Abstract
Unconventional feed, which is abundant in China, contains anti-nutritional factors and toxins; however, these can be greatly reduced with microbial fermentation, thus improving the nutrient content of the feed, enhancing animal appetites, and ultimately significantly improving the intestinal health and growth performance of animals. When oxidative stress occurs, fermented feed can effectively reduce the damage caused by stress to the gastrointestinal tract, accelerate the removal of gastrointestinal abnormalities, improve the ability to resist intestinal stress, and ensure the efficient production of animals. This review introduces the application of unconventional fermented feed in animal production, and expounds upon the function of unconventional fermented feed in animals with oxidative stress symptoms, so as to provide a theoretical reference for the development and application of unconventional fermented feed in antioxidative stress reduction.
1. Introduction
Unconventional feed refers to feed that is different in terms of the raw material source or preparation process compared to conventional feed. This kind of feed is usually obtained from diversified raw materials such as agricultural and sideline products, aquatic product by-products, and industrial by-products, which are obtained through special processing or treatment; China possesses rich resources of such materials [1,2]. However, the nutrient composition of unconventional feed is complex, and it has shortcomings such as a high content of anti-nutritional factors and poisons, poor palatability, unstable nutrient composition, and significant quality variations [3]. Therefore, the comprehensive utilization level of unconventional feed is low, resulting in a waste of resources, environmental pollution, and other problems. At present, unconventional feed processed through microbial fermentation technology, crushing, heating, hydrolysis, drying, and other methods, in order to degrade the anti-nutritional factors, toxins, crude fiber, lignin, and other substances present in it and reflect its high nutritional value [4] in terms of protein, minerals, and trace elements required for livestock supplementation, is called “special feed” or “alternative feed”. Therefore, unconventional feed is often used, in part, to replace conventional feed to reduce feed costs, improve the economic value, and achieve sustainable development in the feed industry. In recent years, the popularity of unconventional feeds has gradually increased.
With the development of the economy and the improvement of people’s living standards, agriculture and animal husbandry have developed rapidly, and the problem of “human and livestock competing for food” is still severe due to the increase in demand for feed and the insufficient supply [5]. At present, “bans of antibiotics” in feed have become an important trend in the development of the livestock and poultry industry, along with studies on several antibiotic alternatives, such as probiotics, antimicrobial peptides, Chinese herbal additives, plant-derived phytochemicals, functional amino acids, organic acids, and other functional additives that pose no potential threat to livestock quality [6,7,8]. At present, research into such alternatives mainly focuses on the application of probiotics in fermented feed and how animal gut health and production performance can be maintained and improved with fermented feed. It has been reported that the fermentation of raw feed materials after inoculation with beneficial microorganisms can decompose organic macromolecules such as proteins and lipids into small molecules such as organic acids; this increases the feed’s nutritional content, allows it to be more easily absorbed by livestock and poultry, and promotes animal intestinal health, thereby improving the growth performance and health of livestock and poultry [9].
Oxidative stress [10] refers to the excessive production of highly reactive molecules, such as reactive oxygen species (ROS) and reactive nitrogen radicals (RNS), which exceeds the scavenging capacity of antioxidants, resulting in the disruption of the balance between the oxidative system and the antioxidant system, and the occurrence of damage to cells and tissues. When animals are faced with external environmental pressure, disease, feed discomfort, transportation, or other adverse stimuli, the body, especially the intestines, may experience oxidative stress, resulting in damage to cell membranes, abnormal organelle functions, the oxidation of DNA, proteins, and lipids, and even the induction of a series of adverse physiological and pathological changes such as inflammatory reactions. These reactions, in turn, affect the health and performance of the animal, in ways such as by reducing immunity, slowing growth rates, and reducing the reproductive capacity. Therefore, the effective control of oxidative stress is essential to maintaining animal health and improving breeding efficiency [11]. Research has shown that the supplementation of fermented feed can effectively help maintain the stability of the intestinal environment and reduce the adverse effects of oxidative stress [12,13]. For example, Jairath et al. [14] found that fermentation also resulted in a significant increase in antioxidant activities. Liu et al. [15] showed that fermented mixed feed was able to regulate the gut microbial community and metabolism of finishing pigs. Hu et al. [16] found that fermented tea residue significantly improved the antioxidant capacity of a juvenile largemouth bass.
Although unconventional feed raw materials have limited nutritional value and contain anti-nutritional factors, they can be transformed into unconventional fermented feed with a high nutritional value after processing via fermentation and other technologies. After fermentation, such unconventional feed not only improves the nutritional value and digestibility of conventional feed, but also reduces the oxidative stress of animals [17] and promotes improvements in livestock and poultry health and production performance [18]. It supports the production of antibiotic-free livestock and poultry products, while expanding feed resources and alleviating raw material shortages. Unconventional fermented feed maintains intestinal health by producing antioxidants and probiotics, and enhances immune function with bioactive substances [19], which is of great significance for achieving antibiotic-free animal husbandry [20], alleviating resource shortage and coping with oxidative stress. Increasing feed diversity is helpful in improving the overall growth performance of livestock and poultry, reducing environmental pollution, and promoting the green development of animal husbandry. In addition, the antioxidants in unconventional fermented feeds can improve the antioxidant capacity of animals and reduce the damage caused by free radicals, thereby further improving the health and production efficiency of animals. These antioxidants may include vitamins, enzymes, peptides, or other small molecule compounds that can neutralize free radicals in the body, reduce oxidative stress, and protect cells from damage. At present, research into the mechanism of fermented feed’s impact on oxidative stress in the intestinal tract is not sufficient, and the evaluation system for such impacts needs to be improved upon. As different fermentation strains, methods, and conditions will affect the quality of the feed, thereby affecting the growth performance and meat quality of animals, it is necessary to optimize the fermentation process and further study its impact on intestinal health, so as to provide scientific support for the application and development of unconventional fermented feed.
2. Types and Applications of Unconventional Feed
The sources of unconventional feed are very extensive, including, but not limited to, grain and oil processing by-products, livestock and poultry processing by-products, aquatic product processing by-products, and other industrial processing by-products. Unconventional raw materials are abundant resources, but their application in animal feeding is limited due to their high amounts of anti-nutritional factors and toxins [21,22]. Therefore, improving the quality of unconventional raw materials and elevating their utilization rate in animal feeding are important topics of current scientific feed research. Current commonly used unconventional raw materials such as wheat bran, rice bran, bean dregs, distiller’s grains, sweet potatoes, straw, and other processing by-products require physical processing, chemical treatment, or microbial fermentation to decompose crude fiber and increase their feed value. Anti-nutritional factors and toxins in unconventional fermented feed can be reduced in a number of ways. During fermentation, microorganisms and enzymes that digest anti-nutritional factors such as phytic acid and cellulose are produced [23], while high-temperature treatment helps to destroy the structure of some toxins. In addition, regulating the pH and microbial metabolism during fermentation can also reduce the toxin content in feed [24]. Physical treatments such as filtration and sedimentation can also be used to reduce toxin levels [25]. By combining these methods, unconventional fermented feed can be safely provided to animals while improving their nutrient availability and health. Table 1 shows the positive effects of the fermentation of unconventional feed on animal health, nutrition and performance, and antioxidant aspects.

Table 1.
Positive effects of the fermentation of unconventional feed on animal health, nutrition and performance, and antioxidant aspects.
2.1. By-Products of Grain and Oil Processing
The utilization of grain and oil processing by-products in animal feed is one of the important links in animal husbandry production. These by-products include rice, wheat, corn, beans, rapeseed, camellia seeds, and other by-products produced in the process of grain and oil processing, such as rice husks, wheat bran, corn stalks, bean dregs, etc. They may be considered unconventional in conventional feed production [35], but they are actually rich in nutrients [36] and can be used in animal feed production through a series of processing and utilization steps [37]. First of all, these by-products are properly processed, such as through milling, crushing, etc., to improve their structure and digestibility and increase their utilization. They can then be used directly as raw materials for animal feed. For example, high-fiber by-products such as wheat bran and corn stover can be used as crude fiber sources [38,39], while protein-rich by-products such as rice bran and okara [40] can be used as protein sources. In addition, some grain and oil processing by-products can also be used as feed additives. For example, cellulose-rich by-products such as rice husks and okara can be used as fiber additives to help improve the digestive health of animals [40]. Zhen et al. [41] found that the content of sugar and glycoside derivatives such as crude protein and D-threitol could be significantly increased by fermenting corn stover with Aspergillus niger, thereby enhancing the antioxidant capacity and nutritional value of fermented corn stover. Finally, these by-products can be blended with other conventional feed ingredients to form a balanced and nutritious feed formulation [42]. Xia et al. [43] showed that with the gradual increase in the proportion of peanut meal replacing soybean meal, the egg production of laying ducks increased, while the weight of the eggs and feed consumption decreased. Valenca et al. [44] found that when peanut meal replaced soybean meal as the feed, the content of oleic acid, pentadecanoic acid, heptadecanoic acid, and eicosapentaenoic acid in lamb meat increased, while the content of palmitic acid decreased, and the total amount of high-cholesterol fatty acids also decreased. These results suggest that peanut meal can improve the lipid profile of lamb meat. Wang et al. [45] found that replacing the 600 g/kg portion of soybean meal in the feed with mixed vegetable proteins (including rapeseed meal, cottonseed meal, and peanut meal) had no adverse effect on the growth performance of Yellow River carp. Azad et al. [46] showed that when soybean meal was partially replaced with 5% fermented cassava residue, piglets could increase catalase, glutathione peroxidase, and hepatic superoxide dismutase levels.
2.2. By-Products of Livestock and Poultry Processing
Livestock and poultry processing by-products refer to the by-products produced during the slaughtering and processing of livestock and poultry, usually including bones, internal organs, fur, blood, and feathers [47,48]. These by-products are rich in nutrients and can be used to make various types of feed, providing comprehensive nutritional support for the animals. For example, bones are rich in minerals such as calcium and phosphorus, which can be used as a supplement to minerals in feed to make bone meal or bone pulp [49], offal is rich in protein and fat, which can be used as raw materials for high-protein feed, and fur can be processed into high-protein animal fur powder as a protein source for animal feed [50]. Suryadi et al. [51] used rice-water microbial fermentation technology to transform snail meat into a new probiotic product that can be used for feed supplementation.
2.3. By-Products of Aquatic Products Processing
Aquatic product processing by-products are the by-products produced during the processing of fish, shrimp, shellfish, and other aquatic products [52], including fish bones [53], fish skins, shrimp shells, etc. [54]. These by-products are rich in nutrients, such as proteins, fats, minerals, etc., and have high nutritional value. These by-products can be made into powdered or granular feed ingredients through appropriate processing, such as drying, crushing, etc. Such feed ingredients can be used as an important source of protein and fat in animal feed, helping to improve the nutritional level of animals, promote their growth and development, and improve their health. Zhu et al. [55] found that the shrimp by-product astaxanthin extract had a positive effect on the antioxidant activity and color difference of readymade shrimp surimi products, which could be used as a potential multifunctional additive for the development of shrimp surimi products.
2.4. Other Processing By-Products
Other industrial processing by-products are by-products produced in some other industrial production processes, such as food processing by-products, wood processing by-products, etc., including mulberry leaves, moringa, chicory, arbor, seaweed, forestry by-products, etc. The crude protein content of mulberry leaves is as high as more than 16%, and mulberry leaves is also rich in a variety of bioactive substances such as flavonoids, alkaloids, polysaccharides, and polyphenols, which makes mulberry leaves have a high medicinal value [56]. Jiayu et al. [57] showed that the feed supplemented with 2% mulberry leaf powder could modify the serum metabolites of piglets, enhance the antioxidant capacity, and reduce the content of potential intestinal pathogens. Studies have shown that chicory can enhance the expression of molecular proteins, folded proteins in proteins, and antioxidant proteins in the cecum and colon of growing pigs [58], optimize the levels of odorin and androstenone in boars, and effectively reduce odor in boars [59]. Chen et al. [60] have shown that feeding piglets xylo-oligosaccharides can reduce malondialdehyde levels and catalase activity in the serum, thereby reducing oxidative damage to the body.
3. The Effects and Mechanism of Fermentation on the Improvement of Unconventional Feed Quality
Unconventional feed comes from a wide range of abundantly produced sources. The development and utilization of unconventional raw materials can not only alleviate the problem of food security, but also serve as a way to consolidate the effect of poverty alleviation. As mentioned above, unconventional raw materials have the advantages of low environmental requirements, a wide growth range, rich nutritional value, and high total output. Nowadays, through the use of microbial fermentation technology, anti-nutritional factors and other substances in unconventional raw materials can be degraded to improve the materials’ nutritional value. Figure 1 shows the expected characteristics of unconventional feed after fermentation, which can effectively reduce the amount of anti-nutritional factors and toxins and increase the flavor substances in raw feed. The antioxidant application of unconventional fermented feed in animal production is a key measure. This type of feed protects animal cells from oxidative damage by providing a rich source of antioxidants [61] such as vitamin C, vitamin E, and polyphenolic compounds that effectively inhibit the production of free radicals. In addition, the preparation of unconventional fermented feed may degrade the anti-nutritional factors and toxins in feed, reduce the load of oxidative stress in animals [62], and further reduce the incidence of oxidative damage. These antioxidants are also able to enhance the immune function of animals, making them more resistant and thus reducing the risk of disease. It is worth noting that unconventional feed has stronger antioxidant activity after fermentation treatment [63], which can not only maintain the feed’s nutrient integrity and extend its shelf life, but can also improve its palatability, digestion, and absorption efficiency, ultimately improving the overall health and production performance of animals. Therefore, the antioxidant application of unconventional fermented feed in animal production is of great significance for improving the sustainable development of the aquaculture industry.
