5-Fluorouracil in Combination with Calcium Carbonate Nanoparticles Loaded with Antioxidant Thymoquinone against Colon Cancer: Synergistically Therapeutic Potential and Underlying Molecular Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CaCO3 NPs from Cockle Shells
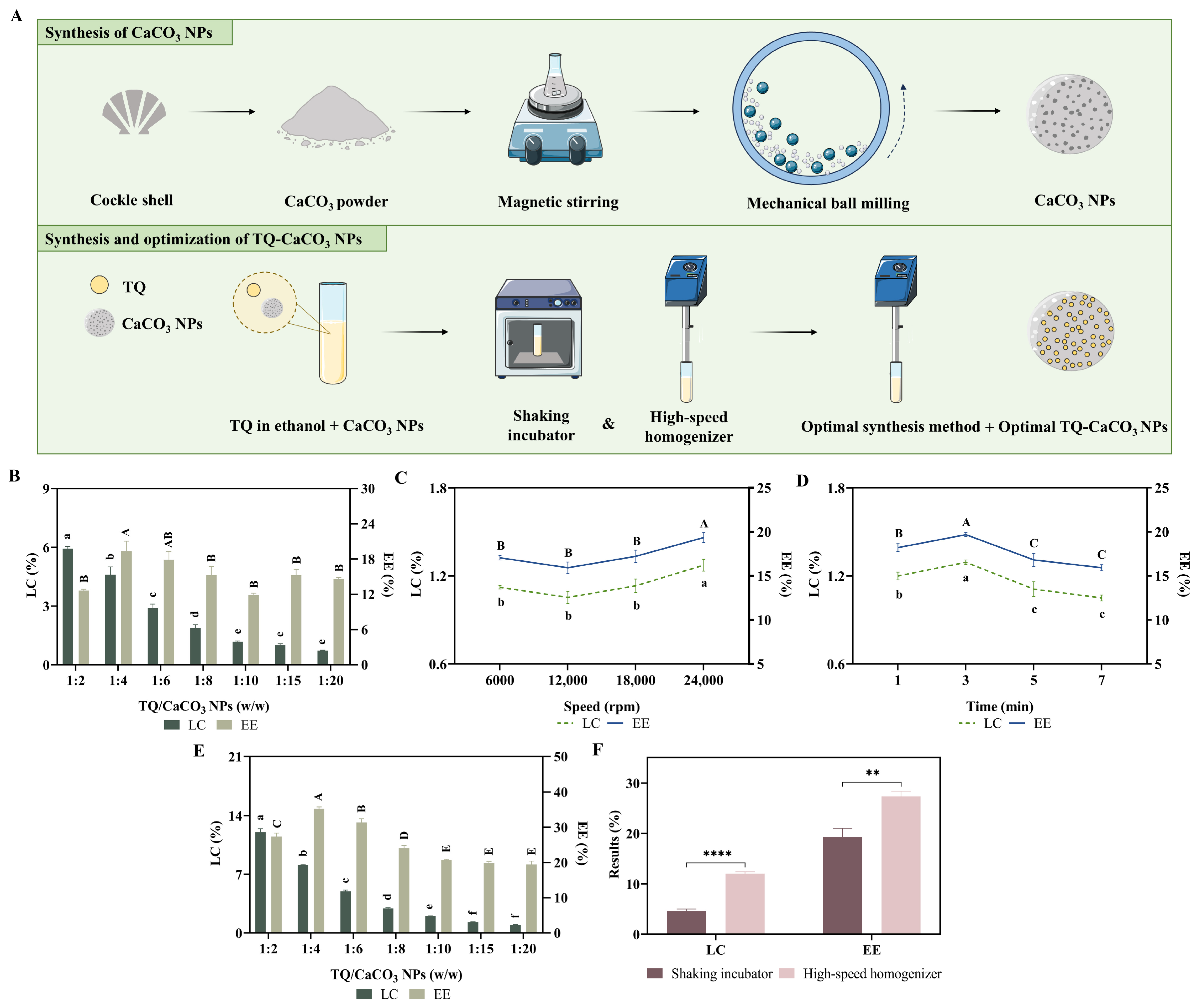
2.3. Synthesis and Optimization of TQ-CaCO3 NPs
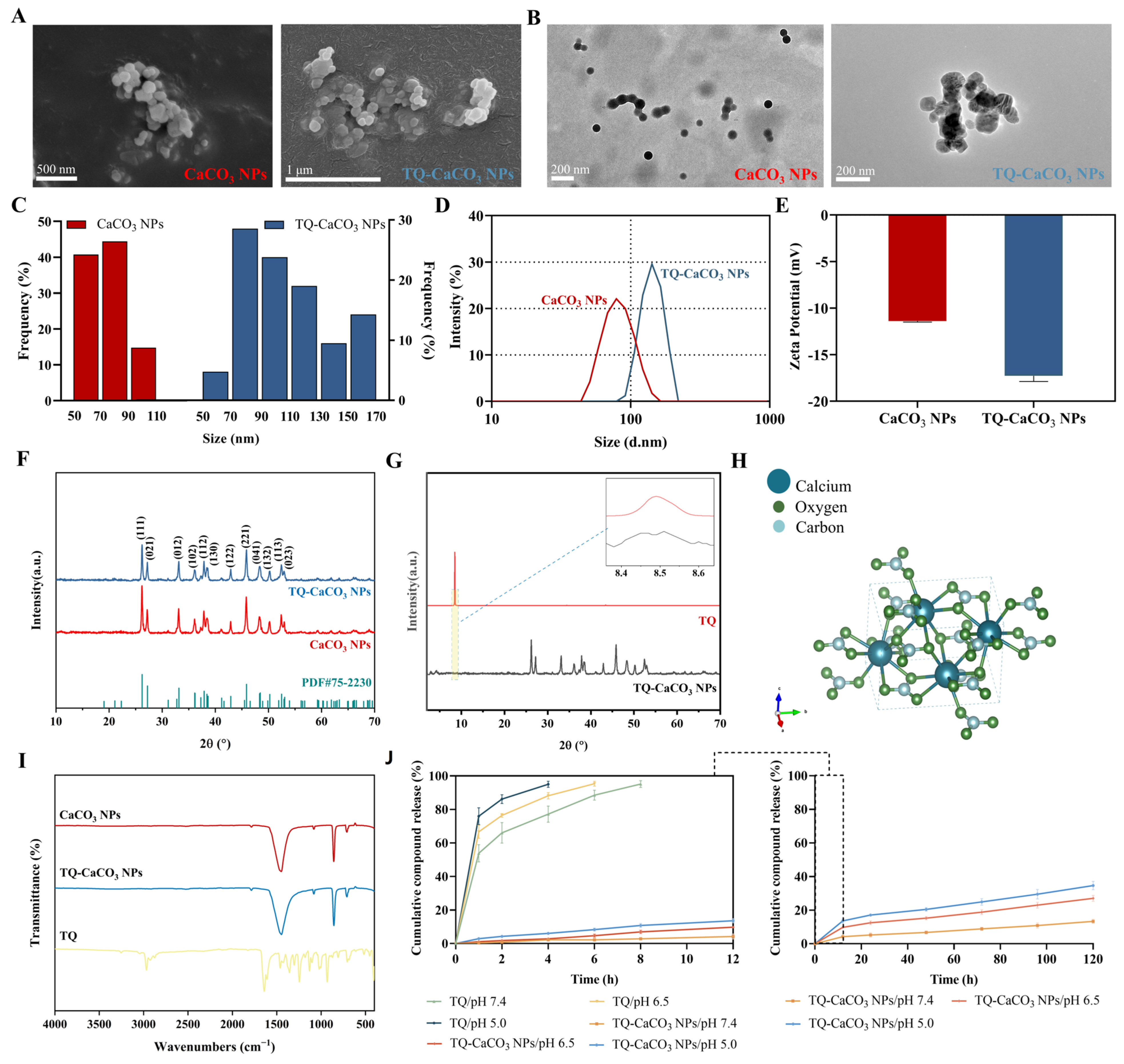
2.4. Physicochemical Characterization of CaCO3 NPs and TQ-CaCO3 NPs
2.4.1. Electron Microscopy and Zetasizer
2.4.2. Energy-Dispersive X-ray Spectroscopy, X-ray Diffractometer, and Fourier Transform Infrared Spectroscopy
2.5. In Vitro Compound Release Study
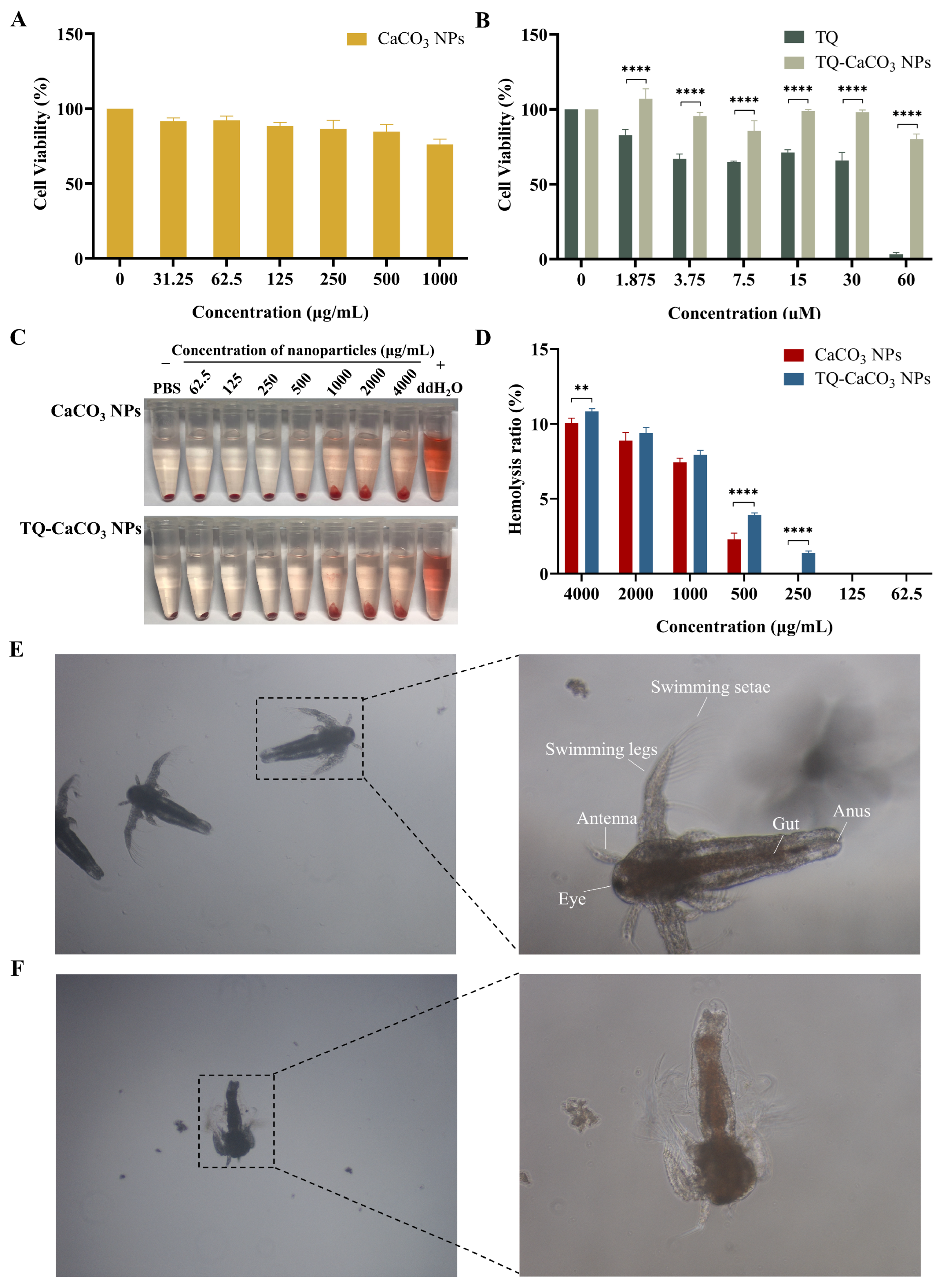
2.6. Biocompatibility Evaluation of CaCO3 NPs and TQ-CaCO3 NPs
2.6.1. Hemocompatibility Analysis
2.6.2. Toxicity Analysis in Brine Shrimp
2.6.3. Cytotoxicity Analysis
2.7. In Vitro Cell Experiments of TQ-CaCO3 NPs in Combination with 5-FU
2.7.1. MTT Assay
2.7.2. Synergism Analysis
2.7.3. Colony-Formation Assay
2.7.4. AO/PI Staining
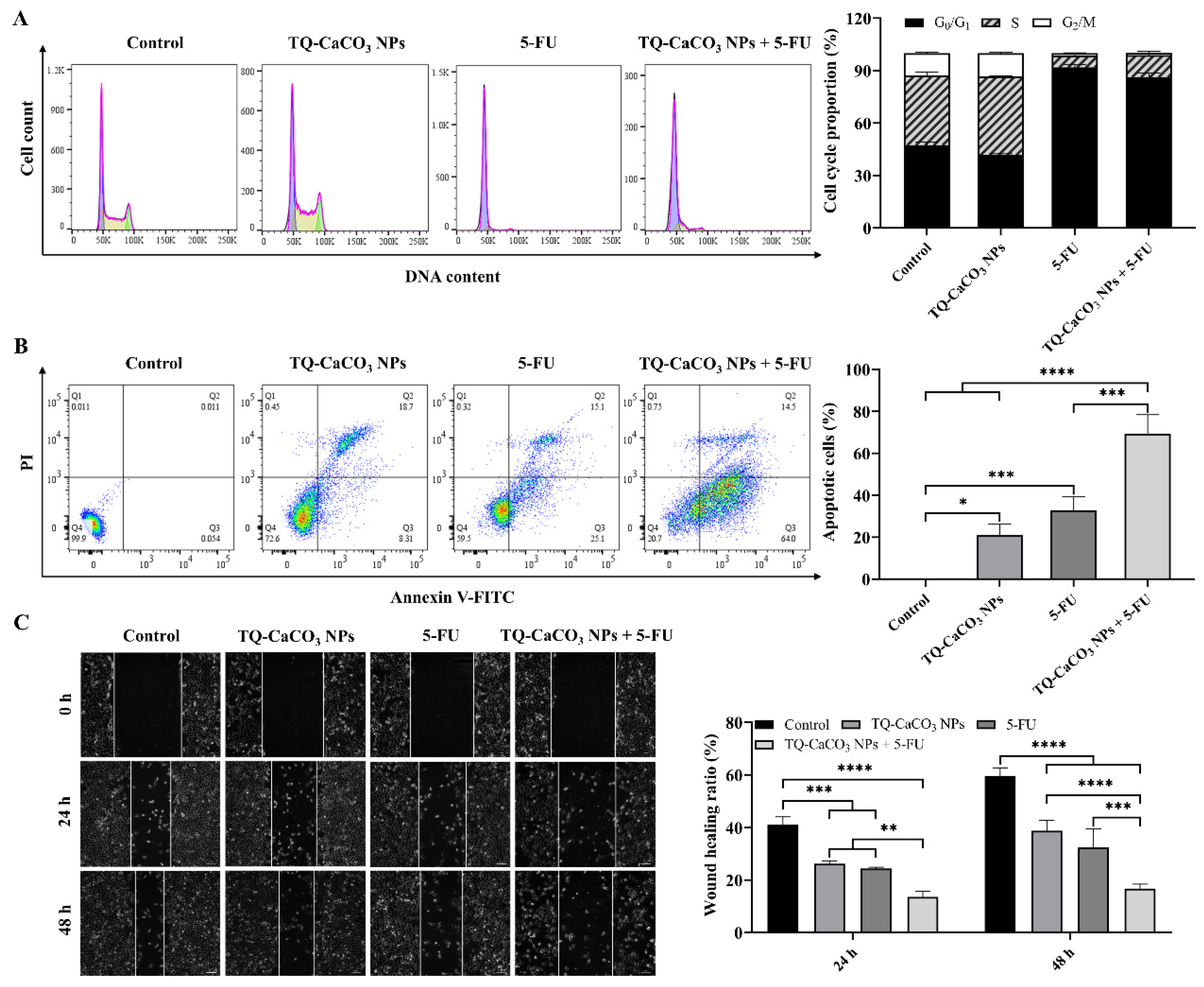
2.7.5. Cell Cycle and Apoptosis Analysis
2.7.6. Wound-Healing Assay
2.7.7. The 3D Spheroid Culture
2.7.8. The 3D Spheroid-Inhibition Assay
2.8. Network Pharmacology
2.8.1. Target Prediction and Screening of 5-FU and TQ
2.8.2. Targets Collection and Screening of Colon Cancer
2.8.3. PPI, GO, and KEGG Analysis
2.9. Molecule Docking
2.10. Statistical Analysis
3. Results
3.1. Synthesis of the Optimal TQ-CaCO3 NPs
3.2. Characterization of CaCO3 NPs and TQ-CaCO3 NPs
3.3. CaCO3 NPs and TQ-CaCO3 NPs Exhibit Favorable Biocompatibility
3.4. TQ-CaCO3 NPs in Combination with 5-FU Inhibits the Proliferation of CT26 Cells
3.5. TQ-CaCO3 NPs in Combination with 5-FU Induces Cell Cycle Arrest and Apoptosis in CT26 Cells
3.6. TQ-CaCO3 NPs in Combination with 5-FU Inhibits the Migration of CT26 Cells
3.7. TQ-CaCO3 NPs in Combination with 5-FU Suppresses the Growth of CT26 Spheroids
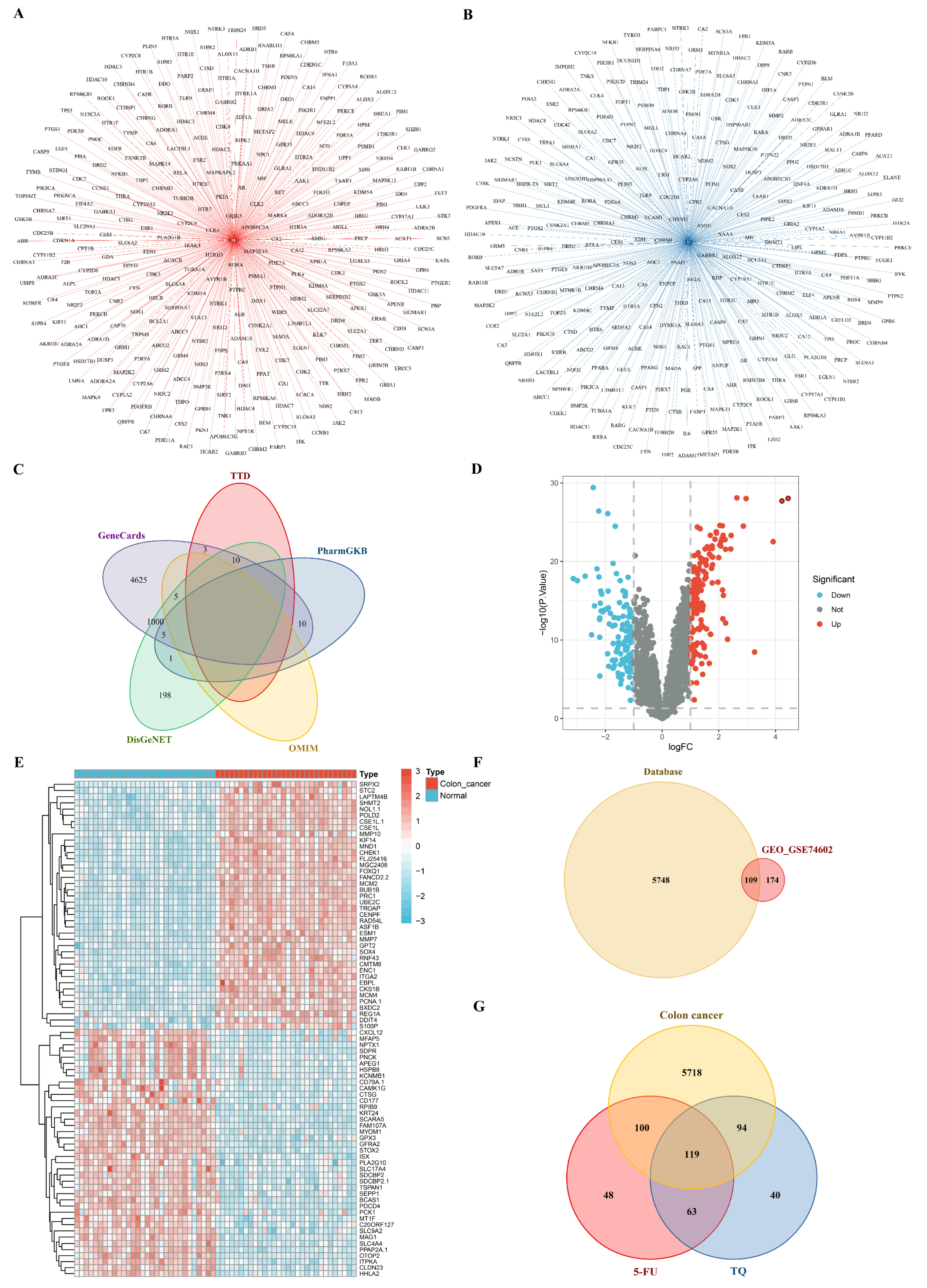
3.8. Network Pharmacology
3.8.1. Potential Targets of Drugs and Colon Cancer
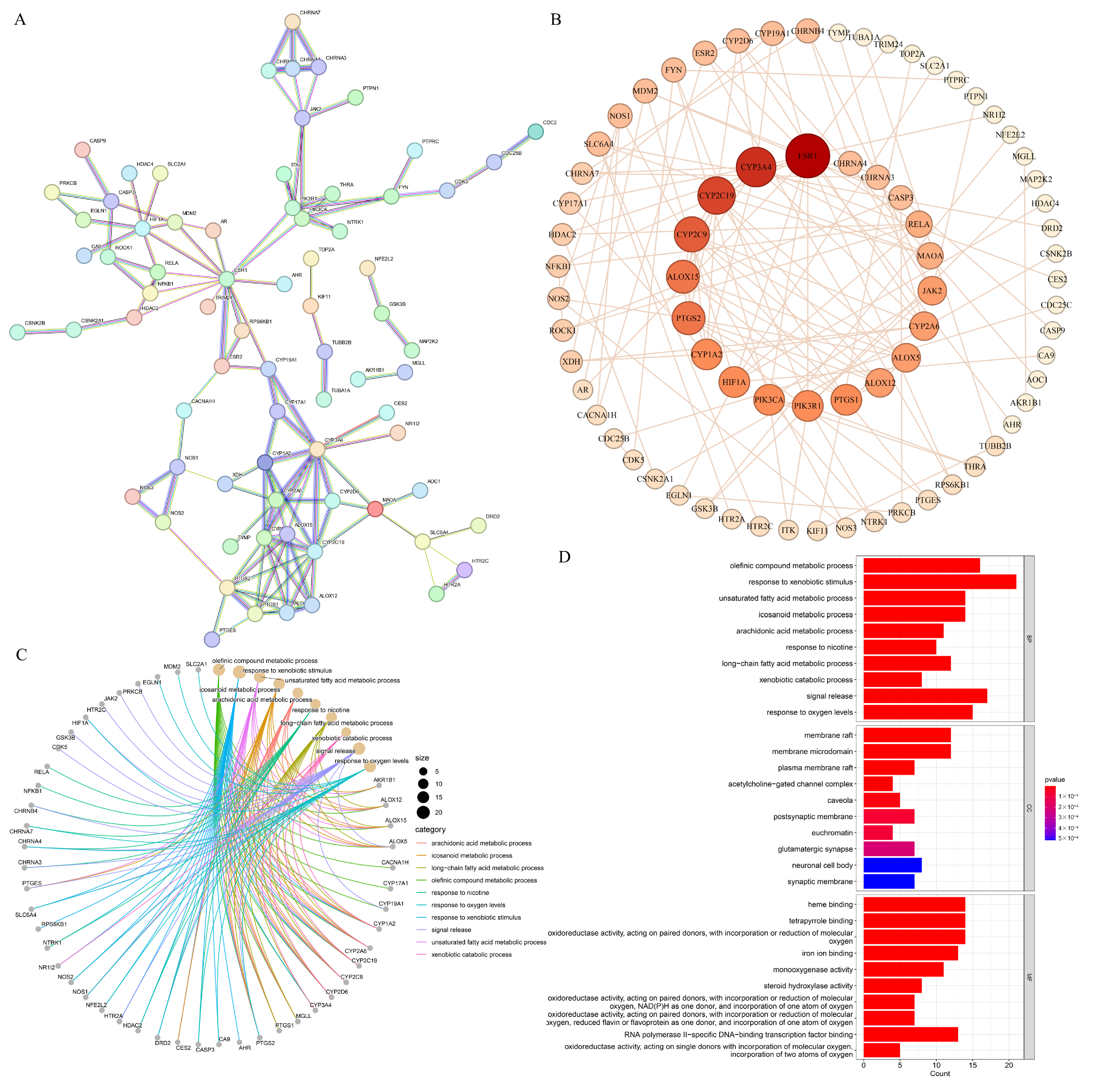
3.8.2. PPI Network and GO Functional Annotation
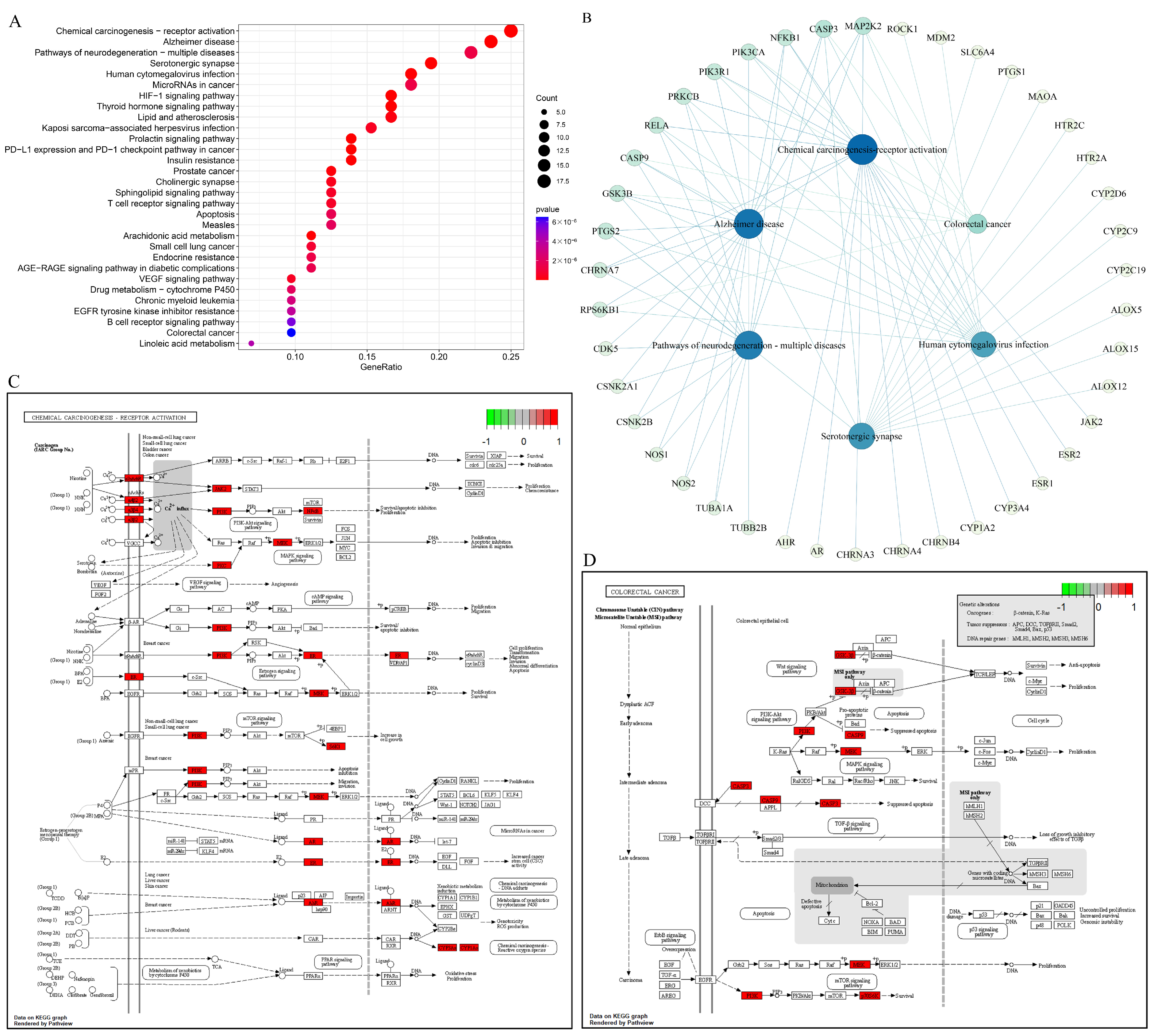
3.8.3. KEGG Pathway Enrichment Analysis
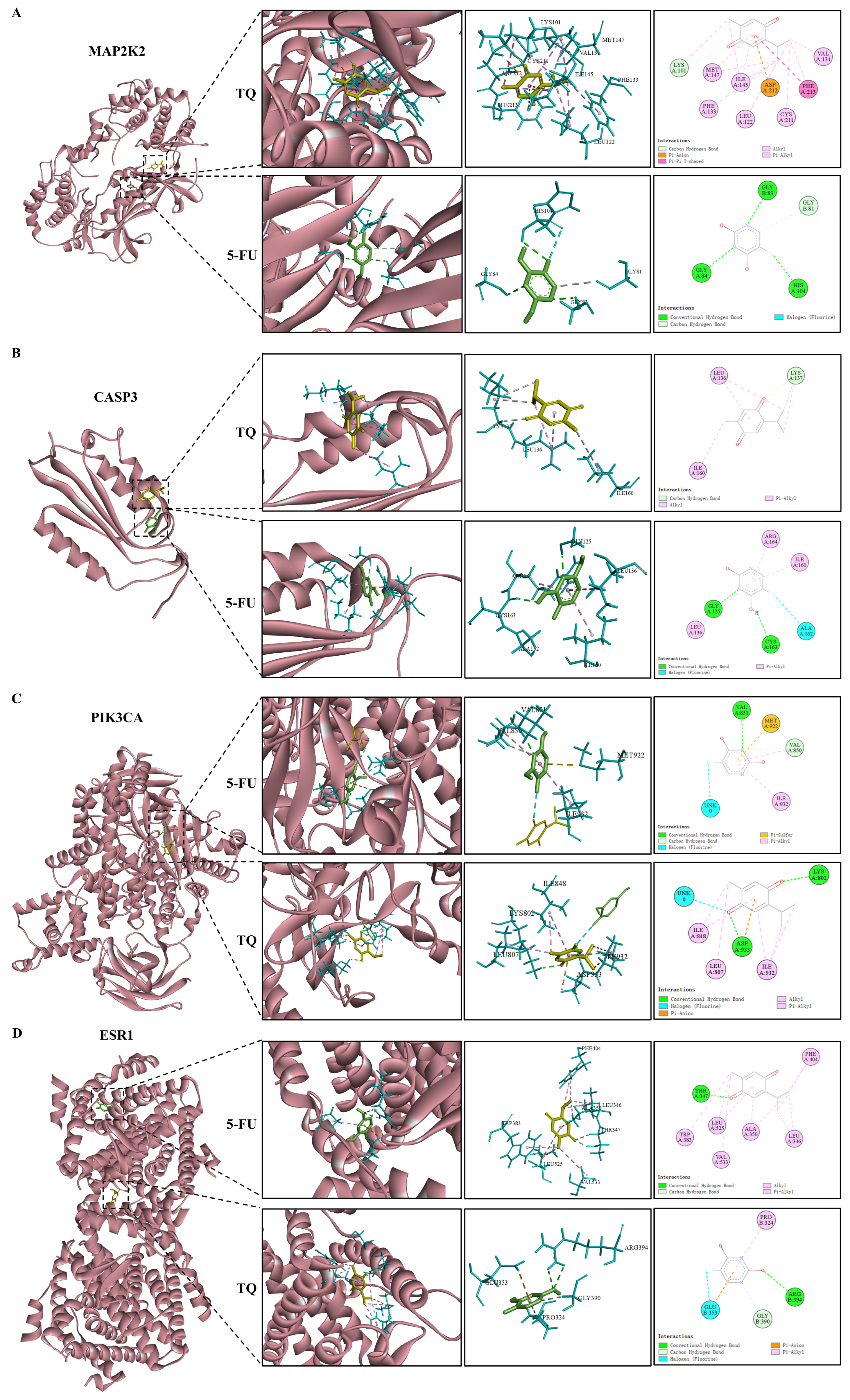
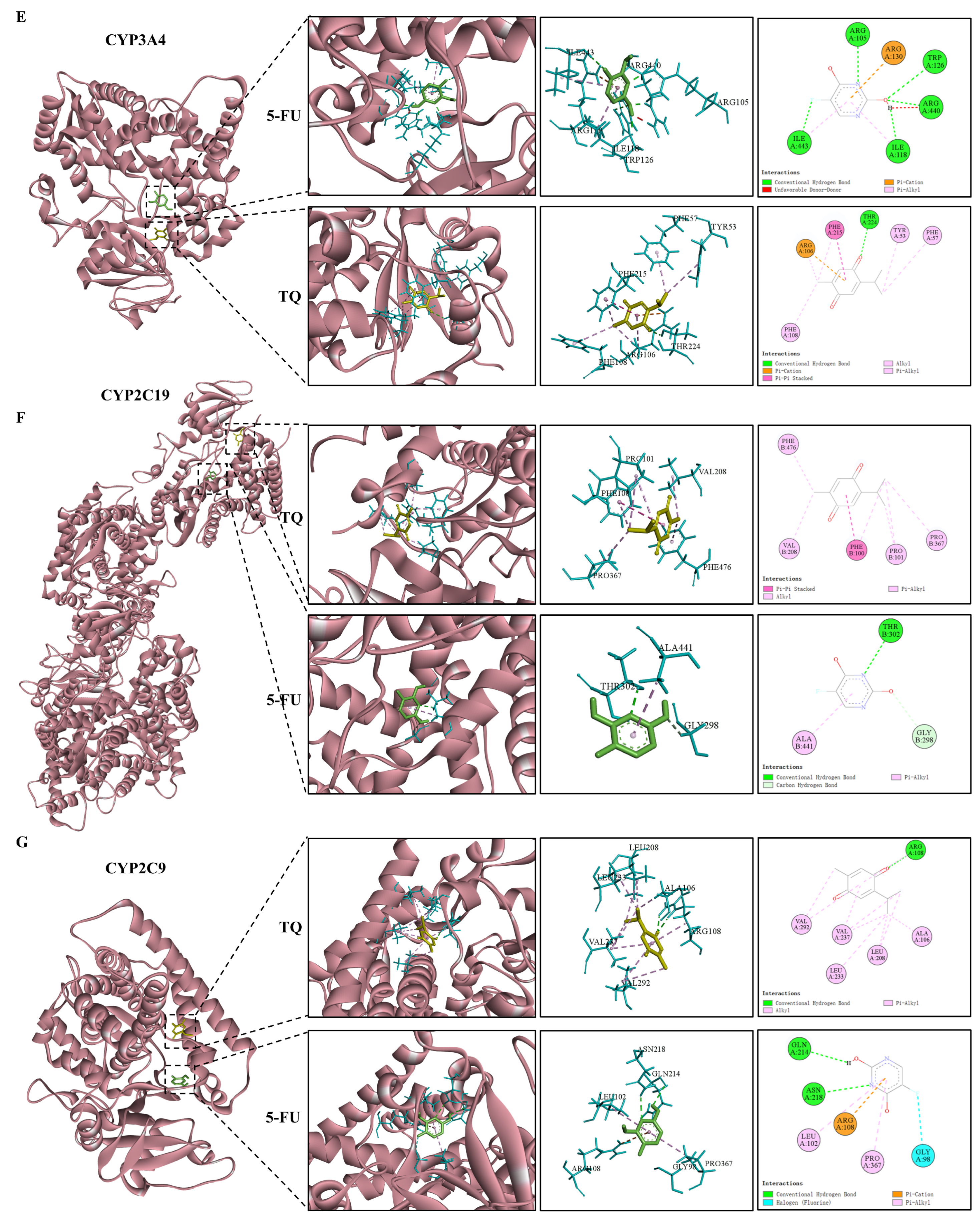
3.9. Molecular Docking
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.; Van der Roest, B.; Besselink, N.; Janssen, R.; Boymans, S.; Martens, J.W.M.; Yaspo, M.L.; Priestley, P.; Kuijk, E.; Cuppen, E.; et al. 5-Fluorouracil Treatment Induces Characteristic T>G Mutations in Human Cancer. Nat. Commun. 2019, 10, 4571. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-Fluorouracil and Other Fluoropyrimidines in Colorectal Cancer: Past, Present and Future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Khan, I.A.; Shahbaz, M.; Qaisrani, T.B.; Fatmawati, S.; Abu-Izneid, T.; Imran, A.; Rahman, K.U.; Gondal, T.A. Thymoquinone: A Novel Strategy to Combat Cancer: A Review. Biomed. Pharmacother. 2018, 106, 390–402. [Google Scholar] [CrossRef]
- Darakhshan, S.; Bidmeshki Pour, A.; Hosseinzadeh Colagar, A.; Sisakhtnezhad, S. Thymoquinone and Its Therapeutic Potentials. Pharmacol. Res. 2015, 95–96, 138–158. [Google Scholar] [CrossRef]
