Oxidised Albumin Levels in Plasma and Skeletal Muscle as Biomarkers of Disease Progression and Treatment Efficacy in Dystrophic mdx Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Procedures
2.2. Taurine Treatment
2.3. Treadmill Exercise
2.4. Histology
2.5. Plasma CK
2.6. Muscle Inflammation
2.7. Muscle Total Protein Thiol Oxidation
2.8. Muscle Albumin Thiol Oxidation Method Development
2.9. Muscle Albumin Thiol Oxidation Method
2.10. Plasma Albumin Thiol Oxidation
2.11. Statistics
3. Results
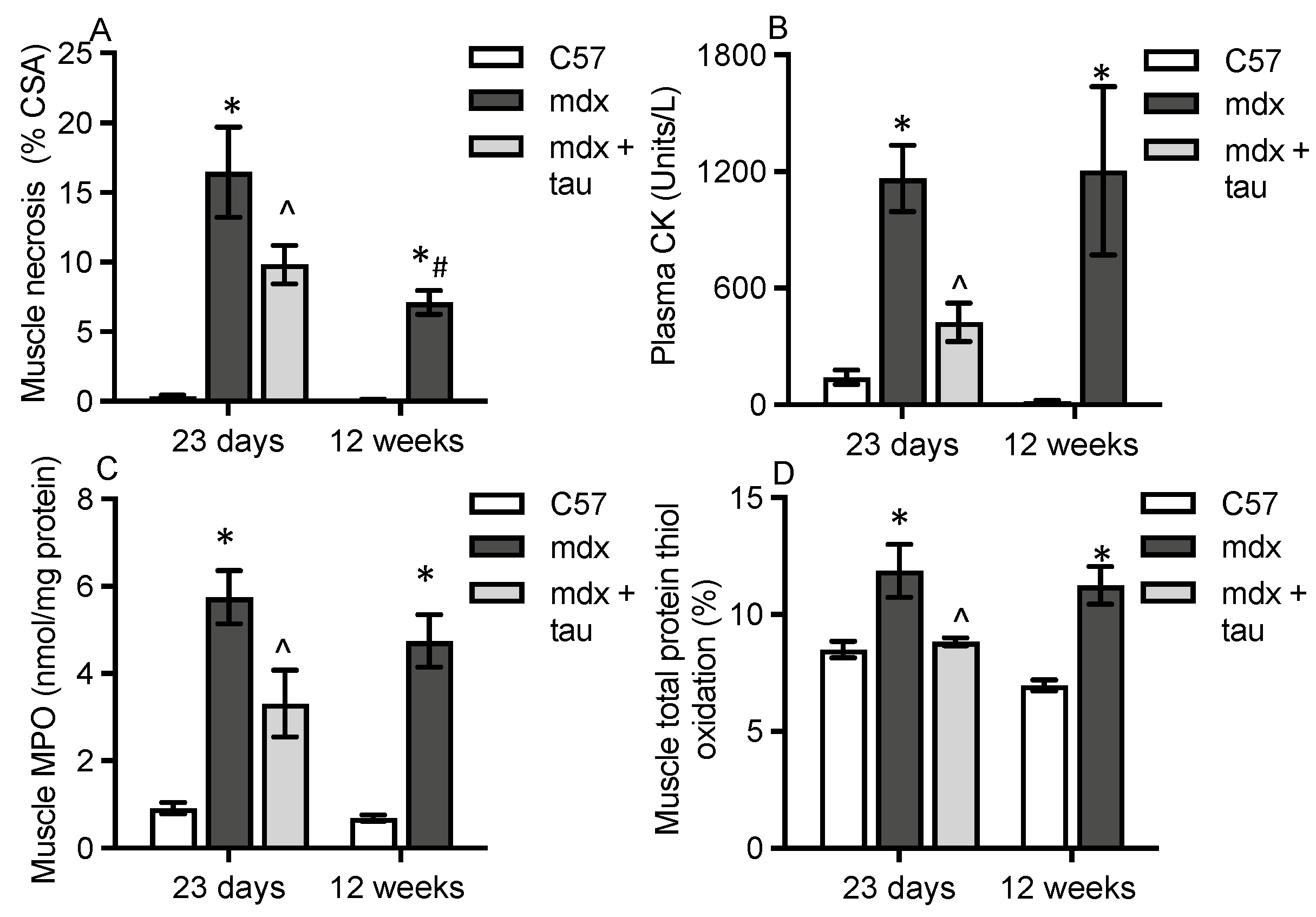
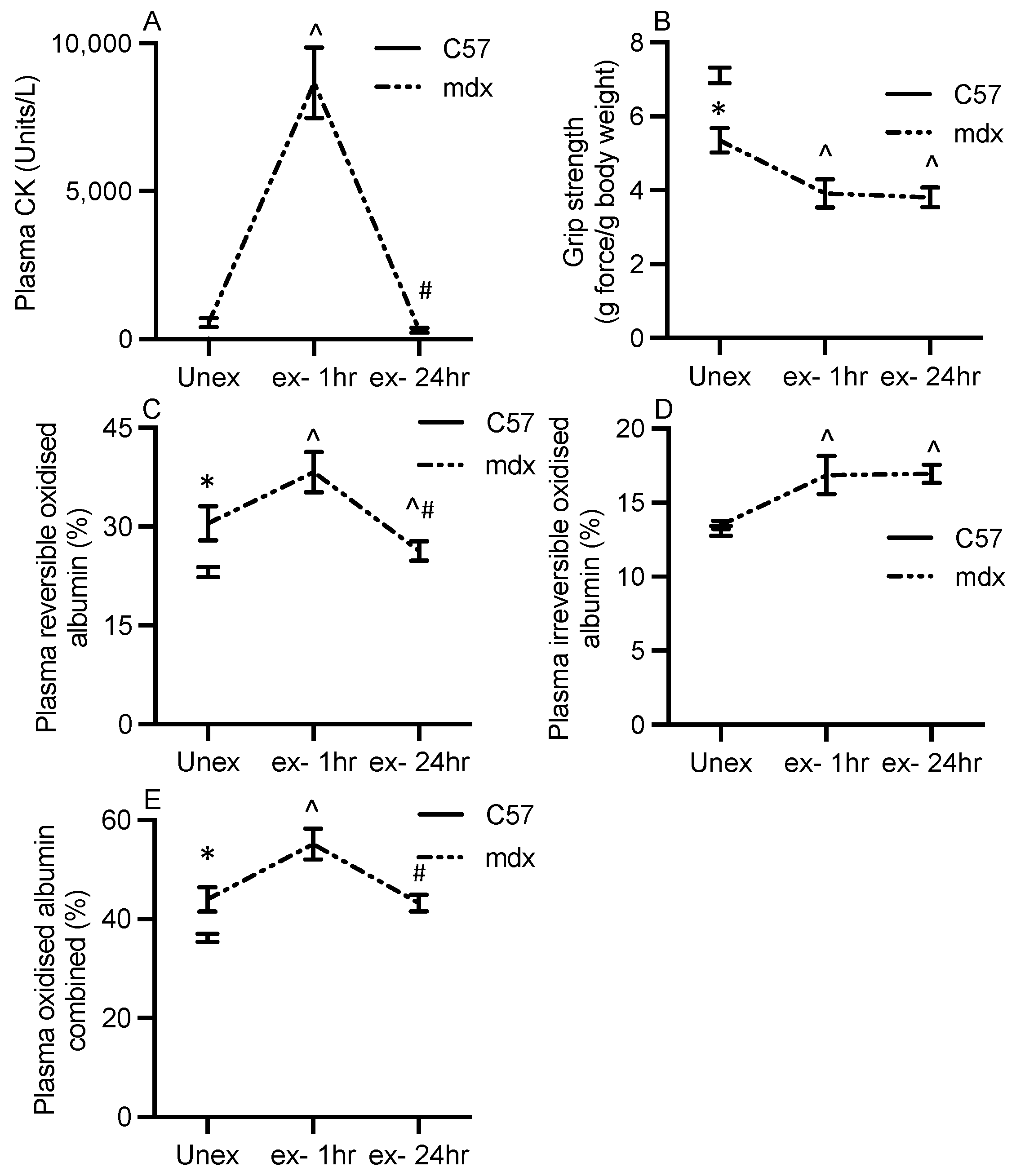
3.1. Characterisation of Dystropathology
3.2. Albumin Thiol Oxidation in mdx Plasma
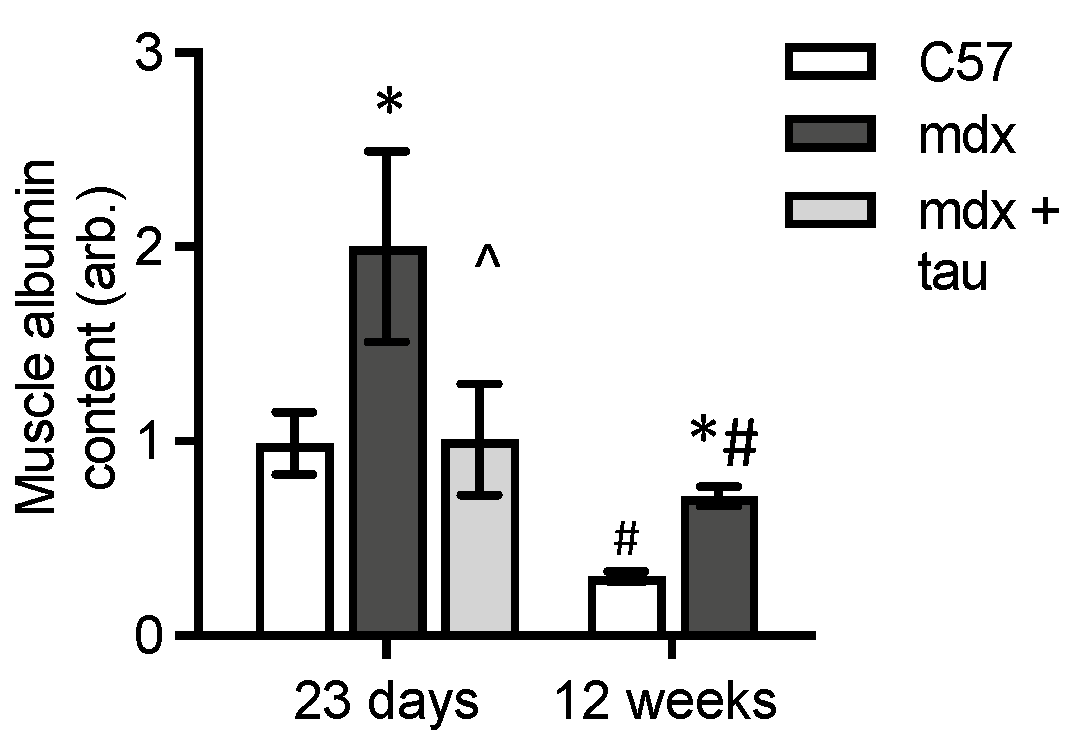
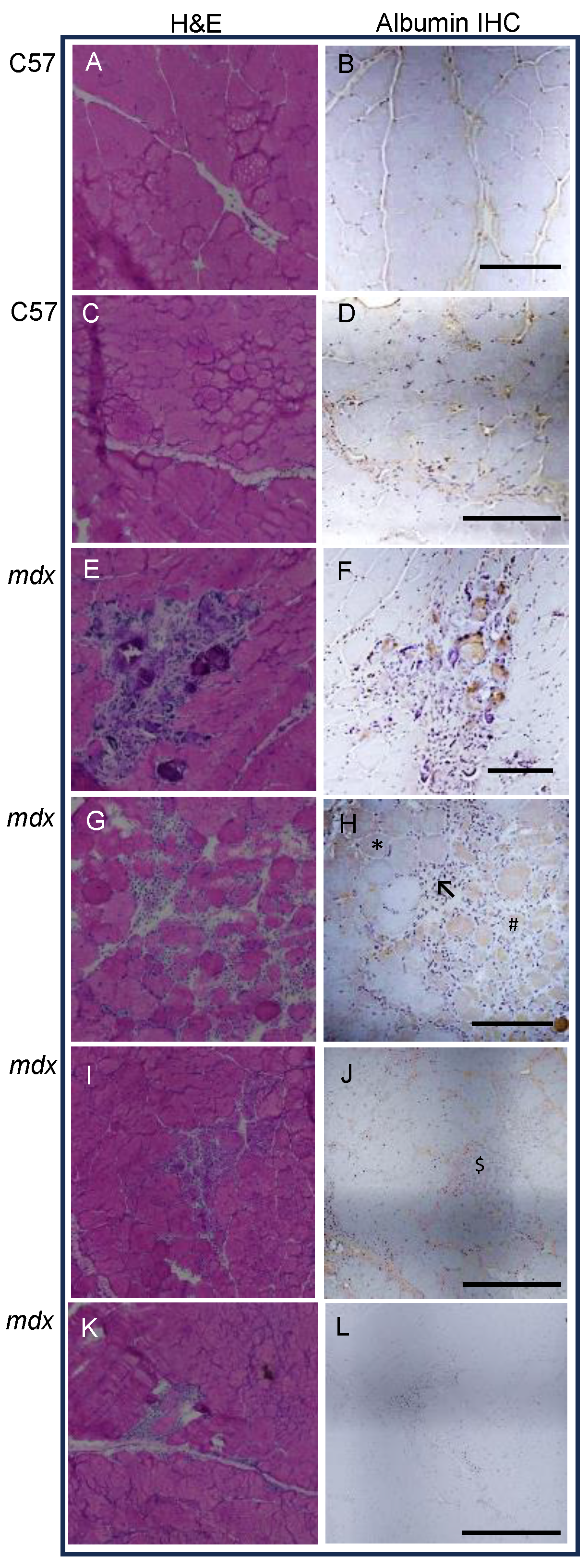
3.3. Levels of Albumin in Dystrophic and Normal Muscles
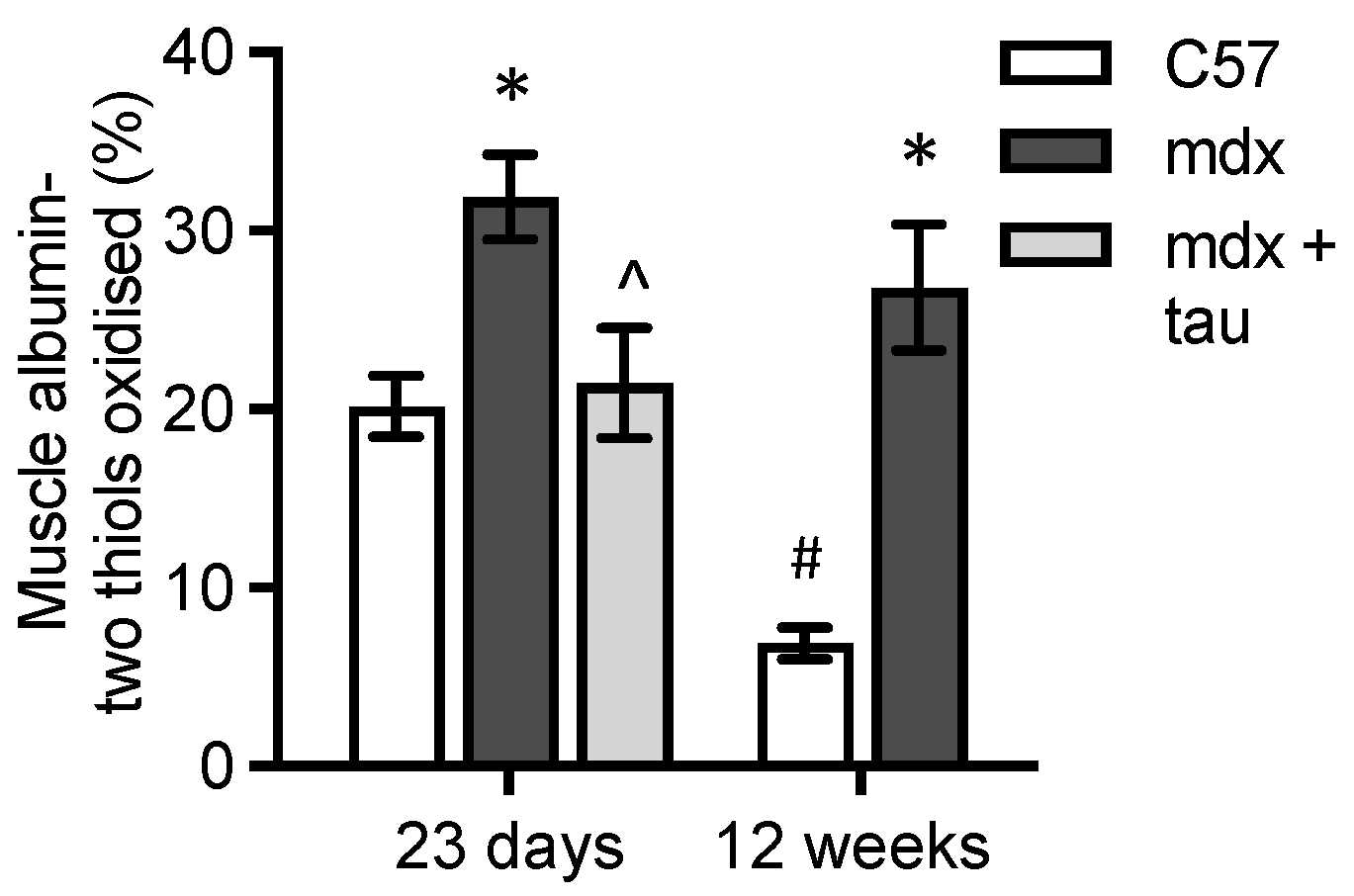
3.4. Levels of Albumin Thiol Oxidation in mdx Muscle
3.5. Tracking Plasma Albumin Thiol Oxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veskoukis, A.S.; Kyparos, A.; Stagos, D.; Kouretas, D. Differential effects of xanthine oxidase inhibition and exercise on albumin concentration in rat tissues. Appl. Physiol. Nutr. Metab. 2010, 35, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Ellmerer, M.; Schaupp, L.; Brunner, G.A.; Sendlhofer, G.; Wutte, A.; Wach, P.; Pieber, T.R. Measurement of interstitial albumin in human skeletal muscle and adipose tissue by open-flow microperfusion. Am. J. Physiol.-Endocrinol. Metab. 2000, 278, E352–E356. [Google Scholar] [CrossRef] [PubMed]
- Belinskaia, D.A.; Voronina, P.A.; Shmurak, V.I.; Jenkins, R.O.; Goncharov, N.V. Serum albumin in health and disease: Esterase, antioxidant, transporting and signaling properties. Int. J. Mol. Sci. 2021, 22, 10318. [Google Scholar] [CrossRef] [PubMed]
- Oettl, K.; Birner-Gruenberger, R.; Spindelboeck, W.; Stueger, H.P.; Dorn, L.; Stadlbauer, V.; Putz-Bankuti, C.; Krisper, P.; Graziadei, I.; Vogel, W.; et al. Oxidative albumin damage in chronic liver failure: Relation to albumin binding capacity, liver dysfunction and survival. J. Hepatol. 