Nanoemulsions of Phoenix dactylifera L. (Decaffeinated) and Coffea arabica L. Extracts as a Novel Approach for the Treatment of Carbon Tetrachloride-Mediated Liver Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Animals
2.2. Extraction of Date Palm Seed Coffee (PSC) and Arabica Seed Coffee (ACS)
2.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
2.4. Preparation of PSCE Nanoemulsions (NE-PSCE) and ACSE Nanoemulsions (NE-ACSE)
2.5. Characterization of Nanoemulsions
2.6. Antioxidant Activity Using DPPH Radical Scavenging Assay
2.7. Experimental Conditions
2.7.1. Experimental Animal Protocol
2.7.2. Calculation of Nutritional Parameters
2.7.3. Biochemical and Enzyme Activities
2.7.4. Histopathological Examination of Liver
2.8. Statistical Analysis
3. Results
3.1. Chemical Compositions of Coffea arabica and Phoenix dactylifera Extracts Obtained Using Gas Chromatography–Mass Spectrometry (GC-MS)
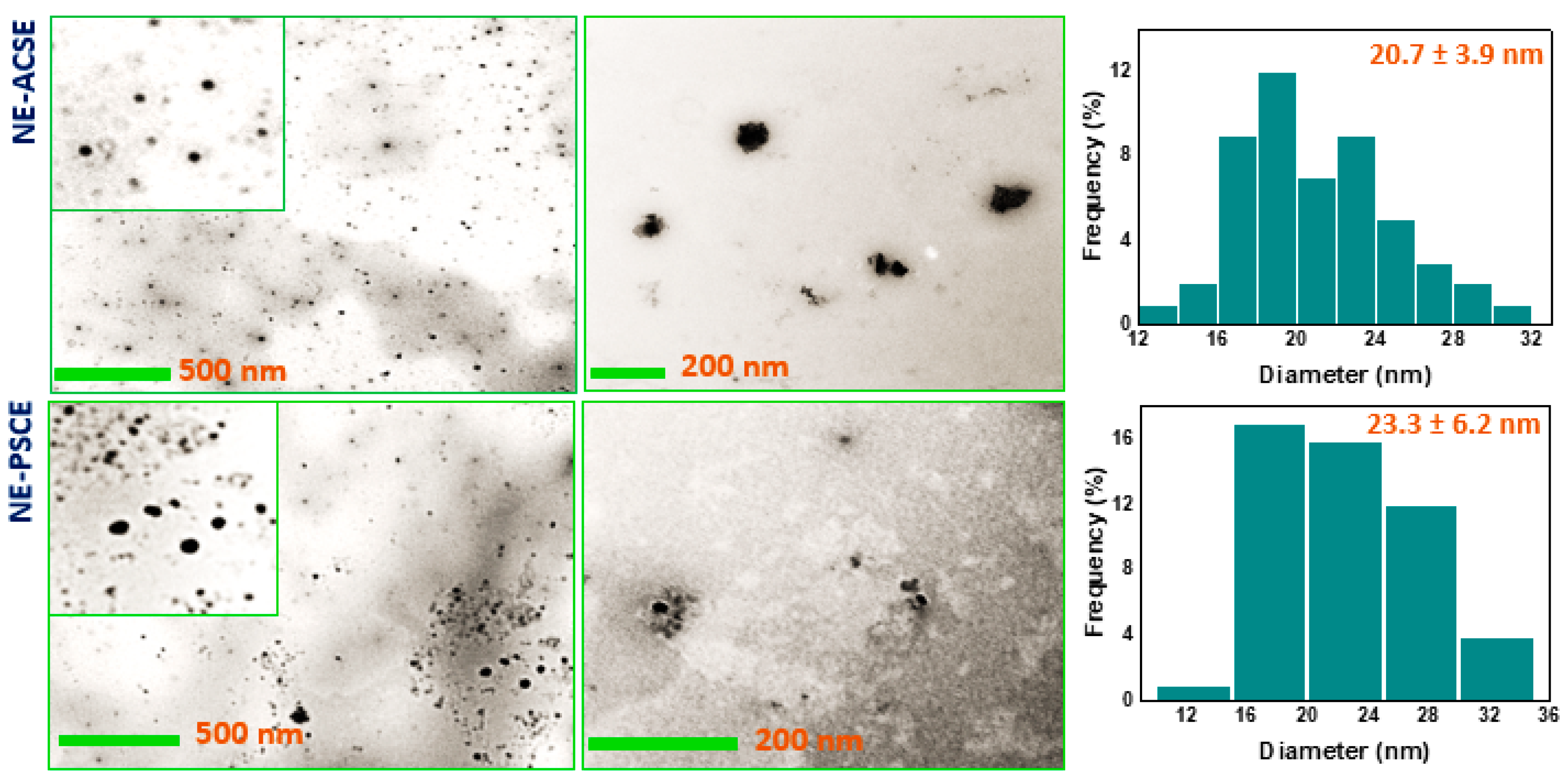
3.2. Morphological Investigation of NE-ACSE and NE-PSCE
3.3. Distribution of Particle Sizes and ζ-Potentials of Nanoemulsions NE-PSCE and NE-ACSE
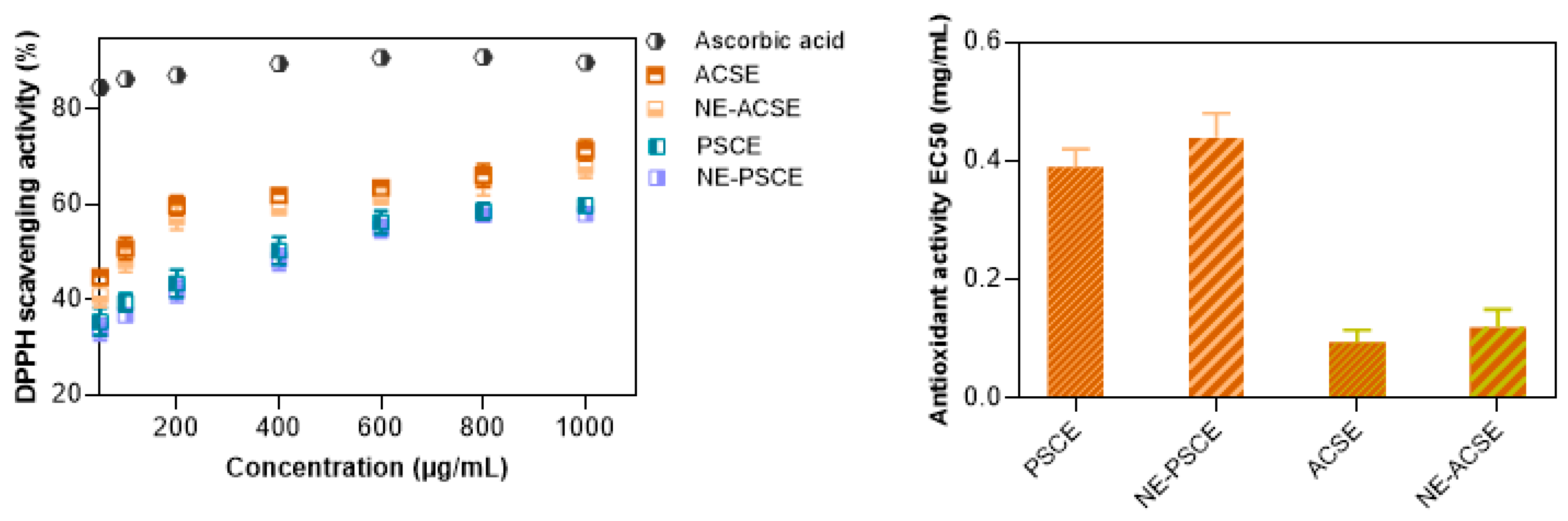
3.4. DPPH Scavenging Activity Measurements of Nanoemulsions
3.5. Effects of NE-PSCE and NE-ACSE on CCL4-Induced Liver Fibrosis in Rat Model
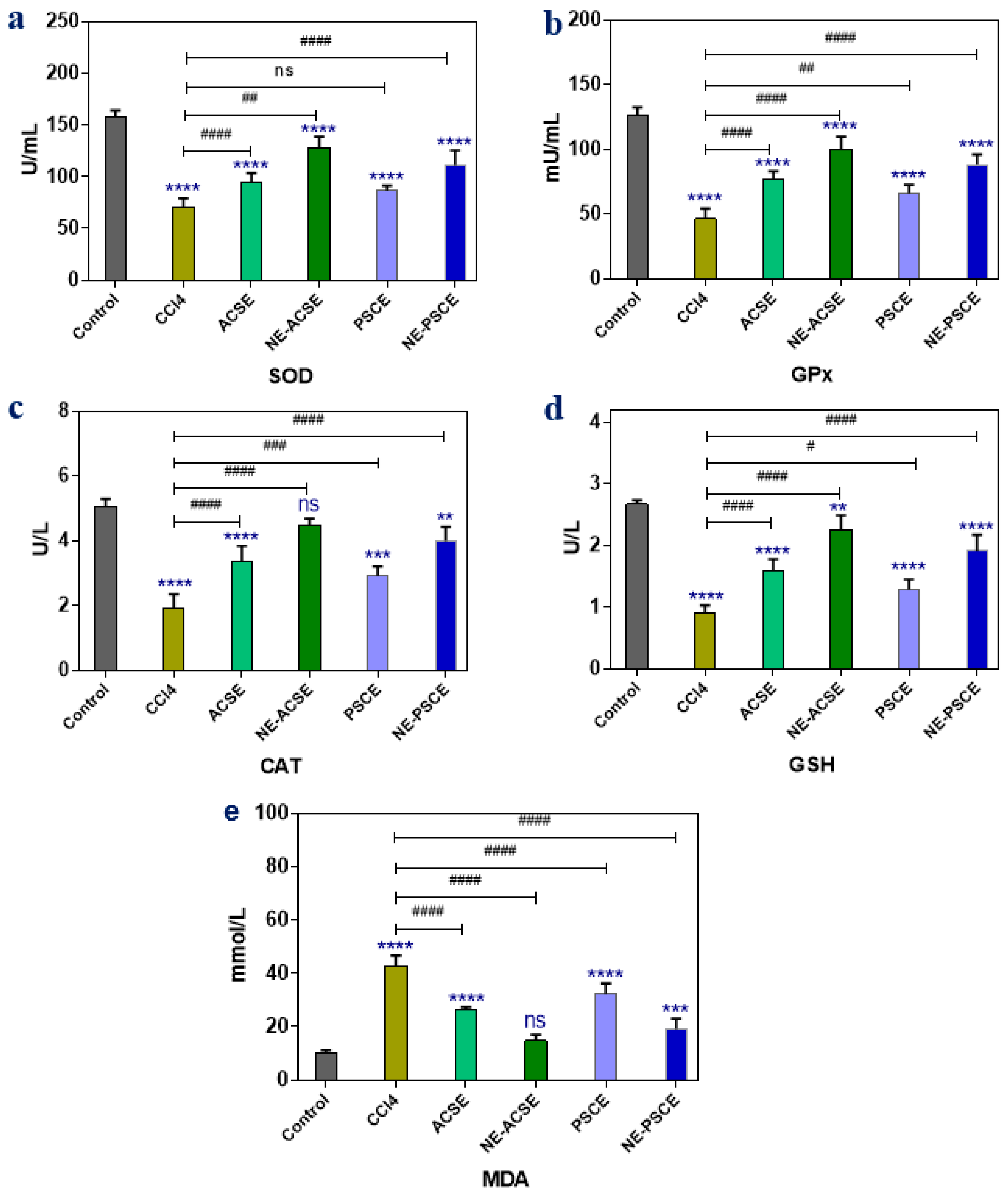
3.5.1. Antioxidant Enzymes and MDA
3.5.2. Liver Functions
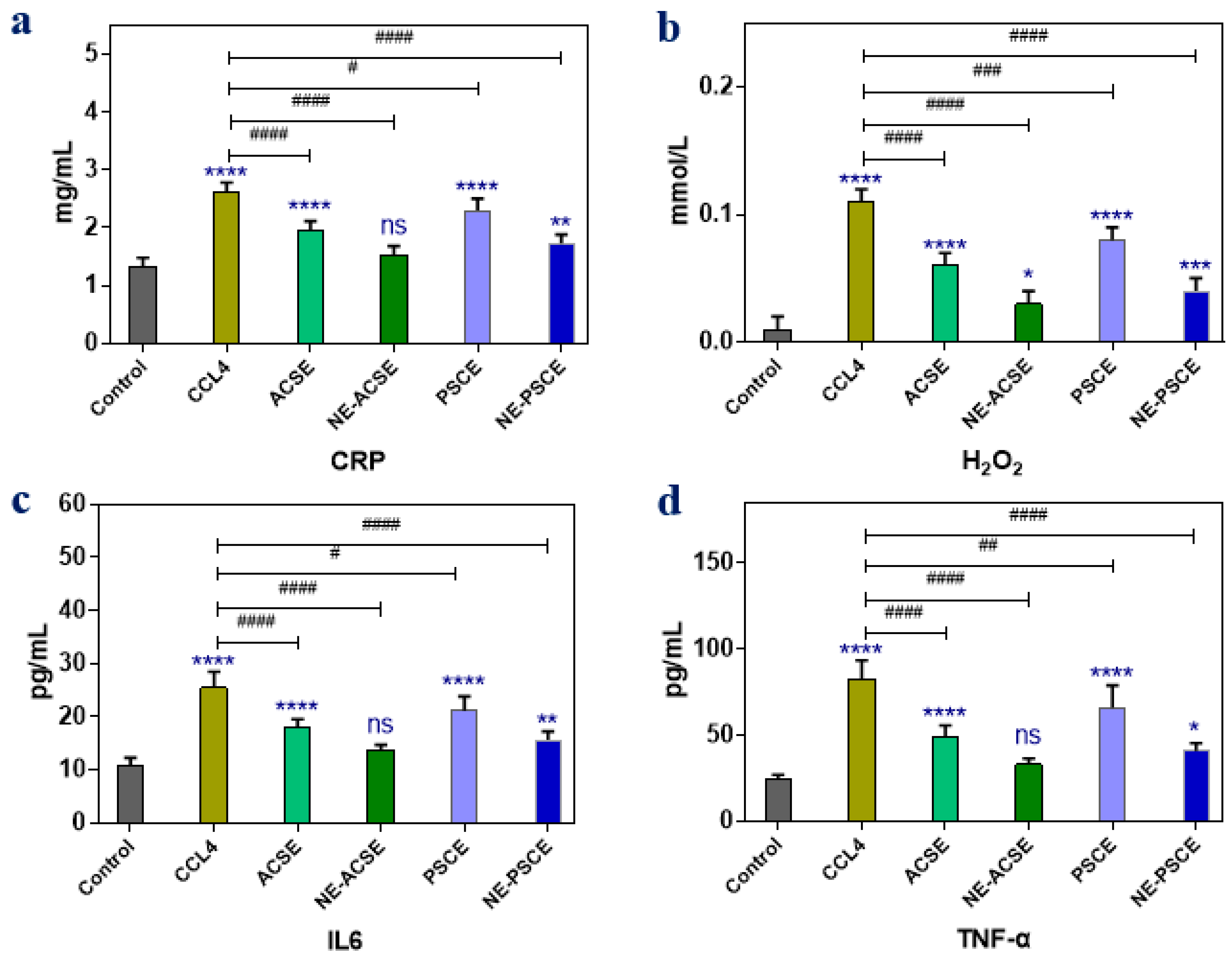
3.5.3. Anti-Inflammatory Parameters
3.5.4. Liver Weight, Relative Liver Weight, Weight Gain, and Food Intake
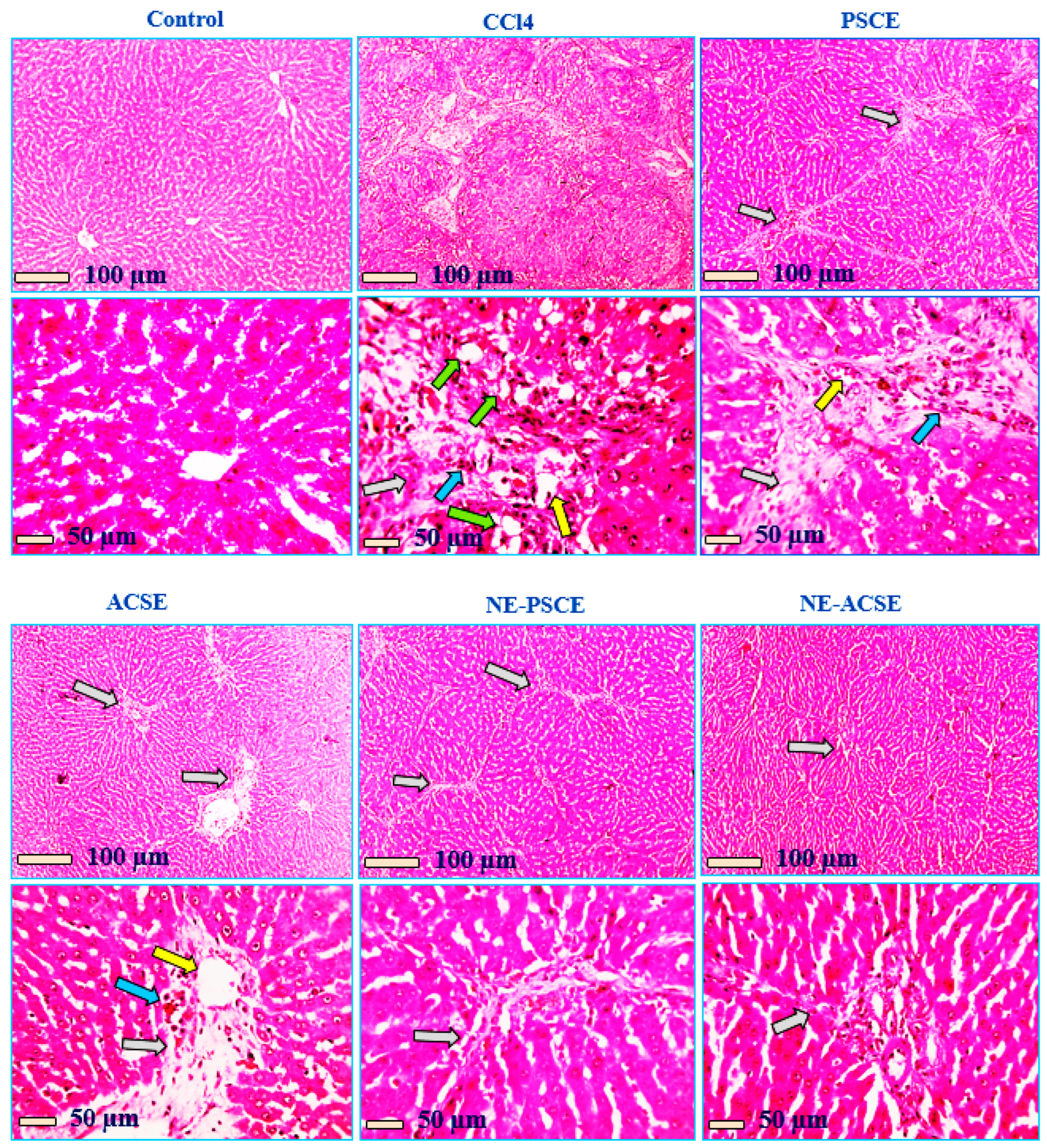
3.5.5. Histopathological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, J.-H.; Yu, Y.-Y.; Xu, X.-Y. Management of chronic liver diseases and cirrhosis: Current status and future directions. Chin. Med. J. 2020, 133, 2647–2649. [Google Scholar] [CrossRef] [PubMed]
- Manka, P.; Zeller, A.; Syn, W.-K. Fibrosis in Chronic Liver Disease: An Update on Diagnostic and Treatment Modalities. Drugs 2019, 79, 903–927. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef]
- Dai, C.; Xiao, X.; Li, D.; Tun, S.; Wang, Y.; Velkov, T.; Tang, S. Chloroquine ameliorates carbon tetrachloride-induced acute liver injury in mice via the concomitant inhibition of inflammation and induction of apoptosis. Cell Death Dis. 2018, 9, 1164. [Google Scholar] [CrossRef]
- Li, J.; Tuo, B. Current and Emerging Approaches for Hepatic Fibrosis Treatment. Gastroenterol. Res. Pract. 2021, 2021, 6612892. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, J.; Xiao, S.; Hu, M.; Cheng, J.; Yao, C.; Zhuang, Q. The impact of liver fibrosis on the progression of hepatocellular carcinoma via a hypoxia-immune-integrated prognostic model. Int. Immunopharmacol. 2023, 125, 111136. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Chen, G.; Yibang, Z.; Hussain, A.; Shafiq, M.; Raza, F.; Liu, D.; Wang, K.; Cao, J.; Qi, X. A new approach based on CXCR4-targeted combination liposomes for the treatment of liver fibrosis. Biomater. Sci. 2022, 10, 2650–2664. [Google Scholar] [CrossRef]
- Poilil Surendran, S.; Thomas, R.; Moon, M.-J.; Jeong, Y. Nanoparticles for the treatment of liver fibrosis. Int. J. Nanomed. 2017, 12, 6997–7006. [Google Scholar] [CrossRef]
- Bai, X.; Su, G.; Zhai, S. Recent Advances in Nanomedicine for the Diagnosis and Therapy of Liver Fibrosis. Nanomaterials 2020, 10, 1945. [Google Scholar] [CrossRef]
- Eftekhari, A.; Arjmand, A.; Asheghvatan, A.; Švajdlenková, H.; Šauša, O.; Abiyev, H.; Ahmadian, E.; Smutok, O.; Khalilov, R.; Kavetskyy, T.; et al. The Potential Application of Magnetic Nanoparticles for Liver Fibrosis Theranostics. Front. Chem. 2021, 9, 674786. [Google Scholar] [CrossRef]
- Suntres, Z.E. Liposomal Antioxidants for Protection against Oxidant-Induced Damage. J. Toxicol. 2011, 2011, 152474. [Google Scholar] [CrossRef]