- Jrah-Harzallah, H.; Ben-Hadj-Khalifa, S.; Almawi, W.Y.; Maaloul, A.; Houas, Z.; Mahjoub, T. Effect of Thymoquinone on 1,2-Dimethyl-Hydrazine-Induced Oxidative Stress during Initiation and Promotion of Colon Carcinogenesis. Eur. J. Cancer 2013, 49, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Homayoonfal, M.; Asemi, Z.; Yousefi, B. Potential Anticancer Properties and Mechanisms of Thymoquinone in Osteosarcoma and Bone Metastasis. Cell Mol. Biol. Lett. 2022, 27, 21. [Google Scholar] [CrossRef]
- Zhu, W.Q.; Wang, J.; Guo, X.F.; Liu, Z.; Dong, W.G. Thymoquinone Inhibits Proliferation in Gastric Cancer via the STAT3 Pathway in Vivo and in Vitro. World J. Gastroenterol. 2016, 22, 4149–4159. [Google Scholar] [CrossRef]
- Zheng, M.; Mei, Z.; Junaid, M.; Tania, M.; Fu, J.; Chen, H.C.; Khan, M.A. Synergistic Role of Thymoquinone on Anticancer Activity of 5-Fluorouracil in Triple Negative Breast Cancer Cells. Anticancer Agents Med. Chem. 2021, 22, 1111–1118. [Google Scholar] [CrossRef]
- Lei, X.; Lv, X.; Liu, M.; Yang, Z.; Ji, M.; Guo, X.; Dong, W. Thymoquinone Inhibits Growth and Augments 5-Fluorouracil-Induced Apoptosis in Gastric Cancer Cells Both in Vitro and in Vivo. Biochem. Biophys. Res. Commun. 2012, 417, 864–868. [Google Scholar] [CrossRef]
- Alkharfy, K.M.; Ahmad, A.; Khan, R.M.A.; Al-Shagha, W.M. Pharmacokinetic Plasma Behaviors of Intravenous and Oral Bioavailability of Thymoquinone in a Rabbit Model. Eur. J. Drug Metab. Pharmacokinet. 2015, 40, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Salmani, J.M.M.; Asghar, S.; Lv, H.; Zhou, J. Aqueous Solubility and Degradation Kinetics of the Phytochemical Anticancer Thymoquinone; Probing the Effects of Solvents, PH and Light. Molecules 2014, 19, 5925–5939. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Ahir, M.; Patra, P.; Mukherjee, S.; Ghosh, S.; Mazumdar, M.; Chattopadhyay, S.; Das, T.; Chattopadhyay, D.; Adhikary, A. PEGylated-Thymoquinone-Nanoparticle Mediated Retardation of Breast Cancer Cell Migration by Deregulation of Cytoskeletal Actin Polymerization through MiR-34a. Biomaterials 2015, 51, 91–107. [Google Scholar] [CrossRef]
- Ibrahim, W.N.; Rosli, L.M.B.M.; Doolaanea, A.A. Formulation, Cellular Uptake and Cytotoxicity of Thymoquinone-Loaded Plga Nanoparticles in Malignant Melanoma Cancer Cells. Int. J. Nanomed. 2020, 15, 8059–8074. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer Nanomedicine: Progress, Challenges and Opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Yakubova, A.A.; Mitusova, K.A.; Darwish, A.; Rogova, A.; Ageev, E.I.; Brodskaia, A.; Muslimov, A.R.; Zyuzin, M.V.; Timin, A.S. Calcium Carbonate Nanoparticles Tumor Delivery for Combined Chemo-Photodynamic Therapy: Comparison of Local and Systemic Administration. J. Control. Release 2023, 359, 400–414. [Google Scholar] [CrossRef]
- Bastos, F.R.; Soares da Costa, D.; Reis, R.L.; Alves, N.M.; Pashkuleva, I.; Costa, R.R. Layer-by-Layer Coated Calcium Carbonate Nanoparticles for Targeting Breast Cancer Cells. Biomater. Adv. 2023, 153, 213563. [Google Scholar] [CrossRef]
- Yu, J.; Wang, L.; Xie, X.; Yu, H.; Xu, J.; Ren, J.; Zhu, W.; Lei, Z.; Lv, L. Multifunctional Nanoparticles Codelivering Doxorubicin and Amorphous Calcium Carbonate Preloaded with Indocyanine Green for Enhanced Chemo-Photothermal Cancer Therapy. Int. J. Nanomed. 2023, 18, 323–337. [Google Scholar] [CrossRef]
- Zhou, F.; Li, H.; Liu, Y.; Deng, H.; Rong, J.; Zhao, J. Hyaluronan Derivative Decorated Calcium Carbonate Nanoparticle as a Potential Platform for Breast Cancer Synergistic Therapy via Blood Coagulation and Drug Delivery. J. Drug Deliv. Sci. Technol. 2023, 83, 104406. [Google Scholar] [CrossRef]
