A Consolidated Understanding of the Contribution of Redox Dysregulation in the Development of Hearing Impairment
Abstract
1. Introduction
2. Contribution of the Anatomy and Biology of the Auditory System to Its Function
3. Established Drivers of the Loss of Auditory Function and Their Sensitivity to Dysregulation of the Redox System
3.1. Genetic Factors and Their Associated Predisposition to the Impairment of Auditory Function
| Gene Symbol | Protein Name | DFN Locus | Type of Hearing Loss | Normal Function | Speculated Involvement in Redox Homeostasis |
|---|---|---|---|---|---|
| Structural Defects | |||||
| MYH14 | Myosin heavy chain 14 | DFNA4 | NS and S | Non-muscle ATP-dependent molecular motors interact with cytoskeletal actin to regulate cell motility and polarity [124]. | miR-499 originates from the MYH14 intronic sequence and is involved in protection of cardiomyocytes [125] and neurons [126] from tissue damage-induced oxidative stress. |
| TECTA | Alpha-tectorin | DFNA8/12/21 | NS | Major non-collagenous structural component of the tectorial membrane [127]. | - |
| COCH | Cochlin | DFNA9 | NS | Major non-collagenous component of the extracellular matrix of the inner ear. Linked to the regulation of bacteria-driven immune response in the inner ear [128,129,130]. | Shear stress which disrupts endothelial homeostasis and promotes oxidative stress leads to multimerization of cochlin and increased interaction with the mechanosensitive potassium channel subfamily K member 2 (TREK-1) in the ocular system [131]. |
| MYO | Myosin, class II and III | DFNA4/11/22/48, DFNB3/30/37 | NS and S | Development and function of the cochlea duct (class II) and stereocilia of the vestibular hair cells (class III) [132,133]. | Myosin are differentially expressed under oxidative stress in diabetic rat brains [134]. |
| COL11A2 | Type XI collagen, called the pro-alpha2(XI) chain | DFNA13 | NS | Minor fibrillar component of the tectorial membrane [135]. | - |
| CDH23 | Cadherin 23 | DFNB12 | NS and S | Calcium-dependent cell-cell adhesion glycoprotein involved in maintaining normal organization of stereocilia bundle [136,137]. | Cadherin 23 regulates purine metabolism [138] involved in the modulation of cellular redox biology [139]. |
| STRC | Stereocilin | DFNB16 | NS | Structural component of the stereocilia involved in the formation of horizontal top connectors of stereocilia and maintenance of the OHC bundle [140]. | - |
| TRIOBP | Trio rho guanine nucleotide exchange factor and F-actin binding protein | DFNB28 | NS | Cytoskeleton-associated protein which organizes actin filaments into uniquely rootlet-like dense bundles that provide durability and rigidity to stereocilia [141]. | Actin is susceptible to oxidation and effects of reactive oxygen species on its functioning [142,143,144]. Specific composition of actin may be important for stereocilia function [145,146]. |
| WHRN | Whirlin | DFNB31 | S | PDZ domain-containing protein expressed at the ankle region of stereocilia. Regulates IHC stereocilia growth and differentiation and OHC stereocilia rigidity and organization during development [147,148]. | - |
| Functional Defects | |||||
| GJB2/3/6 | Gap junction protein 2/3/6 or connexin 26/30/31 | DFNA2B/3A/3B | NS | Formation of hemichannels in the sensory epithelium, required for the formation of endolymphatic potential, which create sufficient driving force for K+ entry and depolarization of hair cells with activation of the MET channel [149]. | Connexin 26 ablation leads to increased oxidative stress in cochlea [45], likely through hemichannel-mediated spread of molecules that trigger redox imbalance in normal cells in the immediate periphery [150]. |
| DIAPH1 | Diaphanous homolog 1 (Drosophila) protein | DFNA1 | S | Regulate actin polymerization and microtubule dynamics to stabilize the cytoskeletal structure of hair cells [151]. | - |
| KCNQ4 | Kv7.4 potassium channel | DFNA2A | NS | Maintaining cochlear ion homoeostasis and regulating hair cell membrane potential [152]. | - |
| SLC17A8 | Solute carrier family 17 member 8 or vesicular glutamate transporter | DFNA25 | NS | Involved in the uptake of glutamate into the synaptic vesicles in IHCs [153]. | - |
| TMC1 | Transmembrane channel-like protein 1 | DFNB7/11 | NS | Ion-conducting pore of the MET channel complex [154,155]. | - |
| SLC26A4 | Solute carrier family 26 member 4 or pendrin | DFNB4 | NS | Transport negatively charged ions across the cell membrane. Involved in the function of the basal and intermediate cells of the stria vascularis to maintain the endocochlear potential [156]. | Pendrin knockout (KO) in mice leads to hyperpigmentation of the stria vascularis due to the increase in pH of the endolymph, which results in inhibition of cysteine uptake and glutathione synthesis by the surrounding cells [157]. Melanin synthesis is linked to oxidative stress in melanocytes [158]. |
| TMPRSS3 | Transmembrane protease serine 3 | DFNB8 | NS | Essential component of hair cell homeostasis and key to their survival. Precise mechanism unclear [159]. | - |
| PJVK | Pejvakin | DFNB59 | NS | Involved in peroxisome proliferation in response to sound. Precise mechanism unclear [160]. | Pejvakin-mediated pexophagy protects auditory hair cells from noise exposure-induced oxidative stress [161]. |
| SLC26A5 | Prestin | DFNB62 | NS | Functions as the molecular motor in OHCs. Generates force of electromotility for the amplification of sound signals in OHCs [162]. | Oxidative stress inhibits the expression of prestin [163]. |
| LHFPL5 | Lipoma high-mobility group protein gene fusion partner tetraspan subfamily member 5 | DFNB67 | NS | Tethers tip link to the MET channel to establish maximal force sensitivity of the MET channel. Required for correct localization of protocadherin related 15 (PCDH15) and TMC1 to the mechanotransduction complex [164,165]. | - |
| LOXHD1 | Lipoxygenase homology polycystin/lipoxygenase/alpha-toxin domains 1 | DFNB77 | NS | Involved in the mechanotranduction process in hair cells. Mechanism unknown [166,167]. | - |
| SERPINB6 | Serine proteinase inhibitor family B member 6 | DFNB91 | NS | Protect hair cells from the leakage of lysosomal content during stress [168]. | Lysosomes are susceptible to oxidative stress-dependent destabilization of membrane, which leads to the release of lysosomal enzymes into the cytosol [169]. |
| CABP2 | Calcium binding protein 2 | DFNB93 | NS | Modulator of IHC Cav1.3 function [170,171]. | CABP2 is a thioredoxin, which contains the redox-active dithiol/disulfide bond involved in defending against oxidative stress [172,173]. |
| Developmental Defects | |||||
| PRPS1 | Phosphoribosyl pyrophosphate synthetase 1 | DFNX1 | NS/S | Catalyze first step of nucleotide synthesis. Involved in fetal auditory system development [174]. | Production of nicotinamide adenine dinucleotide (NAD) is phosphoribosyl pyrophosphate (PRPP)-dependent, and pyridine nucleotides are severely reduced in erythrocytes of patients with PRPS-1 superactivity [175]. |
| POU3F4 | Pit-1/Oct-1/ Oct-2/unc-86 class 3 homeobox 4 | DFNX2 | NS | Involved in the development of the middle and inner ear [176]. | The related POU3F1 is degraded in the presence of oxidative stress [177]. |
| EYA4 | Eyes absent transcriptional coactivator and phosphatase 4 | DFNA10 | S | Involved in embryonic auditory system development and mature inner ear function [178,179]. | Reduced EYA4 expression decreases single-stranded DNA accumulation following DNA damage and impairs homologous recombination [180]. |
| GRXCR1 | Glutaredoxin and cysteine-rich domain containing 1 | DFNB25 | NS | Required for the morphogenesis of stereocilia in hair cells [181]. | - |
| ESRRB | Estrogen-related receptor beta | DFNB35 | S | Essential for inner ear development and function [182]. | ERRB is a negative regulator of NF-E2-related factor 2 (Nrf2) [183], involved in the expression of detoxifying enzyme and antioxidant proteins against oxidative stress [184,185]. |
| HGF | Hepatocyte growth factor | DFNB39 | NS | Involved in the development of stria vascularis of the cochlear epithelium [186]. | HGF attenuates angiotensin II–induced oxidative stress in vascular smooth muscle cells [187] and protects retinal pigment epithelial cells from oxidative stress [188]. |
| PTPRQ | Protein tyrosine phosphatase receptor type Q | DFNB84 | NS | Essential for the maturation and function of the hair bundle in the cochlea [189]. | Increase in expression of the related PTPRO increases reactive oxygen species production and promotes apoptosis through the toll-like receptor 4 (TLR4)/ nuclear factor kappa light chain-enhancer of activated B cell (NF-κB) pathway [190]. |
3.2. Noise-Induced Hearing Loss
3.3. Exposure to Ototoxic Chemicals and Compounds
3.4. Impact of Inflammatory Events on Auditory System Functioning and the Age-Related Decline of Auditory Function
4. Targeting Redox Imbalance-Driven Hearing Impairment with Antioxidants
4.1. Natural Product-Based Antioxidant Therapies
4.2. Supplemental Nutrients
4.3. Ototoxic and Novel Drugs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martinez-Conde, S.; Macknik, S.L.; Hubel, D.H. The Role of Fixational Eye Movements in Visual Perception. Nat. Rev. Neurosci. 2004, 5, 229–240. [Google Scholar] [CrossRef]
- Colenbrander, A. Visual Functions and Functional Vision. Int. Congr. Ser. 2005, 1282, 482–486. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, R.; Aier, I.; Semwal, R.; Tyagi, P.; Varadwaj, P. Sense of Smell: Structural, Functional, Mechanistic Advancements and Challenges in Human Olfactory Research. Curr. Neuropharmacol. 2019, 17, 891–911. [Google Scholar] [CrossRef]
- Su, C.-Y.; Menuz, K.; Carlson, J.R. Olfactory Perception: Receptors, Cells, and Circuits. Cell 2009, 139, 45–59. [Google Scholar] [CrossRef]
- Pereira, A. Biophysical Mechanisms Supporting Conscious Perception: Prospects for an Artificial Astrocyte. Nat. Prec. 2011. [Google Scholar] [CrossRef]
- Liang, M.; Mouraux, A.; Hu, L.; Iannetti, G.D. Primary Sensory Cortices Contain Distinguishable Spatial Patterns of Activity for Each Sense. Nat. Commun. 2013, 4, 1979. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.; Morceau, S.; Folkard, R.; Martin-Cortecero, J.; Groh, A. A Thalamic Bridge from Sensory Perception to Cognition. Neurosci. Biobehav. Rev. 2021, 120, 222–235. [Google Scholar] [CrossRef]
