Long-Term Effect of Maternal Antioxidant Supplementation on the Lipid Profile of the Progeny According to the Sow’s Parity Number
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Ethics Statement
2.3. Animals, Experimental Procedures and Diets
2.4. Sample Collection
2.5. Laboratory Analysis
2.5.1. Fat Content and Fatty Acid Profile of Plasma, Muscle, and Liver Samples
2.5.2. Tocopherol Quantification in Longissimus Dorsi Muscle and Liver Samples
2.5.3. Iron-Induced Lipid Oxidation in Muscle and Liver Samples
2.6. Statistical Analysis
3. Results
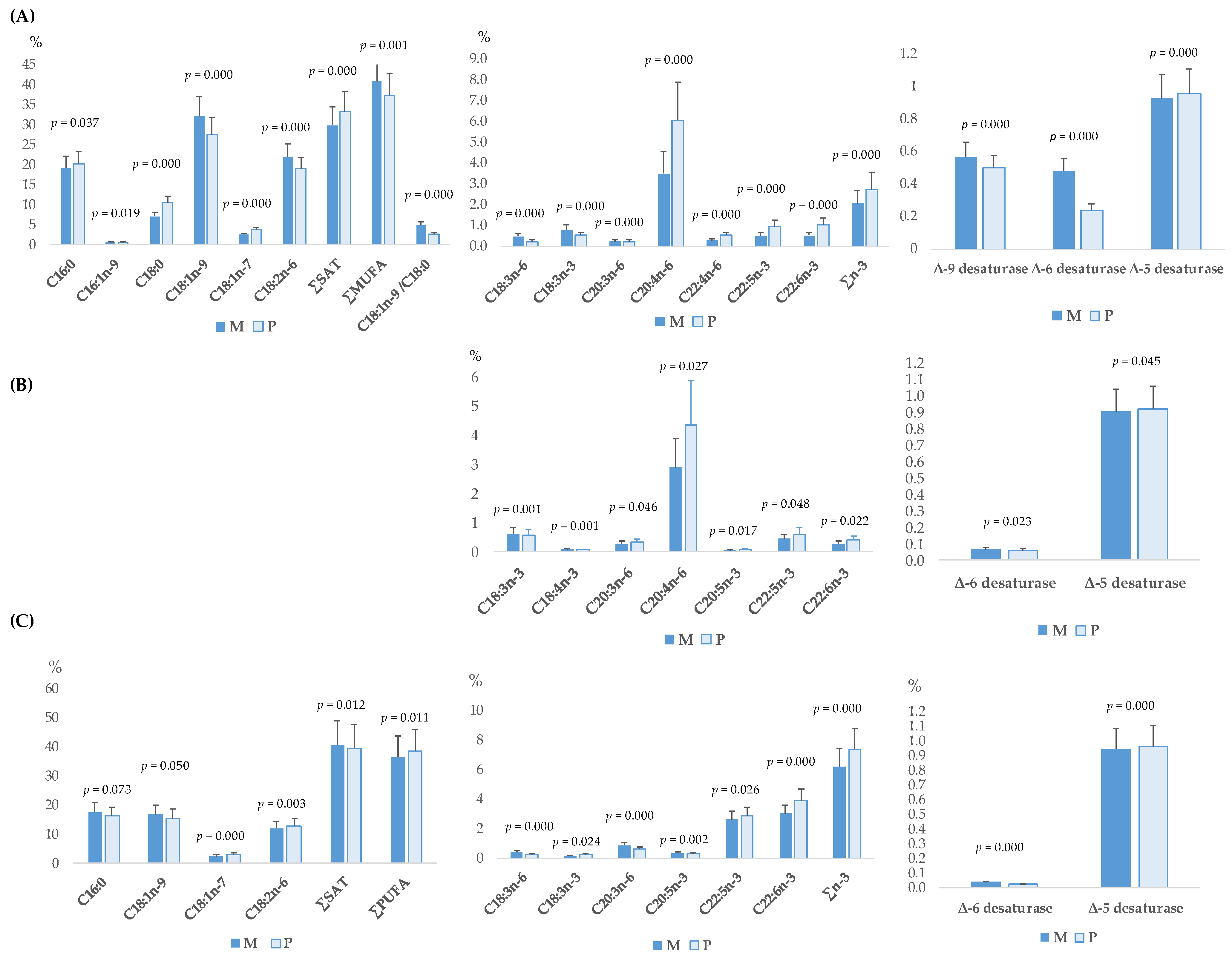
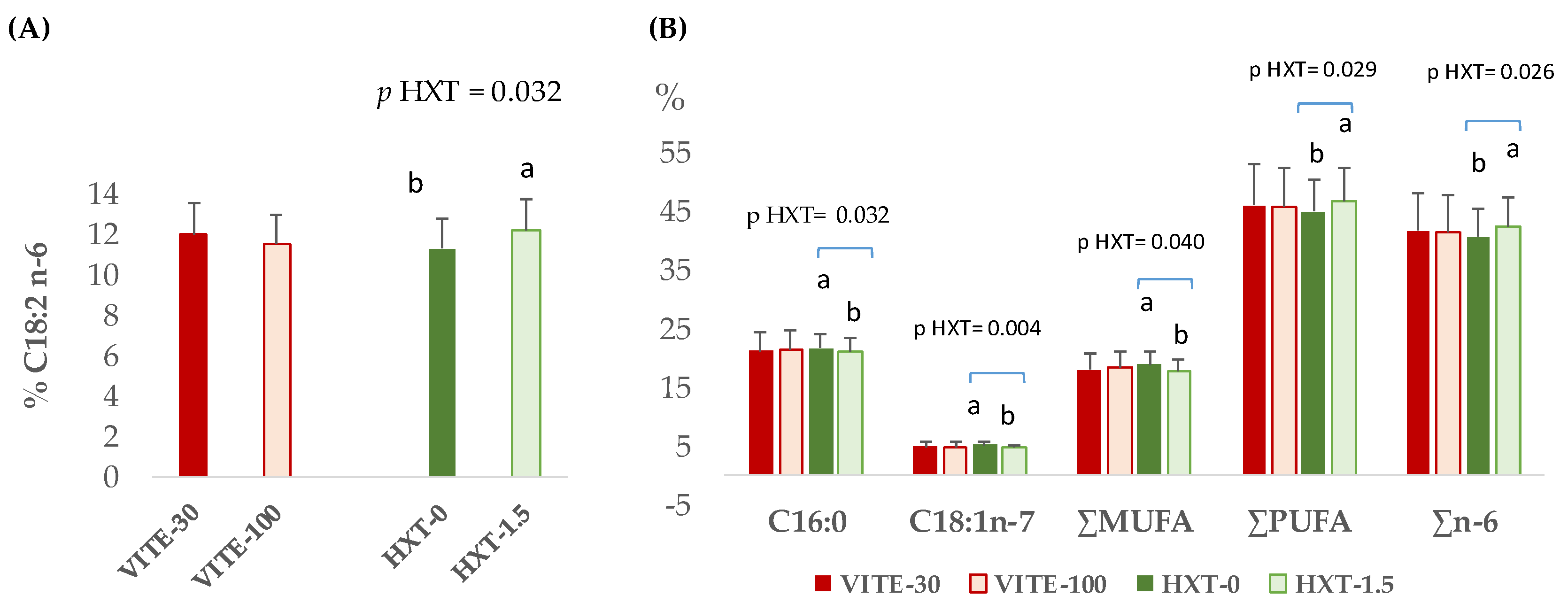
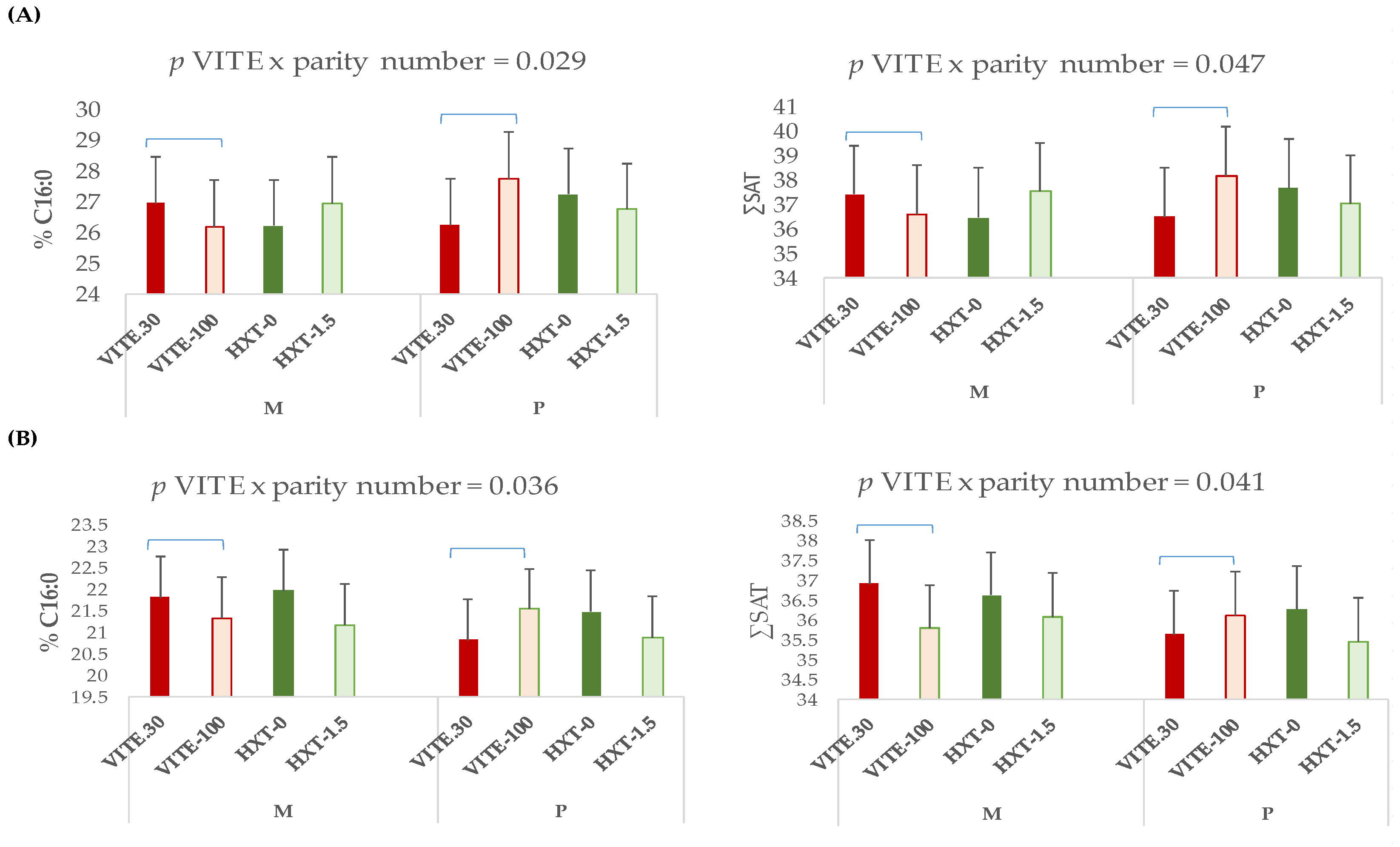
3.1. Total Fatty Acid Profile of Plasma, Longissimus Dorsi Muscle, and Liver of Piglets
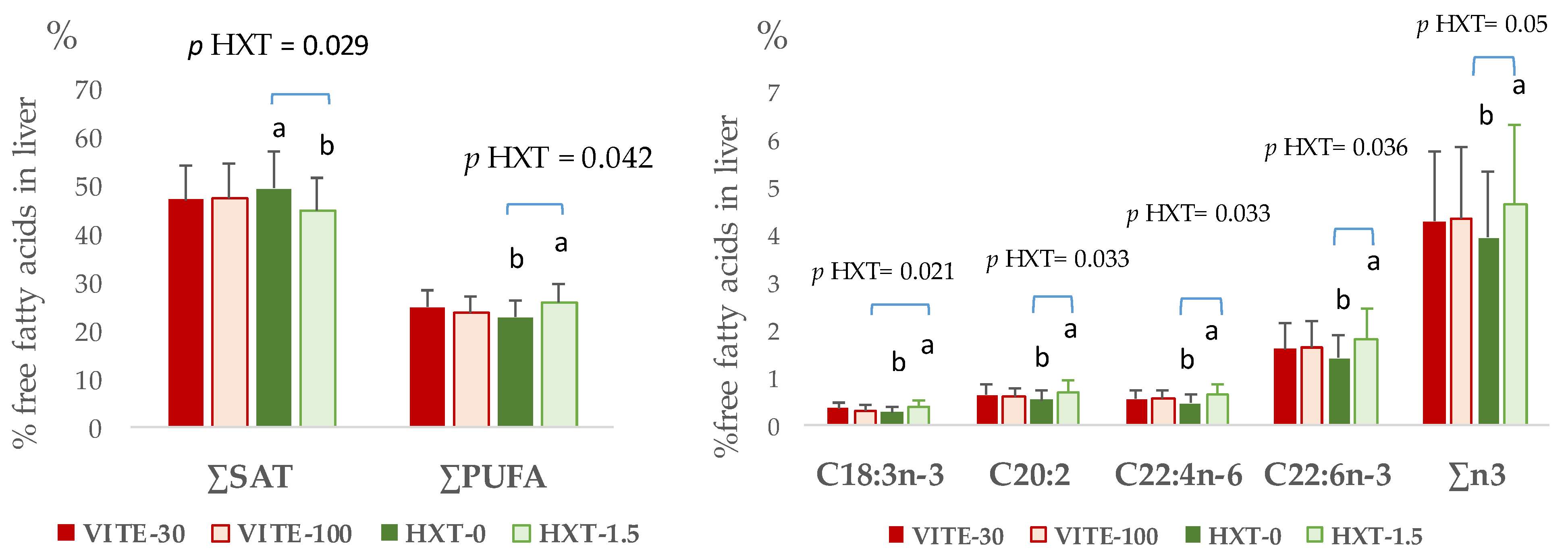
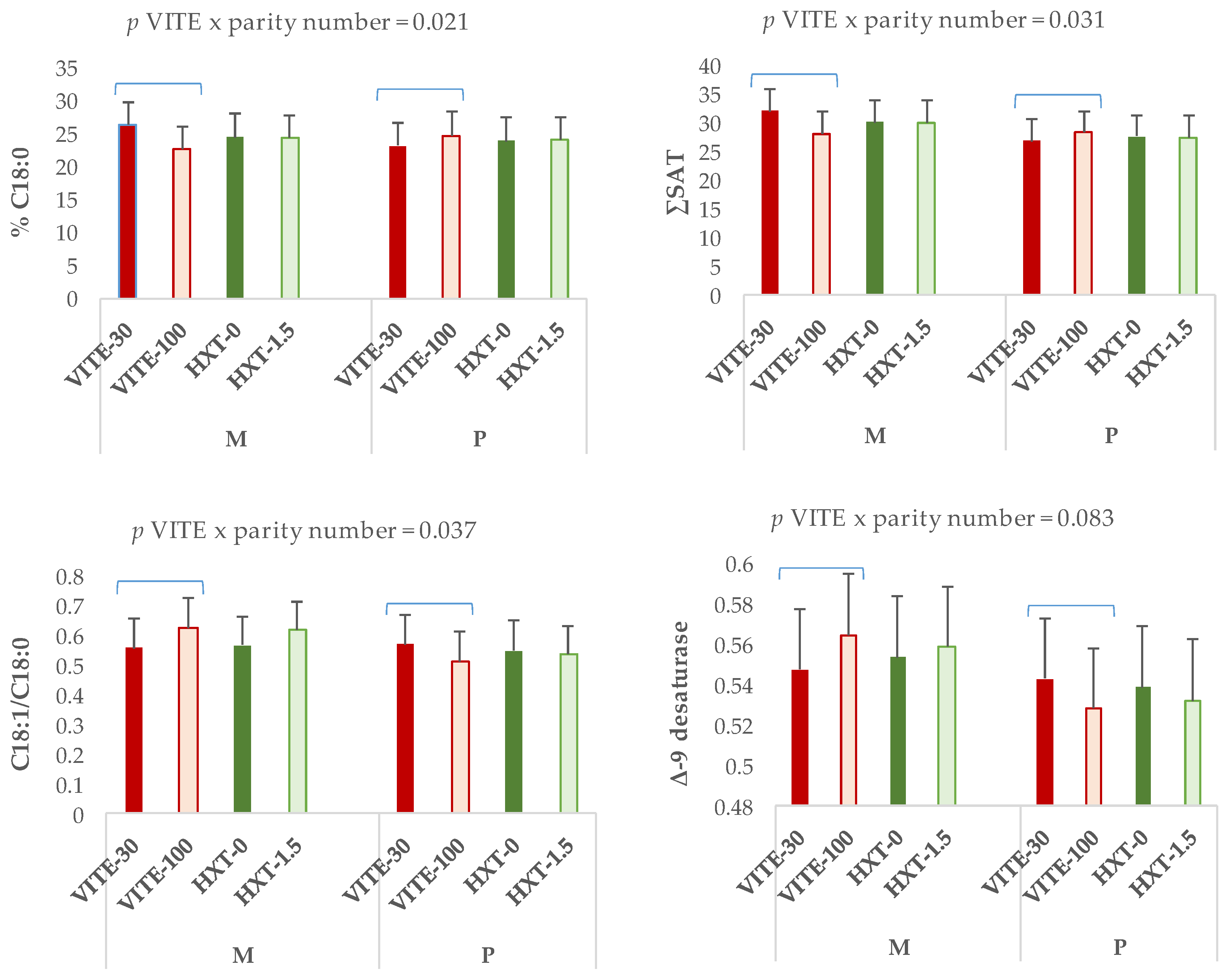
3.2. Fatty Acid Fractions of Longissimus Dorsi Muscle and Liver of Piglets
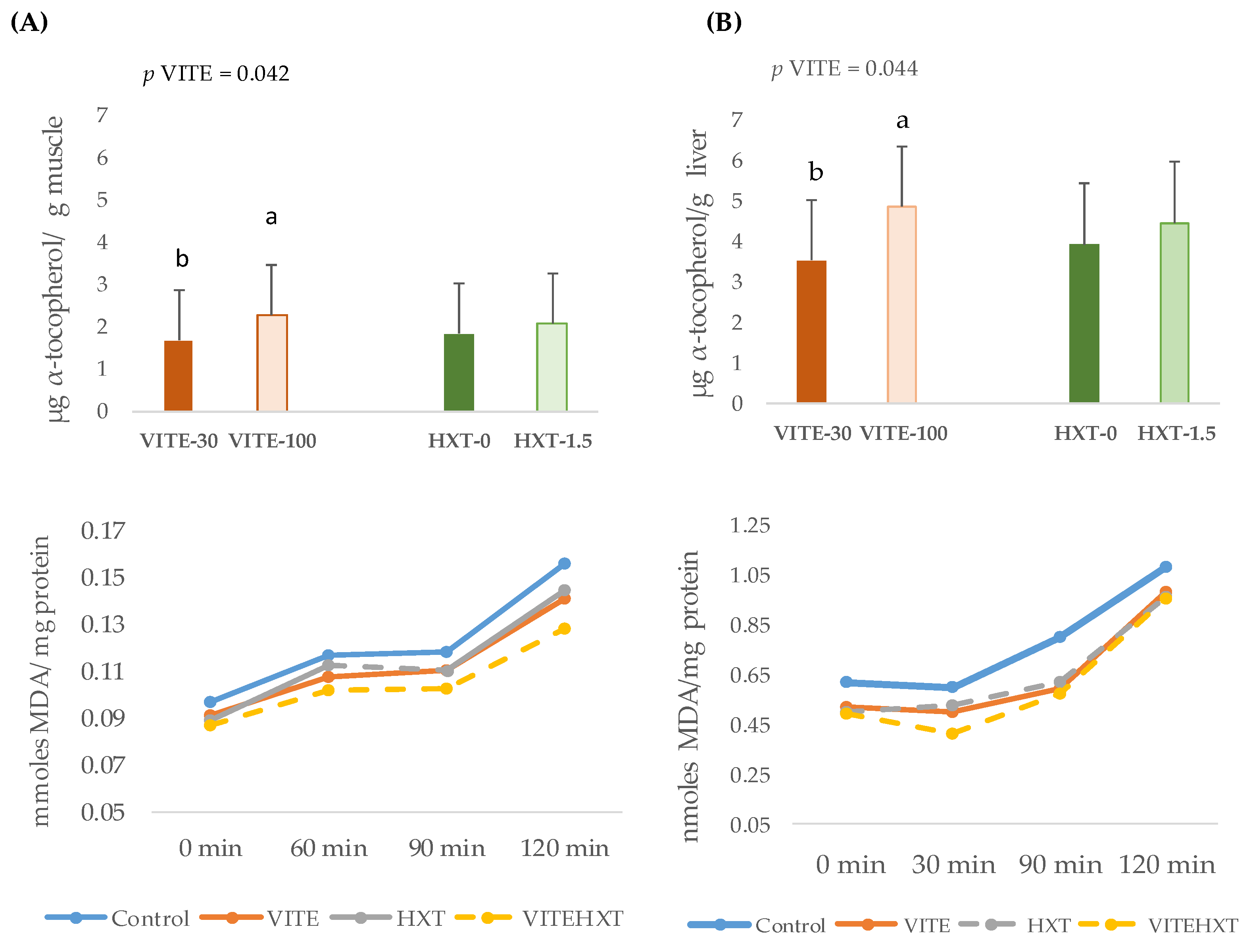
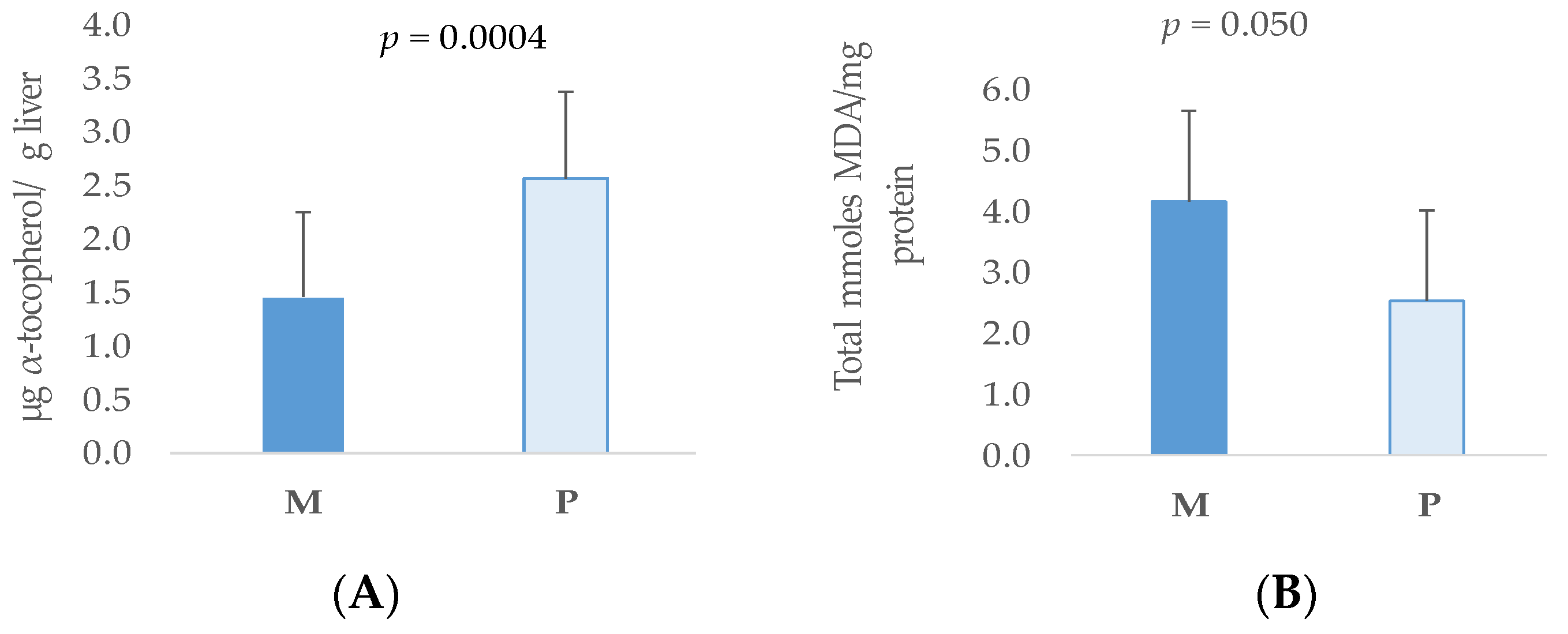
3.3. Lipid Stability in Tissues of Weaned Piglets
3.4. Final Weights and Carcass Characteristics of Pigs after Fattening
3.5. Fatty Acid Profile of Intramuscular Fat of Muscle after Fattening under Free-Range Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Analyzed Composition 1 | CONTROL | VE2 | HXT | HXT + VE |
|---|---|---|---|---|
| Dry matter, % | 90.8 | 89.8 | 91.1 | 91.2 |
| Crude Protein, % | 13.1 | 14.0 | 15.0 | 14.5 |
| Fat, % | 4.3 | 3.6 | 3.8 | 3.7 |
| Ash, % | 6.5 | 6.4 | 6.9 | 6.0 |
| Fiber, % | 4.3 | 4.4 | 4.3 | 4.4 |
| Starch, % | 49.0 | 46.1 | 41.1 | 43.0 |
| Vitamin E, mg/kg | 70.5 | 103.6 | 65.4 | 114.1 |
| Fatty acid composition | ||||
| C14:0 | 0.60 | 0.66 | 0.56 | 0.60 |
| C16:0 | 19.80 | 21.48 | 19.10 | 20.11 |
| C16:1n-9 | 0.12 | 0.15 | 0.12 | 0.12 |
| C16:1n-7 | 0.79 | 0.87 | 0.73 | 0.78 |
| C18:0 | 4.77 | 4.80 | 4.45 | 4.54 |
| C18:1n-9 | 28.97 | 25.26 | 30.33 | 26.94 |
| C18:1n-7 | 1.92 | 1.49 | 1.57 | 1.60 |
| C18:2n-6 | 38.97 | 40.81 | 38.97 | 40.89 |
| C18:3n-3 | 3.04 | 3.44 | 3.15 | 3.36 |
| C20:0 | 0.30 | 0.26 | 0.30 | 0.31 |
| C20:1n-9 | 0.52 | 0.58 | 0.55 | 0.58 |
| ∑SAT | 25.47 | 27.20 | 24.41 | 25.55 |
| ∑MUFA | 32.33 | 28.35 | 33.29 | 30.02 |
| ∑PUFA | 42.01 | 44.25 | 42.11 | 44.25 |
| 10–90 Days 1 | 91–150 Days 2 | 151–240 Days 3 | |
|---|---|---|---|
| Humidity, % | 10.52 | 11.24 | 10.79 |
| Crude Protein, % | 14.46 | 11.50 | 10.42 |
| Fat, % | 4.68 | 3.17 | 4.66 |
| Fiber, % | 3.71 | 4.53 | 5.71 |
| Ash, % | 4.80 | 4.90 | 5.22 |
| Starch, % | 45.55 | 47.19 | 45.57 |
| Sugars, % | 4.09 | 2.00 | 2.78 |
| Met, % | 0.31 | 0.25 | 0.20 |
| Met+ Cys, % | 0.57 | 0.49 | 0.42 |