- Xu, D.; Jin, T.; Song, L.; Zhu, N.; Han, L.; Hong, H. Synthesis of Stable Calcium Carbonate Nanoparticles for PH-Responsive Controlled Drug Release. Mater. Lett. 2023, 333, 133635. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, W.; Liu, Z.; Guo, H.; Zhang, P. Smart Responsive-Calcium Carbonate Nanoparticles for Dual-Model Cancer Imaging and Treatment. Ultrasonics 2020, 108, 106198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zheng, Y.; Zhu, Y.; Ding, K.; Zhou, M.; Liu, T. Co-Delivery of Gemcitabine and Triapine by Calcium Carbonate Nanoparticles against Chemoresistant Pancreatic Cancer. Int. J. Pharm. 2023, 636, 122844. [Google Scholar] [CrossRef]
- Yang, T.; Wan, Z.; Liu, Z.; Li, H.; Wang, H.; Lu, N.; Chen, Z.; Mei, X.; Ren, X. In Situ Mineralization of Anticancer Drug into Calcium Carbonate Monodisperse Nanospheres and Their PH-Responsive Release Property. Mater. Sci. Eng. C 2016, 63, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Feng, L.; Hao, Y.; Li, Q.; Chen, M.; Yang, Z.; Zhao, H.; Liu, Z. Synthesis of CaCO3-Based Nanomedicine for Enhanced Sonodynamic Therapy via Amplification of Tumor Oxidative Stress. Chem 2020, 6, 1391–1407. [Google Scholar] [CrossRef]
- Isa, T.; Zakaria, Z.A.B.; Rukayadi, Y.; Hezmee, M.N.M.; Jaji, A.Z.; Imam, M.U.; Hammadi, N.I.; Mahmood, S.K. Antibacterial Activity of Ciprofloxacin-Encapsulated Cockle Shells Calcium Carbonate (Aragonite) Nanoparticles and Its Biocompatability in Macrophage J774A.1. Int. J. Mol. Sci. 2016, 17, 713. [Google Scholar] [CrossRef]
- Chemmalar, S.; Intan-Shameha, A.R.; Abdullah, C.A.C.; Razak, N.A.A.; Yusof, L.M.; Ajat, M.; Gowthaman, N.S.K.; Bakar, M.Z.A. Synthesis and Characterization of Gefitinib and Paclitaxel Mono and Dual Drug-Loaded Blood Cockle Shells (Anadara Granosa)-Derived Aragonite CaCO3 Nanoparticles. Nanomaterials 2021, 11, 1988. [Google Scholar] [CrossRef]
- Tsuzuki, T. Commercial Scale Production of Inorganic Nanoparticles. Int. J. Nanotechnol. 2009, 6, 567–568. [Google Scholar] [CrossRef]
- Islam, K.N.; Bakar, M.Z.B.A.; Ali, M.E.; Hussein, M.Z.B.; Noordin, M.M.; Loqman, M.Y.; Miah, G.; Wahid, H.; Hashim, U. A Novel Method for the Synthesis of Calcium Carbonate (Aragonite) Nanoparticles from Cockle Shells. Powder Technol. 2013, 235, 70–75. [Google Scholar] [CrossRef]
- Banti, C.N.; Hadjikakou, S.K. Evaluation of Toxicity with Brine Shrimp Assay. Bio Protoc. 2021, 11, e3895. [Google Scholar] [CrossRef]
- Chou, T.-C. Preclinical versus Clinical Drug Combination Studies. Leuk. Lymphoma 2008, 49, 2059–2080. [Google Scholar] [CrossRef] [PubMed]
- Trushina, D.B.; Borodina, T.N.; Belyakov, S.; Antipina, M.N. Calcium Carbonate Vaterite Particles for Drug Delivery: Advances and Challenges. Mater. Today Adv. 2022, 14, 100214. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Madan, P.; Lin, S. Effect of Process and Formulation Variables on the Preparation of Parenteral Paclitaxel-Loaded Biodegradable Polymeric Nanoparticles: A Co-Surfactant Study. Asian J. Pharm. Sci. 2016, 11, 404–416. [Google Scholar] [CrossRef]
- Mo, R.; Gu, Z. Tumor Microenvironment and Intracellular Signal-Activated Nanomaterials for Anticancer Drug Delivery. Mater. Today 2016, 19, 274–283. [Google Scholar] [CrossRef]
