Biochemical Characterization of New Sweet Orange Mutants Rich in Lycopene and β-Carotene Antioxidants
Abstract
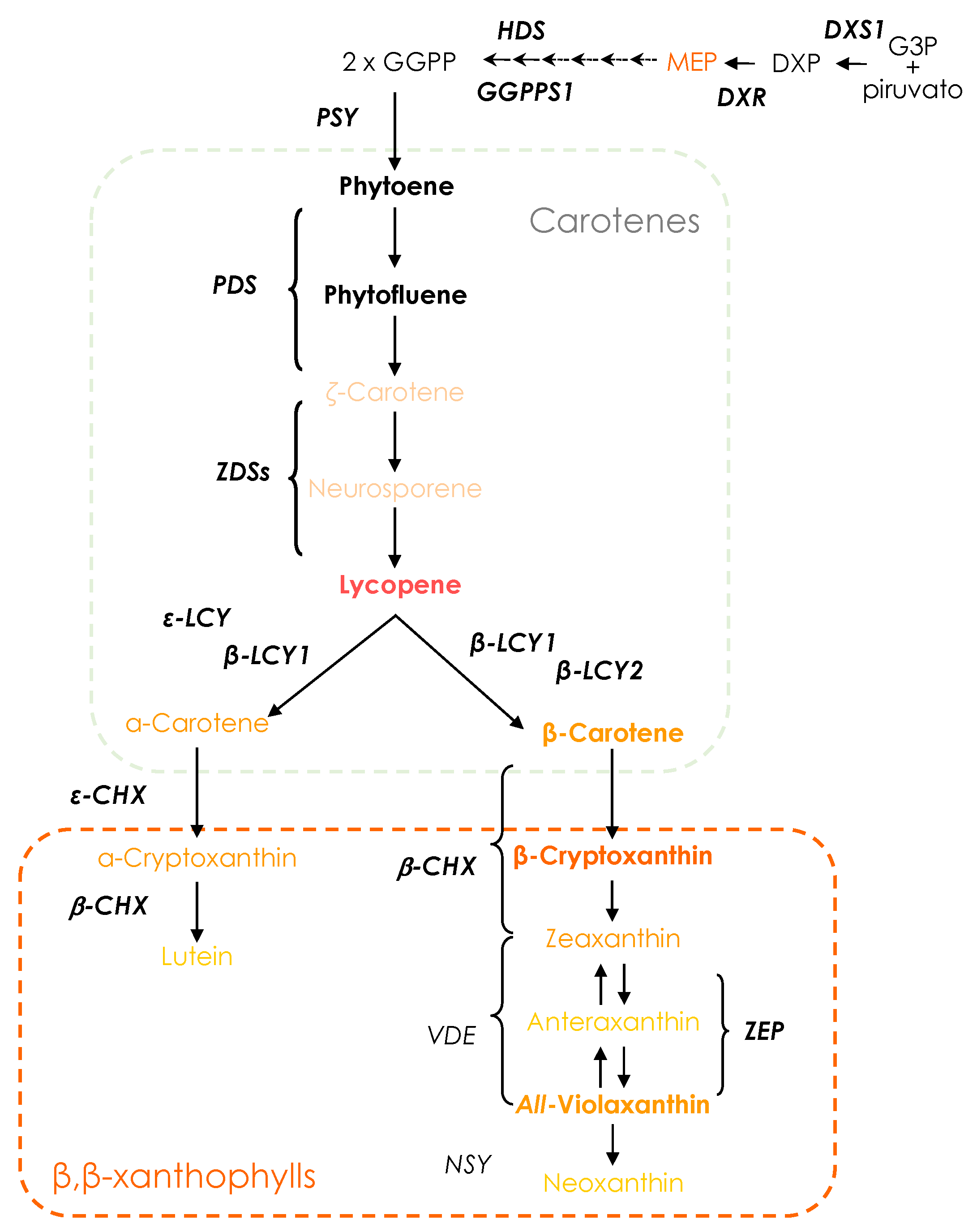
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Analysis of Fruit Quality
2.3. Carotenoid Extraction and UPLC Analysis of Individual Carotenoids
2.4. Total RNA Isolation and Quantitative RT-PCR Analysis
3. Results
3.1. Fruit Appearance at Different Developmental Stages and Fruit Quality Parameters of New Red-Fleshed Sweet Orange Mutants
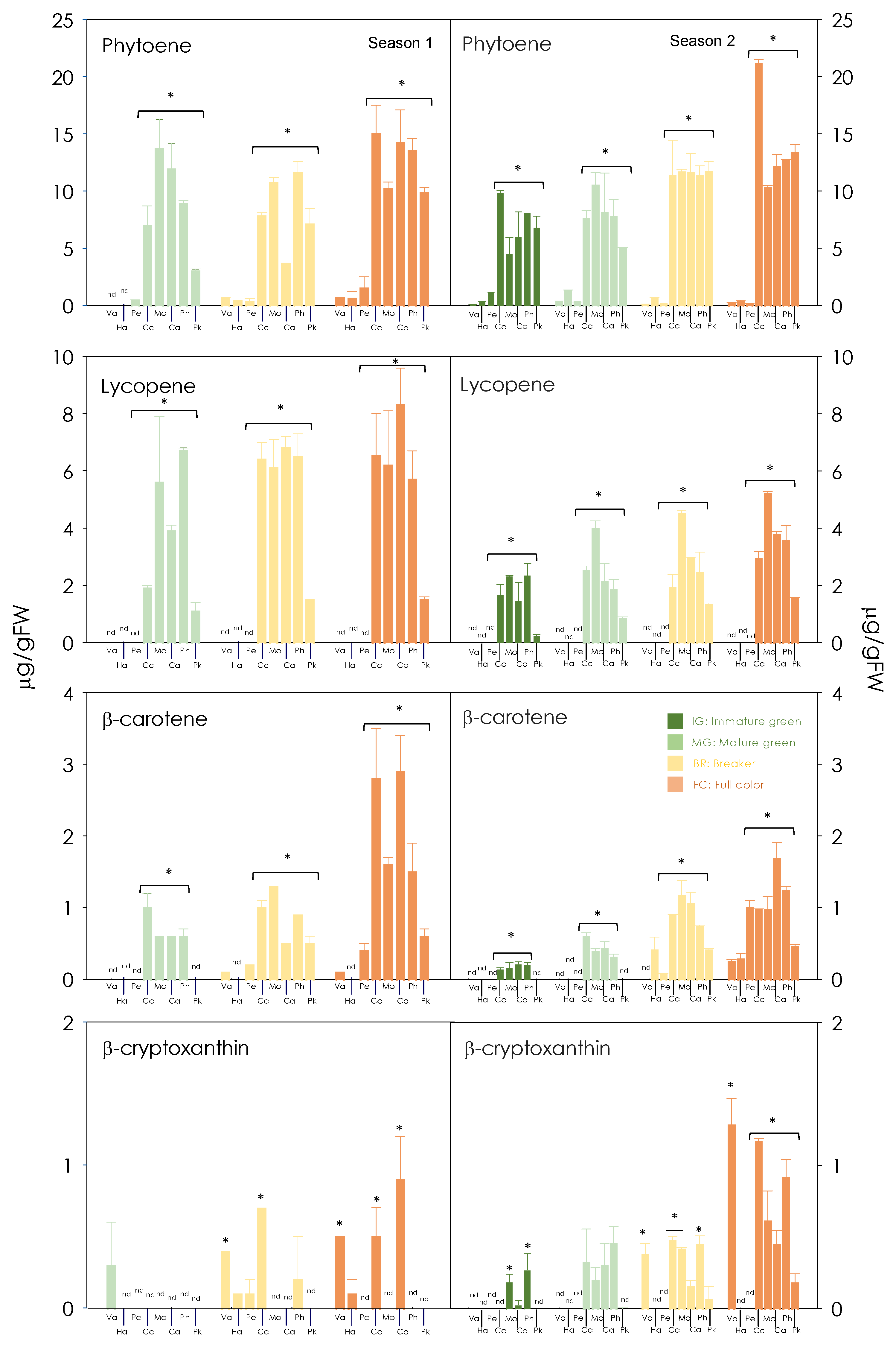
3.2. Carotenoid Composition of Red-Fleshed Sweet Orange Pulp during Maturation
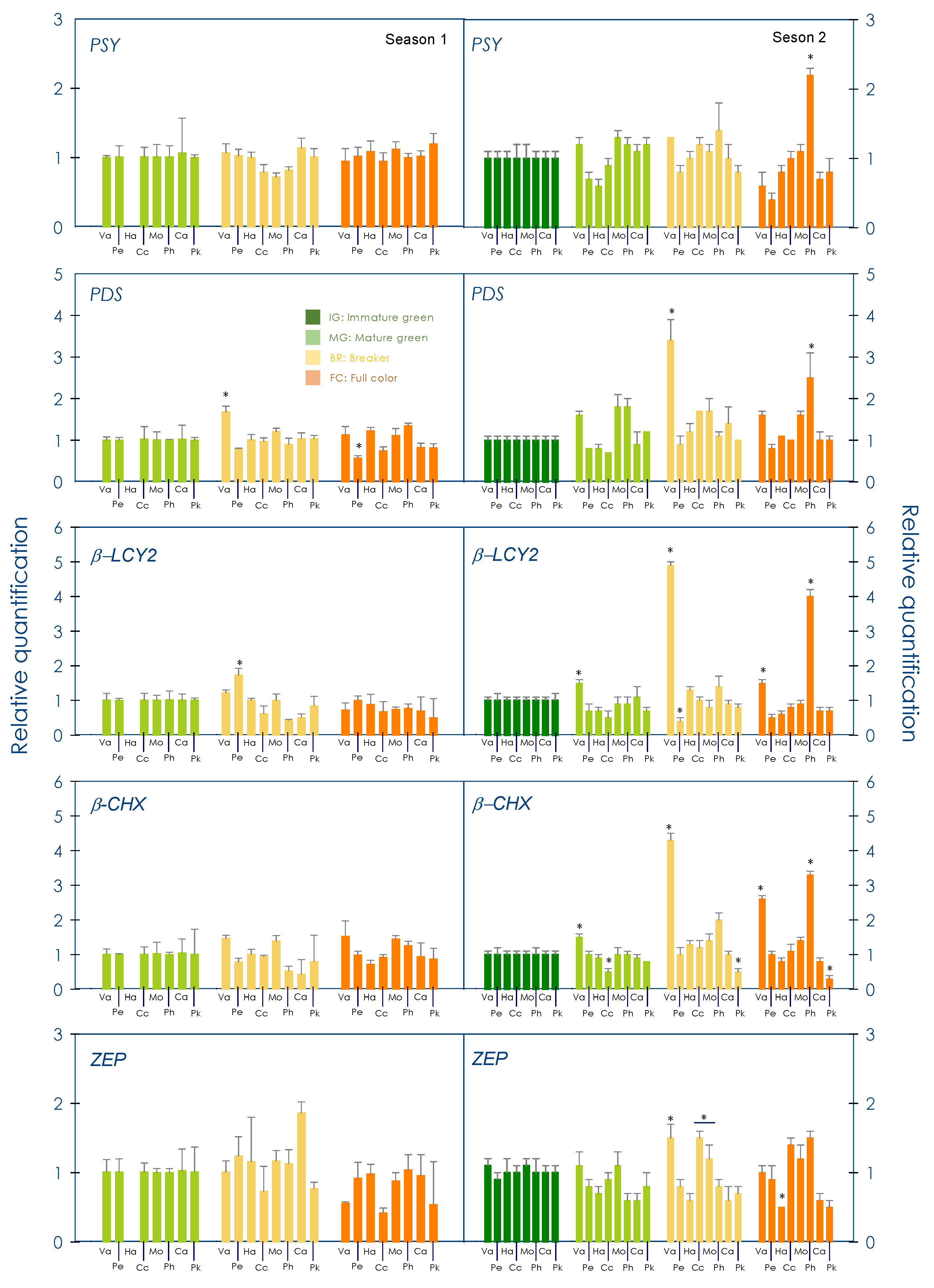
3.3. Gene Expression Profile of Carotenogenic Genes during Development and Maturation in the Pulp of Red-Fleshed Sweet Oranges
