Multitarget Pharmacology of Sulfur–Nitrogen Heterocycles: Anticancer and Antioxidant Perspectives
Abstract
1. Introduction
1.1. Cancer and Oxidative Stress: An Interdependent Relationship Fraught with Complexity
1.2. Multifunctional Drugs: A Highly Beneficial Therapeutic Approach
2. New Sulfur-Nitrogen Heterocyclic Derivatives: Anticancer and Antioxidant Properties
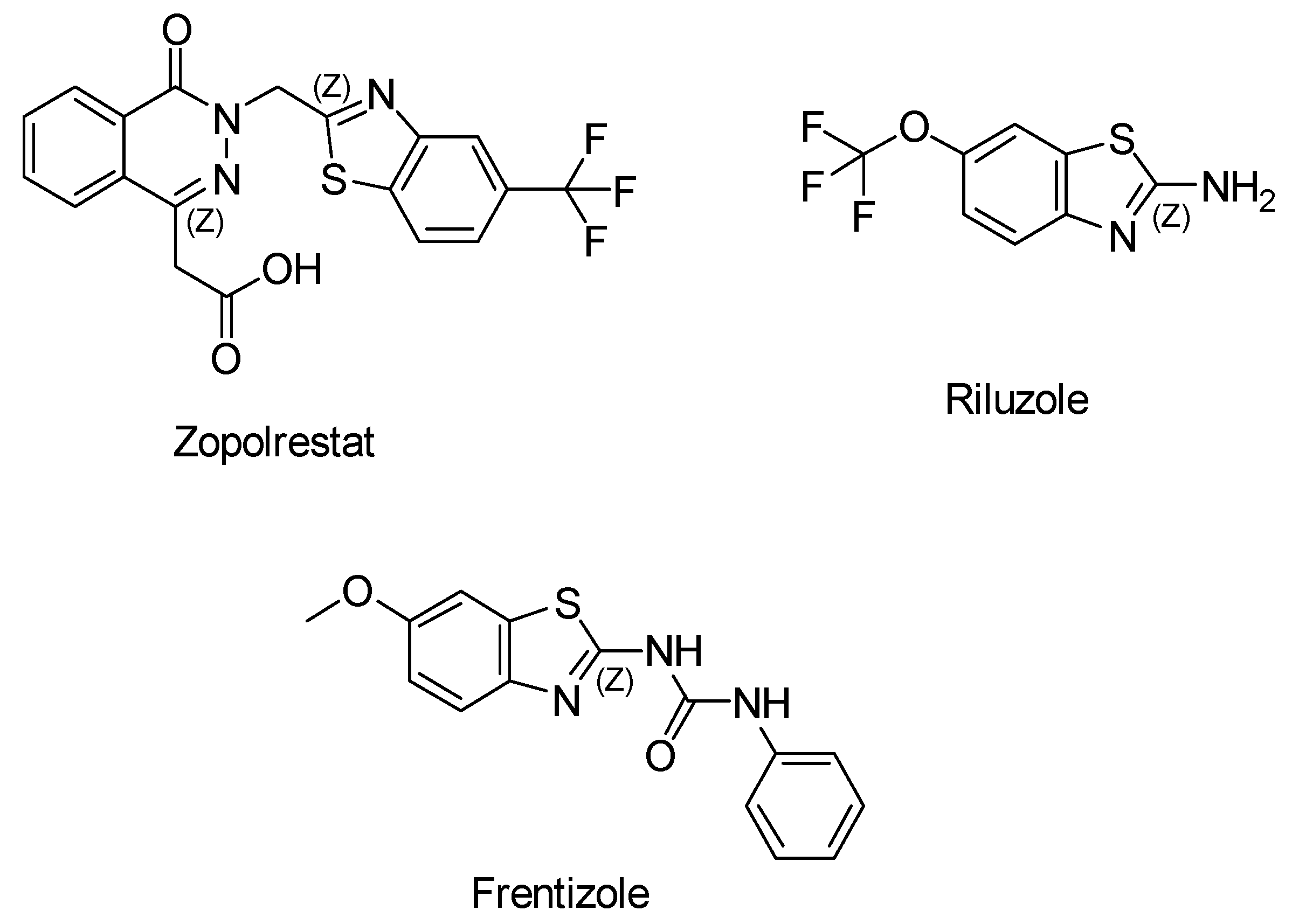
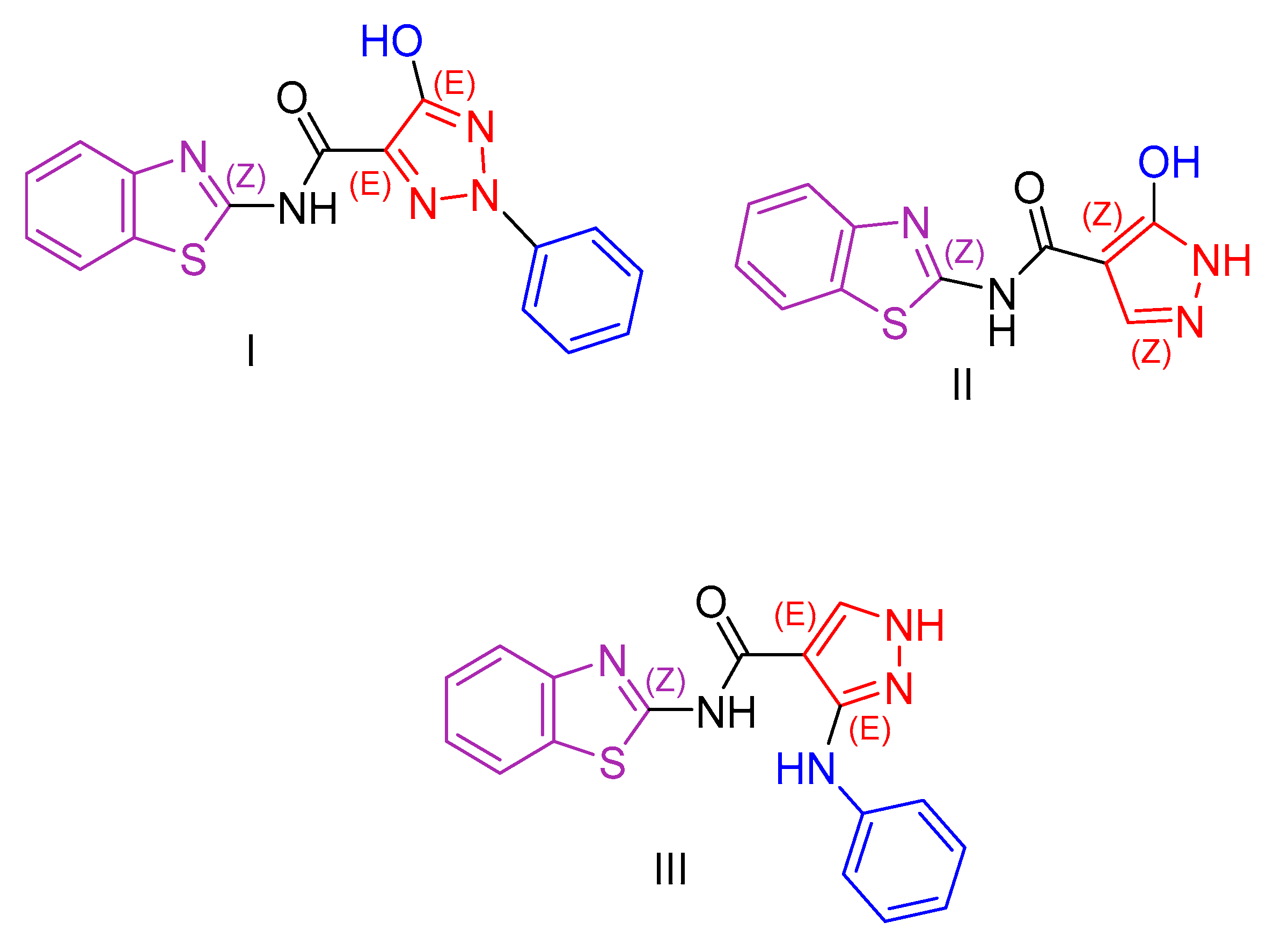
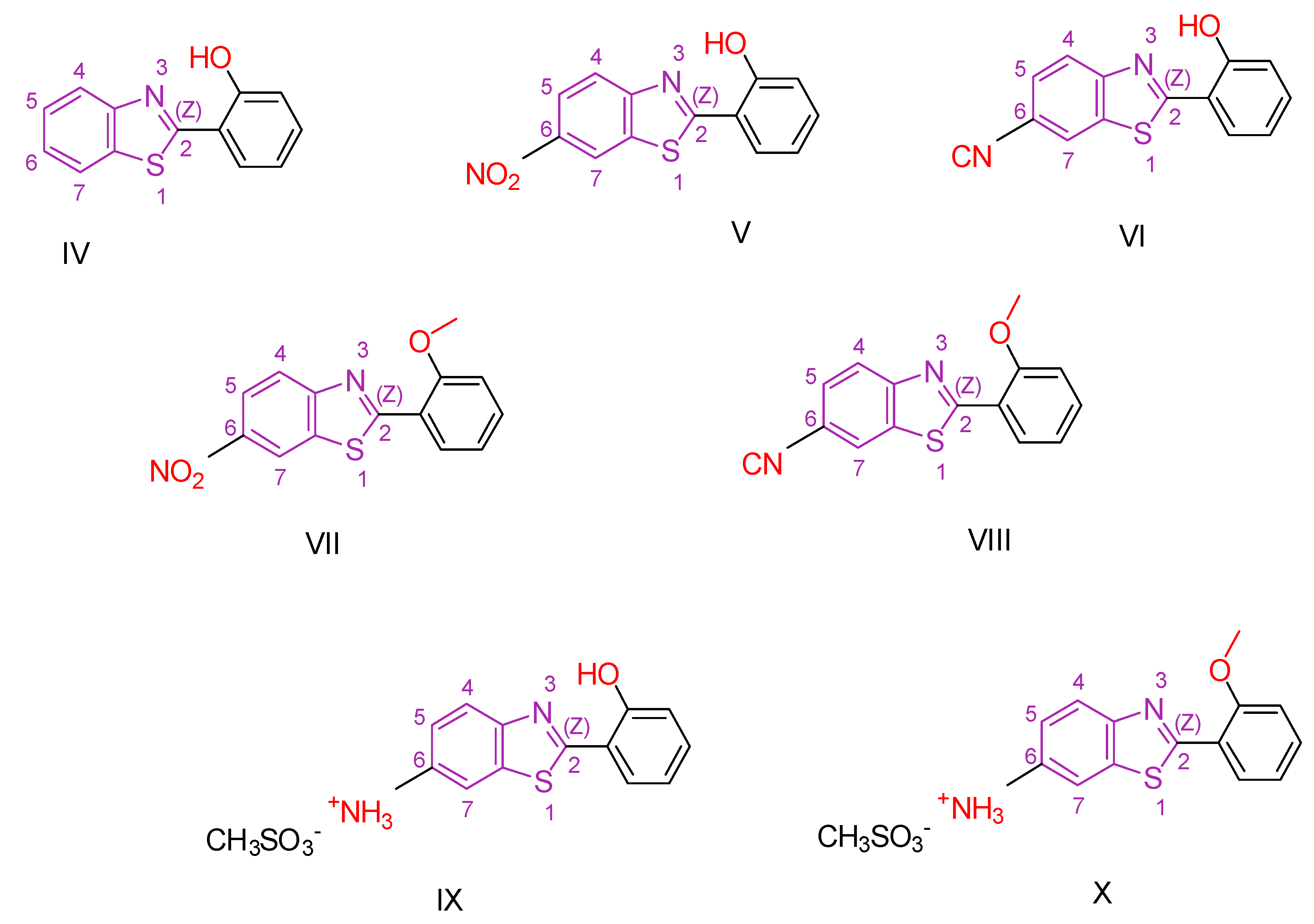
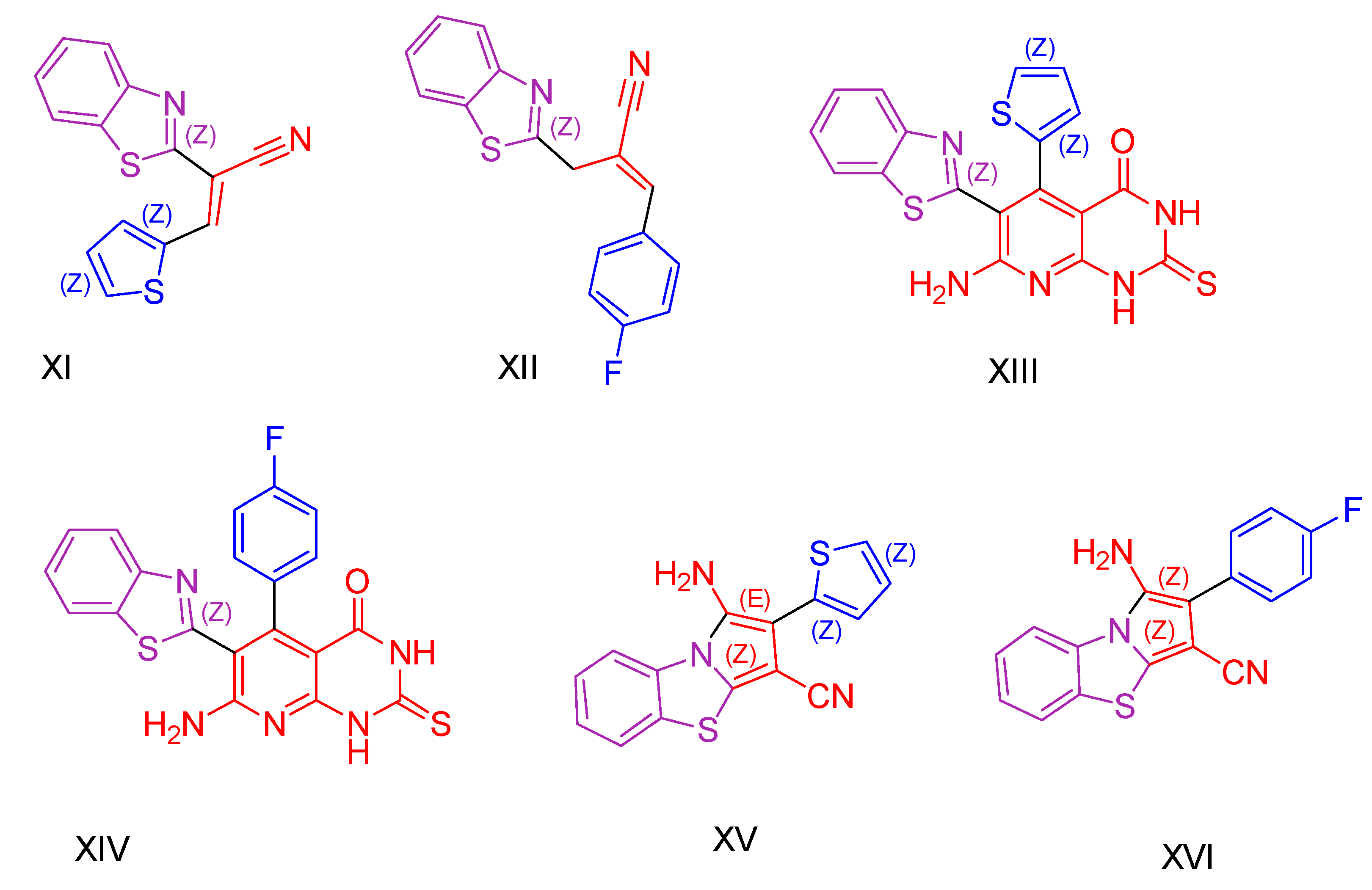
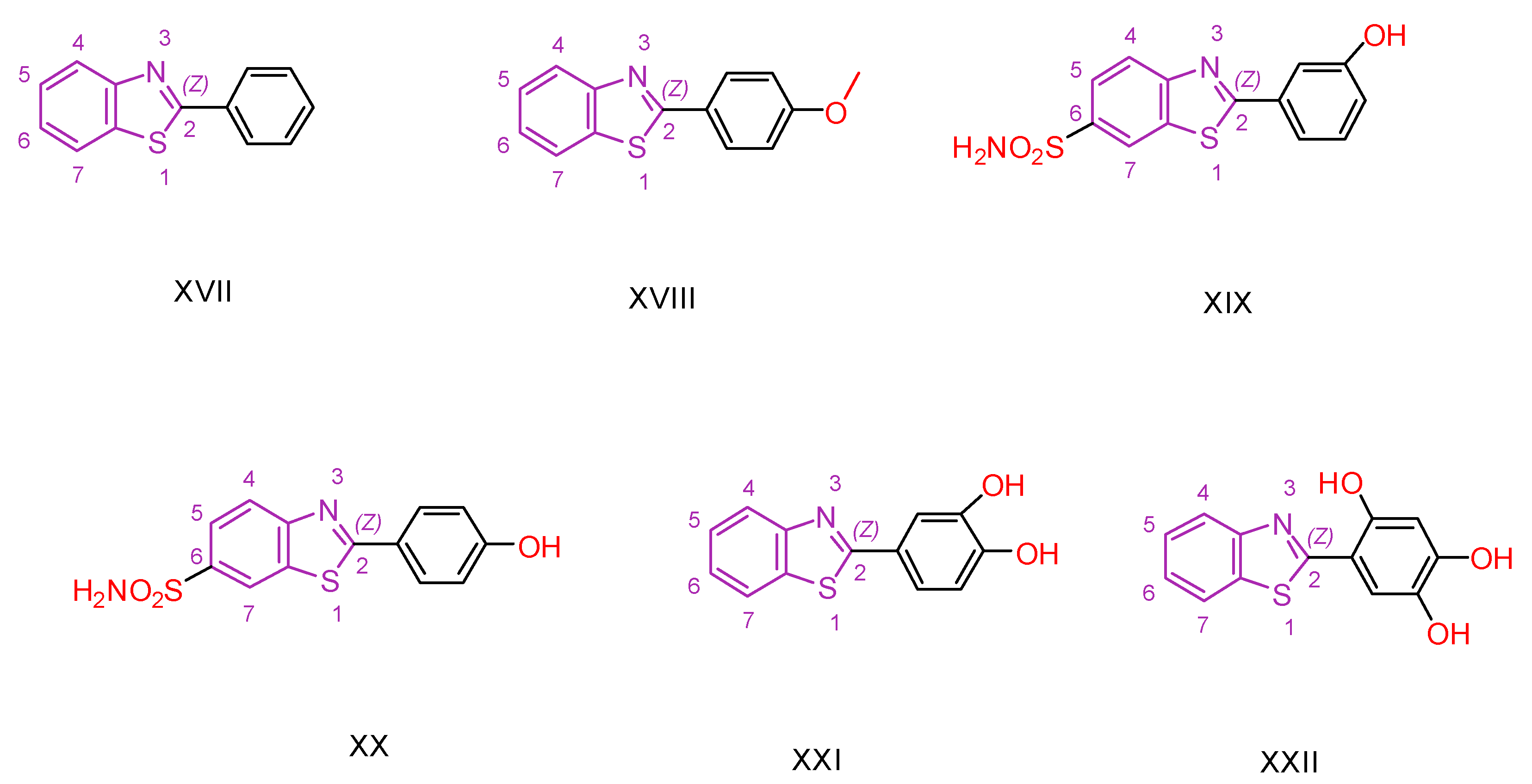
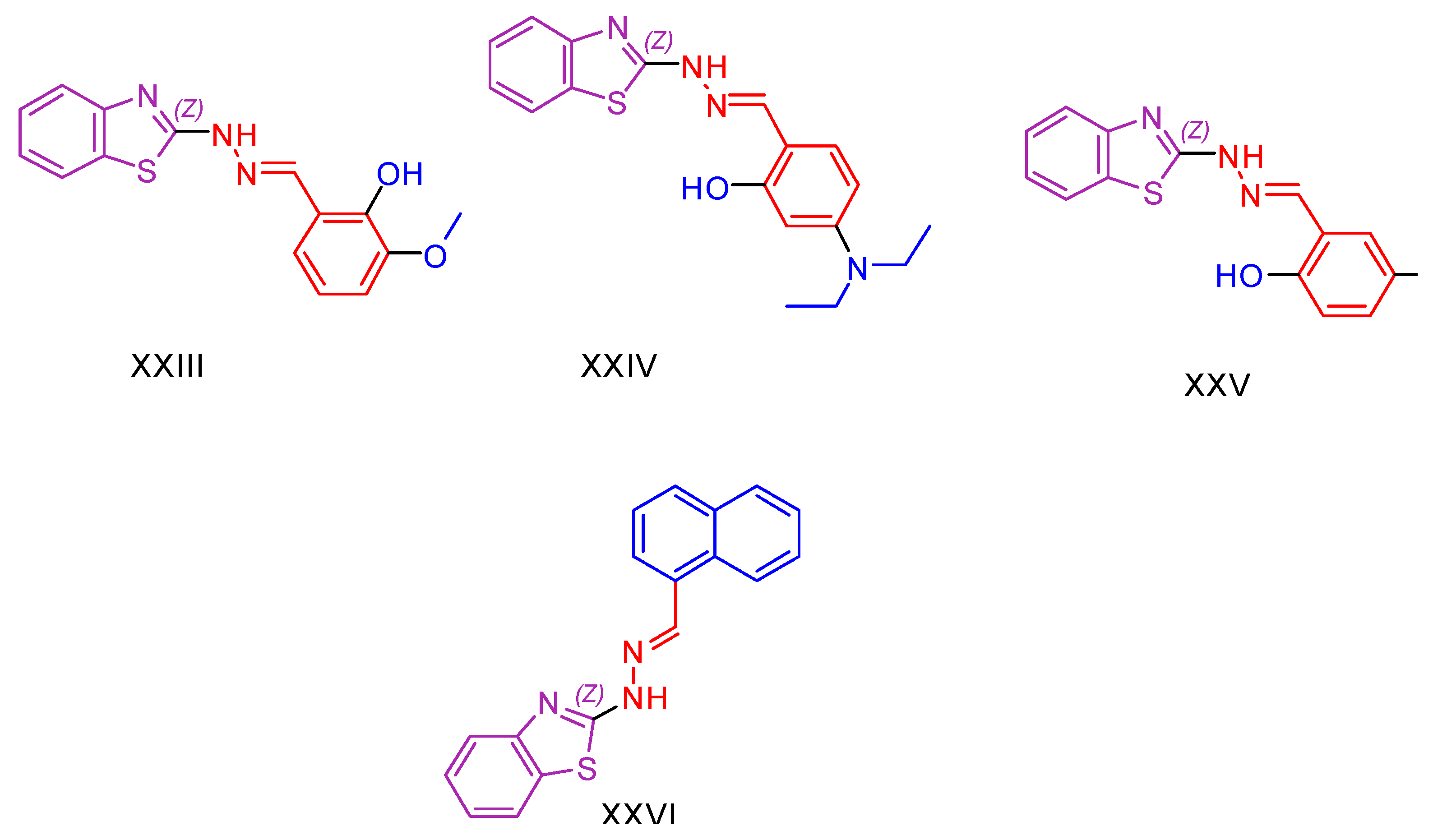
2.1. Benzothiazole Derivatives
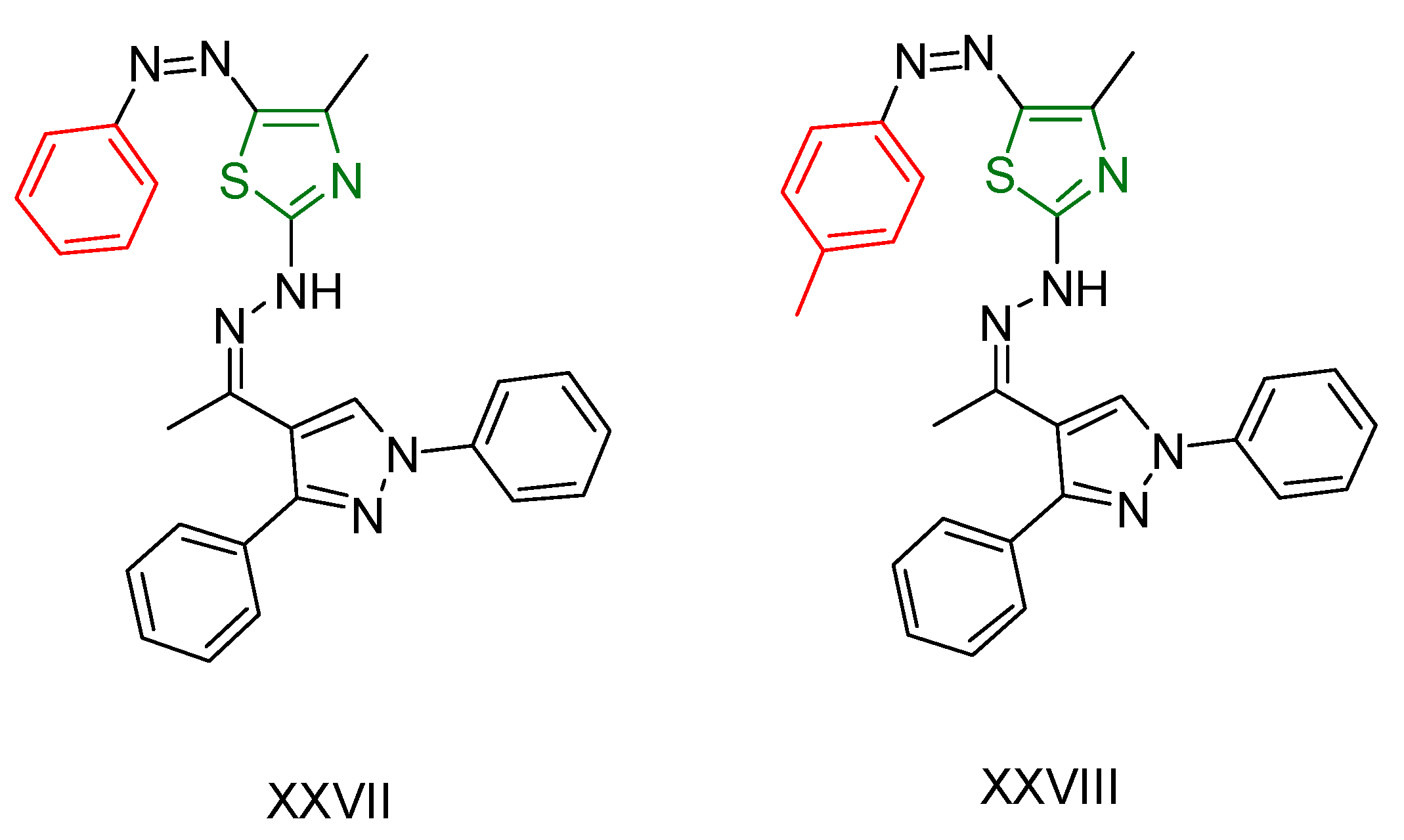
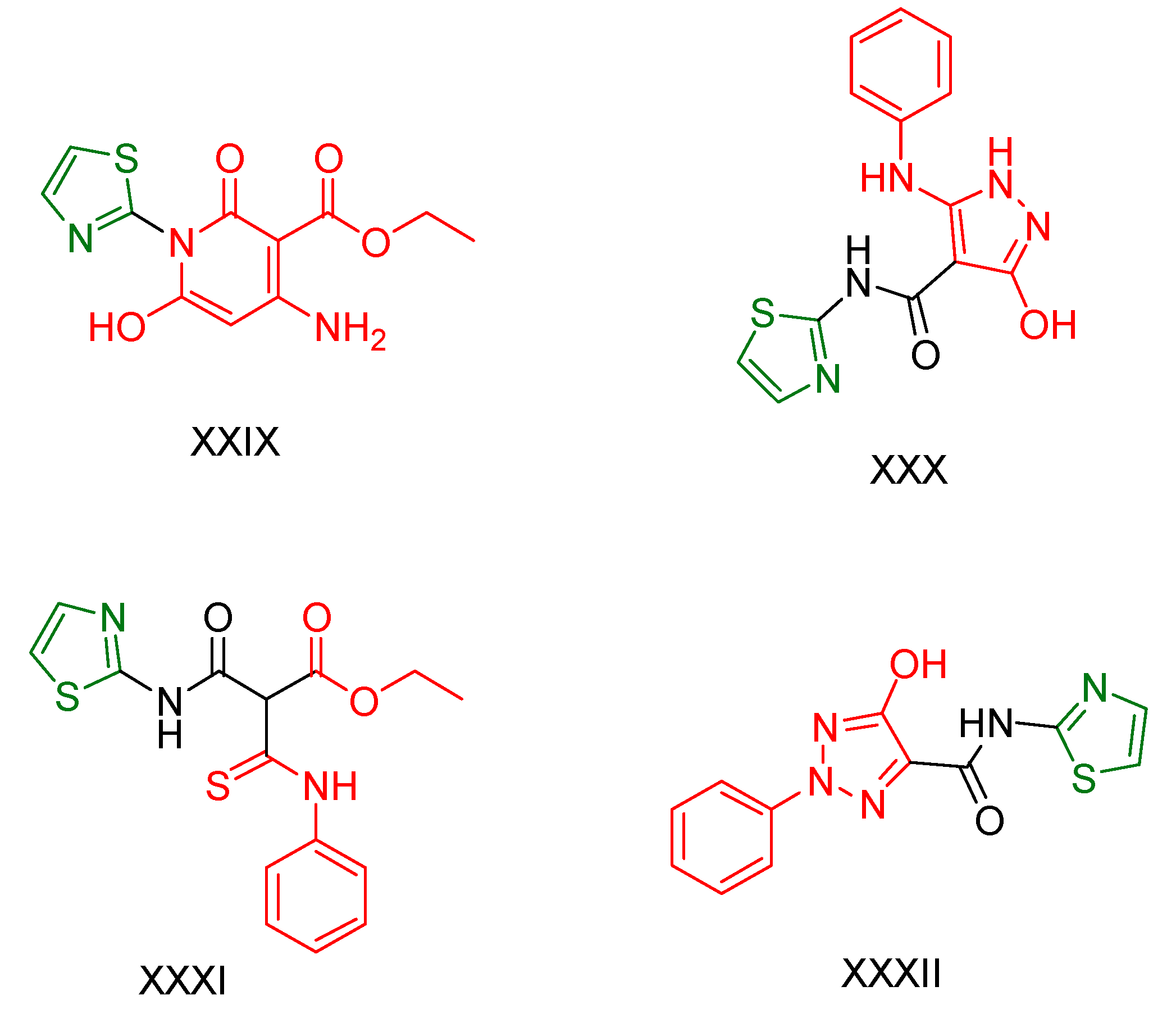
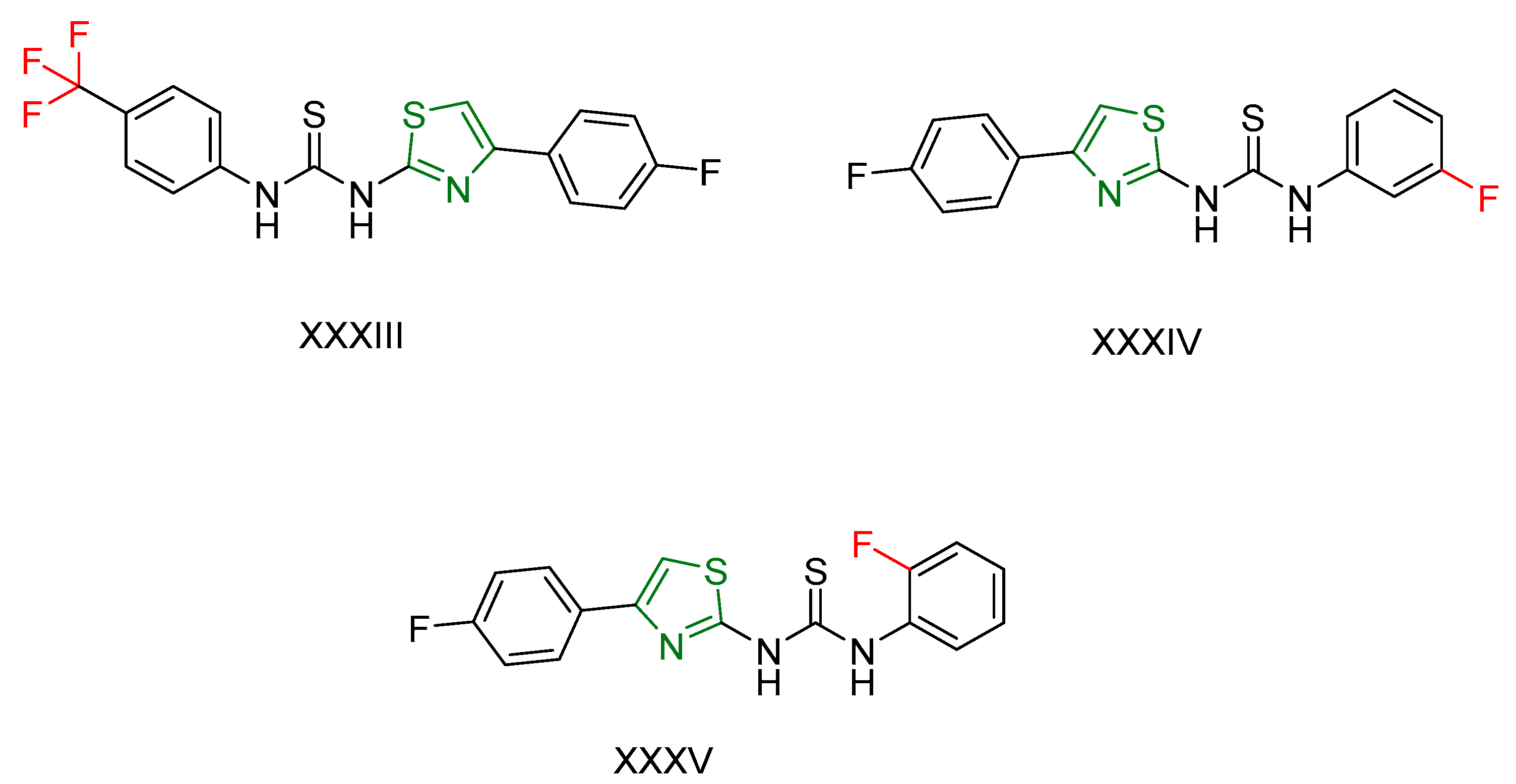
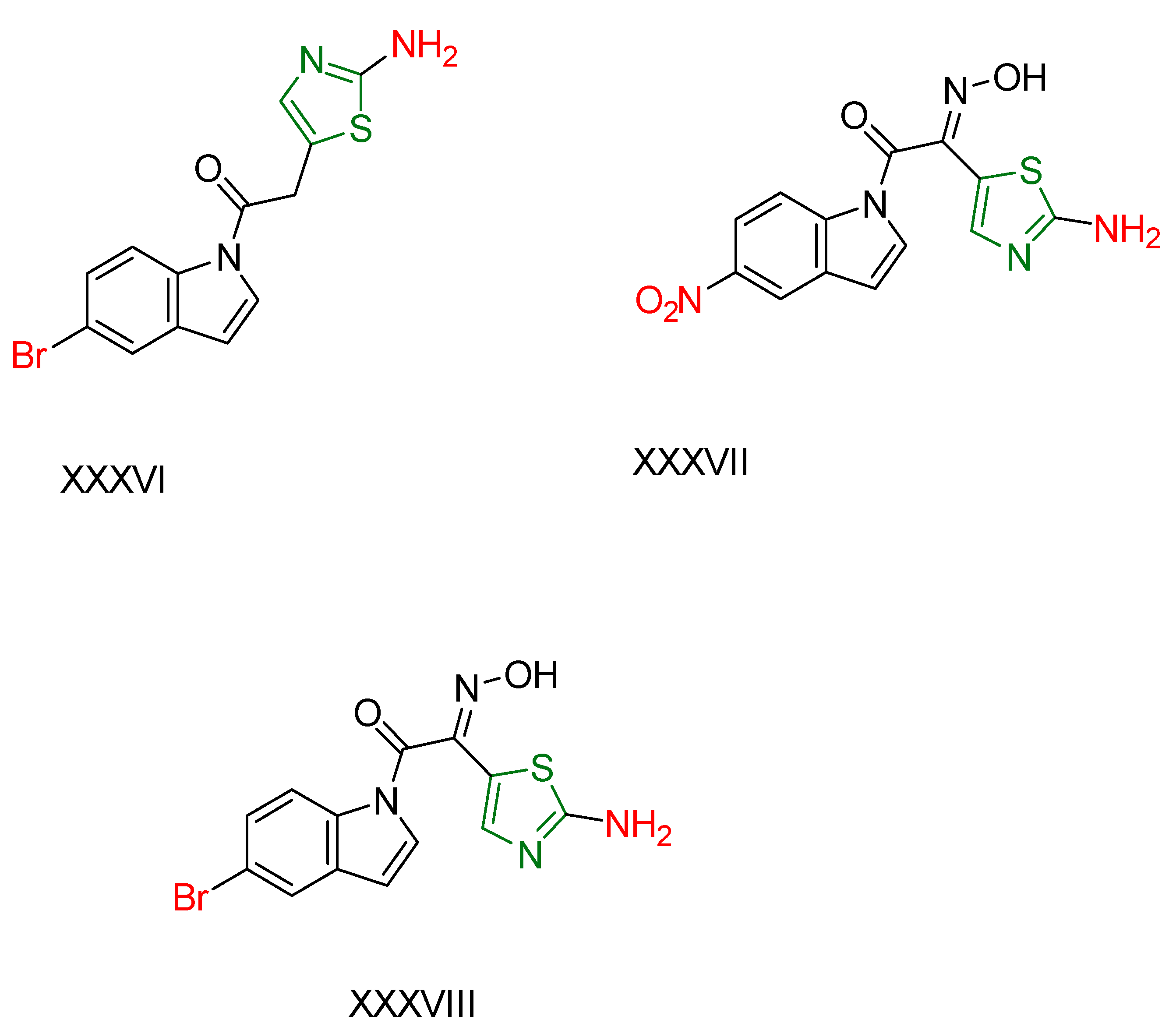
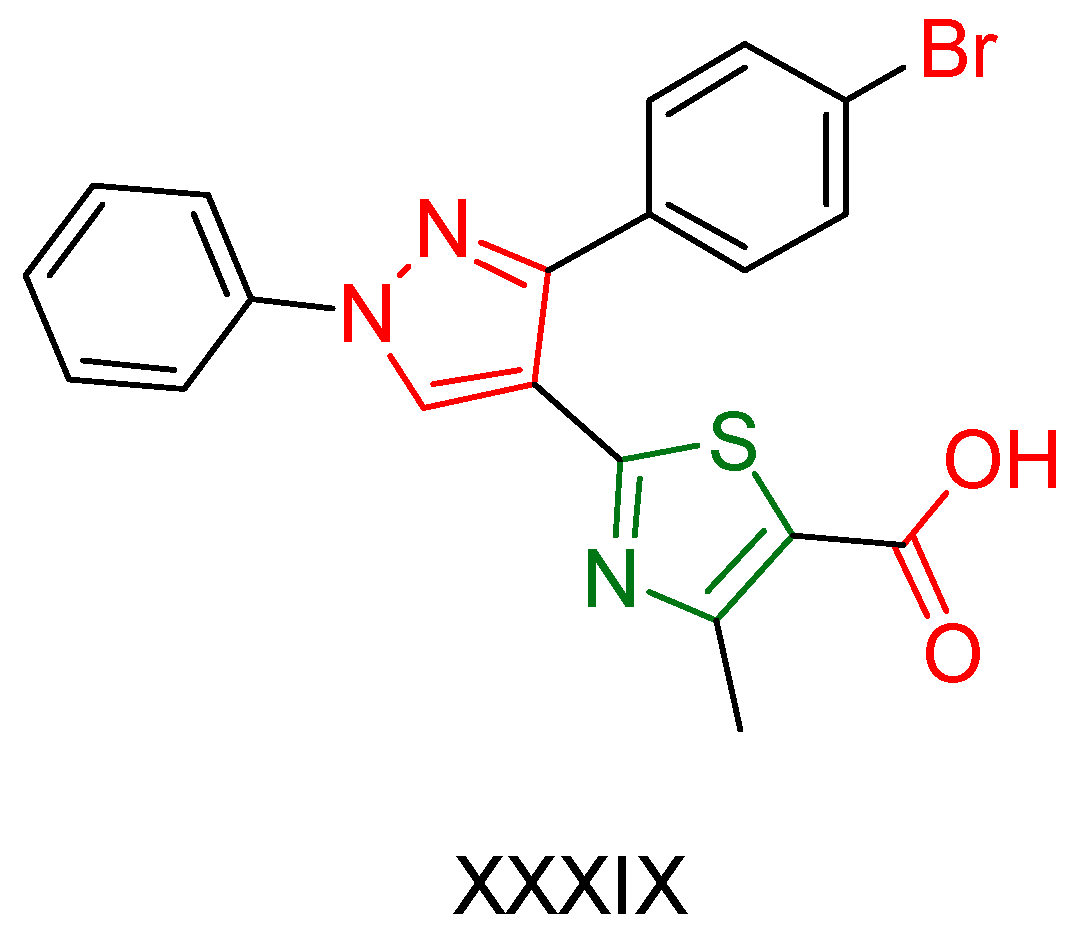
2.2. Thiazole Derivatives
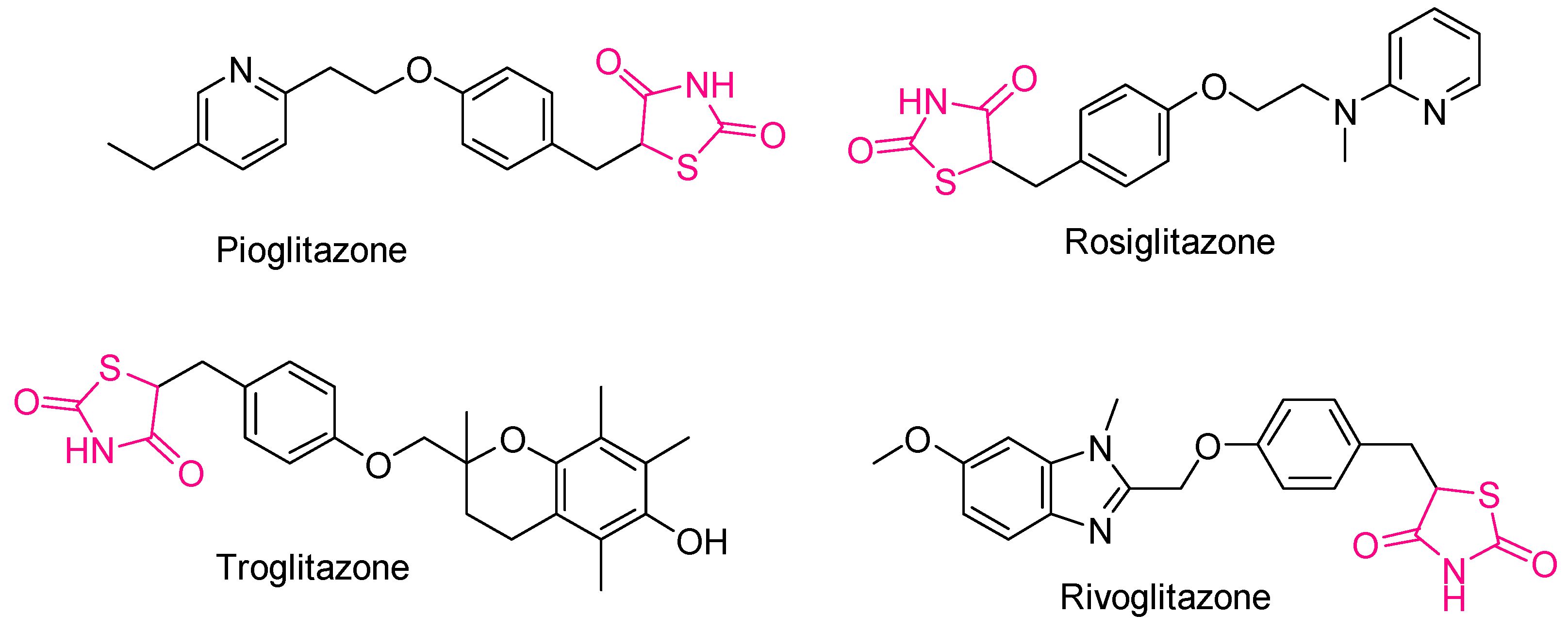
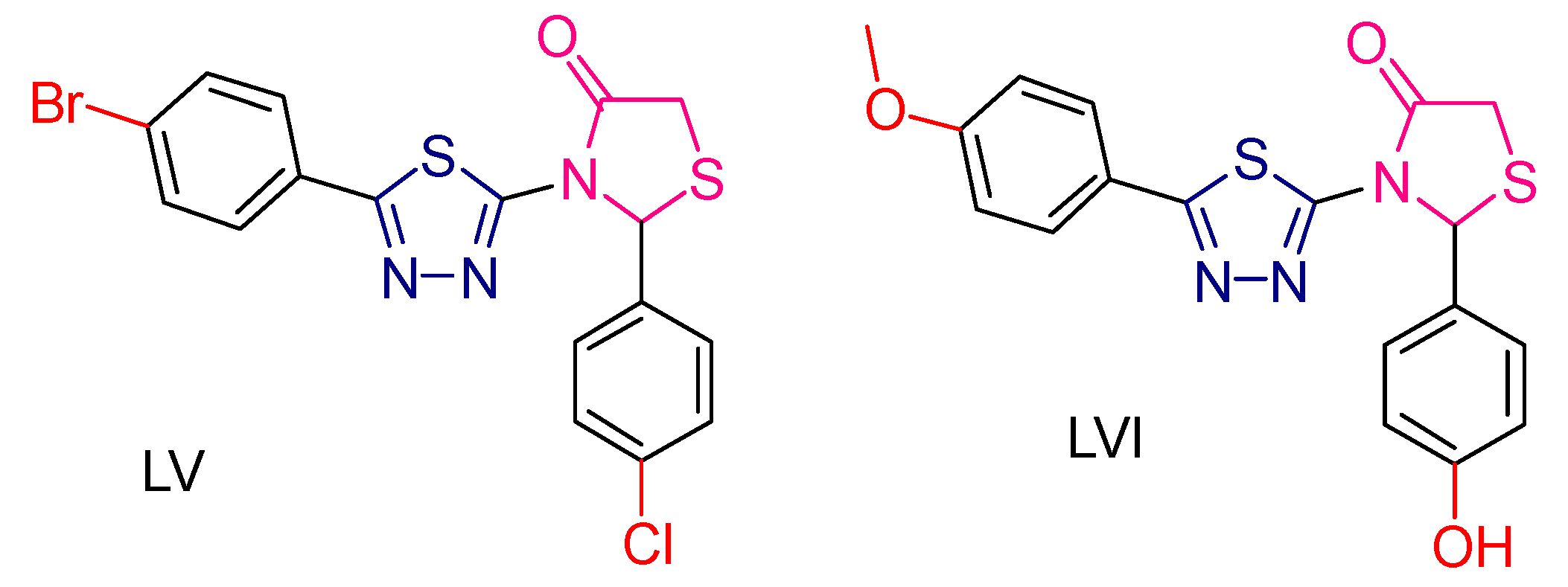
2.3. Thiazolidines and Thiazolidinediones
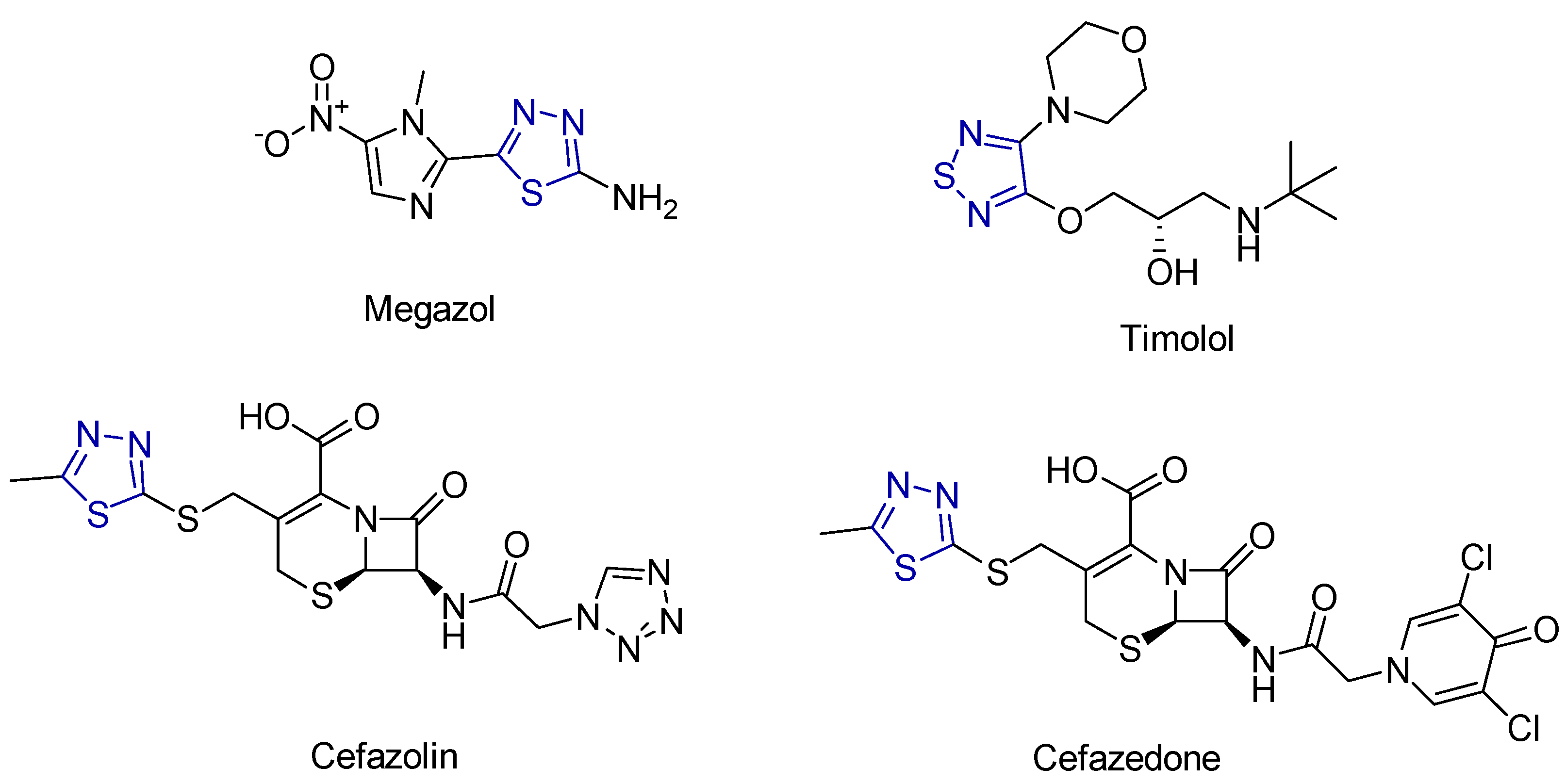
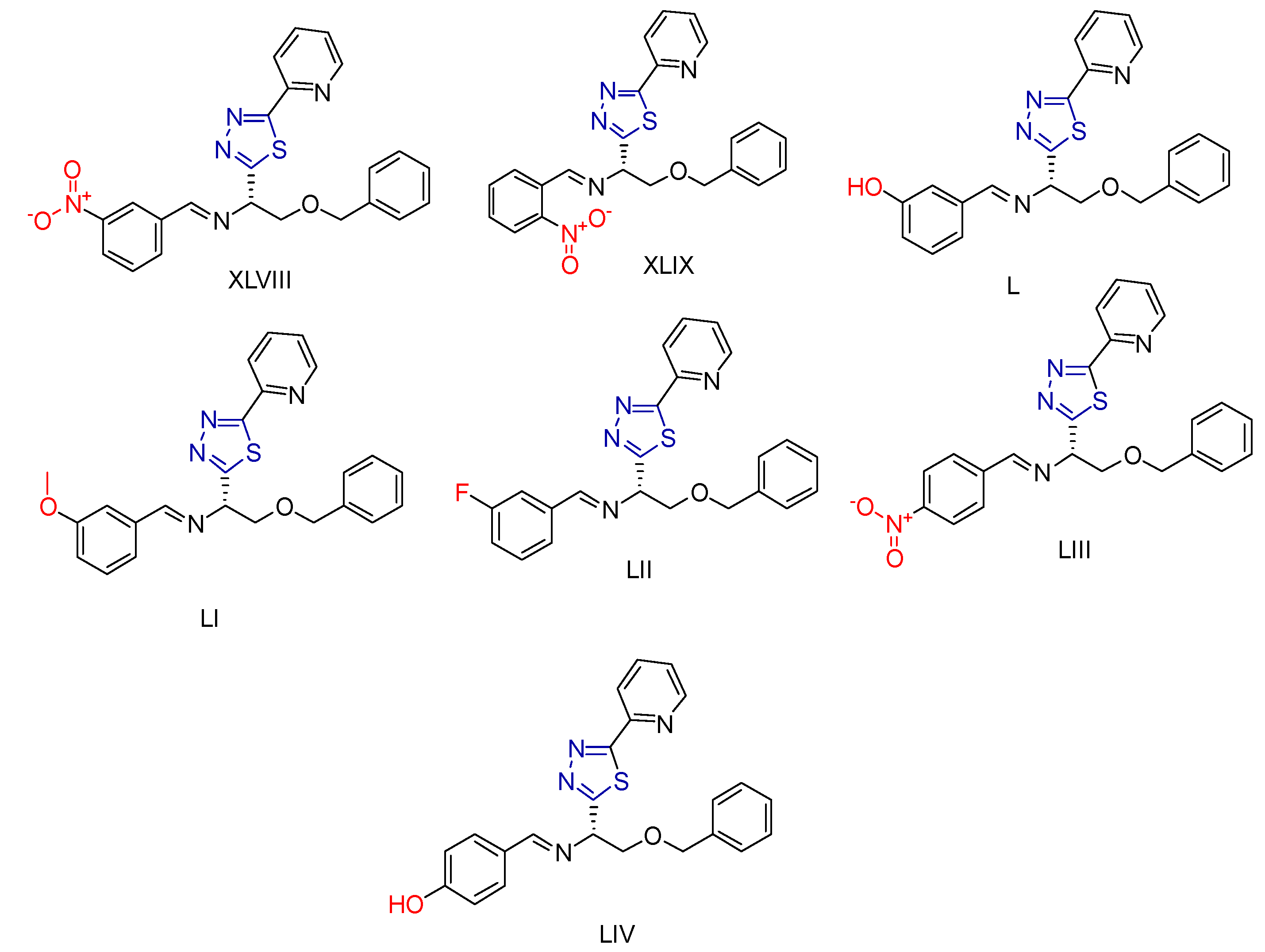
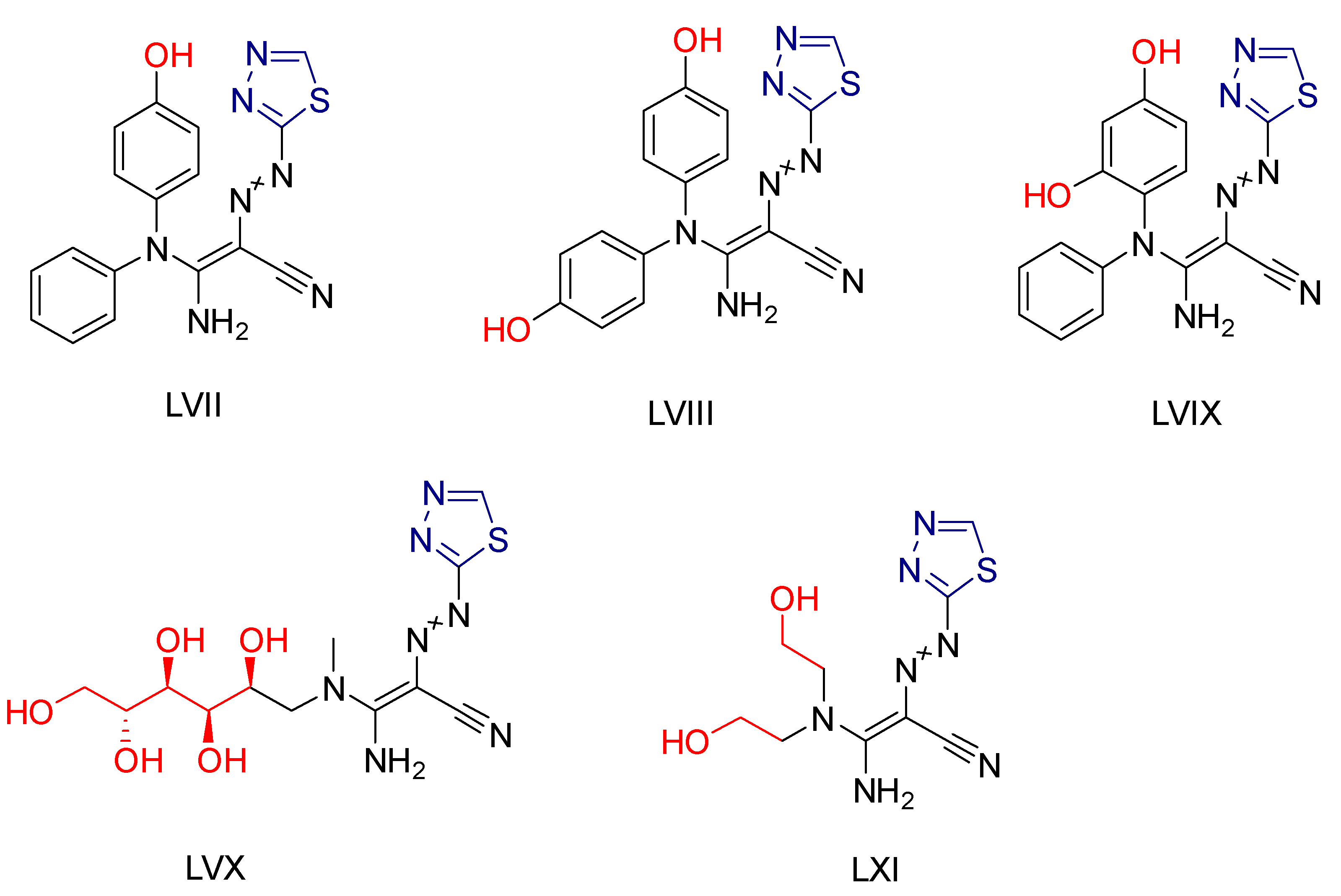
2.4. Thiadiazole Derivatives
2.5. Phenothiazine Derivatives
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harris, I.S.; DeNicola, G.M. The Complex Interplay between Antioxidants and ROS in Cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2015; ISBN 0198717482. [Google Scholar]
- Pourova, J.; Kottova, M.; Voprsalova, M.; Pour, M. Reactive oxygen and nitrogen species in normal physiological processes. Acta Physiol. 2010, 198, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Figge, F.H.J. Cosmic radiation and cancer. Science 1947, 105, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.A. Free Radicals in Biology: Oxidative Stress and the Effects of Ionizing Radiation. Int. J. Radiat. Biol. 1994, 65, 27–33. [Google Scholar] [CrossRef]
- Rose Li, Y.; Halliwill, K.D.; Adams, C.J.; Iyer, V.; Riva, L.; Mamunur, R.; Jen, K.-Y.; del Rosario, R.; Fredlund, E.; Hirst, G.; et al. Mutational signatures in tumours induced by high and low energy radiation in Trp53 deficient mice. Nat. Commun. 2020, 11, 394. [Google Scholar] [CrossRef]
- Veskoukis, A.S.; Tsatsakis, A.M.; Kouretas, D. Dietary oxidative stress and antioxidant defense with an emphasis on plant extract administration. Cell Stress. Chaperones 2012, 17, 11–21. [Google Scholar] [CrossRef]
- Kapiszewska, M.; Kalemba, M.; Grzesiak, A.; Kocemba, K. The level of endogenous DNA damage in lymphocytes isolated from blood is associated with the fluctuation of 17beta-estradiol concentration in the follicular phase of healthy young women. Acta Biochim. Pol. 2005, 52, 535–539. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Sheng, Y.; Abreu, I.A.; Cabelli, D.E.; Maroney, M.J.; Miller, A.-F.; Teixeira, M.; Valentine, J.S. Superoxide Dismutases and Superoxide Reductases. Chem. Rev. 2014, 114, 3854–3918. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Et Biophys. Acta (BBA)-General. Subj. 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Elko, E.A.; Cunniff, B.; Seward, D.J.; Chia, S.B.; Aboushousha, R.; van de Wetering, C.; van der Velden, J.; Manuel, A.; Shukla, A.; Heintz, N.H.; et al. Peroxiredoxins and Beyond; Redox Systems Regulating Lung Physiology and Disease. Antioxid. Redox Signal 2019, 31, 1070–1091. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.G.; Piskounova, E.; Morrison, S.J. Cancer, Oxidative Stress, and Metastasis. Cold Spring Harb. Symp. Quant. Biol. 2016, 81, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Ingrassia, R.; Cavadini, P. Ferritins: A family of molecules for iron storage, antioxidation and more. Biochim. Et Biophys. Acta (BBA)-General. Subj. 2009, 1790, 589–599. [Google Scholar] [CrossRef]
- Pietrangelo, A. Ferroportin disease: Pathogenesis, diagnosis and treatment. Haematologica 2017, 102, 1972–1984. [Google Scholar] [CrossRef] [PubMed]
- Oppermann, U. Carbonyl Reductases: The Complex Relationships of Mammalian Carbonyl- and Quinone-Reducing Enzymes and Their Role in Physiology. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 293–322. [Google Scholar] [CrossRef]
- Jin, Y.; Penning, T.M. Aldo-Keto Reductases and Bioactivation/Detoxication. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 263–292. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.; Viana, S.D.; Nunes, S.; Rolo, A.P.; Palmeira, C.M.; Reis, F. The yin and yang faces of the mitochondrial deacetylase sirtuin 3 in age-related disorders. Ageing Res. Rev. 2020, 57, 100983. [Google Scholar] [CrossRef]
- Meng, H.; Yan, W.-Y.; Lei, Y.-H.; Wan, Z.; Hou, Y.-Y.; Sun, L.-K.; Zhou, J.-P. SIRT3 Regulation of Mitochondrial Quality Control in Neurodegenerative Diseases. Front. Aging Neurosci. 2019, 11, 00313. [Google Scholar] [CrossRef]
- Chu, F.-F.; Esworthy, R.S.; Chu, P.G.; Longmate, J.A.; Huycke, M.M.; Wilczynski, S.; Doroshow, J.H. Bacteria-Induced Intestinal Cancer in Mice with Disrupted Gpx1 and Gpx2 Genes. Cancer Res. 2004, 64, 962–968. [Google Scholar] [CrossRef]
- Hampton, M.B.; Vick, K.A.; Skoko, J.J.; Neumann, C.A. Peroxiredoxin Involvement in the Initiation and Progression of Human Cancer. Antioxid. Redox Signal 2018, 28, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Neumann, C.A.; Krause, D.S.; Carman, C.V.; Das, S.; Dubey, D.P.; Abraham, J.L.; Bronson, R.T.; Fujiwara, Y.; Orkin, S.H.; Van Etten, R.A. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature 2003, 424, 561–565. [Google Scholar] [CrossRef]
- Hwang, I.; Lee, J.; Huh, J.Y.; Park, J.; Lee, H.B.; Ho, Y.-S.; Ha, H. Catalase Deficiency Accelerates Diabetic Renal Injury Through Peroxisomal Dysfunction. Diabetes 2012, 61, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Reczek, C.R.; Birsoy, K.; Kong, H.; Martínez-Reyes, I.; Wang, T.; Gao, P.; Sabatini, D.M.; Chandel, N.S. A CRISPR screen identifies a pathway required for paraquat-induced cell death. Nat. Chem. Biol. 2017, 13, 1274–1279. [Google Scholar] [CrossRef]
- Blackburn, A.C.; Matthaei, K.I.; Lim, C.; Taylor, M.C.; Cappello, J.Y.; Hayes, J.D.; Anders, M.W.; Board, P.G. Deficiency of Glutathione Transferase Zeta Causes Oxidative Stress and Activation of Antioxidant Response Pathways. Mol. Pharmacol. 2006, 69, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Johansson, E.; Fan, Y.; Shertzer, H.G.; Vasiliou, V.; Nebert, D.W.; Dalton, T.P. Early onset senescence occurs when fibroblasts lack the glutamate–cysteine ligase modifier subunit. Free Radic. Biol. Med. 2009, 47, 410–418. [Google Scholar] [CrossRef]
- Patterson, A.D.; Carlson, B.A.; Li, F.; Bonzo, J.A.; Yoo, M.H.; Krausz, K.W.; Conrad, M.; Chen, C.; Gonzalez, F.J.; Hatfield, D.L. Disruption of thioredoxin reductase 1 protects mice from acute acetaminophen-induced hepatotoxicity through enhanced NRF2 activity. Chem. Res. Toxicol. 2013, 26, 1088–1096. [Google Scholar] [CrossRef]
- Zheng, L.; Cardaci, S.; Jerby, L.; Mackenzie, E.D.; Sciacovelli, M.; Johnson, T.I.; Gaude, E.; King, A.; Leach, J.D.G.; Edrada-Ebel, R.; et al. Fumarate induces redox-dependent senescence by modifying glutathione metabolism. Nat. Commun. 2015, 6, 6001. [Google Scholar] [CrossRef] [PubMed]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.K.; Webb, A.E. Regulation of FOXO Factors in Mammalian Cells. Curr. Top. Dev. Biol. 2018, 127, 165–192. [Google Scholar] [CrossRef] [PubMed]
- Rojo de la Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Davoli, T.; Xu, A.W.; Mengwasser, K.E.; Sack, L.M.; Yoon, J.C.; Park, P.J.; Elledge, S.J. Cumulative Haploinsufficiency and Triplosensitivity Drive Aneuploidy Patterns and Shape the Cancer Genome. Cell 2013, 155, 948–962. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Benhar, M. Oxidants, Antioxidants and Thiol Redox Switches in the Control of Regulated Cell Death Pathways. Antioxidants 2020, 9, 309. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Chen, H.-Y.; Harris, I.S.; Stover, D.G.; Selfors, L.M.; Bronson, R.T.; Deraedt, T.; Cichowski, K.; Welm, A.L.; Mori, Y.; et al. Cancer Cells Co-opt the Neuronal Redox-Sensing Channel TRPA1 to Promote Oxidative-Stress Tolerance. Cancer Cell 2018, 33, 985–1003.e7. [Google Scholar] [CrossRef]