- Kumar, N.; Verma, A.; Mandal, A. Formation, characteristics and oil industry applications of nanoemulsions: A review. J. Pet. Sci. Eng. 2021, 206, 109042. [Google Scholar] [CrossRef]
- Kumar, N.; Mandal, A. Surfactant Stabilized Oil-in-Water Nanoemulsion: Stability, Interfacial Tension, and Rheology Study for Enhanced Oil Recovery Application. Energy Fuels 2018, 32, 6452–6466. [Google Scholar] [CrossRef]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef]
- Wilson, R.J.; Li, Y.; Yang, G.; Zhao, C.-X. Nanoemulsions for drug delivery. Particuology 2022, 64, 85–97. [Google Scholar] [CrossRef]
- Ansari, V.; Ahmad, U.; Upadhyay, T.; Sultana, N.; Akhtar, J. Exploring Nanoemulsion for Liver Cancer Therapy. Curr. Cancer Ther. Rev. 2020, 16, 260–268. [Google Scholar] [CrossRef]
- Sravanthi, V.; Preethi Pallavi, M.C.; Bonam, S.R.; Sathyabama, S.; Sampath Kumar, H.M. Oleic acid nanoemulsion for nasal vaccination: Impact on adjuvanticity based immune response. J. Drug Deliv. Sci. Technol. 2015, 28, 56–63. [Google Scholar] [CrossRef]
- de Lourdes Pérez-González, M.L.; González-de la Rosa, C.H.; Pérez-Hernández, G.; Beltrán, H.I. Nanostructured oleic acid/polysorbate 80 emulsions with diminished toxicity in NL-20 cell line: Insights of potential drug carriers. Colloids Surf. B Biointerfaces 2020, 187, 110758. [Google Scholar] [CrossRef] [PubMed]
- Metoui, M.; Essid, A.; Bouzoumita, A.; Ferchichi, A. Chemical Composition, Antioxidant and Antibacterial Activity of Tunisian Date Palm Seed. Pol. J. Environ. Stud. 2018, 28, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Fikry, M.; Yusof, Y.; Al-awaadh, A.; Abdul Rahman, R.; Chin, N. Prediction of the shelf-life of date seeds brew by integration of acceptability and quality indices. J. Food Meas. Charact. 2020, 14, 1158–1171. [Google Scholar] [CrossRef]
- Souda, B.; Rami, R.; Jalloul, B.; Mohamed, D. Roasted date palm seeds (Phoenix dactylifera) as an alternative coffee: Chemical composition and bioactive properties. Biomass Convers. Biorefinery 2022, 12, 3771–3781. [Google Scholar] [CrossRef]
- Niazi, S.; Khan, I.; Rasheed, S.; Niazi, F.; Shoaib, M.; Raza, H.; Iqbal, M. An Overview: Date Palm Seed Coffee, a Functional Beverage. Int. J. Public Health 2017, 2, 18–25. [Google Scholar]
- Fikry, M.; Yusof, Y.A.; Al-Awaadh, A.M.; Abdul Rahman, R.; Chin, N.L.; Ghazali, H.M. Antioxidative and Quality Properties of Full-Fat Date Seeds Brew as Influenced by the Roasting Conditions. Antioxidants 2019, 8, 226. [Google Scholar] [CrossRef] [PubMed]
- Bentrad, N.; Hamida-Ferhat, A. Chapter 22—Date palm fruit (Phoenix dactylifera): Nutritional values and potential benefits on health. In The Mediterranean Diet, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 239–255. [Google Scholar] [CrossRef]
- Dghaim, R.; Hammami, Z.; Al Ghali, R.; Smail, L.; Haroun, D. The Mineral Composition of Date Palm Fruits (Phoenix dactylifera L.) under Low to High Salinity Irrigation. Molecules 2021, 26, 7361. [Google Scholar] [CrossRef] [PubMed]
- Nigra, A.D.; Teodoro, A.J.; Gil, G.A. A Decade of Research on Coffee as an Anticarcinogenic Beverage. Oxid. Med. Cell. Longev. 2021, 2021, 4420479. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.K.; Mhd Rodzi, N.A. Addressing the Neuroprotective Actions of Coffee in Parkinson’s Disease: An Emerging Nutrigenomic Analysis. Antioxidants 2022, 11, 1587. [Google Scholar] [CrossRef]
- Degertekin, B. Regular coffee intake improves liver enzyme levels and liver histology in patients with chronic alcohol consumption, non-alcoholic fatty liver and non-alcoholic steatohepatitis: Report of 259 cases. Hepatol. Forum 2020, 1, 88–96. [Google Scholar] [CrossRef]
- Heath, R.; Brahmbhatt, M.; Tahan, A.; Ibdah, J.; Tahan, V. Coffee: The magical bean for liver diseases. World J. Hepatol. 2017, 9, 689. [Google Scholar] [CrossRef]
- Kolb, H.; Martin, S.; Kempf, K. Coffee and Lower Risk of Type 2 Diabetes: Arguments for a Causal Relationship. Nutrients 2021, 13, 1144. [Google Scholar] [CrossRef]