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demmig-Adams, B.; Gilmore, A.M.; Iii, W.W.A. Carotenoids 3: In Vivo Function of Carotenoids in Higher Plants. FASEB J. 1996, 10, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.D.; Bramley, P.M. The Biosynthesis and Nutritional Uses of Carotenoids. Prog. Lipid Res. 2004, 43, 228–265. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Murakoshi, M.; Tokuda, H.; Satomi, Y. Cancer Prevention by Carotenoids. Arch. Biochem. Biophys. 2009, 483, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Uchiyama, S. Osteoporosis Prevention by β-Cryptoxanthin. ACS Symp. Ser. 2008, 993, 408–418. [Google Scholar]
- Bohn, T.; Balbuena, E.; Ulus, H.; Iddir, M.; Wang, G.; Crook, N.; Eroglu, A. Carotenoids in Health as Studied by Omics-Related Endpoints. Adv. Nutr. 2023, 14, 1538–1578. [Google Scholar] [CrossRef]
- Terao, J. Revisiting Carotenoids as Dietary Antioxidants for Human Health and Disease Prevention. Food Funct. 2023, 14, 7799–7824. [Google Scholar] [CrossRef] [PubMed]
- Edem, D.O. Vitamin A: A Review. Asian J. Clin. Nutr. 2009, 1, 65–82. [Google Scholar] [CrossRef]
- Burri, B.J. Beta-Cryptoxanthin as a Source of Vitamin A. J. Sci. Food Agric. 2015, 95, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. Color and Carotenoid Profile of Spanish Valencia Late Ultrafrozen Orange Juices. Food Res. Int. 2005, 38, 931–936. [Google Scholar] [CrossRef]
- Hirschberg, J. Carotenoid Biosynthesis in Flowering Plants. Curr. Opin. Plant Biol. 2001, 4, 210–218. [Google Scholar] [CrossRef]
- Dellapenna, D.; Pogson, B.J. Vitamin Synthesis in Plants: Tocopherols and Carotenoids. Annu. Rev. Plant Biol. 2006, 57, 711–738. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/ (accessed on 1 June 2023).