| Lys, % | 0.98 | 0.81 | 0.65 |
| Thr, % | 0.62 | 0.53 | 0.42 |
| Thp, % | 0.70 | 0.15 | 0.11 |
| Ca, % | 0.70 | 0.80 | 1.00 |
| P, % | 0.43 | 0.44 | 0.39 |
| ME (Kcal/kg) | 3250 | 3100 | 3100 |
References
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Sheashea, M.; Xiao, J.; Farag, M.A. MUFA in metabolic syndrome and associated risk factors: Is MUFA the opposite side of the PUFA coin? Food Funct. 2021, 12, 12221–12234. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Colmenero, F.; Carballo, J.; Cofrades, S. Healthier meat and meat products: Their role as functional foods. Meat Sci. 2001, 59, 5–13. [Google Scholar] [CrossRef]
- Paton, C.M.; Ntambi, J.M. Biochemical and physiological function of stearoyl-Coa-A desaturase. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E28–E37. [Google Scholar] [CrossRef]
- Zhou, Y.E.; Egeland, G.M.; Meltzer, S.J.; Kubow, S. The association of desaturase 9 and plasma fatty acid composition with insulin resistance-associated factors in female adolescents. Metabolism 2009, 58, 158–166. [Google Scholar] [CrossRef]
- Tejerina, D.; García-Torres, S.; Cabeza de Vaca, M.; Vázquez, F.M.; Cava, R. Effect of production system on physical-chemical, antioxidant and fatty acids composition of Longissimus dorsi and Serratus ventralis muscles from Iberian pigs. Food Chem. 2012, 133, 293–299. [Google Scholar] [CrossRef]
- Ayuso, D.; González, A.; Peña, F.; Hernández-García, F.I.; Izquierdo, M. Effect of fattening period length on intramuscular and subcutaneous fatty acid profiles in Iberian Pigs finished in the Montanera sustainable system. Sustainability 2020, 12, 7937. [Google Scholar] [CrossRef]
- Sarmiento-García, A.; Vieira-Aller, C. Improving Fatty Acid Profile in Native Breed Pigs Using Dietary Strategies: A Review. Animals 2023, 13, 1696. [Google Scholar] [CrossRef]
- Rey, A.I.; Daza, A.; López-Carrasco, C.; López-Bote, C.J. Quantitative study of the alpha- and gamma-tocopherols accumulation in muscle and backfat from Iberian pigs kept free-range as affected by time of free-range feeding or weight gain. Anim. Sci. 2006, 82, 901–908. [Google Scholar] [CrossRef]
- Morales, J.; Baucells, M.D.; Pérez, J.F.; Mourot, J.; Gasa, J. Body Fat Content, Composition and Distribution in Landrace and Iberian Finishing Pigs given Ad Libitum Maize- and Acorn-Sorghum-Maize-Based Diets. Anim. Sci. 2003, 77, 215–224. [Google Scholar] [CrossRef]
- Horrillo, A.; Gaspar, P.; Muñoz, Á.; Escribano, M.; González, E. Fattening Iberian Pigs Indoors vs. Outdoors: Production Performance and Market Value. Animals 2023, 13, 506. [Google Scholar] [CrossRef]
