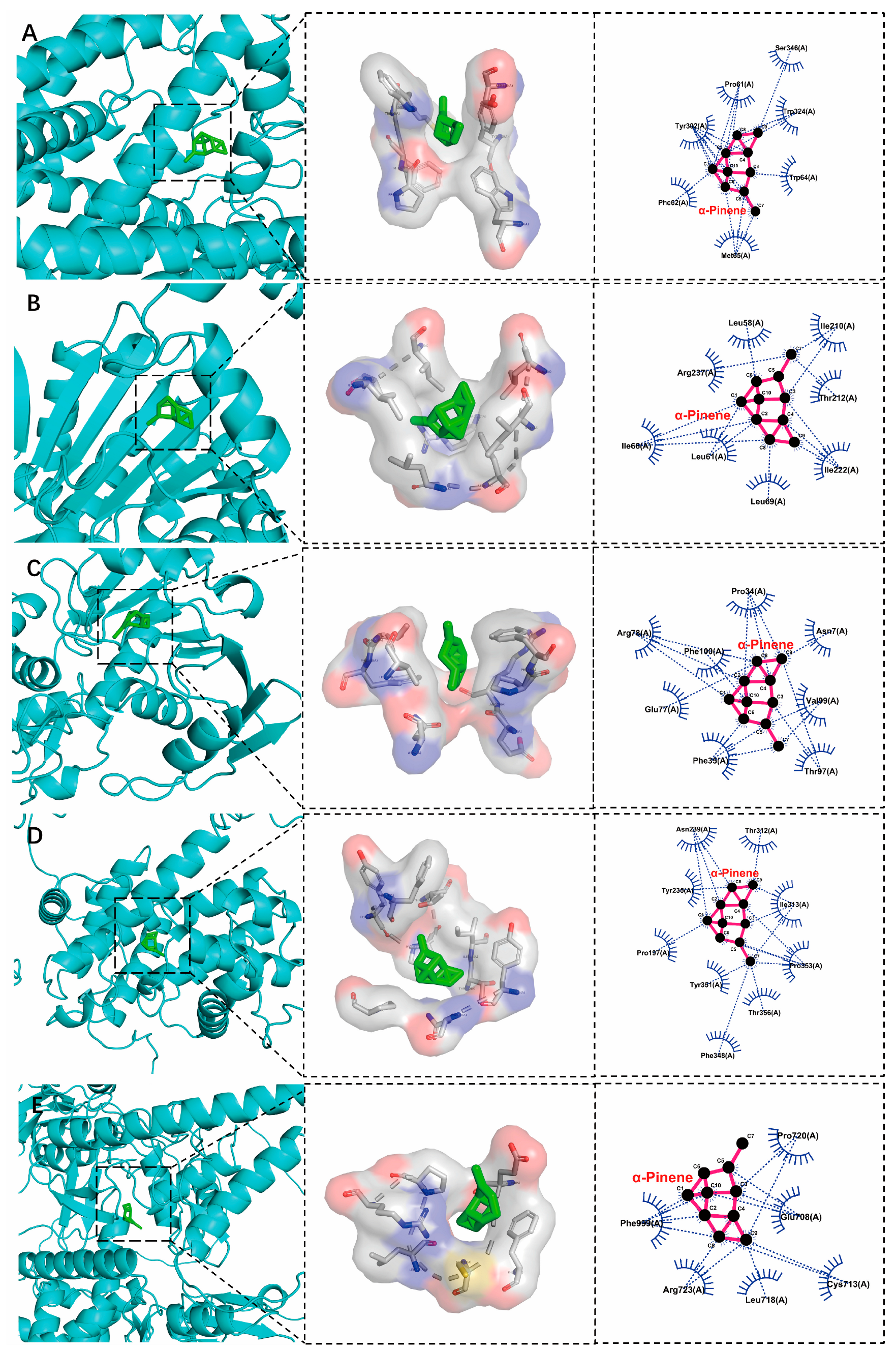
3.4. Analysis of Hepatobiliary Disease Key Target Genes and Molecular Docking of EOA
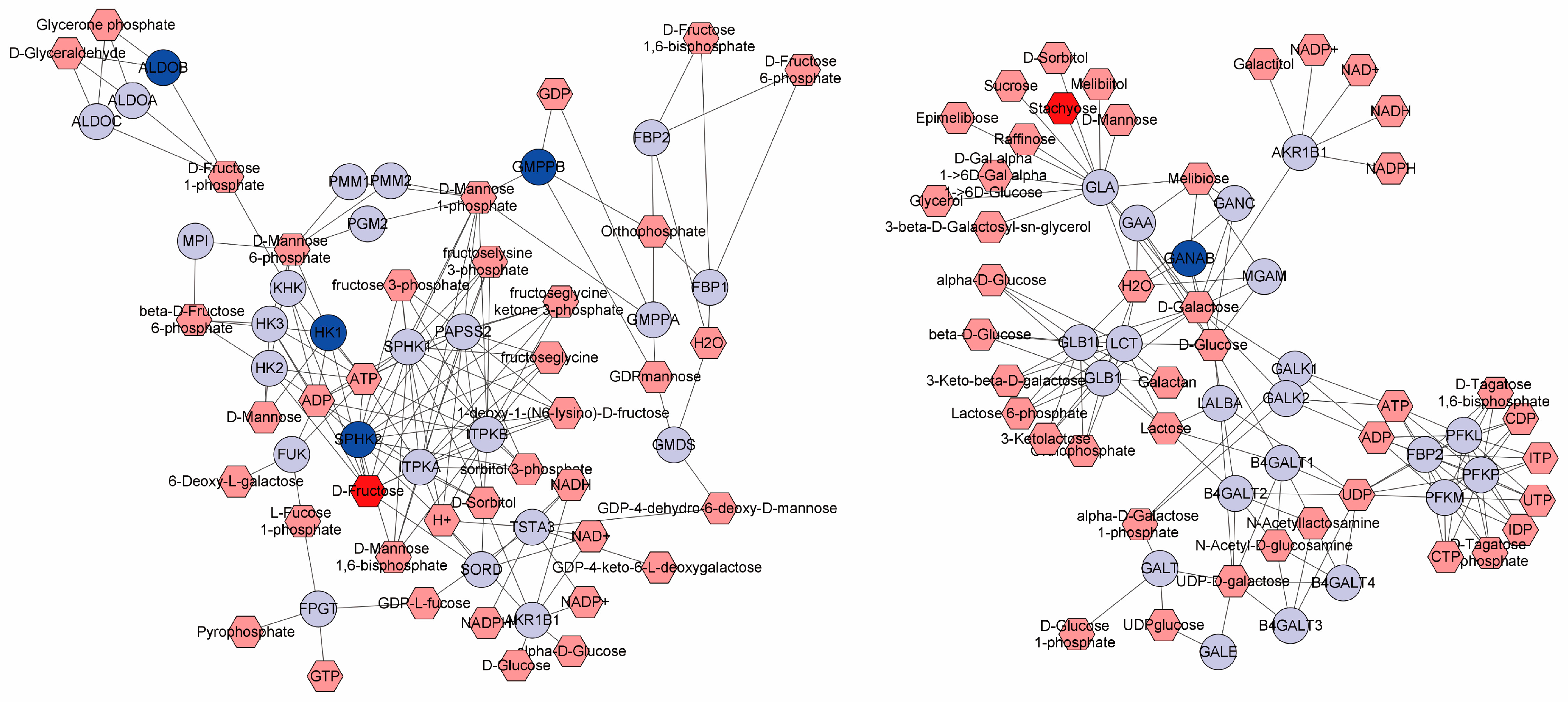
3.5. Metabolomic Alterations by Dietary EOA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, F.; Zhong, H.; Wu, C.; Wang, S.; Guo, Z.; Tao, M.; Zhang, C.; Gong, D.; Gao, X.; Tang, C.; et al. Development of fisheries in China. Reprod. Breed. 2021, 1, 64–79. [Google Scholar] [CrossRef]
- Dong, S.; Dong, Y.; Cao, L.; Verreth, J.; Olsen, Y.; Liu, W.; Fang, Q.; Zhou, Y.; Li, L.; Li, J.; et al. Optimization of aquaculture sustainability through ecological intensification in China. Rev. Aquac. 2022, 14, 1249–1259. [Google Scholar] [CrossRef]
- Chen, J.; Wu, S.; Wu, R.; Ai, H.; Lu, X.; Wang, J.; Luo, Y.; Li, L.; Cao, J. Essential oil from Artemisia argyi alleviated liver disease in zebrafish (Danio rerio) via the gut-liver axis. Fish Shellfish. Immunol. 2023, 140, 108962. [Google Scholar] [CrossRef]
- Kong, Y.; Li, M.; Xia, C.; Liu, X.; Wang, G. A novel model construction of lithocholic acid-induced cholestasis and transcriptome analysis in snakehead fish (Channa argus). Aquaculture 2021, 543, 737014. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, F.; Yao, F.; Ma, R.; Zhang, Y.; Mao, S.; Hu, B.; Ma, G.; Zhu, Y. Study of dietary curcumin on the restorative effect of liver injury induced by carbon tetrachloride in common carp, Cyprinus carpio. Aquac. Rep. 2021, 21, 100825. [Google Scholar] [CrossRef]
- El-Sayed, A.M.; Fitzsimmons, K. From Africa to the world—The journey of Nile tilapia. Rev. Aquac. 2023, 15, 6–21. [Google Scholar] [CrossRef]
- Prabu, E.; Rajagopalsamy, C.; Ahilan, B.; Jeevagan, I.; Renuhadevi, M. Tilapia—An excellent candidate species for world aquaculture: A review. Annu. Res. Rev. Biol. 2019, 31, 1–14. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, Y.; Dai, Y.; Gong, Y. Economic profitability of tilapia farming in China. Aquac. Int. 2017, 25, 1253–1264. [Google Scholar] [CrossRef]
- Kiron, V.; Park, Y.; Siriyappagouder, P.; Dahle, D.; Vasanth, G.K.; Dias, J.; Fernandes, J.M.O.; Sørensen, M.; Trichet, V.V. In-testinal Transcriptome Analysis Reveals Soy Derivative-Linked Changes in Atlantic Salmon. Front. Immunol. 2020, 11, 596514. [Google Scholar] [CrossRef]