2013, 59, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, D.; Ulivelli, M.; Bartalini, S.; Battistini, S.; Cerase, A.; Passero, S.; Summa, D.; Frosali, S.; Priora, R.; Margaritis, A.; et al. Regulation of redox forms of plasma thiols by albumin in multiple sclerosis after fasting and methionine loading test. Amino Acids 2010, 38, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Turell, L.; Radi, R.; Alvarez, B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free Radic. Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Tabata, F.; Wada, Y.; Kawakami, S.; Miyaji, K. Serum albumin redox states: More than oxidative stress biomarker. Antioxidants 2021, 10, 503. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Hayashi, T.; Negawa, T.; Nakamura, K.; Tomida, M.; Koda, K.; Tajima, T.; Koda, Y.; Suda, K.; Era, S. Strenuous exercise-induced change in redox state of human serum albumin during intensive kendo training. Jpn. J. Physiol. 2002, 52, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Clerici, M.; Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. Redox albuminomics: Oxidized albumin in human diseases. Antioxid. Redox Signal. 2012, 17, 1515–1527. [Google Scholar] [CrossRef]
- Rasheed, Z.; Ali, R. Reactive oxygen species damaged human serum albumin in patients with type 1 diabetes mellitus: Biochemical and immunological studies. Life Sci. 2006, 79, 2320–2328. [Google Scholar] [CrossRef]
- Bocedi, A.; Cattani, G.; Stella, L.; Massoud, R.; Ricci, G. Thiol disulfide exchange reactions in human serum albumin: The apparent paradox of the redox transitions of Cys(34). FEBS J. 2018, 285, 3225–3237. [Google Scholar] [CrossRef] [PubMed]
- Terawaki, H.; Yoshimura, K.; Hasegawa, T.; Matsuyama, Y.; Negawa, T.; Yamada, K.; Matsushima, M.; Nakayama, M.; Hosoya, T.; Era, S. Oxidative stress is enhanced in correlation with renal dysfunction: Examination with the redox state of albumin. Kidney Int. 2004, 66, 1988–1993. [Google Scholar] [CrossRef] [PubMed]
- Oettl, K.; Reibnegger, G.; Schmut, O. The redox state of human serum albumin in eye diseases with and without complications. Acta Ophthalmol. 2011, 89, e174–e179. [Google Scholar] [CrossRef] [PubMed]