- Igelmann, S.; Neubauer, H.; Ferbeyre, G. STAT3 and STAT5 Activation in Solid Cancers. Cancers 2019, 11, 1428. [Google Scholar] [CrossRef]
- Magrì, A.; Germano, G.; Lorenzato, A.; Lamba, S.; Chilà, R.; Montone, M.; Amodio, V.; Ceruti, T.; Sassi, F.; Arena, S.; et al. High-dose vitamin C enhances cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaay8707. [Google Scholar] [CrossRef]
- Bagati, A.; Moparthy, S.; Fink, E.E.; Bianchi-Smiraglia, A.; Yun, D.H.; Kolesnikova, M.; Udartseva, O.O.; Wolff, D.W.; Roll, M.V.; Lipchick, B.C.; et al. KLF9-dependent ROS regulate melanoma progression in stage-specific manner. Oncogene 2019, 38, 3585–3597. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.C.; DeNicola, G.M.; Nixon, C.; Blyth, K.; Labuschagne, C.F.; Tuveson, D.A.; Vousden, K.H. Dynamic ROS Control by TIGAR Regulates the Initiation and Progression of Pancreatic Cancer. Cancer Cell 2020, 37, 168–182.e4. [Google Scholar] [CrossRef] [PubMed]
- Cemerski, S.; Cantagrel, A.; van Meerwijk, J.P.M.; Romagnoli, P. Reactive Oxygen Species Differentially Affect T Cell Receptor-signaling Pathways*. J. Biol. Chem. 2002, 277, 19585–19593. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, J.; Zhang, X.; Chen, W. Glutathione reductase-mediated thiol oxidative stress suppresses metastasis of murine melanoma cells. Free Radic. Biol. Med. 2018, 129, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wu, H.; Lei, R.; Chong, R.A.; Wei, Y.; Lu, X.; Tagkopoulos, I.; Kung, S.-Y.; Yang, Q.; Hu, G.; et al. Transcriptional Network Analysis Identifies BACH1 as a Master Regulator of Breast Cancer Bone Metastasis. J. Biol. Chem. 2012, 287, 33533–33544. [Google Scholar] [CrossRef]
- Paoli, P.; Giannoni, E.; Chiarugi, P. Anoikis molecular pathways and its role in cancer progression. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2013, 1833, 3481–3498. [Google Scholar] [CrossRef] [PubMed]
- Hawk, M.A.; Schafer, Z.T. Mechanisms of redox metabolism and cancer cell survival during extracellular matrix detachment. J. Biol. Chem. 2018, 293, 7531–7537. [Google Scholar] [CrossRef] [PubMed]
- Wiel, C.; Le Gal, K.; Ibrahim, M.X.; Jahangir, C.A.; Kashif, M.; Yao, H.; Ziegler, D.V.; Xu, X.; Ghosh, T.; Mondal, T.; et al. BACH1 Stabilization by Antioxidants Stimulates Lung Cancer Metastasis. Cell 2019, 178, 330–345.e22. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.S.; Wilkes, J.G.; Schroeder, S.R.; Buettner, G.R.; Wagner, B.A.; Du, J.; Gibson-Corley, K.; O’Leary, B.R.; Spitz, D.R.; Buatti, J.M.; et al. Pharmacologic Ascorbate Reduces Radiation-Induced Normal Tissue Toxicity and Enhances Tumor Radiosensitization in Pancreatic Cancer. Cancer Res. 2018, 78, 6838–6851. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhou, L.; Huang, Z.; Li, B.; Nice, E.C.; Xu, J.; Huang, C. Antioxidant Therapy in Cancer: Rationale and Progress. Antioxidants 2022, 11, 1128. [Google Scholar] [CrossRef]
- Cockfield, J.A.; Schafer, Z.T. Antioxidant Defenses: A Context-Specific Vulnerability of Cancer Cells. Cancers 2019, 11, 1208. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Abrahamse, H. Redox Potential of Antioxidants in Cancer Progression and Prevention. Antioxidants 2020, 9, 1156. [Google Scholar] [CrossRef] [PubMed]
- Dienstmann, R.; Rodon, J.; Serra, V.; Tabernero, J. Picking the point of inhibition: A comparative review of PI3K/AKT/mTOR pathway inhibitors. Mol. Cancer Ther. 2014, 13, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Stefil, M.; Dixon, M.; Bahar, J.; Saied, S.; Mashida, K.; Heron, O.; Shantsila, E.; Walker, L.; Akpan, A.; Lip, G.Y.; et al. Polypharmacy in Older People with Heart Failure: Roles of the Geriatrician and Pharmacist. Card. Fail. Rev. 2022, 8, e34. [Google Scholar] [CrossRef]
- Han, S.; Kim, S.-Y.; Jung, Y.-E.; Kim, W.; Seo, J.S.; Sohn, I.; Lee, K.; Lee, J.H.; Chung, S.-K.; Lee, S.-Y.; et al. Patient’s Perspective on Psychiatric Drugs: A Multicenter Survey-Based Study. Psychiatry Investig. 2024, 21, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Petrilla, A.A.; Benner, J.S.; Battleman, D.S.; Tierce, J.C.; Hazard, E.H. Evidence-based interventions to improve patient compliance with antihypertensive and lipid-lowering medications. Int. J. Clin. Pract. 2005, 59, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Eisen, S.A.; Miller, D.K.; Woodward, R.S.; Spitznagel, E.; Przybeck, T.R. The effect of prescribed daily dose frequency on patient medication compliance. Arch. Intern. Med. 1990, 150, 1881–1884. [Google Scholar] [CrossRef] [PubMed]
- Shirbhate, E.; Singh, V.; Jahoriya, V.; Mishra, A.; Veerasamy, R.; Tiwari, A.K.; Rajak, H. Dual inhibitors of HDAC and other epigenetic regulators: A novel strategy for cancer treatment. Eur. J. Med. Chem. 2024, 263, 115938. [Google Scholar] [CrossRef] [PubMed]
- Maddeboina, K.; Yada, B.; Kumari, S.; McHale, C.; Pal, D.; Durden, D.L. Recent advances in multitarget-directed ligands via in silico drug discovery. Drug Discov. Today 2024, 29, 103904. [Google Scholar] [CrossRef]
- Fu, R.; Sun, Y.; Sheng, W.; Liao, D. fang Designing multi-targeted agents: An emerging anticancer drug discovery paradigm. Eur. J. Med. Chem. 2017, 136, 195–211. [Google Scholar] [CrossRef]
- Morphy, R.; Kay, C.; Rankovic, Z. From magic bullets to designed multiple ligands. Drug Discov. Today 2004, 9, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Wermuth, C.G. Multitargeted drugs: The end of the “one-target-one-disease” philosophy? Drug Discov. Today 2004, 9, 826–827. [Google Scholar] [CrossRef]
- Morphy, R.; Rankovic, Z. Fragments, network biology and designing multiple ligands. Drug Discov. Today 2007, 12, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L.; Mason, J.S.; Overington, J.P. Can we rationally design promiscuous drugs? Curr. Opin. Struct. Biol. 2006, 16, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, D.B.; do Bomfim, M.R.; de Oliveira, T.A.; da Silva, A.M.; Taranto, A.G.; Cruz, J.N.; de Carvalho, P.B.; Campos, J.M.; Santos, C.B.R.; Leite, F.H.A. Development of Potential Multi-Target Inhibitors for Human Cholinesterases and Beta-Secretase 1: A Computational Approach. Pharmaceuticals 2023, 16, 1657. [Google Scholar] [CrossRef] [PubMed]
- Faivre, S.; Djelloul, S.; Raymond, E. New Paradigms in Anticancer Therapy: Targeting Multiple Signaling Pathways with Kinase Inhibitors. Semin. Oncol. 2006, 33, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Melisi, D.; Piro, G.; Tamburrino, A.; Carbone, C.; Tortora, G. Rationale and clinical use of multitargeting anticancer agents. Curr. Opin. Pharmacol. 2013, 13, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Garuti, L.; Roberti, M.; Bottegoni, G. Multi-kinase inhibitors. Curr. Med. Chem. 2015, 22, 695–712. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhao, Y.; Luo, Q.; Zhang, Y.; Wu, K.; Wang, F. Multi-Targeted Anticancer Agents. Curr. Top. Med. Chem. 2017, 17, 3084–3098. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, M.; Ferraro, M.G.; Iazzetti, F.; Santamaria, R.; Irace, C. Insight into Iron, Oxidative Stress and Ferroptosis: Therapy Targets for Approaching Anticancer Strategies. Cancers 2024, 16, 1220. [Google Scholar] [CrossRef]
- Covell, L.L.; Ganti, A.K. Treatment of advanced thyroid cancer: Role of molecularly targeted therapies. Target. Oncol. 2015, 10, 311–324. [Google Scholar] [CrossRef] [PubMed]
- De Sá Junior, P.L.; Câmara, D.A.D.; Porcacchia, A.S.; Fonseca, P.M.M.; Jorge, S.D.; Araldi, R.P.; Ferreira, A.K. The Roles of ROS in Cancer Heterogeneity and Therapy. Oxid. Med. Cell Longev. 2017, 2017, 2467940. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef] [PubMed]