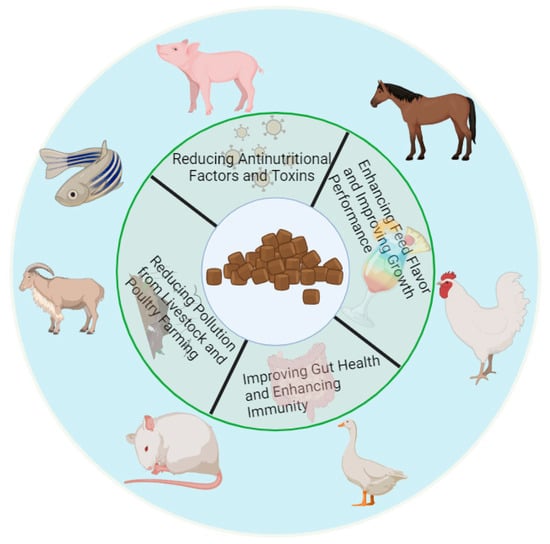
Figure 1.
Expected characteristics of unconventional feed after fermentation. After fermentation, the anti-nutritional factors and toxins present in unconventional feed are greatly reduced, and the flavor substances of the feed are significantly increased. By eating fermented feed, animals can significantly improve their growth performance, improve their intestinal health, enhance their immunity, and reduce environmental pollution in the process of livestock and poultry breeding.
3.1. Reductions in Anti-Nutritional Factors and Toxins
Unconventional fermented feed uses a variety of effective measures to reduce anti-nutritional factors and toxins. Through microbial action [64], enzyme action [65], and acid base treatment, the microflora and enzymes in the fermentation process can decompose, transform, and reduce the contents of anti-nutritional factors and toxins. At the same time, proper heat treatment [66] and the screening of raw materials can further reduce the presence of these harmful substances. The process of microbial fermentation can effectively decompose anti-nutritional factors and toxins in feed [67], thereby reducing their content, and has the advantages of high treatment efficiency, no reagent residue, safe use, and less nutrient loss, so fermented feed has been widely used in actual livestock and poultry production. Fermentation improves not only the nutritional value, but also the safety of feed, making it more suitable for the digestion and absorption of livestock and poultry. Yeast cell walls can adsorb mycotoxins such as aflatoxin B1, reduce the absorption of mycotoxins by the digestive tract, and protect animals from the toxic effects of mycotoxins [68]. Liu et al. [69] found that microbial fermented feed could further improve the growth performance and immune indexes of offspring mice. Wang et al. [70] showed that fermented kelp residue can promote the growth of sea cucumbers, enhance the antioxidant capacity, and maintain the stability of intestinal flora.
3.2. Increases Feed Flavor and Improves Growth Performance
Fermentation produces a large number of organic acids, small peptides, amino acids, enzymes, and various metabolites that increase the natural flavor of feed, improve the palatability of feed, and promote the appetite of animals [71]. For example, lactic acid, butyric acid, acetic acid, propionic acid, and other organic acids [72] can reduce the pH value of feed and inhibit the growth of harmful microorganisms; small peptides and amino acids [73] provide nutrients that help animals grow and develop; and phytase, cellulose, and other enzymes [74] can help to decompose complex polysaccharides and proteins and improve the digestion and absorption rate of feed. Taken together, these effects promote the digestion and absorption of nutrients and improve animal growth performance [75,76]. Yeast produces alcohol and other volatile compounds with a distinctive wine aroma during fermentation. Lactic acid bacteria produce a large amount of lactic acid, which has a sour aroma, during fermentation. Both different types of fermented feed can increase the appetite of animals while also having an increased nutritional value. Studies have shown that fermented feed can increase the contents of unsaturated fatty acids and amino acids in the muscles of fattening pigs during the growth period, enhance the antioxidant capacity, and improve pork quality [77,78,79]. Liu et al. [80] found that fermented mixed feed could up-regulate the expression of unsaturated fatty acid synthesis (acetyl-coenzyme A acyltransferase 1 and fatty acid desaturase 2), enhance the antioxidant capacity, and improve meat quality in fattening pigs. Zhu et al. [28] found that fermented feed could improve the growth performance, immune organ development and ability, and intestinal antioxidant capacity of broiler chickens.
3.3. Improves Intestinal Health and Strengthens Immunity
Unconventional fermented feed plays an important role in improving gut health and boosting immunity. First of all, the beneficial microorganisms and metabolites produced by the fermentation process help to maintain the intestinal microecological balance, inhibit the growth of harmful bacteria, and reduce the invasion of pathogenic bacteria to the intestine, thereby improving intestinal health [81]. Secondly, the bioactive substances in fermented feed, such as organic acids, enzymes, and peptides, can promote the growth and repair of intestinal mucosal cells, enhance intestinal barrier function, and prevent the penetration of harmful substances [82]. In addition, the bioactive ingredients rich in fermented feed can also regulate the function of the immune system, promote the generation and activity of immune cells, and improve the resistance and immunity of animals [83]. Beneficial microorganisms in fermented feed can improve the structure of the intestinal microbial communities of animals [84,85], promote intestinal health, and improve the digestion and absorption of nutrients. Microbial fermented feed can stimulate the development of immune organs, improve immune function, and increase the weight of immune organs, thereby strengthening the immunity of animals [86,87,88]. Studies have shown that fermented feed has a positive impact on improving the production efficiency of laying hens, improving egg quality and gut health [89,90]. Lactation is an important stage of pig reproduction [91], and Wang et al. [92] found that the addition of 15% Bacillus subtilis and Enterococcus faecalis to a fermented corn–soybean meal mixture significantly increased the average daily gain (ADG), litter gain, and individual piglet gain during lactation. With the addition of Bacillus mesenterica, Enterococcus faecalis, and Clostridium butyricum to fermented feed, the productivity of sows and offspring was effectively improved. In addition, Grela et al. [93] showed that the fermented rapeseed had a positive effect on nutrient digestibility during lactation in sows. Qiang et al. [94] found that the fermentation of wolfberry through modern fermentation technology can increase the levels of a variety of nutrients and bioactive components, as well as improve the antioxidant capacity.
3.4. Reduces Pollution from Livestock and Poultry Farming
Unconventional fermented feed plays an important role in reducing pollution from livestock and poultry farming. First of all, the use of unconventional raw materials as the main component of fermented feed can effectively use resources such as agricultural and sideline products and aquatic product by-products, reduce the demand for traditional raw feed materials, and thus reduce resource consumption and environmental pressure in the agricultural production process [27]. Secondly, the microorganisms and enzymes in the fermentation process can degrade organic matter in organic waste, such as straw and manure, which reduces the emission of organic waste and reduces the pollution of livestock and poultry breeding to the environment [95]. In addition, the antioxidants and bioactive components in fermented feed can also improve the digestion and absorption capacity of animals, reduce the emission of nutrients in manure, and further reduce the pollution of water and soil by livestock and poultry farming. Pathogens in feces can pollute the farm environment, including the water and soil, which is harmful to human and animal health [96]. The main pathogen on farms is Enterobacteriaceae, which leads to impaired barrier function and malnutrition by colonizing the intestinal mucosa of pigs [97]. The use of fermented feed in livestock and poultry farming can significantly reduce environmental impacts. This is mainly due to the fact that fermented feed can reduce the discharge of manure, eliminate the foul smell of manure, and reduce the number of mosquitoes and flies, thus effectively reducing environmental pollution. A study by Hăbeanu et al. [98] showed that when a mixture of puffed flaxseed and walnut powder (50:50) was added to a pig’s diet, the amount of nitrogen excreted by the pig was significantly reduced. Additionally, feeding poultry with probiotic fermented feed was shown to reduce nitrogen and phosphorus emissions in manure.
4. Unconventional Fermented Feed Improves Oxidative Stress by Regulating Gut Health
The highly intensive development of modern animal husbandry is prone to result in oxidative stress in animals [99], which can cause damage to intestinal health, including destroying intestinal mucosal structures and affecting the absorption function of the intestine, thus affecting the growth performance of animals. The development of ROS in the gastrointestinal tract is shown in Figure 2. Preventing oxidative stress in animals is a significant issue. The use of probiotic fermented feed to feed animals has been recognized and widely used in animal husbandry production [100]. Many studies have shown that fermented feed can affect the stability of the gut microbiota and the proper function of the gut [101,102]. Moreover, dietary fiber in fermented feed plays an important role in regulating the lipid metabolism and antioxidant stress response, and it has significant effects in improving cardiovascular and cerebrovascular diseases, adjusting the structure of intestinal flora, and affecting the energy balance through the gut microbiota gut–brain axis, thereby having a positive impact on health [103,104,105]. Fermented feed plays a key role in antioxidant activity [22,106,107] as it is rich in antioxidants (e.g., vitamin C, vitamin E, and polyphenols), and some microbial metabolites produced during fermentation also have an antioxidant capacity, which helps maintain the redox balance in the body [108]. In addition, other significant advantages of fermented feed are that it improves the utilization rate of feed nutrients, effectively improves the nutritional level of animals, and enhances the ability to resist oxidative stress [109,110]. Indeed, the use of fermented feed not only provides antioxidants and microbial metabolites, but also promotes the absorption of nutrients, which is an important breeding strategy that is beneficial to animal health and physiological balance.
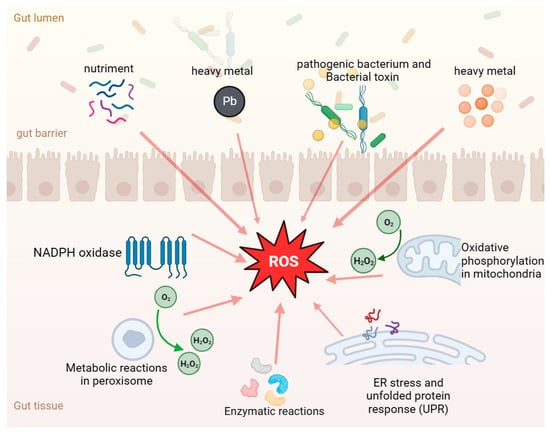
Figure 2.
Development of ROS in the gastrointestinal tract. The orange arrows indicate that ROS can be caused by a variety of factors, such as intestinal nutrient stimulation, antigens, pathogenic bacteria, mycotoxin contamination, and heavy metal pollution, as well as autometabolism by-products such as peroxidase and oxidative phosphorylation.
4.1. Gut Health and Oxidative Stress
Gut health is an important foundation for animal health and growth performance, and the gut is one of the most important sources of oxygen free radicals, which can be produced in the intestinal lumen and intestinal mucosa [111]. When the intestinal environment changes, intestinal oxidative stress may be triggered, which may result in changes in the nutrient content, increases in the number of antigens, the invasion of pathogenic bacilli, mycotoxin contamination, and heavy metal contamination. These factors can upset the balance of microbes in the gut, leading to an increase in harmful microflora, which can trigger intestinal inflammation and damage. In addition, the metabolites and free radicals produced during autometabolism may also cause damage to intestinal cells, leading to the occurrence of oxidative stress. Oxidative stress causes an imbalance in the redox balance within cells, increases the production of free radicals, and leads to the lipid peroxidation of cell membranes, the oxidation of proteins, and damage to DNA [112,113]. The damage of ROS to the intestine is affected by a variety of factors, such as the amount of free radicals and the antioxidant capacity of the body. In general, the ROS produced by the body are quickly removed by the body’s antioxidant system, which helps to maintain oxidative homeostasis and avoid oxidative damage to the body. However, if too much ROS are produced or the antioxidant system is less able to scavenge ROS, the homeostasis between oxidation and antioxidation will be disrupted. In this case, ROS cannot be removed in time and will accumulate in large quantities in the body, which may eventually lead to oxidative stress damage, especially in the intestines [114]. Studies have shown that macromolecular degradation by-products such as respiratory metabolites, lipids, and proteins, metabolites produced by macrophages when they engulf viruses, and the cytochrome P450 system are the main sources of ROS production in the gut [115,116,117]. When an oxidative response is triggered, the regenerating digestive tract epithelial cells are particularly sensitive to free radicals and the unsaturated fatty acids in the cell membrane easily react with these free radicals, resulting in lipid peroxidation, a decline of microbial diversity in the intestinal flora, a decrease in the number of beneficial bacteria, an increase in the number of pathogenic bacteria, a reduction in the ability of beneficial bacteria to produce metabolites such as antioxidant enzymes or short-chain fatty acids (SCFAs), and the change of the free radicals in the chemical structure of the tight junction of digestive tract epithelial cells, which results in the loss of the barrier function; all of these processes are significant hidden dangers to animals. At this point, an animal can reduce the damage caused by oxidative stress by ingesting substances with antioxidant activity (Figure 3). Wang et al. [118] found that spermidine could improve the intestinal health of a Sichuan white goose by improving its intestinal morphology, increasing the antioxidant capacity, and regulating intestinal microbiota structures. Liu et al. [119] showed that cysteine could inhibit the intestinal nuclear factor-κB (NF-κB)/inhibitor of κB kinase/inhibitor of nf-κB signaling pathway and pro-inflammatory cytokine mRNA levels, as well as increase the diversity and relative abundance of intestinal microbiota in golden pomfret. Li et al. [120] found that probiotics inhibited inflammation and oxidative stress by improving intestinal SCFA and inhibiting the p38 mitogen-activated protein kinase/NF-κB pathway in colon tissue.