- Yildirim, S.T.; Oztop, M.H.; Soyer, Y. Cinnamon oil nanoemulsions by spontaneous emulsification: Formulation, characterization and antimicrobial activity. LWT 2017, 84, 122–128. [Google Scholar] [CrossRef]
- Wang, S.; Liang, X.; Zhao, W.; Mi, X.; Zhang, C.; Zhang, W.; Cheng, Y.; Wang, L.; Jiang, Y. Preparation of nanoemulsion of grapefruit seed extract and evaluation of its antibacterial activity. J. Food Process. Preserv. 2022, 46, e16197. [Google Scholar] [CrossRef]
- Singh, R.; Murthy, K.; Jayaprakasha, G. Studies on the Antioxidant Activity of Pomegranate (Punica granatum) Peel and Seed Extracts Using In Vitro Models. J. Agric. Food Chem. 2002, 50, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Brem, B.; Seger, C.; Pacher, T.; Hartl, M.; Hadacek, F.; Hofer, O.; Vajrodaya, S.; Greger, H. Antioxidant dehydrotocopherols as a new chemical character of Stemona species. Phytochemistry 2004, 65, 2719–2729. [Google Scholar] [CrossRef] [PubMed]
- Iredale, J.P.; Benyon, R.C.; Pickering, J.; McCullen, M.; Northrop, M.; Pawley, S.; Hovell, C.; Arthur, M.J. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J. Clin. Investig. 1998, 102, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Elzoheiry, A.; Ayad, E.; Omar, N.; Elbakry, K.; Hyder, A. Anti-liver fibrosis activity of curcumin/chitosan-coated green silver nanoparticles. Sci. Rep. 2022, 12, 18403. [Google Scholar] [CrossRef]
- Ozden, M.; Maral, H.; Akaydın, D.; Cetınalp, P.; Kalender, B. Erythrocyte glutathione peroxidase activity, plasma malondialdehyde and erythrocyte glutathione levels in hemodialysis and CAPD patients. Clin. Biochem. 2002, 35, 269–273. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. In Oxygen Radicals in Biological Systems; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Nishikimi, M.; Appaji Rao, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Mousavizadeh, K.; Rahimian, R.; Fakhfouri, G.; Aslani, F.S.; Ghafourifar, P. Anti-inflammatory effects of 5-HT3 receptor antagonist, tropisetron on experimental colitis in rats. Eur. J. Clin. Investig. 2009, 39, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Sahebkar, A.; Parvin, S.; Saadat, A. A randomized controlled trial on the anti-inflammatory effects of curcumin in patients with chronic sulphur mustard-induced cutaneous complications. Ann. Clin. Biochem. 2012, 49, 580–588. [Google Scholar] [CrossRef]
- Pappas, N.J. Enhanced Cardiac Enzyme Profile. Clin. Lab. Med. 1989, 9, 689–716. [Google Scholar] [CrossRef] [PubMed]
- Doumas, B.T.; Biggs, H.G.; Arends, R.L.; Pinto, P.V.C. Determination of Serum Albumin. In Standard Methods of Clinical Chemistry; Cooper, G.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1972; pp. 175–188. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 4–7. [Google Scholar]
- Kell, D.B.; Brown, M.; Davey, H.M.; Dunn, W.B.; Spasic, I.; Oliver, S.G. Metabolic footprinting and systems biology: The medium is the message. Nat. Rev. Microbiol. 2005, 3, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Trethewey, R.N.; Krotzky, A.J.; Willmitzer, L. Metabolite profiling: From diagnostics to systems biology. Nat. Rev. Mol. Cell Biol. 2004, 5, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Vijayabaskar, G.; Elango, V. Determination of phytocompounds in Withania somnifera and Smilax china using GC-MS technique. J. Pharmacogn. Phytochem. 2018, 7, 554–557. [Google Scholar]
- Al-Shahib, W.; Marshall, R. Fatty acid content of the seeds from 14 varieties of date palm Phoenix dactylifera L. Int. J. Food Sci. Technol. 2003, 38, 709–712. [Google Scholar] [CrossRef]
- Al-Hooti, S.; Sidhu, J.S.; Qabazard, H. Chemical composition of seeds of date fruit cultivars of United Arab Emirates. J. Food Sci. Technol. 1998, 35, 44–46. [Google Scholar]
- Santamaría, E.; Maestro, A.; Vilchez, S.; González, C. Study of nanoemulsions using carvacrol/MCT-(Oleic acid-potassium oleate)/Tween 80®—Water system by low energy method. Heliyon 2023, 9, e16967. [Google Scholar] [CrossRef]
