Impact of Intermittent Fasting and Dietary Restriction on Redox State, Energetic Metabolism, and Liver Injury in Common Bile Duct Ligation Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
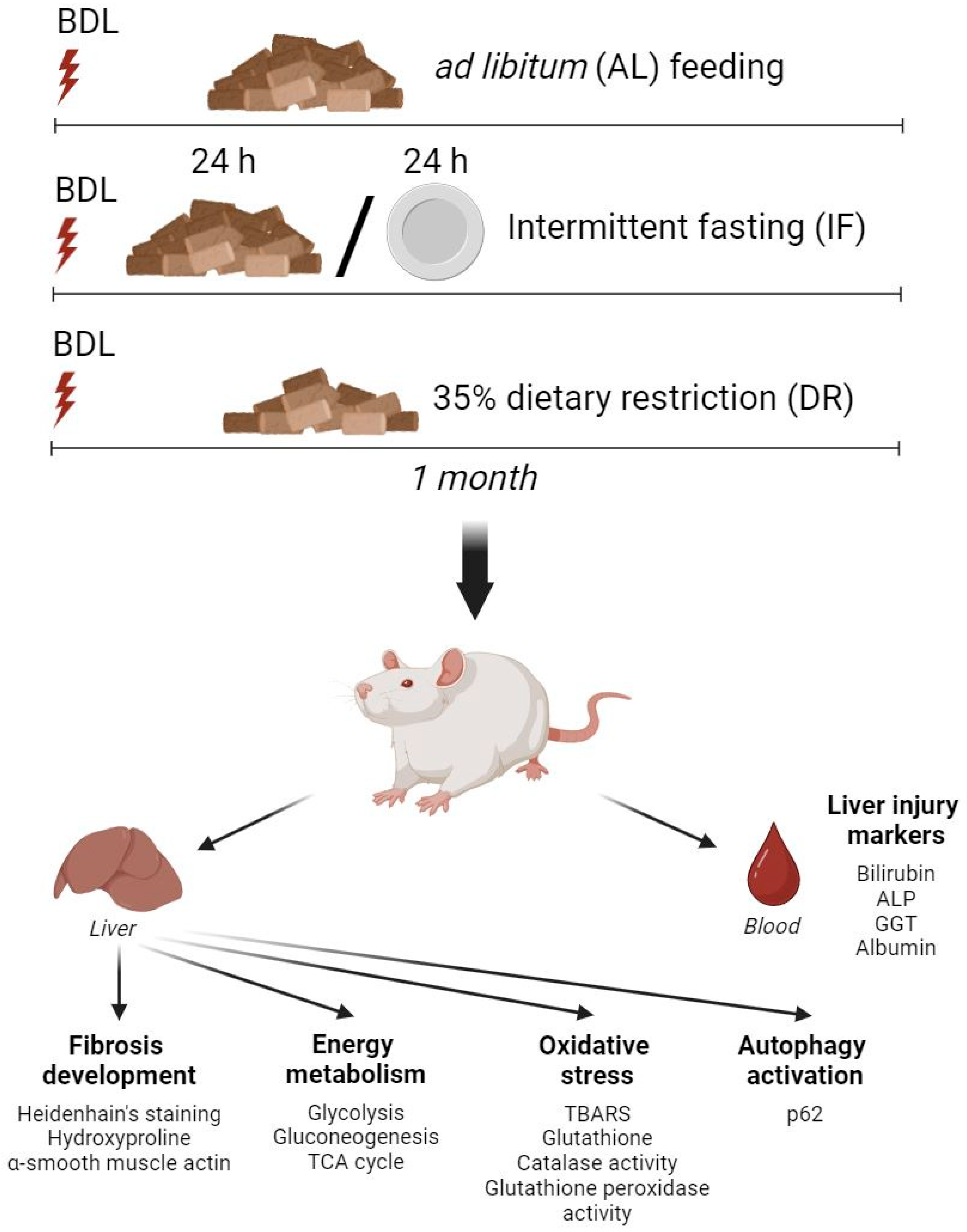
2.2. BDL Model and Dietary Treatment
2.3. Determination of Liver Damage Markers in Blood Serum
2.4. Histological Analysis and Hydroxyproline Measurement
2.5. Determination of the Activity of Energy and Glucose Metabolism Enzymes
2.6. Determination of Parameters of Oxidative Stress and Antioxidant Systems
2.7. Western Blot Analysis
2.8. Bioinformatic Analysis
2.9. Statistical Analysis
3. Results
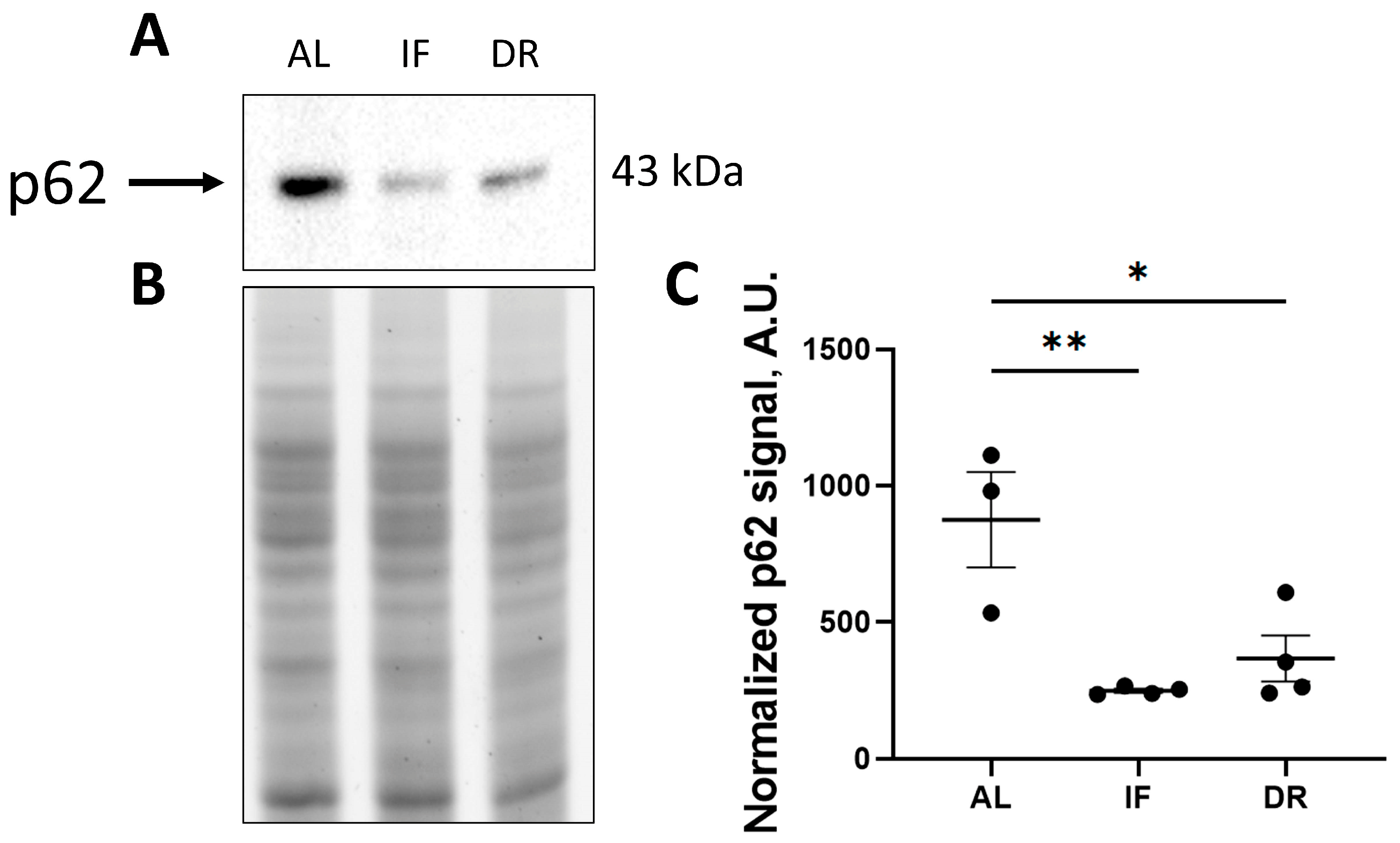
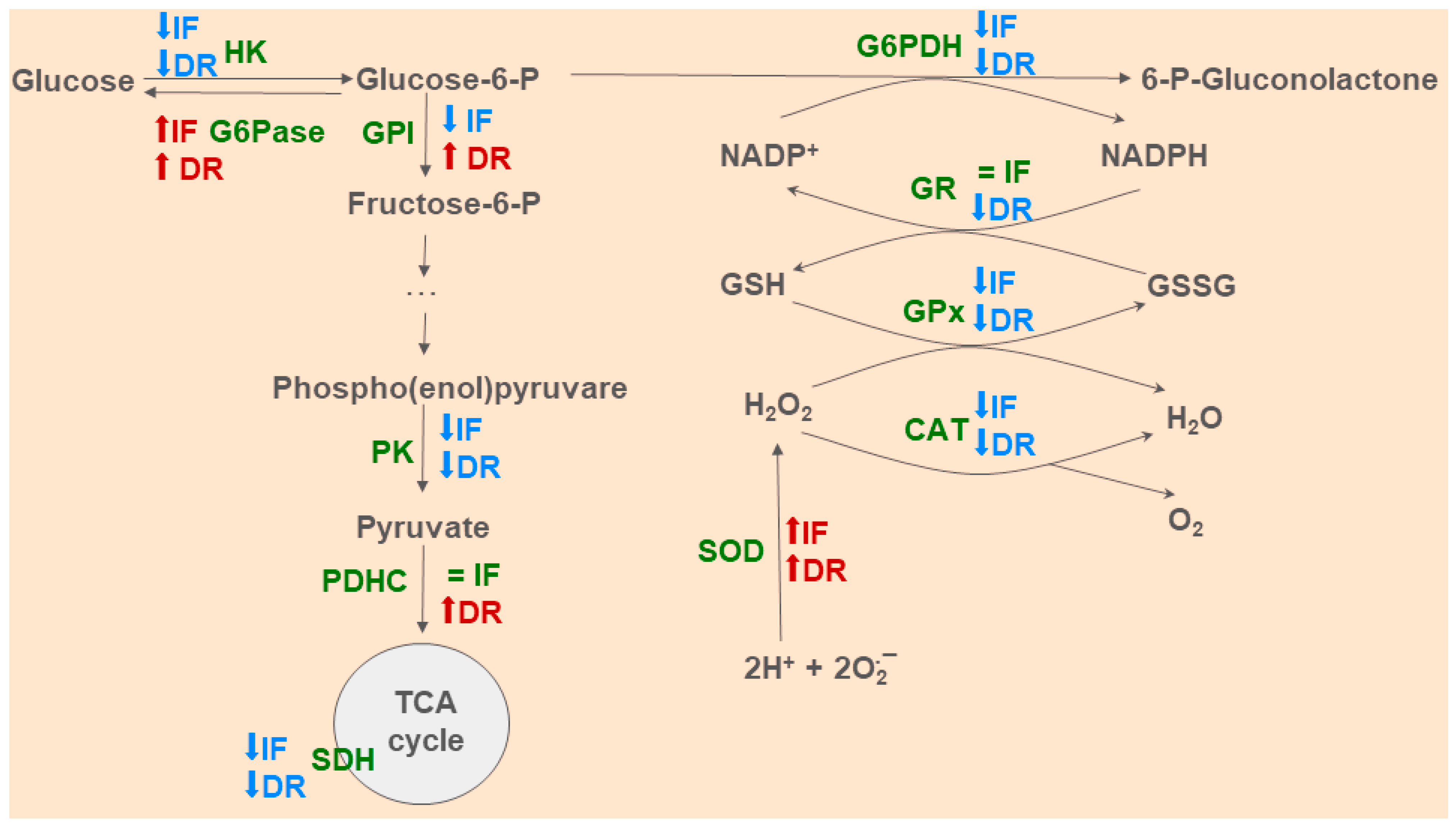
3.1. Effects of Dietary Restrictions on Liver Metabolism without BDL Exposure
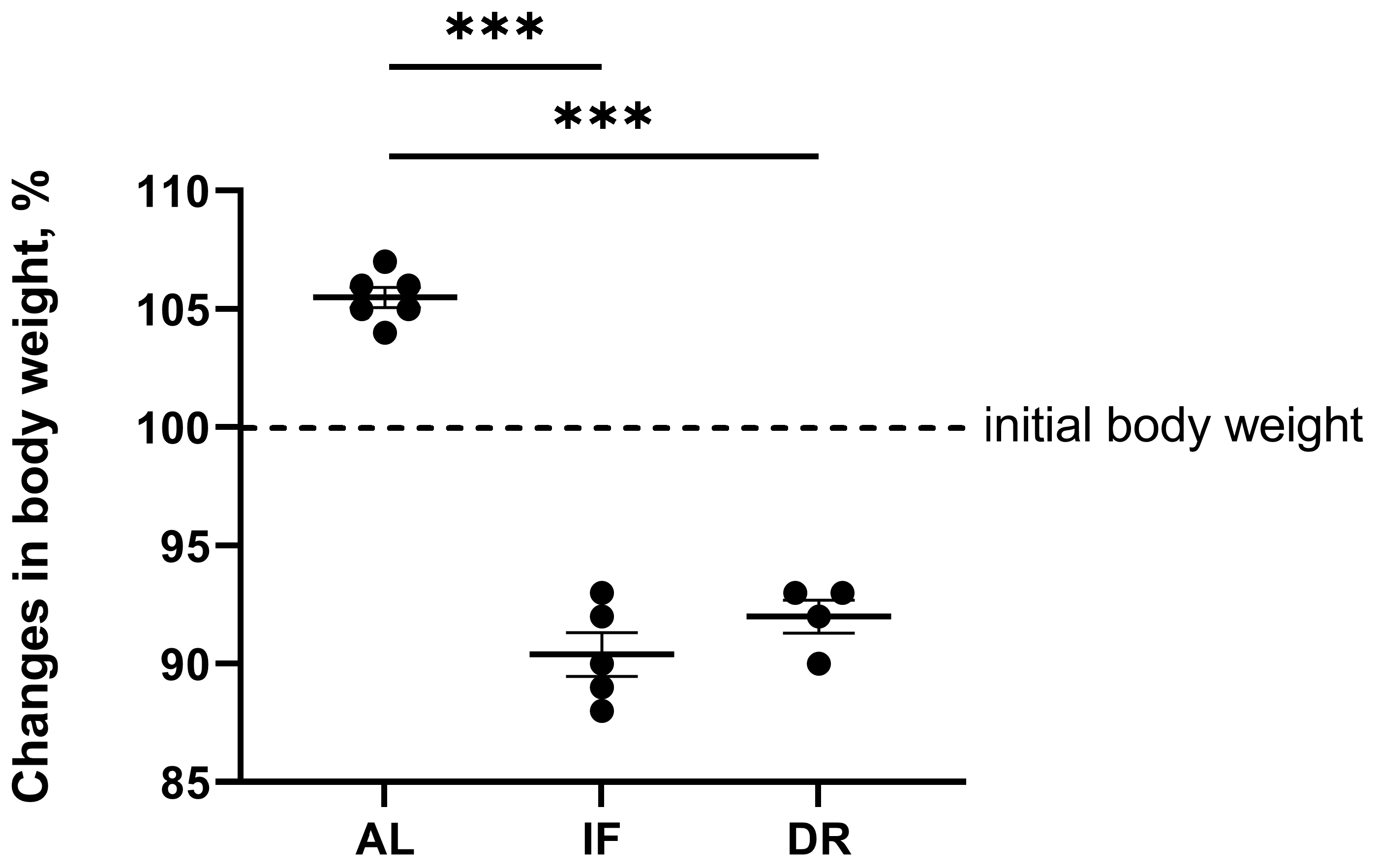
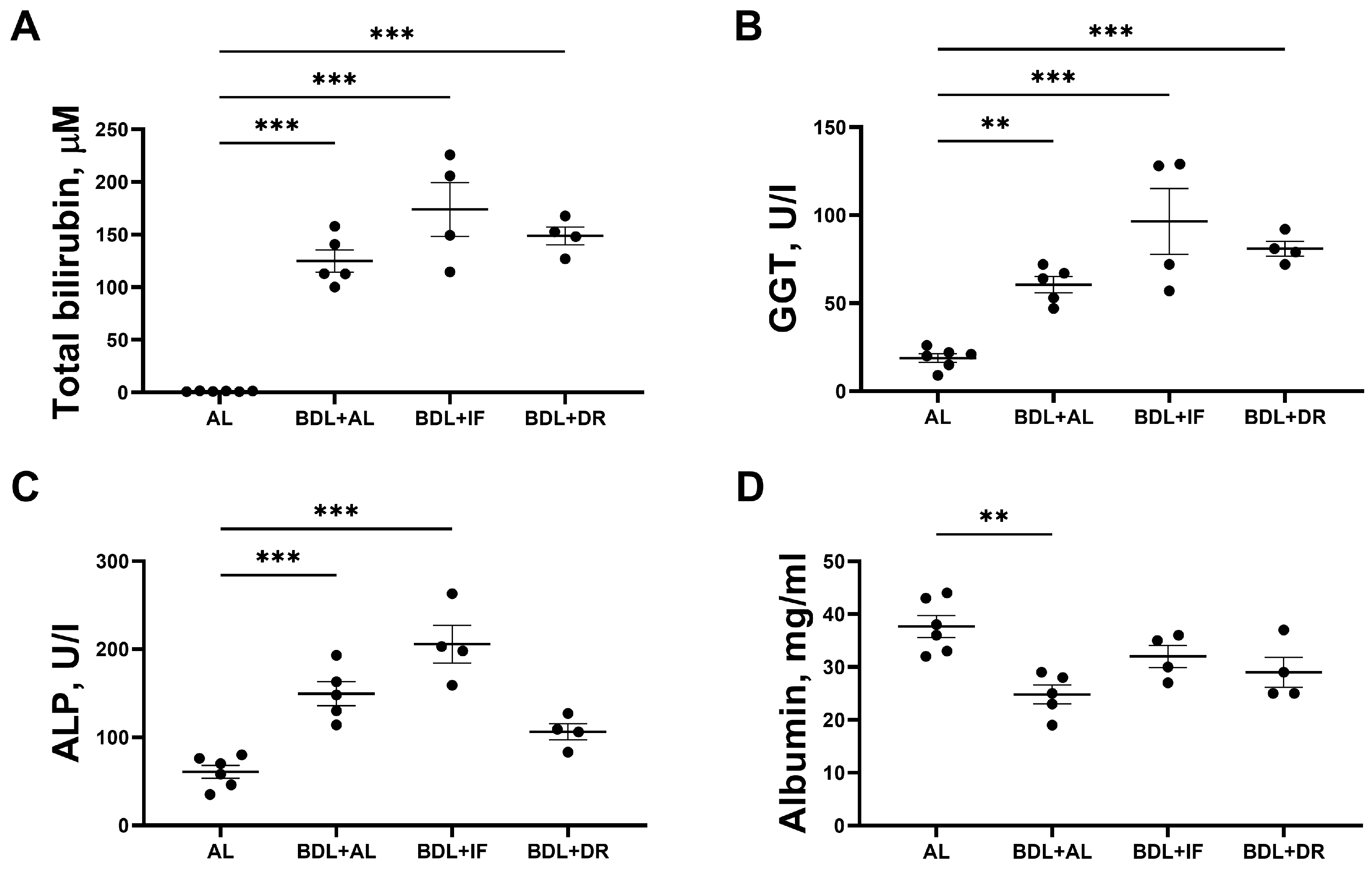
3.2. Effects of BDL and Consequent Various Diets on Liver Damage
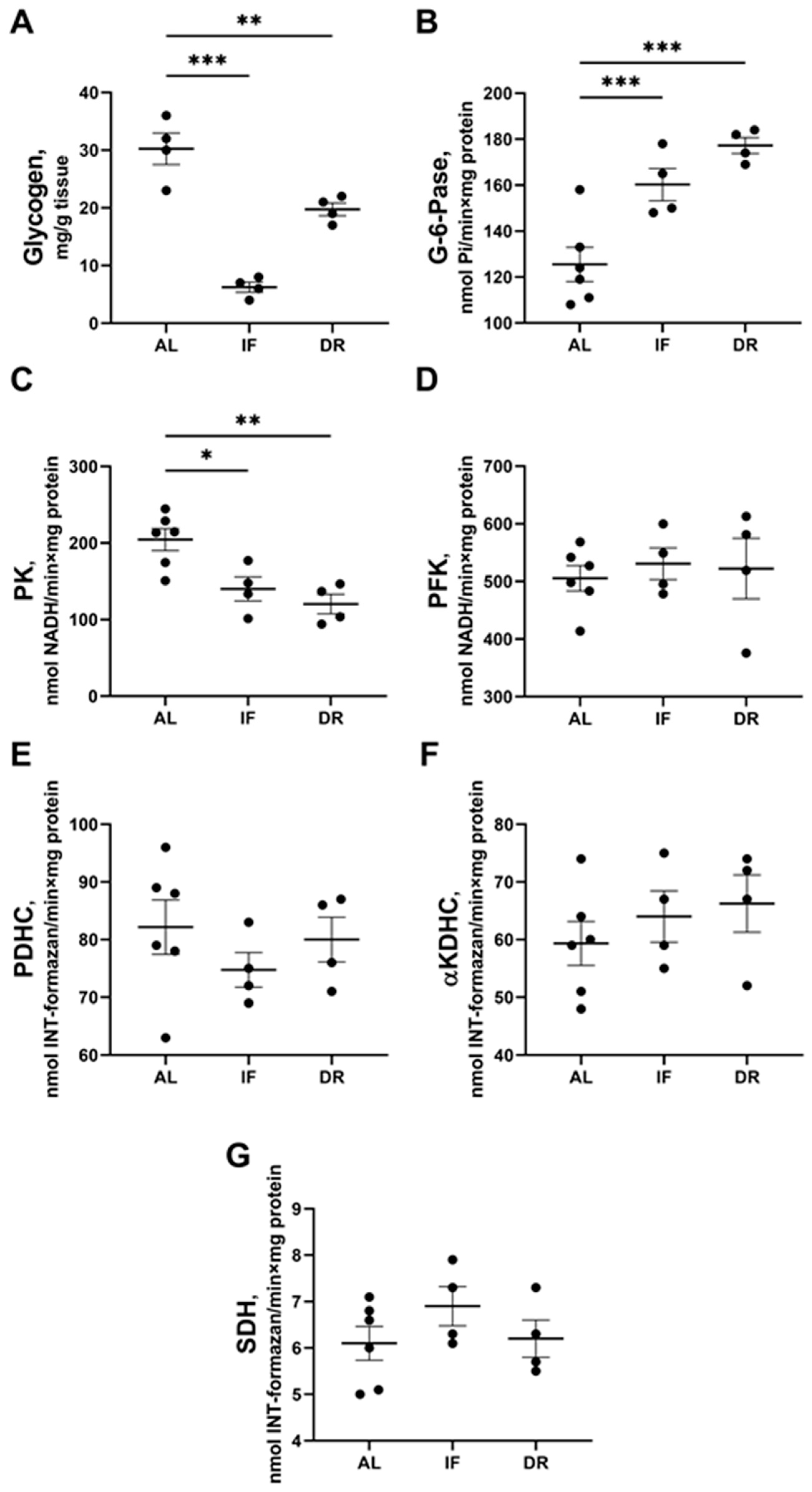
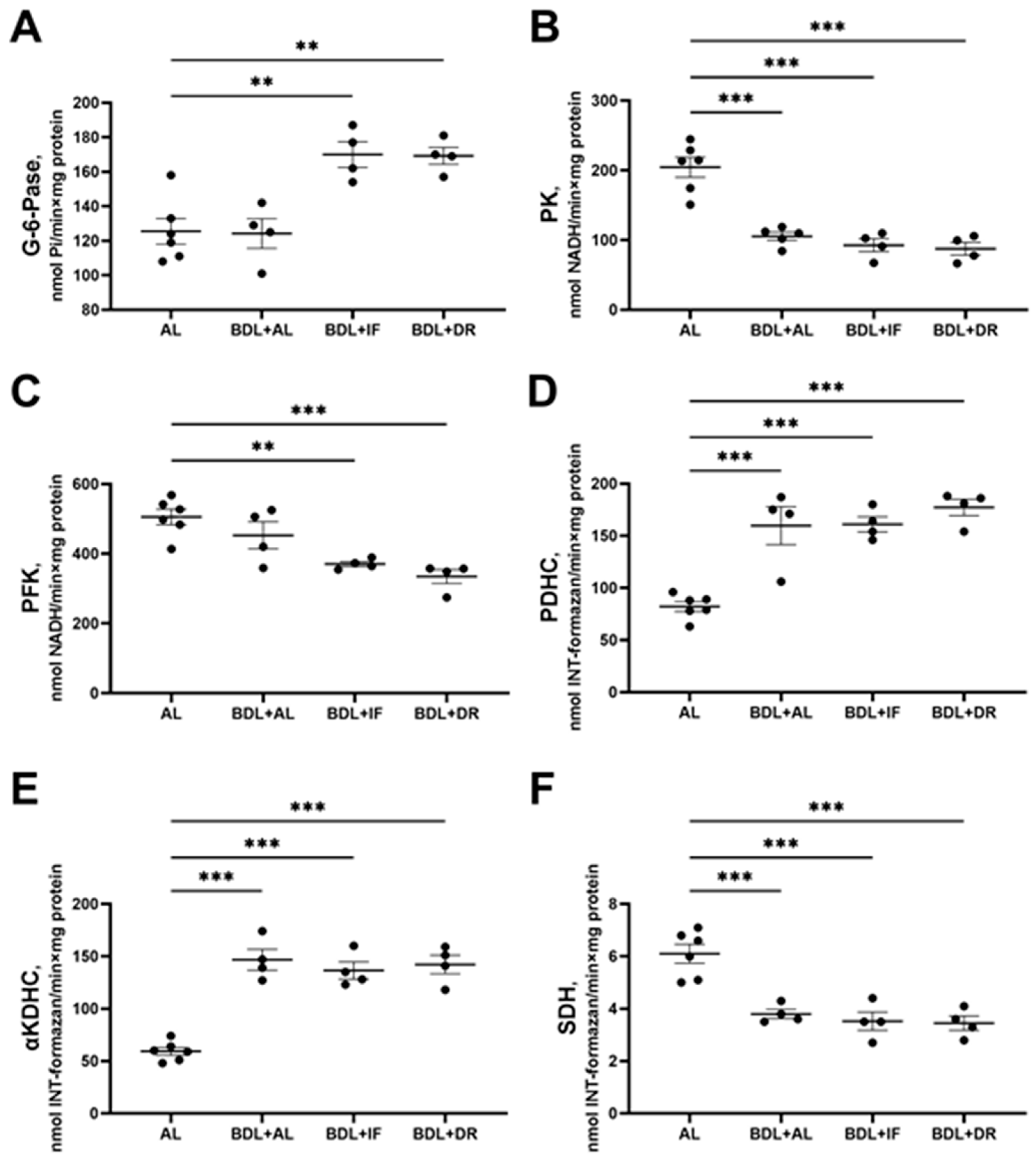
3.3. Changes in Hepatic Energy Metabolism after BDL and Different Diets
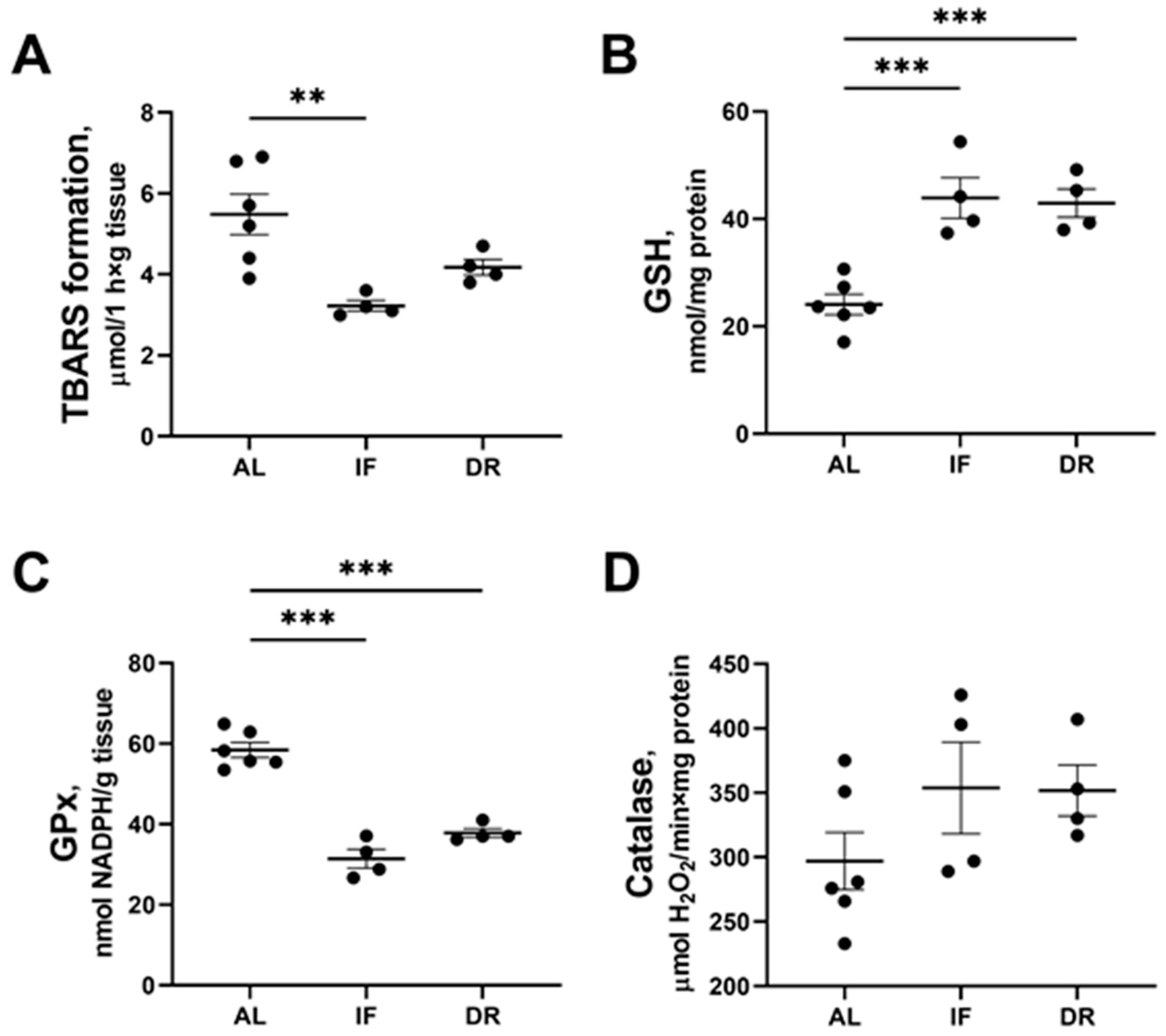
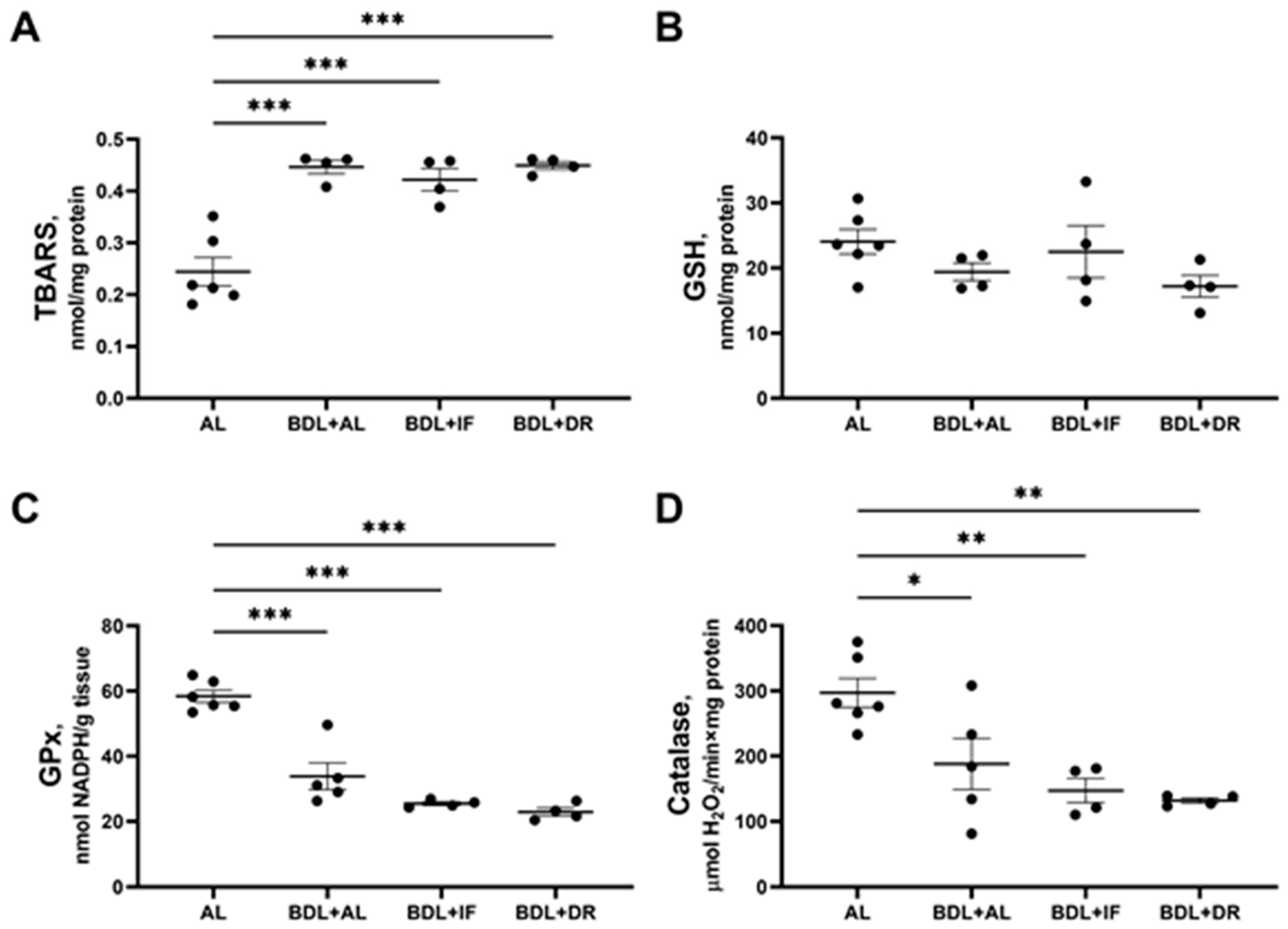
3.4. Oxidative Stress and Activity of Antioxidant Enzymes in the Liver
3.5. Bioinformatic Analysis of the Metabolic Profile of the Liver
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asensio, M.; Ortiz-Rivero, S.; Morente-Carrasco, A.; Marin, J.J.G. Etiopathogenesis and Pathophysiology of Cholestasis. Explor. Dig. Dis. 2022, 1, 97–117. [Google Scholar] [CrossRef]
- Trauner, M.; Fuchs, C.D. Novel Therapeutic Targets for Cholestatic and Fatty Liver Disease. Gut 2022, 71, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Hofer, S.J.; Carmona-Gutierrez, D.; Mueller, M.I.; Madeo, F. The Ups and Downs of Caloric Restriction and Fasting: From Molecular Effects to Clinical Application. EMBO Mol. Med. 2022, 14, e14418. [Google Scholar] [CrossRef] [PubMed]