- González, E.; Tejeda, J.F.; Motilva, M.J.; Romero, M.P. Phenolic compounds in subcutaneous adipose tissue from Iberian pigs. Options Mediterr. Ser. A Semin. Mediterr. 2007, 76, 115–118. [Google Scholar]
- Ventanas, S.; Estevez, M.; Tejeda, J.F.; Ruiz, J. Protein and Lipid Oxidation in Longissimus Dorsi and Dry Cured Loin from Iberian Pigs as Affected by Crossbreeding and Diet. Meat Sci. 2006, 72, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Carrapiso, A.I.; Ventanas, J.; Garcia, C. Characterization of the most odor-active compounds of Iberian ham headspace. J. Agric. Food Chem. 2002, 50, 1996–2000. [Google Scholar] [CrossRef] [PubMed]
- Muriel, E.; Antequera, T.; Petron, M.J.; Martin, D.; Ruiz, J. Volatile compounds on the surface and within Iberian dry-cured loin. Eur. Food Res. Technol. 2004, 219, 445. [Google Scholar] [CrossRef]
- Daza, A.; Mateos, A.; Rey, A.I.; López-Bote, C.J. Feeding level in the period previous to the late fattening phase influences fat composition at slaughter in free-ranged Iberian pigs. Archiv. Anim. Nutr. 2005, 59, 227–236. [Google Scholar] [CrossRef]
- González, E.; Tejeda, J.F. Effects of dietary incorporation of different antioxidant extracts and free-range rearing on fatty acid composition and lipid oxidation of Iberian pig meat. Animal 2007, 1, 1060–1067. [Google Scholar] [CrossRef]
- Moreno, I.; Ladero, L.; Cava, R. Effect of the Iberian pig rearing system on blood plasma antioxidant status and oxidative stress biomarkers. Livest. Sci. 2020, 235, 104006. [Google Scholar] [CrossRef]
- Lauridsen, C.; Engel, H.; Jensen, S.K.; Craig, A.M.; Traber, M. Lactating sows and suckling piglets preferentially incorporate RRR- over all-rac-α-tocopherol into milk, plasma and tissues. J. Nutr. 2002, 132, 1258–1264. [Google Scholar] [CrossRef]
- Amazan, D.; Cordero, G.; López-Bote, C.J.; Lauridsen, C.; Rey, A.I. Effects of oral micellized natural vitamin E (d-α-tocopherol) vs. synthetic vitamin E (dl-α-tocopherol) in feed on α-tocopherol levels, stereoisomer distribution, oxidative stress and the immune response in piglets. Animal 2014, 8, 410–4419. [Google Scholar] [CrossRef]
- Laviano, H.; Gómez, G.; Muñoz, M.; García-Casco, J.; Nuñez, Y.; Escudero, R.; Heras, A.; González-Bulnes, A.; Óvilo, C.; López-Bote, C.; et al. Dietary Vitamin E and/or Hydroxytyrosol Supplementation to Sows during Late Pregnancy and Lactation Modifies the Lipid Composition of Colostrum and Milk. Antioxidants 2023, 12, 1039. [Google Scholar] [CrossRef] [PubMed]
