Use of Optical Redox Imaging to Quantify Alveolar Macrophage Redox State in Infants: Proof of Concept Experiments in a Murine Model and Human Tracheal Aspirates Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Alveolar Macrophage Cell Line Culture and Treatment
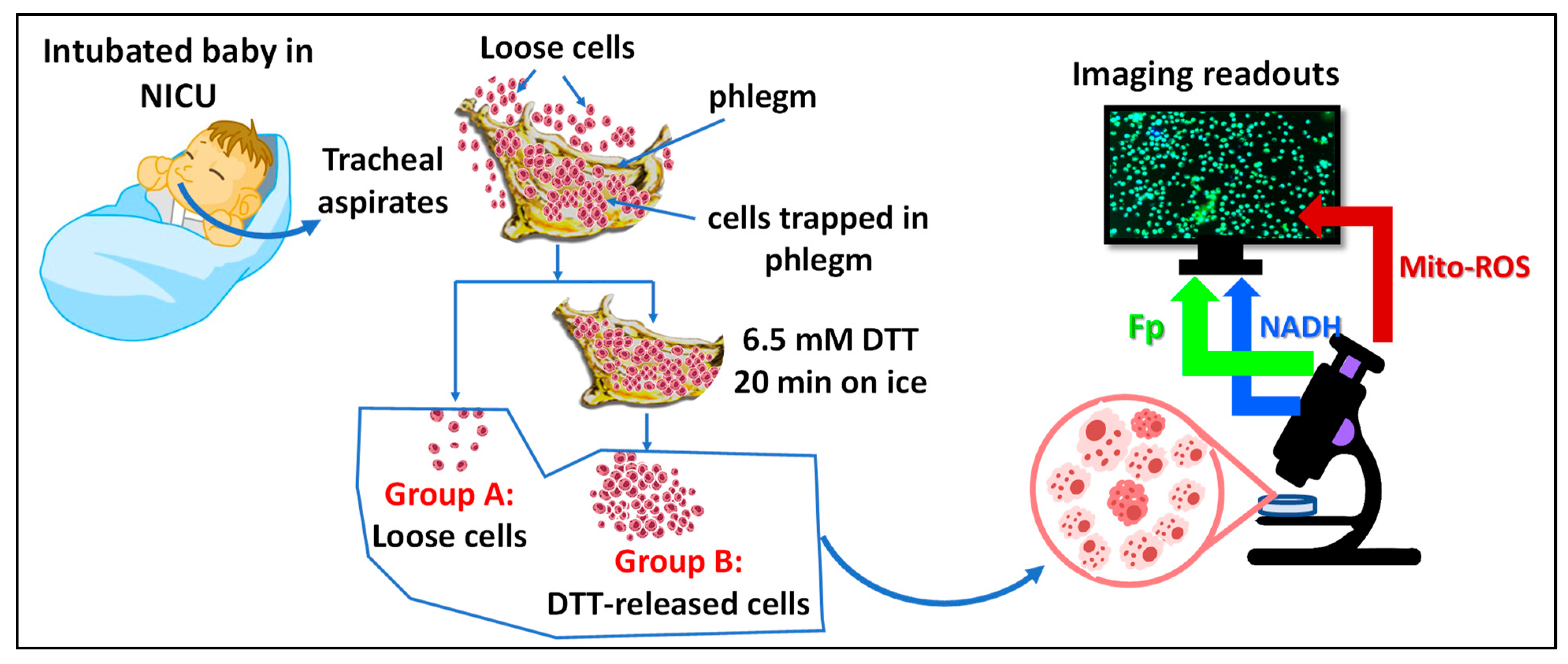
2.2. TA Sample Collection and Processing
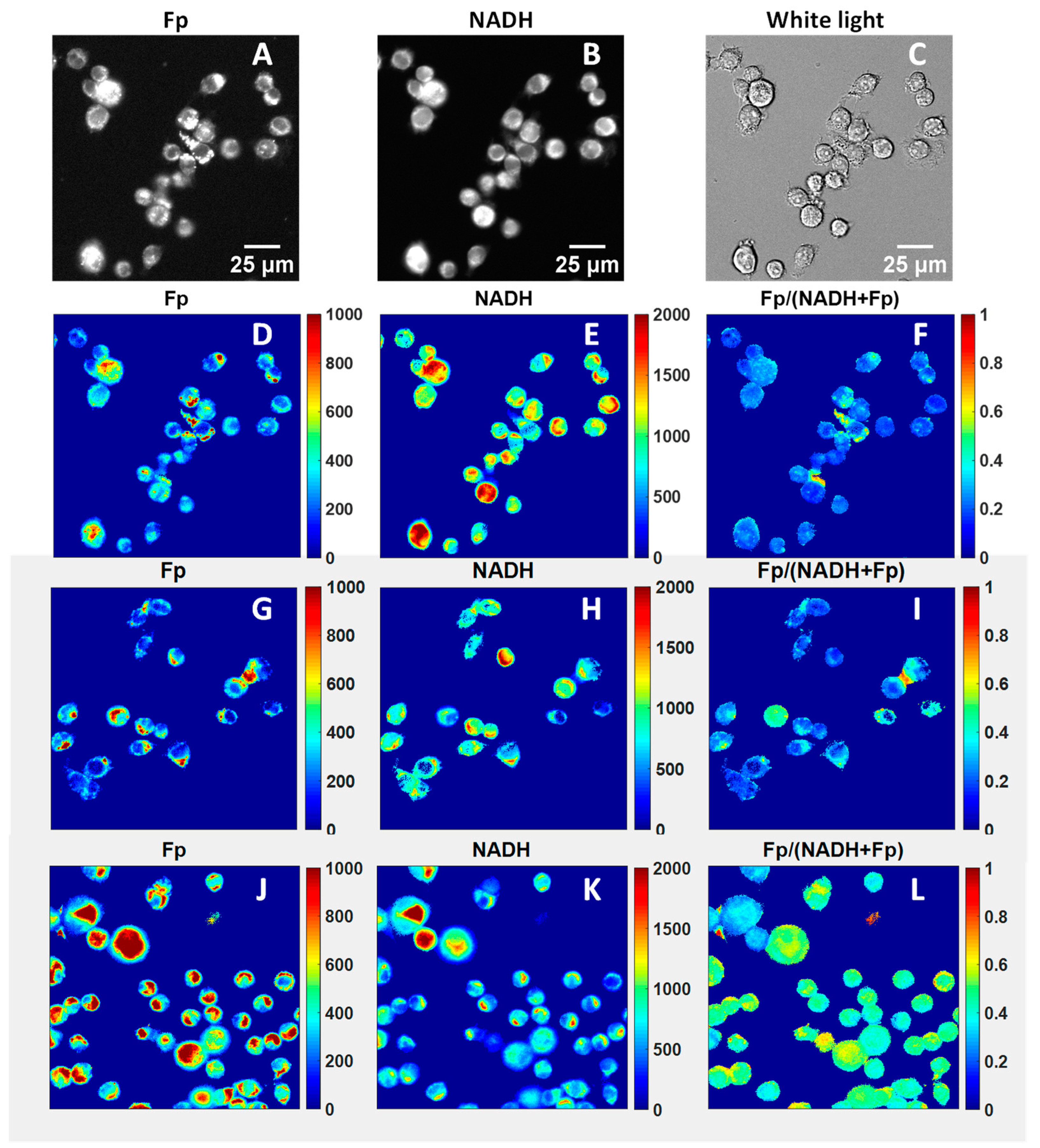
2.3. Optical Redox Imaging and Data Processing
2.4. Statistics
3. Results
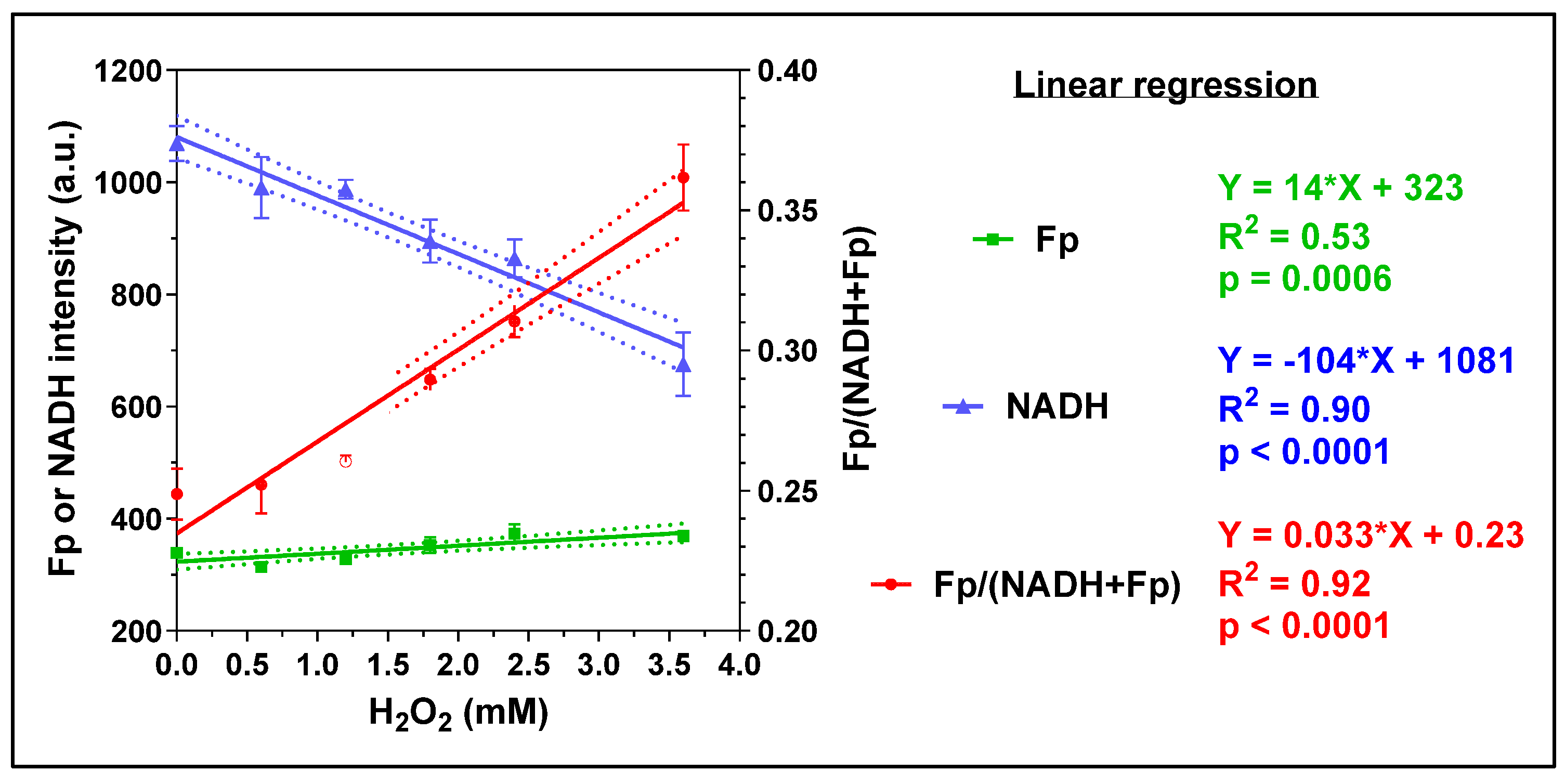
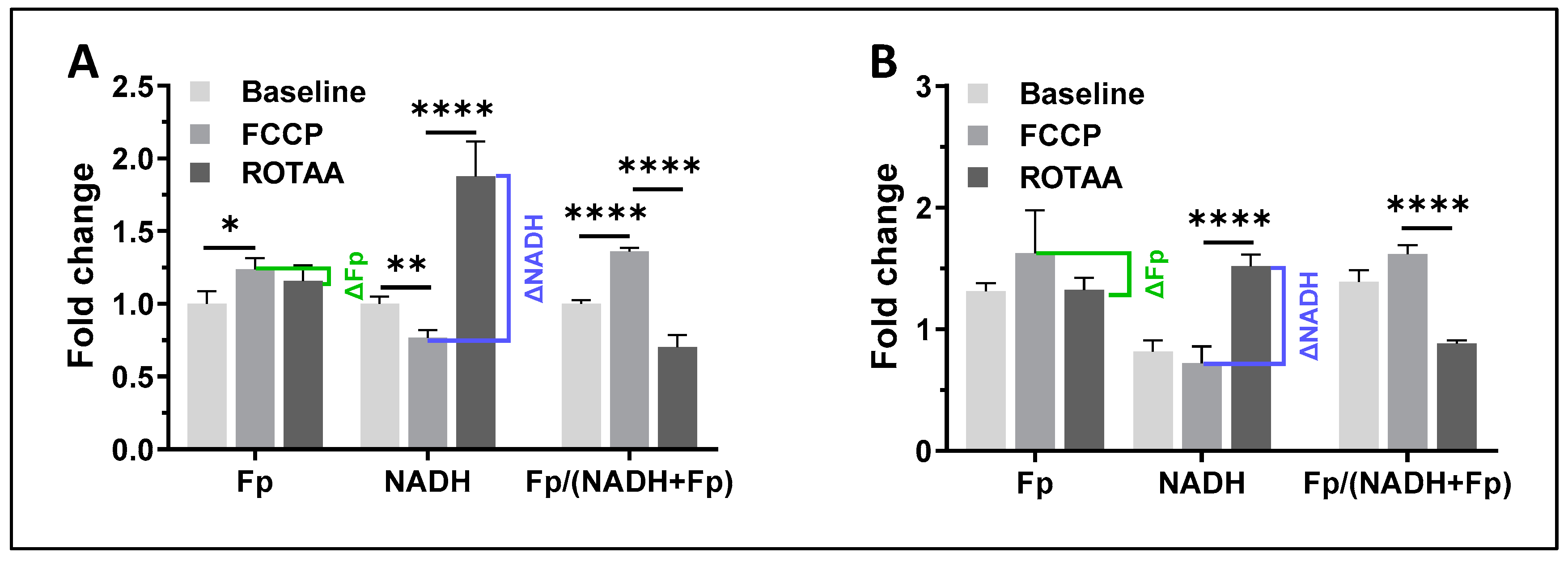
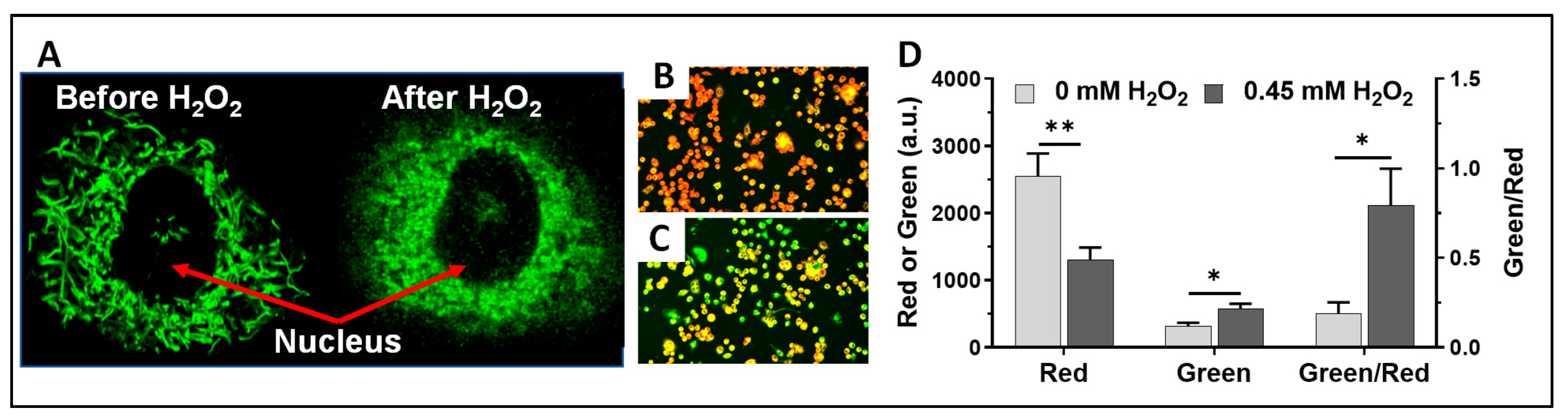
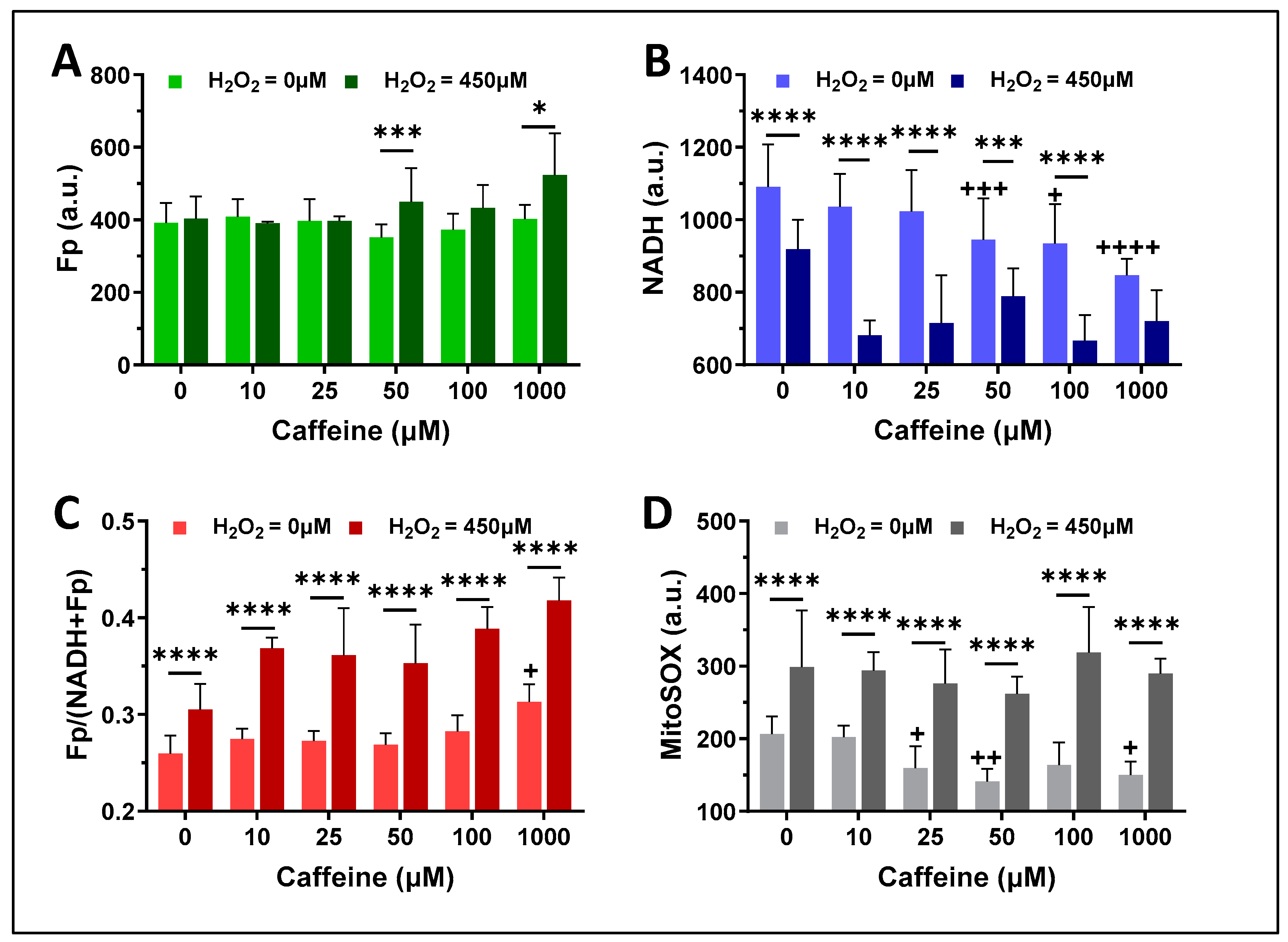
3.1. The Effects of H2O2 and Caffeine on Alveolar Macrophages In Vitro
3.1.1. Acute H2O2 Effects
3.1.2. Caffeine Effects
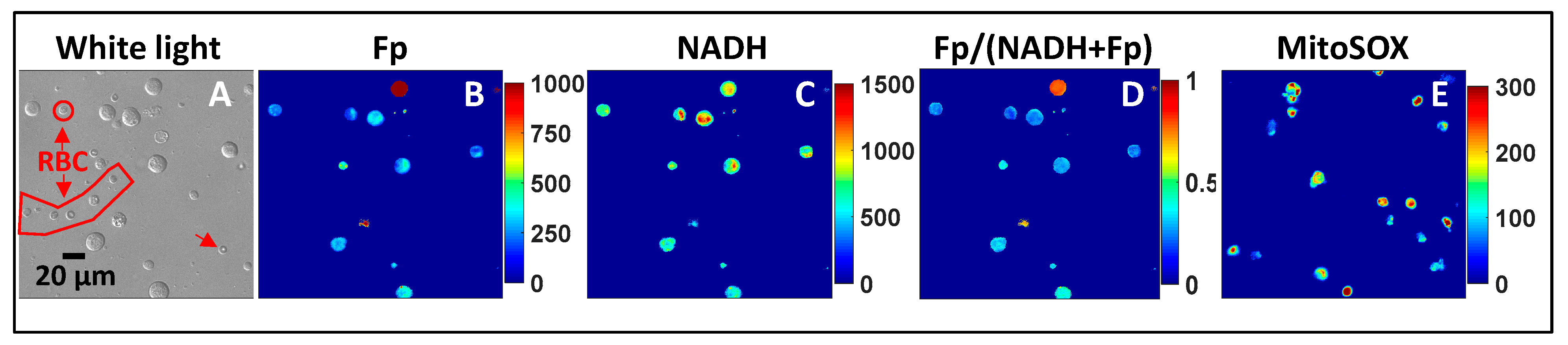
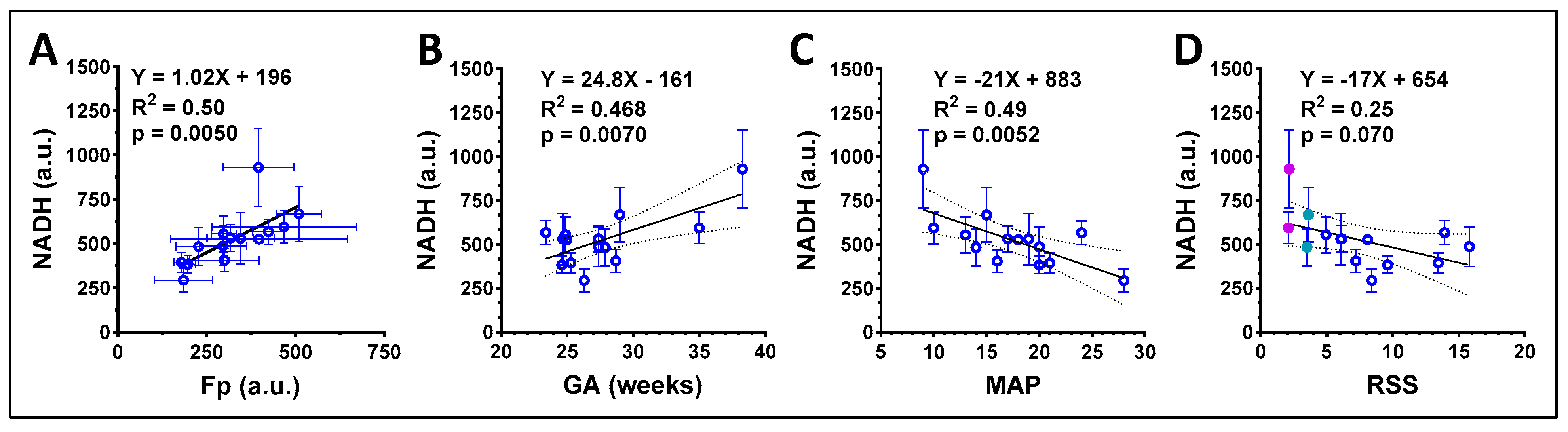
3.2. ORI of Infant Tracheal Aspirate Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osterman, M.J.K.; Hamilton, B.E.; Martin, J.A.; Driscoll, A.K.; Valenzuela, C.P. Births: Final Data for 2021; CDC: Atlanta, GA, USA, 2023; Volume 71. [Google Scholar]
- Jensen, E.A.; Edwards, E.M.; Greenberg, L.T.; Soll, R.F.; Ehret, D.E.Y.; Horbar, J.D. Severity of Bronchopulmonary Dysplasia Among Very Preterm Infants in the United States. Pediatrics 2021, 148, e2020030007. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.A.; Schmidt, B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Res. A Clin. Mol. Teratol. 2014, 100, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Thébaud, B.; Goss, K.N.; Laughon, M.; Whitsett, J.A.; Abman, S.H.; Steinhorn, R.H.; Aschner, J.L.; Davis, P.G.; McGrath-Morrow, S.A.; Soll, R.F.; et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Prim. 2019, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.A.; Dysart, K.; Gantz, M.G.; McDonald, S.; Bamat, N.A.; Keszler, M.; Kirpalani, H.; Laughon, M.M.; Poindexter, B.B.; Duncan, A.F.; et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. Am. J. Respir. Crit. Care Med. 2019, 200, 751–759. [Google Scholar] [CrossRef] [PubMed]
- U.S. Burden of Disease Collaborators. The State of US Health, 1990–2010: Burden of Diseases, Injuries, and Risk Factors. JAMA 2013, 310, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Humayun, J.; Löfqvist, C.; Ley, D.; Hellström, A.; Gyllensten, H. Systematic review of the healthcare cost of bronchopulmonary dysplasia. BMJ Open 2021, 11, e045729. [Google Scholar] [CrossRef]