- Farid, R.M.; El-Salamouni, N.S.; El-Kamel, A.H.; El-Gamal, S.S. Chapter 16—Lipid-based nanocarriers for ocular drug delivery. In Micro and Nano Technologies; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 495–522. [Google Scholar] [CrossRef]
- Ajitha, B.; Kumar Reddy, Y.A.; Reddy, P.S.; Jeon, H.J.; Ahn, C.W. Role of capping agents in controlling silver nanoparticles size, antibacterial activity and potential application as optical hydrogen peroxide sensor. RSC Adv. 2016, 6, 36171–36179. [Google Scholar] [CrossRef]
- Mohamady Hussein, M.A.; Baños, F.G.D.; Grinholc, M.; Abo Dena, A.S.; El-Sherbiny, I.M.; Megahed, M. Exploring the physicochemical and antimicrobial properties of gold-chitosan hybrid nanoparticles composed of varying chitosan amounts. Int. J. Biol. Macromol. 2020, 162, 1760–1769. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef]
- Sari, T.P.; Mann, B.; Kumar, R.; Singh, R.R.B.; Sharma, R.; Bhardwaj, M.; Athira, S. Preparation and characterization of nanoemulsion encapsulating curcumin. Food Hydrocoll. 2015, 43, 540–546. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Tan, H.; Zhang, R.; McClements, D.J. Vitamin E Encapsulation within Oil-in-Water Emulsions: Impact of Emulsifier Type on Physicochemical Stability and Bioaccessibility. J. Agric. Food Chem. 2019, 67, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Inhibition of β-carotene degradation in oil-in-water nanoemulsions: Influence of oil-soluble and water-soluble antioxidants. Food Chem. 2012, 135, 1036–1043. [Google Scholar] [CrossRef]
- Donsì, F.; Sessa, M.; Mediouni, H.; Mgaidi, A.; Ferrari, G. Encapsulation of bioactive compounds in nanoemulsion-based delivery systems. Procedia Food Sci. 2011, 1, 1666–1671. [Google Scholar] [CrossRef]
- Ahmed, G.M.; El-Ghamery, H.E.; Samy, M.F. Effect of Green and Degree of Roasted Arabic Coffee on Hyperlipidemia and Antioxidant Status in Diabetic Rats. Adv. J. Food Sci. Technol. 2013, 5, 619–626. [Google Scholar] [CrossRef]
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Chu, Y.-F. Coffee: Emerging Health Effects and Disease Prevention; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Farah, A. Coffee Constituents. In Coffee; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 21–58. [Google Scholar] [CrossRef]
- Saeed, M.; Naveed, M.; BiBi, J.; Ali Kamboh, A.; Phil, L.; Chao, S. Potential nutraceutical and food additive properties and risks of coffee: A comprehensive overview. Crit. Rev. Food Sci. Nutr. 2019, 59, 3293–3319. [Google Scholar] [CrossRef] [PubMed]
- López-Froilán, R.; Ramírez-Moreno, E.; Podio, N.S.; Pérez-Rodríguez, M.L.; Cámara, M.; Baroni, M.V.; Wunderlin, D.A.; Sánchez-Mata, M.C. In vitro assessment of potential intestinal absorption of some phenolic families and carboxylic acids from commercial instant coffee samples. Food Funct. 2016, 7, 2706–2711. [Google Scholar] [CrossRef]
- Ősz, B.-E.; Jîtcă, G.; Ștefănescu, R.-E.; Pușcaș, A.; Tero-Vescan, A.; Vari, C.-E. Caffeine and Its Antioxidant Properties—It Is All about Dose and Source. Int. J. Mol. Sci. 2022, 23, 13074. [Google Scholar] [CrossRef] [PubMed]
- Heckman, M.A.; Weil, J.; De Mejia, E.G. Caffeine (1, 3, 7-trimethylxanthine) in Foods: A Comprehensive Review on Consumption, Functionality, Safety, and Regulatory Matters. J. Food Sci. 2010, 75, R77–R87. [Google Scholar] [CrossRef]
- Guth, I.; Matos-Pardal, C.F.; Ferreira-Lima, R.; Loureiro-Rebouças, R.; Sobral, A.C.; Moraes-Marques, C.A.; Kubrusly, L.F. Caffeine attenuates liver damage and improves neurologic signs in a rat model of hepatic encephalopathy. Rev. Gastroenterol. México (Engl. Ed.) 2022, 87, 159–169. [Google Scholar] [CrossRef]