- Gómez, G.; Laviano, H.D.; García-Casco, J.M.; Escudero, R.; Muñoz, M.; Heras-Molina, A.; González-Bulnes, A.; Ovilo, C.; López-Bote, C.J.; Rey, A.I. Different effect of dietary vitamin E or hydroxytyrosol supplementation to sow’s diet on oxidative status and performances of weaned piglets. Antioxidants 2023, 12, 1504. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J. Oxidative processes in meat and meat products: Quality implications. Meat Sci. 1994, 36, 169–185. [Google Scholar] [CrossRef]
- Rey, A.I.; de-Cara, A.; Calvo, L.; Puig, P.; Hechavarría, T. Changes in Plasma Fatty Acids, Free Amino Acids, Antioxidant Defense, and Physiological Stress by Oleuropein Supplementation in Pigs Prior to Slaughter. Antioxidants 2020, 9, 56–74. [Google Scholar] [CrossRef]
- Rey, A.I.; de-Cara, A.; Segura, J.F.; Martí, P.; Hechavarría, T.; Calvo, L. Dietary oleuropein extract supplementation and their combination with α-tocopheryl acetate and selenium modifies free fatty acid profile and improves stability of pork. J. Sci. Food Agric. 2020, 101, 2337–2344. [Google Scholar] [CrossRef]
- Palma-Granados, P.; García-Casco, J.M.; Fernández-Barroso, M.A.; López-García, A.; Martínez-Tores, M.; Muñoz, M.; González-Sánchez, E. Inclusion of olive by-products in growing diets causes minor effects on meat quality of Iberian pigs fattened in a traditional system. Span. J. Agric. Res. 2022, 20, e0607. [Google Scholar] [CrossRef]
- Furukaw, E.; Chen, Z.; Kubo, T.; Wu, Y.; Ueda, K.; Chelenga, M.; Chiba, H.; Yanagawa, Y.; Katagiri, S.; Nagano, M.; et al. Simultaneous free fatty acid elevations and accelerated desaturation in plasma and oocytes in early postpartum dairy cows under intensive feeding management. Therigenology 2022, 193, 20–29. [Google Scholar] [CrossRef]
- Willms, J.N.; Hare, K.S.; Fischer-Tlustos, J.; Vahmani, P.; Dugan, M.E.R.; Leal, L.N.; Steele, M.A. Fatty acid profile characterization in colostrum, transition milk, and mature milk of primi- and multiparous cows during the first week of lactation. J. Dairy Sci. 2022, 105, 2612–2630. [Google Scholar] [CrossRef]
- BOE. RD 53/2013, de 21 de octubre por la que se establecen las normas básicas aplicables para la protección de los animales utilizados en experimentación y otros fines científicos, incluyendo la docencia, Spain. Bol. Oficial. Del Estado 2013, 252, 34367–34391. [Google Scholar]
- EC. Council Regulation (EC) No 2010/63/CE of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union. 2010, 276, 33–79. [Google Scholar]
- National Research Council. Nutrient Requirement of Swine, 11th ed.; NRC, National Academy Press: Washington, DC, USA, 2012.
- Normas FEDNA: Necesidades Nutricionales para Ganado Porcino, 2nd ed.; Fundación Española para el Desarrollo de la Nutrición Animal: Madrid, Spain, 2013.