- Sillers, L.; Alexiou, S.; Jensen, E.A. Lifelong pulmonary sequelae of bronchopulmonary dysplasia. Curr. Opin. Pediatr. 2020, 32, 252–260. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sánchez, P.J.; Van Meurs, K.P.; Wyckoff, M.; et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. Jama 2015, 314, 1039–1051. [Google Scholar] [CrossRef]
- Lee, S.M.; Sie, L.; Liu, J.; Profit, J.; Lee, H.C. Evaluation of trends in Bronchopulmonary Dysplasia and Respiratory Support Practice for Very Low Birth Weight Infants: A Population-Based Cohort Study. J. Pediatr. 2021, 243, 47–52.e2. [Google Scholar] [CrossRef]
- Siddaiah, R.; Oji-Mmuo, C.N.; Montes, D.T.; Fuentes, N.; Spear, D.; Donnelly, A.; Silveyra, P. MicroRNA Signatures Associated with Bronchopulmonary Dysplasia Severity in Tracheal Aspirates of Preterm Infants. Biomedicines 2021, 9, 257. [Google Scholar] [CrossRef]
- Heydarian, M.; Schulz, C.; Stoeger, T.; Hilgendorff, A. Association of immune cell recruitment and BPD development. Mol. Cell Pediatr. 2022, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Lu, X.; Dennery, P.A.; Yao, H. Metabolic dysregulation in bronchopulmonary dysplasia: Implications for identification of biomarkers and therapeutic approaches. Redox Biol. 2021, 48, 102104. [Google Scholar] [CrossRef] [PubMed]
- Milan, A.; Priante, E.; Nardo, D.; Tosato, F.; Pantano, G.; Baraldi, E.; Zaramella, P. Early Macrophage Activation in Preterm Newborns and Respiratory Disease. J. Child. Sci. 2017, 07, e110–e119. [Google Scholar] [CrossRef][Green Version]
- Sahoo, D.; Zaramela, L.S.; Hernandez, G.E.; Mai, U.; Taheri, S.; Dang, D.; Stouch, A.N.; Medal, R.M.; McCoy, A.M.; Aschner, J.L.; et al. Transcriptional profiling of lung macrophages identifies a predictive signature for inflammatory lung disease in preterm infants. Commun. Biol. 2020, 3, 259. [Google Scholar] [CrossRef] [PubMed]
- Clement, A.; Chadelat, K.; Sardet, A.; Grimfeld, A.; Tournier, G. Alveolar macrophage status in bronchopulmonary dysplasia. Pediatr. Res. 1988, 23, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Aghai, Z.H.; Kode, A.; Saslow, J.G.; Nakhla, T.; Farhath, S.; Stahl, G.E.; Eydelman, R.; Strande, L.; Leone, P.; Rahman, I. Azithromycin Suppresses Activation of Nuclear Factor-kappa B and Synthesis of Pro-inflammatory Cytokines in Tracheal Aspirate Cells From Premature Infants. Pediatr. Res. 2007, 62, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Booth, G.R.; Al-Hosni, M.; Ali, A.; Keenan, W.J. The utility of tracheal aspirate cultures in the immediate neonatal period. J. Perinatol. 