Strain-Specific Benefits of Bacillus on Growth, Intestinal Health, Immune Modulation, and Ammonia-Nitrogen Stress Resilience in Hybrid Grouper
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Acclimation
2.2. Preparation of Probiotic Diets
2.3. Fish Rearing and Experimental Design
2.4. Sampling Procedure
2.5. Growth Indices
- WG (g) = FBWt − IBW
- FCR (%) = WG (g)/Dry feed intake (g)
- ADG (g) = WG (g)/days (d) × 100
- CF (g/cm3) = FBWt (g)/(FL)3 (cm)3 × 100
- where IBW, FBWt, and FL refer to the initial weight, final weight, and final length, respectively.
2.6. Serum Antioxidant Activities and Malondialdehyde Levels
2.7. Intestinal Morphology Parameters
2.8. Intestinal Digestive Enzyme Activity
2.9. Hepatic Immune-Related Gene mRNA Expression
2.10. Ammonia-Nitrogen Stress Test
2.11. Data Analysis
3. Results
3.1. Growth Performance
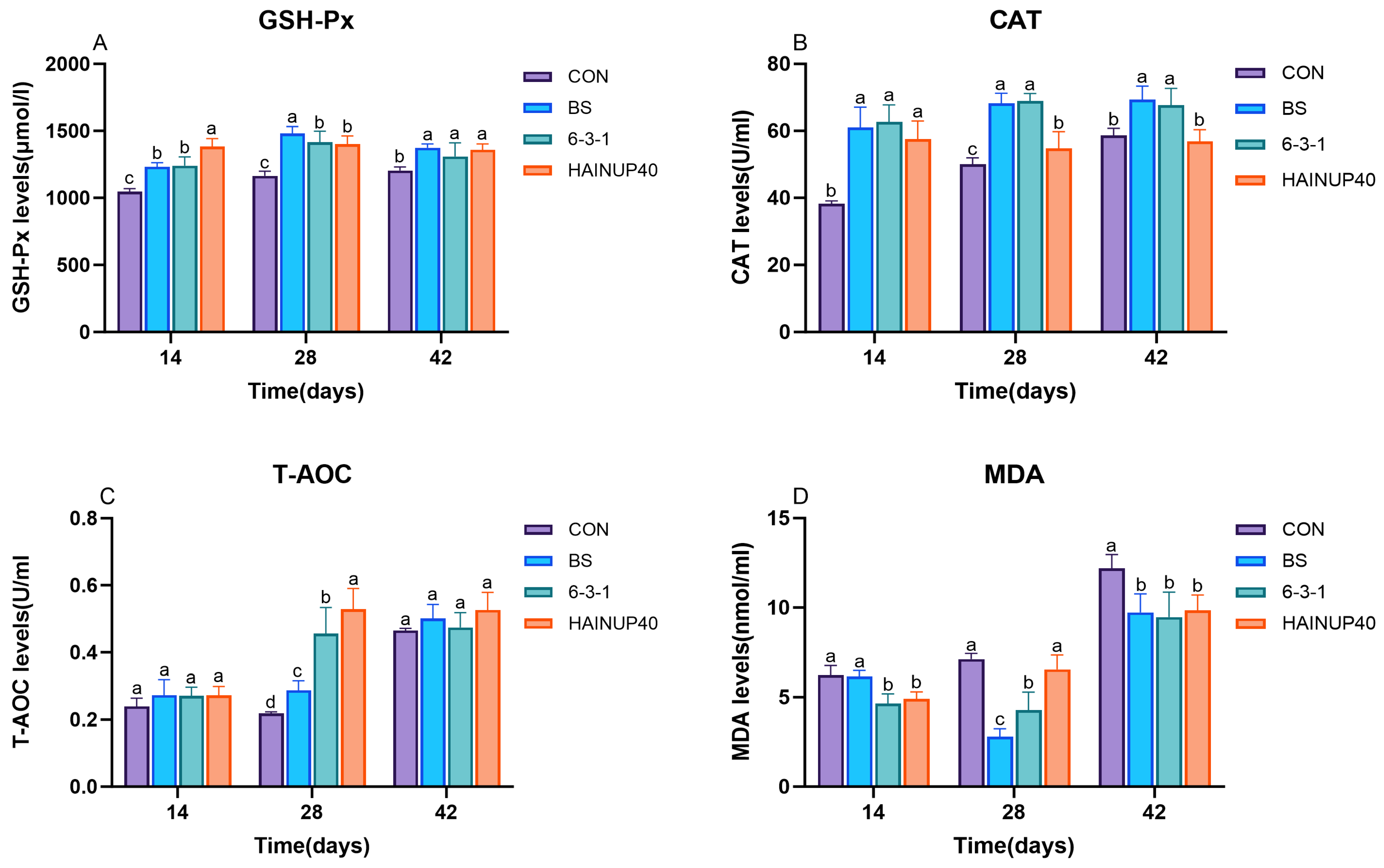
3.2. Serum Antioxidant Activities and Malondialdehyde Levels
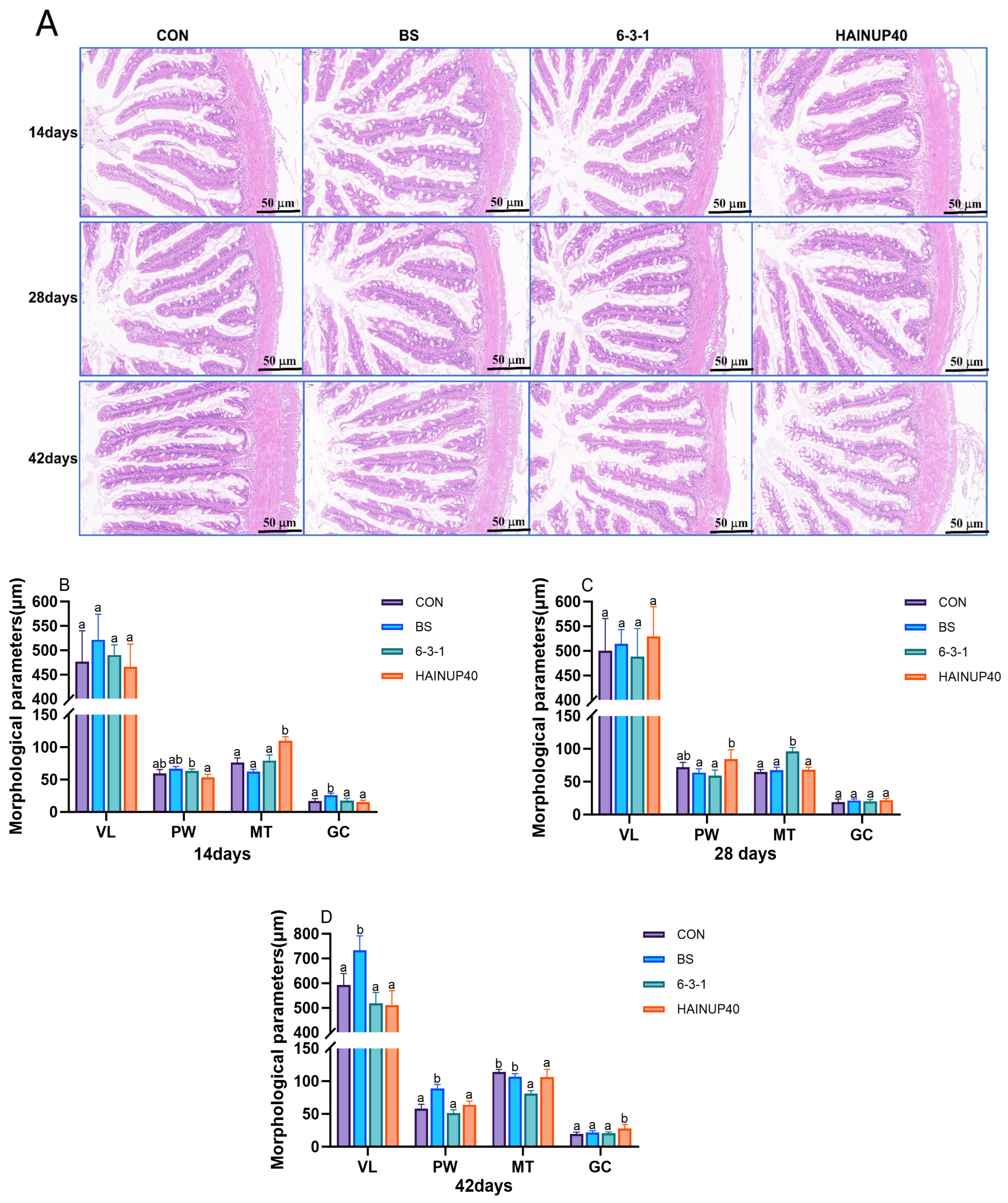
3.3. Intestinal Morphology
3.4. Intestinal Digestive Enzyme Activity
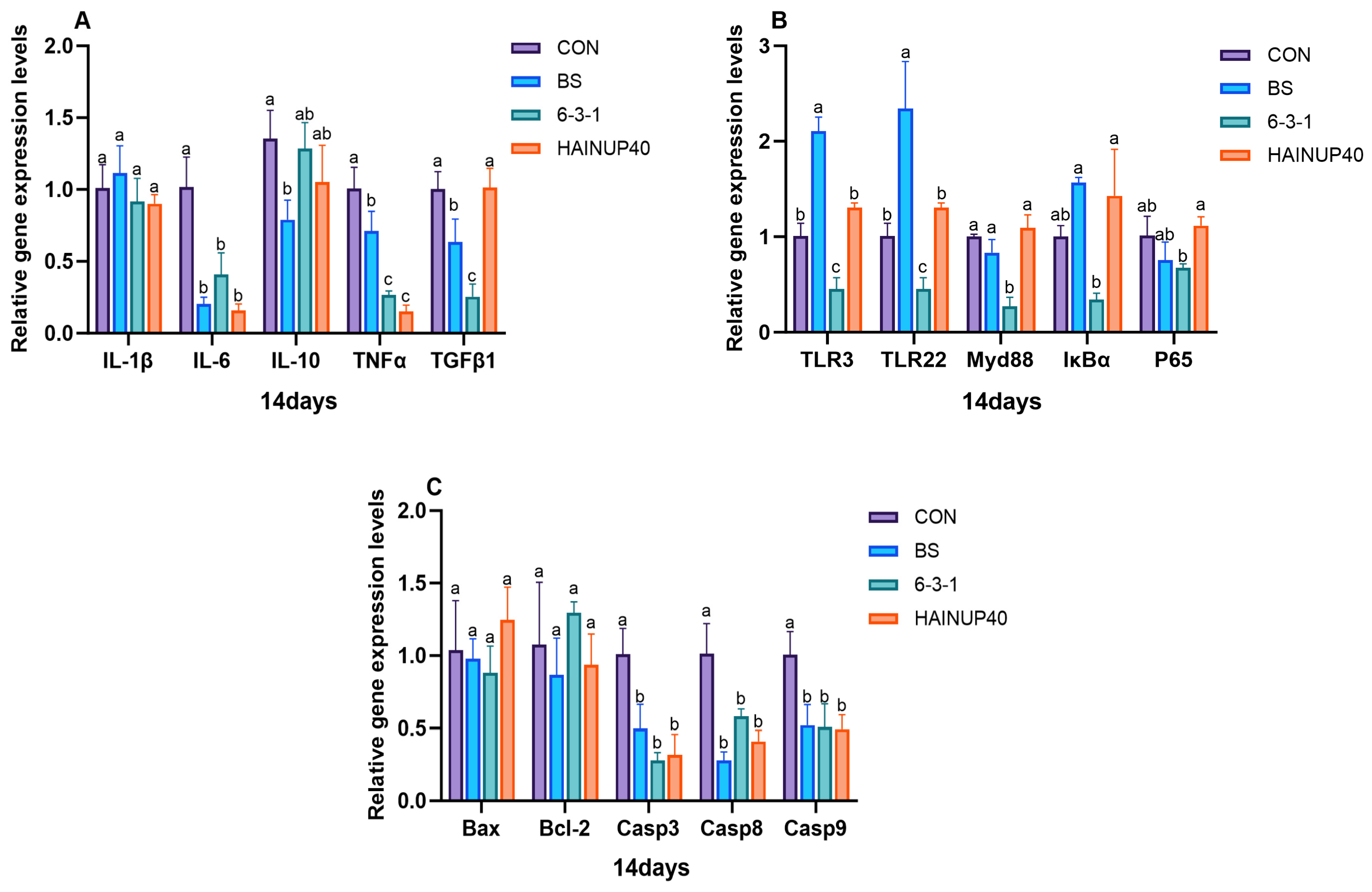
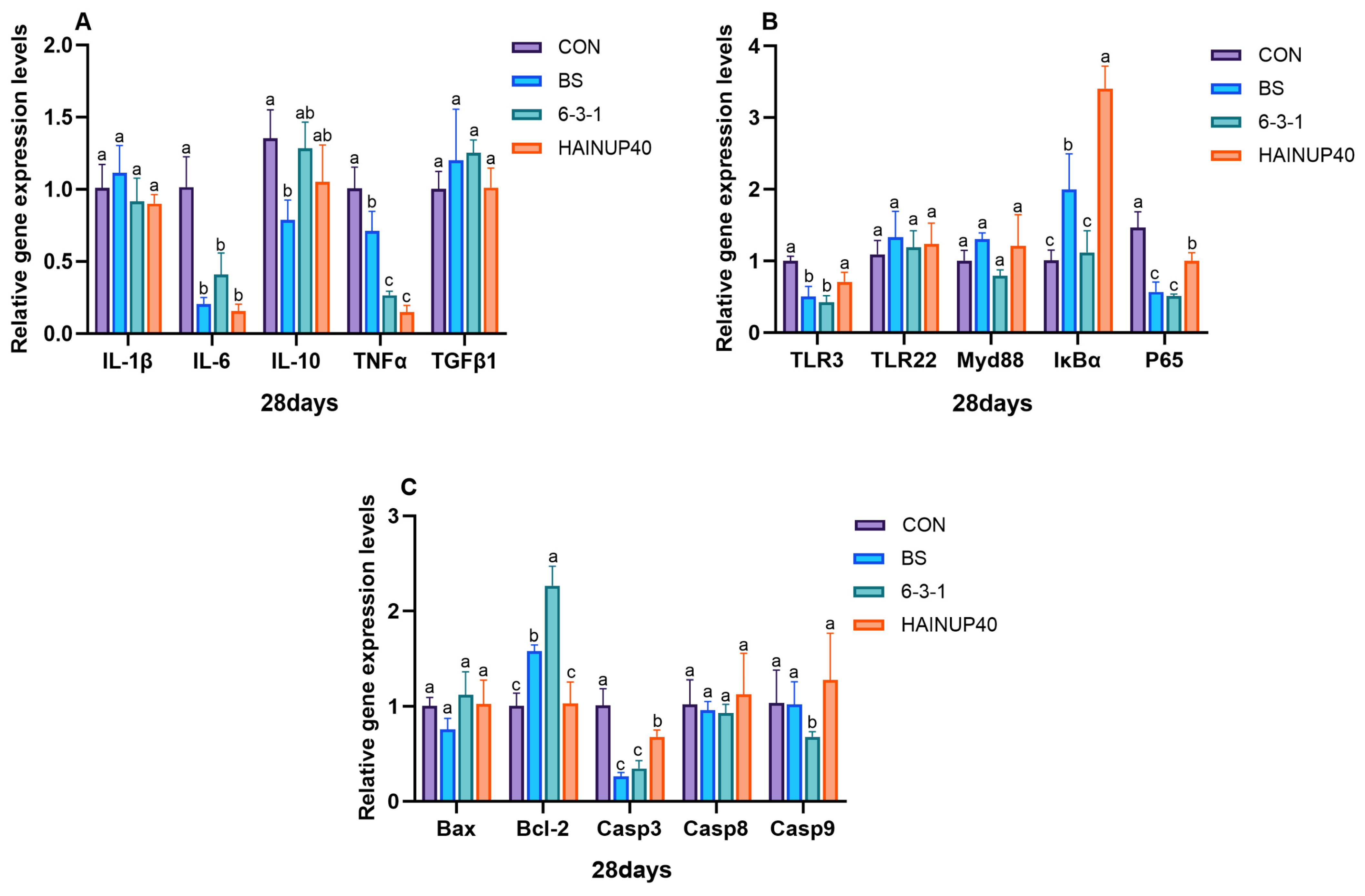
3.5. Expression of Immune-Related Genes in the Liver
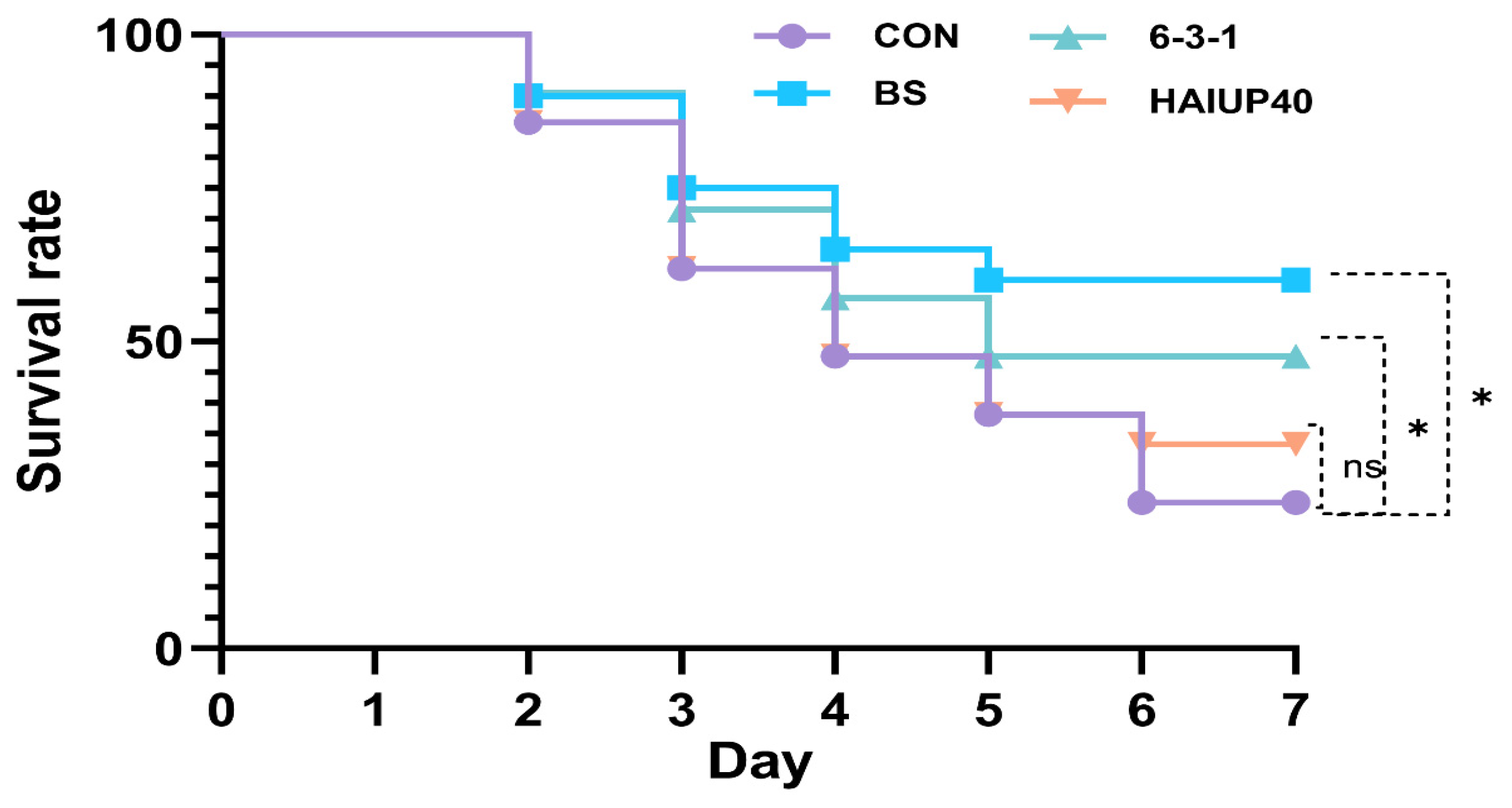
3.6. Ammonia-Nitrogen Stress Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pounds, A.; Kaminski, A.M.; Budhathoki, M.; Gudbrandsen, O.; Kok, B.; Horn, S.; Malcorps, W.; Mamun, A.-A.; McGoohan, A.; Newton, R. More than fish—Framing aquatic animals within sustainable food systems. Foods 2022, 11, 1413. [Google Scholar] [CrossRef]
- Giri, S.S.; Sen, S.S.; Jun, J.W.; Sukumaran, V.; Park, S.C. Role of Bacillus licheniformis VS16-derived biosurfactant in mediating immune responses in Carp Rohu and its application to the food industry. Front. Microbiol. 2017, 8, 514. [Google Scholar] [CrossRef]
- Benli, A.Ç.K.; Köksal, G.; Özkul, A. Sublethal ammonia exposure of Nile tilapia (Oreochromis niloticus L.): Effects on gill, liver and kidney histology. Chemosphere 2008, 72, 1355–1358. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Gao, Q.; Peng, S.; Zhang, C. Dietary vitamin E level affects the response of juvenile Epinehelus moara to ammonia nitrogen stress. Chin. J. Anim. Nutr. 2015, 27, 1596–1604. [Google Scholar]
- Kim, J.-H.; Kang, Y.J.; Lee, K.M. Effects of Nitrite Exposure on the Hematological Properties, Antioxidant and Stress Responses of Juvenile Hybrid Groupers, Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀. Antioxidants 2022, 11, 545. [Google Scholar] [CrossRef]
- Martin, D.M.; Johnson, F.A. Incorporating uncertainty and risk into decision making to reduce nitrogen inputs to impaired waters. J. Environ. Manag. 2019, 249, 109380. [Google Scholar] [CrossRef]
- Hernández-Sandoval, F.E.; Bustillos-Guzmán, J.J.; Band-Schmidt, C.J.; Núñez-Vázquez, E.J.; López-Cortés, D.J.; Fernández-Herrera, L.J.; Poot-Delgado, C.A.; Moreno-Legorreta, M. Effect of Different N: P Ratios on the Growth, Toxicity, and Toxin Profile of Gymnodinium catenatum (Dinophyceae) Strains from the Gulf of California. Toxins 2022, 14, 501. [Google Scholar] [CrossRef]
