Aerobic Exercise Protects against Cardiotoxin-Induced Skeletal Muscle Injury in a DDAH1-Dependent Manner
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Mouse Experiments
2.3. Histopathological Analysis
2.4. Quantitative Real-Time PCR Analysis
2.5. Western Blotting
2.6. Statistical Analysis
3. Results
3.1. Aerobic Exercise Improves CTX-Induced Skeletal Muscle Regeneration
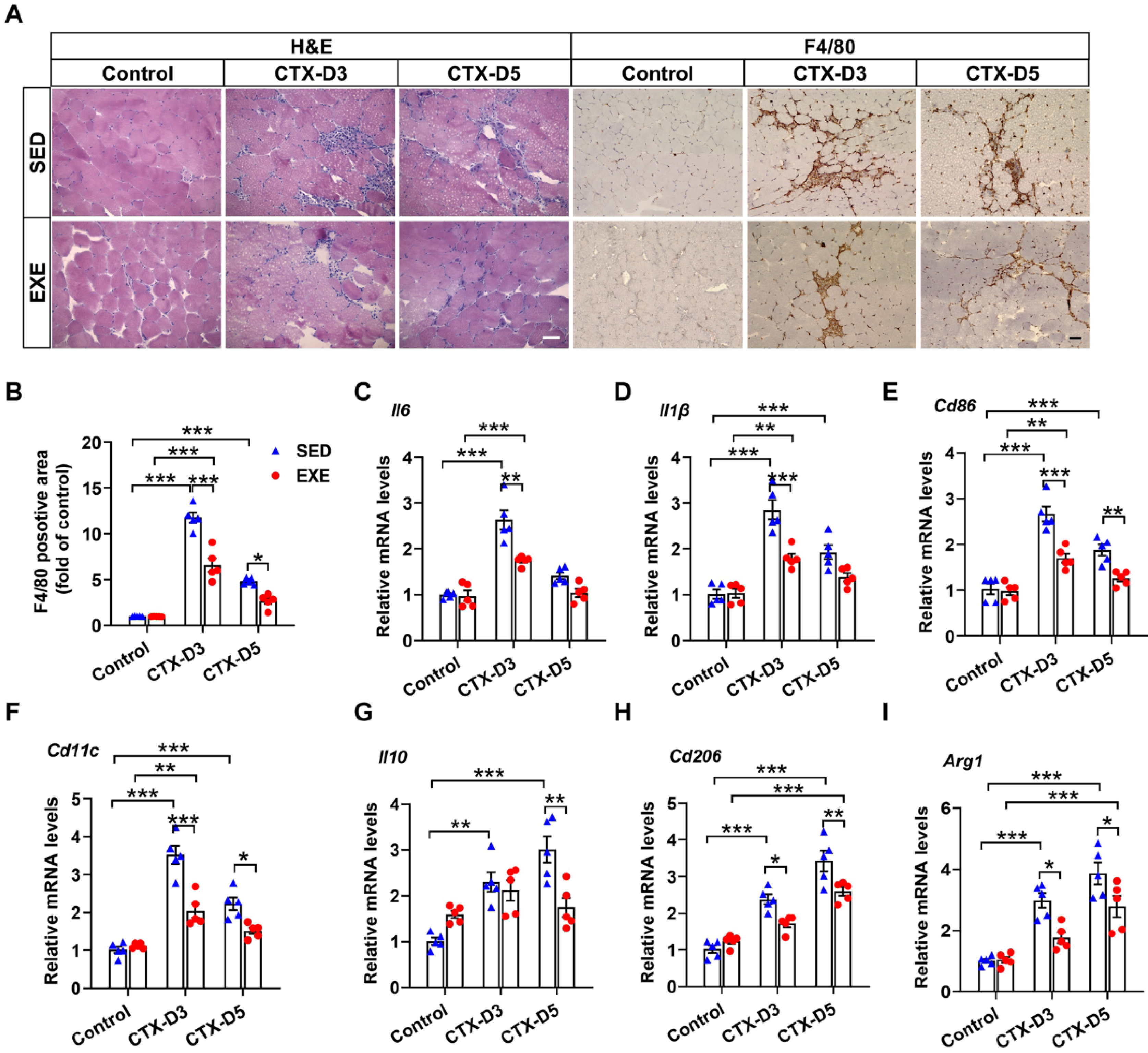
3.2. Aerobic Exercise Attenuated the Inflammatory Response after CTX Treatment
3.3. Aerobic Exercise Reduced the Apoptosis and Oxidative Stress Induced by CTX
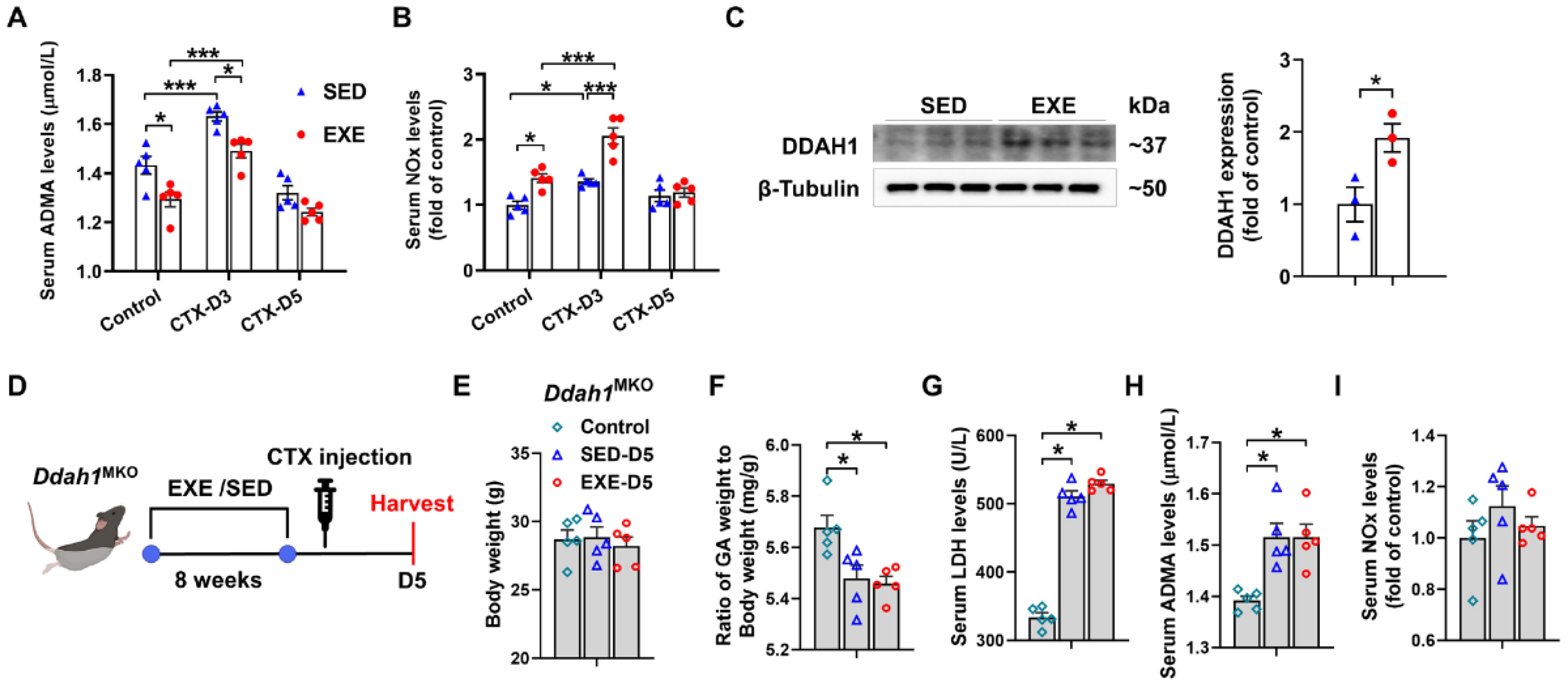
3.4. The Protective Effects of Aerobic Exercise on CTX-Induced Skeletal Muscle Injury Were Diminished in Skeletal Muscle-Specific Ddah1 Knockout Mice
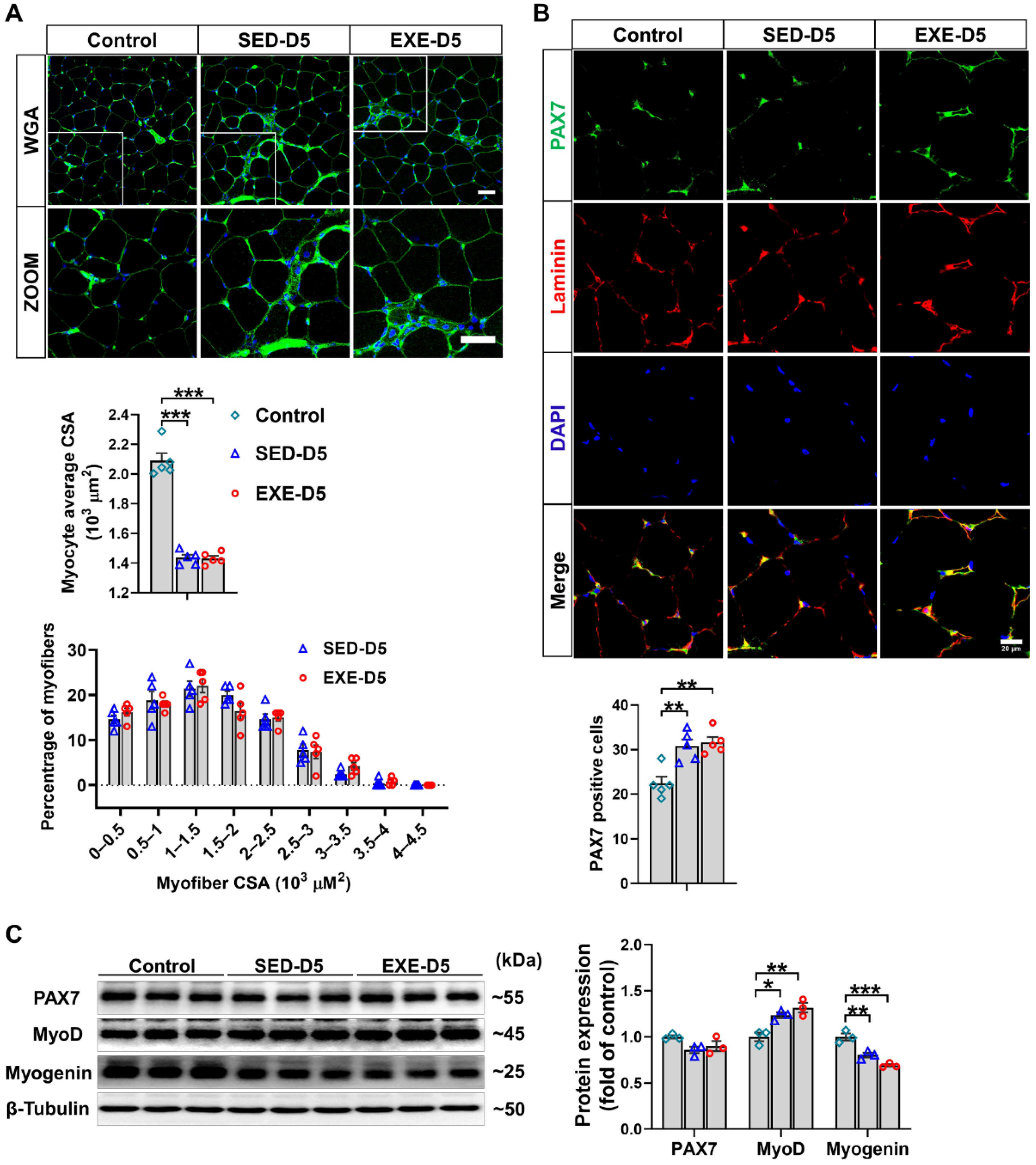
3.5. Aerobic Exercise Had No Obvious Effect on CTX-Induced Skeletal Muscle Regeneration in Ddah1MKO Mice
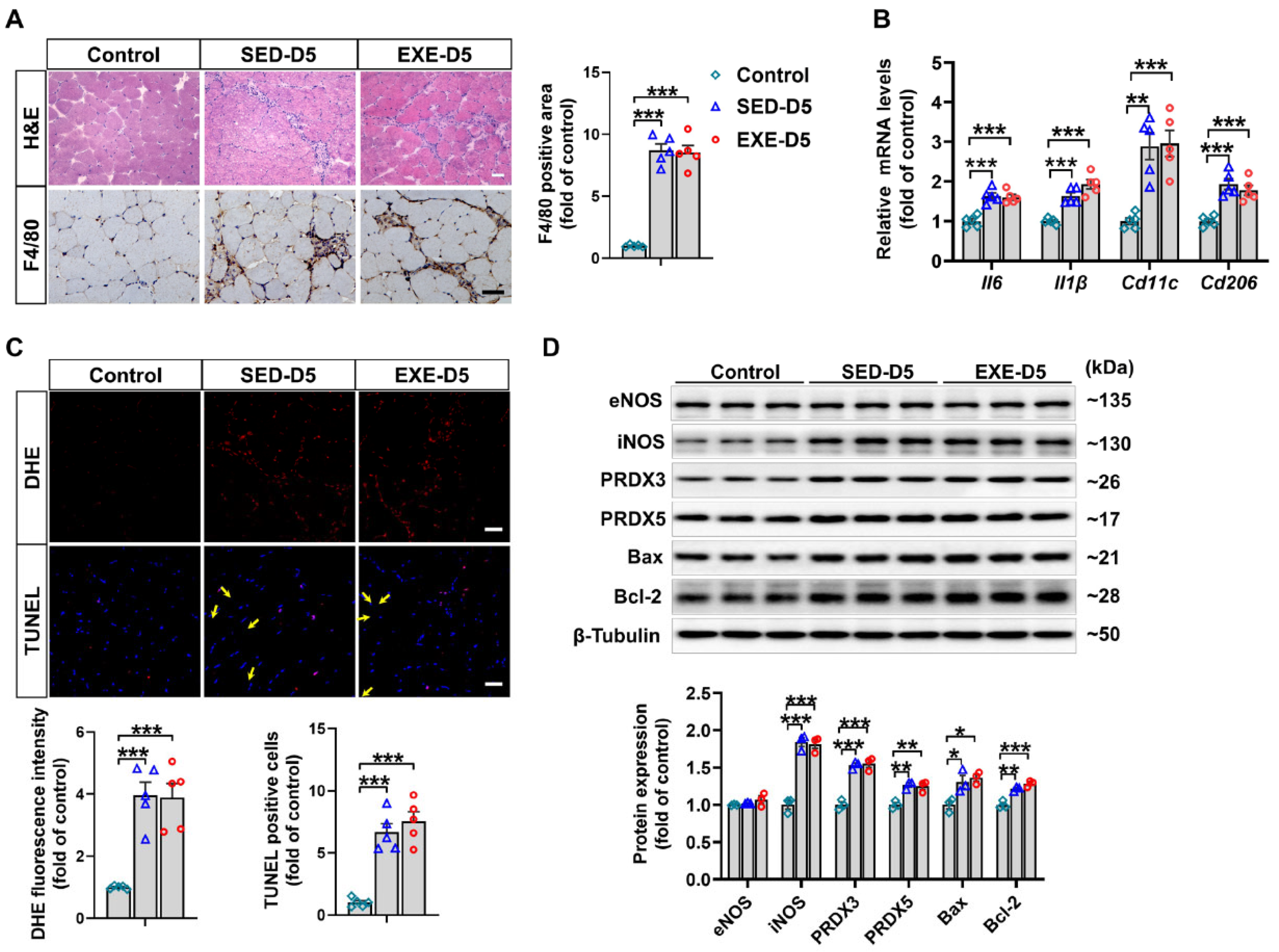
3.6. Aerobic Exercise Did Not Affect CTX-Induced Oxidative Stress or Apoptosis in the GA Muscle of Ddah1MKO Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huard, J.; Li, Y.; Fu, F.H. Muscle injuries and repair: Current trends in research. J. Bone Jt. Surg. Am. 2002, 84, 822–832. [Google Scholar] [CrossRef]
- Jarvinen, T.A.; Jarvinen, T.L.; Kaariainen, M.; Kalimo, H.; Jarvinen, M. Muscle injuries: Biology and treatment. Am. J. Sports Med. 2005, 33, 745–764. [Google Scholar] [CrossRef]
- Birnkrant, D.J.; Bello, L.; Butterfield, R.J.; Carter, J.C.; Cripe, L.H.; Cripe, T.P.; McKim, D.A.; Nandi, D.; Pegoraro, E. Cardiorespiratory management of Duchenne muscular dystrophy: Emerging therapies, neuromuscular genetics, and new clinical challenges. Lancet Respir. Med. 2022, 10, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Cossu, G.; Sampaolesi, M. New therapies for Duchenne muscular dystrophy: Challenges, prospects and clinical trials. Trends Mol. Med. 2007, 13, 520–526. [Google Scholar] [CrossRef]
- Picavet, H.S.; Hoeymans, N. Health related quality of life in multiple musculoskeletal diseases: SF-36 and EQ-5D in the DMC3 study. Ann. Rheum. Dis. 2004, 63, 723–729. [Google Scholar] [CrossRef]