- Tauste Campo, A.; Vázquez, Y.; Álvarez, M.; Zainos, A.; Rossi-Pool, R.; Deco, G.; Romo, R. Feed-Forward Information and Zero-Lag Synchronization in the Sensory Thalamocortical Circuit Are Modulated during Stimulus Perception. Proc. Natl. Acad. Sci. USA 2019, 116, 7513–7522. [Google Scholar] [CrossRef]
- Kong, H.H.; Shin, K.; Won, C.W. Association of Dual Sensory Impairment with Declining Physical Function in Community-Dwelling Older Adults. Int. J. Environ. Res. Public Health 2023, 20, 3546. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hu, Y.; Luo, J.; Li, Y.; Liu, H.; Sun, X.; Zhou, M. Association between Sensory Loss and Falls among Middle-Aged and Older Chinese Population: Cross-Sectional and Longitudinal Analyses. Front. Med. 2022, 8, 810159. [Google Scholar] [CrossRef]
- Schubert, C.R.; Cruickshanks, K.J.; Fischer, M.E.; Chen, Y.; Klein, B.E.K.; Klein, R.; Pinto, A.A. Sensory Impairments and Cognitive Function in Middle-Aged Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1087–1090. [Google Scholar] [CrossRef] [PubMed]
- Azeem, A.; Julleekeea, A.; Knight, B.; Sohail, I.; Bruyns-Haylett, M.; Sastre, M. Hearing Loss and Its Link to Cognitive Impairment and Dementia. Front. Dement. 2023, 2, 1199319. [Google Scholar] [CrossRef]
- Varadaraj, V.; Munoz, B.; Deal, J.A.; An, Y.; Albert, M.S.; Resnick, S.M.; Ferrucci, L.; Swenor, B.K. Association of Vision Impairment with Cognitive Decline Across Multiple Domains in Older Adults. JAMA Netw. Open 2021, 4, e2117416. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, W.J.; Liu, P.L.; Sloane, R.; Van Houtven, C.; Pieper, C.F.; Hastings, S.N.; Cohen, H.J.; Whitson, H.E. Association of Sensory and Cognitive Impairment With Healthcare Utilization and Cost in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Kiely, K.M.; Anstey, K.J.; Luszcz, M.A. Dual Sensory Loss and Depressive Symptoms: The Importance of Hearing, Daily Functioning, and Activity Engagement. Front. Hum. Neurosci. 2013, 7, 837. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-C.; Gau, B.-S.; Liu, T.-C.; Hsieh, Y.-S.; Huang, G.-S.; Lou, M.-F. Association between Sensory Impairments and Restricted Social Participation in Older Adults: A Cross-Sectional Study. Collegian 2022, 29, 850–859. [Google Scholar] [CrossRef]
- Hutmacher, F. What Is Our Most Important Sense? Front. Young Minds 2021, 9, 548120. [Google Scholar] [CrossRef]
- Peterson, D.C.; Reddy, V.; Launico, M.V.; Hamel, R.N. Neuroanatomy, Auditory Pathway. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Aibara, R.; Welsh, J.T.; Puria, S.; Goode, R.L. Human Middle-Ear Sound Transfer Function and Cochlear Input Impedance. Hear. Res. 2001, 152, 100–109. [Google Scholar] [CrossRef]
- Fettiplace, R. Hair Cell Transduction, Tuning, and Synaptic Transmission in the Mammalian Cochlea. Compr. Physiol. 2017, 7, 1197–1227. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Jaruga, P.; Birincioglu, M.; Rodriguez, H. Free Radical-Induced Damage to DNA: Mechanisms and Measurement. Free Radic. Biol. Med. 2002, 32, 1102–1115. [Google Scholar] [CrossRef]
- Valgimigli, L. Lipid Peroxidation and Antioxidant Protection. Biomolecules 2023, 13, 1291. [Google Scholar] [CrossRef] [PubMed]
- Salvi, A.; Carrupt, P.-A.; Tillement, J.-P.; Testa, B. Structural Damage to Proteins Caused by Free Radicals: Asessment, Protection by Antioxidants, and Influence of Protein binding11Abbreviations: AAPH, 2,2′-Azobis(2-Amidinopropane) 2 HCl; and HSA, Human Serum Albumin. Biochem. Pharmacol. 2001, 61, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Sinskey, A.J.; Lodish, H.F. Oxidized Redox State of Glutathione in the Endoplasmic Reticulum. Science 1992, 257, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.; Piemonte, F. S-Glutathionylation Signaling in Cell Biology: Progress and Prospects. Eur. J. Pharm. Sci. 2012, 46, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant Responses and Cellular Adjustments to Oxidative Stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Glutathione Synthesis. Biochim. Et Biophys. Acta (BBA)—Gen. Subj. 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, A. Antioxidant Function of Thioredoxin and Glutaredoxin Systems. Antioxid. Redox Signal. 2000, 2, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Weiss, S.J.; Levine, R.L. Methionine Oxidation and Reduction in Proteins. Biochim. Biophys. Acta 2014, 1840, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Wouters, M.A.; Fan, S.W.; Haworth, N.L. Disulfides as Redox Switches: From Molecular Mechanisms to Functional Significance. Antioxid. Redox Signal. 2010, 12, 53–91. [Google Scholar] [CrossRef]
- Napolitano, G.; Fasciolo, G.; Venditti, P. Mitochondrial Management of Reactive Oxygen Species. Antioxidants 2021, 10, 1824. [Google Scholar] [CrossRef]
- Ferrari, C.K.B. Effects of Xenobiotics on Total Antioxidant Capacity. Interdiscip. Toxicol. 2012, 5, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.J.; Harb, H.L. L-γ-Glutamyl-l-Cysteinyl-Glycine (Glutathione; GSH) and GSH-Related Enzymes in the Regulation of pro- and Anti-Inflammatory Cytokines: A Signaling Transcriptional Scenario for Redox(y) Immunologic Sensor(s)? Mol. Immunol. 2005, 42, 987–1014. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K.; Jimenez, L.A.; Torres, M.; Forman, H.J. Glutathione, Stress Responses, and Redox Signaling in Lung Inflammation. Antioxid. Redox Signal. 2005, 7, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Puschner, B.; Schacht, J. Energy Metabolism in Cochlear Outer Hair Cells in Vitro. Hear. Res. 1997, 114, 102–106. [Google Scholar] [CrossRef]
- Rangaraju, V.; Calloway, N.; Ryan, T.A. Activity-Driven Local ATP Synthesis Is Required for Synaptic Function. Cell 2014, 156, 825–835. [Google Scholar] [CrossRef]
- Caprara, G.A.; Peng, A.W. Mechanotransduction in Mammalian Sensory Hair Cells. Mol. Cell. Neurosci. 2022, 120, 103706. [Google Scholar] [CrossRef]
- Nothwang, H.G.; Engel, J.; Knipper, M.; Friauf, E. L-Type Calcium Channels in the Auditory System. e-Neuroforum 2014, 5, 60–66. [Google Scholar] [CrossRef]
- Finkel, T.; Menazza, S.; Holmström, K.M.; Parks, R.J.; Liu, J.; Sun, J.; Liu, J.; Pan, X.; Murphy, E. The Ins and Outs of Mitochondrial Calcium. Circ. Res. 2015, 116, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How Mitochondria Produce Reactive Oxygen Species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Fetoni, A.R.; Paciello, F.; Rolesi, R.; Paludetti, G.; Troiani, D. Targeting Dysregulation of Redox Homeostasis in Noise-Induced Hearing Loss: Oxidative Stress and ROS Signaling. Free Radic. Biol. Med. 2019, 135, 46–59. [Google Scholar] [CrossRef]
- Rogers, L.K. Cellular Targets of Oxidative Stress. Curr. Opin. Toxicol. 2020, 20–21, 48–54. [Google Scholar] [CrossRef]
- Kannan, K.; Jain, S.K. Oxidative Stress and Apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, C.; Yuan, L.; Guo, M.; Xu, X.; Shao, A.; Zhang, A.; Zhou, D. Redox Homeostasis Dysregulation in Noise-Induced Hearing Loss: Oxidative Stress and Antioxidant Treatment. J. Otolaryngol—Head Neck Surg. 2023, 52, 78. [Google Scholar] [CrossRef] [PubMed]
- Fetoni, A.R.; Zorzi, V.; Paciello, F.; Ziraldo, G.; Peres, C.; Raspa, M.; Scavizzi, F.; Salvatore, A.M.; Crispino, G.; Tognola, G.; et al. Cx26 Partial Loss Causes Accelerated Presbycusis by Redox Imbalance and Dysregulation of Nfr2 Pathway. Redox Biol. 2018, 19, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Talaska, A.E.; Schacht, J.; Sha, S.-H. Oxidative Imbalance in the Aging Inner Ear. Neurobiol. Aging 2007, 28, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.J.T.; Song, L. Role of Mitochondrial Dysfunction and Oxidative Stress in Sensorineural Hearing Loss. Hear. Res. 2023, 434, 108783. [Google Scholar] [CrossRef]
- Forouzanfar, F.; Asgharzade, S. MicroRNAs in Noise-Induced Hearing Loss and Their Regulation by Oxidative Stress and Inflammation. CDT 2020, 21, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Litovsky, R. Development of the Auditory System. Handb. Clin. Neurol. 2015, 129, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, H.; Nishimura, T.; Shimokura, R.; Kitahara, T. Cartilage Conduction as the Third Pathway for Sound Transmission. Auris Nasus Larynx 2019, 46, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.X.; Yoon, Y.-J.; Kim, N. Mechanism of Bone-Conducted Hearing: Mathematical Approach. Biomech. Model. Mechanobiol. 2018, 17, 1731–1740. [Google Scholar] [CrossRef]
- Gan, R.Z.; Reeves, B.P.; Wang, X. Modeling of Sound Transmission from Ear Canal to Cochlea. Ann. Biomed. Eng. 2007, 35, 2180–2195. [Google Scholar] [CrossRef] [PubMed]
- Hüttenbrink, K.B. The Mechanics of the Middle-Ear at Static Air Pressures: The Role of the Ossicular Joints, the Function of the Middle-Ear Muscles and the Behaviour of Stapedial Prostheses. Acta Oto-Laryngol. 1988, 105, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Hüttenbrink, K.B. The mechanics and function of the middle ear. Part 1: The ossicular chain and middle ear muscles. Laryngorhinootologie 1992, 71, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Stenfelt, S.; Hato, N.; Goode, R.L. Fluid Volume Displacement at the Oval and Round Windows with Air and Bone Conduction Stimulation. J. Acoust. Soc. Am. 2004, 115, 797–812. [Google Scholar] [CrossRef] [PubMed]
- Koike, T.; Sakamoto, C.; Sakashita, T.; Hayashi, K.; Kanzaki, S.; Ogawa, K. Effects of a Perilymphatic Fistula on the Passive Vibration Response of the Basilar Membrane. Hear. Res. 2012, 283, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Müller, U. Mechanically Gated Ion Channels in Mammalian Hair Cells. Front. Cell. Neurosci. 2018, 12, 100. [Google Scholar] [CrossRef] [PubMed]
- Zdebik, A.A.; Wangemann, P.; Jentsch, T.J. Potassium Ion Movement in the Inner Ear: Insights from Genetic Disease and Mouse Models. Physiology 2009, 24, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.-H.; Oghalai, J.S. Perilymph Osmolality Modulates Cochlear Function. Laryngoscope 2008, 118, 1621–1629. [Google Scholar] [CrossRef]