- Garcés, R.; Mancha, M. One-step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues. Anal. Biochem. 1993, 211, 139–143. [Google Scholar] [CrossRef]
- Kornbrust, D.J.; Mavis, R.D. Relative susceptibility of microsomes from lung, heart, liver, kidney, brain and testes to lipid peroxidation: Correlation with vitamin E content. Lipids 1980, 15, 315–322. [Google Scholar] [CrossRef]
- Maniongul, C.; Blond, J.P.; Ulmann, L.; Duand, G.; Poisson, J.P.; Bézard, J. Age-related changes in ∆6 and ∆5 desaturase activities in rat liver microsomes. Lipids 1993, 28, 291–297. [Google Scholar] [CrossRef]
- Clandinin, M.T.; Wong, K.; Hacker, R.R. Synthesis of chain elongation-desaturation products of linoleic acid by liver and brain microsomes during development of the pig. Biochem. J. 1985, 226, 305–309. [Google Scholar] [CrossRef]
- Skiba, G.; Raj, S.; Poławska, E. Profile of fatty acids and activity of elongase and Δ5 and Δ9 desaturase of growing pigs differ in concentration of intramuscular fat in musculus longissimus dorsi. Anim. Sci. Pap. Rep. 2013, 31, 123–137. [Google Scholar]
- Dobrzyn, A.; Ntambi, J.M. The role of stearoyl-CoA desaturase in the control of metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 35–41. [Google Scholar] [CrossRef]
- Haman, N.; Romano, A.; Asaduzzaman, M.; Ferrentino, G.; Biasioli, F.; Scampicchio, M. A microcalorimetry study on the oxidation of linoleic acid and the control of rancidity. Talanta 2017, 164, 407–412. [Google Scholar] [CrossRef]
- Wood, J.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Sheard, P.R.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef]
- Dias, K.M.M.; Oliveira, C.H.; Calderano, A.A.; Rostagno, H.S.; O’Connor, K.E.; Davis, R.; Walsh, M.; Britton, J.; Altieri, E.A.; Albino, L.F.T. Effects of Hydroxytyrosol Supplementation on Performance, Fat and Blood Parameters of Broiler Chickens. Animals 2024, 14, 119. [Google Scholar] [CrossRef]
- Drira, R.; Chen, S.; Sakamoto, K. Oleuropein and hydroxytyrosol inhibit adipocyte differentiation in 3T3-L1 cells. Life Sci. 2011, 89, 708–716. [Google Scholar] [CrossRef]
- López de las Hazas, M.-C.; del Saz-Lara, A.; Chapado, L.A.; Dávalos, A. Hydroxytyrosol induces dyslipidemia in an ApoB100 humanized mouse model. Mol. Nutr. Food Res. 2024, 68, 2300508. [Google Scholar] [CrossRef] [PubMed]