- Valovich McLeod, T.C.; Bay, R.C.; Parsons, J.T.; Sauers, E.L.; Snyder, A.R. Recent injury and health-related quality of life in adolescent athletes. J. Athl. Train. 2009, 44, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Perrot, S.; Russell, I.J. More ubiquitous effects from non-pharmacologic than from pharmacologic treatments for fibromyalgia syndrome: A meta-analysis examining six core symptoms. Eur. J. Pain 2014, 18, 1067–1080. [Google Scholar] [CrossRef]
- Hoogeboom, T.J.; Oosting, E.; Vriezekolk, J.E.; Veenhof, C.; Siemonsma, P.C.; de Bie, R.A.; van den Ende, C.H.; van Meeteren, N.L. Therapeutic validity and effectiveness of preoperative exercise on functional recovery after joint replacement: A systematic review and meta-analysis. PLoS ONE 2012, 7, e38031. [Google Scholar] [CrossRef] [PubMed]
- López-Torres Hidalgo, J. Effectiveness of physical exercise in the treatment of depression in older adults as an alternative to antidepressant drugs in primary care. BMC Psychiatry 2019, 19, 21. [Google Scholar] [CrossRef]
- Kirkman, D.L.; Lee, D.C.; Carbone, S. Resistance exercise for cardiac rehabilitation. Prog. Cardiovasc. Dis. 2022, 70, 66–72. [Google Scholar] [CrossRef]
- Tyml, K.; Swarbreck, S.; Pape, C.; Secor, D.; Koropatnick, J.; Feng, Q.; Veldhuizen, R.A.W.; Gill, S.E. Voluntary running exercise protects against sepsis-induced early inflammatory and pro-coagulant responses in aged mice. Crit. Care 2017, 21, 210. [Google Scholar] [CrossRef] [PubMed]
- Hyldahl, R.D.; Chen, T.C.; Nosaka, K. Mechanisms and Mediators of the Skeletal Muscle Repeated Bout Effect. Exerc. Sport. Sci. Rev. 2017, 45, 24–33. [Google Scholar] [CrossRef]
- Smuder, A.J. Exercise stimulates beneficial adaptations to diminish doxorubicin-induced cellular toxicity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R662–R672. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Duarte, J.A.; Le Nguyen, B.; Hyatt, H. Endurance exercise protects skeletal muscle against both doxorubicin-induced and inactivity-induced muscle wasting. Pflug. Arch. 2019, 471, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Brett, J.O.; Arjona, M.; Ikeda, M.; Quarta, M.; de Morree, A.; Egner, I.M.; Perandini, L.A.; Ishak, H.D.; Goshayeshi, A.; Benjamin, D.I.; et al. Exercise rejuvenates quiescent skeletal muscle stem cells in old mice through restoration of Cyclin D1. Nat. Metab. 2020, 2, 307–317. [Google Scholar] [CrossRef]
