Mitogen-Activated Protein Kinase Kinase OsMEK2 Positively Regulates Ca2+ Influx and Ferroptotic Cell Death during Rice Immune Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Fungal Cultures and Growth Conditions
2.3. Fungal Inoculation and Infection Evolution
2.4. Treatment of Pharmacological Compounds
2.5. Detection and Quantification of Ca2+ and H2O2
2.6. Prussian Blue Staining
2.7. Glutathione Measurement
2.8. MDA Quantification
2.9. RNA Extraction and Gene Expression Analysis
3. Results
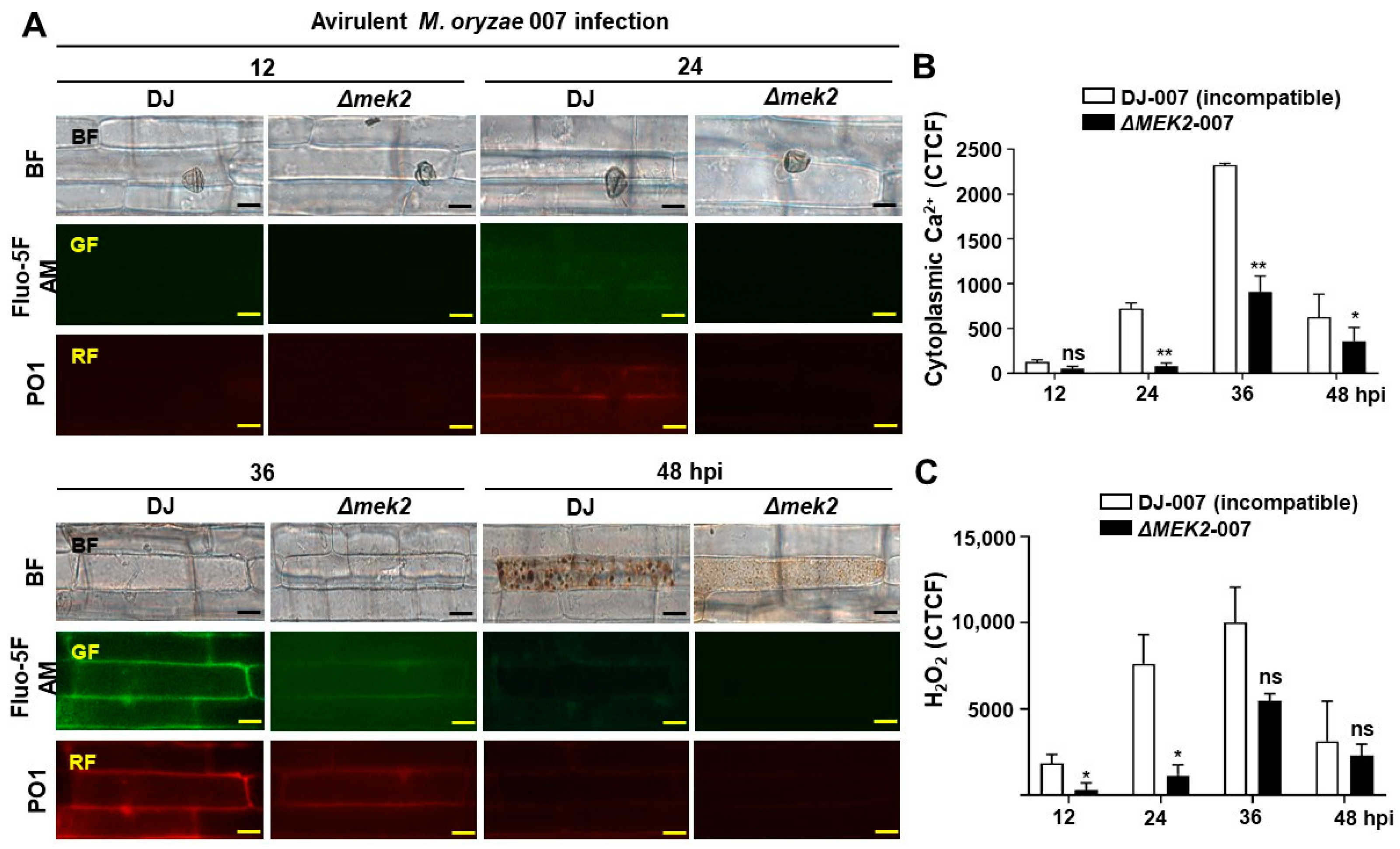
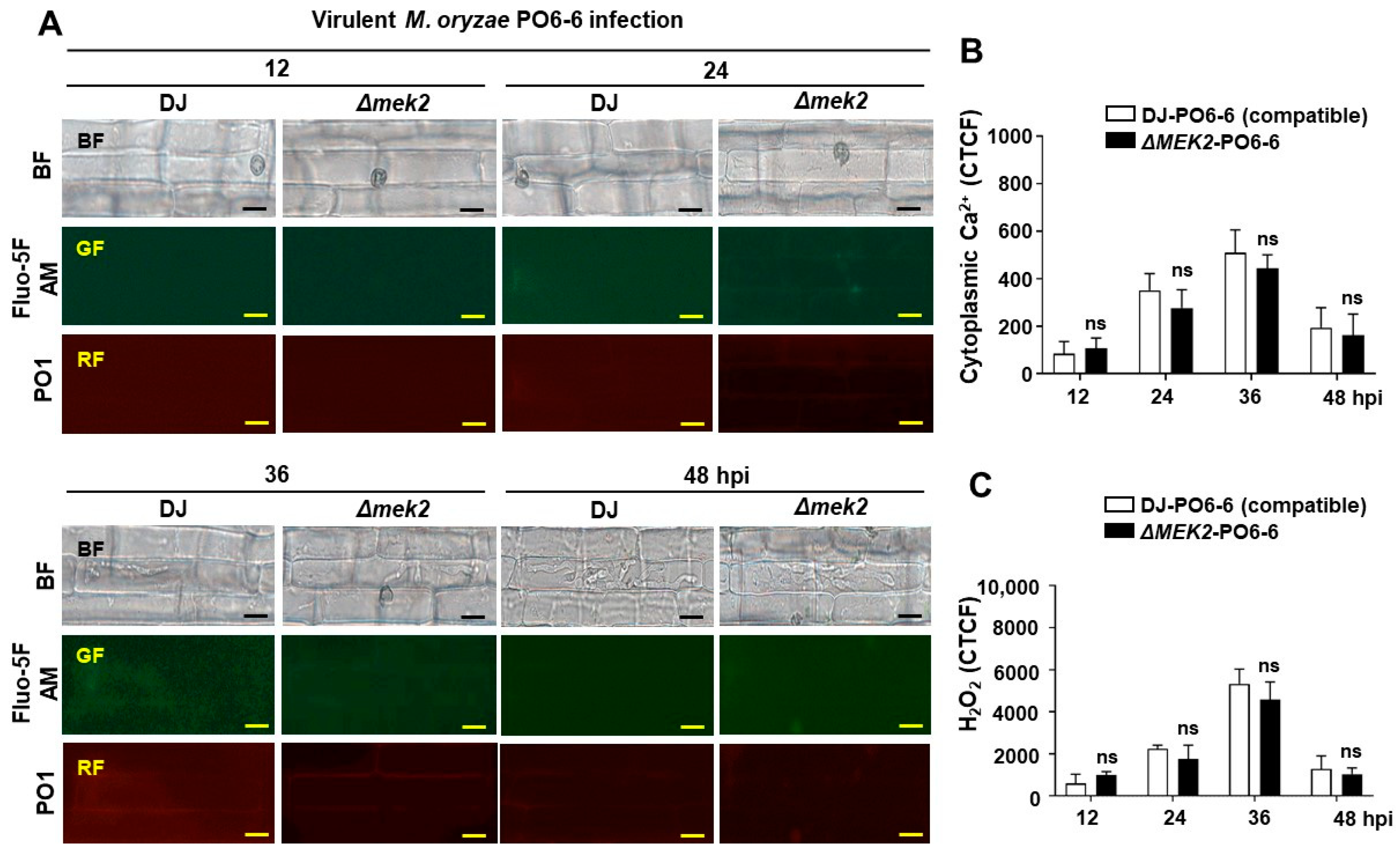
3.1. OsMEK2 Positively Regulates Cytoplasmic Ca2+ and H2O2 Levels during Avirulent M. oryzae Infection
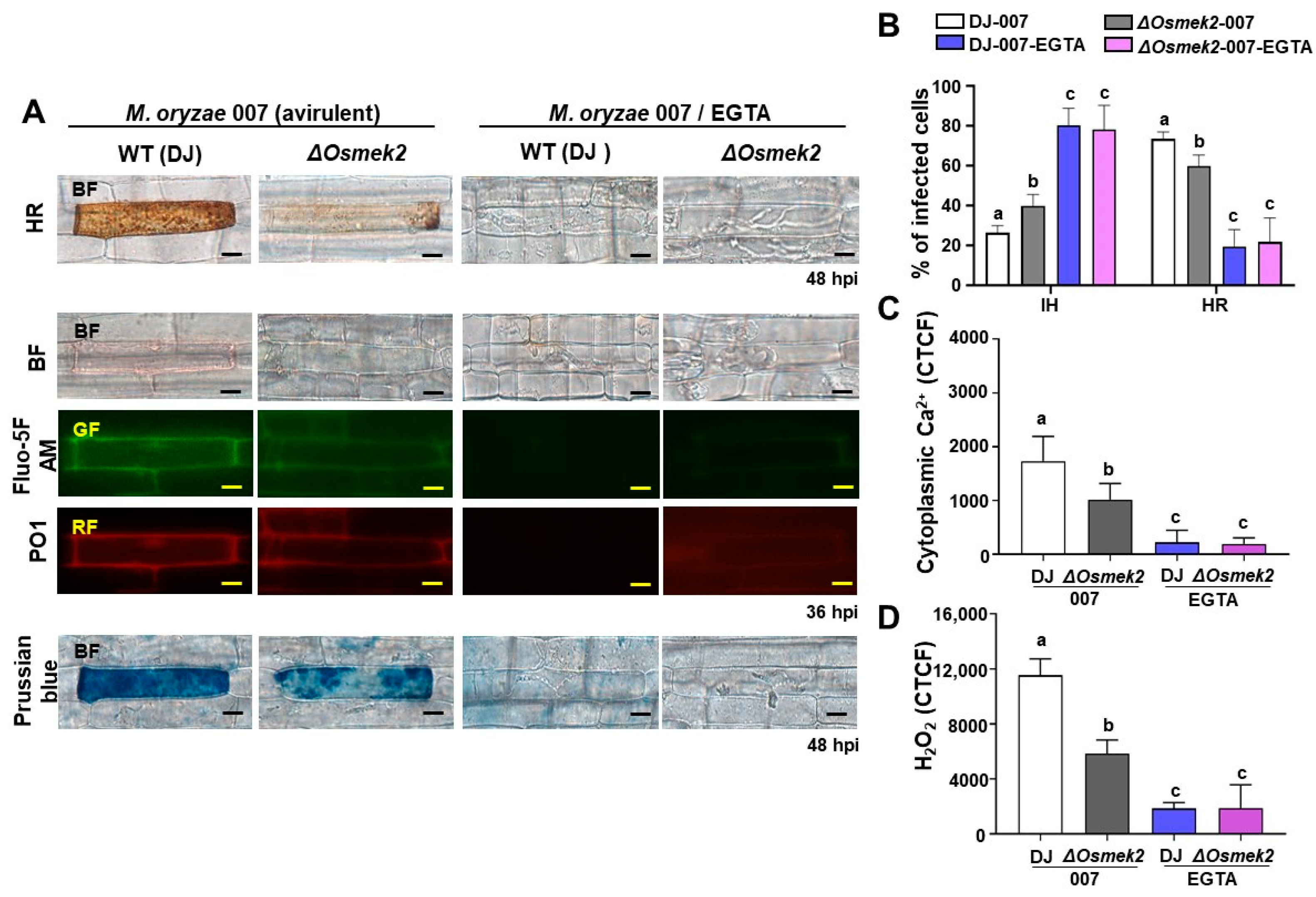
3.2. EGTA Abrogates Ca2+, ROS Increase, Iron Accumulation, and HR Cell Death in WT and ΔOsmek2 Mutant Rice
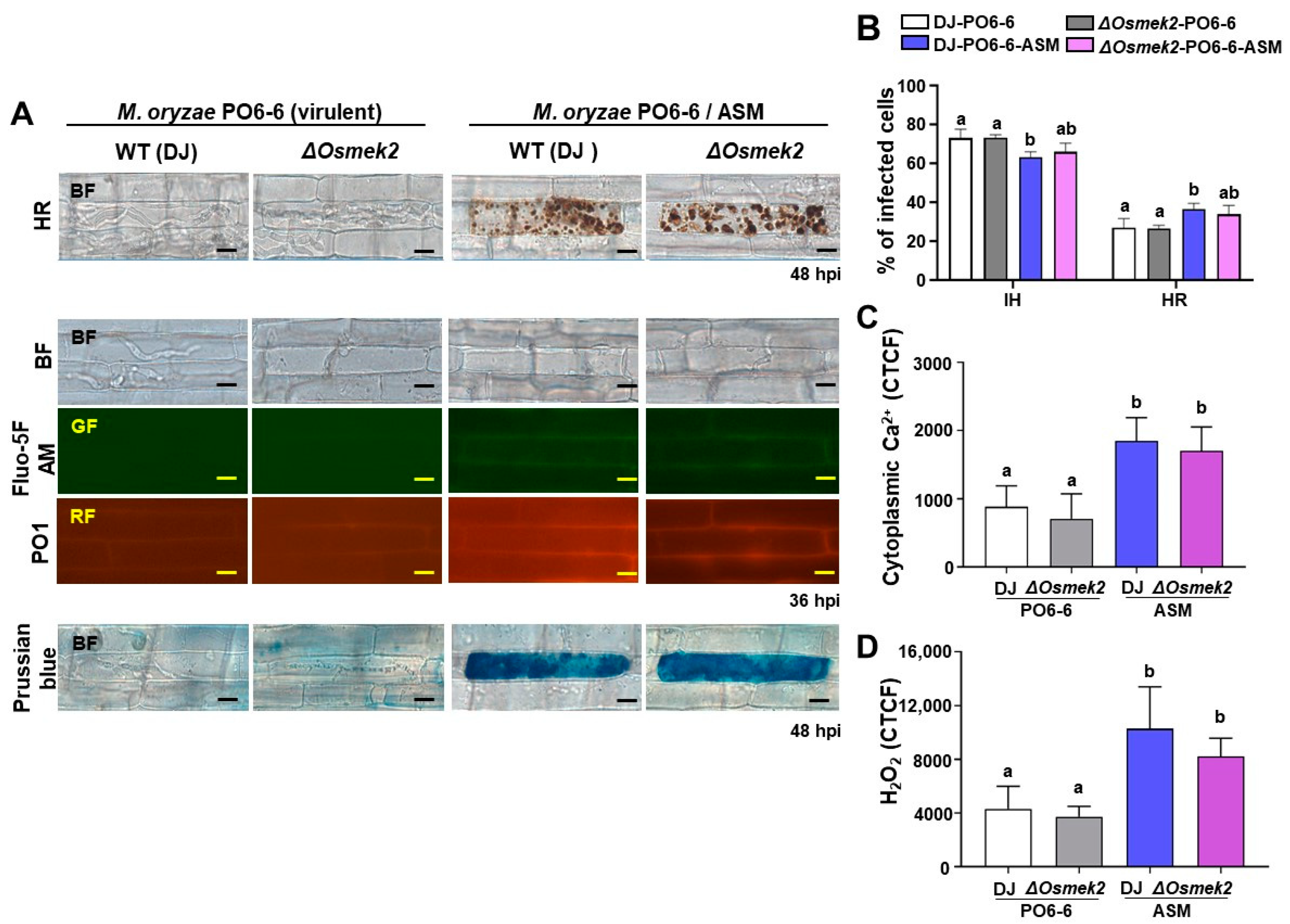
3.3. ASM Enhanced Ca2+, ROS Increase, Iron Accumulation, and HR Cell Death in WT and ΔOsmek2 Mutant Rice
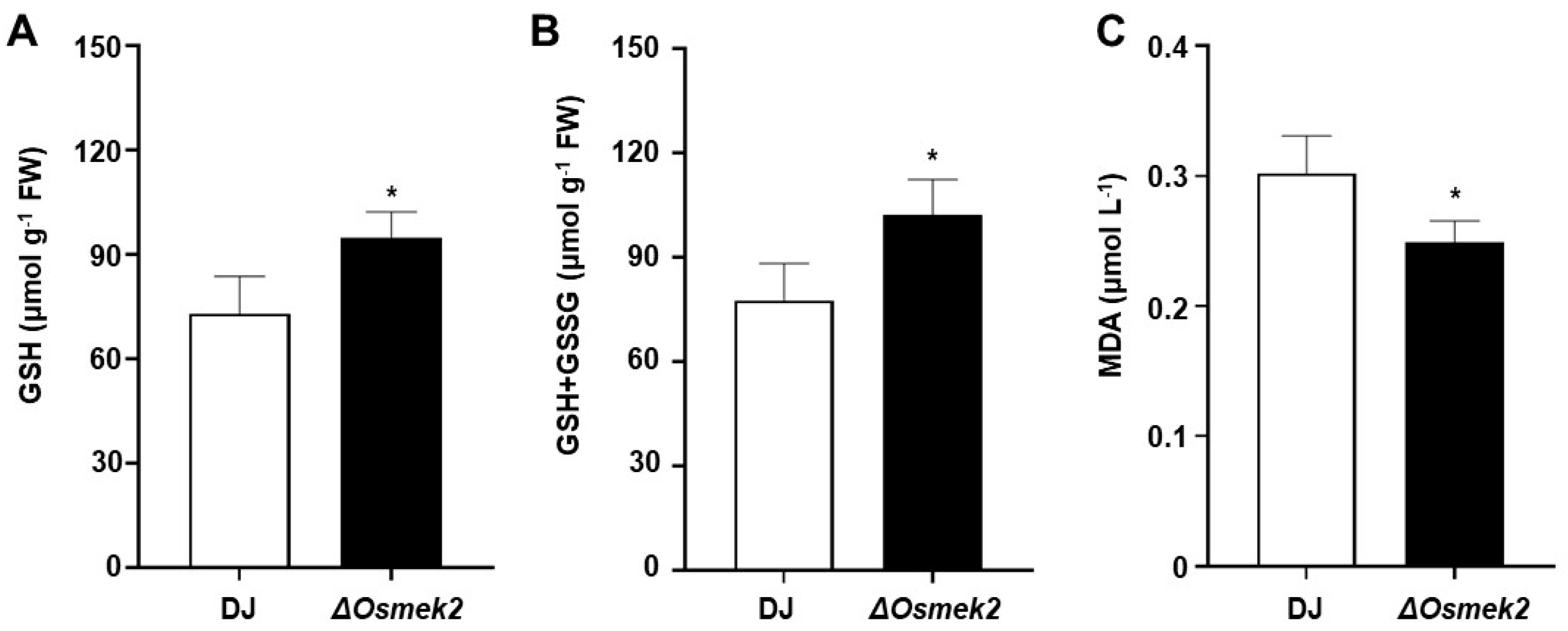
3.4. OsMEK2 Increased Glutathione Content and Impaired Lipid Peroxidation during Avirulent M. oryzae Infection
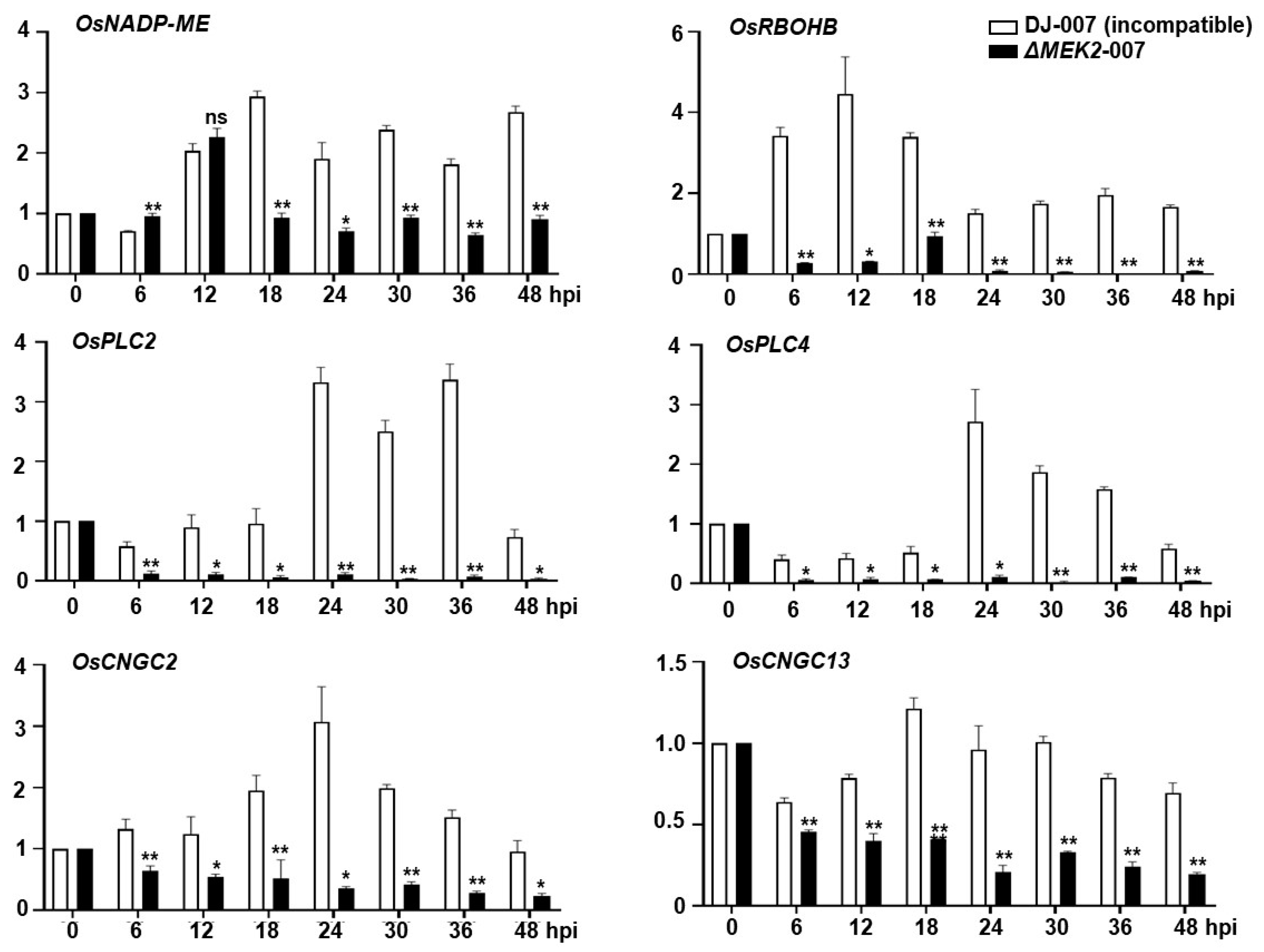
3.5. Defense-Related Genes Were Downregulated in the ΔOsmek2 Mutant Rice during Avirulent M. oryzae Infection
4. Discussion
4.1. OsMEK2 Regulated the Accumulation of Cytoplasmic Ca2+ and H2O2 Induced by Avirulent M. oryzae
4.2. EGTA Blocked Ca2+ Influx, ROS Accumulation, Iron Accumulation, and HR Cell Death in WT and ΔOsmek2 Mutant Rice
4.3. ASM Enhanced Ca2+ Influx, ROS Accumulation, Iron Accumulation, and HR Cell Death in WT and ΔOsmek2 Mutant Rice
4.4. OsMEK2 Regulated Glutathione Depletion and Impaired Lipid Peroxidation during Avirulent M. oryzae Infection
4.5. Time-Course Expression of Defense-Related Genes in WT and ΔOsmek2 Mutant Rice during M. oryzae Infection
4.6. Model of OsMEK2-Mediated Rice Ferroptotic Cell Death and Plant Immune Response