- Meléndez-Martínez, A.; Vicario, I.M.; Heredia, F.J. Carotenoid Pigments: Structural and Physicochemical Considerations. Arch. Latinoam. Nutr. 2007, 57, 109–117. [Google Scholar] [PubMed]
- Stewart, I. Provitamin A and Carotenoid Content of Citrus Juices. J. Am. Chem. Soc. Chromatogr. 1976, 77, 1132–1137. [Google Scholar] [CrossRef]
- Stewart, I.; Wheaton, T.A. Conversion of β-Citraurin to Reticulataxanthin and β-Apo-8′-Carotenal to Citranaxanthin during the Isolation of Carotenoids from Citrus. Phytochemistry 1973, 12, 2947–2951. [Google Scholar] [CrossRef]
- Gross, J. Pigments in Fruits; Academic Press: London, UK, 1987. [Google Scholar]
- Alquézar, B.; Jesús Rodrigo, M.; Zacarías, L. Carotenoid Biosynthesis and Their Regulation in Citrus Fruits. Tree For. Sci. Biotechnol. 2008, 2, 23–35. [Google Scholar]
- Ikoma, Y.; Matsumoto, H.; Kato, M. Diversity in the Carotenoid Profiles and the Expression of Genes Related to Carotenoid Accumulation among Citrus Genotypes. Breed. Sci. 2016, 66, 139–147. [Google Scholar] [CrossRef]
- Römer, S.; Fraser, P.D. Recent Advances in Carotenoid Biosynthesis, Regulation and Manipulation. Planta 2005, 221, 305–308. [Google Scholar] [CrossRef]
- Kato, M.; Ikoma, Y.; Matsumoto, H.; Sugiura, M.; Hyodo, H.; Yano, M. Accumulation of Carotenoids and Expression of Carotenoid Biosynthetic Genes during Maturation in Citrus Fruit. Plant Physiol. 2004, 134, 824–837. [Google Scholar] [CrossRef]
- Fanciullino, A.L.; Dhuique-Mayer, C.; Luro, F.; Morillon, R.; Ollitrault, P. Carotenoid Biosynthetic Pathway in the Citrus Genus: Number of Copies and Phylogenetic Diversity of Seven Genes. J. Agric. Food Chem. 2007, 55, 7405–7417. [Google Scholar] [CrossRef]
- Mendes, A.F.D.S.; Soares, V.L.F.; Costa, M.G.C. Carotenoid Biosynthesis Genomics. In Pigments in Fruits and Vegetables; Springer: New York, NY, USA, 2015; pp. 9–29. [Google Scholar]
- Rodríguez-Concepció, M.; Boronat, A. Elucidation of the Methylerythritol Phosphate Pathway for Isoprenoid Biosynthesis in Bacteria and Plastids. Plant Physiol. 2002, 130, 1079–1089. [Google Scholar] [CrossRef]
- Rodríguez-Concepción, M. Early Steps in Isoprenoid Biosynthesis: Multilevel Regulation of the Supply of Common Precursors in Plant Cells. Phytochem. Rev. 2006, 5, 1–15. [Google Scholar] [CrossRef]
- James, S. Citrus Varieties of the World: An Illustrated Guide; Sinclair International Ltd.: Norwich, UK, 2000. [Google Scholar]
- Saini, R.K.; Rengasamy, K.R.R.; Mahomoodally, F.M.; Keum, Y.S. Protective Effects of Lycopene in Cancer, Cardiovascular, and Neurodegenerative Diseases: An Update on Epidemiological and Mechanistic Perspectives. Pharmacol. Res. 2020, 155, 104730. [Google Scholar] [CrossRef] [PubMed]
- Shafe, M.O.; Gumede, N.M.; Nyakudya, T.T.; Chivandi, E. Lycopene: A Potent Antioxidant with Multiple Health Benefits. J. Nutr. Metab. 2024, 2024, 6252426. [Google Scholar] [CrossRef] [PubMed]