- Inoue, A.; Cheng, X.W.; Huang, Z.; Hu, L.; Kikuchi, R.; Jiang, H.; Piao, L.; Sasaki, T.; Itakura, K.; Wu, H.; et al. Exercise restores muscle stem cell mobilization, regenerative capacity and muscle metabolic alterations via adiponectin/AdipoR1 activation in SAMP10 mice. J. Cachexia Sarcopenia Muscle 2017, 8, 370–385. [Google Scholar] [CrossRef]
- Abreu, P.; Kowaltowski, A.J. Satellite cell self-renewal in endurance exercise is mediated by inhibition of mitochondrial oxygen consumption. J. Cachexia Sarcopenia Muscle 2020, 11, 1661–1676. [Google Scholar] [CrossRef]
- Anderson, J.E. A role for nitric oxide in muscle repair: Nitric oxide-mediated activation of muscle satellite cells. Mol. Biol. Cell 2000, 11, 1859–1874. [Google Scholar] [CrossRef]
- Buono, R.; Vantaggiato, C.; Pisa, V.; Azzoni, E.; Bassi, M.T.; Brunelli, S.; Sciorati, C.; Clementi, E. Nitric oxide sustains long-term skeletal muscle regeneration by regulating fate of satellite cells via signaling pathways requiring Vangl2 and cyclic GMP. Stem Cells 2012, 30, 197–209. [Google Scholar] [CrossRef]
- Hu, X.; Atzler, D.; Xu, X.; Zhang, P.; Guo, H.; Lu, Z.; Fassett, J.; Schwedhelm, E.; Böger, R.H.; Bache, R.J.; et al. Dimethylarginine dimethylaminohydrolase-1 is the critical enzyme for degrading the cardiovascular risk factor asymmetrical dimethylarginine. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1540–1546. [Google Scholar] [CrossRef]
- Achan, V.; Broadhead, M.; Malaki, M.; Whitley, G.; Leiper, J.; MacAllister, R.; Vallance, P. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1455–1459. [Google Scholar] [CrossRef]
- Feng, F.; Cui, B.; Fang, L.; Lan, T.; Luo, K.; Xu, X.; Lu, Z. DDAH1 Protects against Cardiotoxin-Induced Muscle Injury and Regeneration. Antioxidants 2023, 12, 1754. [Google Scholar] [CrossRef]
- Shi, X.; Luo, X.; Xu, X. Dimethylarginine dimethylaminohydrolase-1 contributes to exercise-induced cardiac angiogenesis in mice. Biosci. Trends 2020, 14, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Hou, L.; Shen, S.; Wu, Y.; Wang, J.; Jie, Z.; Zhao, X.; Li, X.; Zhang, X.; Chen, J.; et al. Mechanical force promotes dimethylarginine dimethylaminohydrolase 1-mediated hydrolysis of the metabolite asymmetric dimethylarginine to enhance bone formation. Nat. Commun. 2022, 13, 50. [Google Scholar] [CrossRef]
- Fu, X.; Li, S.; Jia, M.; Yang, W.; Hu, P. Induction of Skeletal Muscle Injury by Intramuscular Injection of Cardiotoxin in Mouse. Bio Protoc. 2023, 13, e4668. [Google Scholar] [CrossRef]