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishad, R.; Ahmed, T.; Rahman, V.J.; Kareem, A. Modulation of plant defense system in response to microbial interactions. Front. Microbiol. 2020, 11, 1298. [Google Scholar] [CrossRef]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling mechanisms in pattern-triggered immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Tsuda, K.; Parker, J.E. Effector-triggered immunity: From pathogen perception to robust defense. Annu. Rev. Plant Biol. 2015, 66, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.W.; Roux, M.; Petersen, M.; Mundy, J. MAP kinase cascades in Arabidopsis innate immunity. Front. Plant Sci. 2012, 3, 169. [Google Scholar] [CrossRef]
- Tripathi, D.; Jiang, Y.L.; Kumar, D. SABP2, a methyl salicylate esterase is required for the systemic acquired resistance induced by acibenzolar-S-methyl in plants. FEBS Lett. 2010, 584, 3458–3463. [Google Scholar] [CrossRef]
- Gao, M.; Liu, J.; Bi, D.; Zhang, Z.; Cheng, F.; Chen, S.; Zhang, Y. MEKK1, MKK1/MKK2 and MPK4 function together in a mitogen-activated protein kinase cascade to regulate innate immunity in plants. Cell Res. 2008, 18, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shao, F.; Li, Y.; Cui, H.; Chen, L.; Li, H.; Zou, Y.; Long, C.; Lan, L.; Chai, J.; et al. A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell Host Microbe 2007, 1, 175–185. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef]
- Ma, H.; Chen, J.; Zhang, Z.; Ma, L.; Yang, Z.; Zhang, Q.; Li, X.; Xiao, J.; Wang, S. MAPK kinase 10.2 promotes disease resistance and drought tolerance by activating different MAPKs in rice. Plant J. 2017, 92, 557–570. [Google Scholar] [CrossRef]
- Singh, R.; Lee, M.O.; Lee, J.E.; Choi, J.; Park, J.H.; Kim, E.H.; Yoo, R.H.; Cho, J.I.; Jeon, J.S.; Rakwal, R.; et al. Rice mitogen-activated protein kinase interactome analysis using the yeast two-hybrid system. Plant Physiol. 2012, 160, 477–487. [Google Scholar] [CrossRef]
- Singh, R.; Jwa, N.S. The rice MAPKK-MAPK interactome: The biological significance of MAPK components in hormone signal transduction. Plant Cell Rep. 2013, 32, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Thulasi Devendrakumar, K.; Li, X.; Zhang, Y. MAP kinase signalling: Interplays between plant PAMP- and effector-triggered immunity. Cell. Mol. Life Sci. 2018, 75, 2981–2989. [Google Scholar] [CrossRef]