- Xie, J.; Li, M.; Ye, W.; Shan, J.; Zhao, X.; Duan, Y.; Liu, Y.; Unger, B.H.; Cheng, Y.; Zhang, W.; et al. Sinomenine Hydrochloride Ameliorates Fish Foodborne Enteritis via α7nAchR-Mediated Anti-Inflammatory Effect Whilst Altering Microbiota Composition. Front. Immunol. 2021, 12, 766845. [Google Scholar] [CrossRef]
- Ao, H.; Wang, J.; Chen, L.; Li, S.; Dai, C. Comparison of Volatile Oil between the Fruits of Amomum villosum Lour. and Amomum villosum Lour. var. xanthioides T. L. Wu et Senjen Based on GC-MS and Chemometric Techniques. Molecules 2019, 24, 1663. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, X.; Wan, M.; Chen, J.; Li, S.; Cao, M.; Zhang, D. Effect of extraction methods on property and bioactivity of water-soluble polysaccharides from Amomum villosum. Carbohydr. Polym. 2015, 117, 632–635. [Google Scholar] [CrossRef]
- Zhang, D.; Li, S.; Xiong, Q.; Jiang, C.; Lai, X. Extraction, characterization and biological activities of polysaccharides from Amomum villosum. Carbohydr. Polym. 2013, 95, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lu, S.H.; Bi, Q.; Liang, L.; Wang, Y.F.; Yang, X.X.; Gu, W.; Yu, J. Volatile Oil from Amomi Fructus Attenuates 5-Fluorouracil-Induced Intestinal Mucositis. Front. Pharmacol. 2017, 8, 786. [Google Scholar] [CrossRef] [PubMed]
- Chenghui, L.; Hongbiao, D.; Xiaoting, Z.; Fukun, G.; Xiangbing, Z.; Junchao, M.; Fei, C.; Jian, C.; Jiasong, Z. Effects of Amomum villosum essential oil on growth, digestion, intestinal antioxidant capacity and serum biochemical indexes of juvenile tilapia (Oreochromis niloticus). South China Fish. Sci. 2023, 19, 51–59. [Google Scholar]
- Pang, A.; Wang, T.; Xie, R.; Wang, Z.; Xin, Y.; Tan, B.; Zhang, W. Effects of soybean meal on immunity and transcriptomics of liver in pearl gentian grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). Aquac. Rep. 2024, 35, 101969. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Zhang, Z.; Tang, Y.; He, Y.; Mai, K.; Ai, Q. Effects of Dietary Fishmeal Replacement with Soybean Meal on Growth Performance, Digestion, Hepatic Metabolism, Antioxidant Capacity, and Innate Immunity of Juvenile Large Yellow Croaker (Larimichthys crocea). Aquac. Res. 2023, 2023, e8842781. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Mozanzadeh, M.T.; Marammazi, J.G.; Safari, O.; Gisbert, E. Dietary replacement of fish meal by soy products (soybean meal and isolated soy protein) in silvery-black porgy juveniles (Sparidentex hasta). Aquaculture 2016, 464, 50–59. [Google Scholar] [CrossRef]