- Jeon, T.I.; Lim, B.O.; Yu, B.P.; Lim, Y.; Jeon, E.J.; Park, D.K. Effect of Dietary Restriction on Age-Related Increase of Liver Susceptibility to Peroxidation in Rats. Lipids 2001, 36, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Hafez, S.M.N.A.; Elbassuoni, E. Dysfunction of Aged Liver of Male Albino Rats and the Effect of Intermitted Fasting; Biochemical, Histological, and Immunohistochemical Study. Int. Immunopharmacol. 2022, 103, 108465. [Google Scholar] [CrossRef] [PubMed]
- Rocha, N.S.; Barbisan, L.F.; de Oliveira, M.L.C.; de Camargo, J.L.V. Effects of Fasting and Intermittent Fasting on Rat Hepatocarcinogenesis Induced by Diethylnitrosamine. Teratog. Carcinog. Mutagen. 2002, 22, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Cheng, Y.; Su, Q.; Ai, W.; Gong, L.; Wang, Y.; Li, L.; Ma, Z.; Pan, Q.; Qiao, Z.; et al. Effects of Intermittent Fasting on Liver Physiology and Metabolism in Mice. Exp. Ther. Med. 2021, 22, 950. [Google Scholar] [CrossRef] [PubMed]
- Song, D.-K.; Kim, Y.-W. Beneficial Effects of Intermittent Fasting: A Narrative Review. J. Yeungnam Med. Sci. 2023, 40, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liang, Y.; Dai, C. Metabolic Regulation of Fibroblast Activation and Proliferation during Organ Fibrosis. Kidney Dis. 2022, 8, 115–125. [Google Scholar] [CrossRef]
- Semenovich, D.S.; Andrianova, N.V.; Zorova, L.D.; Pevzner, I.B.; Abramicheva, P.A.; Elchaninov, A.V.; Markova, O.V.; Petrukhina, A.S.; Zorov, D.B.; Plotnikov, E.Y. Fibrosis Development Linked to Alterations in Glucose and Energy Metabolism and Prooxidant-Antioxidant Balance in Experimental Models of Liver Injury. Antioxidants 2023, 12, 1604. [Google Scholar] [CrossRef]