- Dangol, S.; Nguyen, N.K.; Singh, R.; Chen, Y.; Wang, J.; Lee, H.G.; Hwang, B.K.; Jwa, N.S. Mitogen-activated protein kinase OsMEK2 and OsMPK1 signaling is required for ferroptotic cell death in rice-Magnaporthe oryzae interactions. Front. Plant Sci. 2021, 12, 710794. [Google Scholar] [CrossRef]
- Dangol, S.; Chen, Y.; Hwang, B.; Jwa, N.-S. Iron- and reactive oxygen species-dependent ferroptotic cell death in rice-Magnaporthe oryzae interactions. Plant Cell 2019, 31, 189–209. [Google Scholar] [CrossRef]
- Wang, J.; Choi, W.G.; Nguyen, N.K.; Liu, D.; Kim, S.H.; Lim, D.; Hwang, B.K.; Jwa, N.S. Cytoplasmic Ca2+ influx mediates iron- and reactive oxygen species-dependent ferroptotic cell death in rice immunity. Front. Plant Sci. 2024, 15, 1339559. [Google Scholar] [CrossRef] [PubMed]
- Moeder, W.; Phan, V.; Yoshioka, K. Ca2+ to the rescue—Ca2+ channels and signaling in plant immunity. Plant Sci. 2019, 279, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, M.; Wang, J.; Qi, J.; Han, Z.; Wang, G.; Qi, Y.; Wang, H.W.; Zhou, J.M.; Chai, J. Reconstitution and structure of a plant NLR resistosome conferring immunity. Science 2019, 364, eaav5870. [Google Scholar] [CrossRef]
- Nagano, M.; Ishikawa, T.; Fujiwara, M.; Fukao, Y.; Kawano, Y.; Kawai-Yamada, M.; Shimamoto, K. Plasma membrane microdomains are essential for Rac1-RbohB/H-mediated immunity in rice. Plant Cell 2016, 28, 1966–1983. [Google Scholar] [CrossRef]
- Bi, G.; Su, M.; Li, N.; Liang, Y.; Dang, S.; Xu, J.; Hu, M.; Wang, J.; Zou, M.; Deng, Y.; et al. The ZAR1 resistosome is a calcium-permeable channel triggering plant immune signaling. Cell 2021, 184, 3528–3541.e3512. [Google Scholar] [CrossRef]
- Forderer, A.; Li, E.; Lawson, A.W.; Deng, Y.N.; Sun, Y.; Logemann, E.; Zhang, X.; Wen, J.; Han, Z.; Chang, J.; et al. A wheat resistosome defines common principles of immune receptor channels. Nature 2022, 610, 532–539. [Google Scholar] [CrossRef]
