Microencapsulation of Betalains Extracted from Garambullo (Myrtillocactus geometrizans) to Produce Active Chitosan–Polyvinyl Alcohol Films with Delayed Release of Bioactive Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Microcapsules Production and Physico-Chemical Characterization
2.2.1. Determination of the Antioxidant Capacity of Microcapsules and Films
Quantification of Betalains in Microcapsules
Phenolic Content
ABTS ((Ácido 2,2′-Azino-bis-(3-etillbenzotiazolin-6-sulfonic)) Antioxidant Activity
DPPH (2,2-Difenil-1-picrilhidrazil) Determination
FRAP (Ferric Reducing Antioxidant Power) Determination
2.3. Scanning Electron Microscopy (SEM) Analysis
2.4. Bio-Polymeric Film Fabrication
2.5. Physical Characterization of Films
2.6. Mechanical Properties
2.7. Color Determination
2.8. Fourier Transform Infrared Spectroscopy (FTIR)
2.9. Differential Scanning Calorimetry (DSC) Analysis
2.10. Antimicrobial Activity
Disc Contact Assay
2.11. Application on Tomato
2.12. Evaluation of the Coating of CS-PVOH and Microcapsules of Betalains on Tomato
2.13. Physico-Chemical Properties of Coated Tomatoes
2.13.1. Weight Loss (WL)
2.13.2. pH Determination
2.13.3. Soluble Solids (SS)
2.13.4. Titratable Acidity (TA)
2.14. Shelf Life Determination
2.15. Statistical Analysis
3. Results and Discussion
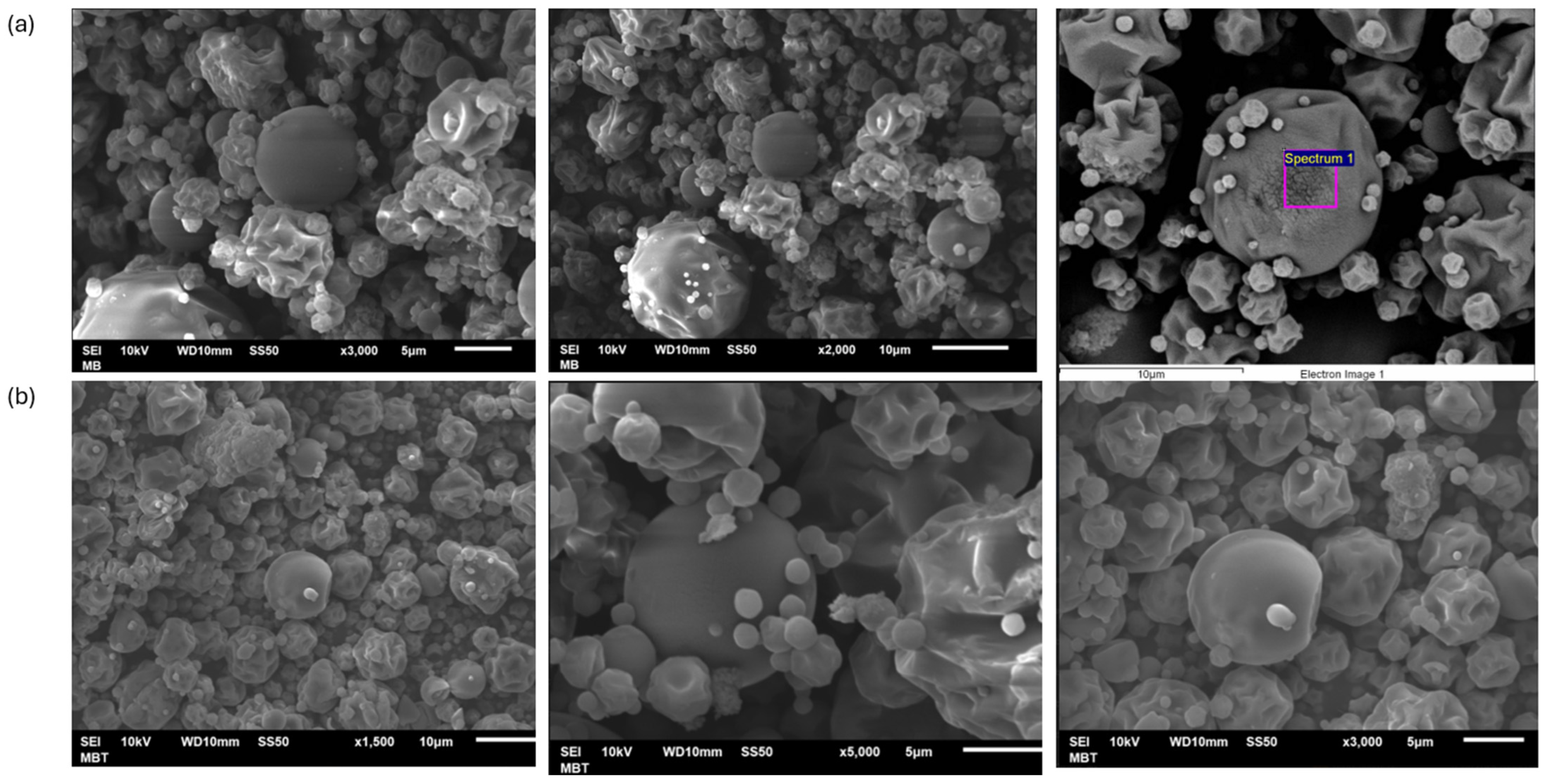