2009, 29, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.K.; Rims, C.R.; Gill, S.E.; McGuire, J.K.; Manicone, A.M. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am. J. Respir. Cell Mol. Biol. 2012, 47, 417–426. [Google Scholar] [CrossRef]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Pearce, E.J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 2016, 213, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.G.; O’Neill, L.A.J. Krebs Cycle Reborn in Macrophage Immunometabolism. Annu. Rev. Immunol. 2020, 38, 289–313. [Google Scholar] [CrossRef] [PubMed]
- Michaeloudes, C.; Bhavsar, P.K.; Mumby, S.; Xu, B.; Hui, C.K.M.; Chung, K.F.; Adcock, I.M. Role of Metabolic Reprogramming in Pulmonary Innate Immunity and Its Impact on Lung Diseases. J. Innate Immun. 2020, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Thapa, B.; Lee, K. Metabolic influence on macrophage polarization and pathogenesis. BMB Rep. 2019, 52, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Langston, P.K.; Shibata, M.; Horng, T. Metabolism Supports Macrophage Activation. Front. Immunol. 2017, 8, 61. [Google Scholar] [CrossRef]
- Miskolci, V.; Tweed, K.E.; Lasarev, M.R.; Britt, E.C.; Walsh, A.J.; Zimmerman, L.J.; McDougal, C.E.; Cronan, M.R.; Fan, J.; Sauer, J.-D.; et al. In vivo fluorescence lifetime imaging of macrophage intracellular metabolism during wound responses in zebrafish. eLife 2022, 11, e66080. [Google Scholar] [CrossRef] [PubMed]
- Ghukasyan, V.V.; Heikal, A.A. Natural Biomarkers for Cellular Metabolism: Biology, Techniques, and Applications; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Heikal, A.A. Intracellular coenzymes as natural biomarkers for metabolic activities and mitochondrial anomalies. Biomark. Med. 2010, 4, 241–263. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.N.; Li, L.Z. Quantitative redox imaging biomarkers for studying tissue metabolic state and its heterogeneity. J. Innov. Opt. Health Sci. 2014, 7, 1430001–1430020. [Google Scholar] [CrossRef]
- Georgakoudi, I.; Quinn, K.P. Optical imaging using endogenous contrast to assess metabolic state. Annu. Rev. Biomed. Eng. 2012, 14, 351–367. [Google Scholar] [CrossRef]
- Chance, B.; Schoener, B.; Oshino, R.; Itshak, F.; Nakase, Y. Oxidation-reduction ratio studies of mitochondria in freeze-trapped samples. NADH and flavoprotein fluorescence signals. J. Biol. Chem. 1979, 254, 4764–4771. [Google Scholar] [CrossRef]
- Podsednik, A.; Jacob, A.; Li, L.Z.; Xu, H.N. Relationship between optical redox status and reactive oxygen species in cancer cells. React. Oxyg. Species 2020, 9, 95–108. [Google Scholar] [CrossRef]