- Jalmi, S.K.; Sinha, A.K. ROS mediated MAPK signaling in abiotic and biotic stress-striking similarities and differences. Front. Plant Sci. 2015, 6, 769. [Google Scholar] [CrossRef]
- Eaddy, A.C.; Schnellmann, R.G. Visualization and quantification of endoplasmic reticulum Ca2+ in renal cells using confocal microscopy and Fluo5F. Biochem. Biophys. Res. Commun. 2011, 404, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, B.C.; Huynh, C.; Chang, C.J. A palette of fluorescent probes with varying emission colors for imaging hydrogen peroxide signaling in living cells. J. Am. Chem. Soc. 2010, 132, 5906–5915. [Google Scholar] [CrossRef]
- Grossi, M.; Morgunova, M.; Cheung, S.; Scholz, D.; Conroy, E.; Terrile, M.; Panarella, A.; Simpson, J.C.; Gallagher, W.M.; O’Shea, D.F. Lysosome triggered near-infrared fluorescence imaging of cellular trafficking processes in real time. Nat. Commun. 2016, 7, 10855. [Google Scholar] [CrossRef]
- Jakic, B.; Buszko, M.; Cappellano, G.; Wick, G. Elevated sodium leads to the increased expression of HSP60 and induces apoptosis in HUVECs. PLoS ONE 2017, 12, e0179383. [Google Scholar] [CrossRef]
- Bora, P.; Gahurova, L.; Masek, T.; Hauserova, A.; Potesil, D.; Jansova, D.; Susor, A.; Zdrahal, Z.; Ajduk, A.; Pospisek, M.; et al. p38-MAPK-mediated translation regulation during early blastocyst development is required for primitive endoderm differentiation in mice. Commun. Biol. 2021, 4, 788. [Google Scholar] [CrossRef]
- Alisik, M.; Neselioglu, S.; Erel, O. A colorimetric method to measure oxidized, reduced and total glutathione levels in erythrocytes. J. Lab. Med. 2019, 43, 269–277. [Google Scholar] [CrossRef]
- Mates, J.M.; Segura, J.A.; Alonso, F.J.; Marquez, J. Intracellular redox status and oxidative stress: Implications for cell proliferation, apoptosis, and carcinogenesis. Arch. Toxicol. 2008, 82, 273–299. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Gao, M.; He, Y.; Yin, X.; Zhong, X.; Yan, B.; Wu, Y.; Chen, J.; Li, X.; Zhai, K.; Huang, Y.; et al. Ca2+ sensor-mediated ROS scavenging suppresses rice immunity and is exploited by a fungal effector. Cell 2021, 184, 5391–5404.e5317. [Google Scholar] [CrossRef]