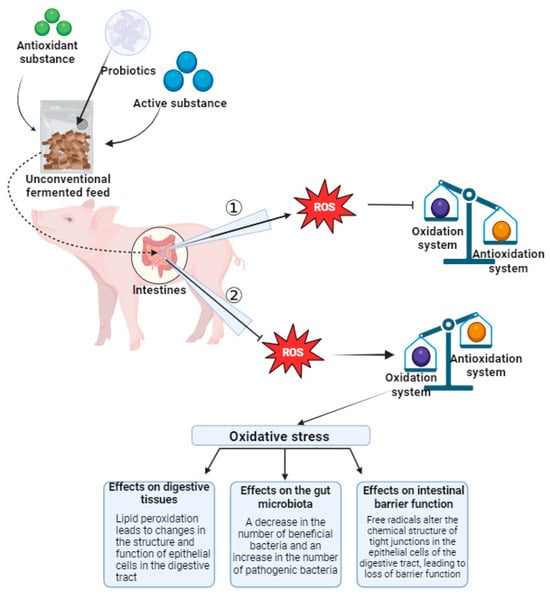
Figure 3.
Unsaturated fatty acids in membranes of the digestive tract epithelium are prone to react with free radicals. Beneficial gut bacteria have a reduced ability to produce metabolites such as antioxidant enzymes or SCFA. The changes of free radicals in the chemical structure of tight junctions in epithelial cells of the digestive tract lead to the loss of barrier function. Arrow ① indicates that feeding animals with unconventional fermented feed can affect the development of ROS, inhibiting the impact of ROS on the oxidative–antioxidative balance system. Arrow ② indicates that animals not fed with unconventional fermented feed are unable to inhibit ROS, thus disrupting the oxidative–antioxidative balance. When the oxidative–antioxidative balance is disrupted, animals experience oxidative stress, which adversely affects digestive tissues, intestinal microbiota, and intestinal barrier function.
4.2. Effects of Unconventional Fermented Feed on the Morphological Structure of Intestinal Mucosa
Changes in intestinal morphology (villi height and crypt depth) are the main causes of functional alterations and decreased intestinal absorptivity. When stress is generated, the level of ROS in the gut is elevated and the microbiome balance is disrupted (Figure 4), which also causes the increased mitosis of intestinal cells and the increased depth of the intestinal crypts. When the rate of intestinal cell migration to villi increases, so does the rate of apical intestinal cell shedding, which leads to an increased loss of mature intestinal cells, thereby reducing enzyme activity and absorption [111]. This may increase the sensitivity of the small intestine to pathogenic microorganisms, such as E. coli, further exacerbating damage to intestinal tissue. Fermented feed can effectively neutralize free radicals in the intestine through the antioxidants and microbial products it contains, slow down the production of oxidative stress, improve the length and width of villi, inhibit inflammation, reduce intestinal permeability [121], and help maintain the stability of the body’s intestinal environment and intestinal morphology [122].
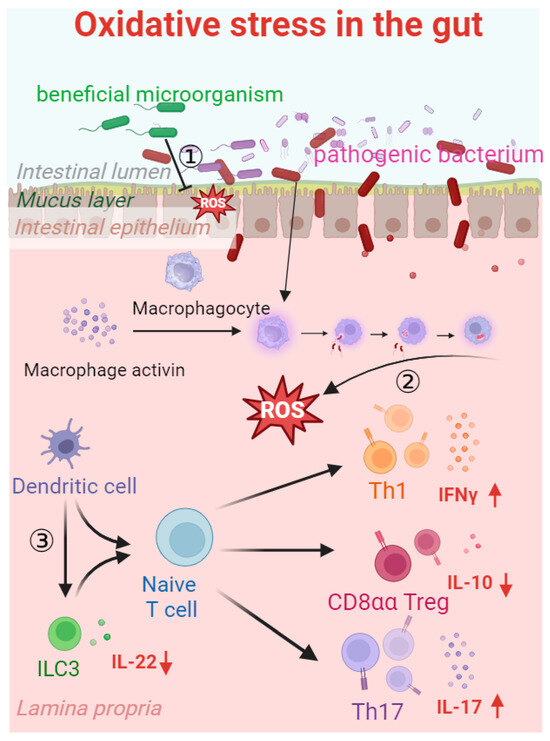
Figure 4.
Oxidative stress in the gut. Pathogenic bacteria enter the internal environment by damaging the gaps between intestinal epithelial cells, triggering an inflammatory response. Inflammatory factors activate macrophages to produce ROS during phagocytosis, which strengthens the inhibition of beneficial bacterial colonization and biological functions. In the case of impaired barrier function, group 3 innate lymphoid cells (ILC3 cells), CD8aa cells, Th17 cells, and Th1 cells are stimulated, thereby reducing the secretion of interleukin-22 (IL-22) (↓) and interleukin-10 (IL-10) (↓) and increasing the secretion of interferon-γ (IFNγ) (↑) and interleukin-17 (IL-17) (↑). Inhibition line ① indicates the definite vegetation inhibition of beneficial microorganisms in the intestine. Arrow ② indicates that ROS are produced when harmful bacteria invade and macrophages initiate phagocytosis. Arrow ③ indicates that dendritic cells are influenced by oxidative stress, leading to the stimulation of ILC3 cells, CD8aa cells, Th17 cells, and Th1 cells, resulting in the decreased secretion of IL-22 and IL-10 and the increased secretion of IFNγ and IL-17.
The effects of unconventional fermented feed on the intestinal health of animals can be seen in a number of ways. First, by influencing the gut microbiota, unconventional fermented feed is able to regulate the structure and composition of the gut microbiota of animals at different stages of growth and to improve the digestibility and overall gastrointestinal function of animals, thereby reducing the risk of diarrhea, helping to maintain a healthy state, and improving the growth performance and antioxidant capacity [26]. Secondly, unconventional feed grows rich in beneficial microorganisms and nutrients after fermentation, which can improve the morphological structure of the intestinal mucosa, increase the height and area of intestinal villi, and further maintain the self-stability of the intestine [123]. The ratio of villi height to crypt depth (V/C) is directly proportional to the body’s ability to absorb nutrients [124]. Dietary supplementation with Bacillus subtilis has been shown to promote the development of small intestinal villi [125]. Sugiharto et al. [126] found that supplementing a basic diet with 10~15% fermented banana peel and the same amount of corn could significantly increase the ileal villi height of Roman broilers. Liu et al. [127] found that feeding layers with fermented mixed feed significantly reduced the depth of crypts in the duodenum and jejunum and increased the ratio of villus height to crypt depth. Wu et al. [128] showed that the duodenal villi height/crypt depth ratio increased after broilers were fed fermented soybean meal (FSBM). Yu et al. [129] found that a level of 6.73~20.18% fermented cottonseed meal in a meat goose diet significantly increased the villi height of the jejunum. Feeding Bacillus subtilis was shown to increase the length of intestinal villi in Nile tilapia [130]. Feeding Nile tilapia with a bacillus-rich diet resulted in an increase in the number of goblet cells [131].
4.3. Effects of Unconventional Fermented Feed on Intestinal Barrier and Absorption Function
The bioactive ingredients and beneficial microorganisms in fermented feed help to enhance the integrity and tightness of the intestinal mucosa, prevent the penetration of harmful substances, reduce the invasion of pathogenic microorganisms, and protect the intestines from damage [81]. Enzymes and metabolites in fermented feed can promote the decomposition and release of nutrients, improve nutrient availability, and thus enhance the ability of animals to digest and absorb nutrients. In addition, fermented feed is rich in antioxidants, which can scavenge free radicals, reduce the impact of oxidative stress on the intestinal barrier and absorption function, protect the health of intestinal mucosal cells and tissues, and maintain normal cell and tissue function [132]. The intestinal mucosa, as the largest interface between the body and the external environment, is easily disturbed by factors such as pathogenic microorganisms, pro-inflammatory factors, and antigens. The mechanical barrier of the intestinal mucosa can prevent damage to the small intestine caused by foreign substances and reduce the permeability of the intestinal mucosa, thereby maintaining the health of the intestine. ROS ultimately damage the intestinal biological barrier by affecting the adhesion and colonization of microorganisms and intestinal epithelial cells and the digestion and metabolism of nutrients between microorganisms and hosts in the gut. The various digestive enzymes produced by probiotics in fermented feed during the fermentation process can decompose macromolecular substances in raw feed materials, such as starch, protein, and cellulose, into small molecule substances for better digestion by animals [133]. These digestive enzymes can also be used as a supplement to the digestive enzymes of animals’ digestive tracts to further break down nutrients, thereby improving the digestion and absorption of nutrients [134,135,136] and improving the growth performance [137]. When piglets are weaned separately from sows, physiological, environmental, and social stresses can lead to multiple stress responses. These stressors include oxidative stress, barrier dysfunction, and disturbances in the gut microbiota. In addition, weaned piglets are susceptible to immune stimulation due to imperfect digestive systems, low digestive enzyme activity, low immunity, etc., resulting in immune imbalance, oxidative stress, diarrhea, and even death. These stress responses may disrupt the development of the intestinal immune system of piglets, inhibiting their growth and development [138]. Xin et al. [139] showed that liquid fermented feed could significantly increase the daily weight gain of weaned piglets, as well as the abundance of lactic acid bacteria in cecal chyme and the level of acetic acid in colonic chyme. Wang et al. [140] reported that fermented feed improved the hematologic profile and serum concentrations of the total protein, albumin, and globulin in weaned piglets. Wang et al. [141] showed that FSBM could alleviate diarrhea symptoms in weaned piglets by modulating the cecal microbiota composition. Guo et al. [142] found that when 6% compound probiotic fermented feed was added to the basal diet of laying hens, the relative mRNA expression level of Occludin in the jejunal mucosa could be significantly increased, and the physical barrier function of the gut of laying hens could be improved. Wang et al. [143] treated piglets with the Lactobacillus plantarum strain ZLP 001 and found that the density of Clostridium spp_1 cells associated with epithelial inflammation in the experimental group was reduced, and the expression and secretion of pro-inflammatory cytokines IL-6 and TNF-α were down-regulated, which strengthened the intestinal barrier function and antioxidant capacity of piglets.
4.4. Effects of Unconventional Fermented Feed on Gut Microbiota
The effect of unconventional fermented feed on intestinal microbiota is significant. Its functions are mainly reflected in regulating the structure and composition of microflora, increasing the number of beneficial microorganisms, promoting the diversity of microflora, and improving the metabolic function of microflora [132]. Through these effects, fermented feed can maintain the balance and stability of the intestinal flora, promote the growth and reproduction of beneficial bacteria, and inhibit the reproduction of harmful bacteria, thereby improving the intestinal health of animals. This combined effect helps to improve the digestion and absorption capacity of animals, enhance immunity, and reduce the occurrence of intestinal diseases such as diarrhea [144]. Fermented feed is rich in organic acids, amino acids, and small peptides after decomposition; these components have a role in regulating dysbiosis [145,146]. Under normal circumstances, the intestinal tract is in a state of microecological balance, and beneficial bacteria such as lactic acid bacteria and bifidobacteria in the intestine adhere to the intestinal epithelial cells to produce bacteriocins, organic acids, and other bacteriostatic substances to inhibit the adhesion, colonization and invasion of pathogenic bacteria. Intestinal microbiota are in a state of reciprocal symbiosis with their host. For example, the transplantation of fecal bacteria has been used to clinically treat diseases caused by intestinal homeostasis imbalance [147,148]. Probiotic supplementation can help alleviate intestinal diseases (such as intestinal inflammation and intestinal cancer diseases), enhance intestinal function, and improve antioxidant capacity [149]. Studies have shown that mice can be supplemented with Lactobacillus gasseri to produce SCFAs [150,151] and inhibit the development of intestinal inflammation-related cancers [152]. These microorganisms can effectively improve the structure of intestinal microbial communities, increase the number of beneficial bacteria in the intestine, reduce the number of harmful bacteria, and improve intestinal health through fermented feed [153]. Xin et al. [139] fed fattening pigs with fermentation broth, and the results showed that the final body weight and ADG of the fattening pigs significantly increased, the total volatile fatty acid content showed an upward trend, and the total intestinal digestibility increased. O’Meara et al. [154] found that the liquid feeding of fermented cereals to fattening pigs could increase the abundance of lactic acid bacteria, reduce the abundance of Enterobacteriaceae bacteria, and improve the composition of intestinal microbiota. Guo et al. [142] showed that adding 6% compound probiotic fermented feed to a diet could regulate the microbial structure of the cecum and improve the intestinal health of laying hens.
4.5. Effects of Unconventional Fermented Feed on Immune Function
Immunomodulatory effects can promote the production and activation of immune cells, enhance the body’s immune response, and reduce the risk of animal disease [132]. Secondly, the anti-inflammatory components in fermented feed can inhibit the occurrence of inflammatory reactions, reduce the burden on the immune system, and protect the body’s tissues from damage. In addition, fermented feed can also promote the development and function of immune organs, as well as enhance the overall function of the immune system. Most importantly, it is rich in antioxidants, which can scavenge free radicals, reduce oxidative stress damage to the immune system, and improve the stability and activity of the immune system [106]. The use of unconventional fermented feed not only increases the contents of immunoactive substances such as cytokines, antibodies, and lysozymes, but also improves the immune capacity of animals by stimulating the development of immune organs, improving immune function, and increasing the weight of immune organs [155], as well as ameliorating the damage of oxidative stress to the intestine [86,87,88]. There are many different microbes in the gut that are involved in different immune defense mechanisms and influence the body’s immune response. Under intestinal oxidative stress, the contents of immunoglobulin A, immunoglobulin G, and immunoglobulin M in the intestine decrease, the number of T cells decreases, and cellular immunity is abnormal. The use of unconventional fermented feed can effectively alleviate the occurrence of this situation. The SCFAs produced during the fermentation of unconventional feed are involved in the regulation of the intestinal mechanical barrier of livestock and poultry, improving the activity of immune substances [156] and reducing the content of harmful bacteria. Studies have shown that the number and activity of immune cells are significantly enhanced when animals ingest microbial fermented feed [157,158]. According to Taranu et al. [159], feeding fermented cassava residue can increase the serum immunoglobulin content in pigs, improving humoral immune activity, and thus enhancing disease resistance. Wu et al. [128] found that the addition of FSBM to broiler diets significantly increased the weight of the bursa and thymus and reduced serum alanine aminotransferase levels. The addition of fermented full-price feed significantly increased the serum levels of interleukin-1β, IL-6, and TNF-a in laying hens [160]. Feeding fermented full-price feed significantly increased the content of insulin-like growth factor-1 (IGF-1) in the sera of finishing pigs [161], promoted the expression of the IGF-1 protein in the liver, and improved growth performance [162]. Cheng et al. [163] showed that fermented feed inhibited the growth of harmful microflora such as E. coli and enterococci, while increasing the number of beneficial microflora such as Bifidobacteria, Lactobacillus, and Ackermansi. As shown in Figure 5, lactic acid bacteria decompose the proteins in feed through the lactic acid bacteria proteases secreted by themselves, so the proteins are converted into small molecule peptides and amino acids and the digestion and absorption of feed by livestock and poultry are improved. The bacteriocins secreted by lactic acid bacteria have the miraculous effect of inhibiting the growth of pathogenic microorganisms present in the intestines after entering the intestines of livestock and poultry. With the colonization of lactic acid bacteria in the intestine, macrophages can be activated to a certain extent, promoting the immune response of immune cells in the intestine, and improving the resistance of livestock and poultry to diseases [4]. Azad et al. [46] showed that when soybean meal was partially replaced with 5% fermented cassava residue, piglets could increase their CAT, GSH-Px, and HSOD levels, as well as enhance their antioxidant capacity.