- Cortassa, S.; O’Rourke, B.; Aon, M.A. Redox-Optimized ROS Balance and the relationship between mitochondrial respiration and ROS. Biochim. Biophys. Acta (BBA) Bioenerg. 2014, 1837, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.N.; Lin, Z.; Gandhi, C.K.; Amatya, S.; Wang, Y.; Li, L.Z.; Floros, J. Sex and SP-A2 dependent NAD(H) Redox Alterations in Mouse Alveolar Macrophages in Response to Ozone Exposure: Potential Implications for COVID-19. Antioxidants 2020, 9, 915. [Google Scholar] [CrossRef]
- Xu, H.N.; Floros, J.; Li, L.Z.; Amatya, S. Imaging NAD(H) Redox Alterations in Cryopreserved Alveolar Macrophages from Ozone-Exposed Mice and the Impact of Nutrient Starvation during Long Lag Times. Antioxidants 2021, 10, 767. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Kayton, A.; Timoney, P.; Vargo, L.; Perez, J.A. A Review of Oxygen Physiology and Appropriate Management of Oxygen Levels in Premature Neonates. Adv. Neonatal Care 2018, 18, 98–104. [Google Scholar] [CrossRef]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W. Caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2006, 354, 2112–2121. [Google Scholar] [CrossRef]
- Jensen, E.A. What is bronchopulmonary dysplasia and does caffeine prevent it? Semin. Fetal Neonatal Med. 2020, 25, 101176. [Google Scholar] [CrossRef]
- Jacob, A.; Xu, H.N.; Stout, A.L.; Li, L.Z. Subcellular analysis of nuclear and cytoplasmic redox indices differentiates breast cancer cell subtypes better than nuclear-to-cytoplasmic area ratio. J. Biomed. Opt. 2022, 27, 086001. [Google Scholar] [CrossRef]
- De la Haba, C.; Morros, A.; Martínez, P.; Palacio, J.R. LPS-Induced Macrophage Activation and Plasma Membrane Fluidity Changes are Inhibited Under Oxidative Stress. J. Membr. Biol. 2016, 249, 789–800. [Google Scholar] [CrossRef]
- Ye, M.; Zhao, Y.; Wang, Y.; Xie, R.; Tong, Y.; Sauer, J.-D.; Gong, S. NAD(H)-loaded nanoparticles for efficient sepsis therapy via modulating immune and vascular homeostasis. Nat. Nanotechnol. 2022, 17, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Schraufstatter, I.U.; Hyslop, P.A.; Hinshaw, D.B.; Spragg, R.G.; Sklar, L.A.; Cochrane, C.G. Hydrogen peroxide-induced injury of cells and its prevention by inhibitors of poly(ADP-ribose) polymerase. Proc. Natl. Acad. Sci. USA 1986, 83, 4908–4912. [Google Scholar] [CrossRef]
- Carson, D.A.; Seto, S.; Wasson, D.B. Lymphocyte dysfunction after DNA damage by toxic oxygen species. A model of immunodeficiency. J. Exp. Med. 1986, 163, 746–751. [Google Scholar] [CrossRef]