- Monselise, S.P.; Halevy, A.H. Detection of Lycopene in Pink Orange Fruit. Science 1961, 133, 1478. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, J.; Liu, Y.; Zhao, X.; Deng, X.; Guo, L.; Gu, J. A Novel Bud Mutation That Confers Abnormal Patterns of Lycopene Accumulation in Sweet Orange Fruit (Citrus sinensis L. Osbeck). J. Exp. Bot. 2007, 58, 4161–4171. [Google Scholar] [CrossRef]
- Lee, H.S. Characterization of Carotenoids in Juice of Red Navel Orange (Cara Cara). J. Agric. Food Chem. 2001, 49, 2563–2568. [Google Scholar] [CrossRef] [PubMed]
- Ollitrault, P. The Genus Citrus, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Pompeu, J. Porta Enxertos. In Citros; Mattos, D., de Negri, J.D., Pio, R.M., Pompeu, J., Eds.; Fundag Campinas, Instituto Agronomico: Campinas, Brazil, 2005; pp. 61–104. [Google Scholar]
- Pinheiro, T.; Nishimura, D.; de Nadai, F.; Figueira, A.; Latado, R. Selection of Reference Genes for Expression Analyses of Red-Fleshed Sweet Orange (Citrus sinensis). Genet. Mol. Res. 2015, 14, 18440–18451. [Google Scholar] [CrossRef] [PubMed]
- Pons, E.; Peris, J.E.; Peña, L. Field Performance of Transgenic Citrus Trees: Assessment of the Long-Term Expression of UidA and NptII Transgenes and Its Impact on Relevant Agronomic and Phenotypic Characteristics. BMC Biotechnol. 2012, 12, 41. [Google Scholar] [CrossRef]
- Carmona, L.; Zacarías, L.; Rodrigo, M.J. Stimulation of Coloration and Carotenoid Biosynthesis during Postharvest Storage of ‘Navelina’ Orange Fruit at 12 °C. Postharvest Biol. Technol. 2012, 74, 108–117. [Google Scholar] [CrossRef]
- de Oliveira Caland, R.B.; Cadavid, C.O.M.; Carmona, L.; Peña, L.; de Paula Oliveira, R. Pasteurized Orange Juice Rich in Carotenoids Protects Caenorhabditis Elegans against Oxidative Stress and β-Amyloid Toxicity through Direct and Indirect Mechanisms. Oxidative Med. Cell. Longev. 2019, 2019, 5046280. [Google Scholar] [CrossRef]
- Alquezar, B.; Rodrigo, M.J.; Zacarías, L. Regulation of Carotenoid Biosynthesis during Fruit Maturation in the Red-Fleshed Orange Mutant Cara Cara. Phytochemistry 2008, 69, 1997–2007. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Marcos, J.F.; Zacarías, L. Biochemical and Molecular Analysis of Carotenoid Biosynthesis in Flavedo of Orange (Citrus sinensis L.) during Fruit Development and Maturation. J. Agric. Food Chem. 2004, 2, 6724–6731. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, M.J.; Marcos, J.F.; Alférez, F.; Mallent, M.D.; Zacarías, L. Characterization of Pinalate, a Novel Citrus Sinensis Mutant with a Fruit-Specific Alteration That Results in Yellow Pigmentation and Decreased ABA Content. J. Exp. Bot. 2003, 54, 727–738. [Google Scholar] [CrossRef]