- Kemp, M.; Donovan, J.; Higham, H.; Hooper, J. Biochemical markers of myocardial injury. Br. J. Anaesth. 2004, 93, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Nakamura, T.; Fujihara, S.; Tanaka, E. Ultrasound modulates the inflammatory response and promotes muscle regeneration in injured muscles. Ann. Biomed. Eng. 2013, 41, 1095–1105. [Google Scholar] [CrossRef]
- Rigamonti, E.; Touvier, T.; Clementi, E.; Manfredi, A.A.; Brunelli, S.; Rovere-Querini, P. Requirement of inducible nitric oxide synthase for skeletal muscle regeneration after acute damage. J. Immunol. 2013, 190, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Stacey, M.; Stein-Streilein, J.; Gordon, S. F4/80: The macrophage-specific adhesion-GPCR and its role in immunoregulation. Adv. Exp. Med. Biol. 2010, 706, 149–156. [Google Scholar]
- Chazaud, B.; Brigitte, M.; Yacoub-Youssef, H.; Arnold, L.; Gherardi, R.; Sonnet, C.; Lafuste, P.; Chretien, F. Dual and beneficial roles of macrophages during skeletal muscle regeneration. Exerc. Sport. Sci. Rev. 2009, 37, 18–22. [Google Scholar] [CrossRef]
- Takahashi, Y.; Shimizu, T.; Kato, S.; Nara, M.; Suganuma, Y.; Sato, T.; Morii, T.; Yamada, Y.; Fujita, H. Reduction of Superoxide Dismutase 1 Delays Regeneration of Cardiotoxin-Injured Skeletal Muscle in KK/Ta-Ins2(Akita) Mice with Progressive Diabetic Nephropathy. Int. J. Mol. Sci. 2021, 22, 5491. [Google Scholar] [CrossRef]
- Wang, Z.G.; Zhu, Z.Q.; He, Z.Y.; Cheng, P.; Liang, S.; Chen, A.M.; Yang, Q. Endogenous conversion of n-6 to n-3 polyunsaturated fatty acids facilitates the repair of cardiotoxin-induced skeletal muscle injury in fat-1 mice. Aging 2021, 13, 8454–8466. [Google Scholar] [CrossRef]
- Karpovich, P.V. Exercise in medicine: A review. Arch. Phys. Med. Rehabil. 1968, 49, 66–76. [Google Scholar]
- Brellenthin, A.G.; Lanningham-Foster, L.M.; Kohut, M.L.; Li, Y.; Church, T.S.; Blair, S.N.; Lee, D.C. Comparison of the Cardiovascular Benefits of Resistance, Aerobic, and Combined Exercise (CardioRACE): Rationale, design, and methods. Am. Heart J. 2019, 217, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: Joint position statement. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Schootemeijer, S.; van der Kolk, N.M.; Bloem, B.R.; de Vries, N.M. Current Perspectives on Aerobic Exercise in People with Parkinson’s Disease. Neurother. J. Am. Soc. Exp. NeuroTherapeutics 2020, 17, 1418–1433. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef]