- Nasif, W.A.; Mukhtar, M.H.; El-Emshaty, H.M.; Alwazna, A.H. Redox State of Human Serum Albumin and Inflammatory Biomarkers in Hemodialysis Patients with Secondary Hyperparathyroidism During Oral Calcitriol Supplementation for Vitamin D. Open Med. Chem. J. 2018, 12, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clemens, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; McDonald, C.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010, 9, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, S.; Sultana, J.; Fontana, A.; Salvo, F.; Messina, S.; Trifirò, G. Global epidemiology of Duchenne muscular dystrophy: An updated systematic review and meta-analysis. Orphanet J. Rare Dis. 2020, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Biggar, W.D. Duchenne muscular dystrophy. Pediatr. Rev. 2006, 27, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Goemans, N.; Takeda, S.; Mercuri, E.; Aartsma-Rus, A. Duchenne muscular dystrophy. Nat. Rev. Dis. Primers 2021, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Kharraz, Y.; Guerra, J.; Pessina, P.; Serrano, A.L.; Munoz-Canoves, P. Understanding the process of fibrosis in Duchenne muscular dystrophy. Biomed. Res. Int. 2014, 2014, 965631. [Google Scholar] [CrossRef]
- Falzarano, M.S.; Scotton, C.; Passarelli, C.; Ferlini, A. Duchenne Muscular Dystrophy: From Diagnosis to Therapy. Molecules 2015, 20, 18168–18184. [Google Scholar] [CrossRef]
- Kourakis, S.; Timpani, C.A.; de Haan, J.B.; Gueven, N.; Fischer, D.; Rybalka, E. Targeting Nrf2 for the treatment of Duchenne Muscular Dystrophy. Redox Biol. 2021, 38, 101803. [Google Scholar] [CrossRef] [PubMed]
- Canton, M.; Menazza, S.; Di Lisa, F. Oxidative stress in muscular dystrophy: From generic evidence to specific sources and targets. J. Muscle Res. Cell Motil. 2014, 35, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, R.J.; Chow, C.K.; Porter, J.D. Oxidative stress as a potential pathogenic mechanism in an animal model of Duchenne muscular dystrophy. Neuromuscul. Disord. 