- Xie, W.; Xu, A.; Yeung, E.S. Determination of NAD+ and NADH in a Single Cell under Hydrogen Peroxide Stress by Capillary Electrophoresis. Anal. Chem. 2009, 81, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Bartolome, F.; Abramov, A.Y. Measurement of mitochondrial NADH and FAD autofluorescence in live cells. Methods Mol. Biol. 2015, 1264, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.N.; Feng, M.; Nath, K.; Nelson, D.; Roman, J.; Zhao, H.; Lin, Z.; Glickson, J.; Li, L.Z. Optical redox imaging of lonidamine treatment response of melanoma cells and xenografts. Mol. Imaging Biol. 2019, 21, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Pignatti, P.; Delmastro, M.; Perfetti, L.; Bossi, A.; Balestrino, A.; Di Stefano, A.; Pisati, P.; Balbi, B.; Moscato, G. Is dithiothreitol affecting cells and soluble mediators during sputum processing? A modified methodology to process sputum. J. Allergy Clin. Immunol. 2002, 110, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Spanevello, A.; Migliori, G.B.; Sharara, A.; Ballardini, L.; Bridge, P.; Pisati, P.; Neri, M.; Ind, P.W. Induced sputum to assess airway inflammation: A study of reproducibility. Clin. Exp. Allergy 1997, 27, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, W.; Morley, C.J.; South, M. Microscopic observations on tracheal aspirates from ventilated neonates. II. The onset of bronchopulmonary dysplasia and other changes. Eur. J. Pediatr. 1992, 151, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R.G.; McDonald, S.A.; Laughon, M.M.; Tanaka, D.; Jensen, E.; Van Meurs, K.; Eichenwald, E.; Brumbaugh, J.E.; Duncan, A.; Walsh, M.; et al. Online clinical tool to estimate risk of bronchopulmonary dysplasia in extremely preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2022, 106, 638–643. [Google Scholar] [CrossRef]
- Capasso, L.; Vento, G.; Loddo, C.; Tirone, C.; Iavarone, F.; Raimondi, F.; Dani, C.; Fanos, V. Oxidative Stress and Bronchopulmonary Dysplasia: Evidences From Microbiomics, Metabolomics, and Proteomics. Front. Pediatr. 2019, 7, 30. [Google Scholar] [CrossRef]
- Perrone, S.; Tataranno, M.L.; Buonocore, G. Oxidative stress and bronchopulmonary dysplasia. J. Clin. Neonatol. 2012, 1, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, V.J.; Rouleau, M.; Poirier, G.G. PARP-1, a determinant of cell survival in response to DNA damage. Exp. Hematol. 2003, 31, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Wang, R.-S.; Handy, D.E.; Loscalzo, J. NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism. Antioxid. Redox Signal. 2017, 28, 251–272. [Google Scholar] [CrossRef]