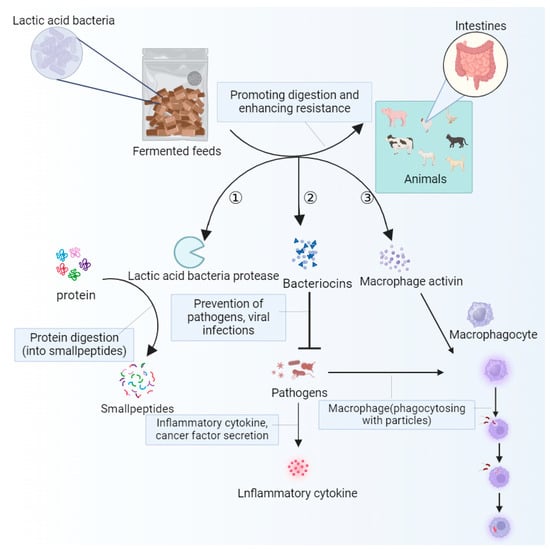
Figure 5.
Lactic acid bacteria promote digestion, inhibit microorganisms, and activate the body’s immune response mechanism. Arrow ① indicates that lactic acid bacteria secrete lactobacillus proteases to break down proteins into peptides; arrow ② indicates that lactic acid bacteria secrete bacteriocin, inhibiting the growth of pathogenic microorganisms, reducing the expression of inflammatory factors, and activating the phagocytic function of macrophages; arrow ③ indicates that lactic acid bacteria secrete macrophage activators, activating the phagocytic function of macrophages and generating immune responses.
4.6. Effects of Unconventional Fermented Feed on Antioxidant Capacity
Unconventional fermented feed has significant effects on the antioxidant capacity of animals as it is rich in antioxidant substances, such as vitamins, polyphenolic compounds, and organic acids, that can effectively remove free radicals in the body, regulate redox balance, enhance antioxidant enzyme activity, and improve the antioxidant capacity of animals [164]. These antioxidants not only protect cells and tissues from oxidative damage, but also improve animal immunity, reduce the risk of disease, and promote the healthy growth and performance of animals [165]. The beneficial microbial metabolites and active substances in unconventional fermented feed have the effects of promoting the activity of antioxidant enzymes, directly or indirectly affecting the activity of antioxidant enzymes, and slowing down the occurrence of oxidative stress [166,167]. Superoxide dismutase (SOD) scavenges ROS in the cytoplasm by catalyzing the disproportionation of two superoxide radicals to H2O2 and oxygen. GSH-Px and CAT [159] convert ROS to water and oxygen. MDA is one of the end products of lipid peroxidative damage by free radicals, and it can be used as a direct indicator of tissue damage caused by oxygen free radicals [168]; the higher the content, the higher the degree of cell damage and the weaker the antioxidant capacity [169]. The level of the total antioxidant capacity can directly reflect the level of antioxidant capacity in animals [170]. Lactic acid bacteria are partially lysed in the intestinal tract and release intracellular substances such as SOD and GSH-Px to reduce the cell damage caused by oxygen free radicals. Dong et al. [171] found that Lactobacillus plantarum KLDS 1.0386 has the ability to scavenge hydroxyl radicals and lipid antioxidants through in vitro tests. Hao et al. [172] found that the use of fermented mixtures was able to increase the activities of SOD and GSH-Px in the sera and longissimus dorsi muscles of fattening pigs. Chen et al. [173] showed that microbial fermentation can alter the structural characteristics of wheat bran polysaccharides and increase their antioxidant activity. FSBM can increase SOD and CAT activities and vitamin C levels in piglet tissues [174]. As the main raw material for the industrial production of natural astaxanthin, astaxanthin can significantly reduce the levels of ROS and MDA in the sera of pigs [175]. Studies have shown that yeast extracts are able to increase the antioxidant capacity of aquatic animals [176]. Long et al. [177] showed that dietary supplementation with Saccharomyces cerevisiae increased serum SOD activity and reduced MDA levels in weaned piglets.
In conclusion, unconventional fermented feed can regulate the structure of the gut and the composition of intestinal microbiota, which can effectively improve the digestibility and overall gastrointestinal function of animals, reduce the risk of oxidative stress, and help maintain a healthy state and improve growth performance [146,178,179].
5. Prospects
The use of unconventional fermented feed has a positive impact on sustainable and environmentally friendly livestock practices in many ways. First of all, this kind of feed uses diversified raw materials such as agricultural and sideline products, aquatic by-products, and industrial by-products, which effectively use resources and reduce resource waste. Secondly, compared with traditional feed production, the production of unconventional fermented feed produces less environmental pollution [59] and greenhouse gas emissions, which helps to reduce the environmental load [180]. In addition, unconventional fermented feed may contain richer nutrients and bioactives than traditional feed, which can improve the growth, development rate [181], immunity, and disease resistance of animals, thereby improving breeding efficiency. Most importantly, the use of unconventional fermented feed promotes the practice of the circular economy, and the goals of waste reduction, resource conservation, and ecological environmental protection can be achieved through waste resource utilization and resource reuse, which is conducive to building a more sustainable and environmentally friendly livestock system.
The rapid development of unconventional fermented feed is important for the livestock industry, but the challenges and limitations require targeted solutions and further research.
- (1)
- Diversified sources of raw materials: Exploring a greater variety of unconventional raw materials, such as agricultural by-products, aquatic by-products, and industrial by-products, can reduce supply instability through inter-regional and inter-seasonal diversification. The introduction of diversified sources of raw materials can not only reduce excessive dependence on a specific raw material, but can also help to improve the diversity and nutritional balance of feed.
- (2)
- Optimization of the fermentation process: By studying and optimizing the fermentation process, such as by controlling parameters such as temperature, humidity, and PH, the stability and efficiency of the fermentation process can be improved. The introduction of automation technology and intelligent monitoring systems can be used to help reduce human error, improve production efficiency, and improve product quality.
- (3)
- Anti-nutritional factor processing technology: The development of efficient treatment technologies, such as enzymatic hydrolysis, heat treatment, and acid–base treatment, can effectively reduce the content of antinutritional factors in raw materials. Further research into the mechanisms of different antinutritional factors in animal digestion and absorption is needed to optimize treatment techniques and ensure the nutritional value of feed.
- (4)
- Economic benefit evaluation: A comprehensive economic benefit evaluation study should be conducted to consider production costs, animal growth performance, feed utilization efficiency, and other factors to provide a more concrete and reliable economic decision-making basis for farmers and breeders. Tailored economic assessments should be conducted for different farming scales and regional characteristics to ensure the practical feasibility and sustainability of solutions.
- (5)
- An in-depth study of animal health and oxidative stress mechanisms: researchers should investigate the mechanism of fermented feed on the intestinal microecological balance, and explore its specific effects on animal immunity and disease resistance in order to further understand the regulatory effects of active ingredients in fermented feed on oxidative stress in animals, as well as the mechanism of their effects on the overall growth performance and meat quality.
Through in-depth research and the continuous improvement of these aspects, the challenges faced by unconventional fermented feed can be better met and its wide application and development in animal husbandry can be promoted.
6. Conclusions
Oxidative stress means that the oxidative substances in animals exceed the scavenging capacity of their antioxidant systems, which may have negative effects on animal health, including affecting the intestinal barrier function and inducing an inflammatory response. As a potential feed alternative to improve animal health, unconventional fermented feed may help reduce the levels of oxidative stress in animals by being rich in antioxidants, maintaining the intestinal microbiota balance, and providing nutrient support. However, they need to be carefully selected and managed to ensure that their effects on animals are positive, and they should be monitored and adjusted on a case-by-case basis.
Therefore, the exploration of the use of unconventional fermented feed to maintain the intestinal health of animals is promising. In future research, it is crucial to further improve the quality of fermented feed and optimize the technological process of fermented feed. At the same time, by studying the mechanism of fermented feed in regulating gut health and the antioxidative capacity, we can provide strong support for the development of more scientific feeding strategies, thereby promoting the overall health and performance of animals. This systematic research will help to continuously improve the application of unconventional fermented feed and promote the development of animal husbandry in a more sustainable and efficient direction.
Author Contributions
X.L.: Writing—original draft; writing—review and editing. M.S.: Writing—review and editing. Y.L.: Writing—review and editing. Q.L.: Writing—review and editing. L.Z.: Funding acquisition; writing—original draft; writing—review and editing. X.L. and L.Z. wrote the manuscript. M.S., Q.L., and Y.L. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2022YFF1100203), the National Natural Science Foundation of China (32372349), the Hunan Provincial Science and Technology Innovation Program (2022RC3056, 2023RC3162), the Natural Science Foundation of Changsha City (kq2208417), the Hunan Provincial Department of Education Outstanding Youth Scientific Research Project (22B0286), and the Open Fund of Key Laboratory of Agro-Ecological Processes in Subtropical Region, Chinese Academy of Sciences (ISA2023102).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liang, J.; Nie, Z.; Zhao, Y.; Qin, S.; Nian, F.; Tang, D. Effects of Jujube Powder on Growth Performance, Blood Biochemical Indices, and Intestinal Microbiota of Broiler. Animals 2023, 13, 3398. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, L.; Zhou, S.; Li, Y.; Wang, Y.; Yang, K.; Chen, W.; Zhao, S. Effects of Different Proportions of Amaranthus hypochondriacus Stem and Leaf Powder Inclusions on Growth Performance, Carcass Traits, and Blood Biochemical Parameters of Broilers. Animals 2023, 13, 2818. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Samak, D.H.; Noreldin, A.E.; Arif, M.; Yaqoob, H.S.; Swelum, A.A. Towards saving freshwater: Halophytes as unconventional feedstuffs in livestock feed: A review. Environ. Sci. Pollut. Res. Int. 2018, 25, 14397–14406. [Google Scholar] [CrossRef] [PubMed]
- Sugiharto, S.; Ranjitkar, S. Recent advances in fermented feeds towards improved broiler chicken performance, gastrointestinal tract microecology and immune responses: A review. Anim. Nutr. 2019, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, G.; Liu, J.; Hao, S.; Huang, S. The Influence of Converting Food Crops to Forage Crops Policy Implementation on Herbivorous Livestock Husbandry Development—Based on Policy Pilot Counties in Hebei, China. Agriculture 2022, 12, 1872. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Han, H.; Liu, Z.; Zeng, X.; Li, T.; Yin, Y. Long-term effects of lysine concentration on growth performance, intestinal microbiome, and metabolic profiles in a pig model. Food Funct. 2018, 9, 4153–4163. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, F.; Yang, H.; Li, J.; Li, Y.; Ding, X.; Xiong, X.; Yin, Y. Effects of dietary supplementation with epidermal growth factor on nutrient digestibility, intestinal development and expression of nutrient transporters in early-weaned piglets. J. Anim. Physiol. Anim. Nutr. (Berl.) 2019, 103, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Sabour, S.; Tabeidian, S.A.; Sadeghi, G. Dietary organic acid and fiber sources affect performance, intestinal morphology, immune responses and gut microflora in broilers. Anim. Nutr. 2019, 5, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shi, C.; Zhang, Y.; Song, D.; Lu, Z.; Wang, Y. Microbiota in fermented feed and swine gut. Appl. Microbiol. Biotechnol. 2018, 102, 2941–2948. [Google Scholar] [CrossRef] [PubMed]