- Gilliham, M.; Dayod, M.; Hocking, B.J.; Xu, B.; Conn, S.J.; Kaiser, B.N.; Leigh, R.A.; Tyerman, S.D. Calcium delivery and storage in plant leaves: Exploring the link with water flow. J. Exp. Bot. 2011, 62, 2233–2250. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Nurnberger, T.; Frachisse, J.M.; Wirtz, W.; Guern, J.; Hedrich, R.; Scheel, D. Receptor-mediated activation of a plant Ca2+-permeable ion channel involved in pathogen defense. Proc. Natl. Acad. Sci. USA 1997, 94, 2751–2755. [Google Scholar] [CrossRef]
- Stab, M.R.; Ebel, J. Effects of Ca2+ on phytoalexin induction by fungal elicitor in soybean cells. Arch. Biochem. Biophys. 1987, 257, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Tavernier, E.; Wendehenne, D.; Blein, J.P.; Pugin, A. Involvement of free calcium in action of cryptogein, a proteinaceous elicitor of hypersensitive reaction in tobacco cells. Plant Physiol. 1995, 109, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Lebrun-Garcia, A.; Ouaked, F.; Chiltz, A.; Pugin, A. Activation of MAPK homologues by elicitors in tobacco cells. Plant J. 1998, 15, 773–781. [Google Scholar] [CrossRef]
- Lecourieux, D.; Mazars, C.; Pauly, N.; Ranjeva, R.; Pugin, A. Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell 2002, 14, 2627–2641. [Google Scholar] [CrossRef]
- Ren, D.; Yang, H.; Zhang, S. Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis. J. Biol. Chem. 2002, 277, 559–565. [Google Scholar] [CrossRef]
- Gilroy, S.; Bialasek, M.; Suzuki, N.; Górecka, M.; Devireddy, A.R.; Karpinski, S.; Mittler, R. ROS, calcium, and electric signals: Key mediators of rapid systemic signaling in plants. Plant Physiol. 2016, 171, 1606–1615. [Google Scholar] [CrossRef]
- Seybold, H.; Trempel, F.; Ranf, S.; Scheel, D.; Romeis, T.; Lee, J. Ca2+ signalling in plant immune response: From pattern recognition receptors to Ca2+ decoding mechanisms. New Phytol. 2014, 204, 782–790. [Google Scholar] [CrossRef]