- Magistretti, J.; Spaiardi, P.; Johnson, S.L.; Masetto, S. Elementary Properties of Ca2+ Channels and Their Influence on Multivesicular Release and Phase-Locking at Auditory Hair Cell Ribbon Synapses. Front. Cell. Neurosci. 2015, 9, 123. [Google Scholar] [CrossRef]
- Krinner, S.; Butola, T.; Jung, S.; Wichmann, C.; Moser, T. RIM-Binding Protein 2 Promotes a Large Number of CaV1.3 Ca2+-Channels and Contributes to Fast Synaptic Vesicle Replenishment at Hair Cell Active Zones. Front. Cell. Neurosci. 2017, 11, 334. [Google Scholar] [CrossRef]
- Usami, S.; Matsubara, A.; Fujita, S.; Takumi, Y.; Ottersen, O.P. Chapter IX Glutamate Neurotransmission in the Mammalian Inner Ear. In Handbook of Chemical Neuroanatomy; Elsevier: Amsterdam, The Netherlands, 2000; Volume 18, pp. 255–271. ISBN 978-0-444-50286-5. [Google Scholar]
- Goutman, J.D.; Elgoyhen, A.B.; Gómez-Casati, M.E. Cochlear Hair Cells: The Sound-Sensing Machines. FEBS Lett. 2015, 589, 3354–3361. [Google Scholar] [CrossRef] [PubMed]
- Weisz, C.J.C.; Lehar, M.; Hiel, H.; Glowatzki, E.; Fuchs, P.A. Synaptic Transfer from Outer Hair Cells to Type II Afferent Fibers in the Rat Cochlea. J. Neurosci. 2012, 32, 9528–9536. [Google Scholar] [CrossRef] [PubMed]
- Weisz, C.; Glowatzki, E.; Fuchs, P. The Postsynaptic Function of Type II Cochlear Afferents. Nature 2009, 461, 1126–1129. [Google Scholar] [CrossRef]
- Xia, A.; Song, Y.; Wang, R.; Gao, S.S.; Clifton, W.; Raphael, P.; Chao, S.; Pereira, F.A.; Groves, A.K.; Oghalai, J.S. Prestin Regulation and Function in Residual Outer Hair Cells after Noise-Induced Hearing Loss. PLoS ONE 2013, 8, e82602. [Google Scholar] [CrossRef] [PubMed]
- Fettiplace, R. Diverse Mechanisms of Sound Frequency Discrimination in the Vertebrate Cochlea. Trends Neurosci. 2020, 43, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Ohn, T.-L.; Rutherford, M.A.; Jing, Z.; Jung, S.; Duque-Afonso, C.J.; Hoch, G.; Picher, M.M.; Scharinger, A.; Strenzke, N.; Moser, T. Hair Cells Use Active Zones with Different Voltage Dependence of Ca2+ Influx to Decompose Sounds into Complementary Neural Codes. Proc. Natl. Acad. Sci. USA 2016, 113, E4716–E4725. [Google Scholar] [CrossRef]
- Sutherland, D.P.; Glendenning, K.K.; Masterton, R.B. Role of Acoustic Striae in Hearing: Discrimination of Sound-Source Elevation. Hear. Res. 1998, 120, 86–108. [Google Scholar] [CrossRef]
- Christov, F.; Nelson, E.G.; Gluth, M.B. Human Superior Olivary Nucleus Neuron Populations in Subjects with Normal Hearing and Presbycusis. Ann. Otol. Rhinol. Laryngol. 2018, 127, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Martínez, M.; Rincón, H.; Gómez-Álvarez, M.; Gómez-Nieto, R.; Saldaña, E. The Nuclei of the Lateral Lemniscus: Unexpected Players in the Descending Auditory Pathway. Front. Neuroanat. 2023, 17, 1242245. [Google Scholar] [CrossRef]
- Gruters, K.G.; Groh, J.M. Sounds and beyond: Multisensory and Other Non-Auditory Signals in the Inferior Colliculus. Front. Neural Circuits 2012, 6, 96. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Ge, S.; Xiong, Q. Medial Geniculate Body and Primary Auditory Cortex Differentially Contribute to Striatal Sound Representations. Nat. Commun. 2019, 10, 418. [Google Scholar] [CrossRef] [PubMed]
- Winer, J.A. The Functional Architecture of the Medial Geniculate Body and the Primary Auditory Cortex. In The Mammalian Auditory Pathway: Neuroanatomy; Webster, D.B., Popper, A.N., Fay, R.R., Eds.; Springer Handbook of Auditory Research; Springer: New York, NY, USA, 1992; Volume 1, pp. 222–409. ISBN 978-0-387-97800-0. [Google Scholar]
- King, A.J.; Hammond-Kenny, A.; Nodal, F.R. Multisensory Processing in the Auditory Cortex. In Multisensory Processes; Lee, A.K.C., Wallace, M.T., Coffin, A.B., Popper, A.N., Fay, R.R., Eds.; Springer Handbook of Auditory Research; Springer International Publishing: Cham, Switzerland, 2019; Volume 68, pp. 105–133. ISBN 978-3-030-10459-7. [Google Scholar]
- Van Bijnen, S.; Parkkonen, L.; Parviainen, T. Activity Level in Left Auditory Cortex Predicts Behavioral Performance in Inhibition Tasks in Children. NeuroImage 2022, 258, 119371. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Hong, S.N.; Kim, H.S.; Han, J.J.; Chung, J.; Seo, M.-W.; Oh, S.-H.; Chang, S.-O.; Lee, J.H. Determinants of Conductive Hearing Loss in Tympanic Membrane Perforation. Clin. Exp. Otorhinolaryngol. 2015, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.J. Cholesteatoma and Otosclerosis: Two Slowly Progressive Causes of Hearing Loss Treatable through Corrective Surgery. Clin. Med. Res. 2003, 1, 151–154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liberman, M.C.; Liberman, L.D.; Maison, S.F. Chronic Conductive Hearing Loss Leads to Cochlear Degeneration. PLoS ONE 2015, 10, e0142341. [Google Scholar] [CrossRef] [PubMed]
- Pillion, J.P.; Vernick, D.; Shapiro, J. Hearing Loss in Osteogenesis Imperfecta: Characteristics and Treatment Considerations. Genet. Res. Int. 2011, 2011, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.C.; Camci, E.D.; Vora, S.; Luquetti, D.V.; Turner, E.E. The Genetics of Auricular Development and Malformation: New Findings in Model Systems Driving Future Directions for Microtia Research. Eur. J. Med. Genet. 2014, 57, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Bartel-Friedrich, S.; Wulke, C. Classification and Diagnosis of Ear Malformations. GMS Curr. Top. Otorhinolaryngol. Head. Neck Surg. 2007, 6, Doc05. [Google Scholar]
- Vijayendra, H.; Parikh, B. Bone Conduction Improvement After Surgery for Conductive Hearing Loss. Indian. J. Otolaryngol. Head. Neck Surg. 2011, 63, 201–204. [Google Scholar] [CrossRef]
- Cheng, H.C.S.; Agrawal, S.K.; Parnes, L.S. Stapedectomy Versus Stapedotomy. Otolaryngol. Clin. N. Am. 2018, 51, 375–392. [Google Scholar] [CrossRef]
- Kakuki, T.; Miyata, R.; Yoshida, Y.; Kaizaki, A.; Kimura, A.; Kurashima, K.; Kuwata, R.; Takano, K. The Effects of Utilizing Cartilage Conduction Hearing Aids among Patients with Conductive Hearing Loss. Audiol. Res. 2023, 13, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Janssen, R.M.; Hong, P.; Chadha, N.K. Bilateral Bone-Anchored Hearing Aids for Bilateral Permanent Conductive Hearing Loss: A Systematic Review. Otolaryngol.—Head Neck Surg. 2012, 147, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Han, B.; Zhou, X.; Huang, S.; Huang, J. Research Progress on the Treatment and Nursing of Sensorineural Hearing Loss. Front. Neurosci. 2023, 17, 1199946. [Google Scholar] [CrossRef] [PubMed]
- Jafari, Z.; Kolb, B.E.; Mohajerani, M.H. Age-Related Hearing Loss and Tinnitus, Dementia Risk, and Auditory Amplification Outcomes. Ageing Res. Rev. 2019, 56, 100963. [Google Scholar] [CrossRef] [PubMed]
- Valero, M.D.; Hancock, K.E.; Liberman, M.C. The Middle Ear Muscle Reflex in the Diagnosis of Cochlear Neuropathy. Hear. Res. 2016, 332, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Starr, A.; Sininger, Y.S.; Pratt, H. The Varieties of Auditory Neuropathy. J. Basic Clin. Physiol. Pharmacol. 2000, 11, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Starr, A.; Picton, T.W.; Sininger, Y.; Hood, L.J.; Berlin, C.I. Auditory Neuropathy. Brain 1996, 119, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Cotanche, D.A.; Lee, K.H. Regeneration of Hair Cells in the Vestibulocochlear System of Birds and Mammals. Curr. Opin. Neurobiol. 1994, 4, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Benkafadar, N.; Sato, M.P.; Ling, A.H.; Janesick, A.; Scheibinger, M.; Jan, T.A.; Heller, S. An Essential Signaling Cascade for Avian Auditory Hair Cell Regeneration. Dev. Cell 2024, 59, 280–291.e5. [Google Scholar] [CrossRef]
- Rubel, E.W.; Furrer, S.A.; Stone, J.S. A Brief History of Hair Cell Regeneration Research and Speculations on the Future. Hear. Res. 2013, 297, 42–51. [Google Scholar] [CrossRef]
- Xu, S.; Yang, N. Research Progress on the Mechanism of Cochlear Hair Cell Regeneration. Front. Cell. Neurosci. 2021, 15, 732507. [Google Scholar] [CrossRef]
- Vona, B.; Nanda, I.; Hofrichter, M.A.H.; Shehata-Dieler, W.; Haaf, T. Non-Syndromic Hearing Loss Gene Identification: A Brief History and Glimpse into the Future. Mol. Cell. Probes 2015, 29, 260–270. [Google Scholar] [CrossRef]
- Kochhar, A.; Hildebrand, M.S.; Smith, R.J.H. Clinical Aspects of Hereditary Hearing Loss. Genet. Med. 2007, 9, 393–408. [Google Scholar] [CrossRef]
- Aldè, M.; Cantarella, G.; Zanetti, D.; Pignataro, L.; La Mantia, I.; Maiolino, L.; Ferlito, S.; Di Mauro, P.; Cocuzza, S.; Lechien, J.R.; et al. Autosomal Dominant Non-Syndromic Hearing Loss (DFNA): A Comprehensive Narrative Review. Biomedicines 2023, 11, 1616. [Google Scholar] [CrossRef] [PubMed]
- Mkaouar-Rebai, E.; Chamkha, I.; Kammoun, T.; Alila-Fersi, O.; Aloulou, H.; Hachicha, M.; Fakhfakh, F. A Novel MT-CO1 m.6498C>A Variation Associated with the m.7444G>A Mutation in the Mitochondrial COI/tRNASer(UCN) Genes in a Patient with Hearing Impairment, Diabetes and Congenital Visual Loss. Biochem. Biophys. Res. Commun. 2013, 430, 585–591. [Google Scholar] [CrossRef]
- Ammar, M.; Tabebi, M.; Sfaihi, L.; Alila-Fersi, O.; Maalej, M.; Felhi, R.; Chabchoub, I.; Keskes, L.; Hachicha, M.; Fakhfakh, F.; et al. Mutational Screening in Patients with Profound Sensorineural Hearing Loss and Neurodevelopmental Delay: Description of a Novel m.3861A>C Mitochondrial Mutation in the MT-ND1 Gene. Biochem. Biophys. Res. Commun. 2016, 474, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, E.M.; Rehman, A.U.; Walsh, T.; Clayton-Smith, J.; Lee, K.; Morell, R.J.; Drummond, M.C.; Khan, S.N.; Naeem, M.A.; Rauf, B.; et al. Perrault Syndrome Is Caused by Recessive Mutations in CLPP, Encoding a Mitochondrial ATP-Dependent Chambered Protease. Am. J. Hum. Genet. 2013, 92, 605–613. [Google Scholar] [CrossRef]
- Oziębło, D.; Leja, M.L.; Jeznach, A.; Orzechowska, M.; Skirecki, T.; Więsik-Szewczyk, E.; Furmanek, M.; Bałdyga, N.; Skarżyński, H.; Ołdak, M. Hearing Loss as the Main Clinical Presentation in NLRP3-Associated Autoinflammatory Disease. Front. Immunol. 2022, 13, 904632. [Google Scholar] [CrossRef] [PubMed]
- Amor, D.J.; Marsh, A.P.L.; Storey, E.; Tankard, R.; Gillies, G.; Delatycki, M.B.; Pope, K.; Bromhead, C.; Leventer, R.J.; Bahlo, M.; et al. Heterozygous Mutations in HSD17B4 Cause Juvenile Peroxisomal D-Bifunctional Protein Deficiency. Neurol. Genet. 2016, 2, e114. [Google Scholar] [CrossRef]