- Idelchik, M.d.P.S.; Begley, U.; Begley, T.J.; Melendez, J.A. Mitochondrial ROS control of cancer. Semin. Cancer Biol. 2017, 47, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Haider, K.; Haider, M.R.; Neha, K.; Yar, M.S. Free radical scavengers: An overview on heterocyclic advances and medicinal prospects. Eur. J. Med. Chem. 2020, 204, 112607. [Google Scholar] [CrossRef]
- Amewu, R.K.; Sakyi, P.O.; Osei-Safo, D.; Addae-Mensah, I. Synthetic and Naturally Occurring Heterocyclic Anticancer Compounds with Multiple Biological Targets. Molecules 2021, 26, 7134. [Google Scholar] [CrossRef]
- Martins, P.; Jesus, J.; Santos, S.; Raposo, L.; Roma-Rodrigues, C.; Baptista, P.; Fernandes, A. Heterocyclic Anticancer Compounds: Recent Advances and the Paradigm Shift towards the Use of Nanomedicine’s Tool Box. Molecules 2015, 20, 16852–16891. [Google Scholar] [CrossRef]
- Lang, D.K.; Kaur, R.; Arora, R.; Saini, B.; Arora, S. Nitrogen-Containing Heterocycles as Anticancer Agents: An Overview. Anticancer Agents Med. Chem. 2020, 20, 2150–2168. [Google Scholar] [CrossRef]
- Ardiansah, B. Chalcones bearing N, O, and S-heterocycles: Recent notes on their biological significances. J. Appl. Pharm. Sci. 2019, 9, 117–129. [Google Scholar] [CrossRef]
- Maji, S.; Debnath, B.; Panda, S.; Manna, T.; Maity, A.; Dayaramani, R.; Nath, R.; Khan, S.A.; Akhtar, M.J. Anticancer potential of the S-heterocyclic ring containing drugs and its bioactivation to reactive metabolites. Chem. Biodivers. 2024, 21, e202400473. [Google Scholar] [CrossRef] [PubMed]
- Pathania, S.; Narang, R.K.; Rawal, R.K. Role of sulphur-heterocycles in medicinal chemistry: An update. Eur. J. Med. Chem. 2019, 180, 486–508. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Yar, M.S.; Khan, A.A.; Ali, Z.; Haider, M.R. Recent Advances in the Synthesis and Anticancer Activity of Some Molecules Other Than Nitrogen Containing Heterocyclic Moeities. Mini-Rev. Med. Chem. 2017, 17, 1602–1632. [Google Scholar] [CrossRef]
- Carradori, S.; Guglielmi, P.; Luisi, G.; Secci, D. Nitrogen- and Sulfur-Containing Heterocycles as Dual Anti-oxidant and Anti-cancer Agents. In Handbook of Oxidative Stress. in Cancer: Mechanistic Aspects; Springer: Singapore, 2021; pp. 1–18. [Google Scholar] [CrossRef]
- Fares, M.; Abou-Seri, S.M.; Abdel-Aziz, H.A.; Abbas, S.E.-S.; Youssef, M.M.; Eladwy, R.A. Synthesis and antitumor activity of pyrido [2,3-d]pyrimidine and pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidine derivatives that induce apoptosis through G1 cell-cycle arrest. Eur. J. Med. Chem. 2014, 83, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Myriagkou, M.; Papakonstantinou, E.; Deligiannidou, G.E.; Patsilinakos, A.; Kontogiorgis, C.; Pontiki, E. Novel Pyrimidine Derivatives as Antioxidant and Anticancer Agents: Design, Synthesis and Molecular Modeling Studies. Molecules 2023, 28, 3913. [Google Scholar] [CrossRef]
- Sayed, E.M.; Hassanien, R.; Farhan, N.; Aly, H.F.; Mahmoud, K.; Mohamed, S.K.; Mague, J.T.; Bakhite, E.A. Nitrophenyl-Group-Containing Heterocycles. I. Synthesis, Characterization, Crystal Structure, Anticancer Activity, and Antioxidant Properties of Some New 5,6,7,8-Tetrahydroisoquinolines Bearing 3(4)-Nitrophenyl Group. ACS Omega 2022, 7, 8767–8776. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Amin, A.; Kumar, M. A Review: Medicinally Important Nitrogen Sulphur Containing Heterocycles. Open Med. Chem. J. 2020, 14, 49–64. [Google Scholar] [CrossRef]
- Keri, R.S.; Patil, M.R.; Patil, S.A.; Budagumpi, S. A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry. Eur. J. Med. Chem. 2015, 89, 207–251. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Syed, M.A.H.; Mohammed, S.M. Therapeutic potential of benzothiazoles: A patent review (2010–2014). Expert Opin. Ther. Pat. 2015, 25, 335–349. [Google Scholar] [CrossRef]
- Cindrić, M.; Jambon, S.; Harej, A.; Depauw, S.; David-Cordonnier, M.-H.; Kraljević Pavelić, S.; Karminski-Zamola, G.; Hranjec, M. Novel amidino substituted benzimidazole and benzothiazole benzo[b]thieno-2-carboxamides exert strong antiproliferative and DNA binding properties. Eur. J. Med. Chem. 2017, 136, 468–479. [Google Scholar] [CrossRef]
- Ashraf, M.; Shaik, T.B.; Malik, M.S.; Syed, R.; Mallipeddi, P.L.; Vardhan, M.V.P.S.V.; Kamal, A. Design and synthesis of cis-restricted benzimidazole and benzothiazole mimics of combretastatin A-4 as antimitotic agents with apoptosis inducing ability. Bioorganic Med. Chem. Lett. 2016, 26, 4527–4535. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Rathore, D.S.; Garg, G.; Khatri, K.; Saxena, R.; Sahu, S.K. Synthesis and Evaluation of Some Benzothiazole Derivatives As Antidiabetic Agents. Int. J. Pharm. Pharm. Sci. 2017, 9, 60. [Google Scholar] [CrossRef]
- Keri, R.S.; Quintanova, C.; Marques, S.M.; Esteves, A.R.; Cardoso, S.M.; Santos, M.A. Design, synthesis and neuroprotective evaluation of novel tacrine–benzothiazole hybrids as multi-targeted compounds against Alzheimer’s disease. Bioorganic Med. Chem. 2013, 21, 4559–4569. [Google Scholar] [CrossRef] [PubMed]
- Alborz, M.; Jarrahpour, A.; Pournejati, R.; Karbalaei-Heidari, H.R.; Sinou, V.; Latour, C.; Brunel, J.M.; Sharghi, H.; Aberi, M.; Turos, E.; et al. Synthesis and biological evaluation of some novel diastereoselective benzothiazole β-lactam conjugates. Eur. J. Med. Chem. 2018, 143, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Padalkar, V.S.; Borse, B.N.; Gupta, V.D.; Phatangare, K.R.; Patil, V.S.; Umape, P.G.; Sekar, N. Synthesis and antimicrobial activity of novel 2-substituted benzimidazole, benzoxazole and benzothiazole derivatives. Arab. J. Chem. 2016, 9, S1125–S1130. [Google Scholar] [CrossRef]
- Patrick, D.A.; Gillespie, J.R.; McQueen, J.; Hulverson, M.A.; Ranade, R.M.; Creason, S.A.; Herbst, Z.M.; Gelb, M.H.; Buckner, F.S.; Tidwell, R.R. Urea Derivatives of 2-Aryl-benzothiazol-5-amines: A New Class of Potential Drugs for Human African Trypanosomiasis. J. Med. Chem. 2017, 60, 957–971. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, D.I.; Okoro, U.C.; Ukoha, P.O.; Gupta, A.; Okafor, S.N. Novel anti-inflammatory and analgesic agents: Synthesis, molecular docking and in vivo studies. J. Enzym. Inhib. Med. Chem. 2018, 33, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Kamboj, P.; Alam, O.; Amir, M. 1,2,4-Triazole-based benzothiazole/benzoxazole derivatives: Design, synthesis, p38α MAP kinase inhibition, anti-inflammatory activity and molecular docking studies. Bioorganic Chem. 2018, 81, 630–641. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, S.; Husain, A. Synthesis and in vivo Anti-inflammatory and Analgesic activities of Oxadiazoles clubbed with Benzothiazole nucleus. Int. Curr. Pharm. J. 2015, 4, 457–461. [Google Scholar] [CrossRef]
- Cressier, D.; Prouillac, C.; Hernandez, P.; Amourette, C.; Diserbo, M.; Lion, C.; Rima, G. Synthesis, antioxidant properties and radioprotective effects of new benzothiazoles and thiadiazoles. Bioorganic Med. Chem. 2009, 17, 5275–5284. [Google Scholar] [CrossRef] [PubMed]
- Weekes, A.; Westwell, A. 2-Arylbenzothiazole as a Privileged Scaffold in Drug Discovery. Curr. Med. Chem. 2009, 16, 2430–2440. [Google Scholar] [CrossRef] [PubMed]
- El-Mekabaty, A.; Sofan, M.A.; Hasel, A.M.; Said, S.B. Concise Synthesis of Some New Benzothiazole-Based Heterocycles as Probable Anticancer and Antioxidant Agents. ChemistrySelect 2021, 6, 2569–2575. [Google Scholar] [CrossRef]
- Saini, M.S.; Kumar, A.; Dwivedi, J.; Singh, R. A review: Biological significances of heterocyclic compounds. Int. J. Pharm. Sci. Res. 2013, 4, 66–77. [Google Scholar]
- Akhtar, J.; Khan, A.A.; Ali, Z.; Haider, R.; Shahar Yar, M. Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anticancer activities. Eur. J. Med. Chem. 2017, 125, 143–189. [Google Scholar] [CrossRef] [PubMed]
- Elattar, K.M.; Mert, B.D.; Monier, M.; El-Mekabaty, A. Advances in the chemical and biological diversity of heterocyclic systems incorporating pyrimido [1,6-a]pyrimidine and pyrimido [1,6-c]pyrimidine scaffolds. RSC Adv. 2020, 10, 15461–15492. [Google Scholar] [CrossRef] [PubMed]