- Dietrich, P.; Moeder, W.; Yoshioka, K. Plant cyclic nucleotide-gated channels: New insights on their functions and regulation. Plant Physiol. 2020, 184, 27–38. [Google Scholar] [CrossRef]
- Lyon, A.M.; Tesmer, J.J. Structural insights into phospholipase C-β function. Mol. Pharmacol. 2013, 84, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.M.; Keppler, L.D.; Orlandi, E.W.; Baker, C.J.; Mischke, C.F. Involvement of plasma membrane calcium influx in bacterial induction of the k/h and hypersensitive responses in tobacco. Plant Physiol. 1990, 92, 215–221. [Google Scholar] [CrossRef]
- Sasabe, M.; Takeuchi, K.; Kamoun, S.; Ichinose, Y.; Govers, F.; Toyoda, K.; Shiraishi, T.; Yamada, T. Independent pathways leading to apoptotic cell death, oxidative burst and defense gene expression in response to elicitin in tobacco cell suspension culture. Eur. J. Biochem. 2000, 267, 5005–5013. [Google Scholar] [CrossRef] [PubMed]
- Salguero-Linares, J.; Coll, N.S. Cell death as a defense strategy against pathogens in plants and animals. PLoS Pathog. 2023, 19, e1011253. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Novianti, F.; Takehara, M.; Fukuhara, T.; Arie, T.; Komatsu, K. Acibenzolar-S-methyl restricts infection of Nicotiana benthamiana by plantago asiatica mosaic virus at two distinct stages. Mol. Plant Microbe Interact. 2019, 32, 1475–1486. [Google Scholar] [CrossRef]
- Brisset, M.-N.; Cesbron, S.; Thomson, S.V.; Paulin, J.-P. Acibenzolar-S-methyl induces the accumulation of defense-related enzymes in apple and protects from fire blight. Eur. J. Plant Pathol. 2000, 106, 529–536. [Google Scholar] [CrossRef]
- Baysal, Ö.; Soylu, E.M.; Soylu, S. Induction of defence-related enzymes and resistance by the plant activator acibenzolar-S-methyl in tomato seedlings against bacterial canker caused by Clavibacter michiganensis ssp. michiganensis. Plant Pathol. 2003, 52, 747–753. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, C.; Hou, J.; Li, Y.; Jiang, C.; Ge, Y. Mitogen-activated protein kinase cascade and reactive oxygen species metabolism are involved in acibenzolar-S-methyl-induced disease resistance in apples. J. Agric. Food Chem. 2020, 68, 10928–10936. [Google Scholar] [CrossRef]