3.1. Microparticle Morphology (SEM)
3.2. Mechanical Characterization of Biopackages
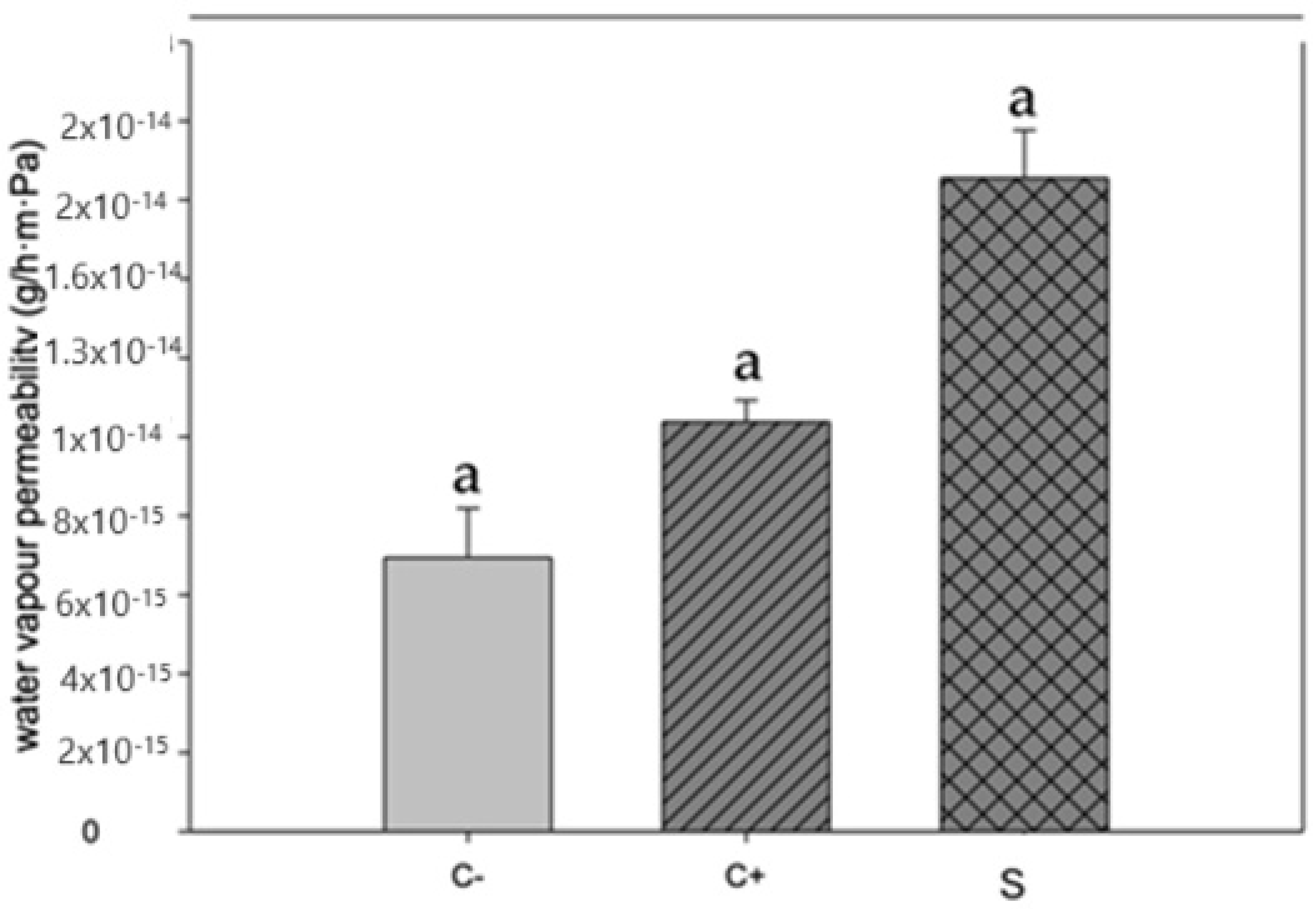
3.3. Water Vapor Permeability
3.4. Puncture Stress and Strain at Extension
3.5. Determination of Color in Biopackaging
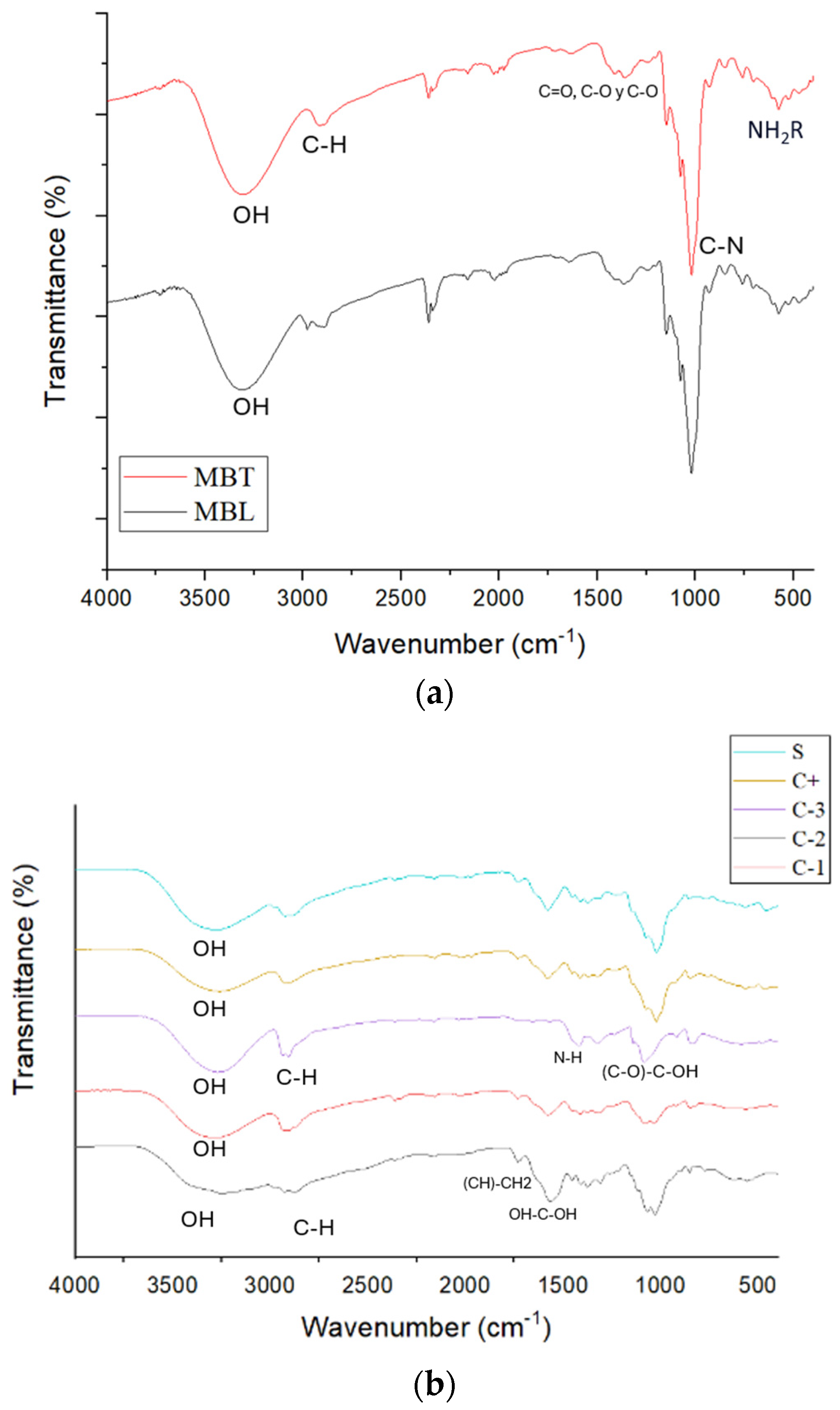
3.6. Fourier Transform Infrared (FTIR) Spectroscopy
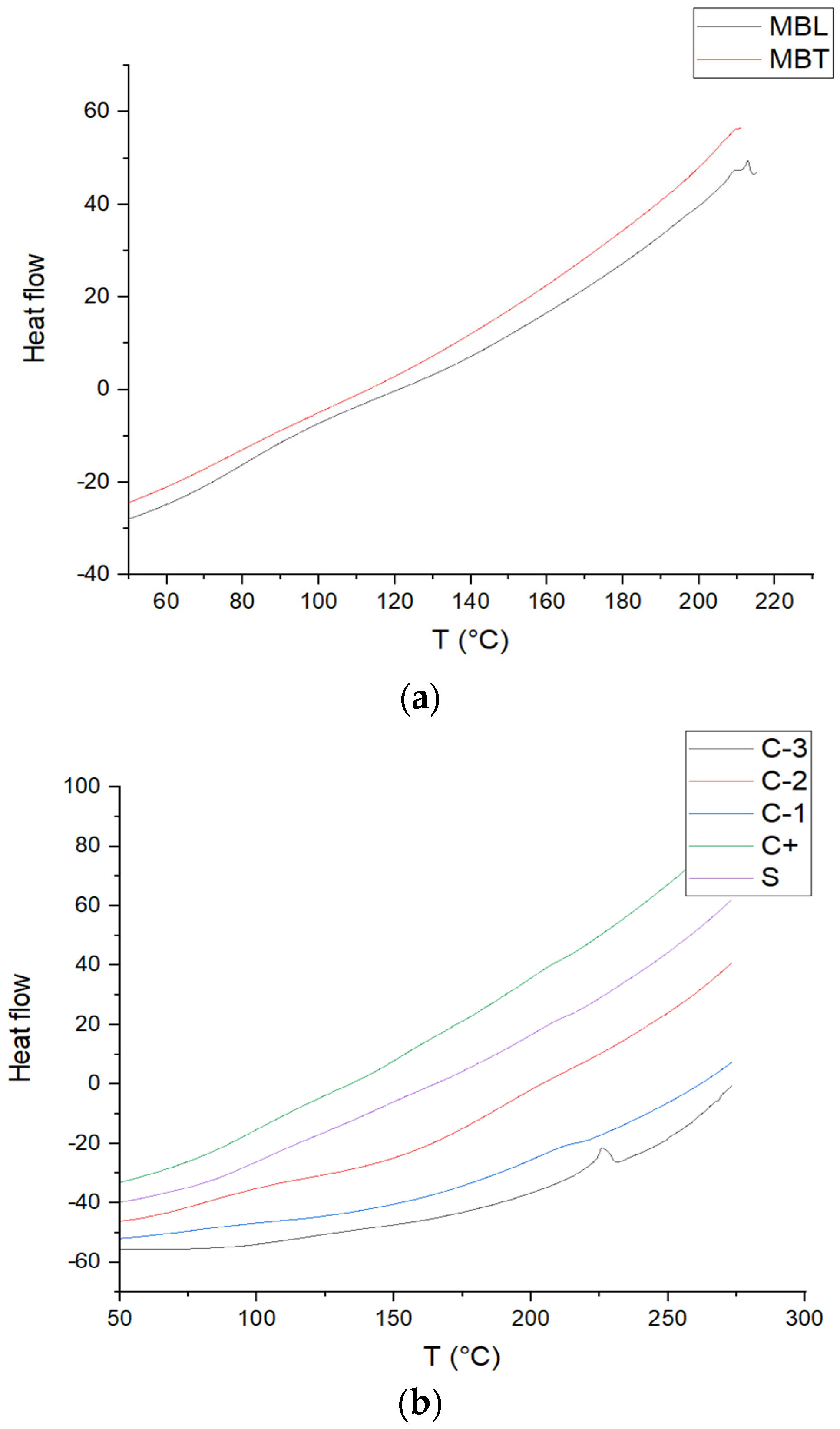
3.7. Determination DSC
3.8. Determination of the Antioxidant Capacity of the Filmogenic Suspension
3.9. Antimicrobial Activity
3.10. Coating Application on Tomato
3.10.1. Physico-Chemical Properties of Tomato
3.10.2. Weight Loss (WL)
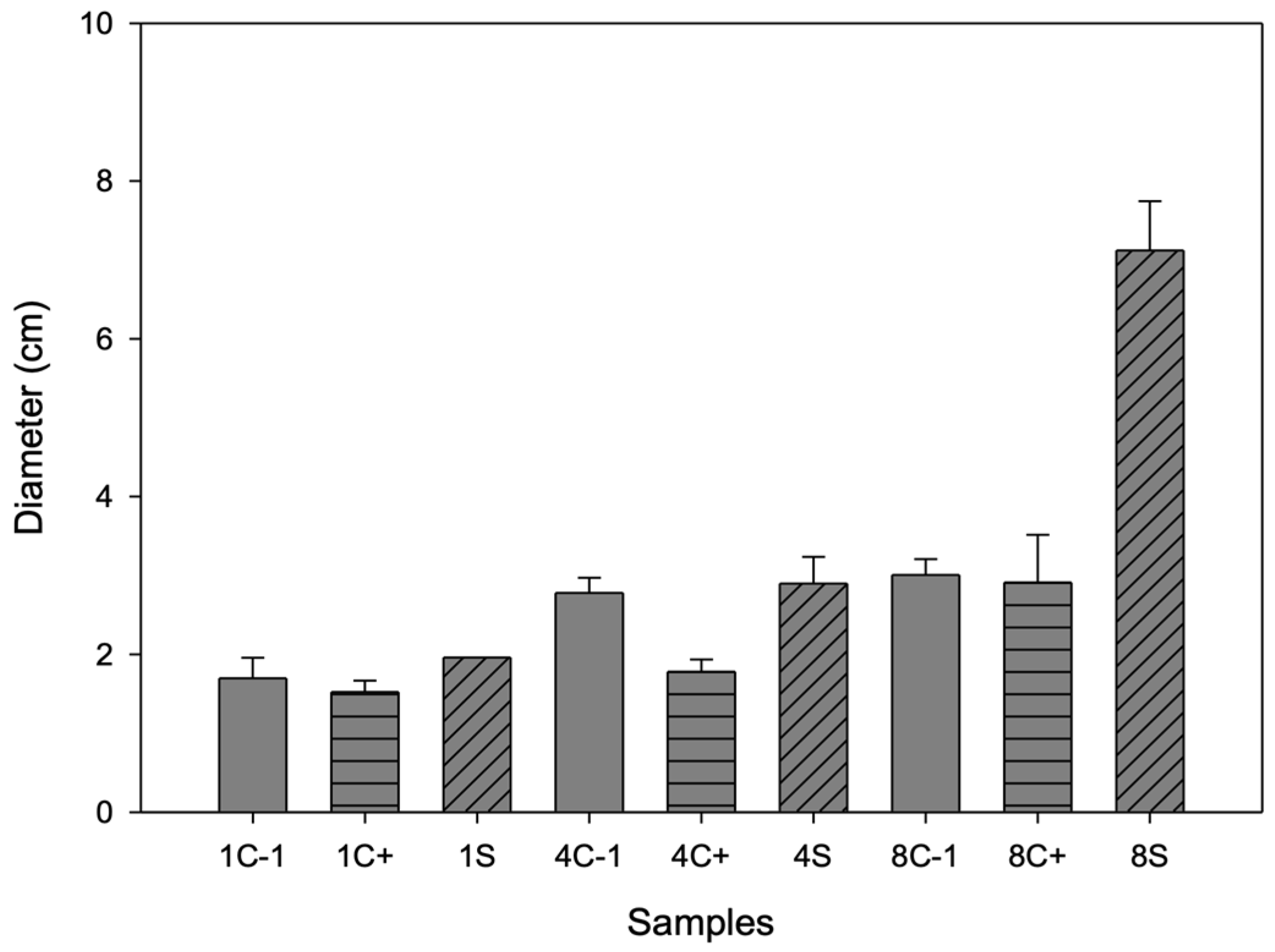
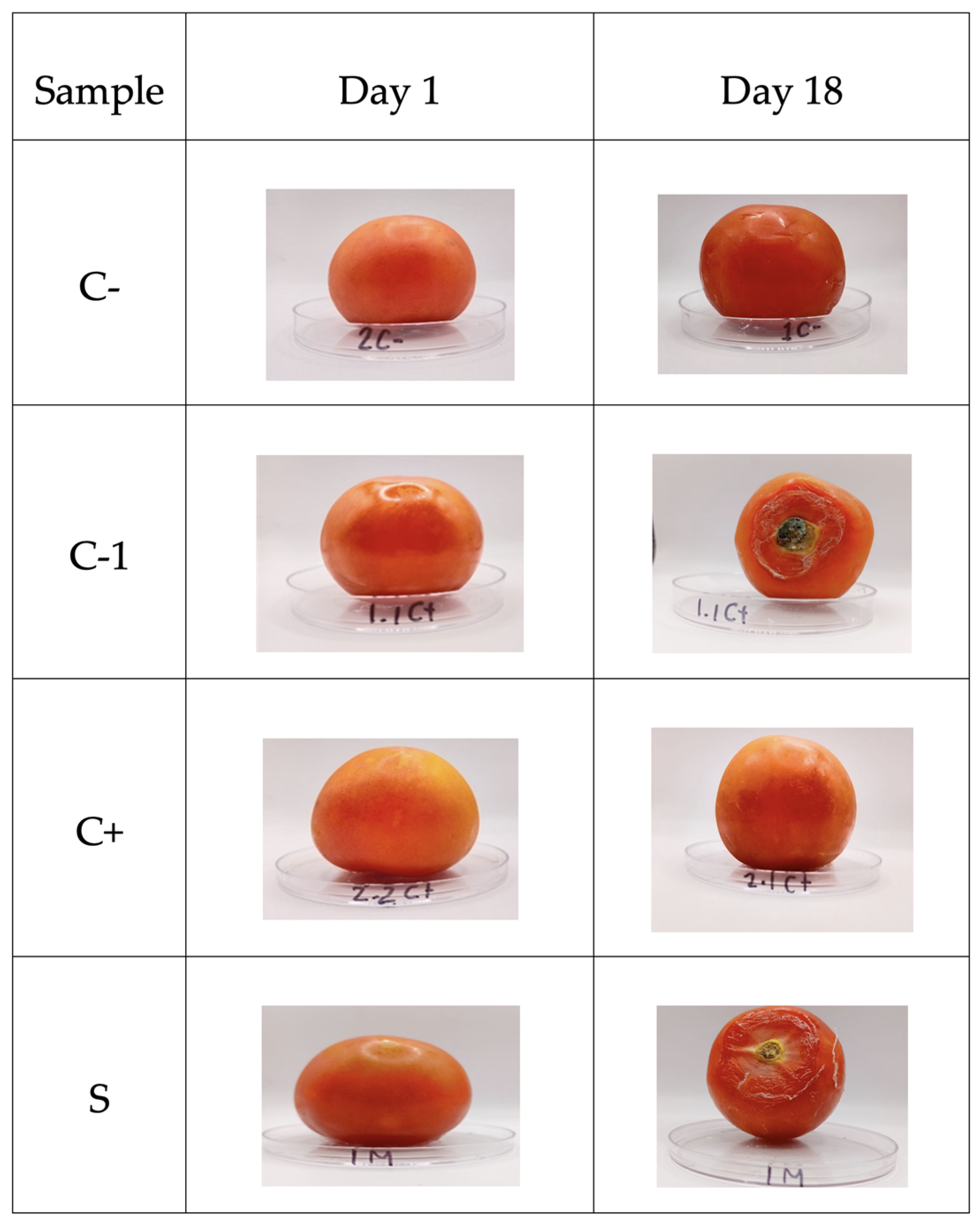
3.10.3. Shelf Life
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreno-Ley, C.M.; Osorio-Revilla, G.; Hernández-Martínez, D.M.; Ramos-Monroy, O.A.; Gallardo-Velázquez, T. Anti-inflammatory activity of betalains: A comprehensive review. Hum. Nutr. Metab. 2021, 25, 200126. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, Y.; Martínez-Huélamo, M.; Pedraza-Chaverri, J.; Ramírez, V.; Martínez-Tagüeña, N.; Trujillo, J. Ethnobotanical, nutritional and medicinal properties of Mexican drylands Cactaceae Fruits: Recent findings and research opportunities. Food Chem. 2020, 312, 126073. [Google Scholar] [CrossRef]