- Rouseff, R.; Raley, L.; Hofsommer, H.J. Application of Diode Array Detection with a C-30 Reversed Phase Column for the Separation and Identification of Saponified Orange Juice Carotenoids. J. Agric. Food Chem. 1996, 44, 2176–2181. [Google Scholar] [CrossRef]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Overview of Carotenoid Biosynthesis. In Biosynthesis and Metabolism; Birkhäuser Verlag: Basel, Switzerland, 1998; Volume 3, pp. 13–148. [Google Scholar]
- Carmona, L.; Alquézar, B.; Marques, V.V.; Peña, L. Anthocyanin Biosynthesis and Accumulation in Blood Oranges during Postharvest Storage at Different Low Temperatures. Food Chem. 2017, 237, 7–14. [Google Scholar] [CrossRef]
- Alós, E.; Cercós, M.; Rodrigo, M.J.; Zacarías, L.; Talón, M. Regulation of Color Break in Citrus Fruits. Changes in Pigment Profiling and Gene Expression Induced by Gibberellins and Nitrate, Two Ripening Retardants. J. Agric. Food Chem. 2006, 54, 4888–4895. [Google Scholar] [CrossRef]
- Lado, J.; Cronje, P.; Alquézar, B.; Page, A.; Manzi, M.; Gómez-Cadenas, A.; Stead, A.D.; Zacarías, L.; Rodrigo, M.J. Fruit Shading Enhances Peel Color, Carotenes Accumulation and Chromoplast Differentiation in Red Grapefruit. Physiol. Plant 2015, 154, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Mafra, V.; Kubo, K.S.; Alves-Ferreira, M.; Ribeiro-Alves, M.; Stuart, R.M.; Boava, L.P.; Rodrigues, C.M.; Machado, M.A. Reference Genes for Accurate Transcript Normalization in Citrus Genotypes under Different Experimental Conditions. PLoS ONE 2012, 7, e31263. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; de Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; de Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, 1–12. [Google Scholar] [CrossRef]
- Kawai, C.; Zhang, Y.; Lukács, G.; Chu, W.; Zheng, C.; Gao, C.; Gozli, D.; Wang, Y.; Ansorge, U. The Good, the Bad, and the Red: Implicit Color-Valence Associations across Cultures. Psychol. Res. 2023, 87, 704–724. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, J.; van Dijk, E.; Li, H.; Schnall, S. The Color Red Is Implicitly Associated with Social Status in the United Kingdom and China. Front. Psychol. 2018, 9, 1902. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.A. Provitamin A Function of Carotenoids: The Conversion of Beta-Carotene into Vitamin A. J. Nutr. 1989, 119, 105–108. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Dias, M.G.; Olmedilla-Alonso, B.; Hornero-Méndez, D.; Mercadante, A.Z.; Osorio, C.; Vargas-Murga, L.; Meléndez-Martínez, A.J. Comprehensive Database of Carotenoid Contents in Ibero-American Foods. A Valuable Tool in the Context of Functional Foods and the Establishment of Recommended Intakes of Bioactives. J. Agric. Food Chem. 2018, 66, 5055–5107. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.J.; Fraser, P.D.; Wang, W.J.; Bramley, P.M. Differences in the Carotenoid Content of Ordinary Citrus and Lycopene-Accumulating Mutants. J. Agric. Food Chem. 2006, 54, 5474–5481. [Google Scholar] [CrossRef] [PubMed]