- Pochiraju, S.S.; Hoppe-Jones, C.; Adams, C.; Weinrich, L. Development and optimization of analytical methods for the detection of 18 taste and odor compounds in drinking water utilities. Water Res. X 2021, 11, 100099. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Y.; Kong, Q.; Dong, W.; Lin, Z. Toxicity of naphthenic acids on the chlorophyll fluorescence parameters and antioxidant enzyme activity of Heterosigma akashiwo. Antioxidants 2021, 10, 1582. [Google Scholar] [CrossRef]
- Tan, C.W.; Malcolm, T.T.; Kuan, C.H.; Thung, T.Y.; Chang, W.S.; Loo, Y.Y.; Premarathne, J.M.; Ramzi, O.B.; Norshafawatie, M.F.; Yusralimuna, N. Prevalence and antimicrobial susceptibility of Vibrio parahaemolyticus isolated from short mackerels (Rastrelliger brachysoma) in Malaysia. Front. Microbiol. 2017, 8, 1087. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, H.; Pan, L.; Guan, W.; Lou, Y. Environmentally relevant concentrations of triclosan exposure promote the horizontal transfer of antibiotic resistance genes mediated by Edwardsiella piscicida. Environ. Sci. Pollut. Res. 2022, 29, 64622–64632. [Google Scholar] [CrossRef]
- Anekwe, J.; Oluseyi, T.; Drage, D.; Harrad, S.; Abdallah, M. Occurrence, seasonal variation and human exposure to pharmaceuticals and personal care products in surface water, groundwater and drinking water in Lagos State, Nigeria. Emerg. Contam. 2020, 6, 124–132. [Google Scholar]
- Xiang, W.; Wan, Y.; Zhang, X.; Tan, Z.; Xia, T.; Zheng, Y.; Gao, B. Adsorption of tetracycline hydrochloride onto ball-milled biochar: Governing factors and mechanisms. Chemosphere 2020, 255, 127057. [Google Scholar] [CrossRef]
- Xu, J.; Han, L.; Yin, W. Research on the ecologicalization efficiency of mariculture industry in China and its influencing factors. Mar. Policy 2022, 137, 104935. [Google Scholar] [CrossRef]
- Liu, J.; Hefni, M.E.; Witthöft, C.M.; Bergström, M.; Burleigh, S.; Nyman, M.; Hållenius, F. Effects of whole brown bean and its isolated fiber fraction on plasma lipid profile, atherosclerosis, gut microbiota, and microbiota-dependent metabolites in Apoe−/− mice. Nutrients 2022, 14, 937. [Google Scholar] [CrossRef]
- Zhang, R.; Kang, X.; Liu, L.; Wang, X.; Li, H.; Zhu, J.; Cao, Y.; Zhu, H. Gut microbiota modulation by plant polyphenols in koi carp (Cyprinus carpio L.). Front. Microbiol. 2022, 13, 977292. [Google Scholar] [CrossRef]
- Chaudhari, A.; Ail, S.K.S.; Misra, C.; De, H.; Rathod, R.; Bhatt, J.; Swain, S. Status of Freshwater Aquaculture in Gujarat: A Trend Analysis and Potential. Int. J. Bio-Resour. Stress Manag. 2023, 14, 059–067. [Google Scholar] [CrossRef]
- Zaineldin, A.I.; Hegazi, S.; Koshio, S.; Ishikawa, M.; Bakr, A.; El-Keredy, A.M.; Dawood, M.A.; Dossou, S.; Wang, W.; Yukun, Z. Bacillus subtilis as probiotic candidate for red sea bream: Growth performance, oxidative status, and immune response traits. Fish Shellfish Immunol. 2018, 79, 303–312. [Google Scholar] [CrossRef]
- Shi, F.; Zi, Y.; Lu, Z.; Li, F.; Yang, M.; Zhan, F.; Li, Y.; Li, J.; Zhao, L.; Lin, L. Bacillus subtilis H2 modulates immune response, fat metabolism and bacterial flora in the gut of grass carp (Ctenopharyngodon idellus). Fish Shellfish Immunol. 2020, 106, 8–20. [Google Scholar] [CrossRef]