- Farzan, M.; Roth, R.; Schoelkopf, J.; Huwyler, J.; Puchkov, M. The Processes behind Drug Loading and Release in Porous Drug Delivery Systems. Eur. J. Pharm. Biopharm. 2023, 189, 133–151. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and Comparison of Dissolution Profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- ISO ISO 10993-5:2009; Biological Evaluation of Medical Devices–Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Mukhopadhyay, S.; Veroniaina, H.; Chimombe, T.; Han, L.; Zhenghong, W.; Xiaole, Q. Synthesis and Compatibility Evaluation of Versatile Mesoporous Silica Nanoparticles with Red Blood Cells: An Overview. RSC Adv. 2019, 9, 35566–35578. [Google Scholar] [CrossRef]
- Sæbø, I.P.; Bjørås, M.; Franzyk, H.; Helgesen, E.; Booth, J.A. Optimization of the Hemolysis Assay for the Assessment of Cytotoxicity. Int. J. Mol. Sci. 2023, 24, 2914. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.; Dannenfelser, R.M. In Vitro Hemolysis: Guidance for the Pharmaceutical Scientist. J. Pharm. Sci. 2006, 95, 1173–1176. [Google Scholar] [CrossRef]
- Rajabi, S.; Ramazani, A.; Hamidi, M.; Naji, T. Artemia Salina as a Model Organism in Toxicity Assessment of Nanoparticles. DARU J. Pharm. Sci. 2015, 23, 20. [Google Scholar] [CrossRef]
- Pinheiro, S.K.D.P.; Lima, A.K.M.; Miguel, T.B.A.R.; Filho, A.G.S.; Ferreira, O.P.; Pontes, M.D.S.; Grillo, R.; Miguel, E.D.C. Assessing Toxicity Mechanism of Silver Nanoparticles by Using Brine Shrimp (Artemia Salina) as Model. Chemosphere 2023, 347, 140673. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, C.; Maharaj, V.J.; Crouch, N.R.; Grace, O.M.; Pillay, P.; Matsabisa, M.G.; Bhagwandin, N.; Smith, P.J.; Folb, P.I. In Vitro Antiplasmodial Activity of Medicinal Plants Native to or Naturalised in South Africa. J. Ethnopharmacol. 2004, 92, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Devi, G.R. Three-Dimensional Culture Systems in Cancer Research: Focus on Tumor Spheroid Model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and Challenges for Use of Tumor Spheroids as Models to Test Drug Delivery and Efficacy. J. Control. Release 2012, 164, 192–204. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of Action and Clinical Strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Cho, Y.H.; Ro, E.J.; Yoon, J.S.; Mizutani, T.; Kang, D.W.; Park, J.C.; Kim, T., II; Clevers, H.; Choi, K.Y. 5-FU Promotes Stemness of Colorectal Cancer via P53-Mediated WNT/β-Catenin Pathway Activation. Nat. Commun. 2020, 11, 5321. [Google Scholar] [CrossRef]
- Vázquez, C.; Orlova, M.; Angriman, F.; Minatta, J.N.; Scibona, P.; Verzura, M.A.; Jáuregui, E.G.; Díaz De Arce, H.; Pallotta, M.G.; Belloso, W.H. Prediction of Severe Toxicity in Adult Patients under Treatment with 5-Fluorouracil: A Prospective Cohort Study. Anticancer Drugs 2017, 28, 1039–1046. [Google Scholar] [CrossRef]
- Helmy, S.A.; El-Mesery, M.; El-Karef, A.; Eissa, L.A.; El Gayar, A.M. Thymoquinone Upregulates TRAIL/TRAILR2 Expression and Attenuates Hepatocellular Carcinoma in Vivo Model. Life Sci. 2019, 233, 116673. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, L.; Liu, H.; Li, M.; Liu, Y.; Yang, F.; Pei, Z. Thymoquinone Reduces Cardiac Damage Caused by Hypercholesterolemia in Apolipoprotein E-Deficient Mice. Lipids Health Dis. 2018, 17, 173. [Google Scholar] [CrossRef]