- Tagliafico, A.S.; Bignotti, B.; Torri, L.; Rossi, F. Sarcopenia: How to measure, when and why. La Radiol. Medica 2022, 127, 228–237. [Google Scholar] [CrossRef]
- Tezze, C.; Sandri, M.; Tessari, P. Anabolic Resistance in the Pathogenesis of Sarcopenia in the Elderly: Role of Nutrition and Exercise in Young and Old People. Nutrients 2023, 15, 4073. [Google Scholar] [CrossRef]
- Short, K.R.; Vittone, J.L.; Bigelow, M.L.; Proctor, D.N.; Nair, K.S. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E92–E101. [Google Scholar] [CrossRef] [PubMed]
- Agostini, D.; Zeppa Donati, S.; Lucertini, F.; Annibalini, G.; Gervasi, M.; Ferri Marini, C.; Piccoli, G.; Stocchi, V.; Barbieri, E.; Sestili, P. Muscle and Bone Health in Postmenopausal Women: Role of Protein and Vitamin D Supplementation Combined with Exercise Training. Nutrients 2018, 10, 1103. [Google Scholar] [CrossRef]
- Kistler-Fischbacher, M.; Weeks, B.K.; Beck, B.R. The effect of exercise intensity on bone in postmenopausal women (part 2): A meta-analysis. Bone 2021, 143, 115697. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, F.; Shi, H.; Xia, H.; Wei, X.; Liu, S.; Wu, T.; Li, Y.; Shu, F.; Chen, M.; et al. Aerobic exercise suppresses CCN2 secretion from senescent muscle stem cells and boosts muscle regeneration in aged mice. J. Cachexia Sarcopenia Muscle 2024. [Google Scholar] [CrossRef] [PubMed]
- Kubat, G.B.; Ulger, O.; Atalay, O.; Fatsa, T.; Turkel, I.; Ozerklig, B.; Celik, E.; Ozenc, E.; Simsek, G.; Tuncer, M. The effects of exercise and mitochondrial transplantation alone or in combination against Doxorubicin-induced skeletal muscle atrophy. J. Muscle Res. Cell Motil. 2024. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Luo, Q.; Song, G. Control of muscle satellite cell function by specific exercise-induced cytokines and their applications in muscle maintenance. J. Cachexia Sarcopenia Muscle 2024, 15, 466–476. [Google Scholar] [CrossRef]
- Koishi, K.; Zhang, M.; McLennan, I.S.; Harris, A.J. MyoD protein accumulates in satellite cells and is neurally regulated in regenerating myotubes and skeletal muscle fibers. Dev. Dyn. 1995, 202, 244–254. [Google Scholar] [CrossRef]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef]