- Di, J.; Chu, Z.; Zhang, S.; Huang, J.; Du, H.; Wei, Q. Evaluation of the potential probiotic Bacillus subtilis isolated from two ancient sturgeons on growth performance, serum immunity and disease resistance of Acipenser dabryanus. Fish Shellfish Immunol. 2019, 93, 711–719. [Google Scholar] [CrossRef]
- Jin, W.; Jiang, L.; Hu, S.; Zhu, A. Effects of Lactobacillus plantarum and Bacillus subtilis on growth, immunity and intestinal flora of largemouth bass (Micropterus salmoides). Aquaculture 2024, 583, 740581. [Google Scholar] [CrossRef]
- Bereded, N.K.; Abebe, G.B.; Fanta, S.W.; Curto, M.; Waidbacher, H.; Meimberg, H.; Domig, K.J. The impact of sampling season and catching site (wild and aquaculture) on gut microbiota composition and diversity of Nile tilapia (Oreochromis niloticus). Biology 2021, 10, 180. [Google Scholar] [CrossRef]
- Amoah, K.; Tan, B.; Zhang, S.; Chi, S.; Yang, Q.; Liu, H.; Yang, Y.; Zhang, H.; Dong, X. Host gut-derived Bacillus probiotics supplementation improves growth performance, serum and liver immunity, gut health, and resistive capacity against Vibrio harveyi infection in hybrid grouper (♀ Epinephelus fuscoguttatus × ♂ E. lanceolatus). Anim. Nutr. 2023, 14, 163–184. [Google Scholar] [CrossRef]
- Ji, Z.; Zhu, C.; Zhu, X.; Ban, S.; Yu, L.; Tian, J.; Dong, L.; Wen, H.; Lu, X.; Jiang, M. Dietary host-associated Bacillus subtilis supplementation improves intestinal microbiota, health and disease resistance in Chinese perch (Siniperca chuatsi). Anim. Nutr. 2023, 13, 197–205. [Google Scholar] [CrossRef]
- Hasan, Z.; Andriani, Y.; Hamdani, H.; Sahidin, A.; Surbakti, S.B. Effect of Probiotics Addition with Different Dosage on Water Quality Performance of Sangkuriang Catfish (Clarias gariepinus) Farming in the Aquaponic System. In Proceedings of the Proceedings of the 1st International Conference on Islam, Science and Technology, ICONISTECH 2019, Bandung, Indonesia, 11–12 July 2019; EAI: Jersey City, NJ, USA, 2021. [Google Scholar]
- Hassan, M.A.; Fathallah, M.A.; Elzoghby, M.A.; Salem, M.G.; Helmy, M.S. Influence of probiotics on water quality in intensified Litopenaeus vannamei ponds under minimum-water exchange. AMB Express 2022, 12, 22. [Google Scholar] [CrossRef]
- Sunitha, K.; Padmavathi, P. Influence of Probiotics on Water Quality and Fish Yield in Fish Ponds. Int. J. Pure Appl. Sci. Technol. 2013, 19, 48–60. [Google Scholar]
- Cao, J.; Mei, J.; Xie, J. Combined effects of hypoxia and ammonia-N exposure on the immune response, oxidative stress, tissue injury and apoptosis of hybrid grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Environ. Sci. Pollut. Res. 2024, 31, 845–856. [Google Scholar] [CrossRef]
- Xue, J.; Shen, K.; Hu, Y.; Hu, Y.; Kumar, V.; Yang, G.; Wen, C. Effects of dietary Bacillus cereus, B. subtilis, Paracoccus marcusii, and Lactobacillus plantarum supplementation on the growth, immune response, antioxidant capacity, and intestinal health of juvenile grass carp (Ctenopharyngodon idellus). Aquac. Rep. 2020, 17, 100387. [Google Scholar] [CrossRef]
- Tang, Y.; Han, L.; Chen, X.; Xie, M.; Kong, W.; Wu, Z. Dietary supplementation of probiotic Bacillus subtilis affects antioxidant defenses and immune response in grass carp under Aeromonas hydrophila challenge. Probiotics Antimicrob. Proteins 2019, 11, 545–558. [Google Scholar] [CrossRef]