- Dorion, S.; Ouellet, J.C.; Rivoal, J. Glutathione metabolism in plants under stress: Beyond reactive oxygen species detoxification. Metabolites 2021, 11, 641. [Google Scholar] [CrossRef]
- Distefano, A.M.; Lopez, G.A.; Setzes, N.; Marchetti, F.; Cainzos, M.; Cascallares, M.; Zabaleta, E.; Pagnussat, G.C. Ferroptosis in plants: Triggers, proposed mechanisms, and the role of iron in modulating cell death. J. Exp. Bot. 2021, 72, 2125–2135. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, Y.; Wang, C.; Liu, Y. Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death Dis. 2018, 9, 753. [Google Scholar] [CrossRef]
- Zechmann, B. Subcellular roles of glutathione in mediating plant defense during biotic stress. Plants 2020, 9, 1067. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, R.L. Iron accumulation, glutathione depletion, and lipid peroxidation must occur simultaneously during ferroptosis and are mutually amplifying events. Med. Hypotheses 2017, 101, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Catala, A.; Diaz, M. Editorial: Impact of lipid peroxidation on the physiology and pathophysiology of cell membranes. Front. Physiol. 2016, 7, 423. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Escriva, C.; Dromant, M.; Borras, C.; Vina, J. Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. Arch. Biochem. Biophys. 2021, 709, 108941. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, W.; Cao, J.; Meng, F.; Yu, Y.; Huang, J.; Jiang, L.; Liu, M.; Zhang, Z.; Chen, X.; et al. Activation of ethylene signaling pathways enhances disease resistance by regulating ROS and phytoalexin production in rice. Plant J. 2017, 89, 338–353. [Google Scholar] [CrossRef]
- Pugin, A.; Frachisse, J.M.; Tavernier, E.; Bligny, R.; Gout, E.; Douce, R.; Guern, J. Early events induced by the elicitor cryptogein in tobacco cells: Involvement of a plasma membrane NADPH oxidase and activation of glycolysis and the pentose phosphate pathway. Plant Cell 1997, 9, 2077–2091. [Google Scholar] [CrossRef]