- Kizir, D.; Karaman, M.; Ceylan, H. Tannic acid may ameliorate doxorubicin-induced changes in oxidative stress parameters in rat spleen. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 3605–3613. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Kiani, A.; Santhiravel, S.; Holman, B.W.B.; Lauridsen, C.; Dunshea, F.R. The Importance of Dietary Antioxidants on Oxidative Stress, Meat and Milk Production, and Their Preservative Aspects in Farm Animals: Antioxidant Action, Animal Health, and Product Quality-Invited Review. Animals 2022, 12, 3279. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, H.; Li, S.; Jiang, Y.; Deng, J.; Yang, C.; Chen, X.; Jiang, L. Effects of different levels of Citri Sarcodactylis Fructus by-products fermented feed on growth performance, serum biochemical, and intestinal health of cyan-shank partridge birds. Sci. Rep. 2023, 13, 20130. [Google Scholar] [CrossRef]
- Fu, Z.; Ao, N.; Liang, X.; Chen, J.; Wang, Y.; Wang, Q.; Fu, J.; Liu, C.; Lu, L. Effects of fermented feed on growth performance, serum biochemical indexes, antioxidant capacity, and intestinal health of lion-head goslings. Front. Vet. Sci. 2023, 10, 1284523. [Google Scholar] [CrossRef]
- Jairath, G.; Verma, A.K.; Rani, D.; Marappan, G.; Bs, Y.; Singh, B.; Mal, G.; Gopinath, D.; Sharma, R.; Katoch, S.; et al. Self-fermented agro-wastes as antioxidant enriched maize grain replacer for sustainable animal feeding. J. Clean. Prod. 2023, 427, 139223. [Google Scholar] [CrossRef]
- Liu, S.; Tu, Y.; Sun, J.; Cai, P.; Zhou, Y.; Huang, Y.; Zhang, S.; Chen, W.; Wang, L.; Du, M.; et al. Fermented mixed feed regulates intestinal microbial community and metabolism and alters pork flavor and umami. Meat Sci. 2023, 201, 109177. [Google Scholar] [CrossRef]
- Hu, M.; Zhou, X.; Wang, Y.; Li, J.; Wu, Q.; Bao, S.; Jiang, L.; Liu, B. Use of fermented tea residues as a feed additive and effects on growth performance, body composition, intestinal enzyme activities, and inflammatory biomarkers in juvenile largemouth bass (Micropterus salmoides). Aquac. Rep. 2023, 31, 101671. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, W.; Xu, X.; Wang, W.; Xu, Y.; Wang, M.; Zhang, J.; Xu, H.; Fu, F. Protective effect of Luffa cylindrica fermentation liquid on cyclophosphamide-induced premature ovarian failure in female mice by attenuating oxidative stress, inflammation and apoptosis. J. Ovarian Res. 2024, 17, 24. [Google Scholar] [CrossRef]
- Bhavna, A.; Zindove, T.J.; Iji, P.A.; Bakare, A.G. Growth performance, carcass characteristics and meat sensory evaluation of broiler chickens fed diets with fermented cassava leaves. Anim. Biosci. 2024, 23, 362. [Google Scholar] [CrossRef]
- Martini, D.; Marino, M.; Venturi, S.; Tucci, M.; Klimis-Zacas, D.; Riso, P.; Porrini, M.; Del Bo, C. Blueberries and their bioactives in the modulation of oxidative stress, inflammation and cardio/vascular function markers: A systematic review of human intervention studies. J. Nutr. Biochem. 2023, 111, 109154. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, W.; Chen, J.; Chen, Z.; Avellán-Llaguno, R.D.; Xu, W.; Duan, Y.; Liu, B.; Huang, Q. Temporal dynamics of microbial composition and antibiotic resistome in fermentation bed culture pig farms across various ages. Sci. Total Environ. 2024, 912, 168728. [Google Scholar] [CrossRef]
- Sun, H.; Kang, X.; Tan, H.; Cai, H.; Chen, D. Progress in Fermented Unconventional Feed Application in Monogastric Animal Production in China. Fermentation 2023, 9, 947. [Google Scholar] [CrossRef]
- Araiza Ponce, K.A.; Gurrola Reyes, J.N.; Martínez Estrada, S.C.; Salas Pacheco, J.M.; Palacios Torres, J.; Murillo Ortiz, M. Fermentation Patterns, Methane Production and Microbial Population under In Vitro Conditions from Two Unconventional Feed Resources Incorporated in Ruminant Diets. Animals 2023, 13, 2940. [Google Scholar] [CrossRef]
- Zhang, X.; Long, J.; Liu, J.; Hua, Y.; Zhang, C.; Li, X. Fermentation Characteristics, Antinutritional Factor Level and Flavor Compounds of Soybean Whey Yogurt. Foods 2024, 13, 330. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Chen, S.; Shao, T.; Tao, X.; Yuan, X. Effect of Lactic Acid Bacteria on the Fermentation Quality and Mycotoxins Concentrations of Corn Silage Infested with Mycotoxigenic Fungi. Toxins 2021, 13, 699. [Google Scholar] [CrossRef]
- Kulabhusan, P.K.; Campbell, K. Physico-chemical treatments for the removal of cyanotoxins from drinking water: Current challenges and future trends. Sci. Total Environ. 2024, 917, 170078. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhao, H.; He, X.; Zhu, F.; Zhang, F.; Liu, B.; Liu, Q. Effects of fermented feed of Pennisetum giganteum on growth performance, oxidative stress, immunity and gastrointestinal microflora of Boer goats under thermal stress. Front. Microbiol. 2022, 13, 1030262. [Google Scholar] [CrossRef]
- Guo, W.; Xu, L.N.; Guo, X.J.; Wang, W.; Hao, Q.H.; Wang, S.Y.; Zhu, B.C. The impacts of fermented feed on laying performance, egg quality, immune function, intestinal morphology and microbiota of laying hens in the late laying cycle. Animal 2022, 16, 100676. [Google Scholar] [CrossRef]
- Zhu, X.; Tao, L.; Liu, H.; Yang, G. Effects of fermented feed on growth performance, immune organ indices, serum biochemical parameters, cecal odorous compound production, and the microbiota community in broilers. Poult. Sci. 2023, 102, 102629. [Google Scholar] [CrossRef]
- Shi, H.T.; Wang, B.Y.; Bian, C.Z.; Han, Y.Q.; Qiao, H.X. Fermented Astragalus in diet improved laying performance, egg quality, antioxidant and immunological status and intestinal microbiota in laying hens. AMB Express 2020, 10, 159. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, K.; Zhang, A.; Chang, W.; Zheng, A.; Chen, Z.; Cai, H.; Liu, G. Effects of Lactobacillus acidophilus on the growth performance, immune response, and intestinal barrier function of broiler chickens challenged with Escherichia coli O157. Poult. Sci. 2021, 100, 101323. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, G.; Meng, X.; Wang, X.; Zhao, Z.; Zhou, S.; Li, G.; Zhang, Q.; Wei, X. Effects of Lactobacillus plantarum and Pediococcus acidilactici co-fermented feed on growth performance and gut microbiota of nursery pigs. Front. Vet. Sci. 2022, 9, 1076906. [Google Scholar] [CrossRef]
- Yu, D.Y.; Oh, S.H.; Kim, I.S.; Kim, G.I.; Kim, J.A.; Moon, Y.S.; Jang, J.C.; Lee, S.S.; Jung, J.H.; Park, J.; et al. Intestinal microbial composition changes induced by Lactobacillus plantarum GBL 16, 17 fermented feed and intestinal immune homeostasis regulation in pigs. J. Anim. Sci. Technol. 2022, 64, 1184–1198. [Google Scholar] [CrossRef]
- Zhang, Q.; Jing, X.; Zhao, Y.; Xia, D.; Liu, S.; Li, D.; Hao, Q.; Wang, M.; Yu, Z.; Li, S.; et al. Partial replacement of pelleted feed by moist fermented feed improved the feed conversion efficiency, liver and intestine health, and gut microbiota structure in common carp (Cyprinus carpio). Aquac. Rep. 2023, 32, 101690. [Google Scholar] [CrossRef]
- Oncul, F.O.; Aya, F.A.; Ali, H.; Seonghun, W.; Geon, L.; Han, K.R.; Bai, S.C. Effects of the dietary fermented tuna by-product meal on growth, blood parameters, nonspecific immune response, and disease resistance in juvenile olive flounder, Paralichthys olivaceus. J. World Aquac. Soc. 2018, 50, 65–77. [Google Scholar] [CrossRef]
- Selim, A.S.M.; Hasan, M.N.; Rahman, M.A.; Rahman, M.M.; Islam, M.R.; Bostami, A.; Islam, S.; Tedeschi, L.O. Nutrient content and in vitro degradation study of some unconventional feed resources of Bangladesh. Heliyon 2022, 8, e09496. [Google Scholar] [CrossRef]
- Wang, J.; Lu, R.; Li, Y.; Lu, J.; Liang, Q.; Zheng, Z.; Huang, H.; Deng, F.; Huang, H.; Jiang, H.; et al. Dietary supplementation with jasmine flower residue improves meat quality and flavor of goat. Front. Nutr. 2023, 10, 1145841. [Google Scholar] [CrossRef]
- Omar, A.E.; Al-Khalaifah, H.S.; Ismail, T.A.; Abd El-Aziz, R.M.; El-Mandrawy, S.A.M.; Shalaby, S.I.; Ibrahim, D. Performance, Serum Biochemical and Immunological Parameters, and Digestive Enzyme and Intestinal Barrier-Related Gene Expression of Broiler Chickens Fed Fermented Fava Bean By-Products as a Substitute for Conventional Feed. Front. Vet. Sci. 2021, 8, 696841. [Google Scholar] [CrossRef]
- Yuchao, H.; Ziqi, M.; Wenwen, W.; Xiran, H.; Yuan, W.; Jingwei, Q. Carcase traits, meat quality, and lipogenic gene expression in muscle of lambs fed wheat bran feruloyl oligosaccharides. Ital. J. Anim. Sci. 2023, 22, 369–378. [Google Scholar]
- Veluri, S.; Gonzalez-Ortiz, G.; Bedford, M.R.; Olukosi, O.A. Interactive effects of a stimbiotic supplementation and wheat bran inclusion in corn- or wheat-based diets on growth performance, ileal digestibility, and expression of nutrient transporters of broilers chickens. Poult. Sci. 2024, 103, 103178. [Google Scholar] [CrossRef]
- Phomkaivon, N.; Pan-utai, W.; Surojanametakul, V.; Varichanan, P.; Kaewtathip, T.; Kanyakam, K.; Klinsoda, J. Isoflavone aglycone-rich powder from soybean residue submerged fermentation using Lactobacillus fermentum 44197. NFS J. 2023, 33, 100157. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, T.; Cai, G.; Huang, X.; Zhong, S.; Li, X.; Zhang, E. Effect of Aspergillus niger Fermentation on the Metabolites in Corn Stalks. Fermentation 2023, 9, 50. [Google Scholar] [CrossRef]
- Nunes, A.J.P.; Dalen, L.L.; Leonardi, G.; Burri, L. Developing sustainable, cost-effective and high-performance shrimp feed formulations containing low fish meal levels. Aquac. Rep. 2022, 27, 101422. [Google Scholar] [CrossRef]
- Xia, W.G.; Abouelezz, K.F.M.; Makled, M.N.; Wang, S.; Chen, W.; Zhang, Y.N.; Elokil, A.A.; Wang, S.L.; Huang, X.B.; Li, K.C.; et al. Effects of dietary substitution of peanut meal for soybean meal on egg production, egg quality, oxidative status, and yolk fatty acid profile in laying ducks. Animal 2022, 16, 100652. [Google Scholar] [CrossRef]
- Valena, R.D.L.; Sobrinho, A.G.d.S.; Romanzini, E.P.; Andrade, N.D.; Oliveira, V.D.S. Peanut meal and crude glycerin in lamb diets: Meat quality and fatty acid profile. Small Rumin. Res. 2020, 185, 106076. [Google Scholar] [CrossRef]
- Wang, T.; Xu, M.; Wang, J.; Wan, W.; Sun, H. A combination of rapeseed, cottonseed and peanut meal as a substitute of soybean meal in diets of Yellow River carp Cyprinus carpio var. Aquac. Nutr. 2020, 26, 1520–1532. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Jiang, H.; Ni, H.; Liu, Y.; Huang, P.; Fang, J.; Kong, X. Diets Partially Replaced With Cassava Residue Modulate Antioxidant Capacity, Lipid Metabolism, and Gut Barrier Function of Huanjiang Mini-Pigs. Front. Vet. Sci. 2022, 9, 902328. [Google Scholar] [CrossRef]
- Santana, T.M.; Dantas, F.d.M.; Monteiro Dos Santos, D.K.; Kojima, J.T.; Pastrana, Y.M.; De Jesus, R.S.; Gonçalves, L.U. Fish Viscera Silage: Production, Characterization, and Digestibility of Nutrients and Energy for Tambaqui Juveniles. Fishes 2023, 8, 111. [Google Scholar] [CrossRef]
- Lasekan, A.; Abu Bakar, F.; Hashim, D. Potential of chicken by-products as sources of useful biological resources. Waste Manag. 2013, 33, 552–565. [Google Scholar] [CrossRef]
- Chen, H.; Gao, S.; Li, Y.; Xu, H.-J.; Li, W.; Wang, J.; Zhang, Y. Valorization of Livestock Keratin Waste: Application in Agricultural Fields. Int. J. Environ. Res. Public Health 2022, 19, 6681. [Google Scholar] [CrossRef]
- Flores Tapia, N.E.; Brito Moina, H. Exploring Tannery Solid Wastes as a Source of Animal Feed. Processes 2023, 11, 2965. [Google Scholar] [CrossRef]
- Suryadi, U.; Hertamawati, R.T.; Imam, A.S. Hydrolyzation of snail (Achatina fulica) meat with rice water as novel probiotic supplements for animal feed. Vet. World 2022, 15, 937–942. [Google Scholar] [CrossRef]