- Gobi, N.; Vaseeharan, B.; Chen, J.-C.; Rekha, R.; Vijayakumar, S.; Anjugam, M.; Iswarya, A. Dietary supplementation of probiotic Bacillus licheniformis Dahb1 improves growth performance, mucus and serum immune parameters, antioxidant enzyme activity as well as resistance against Aeromonas hydrophila in tilapia Oreochromis mossambicus. Fish Shellfish Immunol. 2018, 74, 501–508. [Google Scholar] [CrossRef]
- Liao, J.; Cai, Y.; Wang, X.; Shang, C.; Zhang, Q.; Shi, H.; Wang, S.; Zhang, D.; Zhou, Y. Effects of a potential host gut-derived probiotic, Bacillus subtilis 6-3-1, on the growth, non-specific immune response and disease resistance of hybrid grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Probiotics Antimicrob. Proteins 2021, 13, 1119–1137. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Cai, Y.; Guo, X.; Cao, Z.; Zhang, Y.; Liu, S.; Yuan, W.; Zhu, W.; Zheng, Y. Dietary administration of Bacillus subtilis HAINUP40 enhances growth, digestive enzyme activities, innate immune responses and disease resistance of tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2017, 60, 326–333. [Google Scholar] [CrossRef]
- Yan, X.; Li, Z.; Dong, X.; Tan, B.; Pan, S.; Li, T.; Long, S.; Huang, W.; Suo, X.; Yang, Y. Degradation of Muscle Quality in Hybrid Grouper (female Epinephelus fuscoguttatus x male Epinephelus lanceolatu) Due to Oxidative Damage Caused by Ingestion of Oxidized Fish Oil. Front. Nutr. 2022, 9, 840535. [Google Scholar] [CrossRef]
- Kong, Y.; Li, M.; Chu, G.; Liu, H.; Shan, X.; Wang, G.; Han, G. The positive effects of single or conjoint administration of lactic acid bacteria on Channa argus: Digestive enzyme activity, antioxidant capacity, intestinal microbiota and morphology. Aquaculture 2021, 531, 735852. [Google Scholar] [CrossRef]
- Valcarce, D.G.; Riesco, M.F.; Martínez-Vázquez, J.M.; Robles, V. Diet supplemented with antioxidant and anti-inflammatory probiotics improves sperm quality after only one spermatogenic cycle in zebrafish model. Nutrients 2019, 11, 843. [Google Scholar] [CrossRef]
- Dong, Y.; Li, L.; Xia, T.; Wang, L.; Xiao, L.; Ding, N.; Wu, Y.; Lu, K. Oxidative Stress Can Be Attenuated by 4-PBA Caused by High-Fat or Ammonia Nitrogen in Cultured Spotted Seabass: The Mechanism Is Related to Endoplasmic Reticulum Stress. Antioxidants 2022, 11, 1276. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Angulo, C.; Estrada, N.; Murillo, Y.; Ascencio-Valle, F. Dietary administration of microalgae alone or supplemented with Lactobacillus sakei affects immune response and intestinal morphology of Pacific red snapper (Lutjanus peru). Fish Shellfish Immunol. 2014, 40, 208–216. [Google Scholar] [CrossRef]
- Beck, B.R.; Kim, D.; Jeon, J.; Lee, S.-M.; Kim, H.K.; Kim, O.-J.; Lee, J.I.; Suh, B.S.; Do, H.K.; Lee, K.H. The effects of combined dietary probiotics Lactococcus lactis BFE920 and Lactobacillus plantarum FGL0001 on innate immunity and disease resistance in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2015, 42, 177–183. [Google Scholar] [CrossRef]