- Yakupova, E.I.; Zorov, D.B.; Plotnikov, E.Y. Bioenergetics of the Fibrosis. Biochemistry 2021, 86, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Hou, Y.; Long, M.; Jiang, L.; Du, Y. Advances in Energy Metabolism in Renal Fibrosis. Life Sci. 2023, 312, 121033. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J. Liver physiology: Metabolism and detoxification. In Pathobiology of Human Disease; McManus, L.M., Mitchell, R.N., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 1770–1782. ISBN 9780123864574. [Google Scholar]
- Reddy, G.K.; Enwemeka, C.S. A Simplified Method for the Analysis of Hydroxyproline in Biological Tissues. Clin. Biochem. 1996, 29, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Hartree, E.F. Determination of Protein: A Modification of the Lowry Method That Gives a Linear Photometric Response. Anal. Biochem. 1972, 48, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Bennett, L.W.; Keirs, R.W.; Peebles, E.D.; Gerard, P.D. Methodologies of Tissue Preservation and Analysis of the Glycogen Content of the Broiler Chick Liver. Poult. Sci. 2007, 86, 2653–2665. [Google Scholar] [CrossRef] [PubMed]
- Hinman, L.M.; Blass, J.P. An NADH-Linked Spectrophotometric Assay for Pyruvate Dehydrogenase Complex in Crude Tissue Homogenates. J. Biol. Chem. 1981, 256, 6583–6586. [Google Scholar] [CrossRef] [PubMed]
- Munujos, P.; Coll-Cantí, J.; González-Sastre, F.; Gella, F.J. Assay of Succinate Dehydrogenase Activity by a Colorimetric-Continuous Method Using Iodonitrotetrazolium Chloride as Electron Acceptor. Anal. Biochem. 1993, 212, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Spinazzi, M.; Casarin, A.; Pertegato, V.; Salviati, L.; Angelini, C. Assessment of Mitochondrial Respiratory Chain Enzymatic Activities on Tissues and Cultured Cells. Nat. Protoc. 2012, 7, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Baginski, E.S.; Foà, P.P.; Zak, B. Glucose-6-Phosphatase. In Methods of Enzymatic Analysis, 2nd ed.; Bergmeyer, H.U., Ed.; Academic Press: Cambridge, MA, USA, 1974; pp. 876–880. ISBN 9780120913022. [Google Scholar]