- Nag, M.; Lahiri, D.; Dey, A.; Sarkar, T.; Pati, S.; Joshi, S.; Bunawan, H.; Mohammed, A.; Edinur, H.A.; Ghosh, S.; et al. Seafood Discards: A Potent Source of Enzymes and Biomacromolecules With Nutritional and Nutraceutical Significance. Front. Nutr. 2022, 9, 879929. [Google Scholar] [CrossRef]
- Terzioğlu, P.; Öğüt, H.; Kalemtaş, A. Natural calcium phosphates from fish bones and their potential biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 899–911. [Google Scholar] [CrossRef]
- Naghdi, S.; Rezaei, M.; Tabarsa, M.; Abdollahi, M. Fish Protein Hydrolysate from Sulfated Polysaccharides Extraction Residue of Tuna Processing By-Products with Bioactive and Functional Properties. Glob. Chall. 2023, 7, 2200214. [Google Scholar] [CrossRef]
- Zhu, K.; Yan, W.; Dai, Z.; Zhang, Y. Astaxanthin Extract from Shrimp (Trachypenaeus curvirostris) By-Products Improves Quality of Ready-to-Cook Shrimp Surimi Products during Frozen Storage at −18 °C. Foods 2022, 11, 2122. [Google Scholar] [CrossRef]
- Ge, Q.; Chen, L.; Tang, M.; Zhang, S.; Liu, L.; Gao, L.; Ma, S.; Kong, M.; Yao, Q.; Feng, F.; et al. Analysis of mulberry leaf components in the treatment of diabetes using network pharmacology. Eur. J. Pharmacol. 2018, 833, 50–62. [Google Scholar] [CrossRef]
- Ma, J.; Wang, J.; Jin, X.; Liu, S.; Tang, S.; Zhang, Z.; Long, S.; Piao, X. Effect of Dietary Supplemented with Mulberry Leaf Powder on Growth Performance, Serum Metabolites, Antioxidant Property and Intestinal Health of Weaned Piglets. Antioxidants 2023, 12, 307. [Google Scholar] [CrossRef]
- Herosimczyk, A.; Lepczyński, A.; Ożgo, M.; Tuśnio, A.; Taciak, M.; Barszcz, M. Effect of dietary inclusion of 1% or 3% of native chicory inulin on the large intestinal mucosa proteome of growing pigs. Animal 2020, 14, 1647–1658. [Google Scholar] [CrossRef]
- Li, X.; Jensen, B.B.; Canibe, N. The Mode of Action of Chicory Roots on Skatole Production in Entire Male Pigs Is neither via Reducing the Population of Skatole-Producing Bacteria nor via Increased Butyrate Production in the Hindgut. Appl. Environ. Microbiol. 2019, 85, e02327-18. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Y.; Zhong, R.; Han, H.; Liu, L.; Chen, L.; Zhang, H.; Beckers, Y.; Everaert, N. Effects of graded levels of xylo-oligosaccharides on growth performance, serum parameters, intestinal morphology, and intestinal barrier function in weaned piglets. J. Anim. Sci. 2021, 99, skab183. [Google Scholar] [CrossRef]
- Zou, S.; Sun, C.; Li, F.; Xie, Y.; Liang, T.; Yang, Y.; Shi, B.; Ma, Q.; Shi, Z.; Chai, S.; et al. Effect of Gardenia Pomace Supplementation on Growth Performance, Blood Metabolites, Immune and Antioxidant Indices, and Meat Quality in Xiangcun Pigs. Animals 2022, 12, 2280. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, C.; Sun, Y.; Chen, Y.; Chen, S.; Han, T.; Wang, J. Excessive Substitution of Fish Meal with Fermented Soybean Meal Induces Oxidative Stress by Impairing Glutathione Metabolism in Largemouth Bass (Micropterus salmoides). Antioxidants 2023, 12, 2096. [Google Scholar] [CrossRef]
- Wu, H.; Barrow, C.; Dunshea, F.R.; Suleria, H.A.R. Phenolic bioaccessibility, antioxidant, and antidiabetic effects of indigenous fermented coffee beans after simulated gastrointestinal digestion and colonic fermentation. Food Biosci. 2023, 54, 102920. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, L.; Li, Y.; Wu, H.; Lu, L.; Zang, R.; Xu, H. Effects of Tail Vegetable Fermented Feed on the Growth and Rumen Microbiota of Lambs. Animals 2024, 14, 303. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.; Li, S.; Zhang, H.; Liu, Z. Effects of tea residues-fermented feed on production performance, egg quality, antioxidant capacity, caecal microbiota, and ammonia emissions of laying hens. Front. Vet. Sci. 2023, 10, 1195074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, F.; Shi, T.; Xiong, Z.; Gao, R.; Yuan, L. Suanyu fermentation strains screening, process optimization and the effect of thermal processing methods on its flavor. Food Res. Int. 2023, 173, 113296. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jin, Z.; Hu, D.; Yang, W.; Yan, Y.; Nie, X.; Lin, J.; Zhang, Q.; Gai, D.; Ji, Y.; et al. Effect of solid-state fermentation with Lactobacillus casei on the nutritional value, isoflavones, phenolic acids and antioxidant activity of whole soybean flour. LWT 2020, 125, 109264. [Google Scholar] [CrossRef]
- Yiannikouris, A.; Apajalahti, J.; Siikanen, O.; Dillon, G.P.; Moran, C.A. Saccharomyces cerevisiae Cell Wall-Based Adsorbent Reduces Aflatoxin B1 Absorption in Rats. Toxins 2021, 13, 209. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; Luo, J.; Chen, T.; Xi, Q.; Sun, J.; Wei, L.; Zhang, Y. Synergism of fermented feed and ginseng polysaccharide on growth performance, intestinal development, and immunity of Xuefeng black-bone chickens. BMC Vet. Res. 2024, 20, 13. [Google Scholar] [CrossRef]
- Wang, G.; Meng, Z.; Chen, L.; Jiang, J.; Feng, Y.; Zhang, B. Effects of kelp residues fermented with probiotics on the culture of sea cucumber, Apostichopus japonicus. Aquac. Res. 2020, 51, 1133–1142. [Google Scholar] [CrossRef]
- Olukomaiya, O.O.; Pan, L.; Zhang, D.; Mereddy, R.; Sultanbawa, Y.; Li, X. Performance and ileal amino acid digestibility in broilers fed diets containing solid-state fermented and enzyme-supplemented canola meals. Anim. Feed Sci. Technol. 2021, 275, 114876. [Google Scholar] [CrossRef]
- Adisa, A.M.; Badejo, A.A.; Ifesan, B.O.T.; Enujiugha, V.N. Phenotypic and molecular differentiation of lactic acid bacteria in fonio millet ogi fermentation and their potential as starter cultures. Food Humanit. 2024, 2, 100230. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, M.; Meng, W.; Wen, W.; Zhang, P.; Fan, B.; Wang, F.; Li, S. Study on the quality of soybean proteins fermented by Bacillus subtilis BSNK-5: Insights into nutritional, functional, safety, and flavor properties. Food Chem. 2024, 443, 138523. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, L.; Liu, Z.; Zhang, H.; Yang, Q.; Zhang, X.; Yao, L.; Li, P.; Chen, X.; Dai, J. Improved umami taste of the enzymatic hydrolysate of soybean protein isolate by Corynebacterium glutamicum P-45 fermentation. Food Biosci. 2024, 58, 103565. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, Y.; Cheng, Q.; Xv, J.; Hou, Y.; Wu, X.; Du, E.; Ding, B. Partial Substitution of Fermented Soybean Meal for Soybean Meal Influences the Carcass Traits and Meat Quality of Broiler Chickens. Animals 2020, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Horng, Y.B.; Chen, W.J.; Hua, K.F.; Dybus, A.; Yu, Y.H. Effect of Fermented Products Produced by Bacillus licheniformis on the Growth Performance and Cecal Microbial Community of Broilers under Coccidial Challenge. Animals 2021, 11, 1245. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, K.; Zhao, X.; Liu, S.; Wang, L.; Yang, X.; Jiang, Z. Fermented Feed Modulates Meat Quality and Promotes the Growth of Longissimus Thoracis of Late-Finishing Pigs. Animals 2020, 10, 1682. [Google Scholar] [CrossRef]
- Lu, J.; Han, Q.; Wang, S.; Wang, Z.; Li, X.; Hu, J.; Yang, G.; Wang, L.; Shi, X. Effect of fermented corn-soybean meal on carcass and meat quality of grower-finisher pigs. J. Anim. Physiol. Anim. Nutr. (Berl.) 2021, 105, 693–698. [Google Scholar] [CrossRef]
- Zhu, J.J.; Gao, M.X.; Song, X.J.; Zhao, L.; Li, Y.W.; Hao, Z.H. Changes in bacterial diversity and composition in the faeces and colon of weaned piglets after feeding fermented soybean meal. J. Med. Microbiol. 2018, 67, 1181–1190. [Google Scholar] [CrossRef]
- Liu, S.; Du, M.; Tu, Y.; You, W.; Chen, W.; Liu, G.; Li, J.; Wang, Y.; Lu, Z.; Wang, T.; et al. Fermented mixed feed alters growth performance, carcass traits, meat quality and muscle fatty acid and amino acid profiles in finishing pigs. Anim. Nutr. 2023, 12, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Neves, N.; De Dea Lindner, J.; Stockhausen, L.; Delziovo, F.R.; Bender, M.; Serzedello, L.; Cipriani, L.A.; Ha, N.; Skoronski, E.; Gisbert, E.; et al. Fermentation of Plant-Based Feeds with Lactobacillus acidophilus Improves the Survival and Intestinal Health of Juvenile Nile Tilapia (Oreochromis niloticus) Reared in a Biofloc System. Animals 2024, 14, 332. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, M.; Song, F.; Zhu, X.; Lu, Q.; Liu, R. Fermentation enhances the amelioration effect of bee pollen on Caco-2 monolayer epithelial barrier dysfunction based on NF-κB-mediated MLCK-MLC signaling pathway. Food Res. Int. 2024, 178, 113938. [Google Scholar] [CrossRef]
- Mirsalami, S.M.; Mirsalami, M. Impact of solid-state fermentation utilizing Saccharomyces boulardii on the chemical composition and bioactive constituents of rice husk. J. Agric. Food Res. 2024, 15, 100957. [Google Scholar] [CrossRef]
- Wang, C.; Shi, C.; Su, W.; Jin, M.; Xu, B.; Hao, L.; Zhang, Y.; Lu, Z.; Wang, F.; Wang, Y.; et al. Dynamics of the Physicochemical Characteristics, Microbiota, and Metabolic Functions of Soybean Meal and Corn Mixed Substrates during Two-Stage Solid-State Fermentation. mSystems 2020, 5, e00501-19. [Google Scholar] [CrossRef]
- Koo, B.; Kim, J.W.; Nyachoti, C.M. Nutrient and energy digestibility, and microbial metabolites in weaned pigs fed diets containing Lactobacillus–fermented wheat. Anim. Feed Sci. Technol. 2018, 241, 27–37. [Google Scholar] [CrossRef]
- Kulprachakarn, K.; Chaipoot, S.; Phongphisutthinant, R.; Paradee, N.; Prommaban, A.; Ounjaijean, S.; Rerkasem, K.; Parklak, W.; Prakit, K.; Saengsitthisak, B.; et al. Antioxidant Potential and Cytotoxic Effect of Isoflavones Extract from Thai Fermented Soybean (Thua-Nao). Molecules 2021, 26, 7432. [Google Scholar] [CrossRef]
- de Oliveira, N.S.; Ha, N.; da Cunha, L.; Cipriani, L.A.; Neto, A.T.; Skoronski, E.; Gisbert, E.; Perez Fabregat, T.E.H. Fermentation of Soybean Meal with Lactobacillus acidophilus Allows Greater Inclusion of Vegetable Protein in the Diet and Can Reduce Vibrionacea in the Intestine of the South American Catfish (Rhamdia quelen). Animals 2022, 12, 690. [Google Scholar] [CrossRef]
- Wang, W.; Tan, Z.; Gu, L.; Ma, H.; Wang, Z.; Wang, L.; Wu, G.; Qin, G.; Wang, Y.; Pang, H. Dynamics Changes of Microorganisms Community and Fermentation Quality in Soybean Meal Prepared with Lactic Acid Bacteria and Artemisia argyi through Fermentation and Aerobic Exposure Processes. Foods 2022, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zeng, N.; Jiang, S.; Wang, X.; Yan, H.; Gao, C. Dietary replacement of soybean meal by fermented feedstuffs for aged laying hens: Effects on laying performance, egg quality, nutrient digestibility, intestinal health, follicle development, and biological parameters in a long-term feeding period. Poult. Sci. 2023, 102, 102478. [Google Scholar] [CrossRef]
- Sun, J.; Li, M.; Tang, Z.; Zhang, X.; Chen, J.; Sun, Z. Effects of Rhodotorula mucilaginosa fermentation product on the laying performance, egg quality, jejunal mucosal morphology and intestinal microbiota of hens. J. Appl. Microbiol. 2020, 128, 54–64. [Google Scholar] [CrossRef]
- Tokach, M.D.; Menegat, M.B.; Gourley, K.M.; Goodband, R.D. Review: Nutrient requirements of the modern high-producing lactating sow, with an emphasis on amino acid requirements. Animal 2019, 13, 2967–2977. [Google Scholar] [CrossRef]
- Wang, C.; Lin, C.; Su, W.; Zhang, Y.; Wang, F.; Wang, Y.; Shi, C.; Lu, Z. Effects of supplementing sow diets with fermented corn and soybean meal mixed feed during lactation on the performance of sows and progeny. J. Anim. Sci. 2018, 96, 206–214. [Google Scholar] [CrossRef]
- Grela, E.R.; Czech, A.; Kiesz, M.; Wlazło, Ł.; Nowakowicz-Dębek, B. A fermented rapeseed meal additive: Effects on production performance, nutrient digestibility, colostrum immunoglobulin content and microbial flora in sows. Anim. Nutr. 2019, 5, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Qiang, X.; Xia, T.; Geng, B.; Zhao, M.; Li, X.; Zheng, Y.; Wang, M. Bioactive Components of Lycium barbarum and Deep-Processing Fermentation Products. Molecules 2023, 28, 8044. [Google Scholar] [CrossRef]