- Velagapudi, R.; Kumar, A.; Bhatia, H.S.; El-Bakoush, A.; Lepiarz, I.; Fiebich, B.L.; Olajide, O.A. Inhibition of Neuroinflammation by Thymoquinone Requires Activation of Nrf2/ARE Signalling. Int. Immunopharmacol. 2017, 48, 17–29. [Google Scholar] [CrossRef]
- Velagapudi, R.; El-Bakoush, A.; Lepiarz, I.; Ogunrinade, F.; Olajide, O.A. AMPK and SIRT1 Activation Contribute to Inhibition of Neuroinflammation by Thymoquinone in BV2 Microglia. Mol. Cell. Biochem. 2017, 435, 149–162. [Google Scholar] [CrossRef]
- Madani, F.; Kazemi, S.; Shirafkan, F.; Lotfi, M.; Hosseini, S.M.; Moghadamnia, A.A. Thymoquinone Protects against 5-Fluorouracil-Induced Mucositis by NF-Κβ and HIF-1 Mechanisms in Mice. J. Biochem. Mol. Toxicol. 2023, 37, e23405. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Younus, H. Thymoquinone Shows the Diverse Therapeutic Actions by Modulating Multiple Cell Signaling Pathways: Single Drug for Multiple Targets. Curr. Pharm. Biotechnol. 2018, 19, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Safavi, M.S.; Shojaosadati, S.A.; Dorkoosh, F.A.; Jo, H.J.; Kwon, Y.; Lee, K.C.; Yang, H.G.; Park, E.J.; Na, D.H. The Synthesis of Tamoxifen-Loaded Albumin Nanoparticles by Homogenizers: Optimization and in Vitro Characterization. J. Drug Deliv. Sci. Technol. 2017, 41, 20–30. [Google Scholar] [CrossRef]
- Dey, D.; Kumar, P.; Samantaray, S. A Review of Nanofluid Preparation, Stability, and Thermo-Physical Properties. Heat Transf. -Asian Res. 2017, 46, 1413–1442. [Google Scholar] [CrossRef]
- Deng, X.; Yang, Z.; Chan, K.W.; Abu Bakar, M.Z. Exploring the Therapeutic Potential of 5-Fluorouracil-Loaded Calcium Carbonate Nanoparticles Combined with Natural Compound Thymoquinone for Colon Cancer Treatment. Pharmaceutics 2024, 16, 1011. [Google Scholar] [CrossRef]
- Haq, A.; Michniak-Kohn, B. Effects of Solvents and Penetration Enhancers on Transdermal Delivery of Thymoquinone: Permeability and Skin Deposition Study. Drug Deliv. 2018, 25, 1943–1949. [Google Scholar] [CrossRef]
- Zorrilla-Veloz, R.I.; Stelzer, T.; López-Mejías, V. Measurement and Correlation of the Solubility of 5-Fluorouracil in Pure and Binary Solvents. J. Chem. Eng. Data 2018, 63, 3809–3817. [Google Scholar] [CrossRef]
- Fang, H.; Ni, K.; Wu, J.; Li, J.; Huang, L.; Reible, D. The Effects of Hydrogen Bonding on the Shear Viscosity of Liquid Water. Int. J. Sediment Res. 2019, 34, 8–13. [Google Scholar] [CrossRef]
- Tadano, T.; Zhu, R.; Muroga, Y.; Hoshi, T.; Sasaki, D.; Yano, S.; Sawaguchi, T. A New Mechanism for the Silica Nanoparticle Dispersion-Agglomeration Transition in a Poly(Methyl Methacrylate)/Silica Hybrid Suspension. Polym. J. 2014, 46, 342–348. [Google Scholar] [CrossRef]
- Di, J.; Gao, X.; Du, Y.; Zhang, H.; Gao, J.; Zheng, A. Size, Shape, Charge and “Stealthy” Surface: Carrier Properties Affect the Drug Circulation Time in Vivo. Asian J. Pharm. Sci. 2021, 16, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Minkowicz, L.; Dagan, A.; Uvarov, V.; Benny, O. Controlling Calcium Carbonate Particle Morphology, Size, and Molecular Order Using Silicate. Materials 2021, 14, 3525. [Google Scholar] [CrossRef] [PubMed]
- Kiranda, H.K.; Mahmud, R.; Abubakar, D.; Zakaria, Z.A. Fabrication, Characterization and Cytotoxicity of Spherical-Shaped Conjugated Gold-Cockle Shell Derived Calcium Carbonate Nanoparticles for Biomedical Applications. Nanoscale Res. Lett. 2018, 13, 1. [Google Scholar] [CrossRef]