- Lim, H.D.; Lee, S.M.; Yun, Y.J.; Lee, D.H.; Lee, J.H.; Oh, S.-H.; Lee, S.-Y. WFS1 Autosomal Dominant Variants Linked with Hearing Loss: Update on Structural Analysis and Cochlear Implant Outcome. BMC Med. Genom. 2023, 16, 79. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, H.; Pan, H.; Guan, J.; Yan, L.; Fan, M.; Zhou, H.; Zhou, X.; Wu, K.; Jia, Z.; et al. AIFM1 Variants Associated with Auditory Neuropathy Spectrum Disorder Cause Apoptosis Due to Impaired Apoptosis-Inducing Factor Dimerization. J. Zhejiang Univ. Sci. B 2023, 24, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Sun, P.; Feng, C.; Chen, Q.; Sun, X.; Chen, Y.; Tian, G. Varying Clinical Phenotypes of Mitochondrial DNA T12811C Mutation: A Case Series Report. Front. Med. 2022, 9, 912103. [Google Scholar] [CrossRef]
- Jazin, E.E.; Cavelier, L.; Eriksson, I.; Oreland, L.; Gyllensten, U. Human Brain Contains High Levels of Heteroplasmy in the Noncoding Regions of Mitochondrial DNA. Proc. Natl. Acad. Sci. USA 1996, 93, 12382–12387. [Google Scholar] [CrossRef] [PubMed]
- Timón-Gómez, A.; Nývltová, E.; Abriata, L.A.; Vila, A.J.; Hosler, J.; Barrientos, A. Mitochondrial Cytochrome c Oxidase Biogenesis: Recent Developments. Semin. Cell Dev. Biol. 2018, 76, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA Cycle Metabolites Control Physiology and Disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Electron-Transport Chains and Their Proton Pumps. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Wedam, R.; Greer, Y.E.; Wisniewski, D.J.; Weltz, S.; Kundu, M.; Voeller, D.; Lipkowitz, S. Targeting Mitochondria with ClpP Agonists as a Novel Therapeutic Opportunity in Breast Cancer. Cancers 2023, 15, 1936. [Google Scholar] [CrossRef] [PubMed]
- Haynes, C.M.; Petrova, K.; Benedetti, C.; Yang, Y.; Ron, D. ClpP Mediates Activation of a Mitochondrial Unfolded Protein Response in C. Elegans. Dev. Cell 2007, 13, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Xiong, Q.; Wei, X.; Wang, Y.; Hu, X.; He, G.; Liu, L.; Lai, Q.; Dai, Z.; Anushesh, D.; et al. Mitochondrial Unfolded Protein Response Gene CLPP Changes Mitochondrial Dynamics and Affects Mitochondrial Function. PeerJ 2019, 7, e7209. [Google Scholar] [CrossRef] [PubMed]
- Shpilka, T.; Haynes, C.M. The Mitochondrial UPR: Mechanisms, Physiological Functions and Implications in Ageing. Nat. Rev. Mol. Cell Biol. 2018, 19, 109–120. [Google Scholar] [CrossRef]
- Gispert, S.; Parganlija, D.; Klinkenberg, M.; Drose, S.; Wittig, I.; Mittelbronn, M.; Grzmil, P.; Koob, S.; Hamann, A.; Walter, M.; et al. Loss of Mitochondrial Peptidase Clpp Leads to Infertility, Hearing Loss plus Growth Retardation via Accumulation of CLPX, mtDNA and Inflammatory Factors. Hum. Mol. Genet. 2013, 22, 4871–4887. [Google Scholar] [CrossRef]
- Pryde, K.R.; Taanman, J.W.; Schapira, A.H. A LON-ClpP Proteolytic Axis Degrades Complex I to Extinguish ROS Production in Depolarized Mitochondria. Cell Rep. 2016, 17, 2522–2531. [Google Scholar] [CrossRef] [PubMed]
- Thelen, M.P.; Wirth, B.; Kye, M.J. Mitochondrial Defects in the Respiratory Complex I Contribute to Impaired Translational Initiation via ROS and Energy Homeostasis in SMA Motor Neurons. Acta Neuropathol. Commun. 2020, 8, 223. [Google Scholar] [CrossRef]
- Tschopp, J.; Schroder, K. NLRP3 Inflammasome Activation: The Convergence of Multiple Signalling Pathways on ROS Production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H.; Kawashima, Y.; Kurima, K.; Chae, J.J.; Ross, A.M.; Pinto-Patarroyo, G.; Patel, S.K.; Muskett, J.A.; Ratay, J.S.; Chattaraj, P.; et al. NLRP3 Mutation and Cochlear Autoinflammation Cause Syndromic and Nonsyndromic Hearing Loss DFNA34 Responsive to Anakinra Therapy. Proc. Natl. Acad. Sci. USA 2017, 114, E7766–E7775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zheng, H.; Pyykko, I.; Zou, J. The TLR-4/NF-κB Signaling Pathway Activation in Cochlear Inflammation of Rats with Noise-Induced Hearing Loss. Hear. Res. 2019, 379, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Scherer, E.Q.; Yang, J.; Canis, M.; Reimann, K.; Ivanov, K.; Diehl, C.D.; Backx, P.H.; Wier, W.G.; Strieth, S.; Wangemann, P.; et al. Tumor Necrosis Factor-α Enhances Microvascular Tone and Reduces Blood Flow in the Cochlea via Enhanced Sphingosine-1-Phosphate Signaling. Stroke 2010, 41, 2618–2624. [Google Scholar] [CrossRef] [PubMed]
- Violante, S.; Achetib, N.; Roermund, C.W.T.; Hagen, J.; Dodatko, T.; Vaz, F.M.; Waterham, H.R.; Chen, H.; Baes, M.; Yu, C.; et al. Peroxisomes Can Oxidize Medium- and Long-chain Fatty Acids through a Pathway Involving ABCD3 and HSD17B4. FASEB J. 2019, 33, 4355–4364. [Google Scholar] [CrossRef]
- Morikawa, S.; Blacher, L.; Onwumere, C.; Urano, F. Loss of Function of WFS1 Causes ER Stress-Mediated Inflammation in Pancreatic Beta-Cells. Front. Endocrinol. 2022, 13, 849204. [Google Scholar] [CrossRef] [PubMed]
- Donaudy, F.; Snoeckx, R.; Pfister, M.; Zenner, H.-P.; Blin, N.; Di Stazio, M.; Ferrara, A.; Lanzara, C.; Ficarella, R.; Declau, F.; et al. Nonmuscle Myosin Heavy-Chain Gene MYH14 Is Expressed in Cochlea and Mutated in Patients Affected by Autosomal Dominant Hearing Impairment (DFNA4). Am. J. Hum. Genet. 2004, 74, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Xu, T.; Ding, W.; Zhang, X.; Ji, X.; Yu, T.; Yu, W.; Lin, Z.; Wang, J. miR-499-5p Attenuates Mitochondrial Fission and Cell Apoptosis via P21 in Doxorubicin Cardiotoxicity. Front. Genet. 2019, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zheng, B.; Pang, L.; Che, Y.; Qi, X. Suppression of MALAT1 Alleviates Neurocyte Apoptosis and Reactive Oxygen Species Production through the miR-499-5p/SOX6 Axis in Subarachnoid Hemorrhage. J. Mol. Histol. 2022, 53, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, K.; Laer, L.V.; Kirschhofer, K.; Legan, P.K.; Hughes, D.C.; Schatteman, I.; Verstreken, M.; Hauwe, P.V.; Coucke, P.; Chen, A.; et al. Mutations in the Human α-Tectorin Gene Cause Autosomal Dominant Non-Syndromic Hearing Impairment. Nat. Genet. 1998, 19, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Robertson, N.G.; Lu, L.; Heller, S.; Merchant, S.N.; Eavey, R.D.; McKenna, M.; Nadol, J.B.; Miyamoto, R.T.; Linthicum, F.H.; Lubianca Neto, J.F.; et al. Mutations in a Novel Cochlear Gene Cause DFNA9, a Human Nonsyndromic Deafness with Vestibular Dysfunction. Nat. Genet. 1998, 20, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Yoo, J.E.; Choe, Y.H.; Park, S.C.; Lee, H.J.; Lee, H.J.; Noh, B.; Kim, S.H.; Kang, G.-Y.; Lee, K.-M.; et al. Cleaved Cochlin Sequesters Pseudomonas Aeruginosa and Activates Innate Immunity in the Inner Ear. Cell Host Microbe 2019, 25, 513–525.e6. [Google Scholar] [CrossRef]
- Hosokawa, S.; Mizuta, K.; Nakanishi, H.; Hashimoto, Y.; Arai, M.; Mineta, H.; Shindo, S.; Ikezono, T. Ultrastructural Localization of Cochlin in the Rat Cochlear Duct. Audiol. Neurotol. 2010, 15, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Carreon, T.A.; Castellanos, A.; Gasull, X.; Bhattacharya, S.K. Interaction of Cochlin and Mechanosensitive Channel TREK-1 in Trabecular Meshwork Cells Influences the Regulation of Intraocular Pressure. Sci. Rep. 2017, 7, 452. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Okano, T.; Ma, X.; Adelstein, R.S.; Kelley, M.W. Myosin II Regulates Extension, Growth and Patterning in the Mammalian Cochlear Duct. Development 2009, 136, 1977–1986. [Google Scholar] [CrossRef]
- Cirilo, J.A.; Gunther, L.K.; Yengo, C.M. Functional Role of Class III Myosins in Hair Cells. Front. Cell Dev. Biol. 2021, 9, 643856. [Google Scholar] [CrossRef] [PubMed]
- Calábria, L.K.; Vieira da Costa, A.; da Silva Oliveira, R.J.; Ramos Deconte, S.; Nascimento, R.; de Carvalho, W.J.; de Oliveira, V.N.; Arcaro Filho, C.A.; Rezende Alves de Oliveira, L.; Goulart, L.R.; et al. Myosins Are Differentially Expressed under Oxidative Stress in Chronic Streptozotocin-Induced Diabetic Rat Brains. ISRN Neurosci. 2013, 2013, 423931. [Google Scholar] [CrossRef]
- Masaki, K.; Gu, J.W.; Ghaffari, R.; Chan, G.; Smith, R.J.H.; Freeman, D.M.; Aranyosi, A.J. Col11a2 Deletion Reveals the Molecular Basis for Tectorial Membrane Mechanical Anisotropy. Biophys. J. 2009, 96, 4717–4724. [Google Scholar] [CrossRef]
- Astuto, L.M.; Bork, J.M.; Weston, M.D.; Askew, J.W.; Fields, R.R.; Orten, D.J.; Ohliger, S.J.; Riazuddin, S.; Morell, R.J.; Khan, S.; et al. CDH23 Mutation and Phenotype Heterogeneity: A Profile of 107 Diverse Families with Usher Syndrome and Nonsyndromic Deafness. Am. J. Hum. Genet. 2002, 71, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Jaiganesh, A.; Narui, Y.; Araya-Secchi, R.; Sotomayor, M. Beyond Cell-Cell Adhesion: Sensational Cadherins for Hearing and Balance. Cold Spring Harb. Perspect. Biol. 2018, 10, a029280. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xie, B.-L.; Dong, X.; Wang, L.; Zhu, G.; Wang, T.; Wu, W.; Lai, R.; Tao, R.; Guan, M.; et al. Cdh23 Affects Congenital Hearing Loss through Regulating Purine Metabolism. Front. Mol. Neurosci. 2023, 16, 1079529. [Google Scholar] [CrossRef] [PubMed]
- Savio, L.E.B.; Leite-Aguiar, R.; Alves, V.S.; Coutinho-Silva, R.; Wyse, A.T.S. Purinergic Signaling in the Modulation of Redox Biology. Redox Biol. 2021, 47, 102137. [Google Scholar] [CrossRef] [PubMed]
- Verpy, E.; Leibovici, M.; Michalski, N.; Goodyear, R.J.; Houdon, C.; Weil, D.; Richardson, G.P.; Petit, C. Stereocilin Connects Outer Hair Cell Stereocilia to One Another and to the Tectorial Membrane. J. Comp. Neurol. 2011, 519, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Kitajiri, S.; Sakamoto, T.; Belyantseva, I.A.; Goodyear, R.J.; Stepanyan, R.; Fujiwara, I.; Bird, J.E.; Riazuddin, S.; Riazuddin, S.; Ahmed, Z.M.; et al. Actin-Bundling Protein TRIOBP Forms Resilient Rootlets of Hair Cell Stereocilia Essential for Hearing. Cell 2010, 141, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Terman, J.R.; González-Billault, C.; Ahmed, G. Actin Filaments—A Target for Redox Regulation. Cytoskeleton 2016, 73, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Milzani, A.; DalleDonne, I.; Colombo, R. Prolonged Oxidative Stress on Actin. Arch. Biochem. Biophys. 1997, 339, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Lassing, I.; Schmitzberger, F.; Björnstedt, M.; Holmgren, A.; Nordlund, P.; Schutt, C.E.; Lindberg, U. Molecular and Structural Basis for Redox Regulation of β-Actin. J. Mol. Biol. 2007, 370, 331–348. [Google Scholar] [CrossRef]