- Krueger, L.A.; Koester, L.R.; Jones, D.F.; Spangler, D.A. Carbon dioxide equivalent emissions from corn silage fermentation. Front. Microbiol. 2022, 13, 1092315. [Google Scholar] [CrossRef]
- Delahoy, M.J.; Wodnik, B.; McAliley, L.; Penakalapati, G.; Swarthout, J.; Freeman, M.C.; Levy, K. Pathogens transmitted in animal feces in low- and middle-income countries. Int. J. Hyg. Environ. Health 2018, 221, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Mollenkopf, D.F.; Mathys, D.A.; Feicht, S.M.; Stull, J.W.; Bowman, A.S.; Daniels, J.B.; Wittum, T.E. Maintenance of Carbapenemase-Producing Enterobacteriaceae in a Farrow-to-Finish Swine Production System. Foodborne Pathog. Dis. 2018, 15, 372–376. [Google Scholar] [CrossRef]
- Hăbeanu, M.; Lefter, N.A.; Gheorghe, A.; Untea, A.; Ropotă, M.; Grigore, D.M.; Varzaru, I.; Toma, S.M. Evaluation of Performance, Nitrogen Metabolism and Tissue Composition in Barrows Fed an n-3 PUFA-Rich Diet. Animals 2019, 9, 234. [Google Scholar] [CrossRef]
- Liao, S.; Liao, L.; Huang, P.; Wang, Y.; Zhu, S.; Wang, X.; Lv, T.; Li, Y.; Fan, Z.; Liu, T.; et al. Effects of Different Levels of Garlic Straw Powder on Growth Performance, Meat Quality, Antioxidant and Intestinal Mucosal Morphology of Yellow-Feathered Broilers. Front. Physiol. 2022, 13, 902995. [Google Scholar] [CrossRef]
- Custódio, L.; Prados, L.F.; Oliveira, I.M.; Resende, F.D.; Pettigrew, J.E.; Yiannikouris, A.; Siqueira, G.R. Do mycotoxin contaminated diets and yeast cell wall adsorbent affect meat quality of Nellore bulls finished in feedlot?-A short communication. Meat Sci. 2019, 158, 107865. [Google Scholar] [CrossRef]
- Lobionda, S.; Sittipo, P.; Kwon, H.Y.; Lee, Y.K. The Role of Gut Microbiota in Intestinal Inflammation with Respect to Diet and Extrinsic Stressors. Microorganisms 2019, 7, 271. [Google Scholar] [CrossRef]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef]
- Patnode, M.L.; Beller, Z.W.; Han, N.D.; Cheng, J.; Peters, S.L.; Terrapon, N.; Henrissat, B.; Gall, S.L.; Saulnier, L.; Hayashi, D.K. Interspecies Competition Impacts Targeted Manipulation of Human Gut Bacteria by Fiber-Derived Glycans. Cell 2019, 179, 59–73.e13. [Google Scholar] [CrossRef]
- Liping, Z.; Feng, Z.; Xiaoying, D.; Guojun, W.; Lam, Y.Y.; Xuejiao, W.; Huaqing, F.; Xinhe, X.; Chunhua, L.; Jilin, M. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar]
- Leprun, P.M.B.; Clarke, G. The gut microbiome and pharmacology: A prescription for therapeutic targeting of the gut-brain axis. Curr. Opin. Pharmacol. 2019, 49, 17–23. [Google Scholar] [CrossRef]
- Xu, F.; Wu, H.; Xie, J.; Zeng, T.; Hao, L.; Xu, W.; Lu, L. The Effects of Fermented Feed on the Growth Performance, Antioxidant Activity, Immune Function, Intestinal Digestive Enzyme Activity, Morphology, and Microflora of Yellow-Feather Chickens. Animals 2023, 13, 3545. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Liu, Z.; Qian, C.; Wu, J.; Sultana, N.; Zhong, X. Potential effect of the microbial fermented feed utilization on physicochemical traits, antioxidant enzyme and trace mineral analysis in rabbit meat. J. Anim. Physiol. Anim. Nutr. (Berl.) 2020, 104, 767–775. [Google Scholar] [CrossRef]
- Zeng, X.; Zheng, Y.; He, Y.; Zhang, J.; Peng, W.; Su, W. Microbial Metabolism of Naringin and the Impact on Antioxidant Capacity. Nutrients 2022, 14, 3765. [Google Scholar] [CrossRef]
- Lee, S.; Ryu, C.H.; Back, Y.C.; Lee, S.D.; Kim, H. Effect of Fermented Concentrate on Ruminal Fermentation, Ruminal and Fecal Microbiome, and Growth Performance of Beef Cattle. Animals 2023, 13, 3622. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tao, L.; Zhang, R.; Yang, G. Effects of fermented feed on growth performance, nutrient metabolism and cecal microflora of broilers. Anim. Biosci. 2022, 35, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; Samak, D.H.; Noreldin, A.E.; El-Naggar, K.; Abdo, M. Probiotics and plant-derived compounds as eco-friendly agents to inhibit microbial toxins in poultry feed: A comprehensive review. Environ. Sci. Pollut. Res. Int. 2018, 25, 31971–31986. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, H.; You, Y.; Zhong, G.; Ruan, Z.; Liao, J.; Zhang, H.; Pan, J.; Tang, Z.; Hu, L. Multi-omics reveals the protective effects of curcumin against AFB1-induced oxidative stress and inflammatory damage in duckling intestines. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2024, 276, 109815. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Ding, J.; Zhang, Y.; Hou, C.; Shen, W.; Wu, X.; Zhu, J. Effects of hypoxia stress on oxidative stress, apoptosis and microorganisms in the intestine of large yellow croaker (Larimichthys crocea). Aquaculture 2024, 581, 740444. [Google Scholar] [CrossRef]
- Wang, H.; Liu, P.P.; Wei, Z.; Wang, X.W. PcEiger links the Imd/Relish pathway to ROS production in the intestine of the red swamp crayfish. EMBO Rep. 2023, 24, e55903. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Rommelaere, S.; Kondo, S.; Lemaitre, B. Renal Purge of Hemolymphatic Lipids Prevents the Accumulation of ROS-Induced Inflammatory Oxidized Lipids and Protects Drosophila from Tissue Damage. Immunity 2020, 52, 374–387.e376. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, J.; Wang, P.; Liang, X.; Li, J.; Wu, C.; Fang, H.; Ding, S.; Shao, S.; Shi, K. Transcriptomic and genetic approaches reveal that low-light-induced disease susceptibility is related to cellular oxidative stress in tomato. Hortic. Res. 2023, 10, uhad173. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, C.; Zeng, J.; Yun, Z.; Liu, Y.; Qu, H.; Jiang, Y.; Duan, X.; Xia, R. MicroRNA528, a hub regulator modulating ROS homeostasis via targeting of a diverse set of genes encoding copper-containing proteins in monocots. New Phytol. 2020, 225, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, D.; Wang, X.; Jiang, Y.; Sun, Q.; Ling, W.; An, X.; Ji, C.; Li, S.; Qi, Y.; et al. Spermidine improves the antioxidant capacity and morphology of intestinal tissues and regulates intestinal microorganisms in Sichuan white geese. Front. Microbiol. 2023, 14, 1292984. [Google Scholar] [CrossRef]
- Liu, J.X.; Zhu, K.C.; Guo, H.Y.; Liu, B.S.; Zhang, N.; Zhang, D.C. Effects of cysteine addition to low-fishmeal diets on the growth, anti-oxidative stress, intestine immunity, and Streptococcus agalactiae resistance in juvenile golden pompano (Trachinotus ovatus). Front. Immunol. 2022, 13, 1066936. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Chen, M.; Guo, X.; Ding, Z. The antioxidant strain Lactiplantibacillus plantarum AS21 and Clostridium butyricum ameliorate DSS-induced colitis in mice by remodeling the assembly of intestinal microbiota and improving gut functions. Food Funct. 2024, 5, 2022–2037. [Google Scholar] [CrossRef]
- Han, F.; Fan, H.; Yao, M.; Yang, S.; Han, J. Oral administration of yeast β-glucan ameliorates inflammation and intestinal barrier in dextran sodium sulfate-induced acute colitis. J. Funct. Foods 2017, 35, 115–126. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Z.; Zhang, S.; Page, G.; Jaworski, N.W. The role of lactose in weanling pig nutrition: A literature and meta-analysis review. J. Anim. Sci. Biotechnol. 2021, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhao, S.; Jiang, L.; Lu, L.; Yang, Q.; Yu, Q. Lactobacillus reuteri Stimulates Intestinal Epithelial Proliferation and Induces Differentiation into Goblet Cells in Young Chickens. J. Agric. Food Chem. 2019, 67, 13758–13766. [Google Scholar] [CrossRef]
- Chuang, W.Y.; Lin, L.J.; Hsieh, Y.C.; Chang, S.C.; Lee, T.T. Effects of Saccharomyces cerevisiae and phytase co-fermentation of wheat bran on growth, antioxidation, immunity and intestinal morphology in broilers. Anim. Biosci. 2021, 34, 1157–1168. [Google Scholar] [CrossRef]
- Jacquier, V.; Nelson, A.; Jlali, M.; Rhayat, L.; Brinch, K.S.; Devillard, E. Bacillus subtilis 29784 induces a shift in broiler gut microbiome toward butyrate-producing bacteria and improves intestinal histomorphology and animal performance. Poult. Sci. 2019, 98, 2548–2554. [Google Scholar] [CrossRef]
- Sugiharto, S.; Yudiarti, T.; Isroli, I.; Widiastuti, E.; Wahyuni, H.I.; Sartono, T.A. Growth performance, haematological responses, intestinal microbiology and carcass traits of broiler chickens fed finisher diets containing two-stage fermented banana peel meal. Trop. Anim. Health Prod. 2020, 52, 1425–1433. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, J.; Wang, Y.; Lv, J.; Li, J.; Guo, L.; Min, Y. Fermented Corn-Soybean Meal Mixed Feed Modulates Intestinal Morphology, Barrier Functions and Cecal Microbiota in Laying Hens. Animals 2021, 11, 3059. [Google Scholar] [CrossRef]
- Wu, P.; Golly, M.K.; Guo, Y.; Ma, H.; He, R.; Luo, X.; Luo, S.; Zhang, C.; Zhang, L.; Zhu, J. Effect of partial replacement of soybean meal with high-temperature fermented soybean meal in antibiotic-growth-promoter-free diets on growth performance, organ weights, serum indexes, intestinal flora and histomorphology of broiler chickens. Anim. Feed Sci. Technol. 2020, 269, 114616. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Z.Y.; Yang, H.M.; Xu, L.; Wan, X.L. Effects of cottonseed meal on growth performance, small intestinal morphology, digestive enzyme activities, and serum biochemical parameters of geese. Poult. Sci. 2019, 98, 2066–2071. [Google Scholar] [CrossRef]
- Won, S.; Hamidoghli, A.; Choi, W.; Park, Y.; Jang, W.J.; Kong, I.S.; Bai, S.C. Effects of Bacillus subtilis WB60 and Lactococcus lactis on Growth, Immune Responses, Histology and Gene Expression in Nile tilapia, Oreochromis niloticus. Microorganisms 2020, 8, 67. [Google Scholar] [CrossRef]
- Kuebutornye, F.K.A.; Tang, J.; Cai, J.; Yu, H.; Wang, Z.; Abarike, E.D.; Lu, Y.; Li, Y.; Afriyie, G. In vivo assessment of the probiotic potentials of three host-associated Bacillus species on growth performance, health status and disease resistance of Oreochromis niloticus against Streptococcus agalactiae. Aquaculture 2020, 527, 735440. [Google Scholar] [CrossRef]
- Chen, S.; Mei, H.; Xu, L.; Zhan, L.; Yang, Y.; Zhao, D.; Bao, G.; Li, X.; Cao, Z. Impact of fermented feed of soybean hulls and rapeseed cake on immunity, antioxidant capacity, and gut microbiota in Chahua chicken. Poult. Sci. 2024, 103, 103451. [Google Scholar] [CrossRef]
- Yaman, M.A.; Yunita, T.; Daud, M.; Jeksi, S. Effect of wet fermented diet containing a combination of maggot flour (Hermetia ilucen) and active digestive enzymes on growth and protein retention of hybrid chickens in the early phase of growth. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Banda Aceh, Indonesia, 1 May 2023; p. 012086. [Google Scholar]
- Koo, B.; Bustamante-García, D.; Nyachoti, C.M. Energy content and nutrient digestibility of diets containing Lactobacillus-fermented barley or wheat fed to weaned pigs. J. Anim. Sci. 2018, 96, 4802–4811. [Google Scholar] [CrossRef]
- Zhang, A.R.; Wei, M.; Yan, L.; Zhou, G.L.; Li, Y.; Wang, H.M.; Yang, Y.Y.; Yin, W.; Guo, J.Q.; Cai, X.H.; et al. Effects of feeding solid-state fermented wheat bran on growth performance and nutrient digestibility in broiler chickens. Poult. Sci. 2022, 101, 101402. [Google Scholar] [CrossRef]
- Kongphitee, K.; Sommart, K.; Phonbumrung, T.; Gunha, T.; Suzuki, T. Feed intake, digestibility and energy partitioning in beef cattle fed diets with cassava pulp instead of rice straw. Asian-Australas. J. Anim. Sci. 2018, 31, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, C.; Du, M.; Wang, Y.; Zhang, G.; Lee, Y. Effects of dietary supplementation with different fermented feeds on performance, nutrient digestibility, and serum biochemical indexes of fattening lambs. Anim. Biosci. 2021, 34, 633–641. [Google Scholar] [CrossRef]