- Blacker, T.S.; Duchen, M.R. Investigating mitochondrial redox state using NADH and NADPH autofluorescence. Free Radic. Biol. Med. 2016, 100, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Dong, W. SIRT1-Related Signaling Pathways and Their Association With Bronchopulmonary Dysplasia. Front. Med. 2021, 8, 595634. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhou, X.; Liu, Y.; Tan, S.; Li, Y. The Role of Sirtuin-1 in Immune Response and Systemic Lupus Erythematosus. Front. Immunol. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Mody, K.; Saslow, J.G.; Kathiravan, S.; Eydelman, R.; Bhat, V.; Stahl, G.E.; Pyon, K.; Bhandari, V.; Aghai, Z.H. Sirtuin1 in tracheal aspirate leukocytes: Possible role in the development of bronchopulmonary dysplasia in premature infants. J. Matern. Fetal Neonatal Med. 2012, 25, 1483–1487. [Google Scholar] [CrossRef]
- Tan, F.; Dong, W.; Lei, X.; Liu, X.; Li, Q.; Kang, L.; Zhao, S.; Zhang, C. Attenuated SUMOylation of sirtuin 1 in premature neonates with bronchopulmonary dysplasia. Mol. Med. Rep. 2018, 17, 1283–1288. [Google Scholar] [CrossRef]
- Pollak, N.; Niere, M.; Ziegler, M. NAD Kinase Levels Control the NADPH Concentration in Human Cells*. J. Biol. Chem. 2007, 282, 33562–33571. [Google Scholar] [CrossRef]
- Owen, J.B.; Butterfield, D.A. Measurement of oxidized/reduced glutathione ratio. Methods Mol. Biol. 2010, 648, 269–277. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef]
- Lavoie, J.-C.; Tremblay, A. Sex-Specificity of Oxidative Stress in Newborns Leading to a Personalized Antioxidant Nutritive Strategy. Antioxidans 2018, 7, 49. [Google Scholar] [CrossRef]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Carnesecchi, S.; Deffert, C.; Pagano, A.; Garrido-Urbani, S.; Métrailler-Ruchonnet, I.; Schäppi, M.; Donati, Y.; Matthay, M.A.; Krause, K.-H.; Argiroffo, C.B. NADPH Oxidase-1 Plays a Crucial Role in Hyperoxia-induced Acute Lung Injury in Mice. Am. J. Respir. Crit. Care Med. 2009, 180, 972–981. [Google Scholar] [CrossRef]
- Carvalho, C.G.; Procianoy, R.S.; Neto, E.C.; Silveira, R.C. Preterm Neonates with Respiratory Distress Syndrome: Ventilator-Induced Lung Injury and Oxidative Stress. J. Immunol. Res. 2018, 2018, 6963754. [Google Scholar] [CrossRef]