- Andrade, L.R. Evidence for Changes in Beta- and Gamma-Actin Proportions during Inner Ear Hair Cell Life. Cytoskeleton 2015, 72, 282–291. [Google Scholar] [CrossRef]
- Patrinostro, X.; Roy, P.; Lindsay, A.; Chamberlain, C.M.; Sundby, L.J.; Starker, C.G.; Voytas, D.F.; Ervasti, J.M.; Perrin, B.J. Essential Nucleotide- and Protein-Dependent Functions of Actb/β-Actin. Proc. Natl. Acad. Sci. USA 2018, 115, 7973–7978. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Du, H.; Ren, R.; Du, T.; Lin, L.; Feng, Z.; Zhao, D.; Wei, X.; Zhai, X.; Wang, H.; et al. Temporal and Spatial Assembly of Inner Ear Hair Cell Ankle Link Condensate through Phase Separation. Nat. Commun. 2023, 14, 1657. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Mathur, P.D.; Zheng, T.; Wang, Y.; Almishaal, A.; Park, A.H.; Yang, J. Individual USH2 Proteins Make Distinct Contributions to the Ankle Link Complex during Development of the Mouse Cochlear Stereociliary Bundle. Hum. Mol. Genet. 2015, 24, 6944–6957. [Google Scholar] [CrossRef] [PubMed]
- Verselis, V.K. Connexin Hemichannels and Cochlear Function. Neurosci. Lett. 2019, 695, 40–45. [Google Scholar] [CrossRef]
- Ramachandran, S.; Xie, L.-H.; John, S.A.; Subramaniam, S.; Lal, R. A Novel Role for Connexin Hemichannel in Oxidative Stress and Smoking-Induced Cell Injury. PLoS ONE 2007, 2, e712. [Google Scholar] [CrossRef] [PubMed]
- Chiereghin, C.; Robusto, M.; Massa, V.; Castorina, P.; Ambrosetti, U.; Asselta, R.; Soldà, G. Role of Cytoskeletal Diaphanous-Related Formins in Hearing Loss. Cells 2022, 11, 1726. [Google Scholar] [CrossRef]
- Gao, Y.; Yechikov, S.; Vázquez, A.E.; Chen, D.; Nie, L. Impaired Surface Expression and Conductance of the KCNQ 4 Channel Lead to Sensorineural Hearing Loss. J. Cell. Mol. Medi 2013, 17, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Jiang, X.; Chai, R.; Liu, D. The Roles of Solute Carriers in Auditory Function. Front. Genet. 2022, 13, 823049. [Google Scholar] [CrossRef] [PubMed]
- Kurima, K.; Ebrahim, S.; Pan, B.; Sedlacek, M.; Sengupta, P.; Millis, B.A.; Cui, R.; Nakanishi, H.; Fujikawa, T.; Kawashima, Y.; et al. TMC1 and TMC2 Localize at the Site of Mechanotransduction in Mammalian Inner Ear Hair Cell Stereocilia. Cell Rep. 2015, 12, 1606–1617. [Google Scholar] [CrossRef]
- Fettiplace, R.; Furness, D.N.; Beurg, M. The Conductance and Organization of the TMC1-Containing Mechanotransducer Channel Complex in Auditory Hair Cells. Proc. Natl. Acad. Sci. USA 2022, 119, e2210849119. [Google Scholar] [CrossRef]
- Royaux, I.E.; Belyantseva, I.A.; Wu, T.; Kachar, B.; Everett, L.A.; Marcus, D.C.; Green, E.D. Localization and Functional Studies of Pendrin in the Mouse Inner Ear Provide Insight about the Etiology of Deafness in Pendred Syndrome. J. Assoc. Res. Otolaryngol. 2003, 4, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Spinelli, S.; Pusch, M.; Sarikas, A.; Morabito, R.; Marino, A.; Dossena, S. Role of SLC4 and SLC26 Solute Carriers during Oxidative Stress. Acta Physiol. 2022, 235, e13796. [Google Scholar] [CrossRef]
- Kamiński, K.; Kazimierczak, U.; Kolenda, T. Oxidative Stress in Melanogenesis and Melanoma Development. Contemp. Oncol. 2022, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.-C.; Alex, A.L.; Nie, J.; Lee, J.; Roth, A.A.; Booth, K.T.; Koehler, K.R.; Hashino, E.; Nelson, R.F. Defective Tmprss3-Associated Hair Cell Degeneration in Inner Ear Organoids. Stem Cell Rep. 2019, 13, 147–162. [Google Scholar] [CrossRef]
- Delmaghani, S.; Defourny, J.; Aghaie, A.; Beurg, M.; Dulon, D.; Thelen, N.; Perfettini, I.; Zelles, T.; Aller, M.; Meyer, A.; et al. Hypervulnerability to Sound Exposure through Impaired Adaptive Proliferation of Peroxisomes. Cell 2015, 163, 894–906. [Google Scholar] [CrossRef]
- Defourny, J.; Aghaie, A.; Perfettini, I.; Avan, P.; Delmaghani, S.; Petit, C. Pejvakin-Mediated Pexophagy Protects Auditory Hair Cells against Noise-Induced Damage. Proc. Natl. Acad. Sci. USA 2019, 116, 8010–8017. [Google Scholar] [CrossRef] [PubMed]
- Dallos, P.; Fakler, B. Prestin, a New Type of Motor Protein. Nat. Rev. Mol. Cell Biol. 2002, 3, 104–111. [Google Scholar] [CrossRef]
- Luo, X.; Xia, Y.; Li, X.-D.; Wang, J.-Y. The Effect of AP-2δ on Transcription of the Prestin Gene in HEI-OC1 Cells upon Oxidative Stress. Cell Mol. Biol. Lett. 2019, 24, 45. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Liang, X.; Llongueras, J.P.; Cunningham, C.; Müller, U. The Tetraspan LHFPL5 Is Critical to Establish Maximal Force Sensitivity of the Mechanotransduction Channel of Cochlear Hair Cells. Cell Rep. 2023, 42, 112245. [Google Scholar] [CrossRef]
- Erickson, T.; Pacentine, I.V.; Venuto, A.; Clemens, R.; Nicolson, T. The Lhfpl5 Ohnologs Lhfpl5a and Lhfpl5b Are Required for Mechanotransduction in Distinct Populations of Sensory Hair Cells in Zebrafish. Front. Mol. Neurosci. 2020, 12, 320. [Google Scholar] [CrossRef]
- Trouillet, A.; Miller, K.K.; George, S.S.; Wang, P.; Ali, N.-E.-S.; Ricci, A.; Grillet, N. Loxhd1 Mutations Cause Mechanotransduction Defects in Cochlear Hair Cells. J. Neurosci. 2021, 41, 3331–3343. [Google Scholar] [CrossRef] [PubMed]
- Grillet, N.; Schwander, M.; Hildebrand, M.S.; Sczaniecka, A.; Kolatkar, A.; Velasco, J.; Webster, J.A.; Kahrizi, K.; Najmabadi, H.; Kimberling, W.J.; et al. Mutations in LOXHD1, an Evolutionarily Conserved Stereociliary Protein, Disrupt Hair Cell Function in Mice and Cause Progressive Hearing Loss in Humans. Am. J. Hum. Genet. 2009, 85, 328–337. [Google Scholar] [CrossRef]
- Sırmacı, A.; Erbek, S.; Price, J.; Huang, M.; Duman, D.; Cengiz, F.B.; Bademci, G.; Tokgöz-Yılmaz, S.; Hişmi, B.; Özdağ, H.; et al. A Truncating Mutation in SERPINB6 Is Associated with Autosomal-Recessive Nonsyndromic Sensorineural Hearing Loss. Am. J. Hum. Genet. 2010, 86, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Pivtoraiko, V.N.; Stone, S.L.; Roth, K.A.; Shacka, J.J. Oxidative Stress and Autophagy in the Regulation of Lysosome-Dependent Neuron Death. Antioxid. Redox Signal 2009, 11, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Oestreicher, D.; Picher, M.M.; Rankovic, V.; Moser, T.; Pangrsic, T. Cabp2-Gene Therapy Restores Inner Hair Cell Calcium Currents and Improves Hearing in a DFNB93 Mouse Model. Front. Mol. Neurosci. 2021, 14, 689415. [Google Scholar] [CrossRef]
- Picher, M.M.; Gehrt, A.; Meese, S.; Ivanovic, A.; Predoehl, F.; Jung, S.; Schrauwen, I.; Dragonetti, A.G.; Colombo, R.; Van Camp, G.; et al. Ca2+ -Binding Protein 2 Inhibits Ca2+ -Channel Inactivation in Mouse Inner Hair Cells. Proc. Natl. Acad. Sci. USA 2017, 114, E1717–E1726. [Google Scholar] [CrossRef] [PubMed]
- Nishinaka, Y.; Masutani, H.; Nakamura, H.; Yodoi, J. Regulatory Roles of Thioredoxin in Oxidative Stress-Induced Cellular Responses. Redox Rep. 2001, 6, 289–295. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The Thioredoxin Antioxidant System. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Mittal, R.; Patel, K.; Mittal, J.; Chan, B.; Yan, D.; Grati, M.; Liu, X.Z. Association of PRPS1 Mutations with Disease Phenotypes. Dis. Markers 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Simmonds, H.; Webster, D.; Lingam, S.; Wilson, J. An Inborn Error of Purine Metabolism, Deafness and Neurodevelopmental Abnormality. Neuropediatrics 1985, 16, 106–108. [Google Scholar] [CrossRef]
- Bernardinelli, E.; Roesch, S.; Simoni, E.; Marino, A.; Rasp, G.; Astolfi, L.; Sarikas, A.; Dossena, S. Novel POU3F4 Variants Identified in Patients with Inner Ear Malformations Exhibit Aberrant Cellular Distribution and Lack of SLC6A20 Transcriptional Upregulation. Front. Mol. Neurosci. 2022, 15, 999833. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Jiapaer, Z.; Weng, R.; Hui, Y.; Jia, W.; Xi, J.; Wang, G.; Zhu, S.; Zhang, X.; Feng, D.; et al. Dysregulation of the SIRT1/OCT6 Axis Contributes to Environmental Stress-Induced Neural Induction Defects. Stem Cell Rep. 2017, 8, 1270–1286. [Google Scholar] [CrossRef] [PubMed]
- Wayne, S. Mutations in the Transcriptional Activator EYA4 Cause Late-Onset Deafness at the DFNA10 Locus. Hum. Mol. Genet. 2001, 10, 195–200. [Google Scholar] [CrossRef]
- Wang, L.; Sewell, W.F.; Kim, S.D.; Shin, J.T.; MacRae, C.A.; Zon, L.I.; Seidman, J.G.; Seidman, C.E. Eya4 Regulation of Na+/K+-ATPase Is Required for Sensory System Development in Zebrafish. Development 2008, 135, 3425–3434. [Google Scholar] [CrossRef] [PubMed]
- de la Peña Avalos, B.; Paquet, N.; Tropée, R.; Coulombe, Y.; Palacios, H.; Leung, J.W.; Masson, J.-Y.; Duijf, P.H.G.; Dray, E. The Protein Phosphatase EYA4 Promotes Homologous Recombination (HR) through Dephosphorylation of Tyrosine 315 on RAD51. Nucleic Acids Res. 2024, 52, 1173–1187. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, B. Murine GRXCR1 Has a Different Function Than GRXCR2 in the Morphogenesis of Stereocilia. Front. Cell. Neurosci. 2021, 15, 714070. [Google Scholar] [CrossRef]
- Collin, R.W.J.; Kalay, E.; Tariq, M.; Peters, T.; Van Der Zwaag, B.; Venselaar, H.; Oostrik, J.; Lee, K.; Ahmed, Z.M.; Çaylan, R.; et al. Mutations of ESRRB Encoding Estrogen-Related Receptor Beta Cause Autosomal-Recessive Nonsyndromic Hearing Impairment DFNB35. Am. J. Hum. Genet. 2008, 82, 125–138. [Google Scholar] [CrossRef][Green Version]
- Zhou, W.; Lo, S.-C.; Liu, J.-H.; Hannink, M.; Lubahn, D.B. ERRβ: A Potent Inhibitor of Nrf2 Transcriptional Activity. Mol. Cell. Endocrinol. 2007, 278, 52–62. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/Small Maf Heterodimer Mediates the Induction of Phase II Detoxifying Enzyme Genes through Antioxidant Response Elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Chanas, S.A.; Jiang, Q.; McMAHON, M.; McWALTER, G.K.; McLELLAN, L.I.; Elcombe, C.R.; Henderson, C.J.; Wolf, C.R.; Moffat, G.J.; Itoh, K.; et al. Loss of the Nrf2 Transcription Factor Causes a Marked Reduction in Constitutive and Inducible Expression of the Glutathione S-Transferase Gsta1, Gsta2, Gstm1, Gstm2, Gstm3 and Gstm4 Genes in the Livers of Male and Female Mice. Biochem. J. 2002, 365, 405–416. [Google Scholar] [CrossRef]