- Wang, W.; Dong, H.; Sun, Y.; Cao, M.; Duan, Y.; Li, H.; Liu, Q.; Gu, Q.; Zhang, J. The efficacy of eugenol and tricaine methanesulphonate as anaesthetics for juvenile Chinese sea bass (Lateolabrax maculatus) during simulated transport. J. Appl. Ichthyol. 2019, 35, 551–557. [Google Scholar] [CrossRef]
- Zeng, X.; Dong, H.; Wu, J.; Wang, W.; Duan, Y.; Chen, J.; Zhang, J. Essential oil of Magnolia denudata is an effective anesthetic for spotted seabass (Lateolabrax maculatus): A test of its effect on blood biochemistry, physiology, and gill morphology. Fish Physiol. Biochem. 2022, 48, 1349–1363. [Google Scholar] [CrossRef]
- Zhang, T.; Yan, Z.; Zheng, X.; Wang, S.; Fan, J.; Liu, Z. Effects of acute ammonia toxicity on oxidative stress, DNA damage and apoptosis in digestive gland and gill of Asian clam (Corbicula fluminea). Fish Shellfish. Immunol. 2020, 99, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, T.; Gu, W.; Yang, X.; Lu, J.; Zhao, R.; Yu, J. Volatile Oil of Amomum villosum Inhibits Nonalcoholic Fatty Liver Disease via the Gut-Liver Axis. BioMed Res. Int. 2018, 2018, e3589874. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, W.; Shen, X.; Li, J.; Yang, S.; Chen, S.; He, Z.; Zhou, R.; Shi, S. Identification and characterization of evolutionarily conserved alternative splicing events in a mangrove genus Sonneratia. Sci. Rep. 2018, 8, 4425. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, O.; Convertini, P.; Zhang, Z.; Wen, Y.; Shen, M.; Falaleeva, M.; Stamm, S. Function of alternative splicing. Gene 2013, 514, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Wang, H.; Wang, M.; Zhang, Y.; Zhang, Z.; Lin, Z. Comparative transcriptomics and network pharmacology analysis to identify the potential mechanism of celastrol against osteoarthritis. Clin. Rheumatol. 2021, 40, 4259–4268. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.-L.; Wang, W.; Wang, G. Recent updates on bioactive properties of α-terpineol. J. Essent. Oil Res. 2023, 35, 274–288. [Google Scholar] [CrossRef]