1997, 7, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kwak, H.B.; Thompson, L.V.; Lawler, J.M. Contribution of oxidative stress to pathology in diaphragm and limb muscles with Duchenne muscular dystrophy. J. Muscle Res. Cell Motil. 2013, 34, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rando, T.A. Oxidative stress and the pathogenesis of muscular dystrophies. Am. J. Phys. Med. Rehabil. 2002, 81, S175–S186. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Terrill, J.R.; Kawarai, K.; Miyata, Y.; Tagami, T.; Maeda, N.; Hasegawa, Y.; Watanabe, T.; Grounds, M.D.; Arthur, P.G. The location of protein oxidation in dystrophic skeletal muscle from the mdx mouse model of Duchenne muscular dystrophy. Acta Histochem. 2022, 124, 151959. [Google Scholar] [CrossRef] [PubMed]
- Bulfield, G.; Siller, W.; Wight, P.; Moore, K. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc. Natl. Acad. Sci. USA 1984, 81, 1189–1192. [Google Scholar] [CrossRef] [PubMed]
- Al-Mshhdani, B.A.; Grounds, M.D.; Arthur, P.G.; Terrill, J.R. A Blood Biomarker for Duchenne Muscular Dystrophy Shows That Oxidation State of Albumin Correlates with Protein Oxidation and Damage in Mdx Muscle. Antioxidants 2021, 10, 1241. [Google Scholar] [CrossRef] [PubMed]
- Terrill, J.R.; Pinniger, G.J.; Graves, J.A.; Grounds, M.D.; Arthur, P.G. Increasing taurine intake and taurine synthesis improves skeletal muscle function in the mdx mouse model for Duchenne muscular dystrophy. J. Physiol. 2016, 594, 3095–3110. [Google Scholar] [CrossRef]
- Terrill, J.R.; Grounds, M.D.; Arthur, P.G. Increased taurine in pre-weaned juvenile mdx mice greatly reduces the acute onset of myofibre necrosis and dystropathology and prevents inflammation. PLoS Curr. 2016, 8. [Google Scholar] [CrossRef]
- Cozzoli, A.; Rolland, J.F.; Capogrosso, R.F.; Sblendorio, V.T.; Longo, V.; Simonetti, S.; Nico, B.; De Luca, A. Evaluation of potential synergistic action of a combined treatment with alpha-methyl-prednisolone and taurine on the mdx mouse model of Duchenne muscular dystrophy. Neuropathol. Appl. Neurobiol. 2011, 37, 243–256. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Pierno, S.; Liantonio, A.; Cetrone, M.; Camerino, C.; Fraysse, B.; Mirabella, M.; Servidei, S.; Ruegg, U.T.; Conte Camerino, D. Enhanced dystrophic progression in mdx mice by exercise and beneficial effects of taurine and insulin-like growth factor-1. J. Pharmacol. Exp. Ther. 2003, 304, 453–463. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Pierno, S.; Liantonio, A.; Cetrone, M.; Camerino, C.; Simonetti, S.; Papadia, F.; Camerino, D.C. Alteration of excitation-contraction coupling mechanism in extensor digitorum longus muscle fibres of dystrophic mdx mouse and potential efficacy of taurine. Br. J. Pharmacol. 2001, 132, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Capogrosso, R.F.; Cozzoli, A.; Mantuano, P.; Camerino, G.M.; Massari, A.M.; Sblendorio, V.T.; De Bellis, M.; Tamma, R.; Giustino, A.; Nico, B.; et al. Assessment of resveratrol, apocynin and taurine on mechanical-metabolic uncoupling and oxidative stress in a mouse model of duchenne muscular dystrophy: A comparison with the gold standard, alpha-methyl prednisolone. Pharmacol. Res. 2016, 106, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Mele, A.; Mantuano, P.; De Bellis, M.; Rana, F.; Sanarica, F.; Conte, E.; Morgese, M.G.; Bove, M.; Rolland, J.-F.; Capogrosso, R.F. A long-term treatment with taurine prevents cardiac dysfunction in mdx mice. Transl. Res. 2019, 204, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Horvath, D.M.; Murphy, R.M.; Mollica, J.P.; Hayes, A.; Goodman, C.A. The effect of taurine and β-alanine supplementation on taurine transporter protein and fatigue resistance in skeletal muscle from mdx mice. Amino Acids 2016, 48, 2635–2645. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.G.; Horvath, D.; van der Poel, C.; Murphy, R.M. Benefits of prenatal taurine supplementation in preventing the onset of acute damage in the Mdx mouse. PLoS Curr. 2017, 9. [Google Scholar] [CrossRef]