- El-Mekabaty, A.; Etman, H.A.; Mosbah, A.; Fadda, A.A. Synthesis and Biological Screening of Some Pyrimidinone-Based Heterocycles from Enamines. ChemistrySelect 2020, 5, 7888–7894. [Google Scholar] [CrossRef]
- El-Mekabaty, A.; Etman, H.A.; Mosbah, A.; Fadda, A.A. Synthesis, In Vitro Cytotoxicity and Bleomycin-Dependent DNA Damage Evaluation of Some Heterocyclic-Fused Pyrimidinone Derivatives. ChemistrySelect 2020, 5, 4856–4861. [Google Scholar] [CrossRef]
- Supino, R. MTT Assays. In In Vitro Toxicity Testing Protocols; Humana Press: Totowa, NJ, USA, 1995; pp. 137–149. [Google Scholar] [CrossRef]
- van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell Sensitivity Assays: The MTT Assay. In Cancer Cell Culture. Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; pp. 237–245. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Racané, L.; Ptiček, L.; Fajdetić, G.; Tralić-Kulenović, V.; Klobučar, M.; Kraljević Pavelić, S.; Perić, M.; Paljetak, H.Č.; Verbanac, D.; Starčević, K. Green synthesis and biological evaluation of 6-substituted-2-(2-hydroxy/methoxy phenyl)benzothiazole derivatives as potential antioxidant, antibacterial and antitumor agents. Bioorganic Chem. 2020, 95, 103537. [Google Scholar] [CrossRef]
- Mistry, B.M.; Patel, R.V.; Keum, Y.S.; Kim, D.H. Chrysin–benzothiazole conjugates as antioxidant and anticancer agents. Bioorganic Med. Chem. Lett. 2015, 25, 5561–5565. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Pérez, L.C.; Padilla-Martínez, I.I.; Cruz, A.; Mendieta-Wejebe, J.E.; Tamay-Cach, F.; Rosales-Hernández, M.C. Evaluation of a new benzothiazole derivative with antioxidant activity in the initial phase of acetaminophen toxicity. Arab. J. Chem. 2019, 12, 3871–3882. [Google Scholar] [CrossRef]
- Durgamma, S.; Muralikrishna, A.; Padmavathi, V.; Padmaja, A. Synthesis and antioxidant activity of amido-linked benzoxazolyl/ benzothiazolyl/benzimidazolyl-pyrroles and pyrazoles. Med. Chem. Res. 2014, 23, 2916–2929. [Google Scholar] [CrossRef]
- Racané, L.; Cindrić, M.; Perin, N.; Roškarić, P.; Starčević, K.; Mašek, T.; Maurić, M.; Dogan, J.; Karminski-Zamola, G. Synthesis and antioxidative potency of novel amidino substituted benzimidazole and benzothiazole derivatives. Croat. Chem. Acta 2017, 90, 187–195. [Google Scholar] [CrossRef]
- Racané, L.; Stojković, R.; Tralić-Kulenović, V.; Cerić, H.; Crossed D Signaković, M.; Ester, K.; Krpan, A.M.; Stojković, M.R. Interactions with polynucleotides and antitumor activity of amidino and imidazolinyl substituted 2-phenylbenzothiazole mesylates. Eur. J. Med. Chem. 2014, 86, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Racané, L.; Kralj, M.; Šuman, L.; Stojković, R.; Tralić-Kulenović, V.; Karminski-Zamola, G. Novel amidino substituted 2-phenylbenzothiazoles: Synthesis, antitumor evaluation in vitro and acute toxicity testing in vivo. Bioorganic Med. Chem. 2010, 18, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Racane, L.; Stojkovic, R.; Tralic-Kulenovic, V.; Karminski-Zamola, G. Synthesis and Antitumor Evaluation of Novel Derivatives of 6-Amino-2-phenylbenzothiazoles. Molecules 2006, 11, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Racanè, L.; Tralić-Kulenović, V.; Kitson, R.P.; Karminski-Zamola, G. Synthesis and antiproliferative activity of cyano and amidino substituted 2-phenylbenzothiazoles. Monatsh Chem. 2006, 137, 1571–1577. [Google Scholar] [CrossRef]
- Hielscher, A.; Gerecht, S. Hypoxia and free radicals: Role in tumor progression and the use of engineering-based platforms to address these relationships. Free Radic. Biol. Med. 2015, 79, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Daskalaki, I.; Gkikas, I.; Tavernarakis, N. Hypoxia and selective autophagy in cancer development and therapy. Front. Cell Dev. Biol. 2018, 6, 409970. [Google Scholar] [CrossRef]
- Ban, H.S.; Kim, B.K.; Lee, H.; Kim, H.M.; Harmalkar, D.; Nam, M.; Park, S.K.; Lee, K.; Park, J.T.; Kim, I.; et al. The novel hypoxia-inducible factor-1α inhibitor IDF-11774 regulates cancer metabolism, thereby suppressing tumor growth. Cell Death Dis. 2017, 8, e2843. [Google Scholar] [CrossRef] [PubMed]
- El-Gazzar, A.B.A.; Aly, A.S.; Zaki, M.E.A.; Hafez, H.N. Synthesis and Preliminary Antimicrobial Activity of New Pyrimido[4,5-b]-quinoline and Pyrido[2,3-d]pyrimidine. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 2119–2138. [Google Scholar] [CrossRef]
- Al-Mutairi, A.A.; Hafez, H.N.; El-Gazzar, A.-R.B.A.; Mohamed, M.Y.A. Synthesis and Antimicrobial, Anticancer and Anti-Oxidant Activities of Novel 2,3-Dihydropyrido [2,3-d]pyrimidine-4-one and Pyrrolo [2,1-b][1,3]benzothiazole Derivatives via Microwave-Assisted Synthesis. Molecules 2022, 27, 1246. [Google Scholar] [CrossRef] [PubMed]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Djuidje, E.N.; Sciabica, S.; Buzzi, R.; Dissette, V.; Balzarini, J.; Liekens, S.; Serra, E.; Andreotti, E.; Manfredini, S.; Vertuani, S.; et al. Design, synthesis and evaluation of benzothiazole derivatives as multifunctional agents. Bioorganic Chem. 2020, 101, 103960. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, J.; Rangarajan, M.; Shao, Y.; LaVoie, E.J.; Huang, T.-C.; Ho, C.-T. Antioxidative Phenolic Compounds from Sage (Salvia officinalis). J. Agric. Food Chem. 1998, 46, 4869–4873. [Google Scholar] [CrossRef]
- Romagnoli, C.; Baldisserotto, A.; Vicentini, C.; Mares, D.; Andreotti, E.; Vertuani, S.; Manfredini, S. Antidermatophytic Action of Resorcinol Derivatives: Ultrastructural Evidence of the Activity of Phenylethyl Resorcinol against Microsporum gypseum. Molecules 2016, 21, 1306. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [PubMed]
- García Rodríguez, L.A.; Hernández-Díaz, S. Nonsteroidal Antiinflammatory Drugs as a Trigger of Clinical Heart Failure. Epidemiology 2003, 14, 240–246. [Google Scholar] [CrossRef]
- Ćaleta, I.; Kralj, M.; Marjanović, M.; Bertoša, B.; Tomić, S.; Pavlović, G.; Pavelić, K.; Karminski-Zamola, G. Novel Cyano- and Amidinobenzothiazole Derivatives: Synthesis, Antitumor Evaluation, and X-ray and Quantitative Structure−Activity Relationship (QSAR) Analysis. J. Med. Chem. 2009, 52, 1744–1756. [Google Scholar] [CrossRef] [PubMed]
- Govindaiah, S.; Naha, S.; Madhuchakrapani Rao, T.; Revanasiddappa, B.C.; Srinivasa, S.M.; Parashuram, L.; Velmathi, S.; Sreenivasa, S. Sulfated magnesium zirconate catalyzed synthesis, antimicrobial, antioxidant, anti-inflammatory, and anticancer activity of benzo[d]thiazole-hydrazone analogues and its molecular docking. Results Chem. 2021, 3, 100197. [Google Scholar] [CrossRef]
- Kashid, B.B.; Ghanwat, A.A.; Khedkar, V.M.; Dongare, B.B.; Shaikh, M.H.; Deshpande, P.P.; Wakchaure, Y.B. Design, Synthesis, In Vitro Antimicrobial, Antioxidant Evaluation, and Molecular Docking Study of Novel Benzimidazole and Benzoxazole Derivatives. J. Heterocycl. Chem. 2019, 56, 895–908. [Google Scholar] [CrossRef]
- Youssef, M.M.; Moharram, H.A.; Youssef, M.M. Methods for Determining the Antioxidant Activity: A Review. Alex. J. Food Sci. Technol. 2014, 11, 31–42. [Google Scholar]
- Shwetha, B.; Sudhanva, M.S.; Jagadeesha, G.S.; Thimmegowda, N.R.; Hamse, V.K.; Sridhar, B.T.; Thimmaiah, K.N.; Ananda Kumar, C.S.; Shobith, R.; Rangappa, K.S. Furan-2-carboxamide derivative, a novel microtubule stabilizing agent induces mitotic arrest and potentiates apoptosis in cancer cells. Bioorganic Chem. 2021, 108, 104586. [Google Scholar] [CrossRef]
- Naha, S.; Govindaiah, S.; Sreenivasa, S.; Prakash, J.K.; Velmathi, S. In Vitro, Molecular Docking, and In Silico Binding Mode Analysis of Organic Compounds for Antimicrobial and Anticancer Activity against Jurkat, HCT116, and A549 Cell Lines. ChemistrySelect 2020, 5, 12807–12818. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Revanasiddappa, B.C.; Ishwarbhat, K.; Kumar, M.V.; D’Souza, L.; Smitha Alva, S. Synthesis and in-vitro anti-inflammatory activity of novel pyrazoline derivatives. Res. J. Pharm. Technol. 2017, 10, 1679. [Google Scholar] [CrossRef]
- Eftekhari-Sis, B.; Zirak, M.; Akbari, A. Arylglyoxals in Synthesis of Heterocyclic Compounds. Chem. Rev. 2013, 113, 2958–3043. [Google Scholar] [CrossRef]