- Mirghaed, A.T.; Yarahmadi, P.; Hosseinifar, S.H.; Tahmasebi, D.; Gheisvandi, N.; Ghaedi, A. The effects singular or combined administration of fermentable fiber and probiotic on mucosal immune parameters, digestive enzyme activity, gut microbiota and growth performance of Caspian white fish (Rutilus frisii kutum) fingerlings. Fish Shellfish Immunol. 2018, 77, 194–199. [Google Scholar] [CrossRef]
- Sewaka, M.; Trullas, C.; Chotiko, A.; Rodkhum, C.; Chansue, N.; Boonanuntanasarn, S.; Pirarat, N. Efficacy of synbiotic Jerusalem artichoke and Lactobacillus rhamnosus GG-supplemented diets on growth performance, serum biochemical parameters, intestinal morphology, immune parameters and protection against Aeromonas veronii in juvenile red tilapia (Oreochromis spp.). Fish Shellfish Immunol. 2019, 86, 260–268. [Google Scholar]
- Liu, R.; Wang, S.; Huang, D.; Huang, Y.; He, T.; Chen, X. The probiotic roles of Lactiplantibacillus plantarum E2 as a dietary supplement in growth promotion and disease resistance of juvenile large yellow croaker (Larimichthys crocea). Aquaculture 2024, 578, 740082. [Google Scholar] [CrossRef]
- Son, V.M.; Chang, C.-C.; Wu, M.-C.; Guu, Y.-K.; Chiu, C.-H.; Cheng, W. Dietary administration of the probiotic, Lactobacillus plantarum, enhanced the growth, innate immune responses, and disease resistance of the grouper Epinephelus coioides. Fish Shellfish Immunol. 2009, 26, 691–698. [Google Scholar] [CrossRef]
- Addo, S.; Carrias, A.A.; Williams, M.A.; Liles, M.R.; Terhune, J.S.; Davis, D.A. Effects of Bacillus subtilis strains on growth, immune parameters, and Streptococcus iniae susceptibility in Nile tilapia, Oreochromis niloticus. J. World Aquac. Soc. 2017, 48, 257–267. [Google Scholar] [CrossRef]
- Feng, J.-C.; Cai, Z.-L.; Zhang, X.-P.; Chen, Y.-Y.; Chang, X.-L.; Wang, X.-F.; Qin, C.-B.; Yan, X.; Ma, X.; Zhang, J.-X. The effects of oral Rehmannia glutinosa polysaccharide administration on immune responses, antioxidant activity and resistance against Aeromonas hydrophila in the common carp, Cyprinus carpio L. Front. Immunol. 2020, 11, 904. [Google Scholar] [CrossRef]
- Zou, C.; Du, L.; Wu, J.; Gan, S.; Li, Q.; Babu, V.S.; Wu, Y.; Lin, L. Saikosaponin d alleviates high-fat-diet induced hepatic steatosis in hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀) by targeting AMPK/PPARα pathway. Aquaculture 2022, 553, 738088. [Google Scholar] [CrossRef]
- Esteban, M.; Cordero, H.; Martínez-Tomé, M.; Jiménez-Monreal, A.; Bakhrouf, A.; Mahdhi, A. Effect of dietary supplementation of probiotics and palm fruits extracts on the antioxidant enzyme gene expression in the mucosae of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 2014, 39, 532–540. [Google Scholar] [CrossRef]
- Giri, S.S.; Sukumaran, V.; Oviya, M. Potential probiotic Lactobacillus plantarum VSG3 improves the growth, immunity, and disease resistance of tropical freshwater fish, Labeo rohita. Fish Shellfish Immunol. 2013, 34, 660–666. [Google Scholar] [CrossRef]