- Lanzetta, P.A.; Alvarez, L.J.; Reinach, P.S.; Candia, O.A. An Improved Assay for Nanomole Amounts of Inorganic Phosphate. Anal. Biochem. 1979, 100, 95–97. [Google Scholar] [CrossRef]
- Pilkis, S.J. Glucokinase of Rat Liver. Methods Enzymol. 1975, 42, 31–39. [Google Scholar] [CrossRef]
- Dror, Y.; Sassoon, H.F.; Watson, J.J.; Johnson, B.C. Glucose 6-Phosphate Dehydrogenase Assay in Liver and Blood. Clin. Chim. Acta 1970, 28, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Gracy, R.W. Glucosephosphate Isomerase from Catfish Muscle and Liver and from Mammalian Tissues. Methods Enzymol. 1982, 89 Pt D, 550–558. [Google Scholar] [CrossRef]
- Kemp, R.G. Phosphofructokinase from Rabbit Skeletal Muscle. Methods Enzymol. 1975, 42, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, J.M. Pyruvate Kinase from Bovine Muscle and Liver. Methods Enzymol. 1982, 90 Pt E, 140–149. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Laszczyca, P.; Kawka-Serwecińska, E.; Witas, I.; Dolezych, B.; Migula, P. Iron Ascorbate-Stimulated Lipid Peroxidation in Vitro. Why Is the Method Controversial? Gen. Physiol. Biophys. 1995, 14, 3–18. [Google Scholar] [PubMed]
- Patsoukis, N.; Georgiou, C.D. Determination of the Thiol Redox State of Organisms: New Oxidative Stress Indicators. Anal. Bioanal. Chem. 2004, 378, 1783–1792. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Jakoby, W.B. Assays for Differentiation of Glutathione S-Transferases. Methods Enzymol. 1981, 77, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Mannervik, B. Measurement of Glutathione Reductase Activity. Curr. Protoc. Toxicol. 2001, 7.2.1–7.2.4. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of Glutathione Peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar] [CrossRef]
- Oppenheimer, L.; Wellner, V.P.; Griffith, O.W.; Meister, A. Glutathione Synthetase. Purification from Rat Kidney and Mapping of the Substrate Binding Sites. J. Biol. Chem. 1979, 254, 5184–5190. [Google Scholar] [CrossRef]