- Tavangar, A.; Tan, B.; Venkatakrishnan, K. Synthesis of Three-Dimensional Calcium Carbonate Nanofibrous Structure from Eggshell Using Femtosecond Laser Ablation. J. Nanobiotechnology 2011, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Febrida, R.; Cahyanto, A.; Herda, E.; Muthukanan, V.; Djustiana, N.; Faizal, F.; Panatarani, C.; Joni, I.M. Synthesis and Characterization of Porous CaCO3 Vaterite Particles by Simple Solution Method. Materials 2021, 14, 4425. [Google Scholar] [CrossRef]
- Niu, B.; Chen, H.; Wu, W.; Fang, X.; Mu, H.; Han, Y.; Gao, H. Co-Encapsulation of Chlorogenic Acid and Cinnamaldehyde Essential Oil in Pickering Emulsion Stablized by Chitosan Nanoparticles. Food Chem. X 2022, 14, 100312. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Y.; Zhou, Y.; Guo, D.; Fan, Y.; Guo, F.; Zheng, Y.; Chen, W. Preparation of 5-Fluorouracil-Loaded Chitosan Nanoparticles and Study of the Sustained Release in Vitro and in Vivo. Asian J. Pharm. Sci. 2017, 12, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Xie, X.; Li, Y.; Lian, S.; Lu, Y.; Jia, L. Cancer Metastasis Chemoprevention Prevents Circulating Tumour Cells from Germination. Signal Transduct. Target. Ther. 2022, 7, 341. [Google Scholar] [CrossRef]
- Jiang, W.G.; Sanders, A.J.; Katoh, M.; Ungefroren, H.; Gieseler, F.; Prince, M.; Thompson, S.K.; Zollo, M.; Spano, D.; Dhawan, P.; et al. Tissue Invasion and Metastasis: Molecular, Biological and Clinical Perspectives. Semin. Cancer Biol. 2015, 35, S244–S275. [Google Scholar] [CrossRef]
- Jubelin, C.; Muñoz-Garcia, J.; Griscom, L.; Cochonneau, D.; Ollivier, E.; Heymann, M.-F.; Vallette, F.M.; Oliver, L.; Heymann, D. Three-Dimensional in Vitro Culture Models in Oncology Research. Cell Biosci. 2022, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Recanatini, M.; Cabrelle, C. Drug Research Meets Network Science: Where Are We? J. Med. Chem. 2020, 63, 8653–8666. [Google Scholar] [CrossRef] [PubMed]
- Kennelly, R.; Kavanagh, D.O.; Hogan, A.M.; Winter, D.C. Oestrogen and the Colon: Potential Mechanisms for Cancer Prevention. Lancet Oncol. 2008, 9, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Topi, G.; Satapathy, S.R.; Ghatak, S.; Hellman, K.; Ek, F.; Olsson, R.; Ehrnström, R.; Lydrup, M.L.; Sjölander, A. High Oestrogen Receptor Alpha Expression Correlates with Adverse Prognosis and Promotes Metastasis in Colorectal Cancer. Cell Commun. Signal. 2024, 22, 198. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.-W.; Liu, J.-Y. Pathological and Pharmacological Functions of the Metabolites of Polyunsaturated Fatty Acids Mediated by Cyclooxygenases, Lipoxygenases, and Cytochrome P450s in Cancers. Pharmacol. Ther. 2024, 256, 108612. [Google Scholar] [CrossRef]
- Lee, S.I.L.; Zuo, X.; Shureiqi, I. 15-Lipoxygenase-1 as a Tumor Suppressor Gene in Colon Cancer: Is the Verdict In? Cancer Metastasis Rev. 2011, 30, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Peng, Z.; Wu, Y.; Moussalli, M.J.; Yang, X.L.; Wang, Y.; Parker-Thornburg, J.; Morris, J.S.; Broaddus, R.R.; Fischer, S.M.; et al. Effects of Gut-Targeted 15-LOX-1 Transgene Expression on Colonic Tumorigenesis in Mice. J. Natl. Cancer Inst. 2012, 104, 709–716. [Google Scholar] [CrossRef]
- Liu, F.; Zuo, X.; Liu, Y.; Deguchi, Y.; Moussalli, M.J.; Chen, W.; Yang, P.; Wei, B.; Tan, L.; Lorenzi, P.L.; et al. Suppression of Membranous LRP5 Recycling, Wnt/β-Catenin Signaling, and Colon Carcinogenesis by 15-LOX-1 Peroxidation of Linoleic Acid in PI3P. Cell Rep. 2020, 32, 108049. [Google Scholar] [CrossRef]