- Siddiqui, N.; Arshad, M.; Ahsan, W.; Alam, M. Thiazoles: A Valuable Insight into the Recent Advances and Biological Activities. Int. J. Pharm. Sci. Drug Res. 2009, 1, 136–143. [Google Scholar]
- Virendra, S.A.; Chaurasia, A.; Chawla, P.A.; Bhagat, D.S.; Bumbrah, G.S. Recent developments in nanocatalyst-mediated ecofriendly synthesis of pyrimidine derivatives. In Nanoparticles in Green Organic Synthesis; Elsevier: Amsterdam, The Netherlands, 2023; pp. 401–419. [Google Scholar] [CrossRef]
- Castells, J.; Lopez-Calahorra, F.; Domingo, L. Postulation of bis(thiazolin-2-ylidene)s as the catalytic species in the benzoin condensation catalyzed by a thiazolium salt plus base. J. Org. Chem. 1988, 53, 4433–4436. [Google Scholar] [CrossRef]
- Dahiya, R.; Dahiya, S.; Fuloria, N.K.; Kumar, S.; Mourya, R.; Chennupati, S.V.; Jankie, S.; Gautam, H.; Singh, S.; Karan, S.K.; et al. Natural Bioactive Thiazole-Based Peptides from Marine Resources: Structural and Pharmacological Aspects. Mar. Drugs 2020, 18, 329. [Google Scholar] [CrossRef] [PubMed]
- Voelker, A.R.; Eliasmith, C. Programming Neuromorphics Using the Neural Engineering Framework. In Handbook of Neuroengineering; Springer: Singapore, 2021; pp. 1–43. [Google Scholar] [CrossRef]
- Abdelhamid, A.O.; El Sayed, I.E.; Zaki, Y.H.; Hussein, A.M.; Mangoud, M.M.; Hosny, M.A. Utility of 5-(furan-2-yl)-3-(p-tolyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide in the synthesis of heterocyclic compounds with antimicrobial activity. BMC Chem. 2019, 13, 48. [Google Scholar] [CrossRef]
- Karegoudar, P.; Karthikeyan, M.S.; Prasad, D.J.; Mahalinga, M.; Holla, B.S.; Kumari, N.S. Synthesis of some novel 2,4-disubstituted thiazoles as possible antimicrobial agents. Eur. J. Med. Chem. 2008, 43, 261–267. [Google Scholar] [CrossRef]
- Güzeldemirci, N.U.; Küçükbasmacı, Ö. Synthesis and antimicrobial activity evaluation of new 1,2,4-triazoles and 1,3,4-thiadiazoles bearing imidazo[2,1-b]thiazole moiety. Eur. J. Med. Chem. 2010, 45, 63–68. [Google Scholar] [CrossRef]
- Bondock, S.; Fadaly, W.; Metwally, M.A. Synthesis and antimicrobial activity of some new thiazole, thiophene and pyrazole derivatives containing benzothiazole moiety. Eur. J. Med. Chem. 2010, 45, 3692–3701. [Google Scholar] [CrossRef]
- Mishra, R.; Sharma, P.K.; Verma, P.K.; Tomer, I.; Mathur, G.; Dhakad, P.K. Biological Potential of Thiazole Derivatives of Synthetic Origin. J. Heterocycl. Chem. 2017, 54, 2103–2116. [Google Scholar] [CrossRef]
- Nickeleit, I.; Zender, S.; Sasse, F.; Geffers, R.; Brandes, G.; Sö Rensen, I.; Steinmetz, H.; Kubicka, S.; Carlomagno, T.; Menche, D.; et al. Cancer Cell Article Argyrin A Reveals a Critical Role for the Tumor Suppressor Protein p27 kip1 in Mediating Antitumor Activities in Response to Proteasome Inhibition. Cancer Cell 2008, 14, 23–35. [Google Scholar] [CrossRef]
- Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M. Novel thiazole derivatives: A patent review (2008–2012; Part 1). Expert. Opin. Ther. Pat. 2014, 24, 201–216. [Google Scholar] [CrossRef]
- Chhabria, M.T.; Patel, S.; Modi, P.; Brahmkshatriya, P.S. Thiazole: A Review on Chemistry, Synthesis and Therapeutic Importance of its Derivatives. Curr. Top. Med. Chem. 2016, 16, 2841–2862. [Google Scholar] [CrossRef]
- Pour-Ali, S.; Hejazi, S. Tiazofurin drug as a new and non-toxic corrosion inhibitor for mild steel in HCl solution: Experimental and quantum chemical investigations. J. Mol. Liq. 2022, 354, 118886. [Google Scholar] [CrossRef]
- Kassem, A.F.; Althomali, R.H.; Anwar, M.M.; El-Sofany, W.I. Thiazole moiety: A promising scaffold for anticancer drug discovery. J. Mol. Struct. 2024, 1303, 137510. [Google Scholar] [CrossRef]
- Lindauer, M.; Hochhaus, A. Dasatinib. In Small Molecules in Oncology ; Springer: Berlin/Heidelberg, Germany, 2014; Volume 201, pp. 27–65. [Google Scholar] [CrossRef]
- Cloud, M.L.; Offen, W.W. Nizatidine versus placebo in gastroesophageal reflux disease. Dig. Dis. Sci. 1992, 37, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Basit, A.W.; Newton, J.M.; Lacey, L.F. Susceptibility of the H2-receptor antagonists cimetidine, famotidine and nizatidine, to metabolism by the gastrointestinal microflora. Int. J. Pharm. 2002, 237, 23–33. [Google Scholar] [CrossRef]
- Adelroth, E.; Inman, M.; Summers, E.; Pace, D.; Modi, M.; Obyrne, P. Prolonged protection against exercise-induced bronchoconstriction by the leukotriene D4–receptor antagonist cinalukast. J. Allergy Clin. Immunol. 1997, 99, 210–215. [Google Scholar] [CrossRef]
- Meini, S.; Pagotto, A.; Longo, B.; Vendramin, I.; Pecori, D.; Tascini, C. Role of Lopinavir/Ritonavir in the Treatment of Covid-19: A Review of Current Evidence, Guideline Recommendations, and Perspectives. J. Clin. Med. 2020, 9, 2050. [Google Scholar] [CrossRef]
- Hussein, A.M.; Al Bahir, A.; Zaki, Y.H.; Ahmed, O.M.; Eweas, A.F.; Elroby, S.A.; Mohamed, M.A. Synthesis, in vitro antioxidant, anticancer activity and molecular docking of new thiazole derivatives. Results Chem. 2024, 7, 101508. [Google Scholar] [CrossRef]
- Kumbhare, M.; Guleha, V.; Sivakumar, T. Estimation of total phenolic content, cytotoxicity and in–vitro antioxidant activity of stem bark of Moringa oleifera. Asian Pac. J. Trop. Dis. 2012, 2, 144–150. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Ahmed, O.M.; Elgendy, H.S. Novel Synthesis of Puriens analougues and Thieno[2,3-b] pyridine derivatives with anticancer and antioxidant activity. J. Pharm. Res. 2014, 8, 1303–1313. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Sofan, M.A.; El-Mekabaty, A.; Hasel, A.M.; Said, S.B. Synthesis, cytotoxicity assessment and antioxidant activity of some new thiazol-2-yl carboxamides. J. Heterocycl. Chem. 2021, 58, 1645–1655. [Google Scholar] [CrossRef]
- Lissi, E.A.; Modak, B.; Torres, R.; Escobar, J.; Urzua, A. Total antioxidant potential of resinous exudates from Heliotropium species, and a comparison of the ABTS and DPPH methods. Free Radic. Res. 1999, 30, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Rahim, F.; Khan, I.U.; Uddin, N.; Farooq, R.K.; Wadood, A.; Rehman, A.U.; Khan, K.M. Synthesis of thiazole-based-thiourea analogs: As anticancer, antiglycation and antioxidant agents, structure activity relationship analysis and docking study. J. Biomol. Struct. Dyn. 2023, 41, 12077–12092. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Walker, G.; Gamcsik, M.P. Assessing MTT and sulforhodamine B cell proliferation assays under multiple oxygen environments. Cytotechnology 2023, 75, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Shakil, M.S.; Rana, Z.; Hanif, M.; Rosengren, R.J. Key considerations when using the sulforhodamine B assay for screening novel anticancer agents. Anticancer Drugs 2022, 33, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesan, S.; Karpagam, S. Novel series of N-acyl substituted indole based piperazine, thiazole and tetrazoles as potential antibacterial, antifungal, antioxidant and cytotoxic agents, and their docking investigation as potential Mcl-1 inhibitors. J. Mol. Struct. 2023, 1271, 134013. [Google Scholar] [CrossRef]
- Moon, J.-H.; Terao, J. Antioxidant Activity of Caffeic Acid and Dihydrocaffeic Acid in Lard and Human Low-Density Lipoprotein. J. Agric. Food Chem. 1998, 46, 5062–5065. [Google Scholar] [CrossRef]
- Kachhot, K.D.; Vaghela, F.H.; Dhamal, C.H.; Vegal, N.K.; Bhatt, T.D.; Joshi, H.S. Water-Promoted Synthesis of Pyrazole-Thiazole-Derivatives as Potent Antioxidants And their Anti-cancer Activity: ADMET and SAR Studies. ChemistrySelect 2024, 9, e202303521. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Abhale, Y.K.; Shinde, A.; Shelke, M.; Nawale, L.; Sarkar, D.; Mhaske, P.C. Synthesis of new 2-(thiazol-4-yl)thiazolidin-4-one derivatives as potential anti-mycobacterial agents. Bioorganic Chem. 2021, 115, 105192. [Google Scholar] [CrossRef]