- Anderson, M.E. Assay of the Enzymes of Glutathione Biosynthesis. Anal. Biochem. 2022, 644, 114218. [Google Scholar] [CrossRef] [PubMed]
- Shangari, N.; O’Brien, P.J. Catalase Activity Assays. Curr. Protoc. Toxicol. 2006, 7.7.1–7.7.15. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, M.A.; Nefedova, E.K.; Mal’tseva, E.M.; Kalensii, A.V.; Zvekov, A.A. A UV-Vis Spectroscopic Study of the Auto-Oxidation of Adrenaline. J. Appl. Spectrosc. 2022, 89, 905–912. [Google Scholar] [CrossRef]
- Taormina, G.; Mirisola, M.G. Calorie Restriction in Mammals and Simple Model Organisms. Biomed. Res. Int. 2014, 2014, 308690. [Google Scholar] [CrossRef] [PubMed]
- López-Lluch, G.; Navas, P. Calorie Restriction as an Intervention in Ageing. J. Physiol. 2016, 594, 2043–2060. [Google Scholar] [CrossRef] [PubMed]
- Sokolović, A.; van Roomen, C.P.A.A.; Ottenhoff, R.; Scheij, S.; Hiralall, J.K.; Claessen, N.; Aten, J.; Oude Elferink, R.P.J.; Groen, A.K.; Sokolović, M. Fasting Reduces Liver Fibrosis in a Mouse Model for Chronic Cholangiopathies. Biochim. Biophys. Acta 2013, 1832, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Menezes-Filho, S.L.; Amigo, I.; Prado, F.M.; Ferreira, N.C.; Koike, M.K.; Pinto, I.F.D.; Miyamoto, S.; Montero, E.F.S.; Medeiros, M.H.G.; Kowaltowski, A.J. Caloric Restriction Protects Livers from Ischemia/reperfusion Damage by Preventing Ca2+-Induced Mitochondrial Permeability Transition. Free Radic. Biol. Med. 2017, 110, 219–227. [Google Scholar] [CrossRef]
- Ramkumar, K.M.; Anuradha, C.V. Short-Term Dietary Restriction Modulates Liver Lipid Peroxidation in Carbon Tetrachloride-Intoxicated Rats. J. Basic Clin. Physiol. Pharmacol. 2005, 16, 245–256. [Google Scholar] [CrossRef]
- Mohammadi, M.; Ghaznavi, R.; Keyhanmanesh, R.; Sadeghipour, H.R.; Naderi, R.; Mohammadi, H. Caloric Restriction Prevents Lead-Induced Oxidative Stress and Inflammation in Rat Liver. Sci. World J. 2014, 2014, 821524. [Google Scholar] [CrossRef]