- Cao, S.; Hou, L.; Sun, L.; Gao, J.; Gao, K.; Yang, X.; Jiang, Z.; Wang, L. Intestinal morphology and immune profiles are altered in piglets by early-weaning. Int. Immunopharmacol. 2022, 105, 108520. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Wang, M.; Xia, Z.; Yu, B.; He, J.; Yu, J.; Mao, X.; Huang, Z.; Luo, Y.; Luo, J.; et al. Fermented Diet Liquid Feeding Improves Growth Performance and Intestinal Function of Pigs. Animals 2021, 11, 1452. [Google Scholar] [CrossRef]
- Wang, H.; Kim, I.H. Evaluation of Dietary Probiotic (Lactobacillus plantarum BG0001) Supplementation on the Growth Performance, Nutrient Digestibility, Blood Profile, Fecal Gas Emission, and Fecal Microbiota in Weaning Pigs. Animals 2021, 11, 2232. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Hao, X.; Duan, Y.; Meng, Z.; An, X.; Qi, J. Dietary fermented soybean meal replacement alleviates diarrhea in weaned piglets challenged with enterotoxigenic Escherichia coli K88 by modulating inflammatory cytokine levels and cecal microbiota composition. BMC Vet. Res. 2020, 16, 245. [Google Scholar] [CrossRef]
- Guo, L.; Lv, J.; Liu, Y.; Ma, H.; Chen, B.; Hao, K.; Feng, J.; Min, Y. Effects of Different Fermented Feeds on Production Performance, Cecal Microorganisms, and Intestinal Immunity of Laying Hens. Animals 2021, 11, 2799. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus plantarum Promotes Intestinal Barrier Function by Strengthening the Epithelium and Modulating Gut Microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, P.; Kong, Q.; Deng, Y.; Zhang, W.; Xu, G.; Tang, H. Effects of Co-Fermented Feed Using Lactobacillus acidophilus, Limosilactobacillus reuteri and Lactiplantibacillus plantarum on Growth, Antioxidant Capacity, Fatty Acids and Gut Microbiota of Largemouth Bass (Micropterus salmoides). Fishes 2023, 8, 433. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, R.; Li, G.; Zeng, T.; Chen, L.; Xu, W.; Gu, T.; Tao, Z.; Du, X.; Lu, L. Microbial fermented feed affects flavor amino acids and yolk trimethylamine of duck eggs via cecal microbiota-yolk metabolites crosstalk. Food Chem. 2024, 430, 137008. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, X.; Zhang, K. Effects of Microbial Fermented Feed on Serum Biochemical Profile, Carcass Traits, Meat Amino Acid and Fatty Acid Profile, and Gut Microbiome Composition of Finishing Pigs. Front. Vet. Sci. 2021, 8, 744630. [Google Scholar] [CrossRef]
- Cook, L.; Rees, W.D.; Wong, M.Q.; Peters, H.; Levings, M.K.; Steiner, T.S. Fecal Microbiota Transplantation for Recurrent Clostridioides difficile Infection Enhances Adaptive Immunity to C difficile Toxin B. Gastroenterology 2021, 160, 2155–2158.e2154. [Google Scholar] [CrossRef]
- Nzabarushimana, E.; Tang, H. Functional profile of host microbiome indicates Clostridioides difficile infection. Gut Microbes 2022, 14, 2135963. [Google Scholar] [CrossRef]
- Hao, X.; Ding, N.; Zhang, Y.; Yang, Y.; Zhao, Y.; Zhao, J.; Li, Y.; Li, Z. Benign regulation of the gut microbiota: The possible mechanism through which the beneficial effects of manual acupuncture on cognitive ability and intestinal mucosal barrier function occur in APP/PS1 mice. Front. Neurosci. 2022, 16, 960026. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.W.; Li, R.Q.; An, J.X.; Xie, T.Q.; Han, Z.Y.; Xu, R.; Fang, Y.; Zhang, X.Z. Prebiotics-Encapsulated Probiotic Spores Regulate Gut Microbiota and Suppress Colon Cancer. Adv. Mater. 2020, 32, e2004529. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, Z.; Zhang, Z.; Liu, T.; Fan, Y.; Liu, T.; Peng, N. Bacillus coagulans in Combination with Chitooligosaccharides Regulates Gut Microbiota and Ameliorates the DSS-Induced Colitis in Mice. Microbiol. Spectr. 2022, 10, e0064122. [Google Scholar] [CrossRef]
- Oh, N.S.; Lee, J.Y.; Kim, Y.T.; Kim, S.H.; Lee, J.H. Cancer-protective effect of a synbiotic combination between Lactobacillus gasseri 505 and a Cudrania tricuspidata leaf extract on colitis-associated colorectal cancer. Gut Microbes 2020, 12, 1785803. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, K.; Guo, W.; Zhang, C.; Chen, H.; Xu, T.; Lu, Y.; Wu, Q.; Li, Y.; Chen, Y. Aspergillus niger fermented Tartary buckwheat ameliorates obesity and gut microbiota dysbiosis through the NLRP3/Caspase-1 signaling pathway in high-fat diet mice. J. Funct. Foods 2022, 95, 105171. [Google Scholar] [CrossRef]
- O’ Meara, F.M.; Gardiner, G.E.; O’Doherty, J.V.; Clarke, D.; Cummins, W.; Lawlor, P.G. Effect of wet/dry, fresh liquid, fermented whole diet liquid, and fermented cereal liquid feeding on feed microbial quality and growth in grow-finisher pigs. J. Anim. Sci. 2020, 98, skaa166. [Google Scholar] [CrossRef]
- Zamojska, D.; Nowak, A.; Nowak, I.; Macierzyńska-Piotrowska, E. Probiotics and Postbiotics as Substitutes of Antibiotics in Farm Animals: A Review. Animals 2021, 11, 3431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, J.; Jin, X.; Liu, C.; Fan, C.; Guo, L.; Liang, Y.; Zheng, J.; Peng, N. Developmental, Dietary, and Geographical Impacts on Gut Microbiota of Red Swamp Crayfish (Procambarus clarkii). Microorganisms 2020, 8, 1376. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Mottaghitalab, M.; Hashemi, M. Effects of microbial fermented sesame meal and enzyme supplementation on the intestinal morphology, microbiota, pH, tibia bone and blood parameters of broiler chicks. Ital. J. Anim. Sci. 2020, 19, 457–467. [Google Scholar] [CrossRef]
- Al-Khalaifah, H.S.; Shahin, S.E.; Omar, A.E.; Mohammed, H.A.; Mahmoud, H.I.; Ibrahim, D. Effects of graded levels of microbial fermented or enzymatically treated dried brewer’s grains on growth, digestive and nutrient transporter genes expression and cost effectiveness in broiler chickens. BMC Vet. Res. 2020, 16, 424. [Google Scholar] [CrossRef] [PubMed]
- Taranu, I.; Nguyen, T.T.; Pham, K.D.; Gras, M.A.; Pistol, G.C.; Marin, D.E.; Rotar, C.; Habeanu, M.; Ho, P.H.; Le, T.M. Rice and Cassava Distillers Dried Grains in Vietnam: Nutritional Values and Effects of Their Dietary Inclusion on Blood Chemical Parameters and Immune Responses of Growing Pigs. Waste Biomass Valorization 2019, 10, 3373–3382. [Google Scholar] [CrossRef]
- Zhu, F.; Zhang, B.; Li, J.; Zhu, L. Effects of fermented feed on growth performance, immune response, and antioxidant capacity in laying hen chicks and the underlying molecular mechanism involving nuclear factor-κB. Poult. Sci. 2020, 99, 2573–2580. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jiang, Z.; Qiu, Y.; Wang, L. 447 Effects of probiotic solid-state fermented complete feed on growth performance and pork quality of finishing pigs. J. Anim. Sci. 2018, 96, 232. [Google Scholar] [CrossRef]
- Fan, L.; Dou, M.; Wang, X.; Han, Q.; Zhao, B.; Hu, J.; Yang, G.; Shi, X.; Li, X. Fermented corn-soybean meal elevated IGF1 levels in grower-finisher pigs. J. Anim. Sci. 2018, 96, 5144–5151. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, T.; Chu, X.; Tang, S.; Cao, W.; Liang, F.; Fang, Y.; Pan, S.; Xu, X. Fermented blueberry pomace with antioxidant properties improves fecal microbiota community structure and short chain fatty acids production in an in vitro mode. LWT 2020, 125, 109260. [Google Scholar] [CrossRef]
- Wang, X.; Hu, K.; Chen, Y.; Lai, J.; Zhang, M.; Li, J.; Li, Q.; Zhao, N.; Liu, S. Effect of Lactiplantibacillus plantarum fermentation on the physicochemical, antioxidant activity and immunomodulatory ability of polysaccharides from Lvjian okra. Int. J. Biol. Macromol. 2024, 257, 128649. [Google Scholar] [CrossRef]
- Duan, W.; Zhou, L.; Ren, Y.; Liu, F.; Xue, Y.; Wang, F.Z.; Lu, R.; Zhang, X.J.; Shi, J.S.; Xu, Z.H.; et al. Lactic acid fermentation of goji berries (Lycium barbarum) prevents acute alcohol liver injury and modulates gut microbiota and metabolites in mice. Food Funct. 2024, 15, 1612–1626. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Wang, S.; Sun, Y.; Zhang, S.; Wen, Y.; Zou, D.; Zhou, D. Deciphering survival strategies: Oxidative stress and microbial interplay in Eisenia fetida under tetracycline contamination. Sci. Total Environ. 2024, 909, 168647. [Google Scholar] [CrossRef]
- Collart, L.; Jiang, D.; Halsey, K.H. The volatilome reveals microcystin concentration, microbial composition, and oxidative stress in a critical Oregon freshwater lake. mSystems 2023, 8, e0037923. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lin, Y.; Miao, L.; Pan, W.; Ge, X. Ferulic acid alleviates lipopolysaccharide-induced acute liver injury in Megalobrama amblycephala. Aquaculture 2021, 532, 735972. [Google Scholar] [CrossRef]
- Dai, X.; Xing, C.; Cao, H.; Luo, J.; Wang, T.; Liu, P.; Guo, X.; Hu, G.; Zhang, C. Alterations of mitochondrial antioxidant indexes and apoptosis in duck livers caused by Molybdenum or/and cadmium. Chemosphere 2018, 193, 574. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, Q.; Liang, H.; Ren, M.; Ge, X.; Ji, K. Effects of stocking density and dietary phosphorus levels on the growth performance, antioxidant capacity, and nitrogen and phosphorus emissions of juvenile blunt snout bream (Megalobrama amblycephala). Aquac. Nutr. 2021, 27, 581–591. [Google Scholar] [CrossRef]
- Dong, J.; Ping, L.; Xie, Q.; Liu, D.; Zhao, L.; Evivie, S.E.; Wang, Z.; Li, B.; Huo, G. Lactobacillus plantarum KLDS1.0386 with antioxidant capacity ameliorates the lipopolysaccharide-induced acute liver injury in mice by NF-κB and Nrf2 pathway. Food Biosci. 2022, 47, 101589. [Google Scholar] [CrossRef]
- Hao, L.; Su, W.; Zhang, Y.; Wang, C.; Lu, Z. Effects of Supplementing with Fermented Mixed Feed on the Performance and Meat Quality in Finishing Pigs. Anim. Feed Sci. Technol. 2020, 266, 114501. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, R.; Wang, Y.; An, X.; Qi, J. Characterization and antioxidant activity of wheat bran polysaccharides modified by Saccharomyces cerevisiae and Bacillus subtilis fermentation. J. Cereal Sci. 2020, 97, 103157. [Google Scholar] [CrossRef]
- Czech, A.; Sembratowicz, I.; Kiesz, M. The Effects of a Fermented Rapeseed or/and Soybean Meal Additive on Antioxidant Parameters in the Blood and Tissues of Piglets. Animals 2021, 11, 1646. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, C.T.; Betancor, M.B.; Torrecillas, S.; Sprague, M.; Larroquet, L.; Véron, V.; Panserat, S.; Izquierdo, M.S.; Kaushik, S.J.; Fontagné-Dicharry, S. More Than an Antioxidant: Role of Dietary Astaxanthin on Lipid and Glucose Metabolism in the Liver of Rainbow Trout (Oncorhynchus mykiss). Antioxidants 2023, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Jin, M.; Yuan, Y.; Luo, J.-X.; Lu, Y.; Zhou, Q.-C.; Tan, Z.-L. Dietary nucleotide-rich yeast supplementation improves growth, innate immunity and intestinal morphology of Pacific white shrimp (Litopenaeus vannamei). Aquac. Nutr. 2018, 24, 1425–1435. [Google Scholar] [CrossRef]
- Long, S.; He, T.; Kim, S.W.; Shang, Q.; Kiros, T.; Mahfuz, S.U.; Wang, C.; Piao, X. Live Yeast or Live Yeast Combined with Zinc Oxide Enhanced Growth Performance, Antioxidative Capacity, Immunoglobulins and Gut Health in Nursery Pigs. Animals 2021, 11, 1626. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Duan, M.; Zhang, R.; Zeng, T.; Xu, W.; Feng, W.; Jiang, C.; Tian, Y.; Chen, L.; Lu, L. Probiotic Fermented Feed Alleviates Liver Fat Deposition in Shaoxing Ducks via Modulating Gut Microbiota. Front. Microbiol. 2022, 13, 928670. [Google Scholar] [CrossRef]
- Peng, W.; Talpur, M.Z.; Zeng, Y.; Xie, P.; Li, J.; Wang, S.; Wang, L.; Zhu, X.; Gao, P.; Jiang, Q.; et al. Influence of fermented feed additive on gut morphology, immune status, and microbiota in broilers. BMC Vet. Res. 2022, 18, 218. [Google Scholar] [CrossRef]
- Hanum, F.; Atsuta, Y.; Daimon, H. Methane Production Characteristics of an Anaerobic Co-Digestion of Pig Manure and Fermented Liquid Feed. Molecules 2022, 27, 6509. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, R.; Lei, H.; Hang, Y.; Xue, H.; Cai, X.; Lu, Y. Supplementation with Fermented Feedstuff Enhances Orexin Expression and Secretion Associated with Increased Feed Intake and Weight Gain in Weaned Pigs. Animals 2022, 12, 1329. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).