- Wang, Q.; Dai, X.; Yang, W.; Wang, H.; Zhao, H.; Yang, F.; Yang, Y.; Li, J.; Lv, X. Caffeine protects against alcohol-induced liver fibrosis by dampening the cAMP/PKA/CREB pathway in rat hepatic stellate cells. Int. Immunopharmacol. 2015, 25, 340–352. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Lv, X.; Wang, Q.; Zhao, H.; Yang, F.; Yang, Y.; Li, J. Involvement of cAMP-PKA pathway in adenosine A1 and A2A receptor-mediated regulation of acetaldehyde-induced activation of HSCs. Biochimie 2015, 115, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.-J.; Zhou, Z.-B.; Nie, F.-Q.; Zi, C.-T.; Fan, J.P. Progress in Phytochemical and Bioactivities of Coffea arabica L. Med. Res. 2020, 4, 200012. [Google Scholar] [CrossRef]
- Rezk, N.; Sameh, A.; Muzaffar, I.; Omar, A.; Ahmed, M. Comparative evaluation of caffeine content in Arabian coffee with other caffeine beverages. Afr. J. Pharm. Pharmacol. 2018, 12, 19–26. [Google Scholar] [CrossRef][Green Version]
- Ky, C.-L.; Louarn, J.; Dussert, S.; Guyot, B.; Hamon, S.; Noirot, M. Caffeine, trigonelline, chlorogenic acids and sucrose diversity in wild Coffea arabica L. and C. canephora P. accessions. Food Chem. 2001, 75, 223–230. [Google Scholar] [CrossRef]
- Moreira, M.E.D.C.; Pereira, R.G.F.A.; Dias, D.F.; Gontijo, V.S.; Vilela, F.C.; de Moraes, G.D.O.I.; Giusti-Paiva, A.; dos Santos, M.H. Anti-inflammatory effect of aqueous extracts of roasted and green Coffea arabica L. J. Funct. Foods 2013, 5, 466–474. [Google Scholar] [CrossRef]
- Duangjai, A.; Suphrom, N.; Wungrath, J.; Ontawong, A.; Nuengchamnong, N.; Yosboonruang, A. Comparison of antioxidant, antimicrobial activities and chemical profiles of three coffee (Coffea arabica L.) pulp aqueous extracts. Integr. Med. Res. 2016, 5, 324–331. [Google Scholar] [CrossRef]
- Makino, T.; Izumi, K.; Hiratsuka, K.; Kano, H.; Shimada, T.; Nakano, T.; Kadomoto, S.; Naito, R.; Iwamoto, H.; Yaegashi, H.; et al. Anti-proliferative and anti-migratory properties of coffee diterpenes kahweol acetate and cafestol in human renal cancer cells. Sci. Rep. 2021, 11, 675. [Google Scholar] [CrossRef]
- Eldesouki, S.; Qadri, R.; Abu Helwa, R.; Barqawi, H.; Bustanji, Y.; Abu-Gharbieh, E.; El-Huneidi, W. Recent Updates on the Functional Impact of Kahweol and Cafestol on Cancer. Molecules 2022, 27, 7332. [Google Scholar] [CrossRef]
- Komes, D.; Bušić, A. Chapter 3—Antioxidants in Coffee. In Processing and Impact on Antioxidants in Beverages; Preedy, V., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 25–32. [Google Scholar] [CrossRef]
- Gallus, S.; Tavani, A.; Negri, E.; La Vecchia, C. Does Coffee Protect Against Liver Cirrhosis? Ann. Epidemiol. 2002, 12, 202–205. [Google Scholar] [CrossRef]
- Kennedy, O.J.; Roderick, P.; Buchanan, R.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Systematic review with meta-analysis: Coffee consumption and the risk of cirrhosis. Aliment. Pharmacol. Ther. 2016, 43, 562–574. [Google Scholar] [CrossRef]
- Liu, F.; Xiwei, W.; Wu, G.; Chen, L.; Hu, P.; Ren, H.; Hu, H. Coffee Consumption Decreases Risks for Hepatic Fibrosis and Cirrhosis: A Meta-Analysis. PLoS ONE 2015, 10, e0142457. [Google Scholar] [CrossRef]
- Vayalil, P.K. Date Fruits (Phoenix dactylifera Linn): An Emerging Medicinal Food. Crit. Rev. Food Sci. Nutr. 2012, 52, 249–271. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Alqahtani, J.; Al-Youssef, H.; Al-Said, M.; Ashour, A.; Al-Sohaibani, M.; Rafatullah, S. Proanthocyanidin-Rich Date Seed Extract Protects against Chemically Induced Hepatorenal Toxicity. J. Med. Food 2015, 18, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-M.; Xu, H.-G.; Wang, L.; Li, Y.-J.; Sun, P.-H.; Wu, X.-M.; Wang, G.-J.; Chen, W.-M.; Ye, W.-C. Betulinic Acid and its Derivatives as Potential Antitumor Agents. Med. Res. Rev. 2015, 35, 1127–1155. [Google Scholar] [CrossRef]
- Pang, K.; Vijayaraghavan, K.; Alsayed, B.; Seyed, M. Betulinic acid-induced expression of nicotinamide adenine dinucleotide phosphate-diaphorase in the immune organs of mice: A possible role of nitric oxide in immunomodulation. Mol. Med. Rep. 2017, 17, 3035–3041. [Google Scholar] [CrossRef]