- Mladenović, D.; Ninković, M.; Aleksić, V.; Šljivančanin, T.; Vučević, D.; Todorović, V.; Stanković, M.; Stanojlović, O.; Radosavljević, T. The Effect of Calorie Restriction on Acute Ethanol-Induced Oxidative and Nitrosative Liver Injury in Rats. Environ. Toxicol. Pharmacol. 2013, 36, 296–302. [Google Scholar] [CrossRef]
- Muchova, L.; Vanova, K.; Zelenka, J.; Lenicek, M.; Petr, T.; Vejrazka, M.; Sticova, E.; Vreman, H.J.; Wong, R.J.; Vitek, L. Bile Acids Decrease Intracellular Bilirubin Levels in the Cholestatic Liver: Implications for Bile Acid-Mediated Oxidative Stress. J. Cell. Mol. Med. 2011, 15, 1156–1165. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Allameh, A.; Niayesh-Mehr, R.; Aliarab, A.; Sebastiani, G.; Pantopoulos, K. Oxidative Stress in Liver Pathophysiology and Disease. Antioxidants 2023, 12, 1653. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Shoda, J.; Taguchi, K.; Maher, J.M.; Ishizaki, K.; Inoue, Y.; Ohtsuki, M.; Goto, N.; Sugimoto, H.; Utsunomiya, H.; et al. Nrf2 Counteracts Cholestatic Liver Injury via Stimulation of Hepatic Defense Systems. Biochem. Biophys. Res. Commun. 2009, 389, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Lamark, T.; Svenning, S.; Johansen, T. Regulation of Selective Autophagy: The p62/SQSTM1 Paradigm. Essays Biochem. 2017, 61, 609–624. [Google Scholar] [CrossRef]
- Gao, L.; Lv, G.; Guo, X.; Jing, Y.; Han, Z.; Zhang, S.; Sun, K.; Li, R.; Yang, Y.; Wei, L. Activation of Autophagy Protects against Cholestasis-Induced Hepatic Injury. Cell Biosci. 2014, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Manley, S.; Ni, H.-M.; Kong, B.; Apte, U.; Guo, G.; Ding, W.-X. Suppression of Autophagic Flux by Bile Acids in Hepatocytes. Toxicol. Sci. 2014, 137, 478–490. [Google Scholar] [CrossRef]
- Panzitt, K.; Jungwirth, E.; Krones, E.; Lee, J.M.; Pollheimer, M.; Thallinger, G.G.; Kolb-Lenz, D.; Xiao, R.; Thorell, A.; Trauner, M.; et al. FXR-Dependent Rubicon Induction Impairs Autophagy in Models of Human Cholestasis. J. Hepatol. 2020, 72, 1122–1131. [Google Scholar] [CrossRef]