- Allen, D.G.; Whitehead, N.P. Duchenne muscular dystrophy–what causes the increased membrane permeability in skeletal muscle? Int. J. Biochem. Cell Biol. 2011, 43, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Radley-Crabb, H.; Terrill, J.; Shavlakadze, T.; Tonkin, J.; Arthur, P.; Grounds, M. A single 30 min treadmill exercise session is suitable for ‘proof-of concept studies’ in adult mdx mice: A comparison of the early consequences of two different treadmill protocols. Neuromuscul. Disord. 2012, 22, 170–182. [Google Scholar] [CrossRef]
- Burch, P.M.; Pogoryelova, O.; Goldstein, R.; Bennett, D.; Guglieri, M.; Straub, V.; Bushby, K.; Lochmüller, H.; Morris, C. Muscle-derived proteins as serum biomarkers for monitoring disease progression in three forms of muscular dystrophy. J. Neuromuscul. Dis. 2015, 2, 241–255. [Google Scholar] [CrossRef]
- Setsukinai, K.; Urano, Y.; Kakinuma, K.; Majima, H.J.; Nagano, T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J. Biol. Chem. 2003, 278, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C.; Vissers, M.C.M.; Kettle, A.J. Myeloperoxidase. Curr. Opin. Hematol. 2000, 7, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C.; Kettle, A.J. Biomarkers of myeloperoxidase-derived hypochlorous acid. Free Radic. Biol. Med. 2000, 29, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Ladner, C.L.; Yang, J.; Turner, R.J.; Edwards, R.A. Visible fluorescent detection of proteins in polyacrylamide gels without staining. Anal. Biochem. 2004, 326, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Grounds, M.D.; Terrill, J.R.; Al-Mshhdani, B.A.; Duong, M.N.; Radley-Crabb, H.G.; Arthur, P.G. Biomarkers for Duchenne muscular dystrophy: Myonecrosis, inflammation and oxidative stress. Dis. Model. Mech. 2020, 13, dmm043638. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Becerril, T.; Rodríguez-Cruz, M.; Sánchez-González, J.R.; Villaldama-Soriano, M.A.; Atilano-Miguel, S.; Villa-Morales, J.; Cárdenas-Conejo, A.; Cárdenas-Vázquez, R. Circulating markers of oxidative stress are associated with a muscle injury in patients with muscular dystrophy Duchenne. Brain Dev. 2021, 43, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.E.; Zerbes, R.; Fournier, P.A.; Arthur, P.G. A fluorescent dual labeling technique for the quantitative measurement of reduced and oxidized protein thiols in tissue samples. Free Radic. Biol. Med. 2011, 50, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Era, S.; Kazuo, K.; Imai, H.; Nakamura, K.; Hayashi, T.; Sogami, M. Age-related change in redox state of human serum albumin. Biochim. Biophys. Acta BBA-Protein Struct. Mol. Enzymol. 