- Al-Qarawi, A.A.; Mousa, H.; Ali, B.; Abdel-Rahman, H.; El-Mougy, S.A. Protective effect of extracts from dates (Phoenix dactylifera L.) on carbon tetrachloride-induced hepatotoxicity in rats. Int. J. Appl. Res. Vet. Med. 2009, 2, 176–180. [Google Scholar]
- Okwuosa, C.; Theophilus Kachi, U.; Umeifekwem, J.; Onuba, A.C.; Anioke, I.; Madubueze, R. Hepatoprotective Effect of Methanolic Fruit Extracts of Phoenix dactylifera (Arecaceae) on Thioacetamide Induced Liver Damage in Rats. Am. J. Phytomedicine Clin. Ther. 2014, 2, 290–300. [Google Scholar]
- Guyton, K.Z.; Kensler, T.W. Oxidative mechanisms in carcinogenesis. Br. Med. Bull. 1993, 49, 523–544. [Google Scholar] [CrossRef]
- Fain, O. Carences en vitamine C. La Rev. Médecine Interne 2004, 25, 872–880. [Google Scholar] [CrossRef]
- Sherman, K.E. Chapter 14—Evaluation of Abnormal Liver Tests. In GI/Liver Secrets Plus, 4th ed.; McNally, P.R., Ed.; Mosby: Philadelphia, PA, USA, 2010; pp. 94–99. [Google Scholar] [CrossRef]
- Boone, L.; Meyer, D.; Cusick, P.; Ennulat, D.; Bolliger, A.; Everds, N.; Meador, V.; Elliott, G.; Honor, D.; Bounous, D.; et al. Selection and interpretation of clinical pathology indicators of hepatic injury in preclinical studies. Vet. Clin. Pathol. 2005, 34, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D. 77—Liver Failure. In Pediatric Gastrointestinal Disease, 4th ed.; Wyllie, R., Hyams, J.S., Eds.; W.B. Saunders: St. Louis, MO, USA, 2011; pp. 840–852.e3. [Google Scholar] [CrossRef]
- Salem, G.A.; Shaban, A.; Diab, H.A.; Elsaghayer, W.A.; Mjedib, M.D.; Hnesh, A.M.; Sahu, R.P. Phoenix dactylifera protects against oxidative stress and hepatic injury induced by paracetamol intoxication in rats. Biomed. Pharmacother. 2018, 104, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Khan, T.J.; Kalamegam, G.; Pushparaj, P.N.; Chaudhary, A.; Abuzenadah, A.; Kumosani, T.; Barbour, E.; Al-Qahtani, M. Anti-cancer effects of Ajwa dates (Phoenix dactylifera L.) in diethylnitrosamine induced hepatocellular carcinoma in Wistar rats. BMC Complement. Altern. Med. 2017, 17, 418. [Google Scholar] [CrossRef]
- Alothaid, H. Evaluation of date palm kernels’ biological activities and possible role in improving liver and kidney functions. J. King Saud Univ. Sci. 2022, 34, 102211. [Google Scholar] [CrossRef]
- Ashaolu, T.J. Nanoemulsions for health, food, and cosmetics: A review. Environ. Chem. Lett. 2021, 19, 3381–3395. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Jafari, S.M.; Esfanjani, A.F.; Akhavan, S. Application of nano-encapsulated olive leaf extract in controlling the oxidative stability of soybean oil. Food Chem. 2016, 190, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Azmi, N.A.; Elgharbawy, A.A.M.; Motlagh, S.R.; Samsudin, N.; Salleh, H.M. Nanoemulsions: Factory for Food, Pharmaceutical and Cosmetics. Processes 2019, 7, 617. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S.; Gandhi, M. Nanoemulsion ingredients and components. Environ. Chem. Lett. 2019, 17, 917–928. [Google Scholar] [CrossRef]



| S. No. | RT | Name of the Compound | Molecular Formula | M.W. | Area % |
|---|---|---|---|---|---|
| 1 | 9.33 | Trimethylsilyl)oxy]phenol | C9H14O2Si | 182 | 0.67 |
| 2 | 11.36 | Niacin, TMS derivative | C9H13NO2Si | 195 | 0.78 |
| 3 | 11.65 | Methoxy-4-vinylphenol | C9H10O2 | 150 | 0.71 |
| 4 | 14.01 | 10,11-Dimethylbicyclo [6.3.0]undec-1(8)-en-9-one | C13H20O | 192 | 0.68 |
| 5 | 14.78 | 2,6-Dicarbomethoxy-4-chloroterahydropyran | C13H20O | 192 | 0.58 |
| 6 | 16.13 | 2,2-Dimethyl-5-[2-(2-trimethylsilylethoxymethoxy)-propyl]-[1,3]dioxolane-4-carboxaldehyde | C15H30O5Si | 318 | 1.27 |
| 7 | 16.95 | Triethylene glycol, 2TMS derivative | C12H30O4Si2 | 294 | 0.63 |
| 8 | 17.34 | 6-D1-androst-5-en-3á-ol | C19H31DO | 227 | 1.43 |
| 9 | 17.99 | 2,2,18,18-Tetramethyl-3,6,10,13,17-pentaoxa-2,18-disilaneonadecane | C16H38O5Si2 | 366 | 0.37 |