- Wang, W.; Yang, J.; Edin, M.L.; Wang, Y.; Luo, Y.; Wan, D.; Yang, H.; Song, C.Q.; Xue, W.; Sanidad, K.Z.; et al. Targeted Metabolomics Identifies the Cytochrome P450 Monooxygenase Eicosanoid Pathway as a Novel Therapeutic Target of Colon Tumorigenesis. Cancer Res. 2019, 79, 1822–1830. [Google Scholar] [CrossRef]
- Beyerle, J.; Holowatyj, A.N.; Haffa, M.; Frei, E.; Gigic, B.; Schrotz-King, P.; Boehm, J.; Habermann, N.; Stiborova, M.; Scherer, D.; et al. Expression Patterns of Xenobiotic-Metabolizing Enzymes in Tumor and Adjacent Normal Mucosa Tissues among Patients with Colorectal Cancer: The Colo Care Study. Cancer Epidemiol. Biomark. Prev. 2020, 29, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Custodio, A.; Feliu, J. Prognostic and Predictive Biomarkers for Epidermal Growth Factor Receptor-Targeted Therapy in Colorectal Cancer: Beyond KRAS Mutations. Crit. Rev. Oncol. Hematol. 2013, 85, 45–81. [Google Scholar] [CrossRef] [PubMed]
- Voisin, L.; Julien, C.; Duhamel, S.; Gopalbhai, K.; Claveau, I.; Saba-El-Leil, M.K.; Rodrigue-Gervais, I.; Gaboury, L.; Lamarre, D.; Basik, M.; et al. Activation of MEK1 or MEK2 Isoform Is Sufficient to Fully Transform Intestinal Epithelial Cells and Induce the Formation of Metastatic Tumors. BMC Cancer 2008, 8, 337. [Google Scholar] [CrossRef]
- Caunt, C.J.; Sale, M.J.; Smith, P.D.; Cook, S.J. MEK1 and MEK2 Inhibitors and Cancer Therapy: The Long and Winding Road. Nat. Rev. Cancer 2015, 15, 577–592. [Google Scholar] [CrossRef]
- Willis, O.; Choucair, K.; Alloghbi, A.; Stanbery, L.; Mowat, R.; Charles Brunicardi, F.; Dworkin, L.; Nemunaitis, J. PIK3CA Gene Aberrancy and Role in Targeted Therapy of Solid Malignancies. Cancer Gene Ther. 2020, 27, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Zehir, A.; Benayed, R.; Shah, R.H.; Syed, A.; Middha, S.; Kim, H.R.; Srinivasan, P.; Gao, J.; Chakravarty, D.; Devlin, S.M.; et al. Mutational Landscape of Metastatic Cancer Revealed from Prospective Clinical Sequencing of 10,000 Patients. Nat. Med. 2017, 23, 703–713. [Google Scholar] [CrossRef]
- Mei, Z.B.; Duan, C.Y.; Li, C.B.; Cui, L.; Ogino, S. Prognostic Role of Tumor PIK3CA Mutation in Colorectal Cancer: A Systematic Review and Meta-Analysis. Ann. Oncol. 2016, 27, 1836–1848. [Google Scholar] [CrossRef]
- Vallejo-Díaz, J.; Chagoyen, M.; Olazabal-Morán, M.; González-García, A.; Carrera, A.C. The Opposing Roles of PIK3R1/P85α and PIK3R2/P85β in Cancer. Trends Cancer 2019, 5, 233–244. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, X.; Li, Z.; Huang, Q.; Li, F.; Li, C.Y. Caspase-3 Regulates the Migration, Invasion and Metastasis of Colon Cancer Cells. Int. J. Cancer 2018, 143, 921–930. [Google Scholar] [CrossRef]
- Eskandari, E.; Eaves, C.J. Paradoxical Roles of Caspase-3 in Regulating Cell Survival, Proliferation, and Tumorigenesis. J. Cell Biol. 2022, 221, e202201159. [Google Scholar] [CrossRef]
- Patel, M.; Horgan, P.G.; McMillan, D.C.; Edwards, J. NF-ΚB Pathways in the Development and Progression of Colorectal Cancer. Transl. Res. 2018, 197, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Giorgi, C.; Siviero, R.; Zecchini, E.; Rizzuto, R. Calcium and Apoptosis: ER-Mitochondria Ca2+ Transfer in the Control of Apoptosis. Oncogene 2008, 27, 6407–6418. [Google Scholar] [CrossRef] [PubMed]