- Leyton, J.; Drury, P.J.; Crawford, M.A. Differential oxidation of saturated and unsaturated fatty acids in vivo in the rat. Br. J. Nutr. 1987, 57, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, T.; Ide, T. Comparative effects of α- and g-linolenic acids on rat liver fatty acid oxidation. Lipids 1998, 33, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, C.; Vazquez-Gomez, M.; Barbero, A.; Pesantez, J.L.; Zinellu, A.; Berlinguer, F.; Gonzalez-Añover, P.; Gonzalez, J.; Encinas, T.; Torres-Rovira, L.; et al. Polyphenols and IUGR Pregnancies: Effects of Maternal Hydroxytyrosol Supplementation on Placental Gene Expression and Fetal Antioxidant Status, DNA-Methylation and Phenotype. Int. J. Mol. Sci. 2019, 20, 1187. [Google Scholar] [CrossRef] [PubMed]
- Mennitti, L.V.; Oliveira, J.L.; Morais, C.A.; Estadella, D.; Oyama, L.M.; Oller do Nascimento, C.M.; Pisani, L.P. Type of fatty acids in maternal diets during pregnancy and/or lactation and metabolic consequences of the offspring. J. Nutr. Biochem. 2015, 26, 99–111. [Google Scholar] [CrossRef]
- Okayasu, T.; Kameda, K.; Ono, T.; Imai, Y. Effect of dietary vitamin B2 and vitamin E on the ∆-9-desaturase and catalase activities in rat liver microsomes. Biochim. Biophys. Acta 1977, 489, 389–402. [Google Scholar]
- Fuhrmann, H.; Sallman, H.P. Phospholipid fatty acid of brain and liver are modified by -tocopherol and dietary fat in growing chicks. Br. J. Nutr. 1996, 76, 109–122. [Google Scholar] [CrossRef][Green Version]
- Gonzalez-Soto, M.; Mutch, D.M. Diet regulation of long-chain OUFA synthesis: Role of macronutrients, micronutrients, and polyphenols on ∆-5/∆-6 desaturases and elongases 2/5. Adv. Nutr. 2021, 12, 980–994. [Google Scholar] [CrossRef]
- Palladini, G.; Di Pasqua, L.; Berardo, C.; Siciliano, V.; Richelmi, P.; Mannucci, B.; Croce, A.C.; Rizzo, V.; Perlini, S.; Vairetti, M.; et al. Fatty acid desaturase involvement in non-alcoholic fatty liver disease rat models: Oxidative stress versus metalloproteinases. Nutrients 2019, 11, 799. [Google Scholar] [CrossRef]
- Infante, J.P. A function for the vitamin E metabolite α-tocopherol quinone as an essential enzyme cofactor for the mitochondrial fatty acid desaturases. FEBS Lett. 1999, 446, 1–5. [Google Scholar] [CrossRef]
- Moretti, D.B.; Santos, C.B.; Alencar, S.M.; Machado-Neto, R. Colostrum from pri-miparous Holstein cows shows higher antioxidant activity than colostrum of multipa-rous ones. J. Dairy Res. 2020, 87, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Araya, J.; Rodrigo, R.; Pettinelli, P.; Araya, A.V.; Poniachik, J.; Videla, L.A. Decreased liver fatty acid ∆-6 and ∆-5 desaturase activity in obese patients. Obesity 2010, 18, 1460–1463. [Google Scholar] [CrossRef]
- Huang, S.-J.; Xu, Y.-M.; Lau, A.T.Y. Epigenetic Effects of the 13 Vitamins. Curr. Pharmacol. Rep. 2018, 4, 453–467. [Google Scholar] [CrossRef]
- Frankel, E.N. Free radical oxidation. In Lipid Oxidation, 2nd ed.; Elsevier: Amsterdam, The Netherlands; Woodhead Publishing Ltd.: Cambridge, UK, 2014; pp. 15–24. [Google Scholar]