- Adachi, H.; Nakano, T.; Miyagawa, N.; Ishihama, N.; Yoshioka, M.; Katou, Y.; Yaeno, T.; Shirasu, K.; Yoshioka, H. WRKY transcription factors phosphorylated by MAPK regulate a plant immune NADPH oxidase in Nicotiana benthamiana. Plant Cell 2015, 27, 2645–2663. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, B.; Ding, H.; Zhang, J.; Li, S. Review: The role of NADP-malic enzyme in plants under stress. Plant Sci. 2019, 281, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, Y.; Kaya, H.; Hiraoka, G.; Yumoto, F.; Kimura, S.; Kadota, Y.; Hishinuma, H.; Senzaki, E.; Yamagoe, S.; Nagata, K.; et al. Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J. Biol. Chem. 2008, 283, 8885–8892. [Google Scholar] [CrossRef] [PubMed]
- Dubiella, U.; Seybold, H.; Durian, G.; Komander, E.; Lassig, R.; Witte, C.P.; Schulze, W.X.; Romeis, T. Calcium-dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc. Natl. Acad. Sci. USA 2013, 110, 8744–8749. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Nguyen, N.K.; Liu, D.; Jwa, N.-S. Mitogen-Activated Protein Kinase Kinase OsMEK2 Positively Regulates Ca2+ Influx and Ferroptotic Cell Death during Rice Immune Responses. Antioxidants 2024, 13, 1013. https://doi.org/10.3390/antiox13081013
Wang J, Nguyen NK, Liu D, Jwa N-S. Mitogen-Activated Protein Kinase Kinase OsMEK2 Positively Regulates Ca2+ Influx and Ferroptotic Cell Death during Rice Immune Responses. Antioxidants. 2024; 13(8):1013. https://doi.org/10.3390/antiox13081013
Chicago/Turabian StyleWang, Juan, Nam Khoa Nguyen, Dongping Liu, and Nam-Soo Jwa. 2024. "Mitogen-Activated Protein Kinase Kinase OsMEK2 Positively Regulates Ca2+ Influx and Ferroptotic Cell Death during Rice Immune Responses" Antioxidants 13, no. 8: 1013. https://doi.org/10.3390/antiox13081013
APA StyleWang, J., Nguyen, N. K., Liu, D., & Jwa, N.-S. (2024). Mitogen-Activated Protein Kinase Kinase OsMEK2 Positively Regulates Ca2+ Influx and Ferroptotic Cell Death during Rice Immune Responses. Antioxidants, 13(8), 1013. https://doi.org/10.3390/antiox13081013