- Ganassi, M.; Badodi, S.; Ortuste Quiroga, H.P.; Zammit, P.S.; Hinits, Y.; Hughes, S.M. Myogenin promotes myocyte fusion to balance fibre number and size. Nat. Commun. 2018, 9, 4232. [Google Scholar] [CrossRef]
- Joanisse, S.; Nederveen, J.P.; Baker, J.M.; Snijders, T.; Iacono, C.; Parise, G. Exercise conditioning in old mice improves skeletal muscle regeneration. FASEB J. 2016, 30, 3256–3268. [Google Scholar] [CrossRef]
- Summan, M.; Warren, G.L.; Mercer, R.R.; Chapman, R.; Hulderman, T.; Van Rooijen, N.; Simeonova, P.P. Macrophages and skeletal muscle regeneration: A clodronate-containing liposome depletion study. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1488–R1495. [Google Scholar] [CrossRef]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef]
- Brechot, N.; Gomez, E.; Bignon, M.; Khallou-Laschet, J.; Dussiot, M.; Cazes, A.; Alanio-Brechot, C.; Durand, M.; Philippe, J.; Silvestre, J.S.; et al. Modulation of macrophage activation state protects tissue from necrosis during critical limb ischemia in thrombospondin-1-deficient mice. PLoS ONE 2008, 3, e3950. [Google Scholar] [CrossRef] [PubMed]
- Patsalos, A.; Pap, A.; Varga, T.; Trencsenyi, G.; Contreras, G.A.; Garai, I.; Papp, Z.; Dezso, B.; Pintye, E.; Nagy, L. In situ macrophage phenotypic transition is affected by altered cellular composition prior to acute sterile muscle injury. J. Physiol. 2017, 595, 5815–5842. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Shi, D.; Ding, M.; Huang, T.; Gu, R.; Xiao, J.; Xian, C.J.; Dong, J.; Wang, L.; Liao, H. Calmodulin-dependent signalling pathways are activated and mediate the acute inflammatory response of injured skeletal muscle. J. Physiol. 2019, 597, 5161–5177. [Google Scholar] [CrossRef] [PubMed]
- Saclier, M.; Yacoub-Youssef, H.; Mackey, A.L.; Arnold, L.; Ardjoune, H.; Magnan, M.; Sailhan, F.; Chelly, J.; Pavlath, G.K.; Mounier, R.; et al. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells 2013, 31, 384–396. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, L. The Many Roles of Macrophages in Skeletal Muscle Injury and Repair. Front. Cell Dev. Biol. 2022, 10, 952249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, X.; Baker, J.S.; Davison, G.W.; Yan, X. Redox signaling and skeletal muscle adaptation during aerobic exercise. iScience 2024, 27, 109643. [Google Scholar] [CrossRef]