- Gray, J.I.; Gomaa, E.A.; Buckley, D.J. Oxidative quality and shelf life of meats. Meat Sci. 1996, 43, S111–S123. [Google Scholar] [CrossRef] [PubMed]
| VITE-30 | VITE-100 | HXT-0 | HXT-1.5 | RMSE 1 | p VITE 2 | p HXT | p VITEXHXT | |
|---|---|---|---|---|---|---|---|---|
| Body weight, kg | 163.46 | 166.63 | 163.92 | 166.17 | 20.355 | 0.105 | 0.544 | 0.113 |
| Carcass weight, kg | 129.74 | 132.59 | 130.24 | 132.09 | 16.197 | 0.106 | 0.586 | 0.134 |
| Left ham weight, kg | 10.69 | 10.61 | 10.50 | 10.81 | 1.042 | 0.837 | 0.289 | 0.989 |
| Right ham weight, kg | 10.78 | 10.79 | 10.65 | 10.91 | 1.084 | 0.812 | 0.414 | 0.796 |
| Left loin weight, kg | 1.55 | 1.54 | 1.53 | 1.56 | 0.169 | 0.988 | 0.372 | 0.903 |
| Right loin weight, kg | 1.55 | 1.53 | 1.52 | 1.56 | 0.168 | 0.541 | 0.182 | 0.624 |
| VITE-30 | VITE-100 | HXT-0 | HXT-1.5 | RMSE 1 | p VITE 2 | p HXT | p VITEXHXT | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IMF 3 | 12.842 | 12.151 | 12.727 | 12.266 | 6.216 | 0.5518 | 0.6912 | 0.9136 | ||||
| C14:0 | 1.465 | 1.476 | 1.447 | 1.493 | 0.257 | 0.8241 | 0.3406 | 0.1426 | ||||
| C15:0 | 0.034 | 0.033 | 0.033 | 0.034 | 0.011 | 0.8050 | 0.5973 | 0.9736 | ||||
| C16:0 | 22.628 | 22.777 | 22.862 | 22.544 | 2.058 | 0.6969 | 0.4078 | 0.2493 | ||||
| C16:1n-9 | 0.257 | a | 0.237 | b | 0.245 | 0.249 | 0.046 | 0.0246 | 0.6141 | 0.0846 | ||
| C16:1n-7 | 3.691 | b | 3.967 | a | 3.853 | 3.805 | 0.680 | 0.0311 | 0.7045 | 0.7540 | ||
| C17:0 | 0.163 | 0.157 | 0.155 | 0.164 | 0.038 | 0.3931 | 0.2039 | 0.6529 | ||||
| C17:1 | 0.244 | 0.245 | 0.250 | 0.239 | 0.071 | 0.9098 | 0.4183 | 0.9598 | ||||
| C18:0 | 10.920 | 10.589 | 10.718 | 10.790 | 1.285 | 0.1685 | 0.7653 | 0.1592 | ||||
| C18:1n-9 | 46.239 | 47.077 | 46.425 | 46.892 | 2.468 | 0.0706 | 0.3116 | 0.1830 | ||||
| C18:1n-7 | 4.827 | 4.754 | 4.859 | 4.722 | 0.855 | 0.6445 | 0.3914 | 0.7541 | ||||
| C18:2n-6 | 6.167 | a | 5.498 | b | 5.832 | 5.834 | 1.716 | 0.0384 | 0.9947 | 0.8210 | ||
| C18:3n-6 | 0.045 | 0.041 | 0.044 | 0.042 | 0.016 | 0.1398 | 0.5532 | 0.9296 | ||||
| C18:3n-3 | 0.195 | 0.194 | 0.185 | b | 0.205 | a | 0.050 | 0.9097 | 0.0372 | 0.7318 | ||
| C18:4n-3 | 0.085 | 0.087 | 0.084 | 0.088 | 0.016 | 0.5937 | 0.1830 | 0.2434 | ||||
| C20:0 | 0.156 | 0.151 | 0.152 | 0.154 | 0.031 | 0.3786 | 0.7754 | 0.3473 | ||||
| C20:1n-9 | 0.820 | 0.832 | 0.819 | 0.833 | 0.101 | 0.5210 | 0.4487 | 0.0663 | ||||
| C20:2n-6 | 0.212 | 0.200 | 0.199 | 0.213 | 0.051 | 0.2072 | 0.1452 | 0.6099 | ||||
| C20:3n-6 | 0.102 | 0.095 | 0.098 | 0.098 | 0.028 | 0.1659 | 0.9285 | 0.3311 | ||||
| C20:4n-6 | 1.750 | 1.591 | 1.740 | 1.601 | 0.507 | 0.0936 | 0.1433 | 0.7696 | ||||