- Mokhtar, D.M. Fish Histology: From Cells to Organs; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- He, Y.; Liang, J.; Dong, X.; Liu, H.; Yang, Q.; Zhang, S.; Chi, S.; Tan, B. Soybean β-conglycinin and glycinin reduced growth performance and the intestinal immune defense and altered microbiome in juvenile pearl gentian groupers Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂. Anim. Nutr. 2022, 9, 193–203. [Google Scholar] [CrossRef]
- Yin, B.; Liu, H.; Tan, B.; Dong, X.; Chi, S.; Yang, Q.; Zhang, S. MHC II-PI3K/Akt/mTOR signaling pathway regulates intestinal immune response induced by soy glycinin in hybrid grouper: Protective effects of sodium butyrate. Front. Immunol. 2021, 11, 615980. [Google Scholar] [CrossRef]
- Wu, D.; Fan, Z.; Li, J.; Zhang, Y.; Xu, Q.; Wang, L.; Wang, L. Low Protein Diets Supplemented With Alpha-Ketoglutarate Enhance the Growth Performance, Immune Response, and Intestinal Health in Common Carp (Cyprinus carpio). Front. Immunol. 2022, 13, 915657. [Google Scholar] [CrossRef]
- Peng, K.; Chen, B.; Zhao, H.; Huang, W. Toxic effects of aflatoxin B1 in Chinese sea bass (Lateolabrax maculatus). Toxins 2021, 13, 844. [Google Scholar] [CrossRef]
- Pan, M.V.; Ferriols, V.M.E.N.; Traifalgar, R.F.M. Synergistic influence of hydrolyzed squid processing by-products and Bacillus probiotics as dietary supplements on growth performance, immunological responses, and gut health of juvenile black tiger shrimp fed fishmeal-free diets. Aquac. Int. 2024, 1–30. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Yang, H.L.; Ma, R.L.; Song, K.; Li, J.S. Effect of Lactococcus lactis and Enterococcus faecium on growth performance, digestive enzymes and immune response of grouper Epinephelus coioides. Aquac. Nutr. 2012, 18, 281–289. [Google Scholar] [CrossRef]
- Dang, Y.; Sun, Y.; Zhou, Y.; Men, X.; Wang, B.; Li, B.; Ren, Y. Effects of probiotics on growth, the toll-like receptor mediated immune response and susceptibility to Aeromonas salmonicida infection in rainbow trout Oncorhynchus mykiss. Aquaculture 2022, 561, 738668. [Google Scholar] [CrossRef]
- Obremski, K.; Trybowski, W.; Wojtacha, P.; Gajęcka, M.; Tyburski, J.; Zielonka, Ł. The effect of zearalenone on the cytokine environment, oxidoreductive balance and metabolism in porcine ileal peyer’s patches. Toxins 2020, 12, 350. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.-G.; Yang, B.-T.; Zheng, Z.-Y.; Liang, Z.-L.; Kang, Y.-H.; Cong, W. Improvement of non-specific immunity, intestinal health and microbiota of crucian carp (Carassius auratus) juvenile with dietary supplementation of Bacillus coagulans BC1. Aquaculture 2024, 580, 740327. [Google Scholar] [CrossRef]
- Al-Hisnawi, A.; Rodiles, A.; Rawling, M.D.; Castex, M.; Waines, P.; Gioacchini, G.; Carnevali, O.; Merrifield, D.L. Dietary probiotic Pediococcus acidilactici MA18/5M modulates the intestinal microbiota and stimulates intestinal immunity in rainbow trout (Oncorhynchus mykiss). J. World Aquac. Soc. 2019, 50, 1133–1151. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Kamilya, D.; Choudhury, T.G.; Rathore, G. Dietary administration of a host-gut derived probiotic Bacillus amyloliquefaciens COFCAU_P1 modulates immune-biochemical response, immune-related gene expression, and resistance of Labeo rohita to Aeromonas hydrophila infection. Aquaculture 2022, 546, 737390. [Google Scholar] [CrossRef]