| 10 | 18.13 | 2,2,18,18-Tetramethyl-3,6,10,13,17-pentaoxa-2,18-disilaneonadecane | C16H38O5Si2 | 366 | 0.49 |
| 11 | 18.23 | Tripropylene glycol monomethyl ether, TMS derivative | C13H30O4Si | 278 | 0.34 |
| 12 | 18.63 | à-L-Galactopyranoside, methyl 6-deoxy-2-O-(trimethylsilyl)-, cyclic methylboronate | C11H23BO5Si | 274 | 0.97 |
| 13 | 20.30 | Dodecanoic acid, TMS derivative | C15H32O2Si | 72 | 0.54 |
| 14 | 21.60 | á-D-Galactopyranoside, methyl 2,6-bis-O-(trimethylsilyl)-, cyclic methylboronate | C14H31BO6Si2 | 362 | 0.56 |
| 15 | 23.22 | 1H-Purine-2,6-dione, 3,7-dihydro-1,3,7-trimethyl- | C8H10N4O2 | 194 | 56.36 |
| 16 | 24.43 | Myristic acid, TMS derivative | C17H36O2Si | 300 | 0.73 |
| 17 | 25.68 | Pentadecanoic acid, 14-methyl-, methyl ester | C17H34O2 | 270 | 0.32 |
| 18 | 26.47 | Hexadecanoic acid | C16H32O2 | 256 | 2.53 |
| 19 | 28.22 | Palmitic Acid, TMS derivative | C19H40O2Si | 328 | 10.81 |
| 20 | 29.44 | 9,12-Octadecadienoic acid (Z,Z)- | C18H32O2 | 280 | 1.29 |
| 21 | 29.62 | trans-13-Octadecenoic acid | C18H34O2 | 282 | 1.65 |
| 22 | 30.08 | Octadecanoic acid | C18H36O2 | 284 | 0.41 |
| 23 | 30.99 | 9,12-Octadecadienoic acid (Z,Z)-, TMS derivative | C21H40O2Si | 352 | 0.94 |
| 24 | 31.14 | 9-Octadecenoic acid, (E)-, TMS derivative | C21H42O2Si | 345 | 1.49 |
| 25 | 31.67 | Stearic acid, TMS derivative | C21H44O2Si | 356 | 0.63 |
| 26 | 34.08 | Creatindial | C20H24O2 | 296 | 0.81 |
| 27 | 34.53 | 5,16,20-Pregnatriene-3beta,20-diol diacetate | C25H34O4 | 389 | 3.23 |
| 28 | 34.78 | (20R)-18,20-Epoxypregn-5-en-3á-yl acetate | C21H30O | 289 | 0.71 |
| 29 | 35.14 | 9-Anthracenol, 1,4,8-trimethoxy-2-methyl- | C18H18O4 | 298 | 3.37 |
| 30 | 35.63 | Aarda-5,20(22)-dienolide, 3,14,19-trihydroxy-, (3á)- | C23H32O5 | 388 | 1.05 |
| 31 | 36.20 | 5-Iodo-6-formyl-3,4-dimethoxy-2,2’-bipyridine | C13H11IN2O3 | 370 | 1.10 |
| 32 | 40.44 | Aucubin, hexakis(trimethylsilyl) ether | C33H70O9Si6 | 778 | 0.33 |
| 33 | 44.22 | Stigmasta-5,22-dien-3-ol | C29H48O | 412 | 0.58 |
| 34 | 44.74 | á-Sitosterol | C29H50O | 414 | 0.93 |
| 35 | 45.23 | 9,12-Octadecadienoic acid (z,z)-, 2,3-bis[(trimethylsilyl)oxy]propyl ester | C27H54O4Si2 | 498 | 0.69 |
| S. No. | RT | Name of the Compound | Molecular Formula | M.W. | Area % |
|---|---|---|---|---|---|
| 1 | 9.32 | à-Phenylbenzenemethyl 4-nitrobenzoateate | C20H15NO4 | 333 | 1.03 |
| 2 | 10.62 | Diethylene glycol, 2TMS derivative | C10H26O3Si2 | 250 | 0.76 |
| 3 | 13.16 | Tetraethylene glycol, TMS derivative | C11H26O5Si | 266 | 5.23 |
| 4 | 14.84 | Tripropylene glycol monomethyl ether, TMS derivative | C13H30O4Si | 278 | 0.80 |
| 5 | 14.94 | Tripropylene glycol monomethyl ether, TMS derivative | C13H30O4Si | 278 | 1.30 |
| 6 | 15.03 | 1-(1-Butoxy-2-propoxy)-2-propanol, TMS derivative | C13H30O3Si | 262 | 1.44 |
| 7 | 15.12 | 1-(1-Butoxy-2-propoxy)-2-propanol, TMS derivative | C13H30O3Si | 262 | 0.97 |
| 8 | 16.96 | Triethylene glycol, 2TMS derivative | C12H30O4Si2 | 294 | 9.98 |
| 9 | 17.75 | Tripropylene glycol monomethyl ether, TMS derivative | C13H30O4Si | 278 | 1.20 |
| 10 | 17.99 | Tripropylene glycol monomethyl ether, TMS derivative | C13H30O4Si | 1.56 | |
| 11 | 18.13 | Tripropylene glycol mono-n-butyl ether, TMS derivative | C16H36O4Si | 320 | 1.99 |
| 12 | 18.24 | Tripropylene glycol monomethyl ether, TMS derivative | C13H30O4Si | 278 | 2.23 |
| 13 | 20.30 | Dodecanoic acid, TMS derivative | C15H32O2Si | 272 | 4.37 |
| 14 | 21.59 | Cyclopentanetridecanoic acid, methyl ester | C19H36O2 | 296 | 0.68 |
| 15 | 24.42 | Myristic acid, TMS derivative | C17H36O2Si | 300 | 3.62 |
| 16 | 25.68 | Pentadecanoic acid, 14-methyl-, methyl ester | C17H34O2 | 270 | 0.67 |
| 17 | 26.41 | Hexadecanoic acid | C16H32O2 | 256 | 0.62 |
| 18 | 26.49 | d-Galactose, 2,3,4,5,6-pentakis-O-(trimethylsilyl)-, o-methyloxyme, (1Z)- | C22H55NO6Si5 | 569 | 0.67 |