- Rahimi, P.; Abedimanesh, S.; Mesbah-Namin, S.A.; Ostadrahimi, A. Betalains, the nature-inspired pigments, in health and diseases. Crit. Rev. Food Sci. Nutr. 2019, 59, 2949–2978. [Google Scholar] [CrossRef]
- Villaño, D.; Garcia-Viguera, C.; Mena, P. Colors: Health Effects. In The Encyclopedia of Food and Health; Caballero, B., Finglas, P., Toldra, F., Eds.; Oxford Academic Press: Oxford, UK, 2016; Volume 2, pp. 265–272. [Google Scholar]
- Mancha MA, F.; Monterrubio, A.L.R.; Vega, R.S.; Martínez, A.C. Estructura y estabilidad de las betalaínas. Interciencia 2019, 44, 318–325. [Google Scholar]
- Otálora, M.C.; Carriazo, J.G.; Iturriaga, L.; Nazareno, M.A.; Osorio, C. Microencapsulation of betalains obtained from cactus fruit (Opuntia ficus-indica) by spray drying using cactus cladode mucilage and maltodextrin as encapsulating agents. Food Chem. 2015, 187, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.; Desale, R.J.; Fulpagare, Y.G. Microencapsulation: Applications in the different dairy products. Int. J. Pharm. Biomed. Eng. 2020, 6, 7–11. [Google Scholar] [CrossRef]
- Choudhury, N.; Meghwal, M.; Das, K. Microencapsulación: Una visión general de conceptos, métodos, propiedades y aplicaciones en alimentos. Front. Aliment. 2021, 2, 426–442. [Google Scholar] [CrossRef]