- Weis, S.; Kesselmeier, M.; Davis, J.S.; Morris, A.M.; Lee, S.; Scherag, A.; Hagel, S.; Pletz, M.W. Cefazolin versus anti-staphylococcal penicillins for the treatment of patients with Staphylococcus aureus bacteraemia. Clin. Microbiol. Infect. 2019, 25, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Singh, R.; Sonar, P.K.; Saraf, S.K. Novel 4-Thiazolidinone Derivatives as Anti-Infective Agents: Synthesis, Characterization, and Antimicrobial Evaluation. Biochem. Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-F.; Zhang, P.-L.; Sui, Y.-F.; Lv, J.-S.; Ansari, M.F.; Battini, N.; Li, S.; Zhou, C.-H.; Geng, R.-X. Ethylenic conjugated coumarin thiazolidinediones as new efficient antimicrobial modulators against clinical methicillin-resistant Staphylococcus aureus. Bioorganic Chem. 2020, 94, 103434. [Google Scholar] [CrossRef] [PubMed]
- Elkerdasy, A.; Alsulis, S.; Ahmed, M.; Mousa, A.A.; Orabi, S.; Mansour, D.; Khalifa, H.; Abd Eldaim, M. A convinient synthesis, anticonvulsant screening and neurotoxicity study of novel quinoline incorporated thiazolidin-4-one derivatives as potential anticonvulsant agents. J. Drug Dev. Health Sci. 2021, 1, 1–13. [Google Scholar]
- Youssef, A.M.; Sydney White, M.; Villanueva, E.B.; El-Ashmawy, I.M.; Klegeris, A. Synthesis and biological evaluation of novel pyrazolyl-2,4-thiazolidinediones as anti-inflammatory and neuroprotective agents. Bioorganic Med. Chem. 2010, 18, 2019–2028. [Google Scholar] [CrossRef] [PubMed]
- Borisova, M.S.; Ivankin, D.I.; Sokolov, D.N.; Luzina, O.A.; Rybalova, T.V.; Tolstikova, T.G.; Salakhutdinov, N.F. Synthesis, antiulcerative, and anti-inflammatory activities of new campholenic derivatives-1,3-thiazolidin-4-ones, 1,3-thiazolidine-2,4-diones, and 1,3-thiazinan-4-ones. Chem. Pap. 2021, 75, 5503–5514. [Google Scholar] [CrossRef]
- Piątkowska-Chmiel, W.; Popiołek, Ł.; Gawrońska-Grzywacz, M.; Natorska-Chomicka, D.; Izdebska, M.; Herbet, M.; Dudka, J.; Poleszak, E.; Wujec, M. The analgesic effect of 1,3-thiazolidin-4-one derivatives as potential modulators of the serotoninergic system. Farmacia 2019, 67, 258–266. [Google Scholar] [CrossRef]
- Carvalho, S.A.; Da Silva, E.F.; Santa-Rita, R.M.; De Castro, S.L.; Fraga, C.A.M. Synthesis and antitrypanosomal profile of new functionalized 1,3,4-thiadiazole-2-arylhydrazone derivatives, designed as non-mutagenic megazol analogues. Bioorganic Med. Chem. Lett. 2004, 14, 5967–5970. [Google Scholar] [CrossRef] [PubMed]
- Almandil, N.B.; Taha, M.; Rahim, F.; Wadood, A.; Imran, S.; Alqahtani, M.A.; Bamarouf, Y.A.; Ibrahim, M.; Mosaddik, A.; Gollapalli, M. Synthesis of novel quinoline-based thiadiazole, evaluation of their antileishmanial potential and molecular docking studies. Bioorganic Chem. 2019, 85, 109–116. [Google Scholar] [CrossRef]
- Saini, N.; Sharma, A.; Thakur, V.K.; Makatsoris, C.; Dandia, A.; Bhagat, M.; Tonk, R.K.; Sharma, P.C. Microwave assisted green synthesis of thiazolidin-4-one derivatives: A perspective on potent antiviral and antimicrobial activities. Curr. Res. Green. Sustain. Chem. 2020, 3, 100021. [Google Scholar] [CrossRef]
- Chitre, T.S.; Patil, S.M.; Sujalegaonkar, A.G.; Asgaonkar, K.D. Designing of Thiazolidin-4-one Pharmacophore using QSAR Studies for Anti-HIV Activity. Indian J. Pharm. Educ. Res. 2021, 55, 581–589. [Google Scholar] [CrossRef]
- Yu, I.; Choi, D.; Lee, H.-K.; Cho, H. Synthesis and Biological Evaluation of New Benzylidenethiazolidine-2,4-dione Derivatives as 15-hydroxyprostaglandin Dehydrogenase Inhibitors to Control the Intracellular Levels of Prostaglandin E2 for Wound Healing. Biotechnol. Bioprocess. Eng. 2019, 24, 464–475. [Google Scholar] [CrossRef]
- Naim, M.J.; Alam, M.J.; Nawaz, F.; Naidu, V.G.M.; Aaghaz, S.; Sahu, M.; Siddiqui, N.; Alam, O. Synthesis, molecular docking and anti-diabetic evaluation of 2,4-thiazolidinedione based amide derivatives. Bioorganic Chem. 2017, 73, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.D.; Pasha, T.Y.; Lunagariya, P.; Shah, U.; Bhambharoliya, T.; Tripathi, R.K.P. A Library of Thiazolidin-4-one Derivatives as Protein Tyrosine Phosphatase 1B (PTP1B) Inhibitors: An Attempt To Discover Novel Antidiabetic Agents. ChemMedChem 2020, 15, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameid, M.K.; Zaki, I.; Mohammed, M.R.; Mohamed, K.O. Design, synthesis, and cytotoxic screening of novel azole derivatives on hepatocellular carcinoma (HepG2 Cells). Bioorganic Chem. 2020, 101, 103995. [Google Scholar] [CrossRef] [PubMed]
- Appalanaidu, K.; Kotcherlakota, R.; Dadmal, T.L.; Bollu, V.S.; Kumbhare, R.M.; Patra, C.R. Synthesis and biological evaluation of novel 2-imino-4-thiazolidinone derivatives as potent anti-cancer agents. Bioorganic Med. Chem. Lett. 2016, 26, 5361–5368. [Google Scholar] [CrossRef] [PubMed]
- Tahlan, S.; Kumar, S.; Ramasamy, K.; Lim, S.M.; Shah, S.A.A.; Mani, V.; Narasimhan, B. In-silico molecular design of heterocyclic benzimidazole scaffolds as prospective anticancer agents. BMC Chem. 2019, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.C.; Pandit, U.P.; Dodiya, A. Thiazolidinedione compounds: A patent review (2010–present). Expert. Opin. Ther. Pat. 2015, 25, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Chadha, N.; Bahia, M.S.; Kaur, M.; Silakari, O. Thiazolidine-2,4-dione derivatives: Programmed chemical weapons for key protein targets of various pathological conditions. Bioorganic Med. Chem. 2015, 23, 2953–2974. [Google Scholar] [CrossRef]
- Nyaki, H.Y.; Mahmoodi, N.O. Synthesis and characterization of derivatives including thiazolidine-2,4-dione/1-H- imidazole and evaluation of antimicrobial, antioxidant, and cytotoxic properties of new synthetic heterocyclic compounds. Res. Chem. Intermed. 2021, 47, 4129–4155. [Google Scholar] [CrossRef]
- Gordon, M.H. The development of oxidative rancidity in foods. In Antioxidants in Food; Elsevier: Amsterdam, The Netherlands, 2001; pp. 7–21. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity using the DPPH.Free Radical Method. LWT-Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Mekhlef, Y.O.; AboulMagd, A.M.; Gouda, A.M. Design, Synthesis, Molecular docking, and biological evaluation of novel 2,3-diaryl-1,3-thiazolidine-4-one derivatives as potential anti-inflammatory and cytotoxic agents. Bioorganic Chem. 2023, 133, 106411. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kumar, D.; Kumar, P.; Thareja, S.; Marwaha, M.G.; Navik, U.; Marwaha, R.K. Synthesis, biological evaluation and in-silico ADME studies of novel series of thiazolidin-2,4-dione derivatives as antimicrobial, antioxidant and anticancer agents. BMC Chem. 2022, 16, 68. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Shah, C.S.; Kumar, S.S.; Alex, A.T.; Maliyakkal, N.; Moorkoth, S.; Mathew, J.E. Synthesis, in vitro anticancer and antioxidant activity of thiadiazole substituted thiazolidin-4-ones. Acta Pharm. 2013, 63, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam-manesh, M.; Beyzaei, H.; Heidari Majd, M.; Hosseinzadegan, S.; Ghazvini, K. Investigation and comparison of biological effects of regioselectively synthesized thiazole derivatives. J. Heterocycl. Chem. 2021, 58, 1525–1530. [Google Scholar] [CrossRef]
- Serban, G.; Stanasel, O.; Serban, E.; Bota, S. 2-Amino-1,3,4-thiadiazole as a potential scaffold for promising antimicrobial agents. Drug Des. Dev. Ther. 2018, 12, 1545–1566. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, J.; Opolski, A. Synthesis and antiproliferative activity of N-substituted 2-amino-5-(2,4-dihydroxyphenyl)-1,3,4-thiadiazoles. Bioorganic Med. Chem. 2006, 14, 4483–4489. [Google Scholar] [CrossRef] [PubMed]
- Çevik, U.A.; Osmaniye, D.; Levent, S.; Saǧlik, B.N.; Çavuşoǧlu, B.K.; Özkay, Y.; Kaplancikl, Z.A. Synthesis and characterization of a new series of thiadiazole derivatives as potential anticancer agents. Heterocycl. Commun. 2020, 26, 6–13. [Google Scholar] [CrossRef]