- Shibata, S.; Miwa, T.; Wu, H.-H.; Levitt, P.; Ohyama, T. Hepatocyte Growth Factor-c-MET Signaling Mediates the Development of Nonsensory Structures of the Mammalian Cochlea and Hearing. J. Neurosci. 2016, 36, 8200–8209. [Google Scholar] [CrossRef]
- Shimizu, K.; Taniyama, Y.; Sanada, F.; Azuma, J.; Iwabayashi, M.; Iekushi, K.; Rakugi, H.; Morishita, R. Hepatocyte Growth Factor Inhibits Lipopolysaccharide-Induced Oxidative Stress via Epithelial Growth Factor Receptor Degradation. ATVB 2012, 32, 2687–2693. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Yaung, J.; Kannan, R.; He, S.; Ryan, S.J.; Hinton, D.R. Hepatocyte Growth Factor Protects RPE Cells from Apoptosis Induced by Glutathione Depletion. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4311. [Google Scholar] [CrossRef] [PubMed]
- Goodyear, R.J.; Jones, S.M.; Sharifi, L.; Forge, A.; Richardson, G.P. Hair Bundle Defects and Loss of Function in the Vestibular End Organs of Mice Lacking the Receptor-Like Inositol Lipid Phosphatase PTPRQ. J. Neurosci. 2012, 32, 2762–2772. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Wang, X.; Hu, J.; Lian, X.; Zhu, T.; Zhang, H.; Gu, N.; Li, J. PTPRO Promotes Oxidized Low-Density Lipoprotein Induced Oxidative Stress and Cell Apoptosis through Toll-Like Receptor 4/Nuclear Factor κB Pathway. Cell Physiol. Biochem. 2017, 42, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.L.; Shin, J.-B. Mechanisms of Hair Cell Damage and Repair. Trends Neurosci. 2019, 42, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Assad, J.A.; Shepherd, G.M.G.; Corey, D.P. Tip-Link Integrity and Mechanical Transduction in Vertebrate Hair Cells. Neuron 1991, 7, 985–994. [Google Scholar] [CrossRef]
- Liberman, M.C. Chronic Ultrastructural Changes in Acoustic Trauma: Serial-Section Reconstruction of Stereocilia and Cuticular Plates. Hear. Res. 1987, 26, 65–88. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Yang, S.; Guo, W.; He, D.Z.Z. Fate of Mammalian Cochlear Hair Cells and Stereocilia after Loss of the Stereocilia. J. Neurosci. 2009, 29, 15277–15285. [Google Scholar] [CrossRef]
- Indzhykulian, A.A.; Stepanyan, R.; Nelina, A.; Spinelli, K.J.; Ahmed, Z.M.; Belyantseva, I.A.; Friedman, T.B.; Barr-Gillespie, P.G.; Frolenkov, G.I. Molecular Remodeling of Tip Links Underlies Mechanosensory Regeneration in Auditory Hair Cells. PLoS Biol. 2013, 11, e1001583. [Google Scholar] [CrossRef]
- Fridberger, A.; Flock, A.; Ulfendahl, M.; Flock, B. Acoustic Overstimulation Increases Outer Hair Cell Ca2+ Concentrations and Causes Dynamic Contractions of the Hearing Organ. Proc. Natl. Acad. Sci. USA 1998, 95, 7127–7132. [Google Scholar] [CrossRef]
- Waqas, M.; Gao, S.; Ali, M.K.; Ma, Y.; Li, W. Inner Ear Hair Cell Protection in Mammals against the Noise-Induced Cochlear Damage. Neural Plast. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Nordmann, A.S.; Bohne, B.A.; Harding, G.W. Histopathological Differences between Temporary and Permanent Threshold Shift. Hear. Res. 2000, 139, 13–30. [Google Scholar] [CrossRef]
- Saunders, J.C.; Flock, Å. Recovery of Threshold Shift in Hair-Cell Stereocilia Following Exposure to Intense Stimulation. Hear. Res. 1986, 23, 233–243. [Google Scholar] [CrossRef]
- Henderson, D.; Bielefeld, E.C.; Harris, K.C.; Hu, B.H. The Role of Oxidative Stress in Noise-Induced Hearing Loss. Ear Hear. 2006, 27, 1–19. [Google Scholar] [CrossRef]
- Kma, L.; Baruah, T.J. The Interplay of ROS and the PI3K/Akt Pathway in Autophagy Regulation. Biotech. App Biochem. 2022, 69, 248–264. [Google Scholar] [CrossRef]
- Agostini, F.; Bisaglia, M.; Plotegher, N. Linking ROS Levels to Autophagy: The Key Role of AMPK. Antioxidants 2023, 12, 1406. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, Z.; Wang, P.; He, S.; He, Y. Autophagy: A Novel Horizon for Hair Cell Protection. Neural Plast. 2021, 2021, 5511010. [Google Scholar] [CrossRef]
- Ohinata, Y.; Miller, J.M.; Schacht, J. Protection from Noise-Induced Lipid Peroxidation and Hair Cell Loss in the Cochlea. Brain Res. 2003, 966, 265–273. [Google Scholar] [CrossRef]
- Rouyère, C.; Serrano, T.; Frémont, S.; Echard, A. Oxidation and Reduction of Actin: Origin, Impact in Vitro and Functional Consequences in Vivo. Eur. J. Cell Biol. 2022, 101, 151249. [Google Scholar] [CrossRef]
- Palma, F.R.; Gantner, B.N.; Sakiyama, M.J.; Kayzuka, C.; Shukla, S.; Lacchini, R.; Cunniff, B.; Bonini, M.G. ROS Production by Mitochondria: Function or Dysfunction? Oncogene 2024, 43, 295–303. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Heinrich, U.-R.; Maurer, J.; Mann, W. Ultrastructural Evidence for Protection of the Outer Hair Cells of the Inner Ear during Intense Noise Exposure by Application of the Organic Calcium Channel Blocker Diltiazem. ORL 1999, 61, 321–327. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, B.; Shin, J.-H.; Lei, D.; Du, Y.; Gao, X.; Wang, Q.; Ohlemiller, K.K.; Piccirillo, J.; Bao, J. Prophylactic and Therapeutic Functions of T-Type Calcium Blockers against Noise-Induced Hearing Loss. Hear. Res. 2007, 226, 52–60. [Google Scholar] [CrossRef]
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of Cell Death: The Calcium-Apoptosis Link. Nat. Rev. Mol. Cell Biol. 2003, 4, 552–565. [Google Scholar] [CrossRef]
- Tretter, L.; Adam-Vizi, V. Generation of Reactive Oxygen Species in the Reaction Catalyzed by α-Ketoglutarate Dehydrogenase. J. Neurosci. 2004, 24, 7771–7778. [Google Scholar] [CrossRef]
- Lamm, K.; Arnold, W. Noise-Induced Cochlear Hypoxia Is Intensity Dependent, Correlates with Hearing Loss and Precedes Reduction of Cochlear Blood Flow. Audiol. Neurootol. 1996, 1, 148–160. [Google Scholar] [CrossRef]
- Arpornchayanon, W.; Canis, M.; Suckfuell, M.; Ihler, F.; Olzowy, B.; Strieth, S. Modeling the Measurements of Cochlear Microcirculation and Hearing Function after Loud Noise. Otolaryngol. Head. Neck Surg. 2011, 145, 463–469. [Google Scholar] [CrossRef]
- Marley, R.; Harry, D.; Anand, R.; Fernando, B.; Davies, S.; Moore, K. 8-Isoprostaglandin F2 Alpha, a Product of Lipid Peroxidation, Increases Portal Pressure in Normal and Cirrhotic Rats. Gastroenterology 1997, 112, 208–213. [Google Scholar] [CrossRef]
- Ohinata, Y.; Miller, J.M.; Altschuler, R.A.; Schacht, J. Intense Noise Induces Formation of Vasoactive Lipid Peroxidation Products in the Cochlea. Brain Res. 2000, 878, 163–173. [Google Scholar] [CrossRef]
- Miller, J.M.; Brown, J.N.; Schacht, J. 8-Iso-Prostaglandin F2α, a Product of Noise Exposure, Reduces Inner Ear Blood Flow. Audiol. Neurotol. 2003, 8, 207–221. [Google Scholar] [CrossRef]
- Vlajkovic, S.M.; Lin, S.C.; Wong, A.C.Y.; Wackrow, B.; Thorne, P.R. Noise-Induced Changes in Expression Levels of NADPH Oxidases in the Cochlea. Hear. Res. 2013, 304, 145–152. [Google Scholar] [CrossRef]
- Yamane, H.; Nakai, Y.; Takayama, M.; Iguchi, H.; Nakagawa, T.; Kojima, A. Appearance of Free Radicals in the Guinea Pig Inner Ear after Noise-Induced Acoustic Trauma. Eur. Arch. Otorhinolaryngol. 1995, 252, 504–508. [Google Scholar] [CrossRef]
- Fetoni, A.R.; De Bartolo, P.; Eramo, S.L.M.; Rolesi, R.; Paciello, F.; Bergamini, C.; Fato, R.; Paludetti, G.; Petrosini, L.; Troiani, D. Noise-Induced Hearing Loss (NIHL) as a Target of Oxidative Stress-Mediated Damage: Cochlear and Cortical Responses after an Increase in Antioxidant Defense. J. Neurosci. 2013, 33, 4011–4023. [Google Scholar] [CrossRef]
- Yang, Z.-J.; Zhao, C.-L.; Liang, W.-Q.; Chen, Z.-R.; Du, Z.-D.; Gong, S.-S. ROS-Induced Oxidative Stress and Mitochondrial Dysfunction: A Possible Mechanism Responsible for Noise-Induced Ribbon Synaptic Damage. Am. J. Transl. Res. 2024, 16, 272–284. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Adding Insult to Injury: Cochlear Nerve Degeneration after “Temporary” Noise-Induced Hearing Loss. J. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef]
- Yan, W.; Liu, W.; Qi, J.; Fang, Q.; Fan, Z.; Sun, G.; Han, Y.; Zhang, D.; Xu, L.; Wang, M.; et al. A Three-Dimensional Culture System with Matrigel Promotes Purified Spiral Ganglion Neuron Survival and Function In Vitro. Mol. Neurobiol. 2018, 55, 2070–2084. [Google Scholar] [CrossRef]
- Wang, Y.; Hirose, K.; Liberman, M.C. Dynamics of Noise-Induced Cellular Injury and Repair in the Mouse Cochlea. J. Assoc. Res. Otolaryngol. 2002, 3, 248–268. [Google Scholar] [CrossRef]
- Hirose, K.; Liberman, M.C. Lateral Wall Histopathology and Endocochlear Potential in the Noise-Damaged Mouse Cochlea. JARO—J. Assoc. Res. Otolaryngol. 2003, 4, 339–352. [Google Scholar] [CrossRef]
- Spoendlin, H. Primary Structural Changes in the Organ of Corti After Acoustic Overstimulation. Acta Oto-Laryngol. 1971, 71, 166–176. [Google Scholar] [CrossRef]
- Puel, J.L.; Ruel, J.; Gervais d’Aldin, C.; Pujol, R. Excitotoxicity and Repair of Cochlear Synapses after Noise-Trauma Induced Hearing Loss. Neuroreport 1998, 9, 2109–2114. [Google Scholar] [CrossRef]
- Pujol, R.; Rebillard, G.; Puel, J.-L.; Lenoir, M.; Eybalin, M.; Recasens, M. Glutamate Neurotoxicity in the Cochlea: A Possible Consequence of Ischaemic or Anoxic Conditions Occurring in Ageing. Acta Oto-Laryngol. 1991, 111, 32–36. [Google Scholar] [CrossRef]
- Baker, K.; Staecker, H. Low Dose Oxidative Stress Induces Mitochondrial Damage in Hair Cells. Anat. Rec. 2012, 295, 1868–1876. [Google Scholar] [CrossRef]
- Bozovic, D. Active Biomechanics of Sensory Hair Bundles. Cold Spring Harb. Perspect. Med. 2019, 9, a035014. [Google Scholar] [CrossRef] [PubMed]
- Gentilin, E.; Cani, A.; Simoni, E.; Chicca, M.; Di Paolo, M.L.; Martini, A.; Astolfi, L. Hydrogen Peroxide Toxicity on Auditory Cells: An in Vitro Study. Chem.-Biol. Interact. 2021, 345, 109575. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Cheng, A.G.; Cunningham, L.L.; Rubel, E.W. Mechanisms of Hair Cell Death and Protection. Curr. Opin. Otolaryngol. Head Neck Surg. 2005, 13, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, Caspase-3 and Caspase-7 Have Distinct Roles during Intrinsic Apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Tran Ba Huy, P.; Meulemans, A.; Wassef, M.; Manuel, C.; Sterkers, O.; Amiel, C. Gentamicin Persistence in Rat Endolymph and Perilymph after a Two-Day Constant Infusion. Antimicrob. Agents Chemother. 1983, 23, 344–346. [Google Scholar] [CrossRef]