- de Sousa, M.S.; Schlogl, A.E.; Estanislau, F.R.; Souza, V.G.L.; dos Reis Coimbra, J.S.; Santos, I.J.B. Nanotecnología en envases para la industria alimentaria: Pasado, presente y futuro. Recubrimientos 2023, 13, 1411. [Google Scholar]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef]
- Román-Doval, R.; Torres-Arellanes, S.P.; Tenorio-Barajas, A.Y.; Gómez-Sánchez, A.; Valencia-Lazcano, A.A. Chitosan: Properties and its application in agriculture in context of molecular weight. Polymers 2023, 15, 2867. [Google Scholar] [CrossRef]
- Barik, M.; BhagyaRaj GV, S.; Dash, K.K.; Shams, R. A thorough evaluation of chitosan-based packaging film and coating for food product shelf-life extension. J. Agric. Food Res. 2024, 16, 101164. [Google Scholar] [CrossRef]
- Elango, J.; Zamora-Ledezma, C.; Alexis, F.; Wu, W.; Maté-Sánchez de Val, J.E. Protein adsorption, calcium-binding ability, and biocompatibility of silver nanoparticle-loaded polyvinyl alcohol (PVA) hydrogels using bone marrow-derived mesenchymal stem cells. Pharmaceutics 2023, 15, 1843. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Novellino, E.; Basile, A. Nutraceutical potential and antioxidant benefits of red pitaya (Hylocereus polyrhizus) extracts. J. Funct. Foods 2012, 4, 129–136. [Google Scholar] [CrossRef]