- Zacarías-García, J.; Cronje, P.J.; Diretto, G.; Zacarías, L.; Rodrigo, M.J. A Comprehensive Analysis of Carotenoids Metabolism in Two Red-Fleshed Mutants of Navel and Valencia Sweet Oranges (Citrus sinensis). Front. Plant Sci. 2022, 13, 1034204. [Google Scholar] [CrossRef]
- Xu, Q.; Yu, K.; Zhu, A.; Ye, J.; Liu, Q.; Zhang, J.; Deng, X. Comparative Transcripts Profiling Reveals New Insight into Molecular Processes Regulating Lycopene Accumulation in a Sweet Orange (Citrus sinensis) Red-Flesh Mutant. BMC Genom. 2009, 10, 540. [Google Scholar] [CrossRef]
- Enfissi, E.M.A.; Nogueira, M.; Bramley, P.M.; Fraser, P.D. The Regulation of Carotenoid Formation in Tomato Fruit. Plant J. 2017, 89, 774–788. [Google Scholar] [CrossRef] [PubMed]
- Bramley, P.M. Regulation of Carotenoid Formation during Tomato Fruit Ripening and Development. J. Exp. Bot. 2002, 53, 2107–2113. [Google Scholar] [CrossRef]
- Alquézar, B.; Zacarías, L.; Rodrigo, M.J. Molecular and Functional Characterization of a Novel Chromoplast-Specific Lycopene β-Cyclase from Citrus and Its Relation to Lycopene Accumulation. J. Exp. Bot. 2009, 60, 1783–1797. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, Y.; Zheng, X.; Zhu, K.; Xu, Q.; Deng, X. Isolation and Functional Characterization of a Lycopene β-Cyclase Gene Promoter from Citrus. Front. Plant Sci. 2016, 7, 1367. [Google Scholar] [CrossRef] [PubMed]
- Crupi, P.; Faienza, M.F.; Naeem, M.Y.; Corbo, F.; Clodoveo, M.L.; Muraglia, M. Overview of the Potential Beneficial Effects of Carotenoids on Consumer Health and Well-Being. Antioxidants 2023, 12, 1069. [Google Scholar] [CrossRef]
- Bin-Jumah, M.N.; Nadeem, M.S.; Gilani, S.J.; Mubeen, B.; Ullah, I.; Alzarea, S.I.; Ghoneim, M.M.; Alshehri, S.; Al-Abbasi, F.A.; Kazmi, I. Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases. Antioxidants 2022, 11, 232. [Google Scholar] [CrossRef] [PubMed]

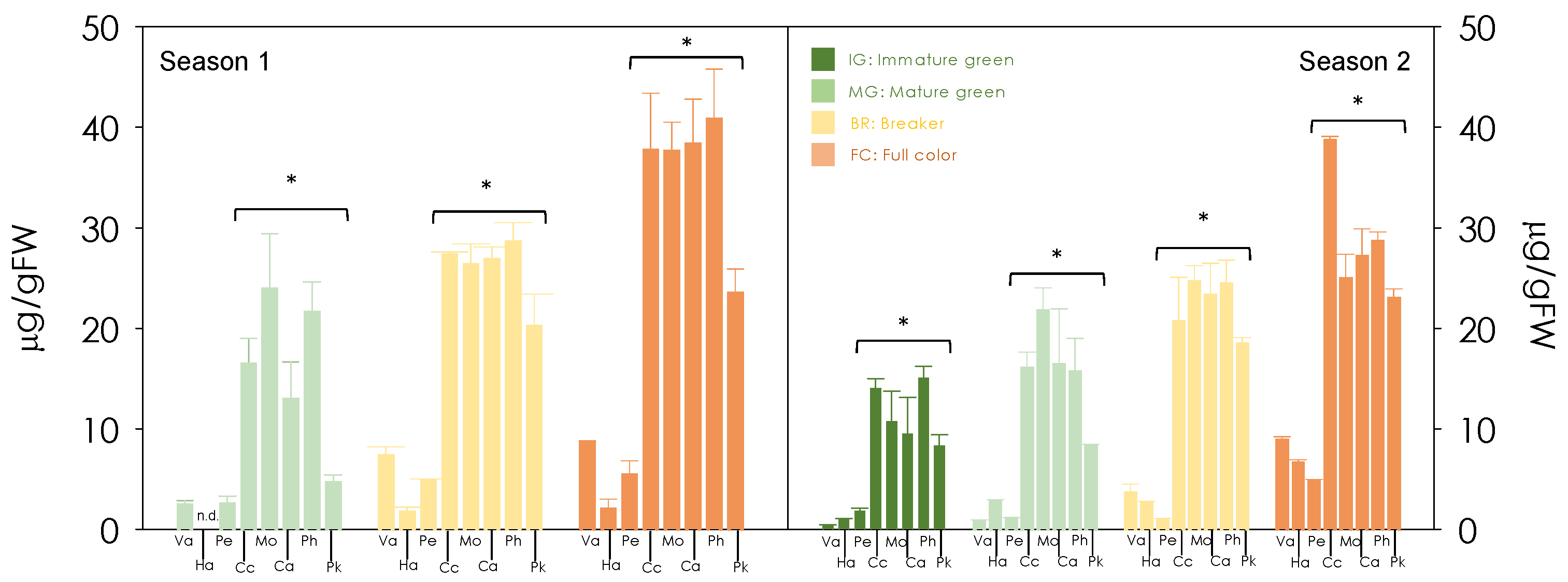
| Variety | Total Soluble Solids (°Brix) (TSS) | Titratable Acidity (g/L) (TA) | Maturity Index (MI) | Juice Content Per Orange (JC) (mL) | Fruit Weight (W) (g) | Percentage Juice Content/Fruit Weight (%) | Fruit Diameter (mm) | Fruit Height (mm) | Cortex (mm) | Peel (mm) | Number of Segments | Number of Seed |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Valencia | 11.7 ± 0.0 | 0.7 ± 0.3 | 13.5 ± 0.3 | 85.0 ± 11.3 | 219.3 ± 20.2 | 38.9 ± 4.6 | 73.7 ± 2.0 | 76.5 ± 3.1 | 4.6 ± 0.2 | 1.7 ± 0.1 | 10.9 ± 0.3 | 56.3 ± 7.4 |
| Hamlim | 10.8 ± 0.3 | 0.8 ± 0.0 | 14.1 ± 0.5 | 61.3 ± 5.3 | 168.8 ± 5.1 | 36.3 ± 3.2 | 68.9 ± 0.9 | 69.4 ± 0.6 | 4.4 ± 0.4 | 1.6 ± 0.1 | 10.5 ± 0.2 | 51.5 ± 6.7 |
| Pera | 12.6 ± 0.1 | 0.7 ± 0.2 | 12.8 ± 0.3 | 88.5 ± 1.5 | 207.7 ± 5.8 | 42.7 ± 1.8 | 71.1 ± 0.7 | 76.1 ± 0.7 | 5.1 ± 0.1 | 1.6 ± 0.1 | 10.6 ± 0.2 | 61.5 ± 6.4 |
| Cara Cara | 9.5 ± 0.0 | 0.5 ± 0.2 | 17.9 ± 0.7 | 38.1 ± 4.5 | 277.0 ± 26.8 | 14.4 ± 2.8 | 86.1 ± 3.8 | 88.9 ± 1.6 | 7.5 ± 0.8 | 2.5 ± 0.4 | 10.7 ± 0.3 | 0.3 ± 0.3 |