- Dulon, D.; Hiel, H.; Aurousseau, C.; Erre, J.P.; Aran, J.M. Pharmacokinetics of Gentamicin in the Sensory Hair Cells of the Organ of Corti: Rapid Uptake and Long Term Persistence. C R. Acad. Sci. III 1993, 316, 682–687. [Google Scholar]
- Aran, J.; Erre, J.; Da Costa, D.L.; Debbarh, I.; Dulon, D. Acute and Chronic Effects of Aminoglycosides on Cochlear Hair Cells. Ann. N. Y. Acad. Sci. 1999, 884, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Hashino, E.; Shero, M.; Salvi, R.J. Lysosomal Targeting and Accumulation of Aminoglycoside Antibiotics in Sensory Hair Cells. Brain Res. 1997, 777, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Priuska, E.M.; Schacht, J. Formation of Free Radicals by Gentamicin and Iron and Evidence for an Iron/Gentamicin Complex. Biochem. Pharmacol. 1995, 50, 1749–1752. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Talaska, A.E.; Schacht, J. New Developments in Aminoglycoside Therapy and Ototoxicity. Hear. Res. 2011, 281, 28–37. [Google Scholar] [CrossRef]
- Karasawa, T.; Steyger, P.S. Intracellular Mechanisms of Aminoglycoside-Induced Cytotoxicity. Integr. Biol. 2011, 3, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Ravi, R.; Somani, S.M.; Rybak, L.P. Mechanism of Cisplatin Ototoxicity: Antioxidant System. Pharmacol. Toxicol. 1995, 76, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Bánfi, B.; Malgrange, B.; Knisz, J.; Steger, K.; Dubois-Dauphin, M.; Krause, K.-H. NOX3, a Superoxide-Generating NADPH Oxidase of the Inner Ear. J. Biol. Chem. 2004, 279, 46065–46072. [Google Scholar] [CrossRef] [PubMed]
- Don Brown, R.; Penny, J.E.; Henley, C.M.; Hodges, K.B.; Kupetz, S.A.; Glenn, D.W.; Jobe, P.C. Ototoxic Drugs and Noise. In Novartis Foundation Symposia; Evered, D., Lawrenson, G., Eds.; Wiley: Hoboken, NJ, USA, 1981; Volume 85, pp. 151–171. ISBN 978-0-470-66395-0. [Google Scholar]
- Forge, A. A Tubulo-Cisternal Endoplasmic Reticulum System in the Potassium Transporting Marginal Cells of the Stria Vascularis and Effects of the Ototoxic Diuretic Ethacrynic Acid. Cell Tissue Res. 1982, 226, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodríguez, R.; Bellido Peti, J.; Palou Redorta, J.; Gómez Ruiz, J.J.; Villavicencio Mavrich, H.; García Lorenzo, J. Diuréticos del asa y ototoxicidad. Actas Urológicas Españolas 2007, 31, 1189–1192. [Google Scholar] [CrossRef]
- Ding, D.; McFadden, S.L.; Woo, J.M.; Salvi, R.J. Ethacrynic Acid Rapidly and Selectively Abolishes Blood Flow in Vessels Supplying the Lateral Wall of the Cochlea. Hear. Res. 2002, 173, 1–9. [Google Scholar] [CrossRef]
- McCabe, P.A.; Dey, F.L. XXVIII The Effect of Aspirin upon Auditory Sensitivity. Ann. Otol. Rhinol. Laryngol. 1965, 74, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.C. The Ototoxic Effect of Toluene and the Influence of Noise, Acetyl Salicylic Acid, or Genotype. A Study in Rats and Mice. Scand. Audiol. Suppl. 1993, 39, 1–40. [Google Scholar] [PubMed]
- Curhan, S.G.; Eavey, R.; Shargorodsky, J.; Curhan, G.C. Analgesic Use and the Risk of Hearing Loss in Men. Am. J. Med. 2010, 123, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.P.; Vernier, L.S.; Dallegrave, E.; Machado, M.S. The Ototoxicity of Chloroquine and Hydroxychloroquine: A Systematic Review. Int. Arch. Otorhinolaryngol. 2022, 26, e167–e177. [Google Scholar] [CrossRef]
- Walker, E.M.; Fazekas-May, M.A.; Bowen, W.R. Nephrotoxic and Ototoxic Agents. Clin. Lab. Med. 1990, 10, 323–354. [Google Scholar] [CrossRef] [PubMed]
- Komune, S.; Snow, J.B. Potentiating Effects of Cisplatin and Ethacrynic Acid in Ototoxicity. Arch. Otolaryngol.—Head Neck Surg. 1981, 107, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, C.A.; Hudson, T.E.; Rybak, L.P. The Effect of Combined Administration of Cadmium and Furosemide on Auditory Function in the Rat. Hear. Res. 1999, 129, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-D.; Chi, L.-H.; Kostyniak, P.J.; Henderson, D. Styrene Induced Alterations in Biomarkers of Exposure and Effects in the Cochlea: Mechanisms of Hearing Loss. Toxicol. Sci. 2007, 98, 167–177. [Google Scholar] [CrossRef][Green Version]
- Campo, P.; Lataye, R.; Loquet, G.; Bonnet, P. Styrene-Induced Hearing Loss: A Membrane Insult. Hear. Res. 2001, 154, 170–180. [Google Scholar] [CrossRef]
- Cruz, S.L.; Mirshahi, T.; Thomas, B.; Balster, R.L.; Woodward, J.J. Effects of the Abused Solvent Toluene on Recombinant N-Methyl-D-Aspartate and Non-N-Methyl-D-Aspartate Receptors Expressed in Xenopus Oocytes. J. Pharmacol. Exp. Ther. 1998, 286, 334–340. [Google Scholar]
- Beckstead, M.J.; Weiner, J.L.; Eger, E.I.; Gong, D.H.; Mihic, S.J. Glycine and Gamma-Aminobutyric Acid(A) Receptor Function Is Enhanced by Inhaled Drugs of Abuse. Mol. Pharmacol. 2000, 57, 1199–1205. [Google Scholar]
- Lopreato, G.F.; Phelan, R.; Borghese, C.M.; Beckstead, M.J.; Mihic, S.J. Inhaled Drugs of Abuse Enhance Serotonin-3 Receptor Function. Drug Alcohol. Depend. 2003, 70, 11–15. [Google Scholar] [CrossRef]
- Bale, A.S.; Meacham, C.A.; Benignus, V.A.; Bushnell, P.J.; Shafer, T.J. Volatile Organic Compounds Inhibit Human and Rat Neuronal Nicotinic Acetylcholine Receptors Expressed in Xenopus Oocytes. Toxicol. Appl. Pharmacol. 2005, 205, 77–88. [Google Scholar] [CrossRef][Green Version]
- Venet, T.; Rumeau, C.; Campo, P.; Rieger, B.; Thomas, A.; Cour, C. Neuronal Circuits Involved in the Middle-Ear Acoustic Reflex. Toxicol. Sci. 2011, 119, 146–155. [Google Scholar] [CrossRef]
- Maguin, K.; Campo, P.; Parietti-Winkler, C. Toluene Can Perturb the Neuronal Voltage-Dependent Ca2+ Channels Involved in the Middle-Ear Reflex. Toxicol. Sci. 2009, 107, 473–481. [Google Scholar] [CrossRef]
- Besser, R.; Krämer, G.; Thümler, R.; Bohl, J.; Gutmann, L.; Hopf, H.C. Acute Trimethyltin Limbic-cerebellar Syndrome. Neurology 1987, 37, 945. [Google Scholar] [CrossRef]
- Yamasoba, T.; Goto, Y.; Komaki, H.; Mimaki, M.; Sudo, A.; Suzuki, M. Cochlear Damage Due to Germanium-Induced Mitochondrial Dysfunction in Guinea Pigs. Neurosci. Lett. 2006, 395, 18–22. [Google Scholar] [CrossRef]
- Crofton, K.M.; Ding, D.-L.; Padich, R.; Taylor, M.; Henderson, D. Hearing Loss Following Exposure during Development to Polychlorinated Biphenyls: A Cochlear Site of Action. Hear. Res. 2000, 144, 196–204. [Google Scholar] [CrossRef]
- Safe, T.M.; Luebke, A.E. Prenatal Low Dosage Dioxin (TCDD) Exposure Impairs Cochlear Function Resulting in Auditory Neuropathy. Hear. Res. 2016, 331, 7–12. [Google Scholar] [CrossRef]
- Woolley, D.E. Toxicological and Pharmacological Studies of Visual and Auditory Potentials Evoked in the Cerebellum of the Rat. Proc. West. Pharmacol. Soc. 1968, 11, 69–73. [Google Scholar]
- Hadjab, S.; Maurel, D.; Cazals, Y.; Siaud, P. Hexachlorobenzene, a Dioxin-like Compound, Disrupts Auditory Function in Rat. Hear. Res. 2004, 191, 125–134. [Google Scholar] [CrossRef]
- Meherali, S.; Campbell, A.; Hartling, L.; Scott, S. Understanding Parents’ Experiences and Information Needs on Pediatric Acute Otitis Media: A Qualitative Study. J. Patient Exp. 2019, 6, 53–61. [Google Scholar] [CrossRef]
- Rosenfeld, R.M.; Kay, D. Natural History of Untreated Otitis Media. Laryngoscope 2003, 113, 1645–1657. [Google Scholar] [CrossRef]
- Verhoeff, M.; Van Der Veen, E.L.; Rovers, M.M.; Sanders, E.A.M.; Schilder, A.G.M. Chronic Suppurative Otitis Media: A Review. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, T.; Koçcan, E.G.; Besler, H.T.; Yilmaz, G.; Gürsel, B. The Role of Oxidants and Antioxidants in Otitis Media with Effusion in Children. Otolaryngol.—Head Neck Surg. 2004, 131, 797–803. [Google Scholar] [CrossRef]
- Sagiroglu, S.; Ates, S.; Tolun, F.; Oztarakci, H. Evaluation of Oxidative Stress and Antioxidants Effect on Turning Process Acute Otitis Media to Chronic Otitis Media with Effusion. Niger. J. Clin. Pract. 2019, 22, 375. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A Role for Mitochondria in NLRP3 Inflammasome Activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, W.; Li, W.; Zhao, Y. NLRP3 Inflammasome: Checkpoint Connecting Innate and Adaptive Immunity in Autoimmune Diseases. Front. Immunol. 2021, 12, 732933. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhai, Y.; Liang, S.; Mori, Y.; Han, R.; Sutterwala, F.S.; Qiao, L. TRPM2 Links Oxidative Stress to NLRP3 Inflammasome Activation. Nat. Commun. 2013, 4, 1611. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, S.; Wang, L.; Li, H.; Wang, Y.; Liu, H.; Wang, X.; Zhu, X.; Liu, Z.; Ye, F.; et al. Mitochondrial Dysfunction in Hearing Loss: Oxidative Stress, Autophagy and NLRP3 Inflammasome. Front. Cell Dev. Biol. 2023, 11, 1119773. [Google Scholar] [CrossRef]
- Mizushima, Y.; Fujimoto, C.; Kashio, A.; Kondo, K.; Yamasoba, T. Macrophage Recruitment, but Not Interleukin 1 Beta Activation, Enhances Noise-Induced Hearing Damage. Biochem. Biophys. Res. Commun. 2017, 493, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Paciello, F.; Fetoni, A.R.; Rolesi, R.; Wright, M.B.; Grassi, C.; Troiani, D.; Paludetti, G. Pioglitazone Represents an Effective Therapeutic Target in Preventing Oxidative/Inflammatory Cochlear Damage Induced by Noise Exposure. Front. Pharmacol. 2018, 9, 1103. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Fujioka, M.; Kanzaki, S.; Okano, H.J.; Shibata, S.; Yamashita, D.; Masuda, M.; Mihara, M.; Ohsugi, Y.; Ogawa, K.; et al. Blockade of Interleukin-6 Signaling Suppressed Cochlear Inflammatory Response and Improved Hearing Impairment in Noise-Damaged Mice Cochlea. Neurosci. Res. 2010, 66, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jędrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar] [CrossRef]
- Srivastava, S. The Mitochondrial Basis of Aging and Age-Related Disorders. Genes 2017, 8, 398. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Trist, B.G.; Hilton, J.B.; Hare, D.J.; Crouch, P.J.; Double, K.L. Superoxide Dismutase 1 in Health and Disease: How a Frontline Antioxidant Becomes Neurotoxic. Angew. Chem. Int. Ed. 2021, 60, 9215–9246. [Google Scholar] [CrossRef]