- Shanmugasundaram, K.; Nayak, B.K.; Friedrichs, W.E.; Kaushik, D.; Rodriguez, R.; Block, K. NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance. Nat. Commun. 2017, 8, 997. [Google Scholar] [CrossRef] [PubMed]
- Larson-Casey, J.L.; Gu, L.; Kang, J.; Dhyani, A.; Carter, A.B. NOX4 regulates macrophage apoptosis resistance to induce fibrotic progression. J. Biol. Chem. 2021, 297, 100810. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Larson-Casey, J.L.; Davis, D.; Hanumanthu, V.S.; Longhini, A.L.F.; Thannickal, V.J.; Gu, L.; Carter, A.B. NOX4 modulates macrophage phenotype and mitochondrial biogenesis in asbestosis. JCI Insight 2019, 4, e126551. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Idol, R.A.; Yang, W.; Rojas Márquez, J.D.; Li, Y.; Huang, G.; Beatty, W.L.; Atkinson, J.J.; Brumell, J.H.; Bagaitkar, J.; et al. Macrophage NOX2 NADPH oxidase maintains alveolar homeostasis in mice. Blood 2022, 139, 2855–2870. [Google Scholar] [CrossRef]
- Menden, H.L.; Xia, S.; Mabry, S.M.; Navarro, A.; Nyp, M.F.; Sampath, V. Nicotinamide Adenine Dinucleotide Phosphate Oxidase 2 Regulates LPS-Induced Inflammation and Alveolar Remodeling in the Developing Lung. Am. J. Respir. Cell Mol. Biol. 2016, 55, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Kimble, A.; Robbins, M.E.; Perez, M. Pathogenesis of Bronchopulmonary Dysplasia: Role of Oxidative Stress from ‘Omics’ Studies. Antioxidants 2022, 11, 2380. [Google Scholar] [CrossRef] [PubMed]
- Venter, G.; Oerlemans, F.T.J.J.; Willemse, M.; Wijers, M.; Fransen, J.A.M.; Wieringa, B. NAMPT-Mediated Salvage Synthesis of NAD+ Controls Morphofunctional Changes of Macrophages. PLoS ONE 2014, 9, e97378. [Google Scholar] [CrossRef] [PubMed]
- Zaramella, P.; Munari, F.; Stocchero, M.; Molon, B.; Nardo, D.; Priante, E.; Tosato, F.; Bonadies, L.; Viola, A.; Baraldi, E. Innate immunity ascertained from blood and tracheal aspirates of preterm newborn provides new clues for assessing bronchopulmonary dysplasia. PLoS ONE 2019, 14, e0221206. [Google Scholar] [CrossRef] [PubMed]
- Piersigilli, F.; Lam, T.T.; Vernocchi, P.; Quagliariello, A.; Putignani, L.; Aghai, Z.H.; Bhandari, V. Identification of new biomarkers of bronchopulmonary dysplasia using metabolomics. Metabolomics 2019, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Hurskainen, M.; Mižíková, I.; Cook, D.P.; Andersson, N.; Cyr-Depauw, C.; Lesage, F.; Helle, E.; Renesme, L.; Jankov, R.P.; Heikinheimo, M.; et al. Single cell transcriptomic analysis of murine lung development on hyperoxia-induced damage. Nat. Commun. 2021, 12, 1565. [Google Scholar] [CrossRef]