- Arredondo, J.M.F.; López, A.P. 2020 Caracterización Física y Bioquímica de Frutos de Garambullo (Myrtillocactus geometrizans). Química, Etnobotánica, Economía y Finanzas, 8. Available online: https://dicea.chapingo.mx/wp-content/uploads/2021/02/Quimica-etno-eco-y-Finanzas.pdf (accessed on 18 March 2023).
- Mathan, L.; Dubey, N.; Verma, S.; Singh, K. Factores de transcripción asociados a la respuesta de defensa contra hongos necrótrofos. In En Factores de Transcripción Para la Tolerancia al Estrés Biótico en Plantas; Editorial Internacional Springer: Cham, Switzerland, 2022; pp. 61–78. [Google Scholar]
- Chaouachi, M.; Marzouk, T.; Jallouli, S.; Elkahoui, S.; Gentzbittel, L.; Ben, C.; Djébali, N. Activity assessment of tomato endophytic bacteria bioactive compounds for the postharvest biocontrol of Botrytis cinerea. Postharvest Biol. Technol. 2021, 172, 111389. [Google Scholar] [CrossRef]
- Castellanos Santiago, E.; Yahia, E.M. Identificationand quantificationo of betalains from the fruits of 10 mexicanpricly pear cultivars by High Performance Liquid Cromatography and Electrospray Ionization Mass Spectometry. J. Agric. Food Chem. 2008, 56, 5758–5764. [Google Scholar] [CrossRef]
- Robles, A.M.V. Contenido de Betalainas y Actividad Antioxidante en Brácteas de Bougainvillea Glabra Choisy. Universidad Técnica de Machala. 2016. Available online: http://repositorio.utmachala.edu.ec/handle/48000/7795 (accessed on 18 March 2023).