| 19 | 28.22 | Palmitic Acid, TMS derivative | C19H40O2Si | 328 | 15.32 |
| 20 | 28.86 | 10-Octadecenoic acid, methyl ester | C19H36O2 | 296 | 1.27 |
| 21 | 29.61 | cis-13-Octadecenoic acid | C18H34O2 | 282 | 2.87 |
| 22 | 29.74 | Benzoxepino [5,4-b]pyridine-3-carbonitrile, 5,6-dihydro-2-methyl-4-(methylthio)- | C16H14N2OS | 282 | 0.86 |
| 23 | 29.90 | 9-octadecenamide | C18H35NO | 281 | 0.48 |
| 24 | 30.91 | Decaethylene glycol, 2TMS derivative | C26H58O11Si2 | 602 | 0.40 |
| 25 | 30.99 | Linoelaidic acid, trimethylsilyl ester | C21H40O2Si | 352 | 2.03 |
| 26 | 31.16 | Oleic Acid, (Z)-, TMS derivative | C21H42O2Si | 354 | 12.63 |
| 27 | 31.67 | Stearic acid, TMS derivative | C21H44O2Si | 356 | 1.90 |
| 28 | 32.08 | Glycidyl palmitate | C19H36O3 | 312 | 0.58 |
| 29 | 32.84 | 9-octadecenamide | C18H35NO | 281 | 1.16 |
| 30 | 34.87 | Glycidyl oleate | C21H38O3 | 338 | 1.10 |
| 31 | 35.86 | Diisooctyl phthalate | C24H38O4 | 390 | 1.22 |
| 32 | 36.57 | 1,3-Dipalmitin, TMS derivative | C38H76O5Si | 640 | 1.02 |
| 33 | 37.94 | 9-Octadecenoic acid (Z)-, 2-hydroxy-1-(hydroxymethyl)ethyl ester | C21H40O4 | 356 | 1.30 |
| 34 | 38.98 | 9-Octadecenoic acid (z)-, 2-[(trimethylsilyl)oxy]-1-[[(trimethylsilyl)oxy]methyl]ethyl ester | C27H56O4Si2 | 500 | 0.88 |
| 35 | 39.10 | 9-Octadecenoic acid (z)-, 2-[(trimethylsilyl)oxy]-1-[[(trimethylsilyl)oxy]methyl]ethyl ester | C27H56O4Si2 | 500 | 2.32 |
| 36 | 40.45 | D-(+)-Trehalose, octakis(trimethylsilyl) ether | C36H86O11Si8 | 918 | 1.52 |
| 37 | 43.20 | 9,12,15-Octadecatrienoic acid, 2,3-bis[(trimethylsilyl)oxy]propyl ester, (z,z,z)- | C27H52O4Si2 | 496 | 1.14 |
| 38 | 43.43 | 2-(dodecanoyloxy)-1-(hydroxymethyl)ethyl laurate # | C27H52O5 | 456 | 0.88 |
| 39 | 43.92 | Ethyl iso-allocholate | C26H44O5 | 436 | 0.69 |
| 40 | 44.15 | Lup-20(29)-en-3-one | C30H48O | 424 | 1.09 |
| 41 | 44.75 | ç-Sitosterol | C29H50O | 414 | 5.87 |
| 42 | 44.87 | Cholest-5-en-3-ol, 24-propylidene-, (3á)- | C30H50O | 426 | 1.26 |
| Group | Weight Gain % | Food Intake (g) |
|---|---|---|
| Control | 30.29 ± 6.5 | 24.48 ± 3.71 |
| CCl4 | 17.48 ± 5.24 *** | 16.32 ± 0.32 **** |
| ACSE | 24.65 ± 3.92 ns | 24.40 ± 0.49 ns |
| NE-ACSE | 29.07 ± 4.37 ns | 25.89 ± 0.34 ns |
| PSCE | 24.21 ± 3.36 ns | 20.39 ± 0.84 ** |
| NE-PSCE | 25.63 ± 1.31 ns | 24.49 ± 0.21 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamri, E.S.; Bayomy, H.M.; Hussein, M.A.M.; Ozaybi, N.A.; Almasoudi, S.E.; Zidan, N.S.; Albalwi, R.A.; Atteia, H.H.; EL-Ezaly, F.M. Nanoemulsions of Phoenix dactylifera L. (Decaffeinated) and Coffea arabica L. Extracts as a Novel Approach for the Treatment of Carbon Tetrachloride-Mediated Liver Fibrosis. Antioxidants 2024, 13, 355. https://doi.org/10.3390/antiox13030355
Alamri ES, Bayomy HM, Hussein MAM, Ozaybi NA, Almasoudi SE, Zidan NS, Albalwi RA, Atteia HH, EL-Ezaly FM. Nanoemulsions of Phoenix dactylifera L. (Decaffeinated) and Coffea arabica L. Extracts as a Novel Approach for the Treatment of Carbon Tetrachloride-Mediated Liver Fibrosis. Antioxidants. 2024; 13(3):355. https://doi.org/10.3390/antiox13030355
Chicago/Turabian StyleAlamri, Eman S., Hala M. Bayomy, Mohamed A. Mohamady Hussein, Nawal A. Ozaybi, Seham E. Almasoudi, Nahla S. Zidan, Renad A. Albalwi, Hebatallah H. Atteia, and Fayza M. EL-Ezaly. 2024. "Nanoemulsions of Phoenix dactylifera L. (Decaffeinated) and Coffea arabica L. Extracts as a Novel Approach for the Treatment of Carbon Tetrachloride-Mediated Liver Fibrosis" Antioxidants 13, no. 3: 355. https://doi.org/10.3390/antiox13030355
APA StyleAlamri, E. S., Bayomy, H. M., Hussein, M. A. M., Ozaybi, N. A., Almasoudi, S. E., Zidan, N. S., Albalwi, R. A., Atteia, H. H., & EL-Ezaly, F. M. (2024). Nanoemulsions of Phoenix dactylifera L. (Decaffeinated) and Coffea arabica L. Extracts as a Novel Approach for the Treatment of Carbon Tetrachloride-Mediated Liver Fibrosis. Antioxidants, 13(3), 355. https://doi.org/10.3390/antiox13030355