- Reports, M.S.-P. Thiadiazole Derivatives as Anticancer Agents; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Gomha, S.M.; Salah, T.A.; Abdelhamid, A.O. Synthesis, characterization, and pharmacological evaluation of some novel thiadiazoles and thiazoles incorporating pyrazole moiety as anticancer agents. Monatsh Chem. 2015, 146, 149–158. [Google Scholar] [CrossRef]
- Gomha, S.M.; Kheder, N.A.; Abdelaziz, M.R.; Mabkhot, Y.N.; Alhajoj, A.M. A facile synthesis and anticancer activity of some novel thiazoles carrying 1,3,4-Thiadiazole moiety. Chem. Cent. J. 2017, 11, 1–9. [Google Scholar] [CrossRef]
- Szeliga, M. Thiadiazole derivatives as anticancer agents. Pharmacol. Rep. 2020, 72, 1079–1100. [Google Scholar] [CrossRef]
- Schenone, S.; Brullo, C.; Bruno, O.; Bondavalli, F.; Ranise, A.; Filippelli, W.; Rinaldi, B.; Capuano, A.; Falcone, G. New 1,3,4-thiadiazole derivatives endowed with analgesic and anti-inflammatory activities. Bioorganic Med. Chem. 2006, 14, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Datar, P.A.; Deokule, T.A. Development of Thiadiazole as an Antidiabetic Agent—A Review. Mini Rev. Med. Chem. 2014, 14, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Küçükgüzel, Ş.G.; Küçükgüzel, İ.; Tatar, E.; Rollas, S.; Şahin, F.; Güllüce, M.; De Clercq, E.; Kabasakal, L. Synthesis of some novel heterocyclic compounds derived from diflunisal hydrazide as potential anti-infective and anti-inflammatory agents. Eur. J. Med. Chem. 2007, 42, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.M.; Krees, S.V.; Mauger, A.B.; Rzeszotarski, W.J.; Wolff, F.W. Some Hypotensive Thiadiazolest. J. Med. Chem. 1972, 15, 1082–1084. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Myers, M.; Gadie, B.; Hale, S.A.; Horsley, A.; Nelson, A.J.; Pape, R.; Seville, J.F.; Doxey, J.C.; Berridge, T.L. Antihypertensive Thiadiazoles. 2. Vasodilator Activity of Some 2-Aryl-5-guanidino-1,3,4-thiadiazoles. J. Med. Chem. 1988, 31, 906–913. [Google Scholar] [CrossRef]
- Turner, S.; Myers, M.; Gadie, B.; Nelson, A.J.; Pape, R.; Seville, J.F.; Doxey, J.C.; Berridge, T.L. Antihypertensive Thiadiazoles. 1. Synthesis of Some 2-Aryl-5-hydrazino-1,3,4-thiadiazoles with Vasodilator Activity. J. Med. Chem. 1988, 31, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Samel, A.B.; Pai, N.R. Synthesis of novel aryloxy propanoyl thiadiazoles as potential antihypertensive agents. J. Chin. Chem. Soc. 2010, 57, 1327–1330. [Google Scholar] [CrossRef]
- El-Enany, W.A.M.A.; Gomha, S.M.; Kamel El-Ziaty, A.; Hussein, W. Synthesis and molecular docking of some new bis-thiadiazoles as anti-hypertensive α-blocking agents. Taylor Fr. 2019, 50, 85–96. [Google Scholar] [CrossRef]
- Luszczki, J.; Karpińska, M.; Matysiak, J.; Niewiadomi, A. Characterization and preliminary anticonvulsant assessment of some 1, 3, 4-thiadiazole derivatives. Pharmacol. Rep. PR 2014, 67, 588–592. [Google Scholar] [CrossRef]
- Sharma, B.; Verma, A.; Prajapati, S.; Sharma, U.K. The anticonvulsant activity of thiadiazoles. Int. J. Med. Chem. 2013, 2013, 348948. [Google Scholar] [CrossRef] [PubMed]
- Harish, K.P.; Mohana, K.N.; Mallesha, L. Synthesis of new 2,5-disubstituted-1,3,4-thiadiazole derivatives and their in vivo anticonvulsant activity. Russ. J. Bioorganic Chem. 2014, 40, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Liesen, A.P.; De Aquino, T.M.; Carvalho, C.S.; Lima, V.T.; De Araújo, J.M.; De Lima, J.G.; De Faria, A.R.; De Melo, E.J.T.; Alves, A.J.; Alves, E.W.; et al. Synthesis and evaluation of anti-Toxoplasma gondii and antimicrobial activities of thiosemicarbazides, 4-thiazolidinones and 1,3,4-thiadiazoles. Eur. J. Med. Chem. 2010, 45, 3685–3691. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kumar, H.; Kumar, V.; Deep, A.; Sharma, A.; Marwaha, M.G.; Marwaha, R.K. Mechanism-based approaches of 1,3,4 thiadiazole scaffolds as potent enzyme inhibitors for cytotoxicity and antiviral activity. Med. Drug Discov. 2023, 17, 100150. [Google Scholar] [CrossRef]
- Siddiqui, N.; Andalip; Bawa, S.; Ali, R.; Afzal, O.; Akhtar, M.; Azad, B.; Kumar, R. Antidepressant potential of nitrogen-containing heterocyclic moieties: An updated review. J. Pharm. Bioallied Sci. 2011, 3, 194. [Google Scholar] [CrossRef]
- Jain, A.K.; Sharma, S.; Vaidya, A.; Ravichandran, V.; Agrawal, R.K. 1,3,4-Thiadiazole and its Derivatives: A Review on Recent Progress in Biological Activities. Chem. Biol. Drug Des. 2013, 81, 557–576. [Google Scholar] [CrossRef]
- Pund, A.A.; Shaikh, M.H.; Chandak, B.G.; Bhosale, V.N.; Magare, B.K. Pyridine-1,3,4-Thiadiazole-Schiff Base Derivatives, as Antioxidant and Antimitotic Agent: Synthesis and in Silico ADME Studies. Polycycl. Aromat. Compd. 2023, 43, 1247–1262. [Google Scholar] [CrossRef]
- Brkanac, S.R.; Vujčić, V.; Cvjetko, P.; Baković, V.; Oreščanin, V. Removal of landfill leachate toxicity and genotoxicity by two treatment methods. Arch. Ind. Hyg. Toxicol. 2014, 65, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Bonciu, E.; Firbas, P.; Fontanetti, C.S.; Wusheng, J.; Karaismailoğlu, M.C.; Liu, D.; Menicucci, F.; Pesnya, D.S.; Popescu, A.; Romanovsky, A.V.; et al. An evaluation for the standardization of the Allium cepa test as cytotoxicity and genotoxicity assay. Caryologia 2018, 71, 191–209. [Google Scholar] [CrossRef]
- Kumar, D.; Aggarwal, N.; Kumar, H.; Kapoor, G.; Deep, A.; Bibi, S.; Sharma, A.; Chopra, H.; Kumar Marwaha, R.; Alshammari, A.; et al. 2-Substituted-3-(5-Substituted-1,3,4-oxadiazol/thiadiazol-2-yl) Thiazolidin-4-one Derivatives: Synthesis, Anticancer, Antimicrobial, and Antioxidant Potential. Pharmaceuticals 2023, 16, 805. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Katouah, H.A. Synthesis, Antioxidant, and Cytotoxic Activities of New 1,3,4-Thiadiazoldiazenylacrylonitrile Derivatives. Polycycl. Aromat. Compd. 2023, 43, 7808–7827. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Salem, M.M.; Elsalam, H.A.A.; Noser, A.A. Design, synthesis, in-silico and biological evaluation of novel 2-Amino-1,3,4-thiadiazole based hydrides as B-cell lymphoma-2 inhibitors with potential anticancer effects. J. Mol. Struct. 2022, 1268, 133673. [Google Scholar] [CrossRef]
- Hamdy, R.; Jones, A.T.; El-Sadek, M.; Hamoda, A.M.; Shakartalla, S.B.; AL Shareef, Z.M.; Soliman, S.S.M.; Westwell, A.D. New Bioactive Fused Triazolothiadiazoles as Bcl-2-Targeted Anticancer Agents. Int. J. Mol. Sci. 2021, 22, 12272. [Google Scholar] [CrossRef] [PubMed]
- Choodamani, B.; Kumar, S.; Gupta, A.K.; Schols, D.; Tahtaci, H.; Karakurt, T.; Kotha, S.; Swapna, B.; Setty, R.; Karki, S.S. Synthesis, molecular docking, and preliminary cytotoxicity study of some novel 2-(naphthalen-1-yl)-methylimidazo[2,1-b][1,3,4]thiadiazoles. J. Mol. Struct. 2021, 1234, 130174. [Google Scholar] [CrossRef]
- Ohlow, M.J.; Moosmann, B. Phenothiazine: The seven lives of pharmacology’s first lead structure. Drug Discov. Today 2011, 16, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Bisht, D.; Singh, A.; Sharma, A.; Parcha, V. Synthesis and antihistaminic potential of some novel substituted dinitrophenothiazine derivatives. J. Rep. Pharm. Sci. 2022, 11, 132–140. [Google Scholar]
- Bhatnagar, A.; Pemawat, G. Recent Developments of Antipsychotic Drugs with Phenothiazine Hybrids: A Review. Chem. Biol. Interface 2022, 12, 77–87. [Google Scholar]
- Egbujor, M.C.; Tucci, P.; Buttari, B.; Nwobodo, D.C.; Marini, P.; Saso, L. Phenothiazines: Nrf2 activation and antioxidant effects. J. Biochem. Mol. Toxicol. 2024, 38, 23661. [Google Scholar] [CrossRef]
- Al Zahrani, N.A.; El-Shishtawy, R.M.; Elaasser, M.M.; Asiri, A.M. Synthesis of Novel Chalcone-Based Phenothiazine Derivatives as Antioxidant and Anticancer Agents. Molecules 2020, 25, 4566. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Doddagaddavalli, M.A.; Bhat, S.S.; Seetharamappa, J. Characterization, crystal structure, anticancer and antioxidant activity of novel N-(2-Oxo-2-(10h-phenothiazin-10-yl)ethyl)piperidine-1-carboxamide. J. Struct. Chem. 2023, 64, 131–141. [Google Scholar] [CrossRef]

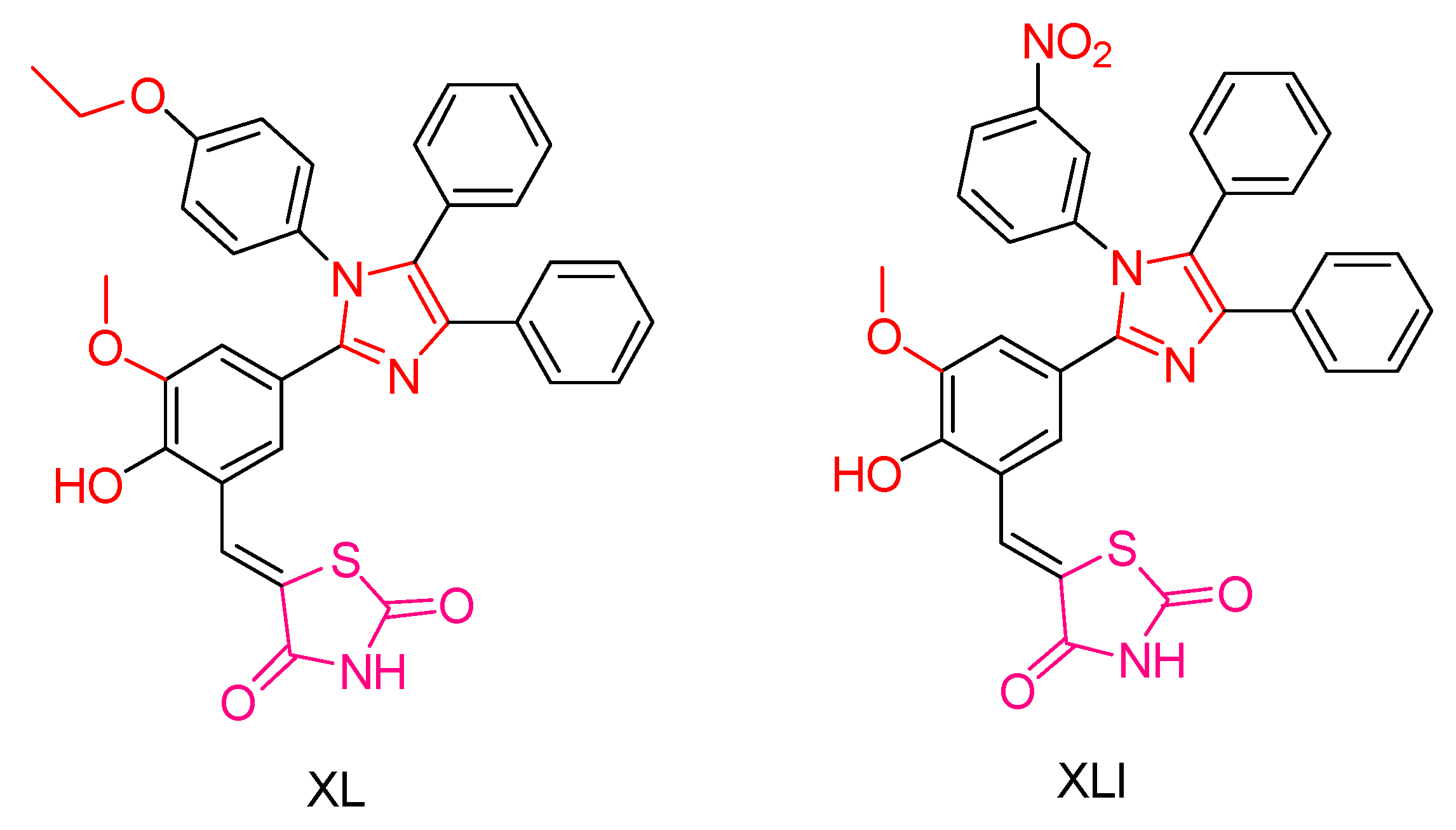
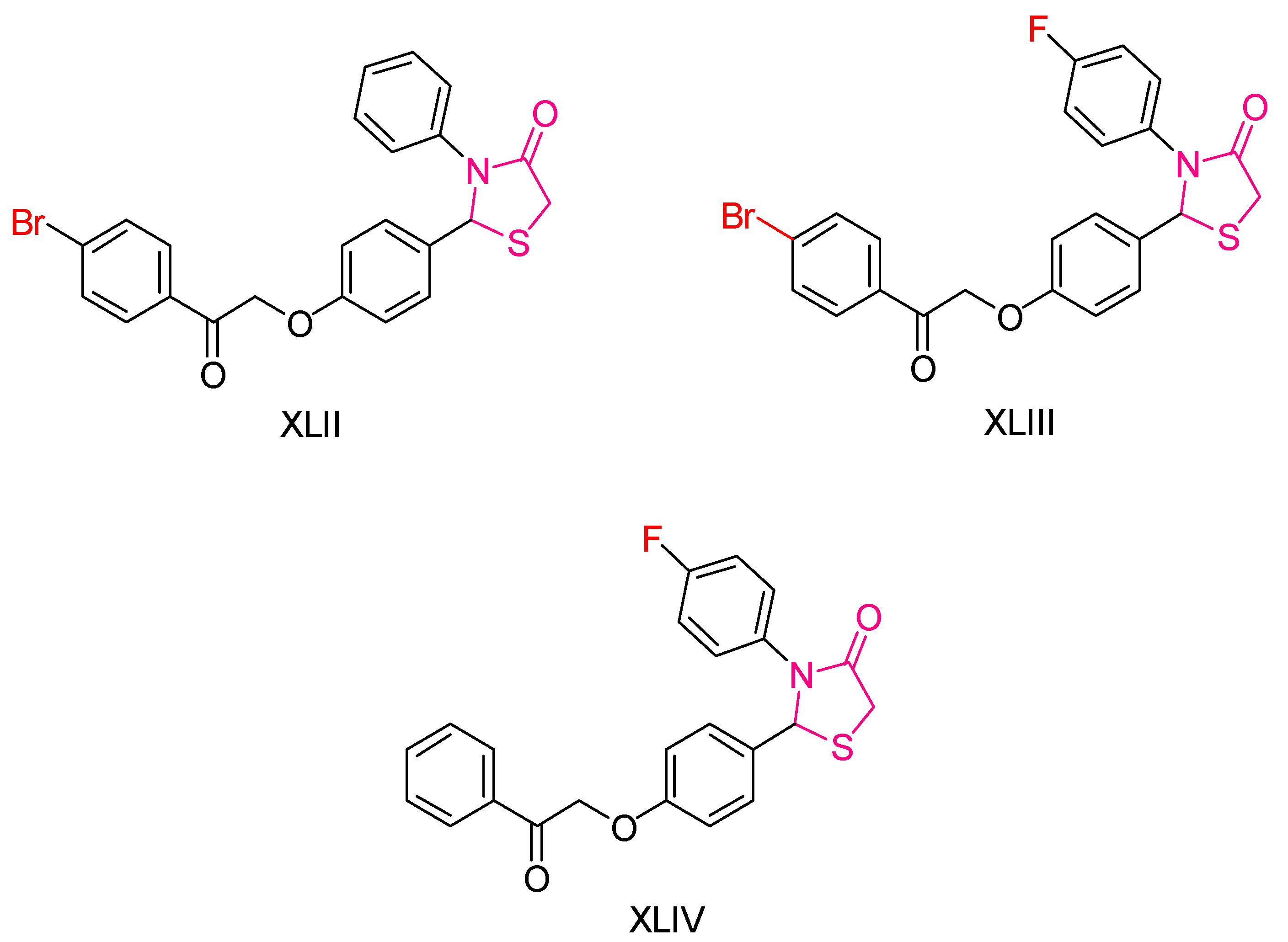
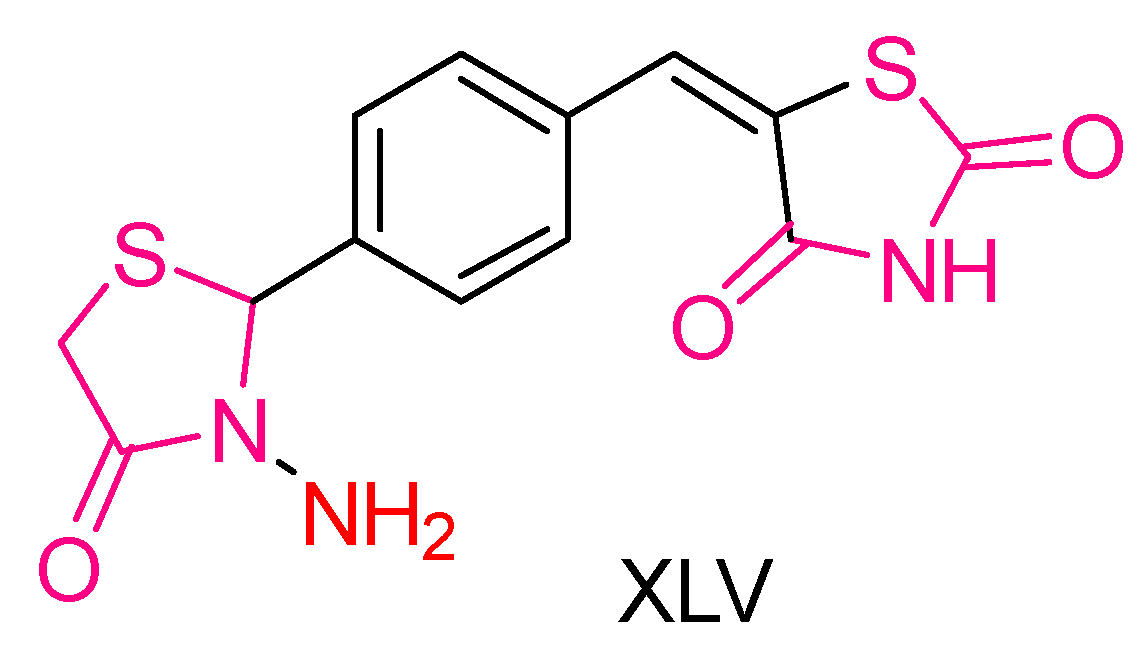