- George, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef]
- González, A.; Guerrero, J. Betalaínas: Importancia, presencia en vegetales y sus aplicaciones en la industria alimentaria. Dep. Ing. Quím. Aliment. 2008, 2008, 1–9. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Yingyuad, S.; Ruamsin, S.; Leekprokok, T.; Douglas, S.; Pongamphai, S.; Siripatrawan, U. Effect of chitosan coating and vacuum packaging on the quality of refrigerated grilled pork. Packag. Technol. Sci. 2006, 19, 149–157. [Google Scholar] [CrossRef]
- Sobral PD, A.; Menegalli, F.C.; Hubinger, M.D.; Roques, M.A. Mechanical, water vapor barrier and thermal properties of gelatin-based edible films. Food Hydrocoll. 2001, 15, 423–432. [Google Scholar] [CrossRef]
- ASTM Subcommittee D20; 10 on Mechanical Properties. Standard Test Method for Tensile Properties of Thin Plastic Sheeting. American Society for Testing and Materials: West Conshohocken, PA, USA, 1995.
- Sebti, I.; Martial-Gros, A.; Carnet-Pantiez, A.; Grelier, S.; Coma, V. Chit|osan polymer as bioactive coating and film against Aspergillus niger contamination. J. Food Sci. 2005, 70, M100–M104. [Google Scholar] [CrossRef]
- Du, W.X.; Olsen, C.W.; Avena-Bustillos, R.J.; McHugh, T.H.; Levin, C.E.; Friedman, M. Antibacterial activity against E. coli O157:H7, physical properties, and storage stability of novel carvacrol-containing edible tomato films. J. Food Sci. 2008, 73, 378–383. [Google Scholar] [CrossRef]
- AOAC. 1995 Method 942.15: Acidity (titratable) of fruit products. In Official Method of Analysis, 16th ed.; AOAC: Arlington, TX, USA, 1995. [Google Scholar]
- Moawad, S.; El-Kalyoubi, M.; Khallaf, M.; Abd El Mageed, M.A.; Ali, H.; Farouk, A. Influence of Carriers on the Functional Properties of Spray-Dried Flavors During Storage. Egypt. J. Food Sci. 2021, 49, 231–238. [Google Scholar] [CrossRef]
- Utpott, M.; Assis, R.Q.; Pagno, C.H.; Pereira Krigger, S.; Rodrigues, E.; de Oliveira Rios, A.; Hickmann Flôres, S. Evaluation of the use of industrial wastes on the encapsulation of betalains extracted from red pitaya pulp (Hylocereus polyrhizus) by spray drying: Powder stability and application. Food Bioprocess Technol. 2020, 13, 1940–1953. [Google Scholar] [CrossRef]
- Fernández-Repetto, A.; Gómez-Maqueo, A.; García-Cayuela, T.; Guajardo-Flores, D.; Cano, M.P. Analysis of hydrocolloid excipients for controlled delivery of high-value microencapsulated prickly pear extracts. Food Hydrocoll. Health 2023, 3, 100115. [Google Scholar] [CrossRef]
- Hu, H.; Yao, X.; Qin, Y.; Yong, H.; Liu, J. Development of multifunctional food packaging by incorporating betalains from vegetable amaranth (Amaranthus tricolor L.) into quaternary ammonium chitosan/fish gelatin blend films. Int. J. Biol. Macromol. 2020, 159, 675–684. [Google Scholar] [CrossRef]
- Yao, X.; Hu, H.; Qin, Y.; YLiu, J. Development of antioxidant, antimicrobial and ammonia-sensitive films based on quaternary ammonium chitosan, polyvinyl alcohol and betalains-rich cactus pears (Opuntia ficusindica) extract. Food Hydrocoll. 2020, 106, 105896. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Assifaoui, A.; Karbowiak, T.; Debeaufort, F.; Chambin, O. Controlled release of tyrosol and ferulic acid encapsulated in chitosan–gelatin films after electron beam irradiation. Radiat. Phys. Chem. 2016, 118, 81–86. [Google Scholar] [CrossRef]
- Kurek, M.; Benbettaieb, N.; Ščetar, M.; Chaudy, E.; Elez-Garofulić, I.; Repajić, M.; Galić, K. Novel functional chitosan and pectin bio-based packaging films with encapsulated Opuntia-ficus indica waste. Food Biosci. 2021, 41, 100980. [Google Scholar] [CrossRef]
- Fernández Hernández, E.; Sandoval-Castilla, O.; Cuevas-Bernardino, J.C.; Pacheco López, N.A. Caracterización de películas bioactivas elaboradas a partir de miel y quitosano. In Factores de la Producción Agrícola; Perez-Soto, F., Figueroa. Hernández, E., Escamilla Garcia, P.E., Garcia-Núñez, R.M., Godínez-Montoya, L., Eds.; Amilla: México City, Mexico, 2022; pp. 9–21. [Google Scholar]
- Jedlińska, A.; Barańska, A.; Witrowa-Rajchert, D.; Ostrowska-Ligęza, E.; Samborska, K. Dehumidified air-assisted spray-drying of cloudy beetroot juice at low temperature. Appl. Sci. 2021, 11, 6578. [Google Scholar] [CrossRef]
- Sadiq, N.M.; Aziz, S.B.; Kadir, M.F. Development of flexible plasticized ion-conducting polymer blend electrolytes based on polyvinyl alcohol (PVA): Chitosan (CS) with high ion transport parameters close to gel-based electrolytes. Gels 2022, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- Chaux-Gutiérrez, A.M. Avaliação da Liofilização Para Produção de Criogéis Como Material de Parede e Sua Aplicação em Encapsulação de Betalaínas. Universidad Estadual Paulista Julio de Mesquita Filho. São José do Rio Preto. 2019. Available online: https://repositorio.unesp.br/server/api/core/bitstreams/41736a2a-dafe-4022-95be-89ce2a41cbc4/content (accessed on 18 March 2023).