- Santos, E.S.; Abrantes Coelho, G.L.; Saraiva Fontes Loula, Y.K.; Saraiva Landim, B.L.; Fernandes Lima, C.N.; Tavares de Sousa Machado, S.; Pereira Lopes, M.J.; Soares Gomes, A.D.; Martins da Costa, J.G.; Alencar de Menezes, I.R.; et al. Hypoglycemic, Hypolipidemic, and Anti-Inflammatory Effects of Beta-Pinene in Diabetic Rats. Evid.-Based Complement. Altern. Med. 2022, 2022, e8173307. [Google Scholar] [CrossRef]
- Allenspach, M.; Valder, C.; Flamm, D.; Grisoni, F.; Steuer, C. Verification of Chromatographic Profile of Primary Essential Oil of Pinus sylvestris L. Combined with Chemometric Analysis. Molecules 2020, 25, 2973. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Türkez, H.; Aydın, E. In vitro assessment of cytogenetic and oxidative effects of α-pinene. Toxicol. Ind. Health 2013, 32, 168–176. [Google Scholar] [CrossRef]
- Özbek, H.; Yılmaz, B.S. Anti-inflammatory and hypoglycemic activities of alpha-pinene. Acta Pharm. Sci. 2017, 55, 7–14. [Google Scholar] [CrossRef]
- Costa, T.E.; Raghavendra, N.M.; Penido, C. Natural heat shock protein 90 inhibitors in cancer and inflammation. Eur. J. Med. Chem. 2020, 189, 112063. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y. Recent advances in nonalcoholic fatty liver disease metabolomics. Clin. Mol. Hepatol. 2021, 27, 553–559. [Google Scholar] [CrossRef]
- Smith, L.; Villaret-Cazadamont, J.; Claus, S.P.; Canlet, C.; Guillou, H.; Cabaton, N.J.; Ellero-Simatos, S. Important Considerations for Sample Collection in Metabolomics Studies with a Special Focus on Applications to Liver Functions. Metabolites 2020, 10, 104. [Google Scholar] [CrossRef]
- Kertys, M.; Grendar, M.; Kosutova, P.; Mokra, D.; Mokry, J. Plasma based targeted metabolomic analysis reveals alterations of phosphatidylcholines and oxidative stress markers in guinea pig model of allergic asthma. Biochim. Et Biophys. Acta (BBA) - Mol. Basis Dis. 2020, 1866, 165572. [Google Scholar] [CrossRef]
- Buchman, A.; Dubin, M.; Jenden, D.; Moukarzel, A.; Roch, M.; Rice, K.; Gornbein, J.; Ament, M.; Eckhert, C. Lecithin increases plasma free choline and decreases hepatic steatosis in long-term total parenteral nutrition patients. Gastroenterology 1992, 102, 1363–1370. [Google Scholar] [CrossRef]
- Cui, L.; Decker, E.A. Phospholipids in foods: Prooxidants or antioxidants? J. Sci. Food Agric. 2015, 96, 18–31. [Google Scholar] [CrossRef]
- Kostetsky, E.Y.; Velansky, P.V.; Sanina, N.M. Phase transitions of phospholipids as a criterion for assessing the capacity for thermal adaptation in fish. Russ. J. Mar. Biol. 2013, 39, 214–222. [Google Scholar] [CrossRef]