| ∑SAT 4 | 35.365 | 35.182 | 35.368 | 35.179 | 2.709 | 0.7177 | 0.7082 | 0.0924 | ||||
| ∑MUFA 5 | 56.078 | b | 57.112 | a | 56.450 | 56.740 | 2.774 | 0.0474 | 0.5757 | 0.1462 | ||
| ∑PUFA 6 | 8.558 | a | 7.706 | b | 8.182 | 8.081 | 2.119 | 0.0328 | 0.7990 | 0.8004 | ||
| ∑n-9 7 | 47.316 | 48.147 | 47.489 | 47.974 | 2.520 | 0.0791 | 0.3029 | 0.1589 | ||||
| ∑n-6 8 | 8.175 | a | 7.330 | b | 7.815 | 7.690 | 2.077 | 0.0307 | 0.7473 | 0.7869 | ||
| ∑n-3 9 | 0.280 | 0.281 | 0.269 | b | 0.292 | a | 0.053 | 0.9571 | 0.0183 | 0.9769 | ||
| ∑n-6:∑n-3 | 29.234 | a | 26.290 | b | 28.911 | a | 26.612 | b | 5.875 | 0.0081 | 0.0376 | 0.6738 |
| C18:1n-9/C18:0 | 4.308 | 4.526 | 4.372 | 4.462 | 0.742 | 0.1169 | 0.5159 | 0.1891 | ||||
| C16:1n-7/C16:0 | 0.163 | 0.175 | 0.169 | 0.170 | 0.034 | 0.0631 | 0.8295 | 0.3960 | ||||
| Δ-9-desaturase 10 | 0.601 | 0.608 | 0.602 | 0.606 | 0.028 | 0.1955 | 0.4868 | 0.0910 | ||||
| Δ-6-desaturase 11 | 0.021 | 0.023 | 0.022 | 0.022 | 0.005 | 0.0698 | 0.9575 | 0.7869 | ||||
| Δ-5-desaturase 12 | 0.942 | 0.941 | 0.944 | 0.939 | 0.019 | 0.7744 | 0.1992 | 0.2422 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez, G.; Laviano, H.D.; García-Casco, J.; Muñoz, M.; Gómez, F.; Sánchez-Esquiliche, F.; González-Bulnes, A.; López-Bote, C.; Óvilo, C.; Rey, A.I. Long-Term Effect of Maternal Antioxidant Supplementation on the Lipid Profile of the Progeny According to the Sow’s Parity Number. Antioxidants 2024, 13, 379. https://doi.org/10.3390/antiox13030379
Gómez G, Laviano HD, García-Casco J, Muñoz M, Gómez F, Sánchez-Esquiliche F, González-Bulnes A, López-Bote C, Óvilo C, Rey AI. Long-Term Effect of Maternal Antioxidant Supplementation on the Lipid Profile of the Progeny According to the Sow’s Parity Number. Antioxidants. 2024; 13(3):379. https://doi.org/10.3390/antiox13030379
Chicago/Turabian StyleGómez, Gerardo, Hernan D. Laviano, Juan García-Casco, Maria Muñoz, Fernando Gómez, Fernando Sánchez-Esquiliche, Antonio González-Bulnes, Clemente López-Bote, Cristina Óvilo, and Ana I. Rey. 2024. "Long-Term Effect of Maternal Antioxidant Supplementation on the Lipid Profile of the Progeny According to the Sow’s Parity Number" Antioxidants 13, no. 3: 379. https://doi.org/10.3390/antiox13030379
APA StyleGómez, G., Laviano, H. D., García-Casco, J., Muñoz, M., Gómez, F., Sánchez-Esquiliche, F., González-Bulnes, A., López-Bote, C., Óvilo, C., & Rey, A. I. (2024). Long-Term Effect of Maternal Antioxidant Supplementation on the Lipid Profile of the Progeny According to the Sow’s Parity Number. Antioxidants, 13(3), 379. https://doi.org/10.3390/antiox13030379