- McFadden, S.L.; Ding, D.; Reaume, A.G.; Flood, D.G.; Salvi, R.J. Age-Related Cochlear Hair Cell Loss Is Enhanced in Mice Lacking Copper/Zinc Superoxide Dismutase. Neurobiol. Aging 1999, 20, 1–8. [Google Scholar] [CrossRef]
- Keithley, E.M.; Canto, C.; Zheng, Q.Y.; Wang, X.; Fischel-Ghodsian, N.; Johnson, K.R. Cu/Zn Superoxide Dismutase and Age-Related Hearing Loss. Hear. Res. 2005, 209, 76–85. [Google Scholar] [CrossRef]
- Ohlemiller, K.K.; McFadden, S.L.; Ding, D.-L.; Lear, P.M.; Ho, Y.-S. Targeted Mutation of the Gene for Cellular Glutathione Peroxidase (Gpx1) Increases Noise-Induced Hearing Loss in Mice. JARO 2000, 1, 243–254. [Google Scholar] [CrossRef]
- Nissanka, N.; Moraes, C.T. Mitochondrial DNA Damage and Reactive Oxygen Species in Neurodegenerative Disease. FEBS Lett. 2018, 592, 728–742. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, R.; Nakahira, K.; Gu, Z. Mitochondrial DNA Mutation, Diseases, and Nutrient-Regulated Mitophagy. Annu. Rev. Nutr. 2019, 39, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.; Zuryn, S. Mitochondrial Genome (mtDNA) Mutations That Generate Reactive Oxygen Species. Antioxidants 2019, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Kujoth, G.C.; Hiona, A.; Pugh, T.D.; Someya, S.; Panzer, K.; Wohlgemuth, S.E.; Hofer, T.; Seo, A.Y.; Sullivan, R.; Jobling, W.A.; et al. Mitochondrial DNA Mutations, Oxidative Stress, and Apoptosis in Mammalian Aging. Science 2005, 309, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Zhang, A.; Zou, T.; Ding, R.; Chen, K.; Pan, Y.; Ji, P.; Ye, B.; Xiang, M. The Influence of Metabolic Syndrome on Age-Related Hearing Loss from the Perspective of Mitochondrial Dysfunction. Front. Aging Neurosci. 2022, 14, 930105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, J.; Sun, H.; Feng, G.; Gao, Z. Interactions between the Hippocampus and the Auditory Pathway. Neurobiol. Learn. Mem. 2022, 189, 107589. [Google Scholar] [CrossRef]
- Chen, A.P.F.; Malgady, J.M.; Chen, L.; Shi, K.W.; Cheng, E.; Plotkin, J.L.; Ge, S.; Xiong, Q. Nigrostriatal Dopamine Pathway Regulates Auditory Discrimination Behavior. Nat. Commun. 2022, 13, 5942. [Google Scholar] [CrossRef]
- Bonna, K.; Finc, K.; Zimmermann, M.; Bola, L.; Mostowski, P.; Szul, M.; Rutkowski, P.; Duch, W.; Marchewka, A.; Jednoróg, K.; et al. Early Deafness Leads to Re-Shaping of Functional Connectivity beyond the Auditory Cortex. Brain Imaging Behav. 2021, 15, 1469–1482. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, P.; Li, P.; Wang, Z.; Zhao, F.; Gao, Z.; Xu, L.; Luo, Y.; Fan, J.; Liu, P. Alterations in Gray Matter Volume Due to Unilateral Hearing Loss. Sci. Rep. 2016, 6, 25811. [Google Scholar] [CrossRef]
- Jafari, Z.; Kolb, B.E.; Mohajerani, M.H. Auditory Dysfunction in Parkinson’s Disease. Mov. Disord. 2020, 35, 537–550. [Google Scholar] [CrossRef]
- Eversfield, C.L.; Orton, L.D. Auditory and Visual Hallucination Prevalence in Parkinson’s Disease and Dementia with Lewy Bodies: A Systematic Review and Meta-Analysis. Psychol. Med. 2019, 49, 2342–2353. [Google Scholar] [CrossRef] [PubMed]
- Golden, H.L.; Nicholas, J.M.; Yong, K.X.X.; Downey, L.E.; Schott, J.M.; Mummery, C.J.; Crutch, S.J.; Warren, J.D. Auditory Spatial Processing in Alzheimer’s Disease. Brain 2015, 138, 189–202. [Google Scholar] [CrossRef]
- Anshu, P.; Jadhav, D.; Agrawal, S.; Durge, V.; Jain, N.; Ravat, S. Case Report of SOD1 ALS Presenting with Prominent Hearing Impairment. J. Neurol. Sci. 2021, 429, 118345. [Google Scholar] [CrossRef]
- Wang, H.-F.; Zhang, W.; Rolls, E.T.; Li, Y.; Wang, L.; Ma, Y.-H.; Kang, J.; Feng, J.; Yu, J.-T.; Cheng, W. Hearing Impairment Is Associated with Cognitive Decline, Brain Atrophy and Tau Pathology. eBioMedicine 2022, 86, 104336. [Google Scholar] [CrossRef]
- Rutherford, B.R.; Brewster, K.; Golub, J.S.; Kim, A.H.; Roose, S.P. Sensation and Psychiatry: Linking Age-Related Hearing Loss to Late-Life Depression and Cognitive Decline. AJP 2018, 175, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Linszen, M.M.J.; Brouwer, R.M.; Heringa, S.M.; Sommer, I.E. Increased Risk of Psychosis in Patients with Hearing Impairment: Review and Meta-Analyses. Neurosci. Biobehav. Rev. 2016, 62, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kadiiska, M.B.; Basu, S.; Brot, N.; Cooper, C.; Saari Csallany, A.; Davies, M.J.; George, M.M.; Murray, D.M.; Jackson Roberts, L.; Shigenaga, M.K.; et al. Biomarkers of Oxidative Stress Study V: Ozone Exposure of Rats and Its Effect on Lipids, Proteins, and DNA in Plasma and Urine. Free Radic. Biol. Med. 2013, 61, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Pak, J.H.; Kim, Y.; Yi, J.; Chung, J.W. Antioxidant Therapy against Oxidative Damage of the Inner Ear: Protection and Preconditioning. Antioxidants 2020, 9, 1076. [Google Scholar] [CrossRef]
- Hazlitt, R.A.; Min, J.; Zuo, J. Progress in the Development of Preventative Drugs for Cisplatin-Induced Hearing Loss: Miniperspective. J. Med. Chem. 2018, 61, 5512–5524. [Google Scholar] [CrossRef]
- Molina, S.J.; Miceli, M.; Guelman, L.R. Noise Exposure and Oxidative Balance in Auditory and Extra-Auditory Structures in Adult and Developing Animals. Pharmacological Approaches Aimed to Minimize Its Effects. Pharmacol. Res. 2016, 109, 86–91. [Google Scholar] [CrossRef]
- Kopke, R.; Slade, M.D.; Jackson, R.; Hammill, T.; Fausti, S.; Lonsbury-Martin, B.; Sanderson, A.; Dreisbach, L.; Rabinowitz, P.; Torre, P.; et al. Efficacy and Safety of N-Acetylcysteine in Prevention of Noise Induced Hearing Loss: A Randomized Clinical Trial. Hear. Res. 2015, 323, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.-S.; Chung, J.-W. Ingestion of Korean Red Ginseng after Noise Exposure Can Potentiate Rapid Recovery of Hearing in Mice. J. Ginseng Res. 2010, 34, 336–341. [Google Scholar] [CrossRef]
- Cunningham, L.L.; Tucci, D.L. Hearing Loss in Adults. N. Engl. J. Med. 2017, 377, 2465–2473. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.S. The Role of Zinc in Growth and Cell Proliferation. J. Nutr. 2000, 130, 1500S–1508S. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Chung, J.W.; Pak, J.H. Zinc Is an Essential Element for the Maintenance of Redox Homeostasis and Cell Cycle in Murine Auditory Hair Cells. J. Nutr. Biochem. 2022, 100, 108901. [Google Scholar] [CrossRef]
- Kamogashira, T.; Fujimoto, C.; Yamasoba, T. Reactive Oxygen Species, Apoptosis, and Mitochondrial Dysfunction in Hearing Loss. BioMed Res. Int. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Novoa, J.M.; Quiros, Y.; Vicente, L.; Morales, A.I.; Lopez-Hernandez, F.J. New Insights into the Mechanism of Aminoglycoside Nephrotoxicity: An Integrative Point of View. Kidney Int. 2011, 79, 33–45. [Google Scholar] [CrossRef]
- Vokes, E.E. Induction Chemotherapy for Head and Neck Cancer: Recent Data. Oncologist 2010, 15, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Alexander, T.H.; Harris, J.P. Incidence of Sudden Sensorineural Hearing Loss. Otol. Neurotol. 2013, 34, 1586–1589. [Google Scholar] [CrossRef]
- Mukherjea, D.; Jajoo, S.; Sheehan, K.; Kaur, T.; Sheth, S.; Bunch, J.; Perro, C.; Rybak, L.P.; Ramkumar, V. NOX3 NADPH Oxidase Couples Transient Receptor Potential Vanilloid 1 to Signal Transducer and Activator of Transcription 1-Mediated Inflammation and Hearing Loss. Antioxid. Redox Signal. 2011, 14, 999–1010. [Google Scholar] [CrossRef]
- Baek, J.-I.; Kim, Y.-R.; Lee, K.-Y.; Kim, U.-K. Mitochondrial Redox System: A Key Target of Antioxidant Therapy to Prevent Acquired Sensorineural Hearing Loss. Front. Pharmacol. 2023, 14, 1176881. [Google Scholar] [CrossRef] [PubMed]
- Freyer, D.R.; Chen, L.; Krailo, M.D.; Knight, K.; Villaluna, D.; Bliss, B.; Pollock, B.H.; Ramdas, J.; Lange, B.; Van Hoff, D.; et al. Effects of Sodium Thiosulfate versus Observation on Development of Cisplatin-Induced Hearing Loss in Children with Cancer (ACCL0431): A Multicentre, Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol. 2017, 18, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Brock, P.R.; Maibach, R.; Childs, M.; Rajput, K.; Roebuck, D.; Sullivan, M.J.; Laithier, V.; Ronghe, M.; Dall’Igna, P.; Hiyama, E.; et al. Sodium Thiosulfate for Protection from Cisplatin-Induced Hearing Loss. N. Engl. J. Med. 2018, 378, 2376–2385. [Google Scholar] [CrossRef] [PubMed]
- Dickey, D.T.; Wu, Y.J.; Muldoon, L.L.; Neuwelt, E.A. Protection against Cisplatin-Induced Toxicities by N-Acetylcysteine and Sodium Thiosulfate as Assessed at the Molecular, Cellular, and in Vivo Levels. J. Pharmacol. Exp. Ther. 2005, 314, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Sun, Q.; Xu, F.; Jiang, N.; Gao, J. Age-Related Hearing Loss and Its Potential Drug Candidates: A Systematic Review. Chin. Med. 2023, 18, 121. [Google Scholar] [CrossRef] [PubMed]
- Pisani, A.; Paciello, F.; Montuoro, R.; Rolesi, R.; Galli, J.; Fetoni, A.R. Antioxidant Therapy as an Effective Strategy against Noise-Induced Hearing Loss: From Experimental Models to Clinic. Life 2023, 13, 1035. [Google Scholar] [CrossRef]
- Bast, A.; Haenen, G.R.M.M. The Toxicity of Antioxidants and Their Metabolites. Environ. Toxicol. Pharmacol. 2002, 11, 251–258. [Google Scholar] [CrossRef]

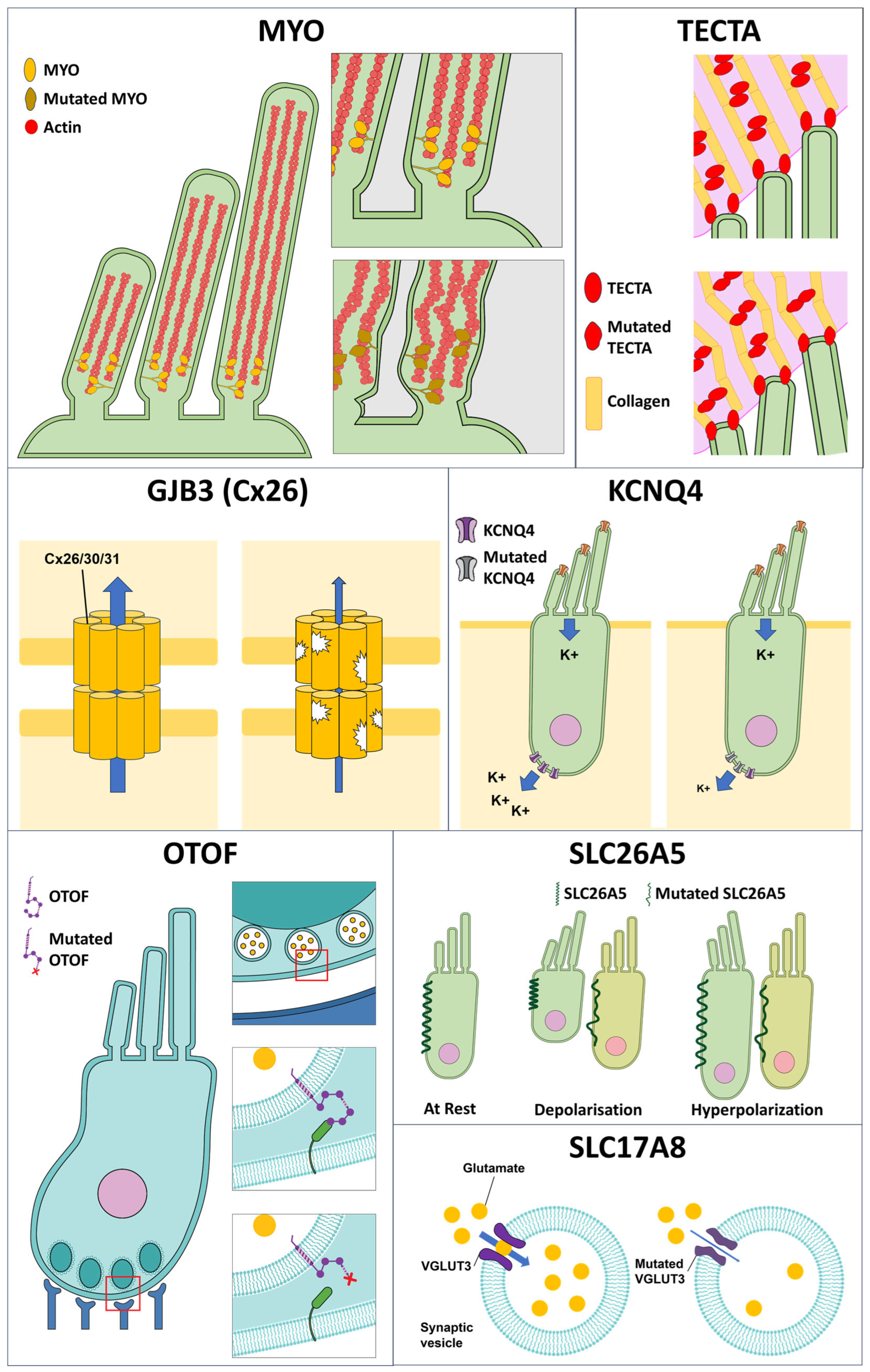

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeo, X.Y.; Kwon, S.; Rinai, K.R.; Lee, S.; Jung, S.; Park, R. A Consolidated Understanding of the Contribution of Redox Dysregulation in the Development of Hearing Impairment. Antioxidants 2024, 13, 598. https://doi.org/10.3390/antiox13050598
Yeo XY, Kwon S, Rinai KR, Lee S, Jung S, Park R. A Consolidated Understanding of the Contribution of Redox Dysregulation in the Development of Hearing Impairment. Antioxidants. 2024; 13(5):598. https://doi.org/10.3390/antiox13050598
Chicago/Turabian StyleYeo, Xin Yi, Soohyun Kwon, Kimberley R. Rinai, Sungsu Lee, Sangyong Jung, and Raekil Park. 2024. "A Consolidated Understanding of the Contribution of Redox Dysregulation in the Development of Hearing Impairment" Antioxidants 13, no. 5: 598. https://doi.org/10.3390/antiox13050598
APA StyleYeo, X. Y., Kwon, S., Rinai, K. R., Lee, S., Jung, S., & Park, R. (2024). A Consolidated Understanding of the Contribution of Redox Dysregulation in the Development of Hearing Impairment. Antioxidants, 13(5), 598. https://doi.org/10.3390/antiox13050598