1995, 1247, 12–16. [Google Scholar] [CrossRef]
- Mullins, R.J.; Bell, D.R. Changes in interstitial volume and masses of albumin and IgG in rabbit skin and skeletal muscle after saline volume loading. Circ. Res. 1982, 51, 305–313. [Google Scholar] [CrossRef]
- Heilig, A.; Pette, D. Albumin in rabbit skeletal muscle. Origin, distribution and regulation by contractile activity. Eur. J. Biochem. 1988, 171, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Lian, D.; Chen, M.-M.; Wu, H.; Deng, S.; Hu, X. The role of oxidative stress in skeletal muscle myogenesis and muscle disease. Antioxidants 2022, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Erstad, B.L. Albumin disposition in critically Ill patients. J. Clin. Pharm. Ther. 2018, 43, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Wooddell, C.I.; Zhang, G.; Griffin, J.B.; Hegge, J.O.; Huss, T.; Wolff, J.A. Use of Evans blue dye to compare limb muscles in exercised young and old mdx mice. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2010, 41, 487–499. [Google Scholar] [CrossRef]
- Hamer, P.; McGeachie, J.i.; Davies, M.; Grounds, M. Evans Blue Dye as an in vivo marker of myofibre damage: Optimising parameters for detecting initial myofibre membrane permeability. J. Anat. 2002, 200, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Turell, L.; Botti, H.; Carballal, S.; Ferrer-Sueta, G.; Souza, J.M.; Durán, R.; Freeman, B.A.; Radi, R.; Alvarez, B. Reactivity of sulfenic acid in human serum albumin. Biochemistry 2008, 47, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Aging-related changes in the thiol/disulfide redox state: Implications for the use of thiol antioxidants. Exp. Gerontol. 2002, 37, 1333–1345. [Google Scholar] [CrossRef]
- Reid, M.B. Invited Review: Redox modulation of skeletal muscle contraction: What we know and what we don’t. J. Appl. Physiol. 2001, 90, 724–731. [Google Scholar] [CrossRef]