- Shin, K.W.; Kim, S.-H.; Kim, J.-H.; Hwang, S.D.; Kang, J.-C. Toxic effects of ammonia exposure on growth performance, hematological parameters, and plasma components in rockfish, Sebastes schlegelii, during thermal stress. Fish. Aquat. Sci. 2016, 19, 1–8. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, X.; Wang, L.; Cai, J.; Shen, J.; Shen, Y.; Li, X.; Zhao, Y. Iron overload accelerated lipid metabolism disorder and liver injury in rats with non-alcoholic fatty liver disease. Front. Nutr. 2022, 9, 961892. [Google Scholar] [CrossRef]
- Liu, B.; Gao, Q.; Liu, B.; Song, C.; Sun, C.; Liu, M.; Liu, X.; Liu, Y.; Li, Z.; Zhou, Q. Application of transcriptome analysis to understand the adverse effects of hypotonic stress on different development stages in the giant freshwater prawn Macrobrachium rosenbergii post-larvae. Antioxidants 2022, 11, 440. [Google Scholar] [CrossRef]
- Zhou, S.; Xia, Y.; Zhu, C.; Chu, W. Isolation of marine Bacillus sp. with antagonistic and organic-substances-degrading activities and its potential application as a fish probiotic. Mar. Drugs 2018, 16, 196. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, H.; Li, X.; Luo, Y.; Xie, M.; Wu, Z.; Chen, X. Regulatory effect of Bacillus subtilis on cytokines of dendritic cells in grass carp (Ctenopharyngodon idella). Int. J. Mol. Sci. 2019, 20, 389. [Google Scholar] [CrossRef]
- Wu, T.; Qi, W.; Shan, H.; Tu, B.; Jiang, S.; Lu, Y.; Wang, F. Ginsenoside Rg1 enhances the healing of injured tendon in achilles tendinitis through the activation of IGF1R signaling mediated by oestrogen receptor. J. Ginseng Res. 2022, 46, 526–535. [Google Scholar] [CrossRef]


| Ingredients | % |
|---|---|
| Red fishmeal | 40.00 |
| Casein | 11.54 |
| Gelatine | 2.85 |
| Wheat flour | 20.00 |
| Fish oil | 4.66 |
| Soy lecithin | 2.00 |
| Calcium monophosphate | 1.00 |
| Vitamin premix a | 0.22 |
| Mineral premix b | 0.56 |
| Antioxidants | 0.06 |
| Choline chloride | 0.50 |
| Vitamin C | 0.09 |
| Cellulose microcrystalline | 16.52 |
| Proximate composition (in dry matter) | |
| Crude protein | 48.86 |
| Crude lipid | 10.75 |
| Moisture | 9.58 |
| Gene | Primer Name | Primer Sequence 5′–3′ | Accession No. |
|---|---|---|---|
| IL-1β | IL-1β-F | CGACATGGTGCGGTTTC | XM_049578640.1 |
| IL-1β-R | TCTGTAGCGGCTGGTGG | ||
| IL-6 | IL-6-F | AGGAAGTCTGGCTGTCAGGA | XM_049603149.1 |
| IL-6-R | GCCCTGAGGCCTTCAAGATT | ||
| IL-10 | IL-10-F | ACACAGCGCTGCTAGACGAG | XM_049580695.1 |
| IL-10-R | GGGCAGCACCGTGTTCAGAT | ||
| TNFα | TNFα-F | GTGGCCTACACGACTGCACC | XM_049582852.1 |
| TNFα-R | TACAAAGGGCCACAGTGAGA | ||
| TGFβ1 | TGFβ1-F | AACATCCCGCTACCTCGCTT | XM_049576571.1 |
| TGFβ1-R | TCCGCTCATCCTCATTCCCT | ||
| TLR3 | TLR3-F | TTCTTAACCATTCGCCCTCC | XM_033624878.1 |
| TLR3-R | GGCCCATATTGCTTCCATC | ||
| TLR22 | TLR22-F | TGTGACGGACAAACCGTGAT | JQ965995.1 |
| TLR22-R | GCGCATATGAGTCCCTTCCC | ||
| IκBα | IκBα-F | GCCAGCAGCACATCACTTCC | XM_049590191.1 |
| IκBα-R | AGCCACCGTAGTTCAAGCAGTT | ||
| Myd88 | Myd88-F | CGAGCCAGGTAAACCCATCA | XM_049565206.1 |
| Myd88-R | CTCATCAAACAGGCGGAAGC | ||
| P65 | P65-F | GGGTGTGTATGGATGGGG | XM_049567828.1 |
| P65-R | TGGCTGGGTGGGTCTTAG | ||
| Bax | Bax-F | CTCCCGAGCTACACTAGACA | XM_049590191.1 |