- Aslam, M.; Raza, Z.A.; Siddique, A. Fabrication and chemo-physical characterization of CuO/chitosan nanocomposite-mediated tricomponent PVA films. Polym. Bull. 2021, 78, 1955–1965. [Google Scholar] [CrossRef]
- Chopra, H.; Bibi, S.; Kumar, S.; Khan, M.S.; Kumar, P.; Singh, I. Preparation and evaluation of chitosan/PVA based hydrogel films loaded with honey for wound healing application. Gels 2022, 8, 111. [Google Scholar] [CrossRef]
- Qiao, C.; Ma, X.; Wang, X.; Liu, L. Structure and properties of chitosan films: Effect of the type of solvent acid. LWT 2021, 135, 109984. [Google Scholar] [CrossRef]
- Goncalves, L.C.; Lopes, N.B.; Augusto, F.A.; Pioli, R.M.; Machado, C.O.; Freitas-Dörr, B.C.; Suffredini, H.B.; Bastos, E.L. Phenolic betalain as antioxidants: Meta means more. Pure Appl. Chem. 2020, 92, 243–253. [Google Scholar] [CrossRef]
- Gómez-Espinoza, D.; Ríos-Fuentes, B.; Aguirre-Mancilla, C.L.; Villaseñor-Ortega, F.; Pérez-Pérez, M.C.I. Microencapsulation of Betalains Obtained from Garambullo Fruit (Myrtillocactus geometrizans) by Spray Drying Microencapsulación de Betalainas Obtenidas del Fruto de Garambullo (Myrtillocactus geometrizans) por Secado por Aspersión. Available online: https://rmiq.org/iqfvp/Numbers/V23/No2/Alim24247.pdf (accessed on 18 March 2023).
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial properties of chitosan and chitosan derivatives in the treatment of enteric infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Biological properties and applications of betalains. Molecules 2021, 26, 2520. [Google Scholar] [CrossRef]
- Vera AE, V.; Vera YA, L.; Mendoza SV, G.; Cedeño SD, R.V.; Chila, C.F.C. Calidad de pitahaya amarilla (Selenicereus megalanthus) en diferentes estados de madurez y temperaturas de conservación. Rev. Espamcienc. 2021, 12, 141–151. [Google Scholar] [CrossRef]
- Machalela, A.A.; Júnior AA, M.; Vatiro, A.; Nanelo, R.F. Production and Characterization of Tomato (Lycopersicon esculentum) Jam. Asian Food Sci. J. 2023, 22, 120–131. [Google Scholar] [CrossRef]
- Candéo, M.; Canteri MH, G.; Macedo DC, D.; Kubaski, E.T.; Tebcherani, S.M. Postharvest durability of tomatoes with PVA covering. Hortic. Bras. 2020, 38, 160–165. [Google Scholar] [CrossRef]
- Kaushal, H. Identifying Possible Quality Deviations and Establishing Critical Quality Points in Perishable Fruits and Vegetables with Bio-Based Packaging Materials; Food Quality and Design (FQD) Wageningen University & Research: Wageningen, The Netherlands, 2020. [Google Scholar]
- Sati, F.; Qubbaj, T. Effect of calcium chloride postharvest treatment in combination with plant natural substance coating on fruit quality and storability of tomato (Solanum lycopersicum) fruits during cold storage. J. Appl. Bot. Food Qual. 2021, 94, 100–107. [Google Scholar]
- Salmas, C.E.; Giannakas, A.E.; Moschovas, D.; Kollia, E.; Georgopoulos, S.; Gioti, C.; Leontiou, A.; Avgeropoulos, A.; Kopsacheili, A.; Avdylaj, L.; et al. Kiwi Fruits Preservation Using Novel Edible Active Coatings Based on Rich Thymol Halloysite Nanostructures and Chitosan/Polyvinyl Alcohol Gels. Gels 2022, 8, 823. [Google Scholar] [CrossRef] [PubMed]
- Salmas, C.E.; Leontiou, A.; Kollia, E.; Zaharioudakis, K.; Kopsacheili, A.; Avdylaj, L.; Georgopoulos, S.; Karabagias, V.K.; Karydis-Messinis, A.; Kehayias, G.; et al. Active Coatings Development Based on Chitosan/Polyvinyl Alcohol Polymeric Matrix Incorporated with Thymol Modified Activated Carbon Nanohybrids. Coatings 2023, 13, 1503. [Google Scholar] [CrossRef]
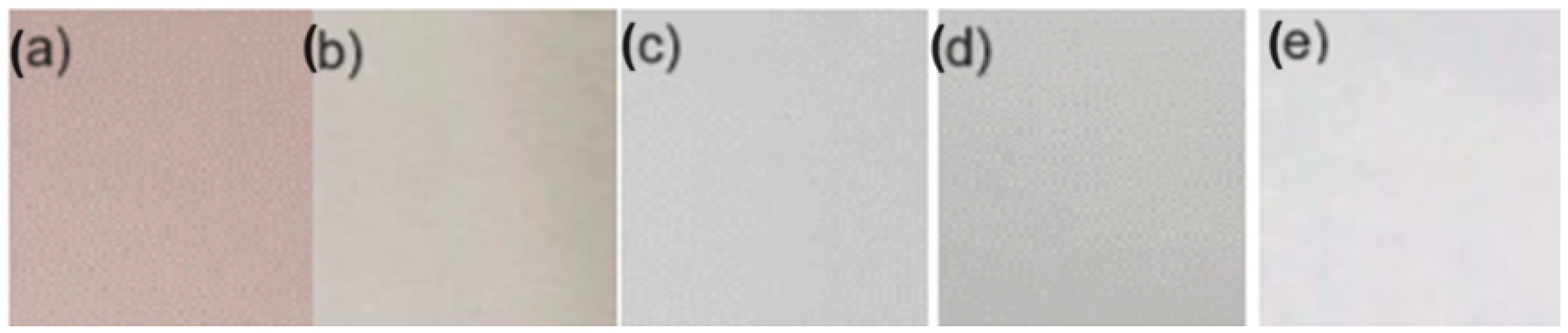


| T (°C) | L | a | b | C | h |
|---|---|---|---|---|---|
| 160 | 87.1 ± 2.4 | 13.7 ± 2.1 | 1.2 ± 0.1 | 13.8 ± 2.1 | 355.0 ± 0.5 |