- Kim, S.; Han, S.Y.; Yu, K.S.; Han, D.; Ahn, H.-J.; Jo, J.-E.; Kim, J.-H.; Shin, J.; Park, H.-W. Impaired Autophagy Promotes Bile Acid-Induced Hepatic Injury and Accumulation of Ubiquitinated Proteins. Biochem. Biophys. Res. Commun. 2018, 495, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Khambu, B.; Li, T.; Yan, S.; Yu, C.; Chen, X.; Goheen, M.; Li, Y.; Lin, J.; Cummings, O.W.; Lee, Y.A.; et al. Hepatic Autophagy Deficiency Compromises Farnesoid X Receptor Functionality and Causes Cholestatic Injury. Hepatology 2019, 69, 2196–2213. [Google Scholar] [CrossRef]
- Kuhla, A.; Hahn, S.; Butschkau, A.; Lange, S.; Wree, A.; Vollmar, B. Lifelong Caloric Restriction Reprograms Hepatic Fat Metabolism in Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 915–922. [Google Scholar] [CrossRef]
- Green, C.L.; Mitchell, S.E.; Derous, D.; Wang, Y.; Chen, L.; Han, J.-D.J.; Promislow, D.E.L.; Lusseau, D.; Douglas, A.; Speakman, J.R. The Effects of Graded Levels of Calorie Restriction: IX. Global Metabolomic Screen Reveals Modulation of Carnitines, Sphingolipids and Bile Acids in the Liver of C57BL/6 Mice. Aging Cell 2017, 16, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Takemori, K.; Kimura, T.; Shirasaka, N.; Inoue, T.; Masuno, K.; Ito, H. Food Restriction Improves Glucose and Lipid Metabolism through Sirt1 Expression: A Study Using a New Rat Model with Obesity and Severe Hypertension. Life Sci. 2011, 88, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Kuykendall, N.S.; Kuykendall, J.R. Ketones in the life sciences—Biochemistry, metabolism, and medicinal significances. In Metabolism—Annual Volume 2024 [Working Title]; IntechOpen: London, UK, 2024. [Google Scholar]
- Newman, J.C.; Covarrubias, A.J.; Zhao, M.; Yu, X.; Gut, P.; Ng, C.-P.; Huang, Y.; Haldar, S.; Verdin, E. Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Metab. 2017, 26, 547–557.e8. [Google Scholar] [CrossRef] [PubMed]