- Can, G.; Akpinar, B.; Baran, Y.; Zhivotovsky, B.; Olsson, M. 5-Fluorouracil Signaling through a Calcium-Calmodulin-Dependent Pathway Is Required for P53 Activation and Apoptosis in Colon Carcinoma Cells. Oncogene 2013, 32, 4529–4538. [Google Scholar] [CrossRef] [PubMed]


| Nanoparticles | C (%) | O (%) | Ca (%) | Na (%) | N (%) | F (%) |
|---|---|---|---|---|---|---|
| CaCO3 NPs | 16.06 | 62.42 | 21.27 | 0.25 | 0 | 0 |
| TQ-CaCO3 NPs | 17.42 | 62.00 | 20.58 | 0 | 0 | 0 |
| TQ | 79.58 | 20.42 | 0 | 0 | 0 | 0 |
| Nanoparticles | a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | Crystallite Size (Å) | Crystallinity (%) |
|---|---|---|---|---|---|---|---|---|
| CaCO3 NPs | 4.96662 | 7.96480 | 5.74860 | 90 | 90 | 90 | 827 | 96.35 |
| TQ-CaCO3 NPs | 4.96283 | 7.96530 | 5.74325 | 90 | 90 | 90 | 848 | 98.18 |
| Peak Assignment | Wavenumber (cm−1) | |
|---|---|---|
| CaCO3 NPs | TQ-CaCO3 NPs | |
| In-plane C-O bending | 707.47 | 707.47 |
| Out of plane C-O bending | 854.80 | 851.60 |
| Symmetric C-O stretching | 1082.21 | 1082.21 |
| Asymmetric C-O stretching | 1450.53 | 1447.33 |
| Symmetric C-O stretching; in-plane C-O bending | 1783.63 | 1783.63 |
| Formulation | pH | Zero-Order Model R2 | First-Order Model R2 | Higuchi Model R2 | Hixon–Crowell Model R2 | Korsmeyer–Peppas Model | |
|---|---|---|---|---|---|---|---|
| R2 | n | ||||||
| TQ-CaCO3 NPs | 7.4 | 0.95013 | 0.94527 | 0.98353 | 0.95486 | 0.98578 | 0.57060 |
| 6.5 | 0.92144 | 0.95302 | 0.98363 | 0.93512 | 0.98266 | 0.53670 | |
| 5.0 | 0.90526 | 0.92793 | 0.98557 | 0.92571 | 0.98765 | 0.45530 | |
| Targets | PDB ID | Ligands | Binding Energy (kcal/Mol) |
|---|---|---|---|
| MAP2K2 | 1S9I | 5-FU | −4.89 |
| TQ | −6.12 | ||
| CASP3 | 3KJF | 5-FU | −4.45 |
| TQ | −4.57 | ||
| PIK3CA | 5DXT | 5-FU | −4.80 |
| TQ | −5.40 | ||
| ESR1 | 6DFN | 5-FU | −5.07 |
| TQ | −6.35 | ||
| CYP3A4 | 8SO1 | 5-FU | −5.31 |
| TQ | −6.79 | ||
| CYP2C19 | 4GQS | 5-FU | −5.08 |
| TQ | −6.25 | ||
| CYP2C9 | 7RL2 | 5-FU | −5.19 |
| TQ | −6.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Yang, Z.; Chan, K.W.; Ismail, N.; Abu Bakar, M.Z. 5-Fluorouracil in Combination with Calcium Carbonate Nanoparticles Loaded with Antioxidant Thymoquinone against Colon Cancer: Synergistically Therapeutic Potential and Underlying Molecular Mechanism. Antioxidants 2024, 13, 1030. https://doi.org/10.3390/antiox13091030
Deng X, Yang Z, Chan KW, Ismail N, Abu Bakar MZ. 5-Fluorouracil in Combination with Calcium Carbonate Nanoparticles Loaded with Antioxidant Thymoquinone against Colon Cancer: Synergistically Therapeutic Potential and Underlying Molecular Mechanism. Antioxidants. 2024; 13(9):1030. https://doi.org/10.3390/antiox13091030
Chicago/Turabian StyleDeng, Xi, Zhongming Yang, Kim Wei Chan, Norsharina Ismail, and Md Zuki Abu Bakar. 2024. "5-Fluorouracil in Combination with Calcium Carbonate Nanoparticles Loaded with Antioxidant Thymoquinone against Colon Cancer: Synergistically Therapeutic Potential and Underlying Molecular Mechanism" Antioxidants 13, no. 9: 1030. https://doi.org/10.3390/antiox13091030
APA StyleDeng, X., Yang, Z., Chan, K. W., Ismail, N., & Abu Bakar, M. Z. (2024). 5-Fluorouracil in Combination with Calcium Carbonate Nanoparticles Loaded with Antioxidant Thymoquinone against Colon Cancer: Synergistically Therapeutic Potential and Underlying Molecular Mechanism. Antioxidants, 13(9), 1030. https://doi.org/10.3390/antiox13091030