- Xie, S.; Yin, P.; Tian, L.; Liu, Y.; Niu, J. Lipid metabolism and plasma metabolomics of juvenile largemouth bass Micropterus salmoides were affected by dietary oxidized fish oil. Aquaculture 2020, 522, 735158. [Google Scholar] [CrossRef]
- Zhao, T.; Ma, A.; Yang, S.; Huang, Z. Integrated metabolome and transcriptome analyses revealing the effects of thermal stress on lipid metabolism in juvenile turbot Scophthalmus maximus. J. Therm. Biol. 2021, 99, 102937. [Google Scholar] [CrossRef]
- Madiraju, P.; Pande, S.V.; Prentki, M.; Madiraju, S.M. Mitochondrial acetylcarnitine provides acetyl groups for nuclear histone acetylation. Epigenetics 2009, 4, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Nasca, C.; Xenos, D.; Barone, Y.; Caruso, A.; Scaccianoce, S.; Matrisciano, F.; Battaglia, G.; Mathé, A.A.; Pittaluga, A.; Lionetto, L.; et al. L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proc. Natl. Acad. Sci. USA 2013, 110, 4804–4809. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Zeng, X.; Zheng, X.; Li, C.; Ming, J.; Zhang, J. The Liver-Protective Effects of the Essential Oil from Amomum villosum in Tilapia (Oreochromis niloticus): Antioxidant, Transcriptomic, and Metabolomic Modulations. Antioxidants 2024, 13, 1118. https://doi.org/10.3390/antiox13091118
Dong H, Zeng X, Zheng X, Li C, Ming J, Zhang J. The Liver-Protective Effects of the Essential Oil from Amomum villosum in Tilapia (Oreochromis niloticus): Antioxidant, Transcriptomic, and Metabolomic Modulations. Antioxidants. 2024; 13(9):1118. https://doi.org/10.3390/antiox13091118
Chicago/Turabian StyleDong, Hongbiao, Xiangbing Zeng, Xiaoting Zheng, Chenghui Li, Junchao Ming, and Jiasong Zhang. 2024. "The Liver-Protective Effects of the Essential Oil from Amomum villosum in Tilapia (Oreochromis niloticus): Antioxidant, Transcriptomic, and Metabolomic Modulations" Antioxidants 13, no. 9: 1118. https://doi.org/10.3390/antiox13091118
APA StyleDong, H., Zeng, X., Zheng, X., Li, C., Ming, J., & Zhang, J. (2024). The Liver-Protective Effects of the Essential Oil from Amomum villosum in Tilapia (Oreochromis niloticus): Antioxidant, Transcriptomic, and Metabolomic Modulations. Antioxidants, 13(9), 1118. https://doi.org/10.3390/antiox13091118