- Solon-Biet, S.M.; Mitchell, S.J.; de Cabo, R.; Raubenheimer, D.; Le Couteur, D.G.; Simpson, S.J. Macronutrients and Caloric Intake in Health and Longevity. J. Endocrinol. 2015, 226, R17–R28. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenovich, D.S.; Zorova, L.D.; Abramicheva, P.A.; Andrianova, N.V.; Elchaninov, A.V.; Petrukhina, A.S.; Pevzner, I.B.; Manskikh, V.N.; Zorov, D.B.; Plotnikov, E.Y. Impact of Intermittent Fasting and Dietary Restriction on Redox State, Energetic Metabolism, and Liver Injury in Common Bile Duct Ligation Model. Antioxidants 2024, 13, 835. https://doi.org/10.3390/antiox13070835
Semenovich DS, Zorova LD, Abramicheva PA, Andrianova NV, Elchaninov AV, Petrukhina AS, Pevzner IB, Manskikh VN, Zorov DB, Plotnikov EY. Impact of Intermittent Fasting and Dietary Restriction on Redox State, Energetic Metabolism, and Liver Injury in Common Bile Duct Ligation Model. Antioxidants. 2024; 13(7):835. https://doi.org/10.3390/antiox13070835
Chicago/Turabian StyleSemenovich, Dmitry S., Ljubava D. Zorova, Polina A. Abramicheva, Nadezda V. Andrianova, Andrey V. Elchaninov, Aleksandra S. Petrukhina, Irina B. Pevzner, Vasily N. Manskikh, Dmitry B. Zorov, and Egor Y. Plotnikov. 2024. "Impact of Intermittent Fasting and Dietary Restriction on Redox State, Energetic Metabolism, and Liver Injury in Common Bile Duct Ligation Model" Antioxidants 13, no. 7: 835. https://doi.org/10.3390/antiox13070835
APA StyleSemenovich, D. S., Zorova, L. D., Abramicheva, P. A., Andrianova, N. V., Elchaninov, A. V., Petrukhina, A. S., Pevzner, I. B., Manskikh, V. N., Zorov, D. B., & Plotnikov, E. Y. (2024). Impact of Intermittent Fasting and Dietary Restriction on Redox State, Energetic Metabolism, and Liver Injury in Common Bile Duct Ligation Model. Antioxidants, 13(7), 835. https://doi.org/10.3390/antiox13070835