| Indices 1 | Indices 2 | r | p |
|---|---|---|---|
| Oxidised plasma albumin | Oxidised muscle albumin | 0.8 | <0.0001 * |
| Oxidised plasma albumin | Muscle albumin | 0.5 | <0.02 * |
| Oxidised plasma albumin | Total muscle protein thiol oxidation | 0.6 | <0.0003 * |
| Oxidised plasma albumin | Muscle necrosis | 0.6 | <0.0003 * |
| Oxidised plasma albumin | Muscle inflammation | 0.6 | <0.0003 * |
| Oxidised plasma albumin | Plasma Ck | 0.7 | <0.0001 * |
| Oxidised muscle albumin | Muscle albumin | 0.6 | <0.001 * |
| Oxidised muscle albumin | Total muscle protein thiol oxidation | 0.6 | <0.003 * |
| Oxidised muscle albumin | Muscle necrosis | 0.6 | <0.006 * |
| Oxidised muscle albumin | Muscle inflammation | 0.6 | <0.0001 * |
| Oxidised muscle albumin | Plasma Ck | 0.7 | <0.0001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terrill, J.R.; Bautista, A.P.R.; Tsioutsias, I.; Grounds, M.D.; Arthur, P.G. Oxidised Albumin Levels in Plasma and Skeletal Muscle as Biomarkers of Disease Progression and Treatment Efficacy in Dystrophic mdx Mice. Antioxidants 2024, 13, 720. https://doi.org/10.3390/antiox13060720
Terrill JR, Bautista APR, Tsioutsias I, Grounds MD, Arthur PG. Oxidised Albumin Levels in Plasma and Skeletal Muscle as Biomarkers of Disease Progression and Treatment Efficacy in Dystrophic mdx Mice. Antioxidants. 2024; 13(6):720. https://doi.org/10.3390/antiox13060720
Chicago/Turabian StyleTerrill, Jessica R., Angelo Patrick R. Bautista, Irene Tsioutsias, Miranda D. Grounds, and Peter G. Arthur. 2024. "Oxidised Albumin Levels in Plasma and Skeletal Muscle as Biomarkers of Disease Progression and Treatment Efficacy in Dystrophic mdx Mice" Antioxidants 13, no. 6: 720. https://doi.org/10.3390/antiox13060720
APA StyleTerrill, J. R., Bautista, A. P. R., Tsioutsias, I., Grounds, M. D., & Arthur, P. G. (2024). Oxidised Albumin Levels in Plasma and Skeletal Muscle as Biomarkers of Disease Progression and Treatment Efficacy in Dystrophic mdx Mice. Antioxidants, 13(6), 720. https://doi.org/10.3390/antiox13060720