| Bax-R | GCATAGGGATCATGGGGGTG | ||
| Bcl-2 | Bcl-2-F | TTAGGTCGCAGTGAGT | XM_033637342.1 |
| Bcl-2-R | CATAGATGGGGAAGAG | ||
| Casp3 | Casp3-F | CGCAAAGAGTAGCGACGGA | XM_049571989.1 |
| Casp3-R | CGATGCTGGGGAAATTCAGAC | ||
| Casp8 | Casp8-F | TGCTTCTTGTGTCGTGATGTTG | XM_049598079.1 |
| Casp8-R | GCGTCGGTCTCTTCTGGTTG | ||
| Casp9 | Casp9-F | TTTTCCTGGTTATGTTTCGTGG | XM_033629367.1 |
| Casp9-R | TTGCTTGTAGAGCCCTTTTGC | ||
| β-actin | β-actin-F | TACGAGCTGCCTGACGGACA | XM_049560987.1 |
| β-actin-R | GGCTGTGATCTCCTTCTGC |
| Group | p-Value | ||||
|---|---|---|---|---|---|
| Item | Control | BS | 6-3-1 | HAINUP40 | |
| IBW, g | 540.80 ± 23.17 | 531.67 ± 37.51 | 531.33 ± 20.55 | 525.33 ± 28.07 | 0.198 |
| FBW, g | 1344.00 ± 39.40 b | 1323.33 ± 24.34 b | 1422.00 ± 14.11 a | 1435.33 ± 21.55 a | <0.001 |
| 14 days | |||||
| WG, g | 331.87 ± 12.43 c | 313.67 ± 10.69 c | 346.67 ± 9.02 b | 422.67 ± 40.13 a | <0.001 |
| FCR, % | 0.84 ± 0.04 a | 0.69 ± 0.02 c | 0.75 ± 0.05 b | 0.59 ± 0.03 d | <0.001 |
| ADG, g | 22.12 ± 0.83 bc | 20.91 ± 0.71 c | 23.11 ± 0.60 b | 28.18 ± 2.68 a | 0.219 |
| CF, g/cm3 | 0.67 ± 0.09 a | 0.70 ± 0.05 a | 0.65 ± 0.09 a | 0.47 ± 0.06 b | 0.103 |
| 28 days | |||||
| WG, g | 510.20 ± 10.81 c | 489.33 ± 19.09 c | 558.33 ± 11.53 b | 616.00 ± 43.09 a | <0.001 |
| FCR, % | 0.91 ± 0.01 a | 0.80 ± 0.02 b | 0.60 ± 0.02 c | 0.58 ± 0.02 c | <0.001 |
| ADG, g | 20.41 ± 0.43 c | 19.57 ± 0.76 c | 22.33 ± 0.06 b | 24.64 ± 1.72 a | 0.023 |
| CF, g/cm3 | 0.81 ± 0.07 a | 0.67 ± 0.08 c | 0.68 ± 0.04 c | 0.73 ± 0.05 bc | <0.001 |
| 42 days | |||||
| WG, g | 803.20 ± 38.30 b | 791.67 ± 23.12 b | 890.67 ± 34.04 a | 910.00 ± 41.79 a | 0.080 |
| FCR, % | 0.94 ± 0.01 a | 0.75 ± 0.02 b | 0.58 ± 0.01 c | 0.61 ± 0.02 c | <0.001 |
| ADG, g | 20.59 ± 0.98 b | 20.30 ± 0.59 b | 22.84 ± 0.10 a | 23.33 ± 0.30 a | <0.001 |
| CF, g/cm3 | 0.79 ± 0.04 ab | 0.83 ± 0.01 a | 0.79 ± 0.04 ab | 0.75 ± 0.04 b | 0.876 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, C.; Shi, H.; Cui, C.; Wang, J.; Li, L.; Bei, W.; Cai, Y.; Wang, S. Strain-Specific Benefits of Bacillus on Growth, Intestinal Health, Immune Modulation, and Ammonia-Nitrogen Stress Resilience in Hybrid Grouper. Antioxidants 2024, 13, 317. https://doi.org/10.3390/antiox13030317
Han C, Shi H, Cui C, Wang J, Li L, Bei W, Cai Y, Wang S. Strain-Specific Benefits of Bacillus on Growth, Intestinal Health, Immune Modulation, and Ammonia-Nitrogen Stress Resilience in Hybrid Grouper. Antioxidants. 2024; 13(3):317. https://doi.org/10.3390/antiox13030317
Chicago/Turabian StyleHan, Congjie, Huizhong Shi, Congcong Cui, Jiawen Wang, Ling Li, Weilie Bei, Yan Cai, and Shifeng Wang. 2024. "Strain-Specific Benefits of Bacillus on Growth, Intestinal Health, Immune Modulation, and Ammonia-Nitrogen Stress Resilience in Hybrid Grouper" Antioxidants 13, no. 3: 317. https://doi.org/10.3390/antiox13030317
APA StyleHan, C., Shi, H., Cui, C., Wang, J., Li, L., Bei, W., Cai, Y., & Wang, S. (2024). Strain-Specific Benefits of Bacillus on Growth, Intestinal Health, Immune Modulation, and Ammonia-Nitrogen Stress Resilience in Hybrid Grouper. Antioxidants, 13(3), 317. https://doi.org/10.3390/antiox13030317