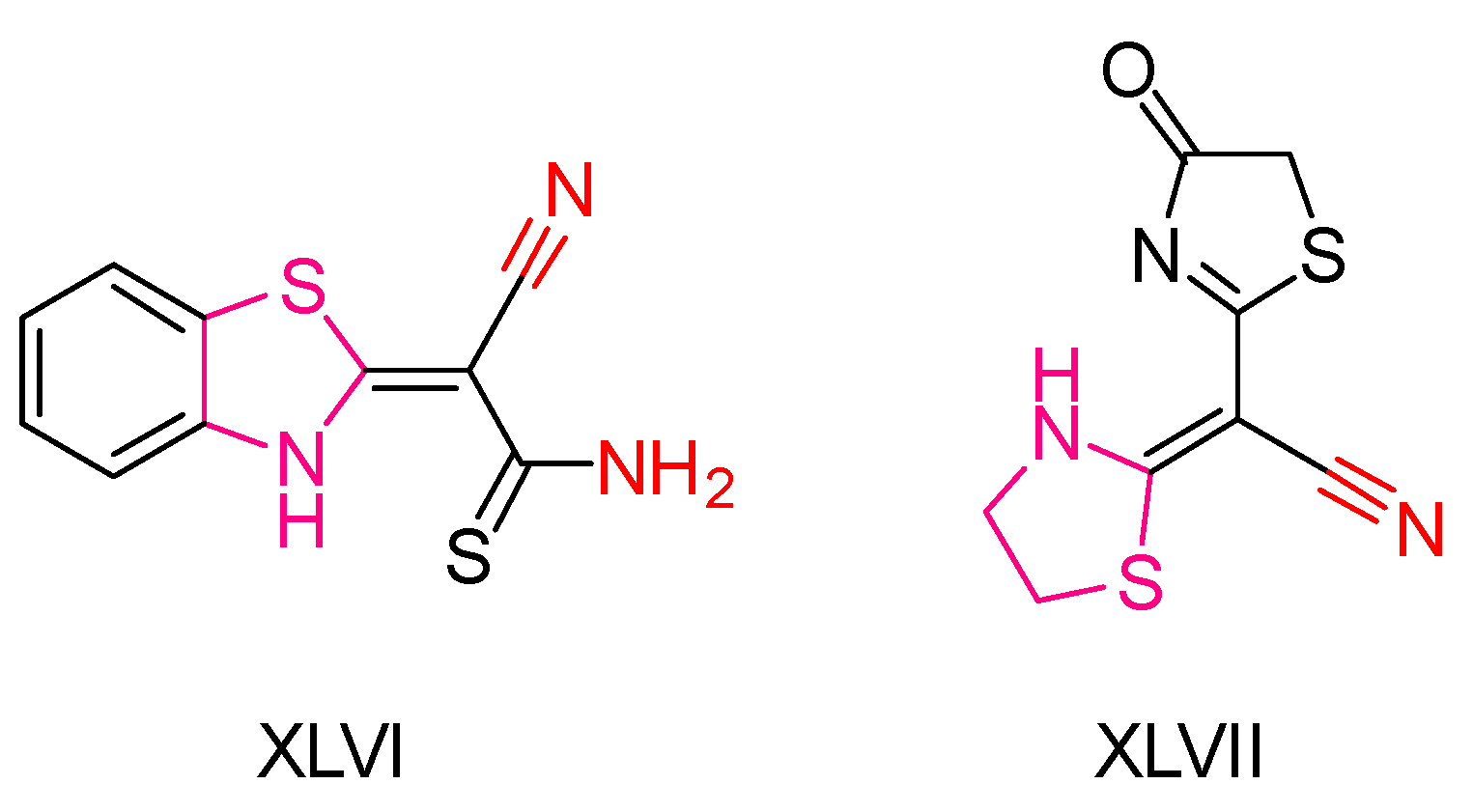
| Compound | Synthesized by | Category | Cytotoxic Assay | IC50 | Antioxidant Assay | IC50 or % Scavenging Ability | Other Activities-COMMENTS |
|---|---|---|---|---|---|---|---|
 | I [105] | Benzothiazoles | MTT assay against HCT-116 | 7.54 ± 0.7 μΜ | ABTS Radical Scavenging Assay | 80–85% | Absence of toxicity to healthy cells WI-38. |
| Reference: Doxorubicin | 5.23 ± 0.3 μΜ | Reference: Ascorbic acid | 88.63% | ||||
 | VIII [114] | Benzothiazoles | Against cervical carcinoma (HeLa) cells | 0.2 μΜ | DPPH | No action | Absence of toxicity to cell lines of human skin fibroblasts (HFF). |
| Reference: 5-fluorouracil | 8.8 ± 1 μΜ | - | - | ||||
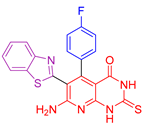 | XIV [127] | Benzothiazoles | MTT assay against colon cancer HCT-116 cells | 0.14 ± 0.004 μΜ | ABTS | 92.8% | Remarkable antimicrobial activity against both Gram-positive and Gram-negative microbes, with efficacy superior to or equal to that of cefotaxime. Ability to repair DNA damage caused by bleomycin. |
| Reference: Doxorubicin | 1.12 ± 0.13 μΜ | Reference: Trolox | 89.5% | ||||
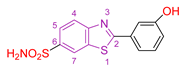 | XIX [130] | Benzothiazoles | MTT against Mia-Paca-2 cells | 5.5 ± 1.1 μΜ | DPPH | 22.73% | Absence of toxicity against normal kidney epithelial cells (HEK 293). Favorable activity against other cancer cell lines. No selectivity. |
| Reference: Unsubstituted Compound ΧVII | >100 | Reference: Compound ΧVII | 43.6% | ||||
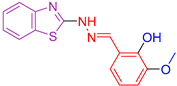 | XXIII [136] | Benzothiazoles | Cytotoxic activity against lung adenocarcinoma (A549) | 7.76 ± 0.5 μΜ | DPPH | IC50: 104 µM (31.16 μg/mL) | Activity against P. aeruginosa, E. coli B. subtilis, S. aureus better than or comparable to Ciprofloxacin. Activity against C. albicans and A. niger is better than Fluconazole. Activity against other cancer lines. |
| Reference: cisplatin | 17.6 ± 0.21 μΜ | Reference: Ascorbic acid | IC50: 140.6 µM (24.76 μg/mL) | ||||
 | XXVII [166] | Thiazoles | MTT assay against hepatocellular carcinoma (HepG2) cell lines | 2.77 μΜ (1.324 μg/mL) | DPPH | 38.03% at a concentration of 26.17µM (12.5 μg/mL) | Form of hydrogen bonds and π interactions with tyrosine kinase. The hydrophobic interactions are attributed to the phenyl moiety. |
| Reference: Gallic acid | 83.1% at a concentration of 26.17µM (12.5 μg/mL) | ||||||
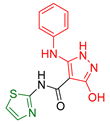 | XXX [168] | Thiazoles | MTT method against lung fibroblast (WI38) cells | 8.91 ± 0.8 μΜ | ABTS method | 73.72% | Activity against human prostate cancer (PC3) too. |
| Reference: Doxorubicin | 6.72 ± 0.5 μΜ | Reference: Ascorbic acid | 88.6% | ||||
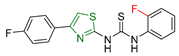 | XXXV [170] | Thiazoles | Sulforhodamine assay against the human breast cancer cell line (MCF7) | 0.10 ± 0.01 μM | DPPH | IC50: 8.90 ± 0.20 μΜ | Good antiglycation inhibitory potential. |
| Reference: Tetrandrine | 1.9 ± 0.1 μM | Reference: n-propyl gallate | IC50: 29.42 ± 0.30 μΜ | ||||
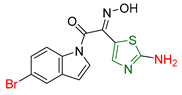 | XXXVI [173] | Thiazoles | Activity against Hela cell line | 0.34 ± 0.1 μΜ | DPPH | EC50: 133.9–148.7 μΜ (45–50 μg/mL) | Absence of toxicity against normal kidney epithelial cells (HEK 293). Favorable activity against other cancer cell lines. High activity against B. cereus, E. coli, and S. aureus. |
| Reference: Doxorubicin | 0.5 ± 0.2 μΜ | Reference: Ascorbic acid | EC50: 33 μΜ (17.95 μg/mL) | ||||
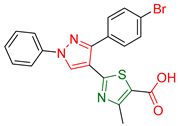 | XXXIX [175] | Thiazoles | Sulforhodamine B colorimetric assay against CNS Cancer cells | 38.58% inhabitation at the concentration of 10−5 M | DPPH | 58.08% at 10 mg/ml | Favorable activity against other cancer cell lines. |
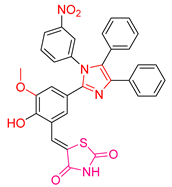 | XLI [197] | Thiazolidinediones | MTT assay against MCF-7 cells | 135.45 μΜ (80 μg/mL) | DPPH | IC50: 1768 μΜ | The compound with the best antitumor capacity and the worst antioxidant activity. Good activity against B. anthracis and P. aeruginosa. |
| Reference: Cisplatin | <64.5 μΜ (20 μg/mL) | Reference: Ascorbic acid | IC50: 971 μΜ | ||||
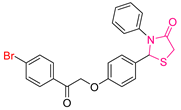 | XLII [200] | Thiazolidinediones | MTT assay against HePG-2 cell line | 2.31 ± 0.1 μΜ | DPPH | IC50: 45.27 ± 2.3 μΜ | Activity against the COX-2 enzyme. |
| Reference: Doxorubicin | 4.50 ± 0.2 μΜ | Reference: Ascorbic acid | IC50: 62.03 ± 3.2 μΜ | ||||
 | XLV [201] | Thiazolidinediones | MTT assay against DU-145 cell line | Cell viability reached 23.792% after 24 h at a 1 mM concentration of the substance | DPPH | IC50: 93.3 μΜ (29.99 μg/mL) | - |
| Reference: Ascorbic acid DPPH | IC50: 227.1 μΜ (40 μg/mL) | ||||||
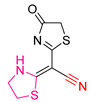 | XLVII [203] | Thiazolidines | MTT assay against MCF-7 breast cancer cells | 112 μM following 72 h exposure | DPPH | IC50: 100.8 μΜ (22.7 μg/mL) | - |
| Reference: Ascorbic acid | IC50: 111.8 μΜ (19.69 μg/mL) | ||||||
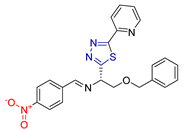 | LIII [226] | Thiadiazoles | Reduction in Allium cepa root length—Concentration: 10 μg/ml | After 96 h 3.8 cm | DPPH | % Inhibition at 150 μg/mL: 70.42% | - |
| Reference: Methotrexate | After 96 h 4 cm | Reference: Ascorbic acid | % Inhibition at 150 μg/mL: 80.40% | ||||
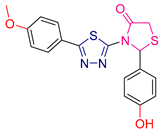 | LVI [229] | Thiadiazoles | MTT assay against human breast cancer (MCF-7 cell line) | 0.5 μΜ | DPPH | IC50: 22.3 μΜ | Combining both actions. |
| Reference: Doxorubicin | 1 μΜ | Reference: Ascorbic acid | IC50: 111.6 μΜ | ||||
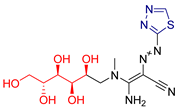 | LVX [231] | Thiadiazoles | MTT assay against human breast cancer (MCF-7 cell line) | 10.7 ± 0.2 μΜ | DPPH | IC50: 8.03 nM (0.003 μg/mL) | Favorable binding energies compared to the native ligand (CK7) of cyclin-dependent kinase 2. |
| Reference: Doxorubicin | 7.7 ± 0.2 μΜ | Reference: Ascorbic acid | IC50: 124.9 nM (0.022 μg/mL) | ||||
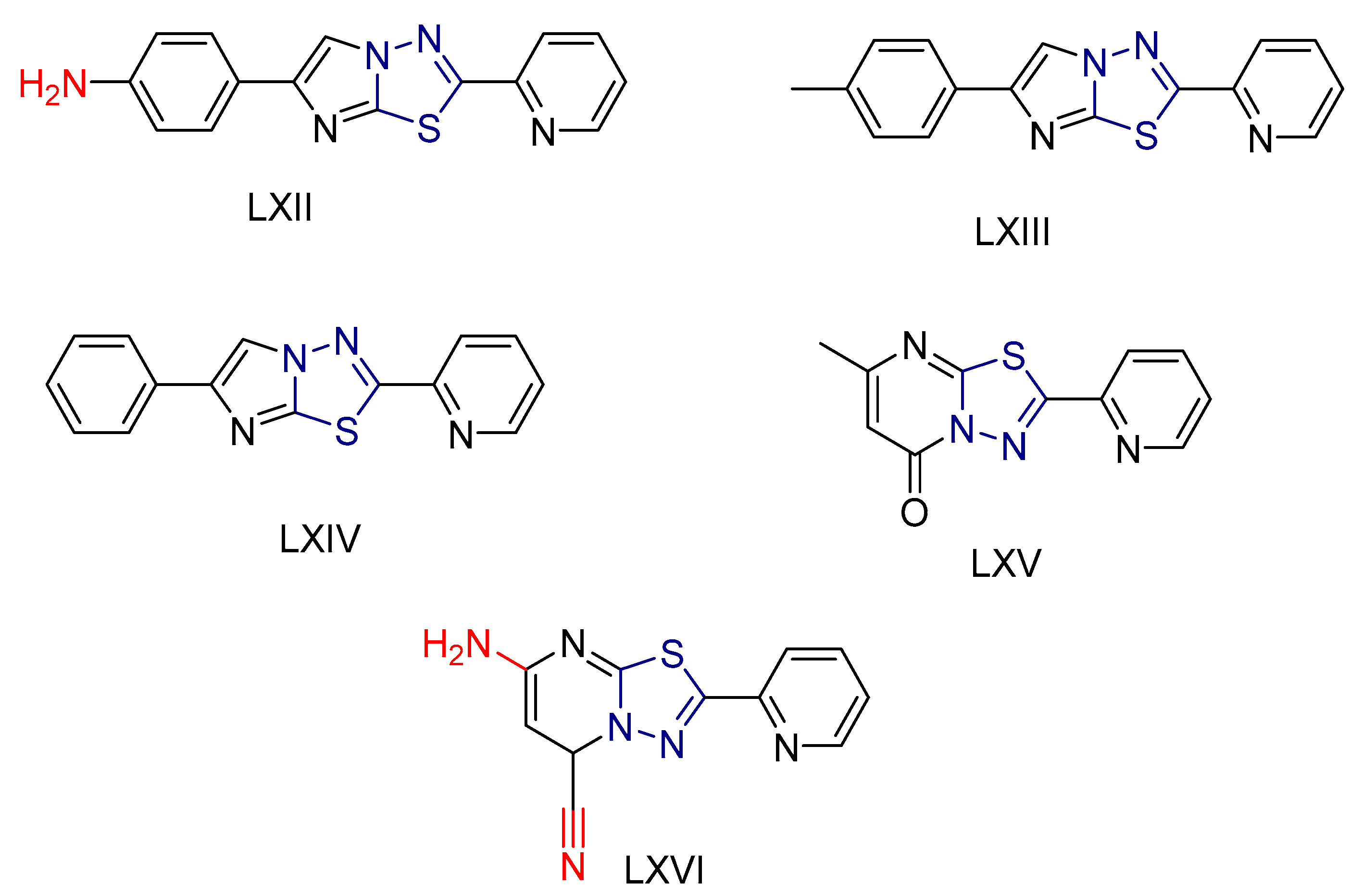
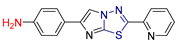 | LXII [232] | Thiadiazoles | MTT assay against triple-negative breast cancer cell line (MDA-231) | 3.92 ± 0.29 μΜ | DPPH | IC50: 41.25 ± 0.5 µM (12.1 ± 0.15 μg/mL) | Absence of toxicity to WISH normal cell line. |
| Reference: Doxorubicin | 2.26 ± 0.1 μΜ | Reference: Ascorbic acid | IC50: 12.32 ± 1μM (2.170 ± 0.21 μg/mL) | ||||
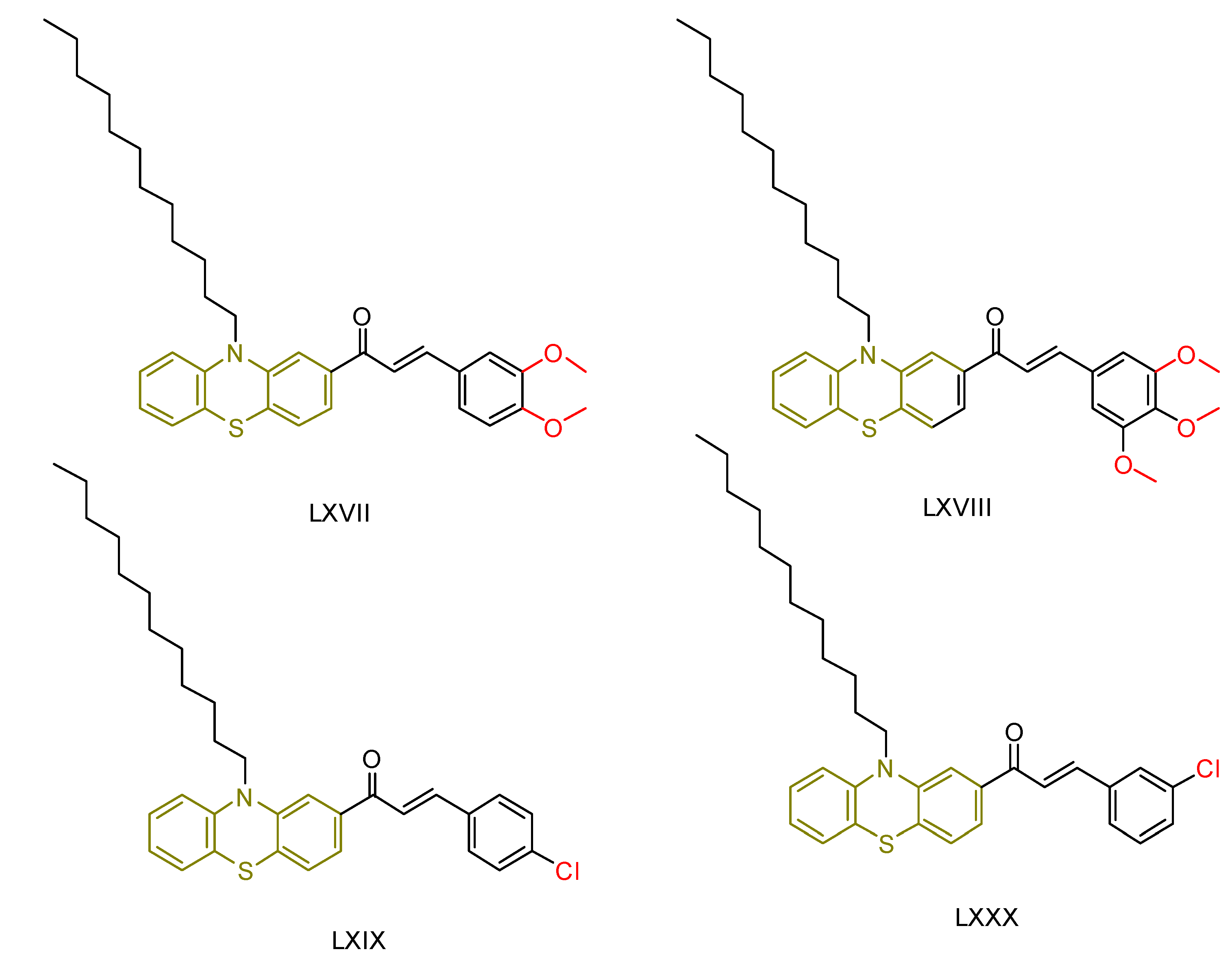
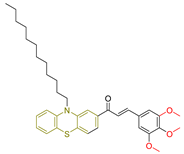 | LXVIII [239] | Phenothiazines | MTT assay against HepG-2 cancer cell line | 12.9 ± 0.3 μM (7.6 ± 0.2 µg/mL) | DPPH scavenging activity of 1 µM of compound | 15–30% | - |
| Reference: Doxorubicin | 66.2 ± 0.07 μM (0.36 ± 0.04 µg/mL) | Reference: Ascorbic acid | 15–30% | ||||
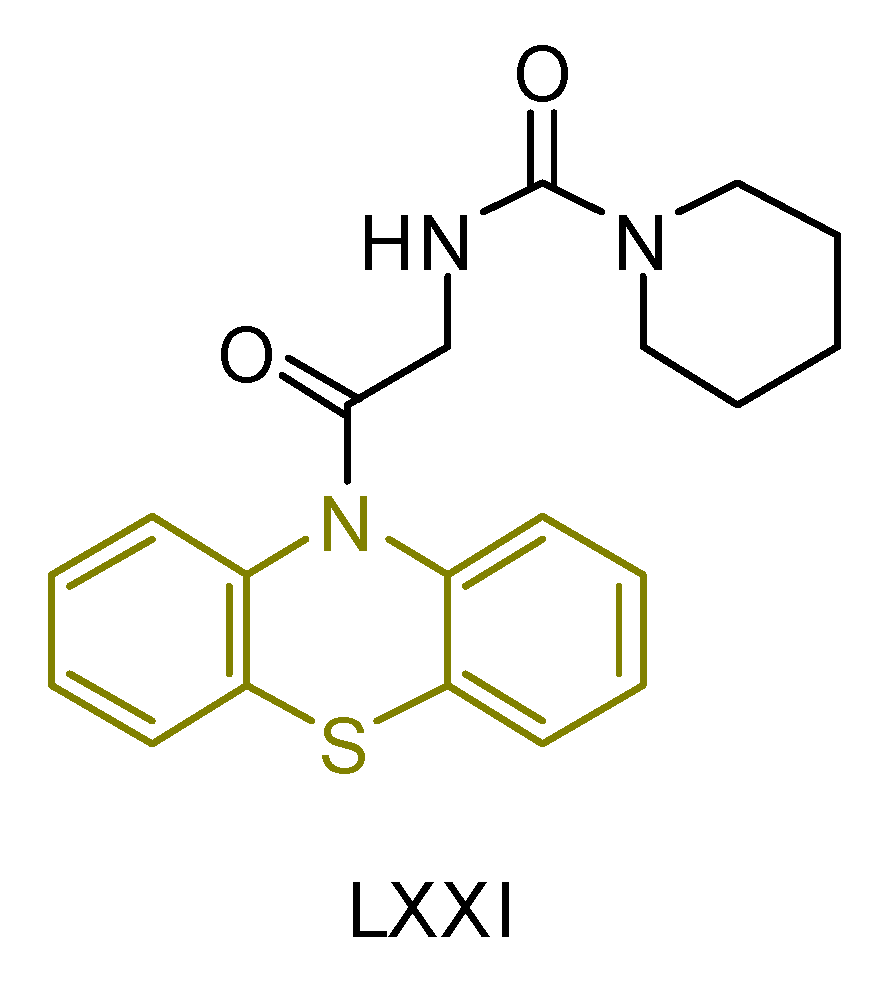
 | LXXI [241] | Phenothiazines | MTT assay against human breast cancer (MCF-7 cell line | 117 ± 3 μM (43.0 ± 1.2 μg/mL) | DPPH | IC50: 44.9 ± 2.7 μM (16.5 ± 1.0 μg/mL) | - |
| Reference: Actinomycin D | 22.4 ± 0.6 μM (28.1 ± 0.8 μg/mL) | Reference: Gallic acid | IC50: 117.6 ± 6.4 μM (20.0 ± 1.1 μg/mL) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drakontaeidi, A.; Papanotas, I.; Pontiki, E. Multitarget Pharmacology of Sulfur–Nitrogen Heterocycles: Anticancer and Antioxidant Perspectives. Antioxidants 2024, 13, 898. https://doi.org/10.3390/antiox13080898
Drakontaeidi A, Papanotas I, Pontiki E. Multitarget Pharmacology of Sulfur–Nitrogen Heterocycles: Anticancer and Antioxidant Perspectives. Antioxidants. 2024; 13(8):898. https://doi.org/10.3390/antiox13080898
Chicago/Turabian StyleDrakontaeidi, Aliki, Ilias Papanotas, and Eleni Pontiki. 2024. "Multitarget Pharmacology of Sulfur–Nitrogen Heterocycles: Anticancer and Antioxidant Perspectives" Antioxidants 13, no. 8: 898. https://doi.org/10.3390/antiox13080898
APA StyleDrakontaeidi, A., Papanotas, I., & Pontiki, E. (2024). Multitarget Pharmacology of Sulfur–Nitrogen Heterocycles: Anticancer and Antioxidant Perspectives. Antioxidants, 13(8), 898. https://doi.org/10.3390/antiox13080898