| Mombuca | 8.3 ± 0.0 | 0.5 ± 0.0 | 18.0 ± 0.6 | 92.0 ± 5.3 | 188.9 ± 8.1 | 48.6 ± 0.9 | 71.0 ± 1.2 | 72.0 ± 1.0 | 4.6 ± 0.2 | 1.7 ± 0.1 | 10.6 ± 0.2 | 144.3 ± 3.7 |
| Carrancas | 7.8 ± 0.0 | 0.4 ± 0.3 | 20.1 ± 0.7 | 55.9 ± 1.6 | 144.6 ± 7.0 | 38.7 ± 1.8 | 64.9 ± 1.3 | 65.2 ± 1.6 | 3.2 ± 0.2 | 1.8 ± 0.1 | 10.2 ± 0.4 | 69.0 ± 5.9 |
| Pinhal | 8.8 ± 0.3 | 0.5 ± 0.2 | 18.9 ± 0.3 | 54.3 ± 3.1 | 157.2 ± 2.0 | 34.5 ± 1.8 | 65.5 ± 1.0 | 68.1 ± 1.6 | 4.9 ± 0.2 | 2.2 ± 0.2 | 10.2 ± 0.2 | 137.5 ± 3.8 |
| Puka | 9.6 ± 0.2 | 0.5 ± 0.1 | 18.6 ± 0.3 | 109.1 ± 4.4 | 209.2 ± 5.4 | 52.2 ± 2.9 | 73.3 ± 0.7 | 72.9 ± 0.9 | 4.7 ± 0.1 | 1.6 ± 0.2 | 10.4 ± 0.3 | 72.5 ± 9.9 |
| Valencia | 11.8 ± 0.3 | 0.7 ± 0.2 | 16.1 ± 0.2 | 92.3 ± 1.9 | 190.6 ± 4.3 | 48.4 ± 1.1 | 70.4 ± 0.8 | 72.5 ± 0.7 | 6.1 ± 1.3 | 1.1 ± 0.1 | 10.8 ± 0.2 | 45.0 ± 5.3 |
| Hamlim | 9.5 ± 0.1 | 0.7 ± 0.2 | 14.5 ± 0.2 | 80.0 ± 0.6 | 152.7 ± 2.2 | 52.4 ± 0.8 | 66.3 ± 0.7 | 69.3 ± 1.1 | 4.4 ± 0.2 | 2.0 ± 0.0 | 10.2 ± 0.2 | 49.3 ± 7.4 |
| Pera | 12.6 ± 0.2 | 0.7 ± 0.3 | 17.9 ± 0.5 | 93.8 ± 2.1 | 181.7 ± 1.2 | 51.6 ± 0.9 | 68.2 ± 0.1 | 73.9 ± 0.7 | 4.7 ± 0.2 | 1.6 ± 0.2 | 9.7 ± 0.2 | 43.5 ± 6.5 |
| Cara Cara | 9.9 ± 0.1 | 0.5 ± 0.1 | 18.6 ± 0.4 | 53.8 ± 3.1 | 225.7 ± 5.6 | 27.1 ± 0.7 | 75.0 ± 0.4 | 74.9 ± 0.8 | 6.2 ± 0.3 | 1.4 ± 0.1 | 10.4 ± 0.2 | 7.0 ± 0.4 |
| Mombuca | 8.5 ± 0.2 | 0.5 ± 0.2 | 17.8 ± 0.4 | 72.3 ± 1.7 | 141.2 ± 1.5 | 51.2 ± 0.8 | 64.8 ± 0.3 | 66.8 ± 0.3 | 5.1 ± 0.1 | 2.2 ± 0.1 | 10.4 ± 0.1 | 147.5 ± 5.5 |
| Carrancas | 8.0 ± 0.4 | 0.4 ± 0.3 | 20.4 ± 0.8 | 74.3 ± 6.7 | 143.9 ± 2.3 | 51.7 ± 5.2 | 64.3 ± 0.7 | 66.0 ± 0.6 | 4.6 ± 0.2 | 1.6 ± 0.1 | 10.4 ± 0.2 | 89.0 ± 11.7 |
| Pinhal | 9.1 ± 0.0 | 0.5 ± 0.0 | 19.7 ± 0.1 | 62.8 ± 2.8 | 131.0 ± 3.0 | 47.9 ± 1.3 | 62.5 ± 0.7 | 64.5 ± 0.6 | 4.8 ± 0.2 | 2.0 ± 0.1 | 10.4 ± 0.2 | 116.8 ± 7.8 |
| Puka | 9.6 ± 0.3 | 0.6 ± 0.2 | 17.1 ± 0.2 | 66.8 ± 5.6 | 160.6 ± 6.6 | 41.9 ± 4.9 | 74.7 ± 0.6 | 65.7 ± 0.8 | 4.4 ± 0.1 | 1.9 ± 0.1 | 10.6 ± 0.1 | 35.5 ± 4.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmona, L.; Alquézar, B.; Peña, L. Biochemical Characterization of New Sweet Orange Mutants Rich in Lycopene and β-Carotene Antioxidants. Antioxidants 2024, 13, 994. https://doi.org/10.3390/antiox13080994
Carmona L, Alquézar B, Peña L. Biochemical Characterization of New Sweet Orange Mutants Rich in Lycopene and β-Carotene Antioxidants. Antioxidants. 2024; 13(8):994. https://doi.org/10.3390/antiox13080994
Chicago/Turabian StyleCarmona, Lourdes, Berta Alquézar, and Leandro Peña. 2024. "Biochemical Characterization of New Sweet Orange Mutants Rich in Lycopene and β-Carotene Antioxidants" Antioxidants 13, no. 8: 994. https://doi.org/10.3390/antiox13080994
APA StyleCarmona, L., Alquézar, B., & Peña, L. (2024). Biochemical Characterization of New Sweet Orange Mutants Rich in Lycopene and β-Carotene Antioxidants. Antioxidants, 13(8), 994. https://doi.org/10.3390/antiox13080994