- Sollanek, K.J.; Burniston, J.G.; Kavazis, A.N.; Morton, A.B.; Wiggs, M.P.; Ahn, B.; Smuder, A.J.; Powers, S.K. Global Proteome Changes in the Rat Diaphragm Induced by Endurance Exercise Training. PLoS ONE 2017, 12, e0171007. [Google Scholar] [CrossRef]
- Vieira Ramos, G.; Choqueta de Toledo-Arruda, A.; Maria Pinheiro-Dardis, C.; Liyoko Suehiro, C.; Luiz de Russo, T.; Vieira, R.P.; Arruda Martins, M.; Salvini, T.F.; Durigan, J.L.Q. Exercise Prevents Diaphragm Wasting Induced by Cigarette Smoke through Modulation of Antioxidant Genes and Metalloproteinases. BioMed Res. Int. 2018, 2018, 5909053. [Google Scholar] [CrossRef]
- Ren, W.; Xu, Z.; Pan, S.; Ma, Y.; Li, H.; Wu, F.; Bo, W.; Cai, M.; Tian, Z. Irisin and ALCAT1 mediated aerobic exercise-alleviated oxidative stress and apoptosis in skeletal muscle of mice with myocardial infarction. Free Radic. Biol. Med. 2022, 193 Pt 2, 526–537. [Google Scholar] [CrossRef]
- Rezaee, N.; Rahmani-Nia, F.; Delfan, M.; Ghahremani, R. Exercise training and probiotic supplementation effects on skeletal muscle apoptosis prevention in type-Iota diabetic rats. Life Sci. 2021, 285, 119973. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kwon, O. Aerobic exercise prevents apoptosis in skeletal muscles of high-fat-fed ovariectomized rats. Phys. Act. Nutr. 2022, 26, 1–7. [Google Scholar] [CrossRef]
- Bao, F.; Zhao, X.; You, J.; Liu, Y.; Xu, Z.; Wu, Y.; Wu, Y.; Xu, Z.; Yu, L.; Li, J.; et al. Aerobic exercise alleviates skeletal muscle aging in male rats by inhibiting apoptosis via regulation of the Trx system. Exp. Gerontol. 2024, 194, 112523. [Google Scholar] [CrossRef]
- Hong, J.; Park, Y. Microvascular Function and Exercise Training: Functional Implication of Nitric Oxide Signaling and Ion Channels. Pulse 2024, 12, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Luo, K.; Yuan, J.; Gao, J.; Cui, B.; Yu, Z.; Lu, Z. Hepatic DDAH1 mitigates hepatic steatosis and insulin resistance in obese mice: Involvement of reduced S100A11 expression. Acta Pharm. Sin. B 2023, 13, 3352–3364. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Lei, T.; Wang, H.; Luo, K.; Wang, Y.; Cui, B.; Yu, Z.; Hu, X.; Zhang, F.; Chen, Y.; et al. Dimethylarginine dimethylaminohydrolase 1 protects PM(2.5) exposure-induced lung injury in mice by repressing inflammation and oxidative stress. Part. Fibre Toxicol. 