| Betacyanin (mg Pigment/100 g Sample) | Betaxanthin (mg Pigment/100 g Sample) | Total Betalain (mg Pigment/100 g Sample) | Water Activity | Condensed Tannins (Eq. of (+) Catechin mg/100 g of Sample) |
|---|---|---|---|---|
| 6.1 × 10−3 ± 1 × 10−3 | 6.3 × 10−3 ± 1 × 10−3 | 1.22 × 10−2 ± 1 × 10−3 | 0.31 ± 0.005 | 1 × 10−2 ± 0 |
| Phenols (AEG mg/100 g of Sample) | DPPH (ET mg/100 g of Sample) | ABTS (ET mg/100 g of Sample) | FRAP (ET mg/100 g of Sample) |
|---|---|---|---|
| 4475 ± 907 | 85,971.4 ± 2439.8 | 21,900 ± 2509.9 | 133,469 ± 11,006 |
| B | Weight (g) | Thickness (m) | Sample Area (m2) | Surface Density (kg/m2) | Density (kg/m3) |
|---|---|---|---|---|---|
| C+ | 0.2 ± 2.9 × 10−3 a | 8.3 × 10−4 ± 7.6 × 10−5 b | 4.4 × 10−3 ± 5 × 10−4 a | 5 × 10−2 ± 9 × 10−3 a | 6.1 × 102 ± 0.92 × 102 a |
| C-1 | 0.2 ± 2.9 × 10−2 a | 8.9 × 10−4 ± 5.8 × 10−5 b | 4.5 × 10−3 ± 5 × 10−4 a | 5.2 × 10−2 ± 8 × 10−3 a | 5.9 × 102 ± 1.20 × 102 a |
| S | 0.2 × 10−2 ± 1.2 × 10−3 b | 1.3 × 10−3 ± 2.6 × 10−5 a | 4.6 × 10−3 ± 7 × 10−4 a | 3.4 × 10−2 ± 3.5 × 10−2 b | 2.7 × 102 ± 0.77 × 102 b |
| B | Young’s Modulus (MPa) | Tensile Strength (MPa) | Puncture Strength (Kg) | Deformation at the Breaking Point | % Elongation |
|---|---|---|---|---|---|
| C-1 | 6.400 ± 2 a | 22.200 ± 0.4 a | 6.870 ± 1.5 × 10−4 c | 0.569 ± 2 × 10−3 b | 5.700 ± 0 a |
| C+ | 6.200 ± 1.4 a | 23.500 ± 2.5 a | 9.810 ± 1.2 × 10−4 b | 1.620 ± 3 × 10−3 a | 4.500 ± 0 c |
| S | 5.500 ± 8 a | 22.800 ± 3 a | 1.262 × 10−3 ± 0 a | 1.620 ± 8 × 10−4 a | 5 ± 0.5 b |
| Color | |||||
|---|---|---|---|---|---|
| B | L | a | b | C | h |
| C-1 | 83.0 ± 0.2 a | 4.0 ± 0.02 b | −0.4 ± 0.5 b | 4 ± 0 b | 19.4 ± 1.4 a |
| C+ | 81.9 ± 0.3 b | 4.2 ± 0.20 a | 1.1 ± 0.6 a | 4.9 ± 0.1 a | 13.8 ± 6.8 a |
| S | 81.7 ± 0.5 b | 4.6 ± 0.01 a | 1.5 ± 0.3 a | 4.8 ± 0.1 a | 17.9 ± 3.4 a |
| Physicochemical Properties | Day 1 S | Day 9 S | Day 9 C+ | Day 9 C-1 |
|---|---|---|---|---|
| pH | 4.5 ± 0.0 b | 4.6 ± 0.0 b | 5.1 ± 0.2 a | 4.6 ± 0.2 b |
| °Brix | 4.5 ± 0 b | 5.5 ± 0.3 a | 4.6 ± 0.1 b | 5.5 ± 0.2 a |
| Titratable acidity g acid/100 mL sample | 1.2 ± 0.2 a | 1.0 ± 0.0 b | 0.9 ± 0.1 b | 0.8 ± 0.0 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Espinoza, D.; Gonzalez-Calderon, J.A.; Rivera-Vázquez, R.; Aguirre-Mancilla, C.L.; Delgado-Alvarado, E.; Herrera-May, A.L.; Pérez-Pérez, M.C.I. Microencapsulation of Betalains Extracted from Garambullo (Myrtillocactus geometrizans) to Produce Active Chitosan–Polyvinyl Alcohol Films with Delayed Release of Bioactive Compounds. Antioxidants 2024, 13, 1031. https://doi.org/10.3390/antiox13091031
Gómez-Espinoza D, Gonzalez-Calderon JA, Rivera-Vázquez R, Aguirre-Mancilla CL, Delgado-Alvarado E, Herrera-May AL, Pérez-Pérez MCI. Microencapsulation of Betalains Extracted from Garambullo (Myrtillocactus geometrizans) to Produce Active Chitosan–Polyvinyl Alcohol Films with Delayed Release of Bioactive Compounds. Antioxidants. 2024; 13(9):1031. https://doi.org/10.3390/antiox13091031
Chicago/Turabian StyleGómez-Espinoza, Daniela, J. A. Gonzalez-Calderon, Ricardo Rivera-Vázquez, César Leobardo Aguirre-Mancilla, Enrique Delgado-Alvarado, Agustín L. Herrera-May, and Ma. Cristina Irma Pérez-Pérez. 2024. "Microencapsulation of Betalains Extracted from Garambullo (Myrtillocactus geometrizans) to Produce Active Chitosan–Polyvinyl Alcohol Films with Delayed Release of Bioactive Compounds" Antioxidants 13, no. 9: 1031. https://doi.org/10.3390/antiox13091031
APA StyleGómez-Espinoza, D., Gonzalez-Calderon, J. A., Rivera-Vázquez, R., Aguirre-Mancilla, C. L., Delgado-Alvarado, E., Herrera-May, A. L., & Pérez-Pérez, M. C. I. (2024). Microencapsulation of Betalains Extracted from Garambullo (Myrtillocactus geometrizans) to Produce Active Chitosan–Polyvinyl Alcohol Films with Delayed Release of Bioactive Compounds. Antioxidants, 13(9), 1031. https://doi.org/10.3390/antiox13091031