- Xu, H.N.; Jacob, A.; Li, L.Z. Optical Redox Imaging Is Responsive to TGFβ Receptor Signalling in Triple-Negative Breast Cancer Cells. Adv. Exp. Med. Biol. 2022, 1395, 269–274. [Google Scholar] [CrossRef]



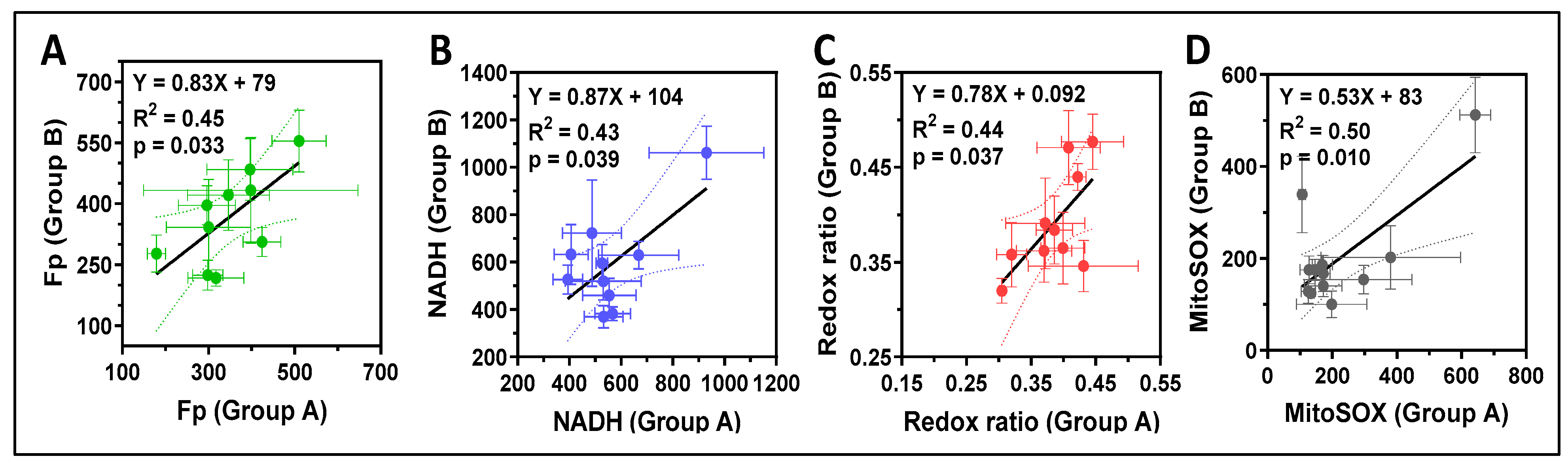
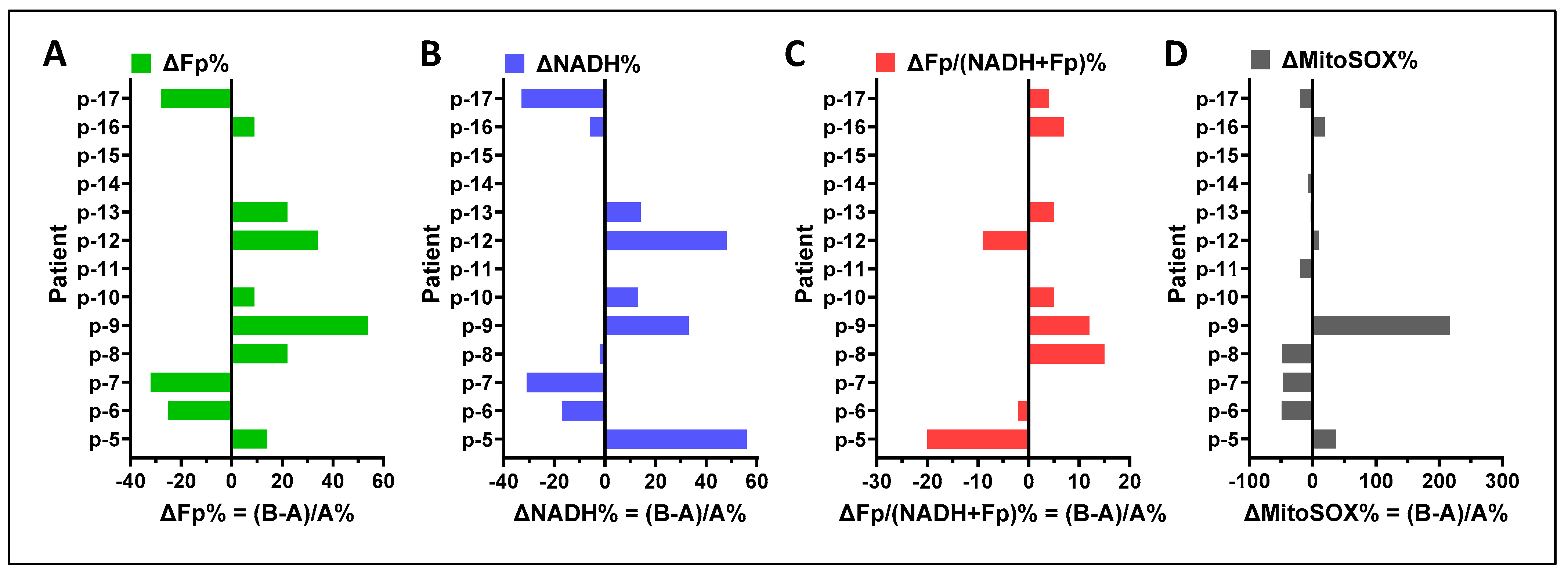

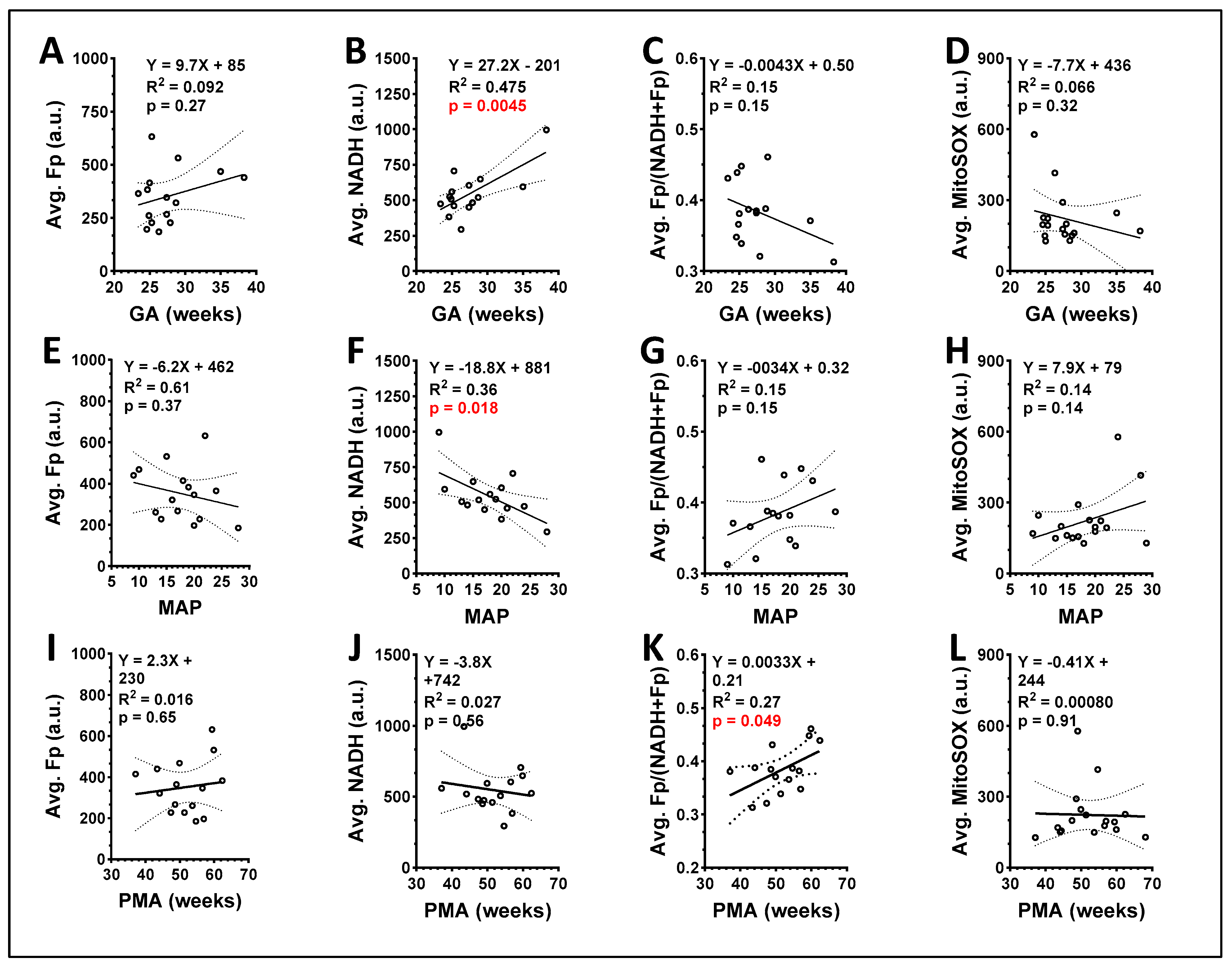
| ID | Gestational Age (GA, Weeks) | Postmenstrual Age at Sample Collection (PMA, Weeks) | FiO2 (%) * | Ventilator Mean Airway Pressure (MAP) | RSS ** |
|---|---|---|---|---|---|
| p-1 | 26.3 | 54.7 | 30 | 28 | 8.4 |
| p-2 | 35.0 | 49.9 | 21 | 10 | 2.1 |
| p-3 | 24.6 | 57.0 | 48 | 20 | 9.6 |
| p-4 | 27.9 | 47.4 | 25 | 14 | 3.5 |
| p-5 | 28.7 | 44.1 | 45 | 16 | 7.2 |
| p-6 | 24.9 | 53.7 | 38 | 13 | 4.9 |
| p-7 | 27.4 | 48.6 | 36 | 17 | 6.1 |
| p-8 | 24.7 | 62.4 | 32 | 19 | 6.1 |
| p-9 | 25.3 | 51.4 | 64 | 21 | 13.4 |
| p-10 | 25.0 | 37.1 | 45 | 18 | 8.1 |
| p-11 | 27.7 | 44.4 | 35 | 17 | 6.0 |
| p-12 | 27.4 | 56.6 | 79 | 20 | 15.8 |
| p-13 | 38.3 | 43.4 | 24 | 9 | 2.2 |
| p-14 | 28.4 | 68.1 | 100 | 29 | 29.0 |
| p-15 | 25.3 | 59.4 | 26 | 22 | 5.7 |
| p-16 | 29.0 | 59.9 | 24 | 15 | 3.6 |
| p-17 | 23.4 | 49.0 | 58 | 24 | 13.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.N.; Gonzalves, D.; Hoffman, J.H.; Baur, J.A.; Li, L.Z.; Jensen, E.A. Use of Optical Redox Imaging to Quantify Alveolar Macrophage Redox State in Infants: Proof of Concept Experiments in a Murine Model and Human Tracheal Aspirates Samples. Antioxidants 2024, 13, 546. https://doi.org/10.3390/antiox13050546
Xu HN, Gonzalves D, Hoffman JH, Baur JA, Li LZ, Jensen EA. Use of Optical Redox Imaging to Quantify Alveolar Macrophage Redox State in Infants: Proof of Concept Experiments in a Murine Model and Human Tracheal Aspirates Samples. Antioxidants. 2024; 13(5):546. https://doi.org/10.3390/antiox13050546
Chicago/Turabian StyleXu, He N., Diego Gonzalves, Jonathan H. Hoffman, Joseph A. Baur, Lin Z. Li, and Erik A. Jensen. 2024. "Use of Optical Redox Imaging to Quantify Alveolar Macrophage Redox State in Infants: Proof of Concept Experiments in a Murine Model and Human Tracheal Aspirates Samples" Antioxidants 13, no. 5: 546. https://doi.org/10.3390/antiox13050546
APA StyleXu, H. N., Gonzalves, D., Hoffman, J. H., Baur, J. A., Li, L. Z., & Jensen, E. A. (2024). Use of Optical Redox Imaging to Quantify Alveolar Macrophage Redox State in Infants: Proof of Concept Experiments in a Murine Model and Human Tracheal Aspirates Samples. Antioxidants, 13(5), 546. https://doi.org/10.3390/antiox13050546