2022, 19, 64. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, P.; Kwak, D.; Fassett, J.; Yue, W.; Atzler, D.; Hu, X.; Liu, X.; Wang, H.; Lu, Z.; et al. Cardiomyocyte dimethylarginine dimethylaminohydrolase-1 (DDAH1) plays an important role in attenuating ventricular hypertrophy and dysfunction. Basic. Res. Cardiol. 2017, 112, 55. [Google Scholar] [CrossRef]


| Genes | Gene ID | Primers | Sequence |
|---|---|---|---|
| Il6 | 16193 | Forward | 5′-AACGATGATGCACTTGCAGA-3′ |
| Reverse | 5′-TGGTACTCCAGAAGACCAGAGG-3′ | ||
| Il1β | 16176 | Forward | 5′-AGGTCAAAGGTTTGGAAGCA-3′ |
| Reverse | 5′-TGAAGCAGCTATGGCAACTG-3′ | ||
| Cd86 | 12524 | Forward | 5′-AGTGATCGCCAACTTCAGTGAACC-3′ |
| Reverse | 5′-GGTGACCTTGCTTAGACGTGCAG-3′ | ||
| Cd11c | 16411 | Forward | 5′-CTGGATAGCCTTTCTTCTGCTG-3′ |
| Reverse | 5′-GCACACTGTGTCCGAACTCA-3′ | ||
| Il10 | 16153 | Forward | 5′-CTTACTGACTGGCATGAGGATCA-3′ |
| Reverse | 5′-GCAGCTCTAGGAGCATGTGG-3′ | ||
| Cd206 | 17533 | Forward | 5′-CTCTGTTCAGCTATTGGACGC-3′ |
| Reverse | 5′-TGGCACTCCCAAACATAATTTGA-3′ | ||
| Arg1 | 11846 | Forward | 5′-GAACACGGCAGTGGCTTTAAC-3′ |
| Reverse | 5′-TGCTTAGCTCTGTCTGCTTTGC-3′ | ||
| 18s | 19791 | Forward | 5′-TTCTGGCCAACGGTCTAGACAAC-3′ |
| Reverse | 5′-CCAGTGGTCTTGGTGTGCTGA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, F.; Luo, K.; Yuan, X.; Lan, T.; Wang, S.; Xu, X.; Lu, Z. Aerobic Exercise Protects against Cardiotoxin-Induced Skeletal Muscle Injury in a DDAH1-Dependent Manner. Antioxidants 2024, 13, 1069. https://doi.org/10.3390/antiox13091069
Feng F, Luo K, Yuan X, Lan T, Wang S, Xu X, Lu Z. Aerobic Exercise Protects against Cardiotoxin-Induced Skeletal Muscle Injury in a DDAH1-Dependent Manner. Antioxidants. 2024; 13(9):1069. https://doi.org/10.3390/antiox13091069
Chicago/Turabian StyleFeng, Fei, Kai Luo, Xinyi Yuan, Ting Lan, Siyu Wang, Xin Xu, and Zhongbing Lu. 2024. "Aerobic Exercise Protects against Cardiotoxin-Induced Skeletal Muscle Injury in a DDAH1-Dependent Manner" Antioxidants 13, no. 9: 1069. https://doi.org/10.3390/antiox13091069
APA StyleFeng, F., Luo, K., Yuan, X., Lan, T., Wang, S., Xu, X., & Lu, Z. (2024). Aerobic Exercise Protects against Cardiotoxin-Induced Skeletal Muscle Injury in a DDAH1-Dependent Manner. Antioxidants, 13(9), 1069. https://doi.org/10.3390/antiox13091069







